Abstract
Background
Chinese herbal medicines have been used for a long time to treat osteoporosis. The evidence of their benefits and harms needs to be systematically reviewed.
Objectives
To assess the beneficial and harmful effects of Chinese herbal medicines as a general experimental intervention for treating primary osteoporosis by comparing herbal treatments with placebo, no intervention and conventional medicine.
Search methods
We searched the following electronic databases to January 2013: the Specialised Register of the Cochrane Complementary Medicine Field, CENTRAL, MEDLINE, EMBASE, LILACS, JICST‐E, AMED, Chinese Biomedical Database and CINAHL.
Selection criteria
Randomised controlled trials of Chinese herbal medicines compared with placebo, no intervention or conventional medicine were included.
Data collection and analysis
Two authors extracted data and assessed risk of bias independently. Disagreement was resolved by discussion.
Main results
One hundred and eight randomised trials involving 10,655 participants were included. Ninety‐nine different Chinese herbal medicines were tested and compared with placebo (three trials), no intervention (five trials) or conventional medicine (61 trials), or Chinese herbal medicines plus western medicine were compared with western medicine (47 trials). The risk of bias across all studies was unclear for most domains primarily due to inadequate reporting of study design. Although we rated the risk of selective reporting for all studies as unclear, only a few studies contributed numerical data to the key outcomes.
Seven trials reported fracture incidence, but they were small in sample size, suffered from various biases and tested different Chinese herbal medicines. These trials compared Kanggusong capsules versus placebo, Kanggusong granule versus Caltrate or ipriflavone plus Caltrate, Yigu capsule plus calcium versus placebo plus calcium, Xianlinggubao capsule plus Caltrate versus placebo plus Caltrate, Bushen Zhuanggu granules plus Caltrate versus placebo granules plus Caltrate, Kanggusong soup plus Caltrate versus Caltrate, Zhuangguqiangjin tablets and Shujinbogu tablets plus calcitonin ampoule versus calcitonin ampoule. The results were inconsistent.
One trial showed that Bushenhuoxue therapy plus calcium carbonate tablets and alfacalcidol had a better effect on quality of life score (scale 0 to 100, higher is better) than calcium carbonate tablets and alfacalcidol (mean difference (MD) 5.30; 95% confidence interval (CI) 3.67 to 6.93).
Compared with placebo in three separate trials, Chinese herbal medicines (Migu decoction, Bushen Yigu soft extract, Kanggusong capsules) showed a statistically significant increase in bone mineral density (BMD) (e.g. Kanggusong capsules, MD 0.06 g/cm3; 95% CI 0.02 to 0.10). Compared with no intervention in five trials, only two showed that Chinese herbal medicines had a statistically significant effect on increase in BMD (e.g. Shigu yin, MD 0.08 g/cm3; 95% CI 0.03 to 0.13). Compared with conventional medicine in 61 trials, 23 showed that Chinese herbal medicines had a statistically significant effect on increase in BMD. In 48 trials evaluating Chinese herbal medicines plus western medication against western medication, 26 showed better effects of the combination therapy on increase in BMD.
No trial reported death or serious adverse events of Chinese herbal medicines, while some trials reported minor adverse effects such as nausea, diarrhoea, etc.
Authors' conclusions
Current findings suggest that the beneficial effect of Chinese herbal medicines in improving BMD is still uncertain and more rigorous studies are warranted.
Plain language summary
Chinese herbal medicines for osteoporosis
Review question
We conducted a review of the effects of Chinese herbal medicines for people with primary osteoporosis. We found 108 studies with 10,655 people.
Background: what is primary osteoporosis and what are Chinese herbal medicines?
Bone is a living, growing part of your body. Throughout your lifetime, new bone cells grow and old bone cells break down to make room for the new, stronger bone. When you have osteoporosis, the old bone breaks down faster than the new bone can replace it. As this happens, the bones lose minerals (such as calcium). This makes bones weaker and more likely to break even after a minor injury, like a little bump or fall.
Chinese herbal medicines are products made from any part of medicinal plants (leaves, stems, buds, flowers or roots). Sometimes non‐plant based components (for example insects, deer horn, snake, various shells and powdered fossil) are included. These can be used in the form of raw plant materials, or water or alcohol extracts of raw plant materials. Herbs can also be taken by mouth as capsules, tablets or liquids, or as injections.
Chinese herbal medicines are widely used in China for primary osteoporosis but their benefits and harms have not been appraised in order to inform clinical practice.
Study characteristics
After searching for all relevant studies up to January 2013, we found 108 studies with 10,655 people with osteoporosis. Ninety‐nine different Chinese herbal medicines were tested and compared with placebo (three trials), no intervention (five trials) or conventional medicine (61 trials), or Chinese herbal medicines plus conventional medicine were compared with conventional medicine (47 trials). The average length of treatment was 5.7 months (ranging from 3 to 12 months).
Key results: what happens to people with osteoporosis who take Chinese herbal medicines?
New fractures
We are uncertain whether Chinese herbal medicines reduce the chance of having a new bone fracture. Seven trials evaluated the incidence of fractures. However, these trials were small and had flaws in their methods.
Quality of life
People who took Bushenhuoxue therapy plus calcium carbonate tablets and alfacalcidol rated their quality of life to be 5.30 points better on a scale of 0 to 100 after three months compared to people who did not take the herbal medicine.
People who took Bushenhuoxue therapy plus calcium carbonate tablets and alfacalcidol rated their quality of life to be 56.05 on a scale of 0 to 100.
People who took calcium carbonate tablets and alfacalcidol rated their quality of life to be 50.75 on a scale of 0 to 100.
Serious side effects or deaths
No serious side effects or deaths occurred in the trials.
We often do not have precise information about side effects and complications. This is particularly true for rare but serious side effects. Possible side effects may include a mild stomach ache or diarrhoea.
Bone mineral density (the amount and type of minerals in the bone)
We found studies that compared Chinese herbal medicines with placebo (fake treatment), with no treatment and with conventional medicine. We also found studies that compared Chinese herbal medicines plus conventional medication with just conventional medication.
Compared to placebo (fake treatment), three studies showed that bone mineral density increased slightly with Chinese herbal medicines.
Compared to no treatment or conventional medicine, some studies showed an increase in bone mineral density with Chinese herbal medicines while others did not.
When Chinese herbal medicines plus conventional medication was compared with just conventional medicine, some studies showed an increase in bone mineral density while others did not.
Quality of the evidence
In people with osteoporosis:
‐ Chinese herbal medicines may improve bone mineral density and quality of life slightly. Further research is likely to change this estimate of how Chinese herbal medicines affect bone mineral density and quality of life.
‐ We are uncertain whether Chinese herbal medicines reduce the chance of having a new bone fracture.
‐ No trial reported death or serious side effects.
Summary of findings
Summary of findings for the main comparison. Chinese herbal medicines versus placebo for osteoporosis.
| Chinese herbal medicines versus placebo for osteoporosis | ||||||
| Patient or population: patients with osteoporosis Settings: inpatients Intervention: Chinese herbal medicine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) |
Comments (95% CI) |
|
| Assumed risk | Corresponding risk | |||||
| Control (placebo) | Chinese herbal medicine | |||||
|
New fractures (Kanggusong capsule versus placebo) Follow‐up: 12 months |
185 per 1000 | 0 per 1000 (0 to 157) | RR 0.05 (0 to 0.85) | 104 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | We are uncertain about the estimate3 |
| Quality of life | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
| Death | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
| Serious adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
|
BMD of lumbar spine (Kanggusong capsule versus placebo) Follow‐up: 12 months Scale from: 0 to 4 |
The mean BMD in the control groups was 0.82 g/cm3 | The mean BMD in the intervention groups was 0.06 higher (0.02 to 0.1 higher) | 140 (1 study) | ⊕⊕⊝⊝ low1,4 | MD 0.06 g/cm3 (0.02 to 0.10) Absolute risk difference 2% (1% to 3%) Relative per cent change 7% (2% to 12%) NNT 5 (3 to 13) |
|
|
BMD of ulna (Bushen Yigu soft extract versus placebo) Follow‐up: 3 months Scale from: 0 to 4 |
The mean BMD in the control groups was 0.59 g/cm3 | The mean BMD in the intervention groups was 0.06 higher (0.02 to 0.1 higher) | 59 (1 study) | ⊕⊕⊝⊝ low1,4 | MD 0.06 g/cm3 (0.02 to 0.10) Absolute risk difference 2% (1% to 3%) Relative per cent change 10% (3% to 17%) NNT 3 (2 to 8) |
|
|
BMD of radius (Bushen Yigu soft extract versus placebo) Follow‐up: 3 months Scale from: 0 to 4 |
The mean BMD in the control groups was 0.58 g/cm3 | The mean BMD in the intervention groups was 0.06 higher (0.03 to 0.09 higher) | 59 (1 study) | ⊕⊕⊝⊝ low1,4 | MD 0.06 g/cm3 (0.03 to 0.09) Absolute risk difference 2% (1% to 2%) Relative per cent change 10% (5% to 16%) NNT 3 (2 to 6) |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; MD: mean difference; NNT: number needed to treat; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1No information on adequate sequence generation and not all the outcomes that were of interest in this review were reported. 2The total population size is less than 300 (a threshold rule‐of‐thumb value). 3Seven trials reported on the outcome of fracture incidence, but these trials were small in sample size, suffered from various biases, tested different Chinese herbal medicines and the results were not consistent. 4The total population size is less than 400 (a threshold rule‐of‐thumb value).
Summary of findings 2. Chinese herbal medicines versus no intervention for osteoporosis.
| Chinese herbal medicines versus no intervention for osteoporosis | ||||||
| Patient or population: patients with osteoporosis Settings: inpatients and outpatients Intervention: Chinese herbal medicine versus no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (no intervention) | Chinese herbal medicine | |||||
| New fractures | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
| Quality of life | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
| Death | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
| Serious adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
|
BMD of lumbar spine
(Bushen Shengsui principle versus no intervention) Follow‐up: 6 months Scale from: 0 to 4 |
The mean BMD in the control groups was 0.82 g/cm3 | The mean BMD in the intervention groups was 0.07 higher (0.06 lower to 0.2 higher) | 61 (1 study) | ⊕⊕⊝⊝ low1,2 | MD 0.07 g/cm3 (‐0.06 to 0.20) Absolute risk difference 2% (‐2% to 5%) Relative per cent change 9% (‐7% to 24%) Not statistically significant |
|
|
BMD of femoral neck (Shigu yin versus no intervention) Follow‐up: 6 months Scale from: 0 to 4 |
The mean BMD in the control groups was 0.60 g/cm3 | The mean BMD in the intervention groups was 0.08 higher (0.03 to 0.13 higher) |
80 (1 study) | ⊕⊕⊝⊝ low1,2 | MD 0.08 g/cm3 (0.03 to 0.13) Absolute risk difference 2% (1% to 3%) Relative per cent change 13% (5% to 22%) NNT 4 (3 to 8) |
|
|
BMD of femoral neck
(Shengu capsule versus no intervention) Follow‐up: 6 months Scale from: 0 to 4 |
The mean BMD in the control groups was 0.62 g/cm3 | The mean in the intervention groups was 0.02 higher (0.06 lower to 0.09 higher) | 34 (1 study) | ⊕⊕⊝⊝ low1,2 | MD 0.02 g/cm3 (‐0.06 to 0.09) Absolute risk difference 1% (‐2% to 2%) Relative per cent change 3% (‐10% to 15%) Not statistically significant |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; MD: mean difference; NNT: number needed to treat; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1No information on adequate sequence generation and not all the outcomes that were of interest in this review were reported. 2The total population size is less than 400 (a threshold rule‐of‐thumb value).
Summary of findings 3. Chinese herbal medicines versus western medicine for osteoporosis.
| Chinese herbal medicines versus western medicine for osteoporosis | ||||||
| Patient or population: patients with osteoporosis Settings: outpatients and inpatients Intervention: Chinese herbal medicine versus western medicine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (western medicine) | Chinese herbal medicine | |||||
|
New fractures (Kanggusong granule versus calcium and vitamin D) Follow‐up: 9 months |
0 per 40 | 0 per 31 | Not estimable | 71 (1 study) | ⊕⊕⊝⊝ very low1,2 | We are uncertain about the estimate3 |
| Quality of life | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
| Death | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
| Serious adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
|
BMD of lumbar spine
(Bushenjianpi Zhuangguyin versus Caltrate and calcitonin) Follow‐up: 12 weeks Scale from: 0 to 4 |
The mean BMD in the control groups was 0.79 g/cm3 | The mean BMD in the intervention groups was 0.16 higher (0.1 to 0.22 higher) |
100 (1 study) | ⊕⊕⊝⊝ low1,2 | MD 0.16 g/cm3 (0.10 to 0.22) Absolute risk difference 4% (3% to 6%) Relative per cent change 20% (13% to 18%) NNT 3 (2 to 4) |
|
|
BMD of lumbar spine
(Liuwei Dihuang pills versus calcium and vitamin D) Follow‐up: 6 months Scale from: 0 to 4 |
The mean BMD in the control groups was 0.89 g/cm3 | The mean BMD in the intervention groups was 0.05 higher (0.03 to 0.07 higher) |
71 (1 study) | ⊕⊕⊝⊝ low1,2 | MD 0.05 g/cm3 (0.03 to 0.07) Absolute risk difference 1% (1% to 2%) Relative per cent change 6% (3% to 8%) NNT 3 (2 to 5) |
|
|
BMD of femoral neck
(Kanggusong granule versus calcium and vitamin D) Follow‐up: 9 months Scale from: 0 to 4 |
The mean BMD in the control groups was 0.71 g/cm3 | The mean BMD in the intervention groups was 0.01 higher (0.03 lower to 0.05 higher) |
71 (1 study) | ⊕⊕⊝⊝ low1,2 | MD 0.01 g/cm3 (‐0.03 to 0.05) Absolute risk difference 0.25% (‐1% to 1%) Relative per cent change 1% (‐4% to 7%) Not statistically significant |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; MD: mean difference; NNT: number needed to treat; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1No information on adequate sequence generation and not all the outcomes that were of interest in this review were reported. 2The total population size is less than 400 (a threshold rule‐of‐thumb value). 3Only one trial out of 61 reported this outcome.
Summary of findings 4. Chinese herbal medicines plus western medicine versus western medicine for osteoporosis.
| Chinese herbal medicines plus western medicine versus western medicine for osteoporosis | ||||||
| Patient or population: patients with osteoporosis Settings: inpatients Intervention: Chinese herbal medicine plus western medicine versus western medicine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (western medicine) | Chinese herbal medicine plus western medicine | |||||
|
New fractures (Yigu capsule plus calcium versus placebo plus calcium) Follow‐up: 6 months |
114 per 1000 | 0 per 1000 (0 to 114) | RR 0.06 (0 to 1) | 140 (1 study) | ⊕⊕⊝⊝ very low1,2 | We are uncertain about the estimate3 |
|
New fractures (Xianlinggubao capsule (low‐dose) plus calcium and vitamin D versus placebo plus calcium and vitamin D) Follow‐up: 12 months |
17 per 1000 | 0 per 1000 (0 to 134) | RR 0.33 (0.01 to 8.02) | 120 (1 study) | ⊕⊕⊝⊝ very low1,2 | We are uncertain about the estimate3 |
|
New fractures (Bushen Zhuanggu granules plus calcium and vitamin D versus placebo granules plus calcium and vitamin D) Follow‐up: 5 years |
104 per 1000 | 46 per 1000 (14 to 149) | RR 0.44 (0.13 to 1.43) | 155 (1 study) | ⊕⊕⊝⊝ very low1,2 | We are uncertain about the estimate3 |
|
New fractures (Kanggusong soup plus calcium and vitamin D versus calcium and vitamin D) Follow‐up: 3 months |
33 per 1000 | 0 per 1000 (0 to 178) | RR 0.22 (0.01 to 5.34) | 75 (1 study) | ⊕⊕⊝⊝ very low1,2 | We are uncertain about the estimate3 |
|
Quality of life (Bushenhuoxue therapy plus calcium carbonate tablets and alfacalcidol versus calcium carbonate tablets and alfacalcidol) Follow‐up: 3 months Scale from: 0 to 100 |
The mean quality of life in the control groups was 50.75 | The mean quality of life in the intervention groups was 5.3 higher (3.67 to 6.93 higher) | 80 (1 study) | ⊕⊕⊝⊝ low1,4 | MD 5.30 g/cm3 (3.67 to 6.93) Absolute risk difference 5% (4% to 7%) Relative per cent change 10% (7% to 14%) NNT 2 (2 to 3) |
|
| Death | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
| Serious adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NNT: number needed to treat; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1No information on adequate sequence generation and not all the outcomes that were of interest in this review were reported. 2The total population size is less than 300 (a threshold rule‐of‐thumb value). 3Five trials reported on the outcome of fracture incidence, but these trials were small in sample size, suffered from various biases, tested different Chinese herbal medicines and the results were not consistent.
4The total population size is less than 400 (a threshold rule‐of‐thumb value).
Background
Description of the condition
Osteoporosis is a worldwide common public health problem with high prevalence (Cole 2008; Woolf 2003). It is defined as a progressive, systemic disease characterised by low bone mass and structural deterioration of bone tissue, leading to bone fragility and an increased susceptibility to fractures (Diez 2002; NOF 2014; Wylie 2010). While osteoporosis is often thought of as an older person's disease, it can strike at any age (Cole 2008; NOF 2014). It is estimated that worldwide osteoporosis is currently a problem for 200 million middle‐aged and elderly persons, and there are 75 million people with osteoporosis in America, Western Europe and Japan. In terms of its incidence rate osteoporosis has been ranked as seventh among common diseases worldwide (Nguyen 2000; Xue 2000). In China, there are already 50 million people with osteoporosis and a large‐scale epidemiological study found that the prevalence in people over 50 years is 50% (Qiu 2001). As life expectancy increases, this number is expected to continue to grow (Bengner 1988; Cumming 1997; Kushner 1998).
Osteoporosis is a highly prevalent disorder associated with sequelae that often have devastating effects on the quality of life of those affected, and may be a high risk factor for death. Morbidity and mortality rates from osteoporosis‐related injuries are high (Diez 2002; Holroyd 2008). Osteoporosis‐related sequelae therefore have a major impact on healthcare costs, both direct and indirect, especially those related to hip fracture (Diez 2002; Holroyd 2008). Three kinds of major fractures are associated with osteoporosis: hip, vertebrae and the distal forearm (such as wrist) (Diez 2002; NOF 2014). Epidemiologic studies from several countries have reported the incidence and impact of hip fractures, and their absolute number is expected to double in the next 25 years, with a projected total of more than six million occurrences per year throughout the world by the year 2050 (Genant 1999). Some studies have found that the mortality rate associated with hip fracture is 15% to 25%, caused by complications such as pneumonia, other infections and cardiac insufficiency. The expense of therapy and hospitalisation is USD 25 billion each year (Xue 2000). It is difficult to calculate the incidence of spinal fractures because many are asymptomatic or may not be diagnosed, however they are also an important fracture in many elderly people (Diez 2002). Therefore, osteoporosis has become an important social and medical care problem.
Osteoporosis is often called the 'silent disease' because bone loss occurs gradually and without symptoms or warning signs until the disease is advanced (Cole 2008; NOF 2014; Wylie 2010). Some people may suffer from chronic or acute pain, muscle fatigue and limited mobility, but they may not know that they have osteoporosis until their bones become so weak that a sudden strain, bump or fall causes a fracture or a vertebra to collapse. Collapsed vertebrae may initially be felt or seen in the form of severe back pain, loss of height or spinal deformities such as kyphosis (stooped posture) (IOF 2014; Xia 2001).
At present, the pathogenesis of osteoporosis is still not clear, but may be associated with heredity, poor nutrition, malabsorption, gonadal insufficiency, inadequate physical activity, lack of sunlight irradiation and smoking (Diez 2002; Raisz 2005; Winsloe 2009; Zhou 2001b).
Description of the intervention
Currently, there are a range of therapeutic approaches for osteoporosis. Conventional medicines include oestrogen, raloxifene, bisphosphonates (such as alendronate, etidronate, risedronate), calcitriol, calcitonin, Caltrate (Confavreux 2012; Diez 2002; Guo 2003a; Guo 2003b; Horst‐Sikorska 2011; Leong 2002; Rizzoli 2012; Sambrook 2000; Silverman 2012; Wells 2008a; Wells 2008b; Wells 2008c; Zhang 2012). Although fluoride has the ability to increase bone mineral density (BMD) at the lumbar spine (Haguenauer 2005), it has been found ineffective for reducing vertebral fractures in postmenopausal women with osteoporosis. Oestrogen therapy has limited effectiveness and long‐term use increases the risks of breast cancer, endometrial haemorrhage and endometrial cancer (Leong 2002). A Cochrane systematic review shows that exercise has the potential to be a safe and effective way to avert bone loss in postmenopausal women (Howe 2011). Although concerted research efforts have been made to identify safe and effective therapies, treatment options are still needed.
In China and other countries, people are looking for alternative modalities to treat osteoporosis and Chinese herbal medicines are one of the popular therapies used. Chinese herbal medicines are defined in this review as products derived from any part of medicinal plants (e.g. leaves, stems, buds, flowers, roots or tubers) used for the treatment of osteoporosis (Rates 2001) and some non‐plant based component (e.g. insects, deer horn, snake, various shells and powdered fossil, etc.) are sometimes included. Chinese herbal medicines can be in the form of raw plant materials, or water or alcohol extracts of raw plant materials, or herbal formulations in capsules, tablets, decoctions or injections.
How the intervention might work
In China, many herbs have been tested in clinical trials and claims made as to their effectiveness in treating osteoporosis (Cao GY 2010; Dong Y 2010; Jian QQ 2010; Wang JM 2008; Zhang XG 2011). For example, Zhang et al found that Zhuangguyin (a formula containing different herbs, such as common yam rhizome, red and white peony root, Chinese angelica, Radix codonopsis pilosulae, liquorice root, etc. plus dry powder of Placenta hominis) improved primary osteoporosis, and proposed that the therapy improved the BMD of the lumbar spine and femoral neck (Zhang XG 2011). One clinical trial found that Gujian capsule (studied and produced by Xiyuan Hospital under the Institute of Traditional Chinese Medicine) improved BMD, increased levels of calcitonin, oestradiol, testosterone, follicle‐stimulating hormone and luteinising hormone, and decreased levels of parathyroid hormone (Meng 2003).
Why it is important to do this review
Cochrane reviews have assessed the efficacy of Chinese herbal medicines in the treatment of many conditions such as atopic eczema (Gu 2013), cholelithiasis (Gan 2013), colorectal cancer (Guo 2012), gastric cancer (Yang 2013), endometriosis (Flower 2012), heart failure (Chen 2012), osteoarthritis (Cameron 2013) and nephrotic syndrome (Chen 2013; Feng 2013), but not osteoporosis. Chinese herbal medicines are widely used in China for the treatment of osteoporosis and many herbal drugs have been approved by the China State Food and Drug Administration (SFDA) for the market. Each year, many clinical trials are published in China, however, there is no critical appraisal of the evidence on the potential benefits and harms of Chinese herbal medicines for treating osteoporosis. The purpose of this review is to systematically identify available randomised clinical trials of Chinese herbal medicines for primary osteoporosis to appraise their benefit and harm in order to inform clinical practice.
Objectives
To assess the beneficial and harmful effects of Chinese herbal medicines as a general experimental intervention for treating primary osteoporosis by comparing herbal treatments with placebo, no intervention and conventional medicine.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials were included irrespective of blinding, publication status or language. For adverse effects associated with medicinal herbs in osteoporosis treatment, we planned to include data from cohort studies for the evaluation of safety.
Types of participants
Study participants with primary osteoporosis or osteopenia regardless of age, gender or ethnic origin were included. Those with secondary osteoporosis including corticosteroid‐induced osteoporosis due to diseases affecting metabolism of bone and the liver, kidney and haematopoietic system, and disability of the heart and cerebral vessels, were excluded.
Osteoporosis could be diagnosed on the basis of one of the following criteria for bone mineral density (BMD) levels. BMD was detected by one of the following methods of examination: single photon absorptiometry (SPA), dual photon absorptiometry (DPA), quantitative computed tomography (QCT), dual energy X‐ray absorptiometry (DXA) or peripheral dual energy X‐ray absorptiometry (pDXA).
The World Health Organization (WHO) have recommended criteria for primary osteoporosis based on BMD levels (WHO 1994):
Normal: BMD is within +1 or ‐1 standard deviations (SD) of the young adult mean.
Osteopenia (low bone mass): BMD is between ‐1 and ‐2.5 SD below the young adult mean.
Osteoporosis: BMD is ‐2.5 SD or more than the young adult mean.
Severe (established) osteoporosis: BMD is more than ‐2.5 SD and one or more osteoporotic fractures have occurred.
The Chinese diagnostic criteria for primary osteoporosis based on BMD levels (Osteoporosis 1999):
Normal: BMD is within +1 or ‐1 SD of peak bone mass of the same race, gender and region.
Osteopenia (low bone mass): BMD is between ‐1 and ‐2 standard deviations below peak bone mass of the same race, gender and region.
Osteoporosis: BMD is ‐2 SD or more than the peak bone mass of the same race, gender and region.
Severe (established) osteoporosis: BMD is more than ‐2 SD and one or more osteoporotic fractures have occurred.
Types of interventions
The experimental interventions included single herbs, combinations of herbs or Chinese proprietary medicines. There was no standard for the classification of Chinese herbal medicines. Therefore, to make it easier, we classified Chinese herbal medicines into two major types: a) Chinese proprietary medicine which was approved by the China State Food and Drug Administration (SFDA) and produced by pharmaceutical companies with good manufacture practices; b) herbal formulae prescribed by Chinese medicine practitioners based on a Chinese medicine diagnosis (i.e. pattern differentiation of symptoms). This type of medicine was usually cooked as decoction by the patients themselves or prepared by a hospital pharmacy. As we predicted great variation in the type, timing, dosage and administration of herbs and their combination with other interventions, we did not limit any of these aspects.
The control intervention could be no treatment, placebo or conventional pharmaceutical medicine (such as hormone replacement therapy, bisphosphonate, calcitonin, calcium and vitamin D), as well as non‐pharmaceutical interventions such as exercise. Co‐intervention was allowed as long as all groups from the randomised allocation received the same co‐intervention.
We excluded studies in which the duration of herbal treatment was less than three months.
Types of outcome measures
Major outcomes
Major outcome measures sought at the end of treatment or at maximal follow‐up:
Number of individuals with fractures and type of fractures (lumbar spine, radius, femoral neck).
Quality of life or symptoms including pain, muscle fatigue and limited mobility.
Death directly or indirectly attributed to osteoporosis.
Adverse effects including serious adverse events and withdrawals: serious adverse events are defined as any untoward medical occurrence that resulted in death or fracture, was life‐threatening, resulted in persistent or significant disability, or any important medical event which may have jeopardised the patient or required intervention to prevent it (ICH GCP 1997). All other adverse effects were considered non‐serious. We recorded reports of adverse effects associated with the herbs used in the treatment of osteoporosis from any type of study in order to evaluate harms.
Minor outcomes
Minor outcomes at the end of treatment or at maximal follow‐up:
Bone mineral density (BMD).
Biochemical indicators: serum calcium (Ca), phosphorus (P), alkaline phosphatase (ALP), oestradiol (E2), parathyroid hormone (PTH), calcitonin (CT), bone Gla protein (BGP), interleukin‐6 (IL‐6).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases irrespective of language or publication status: the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2012, Issue 12), MEDLINE, EMBASE, LILACS, JICST‐E (Japan Information Center for Science and Technology), AMED, the database of the Cochrane Complementary Medicine Field, Chinese Biomedical Database (CBM), CINAHL (Cumulative Index to Nursing and Allied Health Literature) and the Chinese Academic Conference Papers Database.
We used the following MeSH and free‐text terms: osteoporosis, medicine‐Chinese‐traditional, plants‐medicinal, drugs‐Chinese‐herbal, plants extracts and herbs. The full search strategies were in Appendix 1 (MEDLINE), Appendix 2 (EMBASE), Appendix 3 (CENTRAL) and Appendix 4 (CBM). All electronic searches were from inception to 9 January 2013.
Searching other resources
We handsearched the following journals published in Chinese: Chinese Journal of Integrated Traditional and Western Medicine (1981 to 2012), Chinese Journal of Osteoporosis (1995 to 2012), Chinese Journal of Geriatrics (1982 to 2012) and Journal of Clinical Orthopaedics (1998 to 2012).
We screened reference lists of all retrieved articles and reviews for possible eligible trials.
Data collection and analysis
Selection of studies
Two authors (Y Liu, Y Xia) independently selected the trials to be included in the review according to the prespecified selection criteria. Any disagreement was resolved by discussion.
Data extraction and management
Two authors (Y Liu, Y Xia) extracted data independently using a self developed data extraction form which was piloted for formal use. Disagreement was resolved by discussion.
We extracted the following characteristics and data from each included trial: primary author, study setting, methodology, mean age, gender and ethnicity of patients, number of randomised patients, reasons and number of patients who dropped out or were lost during the follow‐up, patient inclusion and exclusion criteria, symptoms of the patients, diagnostic criteria, type of herb or herbs, route of administration, dosage and duration of the herb, details of comparison regime, outcome measures (end of treatment and follow‐up), and number and type of adverse effects.
If the above data were not available in the trial reports, we sought further information by correspondence with the principal author. If the information was still lacking after the author was contacted, we recorded the last reported observed response.
Assessment of risk of bias in included studies
Based on the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8: Assessing risk of bias in included studies), we made separate critical assessments for seven specific domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and 'other' issues (the baseline comparability between groups) (Higgins 2011). Two authors (Y Liu, Y Xia) assessed the risk of bias independently and assigned a judgement of 'low' risk of bias, 'high' risk of bias or 'unclear' risk of bias. Any disagreement was resolved by discussion.
Measures of treatment effect
We presented dichotomous data as risk ratio (RR) and continuous outcomes as mean difference (MD), both with 95% confidence intervals (CI).
Unit of analysis issues
For a trial with multiple treatment groups, in order to overcome a unit of analysis error, we selected the following approaches accordingly.
Combining groups of the same Chinese herbal medicine with different dosages to create a single pair‐wise comparison.
Selecting one pair of interventions and excluding the others.
Splitting the 'shared' group into two or more groups with smaller sample size, and including two or more (reasonably independent) comparisons.
Dealing with missing data
Based on the recommendations in theCochrane Handbook for Systematic Reviews of Interventions (Chapter 16.1: Missing data), we used two options for dealing with missing data (Higgins 2011).
Analysing only the available data when data were assumed to be missing at random (i.e. ignoring the missing data).
Imputing the missing data with replacement values (such as a poor outcome) when data were not assumed to be missing at random.
Assessment of heterogeneity
We assessed heterogeneity using the I2 statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) (Higgins 2011). A rough guide to interpretation of I2 is as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We planned to detect reporting biases with funnel plots, which is a simple scatter plot of the intervention effect estimates from individual studies against some measure of each study's size or precision (Higgins 2011).
Data synthesis
We compared types of Chinese herbal medicines with each control (such as placebo) regardless of the treatment regimen. We performed meta‐analysis within comparisons of the same herb versus the similar control intervention. Whenever there was significant heterogeneity (I2 > 50%), we used the random‐effects model; otherwise we reported fixed‐effect models (Altman 2003). We carried out the statistical analyses using Review Manager 5.2 (Cochrane software) (RevMan 2012).
We tabulated the following comparisons when data were available:
Chinese herbal medicines versus no intervention or placebo;
Chinese herbal medicines versus conventional medicine (such as hormone replacement therapy, bisphosphonate);
Chinese herbal medicines versus non‐pharmaceutical intervention.
We presented trials of herbs plus active intervention versus active intervention alone as a separate comparison.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses on different Chinese herbal medicines and on body parts of participants for BMD measurement. However, due to the limited number of randomised trials for individual Chinese herbal medicines, the following subgroup analyses were not performed: duration of the herbal treatment, types of osteoporosis (for example, senile or postmenopausal osteoporosis) and severity of osteoporosis.
Sensitivity analysis
Similarly, due to the heterogeneity of the interventions, we were unable to pool results into a meta‐analysis and therefore could not undertake a sensitivity analysis to explore the influence of trial methodological quality on effect estimates.
'Summary of findings' tables
We created 'Summary of findings' tables for different comparisons and included the following patient‐important outcomes (fractures, quality of life, death, serious adverse events, BMD). We assessed the overall quality of the evidence by outcome using the GRADE approach and incorporated this in the tables.
Results
Description of studies
Results of the search
Our initial searches identified 4427 references: 4378 from electronic searches and 49 from handsearches. After reading titles and abstracts, 4176 of these articles were excluded because they were duplicates, non‐clinical studies, without a control group, traditional reviews, animal studies, case reports or had study objectives different from this review. We retrieved a total of 251 references published in Chinese or English for further assessment. Of these, 143 references were excluded because they did not meet our inclusion criteria. Reasons for exclusion were shown in the Characteristics of excluded studies table.
In this review, 108 randomised clinical trials met the inclusion criteria. All trials were conducted in China between 1996 and 2012. We did not find any cohort studies for the evaluation of the safety of Chinese herbal medicines for osteoporosis. The included studies reported random allocation of patients with osteoporosis to Chinese herbal medicines versus control (placebo in three trials, no treatment in five trials, conventional pharmaceutical medicine (such as calcium and vitamin D, calcium gluconate, tibolone tablets, nilestriol, calcitonin, alendronate, calcium, vitamin D, etc.) in 61 trials, Chinese herbal medicines plus co‐intervention versus the same co‐intervention in 47 trials). No trial compared Chinese herbal medicines with non‐pharmaceutical interventions. Eighteen trials (An SJ 2000; Chen ZX 2002; Dai Y 2007; Gong L 2001; Liao L 2004; Miu JQ 2008; Peng T 2002; Ruan XY 2006; Song XW 2000; Wang XY 2000; Wu MS 2007; Xiong YH 2008; Xu H 2010; Xu M 2009; Zhan HS 2009; Zhang YS 2003; Zhang YP 2007; Zhu HM 2012) had more than two arms. Attributes of the 108 included studies are shown in the Characteristics of included studies table. Four trials were published in English (Deng WM 2012; Qiu RB 2004; Xie J 2004; Zhu HM 2012) and 104 studies were published in Chinese.
The study flow diagram is shown in Figure 1.
1.
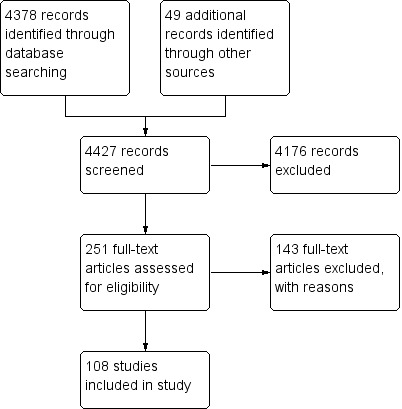
The study screening flow diagram.
Included studies
Participants
A total of 10,655 patients with osteoporosis were randomised in the 108 trials, among which 65 trials included postmenopausal osteoporosis patients, eight trials included senile osteoporosis patients and 35 trials included primary osteoporosis patients unspecified as to life‐stage or co‐morbidity. The race/ethnicity of all patients studied was Chinese in all 108 trials. One hundred and five randomised clinical trials included adults with a mean age of 61.3 years. Three trials did not report data on gender or age or both (Lin W 2000; Xiong YH 2008; Zhao LN 2003). The average number of participants in the trials was 98, ranging from 20 to 600 patients per trial.
Diagnosis
Among the 108 included trials, the diagnostic criteria for osteoporosis in 18 trials were based on WHO criteria (An SJ 2000; Cao W 2010; Chen NQ 2006; Chen ZX 2002; Cui TH 1999; Dai Y 2007; Gang PH 2001; Hu J 2012; Li HW 2004; Li YH 2008; Miu JQ 2008; Wang SW 2003; Ye AN 1998; Zhang RH 2004; Zhang YL 1996; Zhao G 2002; Zheng WK 2007; Zhou LZ 2001), and in the others the Chinese diagnostic criteria for osteoporosis were used. BMD was detected by dual energy X‐ray absorptiometry (DXA), single photon absorptiometry (SPA) or CUBA ultrasound bone densitometer in all of the studies.
Interventions
Ninety‐nine different Chinese herbal medicines were tested in 108 randomised trials. Only eight Chinese herbal medicines were tested twice or more, including Kanggusong granule in two trials (An SJ 2000; Wu MS 2001), Bushen Qiangshen pill in two trials (Mu G 2001; Mu G 2001a), Migu tablets in two trials (Dai Y 2007; Xie J 2004), Gukang oral liquid in three trials (Chen JP 1999; Shao M 2003; Wang JM 2008), Gushukang granule in three trials (Chen ZX 2002; Wu MS 2007; Zhang YS 2003), Qianggu capsule in three trials (Ruan XY 2006; Wang J 2007; Xu H 2010), Liuwei Dihuang pills in two trials (Ma C 2011; Zhang J 2003) and Xianlinggubao capsule in seven trials (Dai Y 2007; Dong Y 2010; Qiu ZX 2010; Wu W 2005; Xu M 2009; Zhang XZ 2004; Zhu HM 2012). However, even when the same Chinese herbal medicines were tested, the control interventions were different for each trial. Therefore, there was no trial which tested exactly the same Chinese herbal medicine and the same control in this review. Seven trials tested two or more different Chinese herbal medicines respectively (Dai Y 2007; Gong L 2001; Wu MS 2007; Xiong YH 2008; Zhan HS 2009; Zhang YS 2003; Zhang ZF 2011). According to the categories of medicinal herbs, two trials tested single herbs (Yang B 2007; Zhao LN 2003) and the remaining trials tested compounds of multiple herbs. Based on our standard classification for Chinese herbal medicines, 45 trials tested Chinese proprietary medicine and the others tested prescribed herbal formulae. The composition and treatment regimens of the Chinese herbal medicines varied (see Table 5: 'The preparation and composition of the Chinese herbal medicines in the included trials'). The median duration of treatment was 5.7 months (ranging from 3 to 12 months).
1. The preparation and composition of the Chinese herbal medicines in the included trials.
| Name of herbs | Preparation | English names (Latin names) of the composition | Study ID |
| Kanggusong granule | Granule | Chinese herbal medicine manufactured by Hebei Handan pharmaceutical factory, which is composed of prepared rhizome of adhesive rehmannia (Radix rehmanniae preparata), Dioscorea batatas (Rhizoma dioscoreae), Cuscuta seed (Semen cuscutae), Epimedium (Herba epimedii), etc. | An SJ 2000; Wu MS 2001 |
| Bushenhuoxue recipe | Decoction | Herbal mixture composed of deer horn glue (Colla cornus cervi), prepared rhizome of adhesive rehmannia (Radix rehmanniae preparata), Epimedium (Herba epimedii), Cuscuta seed (Semen cuscutae), Psoralea corylifolia (Fructus psoraleae), Eucommia bark (Cortex eucommiae), membranous milk vetch root (Radix astragali), Danshen root (Radix salviae miltiorrhizae), Rhizoma corydalis (Rhizoma corydalis), Fortune's drynaria rhizome (Rhizoma drynariae), Liquorice root (Radix glycyrrhizae), Frankincense (olibanum), Myrrh (Commiphora molmol), Dragon's bones (Os Draconis) and oyster shell (Concha ostreae) | Cao GY 2010 |
| Chinese medicine (combinations of 10 herbs) | Decoction | Herbal mixture composed of Psoralea corylifolia (Fructus psoraleae), Fortune's drynaria rhizome (Rhizoma drynariae), Glossy privet fruit (Fructus ligustri lucidi), Epimedium (Herba epimedii), liquorice root (Radix glycyrrhizae), Szechuan lovage rhizome (Rhizoma chuanxiong), Chinese angelica (Radix angelicae sinensis), Himalayan teasel root (Radix dipsaci), Ground beetle (Eupolyphaga seu steleophaga) and liquorice root (Radix glycyrrhizae) | Cao W 2010 |
| Gushen decoction | Decoction | Herbal mixture composed of 6 herbs: Epimedium (Herba epimedii), King Solomon's seal rhizome (Rhizoma polygonati), Barbary wolfberry fruit (Fructus lycii), human placenta (Placenta hominis), Rehmanniae radix preparata and clematis root (Radix clematidis) | Chen FS 2001 |
| Gukang oral liquid | Oral liquid | Herbal mixture manufactured by affiliated orthopedics hospital of Guangzhou University of Traditional Chinese Medicine. It is a combined medicine composed of Epimedium (Herba epimedii), desert‐living cistanche (Herba cistanches), liquorice root (Radix glycyrrhizae), Danshen root (Radix salviae miltiorrhizae), etc. | Chen JP 1999; Shao M 2003; Wang JM 2008 |
| Shenyao capsules | Capsule | Chinese patent herbal medicine composed of Red leaves to kidney (Trachelos permum jasminoides Lem. Var. heterophyllum Tsiang), White leaves to kidney (euonymus fortunei Hand. Mazz), Cotton kidney (Urena procumbens L), Litchi kidney (Agrimonia pilosa Ledeb Var japonica Nakai), Dragon buds kidney (Salvia substolonifera Stib), Fagopyrum esculentum kidney (Fagopyrum cymosum Meisn), etc. | Chen NQ 2006 |
| Gukang dection | Decoction | Herbal mixture composed of Epimedium (Herba epimedii), Psoralea corylifolia (Fructus psoraleae), Rehmanniae radix preparata, Danshen root (Radix salviae miltiorrhizae), desert‐living cistanche (Herba cistanches), liquorice root (Radix glycyrrhizae), Chinese angelica (Radix angelicae sinensis), white peony root (Radix paeoniae alba), Cuscuta seed (Semen cuscutae), Chinese date (Fructus jujubae), etc. | Chen X 2008 |
| Heche Dazao pill | Decoction | Herbal mixture composed of human placenta (Placenta hominis), Rehmanniae radix preparata, Radix asparagi, Radix ophiopogonis (Radix ophiopogonis), Eucommia bark (Cortex eucommiae), two‐tooth achyranthes root (Radix achyranthis bidentatae), Amur cork tree bark (Cortex phellodendri), tortoise shell (Carapax et plastrum testudinis), liquorice root (Radix glycyrrhizae), Psoralea corylifolia (Fructus psoraleae), Dioscorea batatas (Rhizoma dioscoreae) and Wolfiporia cocos (Poria) | Chen XL 2010 |
| Jiarong tablet | Tablet | Chinese patent herbal medicine manufactured by Xi'an Boai Medicine limited company | Chen YQ 2001 |
| Gushukang granule | Granule | Chinese patent herbal medicine composed of Epimedium (Herba epimedii), Rehmanniae radix preparata, liquorice root (Radix glycyrrhizae), Danshen root (Radix salviae miltiorrhizae), Fortune's drynaria rhizome (Rhizoma drynariae), etc. | Chen ZX 2002; Wu MS 2007; Zhang YS 2003 |
| Strong Bone capsule | Capsule | Herbal mixture composed of deer horn glue (Colla cornus cervi), desert‐living cistanche (Herba cistanches), prepared rhizome of rehmannia (Radix rehmanniae preparata), tortoise shell (Carapax et plastrum testudinis), Eucommia bark (Cortex eucommiae), bitter orange (Fructus aurantii), Suberect spatholobus stem (Caulis spatholobi), etc. | Cui TH 1999 |
| Xianlinggubao capsule | Capsule | Chinese patent herbal medicine composed of Epimedium (Herba epimedii), Himalayan teasel root (Radix dipsaci), Psoralea corylifolia (Fructus psoraleae), etc. | Dai Y 2007; Dong Y 2010; Qiu ZX 2010; Wu W 2005; Xu M 2009; Zhang XZ 2004; Zhu HM 2012 |
| Migu tablet | Tablet | The medicine was developed by Union Hospital of Tongji Medical College and composed of Epimedium (Herba epimedii), Eucommia bark (Cortex eucommiae), Psoralea corylifolia (Fructus psoraleae), etc. | Dai Y 2007; Xie J 2004 |
| Herba epimedii prescription | Decoction | Herbal mixture composed of Epimedium (Herba epimedii), Psoralea corylifolia (Fructus psoraleae), Eucommia bark (Cortex eucommiae), oyster shell (Concha ostreae), common monkshood mother root, processed radix aconiti (Radix aconiti preparata), Kusnezoff monkshood root (Radix aconiti kusnezoffii), Chinese angelica (Radix angelicae sinensis) and Szechuan lovage rhizome (Rhizoma chuanxiong) | Dong YF 2004 |
| Gengnian Anyi tablet | Tablet | Chinese patent herbal medicine manufactured by Hubei traditional Chinese medicine company. The main composition was Rehmanniae radix preparata, Dioscorea batatas (Rhizoma dioscoreae), Eucommia bark (Cortex eucommiae), desert‐living cistanche (Herba cistanches), etc. | Fan HX 2004 |
| Gumiling granule | Granule | Self developed herbal mixture (no details on composition) | Gang PH 2001 |
| Shengu capsule | Capsule | Chinese patent medicine manufactured by Beijing Tianjiu Medicine limited company. The main composition was oyster shell (Concha ostreae) | Gong L 2001 |
| Yishen Zhuanggu mixture | Decoction | Chinese patented medicine manufactured by Beijing Xuanwu hospital. It is a combined medicine composed of Psoralea corylifolia (Fructus psoraleae), Radix codonopsis pilosulae (Radix codonopsis), Fortune's drynaria rhizome (Rhizoma drynariae), Rehmanniae radix preparata, liquorice root (Radix glycyrrhizae), etc. | Gong L 2001 |
| Bushen Kangsong pill | Pill | Chinese patent medicine manufactured by the People's Liberation Army General Hospital of Nanjing Military Region. It is composed of Rehmanniae radix preparata, Chinese taxillus twig (Herba taxilli), Dioscorea batatas (Rhizoma dioscoreae), common macrocarpium fruit (Fructus corni), Barbary wolfberry fruit (Fructus lycii), hairy antler (Corn Cervi Pantotrichum), Himalayan teasel root (Radix dipsaci), Psoralea corylifolia (Fructus psoraleae), Eucommia bark (Cortex eucommiae), Epimedium (Herba epimedii), desert‐living cistanche (Herba cistanches), two‐tooth achyranthes root (Radix achyranthis bidentatae), Cuscuta seed (Semen cuscutae), cassia bark (Cortex cinnamomi), Chinese angelica (Radix angelicae sinensis), liquorice root (Radix glycyrrhizae), Dragon's bones (Os Draconis), oyster shell (Concha ostreae), hawthorn fruit (Fructus crataegi), liquorice root (Radix glycyrrhizae), etc. | Guo JH 2008 |
| Shugan zishen huoxue tang | Decoction | Herbal mixture composed of Chinese thorowax root (Radix bupleuri), white peony root (Radix paeoniae alba), Nutgrass galingale rhizome (Rhizoma cyperi), bitter orange (Fructus aurantii), Pinellia tuber (Rhizoma pinelliae), Wolfiporia cocos (Poria), prepared rhizome of adhesive rehmannia (Radix rehmanniae preparata), common macrocarpium fruit (Fructus corni), tortoise shell (Carapax et plastrum testudinis), East Asian tree fern rhizome (Rhizoma cibotii), Fructus chaenomelis (Chaenomeles speciosa Nakai), prepared frankincense (olibanum), Myrrh preparata (Commiphora molmol) and Szechuan lovage rhizome (Rhizoma chuanxiong) | Han XL 2007 |
| Bushen Jianpi Huoxue recipe | Decoction | Herbal mixture composed of Psoralea corylifolia (Fructus psoraleae), Epimedium (Herba epimedii), desert‐living cistanche (Herba cistanches), Rehmanniae radix preparata, white peony root (Radix paeoniae alba), liquorice root (Radix glycyrrhizae), Cuscuta seed (Semen cuscutae), Danshen root (Radix salviae miltiorrhizae), Chinese angelica (Radix angelicae sinensis) and Chinese date (Fructus jujubae) | He MT 2007 |
| Jiawei Zhuangyao Jianshen tang | Decoction | Herbal mixture composed of East Asian tree fern rhizome (Rhizoma cibotii), Cherokee rose fruit (Fructus rosae laevigatae), Chinese taxillus twig (Herba taxilli), suberect spatholobus stem (Caulis spatholobi), Philippine flemingia root (Moghania philippinensis li.), beautiful millettia root (Millettia speciosa champ), Cuscuta seed (Semen cuscutae), glossy privet fruit (Fructus ligustri lucidi), Rhizoma corydalis (Rhizoma corydalis), Chinese angelica (Radix angelicae sinensis), red peony root (Radix paeoniae rubra) and largehead atractylodes rhizome (Rhizoma atractylodis macrocephalae) | He N 2006 |
| Bushen Yangxue tang | Decoction | Herbal mixture composed of medicinal Indian mulberry root (Radix morindae officinalis), Epimedium (Herba epimedii), Cherokee rose fruit (Fructus rosae laevigatae), Barbary wolfberry fruit (Fructus lycii), Chinese date (Fructus jujubae), Chinese angelica (Radix angelicae sinensis), white peony root (Radix paeoniae alba) and tuber fleece flower root (Radix polygoni multiflori), etc. | He XQ 2006; Zhan ML 2007 |
| Shangke Yishen Zhuanggu pill | Pill | Herbal mixture composed of Rehmanniae radix preparata, Chinese angelica (Radix angelicae sinensis), two‐tooth achyranthes root (Radix achyranthis bidentatae), Sanqi (Radix notoginseng), Wolfiporia cocos (Poria), Danshen root (Radix salviae miltiorrhizae), common macrocarpium fruit (Fructus corni), Szechuan lovage rhizome (Rhizoma chuanxiong), membranous milk vetch root (Radix astragali), etc. | Hu J 2012 |
| Erxian Yanggu tang | Decoction | Herbal mixture composed of medicinal Indian mulberry root (Radix morindae officinalis), common curculigo rhizome (Rhizoma curculiginis), Epimedium (Herba epimedii), Barbary wolfberry fruit (Fructus lycii), Eucommia bark (Cortex eucommiae), Fortune's drynaria rhizome (Rhizoma drynariae), Amur cork tree bark (Cortex phellodendri), common anemarrhena rhizome (Rhizoma anemarrhenae), deer horn glue (Colla cornus cervi), tortoise shell glue (Colla carapacis et plastri testudinis), paniculate swallow wort root (Radix Cynanchi Paniculati), white peony root (Radix paeoniae alba), Chinese angelica (Radix angelicae sinensis), Radix astragali preparata and bitter orange (Fructus aurantii) | Huang JW 2008 |
