Abstract
Methylene Blue (MB) is a brain-penetrating drug with putative neuroprotective, antioxidant and metabolic enhancing effects. In vitro studies suggest that MB enhances mitochondrial complexes activity. However, no study has directly assessed the metabolic effects of MB in the human brain. We used in vivo neuroimaging to measure the effect of MB on cerebral blood flow (CBF) and brain metabolism in humans and in rats. Two doses of MB (0.5 and 1 mg/kg in humans; 2 and 4 mg/kg in rats; iv) induced reductions in global cerebral blood flow (CBF) in humans (F(1.74, 12.17)5.82, p = 0.02) and rats (F(1,5)26.04, p = 0.0038). Human cerebral metabolic rate of oxygen (CMRO2) was also significantly reduced (F(1.26, 8.84)8.01, p = 0.016), as was the rat cerebral metabolic rate of glucose (CMRglu) (t = 2.6(16) p = 0.018). This was contrary to our hypothesis that MB will increase CBF and energy metrics. Nevertheless, our results were reproducible across species and dose dependent. One possible explanation is that the concentrations used, although clinically relevant, reflect MB’s hormetic effects, i.e., higher concentrations produce inhibitory rather than augmentation effects on metabolism. Additionally, here we used healthy volunteers and healthy rats with normal cerebral metabolism where MB’s ability to enhance cerebral metabolism might be limited.
Keywords: Cerebral blood flow, cerebral oxygen metabolism, cerebral glucose metabolism, imaging, Methylene Blue
Introduction
Alterations in mitochondria-dependent energy production have been implicated in the pathophysiology of numerous neurological and psychiatric diseases, including Alzheimer’s and Parkinson’s disease, multiple sclerosis, bipolar disorder and schizophrenia.1–6 Compounds used as routine clinical treatments for these conditions do not address the associated neuro-energetic deficits or reverse the observed metabolic decline. This unmet need led to a recent growth of interest in identification of neuroprotective strategies aiming to revert the dysfunction of neuronal mitochondrial respiration.7–10 To this end, one plausible strategy is to enhance cerebral oxygen consumption via boosting the mitochondrial oxidative phosphorylation and adenosine triphosphate (ATP) production.
Methylthioninium chloride, also known as methylene blue (MB), is highly brain penetrant11,12 and hypothesised to have brain metabolic enhancing and neuroprotective properties. MB is one of the oldest known drugs, first synthesised in 1876, as a blue chemical dye and since used as a medical dye for pre-surgical staining. 13 Other uses for MB 11 include that as antimalarial agent, treatment for methemoglobinemia, cyanide poisoning 14 and vasoplegic syndrome, 15 as well as a more novel indication as tau-aggregation inhibitor, for treatment of Alzheimer’s Disease, that has been extended to clinical trials. 16 Converging evidence from newer human and animal research studies suggest that MB also has antidepressant, anxiolytic, antipsychotic and mood-stabilising properties. 17 In accordance with this proposed ‘neurotropic’ efficacy, MB was also shown to dose-dependently enhance cognitive performance, learning and memory in both humans and in experimental animals.18–28
It is likely that many of MB clinical properties rest on its ability to act as an alternate electron acceptor in the mitochondrial electron transport chain, increasing mitochondrial oxygen consumption and boosting ATP production. 29 These effects of MB are dose-dependent, yet not linear: lower doses (0.5–4 mg/kg, calculated based on animal studies) appear to offer metabolic enhancement, while higher doses (>7 mg/kg) produce the opposite effects and may even slow or inhibit mitochondrial respiration 18 (see also review by Rojas et al. 30 ) However, the effects of MB on CNS may also be linked to its dual effect on oxidation (both inhibitory and enhancing), as well as the engagement with numerous other pharmacological targets. For example, MB is thought to be an inhibitor of soluble guanylate cyclase (sGC) and of nitric oxide synthase (NOS) enzymes, 31 both of which modulate the NO-mediated vasodilation and could affect cerebral perfusion. 32 Furthermore, MB is also a potent inhibitor of monoamine oxidase A (MAO-A) which could partially explain its proposed mood stabilising and anti-depressant properties. 33 These and other molecular targets of MB have been reviewed elsewhere. 11
Despite the large body of literature supporting MB’s neurotropic effects in the CNS, data on how it affects the living brain are limited. Studies in rodents suggest that a low dose of MB (0.5 mg/kg) increases regional cerebral blood flow (CBF), oxygen extraction fraction (OEF) and cerebral metabolic rate of oxygen (CMRO2) under resting conditions 34 and even more so under hypoxic conditions coupled with sensory stimulation. 35 These data provided initial corroboration of MB’s mitochondrial enhancing effects in animals in vivo, while the effects of MB on human brain oxygenation and metabolism have not yet been established. Interestingly, in contrast to the animal studies, Rodriguez et al. 36 reported a trend toward an (4 mg/kg) MB-induced reduction in human CBF, despite the apparent increase in network connectivity, providing contradictory evidence of reduced oxygen delivery to the tissue.
Given the paucity of animal data and a lack of direct evidence from human studies, we used quantitative neuroimaging to study the interactions of MB with CBF and metabolism in vivo, in both humans and rats. We reasoned that although preclinical evidence suggested that MB should enhance brain oxygenation and metabolism, owing to the known complexity of MB pharmacology, multiple mechanisms of action and suggested hormetic effects, 37 as well as potential species-specific effects, a direct investigation and comparison of MB in both humans and animals was warranted.
We conducted two studies, in healthy humans and in rats, respectively. For the human study, we studied the effects of two doses of MB (0.5 mg/kg and 1.0 mg/kg) on quantitative MRI parameters. These included estimation of CBF and OEF, which enabled calculation of a parameter closely related to CMRO2. CMRO2 is a measure of the rate of the oxidative phosphorylation processes, and therefore represents an index of brain mitochondrial respiration. For the rat studies, we measured dose-dependent changes in CBF induced by 2 and 4 mg/kg of MB, during both stimulated and rest conditions. The rats were imaged under anaesthesia, as is customarily conducted in rodents to minimise stress, movement and regulate physiology. Indeed, others studying the effects of MB on brain of rats in vivo also used anaesthesia.34,35 Instead of MR-based measurement of oxygen or glucose metabolism, which are technically more challenging at high magnetic fields (e.g. 9.4T used here) 38 we measured brain glucose metabolism by a classic method of 14C-2-deoxyglucose autoradiography (2DG) estimating the rate of glucose utilisation, CMRglu.39,40 As we performed 2-DG in both awake and anaesthetized rats, this additionally allowed us to rule out the confounding interference of anaesthesia on our metabolic measurements.
