Abstract
Background/Aims
Sharing trial results with participants is an ethical imperative but often does not happen. Show RESPECT (ISRCTN96189403) tested ways of sharing results with participants in an ovarian cancer trial (ISRCTN10356387). Sharing results via a printed summary improved patient satisfaction. Little is known about staff experience and the costs of communicating results with participants. We report the costs of communication approaches used in Show RESPECT and the views of site staff on these approaches.
Methods
We allocated 43 hospitals (sites) to share results with trial participants through one of eight intervention combinations (2 × 2 × 2 factorial; enhanced versus basic webpage, printed summary versus no printed summary, email list invitation versus no invitation). Questionnaires elicited data from staff involved in sharing results. Open- and closed-ended questions covered resources used to share results and site staff perspectives on the approaches used. Semi-structured interviews were conducted. Interview and free-text data were analysed thematically. The mean additional site costs per participant from each intervention were estimated jointly as main effects by linear regression.
Results
We received questionnaires from 68 staff from 41 sites and interviewed 11 site staff. Sites allocated to the printed summary had mean total site costs of sharing results £13.71/patient higher (95% confidence interval (CI): −3.19, 30.60; p = 0.108) than sites allocated no printed summary. Sites allocated to the enhanced webpage had mean total site costs £1.91/patient higher (95% CI: −14, 18.74; p = 0.819) than sites allocated to the basic webpage. Sites allocated to the email list had costs £2.87/patient lower (95% CI: −19.70, 13.95; p = 0.731) than sites allocated to no email list. Most of these costs were staff time for mailing information and handling patients’ queries. Most site staff reported no concerns about how they had shared results (88%) and no challenges (76%). Most (83%) found it easy to answer queries from patients about the results and thought the way they were allocated to share results with participants would be an acceptable standard approach (76%), with 79% saying they would follow the same approach for future trials. There were no significant effects of the randomised interventions on these outcomes. Site staff emphasised the importance of preparing patients to receive the results, including giving opt-in/opt-out options, and the need to offer further support, particularly if the results could confuse or distress some patients.
Conclusions
Adding a printed summary to a webpage (which significantly improved participant satisfaction) may increase costs to sites by ~£14/patient, which is modest in relation to the cost of trials. The Show RESPECT communication interventions were feasible to implement. This information could help future trials ensure they have sufficient resources to share results with participants.
Keywords: Feedback of results, communicating results, researcher perspective, researcher–participant relations, trial conduct, trial ethics, mixed methods
Background/aims
Offering trial participants the results of trials they have participated in is an important part of the ethical conduct of clinical trials.1,2 There is evidence from a broad range of studies that most trial participants want to receive trial results.3–7 However, in practice, it often does not happen or is done poorly. The 2021 UK Health Research Authority research transparency report states that ‘90% of clinical trials have not told participants about findings’. 8 A 2016 survey of authors of clinical trials publications from 2014 to 2015 found that only 27% of them reported disseminating results to participants. 9
Barriers to communicating results include practical challenges; concern about the impact of sharing results; uncertainty about how to do it; lack of guidance or incentives9,10 or researchers simply not thinking about it. 11 Cost is a major barrier to sharing results with participants, with many trials not having budgeted for this activity.9,12–16 Sharing of results often occurs after the trial funding period, making it difficult to cover these costs. Trial staff may also have moved to work on new projects, leaving insufficient human resources for this activity.9,15–17
The literature on the feasibility and resource requirements for different approaches to sharing results with trial participants is sparse. The US Center for Information and Study on Clinical Research Participation reported that, when results were returned, each site spent around 30 min to 2 h sending out result summaries to trial participants 18 ; although the number of participants at these sites was not discussed. Another study reported the costs of £1624 for running an online meeting for participants and other stakeholders. 19 However, this cost estimate does not include the 40 h of staff time required to organise the event. The other cost estimate reported in the literature is from a study which sent out leaflets by post, with printing and postage cost coming to £1.22 per participant, excluding the leaflet development and mailing time. 5 Better information on what are the resource requirements for approaches to sharing results could help researchers plan appropriately.
The Show RESPECT study elucidated how to share trial results with participants, testing three approaches in a cluster randomised factorial trial,7,20 within the context of the ICON8 ovarian cancer trial. 21 Data collected from trial participants showed that mailing printed summaries, alongside a link to a webpage ± email list, resulted in higher satisfaction with how the results were shared than the link to a webpage ± email list without the printed summary.7,20 Full results from the Show RESPECT study have been published as part of a doctoral thesis. 20 This article aims to explore the resource implications and views of site staff on the acceptability and feasibility of these approaches to sharing results.
Methods
We used a mixed-methods approach to collect data from site staff on the resources required for sharing results (reported at cluster (site) level) and their views on the process, concerns and challenges faced (reported at individual level). The qualitative and quantitative data have equal weight in their contribution to addressing the research aims. For clarity, we refer to ICON8 participants as ‘patients’ and site staff who contributed data to Show RESPECT as ‘site staff’. The study protocol is available online. 22
Quantitative methods
Supplemental Table S1 contains the Consolidated Standards of Reporting Trials (CONSORT) 2010 checklist.
Trial design
Show RESPECT was a cluster randomised 2 × 2 × 2 factorial trial within the ICON8 ovarian cancer trial (ISRCTN10356387). 21 We randomised each UK trial site (secondary or tertiary hospital) (cluster) in ICON8 that agreed to take part in Show RESPECT to a combination of interventions to communicate ICON8 results to participants. 7 Sites were stratified for randomisation by the number of ICON8 patients alive at the time the results were available (small < 6, medium 6–11 and large ≥ 12). The primary outcome for the study overall, reported previously, was patient satisfaction with how the results were shared.7,20 This article reports results around site staff perspectives on communicating results to participants.
