Abstract
Endometriosis is a prevalent gynecological condition associated with pelvic pain and infertility. Despite more than a century of research, the etiology of endometriosis still eludes scientific consensus. This lack of clarity has resulted in suboptimal prevention, diagnosis, and treatment options. Evidence of genetic contributors to endometriosis is interesting but limited; however, significant progress has been made in recent years in identifying an epigenetic role in the pathogenesis of endometriosis through clinical studies, in vitro cell culture experiments, and in vivo animal models. The predominant findings include endometriosis-related differential expression of DNA methyltransferases and demethylases, histone deacetylases, methyltransferases, and demethylases, and regulators of chromatin architecture. There is also an emerging role for miRNAs in controlling epigenetic regulators in the endometrium and endometriosis. Changes in these epigenetic regulators result in differential chromatin organization and DNA methylation, with consequences for gene expression independent of a genetic sequence. Epigenetically altered expression of genes related to steroid hormone production and signaling, immune regulation, and endometrial cell identity and function have all been identified and appear to play into the pathophysiological mechanisms of endometriosis and resulting infertility. This review summarizes and critically discusses early seminal findings, the ever-growing recent evidence of epigenetic contributions to the pathophysiology of endometriosis, and implications for proposed epigenetically targeted therapeutics.
Keywords: epigenetics, endometriosis
Graphical Abstract
Graphical Abstract.
Essential Points.
Endometriosis is a prevalent gynecological condition associated with pelvic pain and infertility. However, the etiology of endometriosis still evades scientific consensus, with no single proposed pathophysiological or molecular mechanism able to explain all cases
Endometriosis-related differential expression of DNA methyltransferases and demethylases, histone deacetylases, methyltransferases, and demethylases, and regulators of chromatin architecture have been studied
Epigenetically altered expression of genes related to steroid hormone production and signaling, immune regulation, and endometrial cell identity and function have been identified and may play into the pathophysiological mechanisms of endometriosis lesion development as well as resulting infertility
Because epigenetic modifications are reversible, there are reasonable possibilities for therapeutics targeted at epigenetic mechanisms that have been supported by the findings of preclinical studies
Endometriosis is classically described as the presence of endometrium-like tissue outside the uterine cavity, but this complex estrogen-dependent, progesterone-resistant inflammatory condition is multifactorial and heterogeneous, making it difficult to simply and comprehensively define (1, 2). Typically, endometriotic lesions are found on pelvic tissues and are likely derived from retrograde menstruation though extra-pelvic lesions are occasionally discovered, potentially arising from misprogrammed stem cells (1). Although the symptoms of endometriosis are variable and do not correlate with disease stage, the most commonly reported clinical manifestations are pelvic pain and infertility (2). Because the typical medical treatment for endometriosis pain is hormonal suppression, which precludes fertility, and surgical removal of lesions has a mixed impact on fertility outcomes, there is need for an efficacious fertility-sparing treatment (1). Retrograde menstruation is common to most if not all reproductive-age women, so it is still an open question why only a subset develop endometriosis (3). Nevertheless, with the estimated 10% of women affected by this condition extrapolating to approximately 190 million women worldwide, understanding and treating endometriosis more successfully is a major priority for women's health.
Though having a family member with endometriosis substantially increases a woman's chances of developing it as well, familial candidate gene studies and genetic association studies have yet to yield clinically meaningful results (1). Numerous genome-wide association studies have searched for single-nucleotide polymorphisms (SNPs) significantly associated with endometriosis in thousands of cases and controls (1). Meta-analyses of multiple studies have identified several loci of interest, including sites near Wnt family member 4, growth-regulating estrogen receptor binding 1, vezatin, lncRNA cyclin-dependent kinase inhibitor 2B antisense RNA 1, inhibitor of DNA binding 4, fibronectin 1, coiled-coil domain containing 170, estrogen receptor 1 (ESR1), spectrin repeat containing nuclear envelope protein 1, and FSH subunit beta (FSHB), many of which play roles in steroid hormone signaling and function (4-6). Despite increasing risk for endometriosis, no mechanistic link between these gene polymorphisms and endometriosis pathophysiology has yet been established. Therefore, although genetics may contribute to the epidemiologically suggested heritability of endometriosis, we and others in the field view epigenetics as an increasingly important consideration in the quest to understand the causes and consequences of endometriosis (7).
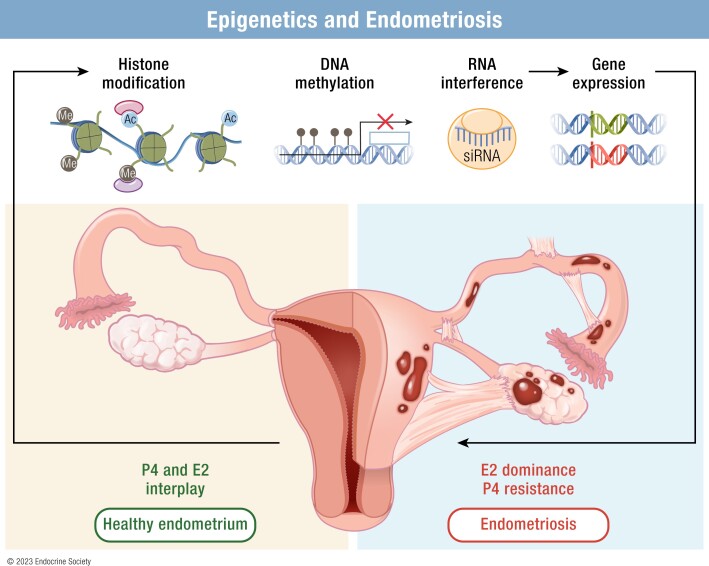
The term “epigenetics” means literally “above genetics” and at its most basic level refers to heritable phenotypes that are not based on DNA sequence (8). The breadth of this definition includes any mechanism that impacts gene expression, limiting its usefulness in a focused examination of a specific disease such as endometriosis. Therefore, to narrow the focus of this review, we have adopted the definition of epigenetics advocated by Deans and Maggert: “the study of phenomena and mechanisms that cause chromosome-bound, heritable changes to gene expression that are not dependent on changes to DNA sequence” (9). Consequently, although many authors use the term “epigenetics” to refer to noncoding RNA regulation of gene expression and transcription factor regulatory networks, we discuss primarily those mechanisms with respect to endometriosis when they are related to “chromosome-bound” mechanisms such as DNA methylation, histone modifications, or other in situ regulators of chromatin organization.
This review provides an update on what is known about epigenetic factor dysregulation in endometriosis with a perspective on gaps in the field, areas of disagreement, and topics requiring further, more rigorous analysis. We will start by covering what is known of endometriosis-related epigenetic regulator disruption before turning to a discussion of the genes and pathways affected by epigenetic mechanisms in endometriosis conditions. We will further analyze the relationship between epigenetics and clinical manifestations of endometriosis as well as the status of epigenetically targeted therapeutics to clarify actionable areas to prioritize further study.
Search Methods
The MEDLINE (PubMed) database from the National Center for Biotechnology Information was used to find peer-reviewed research articles and reviews using the following search terms: endometriosis AND (epigenetics OR chromatin OR methylation OR acetylation OR DNA modification OR histone). These primary search results were supplemented with searches for more specific keywords related to the subject area such as the names of specific molecules of interest as well as with manual searches in reference lists from previous publications. We only included peer-reviewed original research articles in the English language. We excluded articles deemed not within the scope of the topic as well as abstracts and conference proceedings. Previous review articles were consulted for identification of original research but not reviewed directly unless they made a significant unique contribution. Relevant publications through the end of April 2022 were critically evaluated and discussed, with a focus on seminal works and articles from the past 5 years.
Epigenetic Dysregulation in Endometriosis
DNA methylation modifiers
One of the most studied and well-characterized epigenetic processes is DNA methylation. The addition of methyl groups to cytosine nucleotides, particularly in gene promoter regions with concentrated cytosine-guanine repeats (CpG islands), tends to impede transcription factor access and promote condensed chromatin, leading to gene silencing (10). This section summarizes the endometriosis-related changes that occur in the enzymes responsible for DNA methylation and demethylation; the genome-wide and gene-specific findings related to differential DNA methylation are discussed later in this review.
DNA methyltransferases
DNA methyltransferases (DNMTs) are responsible for adding methyl groups to cytosines, with DNMT1 being the primary maintenance methyltransferase and DNMT3A and DNMT3B catalyzing de novo methylation (10). An early foray into analyzing the epigenetics of endometriosis by Wu et al analyzed the expression levels of DNMTs in primary epithelial cells isolated from ectopic lesions, eutopic endometria from women with endometriosis, and control endometria (11). This study found that DNMT1, DNMT3A, and DNMT3B mRNA levels were upregulated in epithelial cells from endometriotic lesions compared with eutopic endometria from normal controls (11). DNMT1 protein upregulation in ectopic lesion samples was confirmed by immunostaining (11). Only DNMT3A was significantly higher in eutopic endometria from women with endometriosis compared with controls, and only DNMT1 was significantly higher in ectopic lesions than eutopic endometria from women with endometriosis (11). The samples were taken from endometria in mixed cycle phases but were in similar proportions in each experimental group, and the expression levels were normalized to proliferating cell nuclear antigen (PCNA) to mitigate differences in cell proliferation levels (11).
Szczepańska et al later supported and expanded the finding that DNMT3A is significantly increased eutopic endometria of endometriosis patients compared with controls by demonstrating this with whole endometrial samples from the mid-secretory phase (12), and Dyson et al showed that primary stromal cells from ovarian endometriotic lesions exhibit higher DNMT3B mRNA and protein than controls in in vitro decidualization conditions (13). These studies did not find significant differences in the other DNMTs (12, 13).
In contrast, van Kaam et al reported DNMT1 and DNMT3B mRNA to be downregulated in ectopic lesions compared with normal control endometria, and the decrease of DNMT1 was also statistically significant in eutopic endometria from endometriosis patients compared with controls (14). In a study analyzing ectopic lesions and paired eutopic endometria from a small cohort of women with endometriosis, Hsiao et al showed a downregulation in DNMT1 mRNA and protein in the lesions compared with the endometria but did not observe significant differences in DNMT3A or DNMT3B (15). Finally, in a study using the largest sample set of any mentioned here (26/group), Wang et al found that mRNA levels of DNMT1, DNMT3A, and DNMT3B were decreased in secretory phase endometriotic lesions and eutopic endometrial samples from women with endometriosis compared with controls but not changed between lesions and eutopic endometria from endometriosis patients (16).
There are several possible reasons for the differences in the findings between these expression profiling studies. One possibility is the cell type of the samples used because some studies used whole endometrial samples, whereas others used isolated epithelial or stromal cells. The studies also varied in which phase of the menstrual cycle the samples were sourced from, and some looked at both mRNA and protein levels, where others only analyzed mRNA. The differences in these sampling factors together with the findings of each study are summarized in Table 1.
Table 1.
Studies analyzing expression of DNA methylation modifiers in human endometriosis: DNMTs and MDB2
| Sample type | Sample size | DNMT1 | DNMT3A | DNMT3B | MBD2 | Reference |
|---|---|---|---|---|---|---|
| Primary epithelial cells from ectopic lesions, eutopic endometria from women with endometriosis, control endometria; mixed but proportionate cycle phases, mostly proliferative | 13 ectopic, 10 eutopic, 8 control | mRNA and protein upregulated in ectopic lesions compared with eutopic and control | mRNA upregulated in ectopic lesions and eutopic compared with control | mRNA upregulated in ectopic lesions compared with control | — | Wu et al (11) |
| Ectopic lesions, eutopic endometria from women with endometriosis, control endometria; proliferative phase | 14 ectopic and eutopic, 20 control | mRNA downregulated in ectopic lesions and eutopic compared with control | — | mRNA downregulated in ectopic lesions compared with control | mRNA downregulated in ectopic lesions and eutopic compared with control | van Kaam et al (14) |
| Eutopic endometria from infertile women with endometriosis, fertile control endometria, endometria from infertile women with tubal occlusion; mid-secretory phase | 18 eutopic, 16 control, 16 infertile with tubal occlusion | No significant change | mRNA upregulated in eutopic from endometriosis | No significant change | — | Szczepańska et al (12) |
| Primary stromal cells from ovarian endometriotic lesions and control endometria treated to induce in vitro decidualization; proliferative phase | 6/group mRNA, 4/group protein | No significant change | No significant change | mRNA and protein upregulated compared with control | — | Dyson et al (13) |
| Ectopic lesions and paired eutopic endometria from women with endometriosis | 6/group | mRNA and protein downregulated in ectopic compared with eutopic | No significant change | No significant change | — | Hsiao et al (15) |
| Ovarian endometriotic lesions, eutopic endometria from women with endometriosis, control endometria; secretory phase | 26/group | mRNA downregulated in ectopic lesions and eutopic compared with control | mRNA downregulated in ectopic lesions and eutopic compared with control | mRNA downregulated in ectopic lesions and eutopic compared with control | mRNA downregulated in ectopic lesions and eutopic compared with control | Wang et al (16) |
Abbreviations: DNMT1, DNA methyltransferase 1; DNMT3A, DNA methyltransferase 3 alpha; DNMT3B, DNA methyltransferase 3B; MBD2, methyl-CpG-binding domain protein 2.
Beyond simple expression profiling, a few studies have looked at potential mechanisms for changes in DNMT expression in endometriosis. For example, Veena et al found SNPs in the DNMT1 and DNMT3B genes to be significantly associated with endometriosis in South Indian women (17). These SNPs could affect the transcription of DNMT1 and DNMT3B and be a plausible explanation for changes in their expression levels. Hsiao et al described a mechanism by which hypoxia in cultured human endometrial stromal cells caused downregulation of DNMT1 mRNA and protein alongside decreasing DNA methylation (15). Rather than transcriptional regulation, the decrease of DNMT1 was shown to be mediated by hypoxia-induced AUF1 binding with miR-148a to DNMT1 mRNA to cause its degradation (15). In a xenograft model of endometriosis in which mixed human immortalized endometriotic epithelial 12Z and stromal 22B cells were transferred to the peritoneal cavities of immunocompromised mice, dual Akt serine/threonine kinase family (AKT) and extracellular signal-regulated protein kinase 1/2 pathway inhibition decreased DNMT1 and DNMT3A expression in the endometrial epithelial cells along with decreasing the number and volume of lesions but did not alter DNMT3B (18). However, it is not clear whether the effects on the endometria were due to direct treatment effects, the reduction in lesion burden, or both. The findings of these studies are just the beginning of serious inquiry into the mechanisms of DNMT dysregulation in endometriosis, but they provide good starting points for further study.