Our main hypothesis was that MB would increase brain oxygen utilisation, by virtue of increase in CBF or OEF, or both. We also predicted a corresponding increase in CMRglu in rats. Such direct assessments of the neurometabolic effects of MB in vivo were expected to provide essential information to facilitate the therapeutic use of MB in neurological and psychiatric diseases underpinned by neuroenergetic deficits. Contrary to our expectations, we detected reductions in blood flow and metabolism in both species, highlighting the complexity of MB and the need for further investigations, which we also discuss below.
Methods
Human study
Ethics
Ethical approval was granted from the Psychiatry, Nursing and Midwifery Research Ethics Subcommittees, King’s College London (Ref HR-16/17-4804) and was sponsored by King’s College London. The study was carried out in compliance with the Declaration of Helsinki and informed consent was obtained from all participants prior to conducting any study procedures.
Study design and procedures
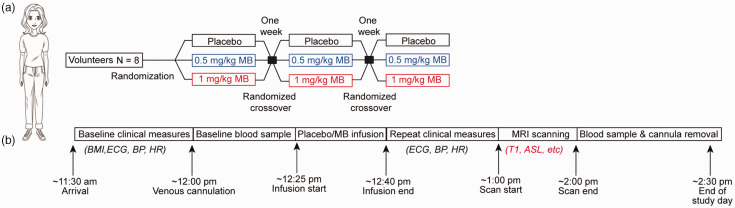
The study adopted a single-blinded, within-subject randomised design (Figure 1). Eight healthy volunteers received infusions of placebo (50 mL of 5% v/v glucose solution), and two MB (Methylthioninium chloride, Martindale Pharmaceuticals Ltd.; also see supplementary materials) doses (0.5 mg/kg and 1 mg/kg, each diluted in 50 mL of 5% v/v glucose solution), in three sessions, on separate days in randomised order. The infusions took place approximately 30 minutes prior to the MRI. Further details on participants selection and experimental methods are reported in the Supplementary Methods.
Figure 1.
Illustration of the design of the human study. (a) Schematic of the overall study design for the human arm of the study and (b) Schematic of the procedures occurring at each study day.
Blood samples were collected on all three visits before and after MB infusion, and haematological parameters including red blood cells (RBC), mean corpuscular haemoglobin concentration (MCHC), packed cell volume (PCV) were measured. Additionally, blood pressure, electrocardiogram (ECG) and heart rate were also monitored pre- and post-infusions (results shown in Supplementary Table 1). Subjective ratings of Energy and Mood were obtained through the administration of Visual Analogue Scales, ranging from 0 to 100, after each infusion. Participants were asked to report any side-effects, including pain ratings on a scale of 0–10 (10 being the highest) experienced during the MB infusion. Subjective ratings are shown in Supplementary Figure 1.
MRI methods and analysis
MR images were acquired on a 3T GE Healthcare MR750 scanner (GE Medical Systems, Milwaukee, WI). The MRI protocol included high-resolution structural T1-weighted (T1w) magnetisation-prepared rapid gradient echo (MPRAGE) images, as well as measurement of regional cerebral blood flow (CBF) by 3D pseudo-continuous Arterial Spin Labelling (3D pCASL), and voxelwise R2′ and OEF by a quantitative BOLD method based on an Asymmetric Spin Echo (ASE) acquisition.41,42 These techniques allow the quasi-simultaneous estimation of CBF and OEF respectively, which in turn enables the computation of a parameter closely related to CMRO2 that is proportional to their product. CBF maps were generated according to standard methods including those recommended by the ASL consensus paper. 43 CMRO2 calculations were carried out using the method outlined by Yablonsky et al., 44 which is based on the Fick’s principle. 45 Further details of MRI acquisitions and image processing are provided in the Supplementary Material.
Rat study
Study design and procedures
The rat study consisted of in vivo MRI and autoradiography experiments. The autoradiography experiment adopted a between-subject randomised design, while the MRI experiment had a mixed design: MB dose was a between-subject factor and time (baseline or MB) was within-subject. Animal experiments were in accordance with the UK Home Office Animals (Scientific Procedures) Act (1986) and were approved by the King’s College London ethical review committee. The reporting of this study complies with the Animal Research Reporting in vivo experiments (ARRIVE) guidelines (https://arriveguidelines.org).
To assess the effect of MB (Methylthioninium chloride, Martindale Pharmaceuticals Ltd.; also see Supplementary Methods) on rat brain perfusion and the BOLD response by MRI under anaesthesia, male adult Sprague-Dawley rats (Charles River, UK) were initially cannulated (tail and femoral vein, for MB and anaesthetic administration) under isoflurane anaesthesia, then switched to α-chloralose anaesthesia (65 mg/kg initial bolus, 30 mg/kg/hr infusion) for the duration of MRI scanning. This anaesthetic regime was chosen due to its well-documented property of preserving the neurovascular coupling, and hence BOLD response, during sensory stimulation paradigms. 46 Each animal’s physiology was maintained and monitored throughout the experiment.
To assess the effect of MB on the rat brain metabolism under both anaesthetised and awake conditions, we undertook in vivo 14C-2-DG autoradiography in two cohorts of male adult Sprague-Dawley rats: one conscious and one anaesthetised with low-level isoflurane. 14C-2-DG enables a quantification of the regional brain glucose utilisation, in a matter analogous to 18F-FDG PET. The method involves timed administration of 14C-labelled glucose analogue deoxyglucose and simultaneous blood sampling. The radioactive brain tissue is subsequently sectioned and exposed to x-ray film for densitometric quantification. For full details of 2-DG procedure see Supplementary Methods.
MRI methods and analysis
BOLD contrast sensitive images were acquired using a GE-EPI sequence, while CBF imaging used a continuous arterial spin-labelling (CASL) method. During the entire scanning protocol, non-noxious sensory forepaw stimulation and rest were alternated, providing BOLD images and CBF data from both resting and stimulated states. The entire combined BOLD/ASL protocol was repeated 5 minutes after intravenous administration of 2 (n = 3) or 4 (n = 4) mg/kg MB. Because we originally aimed to replicate the forepaw stimulation result on BOLD MRI 35 using 0.5 mg/kg MB, we additionally acquired BOLD (but not CBF) data with this dose (n = 6). For full details of MRI protocols, sequence parameters for BOLD and ASL, sensory stimulation and drug/animal combinations see Supplementary materials.
