Abstract
Background
In advanced non‐small cell lung cancer (NSCLC), the effectiveness of standard cytotoxic chemotherapy seems to have reached a 'plateau', and there is a continuous need for new treatments to further improve the prognosis. Cetuximab is a monoclonal antibody targeted at the epidermal growth factor receptor (EGFR) signalling pathway. Basically, it is designed to inhibit the growth and metastasis among other biological processes of cancer. In combination with chemotherapy, it has been evaluated as a first‐line treatment for advanced NSCLC in some randomised controlled trials (RCTs), with inconsistent results.
Objectives
To evaluate the efficacy and toxicity of chemotherapy plus cetuximab, compared with chemotherapy alone, for advanced non‐small cell lung cancer (NSCLC) previously untreated with chemotherapy or epidermal growth factor receptor (EGFR)‐targeted drugs.
Search methods
We systematically searched the Cochrane Lung Cancer Review Group's Specialized Register (from inception to 17 December 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 12), MEDLINE (accessed through PubMed, 1966 to 17 December 2013), EMBASE (1980 to 17 December 2013), ClinicalTrials.gov (from inception to 17 December 2013), and the World Health Organization (WHO) International Clinical Trials Registry Platform (from inception to 17 December 2013). We also handsearched the proceedings related to lung cancer from the American Society of Clinical Oncology and European Society of Medical Oncology (2000 to 17 December 2013). We checked the reference lists of all eligible primary studies and review articles for additional potentially eligible studies.
Selection criteria
Eligible studies were RCTs that compared chemotherapy plus cetuximab with the same chemotherapy alone, in advanced NSCLC, previously untreated with chemotherapy or EGFR‐targeted drugs, and measured at least one of the following: overall survival, progression‐free survival, one‐year survival rate, objective response rate, quality of life, or serious adverse events.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration. We extracted the following data from each study: publication details, participant characteristics, regimens for intervention and control arms, outcome measures and effect size, and information related to the methodological quality of the study. We measured the treatment effects on dichotomous and time‐to‐event outcomes by risk ratio (RR) and hazard ratio (HR), with 95% confidence intervals (CIs), respectively. We conducted meta‐analyses with Review Manager 5 using the random‐effects model. We employed the Mantel‐Haenszel method to combine RRs and the inverse‐variance method to combine HRs.
Main results
We included four trials, containing 2018 patients. The subjects were mostly white people (female: 26% to 56%), with a median age of 58 to 66 years. About half of them had histologically proven adenocarcinoma. Of the 2018 patients, 83% to 99% had their status measured using the Eastern Cooperative Oncology Group performance status, and had a score of 0 to 1 (which is usually considered as physically "fit").
All four studies provided data on overall survival, progression‐free survival, one‐year survival rate, objective response rate, and serious adverse events, with two studies (1901 patients) investigating the effect of cetuximab on quality of life as well. The risk of bias was low for the data on overall survival and one‐year survival rate, and high for the data on all other outcomes, mainly due to lack of blinding. Compared with chemotherapy alone, chemotherapy plus cetuximab improved overall survival (10.5 months versus 8.9 months; HR 0.87, 95% CI 0.79 to 0.96), one‐year survival rate (45% versus 40%; RR 1.13, 95% CI 1.02 to 1.25), and objective response rate (30% versus 23%; RR 1.31, 95% CI 1.14 to 1.51). The difference in progression‐free survival was at the limit of the statistical significance (4.9 months versus 4.4 months; HR 0.91, 95% CI 0.83 to 1.00). No significant difference in quality of life between the two treatment arms was reported by the two relevant studies. Patients in the cetuximab group experienced more acneiform rash (11.2% versus 0.3%; RR 37.36, 95% CI 10.66 to 130.95), hypomagnesemia (5.3% versus 0.8%; RR 6.57, 95% CI 1.13 to 38.12), infusion reaction (3.9% versus 1.1%; RR 3.50, 95% CI 1.76 to 6.94), diarrhoea (4.8% versus 2.3%; RR 2.10, 95% CI 1.26 to 3.48), hypokalaemia (6.3% versus 3.6%; RR 1.74, 95% CI 1.02 to 2.99), febrile neutropenia (10.6% versus 7.6%; RR 1.40, 95% CI 1.10 to 1.77), and leukopenia (58.1% versus 42.7%; RR 1.36, 95% CI 1.17 to 1.58) than did those in the control group. The difference in other adverse events did not reach statistical significance. According to the reports of original studies, the adverse events were generally manageable. There were no cetuximab‐related deaths.
The quality of the evidence is high for overall survival and one‐year survival rate, but low for most secondary outcomes.
Authors' conclusions
The combination of chemotherapy plus cetuximab is better than chemotherapy alone as the first‐line treatment of advanced NSCLC in improving overall survival, while inducing higher rates of some reportedly manageable adverse events.
Plain language summary
Cetuximab: a new treatment for advanced non‐small cell lung cancer
Lung cancer is the most common cancer in the world. Advanced non‐small cell lung cancer (NSCLC) accounts for about 60% of all lung cancer cases. Since the effectiveness of current standard treatment for advanced NSCLC (i.e. chemotherapy) has reached a ceiling, there is a continuous need for new, more effective treatments to further improve the outcome of patients with the disease. This review of 2018 patients, from four trials, found that adding cetuximab (a newly developed agent) to standard treatment, prolonged the survival time of advanced NSCLC patients by about 1.5 months, and deferred the progression of cancer by about 0.5 month. One year after the treatment, 45% of the patients receiving standard treatment plus cetuximab, and 40% of the patients receiving standard treatment alone were still alive. However, the effects of cetuximab on quality of life of patients were uncertain. Seven types of adverse events, mainly involving skin and blood, occurred much more in the patients receiving cetuximab, while other adverse events seemed to occur equally in both groups. The adverse events were reported as generally manageable. No deaths related to cetuximab were reported. In summary, high quality evidence shows that the use of cetuximab combined with standard treatment leads to better survival than standard treatment alone, in improving survival of patients with advanced NSCLC.
Summary of findings
for the main comparison.
| Chemotherapy plus cetuximab compared with chemotherapy alone for chemotherapy‐naive advanced non‐small cell lung cancer | ||||||
|
Patient or population: Patients with advanced non‐small cell lung cancer Settings: First‐line treatment Intervention: Chemotherapy plus cetuximab Comparison: Chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Chemotherapy alone | Chemotherapy plus cetuximab | |||||
| Overall survival1 | 8.9 months | 10.5 months | HR 0.87 (0.79 to 0.96) | 2018 (4 studies) | ⊕⊕⊕⊕ high | |
| Progression‐free survival1 | 4.4 months | 4.9 months | HR 0.91 (0.83 to 1.00) | 2018 (4 studies) | ⊕⊕⊝⊝ low5 | |
| One‐year survival rate2 | 40 per 100 |
45 per 100 (41 to 50) |
RR 1.13 (1.02 to 1.25) | 2018 (4 studies) | ⊕⊕⊕⊕ high | |
| Objective response rate2 | 23 per 100 |
30 per 100 (26 to 35) |
RR 1.31 (1.14 to 1.51) | 2018 (4 studies) | ⊕⊕⊕⊝ low6 | |
| Quality of life3 | See comment | See comment | Not estimable | 1801 (2 studies) | ⊕⊕⊝⊝ low5 | Both studies reported that there were no significant differences in the change of quality of life between the two treatment arms, but no detailed data were reported |
| Serious adverse events2,4 | 1. acneiform rash: 0.3 per 100 2. hypomagnesemia: 0.8 per 100 3. infusion reaction: 1.1 per 100 4. diarrhoea: 2.3 per 100 5. hypokalaemia: 3.6 per 100 6. febrile neutropenia: 7.6 per 100 7.leukopenia: 42.7 per 100 |
1. acneiform rash: 11.2 per 100 (3.2 to 39.3) 2. hypomagnesemia: 5.3 per 100 (0.9 to 30.5) 3. infusion reaction: 3.9 per 100 (1.9 to 7.6) 4. diarrhoea: 4.8 per 100 (2.9 to 8.0) 5. hypokalaemia: 6.3 per 100 (3.7 to 10.8) 6. febrile neutropenia: 10.6 per 100 (8.4 to 13.5) 7.leukopenia: 58.1 per 100 (50.0 to 67.5) |
1. acneiform rash: RR 37.36 (10.66 to 130.95) 2. hypomagnesemia: RR 6.57 (1.13 to 38.12) 3. infusion reaction: RR 3.50 (1.76 to 6.94) 4. diarrhoea: RR 2.10 (1.26 to 3.48) 5. hypokalaemia: RR 1.74 (1.02 to 2.99) 6. febrile neutropenia: RR 1.40 (1.10 to 1.77) 7.leukopenia: RR 1.36 (1.17 to 1.58) |
1. acneiform rash: 1970 (4 studies) 2. hypomagnesemia: 775 (2 studies) 3. infusion reaction: 1885 (3 studies) 4. diarrhoea: 1885 (3 studies) 5. hypokalaemia: 1110 (1 study) 6. febrile neutropenia: 1755 (2 studies) 7.leukopenia: 1755 (2 studies) |
⊕⊕⊝⊝ low5 | For other adverse events, there were no significant differences between the two treatment arms |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard ratio; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 For time‐to‐event outcomes, e.g. overall survival, the assumed risk was obtained by calculating the median value of the "median survival time of the control arm" reported by different studies. The corresponding risk was obtained in a similar way, i.e. by calculating the median value of the "median survival time of the intervention arm" reported by different studies.
2 For dichotomous outcomes, e.g. one‐year survival rate, the assumed risk was obtained by meta‐analysis of the one‐year survival rates of control arms from all relevant studies.
3 For the assessment of quality of life: In Lynch 2010, the FACT‐LCS5 questionnaire was used; in Pirker 2009, the European Organisation for Research and Treatment of Cancer quality of life questionnaire C30 (version 3.0), EORTC lung cancer specific QLQ‐LC13, and EuroQoL (EQ‐5D) questionnaires were used.
4 The overall risk of serious adverse events was not available. Thus, specific adverse events that occurred with significantly different frequencies in the two arms were summarised instead.
5 The quality of evidence is downgraded by two factors, i.e. study limitations and imprecision, according to the guidelines of the GRADE Working Group.
6 The quality of evidence is downgraded by one factor, i.e. study limitations, according to the guidelines of the GRADE Working Group.