Ten-eleven translocation methylcytosine dioxygenases
Ten-eleven translocation methylcytosine dioxygenases (TETs) play a central role in DNA demethylation by DNA repair at 5-methylcytosine CpG sites. The TET family enzymes TET1, TET2, and TET3 hydroxylate 5-methylcytosine to form 5-hydroxymethylcytosine (5hmC), which can be converted into other intermediates and replaced by base excision repair with an unmethylated cytosine (19). The first study to investigate the potential role of TETs in the female reproductive tract by Roca et al found that TET1, TET2, and TET3 mRNA levels were significantly downregulated in ectopic lesions compared with control endometria (20). TET3 was also significantly lower in ectopic lesions compared with eutopic endometria from women with endometriosis (20). There were no significant differences in comparisons between eutopic endometria from endometriosis patients and control whole-tissue samples, but cultured eutopic endometrial stromal cells from women with endometriosis had significantly less TET1, TET2, and TET3 mRNA than control stromal cells (20). Surprisingly, 5hmC, the product of TET enzymatic activity, was significantly upregulated in ectopic lesion tissues compared with control endometria but decreased in blood samples (20). The findings of this study are somewhat weakened by the small sample size, and confounding factors as controls were older and more frequently from the secretory phase than endometriosis patients.
Later studies by Szczepańska et al and Adamczyk et al that focused on the eutopic endometrium also observed decreases in TET1 mRNA and protein and TET2 mRNA in endometria from endometriosis patients compared with controls, but no significant changes were found in TET3 (21, 22). Interestingly, both studies reported that TET1 mRNA and protein levels were downregulated in mid-secretory endometrial samples from infertile women with endometriosis compared with controls, but proliferative phase levels were not significantly changed (21, 22). Elucidating a potential mechanism for TET1 expression decrease, DNA methylation levels within the TET1 gene were found to be significantly higher in endometriosis patients than controls in secretory phase samples but not in proliferative-stage samples (22). Conversely to TET1, TET2 levels were downregulated in infertile women with endometriosis at the proliferative phase but either not changed or upregulated at the mid-secretory phase depending on the study, demonstrating differential cycle-phase dependency (21, 23). In addition to backing up their mRNA observations with protein analysis, Xiao et al found that increased TET2 levels in the mid-secretory phase corresponded to decreased miR22-5p levels, which they showed in culture directly targets and downregulates TET2, resulting in lower 5hmC levels.
Returning to a focus on ectopic endometriosis lesions, Wu et al observed an increase in TET1 protein and 5hmC levels in glandular epithelial cells of ovarian endometriomas compared with matched eutopic endometrium and normal controls (24). The stromal expression pattern appeared to be opposite, but the staining pattern was not quantified because of histological differences, which could explain why the findings contrasted with Roca et al, who looked at total mRNA levels in lesions (20, 24). Furthermore, TET1 expression negatively correlated with E-cadherin and positively correlated with N-cadherin, suggesting that epithelial-mesenchymal transition (EMT) may occur at the same time and cell type that TET1 expression increases (24). In the Ishikawa endometrial epithelial cancer cell line, hypoxia increased TET1 protein levels in concert with HIF-1 alpha and vimentin increases and an E-cadherin decrease. Moreover, TET1 knockdown mitigated the EMT-producing effects of hypoxia (24). Therefore, hypoxia may be a driver of both DNMT1 downregulation and TET1 upregulation in endometriosis conditions. The findings of each study focusing on TET expression in endometriosis are summarized in Table 2.
Table 2.
Studies analyzing expression of DNA methylation modifiers in human endometriosis: TETs
| Sample type | Sample size | TET1 | TET2 | TET3 | References |
|---|---|---|---|---|---|
| Ectopic lesions, eutopic endometria from women with endometriosis, control endometria; mixed cycle phases | 6 ectopic, 5 eutopic, 7 control | mRNA downregulated in ectopic lesions compared with control; downregulated in isolated stromal cells from eutopic compared with control but not total tissue | mRNA downregulated in ectopic lesions compared with control; downregulated in isolated stromal cells from eutopic compared with control but not total tissue | mRNA downregulated in ectopic lesions compared with control and eutopic; downregulated in isolated stromal cells from eutopic compared with control but not total tissue | Roca et al (20) |
| Eutopic endometria from women with endometriosis, control endometria; mid-secretory and proliferative phases (separately) | 15 eutopic, 9 control (mid-luteal); 23 eutopic, 9 control (mid-follicular) | mRNA and protein downregulated in eutopic from endometriosis at mid-secretory but not proliferative phase | mRNA downregulated in eutopic endometrium from endometriosis patients at the proliferative phase but not mid-secretory phase | No significant change | Szczepańska et al (21) |
| Ovarian endometriotic lesions, eutopic endometria from women with endometriosis, control endometria; proliferative phase | 15/group | Protein upregulated in glandular epithelial cells of ectopic lesions compared with eutopic and control | — | — | Wu et al (24) |
| Eutopic endometria from infertile women with endometriosis and infertile women without endometriosis; secretory phase | 11-13/group mRNA, 5-6/group protein | — | mRNA and protein upregulated in eutopic endometrium from endometriosis patients | — | Xiao et al (23) |
| Eutopic endometria from infertile women with endometriosis, infertile women without endometriosis, and fertile controls; proliferative and secretory phases (separately) | 44 infertile with endometriosis, 38 infertile without endometriosis, 39 fertile control | mRNA and protein downregulated in eutopic endometrial samples from infertile women with endometriosis compared with fertile controls and women with idiopathic infertility | — | — | Adamczyk et al (22) |
Abbreviations: TET1, ten-eleven translocation methylcytosine dioxygenase 1; TET2, tet methylcytosine dioxygenase 2, TET3, tet methylcytosine dioxygenase 3.
Methyl-CpG-Binding domain proteins
Methyl-CpG-binding domain proteins (MBDs) are not actually methylation modifiers, strictly speaking, but rather transcriptional repressors that can interact with methylated DNA to recruit other repressors and silence genes as well as link to chromatin remodelers (25, 26). Study of these proteins in the context of endometriosis is limited, but 2 studies that looked at DNMTs have noted differences in MBD expression levels as well (Table 1). First, van Kaam et al reported decreased mRNA levels of methyl-CpG-binding protein 2 in eutopic endometria from endometriosis cases compared with controls, decreased MBD1 in ectopic lesions compared with eutopic endometria from endometriosis cases and controls, and decreased MBD2 in both ectopic and eutopic samples from endometriosis cases compared with controls (14).
In support, Wang et al confirmed with a larger sample set that MBD2 expression levels were decreased in endometriotic lesions and eutopic endometrium samples from women with endometriosis but not changed between eutopic endometria from endometriosis patients and lesions at the mRNA level (16). Though the biological function of MBDs is still being explored, these initial data prompt the speculation that reduced MBD2 in endometriosis conditions could prevent or minimize transcriptional repression of methylated genes that would have been targeted by it.
Histone modifiers
Histones are proteins around which DNA is wound to make up the nucleosome, the basic unit of chromatin. Each nucleosome core consists of a histone octamer made up of 2 units of the histones H2A, H2B, H3, and H4 (27). Posttranslational modifications of the histone proteins with types of marks such as acetylation, methylation, ubiquitination, and many others can change chromatin structure and alter its function in the processes of transcription, replication, and repair (27). The enzymes that make these modifications include histone deacetylases (HDACs), histone acetyltransferases (HATs), histone methyltransferases (HMTs), and histone demethylases (28). In this section, we will review what is currently known about the dysregulation of these enzymes and their marks in endometriosis conditions.
Histone deacetylases and acetyltransferases
Most studies that have analyzed histone acetylation modifiers in endometriosis have focused on HDACs. There are 4 classes of HDAC enzymes in humans: class I (HDAC1, HDAC2, HDAC3, HDAC8), class II (HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, HDAC10), class III Sir2-like proteins (SIRT1, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, SIRT7), and class IV (HDAC11) (29). Unlike histone methylation modifiers, the target specificity for most histone acetylation modifiers has not been clearly defined, making it difficult to match HDAC and HAT protein levels with actual histone acetylation levels in a specific manner (29). Nevertheless, several studies have observed perturbations in histone modifier levels and tied them to physiological dysfunctions in endometriosis.
It was shown in several studies more than a decade ago that HDAC inhibitors led to decreased proliferation and reversal of endometriosis-related gene expression in cultured endometrial and endometriotic cells (30-32). An early expression profiling study of histone acetylation modifiers in endometriosis by Xiaomeng et al reported that mRNA levels of HDAC1 decreased in ectopic sites, HDAC2 was increased in eutopic endometria from women with endometriosis, and SIRT1 was increased in ectopic lesions compared with eutopic endometria from endometriosis patients (33). HDAC4, HDAC5, and HDAC7 levels did not significantly change in any group (33). This study also reported upregulation in ectopic lesions of the HAT p300/CREB-binding protein-associated factor, which was also shown in eutopic endometria of women with endometriosis by another group (33, 34). In this same study, global histone H4 acetylation was significantly downregulated in both lesions and endometriosis-affected eutopic endometria; global H3 acetylation was not significantly changed (33). Global acetylation levels did not match the general pattern of HDAC and HAT levels, but it is difficult to match histone acetylation modifiers to acetylation levels given the uncertainties of their target specificities. To complicate things more, when Monteiro et al analyzed global histone acetylation levels, they found that H3 acetylation was significantly decreased in endometriotic lesions compared with control endometria but that H4 acetylation was not significantly changed, which highlights the variation among patient samples (35). Narrowing the analysis to the active chromatin-associated H3K9 and H4K16 acetylation levels, both were significantly decreased in ectopic and eutopic endometria from endometriosis patients, indicative of generalized repressed heterochromatin (35).
Regarding HDAC levels in endometriosis, another study reported that mRNA and protein levels of HDAC1 and HDAC2 were upregulated in endometriotic stromal cells compared with control cells (36). Estrogen and progesterone treatment modulated HDAC1 in endometrial epithelial cells and HDAC2 in endometrial stromal cells but did not change their expression in endometriotic cells (36). In this study, HDAC1 and HDAC2 protein levels varied based on lesion location and trended up in some endometriotic lesions compared with controls, but there were no statistically significant differences between groups (36). A third expression profiling study reported HDAC1 protein levels to be significantly higher in the proliferative phase stroma and epithelium of ovarian and peritoneal endometriotic lesions compared with controls, but no significant differences were observed in HDAC2 and HDAC3 levels (37). Given the varying results reported by different groups, the need for more focused and detailed mechanistic study is clear, and several groups have done just that.
Taking a closer look at the role of HDAC1 in endometriosis, Zhang and colleagues found that the lncRNA HOX transcript antisense RNA (HOTAIR) was overexpressed in endometriotic lesions in concert with decreased of miR-761 compared with control and eutopic endometrial tissue from patients with endometriosis (38). In cultured primary endometrial stromal cells, they showed that overexpression of HOTAIR in exosomes led to increased HDAC1 expression, whereas a miR-761 mimic repressed HDAC1 (38). Furthermore, exosomal HOTAIR mediated increased proliferation, migration, and invasion of endometrial stromal cells, which was reversed by experimental upregulation miR-761 or by HDAC1 silencing (38). Some of the cellular effects of the HOTAIR-miR-761-HDAC1 axis were also mediated by activation of signal transducer and activator of transcription 3 (STAT3) and STAT3-related proinflammatory cytokines (38). This mechanism was confirmed in vivo using an endometriosis mouse model, where overexpressing exosomal HOTAIR led to increased HDAC1, active STAT3, and elevated proinflammatory cytokines (38). Thus, it appears that HDAC1 upregulation in lesions could worsen endometriosis-related inflammatory conditions.
Focusing on HDAC2, Zhao et al found that valproic acid treatment and combined valproic acid plus levo-tetrahydropalmatine treatment of rats with induced endometriosis decreased HDAC2 expression in lesions and dorsal root ganglia, diminished lesion size, and reduced endometriosis-associated hyperalgesia (39). More recently, Mai et al showed that HDAC2 protein was significantly upregulated in ovarian endometriotic lesion tissue compared with controls (40). Using the hEM15A immortalized eutopic endometrial stromal cell line, small RNA silencing of HDAC2 reduced the proliferation and invasion and increased apoptosis through upregulation of hepatocyte nuclear factor 4 alpha (HNF4A) (40, 41). In endometriotic tissues, HDAC2 and HNF4A were negatively correlated, and HDAC2 was enriched at the promoter region of the HNF4A gene. Overexpression of HDAC2 in vitro decreased the acetylation of HNF4A, revealing a direct mechanism for HDAC2 control of HNF4A expression (40). Furthermore, HNF4A was shown to bind to the promoter of the AT-rich interaction domain 1A (ARID1A) gene and regulate its expression in vitro, providing a plausible mechanism where overexpression of HDAC2 in endometriosis leads to downregulation of HNF4A and subsequent downregulation of ARID1A, a chromatin remodeler important in endometriosis and discussed in detail later (40). In an endometriosis mouse model, HDAC2 silencing led to augmented HNF4A and ARID1A expression together with reduced size and weight of lesions (40). Therefore, increased HDAC2 expression in endometriosis may lead to decreased HNF4A and ARID1A mediating increased proliferation and invasiveness of affected endometrial tissue.
Our group has reported that HDAC3 protein levels were significantly reduced in a set of eutopic endometria from infertile women with endometriosis compared with controls, in both the proliferative and secretory phases (42). Stromal HDAC3 was also decreased in baboon and mouse models of induced endometriosis (42). Deletion of uterine Hdac3 in mice led to infertility from implantation, decidualization, and endometrial receptivity defects (42). Both Hdac3 uterine knockout mice and infertile women with endometriosis exhibited increased endometrial type I collagen expression in all cycle phases, consistent with fibrosis (42). HDAC3 siRNA knockdown in endometrial stromal cells compromised in vitro decidualization along with upregulation of collagen type I alpha 1 chain (COL1A1) and COL1A2, and this appeared to occur through recruitment of the HAT p300 to the HDAC3-binding element in place of HDAC3 (42). Together, the experiments in this study showed that reduced HDAC3 in endometria of women with endometriosis may promote aberrant transcriptional activation of collagen genes and result in a fibrotic endometrium that is nonreceptive to pregnancy (42).
Another interesting similar phenotype with a connection to HDAC1 and HDAC2 was identified in a series of studies by Daftary and colleagues. They showed that the transcription factor Kruppel-like factor 11 (KLF11) was diminished in endometriotic lesions compared with control endometrium, particularly in fibrotic regions (43, 44). Furthermore, KLF11 knockout mice induced with endometriosis developed larger, more fibrotic lesions compared with controls, and chromatin immunoprecipitation using endometrial stromal cells showed that KLF11 bound pro-fibrotic genes including COL1A1 and COL1A2 (43). More detailed in vitro analysis revealed that KLF11 represses COL1A1 through cooperation with SIN3A, HDAC1, and HDAC2, resulting in promoter histone deacetylation; this finding was borne out in vivo in an endometriosis mouse model in which HDAC inhibitor treatment resulted in increased lesion fibrosis similar to what was observed in KLF11-deficient mice (44). Therefore, appropriate activity levels of HDAC1, HDAC2, and HDAC3 all appear necessary for regulation of collogen genes to prevent endometrial fibrosis in the context of endometriosis.