Interventions
Staff at participating sites mailed a Patient Update Information Sheet to all ICON8 patients who were known to be still alive informing them that trial results were available together with access information, including the URL of their randomised webpage (basic or enhanced) and email list sign-up instructions if randomised to the email list. The Patient Update Information Sheet told patients at sites randomised to the printed summary that they would be mailed a printed summary of the results after 3 weeks and how to opt out of receiving it. A full description of the interventions is contained in our previous report.7,20
Participants
For the results presented in this report, participants were site staff who had been involved in sharing the ICON8 results with people taking part in ICON8 (e.g. through mailing information or answering queries).
Outcomes
The primary outcome for this sub-study of Show RESPECT was the cost to site per participant. This is a composite endpoint made up of
An estimate of the cost of the time taken to deliver the interventions at a site (multiplying the reported time taken by the cost per hour for the job role of the staff member(s) who delivered the interventions taken from the National Institute of Health Research Schedule of Events Cost Attribution Template, assuming a medical research charity funder);
An estimate of the cost of the time taken to deal with queries;
Any non-staff costs associated with result dissemination incurred by sites.
These costs were divided by the number of ICON8 patients at the site who were alive at the time results were shared.
Secondary outcomes from site staff data are listed in the S2 Text of the Supplemental Material.
Quantitative data were elicited from site staff by one questionnaire completed immediately after sharing results and another completed 2–3 months later (S3 Text of the Supplemental Material). Site staff involved in sending out printed information to patients were asked to complete the first questionnaire, and site staff involved in dealing with patient queries were asked to complete the second questionnaire. For some sites, this meant both questionnaires were completed by the same people, and for other sites, different people completed the two questionnaires. Data were collected from December 2018 to September 2019. All site staff involved in sharing results were asked to complete questionnaires while recording the time they (individually) spent on this. Where more than one member of staff returned questionnaires, the time (and costs, estimated based on their job role) was added together to estimate the total time/cost for that site.
The trial ended 4 months after the last batch of clusters was randomised, as further data were unlikely.
Sample size
Show RESPECT was powered based on the primary outcome (patient satisfaction). 7 No sample size calculations were carried out to assess power for outcomes collected from site staff.
Randomisation
Sites in Show RESPECT were cluster randomised using a factorial approach to a combination of enhanced versus basic webpage; printed summary versus no printed summary; email list invitation versus no invitation. Sites were randomised in blocks of eight through random permutations within blocks. Full details of our randomisation approach can be found in our previous report. 7
Blinding
It was not feasible to blind site staff to their site’s intervention allocation.
Statistical analysis
This analysis was conducted under the intention to treat (ITT) principle. We analysed data from site staff data according to the interventions their cluster was allocated to. The prior assumption in the trial design was that there would not be any important interactions between the webpages, printed summary and email list. Therefore, our analysis is of the main effects of each intervention, adjusting for the others in the regression models (alpha = 0.05). To reflect the way in which randomisation was carried out, we adjusted for site size stratum by including this as a variable in the regression models. We did not impute any missing outcomes, as we had no information to inform our imputation.
We estimated effect measures for the interventions based on regression models, using linear regression to analyse the costs of the interventions, ordinal logistic regression for ordinal outcomes and logistic regression for binary outcomes. Models include random effects for site. We used mean differences to summarise the effects for continuous outcome measures and odds ratios for binary and ordinal outcomes. As the population for this analysis is small, no subgroup analyses were performed.
Statistical analyses were performed using Stata version 16.1 (StataCorp LLC, College Station, TX).
Qualitative methods
Supplemental Table S4 shows the Consolidated Criteria for Reporting Qualitative Studies (COREQ) checklist.
Qualitative sampling
Invitations to take part in interviews were sent by email to site staff involved in Show RESPECT. Purposive sampling included staff from sites offered the range of Show RESPECT interventions and different job roles (nursing, oncologist and administrative roles).
Qualitative data collection
Semi-structured interviews with site staff were carried out either face-to-face (on-site) or by telephone by A.S. S5 Text of the Supplemental Material contains more details about the research team and reflexivity. Semi-structured interviews were informed by a topic guide (S6 Text of the Supplemental Material), which was revised as interviews proceeded to explore emergent issues. 23 Only the interviewee and interviewer were present during interviews. The interviews were audio recorded and transcribed verbatim. Field notes were made immediately afterwards. Transcripts were checked against the recordings for accuracy, and identifying data were redacted. Transcripts were not returned to interviewees for checking. Repeat interviews were not conducted. Free-text questions within the questionnaires invited comments alongside the quantitative data.
Qualitative analysis
A thematic analysis 24 of qualitative data, taking a critical realist stance, was conducted by A.S. in Atlas.ti version 8.4 (ATLAS.ti Scientific Software Development GmbH), as detailed previously. 7 Inductive thematic saturation was reached at the 11th interview, as was data saturation. Meetings were held with site staff once the initial analysis had been carried out to check our interpretation and messages (rather than for data collection). A ‘following the thread’ approach was used to triangulate the results of the qualitative and quantitative components of the study at the analysis stage: The initial analysis of the two types of data was carried out to identify key themes and questions, then the other type of data was examined to see what light it could shed on those themes and questions. 25
Ethics
Show RESPECT obtained ethics approval from the London-Chelsea Research Ethics Committee, MREC number 18/LO/1011. Both questionnaires contained an embedded informed consent element, in line with the UK Health Research Authority’s guidance on proportionate approaches to informed consent for self-administered questionnaire-based research, 26 with completion and return of the questionnaire taken to indicate consent to use the data has been given. Qualitative interviewees gave written informed consent before the interview started.