Background
Description of the condition
Lung cancer is one of the most common cancers in the world, in terms of both incidence and mortality. In 2008, there were over 1.6 million new cases worldwide; and there were about 1.4 million deaths due to lung cancer in the same year, accounting for 18% of all cancer deaths (IARC 2010). Approximately 85% of all lung cancers are diagnosed as non‐small cell lung cancer (NSCLC), which consists mainly of squamous cell carcinoma, adenocarcinoma, and large‐cell carcinoma (Govindan 2006). Nearly 70% of all NSCLCs have spread either locally or to distant regions of the body at the time of diagnosis, which is referred to as advanced NSCLC (stage IIIB‐IV).
The chemotherapy standard for advanced NSCLC is a platinum agent in combination with a second agent, generally paclitaxel, gemcitabine, vinorelbine, docetaxel, or pemetrexed (Stinchcombe 2009). However, the response rate is only about 20%, corresponding to an increase of three months in median survival, with no significant difference between different regimens (Marino 1994; Schiller 2002). It seems that a 'therapeutic plateau' has been reached using standard cytotoxic chemotherapy, and the prognosis of advanced NSCLC remains poor, with a five‐year survival rate of about 15% (Jemal 2010). Thus, there is a continuous need for new treatments to improve survival. Against this background, targeted therapies have attracted tremendous attention in the past few years. Some of them, such as gefitinib and erlotinib (FDA 2005; FDA 2010), two epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors, and bevacizumab (FDA 2004), a humanised monoclonal antibody that inhibits vascular endothelial growth factor A, have been developed and approved for the treatment of advanced NSCLC.
Like many other anticancer medications, targeted therapies show efficacy in only a subset of recipients. Since these therapies are directed against specific signalling pathways, molecular events of the pathways have been heavily investigated to examine their capacity of predicting the efficacy of the treatment, so as to identify beforehand, those people who are most likely to benefit. As a result several important biomarkers have indeed been discovered, such as KRAS mutations as a predictor of resistance to monoclonal antibodies for metastatic colorectal cancer (Dahabreh 2011) and EGFR mutations (Eberhard 2005) for predicting response to gefitinib and erlotinib in NSCLC.
Description of the intervention
Cetuximab is an IgG1 chimeric mouse‐human monoclonal antibody targeted at the EGFR signalling cascade. Randomised controlled trials (RCTs) confirmed that it was effective in combination with chemotherapy or as a single agent for the treatment of metastatic colorectal cancer and squamous cell head and neck cancer in terms of both response and survival (Reeves 2011; Tol 2010). In 2011, cetuximab had been approved by the Food and Drug Administration (FDA) for treating the two diseases (FDA 2011; National Cancer Institute 2011).
How the intervention might work
Cetuximab can compete with epidermal growth factor in binding to EGFR protein on the cell surface, thus blocking the EGFR signalling pathway that is critical to the growth and spread of cancer cells.
Why it is important to do this review
In patients with advanced NSCLC, a number of RCTs have been conducted to assess the efficacy and toxicity of cetuximab plus chemotherapy, compared with chemotherapy alone, as the first‐line therapy. However, their results are inconsistent, and the role of cetuximab remains to be clarified. For example, the FLEX trial (Pirker 2009) showed that the addition of cetuximab significantly increased the overall survival of patients with advanced NSCLC. By contrast, the BMS099 trial (Lynch 2010) failed to demonstrate a discernible survival benefit from the same treatment. Did the conflicting results arise from clinical or methodological heterogeneity, or purely from chance? To understand the existing evidence, and to better facilitate the translation of knowledge to practice, we conducted the present Cochrane review.
Objectives
To evaluate the efficacy and toxicity of chemotherapy plus cetuximab, compared with chemotherapy alone, for advanced non‐small cell lung cancer (NSCLC) previously untreated with chemotherapy or epidermal growth factor receptor (EGFR)‐targeted drugs.
Methods
Criteria for considering studies for this review
Types of studies
We only included RCTs. We did not impose any restrictions on publication type (abstract or full article) or language.
Types of participants
Patients with histologically‐ or cytologically‐confirmed advanced NSCLC (previously untreated with chemotherapy or EGFR‐targeted drugs).
Types of interventions
Cetuximab plus chemotherapy (such as gemcitabine, cisplatin, carboplatin, vinorelbine, or taxane) compared with the same chemotherapy alone. The dose and duration of cetuximab and chemotherapy did not necessarily have to be the same in different studies. However, in an eligible study, the interventions should not be evaluated as maintenance therapy.
Types of outcome measures
Primary outcomes
The primary outcome measure was overall survival, defined as the time from randomisation to death from any cause.
Secondary outcomes
The secondary outcome measures included progression‐free survival (time to disease progression or death), one‐year survival rate, objective response rate (the proportion of patients whose target lesions decrease to a prespecified level or disappear, as assessed according to the WHO (Miller 1981), RECIST (Response Evaluation Criteria In Solid Tumours) (Therasse 2000), or individual study criteria), quality of life, and serious adverse events (grade 3 and 4, according to WHO toxicity guidelines or the National Cancer Institute's Common Toxicity Criteria).
If a study fulfilled all the inclusion criteria, except that no data on relevant outcomes were reported, we would firstly try to find the protocol of the study (e.g. by visiting clinicaltrials.gov or www.who.int/ictrp) to check if it intended to measure the outcomes. Only when the protocol clearly showed that the study did not intend to measure the outcomes of our interest, did we exclude the study; otherwise, we classified the study under "studies awaiting classification" or "ongoing studies" as appropriate, rather than excluding it.
Search methods for identification of studies
Electronic searches
We conducted a systematic literature search in the following electronic databases, with no restrictions on the language of publication.
The Cochrane Lung Cancer Review Group Specialized Register (from inception to 17 December 2013) (Appendix 1).
The Cochrane Central Register of Controlled Trials (CENTRAL, from inception to 17 December 2013) (Appendix 2).
MEDLINE (access through PubMed (1966 to 17 December 2013)) (Appendix 3).
EMBASE (1980 to 17 December 2013) (Appendix 4).
Clinical trial registries (from inception to 17 December 2013) (Appendix 5).
Searching other resources
We handsearched the proceedings related to lung cancer from the American Society of Clinical Oncology and European Society of Medical Oncology (2000 to 17 December 2013).
We checked the reference lists of all eligible primary studies and review articles for additional potentially eligible studies.
Data collection and analysis
Selection of studies
We used EndNote software to manage the bibliographic references identified by the above searches. Two review authors (ZYY, LL) independently screened the titles and abstracts to judge study relevance. We obtained the full texts of all studies seemingly eligible for this review for closer examination. For trials published as abstracts only, we contacted the study investigators if the information needed to determine eligibility was lacking. After that, if it was still unclear whether the trials fulfilled the inclusion criteria or not, which could be due to the authors' failure to reply, among other reasons, we had to exclude them (simply because the inclusion of them would be groundless). Any disagreements were resolved by discussion of the two review authors. Unresolved disagreements were subject to the judgement of a third review author (JLT), whose opinion was considered as final.
Data extraction and management
Data extraction was performed independently by two review authors (ZYY, LL) using a standard, pilot‐tested form. We collected the following data from each study: publication details, participant characteristics, regimens for intervention and control arms, outcome measures and effect size, information related to the methodological quality of the study, and if available, data useful for assessing the predictive value of KRAS and EGFR mutations.
Assessment of risk of bias in included studies
We assessed the risk of bias of each eligible study using the criteria outlined in Table 8.5.c of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or by involving a third review author.
We assessed risk of bias according to the:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting (reporting bias); and
other bias.
Within each study, we assessed 'random sequence generation', 'allocation concealment', 'selective outcome reporting', and 'other bias' globally for all outcomes while we assessed 'blinding of participants and personnel' and 'blinding of outcome assessment' separately for 'objective' (i.e. overall survival and one‐year survival rate) and 'subjective' (all of the remaining) outcomes (Higgins 2011). We assessed 'incomplete outcome data' separately for different outcomes, but in this review the assessment results with regard to this item were the same for all outcomes across all studies. Thus, within each study, there is only one global assessment result for this item, similar to the situation of 'random sequence generation', 'allocation concealment', 'selective outcome reporting', and 'other bias'. We graded each potential source of bias as high, low, or unclear (for details, see Table 8.5.c of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
Measures of treatment effect
We measured the treatment effect on dichotomous outcomes, such as objective response and adverse events by risk ratio (RR) with 95% confidence interval (CI). We measured the treatment effect on time‐to‐event outcomes, such as overall survival and progression‐free survival, by hazard ratio (HR) with 95% CI.
Unit of analysis issues
Our pilot search found that existing studies eligible for this review were usually individually randomised, non‐cross‐over trials, without multiple intervention groups. Therefore, the unit of analysis issues related to cluster‐randomised trials, cross‐over trials, and multiple intervention groups seemed unlikely to arise. Still, we tried to avoid any potential unit of analysis error by extracting and analysing the data carefully according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We contacted investigators or study sponsors as needed, in order to verify key study characteristics and obtain missing numerical outcome data. If no additional data necessary for meta‐analysis could be obtained in this way, we tried to impute values from reported data (e.g. estimating the HR from published survival curves) and conducted an intention‐to‐treat analysis where possible and appropriate.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each meta‐analysis. We investigated substantial heterogeneity (I2 ≥ 50%) by prespecified subgroup analyses.
Assessment of reporting biases
If we suspected reporting bias (see Assessment of risk of bias in included studies), we attempted to contact study authors, asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to be able to introduce serious bias, we evaluated the impact of including such studies in the overall analysis by conducting a sensitivity analysis. As appropriate, we visually inspected funnel plots to see if there was a possibility of publication bias.
Data synthesis
We performed meta‐analysis with Review Manager 5 (RevMan 2014) for each outcome using the random‐effects model. We employed the Mantel‐Haenszel method to combine RRs and the inverse‐variance method to combine HRs. For eligible studies that lacked numerical outcome data suitable for meta‐analysis, even after contacting authors, we had to present them descriptively (RevMan 2014).