Outside of class I HDACs, the other HDAC that has been extensively studied in the context of endometriosis is the class III HDAC Sirtuin 1 (SIRT1). In addition to the finding that SIRT1 was increased in ectopic lesions compared with eutopic endometria from endometriosis patients, our group reported that SIRT1 was significantly overexpressed in endometria of women with endometriosis compared with controls (33, 45). This observation was supported by studies in nonhuman primate model of induced endometriosis where there was endometriosis-specific upregulation of endometrial SIRT1 expression (45). Further study revealed that uterus-specific overexpression of SIRT1 in mice resulted in subfertility and exacerbation of induced endometriosis development that was reversible by pharmacological inhibition of SIRT1 (46). Interestingly, uterus-specific deletion of Sirt1 also compromised fertility, showing that sufficient SIRT1 activity is also required for normal reproductive function (47).
SIRT1 overexpression studies indicate that SIRT1 coordinates with corepressor B-cell lymphoma 6 (BCL6) to transcriptionally repress the progesterone receptor (PGR) signaling target and Indian Hedgehog pathway gene GLI1 (45). Moreover, SIRT1 was shown to physically interact with progesterone receptor A isoform (PR-A) and not PR-B, without modulating the overall in vivo PGR level, indicating that upregulated SIRT1 may repress PGR targets and cause progesterone resistance in endometriosis via these protein–protein interaction (46). However, these studies did not look at the effects of SIRT1 overexpression on global or gene-specific histone acetylation levels, which is important for understanding the mechanistic role of SIRT1 in the epigenetics of endometriosis. Finally, SIRT1 and BCL6 have been tested as candidate biomarkers for endometriosis; direct analysis of BCL6 endometrial biopsies seems to have a diagnostic potential for diagnosis of endometriosis (48), serum SIRT1 protein was also significantly elevated in patients with advanced-stage endometriosis (49). Once again, these SIRT1 data together support the conclusion that HDAC expression in the female reproductive tract is tightly regulated and appropriate levels of expression required for normal reproductive function.
Histone methyltransferases and demethylases
In agreement with an earlier in vitro report, Zhang et al showed that enhancer of Zeste homolog 2 (EZH2), the catalytic subunit of the HMT polycomb repressive complex 2 (PRC2), was significantly upregulated in both the ectopic lesions and eutopic endometria of endometriosis patients compared with controls in conjunction with increased H3K27me3 and H3K9me3, its catalytic outputs (35, 50, 51). Knockdown or inhibition of EZH2 in endometriotic epithelial 11Z cells led to decreased EMT markers as well as reduced migration and invasiveness, whereas overexpression of EZHT upregulated EMT markers (51). In vivo, EZH2 inhibitor administration reduced lesion weight, H3K27me3, H3K9me3, and collagen I levels while increasing E-cadherin in a mouse model of endometriosis (51). Colón-Caraballo et al came to similar conclusions in a very similar study, although they reported the increase in EZH2 in eutopic endometria of endometriosis patients to be specific to the secretory-phase glands, and they observed a significant decrease in the proliferation but not the invasiveness of endometriotic epithelial 12Z cells after EZH2 inhibition (52).
In support using a distinct model system, Seguinot-Tarafa et al showed that inhibition of EZH2 decreased ectopic site number and size in a rat model of endometriosis (53). Brunty et al were also able to demonstrate a significant upregulation of EZH2 protein in endometriotic lesions compared with control endometria (54). In their study, they tested the hypothesis that miR-155, a putative regulator of the PRC2 complex component Jumonji and AT-Rich interaction domain containing 2 (JARID2), mediates crosstalk between peritoneal fluid and endometrial cells in women with endometriosis (54). Although they showed that transfection of miR-155 mimic into the Ishikawa endometrial cancer cell line did not significantly decrease JARID2 protein after treatment with peritoneal fluid from endometriosis patients like it did with peritoneal fluid from controls, most of their experiments were not powered highly enough to demonstrate statistically significant differences (54). Nevertheless, they did identify interesting interactions between JARID2 and the ARID1A gene and EZH2 and the DNMT3B gene in endometriosis peritoneal fluid-treated cells compared with controls using chromatin immunoprecipitation-qualitative PCR (54). These findings suggest possible crosstalk between the PRC2 complex and other distinct epigenetic regulators.
A mouse study by Nanjuppa et al examined the function of EZH2 in uterine development and function, finding that uterine EZH2 expression was estrogen driven and suppressed by progesterone in adults (55). Uterine-specific Ezh2 knockout mice had compromised fertility and developed endometrial hyperplasia with hormone-independent epithelial proliferation, demonstrating the requirement of EZH2 to regulate endometrial epithelial homeostasis (55). The findings of this study are noteworthy because the estrogen-driven nature of uterine EZH2 expression provides a possible causative explanation for EZH2 upregulation in endometriosis because it is an estrogen-driven condition.
Though EZH2 has been more intensely studied in the context of endometriosis, changes in other histone methylation modifiers have been noted. The HMT mixed lineage leukemia 1 (MLL1) catalyzes deposition of H3K4me3, an active promoter mark, and was observed by Wen et al to be diminished in the endometria of a patient with endometriosis in conjunction with decreased H3K4me3, particularly in the secretory phase (56). Mice induced with endometriosis also exhibited endometrial MLL1 loss with decreased fertility (56). Inhibiting MLL1 action in vivo in mice disrupted the uterine decidualization response, and siRNA knockdown of MML1 in immortalized human endometrial stromal cells also compromised their ability to decidualize (56). Interestingly, hormone treatments in cultured stromal cells revealed that MML1 was progesterone-induced through PGR, which may explain the reduced expression of MML1 in the eutopic endometria of women with endometriosis that also exhibited PGR reduction in this study (56).
Although not significantly changed in eutopic endometria, the HMTs SUV31H1, SUV39H2, and G9a were also reportedly decreased in ectopic lesions at the mRNA level (33). These HMT levels also significantly correlated with the H3K9 methylation they are known to catalyze, which were globally decreased in lesions (33). However, this finding is controversial because other groups reported increases of H3K9 methylation in endometriotic lesions (35, 51).
Lysine-specific demethylase 1 (LSD1) performs the directionally opposite molecular function by demethylating mono- or di-methylated H3K4 and H3K9 (57). Concordantly, LSD1 mRNA and protein expression levels were shown to be elevated in both ectopic lesions and eutopic endometria from women with endometriosis compared with controls, consistent with the independent finding that global H3K4 methylation was decreased in both eutopic endometria and lesions from women with endometriosis (33, 58). Yet again, a separate study cast doubt on this finding by showing an increase of H3K4 methylation in similar sample types (35). Inhibiting LSD1 in cultured primary ectopic endometrial stromal cells decreased their proliferation and invasiveness (58). In an in vivo study by the same group using an endometriosis mouse model, LSD1 was again elevated in lesions, and the same LSD1 inhibitor reduced lesion size and endometriosis-associated hyperalgesia in a dose-dependent manner while increasing H3K4me1 and H3K4me2 and reversing EMT markers (59).
Despite the lack of clarity on whether H3K4me3 levels are globally altered in endometriosis, a recent study by Rytkönen et al suggested that H3K4me3 levels at particular genomic locations in endometrial cells are important for defining the cell stress response in hypoxia, which is likely involved in endometriosis pathophysiology (60). Overall H3K4me3 levels decreased in hypoxia, but H3K4me3 peak breadth rather than height or location correlated best with transcriptional outputs at functional gene sets (60). In other words, genes that retained broader H3K4me3 promoter marks better maintained expression in hypoxia, and these included genes known to have important endometrial function (60). Based on these findings, H3K4me3 dynamics may play a protective role for endometrial function in the hypoxia that can be associated with endometriosis (60).
In addition to the studies focusing on endometriotic lesions and eutopic endometrial tissue, one study by Baumann et al also discovered a role for histone methyltransferases in potentially compromised ovarian function of endometriosis (61). Specifically, they identified striking decreases in the arginine methyltransferases CARM1, PRMT2, and PRMT8 in baboon ovaries after endometriosis induction as assessed by mRNA PCR array (61). CARM1 protein was decreased in the ovaries and endometria of baboons induced with endometriosis, and the PRMT8 promoter was hypomethylated in endometriosis-affected ovaries (61). Even though more research needs to be done to confirm these results, they suggest a mechanism how endometriosis may be linked to low-quality oocytes through an epigenetic mechanism.
In summary, there is consensus that endometriosis is associated with increasing endometrial EZH2 with its H3K27me3 output. Other HMTs appear decreased, whereas the demethylase LSD1 increased; however, conflicting findings make it difficult to draw firm conclusions regarding changes in global H3K4 and H3K9 methylation levels. Experiments that carefully differentiate between endometrial cell type and cycle phase with larger sample sizes will be necessary to make definitive determinations. To generalize, the global methylation patterns are, like global hypoacetylation, suggestive of repressed heterochromatin in endometriotic lesions and endometrial samples from subjects with endometriosis, but it will be important to examine specific genes with pathophysiological relevance that are either repressed or activated to gain a more complete understanding of these phenomena.
Chromatin architecture modifiers
In addition to control by DNA and histone modifications, chromatin conformation is subject to regulation by other proteins and complexes as well. We will highlight 2 of these factors here that have been linked to endometriosis pathophysiology.
ARID1A
ARID1A is a 250-kDa subunit of the mammalian SWI/SNF BRG1-associated factor complex that together binds at many loci in the genome to maintain chromatin accessibility or remodel nucleosome structure (62). As a result, these changes in structure affect the ability of transcription factors to access particular regions of the genome to facilitate expression of the genes therein. ARID1A's biochemical function is to provide rigidity of structure to the complex, nonspecifically bind DNA, and facilitate the BRG1-associated factor complex's ability to slide along DNA (63, 64).
ARID1A is essential for embryonic development because Arid1a-null mouse embryos fail to gastrulate (65). It is also necessary for maintenance of pluripotency in embryonic stem cells, and it suppresses cell proliferation and tissue regeneration (65, 66). Of all the SWI/SNF subunits, ARID1A is the most frequently mutated in cancers, with substantial percentages of ovarian, gastric, bladder, endometrial, and lung tumors, to name a few, harboring inactivating mutations with loss of ARID1A protein expression (63). Gynecological cancers including clear-cell ovarian carcinoma and endometrioid ovarian carcinoma, which are strongly associated with endometriosis, are among the most common cancer types to display ARID1A mutations (67). ARID1A inactivation is not, on its own, enough to drive malignant transformation; rather, ARID1A mutations occur as later events, contributing to the progression rather than initial development of tumors, particularly in the case of endometrial cancers (67).
Not all ARID1A mutations are associated with cancer. Inactivating mutations that cause expression loss have been identified in nonmalignant deeply infiltrating endometriotic lesions (68) and ovarian endometriomas (69) and mutations in normal, apparently healthy endometrial tissues have also been reported (70). We showed that the ARID1A protein expression levels are reduced in eutopic endometrial samples from women with endometriosis compared with controls (71). ARID1A mutations in benign endometrial tissue are very rare (69, 70), and no endometriosis-associated SNPs have been reported in the ARID1A gene despite 1 group specifically looking for them in a genome-wide association study (72). Therefore, although somatic ARID1A mutations may contribute to endometriotic lesion progression in some cases, there is currently no evidence for a genetic basis of ARID1A-related endometrial dysfunction in endometriosis, and the decrease in ARID1A expression in the endometrium of women with endometriosis likely stems from dysfunction at the epigenetic, transcriptional, or posttranscriptional level. As previously mentioned, HDAC2 may be transcriptionally regulating ARID1A expression, indirectly through control of HNF4A (40). The PGR-regulated transcription factor SOX17 has also been identified as a possible upstream regulator of ARID1A expression in the endometrium; thus, reduced SOX17 may precipitate ARID1A loss (73). Additionally, oxidative stress may be a cause of ARID1A downregulation in endometriosis via promoter hypermethylation (74, 75).
Study of ARID1A's molecular function in endometriotic epithelial cells has shown that, in this cell context, ARID1A promotes chromatin accessibility and maintenance of epithelial cell identity, whereas ARID1A loss leads to the accessibility and expression of epithelial to mesenchymal transition-related genes (76). Furthermore, ARID1A bound regions of the genome in sorted mouse endometrial epithelial cells were associated with accessible chromatin near promoters of genes related to inflammation and apoptosis (77).
As demonstrated in mice, ARID1A is required for normal physiological female reproductive function through enabling embryo implantation and uterine stromal decidualization (71). To maintain uterine receptivity during early pregnancy, we showed that uterine ARID1A must be present to enable epithelial PGR signaling and suppress estrogen-induced epithelial proliferation (71). ARID1A also colocalized with PGR in both the human and mouse endometrium, and ARID1A levels correlated with PGR in endometrium affected by endometriosis (71, 78). Furthermore, mice with uterine-specific deletion of either Arid1a or Pgr experienced increased lesion development when endometriosis was induced (78).
In addition to its PGR-associated function, we also showed that ARID1A directly regulates the forkhead box protein A2 (Foxa2) gene in mice during early pregnancy to facilitate leukemia inhibitory factor (LIF) secretion (79). LIF was the first gene discovered to be required for normal fertility and implantation (80). Attenuation of Arid1a in the endometrial epithelium resulted in severe subfertility because of implantation failure, incomplete decidualization, and a nonreceptive endometrium (79). Without sufficient FOXA2 and LIF expression, STAT3 does not become activated and EGR1 is not sufficiently expressed to allow healthy establishment of pregnancy (79). Furthermore, a translational study of infertile women with endometriosis revealed that endometrial FOXA2 is downregulated in correlation with reduced ARID1A (79). In a baboon model of endometriosis, we observed that experimental induction of lesion growth led to simultaneous reduction of both endometrial ARID1A and FOXA2 (79). Therefore, endometrial ARID1A loss may contribute to endometriosis-related infertility through multiple molecular mechanisms.