Results
Participation in Show RESPECT
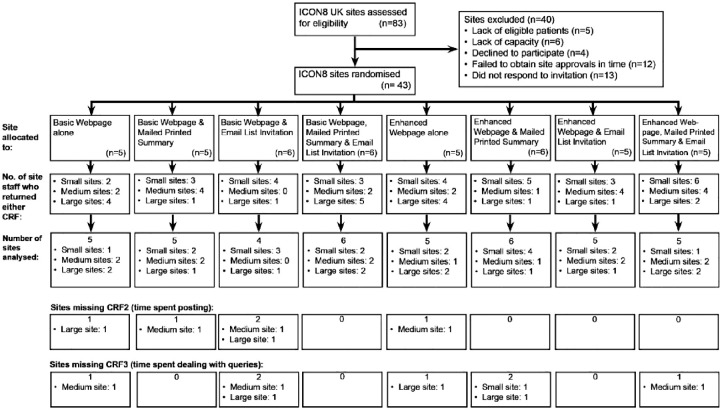
Show RESPECT randomised 43 sites. We received at least one questionnaire from 68 staff members from 41 sites. No questionnaires were returned from two randomised sites due to staff turnover. Figure 1 shows the CONSORT diagram, including the number of sites where no responses were received for the two site staff questionnaires by each of the eight combinations of interventions. Table 1 shows the number of sites and questionnaires received by the three factorial randomisations.
Figure 1.
CONSORT diagram.
Table 1.
Recruitment of sites and site staff respondents.
| Overall | Webpage | Printed summary | Email list | ||||
|---|---|---|---|---|---|---|---|
| n (%) | Basic webpage, n (%) | Enhanced webpage, n (%) | No printed summary, n (%) | Printed summary, n (%) | No invitation, n (%) | Invitation, n (%) | |
| Number of sites | |||||||
| Total | 43 (100) | 22 | 21 | 21 | 22 | 21 | 22 |
| Small sites a | 17 (40) | 8 (36) | 9 (43) | 8 (38) | 9 (41) | 9 (43) | 8 (36) |
| Medium sites a | 13 (30) | 7 (32) | 6 (29) | 6 (29) | 7 (32) | 6 (29) | 7 (32) |
| Large sites a | 13 (30) | 7 (32) | 6 (29) | 7 (33) | 6 (27) | 6 (29) | 7 (32) |
| Number of site staff who returned either Case Report Form (CRF) | |||||||
| Total | 68 | 35 | 33 | 33 | 35 | 35 | 33 |
| Small sites | 28 (41) | 14 (40) | 14 (42) | 13 (39) | 15 (43) | 14 (40) | 14 (42) |
| Medium sites | 19 (28) | 8 (23) | 11 (33) | 8 (24) | 11 (31) | 9 (26) | 10 (30) |
| Large sites | 21 (31) | 13 (37) | 8 (24) | 12 (36) | 9 (26) | 12 (34) | 9 (27) |
| Minimum number b of sites missing CRF2s (as % of sites) – time spent posting information | |||||||
| Total | 5 (12) | 5 (23) | 0 (0) | 4 (19) | 1 (5) | 3 (14) | 2 (9) |
| Small sites | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Medium sites | 3 (23) | 3 (43) | 0 (0) | 2 (33) | 1 (14) | 2 (33) | 1 (14) |
| Large sites | 2 (15) | 2 (29) | 0 (0) | 2 (29) | 0 (0) | 1 (17) | 1 (14) |
| Minimum number c of sites missing CRF3s (as % of sites) – time spent answering queries | |||||||
| Total | 6 (14) | 3 (14) | 3 (14) | 4 (19) | 2 (9) | 3 (14) | 3 (14) |
| Small sites | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Medium sites | 3 (23) | 2 (29) | 1 (17) | 2 (33) | 1 (14) | 1 (17) | 2 (29) |
| Large sites | 3 (23) | 1 (14) | 2 (33) | 2 (29) | 1 (17) | 2 (33) | 1 (14) |
| Number of sites missing both CRFs (as % of sites) | |||||||
| Total | 2 (5) | 2 (9) | 0 (0) | 2 (10) | 0 (0) | 0 (0) | 2 (9) |
| Small sites | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Medium sites | 1 (8) | 1 (14) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (14) |
| Large sites | 1 (8) | 1 (14) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 1 (14) |
Sites were classified as small if they had fewer than 6 ICON8 participants alive at the time results were available, medium if they had 6–11 participants and large if they had 12 or more participants.
Number of sites for which we had no responses (at least one response was expected from each site).
Number of sites for which we had no responses, who had not confirmed that they received no queries from participants (CRF3 was only expected for sites that had received queries from participants).
Most respondents were in nursing (63%, 42) or administrative (30%, 20) roles. Most respondents worked on multiple trials simultaneously, with 72% (49) working on more than 10 trials at the time they completed the questionnaire. Around half (33) had been working on ICON8 for 2 years or less, while 38% (26) had worked on ICON8 for more than 5 years. Table 2 shows site staff respondent characteristics.
Table 2.
Respondent characteristics.