In vivo autoradiography methods and analysis
All rats were initially anaesthetised with isoflurane to cannulate their femoral vein and artery. After this, animals undergoing conscious 2-DG were loosely restrained by wrapping their torso and lower limbs in plaster cast, and allowed to recover for 90 minutes to eliminate residual effects of isoflurane. 47 Rats were intravenously administered 2 mg/kg MB (n = 10 conscious; n = 7 anaesthetised), or vehicle (n = 8 conscious; n = 7 anaesthetised), 30 minutes before 14C-2-DG to maximise the likelihood of MB accumulation in the brain. 12 Brain glucose utilisation (GU) quantification was carried out according to the methodology previously described38,40 using MCID software (Interfocus, UK). Six ellipsoid ROIs across an entire hemisphere at 4 brain levels were averaged to estimate whole brain glucose utilisation.
Results
MB (0.5–1mg/kg iv) reduces CBF, OEF and CMRO2 in humans
Eight female, healthy participants took part in the study. They were non-smokers, with an average age 27 ± 8 years and had a body mass index of 22.5 ± 1.3 (mean ± SD). Haematological and other clinical parameters (blood pressure and ECG, and subjective ratings of Energy, Mood and Pain) are summarised in Supplementary Table 1 and Supplementary Figure 1.
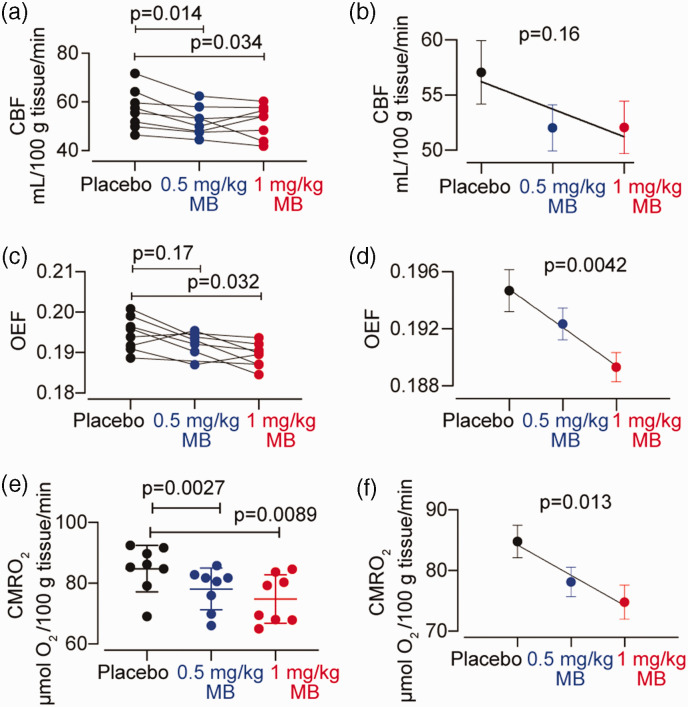
We measured global CBF, OEF and CMRO2 after two doses of MB and after placebo (Figure 2). We observed significant MB-induced reductions in global CBF (mean CBF in placebo was 57.08 ± 8.18, in 0.5 mg/kg 52.05 ± 5.91, and in 1 mg/kg 52.08 ± 6.71 ml/100 g tissue/min; F(1.74, 12.17) = 5.82, p = 0.02). The 0.5 and 1 mg/kg doses of MB reduced CBF by 8.36% (p = 0.014), and 8.3% (p = 0.034) respectively, and no dose-dependent relationship was observed (p = 0.16). We also observed significant MB-induced reductions in global (grey matter) OEF in humans relative to placebo (F(1.64, 10.67) = 6.761, p = 0.016). One value in the 0.5 mg/kg MB group was detected to be an outlier and was removed from this analysis. The 0.5 mg/kg MB dose did not significantly change OEF (−1.6% change relative to placebo, p = 0.17), whilst the 1 mg/kg MB dose caused a 2.7% reduction in OEF (p = 0.032). OEF reduction by MB was dose-dependent (p = 0.0042).
Figure 2.
Effect of MB on CBF, OEF and CMRO2 in healthy human subjects (n = 8). (a, c) Effects of 0.5 mg/kg and 1 mg/kg MB on CBF and OEF respectively, shown for individual subjects. (b) group CBF means ± SEM values and regression line. Both MB doses significantly decreased CBF but no dose-dependent effect was observed. (e) CMRO2 values derived from CBF and OEF for individual participants, with both MB doses showing significant reduction from placebo (d, f) group OEF and CMRO2 means ± SEM values and regression line. MB-induced reductions relative to placebo were dose-dependent.
MB treatment also significantly reduced CMRO2 relative to placebo (mean CMRO2 in placebo was 2.19 ± 0.2, in 0.5 mg/kg 2.02 ± 0.18 and in 1 mg/kg 1.93 ± 0.21; F(1.26, 8.84) = 8.01, p = 0.016). The reductions in CMRO2 were 7.9% (p = 0.0027) and 11.8% (p = 0.0089) after 0.5 and 1 mg/kg MB, respectively. The CMRO2 reduction observed was dose-dependent (p = 0.013).
MB did not have any significant effects on blood pressure or heart rate, nor any of the haematological parameters, including the haematocrit, red blood cells or mean corpuscular haemoglobin concentration (MCHC) which are used in the calculation of CMRO2 (Supplementary Table 1).
MB (2–4 mg/kg iv) reduces CBF and glucose utilisation in rats
We measured resting CBF in anaesthetised rats (Figure 3), and we also measured cerebral glucose metabolism in both anaesthetised and conscious rats (Figure 4).
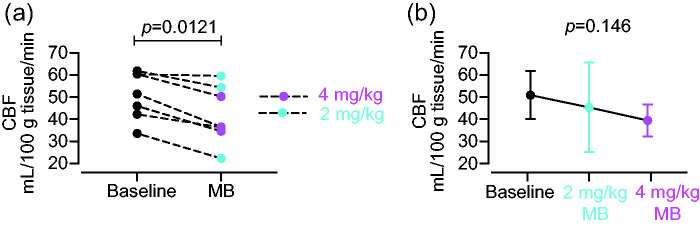
Figure 3.