CCCTC-binding factor
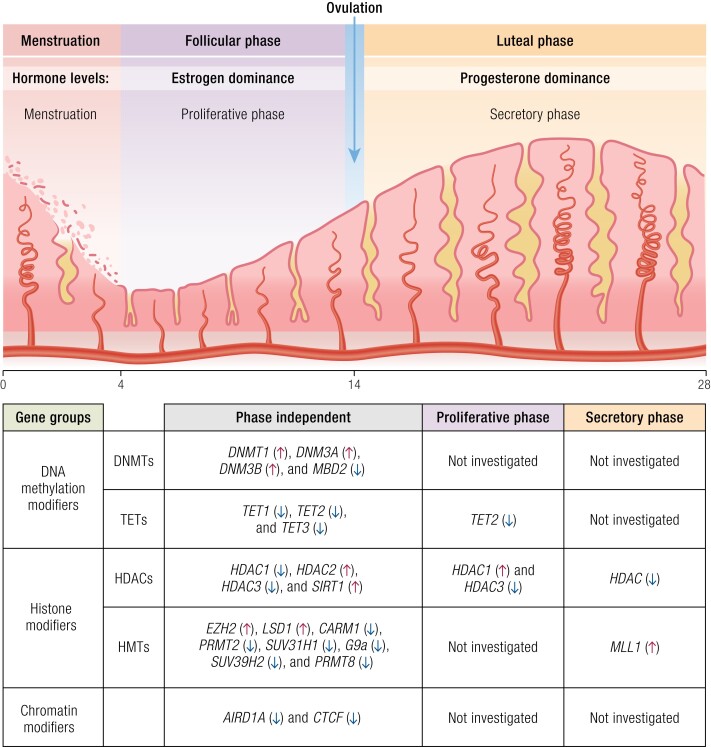
CCCTC-binding factor (CTCF) is a transcription factor known to regulate large-scale chromatin structure by working with cohesin to dynamically facilitate DNA looping and thereby control promoter–enhancer interactions (81). At least 2 studies have identified somatic CTCF mutations in endometriotic lesions, which, despite being infrequent, were enriched above that seen in normal endometria (82, 83). In vitro work has implicated CTCF in the cellular estrogen response, which is a potential direct link to the pathophysiology of endometriosis (84). Interest in this was heightened by the observation that CTCF regulation through epigenetic mechanisms has been implicated in other uterine diseases such as fibroids and endometrial cancer (85, 86). Thus, although evidence implicating CTCF in endometriosis pathophysiology is currently sparse, it is a molecule of interest for future study. A summary of epigenetic regulatory genes expressed differentially in the endometrium during the menstrual cycle is shown in Fig. 1.
Figure 1.
List of epigenetic regulatory genes expressed differentially in the endometrium during the menstrual cycle.
Endometrial genes altered through epigenetic mechanisms
There is a vast array of genes that are altered in the endometrium of women with endometriosis (87, 88). A growing body of work has also described epigenetic regulatory mechanisms impacting gene expression of factors with previously known functions in endometrial tissues. Such implicated mechanisms include hypermethylation of nuclear receptor genes PR-B (89) and ESR1 (90) as well as differential methylation of GATA family genes (91), hypermethylation of (homeobox 10A) HOXA10 (92), aberrant methylation of the E-cadherin gene promoter (93, 94), and hypermethylation of SF-1 (95, 96).
Genome-wide methylation changes
Direct DNA modification has been implicated in endometriosis. As an example, a recent Illumina methylation array-based study reported 12 159 differentially methylated CpG sites and 375 differentially methylated promoter regions in human endometriotic lesions and showed differential expression of DNMTs in the same study participants (16). Furthermore, a mechanism was described where upregulation of DNMT1 results in malignant transformation of endometriosis involving RUNX3 in primary human cells (97).
Bulun and colleagues mapped genome-wide differences in DNA methylation between normal healthy endometrium and that from endometriosis. They identified 42 248 differentially methylated CpG islands, typically correlated with negative gene expression (91). Of note, they reported alterations in the GATA family of transcription factors, specifically finding a switch between GATA2 in healthy endometrium and GATA4/6 in endometriosis. GATA2 is important for endometrial stromal decidualization and successful pregnancy involving PGR (98). In endometriosis, GATA6 predominated, with direct effects on progesterone resistance, altered estrogen metabolism, and mesodermal differentiation. These changes in DNA methylation pattern appear to explain in part the role of inflammation on epigenetic phenomena that promote the pathogenic pathways seen in endometriosis.
Epigenetic Implications for Pathophysiology
Endometriotic implants (ectopic endometrium) represent an aberrant endpoint for endometrial development, distinctly different from normal healthy eutopic endometrium in terms of steroid hormone receptors, signal transduction, transcription factors, and extracellular and architectural milieu. The inflammatory changes in eutopic endometrium take time to be established, as shown with induction studies in both mouse and nonhuman primates (88). Global differences in the endometrium of women with endometriosis are likely epigenetically driven and serve as both biomarkers for the diagnosis of endometriosis as well as a roadmap for novel therapeutic approaches for this disease.
Epigenetics has been increasingly described as having a role in the physiology and pathophysiology of endometrial function (42, 99-104). Although lesions vary in size, location, and character, their presence has been associated with changes in endometrial function and altered gene expression (105, 106). Many of these changes have collectively been attributed to progesterone resistance and include alterations in a surprising number of proteins and pathways (7, 88, 106), increasingly attributed to epigenetic causes (91, 95, 107-109). Alterations in normal gene expression not only hampers implantation, leading to infertility and pregnancy loss, but through progesterone resistance promotes a proliferative and invasive phenotype of shed endometrium that further contributes to the ongoing pathogenesis of this disease.
Inflammation is a common theme in discussions around epigenetics in general (110-112) and endometriosis, specifically (113-118). Endometriosis represents the shedding of retrograde menstrual effluvium with attachment and subsequent growth of ectopic endometrium at distant sites, most frequently on the peritoneal surfaces surrounding the uterus (115). Lesions generate an inflammatory response, noted early by the influx of activated peritoneal macrophages (119-121). Although inflammation might lead to scarring or adhesion formation, infertility is often associated with milder forms of the disease, suggesting an endometrial effect secondary to this inflammatory response (118).
Inflammation of the endometrium is normal and essential for establishment of pregnancy and menstruation (122-124) and is regulated by steroid hormone fluctuations and changes in steroid receptor concentration during the normal endometrial cycle (125, 126) but chronic inflammatory changes represent a threat to normal fecundity (127). Progesterone withdrawal occurs locally in the epithelial compartment during the window of implantation around day 20 of the normal menstrual cycle (128) and at menses (125). This drop in PR-B with residual PR-A reflects the decline in epithelial PGR previously reported using immunohistochemistry by our group and others in the epithelial, but not stromal compartment (129, 130). Successful pregnancy requires continued progesterone support and normal decidualization, which is a progesterone dependent process (131). In lieu of pregnancy, progesterone withdrawal occurs globally in the endometrium initiating menstruation with the demise of the corpus luteum (132), reflecting the loss of progesterone rather than down-regulation of PGR. Shedding and renewal of the endometrial lining depends on complex cell–cell interactions and alterations in immune function (125). The intricacies of tissue differentiation as well as tissue repair requires sophisticated and complicated control of gene expression that goes beyond steroid receptor involvement and likely depends on epigenetic mechanisms.
DNA methylation has been shown to play a role in normal endometrial function and epigenetic pathophysiology of endometriosis. The DNMTs are enzymes that mediate the methylation process have been reported to be aberrantly expressed in endometriotic cells compared with normal endometrium. DNMT3B was elevated and not down-regulated normally in in vitro decidualization, suggesting a role in endometriosis. As discussed later, this is a major targeting mechanism for specific gene modulation with hypermethylation decreasing expression and hypomethylation increasing gene expression as a rule.
Epigenetic mechanisms abound in the endometrium (7, 102, 108, 114). Endometrial PGRs are dynamically regulated throughout the menstrual cycle (129, 133) and epigenetically dysregulated in the setting of endometriosis (101, 115, 134). Normal actions of PR-B are anti-inflammatory (135) and more likely than PR-A to promote gene transcription. Overall, PGR is reduced in endometriotic tissues and shows less cyclic variability in lesions compared with normal tissue (136, 137). PR-B levels are specifically reduced, which is thought to occur through hypermethylation of the promoter (89, 138, 139) and through modulation of AKT or MEK1/2 activity (140), greater in lesions than eutopic endometrium (141). Overabundance of PR-A also inhibits transcription of PR-B receptor (135). The epigenetic alterations aimed at steroid receptors in the endometrium were recently reviewed (101).
Genome-wide DNA methylation studies have suggested that epigenetic mechanisms activate several signaling pathways including PI3K/phosphatase and TENsin homolog deleted on chromosome 10/AKT signaling (142). Endometriotic tissues exhibit multiple somatic cancer-driver mutations (68, 143, 144) that commonly affect components of MAPK/Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) and PI3K-AKT-mTOR signaling. In incisional endometriosis, Lac et al reported mutations in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, KRAS, and PPP2R1A and loss of function ARID1A mutations in 25% of cases (143). Normally, superenhancers marked by high H3K27 acetylation are associated with ARID1A binding. ARID1A loss promotes H3K27 hyperacetylation and increased chromatin accessibility (145). Interestingly, ARID1A appears to be downregulated by reactive oxygen species via promoter methylation in endometriosis (75). Further, ARID1A is activated by HNF4A/AT-rich interactive domain 1A, and both are down-regulated in endometriosis through the histone deacetylase HDAC2 (40). Silencing HDAC2 increased ARID1A and reduced the size of endometriotic implantation in the endometriotic cell line hCM15A (40).
KRAS-activating mutations were noted in deep infiltrating endometriosis and other somatic driver mutations were present in 26% of cases (68). We recently identified KRAS as a main driver of the histone deacetylase SIRT1 expression (45), and nuclear SIRT1 was increased in ovarian cancers associated specifically with endometriosis (146). KRAS activation targets SIRT1 expression and likely contributes to the pathogenesis of endometriosis (45). SIRT1 is dramatically overexpressed in the eutopic endometrium of women with endometriosis and FOXO1, a target of SIRT1 in downregulated (147, 148). Interestingly, miRNA-34a appears to regulate SIRT1 though p53 modulation (148). A decrease in miRNA 199a promotes both HIF1a and SIRT1 expression in cardiac myocytes (149), suggesting a possible link between SIRT1 and HIF1a overexpression seen in endometriosis. Overexpression of SIRT1 worsens endometriosis and contributes to implantation failure, whereas SIRT1 inhibition reverses these effects (147). Progesterone resistance and estrogen dominance further promotes SIRT1 expression (150). Estradiol upregulation of SIRT1 appears to be related to degradation of PPAR-gamma mediated by NEDD4-1 (150). NEDD4 has been promoted as a promising target in cancer therapy (151) and might be exploited for treatment of endometriosis.
Epigenetic Implications for Clinical Outcomes
Endometriosis has been characterized as an inflammatory disease (152) that contributes to infertility (153) and pelvic pain (154). Genome-wide DNA methylation profiling in both ectopic and eutopic endometrium has revealed alterations in a myriad of genes with direct implications to the pathogenesis of endometriosis (91, 142, 155, 156). Hypermethylation of promoter regions that has the potential to silence genes have been noted for steroid receptors, steroid biosynthesis pathways, prostaglandins and cyclooxygenase 2 (COX-2) expression, and transcription factors including HOXA10 and HOXA11 and other signaling pathways that appear critical for embryo implantation. Alterations in steroid receptors and associated proteins have been attributed to progesterone resistance, shown to be linked to both the pathogenesis of this disorder as well as the clinical outcomes of pain and infertility (108, 157-159).
An extensive list of gene changes associated with progesterone resistance associated with endometriosis was published in 2007 using DNA microarray technology (106), demonstrating a wide variety of progesterone-regulated genes that are altered in eutopic endometrium of women with this disease. These include FOXO1 (106) and retinoic acid signaling (160) and genes essential for establishment of pregnancy and for stromal decidualization (161) and implantation specific including HOXA10, ανβ3 integrin, IGFBP-1 (162), and CRABPII (163) among many others (164).
Among the first to recognize the implications of epigenetic mechanisms in endometriosis was Wu et al, in 2005, who suggested aberrant methylation of the HOXA10 gene may be responsible for its reduction in expression seen in endometriosis (165). Brosens and coworkers predicted early on that the mechanisms of endometrial progesterone resistance associated with endometriosis was based on epigenetic phenomena that “determines the intrinsic responsiveness of endometrial cells to differential cues” including inflammation (117).
Progesterone is essential for decidualization, which is critical for successful implantation (131) and endometrial stromal cells from women with endometriosis exhibit decidualization defects (166). Bulun and coworkers have demonstrated that GATA2 interacts specifically with PGR to coregulate decidualization (98). GATA2 is a member of the GATA family of zinc-finger transcription factors. In mice, Gata2 knockout animals are infertile and exhibit excessive estrogen activity and reduced PGR and progesterone signaling defects in early pregnancy (167). GATA2 is expressed at the time of implantation in the mouse uterus and colocalizes with PGR (168).
Progesterone is essential for pregnancy and acts through 2 receptors, PR-A and PR-B, both encoded by a single gene (169, 170). Estrogen promotes PGR expression and contains an estrogen response element in its promoter. Unliganded receptors reside in the cytoplasm bound to heat-shock proteins. In the presence of progesterone, PGR dimerizes and translocates to the nucleus and binds to specific progesterone response elements. PR-B contains an additional segment (AF-3) that exerts differential activity on PR-B over PR-A. For example, PR-B is a more potent activator of gene expression, whereas PR-A is more of a repressor, including repression of PR-B (171, 172). In normal endometrium, PR-A and PR-B are present in the preovulatory endometrial glands, but only PR-B persists at the time of implantation and glandular secretion, whereas PR-A is dominant in the stroma throughout the cycle (133). PR-A exerts antagonistic effects on estrogen-induced proliferation, whereas PR-B promotes more gene expression (173). PR-B exhibits reduced expression in endometriotic stromal cells compared with normal eutopic endometrium (141) through selective hypermethylation (139). Silencing PR-B results in significant increase in proliferation (174).
Controversy exists about PGR being the cause of progesterone resistance, related in part to which frame of reference one takes. In ectopic endometrial stromal cells, PR-A is the dominant receptor; in eutopic endometrium of the same individual, both PR-A and PR-B are present (141). In normal eutopic endometrium, epithelial but not stromal PGR is normally down-regulated at the time of implantation (129, 130). In both mice and humans, this down-regulation of PGR is critical for normal implantation (175). In endometriosis, however, PGR expression appears to persist rather than disappear (176). Because progesterone down-regulates its own receptor, this persistence of PGR is evidence of progesterone resistance, paradoxically showing elevated PGR is present in the setting of endometriosis. Although loss of PR-B from stromal compartment might explain progesterone resistance in endometriotic lesions, epigenetic factors likely contribute to the progesterone resistance in eutopic endometrium, which is associated with infertility and pregnancy loss.
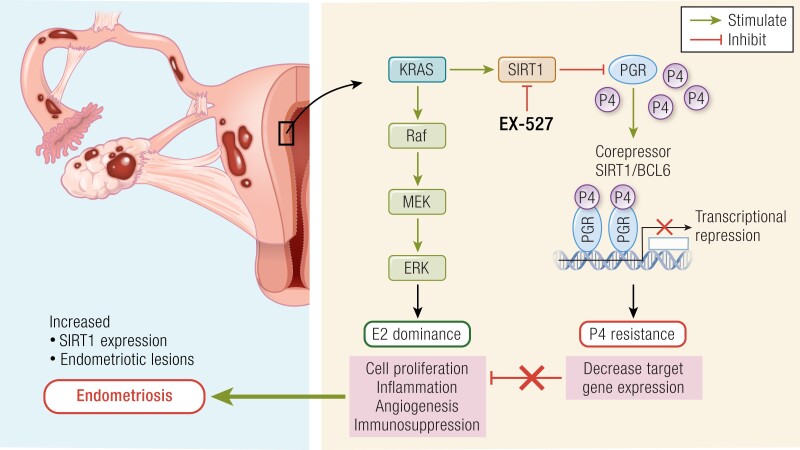
Inflammation has been linked to infertility and pregnancy loss in multiple scenarios including endometriosis, endometritis, hydrosalpinges, endometrial polyps, and polycystic ovarian syndrome (177, 178). KRAS is among the known cancer-driver mutations reported in endometriosis (68, 115). As shown in Fig. 2, KRAS is a central regulator of the histone deacetylase SIRT1 (45), which specifically targets several genes involved in implantation and endometrial health. We reported that SIRT1 is induced by inflammation through KRAS and is a highly specific biomarker for the presence of all stages of endometriosis (45). Both SIRT1 and KRAS are induced over time in the baboon endometriosis model, and we showed that SIRT1 blocks expression of GLI1 as part of a key progesterone signaling pathway. SIRT1 also targets COUP-TFII (179), a cofactor central to progesterone signaling (180). There is an established p53/miRNA34/SIRT1 interconnection that influences action of SIRT1 that may have implications in the infertility associated with SIRT1 overexpression. SIRT1 down-regulates p53 that in turn regulates LIF expression (181). LIF was one of the first proteins shown to be essential for embryo implantation (182) and is regulated in part through progesterone action in mice (175). LIF is present in human endometrium at the opening of the window of implantation with expression beginning with the downregulation of PGR (183).