| Zishen Gukang pill | Pill | Chinese patent medicine manufactured by the Traditional Chinese Medicine Hospital of Henan province pharmacy. It is composed of Epimedium (Herba epimedii), Chinese angelica (Radix angelicae sinensis), prepared rhizome of adhesive rehmannia (Radix rehmanniae preparata), Psoralea corylifolia (Fructus psoraleae), common macrocarpium fruit (Fructus corni), two‐tooth achyranthes root (Radix achyranthis bidentatae), etc. | Huang ZJ 2008 |
| Shengsuiyin | Decoction | Herbal mixture composed of medicinal Indian mulberry root (Radix morindae officinalis), Chinese taxillus twig (Herba taxilli), Cuscuta seed (Semen cuscutae), tortoise shell glue (Colla carapacis et plastri testudinis), Epimedium (Herba epimedii), common macrocarpium fruit (Fructus corni), Radix codonopsis pilosulae (Radix codonopsis), Dioscorea batatas (Rhizoma dioscoreae), Wolfiporia cocos (Poria) and glossy privet fruit (Fructus ligustri lucidi) | Jian QQ 2010 |
| Guli powder | Powder | Chinese patent medicine manufactured by drug manufactory of the research institute of traditional Chinese medicine of Sichuan province. It is composed of Radix codonopsis pilosulae (Radix codonopsis), large head atractylodes rhizome (Rhizoma atractylodis macrocephalae), Wolfiporia cocos (Poria), Radix angelicae (Radix angelicae dahuricae), Epimedium (Herba epimedii), common cnidium fruit (Fructus cnidii), Psoralea fruits (Fructus psoraleae), Rehmanniae radix preparata, two‐tooth achyranthes root (Radix achyranthis bidentatae), etc. | Lan ZX 2006 |
| Jianshenfang granule | Granule | Chinese patent medicine manufactured by Guangzhou Yuexiu District Orthopedic Hospital. It is composed of Himalayan teasel root (Radix dipsaci), clematis root (Radix clematidis), Cherokee rose fruit (Fructus rosae laevigatae), East Asian tree fern rhizome (Rhizoma cibotii), Philippine flemingia root (Moghania philippinensis li.), two‐tooth achyranthes root (Radix achyranthis bidentatae), etc. | Li BL 2007 |
| Ziyin Bushen Zhuanggu prescription | Decoction | Investigator‐prescribed herbal mixture composed of Eucommia bark (Cortex eucommiae), Rehmanniae radix preparata, deer horn glue (Colla cornus cervi), liquorice root (Radix glycyrrhizae), Chinese angelica (Radix angelicae sinensis), Rhizoma corydalis (Rhizoma corydalis), two‐tooth achyranthes root (Radix achyranthis bidentatae), Danshen root (Radix salviae miltiorrhizae), Psoralea corylifolia (Fructus psoraleae), Wolfiporia cocos (Poria), etc. | Li HW 2004 |
| Zhuanggu capsule | Capsule | Herbal mixture composed of Epimedium (Herba epimedii), common cnidium fruit (Fructus cnidii), human placenta (Placenta hominis), liquorice root (Radix glycyrrhizae), Sanqi (Radix notoginseng), etc. | Li SL 2004 |
| Jingujian granule | Granule | Chinese patent medicine manufactured by Xiyuan Hospital of Chinese Academy of Traditional Chinese Medicine pharmacy. It is composed of Fortune's drynaria rhizome (Rhizoma drynariae), Eucommia bark (Cortex eucommiae), Szechuan lovage rhizome (Rhizoma chuanxiong), large head atractylodes rhizome (Rhizoma atractylodis macrocephalae), Manchurian wild ginger (Herba asari), etc. | Li YH 2008 |
| Jingujian granule (1,2,3) | Granule | Jingujian granule 1: Rehmanniae radix preparata, common macrocarpium fruit (Fructus corni), Cuscuta seed (Semen cuscutae), tortoise shell (Carapax et plastrum testudinis), two‐tooth achyranthes root (Radix achyranthis bidentatae), Fortune's drynaria rhizome (Rhizoma drynariae), Eucommia bark (Cortex eucommiae), etc. Jingujian granule 2: Psoralea corylifolia (Fructus psoraleae), Eucommia bark (Cortex eucommiae), two‐tooth achyranthes root (Radix achyranthis bidentatae), Radix codonopsis pilosulae (Radix codonopsis), large‐head atractylodes rhizome (Rhizoma atractylodis macrocephalae), etc. Jingujian granule 3: Eucommia bark (Cortex eucommiae), Fortune's drynaria rhizome (Rhizoma drynariae), Medicinal cyathula root (Radix cyathulae), two‐tooth achyranthes root (Radix achyranthis bidentatae), Manchurian wild ginger (Herba asari), large‐head atractylodes rhizome (Rhizoma atractylodis macrocephalae), Szechuan lovage rhizome (Rhizoma chuanxiong) | Li YH 2010 |
| Kanggusong soup | Decoction | Herbal mixture composed of Rehmanniae radix preparata 18 g, Barbary wolfberry fruit (Fructus lycii) 9 g, Epimedium (Herba epimedii) 18 g, Himalayan teasel root (Radix dipsaci) 9 g, membranous milk vetch root (Radix astragali) 9 g, Wolfiporia cocos (Poria) 9 g, Danshen root (Radix salviae miltiorrhizae) 18 g, Radix puerariae (Radix puerariae) 9 g, suberect spatholobus stem (Caulis spatholobi) 18 g, etc. | Li ZP 2011 |
| Xianling Gusong capsule | Capsule | The capsule included Epimedium (Herba epimedii), common curculigo rhizome (Rhizoma curculiginis), medicinal Indian mulberry root (Radix morindae officinalis), ginseng (Radix ginseng), Chinese angelica (Radix angelicae sinensis), human placenta (Placenta hominis) and common anemarrhena rhizome (Rhizoma anemarrhenae). | Li ZY 2006 |
| Bushenhuoxue therapy | Decoction | Herbal mixture composed of Rehmanniae radix preparata 24 g, Epimedium (Herba epimedii) 18 g, membranous milk vetch root (Radix astragali) 15 g, common macrocarpium fruit (Fructus corni) 12 g, Dioscorea batatas (Rhizoma dioscoreae) 12 g, Eucommia bark (Cortex eucommiae) 12g, Danshen root (Radix salviae miltiorrhizae) 12 g, tree peony bark (Cortex moutan) 9 g, Wolfiporia cocos (Poria) 9 g, oriental water plantain rhizome (Rhizoma alismatis) 9 g, safflower (Flos carthami) 9 g, Sanqi (Radix notoginseng) 3 g | Liang DB 2012 |
| Bushen Shengsui principle | Decoction | Investigator‐prescribed herbal mixture composed of Psoralea corylifolia (Fructus psoraleae), Epimedium (Herba epimedii), Eucommia bark (Cortex eucommiae) and glossy privet fruit (Fructus ligustri lucidi) | Liao L 2004 |
| Migu decoction | Decoction | Self developed herbal mixture (no details on composition) | Lin W 2000 |
| Bushen Zhuanggu tang | Decoction | Herbal mixture composed of East Asian tree fern rhizome (Rhizoma cibotii), Himalayan teasel root (Radix dipsaci), medicinal Indian mulberry root (Radix morindae officinalis), Epimedium (Herba epimedii), Psoralea corylifolia (Fructus psoraleae), liquorice root (Radix glycyrrhizae), white peony root (Radix paeoniae alba), Chinese angelica (Radix angelicae sinensis), Szechuan lovage rhizome (Rhizoma chuanxiong), safflower (Flos carthami) and two‐tooth achyranthes root (Radix achyranthis bidentatae) | Ling JY 2008 |
| Gukang tablet | Tablet | Chinese patent medicine manufactured by Laiyang biological chemical pharmaceutical factory. It is composed of Rehmanniae radix preparata, Epimedium (Herba epimedii), suberect spatholobus stem (Caulis spatholobi), Chinese Pyrola Herb (Herba Pyrolae), Eucommia bark (Cortex eucommiae), medicinal Indian mulberry root (Radix morindae officinalis), common curculigo rhizome (Rhizoma curculiginis), desert‐living cistanche (Herba cistanches), two‐tooth achyranthes root (Radix achyranthis bidentatae), Chinese angelica (Radix angelicae sinensis), safflower (Flos carthami), etc. | Liu JM 2012 |
| Jiawei Bushen Zhuangjintang | Decoction | Investigator‐prescribed herbal formulation composed of Rehmanniae radix preparata, Chinese angelica (Radix angelicae sinensis), two‐tooth achyranthes root (Radix achyranthis bidentatae), Asiatic cornelian cherry fruit (Fructus corni), Wolfiporia cocos (Poria), Himalayan teasel root (Radix dipsaci), Eucommia bark (Cortex eucommiae), white peony root (Radix paeoniae alba), green tangerine peel (Pericarpium citri reticulatae viride), Slenderstyle acanthopanax bark (Cortex acanthopanacis), hairy antler (Corn Cervi Pantotrichum), etc. | Lv ZH 2002 |
| Yiyuanjiangu decoction | Decoction | Herbal mixture composed of Rehmanniae radix preparata 15 g, Psoralea corylifolia (Fructus psoraleae) 15 g, Epimedium (Herba epimedii) 10 g, Radix angelicae pubescentis 10 g, Barbary wolfberry fruit (Fructus lycii) 10 g, East Asian tree fern rhizome (Rhizoma cibotii) 10 g, Chinese angelica (Radix angelicae sinensis) 10 g, membranous milk vetch root (Radix astragali) 20 g, two‐tooth achyranthes root (Radix achyranthis bidentatae) 20 g, Chinese taxillus twig (Herba taxilli) 20 g, Himalayan teasel root (Radix dipsaci) 12 g, etc. | Ma CZ 2011 |
| Yishen Zhuanggu decoction | Decoction | Herbal mixture composed of Rehmanniae radix preparata 24 g, Fortune's drynaria rhizome (Rhizoma drynariae) 20g, Himalayan teasel root (Radix dipsaci) 20 g, Epimedium (Herba epimedii) 30 g, Chinese angelica (Radix angelicae sinensis) 15 g, white peony root (Radix paeoniae alba) 10 g, Eucommia bark (Cortex eucommiae) 20 g, Chinese taxillus twig (Herba taxilli) 30g, membranous milk vetch root (Radix astragali) 30g, Dioscorea batatas (Rhizoma dioscoreae) 2 0g, tuber fleece flower root (Radix polygoni multiflori) 20 g and Cuscuta seed (Semen cuscutae) 15 g | Ma YJ 2011 |
| Bushen Jianpi Jingu decoction | Decoction | Herbal mixture composed of Rehmanniae radix preparata 20 g, Dioscorea batatas (Rhizoma dioscoreae) 15 g, common macrocarpium fruit (Fructus corni) 12 g, Barbary wolfberry fruit (Fructus lycii) 12 g, Eucommia bark (Cortex eucommiae) 15 g, Psoralea corylifolia (Fructus psoraleae) 15 g, Fortune's drynaria rhizome (Rhizoma drynariae) 15 g, Radix codonopsis pilosulae (Radix codonopsis) 20 g, membranous milk vetch root (Radix astragali) 20 g, large‐head atractylodes rhizome (Rhizoma atractylodis macrocephalae) 10 g, Wolfiporia cocos (Poria) 10 g and liquorice root (Radix glycyrrhizae) 10 g | Mao YF 2011 |
| Gujian capsule | Capsule | Developed by Xiyuan hospital of China academy of TCM (no details on composition) | Meng XD 2003 |
| Traditional Chinese capsule | Capsule | The capsule included Epimedium (Herba epimedii), Fortune's drynaria rhizome (Rhizoma drynariae), liquorice root (Radix glycyrrhizae), King Solomon's seal rhizome (Rhizoma polygonati), Radix puerariae (Radix puerariae), Rhizoma corydalis (Rhizoma corydalis), etc. | Miu JQ 2008 |
| Bushen Qiangshen pill | Pill | Chinese patented medicine composed of ginseng (Radix ginseng), hairy antler (Cornu cervi pantotrichum), desert‐living cistanche (Herba cistanches), liquorice root (Radix glycyrrhizae), Barbary wolfberry fruit (Fructus lycii), etc. | Mu G 2001; Mu G 2001a |
| Radix rehmanniae preparata and Radix astragali | Decoction | Herbal mixture composed of Rehmanniae radix preparata 30 g, membranous milk vetch root (Radix astragali) 30 g, etc. | Ou L 2011 |
| Qianggu soft extract | Slurry | Herbal mixture composed of medicinal Indian mulberry root (Radix morindae officinalis), Cuscuta seed (Semen cuscutae), liquorice root (Radix glycyrrhizae), Chinese angelica (Radix angelicae sinensis), etc. | Peng T 2002 |
| Bushen Qianggutang | Decoction | Investigator‐prescribed formula composed of Himalayan teasel root (Radix dipsaci), Cuscuta seed (Semen cuscutae), Psoralea corylifolia (Fructus psoraleae), Fortune's drynaria rhizome (Rhizoma drynariae), common macrocarpium fruit (Fructus corni), Barbary wolfberry fruit (Fructus lycii), glossy privet fruit (Fructus ligustri lucidi), Dioscorea batatas (Rhizoma dioscoreae), Wolfiporia cocos (Poria), etc. | Qi ZX 1998 |
| Yanghuo Sanzi tang | Decoction | Herbal mixture composed of Epimedium (Herba epimedii), Fructus Schisandrae (Fructus schisandrae chinensis), common macrocarpium fruit (Fructus corni), Psoralea corylifolia (Fructus psoraleae), mulberry fruit (Fructus mori), Barbary wolfberry fruit (Fructus lycii), oyster shell (Concha ostreae), prepared fleece flower root (Radix polygoni multiflori preparata) and red ginseng (Radix ginseng rubra) | Qiu RB 2004 |
| Jiangu recipe | Decoction | Herbal mixture composed of Epimedium (Herba epimedii), medicinal Indian mulberry root (Radix morindae officinalis), common cnidium fruit (Fructus cnidii), liquorice root (Radix glycyrrhizae), Fortune's drynaria rhizome (Rhizoma drynariae), deer horn glue (Colla cornus cervi), hawthorn fruit (Fructus crataegi), common macrocarpium fruit (Fructus corni) and Psoralea corylifolia (Fructus psoraleae) | Qiu RB 2008 |
| Qianggu capsule | Capsule | Chinese patent medicine manufactured by Beijing Qihuang Pharmaceutical Limited Company; the main composition was Fortune's drynaria rhizome (Rhizoma drynariae) | Ruan XY 2006; Wang J 2007; Xu H 2010 |
| Kangshu Jiangu granule | Granule | Chinese patent medicine manufactured by Shanxi college of traditional Chinese medicine pharmaceutical factory. It is composed of Epimedium (Herba epimedii) 20 g, Eucommia bark (Cortex eucommiae) 15 g, two‐tooth achyranthes root (Radix achyranthis bidentatae) 15 g, Danshen root (Radix salviae miltiorrhizae) 15 g, large‐head atractylodes rhizome (Rhizoma atractylodis macrocephalae) 12 g, etc. | Shi CD 2012 |
| Kidney‐tonifying herbs | Granule | Herbal mixture composed of Epimedium (Herba epimedii), desert‐living cistanche (Herba cistanches), Psoralea corylifolia (Fructus psoraleae), tuber fleece flower root (Radix polygoni multiflori), Chinese angelica (Radix angelicae sinensis), safflower (Flos carthami), common aucklandia root (Radix aucklandiae), etc. | Song XW 2000 |
| Bushen Qianggu Huoxue therapy | Decoction | Herbal mixture composed of Psoralea corylifolia (Fructus psoraleae) 15 g, Fortune's drynaria rhizome (Rhizoma drynariae) 10 g, Eucommia bark (Cortex eucommiae) 15 g, Epimedium (Herba epimedii) 10 g, Barbary wolfberry fruit (Fructus lycii) 15 g, Rehmanniae radix preparata 15 g, desert‐living cistanche (Herba cistanches) 10 g, deer horn glue (Colla cornus cervi) 10 g, medicinal Indian mulberry root (Radix morindae officinalis) 10 g, Cuscuta seed (Semen cuscutae) 15 g, East Asian tree fern rhizome (Rhizoma cibotii) 10 g, Himalayan teasel root (Radix dipsaci) 10 g, Sanqi (Radix notoginseng) 10 g, Danshen root (Radix salviae miltiorrhizae) 10 g, suberect spatholobus stem (Caulis spatholobi) 15 g, etc. | Tang ZA 2012 |
| Gumikang capsule | Capsule | Herbal mixture composed of human placenta (Placenta hominis), hairy antler (Cornu cervi pantotrichum), etc. | Wang CC 2005 |
| Qianggu paste | Paste | Chinese patent medicine manufactured by Affiliated Hospital of Wuhan University of Technology pharmacy, composed of flat stem milk vetch seed (Semen astragali complanati), Psoralea corylifolia (Fructus psoraleae), Epimedium (Herba epimedii), Chinese angelica (Radix angelicae sinensis), liquorice root (Radix glycyrrhizae), etc. | Wang H 2007 |
| Antai capsule | Capsule | Chinese herbal medicine manufactured by the medicine research institute of Fourth Military Medical University. | Wang SW 2003 |
| Hugu capsule | Capsule | Chinese patent medicine manufactured by Dongguan Super Success Pharmaceutical Co., Ltd. It is composed of tuber fleece flower root (Radix polygoni multiflori), Epimedium (Herba epimedii), Rehmanniae radix preparata, etc. | Wang XD 2011 |
| Bushen Yigu soft extract | Oral liquid | Herbal mixture composed of prepared rhizome of adhesive rehmannia (Radix rehmanniae preparata), Epimedium (Herba epimedii), large‐head atractylodes rhizome (Rhizoma atractylodis macrocephalae), oriental water plantain rhizome (Rhizoma alismatis), etc. | Wang XY 2000 |
| Jiangu capsule | Capsule | Chinese patent medicine manufactured by Affiliated Hospital of Luohe Medical College pharmacy, composed of liquorice root (Radix glycyrrhizae), Danshen root (Radix salviae miltiorrhizae), Szechuan lovage rhizome (Rhizoma chuanxiong), Cuscuta seed (Semen cuscutae), Asiatic cornelian cherry fruit (Fructus corni), Dragon's bones (Os Draconis), oyster shell (Concha ostreae) and medicinal Indian mulberry root (Radix morindae officinalis) | Wang YZ 2008 |
| Bushen Jianpi Migu prescription | Decoction | Investigator‐prescribed herbal formulation composed of liquorice root (Radix glycyrrhizae), Cuscuta seed (Semen cuscutae), Epimedium (Herba epimedii), Asiatic cornelian cherry fruit (Fructus corni), Dragon's bones (Os Draconis), oyster shell (Concha ostreae), deer horn glue (Colla cornus cervi), Rehmanniae radix preparata, desert‐living cistanche (Herba cistanches), Eucommia bark (Cortex eucommiae), suberect spatholobus stem (Caulis spatholobi), medicinal Indian mulberry root (Radix morindae officinalis), Szechuan lovage rhizome (Rhizoma chuanxiong), liquorice root (Radix glycyrrhizae) | Wang ZK 2004 |
| Kanggusong capsule (self developed) | Capsule | Manufactured by the First Attached Hospital of Hebei Northern Institute, composed of Epimedium (Herba epimedii), Himalayan teasel root (Radix dipsaci), Rehmanniae radix preparata, Barbary wolfberry fruit (Fructus lycii), liquorice root (Radix glycyrrhizae), Wolfiporia cocos (Poria), Danshen root (Radix salviae miltiorrhizae), Radix puerariae (Radix puerariae) and suberect spatholobus stem (Caulis spatholobi) | Wei RY 2011 |
| Erxian soup | Liquid | Herbal mixture composed of common curculigo rhizome (Rhizoma curculiginis), Epimedium (Herba epimedii), medicinal Indian mulberry root (Radix morindae officinalis), Chinese angelica (Radix angelicae sinensis), common anemarrhena rhizome (Rhizoma anemarrhenae), Amur cork tree bark (Cortex phellodendri) | Wu JZ 2010 |
| Urinary bladder meridian sticking | Patch | Herbal mixture composed of Rehmannia root (Radix rehmanniae), Epimedium (Herba epimedii), Dioscorea batatas (Rhizoma dioscoreae), Danshen root (Radix salviae miltiorrhizae), Fortune's drynaria rhizome (Rhizoma drynariae), Radix angelicae pubescentis, etc. | Wu MS 2007 |
| Kidney meridian sticking | Patch | Herbal mixture composed of Rehmannia root (Radix rehmanniae), Epimedium (Herba epimedii), Dioscorea batatas (Rhizoma dioscoreae), Danshen root (Radix salviae miltiorrhizae), Fortune's drynaria rhizome (Rhizoma drynariae), Radix angelicae pubescentis, etc. | Wu MS 2007 |
| Prescription for tonifying kidney | Pill | Herbal mixture composed of Rehmannia root (Radix rehmanniae), Epimedium (Herba epimedii), Dioscorea batatas (Rhizoma dioscoreae), Danshen root (Radix salviae miltiorrhizae), Fortune's drynaria rhizome (Rhizoma drynariae), Radix angelicae pubescentis, etc. | Wu MS 2007 |
| Gusong fang | Decoction | Herbal mixture composed of nutmeg, Chinese angelica (Radix angelicae sinensis), liquorice root (Radix glycyrrhizae), Psoralea corylifolia (Fructus psoraleae), Epimedium (Herba epimedii), desert‐living cistanche (Herba cistanches), etc. | Xiao W 2008 |
| Bugu Shengsui capsule | Capsule | Herbal mixture composed of Psoralea corylifolia (Fructus psoraleae), East Asian tree fern rhizome (Rhizoma cibotii), Sanqi (Radix notoginseng), ginseng (Radix ginseng), etc. | Xie YM 1997 |
| Jiangu granule | Granule | Chinese patent medicine manufactured by Ruijin hospital pharmacy, composed of Epimedium (Herba epimedii), Radix codonopsis pilosulae (Radix codonopsis), large‐head atractylodes rhizome (Rhizoma atractylodis macrocephalae), glossy privet fruit (Fructus ligustri lucidi), etc. | Xiong YH 2008 |
| Gusongbao granule | Granule | Chinese patent medicine composed of Epimedium (Herba epimedii), Rehmanniae radix preparata, oyster shell (Concha ostreae), etc. | Xiong YH 2008; Zhan HS 2009 |
| Yishen Yanggan mixture | Oral liquid | Herbal mixture composed of liquorice root (Radix glycyrrhizae), common macrocarpium fruit (Fructus corni), Epimedium (Herba epimedii), desert‐living cistanche (Herba cistanches), Psoralea corylifolia (Fructus psoraleae), Chinese angelica (Radix angelicae sinensis), etc. | Xu W 2005 |
| Acupoint sticking of Migudan | Patch | Herbal mixture composed of Psoralea corylifolia (Fructus psoraleae), Fortune's drynaria rhizome (Rhizoma drynariae), Himalayan teasel root (Radix dipsaci), Szechuan lovage rhizome (Rhizoma chuanxiong), processed radix aconiti (Radix aconiti preparata), tuberculate Speranskia herb (Garden balsam stem), two‐tooth achyranthes root (Radix achyranthis bidentatae), Manchurian wild ginger (Herba asari), etc. | Xu YL 2007 |
| Huangqi | Decoction | Single herb of liquorice root (Radix glycyrrhizae) | Yang B 2007 |
| Bushen Zhuanggutang | Decoction | Investigator‐prescribed formula composed of Eucommia bark (Cortex eucommiae), Rehmanniae radix preparata, Fortune's drynaria rhizome (Rhizoma drynariae), Barbary wolfberry fruit (Fructus lycii), Epimedium (Herba epimedii), Radix codonopsis pilosulae (Radix codonopsis), liquorice root (Radix glycyrrhizae), common macrocarpium fruit (Fructus corni) and Sanqi (Radix notoginseng) | Ye AN 1998 |
| Shangke Jiegu tablet | Tablet | Chinese patent herbal medicine composed of Sanqi (Radix notoginseng), safflower (Flos carthami), frankincense (olibanum), myrrh (Commiphora molmol), ground beetle (Eupolyphaga seu steleophaga), etc. | Yuan YN 2000 |
| Xuduanzhuanggu capsule | Capsule | Chinese patent medicine manufactured by Zhejiang Di'er medicine limited company | Zhan HS 2009 |
| Bushenyiqihuoxue soup | Decoction | Herbal mixture composed of Psoralea corylifolia (Fructus psoraleae), Fortune's drynaria rhizome (Rhizoma drynariae), glossy privet fruit (Fructus ligustri lucidi), Epimedium (Herba epimedii), liquorice root (Radix glycyrrhizae), white peony root (Radix paeoniae alba), Chinese angelica (Radix angelicae sinensis), Himalayan teasel root (Radix dipsaci), ground beetle (Eupolyphaga seu steleophaga) and liquorice root (Radix glycyrrhizae) | Zhang DS 2011 |
| Liuwei Dihuang pill | Pill | Chinese patent medicine | Ma C 2011; Zhang J 2003 |
| Yigu capsule | Capsule | Herbal mixture composed of Epimedium (Herba epimedii), Barbary wolfberry fruit (Fructus lycii), Chinese angelica (Radix angelicae sinensis), two‐tooth achyranthes root (Radix achyranthis bidentatae), etc. | Zhang RH 2004 |
| Bushenjianpi Zhuangguyin | Decoction | Herbal mixture composed of human placenta (Placenta hominis), Dioscorea batatas (Rhizoma dioscoreae), red peony root (Radix paeoniae rubra), white peony root (Radix paeoniae alba), Chinese angelica (Radix angelicae sinensis), Radix codonopsis pilosulae (Radix codonopsis), liquorice root (Radix glycyrrhizae), large‐head atractylodes rhizome (Rhizoma atractylodis macrocephalae), common macrocarpium fruit (Fructus corni), common cnidium fruit (Fructus cnidii), Chinese thorowax root (Radix bupleuri), Eucommia bark (Cortex eucommiae), two‐tooth achyranthes root (Radix achyranthis bidentatae), Amur cork tree bark (Cortex phellodendri), tortoise shell (Carapax et plastrum testudinis), liquorice root (Radix glycyrrhizae), Psoralea corylifolia (Fructus psoraleae), Wolfiporia cocos (Poria), scorpion, Epimedium (Herba epimedii), Sanqi (Radix notoginseng), leech (Hirudo), etc. | Zhang XG 2011 |
| Bushen Huoxue capsule | Capsule | Herbal mixture composed of Epimedium (Herba epimedii), Eucommia bark (Cortex eucommiae), Himalayan teasel root (Radix dipsaci), deer horn glue (Colla cornus cervi), common macrocarpium fruit (Fructus corni), liquorice root (Radix glycyrrhizae), Dioscorea batatas (Rhizoma dioscoreae), Chinese angelica (Radix angelicae sinensis), Safflower (Flos carthami), etc. | Zhang XJ 2008 |
| TPF capsule | Capsule | Chinese herbal medicine composed of cake uterine, Colla corii asini, Corium stomachium galli | Zhang YL 1996 |
| Shigu yin | Liquid | Chinese patent medicine manufactured by Third Affiliated Hospital of Luohe Medical College pharmacy, composed of Rehmanniae radix preparata, East Asian tree fern rhizome (Rhizoma cibotii), Epimedium (Herba epimedii), Psoralea corylifolia (Fructus psoraleae), oyster shell (Concha ostreae), Dioscorea batatas (Rhizoma dioscoreae), etc. | Zhang YP 2007 |
| Huoluo Gukang pill | Pill | Condensed pill composed of Psoralea corylifolia (Fructus psoraleae), Fortune's drynaria rhizome (Rhizoma drynariae), liquorice root (Radix glycyrrhizae), safflower (Flos carthami), Zedoary (Rhizoma curcumae), deer horn glue (Colla cornus cervi), Lumbricus (Pheretima), etc. | Zhang YS 2003 |
| Zhuangguqiangjin tablet | Tablet | Manufactured by Dongwan Hospital of Traditional Chinese Medicine, composed of Slenderstyle acanthopanax bark (Cortex acanthopanacis), deer horn glue (Colla cornus cervi), desert‐living cistanche (Herba cistanches), Philippine flemingia root (Moghania philippinensis li.), Eucommia bark (Cortex eucommiae), Epimedium (Herba epimedii), two‐tooth achyranthes root (Radix achyranthis bidentatae), Radix codonopsis pilosulae (Radix codonopsis), Chinese angelica (Radix angelicae sinensis), tortoise shell (Carapax et plastrum testudinis), tuber fleece flower root (Radix polygoni multiflori), Amur cork tree bark (Cortex phellodendri), Cortex lycii radicis (Cortex lycii), incised notopterygium rhizome (Rhizoma et radix notopterygii) and Radix angelicae pubescentis | Zhang ZF 2011 |
| Shujinbogu tablet | Tablet | Manufactured by Dongwan Hospital of Traditional Chinese Medicine, composed of tree peony bark (Cortex moutan), rhubarb (Radix et rhizoma rhei), two‐tooth achyranthes root (Radix achyranthis bidentatae), tuberculate Speranskia herb (Garden balsam stem), Szechuan lovage rhizome (Rhizoma chuanxiong), Chinese angelica (Radix angelicae sinensis), Rehmannia root (Radix rehmanniae), mulberry twig (Ramulus mori), ground beetle (Eupolyphaga seu steleophaga), common cnidium fruit (Fructus cnidii), common aucklandia root (Radix aucklandiae), Fortune's drynaria rhizome (Rhizoma drynariae) and Flos caryophyllata (Flos caryophylli) | Zhang ZF 2011 |
| Zishen prescription | Decoction | Investigator‐prescribed formula composed of Rehmanniae radix preparata, common macrocarpium fruit (Fructus corni), Dioscorea batatas (Rhizoma dioscoreae), Wolfiporia cocos (Poria), Barbary wolfberry fruit (Fructus lycii), tortoise shell glue (Colla carapacis et plastri testudinis), crude Dragon's bones (Os Draconis) and crude oyster shell (Concha ostreae) | Zhao G 2002 |
| Kanggusong capsule | Capsule | Chinese patent medicine manufactured by Shenzhen Sanjiu medicine limited company, composed of hairy antler (Corn Cervi Pantotrichum), Epimedium (Herba epimedii), desert‐living cistanche (Herba cistanches), Psoralea corylifolia (Fructus psoraleae), common cnidium fruit (Fructus cnidii), etc. | Zhao HX 2001 |
| Yinyanghuo | Decoction | Single herb of Epimedium (Herba epimedii) | Zhao LN 2003 |
| Jinwugutong capsule | Capsule | Chinese patent medicine manufactured by Guizhou Shenqi Pharmaceutical limited company, composed of Epimedium (Herba epimedii), two‐tooth achyranthes root (Radix achyranthis bidentatae), Cibotium barometz (Rhizoma cibotii), Zaocys dhumnade (Zaocys), Psoralea corylifolia (Fructus psoraleae), etc. | Zheng WK 2007 |
| Gushen Yijing tang | Decoction | Herbal mixture composed of Fortune's drynaria rhizome (Rhizoma drynariae), Psoralea corylifolia (Fructus psoraleae), Barbary wolfberry fruit (Fructus lycii), liquorice root (Radix glycyrrhizae), prepared rhizome of adhesive rehmannia (Radix rehmanniae preparata), white peony root (Radix paeoniae alba), glossy privet fruit (Fructus ligustri lucidi), Dioscorea batatas (Rhizoma dioscoreae), Wolfiporia cocos (Poria), Chinese date (Fructus jujubae), etc. | Zhong RQ 2007 |
| Tongbu Qianggutang | Decoction | Herbal mixture composed of Epimedium (Herba epimedii), glossy privet fruit (Fructus ligustri lucidi), liquorice root (Radix glycyrrhizae), Sanqi (Radix notoginseng), wine rhubarb (Radix et rhizoma rhei), etc. | Zhou LZ 2001 |
| Guilu Erxiantang | Decoction | Herbal mixture composed of tortoise shell (Carapax et plastrum testudinis), hairy antler (Corn Cervi Pantotrichum), Epimedium (Herba epimedii), clematis root (Radix clematidis), prepared rhizome of adhesive rehmannia (Radix rehmanniae preparata), desert‐living cistanche (Herba cistanches), medicinal Indian mulberry root (Radix morindae officinalis), liquorice root (Radix glycyrrhizae), Radix codonopsis pilosulae (Radix codonopsis), Chinese angelica (Radix angelicae sinensis) and safflower (Flos carthami) | Zhou XT 2001 |
| Huangqi Sanxian tang | Decoction | Herbal mixture composed of common curculigo rhizome (Rhizoma curculiginis), Epimedium (Herba epimedii), liquorice root (Radix glycyrrhizae), Sanqi (Radix notoginseng), etc. | Zhou ZK 2006 |
| Bushen Tianjing Huoxue therapy | Decoction | Herbal mixture composed of Eucommia bark (Cortex eucommiae) 15 g, Himalayan teasel root (Radix dipsaci) 15 g, Chinese taxillus twig (Herba taxilli) 10 g, Epimedium (Herba epimedii) 15 g, Psoralea corylifolia (Fructus psoraleae) 15 g, common macrocarpium fruit (Fructus corni) 10 g, Danshen root (Radix salviae miltiorrhizae) 10g, suberect spatholobus stem (Caulis spatholobi) 30 g, Fortune's drynaria rhizome (Rhizoma drynariae) 30 g, two‐tooth achyranthes root (Radix achyranthis bidentatae) 30 g, membranous milk vetch root (Radix astragali) 30 g, large‐head atractylodes rhizome (Rhizoma atractylodis macrocephalae) 10 g, Wolfiporia cocos (Poria) 20 g and liquorice root (Radix glycyrrhizae) 5 g | Zhu HJ 2011 |
| Guben Zhuanggu capsule | Capsule | Chinese patent medicine manufactured by Zhejiang Di'er medicine limited company | Zou JJ 2005 |
| Bushen Zhuanggu granules | Granule | Chinese patent medicine manufactured by Yifan Pharmacy Company of Guangdong Province, with the following seven herbal compounds: Epimedium (Herba epimedii), Rehmannia (Rehmannia), Dioscorea batatas (Rhizoma dioscoreae), Cornus officinalis (Fructus corni), Cinnamomum cassia (Cortex cinnamomi), Drynaria fortunei (Rhizoma drynariae) and Morinda officinalis (Radix morindae officinalis) | Deng WM 2012 |
The control interventions included placebo in three trials and no treatment in five trials. In the other trials the control interventions were conventional pharmaceutical medicine or Chinese herbal medicines plus co‐intervention versus the same co‐intervention.
The interventions in the included trials are described in the Characteristics of included studies table.
Outcomes
Seven trials reported fracture incidence (An SJ 2000; Deng WM 2012; Li ZP 2011; Zhang RH 2004; Zhang ZF 2011; Zhao HX 2001; Zhu HM 2012). None of the 108 trials reported death directly or indirectly caused by osteoporosis. The outcomes reported were mainly surrogate outcomes, including BMD, biochemical indicators (serum calcium, phosphorus, alkaline phosphatase, oestradiol, parathyroid hormone, calcitonin, bone Gla protein, interleukin‐6, etc.), the improvement of lumbago and backache, and adverse effects. Only two trials reported quality of life and medical expenses (Liang DB 2012; Zhang ZF 2011). Adverse effects were reported in 44 trials and included nausea, diarrhoea, dry mouth, constipation, tiredness, dental ulcer, epigastric discomfort, breast pain, dizziness, stomach discomfort, etc. All the reported outcomes were measured at the end of treatment.
Risk of bias in included studies
In this review, all 108 trials were reported as parallel‐group randomised trials and three trials were multicentre randomised trials (Deng WM 2012; Xiong YH 2008; Zhu HM 2012). Of the 108, only 27 reported the methods used for the generation of the allocation sequence. Among them, four used random numbers generated by a computer or calculator (Xu YL 2007; Zhang RH 2004; Zhao HX 2001; Zhu HM 2012), 20 used a random numbers table (Chen XL 2010; Chen ZX 2002; Dai Y 2007; He N 2006; Hu J 2012; Li BL 2007; Li HW 2004; Li YH 2008; Ma C 2011; Qiu RB 2008; Wang CC 2005; Wu MS 2007; Wu W 2005; Xiong YH 2008; Xu H 2010; Xu M 2009; Zhang DS 2011; Zhang XZ 2004; Zhao G 2002; Zhu HJ 2011) and one used random drawings (Wang ZK 2004).
Only one trial (Zhu HM 2012) provided information about allocation concealment.
Double‐blinding was reported in 10 trials (An SJ 2000; Lin W 2000; Wang SW 2003; Wang XD 2011; Xiong YH 2008; Zhan HS 2009; Zhao HX 2001; Zhao LN 2003; Zheng WK 2007; Zhu HM 2012). Eight trials reported use of single‐blinding (Chen JP 1999; Chen NQ 2006; Mao YF 2011; Wang XY 2000; Xie YM 1997; Yuan YN 2000; Zhou ZK 2006; Zou JJ 2005).
Two trials (Deng WM 2012; Zhu HM 2012) reported a pre‐trial estimation of sample size and reported performing intention‐to‐treat analysis. Nineteen trials reported withdrawals (Deng WM 2012; He MT 2007; Li SL 2004; Li YH 2010; Ma C 2011; Meng XD 2003; Peng T 2002; Ruan XY 2006; Wang CC 2005; Wang JM 2008; Xiong YH 2008; Xu YL 2007; Zhan HS 2009; Zhang XJ 2008; Zhang RH 2004; Zhang ZF 2011; Zhao HX 2001; Zheng WK 2007; Zhu HM 2012).
None of the 108 trials reported all outcomes of interest in this review.
Seventy trials appeared to be free of other sources of bias, while the 38 other trials did not report the baseline comparability between groups in detail.
The risk of bias in the included studies is shown in detail in Figure 2 and Figure 3.
2.
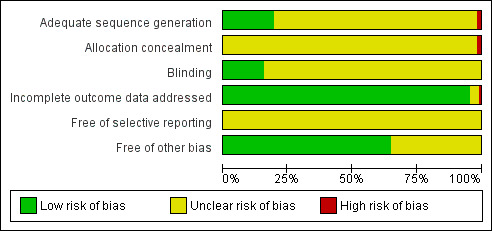
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
The poor availability of information about study design meant that we rated most items as having unclear risk of bias. Based on The Cochrane Collaboration's recommended summary assessments for risk of bias (Higgins 2011), the 'Risk of bias' assessment should be for the important outcomes, therefore for the outcome of fracture we evaluated the risk of bias as 'unclear' for sequence generation, allocation concealment, blinding and selective outcome reporting in those seven studies that reported it (Figure 3).
Assessment of reporting biases
Due to the small number of trials testing the same intervention and same outcome, a meaningful funnel plot analysis could not be conducted.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Chinese herbal medicines versus placebo (Comparison 01)
Three trials (involving 219 patients) compared the effects of Chinese herbal medicines with placebo (Lin W 2000; Wang XY 2000; Zhao HX 2001). Based on our standard classification for Chinese herbal medicines, the tested Chinese herbal medicines included two prescribed herbal formulae (Migu decoction and Bushen Yigu soft extract), and one Chinese proprietary medicine (Kanggusong capsules). The reported outcomes included BMD of the lumbar spine, radius and ulna, and the levels of oestradiol (E2). We were not able to pool the data due to the heterogeneity of the Chinese herbal medicines that were studied.
Based on the included trials, we created a 'Summary of findings' table for patient‐important outcomes (fractures, quality of life, death, serious adverse events, BMD) (see Table 1).
Fractures
In three trials of Chinese herbal medicines versus placebo, one trial reported new compression fractures of the backbone in the placebo group (0/50 versus 10/54) (RR 0.05; 95% CI 0.00 to 0.85) (Analysis 1.1) (Zhao HX 2001).
1.1. Analysis.
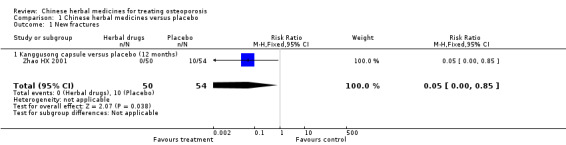
Comparison 1 Chinese herbal medicines versus placebo, Outcome 1 New fractures.
Quality of life or symptoms including pain, muscle fatigue and limited mobility
No trials reported quality of life.
One trial reported improvement in bone pain in the group treated with Chinese herbal medicines compared to placebo (49/50 versus 44/54) (RR 1.20; 95% CI 1.05 to 1.37) (Analysis 1.4) (Zhao HX 2001).
1.4. Analysis.
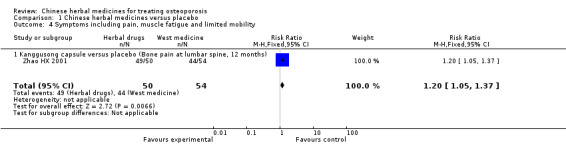
Comparison 1 Chinese herbal medicines versus placebo, Outcome 4 Symptoms including pain, muscle fatigue and limited mobility.
Death
No trials reported this outcome.
Adverse effects
In three trials of Chinese herbal medicines versus placebo, one trial reported some mild adverse effects in the Chinese herbal medicines group, including stomach/intestinal upset, headache and dizziness (Zhao HX 2001). Two trials did not report on adverse effects (Lin W 2000; Wang XY 2000).
Bone mineral density (BMD)
BMD of the lumbar spine
Compared with placebo, the group treated with Migu decoction showed a statistically significant increase in BMD of the lumbar spine after four months treatment (MD 0.16 g/cm3; 95% CI 0.06 to 0.26) (Analysis 1.2) (Lin W 2000). The group treated with Kanggusong capsules had a statistically significant increase in BMD when compared to the placebo group after 12 months treatment (MD 0.06 g/cm3; 95% CI 0.02 to 0.10) (Analysis 1.2) (Zhao HX 2001).
1.2. Analysis.
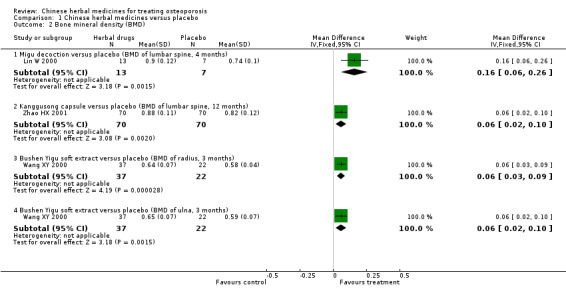
Comparison 1 Chinese herbal medicines versus placebo, Outcome 2 Bone mineral density (BMD).
BMD of the radius
The group treated with Bushen Yigu soft extract showed a statistically significant increase in BMD of the radius compared to a group treated with placebo after three months treatment (MD 0.06 g/cm3; 95% CI 0.03 to 0.09) (Analysis 1.2) (Wang XY 2000).
BMD of the ulna
Those treated with Bushen Yigu soft extract showed a statistically significant increase in BMD of the ulna when compared to a placebo group after three months treatment (MD 0.06 g/cm3; 95% CI 0.02 to 0.10) (Analysis 1.2) (Wang XY 2000).
Biochemical indicators
The effects of the interventions on biochemical indicators are shown in Appendix 5.
Summary: Comparison 01
In summary, compared with placebo, Migu decoction and Kanggusong capsule seemed to improve the BMD of the lumbar spine; Bushen Yigu soft extract improved the BMD of the radius and the ulna.
Chinese herbal medicines versus no intervention (Comparison 02)
Five trials (involving 628 patients) compared seven different Chinese herbal medicines with no intervention (Gong L 2001; Liao L 2004; Peng T 2002; Zhang YP 2007; Zhang YS 2003). Two trials with two arms compared different herbs with no intervention (Gong L 2001; Zhang YS 2003). The tested herbs included Yishen Zhuanggu mixture, Shengu capsule, Bushen Shengsui principle, Qianggu soft extract, Huoluo Gukang pills, Shigu yin and Gushukang granule. Based on our standard classification for Chinese herbal medicines, five herbs were Chinese proprietary medicine, and two were prescribed herbal formulae. The reported outcomes included BMD of the lumbar spine and femoral neck, and the levels of oestradiol (E2) and osteocalcin, also known as bone Gla protein (BGP), but we were not able to pool the data due to the heterogeneity of the Chinese herbal medicines that were studied.
Based on the included trials, we created a 'Summary of findings' table for patient‐important outcomes (fractures, quality of life, death, serious adverse events, BMD) (see Table 2).
Fractures
None of the five trials reported the outcome fractures.
Quality of life or symptoms including pain, muscle fatigue and limited mobility
None of the five trials reported these outcomes.
Death
None of the five trials reported this outcome.
Adverse effects
Four trials reported some mild adverse effects, such as mild stomach discomfort, intestinal upset or constipation, but this did not affect the continuation of treatment (Gong L 2001; Peng T 2002; Zhang YS 2003; Zhang YP 2007). One trial reported no adverse effects (Liao L 2004).
Bone mineral density (BMD)
BMD of lumbar spine
Compared with no intervention, treatment with Bushen Shengsui principle showed no evidence of effect after six months treatment (MD 0.07 g/cm3; 95% CI ‐0.06 to 0.20) (Analysis 2.1) (Liao L 2004).
2.1. Analysis.
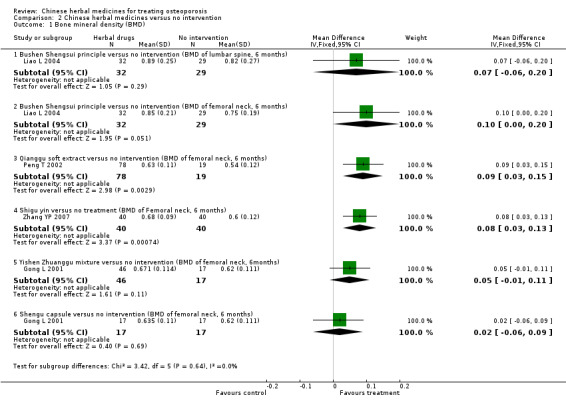
Comparison 2 Chinese herbal medicines versus no intervention, Outcome 1 Bone mineral density (BMD).
BMD of femoral neck
Compared with no intervention, treatment with Bushen Shengsui principle did not increase the BMD of the femoral neck after six months (MD 0.10 g/cm3; 95% CI 0.00 to 0.20) (Analysis 2.1) (Liao L 2004), and nor did treatment with Yishen Zhuanggu mixture after six months (MD 0.05 g/cm3; 95% CI ‐0.01 to 0.11) (Analysis 2.1) (Gong L 2001) or Shengu capsule (MD 0.02 g/cm3; 95% CI ‐0.06 to 0.09) (Analysis 2.1) (Gong L 2001). Treatment with Qianggu soft extract resulted in a statistically significant increase in the BMD of the femoral neck after six months treatment (MD 0.09 g/cm3; 95% CI 0.03 to 0.15) (Analysis 2.1) (Peng T 2002), as did Shigu yin after six months treatment (MD 0.08 g/cm3; 95% CI 0.03 to 0.13) (Analysis 2.1) (Zhang YP 2007).
Biochemical indicators
The effects of the interventions on biochemical indicators are shown in Appendix 5.
Summary: Comparison 02
In summary, compared with no intervention, Qianggu soft extract appeared to improve the BMD of the femoral neck significantly. Shigu yin also seemed to improve the BMD of the femoral neck. However, some groups treated with Chinese herbal medicines showed no significant difference from those receiving no intervention, including Bushen Shengsui principle (BMD of the lumbar spine and femoral neck), Yishen Zhuanggu mixture (BMD of the femoral neck) and Shengu capsule (BMD of the femoral neck) after six months of treatment.
Chinese herbal medicines versus western medicine (Comparison 03)
Sixty‐one trials (involving 4805 patients) compared 56 different Chinese herbal medicines with western medicine (An SJ 2000; Chen JP 1999; Chen NQ 2006; Chen X 2008; Chen ZX 2002; Cui TH 1999; Dong YF 2004; Gang PH 2001; He MT 2007; He N 2006; Huang ZJ 2008; Lan ZX 2006; Li BL 2007; Li YH 2008; Li YH 2010; Li ZY 2006; Liao L 2004; Ling JY 2008; Lv ZH 2002; Ma C 2011; Mao YF 2011; Meng XD 2003; Mu G 2001a; Ou L 2011; Peng T 2002; Qi ZX 1998; Qiu RB 2004; Qiu RB 2008; Ruan XY 2006; Shao M 2003; Shi CD 2012; Song XW 2000; Wang CC 2005; Wang H 2007; Wang J 2007; Wang JM 2008; Wang XY 2000; Wang YZ 2008; Wang ZK 2004; Wei RY 2011; Wu JZ 2010; Wu MS 2001; Wu MS 2007; Xie YM 1997; Xu H 2010; Xu M 2009; Xu W 2005; Xu YL 2007; Yang B 2007; Yuan YN 2000; Zhan ML 2007; Zhang XG 2011; Zhang YP 2007; Zhang J 2003; Zhang XJ 2008; Zhang YL 1996; Zhao LN 2003; Zhong RQ 2007; Zhou LZ 2001; Zhou ZK 2006; Zhu HJ 2011). Among them, two trials compared herbs with two different Western pharmaceutical medicines (An SJ 2000; Song XW 2000). One trial (Wu MS 2007) compared two herbs with western medicine.
The tested herbal preparations included Acupoint sticking of Migudan, Bugu Shengsui capsule, Bushen Huoxue capsules, Bushen Jianpi Huoxue recipe, Bushen Jianpi Jingu decoction, Bushen Jianpi Migu prescription, Bushen Qianggutang, Bushen Qiangshen pill, Bushen Tianjing Huoxue therapy, Bushen Shengsui principle, Bushen Yangxue tang, Bushen Yigu soft extract, Bushen Zhuanggu tang, Bushenjianpi Zhuangguyin, Erxian soup, Gujian capsule, Gukang decoction, Gukang oral liquid, Guli powder, Gumikang capsule, Gumiling granule, Gushen Yijing tang, Gushukang granule, Huangqi, Huangqi Sanxian tang, Jiangu capsule, Jiangu recipe, Jianshenfang granule, Jiawei Bushen Zhuangjintang, Jiawei Zhuangyao Jianshen tang, Jingujian granule, Jingujian granule (1,2,3), Kanggusong capsule, Kanggusong granule, Kangshu Jiangu granule, kidney‐tonifying herbs, Liuwei Dihuang pills, prescription for tonifying kidney, Qianggu capsule, Qianggu paste, Qianggu soft extract, Radix rehmanniae preparata and Radix astragali, Shangke Jiegu tablet, Shenyao capsules, Shigu yin, Strong Bone capsule, TPF capsule, Tongbu Qianggutang, urinary bladder (Kidney) meridian sticking, Xianling Gusong capsules, Xianlinggubao capsule, Yanghuo Sanzi tang, Yinyanghuo, Herba epimedii prescription (including Herba epimedii, Fructus psoraleae, Cortex eucommiae, Concha ostreae, Radix aconiti preparata, Radix aconiti kusnezoffii, Radix angelicae sinensis and Rhizome chuanxiong), Yishen Yanggan mixture and Zishen Gukang pill. All these herbal preparations except Yinyanghuo and Huangqi (Radix Astragali) are mixtures of herbs. Based on our standard classification for Chinese herbal medicines, 22 herbs were Chinese proprietary medicine, and 34 were prescribed herbal formulae.
The reported outcomes included BMD of the lumbar spine, radius, ulna, femoral neck, Ward's triangle, trochanter, hipbone, distal radius and calcaneus. Levels of oestradiol (E2), serum calcium (Ca), phosphorus (P), alkaline phosphatase (ALP), bone Gla protein (BGP), interleukin‐6 (IL‐6), parathyroid hormone (PTH) and calcitonin (CT) were also measured, as was the improvement of lumbago and backache.
We were not able to pool the data due to the variations in the herbal preparations and control interventions that were used in the studies.
Based on the included trials, we created a 'Summary of findings' table for patient‐important outcomes (fractures, quality of life, death, serious adverse events, BMD) (see Table 3).
Fractures
Of the 61 trials, one trial reported that no new fracture occurred (An SJ 2000).
Quality of life or symptoms including pain, muscle fatigue and limited mobility
No trials reported the outcome of quality of life.
Twenty trials out of 61 reported the improvement of lumbago and backache. Among these 20 trials, eight reported that Chinese herbal medicines were better than western medicines without reporting the number of cases with ache improvement in each group (Chen NQ 2006; Dong YF 2004; Ou L 2011; Qi ZX 1998; Wang ZK 2004; Xie YM 1997; Xu H 2010; Zhang XJ 2008). The other 12 trials reported as follows:
46/48 versus 24/42: RR 1.68; 95% CI 1.28 to 2.19 (Analysis 3.4) (Lv ZH 2002);
76/78 versus 11/21: RR 1.86; 95% CI 1.24 to 2.80 (Analysis 3.4) (Peng T 2002);
24/30 versus 15/30: RR 1.60; 95% CI 1.07 to 2.39 (Analysis 3.4) (Wu MS 2001);
53/60 versus 26/50: RR 1.70; 95% CI 1.28 to 2.25 (Analysis 3.4) (Xu W 2005);
13/15 versus 9/10: RR 0.96; 95% CI 0.72 to 1.28 (Analysis 3.4) (Zhao LN 2003);
15/15 versus 2/10: RR 4.26; 95% CI 1.43 to 12.72 (Analysis 3.4) (Gang PH 2001);
25/29 versus 20/29: RR 1.25; 95% CI 0.94 to 1.66 (Analysis 3.4) (He N 2006);
28/30 versus 22/30: RR 1.27; 95% CI 1.01 to 1.61 (Analysis 3.4) (Huang ZJ 2008);
52/60 versus 11/40: RR 3.15; 95% CI 1.89 to 5.26 (Analysis 3.4) (Li YH 2008);
18/21 versus 12/22: RR 1.57; 95% CI 1.03 to 2.39 (Analysis 3.4) (Li ZY 2006);
43/48 versus 19/32: RR 1.51; 95% CI 1.12 to 2.04 (Analysis 3.4) (Wei RY 2011);
56/60 versus 34/60: RR 1.65; 95% CI 1.31 to 2.08 (Analysis 3.4) (Qiu RB 2008).
3.4. Analysis.
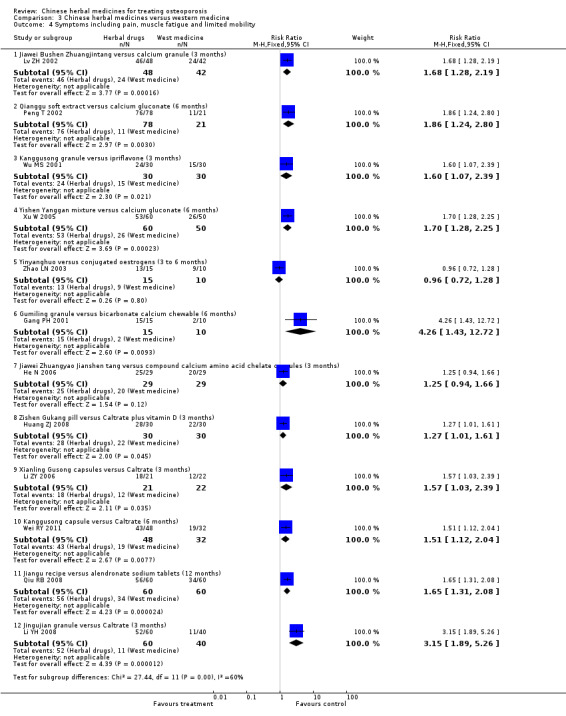
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 4 Symptoms including pain, muscle fatigue and limited mobility.
Death
No trials reported this outcome.
Adverse effects
Twenty‐three trials out of 61 reported adverse effects. These included:
two patients with midsection discomfort in the herbal group (Cui TH 1999);
a few patients with dizziness, rash, nausea, vomiting and cardiopalmus at the beginning of therapy, but no significant difference in comparison groups (Dong Y 2010);
11 patients with dry mouth symptoms, five with stomach discomfort, two with constipation and one with cardiopalmus in the herbal group, and nine patients with stomach discomfort and one with constipation in the western medicine group (Li YH 2010);
three patients with spotting vaginal bleeding at the beginning of treatment, which did not affect observation, and five cases with breast pain who withdrew from the western medicine group (Liao L 2004);
six patients with constipation and five with dry mouth symptoms in the herbal group, which did not affect observation (Meng XD 2003);
two patients with abdominal distention and sour regurgitation in the herbal group, and three with gastrointestinal symptoms in the western medicine group (Ma C 2011);
three patients in the herbal group and one in the western medicine group with stomach discomfort (Peng T 2002);
one patient with stomach discomfort in the western medicine group (Wang CC 2005);
five patients with stomach discomfort in the herbal group and two in the western medicine group (Wang H 2007);
two patients with mild constipation and one with dry mouth symptoms in the herbal group (Wang J 2007);
a few patients with nausea, stomach ache and dizziness in the western medicine group (Wu MS 2001);
two patients with dry and hard stools during treatment in the herbal group (Xie YM 1997);
five patients with nausea in the herbal group and three in the western medicine group (Xu H 2010);
two patients with nausea in the herbal group and three in the western medicine group (Xu M 2009);
three patients with gastrointestinal reactions in the western medicine group (Chen X 2008);
one patient with palpitations, two patients with constipation and four patients with stomach discomfort in the Jingujian granule group, and five patients with constipation and two patients with abdominal distention in the western medicine group (Li YH 2008); and
one trial (Zhang YP 2007) which reported patients with constipation in the control group without reporting data.
Six trials reported no adverse effects (Chen NQ 2006; Gang PH 2001; Xu YL 2007; Yuan YN 2000; Zhang XJ 2008; Zhou LZ 2001).
Most of the adverse effects did not affect continuation of treatment. Thirty‐eight trials did not report adverse effects.
Bone mineral density (BMD)
BMD of the lumbar spine
No significant difference between groups in the BMD of the lumbar spine was reported for any of the following comparisons of treatment groups:
Kanggusong granule versus ipriflavone plus Caltrate (MD 0.00 g/cm3; 95% CI ‐0.05 to 0.05), Kanggusong granule versus Caltrate after nine months treatment (MD 0.04 g/cm3; 95% CI ‐0.02 to 0.10) (Analysis 3.2) (An SJ 2000);
Strong Bone capsule versus Caltrate after three months treatment (MD ‐0.01 g/cm3; 95% CI ‐0.08 to 0.06) (Analysis 3.2) (Cui TH 1999);
Herba epimedii prescription versus vitamin D plus Caltrate after six months treatment (MD 0.03 g/cm3; 95% CI 0.00 to 0.06) (Analysis 3.2) (Dong YF 2004);
Bushen Shengsui principle versus conjugated oestrogens plus medroxyprogesterone after six months treatment (MD 0.00 g/cm3; 95% CI ‐0.12 to 0.12) (Analysis 3.2) (Liao L 2004);
Bushen Jianpi Jingu decoction versus calcitonin after three months treatment (MD 0.02 g/cm3; 95% CI ‐0.09 to 0.13) (Analysis 3.2) (Mao YF 2011);
Gukang oral liquid versus alendronate after six months treatment (MD 0.01 g/cm3; 95% CI ‐0.03 to 0.05) (Analysis 3.2) (Shao M 2003);
kidney‐tonifying herbs versus nilestriol (MD ‐0.02 g/cm3; 95% CI ‐0.09 to 0.05), kidney‐tonifying herbs versus calcium (MD 0.02 g/cm3; 95% CI ‐0.04 to 0.08) after six months treatment (Analysis 3.2) (Song XW 2000);
Bushen Jianpi Huoxue recipe versus alendronate sodium tablets (MD 0.02 g/cm3; 95% CI ‐0.01 to 0.05) after six months treatment (Analysis 3.2) (He MT 2007);
Huangqi Sanxian tang versus nilestriol after three months treatment (MD 0.00 g/cm3; 95% CI ‐0.03 to 0.04) (Analysis 3.2) (Zhou ZK 2006);
Qianggu capsule versus active vitamin D3 after six months treatment (MD 0.04 g/cm3; 95% CI ‐0.01 to 0.09) (Analysis 3.2) (Wang J 2007);
Jianshenfang granule versus alendronate sodium after six months treatment (MD 0.09 g/cm3; 95% CI ‐0.03 to 0.21) (Analysis 3.2) (Li BL 2007);
Jiangu recipe versus alendronate sodium after 12 months treatment (MD 0.02 g/cm3; 95% CI 0.00 to 0.04) (Analysis 3.2) (Qiu RB 2008);
Gukang fang versus alendronate sodium tablets after four months treatment (MD 0.00 g/cm3; 95% CI ‐0.03 to 0.03) (Analysis 3.2) (Chen X 2008);
Qianggu capsule versus alendronate (MD 0.01 g/cm3; 95% CI ‐0.04 to 0.05) after six months treatment (Analysis 3.2) (Xu H 2010);
Bushen Huoxue capsules versus Caltrate and calcitonin (MD 0.02 g/cm3; 95% CI ‐0.05 to 0.09) after 12 months treatment (Analysis 3.2) (Zhang XJ 2008);
Yinyanghuo versus conjugated oestrogens (MD 0.03 g/cm3; 95% CI ‐0.04 to 0.10) after six months treatment (Analysis 3.2) (Zhao LN 2003);
Shenyao capsules versus Caltrate (MD 0.00 g/cm3; 95% CI ‐0.05 to 0.05) after six months treatment (Analysis 3.2) (Chen NQ 2006);
Guli powder versus Caltrate after three months treatment (male: MD 0.02 g/cm3; 95% CI ‐0.01 to 0.05; female: MD 0.04 g/cm3; 95% CI ‐0.07 to 0.15) (Analysis 3.2) (Lan ZX 2006);
Gushen Yijing tang versus Caltrate after six months treatment (MD 0.06 g/cm3; 95% CI ‐0.02 to 0.14) (Analysis 3.2) (Zhong RQ 2007);
Bugu Shengsui capsule versus vitamin D2 plus calcium tablet after six months treatment (MD 0.12 g/cm3; 95% CI ‐0.01 to 0.26) (Analysis 3.2) (Xie YM 1997);
Xianling Gusong capsules versus Caltrate after six months treatment (MD ‐0.03 g/cm3; 95% CI ‐0.08 to 0.02) (Analysis 3.2) (Li ZY 2006);
Zishen Gukang pill versus Caltrate plus vitamin D after three months treatment (MD ‐0.00 g/cm3; 95% CI ‐0.05 to 0.04) (Analysis 3.2) (Huang ZJ 2008);
Gukang decoction versus alendronate sodium tablets after four months treatment (MD ‐0.00 g/cm3; 95% CI ‐0.03 to 0.03) (Analysis 3.2) (Chen X 2008);
Gumiling granule versus chewable bicarbonate calcium after six months treatment (MD 0.04 g/cm3; 95% CI ‐0.02 to 0.09) (Analysis 3.2) (Gang PH 2001);
Gujian capsule versus alfacalcidol after three months treatment (MD 0.08 g/cm3; 95% CI ‐0.02 to 0.18) (Analysis 3.2) (Meng XD 2003);
Xianlinggubao capsule versus alendronate after six months treatment (MD 0.00 g/cm3; 95% CI ‐0.02 to 0.02) (Analysis 3.2) (Xu M 2009);
Bushen Tianjing Huoxue therapy versus Caltrate and calcitriol soft capsule after six months treatment (MD 0.04 g/cm3; 95% CI 0.00 to 0.07) (Analysis 3.2) (Zhu HJ 2011).