We created a 'Summary of findings' table using the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and using GRADEpro software (GRADEpro 2014). We included the following outcomes: overall survival, progression‐free survival, one‐year survival rate, objective response, and adverse events. The assumed risks, with control and experimental interventions, respectively, for each outcome were estimated based on the assessments by different studies. For example, for a given dichotomous or continuous outcome, we meta‐analysed the rates or means reported by different studies to obtain a weighted mean for the control and intervention groups, respectively. For a time‐to‐event outcome such as overall survival, we calculated the median value of the lengths reported by different studies to represent the assumed risk. For adverse events, if available, we used a summary end point (e.g. total risk for all serious adverse events) in the table. If no summary end point was available, we instead summarised specific adverse events that occurred with significantly different frequencies in the two arms.
Subgroup analysis and investigation of heterogeneity
If the data allowed us to do so, we performed subgroup analyses according to the following characteristics of patients: (1) histology of cancer (adenocarcinoma versus other histological types), (2) KRAS mutation status (mutant versus wild‐type), (3) EGFR mutation status (mutant versus wild‐type), and (4) Eastern Cooperative Oncology Group (ECOG) performance status (0 to 1 versus 2 or more).
Sensitivity analysis
Where appropriate, we conducted the following sensitivity analyses to check the robustness of the results of each meta‐analysis.
We excluded the studies with high or unclear risk of bias.
If there was no significant between‐study heterogeneity in a given meta‐analysis, then we estimated the pooled HR or RR with the fixed‐effects model and compared that with the random‐effects model to see if they differed significantly.
We considered both subgroup and sensitivity analyses only for the primary outcome, i.e. overall survival. For secondary outcomes, especially for various types of adverse events, subgroup and sensitivity analyses would probably produce false positive results due to multiple testing, and thus we did not consider conducting such analyses.
Results
Description of studies
Results of the search
In total, we identified 3547 references, 3445 of which were from three electronic databases, 80 from two clinical trial registries, and 22 from the conference abstracts of the American Society of Clinical Oncology and the European Society of Medical Oncology. The flow chart of study selection is shown in Figure 1. Of the 3547 references we identified, we excluded 549 duplicates, and further excluded 2970 after reviewing their titles and abstracts. Among the 28 studies we retrieved for further evaluation, we excluded 24, leaving four eligible studies for final analysis (Butts 2007; Lynch 2010; Pirker 2009; Rosell 2008).
1.
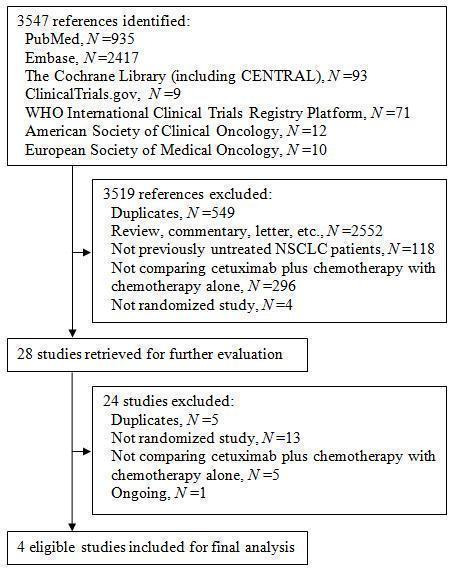
Figure 1. The flow chart of study selection
Included studies
The characteristics of the four eligible studies are shown in Characteristics of included studies. All four studies were parallel group RCTs. The studies were mainly conducted in the United States and Europe, and included 2018 subjects in total (1003 received chemotherapy plus cetuximab and 1015 received chemotherapy alone). The sample size of the studies ranged from 86 to 1125. The proportion of females ranged from 26% to 56%. The comparability of intervention and control arms within these studies were generally good, except that the proportion of female patients was seemingly higher (61.5% versus 50%) in the cetuximab arm in one study (Butts 2007). The median age of subjects ranged from 58 years to 66 years. The subjects were mostly white people (83% to 100% in different studies). The patients with ECOG performance status 0 to 1, which is considered as "fit" (Gridelli 2004), accounted for 83% to 99% of all patients. Most patients had stage IV lung cancer, and about half of them had histologically proven adenocarcinoma. Two studies (Pirker 2009; Rosell 2008) included patients with EGFR expression only, while the others did not select patients on the basis of EGFR expression status. Those who had never smoked accounted for 8% to 22% of patients. None of the studies provided information on the EGFR and KRAS mutation status of patients. The chemotherapies used in the four studies included gemcitabine plus cisplatin or plus carboplatin (Butts 2007), carboplatin plus paclitaxel or plus docetaxel (Lynch 2010), and cisplatin plus vinorelbine (Pirker 2009; Rosell 2008). Cetuximab was given at an initial dose of 400 mg/m2 and then 250 mg/m2 per week intravenously until disease progression or unacceptable toxicity occurred in all four studies.
Excluded studies
The excluded studies and reasons for exclusion are shown in Characteristics of excluded studies. Briefly, we excluded 13 studies for not being randomised studies, five for not comparing cetuximab plus chemotherapy with chemotherapy alone, and five for duplicating other studies. In addition, we did not include one potentially eligible study (NCT00946712) in the present review as it is an ongoing trial and will not be completed until December 2017.
Risk of bias in included studies
Detailed information on the risk of bias are shown in Characteristics of included studies. We presented separately the results of risk of bias assessment for objective outcomes (i.e. overall survival and one‐year survival rate) and subjective ones (all other outcomes). For the data on objective outcomes, we graded all studies at "low risk" or "unclear risk" (Figure 2). We judged the overall risk of bias as "low" (Figure 3). For the data on subjective outcomes, we graded all studies at "high risk", mainly due to lack of blinding. We judged the overall risk of bias as "high". Details about the assessment are described below.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
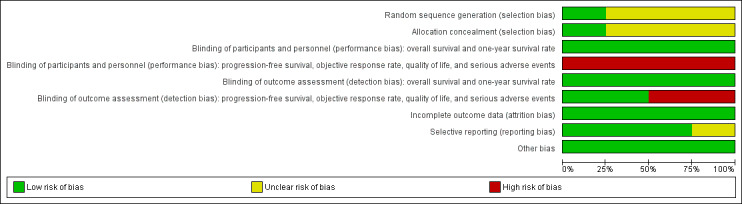
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Although all four eligible trials reported that the participants were "randomised" into different treatment arms, only one of them provided details about the randomisation and concealment procedure. Specifically, in the study of Pirker 2009, patients were randomised centrally using a computer interactive voice response system, and "physicians and study monitors did not have access to the code". We considered Pirker 2009 to have a low risk of selection bias, while the other three studies (Butts 2007; Lynch 2010; Rosell 2008) had an unclear risk of selection bias due to lack of relevant information.
Blinding
All included trials were "open‐label", without masking either participants or personnel (those who gave treatment). However, the results on overall survival and one‐year survival rate were mainly determined by the biological, objective effect of treatments and were unlikely to be affected by the participants' and personnel knowledge of the assignment status. Thus, we considered the risk of performance bias as low for the data on the two outcomes. Nevertheless, progression‐free survival, objective response rate, quality of life, and serious adverse events are not objective outcomes and could be affected if the participants, or personnel, or both were aware of the assignment status. Thus, we considered the results on the four outcomes at high risk of performance bias.
The outcomes were assessed by blinded or independent reviewers in the studies of Butts 2007 and Lynch 2010. Thus, we considered the risk of detection bias in the two studies as low. However, in the studies of Pirker 2009 and Rosell 2008, there were no blinding of outcome assessors. Although the results on overall survival and one‐year survival rate were unlikely to be affected by this (as "death" is an objective, "hard" outcome), the assessments of other outcomes such as progression‐free survival, objective response rate, adverse events, and quality of life involved subjective judgements and were vulnerable to the performance of assessors who were aware of the assignment status. Thus, in the studies of Pirker 2009 and Rosell 2008, we considered the data on the four outcomes at high risk of detection bias.
Incomplete outcome data
All four eligible trials conducted efficacy analyses on an intention‐to‐treat basis and restricted safety analyses to treated patients only. The patients available for safety analyses accounted for 95.4% (Lynch 2010) to 99.2% (Butts 2007) of the randomised patients. Thus, we believe the comparability between the treatment arms is unlikely to have been affected, and the risk of attrition bias is low.
Selective reporting
Data on overall survival, progression‐free survival, one‐year survival rate, objective response rate, and serious adverse events were available from all four eligible studies. Thus, for the data on these outcomes, selective reporting bias is unlikely to exist. Lynch 2010 and Pirker 2009 reported data on quality of life, while Butts 2007 and Rosell 2008) did not. Examination of the protocol of Butts 2007 showed that it had no plan to study quality of life (Butts 2005). Thus, we considered the risk of selective reporting bias as low in the study.
For the study of Rosell 2008, we could not find a protocol or registration, and it is difficult to say whether quality of life is one of the prespecified outcomes of the study. Thus, we considered it had an unclear risk for selective reporting bias.
Other potential sources of bias
We found no evidence of other bias. Although the unbalance in poststudy treatments could be a source of potential bias, we argue that it would not undermine our overall conclusion. Specifically, three studies (Butts 2007; Lynch 2010; Pirker 2009) reported the percentage of patients receiving poststudy cetuximab in the chemotherapy alone group and the percentage of patients receiving poststudy chemotherapy in the chemotherapy plus cetuximab group. In the three studies, the percentage using poststudy cetuximab was higher than that using poststudy chemotherapy alone, which could have "diluted" the effect of cetuximab on overall survival. Thus, the observed efficacy of cetuximab is actually a "conservative" estimate. This would not undermine, but strengthen, our conclusion.
Effects of interventions
See: Table 1
The data on overall survival, progression‐free survival, one‐year survival rate, objective response rate, and serious adverse events were available from all four eligible studies. Quality of life was investigated in two studies (1901 patients) (Lynch 2010; Pirker 2009).
Overall survival
The median overall survival with chemotherapy plus cetuximab ranged from 8.3 months (Rosell 2008) to 12.0 months (Butts 2007) (median: 10.5 months), while the median overall survival with chemotherapy alone ranged from 7.3 months (Rosell 2008) to 10.1 months (Pirker 2009) (median: 8.9 months). The median overall survival with chemotherapy plus cetuximab was longer than that with chemotherapy alone in all four studies. The HR for death (chemotherapy plus cetuximab versus chemotherapy alone) ranged from 0.71 (95% CI 0.48 to 1.05) (Rosell 2008) to 0.89 (95% CI 0.75 to 1.05) (Lynch 2010) in different studies. The pooled HR was 0.87 (95% CI 0.79 to 0.96; P = 0.004), indicating that the efficacy of chemotherapy plus cetuximab was better than that of chemotherapy alone in terms of overall survival (Analysis 1.1; Figure 4). We did not observe any statistical heterogeneity among the studies (P = 0.78, I2 = 0%).