| Variable | Overall | Webpage | Printed summary | Email list | |||
|---|---|---|---|---|---|---|---|
| n (%) | Basic webpage, n (%) | Enhanced webpage, n (%) | No printed summary, n (%) | Printed summary, n (%) | No invitation, n (%) | Invitation, n (%) | |
| Job role | |||||||
| Medical | 5 (7) | 3 (9) | 2 (6) | 3 (9) | 2 (6) | 3 (9) | 2 (6) |
| Nursing | 42 (63) | 23 (66) | 19 (59) | 22 (69) | 20 (57) | 23 (66) | 19 (59) |
| Administrative | 20 (30) | 9 (26) | 11 (34) | 7 (22) | 13 (37) | 9 (26) | 11 (34) |
| Years of experience working in trials | |||||||
| Less than 1 year | 9 (13) | 4 (11) | 5 (15) | 4 (12) | 5 (14) | 4 (11) | 5 (15) |
| 1–5 years | 24 (35) | 11 (31) | 13 (39) | 14 (42) | 10 (29) | 12 (34) | 12 (36) |
| 6–10 years | 16 (24) | 9 (26) | 7 (21) | 6 (18) | 10 (29) | 9 (26) | 7 (21) |
| More than 10 years | 19 (28) | 11 (31) | 8 (24) | 9 (27) | 10 (29) | 10 (39) | 9 (27) |
| Number of trials they currently work on | |||||||
| 1–5 | 7 (10) | 5 (14) | 2 (6) | 3 (9) | 4 (11) | 3 (9) | 4 (12) |
| 6–10 | 12 (18) | 6 (17) | 6 (18) | 7 (21) | 5 (14) | 4 (11) | 8 (24) |
| More than 10 | 49 (72) | 24 (69) | 25 (76) | 23 (70) | 26 (74) | 28 (80) | 21 (64) |
| Time currently spent working on ICON8 | |||||||
| Almost none | 45 (67) | 21 (62) | 24 (73) | 21 (66) | 24 (69) | 19 (56) | 26 (79) |
| Around one day per week | 21 (31) | 13 (38) | 8 (24) | 10 (31) | 11 (31) | 14 (41) | 7 (21) |
| Around two days per week | 1 (1) | 0 (0) | 1 (3) | 1 (3) | 0 (0) | 1 (3) | 0 (0) |
| Number of years they have worked on ICON8 | |||||||
| Less than 1 year | 16 (24) | 6 (17) | 10 (30) | 9 (27) | 7 (20) | 6 (17) | 10 (30) |
| 1–2 years | 17 (25) | 10 (29) | 7 (21) | 12 (36) | 5 (14) | 12 (34) | 5 (15) |
| 3–4 years | 9 (13) | 3 (9) | 6 (18) | 5 (15) | 4 (11) | 5 (14) | 4 (12) |
| 5 years or more | 26 (38) | 16 (46) | 10 (30) | 7 (21) | 19 (54) | 12 (34) | 14 (42) |
| Involvement in sharing the ICON8 results | |||||||
| Sending information by post | 55 (81) | 26 (74) | 29 (88) | 28 (85) | 27 (77) | 31 (89) | 24 (73) |
| Answering patient queries | 43 (63) | 24 (69) | 19 (58) | 23 (70) | 20 (57) | 18 (51) | 25 (76) |
| Other | 7 (10) | 3 (9) | 4 (12) | 3 (9) | 4 (11) | 2 (6) | 5 (15) |
More than one respondent per site was allowed if more than one member of staff was involved in sharing results with participants.
We interviewed 11 site staff from 12 sites (mean duration 52 min, range: 35–75 min): two from small strata sites, five from medium strata sites and five from large strata sites. Most interviewees were in nursing (5) or administrative (4) roles, while two were oncologists. Table 3 shows the characteristics of interviewees. We recruited at least the target number of interviewees in all parts of the sampling frame.
Table 3.
Site staff who were interviewed for the qualitative study.
| Characteristics | No. of interviewees |
|---|---|
| Total interviewed | 11 |
| Show RESPECT randomisation a | |
| Works at site randomised to printed summaries | 6 |
| Works at site not randomised to printed summaries | 6 |
| Site strata (based on number of ICON8 participants) a | |
| Small | 2 |
| Medium | 5 |
| Large | 5 |
| Staff job role | |
| Oncologist | 2 |
| Nursing | 5 |
| Administrative | 4 |
One interviewee worked at two sites of different sizes, which were randomised to different interventions.
Resources required from sites for sharing results with patients
The resources required from sites to share the results with patients include staff time for posting information and dealing with patient queries and the costs of postage and stationery (printed copies of materials were provided by the clinical trials unit). Table 4 shows the estimated total costs per patient and a breakdown by the different types of resources required. Total costs were £13.71/patient higher (95% confidence interval (CI): −3.19, 30.60) in the printed summary group than that in the no printed summary group (where only the Patient Update Information Sheet, informing patients how to access the webpage ± the email list, was mailed), but this difference was not statistically significant (p = 0.108). The differences in costs of the other comparisons were smaller and also not statistically significant. There was no evidence of interaction between interventions. Supplemental Table S7 shows the average costs to site per patient by each of the eight factorial combinations. The biggest component of the total costs was staff time to send out the printed information, taking, on average, 11 min/patient (range 0–35) in the no printed summary group compared with the 46 min/patient in the printed summary group (range 0–144). This translated into a £16.72 higher (95% CI: 5.43, 28.01) cost per patient for time spent posting information in the printed summary group compared to no printed summary (p = 0.005). The amount of time spent dealing with queries from patients was about 10 min per patient (range 0–70), translating into a £2.85/patient lower (95% CI: −13.06, 7.45) cost of dealing with queries in the enhanced webpage group compared to the basic webpage group (p = 0.583) and to £3.63/patient lower (95% CI: −13.92, 6.67) cost in the printed summary group compared to the no printed summary group (p = 0.480). Other costs made up a small proportion of the overall costs, around £0.61/patient higher (95% CI: 0.46, 0.76) in the printed summary group compared to the no printed summary group (p < 0.001). S8 Text of the Supplemental Material summarises the resources used by the clinical trials unit in implementing the Show RESPECT interventions.
Table 4.
Resources used by sites to share results with patient participants, per participant.
