Abstract
Gemella haemolysans is a gram-positive coccus, and commensal of the upper respiratory tract and oral mucosa. In rare cases, it has been identified as an opportunistic pathogen in the development of endocarditis. Here, we describe a case of Gemella haemolysans endocarditis in a patient with bicuspid aortic valve. A 14-year-old male presented to our hospital with a 1-month history of intermittent fever. Gemella haemolysans was isolated from the patient’s blood cultures. Transesophageal echocardiography revealed severe aortic stenosis and a pseudoaneurysm of the mitral–aortic intervalvular fibrosa. The patient underwent aortic valve replacement with pseudoaneurysm of the mitral–aortic intervalvular fibrosa repair and remained symptom-free during follow-up. This case highlights the importance of considering atypical pathogens as causative agents of infective endocarditis.
Keywords: Gemella haemolysans, infective endocarditis, pediatrics, antimicrobial therapy
Introduction
Infective endocarditis (IE) is a life-threatening disease still associated with a high mortality rate.1–3 The vast majority of IE cases stem from streptococci, staphylococci, and enterococci infection, with Staphylococcus aureus specifically responsible for around 30% of cases in the developed world. 4 Changes in the causative organisms have been noted in recent years with emerging species that are often difficult to grow.5,6 Gemella haemolysans is a facultative anaerobic gram-positive coccus that normally resides in the upper respiratory tract and oral cavity of humans. 7 Gemella haemolysans is able to cause severe and generalized infections as opportunistic pathogens, and it has become an emerging bacterial etiology in IE. 8 Most documented cases of Gemella haemolysans endocarditis occur in patients with underlying mitral or aortic valve disease (including prosthetic valves) and/or poor dentition or dental manipulation. 9 We present a case of IE caused by Gemella haemolysans in a pediatric patient with bicuspid aortic valve.
Case presentation
A 14-year-old male was referred to our hospital with intermittent fever of 1-month duration. The patient had a medical history of congenital aortic stenosis due to bicuspid aortic valve, and underwent balloon valvuloplasty after birth.
Investigations
Upon admission, a physical examination revealed the patient to be in good general condition. Vital signs were as follows: body temperature of 38.8°C, respiratory rate of 19 breaths/min, heart rate of 114 bpm, and blood pressure of 110/70 mmHg. Oxygen saturation was 99% while breathing room air. The cardiovascular examination showed no jugular venous distension, pitting edema, or systolic murmurs. There were no mucocutaneous lesions, petechiae, Osler nodes, Janeway lesions or subungual hemorrhages. Ophthalmoscopy and examination of the mouth, ears, nose, throat, and nervous system were normal. Laboratory tests revealed moderate normocytic-normochromic anemia, an increased C-reactive protein level (13.1 mg/dL), a white blood cell count of 9.130/μL with 75.7% neutrophils, and a hemoglobin level of 9.8 g/dL. Hepatic and renal functions were within the normal range. The serum immunoglobulin A level was elevated (437 mg/dL; normal values 70–400). Serum C3 level, titers for antistreptolysin O, and anti-deoxyribonuclease B were within normal limits, and searches for rheumatoid factor, antinuclear antibodies, antineutrophil cytoplasmic antibodies, and cryoglobulins were negative. The chest radiograph was unremarkable, and abdominal ultrasound revealed no hepatic, renal, or splenic abscesses. Serological testing for Toxoplasma, human immunodeficiency virus, and Cytomegalovirus were negative.