1.1. Analysis.
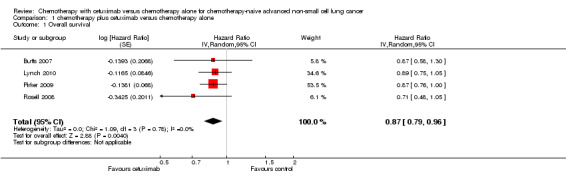
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 1 Overall survival.
4.
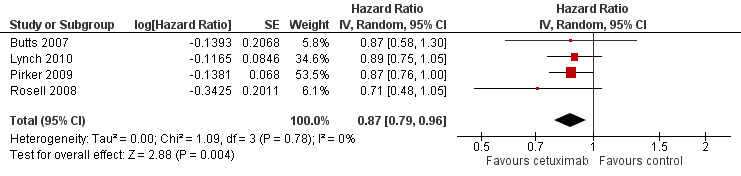
Forest plot of comparison: 1 chemotherapy plus cetuximab versus chemotherapy alone, outcome: 1.1 Overall survival.
Progression‐free survival
The median progression‐free survival with chemotherapy plus cetuximab ranged from 4.4 months (Lynch 2010) to 5.1 months (Butts 2007) (median: 4.9 months), while the median progression‐free survival with chemotherapy alone ranged from 4.2 months (Butts 2007) to 4.8 months (Pirker 2009) (median: 4.4 months). The median progression‐free survival with chemotherapy plus cetuximab was equal to or longer than that with chemotherapy alone in individual studies. The HR for progression (chemotherapy plus cetuximab versus chemotherapy alone) ranged from 0.71 (95% CI 0.41 to 1.23) (Rosell 2008) to 0.94 (95% CI 0.83 to 1.08) (Pirker 2009). The pooled HR was 0.91 (95% CI 0.83 to 1.00; P = 0.06), indicating that the superiority of chemotherapy plus cetuximab over chemotherapy alone in prolonging progression‐free survival did not reach statistical significance (Analysis 1.2; Figure 5). We did not observe any statistical heterogeneity among the studies (P = 0.72, I2 = 0%).
1.2. Analysis.
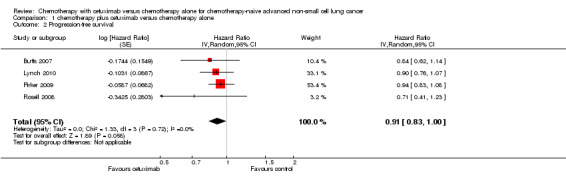
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 2 Progression‐free survival.
5.
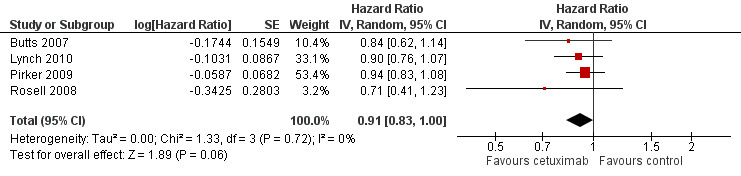
Forest plot of comparison: 1 chemotherapy plus cetuximab versus chemotherapy alone, outcome: 1.2 Progression‐free survival.
Objective response rate
The objective response rate with chemotherapy plus cetuximab ranged from 26% (Lynch 2010) to 36% (Pirker 2009) (weighted rate: 30%), while the objective response rate with chemotherapy alone ranged from 17% (Lynch 2010) to 29% (Pirker 2009) (weighted rate: 23%). The objective response rate with chemotherapy plus cetuximab was higher than that with chemotherapy alone in all studies. The RR for response (chemotherapy plus cetuximab versus chemotherapy alone) ranged from 1.25 (95% CI 0.67 to 2.35) (Rosell 2008) to 1.52 (95% CI 0.80 to 2.90) (Butts 2007). The pooled RR was 1.31 (95% CI 1.14 to 1.51; P = 0.0001), indicating that the efficacy of chemotherapy plus cetuximab was better than that of chemotherapy alone in terms of objective response rate (Analysis 1.3; Figure 6). We did not observe any statistical heterogeneity among the studies (P = 0.71, I2 = 0%).
1.3. Analysis.
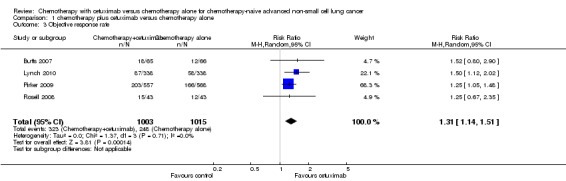
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 3 Objective response rate.
6.
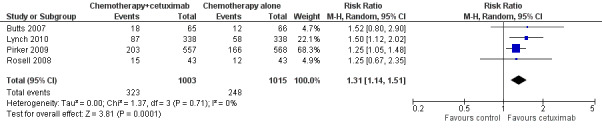
Forest plot of comparison: 1 chemotherapy plus cetuximab versus chemotherapy alone, outcome: 1.3 Objective response rate.
One‐year survival rate
The one‐year survival rate with chemotherapy plus cetuximab ranged from 33% (Rosell 2008) to 50% (Butts 2007) (weighted rate: 45%), while the one‐year survival rate with chemotherapy alone ranged from 26% (Rosell 2008) to 42% (Pirker 2009) (weighted rate: 40%). The one‐year survival rates with chemotherapy plus cetuximab were higher than those with chemotherapy alone in all studies. The RR for survival at one year (chemotherapy plus cetuximab versus chemotherapy alone) ranged from 1.12 (95% CI 0.94 to 1.32) (Lynch 2010) to 1.30 (95% CI 0.88 to 1.93) (Butts 2007). The pooled RR was 1.13 (95% CI 1.02 to 1.25; P = 0.02), indicating that the efficacy of chemotherapy plus cetuximab was better than that of chemotherapy alone in terms of one‐year survival rate (Analysis 1.4). We did not observe any statistical heterogeneity among the studies (P = 0.88, I2 = 0%).
1.4. Analysis.
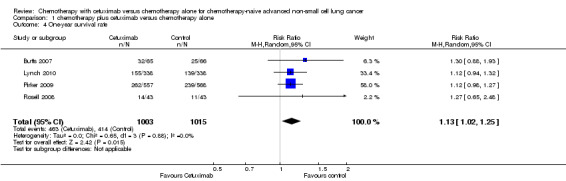
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 4 One‐year survival rate.
Quality of life
Quality of life was assessed in two studies with different questionnaires. In Lynch 2010, the FACT‐LCS5 questionnaire was used. In Pirker 2009, the European Organisation for Research and Treatment of Cancer quality of life questionnaire C30 (version 3.0), EORTC lung cancer specific QLQ‐LC13, and EuroQoL (EQ‐5D) questionnaires were used. Although no detailed data were reported by the two studies, both of them found no significant differences in the change of quality of life between the two treatment arms.
Serious adverse events
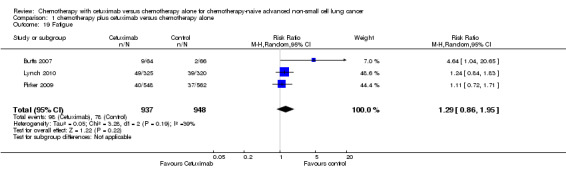
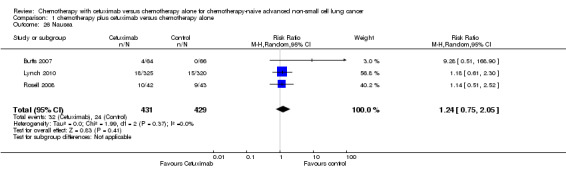
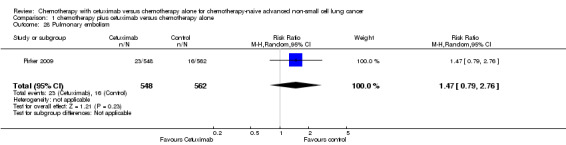
Serious adverse events were reported in all four trials. In total, there were five types of haematological adverse events and 30 types of non‐haemotological adverse events. There were no cetuximab‐related deaths. Patients receiving chemotherapy plus cetuximab experienced more acneiform rash (weighted rate: 11.2% versus 0.3%; RR 37.36, 95% CI 10.66 to 130.95) (Analysis 1.6), diarrhoea (weighted rate: 4.8% versus 2.3%; RR 2.10, 95% CI 1.26 to 3.48) (Analysis 1.15), hypokalaemia (weighted rate: 6.3% versus 3.6%; RR 1.74, 95% CI 1.02 to 2.99) (Analysis 1.20), hypomagnesemia (weighted rate: 5.3% versus 0.8%; RR 6.57, 95% CI 1.13 to 38.12) (Analysis 1.21), infusion reaction (weighted rate: 3.9% versus 1.1%; RR 3.50, 95% CI 1.76 to 6.94) (Analysis 1.24), febrile neutropenia (weighted rate: 10.6% versus 7.6%; RR 1.40, 95% CI 1.10 to 1.77) (Analysis 1.36), and leukopenia (weighted rate: 58.1% versus 42.7%; RR 1.36, 95% CI 1.17 to 1.58) (Analysis 1.37) than did those who received chemotherapy alone. Despite some trends, the difference in the incidence of other serious adverse events between the two treatment arms did not reach statistical significance.
1.6. Analysis.
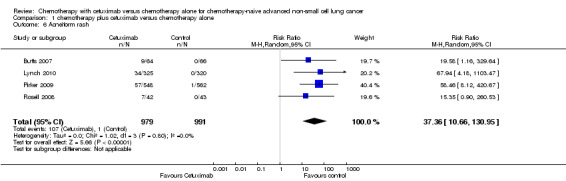
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 6 Acneiform rash.
1.15. Analysis.
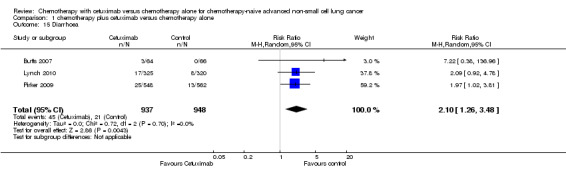
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 15 Diarrhoea.