Figure 2.
A summary of the role of the epigenetic modulator Sirtuin-1 (SIRT1), which is linked to progesterone resistance, in developing endometriosis. In healthy women, uterine expression of SIRT1 is limited during menses; however, a dramatically higher expression of SIRT1 is observed in women with endometriosis. Indeed, the aberrant expression of SIRT1 is related to the upregulation of Raf/MEK/extracellular signal-regulated protein kinase activity associated with the conditions (cell proliferation, inflammation, immunosuppression, etc.) that favor the development and progression of endometriosis. Therefore, treatment with EX-527, a SIRT1 inhibitor, can reduce endometriotic lesions in the mouse endometriosis model, which can be a promising therapeutic for women suffering from endometriosis.
Epigenetic Implications for Therapeutics
Recently, endometriosis has been shown to be an epigenetic disease (8). Epigenetic alterations in endometriosis are indeed complex and widespread and appear to be directly related to the pathophysiology of the disease. Therefore, the epigenetic basis provides a clue to potential therapeutic options for the treatment of endometriosis. Although surgery to diagnose and treat endometriosis is recommendation for women with suspected endometriosis and pelvic pain, this approach has been questioned (99). Medical therapy including suppression of menstruation, progestins, COX-2 inhibitors, and gonadotropin-releasing factor agonists and antagonists have recently been reviewed (134, 184).
Treatment with anti-inflammatory agents including COX-2 inhibitors have long been a mainstay for treatment of dysmenorrhea and pelvic pain associated with gynecologic ailments, including endometriosis (185-187). Overexpression of COX-2 occurs in both ectopic and eutopic endometrium and in adenomyosis (188) and is thought to have an epigenetic basis because it is hypomethylated in the eutopic endometrium of women with endometriosis (189). In the pathophysiology of endometriosis progression, increased ESR2 promotes COX-2 expression, increasing prostaglandin E2 (PGE2), which stimulates SF-1 and aromatase expression. COX-2-specific inhibitors are safe and effective in controlling pain in women with endometriosis (186) and may also be a useful means of reducing the epigenetic instability associated with endometriosis (190, 191).
The female sex steroids estrogen and progesterone promote endometriotic cell progression or suppression, respectively. Estrogen promotes the progression of endometriosis, whereas progesterone suppresses its growth (192). The use of estrogen-progestin oral contraceptive pills (OCPs) is an option for treating symptomatic endometriosis that has been extensively studied (193, 194). One solitary randomized controlled trial with limited number of cases showed the benefit of OCPs over placebo (195). The Cochrane Review concluded that OCPs were of limited use relative to other modalities (196). In fact, progesterone-only pills have a logical role given their targeted action of antiestrogenic effects. The use of progestins over combined OCPs for suppression of the hypothalamic-pituitary-ovarian axis have been advocated because of its direct effect on the endometrium, endometriotic lesions, and potential ability to overcome progesterone resistance associated with endometriosis (134, 197-199). The breakdown of epigenetic homeostasis has been linked to estrogen dominance and progesterone resistance (200), both of which can lead to endometriosis (42). This is because epigenetic alterations related to the transcription factors of estrogen and progesterone signaling are robust in endometriotic tissue (200). However, the evidence that OCP or progestin can reverse the epigenetic modifications attributed to endometriosis is still inconclusive, suggesting further investigation in this field.
As elaborated previously, estrogen dominance and progesterone resistance are epigenetic sequelae of endometriosis and serve as specific targets for therapies targeting endometriosis (193). Resistance to progesterone may be the reason why up to one third of women with endometriosis do not respond to progestin-only therapies (201). For the endometrium, estrogen is inflammatory, whereas progestins are anti-inflammatory. Therefore, the standard of care for endometriosis is based on limiting estrogen exposure and thereby reducing inflammation, as originally proposed in Barbieri's estrogen threshold theory. The use of GnRH agonists (202, 203) and now GnRH antagonists (204-208) have proven to be effective therapies for reducing the activity and actions of endometriosis on women with pelvic pain, as well as a treatment for infertility and implantation failure (209). An important mechanism underlying these treatments is hypoestrogenism, which reduces the size and activity of ectopic lesions and the inflammatory changes that lead to prostaglandin formation and subsequent effects.
Histone methylation is a key epigenetic regulator of chromatin structure and transcriptional activity (210). Hypermethylation and hypomethylation are associated with up- and down-regulation of gene expression and have been reported for several key genes that are associated with the pathophysiology of endometriosis (8), including PR-B (139), ESR2 (211), SF-1 (212), HOXA10 (165), and aromatase (213). Genome-wide methylation profiling in eutopic and ectopic endometrium has demonstrated that methylation differences are greater between ectopic and controls than eutopic (endometriosis) and controls (142). Studies have found more than 12 000 differentially methylated CpG sites and 375 differentially methylated promoter regions in endometriotic lesions compared with eutopic endometrium (16) associated with immune function, steroid hormone action, cell adhesion, and MAPK activation. These results suggest that changes in DNA methylation play an important role in the pathophysiology and progression of endometriosis, which may be a viable target for treating the disease.
Flores and others have documented hypermethylation of HCK4, K3K9, and H3K27 histones in endometriosis (35, 50). Epigenetic targeting for therapeutics in cancer also has shown clinical benefits (214). Multiple preclinical studies have suggested therapeutic approaches that target DNA methylation and histone deacetylation as epigenetic targets for the treatment of endometriosis (215-218). Histone methylation/demethylation approaches may have higher specificity and are increasingly being investigated (219). Suppression of methyltransferase EZH2 was shown to suppress endometriotic vesicles in a rat model of endometriosis (53). Endometriotic lesions were highly enriched in H3K27me3 within promoter regions of endometriosis-related genes (52). The endometriotic epithelial cell line 12Z showed decreased migration and proliferation when EZH2 was inhibited by a specific pharmacological inhibitor that also decreases H3K27me3 levels. Histone acetylation is regulated by HATs and HDACs. Changes in HDACs have been noted in endometrial cancer and endometriosis and are associated with infertility (36, 220).
Gujral et al (100), found that acetylation and deacetylation of histones in the cyclic endometrium is important for normal endometrial function, including implantation, decidualization, and cell differentiation. Because HDAC3 controls ESR1 stability, loss of HDAC3 predisposes to infertility and implantation failure (42). HDAC3 regulates stability of the ESR1 mRNA. Targeted DNA methylation in endometriotic cell line 12Z by inhibition of PGE2 receptors EP2 and EP4 results in widespread suppression of epigenetic regulators such as HDAC1, HDAC3, and others (221).
As discussed previously, KRAS and SIRT1 are 2 epigenetic mediators turned on by inflammation and can be found in the endometrium of women with endometriosis and progesterone resistance (45, 146, 147). We recently reported that SIRT1 is normally expressed at menstruation in normal control eutopic endometrium but expressed throughout the menstrual cycle in women with endometriosis (147). Recent evidence suggests miRNA 34a may regulate SIRT1 expression and found to have reduced expression in endometriosis (148). Other genes, such as FOXO1, which is needed for implantation and is a target of SIRT1, are also thought to have been turned down. Sirtuins have been recently suggested as therapeutic targets for infertility (222). In mice, overexpression of SIRT1 induces implantation failure and worsening endometriosis; treatment with the SIRT1 inhibitor EX-527 reverses these defects (147) (Fig. 2). Let-7 has been shown to be downregulated in endometriosis, and polymorphism of the let-7 mRNA-binding site in the KRAS 3′-UTR results in abnormal KRAS expression (223). Interestingly, the aromatase inhibitor, letrozole, has been shown to increase let-7f levels in Ishikawa cells and decrease migration of these endometrial cells (224), again suggesting therapeutic approaches may target epigenetic mechanisms in endometriosis. A summary of the protective effects of epigenetic modulators and targeted therapies against endometriosis is provided in Table 3.
Table 3.
Protective epigenetic effects of nonsurgical therapies for endometriosis
| Therapies | Epigenetic roles | Active ingredient | Patient/objects | Clinical outcome | References |
|---|---|---|---|---|---|
| COX-2 inhibitors | Diminishes COX-2 hypomethylation in the eutopic endometriosis | Rofecoxib | Women | Endometriosis-related pelvic pains significantly decreased with no recurrence | Cobellis et al (186); Ota et al (188) |
| Celecoxib | Human endometrial cells | Decrease COX-2 activity via a decrease in the production of PGE2 and VEGF | Cobellis et al (186) | ||
| Nimesulide | Endometrial stromal cells | Prevent ectopic endometrial stromal cell proliferation | Kong et al (225) | ||
| Aromatase inhibitor | Counters estrogen-driven epigenetic changes | Letrozole | Women | Improve endometrium receptivity and uterine health | Steiner et al (209) |
| P4-based therapy | Help maintain epigenetic homeostasis linked to endometriosis | OCP | Women | The endometrioma volume dramatically decreased in the OCP group compared with the placebo | Harada et al (195) |
| Progestin | Women | Trigger the HPO and consequently influence the pathophysiology of the endometriosis | Casper et al (198); Buggio et al (197) | ||
| GnRH antagonists | Help maintain epigenetic homeostasis linked to hypoestrogenism in endometriosis and endometrial fibrosis | Leuprolide acetate | Women | Improve endometrium receptivity and subside undiagnosed endometriosis | Steiner et al (209) |
| Linzagolix | Women | Significantly reduced pain related to endometriosis | Dababou et al (204) | ||
| Relugolix | Women | Significantly improved endometriosis-associated pain | Giudice et al (205) | ||
| Elagolix | Women | Effectively alleviate endometriosis lesions and endometriosis-associated pelvic pain | Diamond et al (207); Taylor et al (206) | ||
| Histone/DNA methylation modulators | Directly related to the pathophysiology and progression of endometriosis | EZH2 antagonist | Human endometrial and endometriotic cells | Reduced migration and proliferation of endometriotic epithelial cells | Colón-Caraballo et al (52) |
| EZH2 inhibitor (tazemetostat) | Female rats | Reduce the number of endometriotic vesicles | Seguinot-Tarafa et al (53) | ||
| Modulates DNA methylation and histone modification-related proteins functions in endometriotic cells | AH 6809 and AH23848 (PGE2 receptors antagonist) | Human endometriotic epithelial and stromal cell | Inhibits adhesion, invasion, growth, and survival endometriotic cells by modifying integrins, MMPs, and TIMPs, cell cycle, survival, and intrinsic apoptotic pathways | Arosh et al (221) | |
| SIRT1 is an epigenetic mediator in the endometrium of women with endometriosis | EX-527 (SIRT1 inhibitor) | Mouse endometriosis model | Endometriotic lesions were diminished significantly with EX-527 treatment via downregulating STRT1 | Rezk et al (148) | |
Abbreviations: AH23848, thromboxane receptor blocker; AH 6809, 6-isopropoxy-9-oxoxanthene-2-carboxylic acid; COX-2, cyclooxygenase 2; EZH2, histone methyltransferase; HPO, hypothalamic-pituitary-ovarian axis; MMP, matrix metalloproteinase; OCP, estrogen-progestin oral contraceptive pill; PGE2, prostaglandin E2; SIRT1, sirtuin-1; TIMP, metalloprotease inhibitor.
Conclusions
A thorough examination of the literature leads to the clear conclusion that epigenetic dysregulation is fundamentally intertwined with endometriosis pathophysiology. Both endometriotic lesions and the eutopic endometria of women with endometriosis exhibit changes in DNMTs, HDACs, HMTs, and other enzymes that do the work of epigenetically modifying chromatin to influence its structure and function. In particular, studies characterizing dysregulation of class I HDACs, SIRT1, EZH2, and ARID1A in endometriosis have yielded important information on mechanisms of lesion growth and endometriosis-related infertility. Dysregulation of the levels of these proteins results in changes to DNA promoter methylation, histone methylation and acetylation, and nucleosome structure to repress and activate different genes. Even when overall levels of chromatin marks remain unchanged, changes in their patterns can affect gene expression in cellular conditions relevant to endometriosis such as oxidative stress or hypoxia. Ultimately, epigenetic dysregulation of genes that code for proteins with important functions in endometrial tissue like ovarian steroid hormone receptors, SF-1, HOXA10, E-cadherin, and others can lead to endometriosis-related pathophysiological states such as estrogen dominance, progesterone resistance, and EMT. Basic understanding of these processes has already led to preclinical testing of epigenetically targeted therapeutics such as HDAC inhibitors and HMT inhibitors, which show some promise. In spite of what we know already, there is a great need to better characterize the mechanistic relationships between specific epigenetic modifications and different lesion types, disease stages, and clinical outcomes. Building on the current foundational knowledge to better understand the dynamic interactions between DNA methylation, histone marks, and nuclear receptors will be key to developing new diagnostic approaches and novel nonhormonal therapies for treatment of endometriosis.
Acknowledgments
Portions of the introduction and the text reviewing the literature on the function and dysfunction of ARID1A in endometriosis were modified from the Michigan State University dissertation titled “The Role of Arid1a in Endometriosis-Related Infertility” by Ryan M. Marquardt, the first author of this paper. The dissertation is open access and distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/), which allows reuse as long as appropriate credit is given. The work has not been published elsewhere.