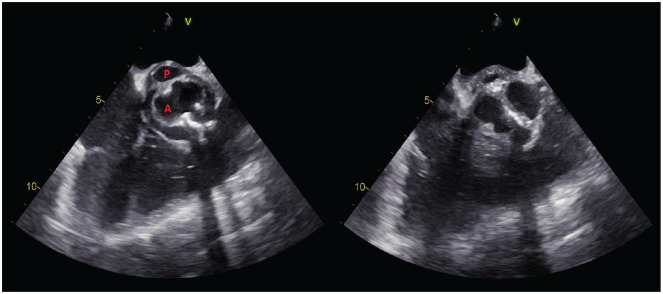
Transthoracic echocardiography (TTE) revealed severe aortic stenosis, but no vegetation was seen. Due to a strong suspicion of IE, TEE was performed. TEE did not reveal any vegetation on the cardiac valves but did identify a pulsatile echo-free cavity, posterior and underneath the aortic valve, measuring approximately 7 mm × 13 mm (Figures 1 and 2). The cavity was recognized as a pseudoaneurysm of the mitral–aortic intervalvular fibrosa (P-MAIVF). Concurrently, one out of two sets of blood cultures obtained upon admission grew G. haemolysans. The isolate was sensitive to penicillin G (0.250 mg/L), ceftriaxone (⩽0.500 mg/L), meropenem (⩽0.5 mg/L), and vancomycin (2 mg/L; Table 1).
Figure 1.
Transesophageal echocardiography showing bicuspid aortic valve (A) and the pseudoaneurysm of the mitral–aortic intervalvular fibrosa (P) in systole (left) and diastole (right).
Figure 2.
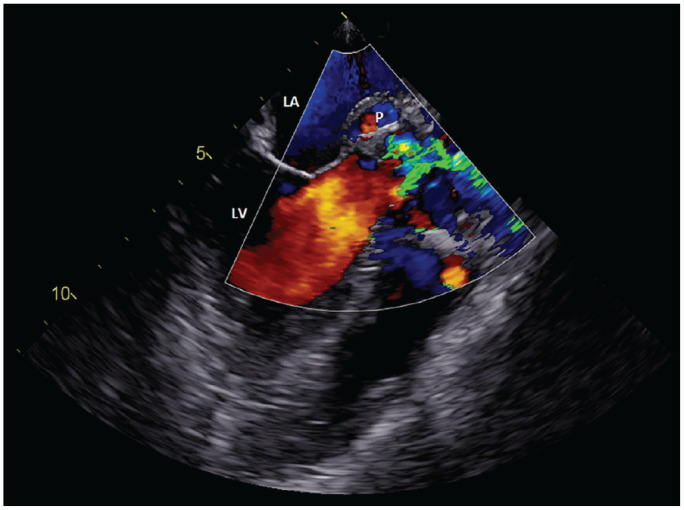
TEE with color Doppler demonstrating the blood flow into the pseudoaneurysm during systole.
P: pseudoaneurysm, LA: left atrium, LV: left ventricle, TTE, transthoracic echocardiography.
Table 1.
Antimicrobial susceptibility testing results for Gemella haemolysans isolated from our patient.
| Antimicrobial agent | MIC (μg/ml) | Susceptibility |
|---|---|---|
| Piperacillin/tazobactam | ⩽0.06 | S |
| Metronidazole | >8 | R |
| Clindamycin | ⩽0.06 | S |
| Penicillin G | 0.250 | S |
| Vancomycin | 2 | S |
| Ertapenem | ⩽0.125 | S |
| Doxycycline | ⩽0.125 | S |
| Ampicillin | 0.250 | S |
| Amoxicillin/clavulanic acid | <0.5 | S |
| Meropenem | ⩽0.5 | S |
| Cefotaxime | 0.500 | S |
| Ceftriaxone | 0.500 | S |
MIC: minimum inhibitory concentration; S: susceptible; R: resistant.
The antimicrobial susceptibility testing was performed by broth microdilution method (MERLIN Diagnostika GmbH, Bornheim, Germany). Interpretative MIC breakpoints were based on the European Committee on Antimicrobial Susceptibility Testing criteria.