3.2. Analysis.
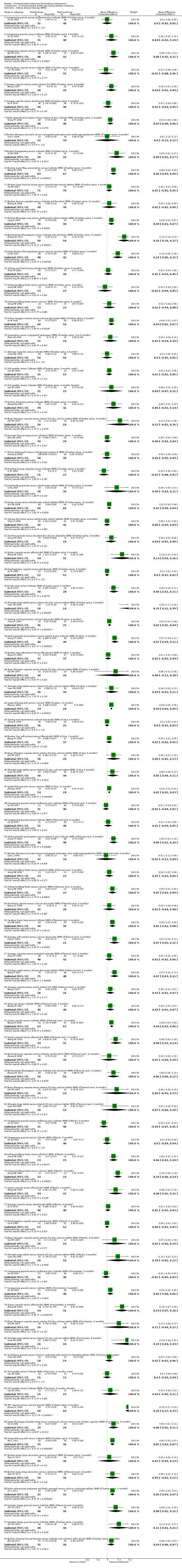
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 2 Bone mineral density (BMD).
In 13 trials Chinese herbal medicines were reported to have statistically significant better effects on the BMD of the lumbar spine when compared with western medicine (calcium supplementation, vitamin D, hormones or different combinations):
Jiawei Bushen Zhuangjintang versus calcium granules after three months treatment (MD 0.10 g/cm3; 95% CI 0.05 to 0.15) (Analysis 3.2) (Lv ZH 2002);
Yishen Yanggan mixture versus calcium gluconate after three months treatment (MD 0.04 g/cm3; 95% CI 0.01 to 0.07) (Analysis 3.2) (Xu W 2005);
Kanggusong granule versus Caltrate after six months treatment (MD 0.06 g/cm3; 95% CI 0.02 to 0.11) (Analysis 3.2) (Wei RY 2011);
Bushen Qianggutang versus Caltrate after three months treatment (MD 0.09 g/cm3; 95% CI 0.01 to 0.17) (Analysis 3.2) (Qi ZX 1998);
Bushen Jianpi Migu prescription versus Caltrate after three months treatment (MD 0.06 g/cm3; 95% CI 0.03 to 0.09) (Analysis 3.2) (Wang ZK 2004);
Bushen Zhuanggu tang versus alendronate sodium tablets after three months treatment (MD 0.04 g/cm3; 95% CI 0.01 to 0.07) (Analysis 3.2) (Ling JY 2008);
Bushenjianpi Zhuangguyin versus Caltrate and calcitonin after 12 weeks treatment (MD 0.16 g/cm3; 95% CI 0.10 to 0.22) (Analysis 3.2) (Zhang XG 2011);
Liuwei Dihuang pills versus Caltrate after six months (MD 0.05 g/cm3; 95% CI 0.03 to 0.07) (Analysis 3.2) (Ma C 2011);
Gujian capsule versus alfacalcidol after six months treatment (MD 0.14 g/cm3; 95% CI 0.03 to 0.26) (Analysis 3.2) (Meng XD 2003);
Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets after six months treatment (MD 0.05 g/cm3; 95% CI 0.03 to 0.07) (Analysis 3.2) (Ou L 2011);
Kangshu Jiangu granule versus Caltrate after six months treatment (MD 0.11 g/cm3; 95% CI 0.01 to 0.21) (Analysis 3.2) (Shi CD 2012);
Erxian soup versus Caltrate after six months treatment (MD 0.06 g/cm3; 95% CI 0.02 to 0.11) (Analysis 3.2) (Wu JZ 2010);
Shangke Jiegu tablet versus vitamin D2 plus calcium tablet after six months treatment (MD 0.25 g/cm3; 95% CI 0.11 to 0.39) (Analysis 3.2) (Yuan YN 2000).
BMD of the radius
Treatment with Liuwei Dihuang pills statistically significantly increased the BMD of the radius as compared to a group supplemented with calcium. The difference at 12 months between before and after treatment was MD 0.04 g/cm3 (95% CI 0.03 to 0.05) (Analysis 3.2) (Zhang J 2003). Another trial of treatment with Gukang oral liquid as compared to treatment with calcium gluconate reported a significant increase in the BMD of the radius after six months treatment (MD 0.02 g/cm3; 95% CI 0.01 to 0.04) (Analysis 3.2) (Chen JP 1999). A third trial showed positive effects of herbal treatments when compared to vitamin D and Caltrate: compared with vitamin D plus Caltrate, Herba epimedii prescription statistically significantly increased the BMD of the radius after six months treatment (MD 0.07 g/cm3; 95% CI 0.04 to 0.11) (Analysis 3.2) (Dong YF 2004).
Other trials comparing herbal treatments to vitamin D and calcium showed no significant difference in the BMD of the radius, including:
Bushen Yigu soft extract versus Alfacalcidol after three months treatment (MD 0.01 g/cm3; 95% CI ‐0.02 to 0.04) (Analysis 3.2) (Wang XY 2000);
Bugu Shengsui capsule versus vitamin D2 and calcium tablets after six months treatment (MD 0.08 g/cm3; 95% CI ‐0.12 to 0.28) (Analysis 3.2) (Xie YM 1997);
Shangke Jiegu tablet versus vitamin D2 and calcium tablets after six months treatment (MD 0.04 g/cm3; 95% CI 0.02 to 0.11) (Analysis 3.2) (Yuan YN 2000).
BMD of the ulna
Four trials showed no significant effects on the BMD of the ulna:
Gukang oral liquid versus calcium gluconate (MD 0.00 g/cm3; 95% CI ‐0.02 to 0.02) (Analysis 3.2) (Chen JP 1999);
Bushen Yigu soft extract versus Alfacalcidol after three months treatment (MD 0.02 g/cm3; 95% CI ‐0.01 to 0.05) (Analysis 3.2) (Wang XY 2000);
Bugu Shengsui capsule versus vitamin D2 plus calcium tablets after six months treatment (MD 0.06 g/cm3; 95% CI ‐0.01 to 0.13) (Analysis 3.2) (Xie YM 1997);
Shangke Jiegu tablet versus vitamin D2 plus calcium tablets after six months treatment (MD 0.05 g/cm3; 95% CI 0.00 to 0.11) (Analysis 3.2) (Yuan YN 2000).
After 12 months treatment with Liuwei Dihuang pills, the BMD of the ulna was statistically significantly increased when compared with calcium supplementation (MD 0.02 g/cm3; 95% CI 0.01 to 0.03) (Analysis 3.2) (Zhang J 2003).
BMD of the femoral neck
Eight trials of Chinese herbal medicines compared with supplementation with calcium or calcium plus other western medicine showed a significant increase in the BMD of the femoral neck:
Qianggu soft extract versus calcium gluconate after six months treatment (MD 0.07 g/cm3; 95% CI 0.02 to 0.12) (Analysis 3.2) (Peng T 2002);
Herba epimedii prescription versus vitamin D plus Caltrate after six months treatment (MD 0.06 g/cm3; 95% CI 0.02 to 0.10) (Analysis 3.2) (Dong YF 2004);
Kangshu Jiangu granule versus Caltrate after six months treatment (MD 0.08 g/cm3; 95% CI 0.01 to 0.15) (Analysis 3.2) (Shi CD 2012);
kidney‐tonifying herbs versus calcium after six months treatment (MD 0.05 g/cm3; 95% CI 0.01 to 0.09) (Analysis 3.2) (Song XW 2000);
Qianggu paste versus calcium gluconate after six months treatment (MD 0.07 g/cm3; 95% CI 0.03 to 0.11) (Analysis 3.2) (Wang H 2007);
Yanghuo Sanzi Tang versus Caltrate after six months treatment (MD 0.05 g/cm3; 95% CI 0.02 to 0.08) (Analysis 3.2) (Qiu RB 2004);
Jiangu capsule versus Caltrate after 12 months treatment (MD 0.04 g/cm3; 95% CI 0.03 to 0.06) (Analysis 3.2) (Wang YZ 2008);
Gujian capsule versus alfacalcidol after six months treatment (MD 0.08 g/cm3; 95% CI 0.03 to 0.14) (Analysis 3.2) (Meng XD 2003).
Ten other trials showed no significant difference in the BMD of the femoral neck for those treated with Chinese herbal medicines when compared to control groups:
Kanggusong granule versus Caltrate after nine months treatment (MD 0.01 g/cm3; 95% CI ‐0.03 to 0.05), Kanggusong granule versus ipriflavone plus Caltrate after nine months treatment (MD ‐0.01 g/cm3; 95% CI ‐0.04 to 0.02) (Analysis 3.2) (An SJ 2000);
Bushen Jianpi decoction versus calcitonin (MD 0.05 g/cm3; 95% CI ‐0.02 to 0.12) (Analysis 3.2) (Mao YF 2011);
Gumikang capsule versus calcium gluconate after six months treatment (MD 0.02 g/cm3; 95% CI ‐0.04 to 0.08) (Analysis 3.2) (Wang CC 2005);
Yanghuo Sanzi Tang versus Caltrate after three months treatment (MD 0.00 g/cm3; 95% CI ‐0.03 to 0.03) (Analysis 3.2) (Qiu RB 2004);
Qianggu capsule versus active vitamin D3 after six months treatment (MD 0.03 g/cm3; 95% CI ‐0.01 to 0.07) (Analysis 3.2) (Wang J 2007);
Shigu yin versus Caltrate after six months treatment (MD 0.03 g/cm3; 95% CI ‐0.01 to 0.07) (Analysis 3.2) (Zhang YP 2007);
Bushen Huoxue capsule versus Caltrate plus calcitriol after 12 months treatment (MD 0.02 g/cm3; 95% CI ‐0.06 to 0.10) (Analysis 3.2) (Zhang XJ 2008);
Bushenjianpi Zhuangguyin versus Caltrate and calcitonin after 12 weeks (MD 0.06 g/cm3; 95% CI 0.00 to 0.12) (Analysis 3.2) (Zhang XG 2011);
Bugu Shengsui capsule versus vitamin D2 plus calcium tablet after six months treatment (MD 0.09 g/cm3; 95% CI ‐0.05 to 0.23) (Analysis 3.2) (Xie YM 1997);
Shangke Jiegu tablet versus vitamin D2 plus calcium tablet after six months treatment (MD 0.07 g/cm3; 95% CI ‐0.06 to 0.20) (Analysis 3.2) (Yuan YN 2000).
Three trials compared Chinese herbal medicines with western medicine and found no significant differences between groups on measurement of BMD of the femoral neck:
Bushen Shengsui principle versus conjugated oestrogens plus medroxyprogesterone after six months treatment (MD ‐0.01 g/cm3; 95% CI ‐0.11 to 0.09) (Analysis 3.2) (Liao L 2004);
kidney‐tonifying herbs versus nilestriol after six months treatment (MD 0.02 g/cm3; 95% CI ‐0.02 to 0.06) (Analysis 3.2) (Song XW 2000);
Qianggu capsules versus oestradiol valerate after six months treatment (MD 0.02 g/cm3; 95% CI ‐0.02 to 0.06) (Analysis 3.2) (Ruan XY 2006).
BMD of Ward's triangle
Two trials showed no evidence of an effect on the BMD of Ward's triangle of Chinese herbal medicines when compared with alendronate after six months treatment:
Qianggu capsule (MD 0.01 g/cm3; 95% CI ‐0.02 to 0.03) (Analysis 3.2) (Xu H 2010);
Xianlinggubao capsule (MD 0.00 g/cm3; 95% CI ‐0.02 to 0.02) (Analysis 3.2) (Xu M 2009).
Two other trials showed no evidence of an effect of Chinese herbal medicines when compared with vitamin D2 plus calcium tablet after six months treatment:
Bugu Shengsui capsule (MD 0.07 g/cm3; 95% CI ‐0.05 to 0.19) (Analysis 3.2) (Xie YM 1997);
Shangke Jiegu capsule (MD 0.10 g/cm3; 95% CI ‐0.01 to 0.22) (Analysis 3.2) (Yuan YN 2000).
There was no significant difference between Chinese herbal medicine (Kanggusong granule) and ipriflavone plus Caltrate (MD ‐0.03 g/cm3; 95% CI ‐0.07 to 0.01), or Caltrate alone (MD 0.00 g/cm3; 95% CI ‐0.04 to 0.04) after nine months treatment (Analysis 3.2) (An SJ 2000). Gujian capsule appeared statistically significantly better than alfacalcidol after six months treatment (MD 0.08 g/cm3; 95% CI 0.01 to 0.15) (Analysis 3.2) (Meng XD 2003).
The trial comparing kidney‐tonifying herbs with treatment with nilestriol (MD 0.06 g/cm3; 95% CI 0.02 to 0.10) or supplementation with calcium (MD 0.10 g/cm3; 95% CI 0.06 to 0.14) (Analysis 3.2) (Song XW 2000) found a significant improvement in the BMD of Ward's triangle.
BMD of the trochanter
One trial showed better treatment effects of Chinese herbal medicines on the BMD of the trochanter when compared with calcium supplementation:
Shangke Jiegu tablet versus vitamin D2 plus calcium tablet after six months treatment (MD 0.19 g/cm3; 95% CI 0.04 to 0.33) (Analysis 3.2) (Yuan YN 2000).
However, two other trials showed no evidence of an effect of Chinese herbal medicines:
Kanggusong granule versus ipriflavone plus Caltrate after nine months treatment (MD ‐0.01 g/cm3; 95% CI ‐0.05 to 0.03) (Analysis 3.2) (An SJ 2000), or versus Caltrate alone (MD 0.03 g/cm3; 95% CI 0.00 to 0.06) (Analysis 3.2) (An SJ 2000);
Bugu Shengsui capsule versus vitamin D2 plus calcium tablet after six months treatment (MD 0.11 g/cm3; 95% CI ‐0.04 to 0.27) (Analysis 3.2) (Xie YM 1997).
There was a significant difference between Gujian capsule and alfacalcidol after six months treatment (MD 0.14 g/cm3; 95% CI 0.07 to 0.20) (Analysis 3.2) (Meng XD 2003).
BMD of the hip bone
There was no significant difference between the Chinese herbal medicine Gushukang granule and Calcium carbonate with vitamin D chewable tablets in the BMD of the hip bone after six months treatment: the MD was 0.02 g/cm3 (95% CI ‐0.02 to 0.06) (Analysis 3.2) (Chen ZX 2002). There was also no significant difference between the Chinese herbal medicine Guli powder when compared with Caltrate after six months treatment (MD 0.03 g/cm3; 95% CI ‐0.05 to 0.11) (Analysis 3.2) (Lan ZX 2006).
BMD of the distal radius
TPF capsule compared to calcium granules statistically significantly increased the BMD of the distal radius (MD 0.20 g/cm3; 95% CI 0.17 to 0.23) (Analysis 3.2) (Zhang YL 1996).
BMD of the calcaneus
Jiawei Zhuangyao Jianshen tang compared to compound calcium amino acid chelate capsules statistically significantly increased the BMD of the calcaneus (MD 0.06 g/cm3; 95% CI 0.01 to 0.11) (Analysis 3.2) (He N 2006).
T score in BMD measurement
Two trials reported T score in bone mineral density measurement:
Jingujian granule versus Caltrate after three months treatment (MD 0.05; 95% CI ‐0.57 to 0.67) (Analysis 3.3) (Li YH 2008);
Jingujian granule versus Caltrate after three months treatment (MD 0.03; 95% CI ‐0.04 to 0.10) (Analysis 3.3) (Li YH 2010).
3.3. Analysis.
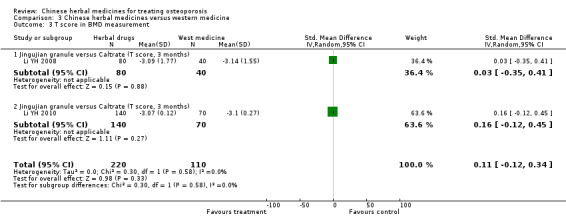
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 3 T score in BMD measurement.
Biochemical indicators
The effects of the interventions on biochemical indicators are shown in Appendix 5.
Summary: Comparison 03
In summary, 61 trials out of the 108 included trials evaluated Chinese herbal medicines compared with conventional western medicines.
The following Chinese herbal medicines increased the BMD of the lumbar spine: Jiawei Bushen Zhuangjintang Yishen Yanggan mixture, Kanggusong granule, Bushen Qianggutang, Bushen Jianpi Migu prescription, Bushen Zhuanggu tang, Bushenjianpi Zhuangguyin, Gujian capsule, Erxian soup, Shangke Jiegu tablet, Liuwei Dihuang pills, Kangshu Jiangu granule, Radix rehmanniae preparata and Radix astragali. However, the following did not increase the BMD of the lumbar spine: Kanggusong granule, Strong Bone capsule, Herba epimedii prescription, Bushen Shengsui principle, Bushen Jianpi Jingu decoction, Gukang oral liquid, kidney‐tonifying herbs, Bushen Jianpi Huoxue recipe, Huangqi Sanxian tang, Qianggu capsule, Jianshenfang granule, Jiangu recipe, Gukang fang, Qianggu capsule, Bushen Huoxue capsules, Yinyanghuo, Shenyao capsules, Guli powder, Gushen Yijing tang, Bugu Shengsui capsule, Xianling Gusong capsules, Zishen Gukang pill, Gukang decoction, Gumiling granule, Gujian capsule, Xianlinggubao capsule and Bushen Tianjing Huoxue therapy.
Gukang oral liquid, Herba epimedii prescription and Liuwei Dihuang pills increased the BMD of the radius, while Bushen Yigu soft extract, Bugu Shengsui capsule and Shangke Jiegu tablet did not.
Liuwei Dihuang pills appeared to increase the BMD of the ulna, while Gukang oral liquid, Bushen Yigu soft extract, Bugu Shengsui capsule and Shangke Jiegu tablet did not.
The following Chinese herbal medicines appeared to improve the BMD of the femoral neck: Qianggu soft extract, Herba epimedii prescription, kidney‐tonifying herbs, Qianggu paste, Yanghuo Sanzi tang, Jiangu capsule, Kangshu Jiangu granule and Gujian capsule. However, the following did not: Kanggusong granule, Bushen Jianpi decoction, Gumikang capsule, Yanghuo Sanzi Tang, Qianggu capsule, Shigu yin, Bushen Huoxue capsule, Bushenjianpi Zhuangguyin, Bugu Shengsui capsule, Shangke Jiegu tablet and Bushen Shengsui principle.
Gujian capsule and kidney‐tonifying herbs seemed to increase the BMD of Ward's triangle, while Qianggu capsule, Xianlinggubao capsule, Bugu Shengsui capsule, Shangke Jiegu capsule and Kanggusong granule did not.
Shangke Jiegu tablet and Gujian capsule appeared to increase the BMD of the trochanter, while Kanggusong granule and Bugu Shengsui capsule did not.
Gushukang granule and Guli powder did not show statistically significant increases in the BMD of the hip bone.
TPF capsule appeared to increase the BMD of the distal radius.
Jiawei Zhuangyao Jianshen tang statistically significantly increased the BMD of the calcaneus.
Jingujian granule did not show a statistically significant increase in the T score of BMD.
Chinese herbal medicines plus western medicine versus western medicine (Comparison 04)
Fourty‐seven trials (involving 5529 patients) compared 42 different Chinese herbal medicines plus western medicine with western medicine (Cao GY 2010; Cao W 2010; Chen FS 2001; Chen XL 2010; Chen YQ 2001; Chen ZX 2002; Dai Y 2007; Deng WM 2012; Dong Y 2010; Fan HX 2004; Guo JH 2008; Han XL 2007; He XQ 2006; Hu J 2012; Huang JW 2008; Jian QQ 2010; Liang DB 2012; Li HW 2004; Li SL 2004; Liu JM 2012; Ma C 2011; Ma CZ 2011; Ma YJ 2011; Miu JQ 2008; Mu G 2001; Qiu ZX 2010; Ruan XY 2006; Tang ZA 2012; Wang SW 2003; Wang XD 2011; Wu W 2005; Xie J 2004; Xiong YH 2008; Xu H 2010; Xu M 2009; Yang B 2007; Ye AN 1998; Zhan HS 2009; Zhang DS 2011; Zhang RH 2004; Zhang XZ 2004; Zhang ZF 2011; Zhao G 2002; Zheng WK 2007; Zhou XT 2001; Zhu HM 2012; Zou JJ 2005).
The tested herbs included Antai capsule, Bushenhuoxue therapy, Bushen Qianggu Huoxue therapy, Bushen Qiangshen pill, Bushen Yangxue tang, Bushen Zhuanggutang, Bushen Zhuanggu granules, Bushen Kangsong pill, Bushenyiqihuoxue soup, Chinese medicine (combination of 10 herbs), Chinese medicine Bushenhuoxue recipe, Erxian Yanggu decoction, Gengnian Anyi tablet, Guben Zhuanggu capsules, Guilu Erxiantang, Gukang tablet, Gushen decoction, Gushukang granule, Gusongbao granule, Heche Dazao pill, Huangqi, Hugu capsule, Jiangu granule, Jiarong tablet, Jinwugutong capsules, Kanggusong soup, Liuwei Dihuang pills, Migu tablet, Qianggu capsule, Shangke Yishen Zhuanggu pill, Shengsuiyin recipe, Shugan zishen huoxue tang, traditional Chinese capsule, Xianlinggubao capsule, Xuduanzhuanggu capsule, Yigu capsule, Yishen Zhuanggu decoction, Yiyuanjiangu decoction, Zhuanggu capsule, Zhuangguqiangjin tablet, Zishen prescription and Ziyin Bushen Zhuanggu prescription. All these Chinese herbal medicines, except Huangqi, were mixtures of herbs. Based on our standard classification for Chinese herbal medicines, 17 herbs were Chinese proprietary medicine, and 25 were prescribed herbal formulae.
The reported outcomes included BMD of the lumbar spine, femoral neck, Ward's triangle, trochanter, hip bone and distal radius. Levels of oestradiol (E2), serum calcium (Ca), phosphorus (P), alkaline phosphatase (ALP), bone Gla protein (BGP) and interleukin‐6 (IL‐6) were also measured, as was the improvement of lumbago and backache. We were not able to pool the data due to the variability of the tested Chinese herbal medicines and control interventions.
Based on the included trials, we created a 'Summary of findings' table for patient‐important outcomes (fractures, quality of life, death, adverse effects) (see Table 4).
Fractures
Five trials reported the outcome fractures. One trial reported that eight new fractures occurred in the control group treated with placebo and calcium (0/70 versus 8/70; RR 0.06; 95% CI 0.00 to 1.00) (Analysis 4.1) (Zhang RH 2004). One trial reported one new fracture in the control group treated with placebo plus calcium and vitamin D (low‐dose group: 0/60 versus 1/60; RR 0.33; 95% CI 0.01 to 8.02; high‐dose group: 0/56 versus 1/60; RR 0.36; 95% CI 0.01 to 8.58) (Analysis 4.1) (Zhu HM 2012). One trial reported four new fractures in the herbal group (Bushen Zhuanggu granules plus calcium and vitamin D) and seven new fractures in the control group (placebo granules plus calcium and vitamin D) (4/88 versus 7/67; RR 0.44; 95% CI 0.13 to 1.43) (Analysis 4.1) (Deng WM 2012). One trial reported one new fracture in the control group (calcium and vitamin D) (0/45 versus 1/30; RR 0.22; 95% CI 0.01 to 5.34) (Analysis 4.1) (Li ZP 2011). One trial reported that no new fracture occurred in any group (Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin ampoule group, and calcitonin ampoule group) (Analysis 4.1) (Zhang ZF 2011).
4.1. Analysis.
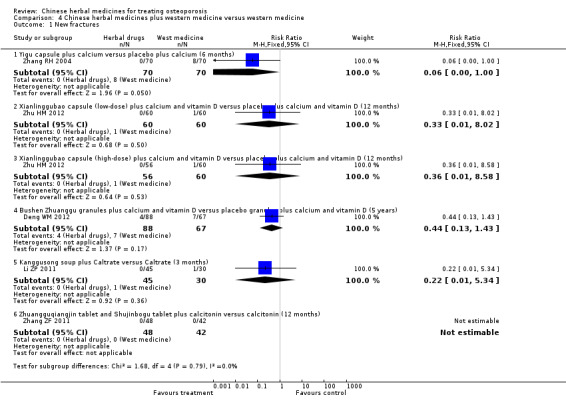
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 1 New fractures.
Quality of life or symptoms including pain, muscle fatigue and limited mobility
Eight of 48 trials reported the improvement of lumbago and backache. Among them, four trials reported that Chinese herbal medicines were better than western medicines without reporting the number of cases with ache improvement in each group (Liang DB 2012; Wang XD 2011; Xu H 2010; Zhan HS 2009). The other three trials reported as follows:
55/57 versus 48/57: RR 1.15; 95% CI 1.01 to 1.30 (Analysis 4.5) (Chen XL 2010);
53/54 versus 51/53: RR 1.33; 95% CI 1.09 to 1.61 (Analysis 4.5) (Dong Y 2010);
40/45 versus 18/30: RR 1.48; 95% CI 1.09 to 2.02 (Analysis 4.5) (Li ZP 2011).
4.5. Analysis.
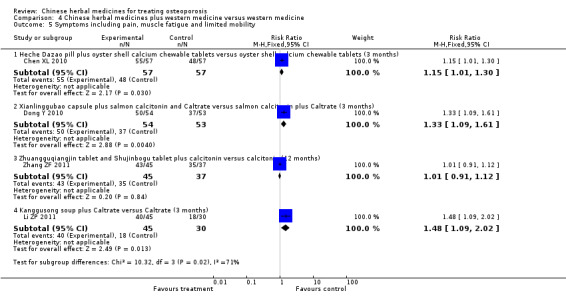
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 5 Symptoms including pain, muscle fatigue and limited mobility.
In another trial, no significant difference was found between comparable groups (43/45 versus 35/37: RR 1.01; 95% CI 0.91 to 1.12) (Analysis 4.5) (Zhang ZF 2011).
Only one trial reported the outcome quality of life: the mean difference in quality of life score was 5.30 (95% CI 3.67 to 6.93) (Analysis 4.4) (Liang DB 2012).
4.4. Analysis.
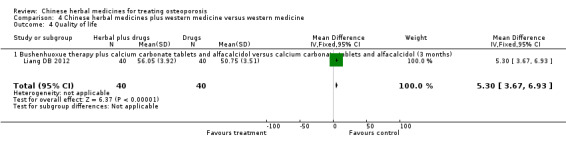
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 4 Quality of life.
Death
No trials reported this outcome.
Adverse effects
Eight trials out of 48 reported no remarkable adverse effects occurring in any groups (Chen FS 2001; Liang DB 2012; Liu JM 2012; Ruan XY 2006; Tang ZA 2012; Xie J 2004; Zheng WK 2007; Zou JJ 2005). Fourteen trials reported some adverse symptoms:
a few patients with nausea, vomiting, etc. after one month of treatment (Cao W 2010);
three patients with constipation and canker sore symptoms (Chen YQ 2001);
21 (23.86%) and 21 (31.34%) adverse effects in the herbal group and control group, respectively, with the predominant effects being liver enzyme abnormality and gastrointestinal complaints (Deng WM 2012);
a few patients with dizziness, rash, nausea, vomiting and cardiopalmus at the beginning of therapy, but no significant difference in comparison groups (Dong Y 2010);
one patient with irregular vaginal bleeding and two with breast tenderness in the western medicine group (Fan HX 2004);
two cases of abdominal distention, acid reflux, etc. in the Liuwei Dihuang pills group, three cases with similar gastrointestinal symptoms in the Liuwei Dihuang pills plus calcium and vitamin D group, and three with similar gastrointestinal symptoms in the calcium and vitamin D group (Ma C 2011);
three patients with mild constipation in the herbal group (Wang XD 2011);
four patients with adverse effects (gastrointestinal reaction, nervous system response, anaphylactic reaction, etc.) in the Jiangu granule group, five in the Gusongbao granule group and five in the western medicine group (Xiong YH 2008);
six patients with nausea in the herbal group and three in the western medicine group (Xu H 2010);
three patients with nausea in the herbal group and three in the western medicine group (Xu M 2009);
three patients with constipation, one with dysthesia, one with elevated ALT, six with abdominal discomfort and one with gum swelling in the Xuduanzhuanggu capsule group; one with dizziness, one with skin petechiae, one with abdominal discomfort and one with gum swelling in the Gusongbao granule group; one with constipation, one with abdominal discomfort, one with sore throat, one with gum swelling and one with electrocardiogram abnormality in the control group (Zhan HS 2009);
five patients with dry mouth symptoms, dreaminess, dryness‐heat in the experimental group, a few with a transient heightened blood calcium in the control group (Zhang RH 2004);
five with flushed face, one with nausea and one with vomiting in 90 patients after using calcitonin (Zhang ZF 2011);
11 (18%), 12 (21%) and 11 (18%) adverse effects in the low‐dose Xianlinggubao capsule group, high‐dose Xianlinggubao capsule group and the control group, respectively, with the predominant effects being liver enzyme abnormality and gastrointestinal complaints (Zhu HM 2012).
The other 26 trials did not report adverse effects.
Bone mineral density (BMD)
BMD of the lumbar spine
Twenty‐one trials showed positive effects of Chinese herbal medicines plus western medicine treatments when compared to the same western medicine on the BMD of the lumbar spine:
Gushen decoction plus Caltrate and Alfacalcidol versus Caltrate and Alfacalcidol after six months treatment (MD 0.06 g/cm3; 95% CI 0.01 to 0.11) (Analysis 4.2) (Chen FS 2001);
Shugan zishen huoxue tang plus Caltrate and alendronate sodium tablets versus Caltrate and alendronate sodium tablets after six months treatment (MD 0.12 g/cm3; 95% CI 0.08 to 0.16) (Analysis 4.2) (Han XL 2007);
Shangke Yishen Zhuanggu pill, plus Caltrate and calcitonin, versus Caltrate and calcitonin after three months treatment (MD 0.04 g/cm3; 95% CI 0.03 to 0.05) (Analysis 4.2) (Hu J 2012);
Gukang tablet plus oyster shell calcium chewable tablets and vitamin A and D capsules versus oyster shell calcium chewable tablets and vitamin A and D capsules after three months treatment (MD 0.24 g/cm3; 95% CI 0.14 to 0.34) (Analysis 4.2) (Liu JM 2012);
Ziyin Bushen Zhuanggu prescription plus Caltrate and calcitonin versus Caltrate plus calcitonin after eight months treatment (MD 0.14 g/cm3; 95% CI 0.08 to 0.20) (Analysis 4.2) (Li HW 2004);
Liuwei Dihuang pills plus Caltrate versus Caltrate after six months treatment (MD 0.05 g/cm3; 95% CI 0.02 to 0.08) (Analysis 4.2) (Ma C 2011);
Xianlinggubao capsule plus Caltrate versus Caltrate after 12 months treatment (MD 0.30 g/cm3; 95% CI 0.25 to 0.35) (Analysis 4.2) (Wu W 2005);
Migu tablet plus Caltrate versus Caltrate after 12 months treatment (MD 0.08 g/cm3; 95% CI 0.07 to 0.09) (Analysis 4.2) (Xie J 2004);
Bushen Zhuanggutang plus Caltrate versus Caltrate after six months treatment (MD 0.14 g/cm3; 95% CI 0.06 to 0.22) (Analysis 4.2) (Ye AN 1998);
Zishen prescription plus Caltrate and calcitonin versus Caltrate plus calcitonin after eight months treatment (MD 0.14 g/cm3; 95% CI 0.08 to 0.20) (Analysis 4.2) (Zhao G 2002);
Guben Zhuanggu capsules plus Caltrate versus Caltrate after six months treatment (MD 0.03 g/cm3; 95% CI 0.01 to 0.05) (Analysis 4.2) (Zou JJ 2005);
Qianggu capsule plus alendronate versus alendronate after six months treatment (MD 0.08 g/cm3; 95% CI 0.03 to 0.12) (Analysis 4.2) (Xu H 2010);
Xianlinggubao capsule plus alendronate versus alendronate after six months treatment (MD 0.06 g/cm3; 95% CI 0.03 to 0.09) (Analysis 4.2) (Xu M 2009);
Xianlinggubao capsule plus Caltrate versus Caltrate after 12 months treatment (MD 0.31 g/cm3; 95% CI 0.28 to 0.34) (Analysis 4.2) (Qiu ZX 2010);
Xianlinggubao capsule plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule after six months treatment (MD 0.18 g/cm3; 95% CI 0.14 to 0.22), and Migu tablet plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule after six months treatment (MD 0.17 g/cm3; 95% CI 0.14 to 0.20) (Analysis 4.2) (Dai Y 2007);
Jinwugutong capsule plus calcium versus placebo plus calcium after six months treatment (MD 0.11 g/cm3; 95% CI 0.07 to 0.15) (Analysis 4.2) (Zheng WK 2007);
Chinese medicine Bushenhuoxue recipe plus conventional western medicine (alfacalcidol soft capsules, bisphosphonate and salmon calcitonin, etc.) versus conventional western medicine after three months treatment (MD 0.16 g/cm3; 95% CI 0.09 to 0.22) (Analysis 4.2) (Cao GY 2010);
Shengsuiyin recipe plus Caltrate versus Caltrate after 12 months treatment (MD 0.31 g/cm3; 95% CI 0.28 to 0.34) (Analysis 4.2) (Jian QQ 2010);
Yigu capsule plus calcium versus placebo plus calcium after six months treatment (MD 0.08 g/cm3; 95% CI 0.04 to 0.12) (Analysis 4.2) (Zhang RH 2004);
Chinese medicine (combination of 10 herbs) plus tamoxifen and Caltrate versus tamoxifen and Caltrate after three months treatment (MD 0.10 g/cm3; 95% CI 0.06 to 0.14) (Analysis 4.2) (Cao W 2010);
Huangqi plus calcium and vitamin D versus calcium and vitamin D after six months treatment (MD 0.06 g/cm3; 95% CI 0.01 to 0.11) (Analysis 4.2) (Yang B 2007).
4.2. Analysis.
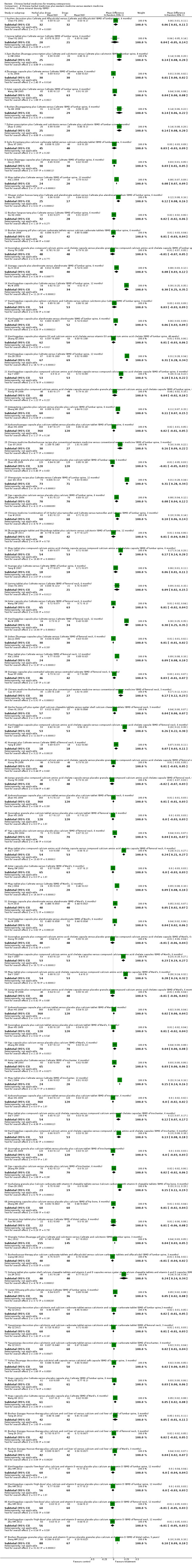
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 2 Bone mineral density (BMD).
No significant difference in the BMD of the lumbar spine was reported for any of the following comparisons of treatment groups:
Jiarong tablet plus Caltrate versus Caltrate (MD 0.04 g/cm3; 95% CI ‐0.06 to 0.14) (Analysis 4.2) (Chen YQ 2001);
Bushen Kangsong pill plus calcium carbonate tablets versus calcium carbonate tablets (MD 0.01 g/cm3; 95% CI ‐0.03 to 0.04) (Analysis 4.2) (Guo JH 2008);
Shugan zishen huoxue tang plus Caltrate and alendronate sodium tablets versus Caltrate and alendronate sodium tablets after three months treatment (MD 0.04 g/cm3; 95% CI ‐0.01 to 0.09) (Analysis 4.2) (Han XL 2007);
Bushen Yangxue tang plus Caltrate versus Caltrate (MD 0.02 g/cm3; 95% CI ‐0.02 to 0.06) (Analysis 4.2) (He XQ 2006);
Bushenhuoxue therapy plus calcium carbonate tablets and alfacalcidol versus calcium carbonate tablets and alfacalcidol (MD ‐0.01 g/cm3; 95% CI ‐0.04 to 0.02) (Analysis 4.2) (Liang DB 2012);
Ziyin Bushen Zhuanggu prescription plus Caltrate and calcitonin versus Caltrate plus calcitonin after four months treatment (MD 0.05 g/cm3; 95% CI 0.00 to 0.10) (Analysis 4.2) (Li HW 2004);
Zhuanggu capsule plus Caltrate versus Caltrate (MD 0.01 g/cm3; 95% CI 0.00 to 0.02) (Analysis 4.2) (Li SL 2004);
Yiyuanjiangu decoction plus calcitonin ampoule and calcium carbonate tablet versus calcitonin ampoule and calcium carbonate tablet (MD 0.02 g/cm3; 95% CI ‐0.01 to 0.05) (Analysis 4.2) (Ma CZ 2011);
Yishen Zhuanggu decoction plus calcitriol soft capsule versus calcitriol soft capsule (MD 0.02 g/cm3; 95% CI 0.00 to 0.05) (Analysis 4.2) (Ma YJ 2011);
Bushen Qianggu Huoxue therapy plus calcium and cod liver oil versus calcium and cod liver oil (MD 0.05 g/cm3; 95% CI ‐0.01 to 0.11) (Analysis 4.2) (Tang ZA 2012);
Antai capsule plus Caltrate versus Caltrate (MD 0.04 g/cm3; 95% CI 0.00 to 0.08) (Analysis 4.2) (Wang SW 2003);
Hugu capsule plus Caltrate versus placebo capsule plus Caltrate (MD 0.03 g/cm3; 95% CI 0.00 to 0.06) (Analysis 4.2) (Wang XD 2011);
Xianlinggubao capsule plus Caltrate versus Caltrate after six months treatment (MD 0.03 g/cm3; 95% CI ‐0.02 to 0.08) (Analysis 4.2) (Wu W 2005);
Zishen prescription plus Caltrate and calcitonin versus Caltrate plus calcitonin after four months treatment (MD 0.05 g/cm3; 95% CI 0.00 to 0.10) (Analysis 4.2) (Zhao G 2002);
Guilu Erxiantang plus calcium carbonate tablet versus calcium carbonate tablet (MD 0.03 g/cm3; 95% CI ‐0.03 to 0.09) (Analysis 4.2) (Zhou XT 2001);
Xianlinggubao capsule (low‐dose) plus calcium and vitamin D versus placebo plus calcium and vitamin D (MD 0.00 g/cm3; 95% CI ‐0.04 to 0.04) and Xianlinggubao capsule (high‐dose) plus calcium and vitamin D versus placebo plus calcium and vitamin D (MD 0.00 g/cm3; 95% CI ‐0.03 to 0.03) (Analysis 4.2) (Zhu HM 2012);
Bushen Kangsong pill plus calcium carbonate tablets versus calcium carbonate tablets after six months treatment (MD 0.01 g/cm3; 95% CI ‐0.03 to 0.04) (Analysis 4.2) (Guo JH 2008);
Gusongbao granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule after six months treatment (MD ‐0.01 g/cm3; 95% CI ‐0.07 to 0.05) (Analysis 4.2) (Xiong YH 2008);
Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate after three months treatment (MD 0.03 g/cm3; 95% CI ‐0.03 to 0.09) (Analysis 4.2) (Dong Y 2010);
Xianlinggubao capsule plus vitamin D3 and calcium amino acid chelate versus vitamin D3 and calcium amino acid chelate after 48 weeks treatment (MD 0.01 g/cm3; 95% CI ‐0.03 to 0.06) (Analysis 4.2) (Zhang XZ 2004);
Jiangu granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule after six months treatment (MD 0.04 g/cm3; 95% CI ‐0.02 to 0.10) (Analysis 4.2) (Xiong YH 2008);
Xuduanzhuanggu capsule plus calcium tablet versus placebo plus calcium tablet after six months treatment (MD 0.02 g/cm3; 95% CI ‐0.01 to 0.05) and Gusongbao granule plus calcium tablet versus placebo plus calcium tablet after six months treatment (MD ‐0.01 g/cm3; 95% CI ‐0.05 to 0.03) (Analysis 4.2) (Zhan HS 2009);
Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin ampoule versus calcitonin ampoule after 12 months treatment (MD 0.01 g/cm3; 95% CI ‐0.04 to 0.06) (Analysis 4.2) (Zhang ZF 2011);
Huangqi plus calcium and vitamin D versus calcium and vitamin D after three months treatment (MD 0.03 g/cm3; 95% CI ‐0.01 to 0.07) (Analysis 4.2) (Yang B 2007).
Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin ampoule versus calcitonin ampoule after 12 months treatment (MD 0.01 g/cm3; 95% CI ‐0.04 to 0.06) (Analysis 4.2) (Zhang ZF 2011);
Huangqi plus calcium and vitamin D versus calcium and vitamin D after three months treatment (MD 0.03 g/cm3; 95% CI ‐0.01 to 0.07) (Analysis 4.2) (Yang B 2007).
BMD of the femoral neck
Seven trials showed positive effects of Chinese herbal medicines plus western medicine treatments when compared to the same western medicine on the BMD of the femoral neck:
Jiarong tablet plus Caltrate versus Caltrate after six months treatment (MD 0.09 g/cm3; 95% CI 0.02 to 0.15) (Analysis 4.2) (Chen YQ 2001);
Xianlinggubao capsule plus Caltrate versus Caltrate after 12 months treatment (MD 0.30 g/cm3; 95% CI 0.25 to 0.35) (Analysis 4.2) (Wu W 2005);
Migu tablet plus Caltrate versus Caltrate (MD 0.09 g/cm3; 95% CI 0.08 to 0.10) (Analysis 4.2) (Xie J 2004);
Chinese medicine Bushenhuoxue recipe plus conventional western medicine (alfacalcidol soft capsules, bisphosphonate and salmon calcitonin, etc.) versus conventional western medicine after three months treatment (MD 0.17 g/cm3; 95% CI 0.12 to 0.23) (Analysis 4.2) (Cao GY 2010);
Xianlinggubao capsule plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule after six months treatment (MD 0.26 g/cm3; 95% CI 0.22 to 0.30) and Migu tablet plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule after six months treatment (MD 0.24 g/cm3; 95% CI 0.21 to 0.27) (Analysis 4.2) (Dai Y 2007);
Huangqi plus calcium and vitamin D versus calcium and vitamin D after six months treatment (MD 0.07 g/cm3; 95% CI 0.03 to 0.11) (Analysis 4.2) (Yang B 2007);
Yigu capsule plus calcium versus placebo plus calcium after six months treatment (MD 0.04 g/cm3; 95% CI 0.01 to 0.07) (Analysis 4.2) (Zhang RH 2004).
No significant difference in the BMD of the femoral neck was reported for any of the following comparisons of treatment groups:
Qianggu capsules plus oestradiol valerate versus oestradiol valerate after six months treatment (MD 0.03 g/cm3; 95% CI ‐0.01 to 0.07) (Analysis 4.2) (Ruan XY 2006);
Antai capsule plus Caltrate versus Caltrate after six months treatment (MD 0.01 g/cm3; 95% CI ‐0.02 to 0.04) (Analysis 4.2) (Wang SW 2003);
Xianlinggubao capsule plus Caltrate versus Caltrate after six months treatment (MD 0.03 g/cm3; 95% CI ‐0.02 to 0.08) (Analysis 4.2) (Wu W 2005);
Guben Zhuanggu capsules plus Caltrate versus Caltrate after six months treatment (MD 0.01 g/cm3; 95% CI ‐0.01 to 0.02) (Analysis 4.2) (Zou JJ 2005);
Heche Dazao pill plus oyster shell calcium chewable tablets versus oyster shell calcium chewable tablets after three months treatment (MD 0.04 g/cm3; 95% CI 0.00 to 0.07) (Analysis 4.2) (Chen XL 2010);
Gusongbao granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule after six months treatment (MD 0.01 g/cm3; 95% CI ‐0.03 to 0.05) and Jiangu granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule after six months treatment (MD ‐0.02 g/cm3; 95% CI ‐0.07 to 0.03) (Analysis 4.2) (Xiong YH 2008);
Xuduanzhuanggu capsule plus calcium tablet versus placebo plus calcium tablet after six months treatment (MD 0.01 g/cm3; 95% CI ‐0.01 to 0.03) and Gusongbao granule plus calcium tablet versus placebo plus calcium tablet after six months treatment (MD 0.00 g/cm3; 95% CI ‐0.03 to 0.03) (Analysis 4.2) (Zhan HS 2009);
Yiyuanjiangu decoction plus calcitonin ampoule and calcium carbonate tablet versus calcitonin ampoule and calcium carbonate tablet after three months treatment (MD 0.01 g/cm3; 95% CI ‐0.01 to 0.03) (Analysis 4.2) (Ma CZ 2011);
Bushen Qianggu Huoxue therapy plus calcium and cod liver oil versus calcium and cod liver oil after three months treatment (MD 0.02 g/cm3; 95% CI ‐0.02 to 0.05) (Analysis 4.2) (Tang ZA 2012);
Xianlinggubao capsule (low‐dose or high‐dose) plus calcium and vitamin D versus placebo plus calcium and vitamin D (MD ‐0.01 g/cm3; 95% CI ‐0.05 to 0.03) (Analysis 4.2) (Zhu HM 2012).
BMD of Ward's triangle
Six trials showed positive effects of Chinese herbal medicines plus western medicine treatments when compared to the same western medicine on the BMD of Ward's triangle:
Migu tablet plus Caltrate versus Caltrate (MD 0.09 g/cm3; 95% CI 0.08 to 0.10) (Analysis 4.2) (Xie J 2004);
Qianggu capsule plus alendronate versus alendronate after six months treatment (MD 0.05 g/cm3; 95% CI 0.02 to 0.07) (Analysis 4.2) (Xu H 2010);
Xianlinggubao capsule plus alendronate versus alendronate after six months treatment (MD 0.04 g/cm3; 95% CI 0.02 to 0.06) (Analysis 4.2) (Xu M 2009);
Xianlinggubao capsule plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule after six months treatment (MD 0.23 g/cm3; 95% CI 0.19 to 0.27) and Migu tablet plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule after six months treatment (MD 0.28 g/cm3; 95% CI 0.24 to 0.32) (Analysis 4.2) (Dai Y 2007);
Bushen Qianggu Huoxue therapy plus calcium and cod liver oil versus calcium and cod liver oil after three months treatment (MD 0.04 g/cm3; 95% CI 0.02 to 0.07) (Analysis 4.2) (Tang ZA 2012);
Hugu capsule plus Caltrate versus placebo capsule plus Caltrate after six months treatment (MD 0.05 g/cm3; 95% CI 0.02 to 0.08) (Analysis 4.2) (Wang XD 2011).
No significant difference in the BMD of Ward's triangle was reported for any of the following comparisons of treatment groups:
Antai capsule plus Caltrate versus Caltrate after six months treatment (MD 0.00 g/cm3; 95% CI ‐0.03 to 0.03) (Analysis 4.2) (Wang SW 2003);
Gusongbao granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule after six months treatment (MD ‐0.01 g/cm3; 95% CI ‐0.06 to 0.04) and Jiangu granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule after six months treatment (MD ‐0.01 g/cm3; 95% CI ‐0.06 to 0.04) (Analysis 4.2) (Xiong YH 2008);
Xuduanzhuanggu capsule plus calcium tablet versus placebo plus calcium tablet after six months treatment (MD 0.02 g/cm3; 95% CI 0.00 to 0.04) and Gusongbao granule plus calcium tablet versus placebo plus calcium tablet after six months treatment (MD 0.01 g/cm3; 95% CI ‐0.02 to 0.04) (Analysis 4.2) (Zhan HS 2009);
Yigu capsule plus calcium versus placebo plus calcium after six months treatment (MD 0.04 g/cm3; 95% CI 0.00 to 0.08) (Analysis 4.2) (Zhang RH 2004).
BMD of the trochanter
Three trials showed positive effects of Chinese herbal medicines plus western medicine treatments when compared to the same western medicine on the BMD of the trochanter:
Migu tablet plus Caltrate versus Caltrate after six months treatment (MD 0.15 g/cm3; 95% CI 0.14 to 0.16) (Analysis 4.2) (Xie J 2004);
Xianlinggubao capsule plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule after six months treatment (MD 0.13 g/cm3; 95% CI 0.08 to 0.18) and Migu tablet plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule after six months treatment (MD 0.12 g/cm3; 95% CI 0.07 to 0.17) (Analysis 4.2) (Dai Y 2007);
Yiyuanjiangu decoction plus calcitonin ampoule and calcium carbonate tablet versus calcitonin ampoule and calcium carbonate tablet after six months treatment (MD 0.02 g/cm3; 95% CI 0.01 to 0.04) (Analysis 4.2) (Ma CZ 2011).
No significant difference in the BMD of the trochanter was reported for any of the following comparisons of treatment groups:
Antai capsule plus Caltrate versus Caltrate after six months treatment (MD 0.03 g/cm3; 95% CI 0.00 to 0.06) (Analysis 4.2) (Wang SW 2003);
Xuduanzhuanggu capsule plus calcium tablet versus placebo plus calcium tablet after six months treatment (MD 0.00 g/cm3; 95% CI ‐0.02 to 0.02) and Gusongbao granule plus calcium tablet versus placebo plus calcium tablet after six months treatment (MD 0.00 g/cm3; 95% CI ‐0.03 to 0.03) (Analysis 4.2) (Zhan HS 2009);
Yigu capsule plus calcium versus placebo plus calcium after six months treatment (MD 0.02 g/cm3; 95% CI ‐0.02 to 0.06) (Analysis 4.2) (Zhang RH 2004).
BMD of the hip bone
Compared with Calcium carbonate with vitamin D chewable tablets, Gushukang granule plus Calcium carbonate with vitamin D chewable tablets showed a statistically significantly better effect on the BMD of the hip bone after six months treatment (MD 0.15 g/cm3; 95% CI 0.11 to 0.19) (Analysis 4.2) (Chen ZX 2002). There was no significant difference in the comparison of Jinwugutong capsules plus calcium and placebo plus calcium regarding the BMD of the hip bone (MD 0.01 g/cm3; 95% CI ‐0.02 to 0.04) (Analysis 4.2) (Zheng WK 2007).
BMD of the distal radius
There was no significant difference in the comparison of Gengnian Anyi tablet plus Caltrate and Caltrate regarding the BMD of the distal radius (MD 0.01 g/cm3; 95% CI ‐0.06 to 0.08) (Analysis 4.2) (Fan HX 2004). However, Bushen Zhuanggu granules plus calcium and vitamin D showed positive effects on the BMD of the distal radius when compared with placebo granules plus calcium and vitamin D after five‐year follow‐up (MD 0.10 g/cm3; 95% CI 0.09 to 0.10) (Analysis 4.2) (Deng WM 2012).
T score in BMD measurement
One trial reported the T score in bone mineral density measurement: Erxian Yanggu decoction plus alendronate sodium tablets versus alendronate sodium tablets (MD of T score 0.91; 95% CI 0.57 to 1.25) (Analysis 4.3) (Huang JW 2008).
4.3. Analysis.
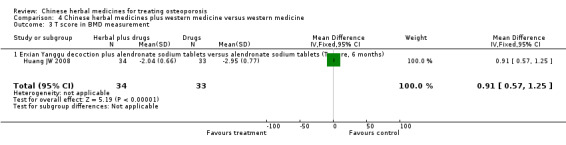
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 3 T score in BMD measurement.
Biochemical indicators
The effects of the interventions on biochemical indicators are shown in Appendix 5.
Summary: Comparison 04
In summary, 37 trials out of 93 included trials evaluated Chinese herbal medicines plus conventional medicine compared with the same conventional medicine.
The results showed that the following Chinese herbal medicines appeared to increase the BMD of the lumbar spine: Gushen decoction, Shugan zishen huoxue tang, Erxian Yanggu decoction, Ziyin Bushen Zhuanggu prescription, Xianlinggubao capsules, Migu tablet, Bushen Zhuanggutang, Zishen prescription, Guben Zhuanggu capsules, Qianggu capsule, Jinwugutong capsule, Shengsuiyin recipe, Yigu capsule and Huangqi. However, the following did not: Jiarong tablet, Bushen Kangsong pill, Shugan zishen huoxue tang, Bushen Yangxue tang, Bushenhuoxue therapy, Ziyin Bushen Zhuanggu prescription, Zhuanggu capsule, Yiyuanjiangu decoction, Yishen Zhuanggu decoction, Bushen Qianggu Huoxue therapy, Antai capsule, Hugu capsule, Xianlinggubao capsule, Zishen prescription, Guilu Erxiantang, Bushen Kangsong pill, Gusongbao granule, Jiangu granule, Zhuangguqiangjin tablet and Shujinbogu tablet, Xuduanzhuanggu capsule and Huangqi.
The following Chinese herbal medicines appeared to increase the BMD of the femoral neck: Jiarong tablet, Xianlinggubao capsule, Migu tablet, Bushenhuoxue recipe, Huangqi and Yigu capsule. However, the following did not: Qianggu capsules, Antai capsule, Xianlinggubao capsule, Guben Zhuanggu capsules, Heche Dazao pill, Gusongbao granule, Jiangu granule, Xuduanzhuanggu capsule, Yiyuanjiangu decoction and Bushen Qianggu Huoxue therapy.
The following Chinese herbal medicines appeared to increase the BMD of Ward's triangle: Migu tablet, Qianggu capsule, Xianling Gubao capsule, Bushen Qianggu Huoxue therapy and Hugu capsule. However, the following did not: Antai capsule, Gusongbao granule, Xuduanzhuanggu capsule and Yigu capsule.
The following Chinese herbal medicines appeared to increase the BMD of the trochanter: Migu tablet, Yiyuanjiangu decoction and Xianlinggubao capsule. However, the following did not: Antai capsule, Xuduanzhuanggu capsule and Yigu capsule.
Gushukang granule seemed to increase the BMD of the hip bone, while Jinwugutong capsules did not.
Bushen Zhuanggu granules appeared to increase the BMD of the distal radius, while Gengnian Anyi tablet dd not.
Erxian Yanggu decoction appeared to increase the T score of BMD.
Discussion
Due to poor study quality and limited data on the individual herbal interventions, no definitive conclusions can be drawn from this review on the effectiveness of any Chinese herbal medicine intervention tested to date.
Summary of main results
One hundred and eight randomised trials were included in this review. Ninety‐nine different Chinese herbal medicines were compared with placebo, no intervention, conventional pharmaceutical medicine or with the same co‐intervention received by the Chinese herbal medicines group. In this review, many of the trials showed a symptomatic benefit of Chinese herbal medicines in patients with osteoporosis either when compared with placebo, no intervention or conventional western medicine. However, there was a lack of replicable evidence because no more than one trial ever compared the same Chinese herbal medicine and control treatment. Thus, the finding of a benefit of Chinese herbal medicine treatment may not be conclusive. Furthermore, the findings of this review should be interpreted with caution due to the small sample sizes and generally low methodological quality of the included studies.
Compared with placebo in three trials, Chinese herbal medicines had a statistically significant effect in increasing bone mineral density (BMD) in each trial. One reported some mild adverse effects (such as stomach/intestinal upset, headache and dizziness) from Chinese herbal medicines, but there were no significant differences between the Chinese herbal medicines and placebo groups.
Five randomised trials compared seven different Chinese herbal medicines with no intervention. Two trials showed Chinese herbal medicines to be significantly effective in increasing BMD, while three others showed no better effect. Four trials reported mild adverse effects (e.g. mild stomach discomfort, intestinal upset, etc.) of Chinese herbal medicines, but this did not affect the continuation of the treatment.
Sixty‐one trials compared 58 different Chinese herbal medicines with western medicine. Twenty‐three trials showed that Chinese herbal medicines were significantly effective in increasing BMD, while 38 showed no better effect. Twenty‐three reported some adverse effects (e.g. dizziness, rash, nausea, vomiting, etc.) from Chinese herbal medicines, but most of them did not affect the continuation of the treatment. Six reported no occurrence of adverse effects and 38 did not report adverse effects.