Abbreviations
- 5hmC
5-hydroxymethylcytosine
- AKT
Akt serine/threonine kinase family
- ARID1A
AT-rich interaction domain 1A
- BCL6
B-cell lymphoma 6
- CTCF
CCCTC-binding factor
- COL1A
collagen type I alpha
- COX-2
cyclooxygenase-2
- CpG
cytosine-guanine repeats
- DNMT
DNA methyltransferase
- EMT
epithelial-mesenchymal transition
- ESR1
estrogen receptor alpha
- EZH2
Zeste homolog 2
- FOXA2
forkhead box protein A2
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- HMT
histone methyltransferase
- HNF-4α
hepatocyte nuclear factor-4 alpha
- HOTAIR
HOX Transcript Antisense RNA
- HOXA10
homeobox 10A
- JARID2
AT-rich interaction domain containing 2
- KLF11
Kruppel-like factor 11
- KRAS
Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
- LIF
leukemia inhibitory factor
- LSD1
lysine-specific demethylase 1
- MBD
methyl-CpG-binding domain protein
- MLL1
HMT mixed lineage leukemia 1
- OCP
oral contraceptive
- PGE2
prostaglandin E2
- PGR
progesterone receptor
- PR-A
progesterone receptor A isoform
- PR-B
progesterone receptor B isoform
- PRC2
polycomb repressive complex 2
- SIRT1
Sirtuin 1
- SNP
single-nucleotide polymorphism
- STAT3
signal transducer and activator of transcription 3
- TET
ten-eleven translocation methylcytosine dioxygenase
Contributor Information
Ryan M Marquardt, Department of Obstetrics, Gynecology & Reproductive Biology, Michigan State University, Grand Rapids, MI, USA.
Dinh Nam Tran, Department of Obstetrics, Gynecology and Women's Health, University of Missouri, Columbia, MO, USA.
Bruce A Lessey, Department of Obstetrics and Gynecology, Wake Forest Baptist Health, Winston-Salem, NC, USA.
Md Saidur Rahman, Department of Obstetrics, Gynecology and Women's Health, University of Missouri, Columbia, MO, USA.
Jae-Wook Jeong, Department of Obstetrics, Gynecology and Women's Health, University of Missouri, Columbia, MO, USA.
Funding
The research and writing of this publication were supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD084478, R01HD102170, R01HD101243, P01HD106485, and R01HD108895 to J.W.J.; F31HD101207, T32HD087166, MSU AgBio Research, and Michigan State University to R.M.M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
The authors have nothing to disclose.
References
- 1. Bulun SE, Yilmaz BD, Sison C, et al. . Endometriosis. Endocr Rev. 2019;40(4):1048‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184(11):2807‐2824. [DOI] [PubMed] [Google Scholar]
- 3. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244‐1256. [DOI] [PubMed] [Google Scholar]
- 4. Nyholt DR, Low SK, Anderson CA, et al. . Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012;44(12):1355‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update. 2014;20(5):702‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sapkota Y, Steinthorsdottir V, Morris AP, et al. . Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 2017;8:15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Pathogenesis of endometriosis: the genetic/epigenetic theory. Fertil Steril. 2019;111(2):327‐340. [DOI] [PubMed] [Google Scholar]
- 8. Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15(10):587‐607. [DOI] [PubMed] [Google Scholar]
- 9. Deans C, Maggert KA. What do you mean, “epigenetic”? Genetics. 2015;199(4):887‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ding L, Yang L, Ren C, et al. . A review of aberrant DNA methylation and epigenetic agents targeting DNA methyltransferases in endometriosis. Curr Drug Targets. 2020;21(11):1047‐1055. [DOI] [PubMed] [Google Scholar]
- 11. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil Steril. 2007;87(1):24‐32. [DOI] [PubMed] [Google Scholar]
- 12. Szczepanska M, Wirstlein P, Skrzypczak J, Jagodzinski PP. Expression of HOXA11 in the mid-luteal endometrium from women with endometriosis-associated infertility. Reprod Biol Endocrinol. 2012;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dyson MT, Kakinuma T, Pavone ME, et al. . Aberrant expression and localization of deoxyribonucleic acid methyltransferase 3B in endometriotic stromal cells. Fertil Steril. 2015;104(4):953‐963.e952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Kaam KJ, Delvoux B, Romano A, D'Hooghe T, Dunselman GA, Groothuis PG. Deoxyribonucleic acid methyltransferases and methyl-CpG-binding domain proteins in human endometrium and endometriosis. Fertil Steril. 2011;95(4):1421‐1427. [DOI] [PubMed] [Google Scholar]
- 15. Hsiao KY, Wu MH, Chang N, et al. . Coordination of AUF1 and miR-148a destabilizes DNA methyltransferase 1 mRNA under hypoxia in endometriosis. Mol Hum Reprod. 2015;21(12):894‐904. [DOI] [PubMed] [Google Scholar]
- 16. Wang L, Zhao J, Li Y, Wang Z, Kang S. Genome-wide analysis of DNA methylation in endometriosis using Illumina human methylation 450 K BeadChips. Mol Reprod Dev. 2019;86(5):491‐501. [DOI] [PubMed] [Google Scholar]
- 17. Veena KV, Siddamalla S, Deenadayal M, Shivaji S, Bhanoori M. DNMT1 And DNMT3B gene variants and their association with endometriosis in South Indian women. Mol Biol Rep. 2022;49(1):321‐329. [DOI] [PubMed] [Google Scholar]
- 18. Arosh JA, Lee J, Banu SK. Effects of dual inhibition of AKT and ERK1/2 pathways on endometrial pro-inflammatory, hormonal, and epigenetic microenvironment in endometriosis. Mol Cell Endocrinol. 2022;539:111446. [DOI] [PubMed] [Google Scholar]
- 19. Bhutani N, Burns DM, Blau HM. DNA Demethylation dynamics. Cell. 2011;146(6):866‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roca FJ, Loomans HA, Wittman AT, Creighton CJ, Hawkins SM. Ten-eleven translocation genes are downregulated in endometriosis. Curr Mol Med. 2016;16(3):288‐298. [DOI] [PubMed] [Google Scholar]
- 21. Szczepanska M, Wirstlein P, Zawadzka M, Wender-Ozegowska E, Jagodzinski PP. Alternation of ten-eleven translocation 1, 2, and 3 expression in eutopic endometrium of women with endometriosis-associated infertility. Gynecol Endocrinol. 2018;34(12):1084‐1090. [DOI] [PubMed] [Google Scholar]
- 22. Adamczyk M, Rawluszko-Wieczorek AA, Wirstlein P, et al. . Assessment of TET1 gene expression, DNA methylation and H3K27me3 level of its promoter region in eutopic endometrium of women with endometriosis and infertility. Biomed Pharmacother. 2022;150:112989. [DOI] [PubMed] [Google Scholar]
- 23. Xiao L, Pei T, Huang W, et al. . MicroRNA22-5p targets ten-eleven translocation and regulates estrogen receptor 2 expression in infertile women with minimal/mild endometriosis during implantation window. PLoS One. 2020;15(7):e0234086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu J, Li X, Huang H, Xia X, Zhang M, Fang X. TET1 may contribute to hypoxia-induced epithelial to mesenchymal transition of endometrial epithelial cells in endometriosis. PeerJ. 2020;8:e9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Devailly G, Grandin M, Perriaud L, et al. . Dynamics of MBD2 deposition across methylated DNA regions during malignant transformation of human mammary epithelial cells. Nucleic Acids Res. 2015;43(12):5838‐5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wood KH, Zhou Z. Emerging molecular and biological functions of MBD2, a reader of DNA methylation. Front Genet. 2016;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Millan-Zambrano G, Burton A, Bannister AJ, Schneider R. Histone post-translational modifications—cause and consequence of genome function. Nat Rev Genet. 2022;23:563‐580. [DOI] [PubMed] [Google Scholar]
- 28. Adamczyk M, Wender-Ozegowska E, Kedzia M. Epigenetic factors in eutopic endometrium in women with endometriosis and infertility. Int J Mol Sci. 2022;23(7):3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6(4):a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imesch P, Fink D, Fedier A. Romidepsin reduces histone deacetylase activity, induces acetylation of histones, inhibits proliferation, and activates apoptosis in immortalized epithelial endometriotic cells. Fertil Steril. 2010;94(7):2838‐2842. [DOI] [PubMed] [Google Scholar]
- 31. Imesch P, Samartzis EP, Schneider M, Fink D, Fedier A. Inhibition of transcription, expression, and secretion of the vascular epithelial growth factor in human epithelial endometriotic cells by romidepsin. Fertil Steril. 2011;95(5):1579‐1583. [DOI] [PubMed] [Google Scholar]
- 32. Kawano Y, Nasu K, Li H, et al. . Application of the histone deacetylase inhibitors for the treatment of endometriosis: histone modifications as pathogenesis and novel therapeutic target. Hum Reprod. 2011;26(9):2486‐2498. [DOI] [PubMed] [Google Scholar]
- 33. Xiaomeng X, Ming Z, Jiezhi M, Xiaoling F. Aberrant histone acetylation and methylation levels in woman with endometriosis. Arch Gynecol Obstet. 2013;287(3):487‐494. [DOI] [PubMed] [Google Scholar]
- 34. Zhu LH, Sun LH, Hu YL, et al. . PCAF impairs endometrial receptivity and embryo implantation by down-regulating beta3-integrin expression via HOXA10 acetylation. J Clin Endocrinol Metab. 2013;98(11):4417‐4428. [DOI] [PubMed] [Google Scholar]
- 35. Monteiro JB, Colon-Diaz M, Garcia M, et al. . Endometriosis is characterized by a distinct pattern of histone 3 and histone 4 lysine modifications. Reprod Sci. 2014;21(3):305‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Colon-Diaz M, Baez-Vega P, Garcia M, et al. . HDAC1 And HDAC2 are differentially expressed in endometriosis. Reprod Sci. 2012;19(5):483‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Samartzis EP, Noske A, Samartzis N, Fink D, Imesch P. The expression of histone deacetylase 1, but not other class I histone deacetylases, is significantly increased in endometriosis. Reprod Sci. 2013;20(12):1416‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang L, Yu Z, Qu Q, Li X, Lu X, Zhang H. Exosomal lncRNA HOTAIR promotes the progression and angiogenesis of endometriosis via the miR-761/HDAC1 axis and activation of STAT3-mediated inflammation. Int J Nanomedicine. 2022;17:1155‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao T, Liu X, Zhen X, Guo SW. Levo-tetrahydropalmatine retards the growth of ectopic endometrial implants and alleviates generalized hyperalgesia in experimentally induced endometriosis in rats. Reprod Sci. 2011;18(1):28‐45. [DOI] [PubMed] [Google Scholar]
- 40. Mai H, Liao Y, Luo S, Wei K, Yang F, Shi H. Histone deacetylase HDAC2 silencing prevents endometriosis by activating the HNF4A/ARID1A axis. J Cell Mol Med. 2021;25(21):9972‐9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li H, Cai E, Cheng H, et al. . FGA Controls VEGFA secretion to promote angiogenesis by activating the VEGFR2-FAK signalling pathway. Front Endocrinol (Lausanne). 2022;13:791860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim TH, Yoo JY, Choi KC, et al. . Loss of HDAC3 results in nonreceptive endometrium and female infertility. Sci Transl Med. 2019;11(474):eaaf7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daftary GS, Zheng Y, Tabbaa ZM, et al. . A novel role of the Sp/KLF transcription factor KLF11 in arresting progression of endometriosis. PLoS One. 2013;8(3):e60165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng Y, Khan Z, Zanfagnin V, Correa LF, Delaney AA, Daftary GS. Epigenetic modulation of collagen 1A1: therapeutic implications in fibrosis and endometriosis. Biol Reprod. 2016;94(4):87. [DOI] [PubMed] [Google Scholar]
- 45. Yoo JY, Kim TH, Fazleabas AT, et al. . KRAS activation and over-expression of SIRT1/BCL6 contributes to the pathogenesis of endometriosis and progesterone resistance. Sci Rep. 2017;7(1):6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim TH, Young SL, Sasaki T, et al. . Role of SIRT1 and progesterone resistance in normal and abnormal endometrium. J Clin Endocrinol Metab. 2021;107(3):788‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hwang YJ, Sung GJ, Marquardt R, et al. . SIRT1 plays an important role in implantation and decidualization during mouse early pregnancy. Biol Reprod. 2022;106(6):1072‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Squatrito M, Blacher S, Henry L, et al. . Comparison of morphological and digital-assisted analysis for BCL6 endometrial expression in women with endometriosis. J Clin Med. 2022;11(20):6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sansone AM, Hisrich BV, Young RB, et al. . Evaluation of BCL6 and SIRT1 as non-invasive diagnostic markers of endometriosis. Curr Issues Mol Biol. 2021;43(3):1350‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Colon-Caraballo M, Monteiro JB, Flores I. H3k27me3 is an epigenetic mark of relevance in endometriosis. Reprod Sci. 2015;22(9):1134‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Q, Dong P, Liu X, Sakuragi N, Guo SW. Enhancer of zeste homolog 2 (EZH2) induces epithelial-mesenchymal transition in endometriosis. Sci Rep. 2017;7(1):6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Colon-Caraballo M, Torres-Reveron A, Soto-Vargas JL, et al. . Effects of histone methyltransferase inhibition in endometriosis. Biol Reprod. 2018;99(2):293‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seguinot-Tarafa I, Luna N, Suarez E, Appleyard CB, Flores I. Inhibition of histone methyltransferase EZH2 suppresses endometriotic vesicle development in a rat model of endometriosis. Reprod Sci. 2020;27(9):1812‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brunty S, Ray Wright K, Mitchell B, Santanam N. Peritoneal modulators of EZH2-miR-155 cross-talk in endometriosis. Int J Mol Sci. 2021;22(7):3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nanjappa MK, Mesa AM, Medrano TI, et al. . The histone methyltransferase EZH2 is required for normal uterine development and function in mice. Biol Reprod. 2019;101(2):306‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wen X, Xiong Y, Liu H, et al. . Decreased mixed lineage leukemia 1 is involved in endometriosis-related infertility. J Mol Endocrinol. 2021;66(1):45‐57. [DOI] [PubMed] [Google Scholar]
- 57. Kim D, Kim KI, Baek SH. Roles of lysine-specific demethylase 1 (LSD1) in homeostasis and diseases. J Biomed Sci. 2021;28(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ding D, Liu X, Guo SW. Overexpression of lysine-specific demethylase 1 in ovarian endometriomas and its inhibition reduces cellular proliferation, cell cycle progression, and invasiveness. Fertil Steril. 2014;101(3):740‐749. [DOI] [PubMed] [Google Scholar]
- 59. Sun Q, Ding D, Liu X, Guo SW. Tranylcypromine, a lysine-specific demethylase 1 (LSD1) inhibitor, suppresses lesion growth and improves generalized hyperalgesia in mouse with induced endometriosis. Reprod Biol Endocrinol. 2016;14(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rytkonen KT, Faux T, Mahmoudian M, et al. . Histone H3K4me3 breadth in hypoxia reveals endometrial core functions and stress adaptation linked to endometriosis. iScience. 2022;25(5):104235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baumann C, Olson M, Wang K, Fazleabas A, De La Fuente R. Arginine methyltransferases mediate an epigenetic ovarian response to endometriosis. Reproduction. 2015;150(4):297‐310. [DOI] [PubMed] [Google Scholar]