Treatment and outcome
The patient was treated with broad-spectrum antibiotics, including intravenous (IV) ceftriaxone (2 g daily) and gentamicin (1 mg/kg IV every 8 h). A cardiovascular surgeon was consulted and the patient was transferred to a children’s hospital to undergo aortic valve replacement with P-MAIVF repair. The postoperative course was uneventful, and the patient has remained symptom-free during follow-up. A detailed timeline of the case presentation is provided in Figure 3.
Figure 3.
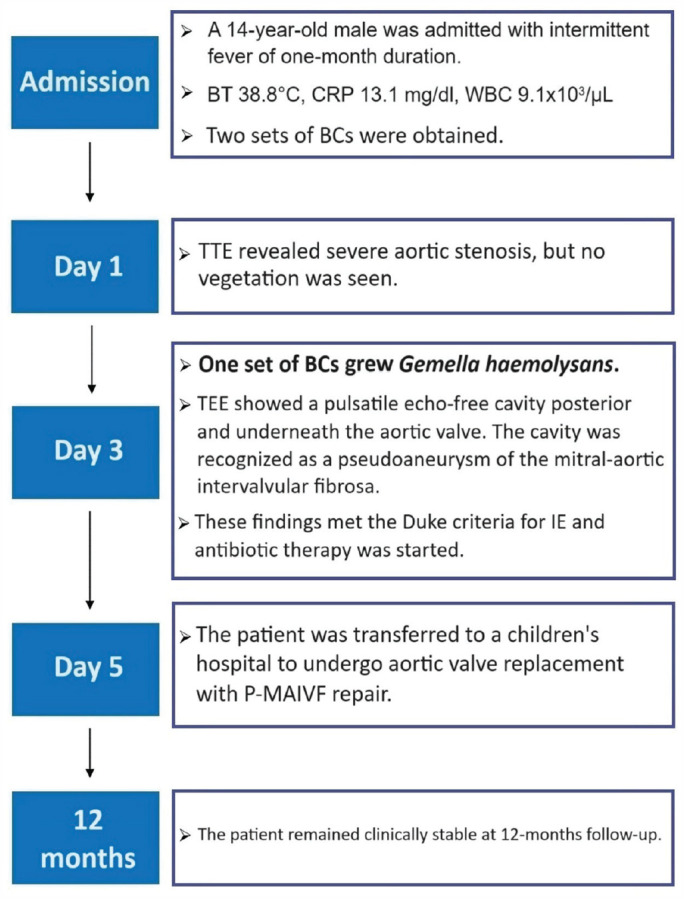
The timeline of the case presentation.
BCs: blood cultures; BT: body temperature; CRP: C-reactive protein; IE: infective endocarditis; P-MAIVF: pseudoaneurysm of the mitral–aortic intervalvular fibrosa.
Discussion
The epidemiology of IE in children has changed in recent years, with congenital heart disease becoming the main predisposing factor in the developed world, while rheumatic heart disease has become less frequent.10,11 Staphylococcus aureus is now the most prevalent cause of IE in most studies, accounting for approximately 26.6% of all cases, followed by viridans group streptococci at 18.7%, other streptococci at 17.5%, and enterococci at 10.5%.5,11 These organisms together account for 80%–90% of all cases of endocarditis. Gemella haemolysans often colonizes the upper respiratory, gastrointestinal, and genitourinary tract as a commensal organism and occasionally causes localized and/or disseminated infections. Most cases of Gemella haemolysans endocarditis involve either the mitral or aortic valve and are found in patients with previous valve damage or those in an immunocompromised state 12 (Table 2). Patients with bicuspid aortic valves not only have a higher risk of IE, but they also face an increased likelihood of developing periannular complications, such as abscess and pseudoaneurysm. 13
Table 2.