1.20. Analysis.
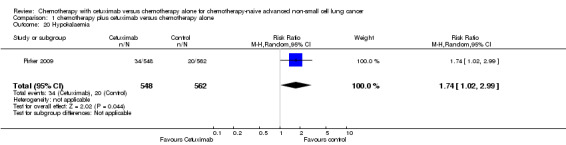
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 20 Hypokalaemia.
1.21. Analysis.
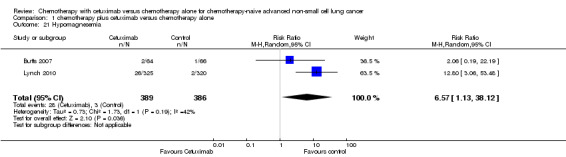
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 21 Hypomagnesemia.
1.24. Analysis.
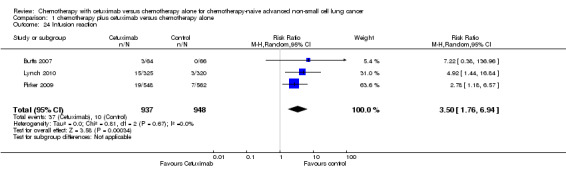
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 24 Infusion reaction.
1.36. Analysis.
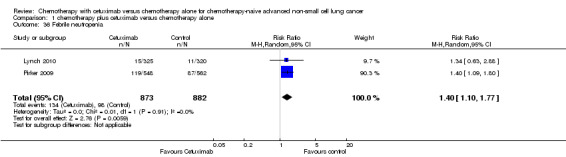
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 36 Febrile neutropenia.
1.37. Analysis.
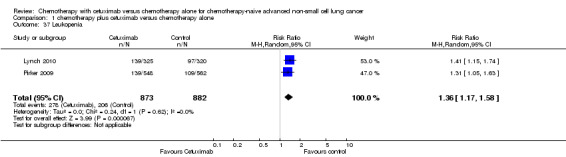
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 37 Leukopenia.
Subgroup and sensitivity analyses
As prespecified, we considered subgroup and sensitivity analyses for the primary outcome (overall survival) only.
Subgroup analysis
We planned to performed subgroup analyses according to histology of cancer (adenocarcinoma versus other histological types), KRAS mutation status (mutant versus wild‐type), EGFR mutation status (mutant versus wild‐type), and ECOG performance status (0 to 1 versus 2 or more). To this end, the included studies should preferably be differentiable according to these factors so that they could be divided into different categories or subgroups. For example, some studies were conducted solely with patients with adenocarcinoma and others solely with those with other histological types; or, some studies had a significantly larger proportion of patients with adenocarcinoma than did others.
However, the fact is that none of the included studies were conducted solely in patients with adenocarcinoma or in those with ECOG performance status 0 to 1. What is more, both the proportion of patients with adenocarcinoma and the proportion of patients with ECOG performance status 0 to 1 did not differ much across the included studies. In other words, the four studies were not differentiable according to the two factors. Thus, it was infeasible to divide them into different subgroups according to the two factors. In addition, none of the included studies provided information on EGFR and KRAS mutation status. Thus, we did not actually conduct any preplanned subgroup analysis.
Sensitivity analysis
As no statistical heterogeneity was observed among the studies (Figure 4), the results from the fixed‐effect model were the same as those from the random‐effects model.
After excluding the studies at high or unclear risk of bias (Butts 2007; Lynch 2010; Rosell 2008), the combined HR in Figure 4 remained unchanged, which was 0.87 (95% CI 0.76 to 1.00) (before excluding the study: 0.87, 95% CI 0.79 to 0.96).
Other analyses
Due to the limited number of eligible studies, we did not construct funnel plots to explore the possibility of publication bias (Higgins 2011).
Discussion
Summary of main results
The main findings of this systematic review are summarised in the Table 1. Briefly, the overall survival, one‐year survival rate, and objective response rate with chemotherapy plus cetuximab were better than those with chemotherapy alone. The addition of cetuximab to chemotherapy reduced the hazard for death by 13% (HR 0.87, 95% CI 0.79 to 0.96), while improving the relative one‐year survival rate and relative objective response rate by 13% and 31%, respectively. These equate to absolute improvements in one‐year survival and objective response rate of 5% and 7%, respectively. There was also a consistent trend towards longer progression‐free survival with the combination treatment, although the results were not statistically significant. No evidence suggested that cetuximab combined with chemotherapy was associated with better quality of life. As expected, some specific adverse events occurred more frequently in the cetuximab group. The adverse events, according to the original reports, were generally manageable.
Overall completeness and applicability of evidence
The data on important patient characteristics, interventions, and almost all outcomes of interest were available in detail from all eligible studies. In addition, all patients were included for efficacy analysis, and 95.4% to 99.2% of randomised patients were available for safety analysis in eligible studies. Thus, the overall completeness of the evidence summarised by this review is good.
According to the inclusion criteria of the four eligible studies, the results of the present review are applicable to chemotherapy‐naive advanced NSCLC patients with ECOG performance status 0 to 1. The subgroup analyses of Pirker 2009 and Lynch 2010 showed that the benefit from cetuximab did not differ significantly across subgroups defined by age, sex, ECOG performance status, and tumour histology. However, it should be noted that about 90% of the patients included in the eligible studies were white people. Pirker 2009 found that cetuximab was better than control in white people, but seemed to do harm in patients of other origins, including Asian people, which warrants further investigation.
In two studies, only the patients with EGFR expression were included (Pirker 2009; Rosell 2008), which did not lead to significant heterogeneity in any of our meta‐analyses. This seems to suggest that cetuximab does not have differential effects in patients with different EGFR expression status. However, a more recent and detailed analysis of the data from Pirker 2012 showed that the survival benefit from cetuximab was greater in the patients with high EGFR expression (median survival 12.0 months versus 9.6 months; HR 0.73, 95% CI 0.58 to 0.93), with no meaningful increase in adverse events, whereas there was no corresponding survival benefit in the patients with low EGFR expression (median survival 9.8 months versus 10.3 months; HR 0.99, 95% CI 0.84 to 1.16). This indicates that the use of cetuximab may be limited to patients with high EGFR expression only. In view of the inconsistency of existing evidence, more studies on the role of EGFR expression are needed before this biomarker can be applied to clinical settings.
Quality of the evidence
The studies included in the present review are all RCTs with data analysed according to the intention‐to‐treat principle and outcomes reported in detail and properly. According to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group (Guyatt 2008), there are five factors that can downgrade the quality of evidence from RCTs, i.e. study limitations, inconsistency of results, indirectness of evidence, imprecision, and publication bias.
Study limitations
In this review, study limitations are mainly reflected by risk of bias, which is low for the data on overall survival and one‐year survival rate, but high for the data on progression‐free survival, objective response rate, quality of life, and serious adverse events, mainly due to the lack of blinding. This issue could have led to potential bias in the results in multiple ways such as by affecting the performance of patients and clinicians (Figure 2; Figure 3). For example, in FLEX (Pirker 2009) and BMS099 (Lynch 2010), the two studies with dominant weights in the meta‐analysis, patients and clinicians were not blinded and 'for unknown reasons', the proportion of censored progression‐free survival data possibly due to patients switching to other treatments before radiologically‐confirmed disease progression (the major endpoint of progression‐free survival) was much higher in the chemotherapy‐alone group than in the chemotherapy‐plus‐cetuximab group. Thus, the observed difference in progression‐free survival between the two groups was putatively smaller than actual, which could at least partly explain why the observed benefit from cetuximab was significant for overall survival but not for progression‐free survival.
Inconsistency of results
For the majority of the meta‐analyses in this review, there was no heterogeneity (I2 = 0%) among studies.
Indirectness of evidence
This review contains no indirect comparison of different treatment regimens, and the population, intervention, comparator, and outcomes of the included studies are similar to those who would be actually treated with chemotherapy plus cetuximab in clinical settings.
Imprecision
For overall survival, one‐year survival rate, and objective response rate, statistically significant results were achieved in this review and the 95% CIs of HRs or RRs were fairly narrow. Thus, the problem of imprecision is unlikely to affect the quality of evidence of these outcomes. However, the HR for progression‐free survival is at the borderline of statistical significance and RRs for most serious adverse events are not statistically significant, which might have resulted from, among other reasons, imprecision due to the relatively small sample size. Addtionally, for some serious adverse events (e.g. acneiform rash and hypomagnesemia), the 95% CIs of RRs are rather wide. For quality of life, the two relevant studies provided narrative description only and no detailed numerical data, precluding a "precise" understanding of the treatment effect on this outcome. Thus, it is justifiable to downgrade the quality of evidence on progression‐free survival, quality of life, and serious adverse events, taking the problem of imprecision into account.
Publication bias
In this review we did not construct funnel plots due to the limited number of studies included. There is no clear evidence for publication bias.
In summary, inconsistency of results, indirectness of evidence, and publication bias are unlikely to have affected the quality of evidence in this review. The quality of evidence is high for overall survival and one‐year survival rate, moderate for objective response rate (downgraded by study limitations), and low for progression‐free survival, quality of life, and serious adverse events (downgraded by study limitations and imprecision).
Potential biases in the review process
As the data sources we searched were all in English, it was not impossible that we missed some non‐English studies. This could lead to language bias if the results of the missing studies contradicted those of the included ones. In addition, due to the limited number of eligible studies, we were unable to construct the preplanned funnel plots, and thus we cannot exclude the possibility that publication bias exists in the present review (Higgins 2011).
Agreements and disagreements with other studies or reviews
Our findings are consistent with a previous phase Ⅱ trial that showed promising efficacy of cetuximab as first‐line treatment of advanced NSCLC (Herbst 2010). RTOG 0324, which was also a phase Ⅱ study, found that cetuximab, when combined with chemoradiation, seemed to be able to significantly improve the overall survival of unresectable stage ⅢA/B NSCLC (Blumenschein 2011). However, as concluded by a previous review, Nieder 2012, the efficacy of cetuximab combined with radiotherapy for stage Ⅲ NSCLC has been uncertain, mainly due to the problem of study design, e.g. lack of randomisation.