- 62. Centore RC, Sandoval GJ, Soares LMM, Kadoch C, Chan HM. Mammalian SWI/SNF chromatin remodeling complexes: emerging mechanisms and therapeutic strategies. Trends Genet. 2020;36(12):936‐950. [DOI] [PubMed] [Google Scholar]
- 63. Mathur R. ARID1A Loss in cancer: towards a mechanistic understanding. Pharmacol Ther. 2018;190:15‐23. [DOI] [PubMed] [Google Scholar]
- 64. He S, Wu Z, Tian Y, et al. . Structure of nucleosome-bound human BAF complex. Science. 2020;367(6480):875‐881. [DOI] [PubMed] [Google Scholar]
- 65. Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci U S A. 2008;105(18):6656‐6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun X, Chuang JC, Kanchwala M, et al. . Suppression of the SWI/SNF component Arid1a promotes mammalian regeneration. Cell Stem Cell. 2016;18(4):456‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Y, Hoang L, Ji JX, Huntsman DG. SWI/SNF complex mutations in gynecologic cancers: molecular mechanisms and models. Annu Rev Pathol. 2020;15(1):467‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Anglesio MS, Papadopoulos N, Ayhan A, et al. . Cancer-associated mutations in endometriosis without cancer. N Engl J Med. 2017;376(19):1835‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Suda K, Nakaoka H, Yoshihara K, et al. . Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Rep. 2018;24(7):1777‐1789. [DOI] [PubMed] [Google Scholar]
- 70. Lac V, Nazeran TM, Tessier-Cloutier B, et al. . Oncogenic mutations in histologically normal endometrium: the new normal? J Pathol. 2019;249(2):173‐181. [DOI] [PubMed] [Google Scholar]
- 71. Kim TH, Yoo JY, Wang Z, et al. . ARID1A is essential for endometrial function during early pregnancy. PLoS Genet. 2015;11(9):e1005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Falconer H, Sundqvist J, Xu H, et al. . Analysis of common variations in tumor-suppressor genes on chr1p36 among Caucasian women with endometriosis. Gynecol Oncol. 2012;127(2):398‐402. [DOI] [PubMed] [Google Scholar]
- 73. Wang X, Li X, Wang T, et al. . SOX17 regulates uterine epithelial-stromal cross-talk acting via a distal enhancer upstream of ihh. Nat Commun. 2018;9(1):4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Winarto H, Tan MI, Sadikin M, Wanandi SI. ARID1A expression is down-regulated by oxidative stress in endometriosis and endometriosis-associated ovarian cancer. Transl Oncogenomics. 2017;9:1177272716689818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xie H, Chen P, Huang HW, Liu LP, Zhao F. Reactive oxygen species downregulate ARID1A expression via its promoter methylation during the pathogenesis of endometriosis. Eur Rev Med Pharmacol Sci. 2017;21(20):4509‐4515. [PubMed] [Google Scholar]
- 76. Wilson MR, Reske JJ, Holladay J, et al. . ARID1A And PI3-kinase pathway mutations in the endometrium drive epithelial transdifferentiation and collective invasion. Nat Commun. 2019;10(1):3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reske JJ, Wilson MR, Holladay J, et al. . Co-existing TP53 and ARID1A mutations promote aggressive endometrial tumorigenesis. PLoS Genet. 2021;17(12):e1009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim HI, Kim TH, Yoo JY, et al. . ARID1A and PGR proteins interact in the endometrium and reveal a positive correlation in endometriosis. Biochem Biophys Res Commun. 2021;550:151‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Marquardt RM, Kim TH, Yoo JY, et al. . Endometrial epithelial ARID1A is critical for uterine gland function in early pregnancy establishment. FASEB J. 2021;35(2):e21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stewart CL. The role of leukemia inhibitory factor (LIF) and other cytokines in regulating implantation in mammals. Ann N Y Acad Sci. 1994;734(1):157‐165. [DOI] [PubMed] [Google Scholar]
- 81. Hansen AS, Pustova I, Cattoglio C, Tjian R, Darzacq X. CTCF and cohesin regulate chromatin loop stability with distinct dynamics. Elife. 2017;6:e25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guo J, Cao B, Xu X, Wu F, Zhu B. Novel CTCF mutations in Chinese patients with ovarian endometriosis. Mol Med Rep. 2018;18(1):1031‐1036. [DOI] [PubMed] [Google Scholar]
- 83. Li X, Zhang Y, Zhao L, et al. . Whole-exome sequencing of endometriosis identifies frequent alterations in genes involved in cell adhesion and chromatin-remodeling complexes. Hum Mol Genet. 2014;23(22):6008‐6021. [DOI] [PubMed] [Google Scholar]
- 84. Zhang Y, Liang J, Li Y, et al. . CCCTC-binding factor acts upstream of FOXA1 and demarcates the genomic response to estrogen. J Biol Chem. 2010;285(37):28604‐28613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. George JW, Fan H, Johnson B, et al. . Integrated epigenome, exome, and transcriptome analyses reveal molecular subtypes and homeotic transformation in uterine fibroids. Cell Rep. 2019;29(12):4069‐4085.e4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Marshall AD, Bailey CG, Champ K, et al. . CTCF genetic alterations in endometrial carcinoma are pro-tumorigenic. Oncogene. 2017;36(29):4100‐4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gordon PB. MRI screening in women with dense breasts. N Engl J Med. 2020;382(13):1283. [DOI] [PubMed] [Google Scholar]
- 88. Kao LC, Germeyer A, Tulac S, et al. . Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870‐2881. [DOI] [PubMed] [Google Scholar]
- 89. Rocha-Junior CV, Da Broi MG, Miranda-Furtado CL, Navarro PA, Ferriani RA, Meola J. Progesterone receptor B (PGR-B) is partially methylated in eutopic endometrium from infertile women with endometriosis. Reprod Sci. 2019;26(12):1568‐1574. [DOI] [PubMed] [Google Scholar]
- 90. Maekawa R, Mihara Y, Sato S, et al. . Aberrant DNA methylation suppresses expression of estrogen receptor 1 (ESR1) in ovarian endometrioma. J Ovarian Res. 2019;12(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dyson MT, Roqueiro D, Monsivais D, et al. . Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10(3):e1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Samadieh Y, Favaedi R, Ramezanali F, Afsharian P, Aflatoonian R, Shahhoseini M. Epigenetic dynamics of HOXA10 gene in infertile women with endometriosis. Reprod Sci. 2019;26(1):88‐96. [DOI] [PubMed] [Google Scholar]
- 93. Li Y, An D, Guan YX, Kang S. Aberrant methylation of the E-cadherin gene promoter region in endometrium and ovarian endometriotic cysts of patients with ovarian endometriosis. Gynecol Obstet Invest. 2017;82(1):78‐85. [DOI] [PubMed] [Google Scholar]
- 94. Guan QH, Shi WJ, Zhou LS, Tao AL, Li L. Effect of epigallocatechin-3-gallate on the status of DNA methylation of E-cadherin promoter region on endometriosis mouse. J Obstet Gynaecol Res. 2020;46(10):2076‐2083. [DOI] [PubMed] [Google Scholar]
- 95. Xue Q, Zhou YF, Zhu SN, Bulun SE. Hypermethylation of the CpG island spanning from exon II to intron III is associated with steroidogenic factor 1 expression in stromal cells of endometriosis. Reprod Sci. 2011;18(11):1080‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xue Q, Xu Y, Yang H, et al. . Methylation of a novel CpG island of intron 1 is associated with steroidogenic factor 1 expression in endometriotic stromal cells. Reprod Sci. 2014;21(3):395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang D, Guo C, Li Y, et al. . Oestrogen up-regulates DNMT1 and leads to the hypermethylation of RUNX3 in the malignant transformation of ovarian endometriosis. Reprod Biomed Online. 2021;44(1):27‐37. [DOI] [PubMed] [Google Scholar]
- 98. Kohlmeier A, Sison CAM, Yilmaz BD, Coon VJ, Dyson MT, Bulun SE. GATA2 And progesterone receptor interaction in endometrial stromal cells undergoing decidualization. Endocrinology. 2020;161(6):bqaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Smolarz B, Szyllo K, Romanowicz H. Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (review of literature). Int J Mol Sci. 2021;22(19):10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gujral P, Mahajan V, Lissaman AC, Ponnampalam AP. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod Biol Endocrinol. 2020;18(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chen H, Malentacchi F, Fambrini M, Harrath AH, Huang H, Petraglia F. Epigenetics of estrogen and progesterone receptors in endometriosis. Reprod Sci. 2020;27(11):1967‐1974. [DOI] [PubMed] [Google Scholar]
- 102. Liu H, Huang X, Mor G, Liao A. Epigenetic modifications working in the decidualization and endometrial receptivity. Cell Mol Life Sci. 2020;77(11):2091‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Borghese B, Zondervan KT, Abrao MS, Chapron C, Vaiman D. Recent insights on the genetics and epigenetics of endometriosis. Clin Genet. 2017;91(2):254‐264. [DOI] [PubMed] [Google Scholar]
- 104. Caplakova V, Babusikova E, Blahovcova E, Balharek T, Zelieskova M, Hatok J. DNA methylation machinery in the endometrium and endometrial cancer. Anticancer Res. 2016;36(9):4407‐4420. [DOI] [PubMed] [Google Scholar]
- 105. Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. 2019;111(4):611‐617. [DOI] [PubMed] [Google Scholar]
- 106. Burney RO, Talbi S, Hamilton AE, et al. . Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814‐3826. [DOI] [PubMed] [Google Scholar]
- 107. Zubrzycka A, Zubrzycki M, Perdas E, Zubrzycka M. Genetic, epigenetic, and steroidogenic modulation mechanisms in endometriosis. J Clin Med. 2020;9(5):1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623‐632. [DOI] [PubMed] [Google Scholar]
- 109. Bulun SE, Monsivais D, Kakinuma T, et al. . Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33(3):220‐224. [DOI] [PubMed] [Google Scholar]
- 110. Zhu X, Chen Z, Shen W, et al. . Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther. 2021;6(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shen J, Abu-Amer Y, O'Keefe RJ, McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. 2017;58(1):49‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. AlAshqar A, Reschke L, Kirschen GW, Borahay MA. Role of inflammation in benign gynecologic disorders: from pathogenesis to novel therapies. Biol Reprod. 2021;105(1):7‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang Y, Nicholes K, Shih IM. The origin and pathogenesis of endometriosis. Ann Rev Pathol. 2020;15(1):71‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bulun SE, Yilmaz BD, Sison C, et al. . Endometriosis. Endocr Rev. 2019;40(4):1048‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Houshdaran S, Nezhat CR, Vo KC, Zelenko Z, Irwin JC, Giudice LC. Aberrant endometrial DNA methylome and associated gene expression in women with endometriosis. Biol Reprod. 2016;95(5):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol. 2012;358(2):208‐215. [DOI] [PubMed] [Google Scholar]
- 118. Halis G, Arici A. Endometriosis and inflammation in infertility. Ann N Y Acad Sci. 2004;1034(1):300‐315. [DOI] [PubMed] [Google Scholar]
- 119. Peros WJ, Gibbons RJ. Evidence suggesting multiple binding sites in experimental pellicles for Streptococcus mutans JBP. J Dent Res. 1986;65(11):1332‐1334. [DOI] [PubMed] [Google Scholar]
- 120. Halme J, Becker S, Wing R. Accentuated cyclic activation of peritoneal macrophages in patients with endometriosis. Am J Obstet Gynecol. 1984;148(1):85‐90. [DOI] [PubMed] [Google Scholar]
- 121. Haney AF, Muscato JJ, Weinberg JB. Peritoneal fluid cell populations in infertility patients. Fertil Steril. 1981;35(6):696‐698. [DOI] [PubMed] [Google Scholar]
- 122. Dekel N, Gnainsky Y, Granot I, Racicot K, Mor G. The role of inflammation for a successful implantation. Am J Reprod Immunol. 2014;72(2):141‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord. 2012;13(4):277‐288. [DOI] [PubMed] [Google Scholar]
- 124. Omatola CA, Okolo MO, Adaji DM, et al. . Coinfection of human immunodeficiency virus-infected patients with hepatitis B virus in Lokoja, north central Nigeria. Viral Immunol. 2020;33(5):391‐395. [DOI] [PubMed] [Google Scholar]
- 125. Critchley HOD, Maybin JA, Armstrong GM, Williams ARW. Physiology of the endometrium and regulation of menstruation. Physiol Rev. 2020;100(3):1149‐1179. [DOI] [PubMed] [Google Scholar]
- 126. Dey SK, Lim H, Das SK, et al. . Molecular cues to implantation. Endocr Rev. 2004;25(3):341‐373. [DOI] [PubMed] [Google Scholar]
- 127. Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med. 2009;27(1):62‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340(23):1796‐1799. [DOI] [PubMed] [Google Scholar]
- 129. Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCartyKS, Jr. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67(2):334‐340. [DOI] [PubMed] [Google Scholar]
- 130. Garcia E, Bouchard P, De Brux J, et al. . Use of immunocytochemistry of progesterone and estrogen receptors for endometrial dating. J Clin Endocrinol Metab. 1988;67(1):80‐87. [DOI] [PubMed] [Google Scholar]
- 131. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28(1):17‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27(1):17‐46. [DOI] [PubMed] [Google Scholar]
- 133. Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1999;84(8):2963‐2971. [DOI] [PubMed] [Google Scholar]
- 134. Reis FM, Coutinho LM, Vannuccini S, Batteux F, Chapron C, Petraglia F. Progesterone receptor ligands for the treatment of endometriosis: the mechanisms behind therapeutic success and failure. Hum Reprod Update. 2020;26(4):565‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21(2):155‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Beliard A, Noel A, Foidart JM. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril. 2004;82(1):80‐85. [DOI] [PubMed] [Google Scholar]
- 137. Lessey BA, Metzger DA, Haney AF, McCartyKS, Jr. Immunohistochemical analysis of estrogen and progesterone receptors in endometriosis: comparison with normal endometrium during the menstrual cycle and the effect of medical therapy. Fertil Steril. 1989;51(3):409‐415. [DOI] [PubMed] [Google Scholar]
- 138. Bedaiwy MA, Dahoud W, Skomorovska-Prokvolit Y, et al. . Abundance and localization of progesterone receptor isoforms in endometrium in women with and without endometriosis and in peritoneal and ovarian endometriotic implants. Reprod Sci. 2015;22(9):1153‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106‐111. [DOI] [PubMed] [Google Scholar]
- 140. Eaton JL, Unno K, Caraveo M, Lu Z, Kim JJ. Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. J Clin Endocrinol Metab. 2013;98(12):E1871‐E1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897‐2902. [DOI] [PubMed] [Google Scholar]
- 142. Barjaste N, Shahhoseini M, Afsharian P, Sharifi-Zarchi A, Masoudi-Nejad A. Genome-wide DNA methylation profiling in ectopic and eutopic of endometrial tissues. J Assist Reprod Genet. 2019;36(8):1743‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lac V, Verhoef L, Aguirre-Hernandez R, et al. . Iatrogenic endometriosis harbors somatic cancer-driver mutations. Hum Reprod. 2019;34(1):69‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wiegand KC, Shah SP, Al-Agha OM, et al. . ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wilson MR, Reske JJ, Holladay J, et al. . ARID1A mutations promote P300-dependent endometrial invasion through super-enhancer hyperacetylation. Cell Rep. 2020;33(6):108366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Teasley HE, Beesley A, Kim TH, et al. . Differential expression of KRAS and SIRT1 in ovarian cancers with and without endometriosis. Reprod Sci. 2020;27(1):145‐151. [DOI] [PubMed] [Google Scholar]