Summary of documented cases of infective endocarditis caused by Gemella haemolysans.
| Author (year) | Age/sex | Associated condition/predisposing cause | Infected valve | Treatment | Outcome/valve replacement |
|---|---|---|---|---|---|
| Chatelain et al. 14 | 62/M | Dental manipulation | Mitral | Cefamandole, gentamicin, benzylpenicillin, and amoxicillin | Cured/no |
| 48/M | Poor dentition and respiratory viral syndrome | Aortic | Benzylpenicillin and gentamicin | Cured/yes | |
| 56/M | Mitral insufficiency and dental manipulation | Mitral | Benzylpenicillin and gentamicin | Cured/no | |
| Kaufhold et al. 15 | 62/F | None | Mitral | Benzylpenicillin, tobramycin, and clindamycin | Cured/no |
| Brack et al. 16 | 74/M | Mitral prolapse | Mitral | Benzylpenicillin, gentamicin, and amoxicillin | Cured/no |
| Morea et al. 17 | 47/M | Bioprosthetic aortic valve and periodontal disease | Aortic | Erythromycin and rifampicin | Cured/yes |
| Devuyst et al. 18 | 53/M | Mitral regurgitation | Mitral | Benzylpenicillin and gentamicin | Cured/yes |
| Frésard et al. 19 | 42/M | Aortic insufficiency and scalp wound | Aortic | Vancomycin, fusidic acid, amoxicillin, and gentamicin | Cured/no |
| Helft et al. 20 | 71/M | Colonic carcinoma | Mitral | Amoxicillin + clavulanic acid and gentamicin | Cured/no |
| Matsis et al. 21 | 20/M | None | Aortic | Benzylpenicillin, gentamicin | Cured/yes |
| Samuel et al. 22 | 34/M | Multiple valve replacements with prosthetic valve, asymptomatic dental sepsis | Aortic | Cefuroxime, tobramycin, ciprofloxacin, and erythromycin | Cured/no |
| Breathnach et al. 23 | 6/M | None | Pulmonary | Amoxicillin and gentamicin | Cured/no |
| La Scola et al. 24 | 63/M | Chronic obstructive bronchitis and poor dental state | Mitral | Amoxicillin and amikacin | Kidney abscess, cured/no |
| Zingaro et al. 25 | 49/M | Glomerulonephritis | Aortic | Piperacillin and ciprofloxacin | Cured/yes |
| Mosquera et al. 26 | 77/M | Hemochromatosis with chronic liver disease |
Aortic | Penicillin and tobramycin | Cured/no |
| Khan et al. 8 | 80/M | Mitral prolapse with regurgitation and denture fixation |
Mitral | Penicillin and gentamicin | Cured/no |
| Avgoustidis et al. 27 | NA | Systemic lupus erythematosus | NA | NA | NA |
| Liu et al. 12 | 87/F | Hypertension, atrial fibrillation, diabetes mellitus type 2, COPD, and multiple myeloma | Aortic | Ampicillin and gentamicin | Cured/no |
| Ando et al. 28 | 24/M | Dental abscess | Aortic and mitral | Clindamycin, penicillin G, vancomycin | Cured/yes |
| Quaesaet et al. 29 | 39/M | Bioprosthetic aortic valve | Aortic | Amoxicillin, and rifampicin | Cured, splenic infarction due to septic emboli/no |
| Youssef et al. 9 | 81/M | Coronary artery disease, hypertension, COPD, and atrial fibrillation | Mitral | Vancomycin, ceftriaxone, and gentamicin | Rupture in the mitral leaflet, died |
| Agrawal et al. 30 | 38/M | None | Aortic | Ampicillin and gentamicin | Cured/no |
| Eslinger et al. 31 | 63/M | Acute myeloid leukemia and poor dental hygiene | Aortic | Vancomycin, cefepime, benzylpenicillin, and gentamicin | Cured/no |
| Rabah et al. 32 | 56/M | Dental procedure | Mitral | Vancomycin and ceftriaxone | Cured/yes |
COPD: chronic obstructive pulmonary disease; NA: not available.