Effect of MB on CBF in rats (n = 7). (a) Effects of 2 mg/kg (n = 3) and 4 mg/kg (n = 4) MB on CBF, comparing baseline and post-MB, in individual rats and (b) Group CBF means ± SEM values and regression line. Both MB doses significantly decreased CBF (paired t-test, p = 0.0021) with a non-significant trend towards a dose-dependent effect (p = 0.146).
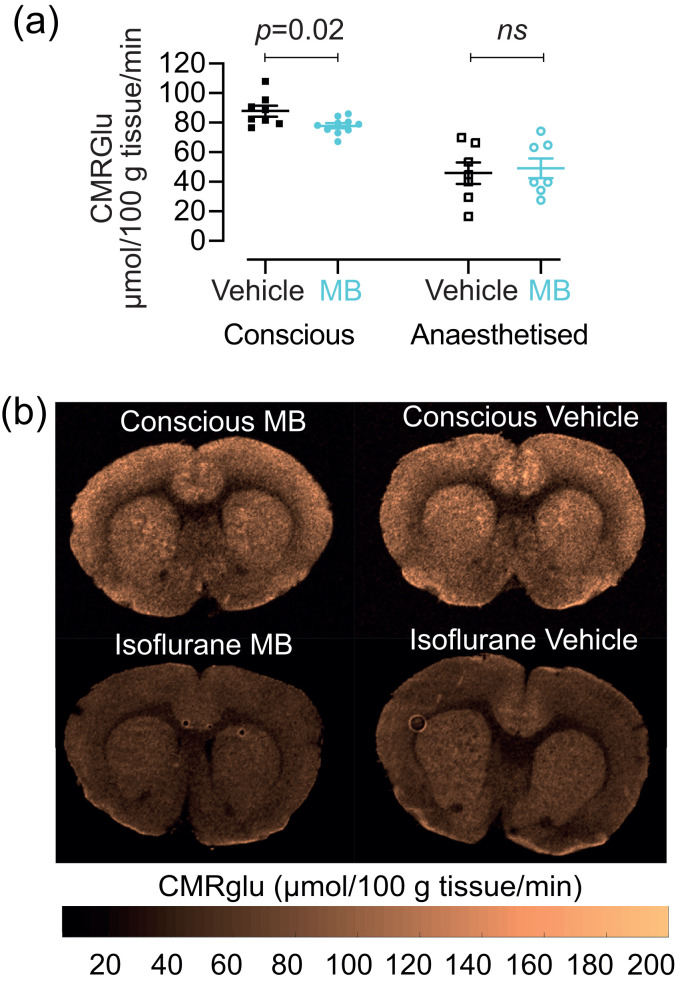
Figure 4.
Effect of 2 mg/kg MB on global CMRglu in conscious (n = 10 MB; n = 8 vehicle) and in anaesthetised rats (n = 7 MB; n = 7 vehicle). (a) There was a significant reduction of CMGglu in conscious rats after MB (p = 0.02) but not in anaesthetised rats and (b) Quantitative CMRglu maps from one slice through the brain of four representative rats.
Because of the small sample size for each of the two groups in our CBF measurements, we combined both doses into one group and observed a significant reduction in global CBF in rats after MB, compared to before MB (mean CBF before MB was 50.93 ± 10.8 and after MB 42.06 ± 13.15 ml/100g tissue/minute; paired t test, p = 0.0021). The CBF reduction was 12.6% and 21.2% for 2 and 4 mg/kg, respectively (Figure 3(a)). Moreover, there was a trend towards a dose-dependent reduction in CBF (p = 0.146, Figure 3 b).
Using in vivo 2-DG autoradiography we next measured the global CMRglu, in response to 2 mg/kg MB or vehicle, in both conscious and in anaesthetised rats. In the conscious rats, we observed a significant reduction in CMRglu with 2 mg/kg MB treatment (87.94 ± 10.26 µmol/100 g tissue/min) compared to vehicle (77.96 ± 5.54 µmol/100 g tissue/min; p = 0.018; Figure 4). We saw no significant difference between vehicle and MB in the anaesthetised rats (49.19 ± 18.05 MB, 45.96 ± 19.19 vehicle). It should however be noted that there was an overall ca. 50% decrease in the global CMRglu between conscious and anaesthetised rats, which is an expected effect of anaesthesia. 48 In this case, the anaesthetised condition also increased the variance of the responses (coefficient of variation in vehicle rats increased from 9.4% in the conscious, to 39% in the anaesthetised), hereby decreasing the power to reject the null hypothesis and likely precluding the possibility to detect any effect of MB in this group of animals.
Representative maps illustrating the effect of MB on CBF and glucose utilisation in rates are also shown in Figure 4(b). In summary, we observed a reduction in all brain CBF and metabolic parameters at rest in both humans and rats after a single administration of iv MB.
MB (2–4 mg/kg iv) does not increase stimulated CBF or BOLD response in the anaesthetised rats
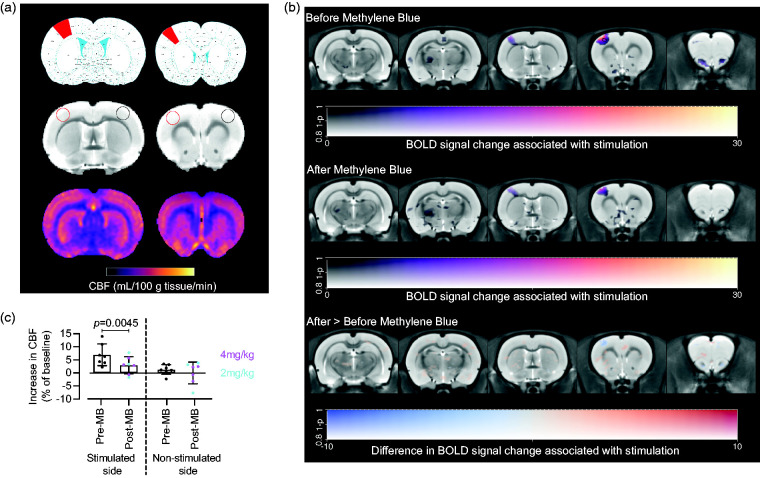
To ascertain whether MB augmented neurovascular responsiveness to sensory stimulation, we measured both CBF and BOLD responses to non-noxious sensory forepaw stimulation, before and after MB. Forepaw stimulation is expected to induce increases in both CBF and in BOLD responses in the S1 forelimb cortex contralateral to the stimulated paw. 35
As expected, we observed a significant, 6.95% increase in contralateral (stimulated) S1 CBF (p = 0.0045, Figure 5(c)) before MB. When repeating the stimulations after MB, the increases were smaller, 2.8% and 3% after 2 or 4 mg/kg dose of MB, respectively; when doses were combined, the overall increase in CBF of 2.94% was not significantly different from baseline (Figure 5(c)). There were no significant changes between stimulated and unstimulated CBF in the ipsilateral (unstimulated) S1, nor in the global (whole slice) CBF, irrespective of MB treatment, as expected.