Forty‐eight trials compared 42 different Chinese herbal medicines plus western medicine with western medicine. Twenty‐six showed better effects in increasing BMD, while 22 did not. Fourteen reported some adverse symptoms (e.g. nausea, vomiting, etc.) from Chinese herbal medicines, but there was no significant difference between comparison groups. Eight reported no remarkable adverse effects and the other 26 did not report adverse effects.
Overall completeness and applicability of evidence
We performed this review to evaluate the benefit and harm of Chinese herbal medicines in patients with osteoporosis. Most of the Chinese herbal medicines evaluated were mixtures of different herbs and most of them were prescribed by the investigators without reporting of the quality standards for the preparations. Interpretation of these findings should therefore be cautious considering that along with the other methodological limitations in the study designs, these studies cannot be replicated. There is a risk that statistically significant findings from a handful of studies at questionable risk of bias are given more prominence than the lack of data for relevant endpoints available from the vast majority of included studies in the review.
The Chinese herbal medicines evaluated generally appeared to be safe. However, we cannot conclude that using Chinese herbal medicines in osteoporosis patients is safe, because adverse effects were not sufficiently and adequately reported in all the included trials. In clinical trials beneficial and harmful effects should receive equal attention, and the recording and reporting of adverse effects must improve if we are to draw any conclusions about safety.
Quality of the evidence
Due to the variations in the Chinese herbal medicines tested and the methodological limitations of the included trials, the evidence for Chinese herbal medicines for the treatment of osteoporosis is not conclusive. In general, Chinese herbal medicines might be a promising treatment. However, it is premature to recommend Chinese herbal medicines for routine use in osteoporosis considering the generally low quality of the evidence.
Most of the included trials suffered from insufficient reporting or inadequate methods of randomisation. The majority of the trials did not report how the random allocation was generated and concealed. Some trials had a significantly skewed distribution of participants among the groups compared which could not be explained if randomisation had been carried out properly. These trials were highly prone to selection bias.
Very few trials used a double‐blinding method. The majority of treatment approaches in the included trials aimed to improve pain symptoms, which is a subjective indicator. If the outcome assessment is not blinded, then performance and detection bias may be a problem.
Most of the included trials were small. Although some data analyses did not demonstrate a statistically significant difference between the groups compared, the results were likely to have been underpowered to demonstrate equivalence. Therefore, the analyses from the small sample trials may not be able to establish with confidence that the two interventions had equivalent effects.
Overall the evidence is limited: potential bias may have occurred in the selection of participants, the administration of treatment and the assessment of outcomes in the included trials, which may obscure or exaggerate treatment effects. Methodologically less rigorous trials have been shown to have significantly larger intervention effects than trials conducted with more rigour (Egger 2003; Moher 1998; Schulz 1995). Therefore, the implications of our findings for clinical practice are very limited.
The trials identified in this review were mostly Chinese and all were conducted in Chinese participants: publication bias might therefore be an issue. An empirical study has shown that Chinese trials are significantly affected by publication bias (Vickers 1998). Furthermore, it is certainly an issue that so few of the included studies actually reported the right outcomes and we downgraded studies for selective reporting. Accordingly, publication bias should be taken into consideration when interpreting the present findings. Moreover, most of the trials were of short duration and were not followed up long‐term, thus they were unable to detect endpoint outcomes such as fracture or long‐term adverse effects of the herbal treatments in patients with osteoporosis. Primary outcomes such as the occurrence of new fractures were reported in only a few trials, so we could not draw any conclusions about efficacy and safety.
Potential biases in the review process
In this review, we comprehensively searched for studies from appropriate databases, and selected trials and extracted data in duplicate. However, we did not find any unpublished studies or studies published in languages other than Chinese or English. Secondly, due to our limited resources, including time, we were not able to contact the original authors to verify unclear information, such as the methods used for random allocation generation and concealment. Furthermore, almost all Chinese herbal medicines in the included trials were tested only once and on small samples. This made it impossible for us to pool data to draw a robust conclusion.
Agreements and disagreements with other studies or reviews
We could not find any other reviews evaluating the benefits and harms of Chinese herbal medicines in patients with osteoporosis. Therefore, this review is the first time this has been reported. Many trials are not included in this review and are listed as excluded studies. Due to the variation among the Chinese herbal medicine interventions, it is not meaningful to compare the results from these excluded trials with those included in this review.
Authors' conclusions
Implications for practice.
Based on this systematic review, some Chinese herbal medicines may have beneficial effects on the bone mineral density at anatomical sites in people with osteoporosis. However, a large number of the included studies have an uncertain risk of bias, there is a lack of relevant clinical endpoints in the available literature and the evidence is of low quality. Further rigorous studies are needed to demonstrate whether these effects are clinically meaningful and to detect other effects.
Implications for research.
The potential benefit of Chinese herbal medicines as a treatment for osteoporosis needs to be further investigated by conducting high‐quality trials to produce convincing evidence. Trials comparing Chinese herbal medicines with conventional pharmaceutical medicine should be designed rigorously. It would also be interesting to verify the additional benefits of Chinese herbal medicines when combined with conventional medicine versus the same medicine by itself. There are a wide range of therapeutic strategies in Traditional Chinese Medicine that address osteoporosis and the related Chinese medicine diagnosis of kidney deficiency. It would be useful for those with expertise in Chinese herbal medicine to group the herbal formulae by therapeutic strategy, so that the Chinese herbal medicines can be tested with reference to the therapeutic framework from which they were derived.
Outcome measures should include both clinical and surrogate outcomes. Standardised monitoring and reporting should be used for assessment of adverse effects. The methodological quality of randomised trials of Chinese herbal medicines for osteoporosis needs to be improved. The following aspects should be addressed: (i) detailed reporting of the methods used to generate the allocation sequence and conceal allocation; (ii) sufficient application of double‐blinding with the use of an adequate placebo; (iii) clear descriptions of withdrawals/drop‐outs during the trial and use of intention‐to‐treat analysis; (iv) reporting of clinically important outcome measures from long‐term follow‐up; and (v) reporting of the trial according to the CONSORT Statement guidelines (www.consort‐statement.org).
History
Protocol first published: Issue 3, 2005 Review first published: Issue 3, 2014
| Date | Event | Description |
|---|---|---|
| 26 August 2008 | Amended | Converted to new review format. C041‐R |
Acknowledgements
The authors wish to acknowledge the helpful comments from Elizabeth Ghogomu, Lara Maxwell and the editorial group and their long‐term support to make this review possible, and Christine Wade's contributions to the protocol and review.
This work is partially supported by the grant number 2011ZX09302‐006(5) from the Ministry of Science and Technology of China. Jian Ping Liu was partially supported by grant number R24 AT001293 from the National Center for Complementary and Alternative Medicine of the US National Institutes of Health.
Appendices
Appendix 1. MEDLINE search strategy
| Database | Time span | Search strategy |
| MEDLINE | 1966 to 2013 | 1. exp bone diseases, metabolic/ 2. osteoporos#s.tw. 3. bone density/ 4. bone densit$.tw. 5. bone mineral densit$.tw. 6. exp bone/ and bones.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 7. bone loss$.tw. 8. osteomalacia.tw. 9. osteodystrophy.tw. 10. exp bone demineralization, pathologic/ 11. (bone adj demineralization).tw. 12. osteopenia.tw. 13. bone mass.tw. 14. exp densitometry/ 15. densitometry.tw. 16. dexa.tw. 17. exp fractures/ 18. fracture$.tw. 19. or/1‐18 20. exp Medicine, Herbal/ 21. exp Plants, Medicinal/ 22. exp Medicine, Traditional/ 23. exp Drugs, Chinese Herbal/ 24. herb$.tw. 25. plant.mp. or plants.tw. [mp=title, original title, abstract, name of substance word, subject heading word] 26. phytomedicine.tw. 27. botanical.tw. 28. weed$.tw. 29. algae.tw. 30. (fungi or fungus).tw. 31. ((traditional or chinese or herbal) adj medicine).tw. 32. ((oriental or chinese) adj tradition$).tw. 33. or/20‐32 34. 19 and 33 |
Appendix 2. EMBASE search strategy
| Database | Time span | Search strategy |
| EMBASE | 1998 to 2013 | 1. exp OSTEOPOROSIS/ 2. exp Metabolic Bone Disease/ 3. osteoporo$.tw. 4. exp Bone Density/ 5. (bone adj2 densit$).tw. 6. exp BONE/ 7. bone loss.tw. 8. osteomalacia.tw. 9. osteodystrophy.tw. 10. exp Bone Demineralization/ 11. (bone adj deminerali#ation).tw. 12. osteopenia.tw. 13. bone mass.tw. 14. exp bone densitometry/ 15. densitometry.tw. 16. dexa.tw. 17. exp Fracture/ 18. fracture$.tw. 19. or/1‐18 20. exp Herbal Medicine/ 21. exp Medicinal Plant/ 22. exp Traditional Medicine/ 23. exp Chinese Medicine/ 24. herb$.tw. 25. (plant or plants).tw. 26. phytomedicine.tw. 27. botanical.tw. 28. weed$.tw. 29. algae.tw. 30. (fungi or fungus).tw. 31. ((traditional or chinese or herbal) adj medicine).tw. 32. ((oriental or chinese) adj tradition$).tw. 33. or/20‐32 34. 19 and 33 35. random$.ti,ab. 36. factorial$.ti,ab. 37. (crossover$ or cross over$ or cross‐over$).ti,ab. 38. placebo$.ti,ab. 39. (doubl$ adj blind$).ti,ab. 40. (singl$ adj blind$).ti,ab. 41. assign$.ti,ab. 42. allocat$.ti,ab. 43. volunteer$.ti,ab. 44. crossover procedure.sh. 45. double blind procedure.sh. 46. randomized controlled trial.sh. 47. single blind procedure.sh. 48. or/35‐47 49. exp animal/ or nonhuman/ or exp animal experiment/ 50. exp human/ 51. 49 and 50 52. 49 not 51 53. 48 not 52 54. 34 and 53 |
Appendix 3. CENTRAL search strategy
| Database | Time span | Search strategy |
| CENTRAL in The Cochrane Library | Issue 12, 2012 | #1 MeSH descriptor Osteoporosis explode all trees in MeSH products #2 MeSH descriptor Bone Diseases, Metabolic explode all trees in MeSH products #3 osteoporo* in All Fields in all products #4 MeSH descriptor Bone Density explode all trees in MeSH products #5 bone near/j2 densit* in All Fields in all products #6 MeSH descriptor Bone and Bones explode all trees in MeSH products #7 bone loss in All Fields in all products #8 osteomalacia in All Fields in all products #9 osteodystrophy in All Fields in all products #10 MeSH descriptor Bone Demineralization, Pathologic explode all trees in MeSH products #11 bone next deminerali* in All Fields in all products #12 osteopenia in All Fields in all products #13 bone mass in All Fields in all products #14 MeSH descriptor Densitometry explode all trees in MeSH products #15 densitometry in All Fields in all products #16 dexa in All Fields in all products #17 MeSH descriptor Fractures, Bone explode all trees in MeSH products #18 fractur* in All Fields in all products #19 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 MeSH descriptor Medicine, Herbal explode all trees in MeSH products #21 MeSH descriptor Plants, Medicinal explode all trees in MeSH products #22 MeSH descriptor Medicine, Traditional explode all trees in MeSH products #23 MeSH descriptor Drugs, Chinese Herbal explode all trees in MeSH products #24 herb* in All Fields in all products #25 plant or plants in All Fields in all products #26 phytomedicine in All Fields in all products #27 botanical in All Fields in all products #28 weed* in All Fields in all products #29 algae in All Fields in all products #30 fungi or fungus in All Fields in all products #31 (traditional or chinese or herbal) next medicine in All Fields in all products #32 (oriental or chinese) next tradition* in All Fields in all products #33 (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32) #34 (#19 AND #33) |
Appendix 4. CBM search strategy
| Database | Time span | Search strategy |
| CBM | 1979 to 2013 | 1 explode "osteoporosis"/cure of Chinese traditional medicine, the combination of Western and traditional medicine 2 osteoporosis/tw 3 Chinese herbal medicine/tw 4 Chinese traditional medicine/tw 5 2 and (3 or 4) 6 1 or 5 |
Appendix 5. Effects of interventions on biochemical indicators
Chinese herbal medicines versus placebo (Comparison 01)
Oestradiol (E2) levels
Compared with the placebo group, those treated with Bushen Yigu soft extract after three months treatment had statistically significantly increased serum levels of oestradiol (mean difference (MD) 122.89 pg/ml; 95% confidence interval (CI) 92.97 to 152.81) (Analysis 1.3) (Wang XY 2000).
1.3. Analysis.
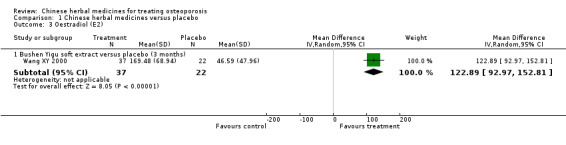
Comparison 1 Chinese herbal medicines versus placebo, Outcome 3 Oestradiol (E2).
Chinese herbal medicines versus no intervention (Comparison 02)
Oestradiol (E2) levels
There was no significant difference in levels of serum or urinary oestradiol between Chinese herbal medicines (Bushen Shengsui principle (Analysis 2.2) (Liao L 2004), Yishen Zhuanggu mixture (Analysis 2.2) (Gong L 2001), Huoluo Gukang pills or Gushukang granule (Analysis 2.2) (Zhang YS 2003)) and no intervention after six months treatment.
2.2. Analysis.
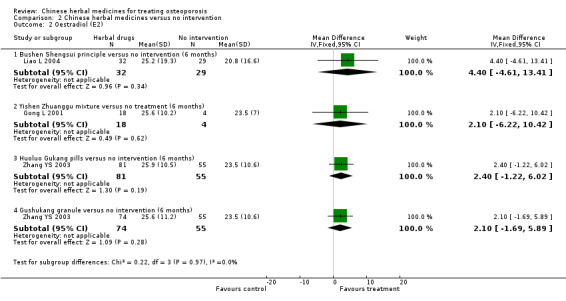
Comparison 2 Chinese herbal medicines versus no intervention, Outcome 2 Oestradiol (E2).
Bone Gla protein (BGP) levels
Compared with the no intervention group, in those treated with Yishen Zhuanggu mixture or Shengu capsule there were no statistically significant effects on BGP after six months treatment (MD ‐1.00 μg/L; 95% CI ‐6.15 to 4.15 and MD ‐5.10 μg/L; 95% CI ‐10.63 to 0.43, respectively) (Analysis 2.3) (Gong L 2001). Those treated with Huoluo Gukang pills or Gushukang granule had statistically significant increases in BGP after six months treatment (MD 2.70 μg/L; 95% CI 1.23 to 4.17 and MD 3.50 μg/L; 95% CI 1.92 to 5.08, respectively) (Analysis 2.3) (Zhang YS 2003). Treatment with Shigu yin also increased BGP after six months treatment (MD 1.20 μg/L; 95% CI 0.17 to 2.23) (Analysis 2.3) (Zhang YP 2007).
2.3. Analysis.
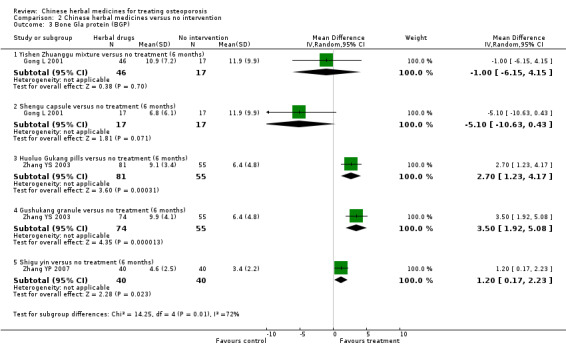
Comparison 2 Chinese herbal medicines versus no intervention, Outcome 3 Bone Gla protein (BGP).
Chinese herbal medicines versus western medicine (Comparison 03)
Oestradiol (E2) levels
Nine trials showed positive effects of herbal treatments on serum or urinary measures of oestradiol when compared to calcium supplementation, or calcium combined with vitamin D supplementation, or calcium combined with synthesised isoflavone (ipriflavone):
Kanggusong granule versus Caltrate after nine months treatment (MD 11.00 pg/ml; 95% CI 6.97 to 15.03), Kanggusong granule versus ipriflavone plus Caltrate after nine months treatment (MD 12.17 pg/ml; 95% CI 8.27 to 16.07) (Analysis 3.5) (An SJ 2000);
Strong Bone capsule versus Caltrate after three months treatment (MD 10.46 pg/ml; 95% CI 4.29 to 16.63) (Analysis 3.5) (Cui TH 1999);
Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets after six months treatment (MD 8.78 pg/ml; 95% CI 0.92 to 16.64) (Analysis 3.5) (Ou L 2011);
Qianggu soft extract versus calcium gluconate after six months treatment (MD 4.20 pg/ml; 95% CI 0.89 to 7.51) (Analysis 3.5) (Peng T 2002);
Gumikang capsule versus calcium gluconate after six months treatment (MD 3.50 pg/ml; 95% CI 1.14 to 5.86) (Analysis 3.5) (Wang CC 2005);
Yanghuo Sanzi tang versus Caltrate after six months treatment (MD 23.06 pg/ml; 95% CI 17.82 to 28.30) (Analysis 3.5) (Qiu RB 2004);
Jingujian granule versus Caltrate after three months treatment (MD 4.85 pg/ml; 95% CI 4.56 to 5.14) (Analysis 3.5) (Li YH 2010);
Qianggu paste versus calcium gluconate tablets after six months treatment (MD 4.20 pg/ml; 95% CI 1.79 to 6.61) (Analysis 3.5) (Wang H 2007);
Bushen Huoxue capsule versus Caltrate plus Rocalirol after 12 months treatment (MD 2.13 pg/ml; 95% CI 0.42 to 3.84) (Analysis 3.5) (Zhang XJ 2008).
3.5. Analysis.
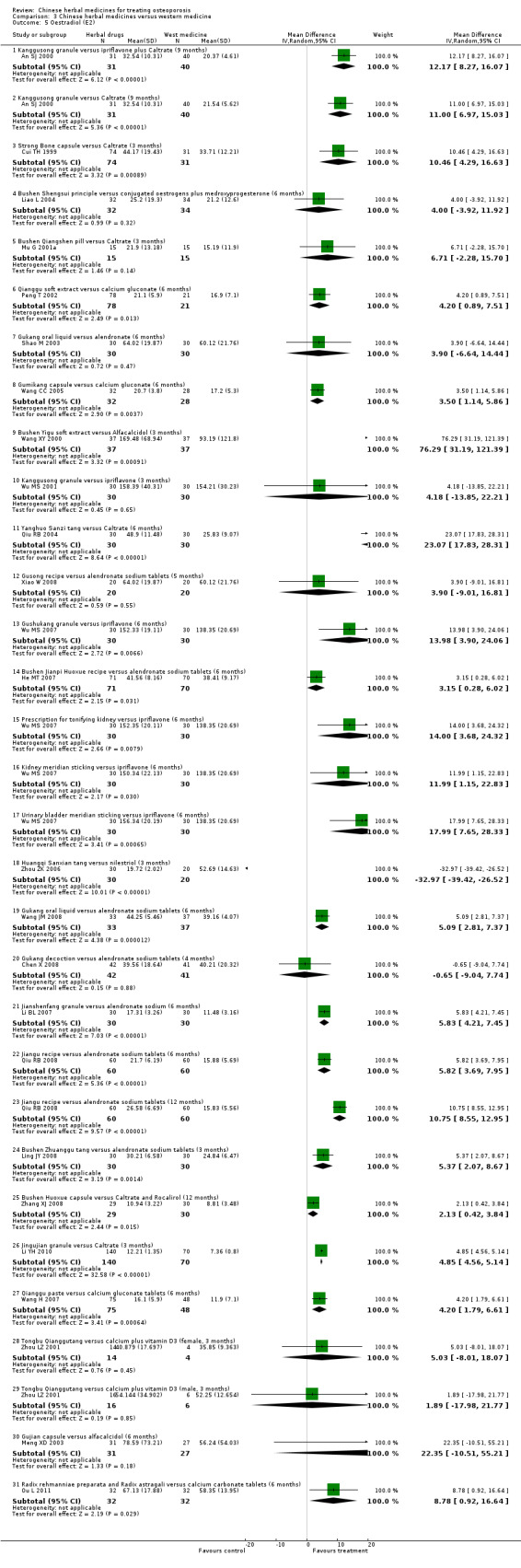
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 5 Oestradiol (E2).
However, in the following two trials, Chinese herbal medicines did not show a significant effect:
Bushen Qiangshen pill versus Caltrate (MD 6.71 pg/ml; 95% CI ‐2.28 to 15.70) (Analysis 3.5) (Mu G 2001a);
Tongbu Qianggutang versus calcium plus vitamin D3 after three months treatment (female MD 5.03 pg/ml; 95% CI ‐8.01 to 18.07; male MD 1.89 pg/ml; 95% CI ‐17.98 to 21.77) (Analysis 3.5) (Zhou LZ 2001).
Seven trials showed a better effect of Chinese herbal medicines when compared with western medicine:
Bushen Jianpi Huoxue recipe versus alendronate sodium tablets after six months treatment (MD 3.15 pg/ml; 95% CI 0.28 to 6.02) (Analysis 3.5) (He MT 2007);
prescription for tonifying kidney versus ipriflavone (MD 14.00 pg/ml; 95% CI 3.68 to 24.32), kidney meridian sticking versus ipriflavone (MD 11.99 pg/ml; 95% CI 1.15 to 22.83), urinary bladder meridian sticking versus ipriflavone (MD 17.99 pg/ml; 95% CI 7.65 to 28.33), Gushukang granule versus ipriflavone (MD 13.98 pg/ml; 95% CI 3.90 to 24.06) after six months treatment (Analysis 3.5) (Wu MS 2007);
Gukang oral liquid versus alendronate sodium tablets after six months treatment (MD 5.09 pg/ml; 95% CI 2.81 to 7.37) (Analysis 3.5) (Wang JM 2008);
Jianshenfang granule versus alendronate sodium tablets after six months treatment (MD 5.83 pg/ml; 95% CI 4.21 to 7.45) (Analysis 3.5) (Li BL 2007);
Jiangu recipe versus alendronate sodium tablets after 12 months treatment (MD 10.75 pg/ml; 95% CI 8.55 to 12.95) (Analysis 3.5) (Qiu RB 2008);
Bushen Zhuanggu tang versus alendronate sodium tablets after three months treatment (MD 5.37 pg/ml; 95% CI 2.07 to 8.67) (Analysis 3.5) (Ling JY 2008);
Bushen Yigu soft extract versus Alfacalcidol after three months treatment (MD 76.29 nmol/L; 95% CI 31.19 to 121.39) (Analysis 3.5) (Wang XY 2000).
However, another five trials found no significant difference between groups in levels of oestradiol:
Bushen Shengsui principle versus conjugated oestrogens plus medroxyprogesterone after six months treatment (MD 4.00 pg/ml; 95% CI ‐3.92 to 11.92) (Analysis 3.5) (Liao L 2004);
Gukang oral liquid versus alendronate after six months treatment (MD 3.90 pg/ml; 95% CI ‐6.64 to 14.44) (Analysis 3.5) (Shao M 2003);
Kanggusong granule versus ipriflavone after three months treatment (MD 4.18 pg/ml; 95% CI ‐13.85 to 22.21) (Analysis 3.5) (Wu MS 2001);
Gukang decoction versus alendronate sodium tablets after four months treatment (MD ‐0.65 pg/ml; 95% CI ‐9.04 to 7.74) (Analysis 3.5) (Chen X 2008);
Gujian capsule versus alfacalcidol after six months treatment (MD 22.35 pg/ml; 95% CI ‐10.51 to 55.21) (Analysis 3.5) (Meng XD 2003).
One trial showed a worse effect of Chinese herbal medicine: Huangqi Sanxian tang versus nilestriol after three months treatment (MD ‐32.97 pg/ml; 95% CI ‐39.42 to ‐26.52) (Analysis 3.5) (Zhou ZK 2006).
Serum calcium (Ca) levels
In 12 trials comparing herbal treatments to calcium and vitamin D and, in one case, isoflavone, there were no significant differences between groups in changes in serum calcium:
Kanggusong granule versus Caltrate after nine months treatment (MD ‐0.03 mmol/L; 95% CI ‐0.11 to 0.05) (Analysis 3.6) (An SJ 2000);
Gushukang granule versus Calcium carbonate with vitamin D chewable tablets after six months treatment (MD ‐0.02 mmol/L; 95% CI ‐0.10 to 0.06) (Analysis 3.6) (Chen ZX 2002);
Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets after six months treatment (MD ‐0.01 mmol/L; 95% CI ‐0.05 to 0.03) (Analysis 3.6) (Ou L 2011);
Bushen Jianpi Migu prescription versus Caltrate after three months treatment (MD 0.01 mmol/L; 95% CI ‐0.05 to 0.07) (Analysis 3.6) (Wang ZK 2004);
Bugu Shengsui capsule versus vitamin D2 plus calcium tablet after six months treatment (MD 0.10 mmol/L; 95% CI ‐0.05 to 0.25) (Analysis 3.6) (Xie YM 1997);
Shenyao capsules versus Caltrate after six months treatment (MD 0.02 mmol/L; 95% CI ‐0.08 to 0.12) (Analysis 3.6) (Chen NQ 2006);
prescription for tonifying kidney versus ipriflavone (MD 0.00 mmol/L; 95% CI ‐0.05 to 0.05), kidney meridian sticking versus ipriflavone (MD ‐0.01 mmol/L; 95% CI ‐0.06 to 0.04), urinary bladder meridian sticking versus ipriflavone (MD ‐0.01 mmol/L; 95% CI ‐0.06 to 0.04), Gushukang granule versus ipriflavone (MD ‐0.02 mmol/L; 95% CI ‐0.07 to 0.03) after six months treatment (Analysis 3.6) (Wu MS 2007);
Gukang oral liquid versus alendronate sodium tablets after six months treatment (MD ‐0.02 mmol/L; 95% CI ‐0.06 to 0.02) (Analysis 3.6) (Wang JM 2008);
Qianggu capsule versus active vitamin D3 after six months treatment (MD 0.01 mmol/L; 95% CI ‐0.06 to 0.08) (Analysis 3.6) (Wang J 2007);
Bushen Yangxue tang versus calcium gluconate after six months treatment (MD 0.13 mmol/L; 95% CI ‐0.00 to 0.26) (Analysis 3.6) (Zhan ML 2007);
Erxian soup versus Caltrate after six months treatment (MD ‐0.02 mmol/L; 95% CI ‐0.25 to 0.21) (Analysis 3.6) (Wu JZ 2010);
Tongbu Qianggutang versus calcium plus vitamin D3 after three months treatment (MD ‐0.02 mmol/L; 95% CI ‐0.10 to 0.06) (Analysis 3.6) (Zhou LZ 2001).
3.6. Analysis.
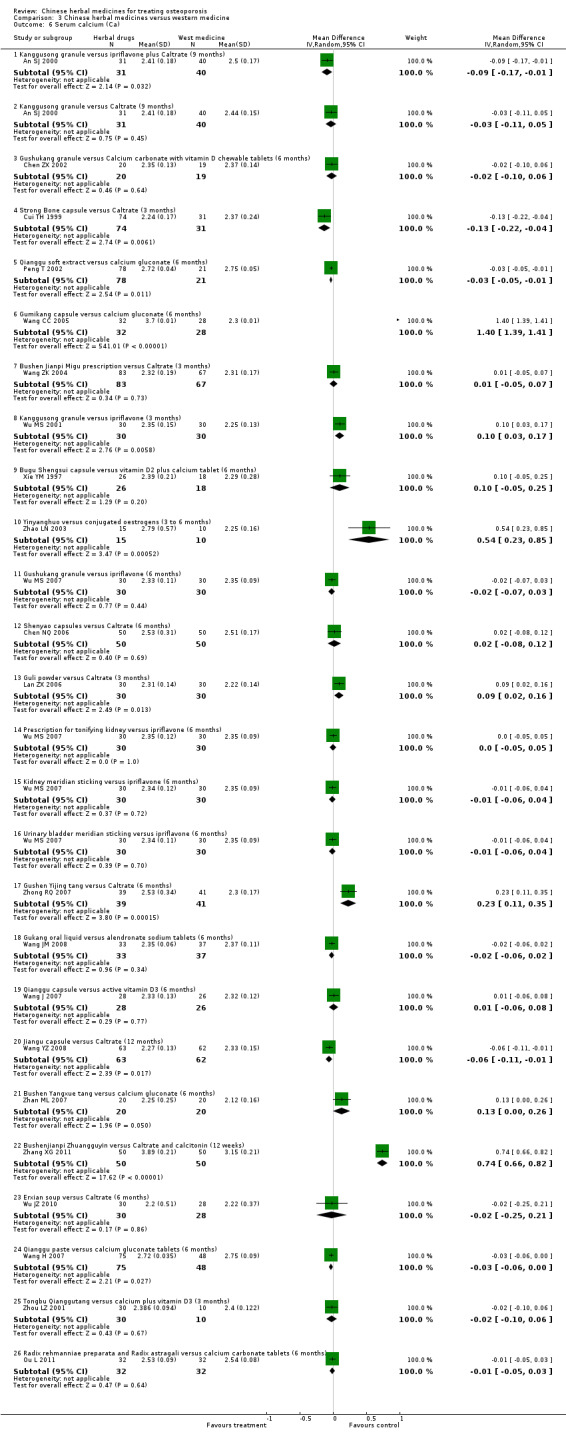
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 6 Serum calcium (Ca).
Patients treated with herbal formulae for three to six months showed significantly higher levels of serum calcium in six trials comparing herbal treatment with calcium supplementation, isoflavone supplementation or conjugated hormones:
Gumikang capsule versus calcium gluconate after six months treatment (MD 1.40 mmol/L; 95% CI 1.39 to 1.41) (Analysis 3.6) (Wang CC 2005);
Kanggusong granule versus ipriflavone after three months treatment (MD 0.10 mmol/L; 95% CI 0.03 to 0.17) (Analysis 3.6) (Wu MS 2001);
Yinyanghuo versus conjugated oestrogens after three to six months treatment (MD 0.54 mmol/L; 95% CI 0.23 to 0.85) (Analysis 3.6) (Zhao LN 2003);
Guli powder versus Caltrate after three months treatment (MD 0.09 mmol/L; 95% CI 0.02 to 0.16) (Analysis 3.6) (Lan ZX 2006);
Gushen Yijing tang versus Caltrate after six months treatment (MD 0.23 mmol/L; 95% CI 0.11 to 0.35) (Analysis 3.6) (Zhong RQ 2007);
Bushenjianpi Zhuangguyin versus Caltrate and calcitonin ampoule after 12 weeks (MD 0.74 mmol/L; 95% CI 0.66 to 0.82) (Analysis 3.6) (Zhang XG 2011).
Five trials reported statistically significantly lower levels of serum calcium in the group treated with Chinese herbal medicines compared to supplementation with calcium or calcium and vitamin D and, in one case, isoflavone plus Caltrate:
Kanggusong granule versus ipriflavone plus Caltrate after nine months treatment (MD ‐0.09 mmol/L; 95% CI ‐0.17 to ‐0.01) (Analysis 3.6) (An SJ 2000);
Strong Bone capsule versus Caltrate after three months treatment (MD ‐0.13 mmol/L; 95% CI ‐0.22 to ‐0.04) (Analysis 3.6) (Cui TH 1999);
Qianggu soft extract versus calcium gluconate (MD ‐0.03 mmol/L; 95% CI ‐0.05 to ‐0.01) (Analysis 3.6) (Peng T 2002);
Qianggu paste versus calcium gluconate tablets after six months treatment (MD ‐0.03 mmol/L; 95% CI ‐0.06 to ‐0.00) (Analysis 3.6) (Wang H 2007);
Jiangu capsule versus Caltrate after 12 months treatment (MD ‐0.06 mmol/L; 95% CI ‐0.11 to ‐0.01) (Analysis 3.6) (Wang YZ 2008).
Phosphorus (P) levels
Eleven trials showed no significant effects of herbal treatments on phosphorus when compared to calcium supplementation, or calcium combined with vitamin D supplementation, or calcium combined with synthesised isoflavone (ipriflavone):
Kanggusong granule versus ipriflavone plus Caltrate (MD 0.06 mmol/L; 95% CI ‐0.03 to 0.15), Kanggusong granule versus Caltrate (MD ‐0.01 mmol/L; 95% CI ‐0.29 to 0.27) after nine months treatment (Analysis 3.7) (An SJ 2000);
Gushukang granule versus Calcium carbonate with vitamin D chewable tablets after six months treatment (MD 0.01 mmol/L; 95% CI ‐0.09 to 0.11) (Analysis 3.7) (Chen ZX 2002);
Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets after six months treatment (MD 0.00 mmol/L; 95% CI ‐0.15 to 0.15) (Analysis 3.7) (Ou L 2011);
Bushen Jianpi Migu prescription versus Caltrate after three months treatment (MD 0.03 mmol/L; 95% CI ‐0.02 to 0.08) (Analysis 3.7) (Wang ZK 2004);
Shenyao capsules versus Caltrate after six months treatment (MD ‐0.01 mmol/L; 95% CI ‐0.08 to 0.06) (Analysis 3.7) (Chen NQ 2006);
Guli powder versus Caltrate after three months treatment (MD ‐0.05 mmol/L; 95% CI ‐0.13 to 0.03) (Analysis 3.7) (Lan ZX 2006);
Gushen Yijing tang versus Caltrate after six months treatment (MD 0.04 mmol/L; 95% CI ‐0.09 to 0.17) (Analysis 3.7) (Zhong RQ 2007);
Qianggu capsule versus active vitamin D3 after six months treatment (MD 0.02 mmol/L; 95% CI ‐0.05 to 0.09) (Analysis 3.7) (Wang J 2007);
Jiangu capsule versus Caltrate after 12 months treatment (MD 0.09 mmol/L; 95% CI ‐0.20 to 0.38) (Analysis 3.7) (Wang YZ 2008);
Erxian soup versus Caltrate after six months treatment (MD ‐0.03 mmol/L; 95% CI ‐0.20 to 0.14) (Analysis 3.7) (Wu JZ 2010);
Tongbu Qianggutang versus calcium plus vitamin D3 after three months treatment (MD ‐0.01 mmol/L; 95% CI ‐0.12 to 0.10) (Analysis 3.7) (Zhou LZ 2001).
3.7. Analysis.
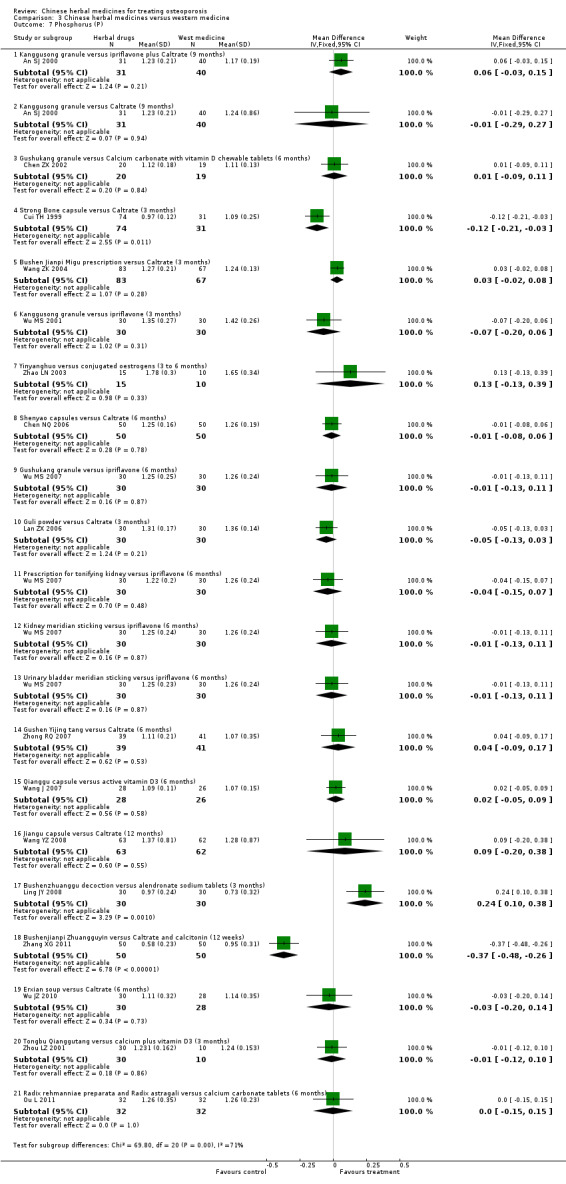
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 7 Phosphorus (P).
Three trials found no significant differences between groups for phosphorus when comparing herbal formulae to conventional western medicines:
Kanggusong granule versus ipriflavone after three months treatment (MD ‐0.07 mmol/L; 95% CI ‐0.20 to 0.06) (Analysis 3.7) (Wu MS 2001);
Yinyanghuo versus conjugated oestrogens after three to six months treatment (MD 0.13 mmol/L; 95% CI ‐0.13 to 0.39) (Analysis 3.7) (Zhao LN 2003);
prescription for tonifying kidney versus ipriflavone (MD ‐0.04 mmol/L; 95% CI ‐0.15 to 0.07), kidney meridian sticking versus ipriflavone (MD ‐0.01 mmol/L; 95% CI ‐0.13 to 0.11), urinary bladder meridian sticking versus ipriflavone (MD ‐0.01 mmol/L; 95% CI ‐0.13 to 0.11), Gushukang granule versus ipriflavone (MD ‐0.01 mmol/L; 95% CI ‐0.13 to 0.11) after six months treatment (Analysis 3.7) (Wu MS 2007).
One trial showed better effects of Bushen Zhuanggu tang compared to alendronate sodium tablets after three months treatment (MD 0.24 mmol/L; 95% CI 0.10 to 0.38) (Analysis 3.7) (Ling JY 2008).
Two other trials showed worse effects of Chinese herbal medicines:
Strong Bone capsule compared with Caltrate after three months treatment (MD ‐0.12 mmol/L; 95% CI ‐0.21 to ‐0.03) (Analysis 3.7) (Cui TH 1999);
Bushenjianpi Zhuangguyin versus Caltrate and calcitonin ampoule after 12 weeks treatment (MD ‐0.37 mmol/L; 95% CI ‐0.48 to ‐0.26) (Analysis 3.7) (Zhang XG 2011).
Alkaline phosphatase (ALP) levels
Eleven trials showed no significant effects of herbal treatments on alkaline phosphatase when compared to calcium supplementation, or calcium combined with vitamin D supplementation, or calcium combined with synthesised isoflavone (ipriflavone):
Gushukang granule versus Calcium carbonate with vitamin D chewable tablets after six months treatment (MD ‐0.50 μmol s‐1/L; 95% CI ‐1.70 to 0.70) (Analysis 3.8) (Chen ZX 2002);
Strong Bone capsule versus Caltrate after three months treatment (MD ‐0.07 μmol s‐1/L; 95% CI ‐0.25 to 0.11) (Analysis 3.8) (Cui TH 1999);
Bushen Qiangshen pill versus Caltrate after three months treatment (MD 0.27 μmol s‐1/L; 95% CI ‐1.27 to 1.81) (Analysis 3.8) (Mu G 2001a);
Bugu Shengsui capsule versus vitamin D2 plus calcium tablet after six months treatment (MD ‐0.29 μmol s‐1/L; 95% CI ‐1.67 to 1.09) (Analysis 3.8) (Xie YM 1997);
Liuwei Dihuang pills versus calcium after 12 months treatment (MD 0.55 μmol s‐1/L; 95% CI ‐0.12 to 1.22) (Analysis 3.8) (Zhang J 2003);
Guli powder versus Caltrate after three months treatment (MD 0.30 μmol s‐1/L; 95% CI ‐2.97 to 3.57) (Analysis 3.8) (Lan ZX 2006);
Qianggu capsule versus active vitamin D3 after six months treatment (MD 0.71 μmol s‐1/L; 95% CI ‐1.17 to 2.59) (Analysis 3.8) (Wang J 2007);
Jingujian granule versus Caltrate after three months treatment (MD 0.53 μmol s‐1/L; 95% CI ‐0.69 to 1.75) (Analysis 3.8) (Li YH 2008);
Bushenjianpi Zhuangguyin versus Caltrate and calcitonin ampoule after 12 weeks treatment (MD ‐0.82 μmol s‐1/L; 95% CI ‐2.83 to 1.19) (Analysis 3.8) (Zhang XG 2011);
Erxian soup versus Caltrate after six months treatment (MD 0.00 μmol s‐1/L; 95% CI ‐0.87 to 0.87) (Analysis 3.8) (Wu JZ 2010);
Tongbu Qianggutang versus calcium plus vitamin D3 after three months treatment (MD 0.18 μmol s‐1/L; 95% CI ‐1.08 to 1.44) (Analysis 3.8) (Zhou LZ 2001).
3.8. Analysis.
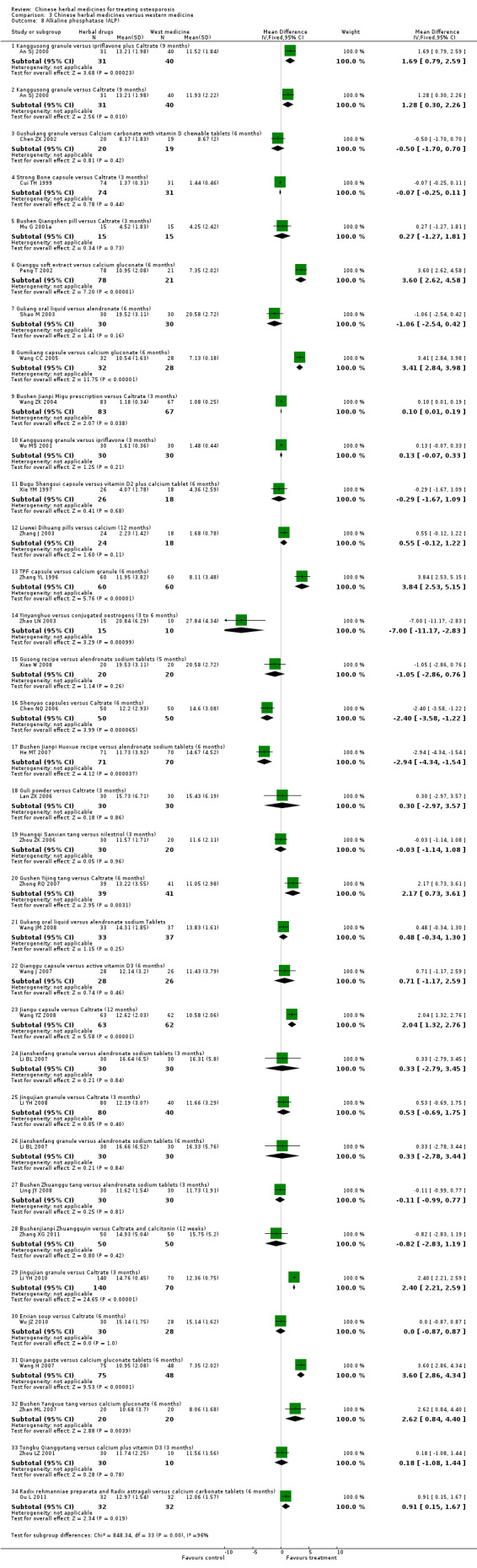
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 8 Alkaline phosphatase (ALP).
Twelve trials showed better effects of herbal treatments on alkaline phosphatase when compared to calcium supplementation, or calcium combined with vitamin D supplementation, or calcium combined with synthesised isoflavone (ipriflavone):
Kanggusong granule versus ipriflavone plus Caltrate (MD 1.69 μmol s‐1/L; 95% CI 0.79 to 2.59), Kanggusong granule versus Caltrate (MD 1.28 μmol s‐1/L; 95% CI 0.30 to 2.26) after nine months treatment (Analysis 3.8) (An SJ 2000);
Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets after six months treatment (MD 0.91 μmol s‐1/L; 95% CI 0.15 to 1.67) (Analysis 3.8) (Ou L 2011);
Qianggu soft extract versus calcium gluconate after six months treatment (MD 3.60 μmol s‐1/L; 95% CI 2.62 to 4.58) (Analysis 3.8) (Peng T 2002);
Gumikang capsule versus calcium gluconate after six months treatment (MD 3.41 μmol s‐1/L; 95% CI 2.84 to 3.98) (Analysis 3.8) (Wang CC 2005);
Bushen Jianpi Migu prescription versus Caltrate after three months treatment (MD 0.10 μmol s‐1/L; 95% CI 0.01 to 0.19) (Analysis 3.8) (Wang ZK 2004);
Liuwei Dihuang pills versus calcium after six months treatment (MD 0.43 μmol s‐1/L; 95% CI 0.11 to 0.75) (Analysis 3.8) (Zhang J 2003);
TPF capsule versus calcium granule after six months treatment (MD 3.84 μmol s‐1/L; 95% CI 2.53 to 5.15) (Analysis 3.8) (Zhang YL 1996);
Gushen Yijing tang versus Caltrate after six months treatment (MD 2.17 μmol s‐1/L; 95% CI 0.73 to 3.61) (Analysis 3.8) (Zhong RQ 2007);
Jiangu capsule versus Caltrate after 12 months treatment (MD 2.04 μmol s‐1/L; 95% CI 1.32 to 2.76) (Analysis 3.8) (Wang YZ 2008);
Jingujian granule versus Caltrate after three months treatment (MD 2.40 μmol s‐1/L; 95% CI 2.21 to 2.59) (Analysis 3.8) (Li YH 2010);
Qianggu paste versus calcium gluconate tablets after six months treatment (MD 3.60 μmol s‐1/L; 95% CI 2.86 to 4.34) (Analysis 3.8) (Wang H 2007);
Bushen Yangxue tang versus calcium gluconate after six months treatment (MD 2.62 μmol s‐1/L; 95% CI 0.84 to 4.40) (Analysis 3.8) (Zhan ML 2007).
Seven trials showed no significant effects of herbal treatments on alkaline phosphatase when compared to conventional western medicines:
Gukang oral liquid versus alendronate sodium tablets after six months treatment (MD ‐1.06 μmol s‐1/L; 95% CI ‐2.54 to 0.42) (Analysis 3.8) (Shao M 2003);
Kanggusong granule versus ipriflavone after three months treatment (MD 0.13 μmol s‐1/L; 95% CI ‐0.07 to 0.33) (Analysis 3.8) (Wu MS 2001);
Huangqi Sanxian tang versus nilestriol after three months treatment (MD ‐0.03 μmol s‐1/L; 95% CI ‐1.14 to 1.08) (Analysis 3.8) (Zhou ZK 2006);
Gukang oral liquid versus alendronate sodium tablets after six months treatment (MD 0.48 μmol s‐1/L; 95% CI ‐0.34 to 1.30) (Analysis 3.8) (Wang JM 2008);
Jianshenfang granule versus alendronate sodium tablets after six months treatment (MD 0.33 μmol s‐1/L; 95% CI ‐2.78 to 3.44) (Analysis 3.8) (Li BL 2007);
Bushen Zhuanggu tang versus alendronate sodium tablets after three months treatment (MD ‐0.11 μmol s‐1/L; 95% CI ‐0.99 to 0.77) (Analysis 3.8) (Ling JY 2008);
Gusong recipe versus alendronate sodium tablets after five months treatment (MD ‐1.05 μmol s‐1/L; 95% CI ‐2.86 to 0.76) (Analysis 3.8) (Xiao W 2008).
Another three trials showed a worse effect of herbal treatments when compared to calcium supplementation, hormones or alendronate sodium tablets:
Shenyao capsules versus Caltrate after six months treatment (MD ‐2.40 μmol s‐1/L; 95% CI ‐3.58 to ‐1.22) (Analysis 3.8) (Chen NQ 2006);
Yinyanghuo versus conjugated oestrogens after three to six months treatment (MD ‐7.00 μmol s‐1/L; 95% CI ‐11.17 to ‐2.83) (Analysis 3.8) (Zhao LN 2003);
Bushen Jianpi Huoxue recipe versus alendronate sodium tablets after six months treatment (MD ‐2.94 μmol s‐1/L; 95% CI ‐4.34 to 1.54) (Analysis 3.8) (He MT 2007).
Bone Gla protein (BGP) levels
One trial found that there was a significant difference in BGP between Chinese herbal and western medicines:
Acupoint sticking of Migudan versus oyster shell calcium chewable tablets after three months treatment (MD 1.55 μg/L; 95% CI 1.24 to 1.86) (Analysis 3.9) (Xu YL 2007).
3.9. Analysis.
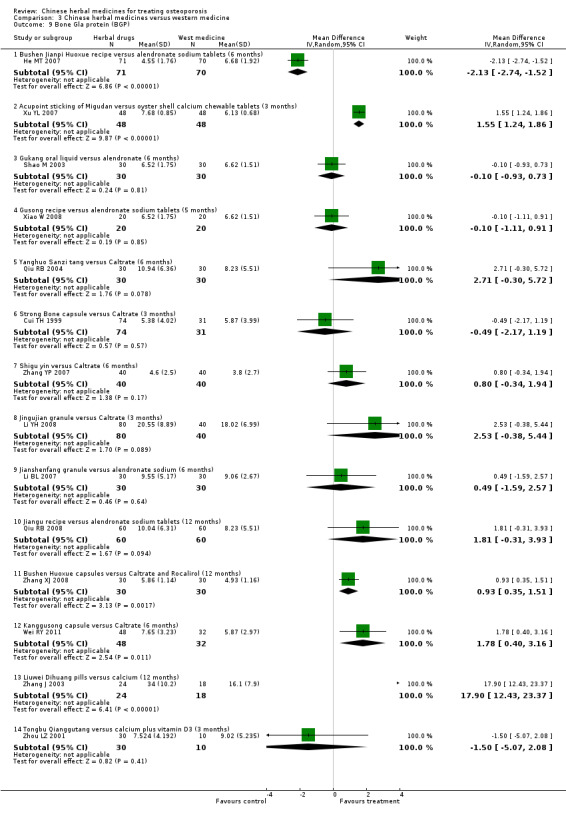
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 9 Bone Gla protein (BGP).
Three trials showed a statistically significantly better effect on BGP when compared with calcium supplementation:
Bushen Huoxue capsule versus Caltrate plus Rocalirol after 12 months treatment (MD 0.93 μg/L; 95% CI 0.35 to 1.51) (Analysis 3.9) (Zhang XJ 2008);
Kanggusong capsule versus Caltrate after six months treatment (MD 1.78 μg/L; 95% CI 0.40 to 3.16) (Analysis 3.9) (Wei RY 2011);
Liuwei Dihuang pills versus calcium after 12 months treatment (MD 17.90 μg/L; 95% CI 12.43 to 23.37) (Analysis 3.9) (Zhang J 2003).
Three trials showed no significant effects of herbal treatments on BGP when compared to western medicines:
Gukang oral liquid versus alendronate after six months treatment (MD ‐0.10 μg/L; 95% CI ‐0.93 to 0.73) (Analysis 3.9) (Shao M 2003);
Jianshenfang granule versus alendronate sodium tablets after six months treatment (MD 0.49 μg/L; 95% CI ‐1.59 to 2.57) (Analysis 3.9) (Li BL 2007);
Jiangu recipe versus alendronate sodium tablets after 12 months treatment (MD 1.81 μg/L; 95% CI ‐0.31 to 3.93) (Analysis 3.9) (Qiu RB 2008).
Five trials showed no significant effects of herbal treatments when compared with calcium supplementation:
Strong Bone capsule versus Caltrate after three months treatment (MD ‐0.49 μg/L; 95% CI ‐2.17 to 1.19) (Analysis 3.9) (Cui TH 1999);
Yanghuo Sanzitang versus Caltrate after six months treatment (MD 2.71 μg/L; 95%CI ‐0.30 to 5.72) (Analysis 3.9) (Qiu RB 2004);
Shigu yin versus Caltrate after six months treatment (MD 0.80 μg/L; 95% CI ‐0.34 to 1.94) (Analysis 3.9) (Zhang YP 2007);
Jingujian granule versus Caltrate after three months treatment (MD 2.53 μg/L; 95% CI ‐0.38 to 5.44) (Analysis 3.9) (Li YH 2008);
Tongbu Qianggutang versus calcium plus vitamin D3 after three months treatment (MD ‐1.50 μg/L; 95% CI ‐5.07 to 2.08) (Analysis 3.9) (Zhou LZ 2001).
Another trial showed a worse effect of Bushen Jianpi Huoxue recipe when compared to alendronate sodium tablets after six months treatment (MD ‐2.13 μg/L; 95% CI ‐2.74 to ‐1.52) (Analysis 3.9) (He MT 2007).
Interleukin‐6 (IL‐6) levels
There was no significant difference between herbal drugs and western medicine in interleukin‐6 in three trials:
Bushen Jianpi Huoxue recipe versus alendronate sodium tablets after six months treatment (MD ‐5.72 pg/ml; 95% CI ‐14.05 to 2.61) (Analysis 3.10) (He MT 2007);
Gukang oral liquid versus alendronate sodium tablets after six months treatment (MD ‐3.50 pg/ml; 95% CI ‐15.93 to 8.93) (Analysis 3.10) (Shao M 2003);
Jianshenfang granule versus alendronate sodium tablets after six months treatment (MD ‐12.56 pg/ml; 95% CI ‐29.26 to 4.14) (Analysis 3.10) (Li BL 2007).
3.10. Analysis.
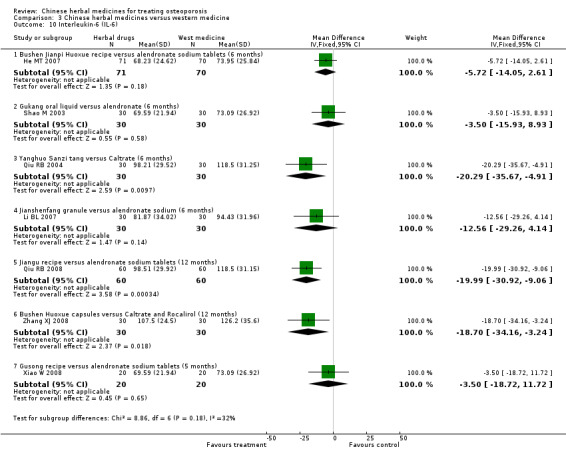
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 10 Interleukin‐6 (IL‐6).
Three trials showed statistically significantly better effects on interleukin‐6:
Jiangu recipe versus alendronate sodium tablets after 12 months treatment (MD ‐19.99 pg/ml; 95% CI ‐30.92 to ‐9.06) (Analysis 3.10) (Qiu RB 2008);
Yanghuo Sanzi tang versus Caltrate after six months treatment (MD ‐20.29 pg/ml; 95% CI ‐35.67 to ‐4.91) (Analysis 3.10) (Qiu RB 2004);
Bushen Huoxue capsule versus Caltrate plus Rocalirol (MD ‐18.70 pg/ml; 95% CI ‐34.16 to ‐3.24) (Analysis 3.10) (Zhang XJ 2008).
Parathyroid hormone (PTH) levels
Four trials showed no significant effects of herbal treatments on PTH when compared to common western medicines:
prescription for tonifying kidney versus ipriflavone (MD ‐13.00 μg/L; 95% CI ‐41.67 to 15.67), kidney meridian sticking versus ipriflavone (MD ‐13.01 μg/L; 95% CI ‐41.95 to 15.93), urinary bladder meridian sticking versus ipriflavone (MD ‐3.01 μg/L; 95% CI ‐31.44 to 25.42), Gushukang granule versus ipriflavone (MD ‐3.02 μg/L; 95% CI ‐30.91 to 24.87) after six months treatment (Analysis 3.11) (Wu MS 2007);
Kanggusong granule versus ipriflavone after three months treatment (MD 11.81 μg/L; 95% CI ‐14.64 to 38.26) (Analysis 3.11) (Wu MS 2001);
Bugu Shengsui capsule versus vitamin D2 plus calcium tablet after six months treatment (MD ‐10.63 μg/L; 95% CI ‐140.92 to 119.66) (Analysis 3.11) (Xie YM 1997);
Gujian capsule versus alfacalcidol after six months treatment (MD ‐22.57 pg/ml; 95% CI ‐60.92 to 15.78) (Analysis 3.11) (Meng XD 2003);
Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets after six months treatment (MD ‐2.06 pg/ml; 95% CI ‐6.74 to 2.62) (Analysis 3.11) (Ou L 2011).