IE remains a clinical challenge, even though there have been strides in the diagnostic techniques and management approaches over the past few decades. 10 In cases of prolonged fever, especially in patients with a history of heart disease, IE should be considered as one of the potential causes. The first step in the diagnosis of IE is to identify risk factors, including prosthetic heart valves, structural cardiac anomalies, intravenous drug use, and recent invasive procedures. Echocardiography continues to be the primary imaging modality for detecting anatomical evidence of IE. According to the modified Duke criteria, 33 our patient fulfilled one major criterion (echocardiography finding of pseudoaneurysm) and three minor criteria (fever of at least 38°C, valvular heart disease as a predisposing heart condition, and positive blood culture for G. haemolysans). Gemella isolates have shown susceptibility to β-lactams. In almost all published cases, patients were successfully treated with antibiotics alone or in combination with cardiac valvular replacement.
TTE is recommended as the first-line imaging modality in suspected IE. 34 TEE must be performed in case of negative TTE, but high clinical suspicion of IE. TEE has a sensitivity of 90%–100% and a specificity of 90% for detection of vegetations, and it is superior to TTE for detection of complications, such as abscess, leaflet perforation, and pseudoaneurysm.35,36
P-MAIVF can be a catastrophic sequela of untreated active IE.37,38 If there is a delay in diagnosis or treatment, the bacteria may invade into the fibrous tissue between the mitral and aortic valves, leading to the development of P-MAIVF. A total of 89 cases were reported in the literature from 1966 to 2009. 38 P-MAIVF appears as a pulsatile echo-free pouch that expands during systole and collapses during diastole. Sudhakar et al. 38 observed that the most common clinical presentations of P-MAIVF were symptoms and/or signs of active endocarditis (39%), followed by dyspnea and heart failure (16%), chest pain (10%), cerebrovascular accident, and systemic embolism (12%). TEE had a higher sensitivity in detecting P-MAIVF compared to TTE (90% vs 43%). 39 However, there are certain clinical scenarios where echocardiography alone may not definitively confirm or exclude the diagnosis of IE. In such cases, newer diagnostic techniques, such as cardiac computed tomography (CCT) can play a crucial role in confirming the diagnosis. In a recent meta-analysis, it was found that CCT had superior sensitivity in detecting abscess or pseudoaneurysm compared to TEE (78% vs 69%), while TEE had significantly higher sensitivity than CCT for the detection of vegetations (94% vs 64%). 40 P-MAIVF is a rare disease in pediatric patients, with only a few case reports and case series available. 38 To the best of our knowledge, this case represents the first association of IE caused by Gemella haemolysans and P-MAIVF.
Conclusion
This case highlights the importance of considering atypical pathogens as causative agents of IE. Gemella haemolysans can cause serious infections in susceptible individuals, particularly those with underlying valvular heart disease. TEE must be performed in the majority of patients with suspected IE, because of its better sensitivity, particularly for the diagnosis of perivalvular involvement. P-MAIVF is a potentially life-threatening condition that is well described in adults but remains rare in the pediatric population.
Acknowledgments
None.