Figure 5.
Rat CBF and BOLD responses to forepaw stimulation (n = 7). (a) ROIs (top – rat brain atlas showing location of forelimb S1 cortex (red); middle – structural images showing location of ROIs – red stimulated side, black non-stimulated side; bottom, representative CBF maps; (b) BOLD responses voxelwise for before (top) and after (middle) MB. The difference between the two conditions was tested (bottom) with a two-way ANOVA with MB dose and condition as factors. The response to stimulation is significant before MB (black contour denotes p < 0.001 uncorrected), but not after, although the difference between the two conditions is not significant and (c) CBF % change from baseline, which was only significantly increased before, but not after MB, in the contralateral forelimb cortex (p = 0.0045, one sample t test, compared to 0).
Raw CBF values from all treatment combinations are given in Table 1. In keeping with the aforementioned observations of decreased resting CBF (Figure 3), MB caused significant decreases in CBF in both S1 areas and in global CBF (post- vs pre-MB differences, Table 1).
Table 1.
Resting and stimulated rat CBF (ml/100 g tissue/min).
| pre MB |
post MB |
|||
|---|---|---|---|---|
| Region | off | on | off | on |
| S1 ipsi | 64 ± 11.6 | 65 ± 12.6 | 50.1 ± 16.3#### | 50.5 ± 17.8#### |
| S1 contra | 64 ± 10.4 | 69 ± 12*** | 51.4 ± 12.4#### | 53.2 ± 13.9*,#### |
| Global | 50.9 ± 10.8 | 50.7 ± 10.7 | 42.1 ± 13.2#### | 42.7 ± 13.0#### |
####p < 0.0001 compared to pre; *p < 0.05, ***p < 0.001 compared to off. 2-way RM ANOVA, mean ± sd, n = 7 per condition (2 and 4 mg/kg MB groups combined).
To characterise the effect of MB on BOLD responses resulting from forepaw stimulation, we also performed voxelwise, whole brain statistical parametric mapping analysis (Figure 5(b)). We observed a characteristic pattern of significantly increased BOLD response in the contralateral S1 area in stimulated rats before MB administration (Figure 5(b); top panel); this cluster appeared after MB administration but was no longer statistically significant (Figure 5(b); middle panel). However, the difference between the MB conditions was not significant (Figure 5(b); bottom panel).
Taken together, the results from rats indicate that MB caused a decrease in both resting CBF and in glucose metabolism, in direct support of our CBF and CMRO2 data from humans. Moreover, and contrary to expectations, MB also did not increase stimulation evoked CBF or BOLD responses, robustly ruling out the originally proposed enhancement of neurovascular coupling in animals.
Discussion
We report the results of quantitative imaging experiments designed to assess the effects of MB on CBF and metabolism in healthy humans and in rats. To the best of our knowledge, this is the first study to report quantifiable changes in human cerebral metabolism in response to MB, despite its clinical use since the 17th century.
Contrary to our original expectations, we show robust reductions of resting CBF after MB administration in both species. We detected a ∼8% decrease in global human CBF, after 0.5–1 mg/kg MB (Figure 2), and 12 and 20% decrease in global rat CBF after 2 and 4 mg/kg MB, respectively (Figure 3). All these results are in sharp contrast with the findings previously reported by Lin et al. 34 who demonstrated an 18% global CBF increase in anaesthetised rats 10 minutes after 0.5 mg/kg MB iv administration. To the best of our knowledge, other than the aforementioned Rodriguez et al. 36 who demonstrated reduced stimulation induced CBF in humans, and an earlier Rodriguez et al. 49 which showed no effect of MB on cerebrovascular reactivity or on stimulation induced CBF, no other human studies have examined acute effects of MB.
We further characterised brain metabolism in both humans and in rats, and we show MB-induced decreases in both. Specifically, we demonstrate that both doses of MB decreased CMRO2 in humans, and that the higher (1 mg/kg) dose also significantly decreased OEF; all parameters also show a significant dose dependency (Figure 2). In conscious rats, 2 mg/kg of MB significantly decreased CMRglu, which is a gold-standard direct measurement of brain metabolism. We did not see the same decrease in anaesthetised rats, but we note that anaesthetic-induced decrease in metabolic rate and a consequent decrease in SNR might have reduced our power to detect an effect. These results are, again in contrast to those by Lin et al. 34 who demonstrated significant increase in both CMRglu and CMRO2 in rats after 0.5 mg/kg MB, albeit both in anaesthetised animals. Finally, and contrary to Huang et al. 35 we did not detect increased BOLD response to somatosensory forepaw stimulation after neither MB dose they originally used (0.5 mg/kg; Supplementary Figure 2) nor after our 2 and 4 mg/kg doses (Figure 5). In other words, in our study MB (0.5–4 mg/kg) did not appear to potentiate stimulated BOLD response in the rat brain.