| Variable | Overall | Webpage | Printed summary | Email list | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (Std dev.) | Basic | Enhanced | Mean difference a (95% CI) p-value | No printed summary | Printed summary | Mean difference a (95% CI) p-value | No invitation | Invitation | Mean difference a (95% CI) p-value | |
| Costs | ||||||||||
| Estimated cost of time spent posting all information, British Pound Sterling (GBP) | 14.10 (19.08) | 11.81 (19.35) | 16.28 (19.03) | 4.71 (−6.53, 15.96) 0.401 | 5.13 (4.65) | 21.85 (23.22) | 16.72 (5.43, 28.01) 0.005 | 15.59 (22.60) | 12.54 (14.96) | −3.25 (−14.50, 7.99) 0.561 |
| Estimated cost of time spent dealing with queries, GBP | 8.00 (16.08) | 9.38 (16.64) | 6.69 (15.81) | −2.85 (−13.06, 7.45) 0.583 | 9.56 (17.02) | 6.65 (15.49) | −3.63 (−13.92, 6.67) 0.480 | 7.80 (18.88) | 8.20 (12.99) | 0.45 (−9.80, 10.71) 0.929 |
| Other costs, GBP | 1.01 (0.38) | 1.01 (0.36) | 1.00 (0.41) | 0.00 (−0.15, 0.15) 0.984 | 0.68 (0.27) | 1.29 (0.19) | 0.61 (0.46, 0.76) <0.001 | 1.04 (0.42) | 0.98 (0.34) | −0.08 (−0.23, 0.07) 0.310 |
| Total costs b , GBP | 23.11 (27.00) | 22.20 (32.73) | 23.97 (20.93) | 1.91 (−14, 18.74) 0.819 | 15.37 (17.13) | 29.79 (32.18) | 13.71 (−3.19, 30.60) 0.108 | 24.43 (33.71) | 21.72 (18.29) | −2.87 (−19.70, 13.95) 0.731 |
| Time | ||||||||||
| Estimated hours sending out all printed information | 0.49 (0.64) | 0.36 (0.55) | 0.61 (0.70) | 0.26 (−0.11, 0.62) 0.161 | 0.18 (0.16) | 0.76 (0.77) | 0.59 (0.22, 0.96) 0.002 | 0.53 (0.73) | 0.45 (0.55) | −0.08 (−0.44, 0.28) 0.657 |
| Estimated hours dealing with queries | 0.17 (0.27) | 0.19 (0.29) | 0.15 (0.25) | −0.04 (−0.21, 0.13) 0.631 | 0.21 (0.26) | 0.14 (0.28) | −0.08 (−0.25, 0.09) 0.343 | 0.12 (0.23) | 0.22 (0.30) | 0.10 (−0.07, 0.26) 0.237 |
| Other | ||||||||||
| Approximate number of participants who had queries | 0.34 (0.49) | 0.44 (0.53) | 0.25 (0.44) | −0.19 (−0.49, 0.11) 0.198 | 0.45 (0.58) | 0.25 (0.38) | −0.23 (−0.52, 0.07) 0.131 | 0.33 (0.46) | 0.35 (0.53) | 0.03 (−0.27, 0.33) 0.840 |
CI: confidence interval.
Adjusted for strata and other randomisations.
Primary outcome for site staff data.
Site staff views on the experience of sharing trial results with patients in Show RESPECT
Overall views of the process of sharing results in Show RESPECT
Site staff described their experiences of sharing results in positive terms, as ‘easy’, ‘not complex’, ‘achievable’, ‘not time-consuming’ and ‘working well’. They appreciated the ‘clear instructions’ provided.
Preparing patients to receive the trial results
The need to prepare patients to receive results was discussed repeatedly in site staff interviews and questionnaires. The importance of preparing patients stemmed partly from recognition that not all would want to know the results, and so it was important to offer the opportunity to opt in or out. Views differed on the timepoint at which this should be done, with some suggesting it should be done when patients join the trial. Others thought patients might change their minds between joining the trial and the results becoming available and so would need to be asked nearer the time:
I don’t believe in just sending information without first asking for their consent or asking them if they’re interested in doing this, because some do want to know and some don’t want to know. (BMRNI04: research burse, medium strata site)
Another driver behind the need to prepare patients prior to sharing results was that results may potentially be upsetting or confusing:
If the results are difficult to interpret or complex you may want to add something like the results are complex and you may want to go through it with your doctor, or something like that. (EBLMCLI02: oncologist, large and medium strata sites)
In Show RESPECT, the Patient Update Information Sheet was designed to prepare patients to receive results and to give them the opportunity to opt-out or access the interventions. Some site staff added an additional preliminary step, telephoning or talking to patients in clinic to inform them that the Patient Update Information Sheet was coming:
We sent the information sheets out, then contacted them and let them know they’re coming … That’s a really good way of doing it because if that had just ended up on their doorstep, they’d have read it and then just probably thrown it in the bin because it didn’t come with any compliment[s] slip or they didn’t really know what it was. (GMTCI02: trial coordinator, medium strata site)
However, not all site staff agreed that this step was needed or that it was a good idea:
I don’t agree with phoning the patients, just because, you know, a lot of our patients, you know, are busy with day-to-day life and it’s not, I don’t think it’s nice just calling them and reminding them of it all. (FLTCI01: trial coordinator, large strata site)
Further follow-up and support
There was recognition that some patients may need further support or have questions about the results. Some site staff phoned patients after they sent the Patient Update Information Sheet or printed summary to see if they had any questions or needed further support. Others included a note with the results inviting patients to make contact with any questions.
Concerns and challenges
Most site staff questionnaire respondents (88%, 60/68) reported having no concerns about how they shared the ICON8 results with patients, with no statistically significant differences between the randomised interventions. Similarly, around three quarters of site staff reported not finding anything challenging about sharing the ICON8 results, again with no statistically significant differences between the arms (Table 5). Some of the site staff were concerned about the emotional impact of sharing results: Some were uncomfortable sharing information on average progression-free survival times, while others were concerned that those who felt they had benefitted from trial participation may be upset to learn that the trial did not find a benefit overall.
Table 5.
Site staff views and experience around sharing the ICON8 results with patient participants.