3.11. Analysis.
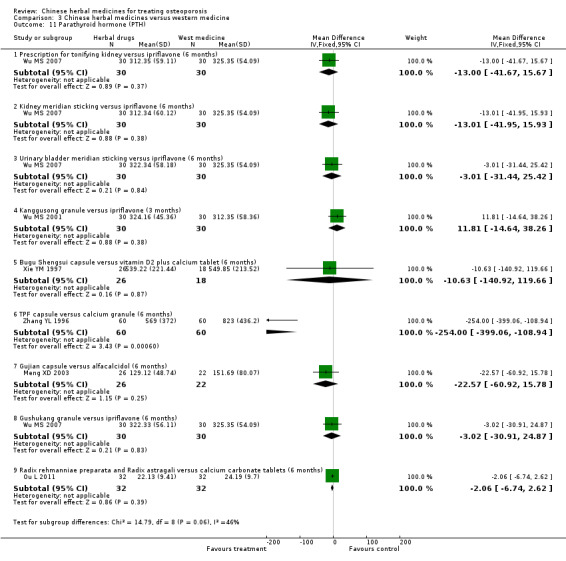
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 11 Parathyroid hormone (PTH).
In another trial, TPF capsule showed a better effect than calcium granule after six months treatment (MD ‐254.00 pg/ml; 95% CI ‐399.06 to ‐108.94) (Analysis 3.11) (Zhang YL 1996).
Calcitonin (CT) levels
Five trials showed no significant effects of herbal treatments on calcitonin when compared to common western medicines:
prescription for tonifying kidney versus ipriflavone (MD 0.96 ng/L; 95% CI ‐3.71 to 5.63), kidney meridian sticking versus ipriflavone (MD ‐1.01 ng/L; 95% CI ‐5.44 to 3.42), urinary bladder meridian sticking versus ipriflavone (MD ‐0.01 ng/L; 95% CI ‐4.68 to 4.66), Gushukang granule versus ipriflavone (MD ‐0.01 ng/L; 95% CI ‐4.44 to 4.42) after six months treatment (Analysis 3.12) (Wu MS 2007);
Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (MD 15.93 ng/L; 95% CI ‐24.16 to 56.02) (Analysis 3.12) (Xie YM 1997);
Tongbu Qianggutang versus calcium plus vitamin D3 after three months treatment (MD ‐6.18 ng/L; 95% CI ‐12.89 to 0.52) (Analysis 3.12) (Zhou LZ 2001);
Gujian capsule versus alfacalcidol after six months treatment (MD ‐12.77 ng/L; 95% CI ‐70.92 to 45.38) (Analysis 3.12) (Meng XD 2003);
Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets after six months treatment (MD 0.11 ng/L; 95% CI ‐0.04 to 0.26) (Analysis 3.12) (Ou L 2011).
3.12. Analysis.
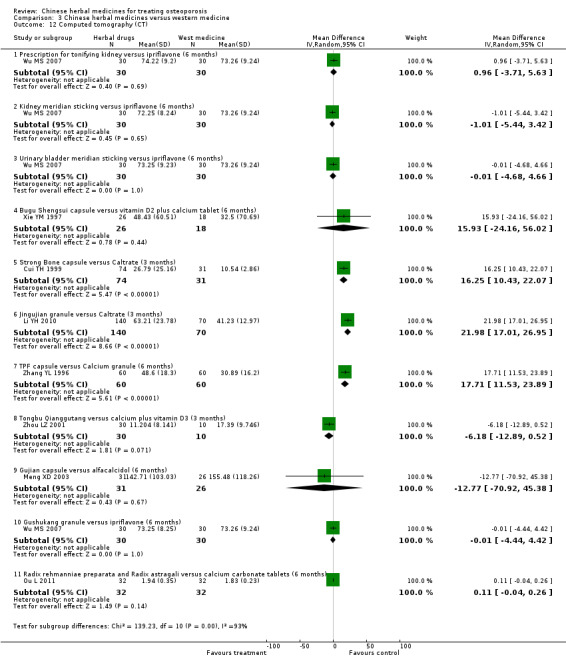
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 12 Computed tomography (CT).
Three trials showed a statistically significantly better effect on calcitonin when compared with calcium supplementation:
Strong Bone capsule versus Caltrate after three months treatment (MD 16.25 ng/L; 95% CI 10.43 to 22.07) (Analysis 3.12) (Cui TH 1999);
Jingujian granule versus Caltrate after three months treatment (MD 21.98 ng/L; 95% CI 17.01 to 26.95) (Analysis 3.12) (Li YH 2010)
TPF capsule versus calcium granule after six months treatment (MD 17.71 ng/L; 95% CI 11.53 to 23.89) (Analysis 3.12) (Zhang YL 1996).
Chinese herbal medicines plus western medicine versus western medicine (Comparison 04)
Oestradiol (E2) levels
Eight trials showed positive effects of Chinese herbal medicines plus western medicine treatments on E2 when compared to the same western medicines:
Gushen decoction plus Caltrate and Alfacalcidol versus Caltrate and Alfacalcidol after six months treatment (MD 4.54 pg/ml; 95% CI 0.47 to 8.61) (Analysis 4.6) (Chen FS 2001);
Shugan zishen huoxue tang plus Caltrate and alendronate sodium tablets versus Caltrate and alendronate sodium tablets after six months treatment (MD 26.99 pg/ml; 95% CI 18.44 to 35.54) (Analysis 4.6) (Han XL 2007);
Ziyin Bushen Zhuanggu prescription plus Caltrate and calcitonin versus Caltrate plus calcitonin after eight months treatment (MD 23.21 pg/ml; 95% CI 14.46 to 31.96) (Analysis 4.6) (Li HW 2004);
Zhuanggu capsule plus Caltrate versus Caltrate after six months treatment (MD 4.06 pg/ml; 95% CI 2.56 to 5.56) (Analysis 4.6) (Li SL 2004);
Antai capsule plus Caltrate versus Caltrate after six months treatment (MD 11.00 pg/ml; 95% CI 8.14 to 13.86) (Analysis 4.6) (Wang SW 2003);
Zishen prescription plus Caltrate and calcitonin versus Caltrate plus calcitonin (MD 23.21 pg/ml; 95% CI 14.46 to 31.96) (Analysis 4.6) (Zhao G 2002);
Xianlinggubao capsule plus vitamin D3 and calcium amino acid chelate versus vitamin D3 and calcium amino acid chelate after 48 weeks treatment (MD 6.04 pg/ml; 95% CI 4.61 to 7.47) (Analysis 4.6) (Zhang XZ 2004);
Yigu capsule plus calcium versus placebo plus calcium after six months treatment (MD 40.85 pg/ml; 95% CI 33.61 to 48.09) (Analysis 4.6) (Zhang RH 2004).
4.6. Analysis.
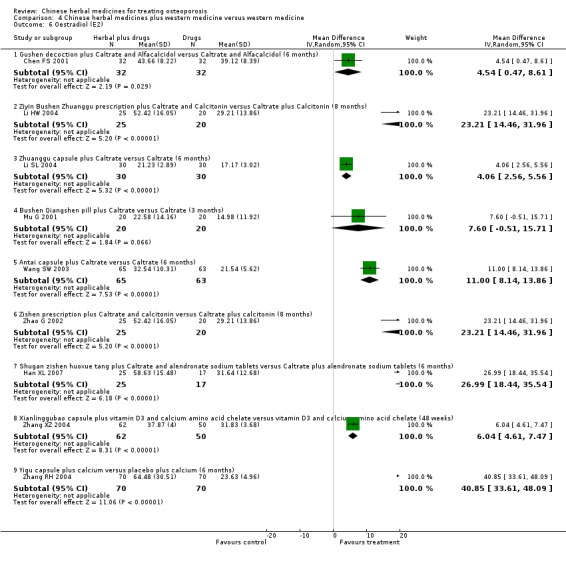
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 6 Oestradiol (E2).
Compared with Caltrate alone, Bushen Qiangshen pill plus Caltrate did not have significant effect on oestradiol (MD 7.60 pg/ml; 95% CI ‐0.51 to 15.71) (Analysis 4.6) (Mu G 2001).
Serum calcium (Ca) levels
Three trials showed positive effects of Chinese herbal medicines plus western medicine treatments on calcium when compared to the same western medicines:
Bushenyiqihuoxue soup plus tamoxifen and Caltrate versus tamoxifen and Caltrate after three months treatment (MD 0.37 mmol/L; 95% CI 0.32 to 0.42) (Analysis 4.7) (Zhang DS 2011);
Chinese medicine (combinations of 10 herbs) plus tamoxifen and Caltrate versus tamoxifen and Caltrate after three months treatment (MD 0.37 mmol/L; 95% CI 0.32 to 0.42) (Analysis 4.7) (Cao W 2010);
Huangqi plus Caltrate versus Caltrate after six months treatment (MD 0.28 mmol/L; 95% CI 0.11 to 0.45) (Analysis 4.7) (Yang B 2007).
4.7. Analysis.
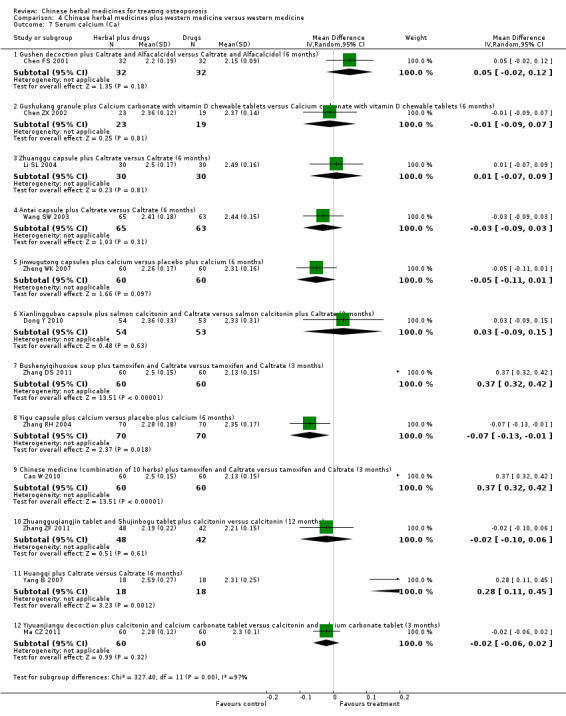
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 7 Serum calcium (Ca).
While Yigu capsule plus calcium versus placebo plus calcium had a negative effect on calcium after six months treatment (MD ‐0.07 mmol/L; 95% CI ‐0.13 to ‐0.01) (Analysis 4.7) (Zhang RH 2004).
Eight trials did not show positive effects of herbal treatments plus western medicines when compared to the same western medicines:
Gushen decoction plus Caltrate and Alfacalcidol versus Caltrate and Alfacalcidol after six months treatment (MD 0.05 mmol/L; 95% CI ‐0.02 to 0.12) (Analysis 4.7) (Chen FS 2001);
Gushukang granule plus Calcium carbonate with vitamin D chewable tablets versus Calcium carbonate with vitamin D chewable tablets after six months treatment (MD ‐0.01 mmol/L; 95% CI ‐0.09 to 0.07) (Analysis 4.7) (Chen ZX 2002);
Zhuanggu capsule plus Caltrate versus Caltrate after six months treatment (MD 0.01 mmol/L; 95% CI ‐0.07 to 0.09) (Analysis 4.7) (Li SL 2004);
Yiyuanjiangu decoction plus calcitonin ampoule and calcium carbonate tablet versus calcitonin ampoule and calcium carbonate tablet after three months treatment (MD ‐0.02 mmol/L; 95% CI ‐0.06 to 0.02) (Analysis 4.7) (Ma CZ 2011);
Antai capsule plus Caltrate versus Caltrate after six months treatment (MD ‐0.03 mmol/L; 95% CI ‐0.09 to 0.03) (Analysis 4.7) (Wang SW 2003);
Jinwugutong capsule plus calcium versus placebo plus calcium after six months treatment (MD ‐0.05 mmol/L; 95% CI ‐0.11 to 0.01) (Analysis 4.7) (Zheng WK 2007);
Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate after three months treatment (MD 0.03 mmol/L; 95% CI ‐0.09 to 0.15) (Analysis 4.7) (Dong Y 2010)
Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin ampoule versus calcitonin ampoule after 12 months treatment (MD ‐0.02 mmol/L; 95% CI ‐0.10 to 0.06) (Analysis 4.7) (Zhang ZF 2011).
Phosphorus (P) levels
Nine trials did not show positive effects of herbal treatments plus western medicines when compared to the same western medicines:
Gushen decoction plus Caltrate and Alfacalcidol versus Caltrate and Alfacalcidol (MD ‐0.13 mmol/L; 95% CI ‐0.27 to 0.01) (Analysis 4.8) (Chen FS 2001);
Gushukang granule plus Calcium carbonate with vitamin D chewable tablets versus Calcium carbonate with vitamin D chewable tablets after six months treatment (MD ‐0.02 mmol/L; 95% CI ‐0.11 to 0.07) (Analysis 4.8) (Chen ZX 2002);
Zhuanggu capsule plus Caltrate versus Caltrate (MD 0.01 mmol/L; 95% CI ‐0.06 to 0.08) (Analysis 4.8) (Li SL 2004);
Yiyuanjiangu decoction plus calcitonin ampoule and calcium carbonate tablet versus calcitonin ampoule and calcium carbonate tablet after three months treatment (MD 0.06 mmol/L; 95% CI ‐0.22 to 0.34) (Analysis 4.8) (Ma CZ 2011);
Antai capsule plus Caltrate versus Caltrate (MD ‐0.01 mmol/L; 95% CI ‐0.23 to 0.21) (Analysis 4.8) (Wang SW 2003);
Jinwugutong capsule plus calcium versus placebo plus calcium after six months treatment (MD ‐0.02 mmol/L; 95% CI ‐0.09 to 0.05) (Analysis 4.8) (Zheng WK 2007);
Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate after three months treatment (MD 0.01 mmol/L; 95% CI ‐0.12 to 0.14) (Analysis 4.8) (Dong Y 2010);
Yigu capsule plus calcium versus placebo plus calcium after six months treatment (MD ‐0.03 mmol/L; 95% CI ‐0.08 to 0.02) (Analysis 4.8) (Zhang RH 2004);
Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin ampoule versus calcitonin ampoule after 12 months treatment (MD ‐0.02 mmol/L; 95% CI ‐0.08 to 0.04) (Analysis 4.8) (Zhang ZF 2011).
4.8. Analysis.
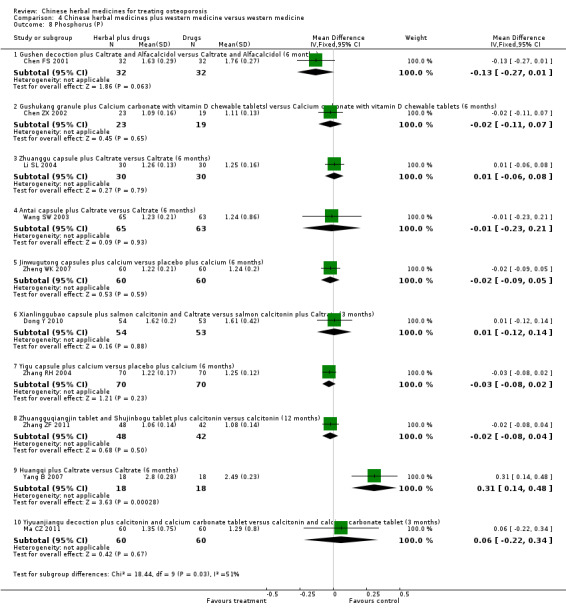
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 8 Phosphorus (P).
Alkaline phosphatase (ALP) levels
Seven trials did not show significant effects of herbal treatments plus western medicines on alkaline phosphatase levels when compared to the same western medicines:
Gushukang granule plus Calcium carbonate with vitamin D chewable tablets versus Calcium carbonate with vitamin D chewable tablets after six months treatment (MD ‐0.50 μmol s‐1/L; 95% CI ‐1.70 to 0.70) (Analysis 4.9) (Chen ZX 2002);
Zhuanggu capsule plus Caltrate versus Caltrate after six months treatment (MD 0.00 μmol s‐1/L; 95% CI ‐1.55 to 1.55) (Analysis 4.9) (Li SL 2004);
Yiyuanjiangu decoction plus calcitonin ampoule and calcium carbonate tablet versus calcitonin ampoule and calcium carbonate tablet after three months treatment (MD 0.31 μmol s‐1/L; 95% CI ‐0.48 to 1.10) (Analysis 4.9) (Ma CZ 2011);
Bushen Qiangshen pill plus Caltrate versus Caltrate after three months treatment (MD ‐0.31 μmol s‐1/L; 95% CI ‐1.72 to 1.10) (Analysis 4.9) (Mu G 2001);
Jinwugutong capsule plus calcium versus placebo plus calcium after six months treatment (MD 1.01 μmol s‐1/L; 95% CI ‐0.04 to 2.06) (Analysis 4.9) (Zheng WK 2007);
Chinese medicine (combinations of 10 herbs) plus tamoxifen and Caltrate versus tamoxifen and Caltrate after three months treatment (MD ‐0.26 μmol s‐1/L; 95% CI ‐1.64 to 1.12) (Analysis 4.9) (Cao W 2010);
Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin ampoule versus calcitonin ampoule after 12 months treatment (MD 0.08 μmol s‐1/L; 95% CI ‐0.54 to 0.70) (Analysis 4.9) (Zhang ZF 2011).
4.9. Analysis.
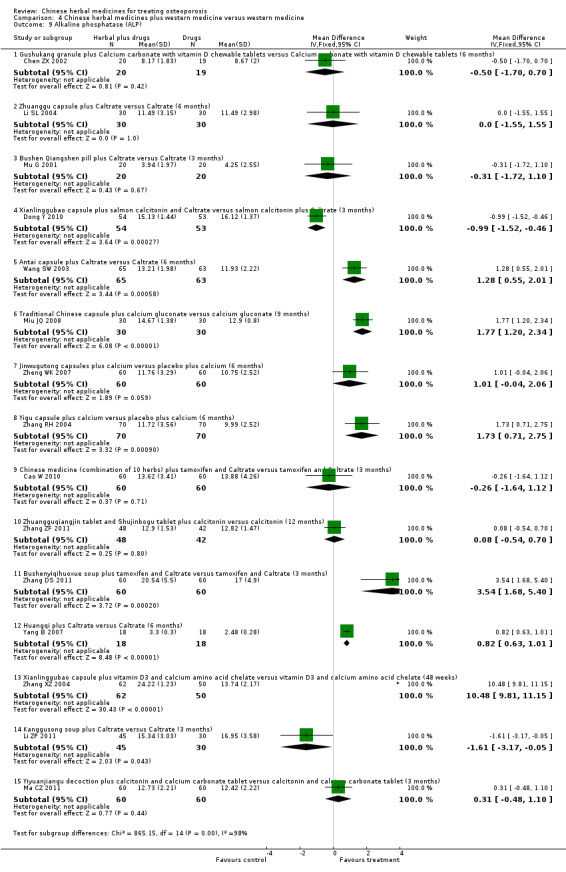
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 9 Alkaline phosphatase (ALP).
Six trials showed that herbal drugs plus western medicine could increase the alkaline phosphatase level when compared with the same western medicine:
Antai capsule plus Caltrate versus Caltrate regarding alkaline phosphatase before and after six months treatment (MD 1.28 μmol s‐1/L; 95% CI 0.55 to 2.01) (Analysis 4.9) (Wang SW 2003);
Chinese decoction plus calcium gluconate versus calcium gluconate after nine months treatment (MD 1.77 μmol s‐1/L; 95% CI 1.20 to 2.34) (Analysis 4.9) (Miu JQ 2008);
Bushenyiqihuoxue soup plus tamoxifen and Caltrate versus tamoxifen and Caltrate after three months treatment (MD 3.54 μmol s‐1/L; 95% CI 1.68 to 5.40) (Analysis 4.9) (Zhang DS 2011);
Yigu capsule plus calcium versus placebo plus calcium after six months treatment (MD 1.73 μmol s‐1/L; 95% CI 0.71 to 2.75) (Analysis 4.9) (Zhang RH 2004);
Huangqi plus Caltrate versus Caltrate after six months treatment (MD 0.82 μmol s‐1/L; 95% CI 0.63 to 1.01) (Analysis 4.9) (Yang B 2007);
Xianlinggubao capsule plus vitamin D3 and calcium amino acid chelate versus vitamin D3 and calcium amino acid chelate after 48 weeks (MD 10.48 μmol s‐1/L; 95% CI 9.81 to 11.15) (Analysis 4.9) (Zhang XZ 2004).
Two trials showed the opposite effect:
Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate after three months treatment (MD ‐0.99 μmol s‐1/L; 95% CI ‐1.52 to ‐0.46) (Analysis 4.9) (Dong Y 2010);
Kanggusong soup plus Caltrate versus Caltrate after three months treatment (MD ‐1.61 μmol s‐1/L; 95% CI ‐3.17 to ‐0.05) (Analysis 4.9) (Li ZP 2011).
Bone Gla protein (BGP) levels
One trial showed a better effect of herbal drugs plus western medicine:
Zhuanggu capsule plus Caltrate versus Caltrate (MD ‐2.30 μg/L; 95% CI ‐4.07 to ‐0.53) (Analysis 4.10) (Li SL 2004).
4.10. Analysis.
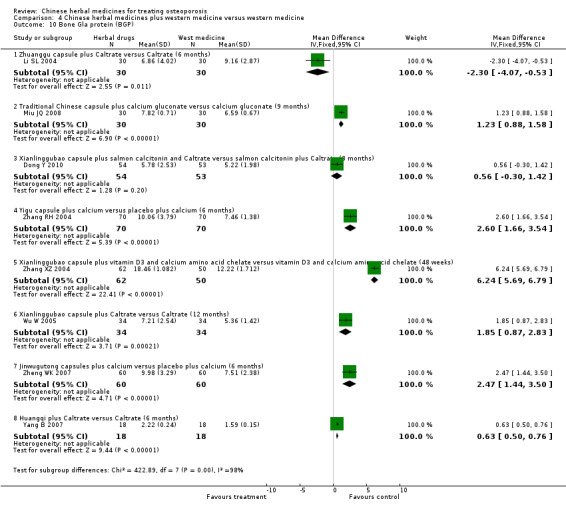
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 10 Bone Gla protein (BGP).
Seven trials showed a worse effect of herbal drugs plus western medicine:
Traditional Chinese capsule plus calcium gluconate versus calcium gluconate after nine months treatment (MD 1.23 μg/L; 95% CI 0.88 to 1.58) (Analysis 4.10) (Miu JQ 2008);
Jinwugutong capsule plus calcium versus placebo plus calcium after six months treatment (MD 2.47 μg/L; 95% CI 1.44 to 3.50) (Analysis 4.10) (Zheng WK 2007);
Yigu capsule plus calcium versus placebo plus calcium after six months treatment (MD 2.60 μg/L; 95% CI 1.66 to 3.54) (Analysis 4.10) (Zhang RH 2004);
Xianlinggubao capsule plus vitamin D3 and calcium amino acid chelate versus vitamin D3 and calcium amino acid chelate after 48 weeks treatment (MD 6.24 μg/L; 95% CI 5.69 to 6.79) (Analysis 4.10) (Zhang XZ 2004);
Xianlinggubao capsules plus Caltrate versus Caltrate after 12 months treatment (MD 1.85 μg/L; 95% CI 0.87 to 2.83) (Analysis 4.10) (Wu W 2005);
Huangqi plus Caltrate versus Caltrate after six months treatment (MD 0.63 μg/L; 95% CI 0.50 to 0.76) (Analysis 4.10) (Yang B 2007).
There was no significant difference between Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate after three months treatment (MD 0.56 μg/L; 95% CI ‐0.30 to 1.42) (Analysis 4.10) (Dong Y 2010).
Interleukin‐6 (IL‐6) levels
Three trials showed a better effect of herbal drugs plus western medicine on interleukin‐6:
Erxian Yanggu tang plus alendronate sodium tablets versus alendronate sodium tablets (MD ‐16.18 pg/ml; 95% CI ‐19.62 to ‐12.74) (Analysis 4.11) (Huang JW 2008);
Kanggusong soup plus Caltrate versus Caltrate after three months treatment (MD ‐16.32 pg/ml; 95% CI ‐31.60 to ‐1.04) (Analysis 4.11) (Li ZP 2011);
Xianlinggubao capsule plus vitamin D3 and calcium amino acid chelate versus vitamin D3 and calcium amino acid chelate after 48 weeks treatment (MD ‐133.30 pg/ml; 95% CI ‐139.94 to ‐126.66) (Analysis 4.11) (Zhang XZ 2004).
4.11. Analysis.
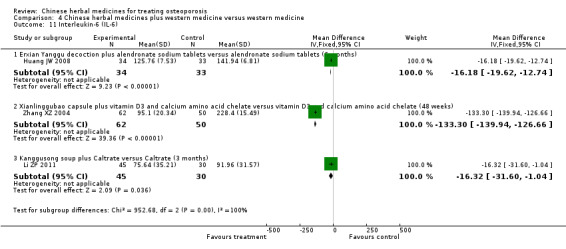
Comparison 4 Chinese herbal medicines plus western medicine versus western medicine, Outcome 11 Interleukin‐6 (IL‐6).
Data and analyses
Comparison 1. Chinese herbal medicines versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 New fractures | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.85] |
| 1.1 Kanggusong capsule versus placebo (12 months) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.85] |
| 2 Bone mineral density (BMD) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Migu decoction versus placebo (BMD of lumbar spine, 4 months) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.06, 0.26] |
| 2.2 Kanggusong capsule versus placebo (BMD of lumbar spine, 12 months) | 1 | 140 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.02, 0.10] |
| 2.3 Bushen Yigu soft extract versus placebo (BMD of radius, 3 months) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.03, 0.09] |
| 2.4 Bushen Yigu soft extract versus placebo (BMD of ulna, 3 months) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.02, 0.10] |
| 3 Oestradiol (E2) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Bushen Yigu soft extract versus placebo (3 months) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 122.89 [92.97, 152.81] |
| 4 Symptoms including pain, muscle fatigue and limited mobility | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.05, 1.37] |
| 4.1 Kanggusong capsule versus placebo (Bone pain at lumbar spine, 12 months) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.05, 1.37] |
Comparison 2. Chinese herbal medicines versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bone mineral density (BMD) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Bushen Shengsui principle versus no intervention (BMD of lumbar spine, 6 months) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.06, 0.20] |
| 1.2 Bushen Shengsui principle versus no intervention (BMD of femoral neck, 6 months) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.00, 0.20] |
| 1.3 Qianggu soft extract versus no intervention (BMD of femoral neck, 6 months) | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.03, 0.15] |
| 1.4 Shigu yin versus no treatment (BMD of Femoral neck, 6 months) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [0.03, 0.13] |
| 1.5 Yishen Zhuanggu mixture versus no intervention (BMD of femoral neck, 6months) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 1.6 Shengu capsule versus no intervention (BMD of femoral neck, 6 months) | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.06, 0.09] |
| 2 Oestradiol (E2) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Bushen Shengsui principle versus no intervention (6 months) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 4.40 [‐4.61, 13.41] |
| 2.2 Yishen Zhuanggu mixture versus no treatment (6 months) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐6.22, 10.42] |
| 2.3 Huoluo Gukang pills versus no intervention (6 months) | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [‐1.22, 6.02] |
| 2.4 Gushukang granule versus no intervention (6 months) | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐1.69, 5.89] |
| 3 Bone Gla protein (BGP) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Yishen Zhuanggu mixture versus no treatment (6 months) | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐6.15, 4.15] |
| 3.2 Shengu capsule versus no treatment (6 months) | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐5.10 [‐10.63, 0.43] |
| 3.3 Huoluo Gukang pills versus no treatment (6 months) | 1 | 136 | Mean Difference (IV, Random, 95% CI) | 2.70 [1.23, 4.17] |
| 3.4 Gushukang granule versus no treatment (6 months) | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 3.5 [1.92, 5.08] |
| 3.5 Shigu yin versus no treatment (6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 1.2 [0.17, 2.23] |
Comparison 3. Chinese herbal medicines versus western medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 New fractures | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Kanggusong granule versus Caltrate (9 months) | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Kanggusong granule versus ipriflavone plus Caltrate (9 months) | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Bone mineral density (BMD) | 51 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Kanggusong granule versus ipriflavone plus Caltrate (BMD of lumbar spine, 9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 2.2 Kanggusong granule versus Caltrate (BMD of lumbar spine, 9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.02, 0.10] |
| 2.3 Kanggusong capsule versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.11] |
| 2.4 Strong Bone capsule versus Caltrate (BMD of lumbar spine, 3 months) | 1 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 2.5 Qianggu capsule versus active vitamin D3 (BMD of lumbar spine, 6 months) | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 2.6 Qianggu capsule versus alendronate (BMD of lumbar spine, 6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.05] |
| 2.7 Herba epimedii prescription versus vitamin D plus Caltrate (BMD of lumbar spine, 6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.00, 0.06] |
| 2.8 Bushen Shengsui principle versus conjugated oestrogens plus medroxyprogesterone (BMD of lumbar spine, 6 months) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 2.9 Bushen Qianggutang versus Caltrate (BMD of lumbar spine, 3 months) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.17] |
| 2.10 Bushen Jianpi Migu prescription versus Caltrate (BMD of lumbar spine, 3 months) | 1 | 150 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.03, 0.09] |
| 2.11 Bushen Jianpi Huoxue recipe versus alendronate sodium tablets (BMD of lumbar spine, 6 months) | 1 | 141 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 2.12 Bushen Huoxue capsules versus Caltrate and Rocalirol (BMD of lumbar spine, 12 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.05, 0.09] |
| 2.13 Bushen Zhuanggu tang versus alendronate sodium tablets (BMD of lumbar spine, 3 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.01, 0.07] |
| 2.14 Bushenjianpi Zhuangguyin versus Caltrate and calcitonin (BMD of lumbar spine, 12 weeks) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.16 [0.10, 0.22] |
| 2.15 Jiawei Bushen Zhuangjintang versus calcium granule (BMD of lumbar spine, 3 months) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.05, 0.15] |
| 2.16 Gukang oral liquid versus alendronate (BMD of lumbar spine, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2.17 Kidney‐tonifying herbs versus nilestriol (BMD of lumbar spine, 6 months) | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 2.18 Kidney‐tonifying herbs versus calcium (BMD of lumbar spine, 6 months) | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 2.19 Yishen Yanggan mixture versus calcium gluconate (BMD of lumbar spine, 6 months) | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.01, 0.07] |
| 2.20 Yinyanghuo versus conjugated oestrogens (BMD of lumbar spine, 3 to 6 months) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 2.21 Shenyao capsules versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 2.22 Guli powder versus Caltrate (BMD of lumbar spine, 3 months, male) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 2.23 Guli powder versus Caltrate (BMD of lumbar spine, 3 months, female) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.07, 0.15] |
| 2.24 Gushen Yijing tang versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.02, 0.14] |
| 2.25 Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (BMD of lumbar spine, 6 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.01, 0.26] |
| 2.26 Huangqi Sanxian tang versus nilestriol (BMD of lumbar spine, 3 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.03, 0.04] |
| 2.27 Zishen Gukang pill versus Caltrate plus vitamin D (BMD of lumbar spine, 3 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 2.28 Xianling Gusong capsules versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.08, 0.02] |
| 2.29 Jianshenfang granule versus alendronate sodium (BMD of lumbar spine, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.03, 0.21] |
| 2.30 Jiangu recipe versus alendronate sodium tablets (BMD of lumbar spine, 12 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.00, 0.04] |
| 2.31 Gukang decoction versus alendronate sodium tablets (BMD of lumbar spine, 4 months) | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 2.32 Gumiling granule versus bicarbonate calcium chewable (BMD of lumbar spine, 6 months) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.02, 0.09] |
| 2.33 Gujian capsule versus alfacalcidol (BMD of lumbar spine, 6 months) | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.03, 0.26] |
| 2.34 Xianlinggubao capsule versus alendronate (BMD of lumbar spine, 6 months) | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 2.35 Erxian soup versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.11] |
| 2.36 Shangke Jiegu tablet versus vitamin D2 plus calcium tablet (BMD of lumbar spine, 6 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.25 [0.11, 0.39] |
| 2.37 Gukang oral liquid versus calcium gluconate (BMD of radius, 6 months) | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.02 [0.01, 0.04] |
| 2.38 Herba epimedii prescription versus vitamin D plus Caltrate (BMD of radius, 6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.04, 0.11] |
| 2.39 Bushen Yigu soft extract versus Alfacalcidol (BMD of radius, 3 months) | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 2.40 Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (BMD of radius, 6 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.12, 0.28] |
| 2.41 Shangke Jiegu tablet versus vitamin D2 plus calcium tablet (BMD of radius, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.02, 0.11] |
| 2.42 Liuwei Dihuang pills versus calcium (BMD of radius, 12 months) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.03, 0.05] |
| 2.43 Gukang oral liquid versus calcium gluconate (BMD of ulna, 6 months) | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 2.44 Bushen Yigu soft extract versus Alfacalcidol (BMD of ulna, 3 months) | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 2.45 Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (BMD of ulna, 6 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.01, 0.13] |
| 2.46 Shangke Jiegu tablet versus vitamin D2 plus calcium tablet (BMD of ulna, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.00, 0.11] |
| 2.47 Liuwei Dihuang pills versus calcium (BMD of ulna, 12 months) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.02 [0.01, 0.03] |
| 2.48 Kanggusong granule versus ipriflavone plus Caltrate (BMD of femoral neck, 9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 2.49 Kanggusong granule versus Caltrate (BMD of femoral neck, 9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2.50 Herba epimedii prescription versus vitamin D plus Caltrate (BMD of femoral neck, 6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 2.51 Bushen Shengsui principle versus conjugated oestrogens plus medroxyprogesterone (BMD of femoral neck, 6 months) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 2.52 Kidney‐tonifying herbs versus nilestriol (BMD of femoral neck, 6 months) | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 2.53 Kidney‐tonifying herbs versus calcium (BMD of femoral neck, 6 months) | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.01, 0.09] |
| 2.54 Gumikang capsule versus calcium gluconate (BMD of femoral neck, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 2.55 Yanghuo Sanzi tang versus Caltrate (BMD of femoral neck, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.02, 0.08] |
| 2.56 Qianggu soft extract versus calcium gluconate (BMD of femoral neck, 6 months) | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.02, 0.12] |
| 2.57 Qianggu capsules versus oestradiol valerate (BMD of femoral neck, 6 months) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 2.58 Qianggu paste versus calcium gluconate tablets (BMD of femoral neck, 6 months) | 1 | 123 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.03, 0.11] |
| 2.59 Qianggu capsule versus active vitamin D3 (BMD of femoral neck, 6 months) | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 2.60 Shigu yin versus Caltrate (BMD of femoral neck, 6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 2.61 Jiangu capsule versus Caltrate (BMD of femoral neck, 12 months) | 1 | 125 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.03, 0.06] |
| 2.62 Gujian capsule versus alfacalcidol (BMD of femoral neck, 6 months) | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.03, 0.14] |
| 2.63 Bushenhuoxue capsules versus Caltrate and Rocalirol (BMD of femoral neck, 12 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.06, 0.10] |
| 2.64 Bushenjianpi Zhuangguyin versus Caltrate and calcitonin (BMD of femoral neck, 12 weeks) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.00, 0.12] |
| 2.65 Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (BMD of femoral neck, 6 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.05, 0.23] |
| 2.66 Shangke Jiegu tablet versus vitamin D2 plus calcium tablet (BMD of femoral neck, 6 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.06, 0.20] |
| 2.67 Kanggusong granule versus ipriflavone plus Caltrate (BMD of Ward's, 9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.07, 0.01] |
| 2.68 Kanggusong granule versus Caltrate (BMD of Ward's, 9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 2.69 Kidney‐tonifying herbs versus nilestriol (BMD of Ward's, 6 months) | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 2.70 Kidney‐tonifying herbs versus calcium (BMD of Ward's, 6 months) | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.06, 0.14] |
| 2.71 Gujian capsule versus alfacalcidol (BMD of Ward's, 6 months) | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.01, 0.15] |
| 2.72 Qianggu capsule versus alendronate (BMD of Ward's, 6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 2.73 Xianlinggubao capsule versus alendronate (BMD of Ward's, 6 months) | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 2.74 Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (BMD of Ward's, 6 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.05, 0.19] |
| 2.75 Shangke Jiegu tablet versus vitamin D2 plus calcium tablet (BMD of Ward's, 6 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.01, 0.22] |
| 2.76 Kanggusong granule versus ipriflavone plus Caltrate (BMD of trochanter, 9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 2.77 Kanggusong granule versus Caltrate (BMD of trochanter, 9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.00, 0.06] |
| 2.78 Gujian capsule versus alfacalcidol (BMD of trochanter, 6 months) | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.07, 0.20] |
| 2.79 Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (BMD of trochanter, 6 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.04, 0.27] |
| 2.80 Shangke Jiegu tablet versus vitamin D2 plus calcium tablet (BMD of trochanter, 6 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.19 [0.04, 0.33] |
| 2.81 Gushukang granule versus Calcium carbonate with vitamin D chewable tablets (BMD of hip bone, 6 months) | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 2.82 Guli powder versus Caltrate (BMD of hip bone, 3 months, male) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 2.83 Guli powder versus Caltrate (BMD of hip bone, 3 months, female) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.05, 0.11] |
| 2.84 TPF capsule versus calcium granule (BMD of distal radius, 6 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.17, 0.23] |
| 2.85 Jiawei Zhuangyao Jianshen tang versus compound calcium amino acid chelate capsules (BMD of calcaneus, 3 months) | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.01, 0.11] |
| 2.86 Liuwei Dihuang pills versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.03, 0.07] |
| 2.87 Bushen Jianpi Jingu decoction versus calcitonin (BMD of lumbar spine, 3 months) | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.09, 0.13] |
| 2.88 Bushen Jianpi Jingu decoction versus calcitonin (BMD of femoral neck, 3 months) | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.02, 0.12] |
| 2.89 Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets (BMD of lumbar spine, 6 months) | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.03, 0.07] |
| 2.90 Kangshu Jiangu granule versus Caltrate (BMD of femoral neck, 6 months) | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.01, 0.15] |
| 2.91 Kangshu Jiangu granule versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.01, 0.21] |
| 2.92 Bushen Tianjing Huoxue therapy versus Caltrate and calcitriol soft capsule (BMD of lumbar spine, 6 months) | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.00, 0.07] |
| 3 T score in BMD measurement | 2 | 330 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.12, 0.34] |
| 3.1 Jingujian granule versus Caltrate (T score, 3 months) | 1 | 120 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.35, 0.41] |
| 3.2 Jingujian granule versus Caltrate (T score, 3 months) | 1 | 210 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.12, 0.45] |
| 4 Symptoms including pain, muscle fatigue and limited mobility | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Jiawei Bushen Zhuangjintang versus calcium granule (3 months) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.28, 2.19] |
| 4.2 Qianggu soft extract versus calcium gluconate (6 months) | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.24, 2.80] |
| 4.3 Kanggusong granule versus ipriflavone (3 months) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [1.07, 2.39] |
| 4.4 Yishen Yanggan mixture versus calcium gluconate (6 months) | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.28, 2.25] |
| 4.5 Yinyanghuo versus conjugated oestrogens (3 to 6 months) | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.28] |
| 4.6 Gumiling granule versus bicarbonate calcium chewable (6 months) | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.26 [1.43, 12.72] |
| 4.7 Jiawei Zhuangyao Jianshen tang versus compound calcium amino acid chelate capsules (3 months) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.94, 1.66] |
| 4.8 Zishen Gukang pill versus Caltrate plus vitamin D (3 months) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.01, 1.61] |
| 4.9 Xianling Gusong capsules versus Caltrate (3 months) | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.03, 2.39] |
| 4.10 Kanggusong capsule versus Caltrate (6 months) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.12, 2.04] |
| 4.11 Jiangu recipe versus alendronate sodium tablets (12 months) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.31, 2.08] |
| 4.12 Jingujian granule versus Caltrate (3 months) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [1.89, 5.26] |
| 5 Oestradiol (E2) | 25 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Kanggusong granule versus ipriflavone plus Caltrate (9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 12.17 [8.27, 16.07] |
| 5.2 Kanggusong granule versus Caltrate (9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 11.0 [6.97, 15.03] |
| 5.3 Strong Bone capsule versus Caltrate (3 months) | 1 | 105 | Mean Difference (IV, Random, 95% CI) | 10.46 [4.29, 16.63] |
| 5.4 Bushen Shengsui principle versus conjugated oestrogens plus medroxyprogesterone (6 months) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐3.92, 11.92] |
| 5.5 Bushen Qiangshen pill versus Caltrate (3 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 6.71 [‐2.28, 15.70] |
| 5.6 Qianggu soft extract versus calcium gluconate (6 months) | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 4.20 [0.89, 7.51] |
| 5.7 Gukang oral liquid versus alendronate (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐6.64, 14.44] |
| 5.8 Gumikang capsule versus calcium gluconate (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 3.5 [1.14, 5.86] |
| 5.9 Bushen Yigu soft extract versus Alfacalcidol (3 months) | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 76.29 [31.19, 121.39] |
| 5.10 Kanggusong granule versus ipriflavone (3 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.18 [‐13.85, 22.21] |
| 5.11 Yanghuo Sanzi tang versus Caltrate (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 23.07 [17.83, 28.31] |
| 5.12 Gusong recipe versus alendronate sodium tablets (5 months) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐9.01, 16.81] |
| 5.13 Gushukang granule versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 13.98 [3.90, 24.06] |
| 5.14 Bushen Jianpi Huoxue recipe versus alendronate sodium tablets (6 months) | 1 | 141 | Mean Difference (IV, Random, 95% CI) | 3.15 [0.28, 6.02] |
| 5.15 Prescription for tonifying kidney versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 14.00 [3.68, 24.32] |
| 5.16 Kidney meridian sticking versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 11.99 [1.15, 22.83] |
| 5.17 Urinary bladder meridian sticking versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 17.99 [7.65, 28.33] |
| 5.18 Huangqi Sanxian tang versus nilestriol (3 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐32.97 [‐39.42, ‐26.52] |
| 5.19 Gukang oral liquid versus alendronate sodium tablets (6 months) | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 5.09 [2.81, 7.37] |
| 5.20 Gukang decoction versus alendronate sodium tablets (4 months) | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐9.04, 7.74] |
| 5.21 Jianshenfang granule versus alendronate sodium (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 5.83 [4.21, 7.45] |
| 5.22 Jiangu recipe versus alendronate sodium tablets (6 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 5.82 [3.69, 7.95] |
| 5.23 Jiangu recipe versus alendronate sodium tablets (12 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 10.75 [8.55, 12.95] |
| 5.24 Bushen Zhuanggu tang versus alendronate sodium tablets (3 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 5.37 [2.07, 8.67] |
| 5.25 Bushen Huoxue capsule versus Caltrate and Rocalirol (12 months) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 2.13 [0.42, 3.84] |
| 5.26 Jingujian granule versus Caltrate (3 months) | 1 | 210 | Mean Difference (IV, Random, 95% CI) | 4.85 [4.56, 5.14] |
| 5.27 Qianggu paste versus calcium gluconate tablets (6 months) | 1 | 123 | Mean Difference (IV, Random, 95% CI) | 4.20 [1.79, 6.61] |
| 5.28 Tongbu Qianggutang versus calcium plus vitamin D3 (female, 3 months) | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 5.03 [‐8.01, 18.07] |
| 5.29 Tongbu Qianggutang versus calcium plus vitamin D3 (male, 3 months) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 1.89 [‐17.98, 21.77] |
| 5.30 Gujian capsule versus alfacalcidol (6 months) | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 22.35 [‐10.51, 55.21] |
| 5.31 Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets (6 months) | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 8.78 [0.92, 16.64] |
| 6 Serum calcium (Ca) | 22 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Kanggusong granule versus ipriflavone plus Caltrate (9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.17, ‐0.01] |
| 6.2 Kanggusong granule versus Caltrate (9 months) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.11, 0.05] |
| 6.3 Gushukang granule versus Calcium carbonate with vitamin D chewable tablets (6 months) | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 6.4 Strong Bone capsule versus Caltrate (3 months) | 1 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.22, ‐0.04] |
| 6.5 Qianggu soft extract versus calcium gluconate (6 months) | 1 | 99 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.05, ‐0.01] |
| 6.6 Gumikang capsule versus calcium gluconate (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.40 [1.39, 1.41] |
| 6.7 Bushen Jianpi Migu prescription versus Caltrate (3 months) | 1 | 150 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.05, 0.07] |
| 6.8 Kanggusong granule versus ipriflavone (3 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.03, 0.17] |
| 6.9 Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (6 months) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.05, 0.25] |
| 6.10 Yinyanghuo versus conjugated oestrogens (3 to 6 months) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.54 [0.23, 0.85] |
| 6.11 Gushukang granule versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 6.12 Shenyao capsules versus Caltrate (6 months) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.08, 0.12] |
| 6.13 Guli powder versus Caltrate (3 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.02, 0.16] |
| 6.14 Prescription for tonifying kidney versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 6.15 Kidney meridian sticking versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 6.16 Urinary bladder meridian sticking versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 6.17 Gushen Yijing tang versus Caltrate (6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.23 [0.11, 0.35] |
| 6.18 Gukang oral liquid versus alendronate sodium tablets (6 months) | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 6.19 Qianggu capsule versus active vitamin D3 (6 months) | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.06, 0.08] |
| 6.20 Jiangu capsule versus Caltrate (12 months) | 1 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.11, ‐0.01] |
| 6.21 Bushen Yangxue tang versus calcium gluconate (6 months) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.00, 0.26] |
| 6.22 Bushenjianpi Zhuangguyin versus Caltrate and calcitonin (12 weeks) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.74 [0.66, 0.82] |
| 6.23 Erxian soup versus Caltrate (6 months) | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.25, 0.21] |
| 6.24 Qianggu paste versus calcium gluconate tablets (6 months) | 1 | 123 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.06, ‐0.00] |
| 6.25 Tongbu Qianggutang versus calcium plus vitamin D3 (3 months) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 6.26 Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets (6 months) | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 7 Phosphorus (P) | 17 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Kanggusong granule versus ipriflavone plus Caltrate (9 months) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.03, 0.15] |
| 7.2 Kanggusong granule versus Caltrate (9 months) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.29, 0.27] |
| 7.3 Gushukang granule versus Calcium carbonate with vitamin D chewable tablets (6 months) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.09, 0.11] |
| 7.4 Strong Bone capsule versus Caltrate (3 months) | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.21, ‐0.03] |
| 7.5 Bushen Jianpi Migu prescription versus Caltrate (3 months) | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.02, 0.08] |
| 7.6 Kanggusong granule versus ipriflavone (3 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.20, 0.06] |
| 7.7 Yinyanghuo versus conjugated oestrogens (3 to 6 months) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.13, 0.39] |
| 7.8 Shenyao capsules versus Caltrate (6 months) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 7.9 Gushukang granule versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.13, 0.11] |
| 7.10 Guli powder versus Caltrate (3 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.13, 0.03] |
| 7.11 Prescription for tonifying kidney versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.15, 0.07] |
| 7.12 Kidney meridian sticking versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.13, 0.11] |
| 7.13 Urinary bladder meridian sticking versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.13, 0.11] |
| 7.14 Gushen Yijing tang versus Caltrate (6 months) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.09, 0.17] |
| 7.15 Qianggu capsule versus active vitamin D3 (6 months) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.09] |
| 7.16 Jiangu capsule versus Caltrate (12 months) | 1 | 125 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.20, 0.38] |
| 7.17 Bushenzhuanggu decoction versus alendronate sodium tablets (3 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.10, 0.38] |
| 7.18 Bushenjianpi Zhuangguyin versus Caltrate and calcitonin (12 weeks) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.48, ‐0.26] |
| 7.19 Erxian soup versus Caltrate (6 months) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.20, 0.14] |
| 7.20 Tongbu Qianggutang versus calcium plus vitamin D3 (3 months) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] |
| 7.21 Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets (6 months) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.15, 0.15] |
| 8 Alkaline phosphatase (ALP) | 32 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Kanggusong granule versus ipriflavone plus Caltrate (9 months) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 1.69 [0.79, 2.59] |
| 8.2 Kanggusong granule versus Caltrate (9 months) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [0.30, 2.26] |
| 8.3 Gushukang granule versus Calcium carbonate with vitamin D chewable tablets (6 months) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.70, 0.70] |
| 8.4 Strong Bone capsule versus Caltrate (3 months) | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| 8.5 Bushen Qiangshen pill versus Caltrate (3 months) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐1.27, 1.81] |
| 8.6 Qianggu soft extract versus calcium gluconate (6 months) | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [2.62, 4.58] |
| 8.7 Gukang oral liquid versus alendronate (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐2.54, 0.42] |
| 8.8 Gumikang capsule versus calcium gluconate (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.41 [2.84, 3.98] |
| 8.9 Bushen Jianpi Migu prescription versus Caltrate (3 months) | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.01, 0.19] |
| 8.10 Kanggusong granule versus ipriflavone (3 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.07, 0.33] |
| 8.11 Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (6 months) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.67, 1.09] |
| 8.12 Liuwei Dihuang pills versus calcium (12 months) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐0.12, 1.22] |
| 8.13 TPF capsule versus calcium granule (6 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 3.84 [2.53, 5.15] |
| 8.14 Yinyanghuo versus conjugated oestrogens (3 to 6 months) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐11.17, ‐2.83] |
| 8.15 Gusong recipe versus alendronate sodium tablets (5 months) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐2.86, 0.76] |
| 8.16 Shenyao capsules versus Caltrate (6 months) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐3.58, ‐1.22] |
| 8.17 Bushen Jianpi Huoxue recipe versus alendronate sodium tablets (6 months) | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐2.94 [‐4.34, ‐1.54] |
| 8.18 Guli powder versus Caltrate (3 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐2.97, 3.57] |
| 8.19 Huangqi Sanxian tang versus nilestriol (3 months) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐1.14, 1.08] |
| 8.20 Gushen Yijing tang versus Caltrate (6 months) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.17 [0.73, 3.61] |
| 8.21 Gukang oral liquid versus alendronate sodium Tablets | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.34, 1.30] |
| 8.22 Qianggu capsule versus active vitamin D3 (6 months) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐1.17, 2.59] |
| 8.23 Jiangu capsule versus Caltrate (12 months) | 1 | 125 | Mean Difference (IV, Fixed, 95% CI) | 2.04 [1.32, 2.76] |
| 8.24 Jianshenfang granule versus alendronate sodium tablets (3 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐2.79, 3.45] |
| 8.25 Jingujian granule versus Caltrate (3 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.69, 1.75] |
| 8.26 Jianshenfang granule versus alendronate sodium tablets (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐2.78, 3.44] |
| 8.27 Bushen Zhuanggu tang versus alendronate sodium tablets (3 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.99, 0.77] |
| 8.28 Bushenjianpi Zhuangguyin versus Caltrate and calcitonin (12 weeks) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐2.83, 1.19] |
| 8.29 Jingujian granule versus Caltrate (3 months) | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [2.21, 2.59] |
| 8.30 Erxian soup versus Caltrate (6 months) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.87, 0.87] |
| 8.31 Qianggu paste versus calcium gluconate tablets (6 months) | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [2.86, 4.34] |
| 8.32 Bushen Yangxue tang versus calcium gluconate (6 months) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.62 [0.84, 4.40] |
| 8.33 Tongbu Qianggutang versus calcium plus vitamin D3 (3 months) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐1.08, 1.44] |
| 8.34 Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets (6 months) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [0.15, 1.67] |
| 9 Bone Gla protein (BGP) | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Bushen Jianpi Huoxue recipe versus alendronate sodium tablets (6 months) | 1 | 141 | Mean Difference (IV, Random, 95% CI) | ‐2.13 [‐2.74, ‐1.52] |
| 9.2 Acupoint sticking of Migudan versus oyster shell calcium chewable tablets (3 months) | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 1.55 [1.24, 1.86] |
| 9.3 Gukang oral liquid versus alendronate (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.93, 0.73] |
| 9.4 Gusong recipe versus alendronate sodium tablets (5 months) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.11, 0.91] |
| 9.5 Yanghuo Sanzi tang versus Caltrate (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 2.71 [‐0.30, 5.72] |
| 9.6 Strong Bone capsule versus Caltrate (3 months) | 1 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐2.17, 1.19] |
| 9.7 Shigu yin versus Caltrate (6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.34, 1.94] |
| 9.8 Jingujian granule versus Caltrate (3 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 2.53 [‐0.38, 5.44] |
| 9.9 Jianshenfang granule versus alendronate sodium (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐1.59, 2.57] |
| 9.10 Jiangu recipe versus alendronate sodium tablets (12 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐0.31, 3.93] |
| 9.11 Bushen Huoxue capsules versus Caltrate and Rocalirol (12 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.93 [0.35, 1.51] |
| 9.12 Kanggusong capsule versus Caltrate (6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 1.78 [0.40, 3.16] |
| 9.13 Liuwei Dihuang pills versus calcium (12 months) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 17.9 [12.43, 23.37] |
| 9.14 Tongbu Qianggutang versus calcium plus vitamin D3 (3 months) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐5.07, 2.08] |
| 10 Interleukin‐6 (IL‐6) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Bushen Jianpi Huoxue recipe versus alendronate sodium tablets (6 months) | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐5.72 [‐14.05, 2.61] |
| 10.2 Gukang oral liquid versus alendronate (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐15.93, 8.93] |
| 10.3 Yanghuo Sanzi tang versus Caltrate (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐20.29 [‐35.67, ‐4.91] |
| 10.4 Jianshenfang granule versus alendronate sodium (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐12.56 [‐29.26, 4.14] |
| 10.5 Jiangu recipe versus alendronate sodium tablets (12 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐19.99 [‐30.92, ‐9.06] |
| 10.6 Bushen Huoxue capsules versus Caltrate and Rocalirol (12 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐18.70 [‐34.16, ‐3.24] |
| 10.7 Gusong recipe versus alendronate sodium tablets (5 months) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐18.72, 11.72] |
| 11 Parathyroid hormone (PTH) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Prescription for tonifying kidney versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐41.67, 15.67] |
| 11.2 Kidney meridian sticking versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐13.01 [‐41.95, 15.93] |
| 11.3 Urinary bladder meridian sticking versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.01 [‐31.44, 25.42] |
| 11.4 Kanggusong granule versus ipriflavone (3 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 11.81 [‐14.64, 38.26] |
| 11.5 Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (6 months) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐10.63 [‐140.92, 119.66] |
| 11.6 TPF capsule versus calcium granule (6 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐254.0 [‐399.06, ‐108.94] |
| 11.7 Gujian capsule versus alfacalcidol (6 months) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐22.57 [‐60.92, 15.78] |
| 11.8 Gushukang granule versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.02 [‐30.91, 24.87] |
| 11.9 Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets (6 months) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐2.06 [‐6.74, 2.62] |
| 12 Computed tomography (CT) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Prescription for tonifying kidney versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.96 [‐3.71, 5.63] |
| 12.2 Kidney meridian sticking versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐5.44, 3.42] |
| 12.3 Urinary bladder meridian sticking versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐4.68, 4.66] |
| 12.4 Bugu Shengsui capsule versus vitamin D2 plus calcium tablet (6 months) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 15.93 [‐24.16, 56.02] |
| 12.5 Strong Bone capsule versus Caltrate (3 months) | 1 | 105 | Mean Difference (IV, Random, 95% CI) | 16.25 [10.43, 22.07] |
| 12.6 Jingujian granule versus Caltrate (3 months) | 1 | 210 | Mean Difference (IV, Random, 95% CI) | 21.98 [17.01, 26.95] |
| 12.7 TPF capsule versus Calcium granule (6 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 17.71 [11.53, 23.89] |
| 12.8 Tongbu Qianggutang versus calcium plus vitamin D3 (3 months) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐6.18 [‐12.89, 0.52] |
| 12.9 Gujian capsule versus alfacalcidol (6 months) | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐12.77 [‐70.92, 45.38] |
| 12.10 Gushukang granule versus ipriflavone (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐4.44, 4.42] |
| 12.11 Radix rehmanniae preparata and Radix astragali versus calcium carbonate tablets (6 months) | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.04, 0.26] |
3.1. Analysis.
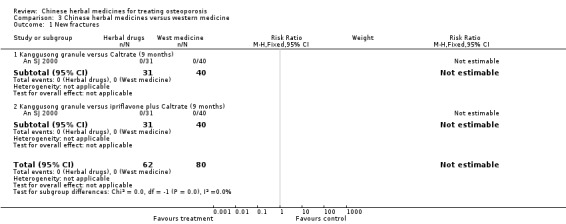
Comparison 3 Chinese herbal medicines versus western medicine, Outcome 1 New fractures.