Footnotes
Author contributions: All authors contributed to this article. G.D., A.V., E.G., F.D.A., M.S., P.G., and F.A. wrote the abstract, introduction, case, discussion, and conclusion. A.V., P.G., and F.A. performed critical edits and final revision of figures. The article has been read and approved by all the named authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Gianluca Dini  https://orcid.org/0000-0003-1572-221X
https://orcid.org/0000-0003-1572-221X
References
- 1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016; 387(10021): 882–893. [DOI] [PubMed] [Google Scholar]
- 2. Hill EE, Herijgers P, Claus P, et al. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J 2007; 28(2): 196–203. [DOI] [PubMed] [Google Scholar]
- 3. Abegaz TM, Bhagavathula AS, Gebreyohannes EA, et al. Short- and long-term outcomes in infective endocarditis patients: a systematic review and meta-analysis. BMC Cardiovasc Disord 2017; 17(1): 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selton-Suty C, Célard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012; 54(9): 1230–1239. [DOI] [PubMed] [Google Scholar]
- 5. Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19): 2070–2076. [DOI] [PubMed] [Google Scholar]
- 6. Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev 2001; 14(1): 177–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Facklam R, Elliott JA. Identification, classification, and clinical relevance of catalase-negative, gram-positive cocci, excluding the streptococci and enterococci. Clin Microbiol Rev 1995; 8(4): 479–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan R, Urban C, Rubin D, et al. Subacute endocarditis caused by Gemella haemolysans and a review of the literature. Scand J Infect Dis 2004; 36(11–12): 885–888. [DOI] [PubMed] [Google Scholar]
- 9. Youssef D, Youssef I, Marroush TS, et al. Gemella endocarditis: a case report and a review of the literature. Avicenna J Med 2019; 9(4): 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baltimore RS, Gewitz M, Baddour LM, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young and the Council on Cardiovascular and Stroke Nursing. Infective Endocarditis in Childhood: 2015 Update: a Scientific Statement From the American Heart Association. Circulation 2015; 132(15): 1487–515. [DOI] [PubMed] [Google Scholar]
- 11. Rosenthal LB, Feja KN, Levasseur SM, et al. The changing epidemiology of pediatric endocarditis at a children’s hospital over seven decades. Pediatr Cardiol 2010; 31(6): 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu D, Bateman T, Carr E, et al. Endocarditis due to Gemella haemolysans in a newly diagnosed multiple myeloma patient. J Community Hosp Intern Med Perspect 2016; 6(4): 32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahveci G, Bayrak F, Pala S, et al. Impact of bicuspid aortic valve on complications and death in infective endocarditis of native aortic valves. Tex Heart Inst J 2009; 36(2): 111–116. [PMC free article] [PubMed] [Google Scholar]
- 14. Chatelain R, Croize J, Rouge P, et al. Isolement de Gemella haemolysans dans trois cas d’endocardites bactériennes, Med Mal Infect 1982; 1: 25–30. [Google Scholar]
- 15. Kaufhold A, Franzen D, Lütticken R. Endocarditis caused by Gemella haemolysans. Infection 1989; 17(6): 385–387. [DOI] [PubMed] [Google Scholar]
- 16. Brack MJ, Avery PG, Hubner PJ, et al. Gemella haemolysans: a rare and unusual cause of infective endocarditis. Postgrad Med J 1991; 67(784): 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morea P, Toni M, Bressan M, et al. Prosthetic valve endocarditis by Gemella haemolysans. Infection 1991; 19(6): 446. [DOI] [PubMed] [Google Scholar]
- 18. Devuyst O, Hainaut P, Gigi J, et al. Rarity and potential severity of Gemella haemolysans endocarditis. Acta Clin Belg 1993; 48(1): 52–53. [DOI] [PubMed] [Google Scholar]
- 19. Frésard A, Michel VP, Rueda X, et al. Gemella haemolysans endocarditis. Clin Infect Dis 1993; 16(4): 586–587. [DOI] [PubMed] [Google Scholar]
- 20. Helft G, Tabone X, Metzger JP, et al. Gemella haemolysans endocarditis with colonic carcinoma. Eur J Med 1993; 2(6): 369–370. [PubMed] [Google Scholar]