| Variable | Overall | Webpage | Printed summary | Email list | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Basic, n (%) | Enhanced, n (%) | Odds ratio (95% CI) p-value | No printed summary, n (%) | Printed summary, n (%) | Odds ratio (95% CI) p-value | No invitation, n (%) | Invitation, n (%) | Odds ratio (95% CI) p-value | |
| Any concerns about how you shared the results? | 0.71 (0.14, 3.58) 0.679 | 2.59 (0.45, 14.83) 0.284 | 3.15 (0.56, 17.59) 0.191 | |||||||
| Yes | 8 (12) | 5 (14) | 3 (9) | 2 (6) | 6 (17) | 2 (6) | 6 (18) | |||
| No | 60 (88) | 30 (86) | 30 (91) | 31 (94) | 29 (83) | 33 (94) | 27 (82) | |||
| Did you find anything challenging about sharing the results? | 5.94 (0.80, 44.27) 0.082 | 3.31 (0.47, 23.52) 0.231 | 2.57 (0.40, 16.59) 0.321 | |||||||
| No | 52 (76) | 30 (86) | 22 (67) | 27 (82) | 25 (71) | 29 (83) | 23 (70) | |||
| Yes | 16 (24) | 5 (14) | 11 (33) | 6 (18) | 10 (29) | 6 (17) | 10 (30) | |||
| How many participants contacted you with queries? a | ||||||||||
| 0 | 11 (27) | 4 (17) | 7 (39) | 4.8 (0.53, 43.13) 0.161 | 3 (16) | 8 (36) | 0.76 (0.07, 8.33) 0.824 | 2 (13) | 9 (36) | 1.23 (0.14, 10.14) 0.851 |
| 1 to 2 | 24 (59) | 17 (74) | 7 (39) | 12 (63) | 12 (55) | 11 (69) | 13 (52) | |||
| 3 to 5 | 6 (15) | 2 (9) | 4 (22) | 4 (21) | 2 (9) | 3 (19) | 3 (12) | |||
| How able did you feel to help with queries? b | ||||||||||
| Very difficult | 0 (0) | 0 (0) | 0 (0) | 0.27 (0.02, 3.56) 0.317 | 0 (0) | 0 (0) | 2.61 (0.24, 28.40) 0.432 | 0 (0) | 0 (0) | 0.75 (0.08, 7.32) 0.802 |
| Quite difficult | 1 (3) | 0 (0) | 1(8) | 1 (6) | 0 (0) | 0 (0) | 1 (6) | |||
| Not sure | 4 (13) | 2 (11) | 2 (17) | 3 (18) | 1 (7) | 0 (0) | 4 (22) | |||
| Quite easy | 15 (48) | 9 (47) | 6 (50) | 9 (53) | 6 (43) | 9 (69) | 6 (33) | |||
| Very easy | 11 (35) | 8 (42) | 3 (25) | 4 (24) | 7 (50) | 4 (31) | 7 (39) | |||
| Should the way you shared results with participants be the standard approach for other trials? | ||||||||||
| No | 16 (24) | 6 (17) | 10 (30) | 0.39 (0.07, 2.42) 0.318 | 9 (27) | 7 (20) | 1.7 (0.32, 9.04) 0.532 | 8 (23) | 8 (24) | 0.84 (0.16, 4.45) 0.838 |
| Yes | 52 (76) | 29 (83) | 23 (70) | 24 (73) | 28 (80) | 27 (77) | 25 (76) | |||
| Would you do anything different for future trials? | ||||||||||
| No | 52 (79) | 28 (82) | 24 (75) | 1.65 (0.48, 5.67) 0.424 | 26 (81) | 26 (76) | 1.43 (0.41, 5.02) 0.573 | 28 (80) | 24 (77) | 1.06 (0.31, 3.62) 0.93 |
| Yes | 14 (21) | 6 (18) | 8 (25) | 6 (19) | 8 (24) | 7 (20) | 7 (23) | |||
CI: confidence interval.
Ordinal odds ratio.
For the ordinal regression analysis, the very and quite difficult and not sure categories were merged.
Oncologists expressed the need for care in how sharing results is done to ensure patients are not unnecessarily distressed or confused. Patient and public involvement in the preparation of information about results for patients was identified as a way of mitigating the risk of causing upset:
We do have a duty to give the patient information, it’s just being wise and careful to give that information well, in a way that patients can understand … And like any information we have to time that well and be sensible about who we’re giving that to. (EBLMCLI02: oncologist, large and medium strata sites)
The other main challenges identified by site staff were the time needed to share results via mailed information. This was particularly an issue for staff at sites with larger numbers of ICON8 patients. Staff at smaller sites recognised that although it may not have been a problem for them in this study, it would be challenging in studies with many patients at a site:
It was just so time-consuming to send, you know, the results out to so many patients. (HLRNI03: research nurse, large strata site)
If site staff had not had contact with a patient for a while and were aware the patient was unwell, this did raise some questions over whether they should send the results. Similarly, if patients had transferred between sites, it could cause confusion over who was responsible for sharing results with that patient. For some site staff, giving information out remotely, rather than face-to-face, was challenging as they could not gauge the reaction of patients.
Dealing with queries from patients
Just over a quarter of site staff questionnaire respondents reported that no patients contacted them with queries, while almost 60% were only contacted by one or two patients (Table 5). There were no significant differences between the randomised arms in the number of patient queries. Eighty-three percent of questionnaire respondents felt it was quite or very easy to deal with queries patients raised, with no significant between-arm differences.
Sharing results in future trials
Most site staff respondents said the way they had shared results with patients in Show RESPECT should be the standard approach for other trials (76%), with no significant differences between the randomised arms. Similarly, 79% said they would not do anything different for future trials (Table 5).
Discussion
Providing results to patients in the form of opt-out printed summaries increased costs to sites of sharing results by around £14 per patient. Most of that increased cost was attributable to staff time in mailing printed information. These costs, and those incurred by the clinical trials unit, are small (1% or less) compared with the overall cost of trials, which previous research has estimated to be around £2987 27 or £7890 28 per participant on average, in the UK.
The processes and methods used in Show RESPECT to share results with patients were seen as both appropriate and feasible by site staff. Preparing patients to receive trial results was an important step in this process (similar to the findings of the BRACELET study). 29 Some site staff viewed the Patient Update Information Sheet alone as sufficient, while others felt more comfortable talking to the patients first to let them know what to expect, and some felt it should have been covered in the informed consent process when patients joined the trial. Mailing out the printed information was generally reported to be easy and not too time-consuming although staff at sites with large numbers of ICON8 patient participants found it more burdensome. Site staff received few queries from patients about the results.