Comparison 4. Chinese herbal medicines plus western medicine versus western medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 New fractures | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Yigu capsule plus calcium versus placebo plus calcium (6 months) | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 1.00] |
| 1.2 Xianlinggubao capsule (low‐dose) plus calcium and vitamin D versus placebo plus calcium and vitamin D (12 months) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 1.3 Xianlinggubao capsule (high‐dose) plus calcium and vitamin D versus placebo plus calcium and vitamin D (12 months) | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.58] |
| 1.4 Bushen Zhuanggu granules plus calcium and vitamin D versus placebo granules plus calcium and vitamin D (5 years) | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.13, 1.43] |
| 1.5 Kanggusong soup plus Caltrate versus Caltrate (3 months) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 5.34] |
| 1.6 Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin versus calcitonin (12 months) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Bone mineral density (BMD) | 43 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Gushen decoction plus Caltrate and Alfacalcidol versus Caltrate and Alfacalcidol (BMD of lumbar spine, 6 months) | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.01, 0.11] |
| 2.2 Jiarong tablet plus Caltrate versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.05, 0.14] |
| 2.3 Ziyin Bushen Zhuanggu prescription plus Caltrate and calcitonin versus Caltrate plus calcitonin (BMD of lumbar spine, 8 months) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.08, 0.20] |
| 2.4 Zhuanggu capsule plus Caltrate versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.01 [0.00, 0.02] |
| 2.5 Antai capsule plus Caltrate versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.00, 0.08] |
| 2.6 Bushen Zhuanggutang plus Caltrate versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.06, 0.22] |
| 2.7 Zishen prescription plus Caltrate and calcitonin versus Caltrate plus calcitonin (BMD of lumbar spine, 8 months) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.08, 0.20] |
| 2.8 Guilu Erxiantang plus calcium carbonate tablet versus calcium carbonate tablet (BMD of lumbar spine, 6 months) | 1 | 85 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.03, 0.09] |
| 2.9 Guben Zhuanggu capsules plus Caltrate versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.03 [0.01, 0.05] |
| 2.10 Migu tablet plus Caltrate versus Caltrate (BMD of Lumbar spine, 12 months) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.07, 0.09] |
| 2.11 Shugan zishen huoxue tang plus Caltrate and alendronate sodium versus Caltrate plus alendronate sodium (BMD of lumbar spine, 6 months) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.08, 0.16] |
| 2.12 Bushen Yangxue tang plus Caltrate versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 2.13 Bushen Kangsong pill plus calcium carbonate tablets versus calcium carbonate tablets (BMD of lumbar spine, 6 months) | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 2.14 Gusongbao granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule (BMD of lumbar spine, 6 months) | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 2.15 Qianggu capsule plus alendronate versus alendronate (BMD of lumbar spine, 6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.03, 0.12] |
| 2.16 Xianlinggubao capsule plus Caltrate versus Caltrate (BMD of lumbar spine, 12 months) | 1 | 68 | Mean Difference (IV, Random, 95% CI) | 0.30 [0.25, 0.35] |
| 2.17 Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate (BMD of lumbar spine, 3 months) | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.03, 0.09] |
| 2.18 Xianlinggubao capsule plus alendronate versus alendronate (BMD of lumbar spine, 6 months) | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.03, 0.09] |
| 2.19 Xianlinggubao capsule plus vitamin D3 and calcium amino acid chelate versus vitamin D3 and calcium amino acid chelate (BMD of lumbar spine, 48 weeks) | 1 | 112 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.06] |
| 2.20 Xianlinggubao capsule plus Caltrate versus Caltrate (BMD of lumbar spine, 12 months) | 1 | 136 | Mean Difference (IV, Random, 95% CI) | 0.31 [0.28, 0.34] |
| 2.21 Xianlinggubao capsule plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule (BMD of lumbar spine, 6 months) | 1 | 106 | Mean Difference (IV, Random, 95% CI) | 0.18 [0.14, 0.22] |
| 2.22 Jiangu granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule (BMD of lumbar spine, 6 months) | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.02, 0.10] |
| 2.23 Jinwugutong capsules plus calcium versus placebo plus calcium (BMD of lumbar spine, 6 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.07, 0.15] |
| 2.24 Xuduanzhuanggu capsule plus calcium tablet versus placebo plus calcium tablet (BMD of lumbar spine, 6 months) | 1 | 480 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 2.25 Chinese medicine Bushenhuoxue recipe plus conventional western medicine versus conventional western medicine (BMD of lumbar spine, 3 months) | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.16 [0.09, 0.22] |
| 2.26 Gusongbao granule plus calcium tablet versus placebo plus calcium tablet (BMD of lumbar spine, 6 months) | 1 | 240 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 2.27 Shengsuiyin recipe plus Caltrate versus Caltrate (BMD of Lumbar spine, 12 months) | 1 | 144 | Mean Difference (IV, Random, 95% CI) | 0.31 [0.28, 0.34] |
| 2.28 Yigu capsule plus calcium versus placebo plus calcium (BMD of lumbar spine, 6 months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.04, 0.12] |
| 2.29 Chinese medicine (combination of 10 herbs) plus tamoxifen and Caltrate versus tamoxifen and Caltrate (BMD of lumbar spine, 3 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.06, 0.14] |
| 2.30 Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin versus calcitonin (BMD of lumbar spine, 12 months) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 2.31 Migu tablet plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule (BMD of lumbar spine, 6 months) | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 0.17 [0.14, 0.20] |
| 2.32 Huangqi plus Caltrate versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.01, 0.11] |
| 2.33 Jiarong tablet plus Caltrate versus Caltrate (BMD of femoral neck, 6 months) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.02, 0.15] |
| 2.34 Antai capsule plus Caltrate versus Caltrate (BMD of femoral neck, 6 months) | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 2.35 Xianlinggubao capsule plus Caltrate versus Caltrate (BMD of femoral neck, 12 months) | 1 | 68 | Mean Difference (IV, Random, 95% CI) | 0.3 [0.25, 0.35] |
| 2.36 Guben Zhuanggu capsules plus Caltrate versus Caltrate (BMD of femoral neck, 6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 2.37 Migu tablet plus Caltrate versus Caltrate (BMD of femoral neck, 12 months) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.08, 0.10] |
| 2.38 Qianggu capsules plus oestradiol valerate versus oestradiol valerate (BMD of femoral neck, 6 months) | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 2.39 Chinese medicine Bushenhuoxue recipe plus conventional western medicine versus conventional western medicine (BMD of femoral neck, 3 months) | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.17 [0.12, 0.23] |
| 2.40 Heche Dazao pill plus oyster shell calcium chewable tablets versus oyster shell calcium chewable tablets (BMD of femoral neck, 3 months) | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.00, 0.07] |
| 2.41 Xianlinggubao capsule plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule (BMD of femoral neck, 6 months) | 1 | 106 | Mean Difference (IV, Random, 95% CI) | 0.26 [0.22, 0.30] |
| 2.42 Huangqi plus Caltrate versus Caltrate (BMD of femoral neck, 6 months) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.03, 0.11] |
| 2.43 Gusongbao granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule (BMD of femoral neck, 6 months) | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2.44 Jiangu granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule (BMD of femoral neck, 6 months) | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 2.45 Xuduanzhuanggu capsule plus calcium tablet versus placebo plus calcium tablet (BMD of femoral neck, 6 months) | 1 | 480 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 2.46 Gusongbao granule plus calcium tablet versus placebo plus calcium tablet (BMD of femoral neck, 6 months) | 1 | 240 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 2.47 Yigu capsule plus calcium versus placebo plus calcium (BMD of femoral neck, 6 months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.01, 0.07] |
| 2.48 Migu tablet plus compound calcium amino acid chelate capsule versus compound calcium amino acid chelate capsule (BMD of femoral neck, 6 months) | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 0.24 [0.21, 0.27] |
| 2.49 Antai capsule plus Caltrate versus Caltrate (BMD of Ward's, 6 months) | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 2.50 Migu tablet plus Caltrate versus Caltrate (BMD of Ward's, 12 months) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.08, 0.10] |
| 2.51 Qianggu capsule plus alendronate versus alendronate (BMD of Ward's, 6 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.02, 0.07] |
| 2.52 Xianlinggubao capsule plus alendronate versus alendronate (BMD of Ward's, 6 months) | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.02, 0.06] |
| 2.53 Gusongbao granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule (BMD of Ward's, 6 months) | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 2.54 Xianlinggubao capsule plus compound calcium amino acid chelate capsules versus compound calcium amino acid chelate capsules (BMD of Ward's, 6 months) | 1 | 106 | Mean Difference (IV, Random, 95% CI) | 0.23 [0.19, 0.27] |
| 2.55 Migu tablet plus compound calcium amino acid chelate capsules versus compound calcium amino acid chelate capsules (BMD of Ward's, 6 months) | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 0.28 [0.24, 0.32] |
| 2.56 Jiangu granule plus compound calcium amino acid chelate capsule versus placebo granule plus compound calcium amino acid chelate capsule (BMD of Ward's, 6 months) | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 2.57 Xuduanzhuanggu capsule plus calcium tablet versus placebo plus calcium tablet (BMD of Ward's, 6 months) | 1 | 480 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.00, 0.04] |
| 2.58 Gusongbao granule plus calcium tablet versus placebo plus calcium tablet (BMD of Ward's, 6 months) | 1 | 240 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 2.59 Yigu capsule plus calcium versus placebo plus calcium (BMD of Ward's, 6 months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.00, 0.08] |
| 2.60 Antai capsule plus Caltrate versus Caltrate (BMD of trochanter, 6 months) | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 0.03 [0.00, 0.06] |
| 2.61 Migu tablet plus Caltrate versus Caltrate (BMD of trochanter, 12 months) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.14, 0.16] |
| 2.62 Xuduanzhuanggu capsule plus calcium tablet versus placebo plus calcium tablet (BMD of trochanter, 6 months) | 1 | 480 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 2.63 Migu tablet plus compound calcium amino acid chelate capsules versus compound calcium amino acid chelate capsules (BMD of trochanter, 6 months) | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.07, 0.17] |
| 2.64 Xianlinggubao capsule plus compound calcium amino acid chelate capsules versus compound calcium amino acid chelate capsules (BMD of trochanter, 6 months) | 1 | 106 | Mean Difference (IV, Random, 95% CI) | 0.13 [0.08, 0.18] |
| 2.65 Gusongbao granule plus calcium tablet versus placebo plus calcium tablet (BMD of trochanter, 6 months) | 1 | 240 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 2.66 Yigu capsule plus calcium versus placebo plus calcium (BMD of trochanter, 6 months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 2.67 Gushukang granule plus Calcium carbonate with vitamin D chewable tablets versus Calcium carbonate with vitamin D chewable tablets (BMD of hip bone, 6 months) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.11, 0.19] |
| 2.68 Jinwugutong capsules plus calcium versus placebo plus calcium (BMD of hip bone, 6 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 2.69 Gengnian Anyi tablet plus Caltrate versus Caltrate (BMD of distal radius, 6 months) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.06, 0.08] |
| 2.70 Shangke Yishen Zhuanggu pill plus Caltrate and calcitonin versus Caltrate and calcitonin (BMD of lumbar spine, 3 months) | 1 | 300 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.03, 0.05] |
| 2.71 Bushenhuoxue therapy plus calcium carbonate tablets and alfacalcidol versus calcium carbonate tablets and alfacalcidol (BMD of lumbar spine, 3 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 2.72 Gukang tablet plus oyster shell calcium chewable tablets and vitamin A and D capsules versus oyster shell calcium chewable tablets and vitamin A and D capsules (BMD of lumbar spine, for 3 months) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.24 [0.14, 0.34] |
| 2.73 Liuwei Dihuang pills plus Caltrate versus Caltrate (BMD of lumbar spine, 6 months) | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.02, 0.08] |
| 2.74 Yiyuanjiangu decoction plus calcitonin and calcium carbonate tablet versus calcitonin and calcium carbonate tablet (BMD of lumbar spine,3 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 2.75 Yiyuanjiangu decoction plus calcitonin and calcium carbonate tablet versus calcitonin and calcium carbonate tablet (BMD of femoral neck, 3 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 2.76 Yiyuanjiangu decoction plus calcitonin and calcium carbonate tablet versus calcitonin and calcium carbonate tablet (BMD of trochanter, 3 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.02 [0.01, 0.04] |
| 2.77 Yishen Zhuanggu decoction plus calcitriol soft capsule versus calcitriol soft capsule (BMD of lumbar spine, 3 months) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.00, 0.05] |
| 2.78 Hugu capsule plus Caltrate versus placebo capsule plus Caltrate (BMD of lumbar spine, 6 months) | 1 | 122 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.00, 0.06] |
| 2.79 Hugu capsule plus Caltrate versus placebo capsule plus Caltrate (BMD of Ward's, 6 months) | 1 | 122 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.02, 0.08] |
| 2.80 Bushen Qianggu Huoxue therapy plus calcium and cod liver oil versus calcium and cod liver oil (BMD of lumbar spine, 3 months) | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.01, 0.11] |
| 2.81 Bushen Qianggu Huoxue therapy plus calcium and cod liver oil versus calcium and cod liver oil (BMD of femoral neck, 3 months) | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.02, 0.05] |
| 2.82 Bushen Qianggu Huoxue therapy plus calcium and cod liver oil versus calcium and cod liver oil (BMD of Ward's, 3 months) | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.02, 0.07] |
| 2.83 Xianlinggubao capsule (low‐dose) plus calcium and vitamin D versus placebo plus calcium and vitamin D (BMD of lumbar spine, 12 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 2.84 Xianlinggubao capsule (high‐dose) plus calcium and vitamin D versus placebo plus calcium and vitamin D (BMD of lumbar spine, 12 months) | 1 | 116 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 2.85 Xianlinggubao capsule (low‐dose) plus calcium and vitamin D versus placebo plus calcium and vitamin D (BMD of femoral neck, 12 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 2.86 Xianlinggubao capsule (high‐dose) plus calcium and vitamin D versus placebo plus calcium and vitamin D (BMD of femoral neck, 12 months) | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 2.87 Bushen Zhuanggu granules plus calcium and vitamin D versus placebo granules plus calcium and vitamin D (BMD of distal radius, 5 years) | 1 | 155 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.09, 0.10] |
| 3 T score in BMD measurement | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [0.57, 1.25] |
| 3.1 Erxian Yanggu decoction plus alendronate sodium tablets versus alendronate sodium tablets (T score, 6 months) | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [0.57, 1.25] |
| 4 Quality of life | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [3.67, 6.93] |
| 4.1 Bushenhuoxue therapy plus calcium carbonate tablets and alfacalcidol versus calcium carbonate tablets and alfacalcidol (3 months) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [3.67, 6.93] |
| 5 Symptoms including pain, muscle fatigue and limited mobility | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Heche Dazao pill plus oyster shell calcium chewable tablets versus oyster shell calcium chewable tablets (3 months) | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.01, 1.30] |
| 5.2 Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate (3 months) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.09, 1.61] |
| 5.3 Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin versus calcitonin (12 months) | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.12] |
| 5.4 Kanggusong soup plus Caltrate versus Caltrate (3 months) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.09, 2.02] |
| 6 Oestradiol (E2) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Gushen decoction plus Caltrate and Alfacalcidol versus Caltrate and Alfacalcidol (6 months) | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 4.54 [0.47, 8.61] |
| 6.2 Ziyin Bushen Zhuanggu prescription plus Caltrate and Calcitonin versus Caltrate plus Calcitonin (8 months) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 23.21 [14.46, 31.96] |
| 6.3 Zhuanggu capsule plus Caltrate versus Caltrate (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.06 [2.56, 5.56] |
| 6.4 Bushen Qiangshen pill plus Caltrate versus Caltrate (3 months) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 7.60 [‐0.51, 15.71] |
| 6.5 Antai capsule plus Caltrate versus Caltrate (6 months) | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 11.0 [8.14, 13.86] |
| 6.6 Zishen prescription plus Caltrate and calcitonin versus Caltrate plus calcitonin (8 months) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 23.21 [14.46, 31.96] |
| 6.7 Shugan zishen huoxue tang plus Caltrate and alendronate sodium tablets versus Caltrate plus alendronate sodium tablets (6 months) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 26.99 [18.44, 35.54] |
| 6.8 Xianlinggubao capsule plus vitamin D3 and calcium amino acid chelate versus vitamin D3 and calcium amino acid chelate (48 weeks) | 1 | 112 | Mean Difference (IV, Random, 95% CI) | 6.04 [4.61, 7.47] |
| 6.9 Yigu capsule plus calcium versus placebo plus calcium (6 months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 40.85 [33.61, 48.09] |
| 7 Serum calcium (Ca) | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Gushen decoction plus Caltrate and Alfacalcidol versus Caltrate and Alfacalcidol (6 months) | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.02, 0.12] |
| 7.2 Gushukang granule plus Calcium carbonate with vitamin D chewable tablets versus Calcium carbonate with vitamin D chewable tablets (6 months) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 7.3 Zhuanggu capsule plus Caltrate versus Caltrate (6 months) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.07, 0.09] |
| 7.4 Antai capsule plus Caltrate versus Caltrate (6 months) | 1 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.09, 0.03] |
| 7.5 Jinwugutong capsules plus calcium versus placebo plus calcium (6 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.11, 0.01] |
| 7.6 Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate (3 months) | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.09, 0.15] |
| 7.7 Bushenyiqihuoxue soup plus tamoxifen and Caltrate versus tamoxifen and Caltrate (3 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.37 [0.32, 0.42] |
| 7.8 Yigu capsule plus calcium versus placebo plus calcium (6 months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.13, ‐0.01] |
| 7.9 Chinese medicine (combination of 10 herbs) plus tamoxifen and Caltrate versus tamoxifen and Caltrate (3 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.37 [0.32, 0.42] |
| 7.10 Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin versus calcitonin (12 months) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 7.11 Huangqi plus Caltrate versus Caltrate (6 months) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.28 [0.11, 0.45] |
| 7.12 Yiyuanjiangu decoction plus calcitonin and calcium carbonate tablet versus calcitonin and calcium carbonate tablet (3 months) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 8 Phosphorus (P) | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Gushen decoction plus Caltrate and Alfacalcidol versus Caltrate and Alfacalcidol (6 months) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.27, 0.01] |
| 8.2 Gushukang granule plus Calcium carbonate with vitamin D chewable tabletsl versus Calcium carbonate with vitamin D chewable tablets (6 months) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 8.3 Zhuanggu capsule plus Caltrate versus Caltrate (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.06, 0.08] |
| 8.4 Antai capsule plus Caltrate versus Caltrate (6 months) | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.23, 0.21] |
| 8.5 Jinwugutong capsules plus calcium versus placebo plus calcium (6 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 8.6 Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate (3 months) | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.12, 0.14] |
| 8.7 Yigu capsule plus calcium versus placebo plus calcium (6 months) | 1 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.08, 0.02] |
| 8.8 Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin versus calcitonin (12 months) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 8.9 Huangqi plus Caltrate versus Caltrate (6 months) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.14, 0.48] |
| 8.10 Yiyuanjiangu decoction plus calcitonin and calcium carbonate tablet versus calcitonin and calcium carbonate tablet (3 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.22, 0.34] |
| 9 Alkaline phosphatase (ALP) | 15 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Gushukang granule plus Calcium carbonate with vitamin D chewable tablets versus Calcium carbonate with vitamin D chewable tablets (6 months) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.70, 0.70] |
| 9.2 Zhuanggu capsule plus Caltrate versus Caltrate (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.55, 1.55] |
| 9.3 Bushen Qiangshen pill plus Caltrate versus Caltrate (3 months) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.72, 1.10] |
| 9.4 Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate (3 months) | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐1.52, ‐0.46] |
| 9.5 Antai capsule plus Caltrate versus Caltrate (6 months) | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [0.55, 2.01] |
| 9.6 Traditional Chinese capsule plus calcium gluconate versus calcium gluconate (9 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.77 [1.20, 2.34] |
| 9.7 Jinwugutong capsules plus calcium versus placebo plus calcium (6 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 1.01 [‐0.04, 2.06] |
| 9.8 Yigu capsule plus calcium versus placebo plus calcium (6 months) | 1 | 140 | Mean Difference (IV, Fixed, 95% CI) | 1.73 [0.71, 2.75] |
| 9.9 Chinese medicine (combination of 10 herbs) plus tamoxifen and Caltrate versus tamoxifen and Caltrate (3 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐1.64, 1.12] |
| 9.10 Zhuangguqiangjin tablet and Shujinbogu tablet plus calcitonin versus calcitonin (12 months) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.54, 0.70] |
| 9.11 Bushenyiqihuoxue soup plus tamoxifen and Caltrate versus tamoxifen and Caltrate (3 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 3.54 [1.68, 5.40] |
| 9.12 Huangqi plus Caltrate versus Caltrate (6 months) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.82 [0.63, 1.01] |
| 9.13 Xianlinggubao capsule plus vitamin D3 and calcium amino acid chelate versus vitamin D3 and calcium amino acid chelate (48 weeks) | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 10.48 [9.81, 11.15] |
| 9.14 Kanggusong soup plus Caltrate versus Caltrate (3 months) | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐1.61 [‐3.17, ‐0.05] |
| 9.15 Yiyuanjiangu decoction plus calcitonin and calcium carbonate tablet versus calcitonin and calcium carbonate tablet (3 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.48, 1.10] |
| 10 Bone Gla protein (BGP) | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Zhuanggu capsule plus Caltrate versus Caltrate (6 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.3 [‐4.07, ‐0.53] |
| 10.2 Traditional Chinese capsule plus calcium gluconate versus calcium gluconate (9 months) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [0.88, 1.58] |
| 10.3 Xianlinggubao capsule plus salmon calcitonin and Caltrate versus salmon calcitonin plus Caltrate (3 months) | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [‐0.30, 1.42] |
| 10.4 Yigu capsule plus calcium versus placebo plus calcium (6 months) | 1 | 140 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [1.66, 3.54] |
| 10.5 Xianlinggubao capsule plus vitamin D3 and calcium amino acid chelate versus vitamin D3 and calcium amino acid chelate (48 weeks) | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 6.24 [5.69, 6.79] |
| 10.6 Xianlinggubao capsule plus Caltrate versus Caltrate (12 months) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 1.85 [0.87, 2.83] |
| 10.7 Jinwugutong capsules plus calcium versus placebo plus calcium (6 months) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 2.47 [1.44, 3.50] |
| 10.8 Huangqi plus Caltrate versus Caltrate (6 months) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [0.50, 0.76] |
| 11 Interleukin‐6 (IL‐6) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Erxian Yanggu decoction plus alendronate sodium tablets versus alendronate sodium tablets (6 months) | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐16.18 [‐19.62, ‐12.74] |
| 11.2 Xianlinggubao capsule plus vitamin D3 and calcium amino acid chelate versus vitamin D3 and calcium amino acid chelate (48 weeks) | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐133.3 [‐139.94, ‐126.66] |
| 11.3 Kanggusong soup plus Caltrate versus Caltrate (3 months) | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐16.32 [‐31.60, ‐1.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
An SJ 2000.
| Methods | Country: China Setting: hospital based Aim: to observe the therapeutic effect and mechanism of kidney‐tonifying herbs on postmenopausal osteoporosis Study design: double‐blind, randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 111 postmenopausal osteoporosis patients enrolled: 31 in Kanggusong granule group, 40 in ipriflavone group, 40 in Caltrate group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels recommended by WHO; BMD detected by DXA Exclusion criteria: secondary osteoporosis |
|
| Interventions | Experimental: Kanggusong granule, 12 g, twice daily, for 9 months Control 1: ipriflavone (200 mg, 3 times a day) and Caltrate (1 tablet per day), for 9 months Control 2: Caltrate (1 tablet per day), for 9 months |
|
| Outcomes | BMD, E2, Ca, P, ALP, fractures | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | It was described as double‐blind but did not describe who was blinded |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Cao GY 2010.
| Methods | Country: China Setting: hospital based Aim: to observe the therapeutic effect of integrated traditional Chinese and western medicine on primary osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese
57 primary osteoporosis patients enrolled: 30 in trial group, 27 in control group Inclusion criteria: WHO diagnostic criteria for osteoporosis based on bone density levels Exclusion criteria: secondary osteoporosis |
|
| Interventions | Experimental: Chinese medicine Bushenhuoxue recipe (1 dose per day, water fried) and conventional western medicine (alfacalcidol soft capsules, bisphosphonate and salmon calcitonin, etc.) based on the patient's condition, for 3 months Control: conventional western medicine based on the patient's condition, for 3 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No detailed description and P value for the baseline comparison not stated |
Cao W 2010.
| Methods | Country: China Setting: hospital based Aim: to observe the therapeutic effect of integrated traditional Chinese and western medicine on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese
120 postmenopausal osteoporosis patients enrolled: 60 in trial group, 60 in control group Inclusion criteria: WHO diagnostic criteria for osteoporosis based on bone density levels Exclusion criteria: serious women's illnesses, malignant tumour, diabetes, etc. |
|
| Interventions | Experimental: Chinese medicine (combinations of herbs) (1 dose per day, water fried 300 ml, twice a day), tamoxifen (20 mg per day) and Caltrate (600 mg, 1 to 2 tablets per day), for 3 months Control: tamoxifen (20 mg per day) and Caltrate (600 mg, 1 to 2 tablets per day), for 3 months |
|
| Outcomes | BMD, Ca, ALP, adverse events | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk'. |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Chen FS 2001.
| Methods | Country: China Setting: outpatients and inpatients Aim: to observe the therapeutic effect of integrated traditional Chinese and western medicine on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 64 postmenopausal osteoporosis patients enrolled: 32 in trial group, 32 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Gushen decoction (1 dose per day), Caltrate (600 mg per day) and Alfacalcidol (0.25 μg per day), for 24 weeks Control: Caltrate (600 mg per day) and Alfacalcidol (0.25 μg per day), for 24 weeks |
|
| Outcomes | BMD, E2, Ca, P, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Chen JP 1999.
| Methods | Country: China Setting: outpatients Aim: to observe the therapeutic effect of Gukang oral liquid on postmenopausal osteoporosis Study design: single‐blind, randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese
61 postmenopausal osteoporosis patients enrolled: 36 in trial group, 25 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by single photon absorptiometry (SPA) Exclusion criteria: secondary osteoporosis, etc. |
|
| Interventions | Experimental: Gukang oral liquid (a basic herbal compound), 10 ml, 3 times a day, for 6 months Control: Tridin, 1 tablet, 3 times a day, for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Single‐blind but did not describe who was blinded |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Chen NQ 2006.
| Methods | Country: China Setting: outpatients Aim: to observe the therapeutic effect of Shenyao capsules on primary osteoporosis Study design: single‐blind, randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 100 primary osteoporosis patients enrolled: 50 in trial group, 50 in control group Inclusion criteria: WHO diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: secondary osteoporosis, endocrine disease |
|
| Interventions | Experimental: Shenyao capsules (2 capsules, 3 times a day) for 6 months Control: Caltrate (600 mg, once a day), for 6 months |
|
| Outcomes | BMD, Ca, P, ALP, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Single‐blind but did not describe who was blinded |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No detailed description and P value for baseline comparison not stated |
Chen X 2008.
| Methods | Country: China Setting: hospital based Aim: to observe the therapeutic effect of Gukang decoction on primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: China 83 primary osteoporosis patients enrolled: 42 in trial group, 41 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Gukang decoction, 1 dose per day, for 4 months Control: alendronate sodium tablets, 10 mg, once a day, for 4 months |
|
| Outcomes | BMD, E2, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Sequence generated by some rule based on clinic time |
| Allocation concealment | High risk | Allocation based on clinic time |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Chen XL 2010.
| Methods | Country: China Setting: outpatients Aim: to observe the therapeutic effect of Heche Dazao pill on primary osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 114 primary osteoporosis patients enrolled: 57 in trial group, 57 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: liver or kidney disease, endocrine diseases, etc. |
|
| Interventions | Experimental: Heche Dazao pill (1 dose per day, water fried twice, twice a day) and Gaitianli (300 mg, 3 times a day), for 3 months Control: Gaitianli (300 mg, 3 times a day), for 3 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Chen YQ 2001.
| Methods | Country: China Setting: outpatients Aim: to observe the therapeutic effect of Jiarong tablet on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 61 postmenopausal osteoporosis patients enrolled: 20 in trial group, 20 in Livial group, 21 in Caltrate group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Jiarong tablet (5 g, 3 times a day) and Caltrate (1 tablet per day), for 6 months Control: Livial (2.5 mg, every other day) and Caltrate (1 tablet per day), or Caltrate (1 tablet per day), for 6 months | |
| Outcomes | BMD, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline comparison |
Chen ZX 2002.
| Methods | Country: China Setting: hospital based Aim: to observe the therapeutic effect of Gushukang granule on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese
62 postmenopausal osteoporosis patients enrolled: 23 in Gushukang granule and Calcium carbonate with vitamin D chewable tablets group, 20 in Gushukang granule group, 19 in Calcium carbonate with vitamin D chewable tablets group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels recommended by WHO, BMD detected by DXA Exclusion criteria: secondary osteoporosis |
|
| Interventions | Experimental: Gushukang granule (10 g, twice a day) and Calcium carbonate with vitamin D chewable tablets (1 tablet, twice a day), or Gushukang granule (10 g, twice a day), for 6 months Control: Calcium carbonate with vitamin D chewable tablets, 1 tablet, twice a day, for 6 months |
|
| Outcomes | BMD, Ca, P, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers table |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Cui TH 1999.
| Methods | Country: China Setting: hospital based Aim: to observe the therapeutic effect of Strong Bone capsule on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 105 postmenopausal osteoporosis patients enrolled: 74 in trial group, 31 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels recommended by WHO; BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Strong Bone capsule (a basic herbal compound), 5 capsules, 3 times a day, for 3 months Control: Caltrate, 1 tablet per day, for 3 months |
|
| Outcomes | BMD, E2, Ca, P, ALP, BGP, CT, adverse effects | |
| Notes | The study was supported by administration of traditional Chinese medicine scientific research funds in Jiangsu Province, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline comparison |
Dai Y 2007.
| Methods | Country: China Setting: hospital based Aim: to observe the therapeutic effect of Migu tablet on BMD, OPG, etc. in patients with postmenopausal osteoporosis Study design: randomised controlled trial Analysis: analysis of variance (F‐test) Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 160 postmenopausal osteoporosis patients enrolled: 54 in Migu tablet trial group, 53 in Xianlinggubao group, 53 in compound calcium amino acid chelate capsules group Inclusion criteria: diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: disease‐affected bone metabolism, endocrine diseases, etc |
|
| Interventions | Experimental: Migu tablet group: Migu tablet, 1 tablet, 3 times a day and compound calcium amino acid chelate capsule, 1 g, once a day; for 24 weeks Xianlinggubao group: Xianlinggubao capsule, 0.5, g 3 times a day and compound calcium amino acid chelate capsule, 1 g, once a day, for 24 weeks Control: compound calcium amino acid chelate capsule, 1 g, once a day, for 24 weeks |
|
| Outcomes | BMD | |
| Notes | The study was supported by a health department funded project in Hubei Province, but no declarations of interest for the primary researchers was reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline comparison |
Deng WM 2012.
| Methods | Country: China Setting: hospital based Aim: to observe the kidney‐tonifying herbal Fufangs with phytoestrogenic Epimedium for prevention of postmenopausal osteoporosis with both BMD and fracture as study endpoints Study design: a multicentre, double‐blind, randomised controlled trial Analysis: F‐test, Chi2 test based on the intention‐to‐treat (ITT) principle Loss to follow‐up: 5‐year follow‐up. At the end of 5 years, 155 participants had completed the study (13 drop‐outs in the treatment group, 26 drop‐outs in the control group); the difference in drop‐out rate was statistically significant between the 2 groups (P < 0.05) |
|
| Participants | Ethnicity: Chinese 194 postmenopausal osteoporosis patients enrolled: 101 in experimental group, 93 in placebo group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: those who used any kind of anti‐osteoporosis drugs or had been under HRT during the past year; current medical disease(s) associated with potential development of metabolic bone diseases, including Paget disease, osteomalacia, bone marrow disease, hereditary disorders of calcium or mineral metabolism, adrenal disorders, poorly controlled diabetes mellitus, severe connective tissue disorders, gastrointestinal diseases and current or past history of clinically significant haematological, endocrinological, cardiovascular, renal, hepatic, gastrointestinal, psychiatric or neurological diseases |
|
| Interventions | Experimental: Bushen Zhuanggu granules (10 g per day, twice per day), plus calcium (600 mg) and vitamin D (400 IU) Control: placebo granules (10 g per day, twice per day), plus calcium (600 mg) and vitamin D (400 IU) |
|
| Outcomes | BMD, fracture, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline comparison |
Dong Y 2010.
| Methods | Country: China Setting: outpatients Aim: to evaluate the efficacy and safety of salmon calcitonin plus Xianlinggubao for osteoporosis and ostealgia in postmenopausal women Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 160 postmenopausal osteoporosis patients enrolled: 54 in Xianlinggubao plus salmon calcitonin group, 53 in Xianlinggubao group, 53 in salmon calcitonin control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: disease‐affected bone metabolism, endocrine diseases, secondary osteoporosis, liver or kidney disease, etc. |
|
| Interventions | Experimental: Xianlinggubao plus salmon calcitonin group: Xianlinggubao (1.5 g, twice a day), salmon calcitonin (500 IU, once a day) and Caltrate (1 tablet per day), for 3 months Xianlinggubao group: Xianlinggubao (1.5 g, twice a day) and Caltrate (1 tablet per day), for 3 months Control: salmon calcitonin (500 IU, once a day) and Caltrate (1 tablet per day), for 3 months |
|
| Outcomes | BMD, ALP, BGP, Ca, P, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Dong YF 2004.
| Methods | Country: China Setting: outpatients Aim: to observe the therapeutic effect of traditional Chinese herbs on primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 80 senile osteoporosis patients enrolled: 40 in trial group, 40 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: endocrine diseases, secondary osteoporosis, etc. |
|
| Interventions | Experimental: traditional Chinese herbs (Longspur Epimedium, etc.), 1 dose per day, for 6 months Control: vitamin D (10000 U, once a day), Caltrate (600 mg, once a day), for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Fan HX 2004.
| Methods | Country: China Setting: outpatients Aim: to observe the therapeutic effect of Gengnian Anyi tablet on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 postmenopausal osteoporosis patients enrolled: 22 in herb trial group, 18 in nilestriol control group, 20 in Caltrate group Inclusion criteria: international diagnostic criteria for osteoporosis, BMD detected by SPA Exclusion criteria: endocrine diseases, etc. |
|
| Interventions | Experimental: Gengnian Anyi tablet (6 tablets, 3 times a day) and Caltrate (1 tablet per day), for 6 months Control: nilestriol (2 mg, once a half month) and Caltrate (1 tablet per day), or Caltrate (1 tablet per day), for 6 months |
|
| Outcomes | BMD, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline comparison |
Gang PH 2001.
| Methods | Country: China Setting: outpatients Aim: to observe the therapeutic effect of Gumiling granule on primary osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 25 primary osteoporosis patients enrolled: 15 in herb trial group, 10 in control group Inclusion criteria: BMD, detected by DXA Exclusion criteria: secondary osteoporosis, liver or kidney disease, etc. |
|
| Interventions | Experimental: Gumiling granule (10 g, 3 times a day), for 6 months Control: bicarbonate calcium chewable(1 g, twice a day), for 6 months |
|
| Outcomes | BMD, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline comparison |
Gong L 2001.
| Methods | Country: China Setting: outpatients Aim: to observe the therapeutic effect of Yishen Zhuanggu mixture on primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, F‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 80 primary osteoporosis patients enrolled: 46 in herb trial group, 17 in herb control group, 17 in blank control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine diseases, etc. |
|
| Interventions | Experimental: Yishen Zhuanggu mixture (25 ml, twice a day) in herb trial group, Shengu capsule (2 capsules, 3 times a day) in herb control group, for 6 months Control: blank |
|
| Outcomes | BMD, BGP, adverse effects | |
| Notes | The study was supported by a Science and Technology Commission funded project in Beijing, but no declarations of interest of the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Guo JH 2008.
| Methods | Country: China Setting: outpatients Aim: to explore the effect of Bushen Kangsong pill on BMD in postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 84 postmenopausal osteoporosis patients enrolled: 42 in trial group, 42 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: disease‐affected bone metabolism, liver or kidney disease, etc. |
|
| Interventions | Experimental: Bushen Kangsong pill, 3 g, 3 times a day, calcium carbonate, 4 tablets, 3 times a day, for 6 months Control: calcium carbonate, 4 tablets, 3 times a day, for 6 months |
|
| Outcomes | BMD | |
| Notes | The study was supported by the Science Foundation for Post‐doctoral Scientists of Jiangsu Province, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Han XL 2007.
| Methods | Country: China Setting: outpatients and inpatients Aim: to explore the effect of Shugan Zishen Huoxue tang on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 42 postmenopausal osteoporosis patients enrolled: 25 in herb trial group, 17 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine diseases, etc. |
|
| Interventions | Experimental: Shugan Zishen Huoxue tang (100 ml, twice a day), Caltrate (1.2 g, twice a day) and alendronate sodium (10 mg, once a day), for 6 months Control: Caltrate (1.2 g, twice a day) and alendronate sodium tablets (10 mg, once a day), for 6 months |
|
| Outcomes | BMD, E2 | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
He MT 2007.
| Methods | Country: China Setting: outpatients Aim: to explore the effect of Bushen Jianpi Huoxue recipe on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 160 postmenoporosis patients enrolled: 80 in herb trial group, 80 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine diseases, fractures, metabolic disease, etc. |
|
| Interventions | Experimental: Bushen Jianpi Huoxue recipe (1 dose per day), for 6 months Control: alendronate sodium tablets (70 mg per week), for 6 months |
|
| Outcomes | BMD, IL‐6, BGP, ALP, E2 | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
He N 2006.
| Methods | Country: China Setting: hospital based Aim: to explore the effect of Jiawei Zhuangyao Jianshen tang on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 58 postmenopausal osteoporosis patients enrolled: 29 in trial group, 29 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Jiawei Zhuangyao Jianshen tang (1 dose per day) for 3 months Control: compound calcium amino acid chelate capsules (1 g, once a day), for 3 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
He XQ 2006.
| Methods | Country: China Setting: outpatients and inpatients Aim: to explore the effect of Bushen Yangxue tang on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 postmenopausal osteoporosis patients enrolled: 32 in trial group, 28 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: endocrine disease, liver or kidney disease |
|
| Interventions | Experimental: Bushen Yangxue tang (1 dose per day) and Caltrate (once a day), for 6 months Control: Caltrate (once a day), for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Hu J 2012.
| Methods | Country: China Setting: inpatients Aim: to explore the effect of Shangke Yishen Zhuanggu pill on primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, F‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 300 primary osteoporosis patients enrolled: 155 in trial group, 145 in control group Inclusion criteria: WHO diagnostic criteria for osteoporosis Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Shangke Yishen Zhuanggu pill (6 g, 3 times a day), Caltrate (1.2 g, twice a day), and miacalcic ampoule (50 U, 3 times a day, intramuscular injection), for 3 months Control: Caltrate (1.2 g, twice a day), and miacalcic ampoule (50 U, 3 times a day, intramuscular injection), for 3 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Huang JW 2008.
| Methods | Country: China Setting: hospital based Aim: to explore the effect of Erxian Yanggu decoction on postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 67 postmenopausal osteoporosis patients enrolled: 34 in trial group, 33 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine diseases, secondary osteoporosis, patients with uterine/breast disease |
|
| Interventions | Experimental: Erxian Yanggu decoction, 1 dose per day, alendronate sodium tablets, 70 mg per week, for 6 months Control: alendronate sodium tablets, 70 mg per week, for 6 months |
|
| Outcomes | IL‐6 | |
| Notes | The study was supported by administration of traditional Chinese medicine scientific research funds in Zhejiang Province, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Huang ZJ 2008.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Zishen Gukang pill in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 postmenopausal osteoporosis patients enrolled: 30 in trial group, 30 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA, DPA, SPA, etc. Exclusion criteria: disease‐affected bone metabolism, etc. |
|
| Interventions | Experimental: Zishen Gukang pill (6 g, 3 times a day) Control: Caltrate (2 pills, once a day), for 3 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Sequence generated by some rule based on clinic time |
| Allocation concealment | High risk | Allocation based on clinic time |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Jian QQ 2010.
| Methods | Country: China Setting: inpatients Aim: to study the effects of Shengsuiyin recipe in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 144 postmenopausal osteoporosis patients enrolled: 73 in trial group, 71 in control group Inclusion criteria: diagnostic criteria for osteoporosis on Obstetrics and Gynecology in Chinese Medicine Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Shengsuiyin recipe (1 dose per day, water fried) and Caltrate (750 mg, once a day), for 12 months Control: Caltrate, 750 mg, once a day, for 12 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Lan ZX 2006.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Guli powder in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 primary osteoporosis patients enrolled: 30 in trial group, 30 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: disease‐affected bone metabolism |
|
| Interventions | Experimental: Guli powder (10 g, 3 times a day), for 3 months Control: Caltrate (once a day), for 3 months |
|
| Outcomes | BMD, Ca, P, ALP | |
| Notes | The study was supported by administration of traditional Chinese medicine scientific research funds in Sichuan Province, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Li BL 2007.
| Methods | Country: China Setting: hospital based Aim: to observe the mechanism of Jianshen decoction in treating patients with postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 postmenopausal osteoporosis patients enrolled: 30 in trial group, 30 in control group Inclusion criteria: diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: endocrine diseases, disease‐affected bone metabolism |
|
| Interventions | Experimental: Jianshen decoction, 1 dose per day, for 6 months Control: alendronate sodium tablets, 10 mg, once a day, for 6 months |
|
| Outcomes | BMD, ALP, BGP, E2, IL‐6 | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Li HW 2004.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Ziyin Bushen Zhuanggu prescription in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 45 postmenopausal osteoporosis patients enrolled: 25 in trial group, 20 in control group Inclusion criteria: international diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: endocrine diseases, etc |
|
| Interventions | Experimental: Ziyin Bushen Zhuanggu prescription (1 dose per day), Caltrate (1.2 g, twice a day) and miacalcin (50 IU, injection, twice per week), for 8 months Control: Caltrate (1.2 g, twice a day) and miacalcin (50 IU, injection, twice a week), for 8 months |
|
| Outcomes | BMD, E2 | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Li SL 2004.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Zhuanggu capsule in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 postmenopausal osteoporosis patients enrolled: 30 in trial group, 30 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine diseases, etc |
|
| Interventions | Experimental: Zhuanggu capsule (4 capsules, 3 times a day) and Caltrate (600 mg, once a day), for 6 months Control: Caltrate (600 mg, once a day), for 6 months |
|
| Outcomes | BMD, E2, Ca, P, ALP, BGP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Li YH 2008.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Jingujian granule in the treatment of senile osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 120 senile osteoporosis patients enrolled: 80 in trial group, 40 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels recommended by WHO Exclusion criteria: secondary osteoporosis, liver or kidney disease |
|
| Interventions | Experimental: Jingujian granule, 8 g, twice a day, for 3 months Control: Caltrate, 600 mg, once a day, for 3 months |
|
| Outcomes | BMD, ALP, BGP, adverse effects | |
| Notes | The study was supported by a China academy of traditional Chinese medicine advantage diseases research project, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Li YH 2010.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Jingujian granule 1, 2 or 3 in the treatment of primary osteoporosis based on epigastralgia traditional Chinese medicine Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: 30 drop‐outs (11 in trial group, 19 in control group) were not included in the analysis |
|
| Participants | Ethnicity: Chinese 210 primary osteoporosis patients enrolled: 140 in trial group (11 drop‐outs), 70 in control group (19 drop‐outs) Inclusion criteria: Chinese diagnostic criteria for osteoporosis and guiding principles for clinical research on new drugs in traditional Chinese medicine, BMD detected by DXA Exclusion criteria: secondary osteoporosis, liver or kidney disease, etc. |
|
| Interventions | Experimental: Jingujian granule 1, 2 or 3 based on epigastralgia traditional Chinese medicine (kidney deficiency; spleen and kidney deficiency; spleen and kidney deficiency, and blood stasis), 8 g, twice a day, for 3 months Control: Caltrate, 600 mg, once a day, for 3 months |
|
| Outcomes | BMD, ALP, E2, CT, BGP, adverse events | |
| Notes | The study was supported by a China academy of traditional Chinese medicine advantage diseases research project, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Li ZP 2011.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Kanggusong soup in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 75 postmenopausal osteoporosis patients enrolled: 45 in trial group, 30 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: secondary osteoporosis, endocrine disease, liver or kidney disease, etc. |
|
| Interventions | Experimental: Kanggusong soup (1 dose per day, twice a day) and Caltrate (1 tablet per night), for 3 months Control: Caltrate (1 tablet per night), for 3 months |
|
| Outcomes | ALP, IL‐6, fractures | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Li ZY 2006.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Xianling Gusong capsules in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 43 postmenopausal osteoporosis patients enrolled: 21 in trial group, 22 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis, liver or kidney disease |
|
| Interventions | Experimental: Xianling Gusong capsules (4 capsules, 3 times a day), for 6 months Control: Caltrate (1 pill, twice a day), for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Liang DB 2012.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Bushenhuoxue therapy in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 80 primary osteoporosis patients enrolled: 40 in trial group, 40 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: secondary osteoporosis, liver or kidney disease, etc. |
|
| Interventions | Experimental: Bushenhuoxue therapy (1 dose, per day), calcium carbonate tablets (1 tablet (0.5 g), twice a day), and alfacalcidol (1 tablet (0.5 μg) per day), for 3 months Control: calcium carbonate tablets (1 tablet (0.5 g), twice a day), and alfacalcidol (1 tablet (0.5 μg) per day), for 3 months |
|
| Outcomes | BMD, quality of life, adverse events | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | No information provided |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Liao L 2004.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Bushen Shengsui principle in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 100 postmenopausal osteoporosis patients enrolled: 32 in herb trial group, 39 in control group, 29 in blank group Inclusion criteria: diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: endocrine diseases, etc. |
|
| Interventions | Experimental: Bushen Shengsui principle, 1 dose per day, for 6 months Control: Premarin (1 tablet per day) and medroxyprogesterone (2.5 mg per day), or blank, for 6 months |
|
| Outcomes | BMD, E2, adverse effects | |
| Notes | The study was supported by a science and technology planning project Zhongshan, Guangdong Province, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Lin W 2000.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Migu decoction in the treatment of postmenopausal osteoporosis Study design: double‐blind, randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 20 postmenopausal osteoporosis patients enrolled: 13 in trial group, 7 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine diseases |
|
| Interventions | Experimental: Migu decoction, for 4 months Control: placebo, for 4 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Double‐blind but did not describe who was blind |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Ling JY 2008.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Bushen Zhuanggu decoction in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 primary osteoporosis patients enrolled: 30 in trial group, 30 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: endocrine diseases, liver or kidney disease, etc |
|
| Interventions | Experimental: Bushen Zhuanggu decoction, 1 dose per day, for 3 months Control: alendronate sodium tablets, 10 mg, once a day, for 3 months |
|
| Outcomes | BMD, E2, ALP, P | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Liu JM 2012.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Gukang tablet in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 80 primary osteoporosis patients enrolled: 40 in trial group, 40 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: endocrine diseases, liver or kidney disease, etc. |
|
| Interventions | Experimental: Gukang tablet (0.5 g per tablet, 6, 3 times a day), Gaitianli tablets (50 mg per tablet, 2 tablets, 3 times a day) and vitamin A and D capsules (1 pill, twice a day), for 3 months Control: Gaitianli tablets (50 mg per tablet, 2 tablets, 3 times a day), and vitamin A and D capsules (1 pill, twice a day), for 3 months |
|
| Outcomes | BMD, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Lv ZH 2002.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Jiawei Bushen Zhuangjintang in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 90 primary osteoporosis patients enrolled: 48 in trial group, 42 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Jiawei Bushen Zhuangjintang, 1 dose per day, for 3 months Control: calcium granule, 5 g, 3 times a day, for 3 months |
|
| Outcomes | BMD | |
| Notes | The study was supported by administration of traditional Chinese medicine scientific research funds in Guangdong Province, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Ma C 2011.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Liuwei Dihuang pills in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: F‐test, Chi2 test Loss to follow‐up: 1 drop‐out in Liuwei Dihuang pills group was not included in the analysis |
|
| Participants | Ethnicity: Chinese 108 postmenopausal osteoporosis patients enrolled: 36 in Liuwei Dihuang pills group, 36 in Caltrate group, 36 in Liuwei Dihuang pills plus Caltrate group Inclusion criteria: diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: endocrine diseases, etc. |
|
| Interventions | Liuwei Dihuang pills group: Liuwei Dihuang pills, 8 pills, 3 times a day, for 6 months Caltrate group: Caltrate, 1 tablet, 3 times a day, for 6 months Liuwei Dihuang pills plus Caltrate group: Liuwei Dihuang pills (8 pills, 3 times a day) and Caltrate (1 tablet, 3 times a day), for 6 months |
|
| Outcomes | BMD, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Ma CZ 2011.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Yiyuanjiangu decoction in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 120 primary osteoporosis patients enrolled: 60 in trial group, 60 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: endocrine diseases, liver or kidney disease, etc. |
|
| Interventions | Experimental: Yiyuanjiangu decoction (1 dose per day), Miacalcin ampoule (50 U, once a day, intramuscular), and calcium carbonate tablet (1 tablet (0.75 g), twice a day), for 3 months Control: Miacalcin ampoule (50 U, once a day, intramuscular) and calcium carbonate tablet (1 tablet (0.75 g), twice a day), for 3 months |
|
| Outcomes | BMD, ALP, Ca, P | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Ma YJ 2011.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Yishen Zhuanggu decoction in the treatment of senile osteoporosis Study design: randomised controlled trial Analysis: T‐test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 100 senile osteoporosis enrolled: 50 in trial group, 50 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: secondary osteoporosis, endocrine diseases, liver or kidney disease, etc. |
|
| Interventions | Experimental: Yishen Zhuanggu decoction (1 dose per day) and calcitriol soft capsule (0.25 mg, twice a day), for 3 months Control: calcitriol soft capsule (0.25 mg, twice a day), for 3 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Mao YF 2011.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Bushen Jianpi Jingu decoction in the treatment of postmenopausal osteoporosis Study design: single‐blind, randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 75 postmenopausal osteoporosis patients enrolled: 39 in trial group, 36 in control group Inclusion criteria: diagnostic criteria for osteoporosis recommend by WHO, BMD detected by DXA Exclusion criteria: liver or kidney disease, disease‐affected bone metabolism, etc. |
|
| Interventions | Experimental: Bushen Jianpi Jingu decoction, 1 dose per day, for 3 months Control: Miacalcin ampoule (50 U), for 3 months |
|
| Outcomes | BGP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Single‐blind but did not describe who was blind |
| Incomplete outcome data addressed All outcomes | Unclear risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Meng XD 2003.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Gujian capsule in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 68 primary osteoporosis patients enrolled: 35 in trial group (1 drop‐out), 33 in control group (2 drop‐out) Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: secondary osteoporosis, liver or kidney disease, etc. |
|
| Interventions | Experimental: Gujian capsule (2 capsules, 3 times a day), for 6 months Control: alfacalcidol (0.5 μg, twice a day), for 6 months |
|
| Outcomes | BMD, PTH, CT, E2, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Miu JQ 2008.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of a traditional Chinese medicine capsule in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 90 postmenopausal osteoporosis patients enrolled: 60 in trial group, 30 in control group Inclusion criteria: diagnostic criteria for osteoporosis recommend by WHO, BMD detected by DXA Exclusion criteria: secondary osteoporosis, liver or kidney disease |
|
| Interventions | Experimental: traditional Chinese medicine capsule (300 mg per pill, 5 pills per day), calcium gluconate, 2 g, 3 times a day, for 9 months Control: calcium gluconate, 2 g, 3 times a day, for 9 months |
|
| Outcomes | BGP, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Mu G 2001.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Bushen Qiangshen pill in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 40 postmenopausal osteoporosis patients enrolled: 20 in trial group, 20 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by CUBA ultrasound absorptiometry Exclusion criteria: endocrine diseases, secondary osteoporosis |
|
| Interventions | Experimental: Bushen Qiangshen pill (1 pill, twice a day) and Caltrate (1 tablet, twice a day), for 12 weeks Control: Caltrate, 1 tablet, twice a day, for 12 weeks |
|
| Outcomes | E2, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Mu G 2001a.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Bushen Qiangshen pill in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 30 postmenopausal osteoporosis patients enrolled: 15 in trial group, 15 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by CUBA ultrasound absorptiometry Exclusion criteria: endocrine diseases, secondary osteoporosis |
|
| Interventions | Experimental: Bushen Qiangshen pill, 1 pill, twice a day, for 12 weeks Control: Caltrate, 1 tablet, twice a day, for 12 weeks |
|
| Outcomes | E2, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Ou L 2011.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Radix rehmanniae preparata, Radix astragali, etc. in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 64 postmenopausal osteoporosis patients enrolled: 32 in trial group, 32 in control group Inclusion criteria: Chinese and WHO diagnostic criteria for osteoporosis Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Radix rehmanniae preparata, Radix astragali, etc. (1 dose per day), for 6 months Control:calcium carbonate tablets (600 mg per day, once a day), for 6 months |
|
| Outcomes | BMD, E2, ALP, Ca, P, PTH, CT | |
| Notes | The study was supported by Shanxi Provincial Department of Education project, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Peng T 2002.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Qianggu soft extract in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 118 postmenopausal osteoporosis patients enrolled: 78 in trial group, 21 in calcium gluconate oral group, 19 in blank control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine diseases |
|
| Interventions | Experimental: Qianggu soft extract, 20 ml, 3 times a day, for 6 months Control: calcium gluconate (500 mg, 3 times a day), or blank, for 6 months |
|
| Outcomes | BMD, E2, Ca, ALP, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Qi ZX 1998.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Bushen Qianggutang in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 40 primary osteoporosis patients enrolled: 20 in trial group, 20 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis |
|
| Interventions | Experimental: Bushen Qianggutang, 1 dose per day, fried, for 3 months Control: Caltrate, 600 mg, twice a day, for 3 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Qiu RB 2004.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Yanghuo Sanzi tang in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 postmenopausal osteoporosis patients enrolled: 30 in trial group, 30 in control group Inclusion criteria: diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Yanghuo Sanzi tang, 150 ml, twice a day, for 6 months Control: Caltrate, 2 tablets per day, for 6 months |
|
| Outcomes | BMD, E2, BGP, IL‐6 | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Qiu RB 2008.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Jiangu prescription in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 120 postmenopausal osteoporosis enrolled: 60 in trial group, 60 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine disease, liver or kidney disease, disease‐affected bone metabolism, etc. |
|
| Interventions | Experimental: Jiangu prescription, 1 dose per day, for 6 months Control: alendronate sodium tablets, 10 mg, once a day, for 6 months |
|
| Outcomes | BMD, BGP, E2, IL‐6 | |
| Notes | The study was supported by a Shenzhen science and technology planning project, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Qiu ZX 2010.
| Methods | Country: China Setting: inpatients Aim: to study the effects of Xianlinggubao capsule in the treatment of senile osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 136 senile osteoporosis patients enrolled: 67 in trial group, 69 in control group Inclusion criteria: BMD Exclusion criteria: other diseases |
|
| Interventions | Experimental: Xianlinggubao capsule (1 g, 3 times a day) and Caltrate (1 tablet, twice a day), for 12 months Control: Caltrate, 1 tablet, twice a day, for 12 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No detailed description of diagnostic standard and P value for the baseline comparison not stated |
Ruan XY 2006.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Qianggu capsules in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 150 postmenopausal osteoporosis or osteopenia patients enrolled: 50 in herbal trial and hormone group, 50 in herbal group, 50 in hormone group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine disease, digestion disease, mental disorder, etc. |
|
| Interventions | Experimental: Qianggu capsules (1 capsule, 3 times a day) and oestradiol valerate (0.5 to 1.5 mg, once a day), for 24 weeks; Qianggu capsules (1 capsule, 3 times a day), for 24 weeks Control: oestradiol valerate (0.5 to 1.5 mg, once a day), for 24 weeks |
|
| Outcomes | BMD, adverse effects | |
| Notes | The study was supported by Beijing administration of traditional Chinese medicine scientific research funds and Capital Foundation of Medical Developments Project, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Shao M 2003.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Gukang oral liquid in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 postmenopausal osteoporosis patients enrolled: 30 in trial group, 30 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis, etc. |
|
| Interventions | Experimental: Gukang oral liquid, 10 ml, 3 times a day, for 6 months Control: alendronate, 1 tablet per day, for 6 months |
|
| Outcomes | BMD, E2, ALP, BGP, IL‐6 | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Shi CD 2012.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Kangshu Jiangu granule in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 76 postmenopausal osteoporosis patients enrolled: 40 in trial group, 36 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: liver or kidney disease, endocrine disease, etc. |
|
| Interventions | Experimental: Kangshu Jiangu granule, 4 g, 3 times a day, for 6 months Control: Caltrate, 1 tablet, once a day, for 6 months |
|
| Outcomes | BMD | |
| Notes | The study was supported by Shanxi Provincial Department of traditional Chinese medicine (TCM) modernisation research plan, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Song XW 2000.