Our findings about the effectiveness of chemotherapy combined with cetuximab are generally consistent with Lin 2010, that systematic review based on the same four trials as included in our review. However, we found that adding cetuximab also improved one‐year survival rate, whereas Lin 2010, found no such improvement. Further examination showed that Lin 2010 included only three studies (Butts 2007; Pirker 2009; Rosell 2008) for the analyses on one‐year survival rate, while our review included all four trials. Regarding serious adverse events, Lin 2010 found that only two types of serious adverse events, i.e. rash and infusion reaction, occurred more in the cetuximab arm (Lin 2010), whereas our review showed that adding cetuximab caused significantly higher rates of seven types of serious adverse events. Close examination of the original papers suggested that the data extracted by us was more complete and accurate than that by Lin 2010.
Authors' conclusions
Implications for practice.
Compared to chemotherapy alone, chemotherapy combined with cetuximab is more effective in improving overall survival, which is the most important outcome. Although the combination treatment could induce much higher rates of some adverse events, studies reported that these events were generally manageable. Thus, provided one accepts the increased risk of adverse events and the significantly increased cost, chemotherapy plus cetuximab may be preferred to chemotherapy alone for the first‐line treatment of chemotherapy‐naive, advanced NSCLC.
Implications for research.
First, as mentioned above, the majority of the patients studied in existing trials were white people. Whether the efficacy of cetuximab varies significantly in other populations, e.g. Asian people, is unclear. The rationale behind this question is that people of different origins may have a different genetic basis that predisposes the patients' sensitivity to a specific treatment. For example, in the EGFR tyrosine kinase inhibitors treatment for advanced NSCLC, Asian patients are more likely to benefit from the treatment than Western counterparts, as they harbour a higher rate of EGFR mutations (Mitsudomi 2011). With regard to cetuximab for advanced NSCLC, the study of Pirker 2009 indicated that ethnicity could be a potential factor that can modify the treatment efficacy, with white people seemingly more likely to benefit than Asian people and others. However, it should be noted that this result was based on one of the many subgroup analyses in the study and limited by the small number of non‐white patients and potential false‐positivity. Thus, this issue is worthy of further research.
Second, as shown by the included studies, cetuximab could be combined with different chemotherapies, such as gemcitabine plus cisplatin or plus carboplatin, carboplatin plus paclitaxel or plus docetaxel, and cisplatin plus vinorelbine. Different chemotherapies, with the addition of cetuximab, may have different efficacy. For example, Lynch 2010 found that overall survival with docetaxel plus cetuximab and that with docetaxel alone was 11.04 months and 10.22 months, respectively, while overall survival with paclitaxel plus cetuximab and that with paclitaxel alone was 9.03 months and 7.69 months, respectively. Paclitaxel appeared to be inferior to docetaxel. However, the evidence has been limited in amount. Which chemotherapy is the best to be used together with cetuximab remains to be clarified.
Third, given the consistent evidence that cetuximab is effective as first‐line treatment, it would be of interest to know whether this treatment is also useful in the second‐line setting (Kim 2009). In addition, the treatment can be further and better individualised by the identification of potential predictive factors for efficacy, including EGFR protein expression, EGFR gene mutations, and so on.
Acknowledgements
We thank Mia Schmidt‐Hansen, Noelle O'Rourke, José Expósito, and Marta Roqué for their comments on the protocol. We thank Ivan Solà for his comments on the search strategy. We thank Sera Tort for her kind editorial assistance and Desiree West (Consumer of the Lung Cancer Group) for her feedback.
Appendices
Appendix 1. The Cochrane Lung Cancer Review Group Specialized Register (from inception to 17 December 2013) search strategy
All records in the Register coded as 'non‐small cell lung cancer' will be searched using the following terms: monoclonal antibody OR monoclonal antibodies OR mab OR mcab OR moab OR cetuximab OR erbitux OR c225 OR c‐225.
Appendix 2. The Cochrane Central Register of Controlled Clinical Trials (CENTRAL, from inception to 17 December 2013) search strategy
1. MeSH descriptor Carcinoma, Non‐Small‐Cell Lung explode all trees
2. "Non‐Small‐Cell Lung Cancer" OR "Non‐Small‐Cell Lung Carcinoma" OR "Non‐Small Cell Lung Cancer" OR "Non‐Small Cell Lung Carcinoma" OR "Non Small‐Cell Lung Cancer" OR "Non Small‐Cell Lung Carcinoma" OR "Non Small Cell Lung Cancer" OR "Non Small Cell Lung Carcinoma" OR NSCLC
3. (#1 OR #2)
4. MeSH descriptor Antibodies, Monoclonal explode all trees/
5. "monoclonal antibody" OR "monoclonal antibodies" OR mab OR mcab OR moab OR cetuximab OR erbitux OR c225 OR c‐225
6. (#4 OR #5)
7. (#3 AND #6)
Appendix 3. MEDLINE (access through PubMed (1966 to 17 December 2013)) search strategy
1. "Carcinoma, Non‐Small‐Cell Lung"[Mesh]
2. "Non‐Small‐Cell Lung Cancer" OR "Non‐Small‐Cell Lung Carcinoma" OR "Non‐Small Cell Lung Cancer" OR "Non‐Small Cell Lung Carcinoma" OR "Non Small‐Cell Lung Cancer" OR "Non Small‐Cell Lung Carcinoma" OR "Non Small Cell Lung Cancer" OR "Non Small Cell Lung Carcinoma" OR NSCLC
3. #1 OR #2
4. "Antibodies, Monoclonal"[Mesh]
5. "cetuximab"[Substance Name]
6. "monoclonal antibody" OR "monoclonal antibodies" OR mab OR mcab OR moab OR cetuximab OR erbitux OR c225 OR c‐225
7. #4 OR #5 OR #6
8. "Clinical Trial" [Publication Type] Field: Title/Abstract
9. random* Field: Title/Abstract
10. placebo Field: Title/Abstract
11. trial Field: Title
12. "Meta‐Analysis" [Publication Type] Field: Title/Abstract
13. "Review" [Publication Type] Field: Title/Abstract
14. #8 OR #9 OR #10 OR #11 OR #12 OR #13
15. #3 AND #7 AND #14
16. #15 Limits: Humans
Appendix 4. EMBASE (1980 to 17 December 2013) search strategy
1. lung non small cell cancer/
2. Non‐Small‐Cell Lung Cancer.af.
3. Non‐Small‐Cell Lung Carcinoma.af.
4. Non‐Small Cell Lung Cancer.af.
5. Non‐Small Cell Lung Carcinoma.af.
6. Non Small‐Cell Lung Cancer.af.
7. Non Small‐Cell Lung Carcinoma.af.
8. Non Small Cell Lung Cancer.af.
9. Non Small Cell Lung Carcinoma.af.
10. NSCLC.af.
11. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
12. monoclonal antibody/
13. cetuximab/
14. monoclonal antibody.af.
15. monoclonal antibodies.af.
16. mab.af.
17. mcab.af.
18. moab.af.
19. cetuximab.af.
20. erbitux.af.
21. c225.af.
22. c‐225.af.
23. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24. 11 and 23
25. limit 24 to (clinical trial or randomized controlled trial or controlled clinical trial)
26. limit 24 to (meta analysis or "systematic review")
27. random*.ab.
28. placebo.ab.
29. trial.ti.
30. 27 or 28 or 29
31. 24 and 30
32. 25 or 26 or 31
33. limit 32 to human
Appendix 5. Clinical trial registries (from inception to 17 December 2013) search strategy
The website of ClinicalTrials.gov (clinicaltrials.gov) will be searched using "cetuximab or erbitux or c225 or c‐225" as "Search Terms" and "lung cancer" as "Conditions" under the "Advanced Search" tab. The website of WHO International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) will also be searched using "cetuximab or erbitux or c225 or c‐225" in the "Title" and "lung cancer" as "Conditions" under the "Advanced Search" tab.