Having based our a priori hypothesis on MB stimulating mitochondrial oxygen consumption and ATP production as suggested by both in vitro and animal studies,29,34 the observed reduction in all cerebral metabolic measures, in both humans and rats, was unexpected. However, various aspects of MB’s pharmacology provide crucial hints for the interpretation of our findings and may explain the discrepancies from the results reported by others. MB’s pharmacological action is biphasic: within the hormetic zone, MB enhances mitochondrial-dependent energy production and ATP synthesis, and in vitro evidence from rat brain homogenates show MB doses between 0.5 and 5 µM increase cytochrome C oxidase activity; however, doses higher than 10 µM result in cytochrome C oxidase activity inhibition, possibly due to oxidising effect of MB on mitochondrial electron transport chain. 37
Clinical observations confirm these hormetic mechanisms and are also relevant to in vivo observations. Use of MB as treatment of methaemoglobinemia indicates antagonistic effects depending on the concentration; i.e. doses up to 4 mg/kg iv convert methaemoglobin to haemoglobin, but doses exceeding 7 mg/kg, which are considered cytotoxic appear to worsen methemoglobinemia.11,50 Behavioural pharmacological studies in rodents report that intermediate MB doses (between 5–20 mg/kg) are associated with maximal behavioural response consistent with memory enhancement effect, while does below 5 mg/kg and over 20 mg/kg have either none or opposite effects. 37 It is worth noting the species differences between humans and rodents where, due to smaller surface to volume ratio and higher metabolic activity, estimated equivalent dose in rats to humans is approximately 6× higher; 51 hence we chose 2 & 4 mg/kg to approximately match our (0.5 and 1 mg/kg) human doses while still in keeping within dose range previously shown to affect brain metabolism in rats.
MB’s poly-pharmacological actions include mechanisms other than its cycling reducing-oxidising action. Of relevance to our study, MB can inhibit nitric oxide-induced vasodilation via blockade of guanylyl cyclase activation and NOS antagonism. 11 Nitric oxide-induced vasodilation is one of regulatory mechanism of CBF, 52 which enables the brain to adjust to increased oxidative phosphorylation and metabolic demand by increasing supply of substrates, namely oxygen and glucose, in response to increased neuronal and glial energetic requirements. If MB antagonises NO-induced vasodilation this could alter normal neurovascular responses. Again, dose consideration is important as MB has little effect on the nitrergic system at very low doses, whilst its anti-vasodilating effect increases with the dose. 31
Based on these arguments, it is plausible to predict that MB concentrations at the lower end of the hormetic zone may cause an increase in CMRO2 resulting from stimulation of oxidative phosphorylation processes, with resulting coupled haemodynamic effects, i.e. an increase in CBF and CMRglu. This would be consistent with the findings of Lin et al. 34 study which used a very low (0.5 mg/kg) dose in rats. However, once MB concentrations raise nearer or beyond the limit of the hormetic zone range, MB’s cumulative effect could be determined by a progressively lower stimulation of the oxidative phosphorylation metabolic rate, and by larger NOS inhibition, resulting in a null or even inhibitory effect on CBF and metabolism.
It is therefore possible that in our human and animal study, the concentration of MB in the brain was different to those previously used in aforementioned studies and may be too high for the expected metabolic enhancing effect. In support of this theory is the fact that a trend toward decrease in fMRI task-related CBF in humans was observed by Rodriguez et al. 36 using a higher dose of 4 mg/kg. The doses we used are in keeping with the current clinical practice, and most beneficial therapeutic effects MB in treatment of methemoglobinemia, cyanide poisoning, and malaria, do correspond to MB doses of 1 to 2 mg/kg.
Despite this, the difference in concentrations between blood and CNS needs to be taken into account. Peter et al. 12 demonstrated an accumulation of MB in CNS resulting in MB concentrations 20 times higher in the brain relative to blood. Considering they administered 1 mg/kg iv resulting in an approximate plasma concentration of about 2 µM, it might be extrapolated that our dose of 0.5–1 mg/kg in humans is very likely to cause a much higher MB concentration in the brain (plausibly in a range between 10 to 40 µM) relative to the concentrations in blood known to induce antioxidant and pro-metabolic effects. Nevertheless, we also measured BOLD responses in rats using 0.5 mg/kg and we did not detect increases after MB, in direct contrast to Lin et al. 34 and Huang et al. 35 who used the same dose in rats.
Interestingly, and in support of our hypothesis, we noticed that the lower MB dose used in the human study was associated with higher energy ratings relative to placebo and the higher dose. There was no effect of any MB dose on mood relative to placebo. Pain ratings instead increased linearly with the dose. We did not observe significant changes in blood related parameters, i.e., the haematocrit, red blood cells or mean corpuscular haemoglobin concentration (MCHC) (Supplementary Table 1). Whilst we could not monitor O2 saturation in our human study, as MB interferes with the light absorption dependent SatO2 signal measured using pulse oximetry, we were able to monitor pO2 throughout scanning in rats by pulse oximetry and the values were within normal range between 97–99%.
There are some limitations in our study. We have only recruited female subjects in our human study, whereas the rats were males, both of which limits the generalisability of our findings, although we are not aware of any evidence of sex-specific effects of MB. Furthermore, our sample size is very small, although the consistent findings we observed across subjects indicates that the direction of the effects observed in our study is not the result of an underpowered analysis.
The OEF values obtained by ASE, and consequently the CMRO2 values derived from the product of CBF and ASE, are lower than those detected in the human brain using older and more validated methods. Discrepancies between ASE-derived OEF estimates and those obtained with invasive and 15O2 PET methods, are likely deriving to the overestimation of deoxygenated blood volume (DBV) known to result from the implementations of the qBOLD model we used. 53 However, this should have not affected our findings due to the study’s within-subject, cross-over design. We have analysed OEF at whole-brain level and therefore we cannot provide analysis of regional distribution of OEF, and consequently of CMRO2, changes. OEF maps obtained with ASE are characterised by low signal to noise (SNR) ratio, 42 and analysis of smaller regions would be problematic and reduce confidence in our results obtained in a small sample. Future studies in larger samples, and using improved modelling of qBOLD data to address OEF underestimation (e.g. by the inclusion of Z-shimming to account for macroscopic field inhomogeneities), and to obtain better SNR of OEF maps, will be needed to explore the distribution of the effects of MB in the brain. 54 Moreover, based on current results we can only infer on acute MB administration effects. Our findings do not preclude the possibility that the chronic use of MB may lead to metabolic enhancements, in line with preliminary evidence of clinical efficacy in a 6-month trial in Bipolar Disorder. 17
In conclusion, we report robust human and rat imaging data suggestive of an inhibitory effect of MB on brain metabolic measures, at doses used clinically for treatment of haematological conditions. Data from clinical trials application of MB provide further support to our hypothesis that the most beneficial effects of MB at CNS level is expected using extremely small MB doses, significantly lower than used for treatment of methaemoglobinemia. Interestingly, a clinical trial of an MB derivative with putative anti-TAU aggregating properties (hydromethylthionine) demonstrated that the lower dose (8 mg/kg) originally meant to be used as control, had significant pro-cognitive and anti-brain atrophy effect, whilst the test doses of 200 mg/day were not associated with any benefit on those parameters.55–57 In an earlier study, investigating mood stabilising effects of MB, a 15 mg/day oral dose originally selected as placebo condition, equating to ∼0.2 mg/kg, and 20 times lower than the putative active dose, was found to be potentially clinically effective. 58
In conclusion, MB, at the doses employed in clinical haematological applications, reduces cerebral perfusion and energy metabolism in rats and humans, at rest. It is possible that the proposed neurometabolic enhancement effect of MB requires lower doses, in both healthy volunteers and in rodents. Further quantitative imaging studies of MB effects on cerebral blood flow and metabolism are necessary to characterise its potential in therapeutic CNS applications.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231157958 for The effects of acute Methylene Blue administration on cerebral blood flow and metabolism in humans and rats by Nisha Singh, Eilidh MacNicol, Ottavia DiPasquale, Karen Randall, David Lythgoe, Ndabezinhle Mazibuko, Camilla Simmons, Pierluigi Selvaggi, Stephanie Stephenson, Federico E Turkheimer, Diana Cash, Fernando Zelaya and Alessandro Colasanti in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We are grateful to the radiographers of the Centre for Neuroimaging Sciences. We thank Mattia Baraldo for his helpful assistance with references, and to Tobias Wood for help with rat study MRI protocols.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: AC, FT, FZ, DC conceived the studies.