- 147. Kim TH, Young SL, Sasaki T, et al. . Role of SIRT1 and progesterone resistance in normal and abnormal endometrium. J Clin Endocrinol Metab. 2022;107(3):788‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rezk NA, Lashin MB, Sabbah NA. MiRNA 34-a regulate SIRT-1 and Foxo-1 expression in endometriosis. Noncoding RNA Res. 2021;6(1):35‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Rane S, He M, Sayed D, et al. . Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104(7):879‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Han L, Wang P, Zhao G, et al. . Upregulation of SIRT1 by 17beta-estradiol depends on ubiquitin-proteasome degradation of PPAR-gamma mediated by NEDD4-1. Protein Cell. 2013;4(4):310‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ye X, Wang L, Shang B, Wang Z, Wei W. NEDD4: a promising target for cancer therapy. Curr Cancer Drug Targets. 2014;14(6):549‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Symons LK, Miller JE, Kay VR, et al. . The immunopathophysiology of endometriosis. Trends Mol Med. 2018;24(9):748‐762. [DOI] [PubMed] [Google Scholar]
- 153. Miller JE, Ahn SH, Monsanto SP, Khalaj K, Koti M, Tayade C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget. 2017;8(4):7138‐7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Houshdaran S, Oke AB, Fung JC, Vo KC, Nezhat C, Giudice LC. Steroid hormones regulate genome-wide epigenetic programming and gene transcription in human endometrial cells with marked aberrancies in endometriosis. PLoS Genet. 2020;16(6):e1008601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Izawa M, Taniguchi F, Terakawa N, Harada T. Epigenetic aberration of gene expression in endometriosis. Front Biosci (Elite Ed). 2013;5(3):900‐910. [DOI] [PubMed] [Google Scholar]
- 157. Marquardt RM, Kim TH, Shin JH, Jeong JW. Progesterone and estrogen signaling in the endometrium: what goes wrong in endometriosis? Int J Mol Sci. 2019;20(15):3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. McKinnon B, Mueller M, Montgomery G. Progesterone resistance in endometriosis: an acquired property? Trends Endocrinol Metab. 2018;29(8):535‐548. [DOI] [PubMed] [Google Scholar]
- 159. Lessey BA, Kim JJ. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil Steril. 2017;108(1):19‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pavone ME, Malpani S, Dyson M, Bulun SE. Altered retinoid signaling compromises decidualization in human endometriotic stromal cells. Reproduction. 2017;154(3):207‐216. [DOI] [PubMed] [Google Scholar]
- 161. Vasquez YM, Wang X, Wetendorf M, et al. . FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet. 2018;14(11):e1007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Cakmak H, Taylor HS. Molecular mechanisms of treatment resistance in endometriosis: the role of progesterone-hox gene interactions. Semin Reprod Med. 2010;28(1):69‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab. 2010;95(11):E300‐E309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011;17(5):637‐653. [DOI] [PubMed] [Google Scholar]
- 165. Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol. 2005;193(2):371‐380. [DOI] [PubMed] [Google Scholar]
- 166. Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin Reprod Med. 2013;31(2):109‐124. [DOI] [PubMed] [Google Scholar]
- 167. Rubel CA, Wu SP, Lin L, et al. . A Gata2-dependent transcription network regulates uterine progesterone responsiveness and endometrial function. Cell Rep. 2016;17(5):1414‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Rubel CA, Franco HL, Jeong JW, Lydon JP, DeMayo FJ. GATA2 is expressed at critical times in the mouse uterus during pregnancy. Gene Expr Patterns. 2012;12(5-6):196‐203. [DOI] [PubMed] [Google Scholar]
- 169. Jacobsen BM, Horwitz KB. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol. 2012;357(1-2):18‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Horwitz KB, Sheridan PL, Wei LL, Krett NL. Human progesterone receptors: synthesis, structure, and phosphorylation. Prog Clin Biol Res. 1990;322:41‐52. [PubMed] [Google Scholar]
- 171. Jacobsen BM, Richer JK, Schittone SA, Horwitz KB. New human breast cancer cells to study progesterone receptor isoform ratio effects and ligand-independent gene regulation. J Biol Chem. 2002;277(31):27793‐27800. [DOI] [PubMed] [Google Scholar]
- 172. Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7(10):1244‐1255. [DOI] [PubMed] [Google Scholar]
- 173. Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011;22(4):145‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Wu Y, Shi X, Guo SW. The knockdown of progesterone receptor isoform B (PR-B) promotes proliferation in immortalized endometrial stromal cells. Fertil Steril. 2008;90(4):1320‐1323. [DOI] [PubMed] [Google Scholar]
- 175. Li R, Wang X, Huang Z, et al. . The role of epithelial progesterone receptor isoforms in embryo implantation. iScience. 2021;24(12):103487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lessey BA, Yeh I, Castelbaum AJ, et al. . Endometrial progesterone receptors and markers of uterine receptivity in the window of implantation. Fertil Steril. 1996;65(3):477‐483. [PubMed] [Google Scholar]
- 177. Garcia-Gomez E, Vazquez-Martinez ER, Reyes-Mayoral C, Cruz-Orozco OP, Camacho-Arroyo I, Cerbon M. Regulation of inflammation pathways and inflammasome by sex steroid hormones in endometriosis. Front Endocrinol (Lausanne). 2019;10:935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17(2):242‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Senawong T, Peterson VJ, Avram D, et al. . Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2-mediated transcriptional repression. J Biol Chem. 2003;278(44):43041‐43050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358(2):155‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Baxter EW, Milner J. P53 regulates LIF expression in human medulloblastoma cells. J Neurooncol. 2010;97(3):373‐382. [DOI] [PubMed] [Google Scholar]
- 182. Stewart CL, Kaspar P, Brunet LJ, et al. . Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992:359(6390):76‐79. [DOI] [PubMed] [Google Scholar]
- 183. Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci U S A. 1996;93(7):3115‐3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Directory of members: Aerospace Medical Association. Aviat Space Environ Med. 1987;58(9 Pt 1):860‐924. [PubMed] [Google Scholar]
- 185. Horne AW, Daniels J, Hummelshoj L, Cox E, Cooper KG. Surgical removal of superficial peritoneal endometriosis for managing women with chronic pelvic pain: time for a rethink? BJOG. 2019;126(12):1414‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Cobellis L, Razzi S, De Simone S, et al. . The treatment with a COX-2 specific inhibitor is effective in the management of pain related to endometriosis. Eur J Obstet Gynecol Reprod Biol. 2004;116(1):100‐102. [DOI] [PubMed] [Google Scholar]
- 187. Hayes EC, Rock JA. COX-2 inhibitors and their role in gynecology. Obstet Gynecol Surv. 2002;57(11):768‐780. [DOI] [PubMed] [Google Scholar]
- 188. Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16(3):561‐566. [DOI] [PubMed] [Google Scholar]
- 189. Koike N, Higashiura Y, Akasaka J, Uekuri C, Ito F, Kobayashi H. Epigenetic dysregulation of endometriosis susceptibility genes (review). Mol Med Rep. 2015;12(2):1611‐1616. [DOI] [PubMed] [Google Scholar]
- 190. Grimstad FW, Decherney A. A review of the epigenetic contributions to endometriosis. Clin Obstet Gynecol. 2017;60(3):467‐476. [DOI] [PubMed] [Google Scholar]
- 191. Maia H Jr., Haddad C, Coelho G, Casoy J. Role of inflammation and aromatase expression in the eutopic endometrium and its relationship with the development of endometriosis. Womens Health (Lond). 2012;8(6):647‐658. [DOI] [PubMed] [Google Scholar]
- 192. Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319‐328. [DOI] [PubMed] [Google Scholar]
- 193. Donnez J, Dolmans MM. Endometriosis and medical therapy: from progestogens to progesterone resistance to GnRH antagonists: a review. J Clin Med. 2021;10(5):1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Vercellini P, Buggio L, Berlanda N, Barbara G, Somigliana E, Bosari S. Estrogen-progestins and progestins for the management of endometriosis. Fertil Steril. 2016;106(7):1552‐1571.e1552. [DOI] [PubMed] [Google Scholar]
- 195. Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008;90(5):1583‐1588. [DOI] [PubMed] [Google Scholar]
- 196. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2014;3:CD009590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Buggio L, Somigliana E, Barbara G, Frattaruolo MP, Vercellini P. Oral and depot progestin therapy for endometriosis: towards a personalized medicine. Expert Opin Pharmacother. 2017;18(15):1569‐1581. [DOI] [PubMed] [Google Scholar]
- 198. Casper RF. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil Steril. 2017;107(3):533‐536. [DOI] [PubMed] [Google Scholar]
- 199. Andres Mde P, Lopes LA, Baracat EC, Podgaec S. Dienogest in the treatment of endometriosis: systematic review. Arch Gynecol Obstet. 2015;292(3):523‐529. [DOI] [PubMed] [Google Scholar]
- 200. Szukiewicz D, Stangret A, Ruiz-Ruiz C, et al. . Estrogen- and progesterone (P4)-mediated epigenetic modifications of endometrial stromal cells (EnSCs) and/or mesenchymal stem/stromal cells (MSCs) in the etiopathogenesis of endometriosis. Stem Cell Rev Rep. 2021;17(4):1174‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone receptor status predicts response to progestin therapy in endometriosis. J Clin Endocrinol Metab. 2018;103(12):4561‐4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron add-back study group. Obstet Gynecol. 1998;91(1):16‐24. [DOI] [PubMed] [Google Scholar]
- 203. Barbieri RL. Primary gonadotropin-releasing hormone agonist therapy for suspected endometriosis: a nonsurgical approach to the diagnosis and treatment of chronic pelvic pain. Am J Manag Care. 1997;3(2):285‐290. [PubMed] [Google Scholar]
- 204. Dababou S, Garzon S, Lagana AS, et al. . Linzagolix: a new GnRH-antagonist under investigation for the treatment of endometriosis and uterine myomas. Expert Opin Investig Drugs. 2021;30(9):903‐911. [DOI] [PubMed] [Google Scholar]
- 205. Osuga Y, Enya K, Kudou K, Hoshiai H. Relugolix, a novel oral gonadotropin-releasing hormone antagonist, in the treatment of pain symptoms associated with uterine fibroids: a randomized, placebo-controlled, phase 3 study in Japanese women. Fertil Steril. 2019;112(5):922‐929.e922. [DOI] [PubMed] [Google Scholar]
- 206. Taylor HS, Giudice LC, Lessey BA, et al. . Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28‐40. [DOI] [PubMed] [Google Scholar]
- 207. Diamond MP, Carr B, Dmowski WP, et al. . Elagolix treatment for endometriosis-associated pain: results from a phase 2, randomized, double-blind, placebo-controlled study. Reprod Sci. 2014;21(3):363‐371. [DOI] [PubMed] [Google Scholar]
- 208. Carr B, Giudice L, Dmowski WP, et al. . Elagolix, an oral GnRH antagonist for endometriosis-associated pain: a randomized controlled study. J Endometr Pelvic Pain Disord. 2013;5(3):105‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Steiner N, Shrem G, Tannus S, et al. . Effect of GnRH agonist and letrozole treatment in women with recurrent implantation failure. Fertil Steril. 2019;112(1):98‐104. [DOI] [PubMed] [Google Scholar]
- 210. Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6(11):838‐849. [DOI] [PubMed] [Google Scholar]
- 211. Xue Q, Lin Z, Cheng YH, et al. . Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77(4):681‐687. [DOI] [PubMed] [Google Scholar]
- 212. Xue Q, Lin Z, Yin P, et al. . Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab. 2007;92(8):3261‐3267. [DOI] [PubMed] [Google Scholar]
- 213. Izawa M, Taniguchi F, Uegaki T, et al. . Demethylation of a nonpromoter cytosine-phosphate-guanine island in the aromatase gene may cause the aberrant up-regulation in endometriotic tissues. Fertil Steril. 2011;95(1):33‐39. [DOI] [PubMed] [Google Scholar]
- 214. Mund C, Lyko F. Epigenetic cancer therapy: proof of concept and remaining challenges. Bioessays. 2010;32(11):949‐957. [DOI] [PubMed] [Google Scholar]
- 215. Wu Y, Starzinski-Powitz A, Guo SW. Trichostatin A, a histone deacetylase inhibitor, attenuates invasiveness and reactivates E-cadherin expression in immortalized endometriotic cells. Reprod Sci. 2007;14(4):374‐382. [DOI] [PubMed] [Google Scholar]
- 216. Wu Y, Guo SW. Histone deacetylase inhibitors trichostatin A and valproic acid induce cell cycle arrest and p21 expression in immortalized human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol. 2008;137(2):198‐203. [DOI] [PubMed] [Google Scholar]
- 217. Wu Y, Guo SW. Suppression of IL-1beta-induced COX-2 expression by trichostatin A (TSA) in human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol. 2007;135(1):88‐93. [DOI] [PubMed] [Google Scholar]
- 218. Wu Y, Guo SW. Inhibition of proliferation of endometrial stromal cells by trichostatin A, RU486, CDB-2914, N-acetylcysteine, and ICI 182780. Gynecol Obstet invest. 2006;62(4):193‐205. [DOI] [PubMed] [Google Scholar]
- 219. Copeland RA, Solomon ME, Richon VM. Protein methyltransferases as a target class for drug discovery. Nat Rev Drug Discov. 2009;8(9):724‐732. [DOI] [PubMed] [Google Scholar]
- 220. Krusche CA, Vloet AJ, Classen-Linke I, von Rango U, Beier HM, Alfer J. Class I histone deacetylase expression in the human cyclic endometrium and endometrial adenocarcinomas. Hum Reprod. 2007;22(11):2956‐2966. [DOI] [PubMed] [Google Scholar]
- 221. Arosh JA, Lee J, Starzinski-Powitz A, Banu SK. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 modulates DNA methylation and histone modification machinery proteins in human endometriotic cells. Mol Cell Endocrinol. 2015;409:51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Tatone C, Di Emidio G, Barbonetti A, et al. . Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update. 2018;24(3):267‐289. [DOI] [PubMed] [Google Scholar]
- 223. Grechukhina O, Petracco R, Popkhadze S, et al. . A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med. 2012;4(3):206‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Cho S, Mutlu L, Zhou Y, Taylor HS. Aromatase inhibitor regulates let-7 expression and let-7f-induced cell migration in endometrial cells from women with endometriosis. Fertil Steril. 2016;106(3):673‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kong B, Tian Y, Zhu W, Su S, Kan Y. Effects of celecoxib and nimesulide on the proliferation of ectopic endometrial stromal cells in vitro. J Int Med Res. 2008;36(5):1032‐1038. [DOI] [PubMed] [Google Scholar]