- 21. Matsis PP, Easthope RN. Gemella haemolysans endocarditis. Aust N Z J Med 1994; 24(4): 417–418. [DOI] [PubMed] [Google Scholar]
- 22. Samuel L, Bloomfield P, Ross P. Gemella haemolysans prosthetic valve endocarditis. Postgrad Med J 1995; 71(833): 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breathnach AS, Gould FK, Bain HH, et al. Gemella haemolysans endocarditis associated with a raised anti-streptolysin-O titre. J Infect 1997; 34(1): 87–88. [DOI] [PubMed] [Google Scholar]
- 24. La Scola B, Raoult D. Molecular identification of Gemella species from three patients with endocarditis. J Clin Microbiol 1998; 36(4): 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zingaro L, Bartoli E, Sechi LA. Post-infectious glomerulonephritis in a patient with Gemella haemolysans endocarditis. Am J Med 1999; 106(1): 125–126. [PubMed] [Google Scholar]
- 26. Mosquera JD, Zabalza M, Lantero M, et al. Endocarditis due to Gemella haemolysans in a patient with hemochromatosis. Clin Microbiol Infect 2000; 6(10): 566–568. [DOI] [PubMed] [Google Scholar]
- 27. Avgoustidis N, Bourantas CV, Anastasiadis GP, et al. Endocarditis due to Gemella haemolysans in a patient with systemic lupus erythematosus. J Heart Valve Dis 2011; 20(1): 107–109. [PubMed] [Google Scholar]
- 28. Ando A, Kagihara J, Chung H, et al. Native bivalvular endocarditis by Gemella haemolysans requiring venovenous extracorporeal membrane oxygenation. BMJ Case Rep 2016; 2016: bcr2016216029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quaesaet L, Jaffuel S, Garo B, et al. Endocardite à Gemella haemolysans chez un patient porteur d’une bioprothèse valvulaire aortique [Gemella haemolysans endocarditis in a patient with a bioprosthetic aortic valve]. Med Mal Infect 2016; 46(1): 61–63. [DOI] [PubMed] [Google Scholar]
- 30. Agrawal T, Irani M, Fuentes Rojas S, et al. A rare case of infective endocarditis caused by Gemella haemolysans. Cureus 2019; 11(11): e6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eslinger LJ, Ahmed T. Gemella haemolysans infective endocarditis in a patient with febrile neutropenia. Cureus 2022; 14(4): e24076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rabah H, El Gharib K, Assaad M, et al. Gemella endocarditis. IDCases 2022; 29: e01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fowler VG, Durack DT, Selton-Suty C, et al. The 2023 Duke-ISCVID criteria for infective endocarditis: updating the modified duke criteria. Clin Infect Dis 2023; 77(4): 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44): 3075–3128. [DOI] [PubMed] [Google Scholar]
- 35. De Castro S, Cartoni D, d’Amati G, et al. Diagnostic accuracy of transthoracic and multiplane transesophageal echocardiography for valvular perforation in acute infective endocarditis: correlation with anatomic findings. Clin Infect Dis 2000; 30(5): 825–826. [DOI] [PubMed] [Google Scholar]
- 36. Daniel WG, Mügge A, Martin RP, et al. Improvement in the diagnosis of abscesses associated with endocarditis by transesophageal echocardiography. N Engl J Med 1991; 324(12): 795–800. [DOI] [PubMed] [Google Scholar]
- 37. Ahmed A, Shivaram P, Zakaria D. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa following endocarditis and aortic valve surgery in an infant-Case report and exhaustive systematic review of pediatric cases. Echocardiography 2020; 37(9): 1495–1505. [DOI] [PubMed] [Google Scholar]
- 38. Sudhakar S, Sewani A, Agrawal M, et al. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa (MAIVF): a comprehensive review. J Am Soc Echocardiogr 2010; 23(10): 1009–1018. [DOI] [PubMed] [Google Scholar]
- 39. Afridi I, Apostolidou MA, Saad RM, et al. Pseudoaneurysms of the mitral-aortic intervalvular fibrosa: dynamic characterization using transesophageal echocardiographic and Doppler techniques. J Am Coll Cardiol 1995; 25(1): 137–145. [DOI] [PubMed] [Google Scholar]
- 40. Oliveira M, Guittet L, Hamon M, et al. Comparative value of cardiac CT and transesophageal echocardiography in infective endocarditis: a systematic review and meta-analysis. Radiol Cardiothorac Imaging 2020; 2(3): e190189. [DOI] [PMC free article] [PubMed] [Google Scholar]