A key strength of these results is that they are from a mixed-methods randomised controlled study within a trial. The randomised design allows us to be confident that the differences we observed were due to the interventions rather than other factors, while the qualitative data allow us to explore the views of site staff on their experience of sharing trial results within the Show RESPECT study. The study included sites of different types and numbers of ICON8 patient participants, allowing us to transfer our results on feasibility and acceptability to other trials with similar site characteristics. However, Show RESPECT only looked at sharing the results of a single trial, and care needs to be taken when transferring the results to other trials in different diseases, health systems and results scenarios. Show RESPECT was not powered to detect differences in secondary outcome measures (which includes the outcomes reported in this article). This means there may have been real differences we were unable to detect. The questionnaires used to collect data on time spent on activities asked respondents to select the category that reflected the time they had spent, rather than giving precise figures. Similarly, our costs of time for different job roles at sites are based on a generic research costing tool, rather than the actual cost of each individual staff member. This means our cost figures are estimates rather than precise reflections of the time and cost of these activities. The resources required at the clinical trials unit level, reported in the S8 Text of the Supplemental Material, do not include the costs of translation, which should be included in future studies to ensure people who have different first languages are able to access the results. 30 Another limitation is our cost estimates do not distinguish between fixed and per-patient costs. Economies of scale mean per-patient costs to the clinical trials units are likely to be lower in trials with more patients. There may also be economies of scale at the site level, meaning average costs to site per patient may be lower at larger sites and in larger trials.
Previously reported results from Show RESPECT showed that printed summaries improved patient satisfaction with how the results were shared and enabled more people who wanted to know the results to find them out. 7 The results reported here show that this approach is feasible for site staff to implement and acceptable to them. This echoes findings from previous research, where study staff reported that disseminating trial results summaries was simple, straightforward and not time-consuming and that patient queries were not common and did not require substantial amounts of time to deal with. 31
We believe this is the first study to provide detailed information about the costs of different approaches to sharing results with trial participants from both a site and clinical trials unit perspective. Our results include the human resource, printing and posting costs and therefore reflect a more accurate cost estimate. These costs should inform those planning results dissemination in future trials to ensure adequate resources are available (both budget and staff time) to share results with patients. Funders have a role to play in encouraging researchers to do this and in ensuring plans for sharing results are implemented. Further research is needed to determine the extent to which approaches to sharing results with trial participants need to differ for different populations, disease states, study questions and settings.
Conclusions
We found that the process and communication approaches used within the Show RESPECT study for sharing results with patients were acceptable and feasible for site staff. The combination of a webpage and printed summary, which resulted in the highest participant satisfaction with how the results were shared, came at a modest cost that could be incorporated into trial budgets. The information on the process and resource requirements for the approaches used in Show RESPECT can guide others seeking to plan for sharing results.
Supplemental Material
Supplemental material, sj-docx-1-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-docx-2-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-docx-5-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-docx-6-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-docx-7-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-docx-8-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-pdf-3-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-pdf-4-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Acknowledgments
We are grateful to the patients and site staff who participated in this study. We dedicate this article to the memory of Amanda Hunn, who was part of the steering group for this study until she passed away in 2022. We thank other members of the steering group for their input into the study, especially Conor Tweed and Liz James. We acknowledge the hard work and diligence of Cara Purvis, the trial manager, and the data managers for Show RESPECT. We also thank the ICON8 trial team for their support, particularly Babasola Popoola, Francesca Schiavone, Jonathan Badrock, Andrew Clamp and Rick Kaplan.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AS, JB, CS, AJC, NJ-H, CD-M, KG, TI and WJC have nothing to declare. MRS reports grants from Clovis; grants and non-financial support from Astellas, Janssen, Novartis, Pfizer and Sanofi; personal fees from Lilly Oncology and Janssen, outside the submitted work. BEB reports receiving consulting fees from Lilly outside the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Show RESPECT study was funded by the Medical Research Council through core grants to MRS at the MRC CTU at UCL for Trial Conduct Methodology (MC_UU12023/24 and MC_UU_00004/08) https://mrc.ukri.org/. The funder had no role in the study design, collection, analysis and interpretation of data, the writing of the report and the decision to submit the article for publication. All authors had full access to the study data, including statistical reports and tables, and take responsibility for the integrity of the data and accuracy of the data analysis.
Trial registration: Show RESPECT is registered on the ISRCTN registry: ISRCTN96189403. The ICON8 trial in which Show RESPECT took place is registered on the ISRCTN registry: ISRCTN10356387.
ORCID iDs: Annabelle South  https://orcid.org/0000-0001-8912-2001
https://orcid.org/0000-0001-8912-2001
Barbara E Bierer  https://orcid.org/0000-0001-6448-8170
https://orcid.org/0000-0001-6448-8170
William J Cragg  https://orcid.org/0000-0002-1274-8521
https://orcid.org/0000-0002-1274-8521
Carlos Diaz-Montana  https://orcid.org/0000-0001-9082-4596
https://orcid.org/0000-0001-9082-4596
Katie Gillies  https://orcid.org/0000-0001-7890-2854
https://orcid.org/0000-0001-7890-2854
Matthew R Sydes  https://orcid.org/0000-0002-9323-1371
https://orcid.org/0000-0002-9323-1371
Andrew J Copas  https://orcid.org/0000-0001-8968-5963
https://orcid.org/0000-0001-8968-5963
Data availability: The individual participant data, qualitative and quantitative, that underlie the results reported in this article, after de-identification, will be available beginning 12 months after publication following the MRC CTU’s standard moderated access approach (details of which are available at https://www.mrcctu.ucl.ac.uk/our-research/other-research-policy/data-sharing/). Applicants will need to state the aims of any analyses and provide a methodologically sound proposal. Applications should be directed to mrcctu.datareleaserequest@ucl.ac.uk. Data requestors will need to sign a data access agreement at an institutional level.
Supplemental material: Supplemental material for this article is available online.