Data and analyses
Comparison 1. chemotherapy plus cetuximab versus chemotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 4 | Hazard Ratio (Random, 95% CI) | 0.87 [0.79, 0.96] | |
| 2 Progression‐free survival | 4 | Hazard Ratio (Random, 95% CI) | 0.91 [0.83, 1.00] | |
| 3 Objective response rate | 4 | 2018 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.14, 1.51] |
| 4 One‐year survival rate | 4 | 2018 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.02, 1.25] |
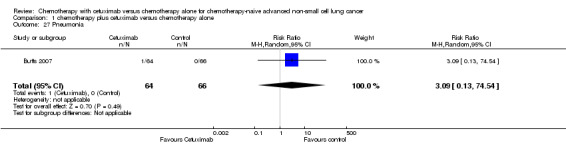
| 5 Abdominal pain | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.13, 74.54] |
| 6 Acneiform rash | 4 | 1970 | Risk Ratio (M‐H, Random, 95% CI) | 37.36 [10.66, 130.95] |
| 7 Anorexia | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 9.28 [0.51, 168.90] |
| 8 Anxiety | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.07, 16.14] |
| 9 Asthenia | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [0.10, 62.68] |
| 10 Back pain | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 5.15 [0.25, 105.31] |
| 11 Bleeding events | 1 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.31, 1.51] |
| 12 Cardiac events | 1 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.69, 1.87] |
| 13 Constipation | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.19, 22.19] |
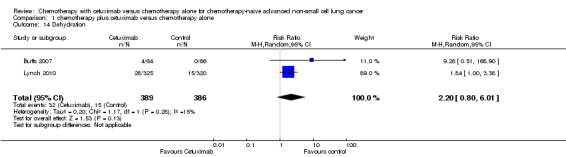
| 14 Dehydration | 2 | 775 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [0.80, 6.01] |
| 15 Diarrhoea | 3 | 1885 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.26, 3.48] |
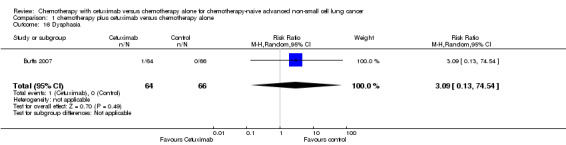
| 16 Dysphasia | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.13, 74.54] |
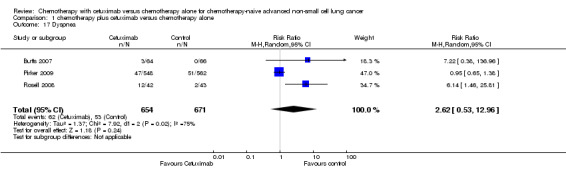
| 17 Dyspnea | 3 | 1325 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [0.53, 12.96] |
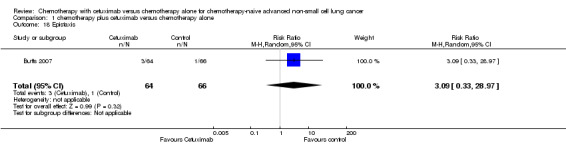
| 18 Epistaxis | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.33, 28.97] |
| 19 Fatigue | 3 | 1885 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.86, 1.95] |
| 20 Hypokalaemia | 1 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.02, 2.99] |
| 21 Hypomagnesemia | 2 | 775 | Risk Ratio (M‐H, Random, 95% CI) | 6.57 [1.13, 38.12] |
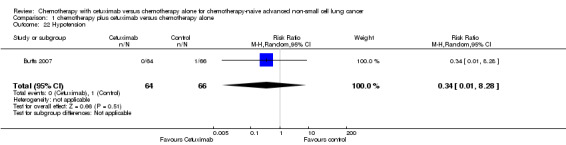
| 22 Hypotension | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.28] |
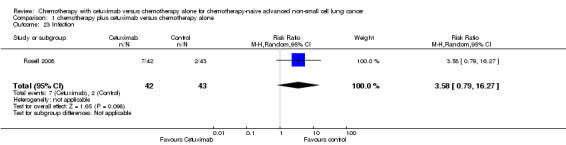
| 23 Infection | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 3.58 [0.79, 16.27] |
| 24 Infusion reaction | 3 | 1885 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [1.76, 6.94] |
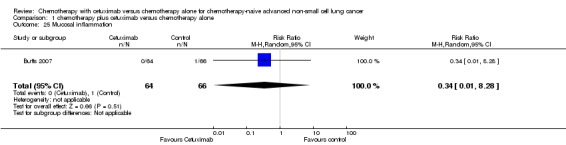
| 25 Mucosal inflammation | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.28] |
| 26 Nausea | 3 | 860 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.75, 2.05] |
| 27 Pneumonia | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.13, 74.54] |
| 28 Pulmonary embolism | 1 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.79, 2.76] |
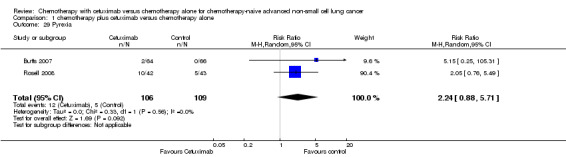
| 29 Pyrexia | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 2.24 [0.88, 5.71] |
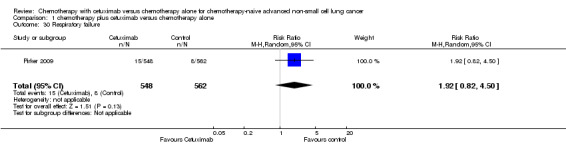
| 30 Respiratory failure | 1 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.82, 4.50] |
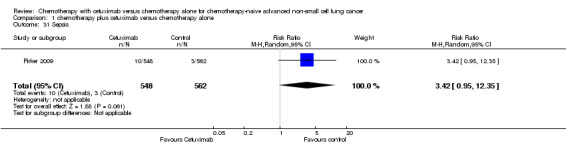
| 31 Sepsis | 1 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 3.42 [0.95, 12.35] |
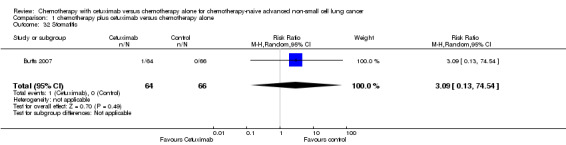
| 32 Stomatitis | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.13, 74.54] |
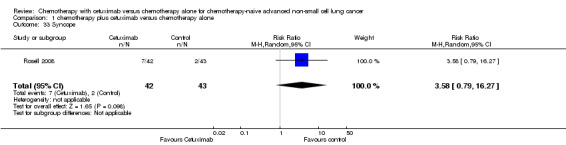
| 33 Syncope | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 3.58 [0.79, 16.27] |
| 34 Vomiting | 3 | 1325 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.69, 1.40] |
| 35 Anaemia | 4 | 1970 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.17] |
| 36 Febrile neutropenia | 2 | 1755 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.10, 1.77] |
| 37 Leukopenia | 2 | 1755 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.17, 1.58] |
| 38 Thrombocytopenia | 3 | 860 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.96, 1.66] |
| 39 Neutropenia | 3 | 1885 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.15] |
1.5. Analysis.
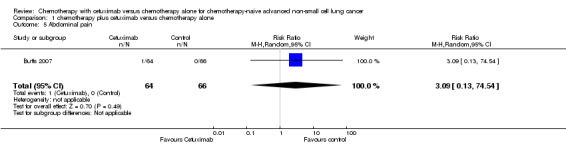
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 5 Abdominal pain.
1.7. Analysis.
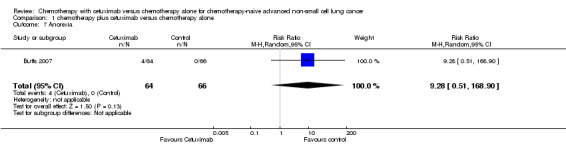
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 7 Anorexia.
1.8. Analysis.
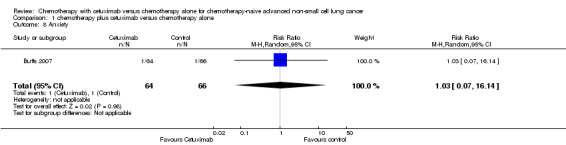
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 8 Anxiety.
1.9. Analysis.
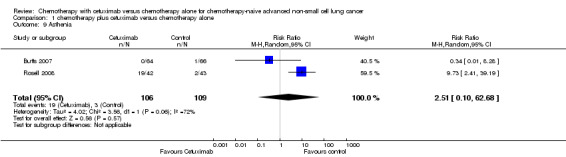
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 9 Asthenia.
1.10. Analysis.
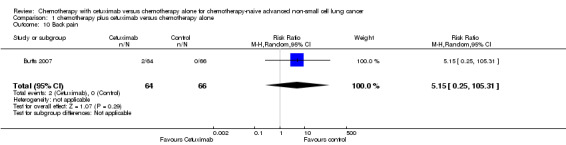
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 10 Back pain.
1.11. Analysis.
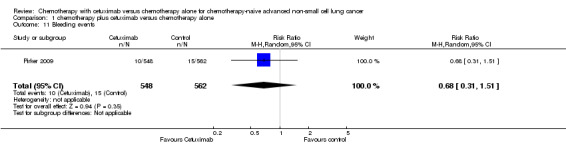
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 11 Bleeding events.
1.12. Analysis.
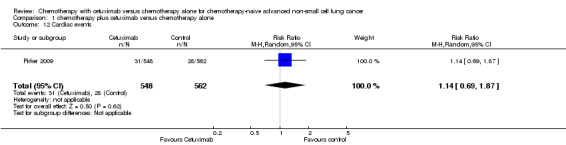
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 12 Cardiac events.
1.13. Analysis.
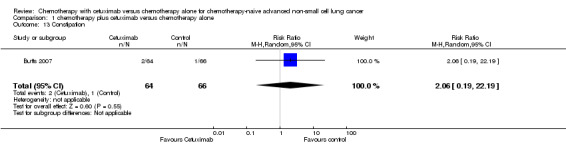
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 13 Constipation.
1.14. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 14 Dehydration.
1.16. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 16 Dysphasia.
1.17. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 17 Dyspnea.
1.18. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 18 Epistaxis.
1.19. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 19 Fatigue.
1.22. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 22 Hypotension.
1.23. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 23 Infection.
1.25. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 25 Mucosal inflammation.
1.26. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 26 Nausea.
1.27. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 27 Pneumonia.
1.28. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 28 Pulmonary embolism.
1.29. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 29 Pyrexia.
1.30. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 30 Respiratory failure.
1.31. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 31 Sepsis.
1.32. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 32 Stomatitis.
1.33. Analysis.
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 33 Syncope.
1.34. Analysis.
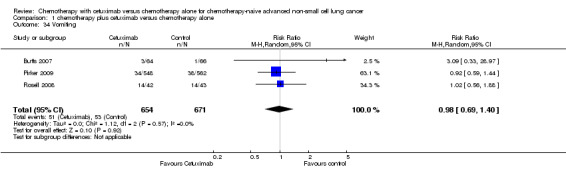
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 34 Vomiting.
1.35. Analysis.
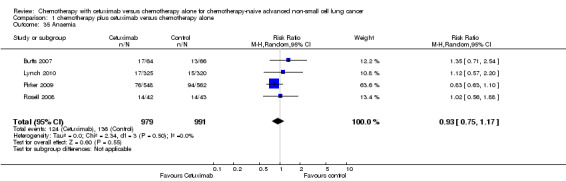
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 35 Anaemia.
1.38. Analysis.
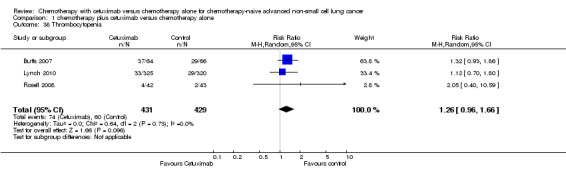
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 38 Thrombocytopenia.