AC, NS, FZ, DL, DC designed the studies.
NS, AC, KR, NM, PS, SS carried out experimental procedures for the human study.
OD, DL, FZ carried out imaging analyses for the human study.
EM, CS, DC carried out experimental procedures and imaging analyses for the rat study.
NS, AC, EM, DC and FZ wrote manuscript draft.
All authors approved the submitted manuscript.
ORCID iDs: Nisha Singh https://orcid.org/0000-0002-1312-688X
Eilidh MacNicol https://orcid.org/0000-0003-3715-7012
Camilla Simmons https://orcid.org/0000-0002-8561-4241
Alessandro Colasanti https://orcid.org/0000-0001-6017-801X
Supplemental material: Supplemental material for this article is available online.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The human study was partly funded by the Mental Health Biomedical Research Centre award of the King’s College London and the South London & Maudsley NHS Trust and by a NARSAD Young Investigator grant from the Brain and Behavior Research Foundation to AC. The rat study was funded by the UK Biotechnology and Biological Sciences Research Council (BB/N009088/1). EM was supported by the UK Medical Research Council (MR/N013700/1) and King’s College London as a member of the MRC Doctoral Training Partnership in Biomedical Sciences. FET was funded by the NIHR Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
References
- 1.Andreazza AC, Shao L, Wang J-F, et al. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry 2010; 67: 360–368. [DOI] [PubMed] [Google Scholar]
- 2.Dutta R, McDonough J, Yin X, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 2006; 59: 478–489. [DOI] [PubMed] [Google Scholar]
- 3.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 2008; 4: 600–609. [DOI] [PubMed] [Google Scholar]
- 4.Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci 2001; 21: 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinna A, Colasanti A. The neurometabolic basis of mood instability: the parvalbumin interneuron link – a systematic review and meta-analysis. Front Pharmacol 2021; 12: 689473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry 2004; 9: 684–697, 643. [DOI] [PubMed] [Google Scholar]
- 7.Broman K, Davis AU, May J, et al. Lifestyle factors, mitochondrial dynamics, and neuroprotection. London: IntechOpen, 2019.
- 8.Chaturvedi RK, Flint Beal M. Mitochondrial diseases of the brain. Free Radic Biol Med 2013; 63: 1–29. [DOI] [PubMed] [Google Scholar]
- 9.Giménez-Palomo A, Dodd S, Anmella G, et al. The role of mitochondria in mood disorders: from physiology to pathophysiology and to treatment. Front Psychiatry 2021; 12: 546801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Youngblood H, Wu C, et al. Mitochondria as a target for neuroprotection: role of methylene blue and photobiomodulation. Transl Neurodegener 2020; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oz M, Lorke DE, Hasan M, et al. Cellular and molecular actions of methylene blue in the nervous system. Med Res Rev 2011; 31: 93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peter C, Hongwan D, Küpfer A, et al. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol 2000; 56: 247–250. [DOI] [PubMed] [Google Scholar]
- 13.Dudley NE. Methylene blue for rapid identification of the parathyroids. Br Med J 1971; 3: 680–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendel WB. The control of methemoglobinemia with methylene blue. J Clin Invest 1939; 18: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanmugam G. Vasoplegic syndrome – the role of methylene blue. Eur J Cardiothorac Surg 2005; 28: 705–710. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier S, Feldman HH, Schneider LS, et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 2016; 388: 2873–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alda M. Methylene blue in the treatment of neuropsychiatric disorders. CNS Drugs 2019; 33: 719–725. [DOI] [PubMed] [Google Scholar]
- 18.Callaway NL, Riha PD, Bruchey AK, et al. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol Biochem Behav 2004; 77: 175–181. [DOI] [PubMed] [Google Scholar]
- 19.Deiana S, Harrington CR, Wischik CM, et al. Methylthioninium chloride reverses cognitive deficits induced by scopolamine: comparison with rivastigmine. Psychopharmacology (Berl) 2009; 202: 53–65. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Lima F, Bruchey AK. Extinction memory improvement by the metabolic enhancer methylene blue. Learn Mem 2004; 11: 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez JL, Jensen RA, Vasquez BJ, et al. Methylene blue alters retention of inhibitory avoidance responses. Psychobiology 1978; 6: 387–390. [Google Scholar]
- 22.Medina DX, Caccamo A, Oddo S. Methylene blue reduces Aβ levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol 2011; 21: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Leary JC, Li Q, Marinec P, et al. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol Neurodegener 2010; 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riha PD, Bruchey AK, Echevarria DJ, et al. Memory facilitation by methylene blue: dose-dependent effect on behavior and brain oxygen consumption. Eur J Pharmacol 2005; 511: 151–158. [DOI] [PubMed] [Google Scholar]
- 25.Telch MJ, Bruchey AK, Rosenfield D, et al. Post-session administration of USP methylene blue facilitates the retention of pathological fear extinction and contextual memory in phobic adults. Am J Psychiatry 2014; 171: 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wrubel KM, Barrett D, Shumake J, et al. Methylene blue facilitates the extinction of fear in an animal model of susceptibility to learned helplessness. Neurobiol Learn Mem 2007; 87: 209–217. [DOI] [PubMed] [Google Scholar]
- 27.Wrubel KM, Riha PD, Maldonado MA, et al. The brain metabolic enhancer methylene blue improves discrimination learning in rats. Pharmacol Biochem Behav 2007; 86: 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callaway NL, Riha PD, Wrubel KM, et al. Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats. Neurosci Lett 2002; 332: 83–86. [DOI] [PubMed] [Google Scholar]
- 29.Atamna H, Nguyen A, Schultz C, et al. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J 2008; 22: 703–712. [DOI] [PubMed] [Google Scholar]