| Methods | Country: China Setting: hospital based Aim: to study the effects of kidney‐tonifying herbs in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 68 postmenopausal osteoporosis patients enrolled: 23 in herb group, 23 in nilestriol group, 22 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis |
|
| Interventions | Experimental: kidney‐tonifying herbs, twice a day, for 6 months Control: nilestriol, 0.2 mg, twice per month; or calcium, 400 mg/d, for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Tang ZA 2012.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Bushen Qianggu Huoxue therapy in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 82 postmenopausal osteoporosis patients enrolled: 42 in trial group, 40 in control group Inclusion criteria: WHO diagnostic criteria for osteoporosis Exclusion criteria: liver or kidney disease, endocrine disease, metabolic disease, etc. |
|
| Interventions | Experimental: Bushen Qianggu Huoxue therapy (1 dose per day), calcium (1000 mg per day) and cod liver oil (1 pill per day), for 3 months Control: calcium (1000 mg per day) and cod liver oil (1 pill per day), for 3 months |
|
| Outcomes | BMD, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Wang CC 2005.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Gumikang capsule in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 postmenopausal osteoporosis patients enrolled: 32 in trial group, 28 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: metabolic disease, endocrine disease |
|
| Interventions | Experimental: Gumikang capsule, 5 capsules, twice a day, for 6 months Control: calcium gluconate, 500 mg, 3 times a day, for 6 months |
|
| Outcomes | BMD, E2, Ca, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Wang H 2007.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Qianggu paste II in the treatment of senile osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 123 senile osteoporosis patients enrolled: 75 in trial group, 48 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: disease‐affected bone metabolism |
|
| Interventions | Experimental: Qianggu paste II (3.0 g, 3 times a day, for 6 months) Control: calcium gluconate (500 mg, 3 times a day, for 6 months) |
|
| Outcomes | BMD, E2, ALP, Ca, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Wang J 2007.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Qianggu capsule in the treatment of senile osteoporosis Study design: randomised controlled trial Analysis: T‐test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 54 postmenopausal osteoporosis patients enrolled: 28 in trial group, 26 in control group Inclusion criteria: diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: disease‐affected bone metabolism |
|
| Interventions | Experimental: Qianggu capsule, 0.25 g, 3 times a day, for 6 months Control: alfa calcidiol capsule, 0.5 μg, twice a day, for 6 months |
|
| Outcomes | BMD, Ca, P, ALP, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Wang JM 2008.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Gukang oral liquid in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 70 postmenopausal osteoporosis patients enrolled: 32 in trial group, 34 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: disease‐affected bone metabolism, secondary osteoporosis |
|
| Interventions | Experimental: Gukang oral liquid,10 ml, 3 times a day, for 6 months Control: alendronate sodium tablets, 10 mg, once a day, for 6 months |
|
| Outcomes | ALP, Ca, E2 | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Wang SW 2003.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Antai capsule in the treatment of postmenopausal osteoporosis Study design: double‐blind, randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 168 postmenopausal osteoporosis patients enrolled: 65 in herbal group, 60 in ipriflavone group, 63 in Caltrate group Inclusion criteria: WHO diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: endocrine disease |
|
| Interventions | Experimental: Antai capsule (3 capsules, 3 times a day) and Caltrate (1 tablet per day), for 6 months Control: ipriflavone (200 mg, 3 times a day) and Caltrate (1 tablet per day), or Caltrate (1 tablet per day), for 6 months |
|
| Outcomes | BMD, E2, Ca, P, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Double‐blind but did not describe who was blind |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Wang XD 2011.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Hugu capsule in the treatment of postmenopausal osteoporosis Study design: double‐blind, placebo‐controlled, randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 122 postmenopausal osteoporosis patients enrolled: 61 in trial group, 61 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: bone metabolic disease, endocrine disease, etc. |
|
| Interventions | Experimental: Hugu capsule (4 capsules, 3 times a day) and Caltrate (1 tablet per day), for 6 months Control: placebo capsule (4 capsules, 3 times a day) and Caltrate (1 tablet per day), for 6 months |
|
| Outcomes | BMD, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Patients were randomly allocated |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Double‐blind |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Wang XY 2000.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Bushen Yigu soft extract in the treatment of osteoporosis Study design: single‐blind, placebo‐controlled, randomised controlled trial Analysis: F‐test, T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 96 osteoporosis patients enrolled: 37 in herbal group, 37 in Alfacalcidol group, 22 in placebo group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by SPA Exclusion criteria: endocrine disease |
|
| Interventions | Experimental: Bushen Yigu soft extract, 20 ml, twice a day, for 3 months Control: Alfacalcidol, 0.25 mg per day, or placebo, for 3 months |
|
| Outcomes | BMD, E2 | |
| Notes | The study was supported by a national department of traditional Chinese medicine (TCM) modernisation research project, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Single‐blind but did not describe who was blind |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Wang YZ 2008.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Jiangu capsule in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 125 primary osteoporosis patients enrolled: 63 in trial group, 62 in control group Inclusion criteria: diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: secondary osteoporosis, metabolic disease, etc. |
|
| Interventions | Experimental: Jiangu capsule, 9 g, 3 times a day, for 12 months Control: Caltrate, 1 tablet per day, for 12 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Wang ZK 2004.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Bushen Jianpi Migu prescription in the treatment of senile osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 150 senile osteoporosis patients enrolled: 83 in trial group, 67 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine disease, secondary osteoporosis |
|
| Interventions | Experimental: Bushen Jianpi Migu prescription, 1 dose per day, for 3 months Control: Caltrate, 1 tablet, twice a day, for 3 months |
|
| Outcomes | BMD, Ca, P, ALP, BMC | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Drawing of lots |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Wei RY 2011.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Kanggusong capsule in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 80 postmenopausal osteoporosis patients enrolled: 48 in trial group, 32 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, the Chinese diagnostic criteria for primary osteoporosis Exclusion criteria: secondary osteoporosis |
|
| Interventions | Experimental: Kanggusong capsule, 3, 3 times a day, for 6 months Control: Caltrate, 1 tablet each night, for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Wu JZ 2010.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Erxian soup in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: F‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 58 primary osteoporosis patients with deficiency of liver and kidney enrolled: 30 in trial group, 28 in control group Inclusion criteria: the Chinese diagnostic criteria for primary osteoporosis and guiding principles for clinical research on new drugs in traditional Chinese medicine Exclusion criteria: bone metabolic disease, endocrine disease, etc. |
|
| Interventions | Experimental: Erxian soup, 1 pack per day, for 6 months Control: Caltrate, 1 tablet per day, for 6 months |
|
| Outcomes | BMD, ALP, Ca, P | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Wu MS 2001.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Kanggusong granule in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: F‐test, T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 90 postmenopausal osteoporosis patients enrolled: 30 in Kanggusong granule Tieji group, 30 in Kanggusong granule group, 30 in ipriflavone group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine diseases, etc. |
|
| Interventions | Experimental: Kanggusong granule, 12 g, twice a day, for 3 months Control: ipriflavone, 1 tablet, 3 times a day, for 3 months |
|
| Outcomes | E2, Ca, P, ALP, PTH | |
| Notes | The study was supported by the National Natural Science Foundation, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Wu MS 2007.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of prescription for tonifying kidney in the treatment of osteoporosis Study design: randomised controlled trial Analysis: T‐test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 180 osteoporosis patients enrolled: 30 in prescription for tonifying kidney group, 30 in kidney meridian sticking group, 30 in urinary bladder meridian sticking group, 30 in ipriflavone group, 30 in Gushukang group, 30 in non‐meridian or acupoint sticking group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis, patients with fractures, etc. |
|
| Interventions | Experimental: prescription for tonifying kidney, 10 pills, 3 times a day; Kanggusong Tieji (kidney meridian sticking or urinary bladder meridian sticking), once per 2 days; for 6 months Control: ipriflavone, 200 mg, 3 times a day, for 6 months |
|
| Outcomes | BMD, Ca, P, E2, PTH, CT, adverse effects | |
| Notes | The study was supported by the National Natural Science Foundation, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Wu W 2005.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Xianling Gubao capsules in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 68 postmenopausal osteoporosis patients enrolled: 34 in trial group, 34 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis |
|
| Interventions | Experimental: Xianling Gubao capsules (1 g, 3 times a day) and Caltrate (1 tablet per day), for 12 months Control: Caltrate, 1 tablet per day, for 12 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Xiao W 2008.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Gusong decoction in the treatment of osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 40 osteoporosis patients enrolled: 20 in trial group, 20 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels Exclusion criteria: secondary osteoporosis |
|
| Interventions | Experimental: Gusong decoction (15 ml, 3 times a day) and alendronate sodium tablet (1 tablet per day), for 5 months Control: alendronate sodium tablet, 1 tablet per day, for 5 months |
|
| Outcomes | BMD, E2, ALP, IL‐6, BGP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Xie J 2004.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Migu tablet in the treatment of senile osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: 21 drop‐outs were not included in the analysis |
|
| Participants | Ethnicity: Chinese 65 senile osteoporosis males enrolled (21 drop‐out): 24 in trial group, 20 in control group Inclusion criteria: diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: metabolic disease, etc. |
|
| Interventions | Experimental: Migu tablet (3 tablets, 3 times a day) and Caltrate (600 mg per day), for 1 year Control: Caltrate, 600 mg per day, for 1 year |
|
| Outcomes | BMD, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | High risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Xie YM 1997.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Bugu Shengsui capsule in the treatment of primary osteoporosis Study design: single‐blind, randomised controlled trial Analysis: T‐test, Chi2 test, Ridit test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 80 primary osteoporosis patients with kidney‐yang deficiency enrolled: 50 in trial group, 30 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by SPA and DXA Exclusion criteria: secondary osteoporosis, endocrine disease, etc. |
|
| Interventions | Experimental: Bugu Shengsui capsule, 3 capsules, 3 times a day, for 6 months Control: vitamin D2 (10,000 U per day) and calcium tablet (500 mg, 3 times a day), for 6 months |
|
| Outcomes | BMD, Ca, ALP, PTH, CT, adverse effects | |
| Notes | The study was supported by the state administration of traditional Chinese medicine youth fund, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Single‐blind but did not describe who was blind |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Xiong YH 2008.
| Methods | Country: China Setting: hospital based Aim: to study the effects of Gusongbao granule in the treatment of postmenopausal osteoporosis Study design: double‐blind, placebo‐controlled, randomised controlled trial Analysis: T‐test Loss to follow‐up: 36 drop‐outs (12 in Gusongbao granule group, 13 in placebo group, 11 in Jiangu granule group) were not included in the analysis |
|
| Participants | Ethnicity: Chinese 144 postmenopausal osteoporosis patients enrolled: 48 (12 drop‐outs) in Gusongbao granule group, 48 (13 drop‐outs) in placebo group, 48 (11 drop‐outs) in Jiangu granule group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis, liver or kidney disease, etc. |
|
| Interventions | Gusongbao granule group: Gusongbao granule, 1 dose, twice a day, Osteoform calcium amino acid chelate compound capsule, 1 pill, twice a day, for 6 months Jiangu granule group: 1 dose, twice a day, Osteoform calcium amino acid chelate compound capsule, 1 pill, twice a day, for 6 months Placebo group: 1 dose, twice a day, Osteoform calcium amino acid chelate compound capsule, 1 pill, twice a day |
|
| Outcomes | BMD, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Xu H 2010.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Qianggu capsule in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 120 postmenopausal osteoporosis patients enrolled: 40 in trial group, 40 in control A group, 40 in control B group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Qianggu capsule (1 capsule, 3 times a day) and alendronate sodium (70 mg per week) Control A: alendronate sodium (70 mg per week) Control B: Qianggu capsule (1 capsule, 3 times a day) |
|
| Outcomes | BMD, adverse events | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Random numbers table |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Xu M 2009.
| Methods | Country: China Setting: outpatients and inpatients Aim: to study the effects of Xianlinggubao capsule in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 156 postmenopausal osteoporosis patients enrolled: 52 in trial group, 52 in control A group, 52 in control B group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis, disease‐affected bone metabolism, endocrine disease, etc. |
|
| Interventions | Experimental: Xianlinggubao capsule (3 capsules, 3 times a day) and alendronate sodium (70 mg per week) Control A: alendronate sodium (70 mg per week) Control B: Xianlinggubao capsule (3 capsules, 3 times a day) |
|
| Outcomes | BMD, adverse events | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Random numbers table |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Xu W 2005.
| Methods | Country: China Setting: outpatients Aim: to study the effects of Yishen Yanggan mixture in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 110 postmenopausal osteoporosis patients with liver‐kidney deficiency enrolled: 60 in trial group, 50 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Yishen Yanggan mixture, 50 ml, twice a day, for 6 months Control: calcium gluconate, 4 tablets, 3 times a day, for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Xu YL 2007.
| Methods | Country: China Setting: outpatients Aim: to study the effects of acupoint sticking of Migudan in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 96 primary osteoporosis patients enrolled: 48 in trial group, 48 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: secondary osteoporosis, endocrine disease |
|
| Interventions | Experimental: acupoint sticking of Migudan (3 times a week), for 3 months Control: Gaitianli (3 tablets, 3 times a day), for 3 months |
|
| Outcomes | BGP, Hyp, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Yang B 2007.
| Methods | Country: China Setting: inpatients Aim: to study the effects of Huangqi in the treatment of osteoporosis Study design: randomised controlled trial Analysis: F‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 58 osteoporosis patients enrolled: 20 in trial group of Huangqi, 20 in control group of Liweiai, 18 in control group of blank Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: endocrine disease, liver or kidney disease, etc. |
|
| Interventions | Experimental: Huangqi, 1 dose per day, for 6 months Control: Liweiai, 2.5 mg, once a day, for 6 months Blank group: no drug |
|
| Outcomes | BMD, ALP, P, Ca | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Ye AN 1998.
| Methods | Country: China Setting: outpatients and inpatients Aim: to observe the effects of Bushen Zhuanggutang in the treatment of senile osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 senile osteoporosis patients enrolled: 31 in trial group, 29 in control group Inclusion criteria: WHO diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis |
|
| Interventions | Experimental: Bushen Zhuanggutang (1 dose per day, fried) and Caltrate (1 tablet per day), for 6 months Control: Caltrate (1 tablet per day), for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Yuan YN 2000.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Shangke Jiegu tablet in the treatment of primary osteoporosis Study design: single‐blinded, randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 primary osteoporosis patients enrolled: 30 in trial group, 30 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by SPA Exclusion criteria: endocrine disease, etc. |
|
| Interventions | Experimental: Shangke Jiegu tablet, 4 tablets, 3 times a day, for 6 months Control: vitamin D2 (10,000 U per day) and calcium tablet (500 mg, 3 times a day), for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Single‐blind but did not describe who was blind |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Zhan HS 2009.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Xuduanzhuanggu capsule in the treatment of primary osteoporosis Study design: double‐blinded, placebo and positive‐controlled, randomised controlled trial Analysis: T‐test Loss to follow‐up: 67 drop‐outs (37 in herb trial group, 18 in positive control group, 21 in placebo group) were not included in the analysis |
|
| Participants | Ethnicity: Chinese 600 primary osteoporosis patients enrolled: 360 in herb trial group (37 drop‐outs), 120 in positive control group (18 drop‐outs), 120 in placebo group (21 drop‐outs) Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: secondary osteoporosis, disease‐affected bone metabolism, metabolic disease, liver or kidney disease, etc. |
|
| Interventions | Experimental: Xuduanzhuanggu capsule (2 capsules, 3 times a day), Gusongbao granule placebo (1 packet, 3 times a day) and calcium tablet (1 tablet, once a day), for 6 months Control: Xuduanzhuanggu capsule placebo (2 capsules, 3 times a day), Gusongbao granule (1 packet, 3 times a day) and calcium tablet (1 tablet, once a day), for 6 months Placebo: Xuduanzhuanggu capsule placebo (2 capsules, 3 times a day), Gusongbao granule placebo (1 packet, 3 times a day) and calcium tablet (1 tablet, once a day), for 6 months |
|
| Outcomes | BMD, adverse events | |
| Notes | The study was supported by a National High Technology Research and Development Program, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding All outcomes | Low risk | Double‐blind but did not describe who was blind |
| Incomplete outcome data addressed All outcomes | Unclear risk | Insufficient information about drop‐outs |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhan ML 2007.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Bushen Yangxue decoction in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 40 postmenopausal osteoporosis patients enrolled: 20 in trial group, 20 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Bushen Yangxue decoction, 1 dose per day, for 6 months Control: calcium gluconate, 4 tablets, 3 times a day, for 6 months |
|
| Outcomes | Ca, P | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhang DS 2011.
| Methods | Country: China Setting: outpatients Aim: to observe the effects of Bushenyiqihuoxue soup in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 120 postmenopausal osteoporosis patients enrolled: 60 in trial group, 60 in control group Inclusion criteria: guiding principles for clinical research on new drugs in traditional Chinese medicine Exclusion criteria: metabolic disease, etc. |
|
| Interventions | Experimental: Bushenyiqihuoxue soup (1 dose per day, water fried 600 ml, twice a day), tamoxifen (20 mg, once a day) and Caltrate (1 tablet, twice a day), for 3 months Control: tamoxifen (20 mg, once a day) and Caltrate (1 tablet, twice a day), for 3 months |
|
| Outcomes | ALP, Ca | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No detailed description of the detection of BMD and biochemical indicators |
Zhang J 2003.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Liuwei Dihuang pills in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 42 postmenopausal osteoporosis patients enrolled: 24 in trial group, 18 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Liuwei Dihuang pills, 8 pills, 3 times a day, for 12 months Control: calcium, 500 mg per day, for 12 months |
|
| Outcomes | BMD, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Zhang RH 2004.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Yigu capsule in the treatment of postmenopausal osteoporosis Study design: double‐blinded, randomised controlled trial Analysis: F‐test, Chi2 test Loss to follow‐up: 10 drop‐outs were not included in the analysis |
|
| Participants | Ethnicity: Chinese 210 postmenopausal osteoporosis patients enrolled: 70 (3 drop‐outs) in herbal trial group, 70 (4 drop‐outs) in control group, 70 (3 drop‐outs) in placebo group Inclusion criteria: WHO diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: metabolic disease, etc. |
|
| Interventions | Experimental: Yigu capsule (4 capsules, 3 times a day) and calcium (510 mg), for 6 months Control: Alfacalcidol (1 capsule per day) and calcium (510 mg), or placebo and calcium (510 mg), for 6 months |
|
| Outcomes | BMD, E2, Ca, P, ALP, BGP, fractures | |
| Notes | The study was supported by the National Science Foundation and China Postdoctoral Science Foundation, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers generated by computer |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhang XG 2011.
| Methods | Country: China Setting: outpatients Aim: to observe the effects of Bushenjianpi Zhuangguyin in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 100 primary osteoporosis patients enrolled: 50 in trial group, 50 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis, disease‐affected bone metabolism, etc. |
|
| Interventions | Experimental: Bushenjianpi Zhuangguyin, 1 dose per day, water fried, twice a day, for 12 weeks Control: Caltrate, 600 mg, twice a day, and Miacalcin ampoule 50 IU, once a day, for 12 weeks |
|
| Outcomes | BMD, Ca, P, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Zhang XJ 2008.
| Methods | Country: China Setting: outpatients and inpatients Aim: to observe the effects of Bushenhuoxue capsule in the treatment of senile osteoporosis Study design: randomised controlled trial Analysis: T‐test, F‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 senile osteoporosis patients enrolled: 30 in trial group, 30 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis, liver or kidney disease, disease‐affected bone metabolism |
|
| Interventions | Experimental: Bushenhuoxue capsule, 4 pills, 3 times a day, for 12 months Control: Caltrate, 600 mg, once a day and Rocaltrol, 0.5 mg, once a day, for 12 months |
|
| Outcomes | BMD, E2, IL‐6, BGP | |
| Notes | The study was supported by the Zhejiang Provincial Department of traditional Chinese medicine (TCM) modernisation research project, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Zhang XZ 2004.
| Methods | Country: China Setting: outpatients and inpatients Aim: to observe the effects of Xianlinggubao capsule in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 178 postmenopausal osteoporosis patients enrolled: 62 in trial group (Xianlinggubao capsule), 66 in control group 1 (tibolone), 50 in control group 2 (Osteoform) Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: liver or kidney disease, disease‐affected bone metabolism, etc. |
|
| Interventions | Experimental: Xianlinggubao capsule and Osteoform, 1.0 g, 3 times a day, for 48 weeks Control 1:tibolone tablets and Osteoform, 1.25 mg, once a day control 2: Osteoform, 1 g, once a day |
|
| Outcomes | BMD, E2, ALP, BGP, IL‐6 | |
| Notes | The study was supported by National Natural Science Foundation, but no declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Random numbers table |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Zhang YL 1996.
| Methods | Country: China Setting: outpatients and inpatients Aim: to observe the effects of TPF capsule in the treatment of senile osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 120 senile osteoporosis patients enrolled: 60 in trial group, 60 in control group Inclusion criteria: WHO diagnostic criteria for osteoporosis based on bone density levels, BMD detected by SPA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: TPF capsule, 3 to 4 capsules, 3 times a day, for 6 months Control: calcium granule, 10 g, twice a day, for 6 months |
|
| Outcomes | BMD, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
Zhang YP 2007.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Shigu yin in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, F‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 120 postmenopausal osteoporosis patients enrolled: 40 in Shigu yin group, 40 in Caltrate group, 40 in blank group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: disease‐affected bone metabolism, metabolic disease, etc. |
|
| Interventions | Experimental: Shigu yin, 200 ml, twice a day, for 6 months Control: Caltrate, 600 mg, twice a day, for 6 months blank: no treatment |
|
| Outcomes | BMD, BGP, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhang YS 2003.
| Methods | Country: China Setting: outpatients Aim: to observe the effects of Huoluo Gukang pills in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 210 primary osteoporosis patients enrolled: 81 in herbal trial group, 74 in herbal control group, 55 in blank group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis, etc. |
|
| Interventions | Experimental: Huoluo Gukang pills, 6.0 g, 3 times a day, for 6 months; Gushukang granule, 10.0 g, twice a day, for 6 months Control: blank (no drug) |
|
| Outcomes | E2, BGP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhang ZF 2011.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Zhuangguqiangjin tablet in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: 8 drop‐outs (3 in trial group, 5 in control group) were not included in the analysis |
|
| Participants | Ethnicity: Chinese 90 primary osteoporosis patients with deficiency of kidney and liver enrolled: 48 in trial group (3 drop‐outs), 42 in control group (5 drop‐outs) Inclusion criteria: Chinese diagnostic criteria for primary osteoporosis, BMD detected by DXA Exclusion criteria: diseases which can affect bone conversion biochemical index, etc. |
|
| Interventions | Experimental: Zhuangguqiangjin tablet (5, 3 times a day for 3 months, then twice a day for 1 year), Shujinbogu tablet (5 tablets, 3 times a day, for 2 to 4 weeks), Miacalcin ampoule (50 IU, injection, once every other day for 10 days; once every 2 days for 10 days; once every 5 days for 10 days. In the 3rd, 6th, 9th and 12th month repeated again) Control: Miacalcin ampoule (50 IU, injection, once every other day for 10 days; once every 2 days for 10 days; once every 5 days for 10 days. In the 3rd, 6th, 9th and 12th month repeated again) |
|
| Outcomes | BMD, Ca, P, ALP, adverse effects, fracture | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhao G 2002.
| Methods | Country: China Setting: outpatients Aim: to observe the effects of Zishen prescription in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 45 postmenopausal osteoporosis patients enrolled: 25 in trial group, 20 in control group Inclusion criteria: international diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: diseases which can affect therapeutic effect |
|
| Interventions | Experimental: Zishen prescription (25 ml, twice a day), Caltrate (1.2 g, twice a day) and Miacalcin (50 IU, injection, twice weekly), for 8 months Control: Caltrate (1.2 g, twice a day) and Miacalcin (50 IU, injection, twice weekly), for 8 months |
|
| Outcomes | BMD, E2 | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhao HX 2001.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Kanggusong capsule in the treatment of postmenopausal osteoporosis Study design: double‐blinded, placebo‐controlled, randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: 35 drop‐outs (11 in trial group, 16 in control group, 8 in opening experimental group) were not included in the analysis |
|
| Participants | Ethnicity: Chinese 198 postmenopausal osteoporosis patients enrolled: 70 (11 drop‐outs) in trial group, 70 (16 drop‐outs) in control group, 58 (8 drop‐outs) in opening experimental group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: diseases which can affect biochemical markers of bone turnover |
|
| Interventions | Experimental: Kanggusong capsule, 3 capsules, 3 times a day, for 1 year Control: placebo, 3 capsules, 3 times a day, for 1 year |
|
| Outcomes | BMD, fractures | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Computer QBASIC procedure |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Double‐blind but did not describe who was blind |
| Incomplete outcome data addressed All outcomes | Unclear risk | No reasons for missing data provided |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhao LN 2003.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Yinyanghuo in the treatment of postmenopausal osteoporosis Study design: double‐blinded, randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 25 postmenopausal osteoporosis patients enrolled: 15 in trial group, 10 in control group Inclusion criteria: diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: not mentioned |
|
| Interventions | Experimental: Yinyanghuo, fried, 200 g per day, 3 times a day, for 3 to 6 months Control: Premarin, 0.625 mg per day, for 3 to 6 months |
|
| Outcomes | BMD, Ca, P, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Double‐blind but did not describe who was blind |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | No information on baseline |
Zheng WK 2007.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Jinwugutong capsules in the treatment of postmenopausal osteoporosis Study design: double‐blinded, placebo‐controlled, randomised controlled trial Analysis: T‐test, F‐test, Chi2 test Loss to follow‐up: 11 drop‐outs (5 in trial group, 6 in control group) were not included in the analysis |
|
| Participants | Ethnicity: Chinese 120 postmenopausal osteoporosis patients enrolled: 60 in trial group, 60 in control group Inclusion criteria: WHO diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: disease‐affected bone metabolism, used medicines that affected bone metabolism |
|
| Interventions | Experimental: Jinwugutong capsules (3 capsules, 3 times a day) plus calcium (510 mg per day), for 6 months Control: placebo capsules (3 capsules, 3 times a day) plus calcium (510 mg per day), for 6 months |
|
| Outcomes | BMD, BGP, ALP, Ca, P, adverse effect | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Double‐blind but did not describe who was blind |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhong RQ 2007.
| Methods | Country: China Setting: outpatients Aim: to observe the effects of Gushen Yijing tang in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 80 primary osteoporosis patients enrolled: 39 in trial group, 41 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: endocrine disease, liver or kidney disease |
|
| Interventions | Experimental: Gushen Yijing tang (1 dose and 2 times a day), for 6 months Control: Caltrate (600 mg, twice a day) for 6 months |
|
| Outcomes | BMD, Ca, P, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhou LZ 2001.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Tongbu Qianggutang in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 40 primary osteoporosis patients enrolled: 30 in trial group, 10 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis, liver or kidney disease, etc. |
|
| Interventions | Experimental: Tongbu Qianggutang (400 ml per day), for 3 months Control: calcium (1.5 g, 3 times a day) plus vitamin D3 (0.25 μg per day), for 3 months |
|
| Outcomes | ALP, CT, BGP, Ca, P, E2, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhou XT 2001.
| Methods | Country: China Setting: outpatients Aim: to observe the effects of Guilu Erxiantang in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 85 primary osteoporosis patients enrolled: 45 in trial group, 40 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis based on bone density levels, BMD detected by DXA Exclusion criteria: secondary osteoporosis |
|
| Interventions | Experimental: Guilu Erxiantang (1 dose per day) and calcium carbonate tablet (300 mg per day), for 6 months Control: calcium carbonate tablet (300 mg per day), for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhou ZK 2006.
| Methods | Country: China Setting: outpatients and inpatients Aim: to observe the effects of Huangqi Sanxian tang in the treatment of postmenopausal osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 50 postmenopausal osteoporosis patients enrolled: 30 in trial group, 20 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: bone metabolic disease induced by diabetes mellitus, liver or kidney disease |
|
| Interventions | Experimental: Huangqi Sanxian tang (1 dose per day), for 3 months Control: nilestriol (2 mg, twice per month), for 3 months |
|
| Outcomes | BMD, E2, ALP | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Single‐blind |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhu HJ 2011.
| Methods | Country: China Setting: inpatients Aim: to observe the effects of Bushen Tianjing Huoxue therapy in the treatment of primary osteoporosis Study design: randomised controlled trial Analysis: T‐test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 61 primary osteoporosis patients enrolled: 31 in trial group, 30 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis Exclusion criteria: secondary osteoporosis, endocrine disease, liver or kidney disease, etc. |
|
| Interventions | Experimental: Bushen Tianjing Huoxue therapy (1 dose per day, twice a day), for 6 months Control: Caltrate (600 mg, once a day) and calcitriol soft capsule (0.25 μg, once a day), for 6 months |
|
| Outcomes | BMD | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zhu HM 2012.
| Methods | Country: China Setting: hospital based Aim: to observe the effects of Bushen Tianjing Huoxue therapy in the treatment of primary osteoporosis Study design: a multicentre, double‐blinded, placebo‐controlled, randomised controlled trial Analysis: F‐test, based on the intention‐to‐treat (ITT) principle Loss to follow‐up: 42 drop‐outs (15 in low‐dose Xianlinggubao group, 14 in high‐dose Xianlinggubao group, 13 in placebo group) |
|
| Participants | Ethnicity: Chinese 180 postmenopausal osteoporosis patients enrolled: 61 in low‐dose Xianlinggubao group, 58 in high‐dose Xianlinggubao group, 61 in placebo group Inclusion criteria: BMD criteria for osteoporosis Exclusion criteria: previous exposure to toremifene, tamoxifen, droloxifene, lasofoxifene or other investigational selective oestrogen receptor modulators; use of bisphosphonates during the past 24 months; history of advanced scoliosis, osteoarthritis or other clinical spinal deformity which would interfere with BMD measurement etc. |
|
| Interventions | Experimental: Xianlinggubao capsule, 3 g (6 capsules) per day in low‐dose group, or 6 g (12 capsules) per day in high‐dose group, plus calcium (500 mg) and vitamin D (200 IU), for 12 months Control: placebo capsule (12 capsules per day), plus calcium (500 mg) and vitamin D (200 IU), for 12 months |
|
| Outcomes | BMD, fracture, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random numbers |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Double‐blind |
| Incomplete outcome data addressed All outcomes | Unclear risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Zou JJ 2005.
| Methods | Country: China Setting: outpatients Aim: to observe the effects of Guben Zhuanggu capsules in the treatment of primary osteoporosis Study design: single‐blinded, randomised controlled trial Analysis: T‐test, Chi2 test Loss to follow‐up: not reported |
|
| Participants | Ethnicity: Chinese 60 primary osteoporosis patients enrolled: 30 in trial group, 30 in control group Inclusion criteria: Chinese diagnostic criteria for osteoporosis, BMD detected by DXA Exclusion criteria: secondary osteoporosis, endocrine disease |
|
| Interventions | Experimental: Guben Zhuanggu capsules (2 capsules, 3 times a day) and Caltrate (600 mg, once a day), for 6 months Control: Guben Zhuanggu capsules simulated (2 capsule, 3 times a day) and Caltrate (600 mg, once a day), for 6 months |
|
| Outcomes | BMD, adverse effects | |
| Notes | No funding sources or declarations of interest for the primary researchers reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Single‐blind |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | Not all the outcomes that were of interest in this review were reported |
| Free of other bias | Unclear risk | P value for the baseline comparison not stated |
ALP: alkaline phosphatase BGP: bone Gla protein BMC: bone mineral content
BMD: bone mineral density Ca: serum calcium CT: computed tomography DPA: dual photon absorptiometry DXA: dual energy X‐ray absorptiometry E2: oestradiol HRT: hormone replacement therapy
Hyp: hydroxyproline IL‐6: interleukin‐6
OPG: osteoprotegerin P: phosphorus PTH: parathyroid hormone SPA: single photon absorptiometry WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ai SC 2003 | Randomised controlled trial comparing herbal application Shenque (CV 8) with sodium etidronate in 70 primary osteoporosis patients. However, the herbal intervention was acupoint patch and did not meet the inclusion criteria |
| Ai SC 2003a | Non‐randomised study comparing herbal application Shenque with sodium etidronate in 70 patients with osteoporosis |
| Bo LY 2003 | Randomised controlled trial comparing Gushukang particles with calcium carbonate plus vitamin D in 94 cases with osteoporotic compression fracture of the spine. However, the outcome measure was general therapeutic effect based on different evaluated standards and did not meet the inclusion criteria |
| Chen AP 2005 | Randomised controlled trial comparing Liuwei Dihuang pills with Caltrate in 40 senile osteoporosis patients. However, the diagnosis of osteoporosis was not based on a BMD test |
| Chen JJ 2010 | Randomised controlled trial comparing Zini Bushenjiangu soup and routine western medicine treatment with routine western medicine treatment in 43 senile osteoporosis patients. However, the treatment duration was less than 3 months |
| Chen XF 2003 | Randomised controlled trial comparing Xianlinggubao plus vitamin D3 and calcium amino acid chelate with calcium gluconate in 150 osteoporosis patients. The trial intervention did not meet the inclusion criteria |
| Chen XF 2007 | Randomised controlled trial comparing Bushen Jianpi mixture with Caltrate in 64 senile primary osteoporosis patients. However, it did not report the part of BMD measured |
| Dai Y 2007a | Randomised controlled trial comparing Migupian (68) and Xianlinggubao (50) with Caltrate (42) in 160 postmenopausal osteoporosis patients. However, the trial was published repeatedly |
| Deng WM 1997 | Randomised controlled trial comparing tonifying kidney to strength bone decoction with calcitonin in 135 postmenopausal osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Deng WM 1997a | Randomised controlled trial comparing kidney‐tonifying herbs with calcitonin in 77 postmenopausal osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Deng WM 2001 | The participants were not osteoporosis patients |
| Deng WM 2002 | Randomised controlled trial comparing Bushen Zhuanggu granule with calcitonin in 175 postmenopausal osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Ding GZ 1995 | Randomised controlled trial comparing Bushen Jiangu capsule with calcium in 34 postmenopausal osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Dong YL 2004 | Randomised controlled trial comparing Liuwei Dihuang pills plus calcium tablet and vitamin C with calcium tablet plus vitamin C in 60 senile osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and did not meet the inclusion criteria |
| Fan GF 2008 | Randomised controlled trial comparing Qiangjin Zhuanggu pill with oyster shell calcium chewable tablets in 248 primary osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Fan ZY 1995 | Randomised controlled trial comparing Yugu pill with nilestriol in 90 primary osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Ge L 2005 | Randomised controlled trial comparing Jiangu pills with Caltrate in 40 primary osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Gong L 2000 | Randomised controlled trial comparing traditional medicine (Yishen Zhuanggu mixture or Shengu capsule) with no treatment in 80 primary osteoporosis patients. However, it was a duplicate publication |
| Gong ZF 2011 | Randomised controlled trial comparing Bushenshujin soup and celecoxib capsules with celecoxib capsules in 90 primary osteoporosis patients. However, the treatment duration was 4 to 12 weeks |
| Gu M 2004 | Randomised controlled trial comparing Fortune's drynariae rhizome with calcium gluconate in 82 primary osteoporosis patients. However, it did not report the part of BMD measured |
| Gu T 2011 | Randomised controlled trial comparing Gushu recipes, calcium carbonate D3 and elcatonin injection with calcium carbonate D3 and elcatonin injection in 43 primary osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Guo F 2003 | Randomised controlled trial comparing Yishen Yanggan decoction with calcium gluconate in 60 osteoporosis patients. However, it did not report the part of BMD measured |
| Guo HN 1998 | Randomised controlled trial comparing Tiankui tang with gonadal hormone in 120 primary osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| He YC 2008 | Randomised controlled trial comparing Xianlinggubao capsule with salmon calcitonin in 88 senile osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Hu XD 2011 | Randomised controlled trial comparing Chinese Traditional Medicine recipe with high calcium tablets and Caltrate in 164 senile osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Jia JH 2010 | The type of interventions did not meet the inclusion criteria |
| Jiang SY 1995 | Randomised controlled trial comparing Gushukang granule with calcium tablet and vitamin D in 400 primary osteoporosis patients. However, it did not report the BMD of the ulna and radius respectively |
| Ke Q 2005 | Randomised controlled trial comparing Jiangu recipe preparation with alendronate sodium tablet in 70 postmenopausal osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Ke Q 2005a | Randomised controlled trial comparing Jiangu recipe preparation with alendronate sodium tablet in 95 primary osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Li BL 2007a | Randomised controlled trial comparing Jianshen prescription with alendronate sodium in 60 postmenopausal osteoporosis patients. However, it was a duplicate publication |
| Li CX 2005 | Randomised controlled trial comparing movement therapy plus Bushen Tongluo tang with Caltrate in 87 senile osteoporosis patients. The therapy intervention did not meet the inclusion criteria |
| Li JF 2008 | Randomised controlled trial comparing Bushen Qianggu tang plus calcium with calcium in 120 primary osteoporosis patients. However, the treatment duration was less than 3 months |
| Li YM 2005 | Non‐randomised study comparing Bushen Huoxue preparation with Caltrate in 60 patients with postmenopausal osteoporosis |
| Lin HY 1998 | Randomised controlled trial comparing Yishen Jiangu tang with Caltrate in 40 primary osteoporosis patients. However, it was published repeatedly |
| Liu HB 2005 | Non‐randomised study comparing medicine‐separated sticking on Shenqu with sodium etidronate in 130 patients with primary osteoporosis |
| Liu HQ 2010 | The type of interventions did not meet the inclusion criteria |
| Liu TS 2008 | Randomised controlled trial comparing Traditional Chinese Medicine plus alfacalcidol soft capsule with alfacalcidol soft capsule in 124 primary osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Liu YS 2011 | Without control group |
| Liu YX 1998 | Randomised controlled trial comparing Fuguning granule with calcium tablet in 100 postmenopausal osteoporosis patients. However, it did not report the BMD of the ulna and radius respectively |
| Lu W 2004 | Randomised controlled trial comparing Bushen Jiangu tang with tibolone tablets in 80 primary osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Lu ZD 2004 | Randomised controlled trial comparing Gushukang with nilestriol in 40 postmenopausal osteoporosis patients. However, it did not report the part of BMD measured |
| Luo QY 2011 | Without control group |
| Pan X 2004 | Non‐randomised study comparing Huangqi injection to acupoint plus Caltrate with Caltrate in 64 patients with primary osteoporosis |
| Peng T 2003 | Non‐randomised study comparing Qianggu extracts with calcium gluconate in 102 patients with postmenopausal osteoporosis |
| Qi ZX 2000 | Randomised controlled trial comparing Shengsuiyin with Caltrate in 40 primary osteoporosis patients. However, it was published repeatedly |
| Ren JH 2011 | Randomised controlled trial comparing Jinwugutong capsule and alendronate with alendronate in 90 postmenopausal osteoporosis patients. However, the outcome measure was general therapeutic effect about pain degree based on visual analogue scale (VAS) and it did not meet the inclusion criteria |
| Shang YM 2007 | Randomised controlled trial comparing Xianlinggubao capsule with conjugated oestrogens or calcium, or both, in 90 postmenopausal osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Shi B 2010 | Randomised controlled trial comparing Traditional Chinese Medicine differentiation with calcium and vitamin D in 110 senile osteoporosis patients. However, it did not report the part of BMD measured |
| Shou ZX 2008 | Randomised controlled trial comparing Qianggu Huoli tablet plus Caltrate with Caltrate in 227 primary osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Shu J 2005 | Randomised controlled trial comparing Bushen Yijing prescription with calcium tablet in 50 cases of osteoporosis. The interventions in both arms were used for 7 weeks (less than 3 months) |
| Shuai B 2008 | Randomised controlled trial comparing Jiangu granule with Gusongbao granule in 96 primary osteoporosis patients. However, the control treatment did not meet the inclusion criteria |
| Song HX 2003 | Randomised controlled trial comparing Bushen Zhuanggu pill with calcium in 84 osteoporosis patients. However, it did not report the part of BMD measured |
| Song XW 1997 | Randomised controlled trial comparing Bushen herbal medicine with calcium in 55 postmenopausal osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Song XW 2001 | Randomised controlled trial comparing Bushenhuoxue recipe with nilestriol or calcium in 68 postmenopausal osteoporosis patients. However, it was published repeatedly |
| Su CH 2004 | Randomised controlled trial comparing strong bone capsule with calcium carbonate plus vitamin D in 250 senile osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Su CH 2005 | Randomised controlled trial comparing strong bone capsule with Caltrate in 105 postmenopausal osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Su YB 2008 | Randomised controlled trial comparing Qianggu Huoxue pill with Caltrate in 61 primary osteoporosis patients. However, the treatment duration was less than 3 months |
| Su ZW 2010 | Randomised controlled trial comparing Bushenzhuanggu soup, calcitriol capsule, calcium supplement with vitamin D chewable tablets, salcatonin injection and bone peptide injection with calcitriol capsule, calcium supplement with vitamin D chewable tablets, salcatonin injection and bone peptide injection in 110 primary osteoporosis patients. However, the treatment duration was less than 3 months |
| Sun JM 2002 | Randomised controlled trial comparing Yiqi Tiansui mixture plus Caltrate with Caltrate in 40 patients. However, this trial had the same data as the trial conducted by Mu G 2001 |
| Sun X 2002 | Randomised controlled trial comparing Jiawei Zuogui pill with calcium in 90 patients. However, this trial had the same data as the trial conducted by Lv ZH |
| Sun YM 2008 | Randomised controlled trial comparing Gegen with alfacalcidol in 44 primary osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Tan X 2010 | The type of interventions did not meet the inclusion criteria |
| Tang JM 1999 | Randomised controlled trial comparing Jiangu powder with activated calcium powder in 98 primary osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Tu PS 2008 | Randomised controlled trial comparing Bushen prescription with no treatment in 60 osteoporosis patients. However, the treatment duration was less than 3 months |
| Wang CH 2003 | Non‐randomised study comparing Gusongkang capsule with calcium lactate in 100 patients with senile osteoporosis |
| Wang GH 2011 | Randomised controlled trial comparing Zhuanggutongluobao with vitamin D2 plus calcium tablet in 65 patients with primary osteoporosis. However, the comparability between groups was bad |
| Wang HM 1998 | Non‐randomised study comparing Bugu capsule with activated calcium powder in 60 patients with senile osteoporosis |
| Wang J 2008 | Randomised controlled trial comparing Bugan Yishen tang with alfacalcidol in 65 postmenopausal osteoporosis patients. However, the treatment duration was less than 3 months |
| Wang JH 2001 | Randomised controlled trial comparing Qianggu pill with calcium lactate plus vitamin D in 120 osteoporosis patients. However, it was not clear that all were primary osteoporosis patients |
| Wang LM 2004 | Randomised controlled trial comparing Jiangu tang with Suplical in 99 senile osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Weng M 2003 | Randomised controlled trial comparing medicine‐separated sticking on Shenque (RN8) with sodium etidronate in 70 primary osteoporosis patients. However, the herbal intervention was an acupoint patch and it did not meet the inclusion criteria |
| Wu CM 2004 | Randomised controlled trial comparing Bushen Zhuanggu capsule with diethylstilbestrol in 71 osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Wu D 2008 | Randomised controlled trial comparing Xiaoyao san plus Caltrate with Caltrate in 60 senile osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Wu XG 2008 | Randomised controlled trial comparing Xianlinggubao capsule with no treatment in 87 osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Xia WF 2006 | Unavailable data |
| Xie J 2001 | The participants were not patients with osteoporosis |
| Xie J 2003 | The participants were not patients with osteoporosis |
| Xu CY 2004 | Randomised controlled trial comparing Bushen Jianpi Tongluo decoction plus Yishen Jiangu tablet and Caltrate with Yishen Jiangu tablet and Caltrate in 125 primary osteoporosis patients. The interventions in both arms were used for 60 days (less than 3 months) |
| Xu LZ 2001 | Randomised controlled trial comparing Bupi Yishen Huoxue prescription with calcium or analgesic discontinuously in 130 osteoporosis patients. However, it was not clear that all were primary osteoporosis patients |
| Xu PH 2001 | Non‐randomised study comparing Bushen Jiangu capsule with Gusongbao particles in 330 patients with osteoporosis |
| Yan XP 2007 | Without control group |
| Yang JP 2007 | Randomised controlled trial comparing Gushibao capsule with calcium tablet plus vitamin D in 105 osteoporosis patients. However, it was not clear that all were primary osteoporosis patients |
| Yang WH 2010 | The type of interventions did not meet the inclusion criteria |
| Yu YH 2011 | Randomised controlled trial comparing Erzhi pill with alendronate sodium tablets, Erzhi pill and alendronate sodium tablets with alendronate sodium tablets, in 60 postmenopausal osteoporosis patients. However, it did not report the part of BMD measured |
| Zhang GM 2001 | Randomised controlled trial comparing Xianguning powder with nilestriol in 80 postmenopausal osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Zhang H 1999 | Randomised controlled trial comparing Gubao capsule with Vita‐D3 or Longmu Zhuanggu powder in 140 primary osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Zhang WY 2005 | Randomised controlled trial comparing Bupi Yishen Huoxue prescription with oestradiol or testosterone in 45 senile osteoporosis patients. However, the outcome measure was general therapeutic effect based on different evaluated standards and it did not meet the inclusion criteria |
| Zhao G 2004 | Randomised controlled trial comparing Qianggu capsule with tibolone tablets in 69 postmenopausal osteoporosis patients. However, the diagnostic method was not reported definitively |
| Zhao Z 2010 | Randomised controlled trial comparing Yanghe capsule with Caltrate in 60 primary osteoporosis patients. However, it did not report the part of BMD measured |
| Zheng NX 2011 | The type of interventions did not meet the inclusion criteria |
| Zheng XH 2004 | Randomised controlled trial comparing Gukangye with calcium tablet in 150 primary osteoporosis patients. However, the diagnostic method was not based on a BMD test |
| Zheng ZW 2010 | Randomised controlled trial comparing Bushenzhuanggu soup plus western medicines (ossification alcohol capsule, calcium supplement with vitamin D chewable tablets, calcitonin and salmon calcitonin injection) with the same western medicines in 110 primary osteoporosis patients. However, the treatment duration was less than 3 months |
| Zhong J 2010 | Randomised controlled trial comparing Zhuanggubushen decoction plus carp calcitonin injection J with carp calcitonin injection in 50 osteoporosis patients. However, the treatment duration was less than 3 months and the participants did not meet the inclusion criteria |
| Zhong RQ 2007a | Randomised controlled trial comparing Gushen Yijing tang with Caltrate in 79 osteoporotic fracture patients. However, the participants did not meet the inclusion criteria |
| Zhong RQ 2007b | Randomised controlled trial comparing Gushen Yijing tang with Caltrate in 124 osteoporotic fracture patients. However, the participants did not meet the inclusion criteria |
| Zhou PQ 1997 | Non‐randomised study comparing Migu tablet with nilestriol in 50 patients with postmenopausal osteoporosis |
| Zhu LP 2008 | Randomised controlled trial comparing Bushen Jianpi tang plus oyster shell calcium chewable tablets with oyster shell calcium chewable tablets in 80 postmenopausal osteoporosis patients. However, the treatment duration was less than 3 months |
BMD: bone mineral density
Differences between protocol and review
Chinese diagnostic criteria for osteoporosis were added, besides the WHO criteria, as many trials used the Chinese criteria (these were adapted from the WHO criteria).
Contributions of authors
Y Liu: drafted the protocol, developed the search strategy, selected trials, assessed trial quality, extracted data, performed data analyses and drafted the review.
Y Xia: updated the searches and selected trials, assessed trial quality, extracted data, performed data analyses and co‐developed the review.
JP Liu: conceived the study, revised the protocol and the review, provided a methodological perspective and performed data analyses.
Sources of support
Internal sources
School of Public Health, Shandong University, China.
Beijing University of Chinese Medicine, China.
External sources
National Basic Research Program ('973' program, grant number 2006CB504602), China.
International Science & Technology Cooperation Project (grant number 2009DFA31460), China.
The Program for Innovative Research Team of Beijing University of Chinese Medicine (no. 2011‐CXTD‐09), China.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony).
New
References
References to studies included in this review
An SJ 2000 {published data only}
- An SJ, Li T, Li E. Effect of kidney‐tonifying herbs on ovary function and bone mass in postmenopausal women. Chinese Journal of Osteoporosis 2000;6(2):55‐9. [Google Scholar]
Cao GY 2010 {published data only}
- Cao GY, Zhang XM. Clinical observation of integrated traditional Chinese and western medicine in the treatment of osteoporosis. Nei Mongol Journal of Traditional Chinese Medicine 2010;29(2):18‐9. [Google Scholar]
Cao W 2010 {published data only}
- Cao W, Li JW. Clinical research of the treatment of postmenopausal osteoporosis with integration of traditional and western medicine. Journal of New Chinese Medicine 2010;42(4):44‐5. [Google Scholar]
Chen FS 2001 {published data only}
- Chen FS, Wei AS, Lang JM. Clinical observation on effect of 32 cases of postmenopausal osteoporosis treated with integrated traditional with western medicine. New Journal of Traditional Chinese Medicine 2001;33(11):42‐3. [Google Scholar]
Chen JP 1999 {published data only}
- Chen JP, Shao M, Du Y, Huang HX, Zhu BH, Lin YF. Clinical observation of the therapeutic effect of 36 cases with postmenopausal osteoporosis treated with Gukang oral solution. New Journal of Traditional Chinese Medicine 1999;31(7):22‐3. [Google Scholar]
Chen NQ 2006 {published data only}
- Chen NQ, An MH, Wu CL. Clinical study of Shenyao capsules on the treatment of 50 cases with primary osteoporosis. Clinical Journal of Traditional Chinese Medicine 2006;18(2):161‐2. [Google Scholar]
Chen X 2008 {published data only}
- Chen X. Curative effect observation of Gukangfang on treatment of primary osteoporosis. Zhejiang Journal of Integrative Traditional and Western Medicine 2008;18(7):402‐4. [Google Scholar]
Chen XL 2010 {published data only}
- Chen XL, Liang XH. Clinical observation of Heche Dazao pill on the treatment of 57 cases with primary osteoporosis. Guiding Journal of Traditional Chinese Medicine and Pharmacy 2010;16(8):40‐1. [Google Scholar]
Chen YQ 2001 {published data only}
- Chen YQ, Huang YH. Clinical study of a Chinese medicine of replenishing kidney in treating postmenopausal osteoporosis. China Contemporary Medicine 2001;7(4):57‐8. [Google Scholar]
Chen ZX 2002 {published data only}
- Chen ZX, Xu XJ, Huang G, Hu JM. Clinical study of the treatment of postmenopausal osteoporosis with Gushukang granule combined with calcium. Chinese Journal of Clinical Rehabilitation 2002;6(23):3541‐2. [Google Scholar]
Cui TH 1999 {published data only}
- Cui TH, Yu JH, Ding S, Wang RP, Liu H, Shi QY, et al. A clinical study in 74 cases of type primary osteoporosis treated with Chinese medicine Strong bone capsule. Suzhou University Journal of Medical Science 1999;19(12):1291‐4. [Google Scholar]
Dai Y 2007 {published data only}
- Dai Y, Shen L, Yang YP, Xie J, Zhou PQ. Effects of Migu tablet on bone mineral density, serum levels of osteoprotegerin and its ligand and bone metabolic markers in patients with postmenopausal osteoporosis. Chinese Journal of Integrative Traditional and Western Medicine 2007;27(8):696‐9. [PubMed] [Google Scholar]
Deng WM 2012 {published data only}
- Deng WM, Zhang P, Huang H, Shen YG, Yang QH, Cui WL, et al. Five‐year follow‐up study of a kidney‐tonifying herbal Fufang for prevention of postmenopausal osteoporosis and fragility fractures. Journal of Bone and Mineral Metabolism 2012;30:517‐24. [DOI] [PubMed] [Google Scholar]
Dong Y 2010 {published data only}
- Dong Y, Lu Y, Hua LX. Efficacy of salmon calcitonin plus Xianlinggubao for osteoporosis and ostealgia in postmenopausal women: a clinical observation. China Pharmacy 2010;21(2):154‐6. [Google Scholar]
Dong YF 2004 {published data only}
- Dong YF. Clinical observation of Herba epimedii prescription in treating osteoporosis. Journal of Chinese Medicinal Materials 2004;27(8):620‐2. [Google Scholar]
Fan HX 2004 {published data only}
- Fan HX, Fan HY, Wang ZH. Clinical observation on the therapeutic effect of Gengnian Anyi tablet on postmenopausal osteoporosis. Tianjin Journal of Traditional Chinese Medicine 2004;21(1):20‐1. [Google Scholar]
Gang PH 2001 {published data only}
- Gang PH, Jiang SY. Clinical observation of Gumiling on osteoporosis. The national academic seminar of osteoporosis and osteoarthritis in 2001. 2001:327‐30.