References
- 1. World Medical Association. World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 2. Fernandez CV, Kodish E, Weijer C. Informing study participants of research results: an ethical imperative. IRB 2003; 25: 12–19. [PubMed] [Google Scholar]
- 3. Partridge AH, Wong JS, Knudsen K, et al. Offering participants results of a clinical trial: sharing results of a negative study. Lancet 2005; 365: 963–964. [DOI] [PubMed] [Google Scholar]
- 4. Donaldson S, Khetani N, Maniatis G, et al. Sharing clinical trial results with adolescent idiopathic scoliosis patients. J Pediatr Orthop 2009; 29: 467–475. [DOI] [PubMed] [Google Scholar]
- 5. Brealey S, Andronis L, Dennis L, et al. Participants’ preference for type of leaflet used to feed back the results of a randomised trial: a survey. Trials 2010; 11: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elzinga KE, Khan OF, Tang AR, et al. Adult patient perspectives on clinical trial result reporting: a survey of cancer patients. Clin Trials 2016; 13: 574–581. [DOI] [PubMed] [Google Scholar]
- 7. South A, Joharatnam-Hogan N, Purvis C, et al. Testing approaches to sharing trial results with participants: the Show RESPECT cluster randomised, factorial, mixed methods trial. PLoS Med 2021; 18: e1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Health Research Authority. Make it public: research transparency annual report 2021. London: Health Research Authority, 2021. [Google Scholar]
- 9. Schroter S, Price A, Malički M, et al. Frequency and format of clinical trial results dissemination to patients: a survey of authors of trials indexed in PubMed. BMJ Open 2019; 9: e032701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hinds PS. Sharing our research findings with study participants. Cancer Nurs 2008; 31: 173–174. [DOI] [PubMed] [Google Scholar]
- 11. Rigby H, Fernandez CV. Providing research results to study participants: support versus practice of researchers presenting at the American Society of Hematology annual meeting. Blood 2005; 106: 1199–1202. [DOI] [PubMed] [Google Scholar]
- 12. Macneil SD, Fernandez CV. Informing research participants of research results: analysis of Canadian university based research ethics board policies. J Med Ethics 2006; 32: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aldinger C, Bierer B, Collyar D, et al. MRCT return of results guidance document. Cambridge, MA: The Multi-regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard, 2016. [Google Scholar]
- 14. Partridge AH, Winer EP. Informing clinical trial participants about study results. JAMA 2002; 288: 363–365. [DOI] [PubMed] [Google Scholar]
- 15. Fernandez CV, Kodish E, Shurin S, et al. Offering to return results to research participants: attitudes and needs of principal investigators in the Children’s Oncology Group. J Pediatr Hematol Oncol 2003; 25: 704–708. [DOI] [PubMed] [Google Scholar]
- 16. Dixon-Woods M, Tarrant C, Jackson CJ, et al. Providing the results of research to participants: a mixed-method study of the benefits and challenges of a consultative approach. Clin Trials 2011; 8: 330–341. [DOI] [PubMed] [Google Scholar]
- 17. MacNeil SD, Fernandez CV. Attitudes of research ethics board chairs towards disclosure of research results to participants: results of a national survey. J Med Ethics 2007; 33: 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Getz K, Hallinan Z. Creating a standard practice for communicating lay language trial results to study volunteers. Res Pract 2014; 10–15. [Google Scholar]
- 19. Bruhn H, Anderson AS, Hickman A, et al. Letter on ‘Sharing trial results directly with trial participants and other stakeholders after the SARS-CoV-2 pandemic hit the UK – experience from the ActWELL trial’. Trials 2021; 22: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Annabelle South. Showing RESPECT: a mixed methods study into communicating the results of a phase iii clinical trial to trial participants. London: UCL, 2023. [Google Scholar]
- 21. Clamp AR, James EC, McNeish IA, et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 2019; 394: 2084–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Show RESPECT team. Show RESPECT: Show RESults to participants engaged in clinical trials: a cluster randomised factorial trial of different modes of communicating results to participants of the ICON8 phase III ovarian cancer trial, https://www.mrcctu.ucl.ac.uk/media/1980/show-respect_protocol_v30_20aug2018_clean.pdf (2018, accessed 20 July 2023).
- 23. Guest G, Namey E, Mitchell M. Chapter 1: qualitative research: defining and designing. In: Guest G, Namey E, Mitchell M. (eds). Collecting qualitative data: a field manual for applied research. London: Sage, 2013. DOI: 10.4135/9781506374680. [DOI] [Google Scholar]
- 24. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psycholy 2006; 3: 77–101. [Google Scholar]
- 25. Moran-Ellis J, Alexander VD, Cronin A, et al. Triangulation and integration: processes, claims and implications. Qual Res 2006; 6: 45–59. [Google Scholar]
- 26. Health Research Authority. Applying a proportionate approach to the process of seeking consent, https://s3.eu-west-2.amazonaws.com/www.hra.nhs.uk/media/documents/applying-proportionate-approach-process-seeking-consent_R3gbJKn.pdf (2017, accessed 20 July 2021).
- 27. Pirosca S, Shiely F, Clarke M, et al. Tolerating bad health research: the continuing scandal. Trials 2022; 23: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hawkes N. UK must improve its recruitment rate in clinical trials, report says. BMJ 2012; 345: e8104. [DOI] [PubMed] [Google Scholar]
- 29. Snowdon C, Brocklehurst P, Tasker R, et al. Death, bereavement and randomised controlled trials (BRACELET): a methodological study of policy and practice in neonatal and paediatric intensive care trials. Health Technol Assess 2014; 18: 1–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dawson S, Banister K, Biggs K, et al. Trial Forge Guidance 3: randomised trials and how to recruit and retain individuals from ethnic minority groups – practical guidance to support better practice. Trials 2022; 23: 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Getz K, Hallinan Z, Simmons D, et al. Meeting the obligation to communicate clinical trial results to study volunteers. Expert Rev Clin Pharmacol 2012; 5: 149–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-docx-2-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-docx-5-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-docx-6-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-docx-7-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-docx-8-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-pdf-3-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials
Supplemental material, sj-pdf-4-ctj-10.1177_17407745231186088 for Site staff perspectives on communicating trial results to participants: Cost and feasibility results from the Show RESPECT cluster randomised, factorial, mixed-methods trial by Annabelle South, Julia Bailey, Barbara E Bierer, Eva Burnett, William J Cragg, Carlos Diaz-Montana, Katie Gillies, Talia Isaacs, Nalinie Joharatnam-Hogan, Claire Snowdon, Matthew R Sydes and Andrew J Copas in Clinical Trials