- 30.Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol 2012; 96: 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer B, Brunner F., Schmidt K. Novel actions of methylene blue. European Heart Journal 1993; 14: 22–26. [PubMed] [Google Scholar]
- 32.Volke V, Wegener G, Vasar E, et al. Methylene blue inhibits hippocampal nitric oxide synthase activity in vivo. Brain Res 1999; 826: 303–305. [DOI] [PubMed] [Google Scholar]
- 33.Ramsay RR, Dunford C, Gillman PK. Methylene blue and serotonin toxicity: inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction. Br J Pharmacol 2007; 152: 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin A-L, Poteet E, Du F, et al. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PloS One 2012; 7: e46585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang S, Du F, Shih Y-YI, et al. Methylene blue potentiates stimulus-evoked fMRI responses and cerebral oxygen consumption during normoxia and hypoxia. NeuroImage 2013; 72: 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez P, Singh AP, Malloy KE, et al. Methylene blue modulates functional connectivity in the human brain. Brain Imaging and Behavior 2017; 11: 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruchey AK, Gonzalez-Lima F. Behavioral, physiological and biochemical hormetic responses to the autoxidizable dye methylene blue. Am J Pharmacol Toxicol 2008; 3: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood TC, Cash D, MacNicol E, et al. Non-Invasive measurement of the cerebral metabolic rate of oxygen using MRI in rodents. Wellcome Open Res 2021; 6: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurumaji A, Dewar D, McCulloch J. Metabolic mapping with deoxyglucose autoradiography as an approach for assessing drug action in the central nervous system. Imaging Drug Action in the Brain. Routledge, 1993, pp. 207–246. [Google Scholar]
- 40.Sokoloff L, Reivich M, Kennedy C, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 1977; 28: 897–916. [DOI] [PubMed] [Google Scholar]
- 41.He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med 2007; 57: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone AJ, Blockley NP. A streamlined acquisition for mapping baseline brain oxygenation using quantitative BOLD. NeuroImage 2017; 147: 79–88. [DOI] [PubMed] [Google Scholar]
- 43.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European Consortium for ASL in dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yablonskiy DA, Sukstanskii AL, He X. Blood oxygenation level-dependent (BOLD)-based techniques for the quantification of brain hemodynamic and metabolic properties – theoretical models and experimental approaches. NMR Biomed 2013; 26: 963–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 1948; 27: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zong X, Kim T, Kim S-G. Contributions of dynamic venous blood volume versus oxygenation level changes to BOLD fMRI. Neuroimage 2012; 60: 2238–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang W, Chini M, Pöpplau JA, et al. Anesthetics fragment hippocampal network activity, alter spine dynamics, and affect memory consolidation. PLOS Biol 2021; 19: e3001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slupe AM, Kirsch JR. Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J Cereb Blood Flow Metab 2018; 38: 2192–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez P, Zhou W, Barrett DW, et al. Multimodal randomized functional MR imaging of the effects of methylene blue in the human brain. Radiology 2016; 281: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vutskits L, Briner A, Klauser P, et al. Adverse effects of methylene blue on the central nervous system. Anesthesiology 2008; 108: 684–692. [DOI] [PubMed] [Google Scholar]
- 51.Nair A, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016; 7: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lourenço CF, Laranjinha J. Nitric oxide pathways in neurovascular coupling under normal and stress conditions in the brain: strategies to rescue aberrant coupling and improve cerebral blood flow. Front Physiol 2021; 12: 729201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone AJ, Holland NC, Berman AJL, et al. Simulations of the effect of diffusion on asymmetric spin echo based quantitative BOLD: an investigation of the origin of deoxygenated blood volume overestimation. NeuroImage 2019; 201: 116035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alda M, McKinnon M, Blagdon R, et al. Methylene blue treatment for residual symptoms of bipolar disorder: randomised crossover study. Br J Psychiatry 2017; 210: 54–60. [DOI] [PubMed] [Google Scholar]
- 55.Schelter BO, Shiells H, Baddeley TC, et al. Concentration-dependent activity of hydromethylthionine on cognitive decline and brain atrophy in mild to moderate Alzheimer's disease. J Alzheimers Dis 2019; 72: 931–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shiells H, Schelter BO, Bentham P, et al. Concentration-dependent activity of hydromethylthionine on clinical decline and brain atrophy in a randomized controlled trial in behavioral variant frontotemporal dementia. J Alzheimers Dis 2020; 75: 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilcock GK, Gauthier S, Frisoni GB, et al. Potential of low dose leuco-methylthioninium bis(hydromethanesulphonate) (LMTM) monotherapy for treatment of mild Alzheimer's disease: cohort analysis as modified primary outcome in a phase III clinical trial. J Alzheimers Dis 2018; 61: 435–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naylor GJ, Martin B, Hopwood SE, et al. A two-year double-blind crossover trial of the prophylactic effect of methylene blue in manic depressive psychosis. Biol Psychiatry 1986; 21: 915–920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231157958 for The effects of acute Methylene Blue administration on cerebral blood flow and metabolism in humans and rats by Nisha Singh, Eilidh MacNicol, Ottavia DiPasquale, Karen Randall, David Lythgoe, Ndabezinhle Mazibuko, Camilla Simmons, Pierluigi Selvaggi, Stephanie Stephenson, Federico E Turkheimer, Diana Cash, Fernando Zelaya and Alessandro Colasanti in Journal of Cerebral Blood Flow & Metabolism