Gong L 2001 {published data only}
- Gong L, Wang MY, Xia P, Cao JQ, Zhou JQ, Hu J. Clinical study of Yishen Zhuanggu mixture in treating 80 cases with primary osteoporosis. Chinese Journal of Osteoporosis 2001;7(2):149‐51. [Google Scholar]
Guo JH 2008 {published data only}
- Guo JH, Zhang YW, Zhao ZM, Yao RB, Shang W, Zang XL, et al. Effects of Bushen Kangsong pellet on bone mineral density in postmenopausal osteoporosis. Journal of Anhui Traditional Chinese Medicine College 2008;27(5):14‐5. [Google Scholar]
Han XL 2007 {published data only}
- Han XL, Lou ZJ, Shang FM, Lin HW. Clinical observation of Shugan zishen huoxue tang on 25 cases with postmenopausal osteoporosis. Liaoning Journal of Traditional Chinese Medicine 2007;34(9):1279‐80. [Google Scholar]
He MT 2007 {published data only}
- He MT, Liang XM, Liang ZJ, Zhang BD. Clinical study of Bushen Huoxue recipe on the treatment of postmenopausal osteoporosis. Shandong Journal of Traditional Chinese Medicine 2007;26(7):447‐9. [Google Scholar]
He N 2006 {published data only}
- He N. Treatment of postmenopausal osteoporosis by modified 'Zhaungyao Jianshen Tang': a therapeutic effect observation. Journal of Traditional Chinese Orthopedics and Traumatology 2006;18(11):10‐1. [Google Scholar]
He XQ 2006 {published data only}
- He XQ. Clinical study of Bushen Yangxue tang on the treatment of postmenopausal osteoporosis and effect on BMD. Journal of New Chinese Medicine 2006;38(4):42‐3. [Google Scholar]
Huang JW 2008 {published data only}
- Huang JW, Chen JC, Huang JH, Lin AJ, Lin XZ. The influence of bone mineral density and cell factors of Erxianyanggu decoction and alendronate sodium tablets for patients with osteoporosis. Journal of Traditional Chinese Orthopedics and Traumatology 2008;20(3):12‐3. [Google Scholar]
Huang ZJ 2008 {published data only}
- Huang ZJ, Chen JX, Bai YB. Clinical research of Zishengukang pill for osteoporosis. Letters in Biotechnology 2008;19(2):263‐4. [Google Scholar]
Hu J 2012 {published data only}
- Hu J, Li C. Clinical observation of Bushenjianpi treatment of primary osteoporosis. Hubei Journal of TCM 2012;34(4):11‐2. [Google Scholar]
Jian QQ 2010 {published data only}
- Jian QQ, Xu Y. Clinical observation on effect of integration of traditional and western medicine in treating postmenopausal osteoporosis. Journal of Sichuan of Traditional Chinese Medicine 2010;28(9):82‐3. [Google Scholar]
Lan ZX 2006 {published data only}
- Lan ZX, Li LZ, Zhang JT, Wang ZQ, Chen C, Liu JY. Clinical study of Guli on treating primary osteoporosis of deficiency in spleen and kidney. Journal of Sichuan Traditional Chinese Medicine 2006;24(6):75‐7. [Google Scholar]
Liang DB 2012 {published data only}
- Liang DB, Zhuang H. Clinical observation of Bushenhuoxue therapy of osteoporosis. Journal of New Chinese Medicine 2012;44(5):53‐5. [Google Scholar]
Liao L 2004 {published data only}
- Liao L, Li XS, Cai QH, Liang YS, Lai RB, Xiao WC, et al. Clinical research of the Bushen Shengsui principle treating postmenopausal osteoporosis. Chinese Journal of Information on traditional Chinese medicine 2004;11(4):287‐90. [Google Scholar]
Li BL 2007 {published data only}
- Li BL, Huang CB, Tan JW, Li YF, Yang B. The Influence of bone metabolism using traditional Chinese medicine Jianshenfang granule in treating of postmenopausal osteoporosis patients. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2007;15(11):15‐7. [Google Scholar]
Li HW 2004 {published data only}
- Li HW, Liu F. Clinical observation on effect of Ziyin Bushen Zhuanggu prescription in treating postmenopausal osteoporosis. The Chinese Medicine Magazine of China 2004;2(3):114‐6. [Google Scholar]
Ling JY 2008 {published data only}
- Ling JY, Liu Q. Clinical study on treatment of postmenopausal osteoporosis by Bushenzhuanggu prescription. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2008;16(4):22‐3. [Google Scholar]
Lin W 2000 {published data only}
- Lin W, Wu HF, Deng LP, Wei XY, Ye GC, Liu QS, et al. Clinical use of traditional Chinese medicine Migu decoction in treating postmenopausal osteoporosis. Chinese Journal of Osteoporosis 2000;6(3):71‐2. [Google Scholar]
Li SL 2004 {published data only}
- Li SL, Fan GJ, Tang AH, Liu CH, Luo GB, Tang XY. Clinical study of the treatment of postmenopausal osteoporosis with Strong bone capsule. Chinese Journal of Osteoporosis 2004;10(3):330‐2. [Google Scholar]
Liu JM 2012 {published data only}
- Liu JM, Xue L, Tian DX. Clinical research of primary osteoporosis treated with Gukang tablets. World Journal of Integrated Traditional and Western Medicine 2012;7(4):323‐5, 354. [Google Scholar]
Li YH 2008 {published data only}
- Li YH, Liu HH, Yang HJ, Li Y, Zhang LF, Liu F, et al. Clinical research of Jingujian granule for old people with osteoporosis. Beijing Journal of Traditional Chinese Medicine 2008;27(10):758‐61. [Google Scholar]
Li YH 2010 {published data only}
- Li YH, Xue L, Zhao FF, Zhu QQ, Li Y, Yang HJ. Effect of Jingujian granule on 129 primary osteoporosis cases. Journal of Traditional Chinese Medicine 2010;51(6):520‐3. [Google Scholar]
Li ZP 2011 {published data only}
- Li ZP, Tong HT, Sun GH, Lao RP, Liu ZJ. The effects of Kanggusong soup plus Caltrate on ALP and IL‐6 of postmenopausal osteoporosis. Journal of Yunnan University of Traditional Chinese Medicine 2011;34(4):37‐8, 46. [Google Scholar]
Li ZY 2006 {published data only}
- Li ZY, Liu BG, Liu YM, Zhang JX. Clinical observation of Xianling Gubao capsules on postmenopausal osteoporosis. Journal of Sichuan Traditional Chinese Medicine 2006;24(1):83‐4. [Google Scholar]
Lv ZH 2002 {published data only}
- Lv ZH, Wen ZJ, Wu SP, Qiu JM, Qiu JX. Clinical observation of Jianwei Bushen Zhuangjintang on the treatment of primary osteoporosis. China Journal of Orthopaedics and Traumatology 2002;15(5):263‐4. [Google Scholar]
Ma C 2011 {published data only}
- Ma C. Clinical analysis on Liuwei Dihuang pills plus calcium in treating postmenopausal osteoporosis. China Medicine and Pharmacy 2011;1(18):74‐5. [Google Scholar]
Ma CZ 2011 {published data only}
- Ma CZ. Clinical observation on combined treatment of traditional Chinese medicine and western medicine in treating osteoporosis. Journal of New Chinese Medicine 2011;43(9):56‐7. [Google Scholar]
Mao YF 2011 {published data only}
- Mao YF. Effect of Bushen Jianpi Jingu decoction on 75 postmenopausal osteoporosis. Beijing Journal of Traditional Chinese Medicine 2011;30(4):292‐3. [Google Scholar]
Ma YJ 2011 {published data only}
- Ma YJ, Ma YJ, Ma YL. A clinical observation on treating senile osteoporosis with Yishen Zhuanggu decoction. Chinese Journal of the Practical Chinese with Modern Medicine 2011;24(5):18‐9. [Google Scholar]
Meng XD 2003 {published data only}
- Meng XD, Xie YM, Huang YZ, Cui TH, Gao R, Tu RS, et al. Clinical study of Gujian capsule in treating primary osteoporosis with kidney‐yang deficiency syndrome. Chinese Journal of Clinical Rehabilitation 2003;7(3):445‐7, 457. [Google Scholar]
Miu JQ 2008 {published data only}
- Miu JQ. Clinical biochemical parameters observation of integrative Chinese and Western medicine for postmenopausal osteoporosis. Chinese Journal of Applied Physiology 2008;24(1):54‐5. [PubMed] [Google Scholar]
Mu G 2001 {published data only}
- Mu G, Mu JJ, Wang P, Zhang K. Clinical observation of Bushen Qiangshen pill plus Caltrate on postmenopausal osteoporosis. Chinese Journal of Integrated Traditional with Western Medicine 2001;21(5):382‐3. [Google Scholar]
Mu G 2001a {published data only}
- Mu G, Wang P, Mu JJ. Clinical study of Bushen Qiangshen pill on postmenopausal osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2001;9(1):20‐2. [Google Scholar]
Ou L 2011 {published data only}
- Ou L, Zeng XH, Zhao P. Clinical observation on Radix rehmanniae preparata and Radix astragali in treating postmenopausal osteoporosis. Beijing Journal of Traditional Chinese Medicine 2011;30(8):605‐6. [Google Scholar]
Peng T 2002 {published data only}
- Peng T, Huang ML, Xiong W, Wang L, Yang YP, Qi K, et al. Clinical and experimental study of Qianggu soft extract in treatment of type postmenopausal osteoporosis. Chinese Journal of Osteoporosis 2002;8(4):337‐41, 364. [Google Scholar]
Qiu RB 2004 {published data only}
- Qiu RB, Shen RZ, Zhang PQ. Treatment of postmenopausal osteoporosis with Yanghuo Sanzi tang‐‐‐A report of 30 cases. Journal of Traditional Chinese Medicine 2004;24(2):102‐4. [PubMed] [Google Scholar]
Qiu RB 2008 {published data only}
- Qiu RB, Shen RZ, Zhang PQ, Chen YL, Ye HP, Zhang Y. Clinical study about treatment of postmenopausal osteoporosis with Jiangu recipe. Chinese Archives of Traditional Chinese Medicine 2008;26(7):1529‐30. [Google Scholar]
Qiu ZX 2010 {published data only}
- Qiu ZX. Observation of the effect of Xianlinggubao capsule on 67 senile osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2010;18(8):52. [Google Scholar]
Qi ZX 1998 {published data only}
- Qi ZX, Lin HY. Clinical observation on Bushen Qianggutang in treating primary osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 1998;6(6):41‐2. [Google Scholar]
Ruan XY 2006 {published data only}
- Ruan XY, Qi JM, Liu YL, Ji Y, Chen BY. Effects of traditional Chinese medicine on bone mineral density and femoral neck strength in postmenopausal women. Chinese Journal of Osteoporosis 2006;12(2):181‐4. [Google Scholar]
Shao M 2003 {published data only}
- Shao M, Huang HX, Zhao J. Clinical observation on effect of Gukang oral preparation in treating postmenopausal osteoporosis. Journal of Traditional Chinese Orthopedics and Traumatology 2003;15(3):11‐2. [Google Scholar]
Shi CD 2012 {published data only}
- Shi CD, Yuan PW, Yang XH. Observation of Kangshu Jiangu granule on postmenopausal osteoporosis, a report of 40 cases. Shanxi Journal of Traditional Chinese Medicine 2012;28(1):16‐7. [Google Scholar]
Song XW 2000 {published data only}
- Song XW, Chen BX, Shen PZ. Clinical study on postmenopausal osteoporosis treated by kidney‐nourishing herbs. Shanghai Journal of Traditional Chinese Medicine 2000;34(7):28‐9. [Google Scholar]
Tang ZA 2012 {published data only}
- Tang ZA. Clinical observation on Bushen Qianggu Huoxue therapy in treating 42 primary osteoporosis. Guiding Journal of Traditional Chinese Medicine and Pharmacy 2012;18(4):50‐1. [Google Scholar]
Wang CC 2005 {published data only}
- Wang CC, Shang LL, Liang JY. Clinical observation on the therapeutic effect of Gumikang capsule plus nuclear honey beans on postmenopausal osteoporosis. Journal of Traditional Chinese Orthopedics and Traumatology 2005;17(8):15‐6. [Google Scholar]
Wang H 2007 {published data only}
- Wang H. Clinical observation of Qianggu paste II for old people with osteoporosis. Hubei Journal of Traditional Chinese Medicine 2007;29(10):54‐5. [Google Scholar]
Wang J 2007 {published data only}
- Wang J, Zhang WK, Wang ZH. Qianggu capsule for postmenopausal osteoporosis in 28 cases. Herald of Medicine 2007;26(11):1325‐7. [Google Scholar]
Wang JM 2008 {published data only}
- Wang JM, Liu T. Clinical observation of Gukang on bone quality in postmenopausal osteoporosis patients. Chinese Journal of Medical Traumatology & Orthopedics 2008;16(11):8‐9. [Google Scholar]
Wang SW 2003 {published data only}
- Wang SW, Zheng BR, Sun JY, Xie YH. Effects of Antai capsule on women with postmenopausal osteoporosis. Journal of the Fourth Military Medical University 2003;24(14):1324‐7. [Google Scholar]
Wang XD 2011 {published data only}
- Wang XD, Deng WM, Hong MJ, Liu F, Wang Q, Sun P. Clinical study on Hugu capsule in treating postmenopausal osteoporosis. Journal of Practical Medicine 2011;27(9):1662‐4. [Google Scholar]
Wang XY 2000 {published data only}
- Wang XY, Zhang CL, Mo LL, Qu YQ, Han LL. The effect of Bushen Jianpi herbs on bone metabolism and estrogen in perimenopausal woman. Journal of Guangzhou University of Traditional Chinese Medicine 2000;17(3):230‐2. [Google Scholar]
Wang YZ 2008 {published data only}
- Wang YZ, Wang HC. Clinical research of Jiangu capsule for osteoporosis. Chinese Journal of Guang Ming Chinese Medicine 2008;23(8):1125‐6. [Google Scholar]
Wang ZK 2004 {published data only}
- Wang ZK. The effects of Bushen Jianpi Migu prescription on pain and relevant biochemical indexes in patients with senile osteoporosis. Chinese Journal of Clinical Rehabilitation 2004;8(27):5930, 5949. [Google Scholar]
Wei RY 2011 {published data only}
- Wei RY, Lao RP, Liu ZJ, Xu GR, Li ZP. Effect of anti‐osteoporosis capsule on ostealgia and bone metabolism of postmenopausal osteoporosis patients. Journal of New Chinese Medicine 2011;43(1):61‐3. [Google Scholar]
Wu JZ 2010 {published data only}
- Wu JZ, Zhou XM, Zheng CX. Clinical observation of Erxian soup for primary osteoporosis. Journal of New Chinese Medicine 2010;42(2):25‐6. [Google Scholar]
Wu MS 2001 {published data only}
- Wu MS, Zhao SZ, Li E. Clinical study of Kanggusong granule in preventing and treating primary osteoporosis. Traditional Chinese Medicine Research 2001;14(1):26‐8. [PubMed] [Google Scholar]
Wu MS 2007 {published data only}
- Wu MS, Li E, Zhao SZ, Bai X, Li AY. Channel tropism of the prescription for kidney tonifying by target‐oriented administration for osteoporosis. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11(27):5336‐40. [Google Scholar]
Wu W 2005 {published data only}
- Wu W, Li DF, Zhi XM, Han MQ. Preventive and therapeutic effects of Xianling Gubao capsule for postmenopausal osteoporosis. Journal of Guangzhou University of Traditional Chinese Medicine 2005;22(3):191‐3. [Google Scholar]
Xiao W 2008 {published data only}
- Xiao W, Huang MQ. Therapeutic effect observation of selfmade Gusong recipe for postmenopausal osteoporosis. Medicine Innovation Research 2008;5(14):67‐8. [Google Scholar]
Xie J 2004 {published data only}
- Xie J, Shen L, Yang YP, Zhou PQ, Gao L. Prevention action of Chinese herbs for tonifying kidney on bone loss in senile males: 1 year follow‐up. Chinese Journal of Clinical Rehabilitation 2004;8(24):5180‐1. [Google Scholar]
Xie YM 1997 {published data only}
- Xie YM, Zhang FZ, Zhou WQ, Gao P, Fu RJ, Zhao TY, et al. Clinical study of Bugu Shengsui capsule in treating primary osteoporosis with kidney‐yang deficiency syndrome. Chinese Journal of Integrated Traditional and Western Medicine 1997;17(9):526‐30. [PubMed] [Google Scholar]
Xiong YH 2008 {published data only}
- Xiong YH, Teng WR, Liu T, Fu SC, Gu PF, Fu WY, et al. Jiangu granules in treatment of postmenopausal osteoporosis: a randomized, double‐blind, double dummy, multicenter clinical trial. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2008;16(4):17‐21. [Google Scholar]
Xu H 2010 {published data only}
- Xu H, Ren DH, Liang Z, Wang J. Clinical observation of Qianggu capsule plus alendronate on postmenopausal osteoporosis. Journal of Zhejiang University of Traditional Chinese Medicine 2010;34(4):503‐4. [Google Scholar]
Xu M 2009 {published data only}
- Xu M, Liu BX, Huang CJ, Tang FY, Lou YM, Liang Z, et al. Clinical observation of Xianlinggubao plus alendronate on postmenopausal osteoporosis. Journal of Liaoning University of Traditional Chinese Medicine 2009;11(1):94‐5. [Google Scholar]
Xu W 2005 {published data only}
- Xu W, Chen YC, Zhang JF. Clinical observation on effect of Yishen Yanggan mixture in treating liver‐kidney deficiency postmenopausal osteoporosis. Chinese Traditional and Herbal Drugs 2005;36(6):898‐9. [Google Scholar]
Xu YL 2007 {published data only}
- Xu YL, Jin JJ, Xu DY, Zheng Y. Effects of acupoint sticking of Migudan on bone Gla protein and hydroxyproline in the patients of primary osteoporosis. Chinese Journal of Information on Traditional Chinese Medicine 2007;14(6):17‐9. [Google Scholar]
Yang B 2007 {published data only}
- Yang B, Yang J. Clinical research of Huangqi for patients with postmenopausal osteoporosis. Sichuan Medical Journal 2007;28(3):291‐3. [Google Scholar]
Ye AN 1998 {published data only}
- Ye AN. Clinical observation of Bushen Zhuanggutang in treating 60 cases with senile osteoporosis. New Journal of Traditional Chinese Medicine 1998;30(7):52‐3. [Google Scholar]
Yuan YN 2000 {published data only}
- Yuan YN, Liu Y, Han P. Treatment of 30 cases of primary osteoporosis with Shanke Jiegu tables. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2000;6(9):31‐2. [Google Scholar]
Zhang DS 2011 {published data only}
- Zhang DS, Cao W, Chen H, Zhang FK. Treatment of integration of traditional and western medicine on 60 cases with postmenopausal osteoporosis. Traditional Chinese Medicinal Research 2011;24(3):34‐6. [Google Scholar]
Zhang J 2003 {published data only}
- Zhang J, Yin SL, Yin HQ, Ling HW, Li W, Li XQ. Clinical observation on effect of Liuwei Dihuang pills in treating postmenopausal osteoporosis. Journal of Clinical Internal Medicine 2003;20(10):558. [Google Scholar]
Zhang RH 2004 {published data only}
- Zhang RH, Chen KJ, Lu DX, Zhu XF, Ma XC. Clinical study on treatment of postmenopausal osteoporosis by Yiigu capsule. Chinese Journal of Integrated Traditional with Western Medicine 2004;24(8):680‐4. [PubMed] [Google Scholar]
Zhang XG 2011 {published data only}
- Zhang XG. Effect of Zhuangguyin on 100 cases with primary osteoporosis. Acta Chinese Medicine and Pharmacology 2011;39(1):109‐11. [Google Scholar]
Zhang XJ 2008 {published data only}
- Zhang XJ, He BR, Nie J. Clinical study on effect of Bushenhuoxue capsules for treatment of osteoporosis in aged males. Chinese Archives of Traditional Chinese Medicine 2008;26(2):307‐11. [Google Scholar]
Zhang XZ 2004 {published data only}
- Zhang XZ, Han JF, Qian GF, He M, Li Y, Gu L. Effects of Xianlinggubao capsule on bone mineral density and cytokines in postmenopausal osteoporotic patients. Chinese Journal of Osteoporosis 2004;10(1):90‐3. [Google Scholar]
Zhang YL 1996 {published data only}
- Zhang YL, Wu SL. Clinical observation of herbal medicine in treating 120 senile male cases with primary osteoporosis. New Journal of Traditional Chinese Medicine 1996;28(2):58‐60. [Google Scholar]
Zhang YP 2007 {published data only}
- Zhang YP, Cui YH, Kang HY, Zhang B. Therapeutic effect observation and influence of bone formation and damage indexes of Shigu yin for patients with postmenopausal osteoporosis. Journal of New Chinese Medicine 2007;39(1):30‐1. [Google Scholar]
Zhang YS 2003 {published data only}
- Zhang YS, Zhai MY, Guo L, Liu N, Han MX, Bai XM. Clinical and experimental study of the treatment of primary osteoporosis with Huoluo Gukang pills. Journal of Traditional Chinese Orthopedics and Traumatology 2003;15(7):9‐11. [Google Scholar]
Zhang ZF 2011 {published data only}
- Zhang ZF, Ye WH. Effect of Zhuangguqiangjin tablet on cases with liver and kidney deficiency osteoporosis. Journal of New Chinese Medicine 2011;43(5):70‐2. [Google Scholar]
Zhan HS 2009 {published data only}
- Zhan HS, Shi YY, Zhao YF, Hu XY, He ZY, Lou XF, et al. Phase clinical investigation of Xuduanzhuanggu capsules to the primary osteoporotic patient. Chinese Journal of Osteoporosis 2009;15(3):197‐200. [Google Scholar]
Zhan ML 2007 {published data only}
- Zhan ML, Yang YF, Zhang J, Li JP. Therapeutic effect observation of Bushenyangxue decoction for postmenopausal osteoporosis. Gansu Journal of Traditional Chinese Medicine 2007;20(2):37‐8. [Google Scholar]
Zhao G 2002 {published data only}
- Zhao G, Cai DF, Dong SF, Fan Y. Clinical study in 25 cases of postmenopausal osteoporosis treated with Zishen prescription. Journal of Traditional Chinese Medicine 2002;43(6):444‐5. [PubMed] [Google Scholar]
Zhao HX 2001 {published data only}
- Zhao HX. Clinical efficacy of Kanggusong capsule in treating postmenopausal osteoporosis. Chinese Journal of Traditional Chinese Medicine and Pharmacy 2001;16(2):31‐3. [Google Scholar]
Zhao LN 2003 {published data only}
- Zhao LN. Preventive and therapeutic effects of Yinyanghuo for osteoporosis. Modern Journal of Integrated Traditional Chinese and Western Medicine 2003;12(9):922‐3. [Google Scholar]
Zheng WK 2007 {published data only}
- Zheng WK, Liu CY, Han CY, Zhou YJ. Clinical study on treatment of postmenopausal osteoporosis by Jinwugutong capsule. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2007;15(3):30‐2. [Google Scholar]
Zhong RQ 2007 {published data only}
- Zhong RQ, Pan GM, Deng WM, Zhang GC. Clinical study of Gushenyijingtang on the treatment of primary osteoporosis of deficiency in kidney yang. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2007;15(9):16‐8. [Google Scholar]
Zhou LZ 2001 {published data only}
- Zhou LZ, Ma Y. Clinical observation of traditional Chinese medicine on primary osteoporosis. The National Academic Seminar of Osteoporosis and Osteoarthritis in 2001. 2001:134‐9.
Zhou XT 2001 {published data only}
- Zhou XT. Clinical research of 45 cases with osteoporosis treated by Guilu Erxiantang. Jiangsu Journal of Traditional Chinese Medicine 2001;22(6):28. [Google Scholar]
Zhou ZK 2006 {published data only}
- Zhou ZK, Zeng HB, Qin JJ, Chen C. Clinical observation of Huangqi Sanxian tang on 30 cases with postmenopausal osteoporosis. Journal of New Chinese Medicine 2006;38(6):40‐1. [Google Scholar]
Zhu HJ 2011 {published data only}
- Zhu HJ, Duan ZZ, Liao KH, Tan N, Huang SG. Clinical observation on Bushen Tianjing Huoxue therapy in treating primary osteoporosis. Guiding Journal of Traditional Chinese Medicine and Pharmacy 2011;17(12):30‐2. [Google Scholar]
Zhu HM 2012 {published data only}
- Zhu HM, Qin L, Garnero P, Genant HK, Zhang G, Dai K, et al. The first multicenter and randomized clinical trial of herbal Fufang for treatment of postmenopausal osteoporosis. Osteoporosis International 2012;23:1317‐27. [DOI] [PubMed] [Google Scholar]
Zou JJ 2005 {published data only}
- Zou JJ, Liu ZM, Zheng JY, Zhao WM, Liu Y, Shi YQ. Clinical investigation of Gu Ben Jian Gu capsules to the primary osteoporotic patient. Journal of Practical Training of Medicine 2005;33(2):96‐8. [Google Scholar]
References to studies excluded from this review
Ai SC 2003 {published data only}
- Ai SC, Yang L, Liao FZ, Chen XL. Influence of herbal application at Shenque (CV 8) on BGP in primary osteoporosis. Shanghai Journal of Acupuncture and Moxibustion 2003;22(3):39‐40. [Google Scholar]
Ai SC 2003a {published data only}
- Ai SC, Wong M, Liao FZ, Chen XL. Influence of herbal application at Shenque on interleukin‐6 in primary osteoporosis. Journal of Sichuan of Traditional Chinese Medicine 2003;21(2):13‐5. [Google Scholar]
Bo LY 2003 {published data only}
- Bo LY, Wu CS, Wang XX. Clinical observation on the use of Gushukang in treating of osteoporotic compression fracture of the spine. Chinese Journal of Bone Tumor and Bone Disease 2003;2(5):298‐300. [Google Scholar]
Chen AP 2005 {published data only}
- Chen AP, Chen YZ. Clinical observation on effect of Liuwei Dihuang pills in treating senile osteoporosis. Journal of Chinese Modern Medicine 2005;2(5):456‐7. [Google Scholar]
Chen JJ 2010 {published data only}
- Chen JJ, Lou Q, Ning JC. Analysis of CT bone mineral density of senile osteoporosis cases treated by integration of traditional Chinese and western medicine. International Medicine & Health Guidance News 2010;16(12):1488‐90. [Google Scholar]
Chen XF 2003 {published data only}
- Chen XF, Jiang YX. Effect of Xianling Gubao plus vitamin D3 and calcium amino acid chelate on bone mineral density and pain in osteoporosis. Chinese Journal of Clinical Rehabilitation 2003;7(27):3769. [Google Scholar]
Chen XF 2007 {published data only}
- Chen XF, Lan TF. Therapeutic effect observation of Bushen Jianpi Huoxue method for patients with senile osteoporosis. Journal of Sichuan of Traditional Chinese Medicine 2007;25(10):60‐1. [Google Scholar]
Dai Y 2007a {published data only}
- Dai Y, Shen L. Effects of Migu tablet on bone mineral density, serum matrix metalloproteinase‐2 level and bone metabolic markers in postmenopausal osteoporosis. China Journal of Chinese Materia Medica 2007;32(22):2409‐12. [PubMed] [Google Scholar]
Deng WM 1997 {published data only}
- Deng WM, He YS, Feng YJ. Clinical study on postmenopausal osteoporosis syndrome treated with tonifying kidney to strength bone decoction. Chinese Journal of Traditional Medical Science and Technology 1997;4(2):70‐2. [Google Scholar]
Deng WM 1997a {published data only}
- Deng WM, He YS, Feng YJ. Clinical effect of kidney‐tonifying herbs on postmenopausal osteoporosis. Chinese Journal of Osteoporosis 1997;3(1):64‐6. [Google Scholar]
Deng WM 2001 {published data only}
- Deng WM, Shen YG, He YS, Wei S. Influences of Bushen Zhuanggu granule on contents of sexual hormones and metabolism of calcium and phosphorous in early menopausal woman. Journal of Guangzhou University of Traditional Chinese Medicine 2001;18(4):301‐3. [Google Scholar]
Deng WM 2002 {published data only}
- Deng WM, Cui WL, Shen YG, Yang QH, He YS, Wei S. Effects of a 2‐year treatment with Bushen Zhuanggu granule for postmenopausal osteoporosis. Journal of Guangzhou University of Traditional Chinese Medicine 2002;19(4):275‐8. [Google Scholar]
Ding GZ 1995 {published data only}
- Ding GZ, Zhang ZL, Zhou Y, Li R. Clinical study on effect of Bushen Jiangu capsule on postmenopausal osteoporosis. Chinese Journal of Integrated Traditional and Western Medicine 1995;15(7):392‐4. [PubMed] [Google Scholar]
Dong YL 2004 {published data only}
- Dong YL. Clinical observation of Liuwei Dihuang pills combined with west medicine in treating 60 senile patients with deficiency of kidney‐YIN osteoporosis. Clinical Journal of Traditional Chinese Medicine 2004;16(2):135‐6. [Google Scholar]
Fan GF 2008 {published data only}
- Fan GF, Qi ZJ, Chen RX, Dong SL, Wang FG, Zhou CJ. Therapeutic effect observation of Qiangjin Zhuanggu pill for patients with primary osteoporotic lumbago and backache. Journal of Traditional Chinese Orthopedics and Traumatology 2008;20(2):13‐4. [Google Scholar]
Fan ZY 1995 {published data only}
- Fan ZY. Clinical observation on effect of Yugu pill in treating osteoporosis. Journal of Sichuan of Traditional Chinese Medicine 1995;13(1):43‐4. [Google Scholar]
Ge L 2005 {published data only}
- Ge L, Dai FF, Cai X. Clinical observation of treating primary osteoporosis of female patients with Jiangu pills. Jiangsu Journal of Traditional Chinese Medicine 2005;26(3):24‐5. [Google Scholar]
Gong L 2000 {published data only}
- Gong L, Wang MY, Cao JQ, Xu F, Zhou PQ, Hu J. Clinical study of Yishen Zhuanggu mixture in treating 17 cases with osteoporosis. Journal of Traditional Chinese Medicine 2000;41(10):606‐7. [Google Scholar]
Gong ZF 2011 {published data only}
- Gong ZF. Effect of Bushenshujing soup and Celecoxib on primary osteoporosis with low back pain. Chinese Journal of Clinical Research 2011;24(2):155‐6. [Google Scholar]
Gu M 2004 {published data only}
- Gu M, Guo JN. Clinical study of the treatment of primary osteoporosis with Fortune's drynaria rhizome. Chinese Journal of Rehabilitation 2004;19(5):297. [Google Scholar]
Guo F 2003 {published data only}
- Guo F. Clinical observation on Yishen Yanggan decoction treating 30 cases of osteoporosis. Modern Diagnosis & Treatment 2003;14(5):268‐9. [Google Scholar]
Guo HN 1998 {published data only}
- Guo HN, Wang WZ, Tian WM. Clinical observation of herbal medicine in treating 120 primary osteoporosis. Journal of Hebei Traditional Chinese Medicine and Pharmacology 1998;13(3):4‐6. [Google Scholar]
Gu T 2011 {published data only}
- Gu T, Jin W. Integration of traditional and western medicine on 26 primary osteoporosis. Yunnan Journal of Traditional Chinese Medicine and Materia Medica 2011;32(4):32. [Google Scholar]
He YC 2008 {published data only}
- He YC, Liao KX, Huang YR, Jiang TB. Curative effect of Xianlinggubao capsule on thoracolumbar burst fracture in senile patients with osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2008;163(312‐13):12‐3. [Google Scholar]
Hu XD 2011 {published data only}
- Hu XD. Clinical discussion on treating 164 cases of patients with senile osteoporosis in TCM. Clinical Journal of Chinese Medicine 2011;3(3):94‐5. [Google Scholar]
Jia JH 2010 {published data only}
- Jia JH, Chen XF, Zhang Z, Wang XP. Shugukang decoction in the treatment of primary osteoporosis: a clinical observation of 60 cases. Journal of Anhui Traditional Chinese Medical College 2010;29(1):28‐31. [Google Scholar]
Jiang SY 1995 {published data only}
- Jiang SY, Shang YP, Wu LS, Liu BY, Zhang L, Guo RY, et al. Clinical observation of Gushukang granule on osteoporosis. Chinese Journal of Osteoporosis 1995;1(2):158, 167‐9. [Google Scholar]
Ke Q 2005 {published data only}
- Ke Q, Liu HQ, Niu W. Effect of kidney‐tonifying herbs on bone mineral density, serum calcium and serum alkaline phosphatase in postmenopausal osteoporosis patients. Chinese Journal of Clinical Rehabilitation 2005;9(31):186, 189. [Google Scholar]
Ke Q 2005a {published data only}
- Ke Q, Liu HQ, Chen XC, Wu DF. Clinical observation of kidney‐tonifying herbs in treating 95 senile male cases with renal deficiency primary osteoporosis. Information on Traditional Chinese Medicine 2005;22(6):26‐7. [Google Scholar]
Li BL 2007a {published data only}
- Li BL, Li YF, Tan JW, Geng WZ, Zhan Q. Effect of bone metabolism index of Jianshen prescription for patients with postmenopausal osteoporotic vertebral fracture. Journal of Traditional Chinese Orthopedics and Traumatology 2007;19(9):4‐5. [Google Scholar]
Li CX 2005 {published data only}
- Li CX. Effect of movement therapy plus herbal medicine on bone mineral density in senile osteoporosis. Chinese Journal of Clinical Rehabilitation 2005;9(7):95, 97. [Google Scholar]
Li JF 2008 {published data only}
- Li JF, Xiao YH. Therapeutic effect observation of Bushen Qianggu tang for primary osteoporosis. Practical Clinical Medicine 2008;9(5):66‐7. [Google Scholar]
Lin HY 1998 {published data only}
- Lin HY, Qi ZX. Clinical observation of Yishen Jiangu tang in treating osteoporosis. Fujian Journal of Traditional Chinese Medicine 1998;29(2):9‐10. [Google Scholar]
Liu HB 2005 {published data only}
- Liu HB, Ai SC, Wu LD. Clinical observation of medicine‐separated sticking on Shenque on sex hormone in primary osteoporosis. Journal of Sichuan of Traditional Chinese Medicine 2005;23(9):74‐5. [Google Scholar]
Liu HQ 2010 {published data only}
- Liu HQ, Qin JJ. Effect of Gukang granule in treating postmenopausal osteoporosis with kidney yang deficiency. Journal of Shandong University of TCM 2010;34(1):48‐9. [Google Scholar]
Liu TS 2008 {published data only}
- Liu TS. Therapeutic effect of integrative Chinese and Western medicine for patients with osteoporosis in 62 cases. Journal of Shanxi College of Traditional Chinese Medicine 2008;9(6):34‐5. [Google Scholar]
Liu YS 2011 {published data only}
- Liu YS. Treatment of 49 cases of postmenopausal osteoporosis with Jiaweierxian soup. Guangming Journal of Chinese Medicine 2011;26(4):698‐9. [Google Scholar]
Liu YX 1998 {published data only}
- Liu YX. Clinical study of Fuguning granule on postmenopausal osteoporosis. Liaoning Journal of Traditional Chinese Medicine 1998;25(6):284‐5. [Google Scholar]
Li YM 2005 {published data only}
- Li YM. Treatment of 60 cases of postmenopausal osteoporosis with Bushen Huoxue preparation. Journal of Nanjing University of Traditional Chinese Medicine 2005;21(1):56‐7. [Google Scholar]
Luo QY 2011 {published data only}
- Luo QY, Wen DH. Treatment of 24 cases of senile osteoporosis with Huoluoxiaoling pill. Journal of Practical Traditional Chinese Medicine 2011;27(4):245. [Google Scholar]
Lu W 2004 {published data only}
- Lu W, Chu XG, Peng TP. Clinical study of the treatment of primary osteoporosis with Bushen Jiangu tang. Jiangxi Journal of Traditional Chinese Medicine 2004;35(259):27. [Google Scholar]
Lu ZD 2004 {published data only}
- Lu ZD, Wang YS. Clinical observation of Gushukang in treating 80 primary osteoporosis patients. Chinese Journal of Clinical Rehabilitation 2004;8(18):3652. [Google Scholar]
Pan X 2004 {published data only}
- Pan X, Wang DY. Treatment of 34 cases of primary osteoporosis with Huangqi injection to acupoint. Shanxi Journal of Traditional Chinese Medicine 2004;25(12):1133‐4. [Google Scholar]
Peng T 2003 {published data only}
- Peng T, Chu ZR, Lu Y, Qi K, Liu JQ, Huang JH. Clinical observation of Qianggu extracts in treating postmenopausal osteoporosis. Acta Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai 2003;17(3):25‐7. [Google Scholar]
Qi ZX 2000 {published data only}
- Qi ZX, Lin HY. The clinical research of Shengsuiyin on the primary osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2000;8(5):21‐2. [Google Scholar]
Ren JH 2011 {published data only}
- Ren JH. Effect of Jinwugutong and alendronate sodium on postmenopausal osteoporosis patients with pain. Guiding Journal of Traditional Chinese Medicine and Pharmacy 2011;17(1):60‐1. [Google Scholar]
Shang YM 2007 {published data only}
- Shang YM, Liu YY, Li HP. Clinical observation of Xianlinggubao capsule on postmenopausal osteoporosis. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care 2007;14(1):55. [Google Scholar]
Shi B 2010 {published data only}
- Shi B. Clinical observation of traditional Chinese Medicine for senile osteoporosis. Chinese Journal of Ethnomedicine and Ethnopharmacy 2010;19(12):39‐40. [Google Scholar]
Shou ZX 2008 {published data only}
- Shou ZX, Shen L, Fan H, Xie J, Yang YP, Zhou PQ, et al. Clinical observation on Qianggu Huoli pills in the treatment of primary osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2008;16(10):1‐2. [Google Scholar]
Shuai B 2008 {published data only}
- Shuai B, Shen L, Yang YP, Xie J, Zhou PQ, Gao L, et al. Clinical study (phase III) on the effects of Jiangu granula in primary osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2008;16(3):27‐30. [Google Scholar]
Shu J 2005 {published data only}
- Shu J. Clinical observation of Bushen Yijing prescription in treating 50 osteoporosis patients. Journal of Occupational Health and Damage 2005;20(2):143‐4. [Google Scholar]
Song HX 2003 {published data only}
- Song HX. Treatment of 84 cases of osteoporosis with Bushen Yijing method. Shanxi Journal of Traditional Chinese Medicine 2003;24(12):1075‐7. [Google Scholar]
Song XW 1997 {published data only}
- Song XW, Song ZJ, Shen PZ. Clinical observation of Bushen herbal medicine in treating postmenopausal osteoporosis. The Practical Journal of Integrating Chinese with Modern Medicine 1997;10(9):892. [Google Scholar]
Song XW 2001 {published data only}
- Song XW, Shen PZ, Chen BX. Clinical study of Bushenhuoxue recipe on postmenopausal osteoporosis. China Journal of Orthopaedics and Traumatology 2001;14(8):476. [Google Scholar]
Su CH 2004 {published data only}
- Su CH, Cui TH, Sang SB, Wu YW, Ding S, Jiang YM, et al. Comparison of strong bone capsule and calcium carbonate combined with vitamin D in the treatment of senile osteoporosis. Chinese Journal of Clinical Rehabilitation 2004;8(29):6429‐31. [Google Scholar]
Su CH 2005 {published data only}
- Su CH, Cui TH, Sang SB, Wu YW, Wang DL, Jiang JW, et al. Clinical study of 105 patients with postmenopausal osteoporosis treated by TCM strong bone capsule and Caltrate. Liaoning Journal of Traditional Chinese Medicine 2005;32(7):701‐3. [Google Scholar]
Sun JM 2002 {published data only}
- Sun JM, Fu GM, Zhang HL. Analysis of the therapeutic effect of Yiqi Tiansui mixture on osteoporosis. Chinese Journal of Clinical Rehabilitation 2002;6(9):1309. [Google Scholar]
Sun X 2002 {published data only}
- Sun X. Clinical observation of the therapeutic effect of Jiawei Zuogui pill on osteoporosis with back pain. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2002;10(5):36‐7. [Google Scholar]
Sun YM 2008 {published data only}
- Sun YM, Wang PM, Zha W, Zhao HQ, Xia JL, Liu XQ, et al. Research on the influence of the manifestations in the primary osteoporosis patients by Ge Gen (Radix Puerariae). Chinese Journal of Traditional Medical Traumatology & Orthopedics 2008;16(4):14‐6. [Google Scholar]
Su YB 2008 {published data only}
- Su YB, Li MX, Lin YF, Zheng YM, Wu ZB, Rao HC, et al. Clinical observation of Qianggu Huoxue tang for primary osteoporosis combined with distal radius fracture. Journal of Traditional Chinese Orthopedics and Traumatology 2008;20(7):17‐8. [Google Scholar]
Su ZW 2010 {published data only}
- Su ZW, Zheng ZY, Jin J. Clinical observation of Bushenzhuanggu soup for primary osteoporosis. Journal of Hebei Traditional Chinese Medicine and Pharmacology 2010;25(1):17‐8. [Google Scholar]
Tang JM 1999 {published data only}
- Tang JM, Liu KJ, Wang GX. Effect of Jiangu powder on bone mineral density in three type of renal deficiency of osteoporosis. Chinese Journal of Information on Traditional Chinese Medicine 1999;6(10):51. [Google Scholar]
Tan X 2010 {published data only}
- Tan X, Huang H, Liu Z, Liu HY, Shao Y, Ceng D, et al. Clinical study of the effect of Bushen Zhuanggu powder on bone transformation in aged males with osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2010;18(7):17‐20. [Google Scholar]
Tu PS 2008 {published data only}
- Tu PS, Deng WQ, Li MJ, Wu XY, Lv ZY, Zhou HJ. Clinical observation of Shuanggu decoction for patients with postmenopausal osteoporosis in 30 cases. Chinese Journal of Traditional Medical Science and Technology 2008;15(6):471‐2. [Google Scholar]
Wang CH 2003 {published data only}
- Wang CH, Tian LM, Jiang YP, Li JC, Chen XF, Li F, et al. Clinical effects of Gusongkang capsule on senile osteoporosis. Journal of the Fourth Military Medical University 2003;24(11):1029‐31. [Google Scholar]
Wang GH 2011 {published data only}
- Wang GH, Wei XX. Clinical observation on primary osteoporosis treated by Zhuanggutongluobao. China Journal of Chinese Medicine 2011;26(8):999‐1000. [Google Scholar]
Wang HM 1998 {published data only}
- Wang HM, Ge JR, Li N. Clinical observation of treating osteoporosis of old people with Bugu capsule. Chinese Journal of Traditional Medical Traumatology & Orthopedics 1998;6(4):13‐4. [Google Scholar]
Wang J 2008 {published data only}
- Wang J, Li WJ, Wang HY, Yang JY, Lin XS, Li A. Therapeutic effect observation of Ganshen Tongzhi method in treating postmenopausal osteoporosis in 38 cases. Journal of New Chinese Medicine 2008;40(6):54‐5. [Google Scholar]
Wang JH 2001 {published data only}
- Wang JH, Li ZG. The investigation on the treatment effect with osteoporosis by Qianggu pill. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 2001;7(5):243‐4. [Google Scholar]
Wang LM 2004 {published data only}
- Wang LM, Guo HS, Guo MX. Clinical observation on effect of Jiangu tang in treating 69 cases with senile osteoporosis. Chinese Journal of Clinical Practical Medicine 2004;5(11):103‐4. [Google Scholar]
Weng M 2003 {published data only}
- Weng M, Ai SC. Influence of medicine‐separated sticking on Shenque (RN8) on calcitonin in primary osteoporosis. Tianjin Journal of Traditional Chinese Medicine 2003;20(5):48‐9. [Google Scholar]
Wu CM 2004 {published data only}
- Wu CM, Zhang SQ, Zhang ZM. Clinical observation on effect of Bushen Zhuanggu capsule in treating osteoporosis. Journal of Sichuan Traditional Chinese Medicine 2004;22(2):76‐7. [Google Scholar]
Wu D 2008 {published data only}
- Wu D, Chen RH, Fang JL. Bushen Tiaogan method for patients with senile osteoporosis in 31 cases. Journal of Shandong University of Traditional Chinese Medicine 2008;32(2):126‐7. [Google Scholar]
Wu XG 2008 {published data only}
- Wu XG, Wu DH. Effects of Xianlinggubao, compound calcium amino acid chelate capsules and salcalcitonin in treating osteoporosis fracture. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2008;16(4):20‐1. [Google Scholar]
Xia WF 2006 {published data only}
- Xia WF, Chen LL. Comparison of traditional Chinese medicine (Qianggu capsule) and risedronate sodium in management of postmenopausal osteoporosis. Chinese Journal of Osteoporosis 2006;12(4):393‐6. [Google Scholar]
Xie J 2001 {published data only}
- Xie J, Shen L, Yang YP, Zhou PQ, Gao L. The effect of Chinese traditional medicine of kidney invigoration on bone mass after Colles' fracture. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2001;9(2):1‐3. [Google Scholar]
Xie J 2003 {published data only}
- Xie J, Shen L, Yang YP, Zhou PQ, Gao L. Clinical study on bone loss preventive effect of Chinese drugs for kidney‐tonifying in aged males. Chinese Journal of Integrated Traditional and Western Medicine 2003;23(1):4‐6. [Google Scholar]
Xu CY 2004 {published data only}
- Xu CY, Li BS, Xin BZ. Effect of the self‐made Bushen Jianpi Tongluo decoction on pain and bone mineral density of patients with osteoporosis. Chinese Journal of Clinical Rehabilitation 2004;8(30):6698‐9. [Google Scholar]
Xu LZ 2001 {published data only}
- Xu LZ, Hou BX, Shi Q, Xie KY. Analysis of the therapeutic effect of Yishen Zhuanggu granules on osteoporosis. Journal of Traditional Chinese Orthopedics and Traumatology 2001;13(9):9‐10. [Google Scholar]
Xu PH 2001 {published data only}
- Xu PH, Huang ZJ, Xiong FL, Zhang HM, Yu DK. Clinical study of Bushen Jiangu capsule in treating liver‐kidney deficiency osteoporosis. Traditional Chinese Drug Research & Clinical Pharmacology 2001;12(4):245‐7, 307. [Google Scholar]
Yang JP 2007 {published data only}
- Yang JP, Wang JB, Ding CG, Huo ST. Effect of Gushibao capsule on bone density and bone metabolism of osteoporosis. Hebei Journal of Traditional Chinese Medicine 2007;29(5):399‐400. [Google Scholar]
Yang WH 2010 {published data only}
- Yang WH, Shi X. Clinical study on Bushen Tongluo decoction plus western medicine in treating primary osteoporosis of kidney deficiency and blood‐stasis. Shanghai Journal of Traditional Chinese Medicine 2010;44(7):36‐7, 46. [Google Scholar]
Yan XP 2007 {published data only}
- Yan XP, Zhu JL, Yan Y, Kong WP, Lu P, Tao QW. Clinical study of Bushen Qiangdu recipe on 102 cases with ankylosing spondylitis, osteoporosis or osteopenia. China Journal of Traditional Chinese Medicine and Pharmacy 2007;22(8):571‐3. [Google Scholar]
Yu YH 2011 {published data only}
- Yu YH, Chen JY, Wang J. Effect of Erzhi pill on postmenopausal osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2011;19(4):42‐3. [Google Scholar]
Zhang GM 2001 {published data only}
- Zhang GM, Guan SY, Wang WZ. Clinical study in postmenopausal osteoporosis treated with Xianguning powder. Acta Chinese Medicine and Pharmacology 2001;29(2):31‐2. [Google Scholar]
Zhang H 1999 {published data only}
- Zhang H, Wei ZY, Dong JW, Cui DZ, Cheng YC. Clinical observation on effect of Gubao capsule in treating primary osteoporosis. Journal of Traditional Chinese Orthopedics and Traumatology 1999;11(11):3‐4, 63. [Google Scholar]
Zhang WY 2005 {published data only}
- Zhang WY. Report of treating 45 cases of senile osteoporosis with Bupi Yishen Huoxue prescription. Gansu Journal of Traditional Chinese Medicine 2005;18(12):22‐3. [Google Scholar]
Zhao G 2004 {published data only}
- Zhao G, Xu ZL, Shao QX, Feng JL, Xue JP, Wang XG, et al. Comparison of tibolone tablets and kidney‐invigorating traditional Chinese medicine in prevention and treatment of postmenopausal osteoporosis. Chinese Journal of Osteoporosis 2004;10(3):337‐9. [Google Scholar]
Zhao Z 2010 {published data only}
- Zhao Z, Xiang K, Sun L. Clinical observation of 30 cases of primary osteoporosis in elderly people treated with Yanghe capsule. World Journal of Integrated Traditional and Western Medicine 2010;5(8):698‐700. [Google Scholar]
Zheng NX 2011 {published data only}
- Zheng NX, Zhang XH, Guo HM, Wang PC, Du HF, Guo HY, et al. Clinical observation of Duhuojishengtang on osteoporosis. Hebei Medicine 2011;17(2):225‐7. [Google Scholar]
Zheng XH 2004 {published data only}
- Zheng XH, Huang L. The clinical research of Gukangye on the primary osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2004;12(3):20‐1. [Google Scholar]
Zheng ZW 2010 {published data only}
- Zheng ZW, Wang Z. Clinical observation of Bushenzhuanggu soup on primary osteoporosis. Family Medicine (Medical Tribune) 2010;2(7):516‐8. [DOI: 10.3969/j.issn.1671-4954.2010.07.030] [DOI] [Google Scholar]
Zhong J 2010 {published data only}
- Zhong J, Tan SS, Xie LS. Zhuanggubushen decoction and acupuncture treat osteoporosis. Journal of Zhejiang University of Traditional Chinese Medicine 2010;34(3):408, 410. [Google Scholar]
Zhong RQ 2007a {published data only}
- Zhong RQ, Pan GM, Deng WM, Zhang GC. Clinical observation on effects of Gushenyijingtang in treating hip osteoporotic fractures. Chinese Journal of Traditional Medical Traumatology & Orthopedics 2007;15(7):50‐2. [Google Scholar]
Zhong RQ 2007b {published data only}
- Zhong RQ, Pan GM, Deng WM, Zhang GC. Clinical observation of Gushenyijing tang on treatment of thoracic vertebral compression osteoporotic fracture. Journal of South Medical University 2007;27(11):1724‐5. [Google Scholar]
Zhou PQ 1997 {published data only}
- Zhou PQ, Shen L, Yang YP, Xie J. Clinical study on effect of Migu tablet on postmenopausal osteoporosis. Chinese Journal of Traditional Medical Traumatology & Orthopedics 1997;15(1):20‐2. [Google Scholar]
Zhu LP 2008 {published data only}
- Zhu LP. Effect of Bushen Jianpi decoction on osteoporosis during climacteric. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine 2008;18(3):139‐41. [Google Scholar]
Additional references
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bengner 1988
- Bengner U, Johnell O, Redlund‐Johnell I. Changes in incidence and prevalence of vertebral fractures during 30 years. Calcified Tissue International 1988;42(5):293‐6. [DOI] [PubMed] [Google Scholar]
Cameron 2013
- Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD010538] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2012
- Chen J, Yao Y, Chen H, Kwong JSW, Chen J. Shengmai (a traditional Chinese herbal medicine) for heart failure. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD005052.pub4] [DOI] [PubMed] [Google Scholar]
Chen 2013
- Chen Y, Gong Z, Chen X, Tang L, Zhao X, Yuan Q, et al. Tripterygium wilfordii Hook F (a traditional Chinese medicine) for primary nephrotic syndrome. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD008568.pub2] [DOI] [PubMed] [Google Scholar]
Cole 2008
- Cole ZA, Dennison EM, Cooper C. Osteoporosis epidemiology update. Current Rheumatology Reports 2008;10(2):92‐6. [DOI] [PubMed] [Google Scholar]
Confavreux 2012
- Confavreux CB, Canoui‐Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat R. Persistence at 1 year of oral anti‐osteoporotic drugs: a prospective study in a comprehensive health insurance database. European Journal of Endocrinology 2012;166(4):735‐41. [DOI] [PubMed] [Google Scholar]
Cumming 1997
- Cumming RG, Nevitt MC, Cummings SR. Epidemiology of hip fractures. Epidemiologic Reviews 1997;19(2):244‐57. [DOI] [PubMed] [Google Scholar]
Diez 2002
- Diez F. Guidelines for the diagnosis of osteoporosis by densitometric methods. Journal of Manipulative and Physiological Therapeutics 2002;25(6):403‐15. [DOI] [PubMed] [Google Scholar]
Egger 2003
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment 2003;7(1):1‐76. [PubMed] [Google Scholar]
Feng 2013
- Feng M, Yuan W, Zhang R, Fu P, Wu T. Chinese herbal medicine Huangqi type formulations for nephrotic syndrome. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD006335.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flower 2012
- Flower A, Liu JP, Lewith G, Little P, Li Q. Chinese herbal medicine for endometriosis. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD006568.pub3] [DOI] [PubMed] [Google Scholar]
Gan 2013
- Gan T, Chen J, Jin SJ, Wang Y. Chinese medicinal herbs for cholelithiasis. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD004547.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Genant 1999
- Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, et al. Interim report and recommendations of the World Health Organization Task‐Force for Osteoporosis. Osteoporosis International 1999;10(4):259‐64. [DOI] [PubMed] [Google Scholar]
Gu 2013
- Gu S, Yang AWH, Xue CCL, Li CG, Pang C, Zhang W, et al. Chinese herbal medicine for atopic eczema. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD008642.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2003a
- Guo SHF. Advances in treatment of osteoporosis (I). Modern Diagnosis & Treatment 2003;14(5):257‐62. [Google Scholar]
Guo 2003b
- Guo SHF. Advances in treatment of osteoporosis (II). Modern Diagnosis & Treatment 2003;14(6):321‐6. [Google Scholar]
Guo 2012
- Guo Z, Jia X, Liu JP, Liao J, Yang Y. Herbal medicines for advanced colorectal cancer. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD004653.pub3] [DOI] [PubMed] [Google Scholar]
Haguenauer 2005
- Haguenauer D, Welch V, Shea B, Tugwell P, Wells G. Fluoride for treating postmenopausal osteoporosis. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD002825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Holroyd 2008
- Holroyd C, Cooper C, Dennison E. Epidemiology of osteoporosis. Best Practice & Research Clinical Endocrinology & Metabolism 2008;22(5):671‐85. [DOI] [PubMed] [Google Scholar]
Horst‐Sikorska 2011
- Horst‐Sikorska W, Wawrzyniak A. The role of hormonal therapy in osteoporosis. Endokrynologia Polska 2011;62(1):61‐4. [PubMed] [Google Scholar]
Howe 2011
- Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD000333] [DOI] [PubMed] [Google Scholar]
ICH GCP 1997
- International Conference on Harmonisation Expert Working Group. Code of Federal Regulations & International Conference on Harmonisation Guidelines. 1. Vol. 1, Pennsylvania: Parexel/Barnett, 1997. [Google Scholar]
IOF 2014
- International Osteoporosis Foundation. What is Osteoporosis?. http://www.iofbonehealth.org/what‐is‐osteoporosis.
Kushner 1998
- Kushner P. Osteoporosis: unmasking the silent epidemic. Hospital Medicine 1998;34:25‐39. [Google Scholar]
Leong 2002
- Leong KH. Medical treatment of osteoporosis‐‐increasing options. Annals of the Academy of Medicine, Singapore 2002;31(1):43‐7. [PubMed] [Google Scholar]
Meng 2003
- Meng XD, Xie YM, Huang YZH, Cui TH, Gao R, Tu RSH, et al. Clinical study of Gu‐Jian capsule in treating primary osteoporosis with kidney‐yang deficiency syndrome. Chinese Journal of Clinical Rehabilitation 2003;7(3):445‐7, 457. [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Nguyen 2000
- Nguyen TV, Center JR, Eisman JA. Association between breast cancer and bone mineral density: the Dubbo Osteoporosis Epidemiology Study. Maturitas 2000;36(1):27‐34. [DOI] [PubMed] [Google Scholar]
NOF 2014
- National Osteoporosis Foundation. What is Osteoporosis?. http://nof.org/articles/7.
Osteoporosis 1999
- Osteoporosis diagnostic criteria subject group in China. The Chinese diagnostic criteria of primary osteoporosis. Chinese Journal of Osteoporosis 1999;5(1):1‐3. [Google Scholar]
Qiu 2001
- Qiu GX. Osteoporosis and fracture. The First Countrywide Osteoporosis Academic Conference of China Medical Association. 2001:12‐9.
Raisz 2005
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. Journal of Clinical Investigation 2005;115(12):3318‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rates 2001
- Rates SMK. Plants as source of drugs. Toxicon 2001;39(5):603‐13. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rizzoli 2012
- Rizzoli R. Bisphosphonates treatment in patients with osteoporosis. Therapeutische Umschau. Revue Thérapeutique 2012;69(3):173‐81. [DOI] [PubMed] [Google Scholar]
Sambrook 2000
- Sambrook P, O'Neill S, Diamond T, Flicker L, MacLennan A. Postmenopausal osteoporosis treatment guidelines. Australian Family Physician 2000;29(8):756‐8. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Silverman 2012
- Silverman S, Christiansen C. Individualizing osteoporosis therapy. Osteoporosis International 2012;23(3):797‐809. [DOI] [PubMed] [Google Scholar]
Vickers 1998
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials 1998;19(2):159‐66. [DOI] [PubMed] [Google Scholar]
Wells 2008a
- Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Welch V, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD001155.pub2] [DOI] [PubMed] [Google Scholar]
Wells 2008b
- Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Welch V, et al. Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003376.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2008c
- Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Welch V, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004523.pub3] [DOI] [PubMed] [Google Scholar]
WHO 1994
- World Health Organization. Assessment of fracture risk and its application to screening postmenopausal osteoporosis. Report of a WHO Study Group. WHO Technical Report Series 843, 1994:1‐129. [PubMed]
Winsloe 2009
- Winsloe C, Earl S, Dennison EM, Cooper C, Harvey NC. Early life factors in the pathogenesis of osteoporosis. Current Osteoporosis Reports 2009;7(4):140‐4. [DOI] [PubMed] [Google Scholar]
Woolf 2003
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bulletin of the World Health Organization 2003;81(9):646‐56. [PMC free article] [PubMed] [Google Scholar]
Wylie 2010
- Wylie CD. Setting a standard for a "silent" disease: defining osteoporosis in the 1980s and 1990s. Studies in History and Philosophy of Biological and Biomedical Sciences 2010;41(4):376‐85. [DOI] [PubMed] [Google Scholar]
Xia 2001
- Xia YX. Medicated diets therapy for osteoporosis. The First Countrywide Osteoporosis Academic Conference of China Medical Association. 2001:171‐2.
Xue 2000
- Xue Y. The retrospect and prospect of osteoporosis. New Century Seminar of Osteoporosis and Arthrosis Prospect and Constructing Convention of Osteoporosis and Osteoarthrosis of China Health Care Scientific and Technological College. 2000:1.
Yang 2013
- Yang J, Zhu L, Wu Z, Wang Y. Chinese herbal medicines for induction of remission in advanced or late gastric cancer. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD005096.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2012
- Zhang H, Huang QR, Gu JM, Hu WW, Liu YJ, Hu YQ, et al. Comparison of the effects of cholecalciferol and calcitriol on calcium metabolism and bone turnover in Chinese postmenopausal women with vitamin D insufficiency. Acta Pharmacologica Sinica 2012;33:490‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2001b
- Zhou LZH, Ma Y. Clinical examining of purgation‐tonifying therapy of Chinese traditional medicine on primary osteoporosis. Academic Seminar of Countrywide Osteoporosis and Osteoarthrosis. 2001:134‐9.