1.39. Analysis.
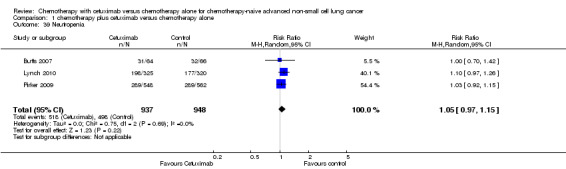
Comparison 1 chemotherapy plus cetuximab versus chemotherapy alone, Outcome 39 Neutropenia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Butts 2007.
| Methods | 1. Design: A multicentre, open‐label, parallel group, randomised phase II trial 2. Centres: 32 US and 9 Canadian sites 3. Randomisation: Patients were assigned to treatment arms in a 1:1 ratio. Randomisation was stratified by site, ECOG PS (0 or 1), and on‐study platinum (cisplatin, carboplatin) | |
| Participants | Inclusion criteria: chemotherapy‐naive patients at least 18 years of age and with an ECOG PS less than 2, with histologically or cytologically documented advanced NSCLC (stage IIIB with pleural effusion or stage IV) of all histologic subtypes 1. Female, n (%): 73 (55.7) 2. Age in years, median (range): 66 (35‐84) 3. White people, n (%): 109 (83.2) 4. ECOG PS 0‐1, n (%): 129 (98.5) 5. Tumour stage IIIB/IV: 123 (93.9) 6. Adenocarcinoma, n (%): 61 (46.6) 7. Never smoked, n (%): 19 (14.5) 8. EGFR expression, n (%): NA 9. KRAS mutations, n (%): NA 10. EGFR mutations, n (%): NA |
|
| Interventions | 1. Arm A (n = 65): gemcitabine + cisplatin + cetuximab, or gemcitabine + carboplatin + cetuximab (21.5% received poststudy chemotherapy)
2. Arm B (n = 66): gemcitabine + cisplatin, or gemcitabine + carboplatin (37.9% received poststudy cetuximab) Cross‐over between treatment arms was not allowed |
|
| Outcomes | 1. Primary: Objective response rate 2. Secondary: Progression‐free survival, overall survival (including data on one‐year survival rate), safety, disease control rate, duration of response, time to response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on the procedure were provided |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of allocation concealment |
| Blinding of participants and personnel (performance bias) overall survival and one‐year survival rate | Low risk | No blinding. However, the results on the two outcomes were mainly determined by the biological, objective effect of treatments and unlikely to be affected by the participants' and personnels' knowledge of the assignment status |
| Blinding of participants and personnel (performance bias) progression‐free survival, objective response rate, quality of life, and serious adverse events | High risk | No blinding. Progression‐free survival, objective response rate, and serious adverse events are not objective outcomes and could be affected by participants' and/or personnels' knowledge of the assignment status. The study did not use quality of life as an outcome |
| Blinding of outcome assessment (detection bias) overall survival and one‐year survival rate | Low risk | Quote: "The sponsor conducted centralized reviews to confirm investigator measurements and to determine best response. These reviews were blinded, as the sponsor reviewer did not receive information as to which treatment the patients were receiving" |
| Blinding of outcome assessment (detection bias) progression‐free survival, objective response rate, quality of life, and serious adverse events | Low risk | Quote: "The sponsor conducted centralized reviews to confirm investigator measurements and to determine best response. These reviews were blinded, as the sponsor reviewer did not receive information as to which treatment the patients were receiving" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Efficacy analyses were performed on an intent‐to‐treat basis. Analyses of safety and dosing data were restricted to treated patients." In effect, the information on safety were available for almost all (130, 99.2%) patients |
| Selective reporting (reporting bias) | Low risk | Data on all outcomes concerned in this review, except quality of life, were reported in the original paper. Examination of the protocol of the trial showed that quality of life was not a pre‐specified outcome (see: http://clinicaltrials.gov/ct2/show/study/NCT00112346) |
| Other bias | Low risk | No evidence about other bias was found |
Lynch 2010.
| Methods | 1. Design: A multicentre, open‐label, parallel group, randomised phase III trial 2. Centres: 96 US centres 3. Randomisation: Patients were randomly assigned 1:1 to cetuximab plus TC or TC alone. Choice of taxane was at the investigator’s discretion on an individual‐patient basis. The random assignment was stratified by study site, ECOG PS (0 or 1), and intended taxane (paclitaxel or docetaxel) | |
| Participants | Inclusion criteria: patients who had histologically or cytologically confirmed stage IV, stage IIIB (with malignant pleural effusion), or recurrent (after radiotherapy or surgery) NSCLC with bidimensionally measurable disease, were ≥ 18 years of age, and had an ECOG PS less than 2 1. Female, n (%): 280 (41.4) 2. Age in years, median (range): 65 (34‐87) 3. White people, n (%): 596 (88.1) 4. ECOG PS 0‐1, n (%): 665 (98.4) 5. Tumour stage IIIB/IV: 646 (95.6) 6. Adenocarcinoma, n (%): 354 (52.4) 7. Never smoked, n (%): 53 (7.8) 8. EGFR expression, n (%): NA 9. KRAS mutations, n (%): NA 10. EGFR mutations, n (%): NA |
|
| Interventions | 1. Arm A (n = 338): taxane (paclitaxel or docetaxel) +carboplatin + cetuximab (24.3% received poststudy chemotherapy) 2. Arm B (n = 338): taxane (paclitaxel or docetaxel) +carboplatin (26% received poststudy cetuximab) Cross over to cetuximab was not permitted |
|
| Outcomes | 1. Primary: Progression‐free survival 2. Secondary: Objective response rate, overall survival (including data on one‐year survival rate), quality of life, safety |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on the procedure were provided |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of allocation concealment |
| Blinding of participants and personnel (performance bias) overall survival and one‐year survival rate | Low risk | No blinding. However, the results on the two outcomes were mainly determined by the biological, objective effect of treatments and unlikely to be affected by the participants' and personnels' knowledge of the assignment status |
| Blinding of participants and personnel (performance bias) progression‐free survival, objective response rate, quality of life, and serious adverse events | High risk | No blinding. The four outcomes are not objective in nature and could be affected by participants' and/or personnels' knowledge of the assignment status |
| Blinding of outcome assessment (detection bias) overall survival and one‐year survival rate | Low risk | The outcomes were assessed by an independent radiologic review committee consisting of two primary radiologist reviewers and a third for adjudication. Final review was conducted by an oncologist, integrating radiologic assessment with clinical information |
| Blinding of outcome assessment (detection bias) progression‐free survival, objective response rate, quality of life, and serious adverse events | Low risk | The outcomes were assessed by an independent radiologic review committee consisting of two primary radiologist reviewers and a third for adjudication. Final review was conducted by an oncologist, integrating radiologic assessment with clinical information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Baseline characteristics and efficacy were analyzed in all randomly assigned patients. Analyses of safety and dosing included only treated patients (patients receiving at least one dose of any study therapy)." Almost all (645, 95.4%) patients were available for safety analysis |
| Selective reporting (reporting bias) | Low risk | Data on all six outcomes concerned in this review were reported in the original paper |
| Other bias | Low risk | No evidence about other bias was found |
Pirker 2009.
| Methods | 1. Design: A multicentre, open‐label, parallel group, randomised phase III trial 2. Centres: 155 centres across the world 3. Randomisation: Patients were randomised centrally using an interactive voice response system. The random allocation schedule was generated using a computer. Randomisation was stratified by the ECOG PS (0–1 vs 2) and tumour stage (IIIB with malignant pleural effusion [wet IIIB] vs IV). Permutated blocks were assigned to each of four randomisation strata | |
| Participants | Inclusion criteria: Chemotherapy‐naive patients with histologically or cytologically proven stage wet IIIB or stage IV NSCLC and immunohistochemical evidence of EGFR expression in at least one positively stained tumour cell 1. Female, n (%): 335 (29.8) 2. Age in years, median (range): 60 (18‐83) 3. White people, n (%): 946 (84.1) 4. ECOG PS 0‐1, n (%): 929 (82.6) 5. Tumour stage IIIB/IV: 1125 (100.0) 6. Adenocarcinoma, n (%): 532 (47.3) 7. Never smoked, n (%): 244 (21.7) 8. EGFR expression, n (%): 1125 (100.0) 9. KRAS mutations, n (%): NA 10. EGFR mutations, n (%): NA |
|
| Interventions | 1. Arm A (n = 557): cisplatin + vinorelbine + cetuximab (17% received poststudy chemotherapy) 2. Arm B (n = 568): cisplatin + vinorelbine (27% received poststudy cetuximab) |
|
| Outcomes | 1. Primary: Overall survival (including data on one‐year survival rate) 2. Secondary: Progression‐free survival, best overall response, quality of life, safety |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised centrally using an interactive voice response system. The random allocation schedule was generated using a computer |
| Allocation concealment (selection bias) | Low risk | Quote: "......generated the random allocation schedule using a computer; physicians and study monitors did not have access to the code" |
| Blinding of participants and personnel (performance bias) overall survival and one‐year survival rate | Low risk | No blinding. However, the results on the two outcomes were mainly determined by the biological, objective effect of treatments and unlikely to be affected by the participants' and personnels' knowledge of the assignment status |
| Blinding of participants and personnel (performance bias) progression‐free survival, objective response rate, quality of life, and serious adverse events | High risk | No blinding. The four outcomes are not objective in nature and could be affected by participants' and/or personnels' knowledge of the assignment status |
| Blinding of outcome assessment (detection bias) overall survival and one‐year survival rate | Low risk | No blinding. However, overall survival and one‐year survival rate were objective, "hard" outcomes and were unlikely to have been affected by the subjective judgement of assessors |
| Blinding of outcome assessment (detection bias) progression‐free survival, objective response rate, quality of life, and serious adverse events | High risk | No blinding. The assessments of these outcomes involved subjective judgements and were vulnerable to the performance of assessors who were aware of the assignment status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Efficacy analysis was by intention to treat". Almost all (1110, 98.7%) patients were available for safety analysis |
| Selective reporting (reporting bias) | Low risk | Data on all six outcomes concerned in this review were reported in the original paper |
| Other bias | Low risk | No evidence about other bias was found |
Rosell 2008.
| Methods | 1. Design: A multicentre, open‐label, parallel group, randomised phase II trial 2. Centres: 16 centres in 6 European countries 3. Randomisation: No details were provided | |
| Participants | Inclusion criteria: Chemotherapy‐naive patients with histologically or cytologically proven NSCLC, stage IV or stage IIIB with documented malignant pleural effusion, according to American Joint Committee on Cancer criteria, and immunohistochemical evidence of EGFR expression in the primary tumour and/or metastases 1. Female, n (%): 22 (25.6) 2. Age in years, median (range): 58 (33‐74) 3. White people, n (%): 86 (100.0) 4. ECOG PS 0‐1, n (%): NA (Karnofsky performance status 80‐100: 78 (92.9) 5. Tumour stage IIIB/IV: 86 (100.0) 6. Adenocarcinoma, n (%): 37 (43.0) 7. Never smoked, n (%): NA 8. EGFR expression, n (%): 86 (100.0) 9. KRAS mutations, n (%): NA 10. EGFR mutations, n (%): NA |
|
| Interventions | 1. Arm A (n = 43): cisplatin + vinorelbine + cetuximab 2. Arm B (n = 43): cisplatin + vinorelbine |
|
| Outcomes | 1. Primary: Overall response rate 2. Secondary: Overall survival (including data on one‐year survival rate), progression‐free survival, time to treatment failure, duration of response, safety |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on the procedure were provided |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of allocation concealment |
| Blinding of participants and personnel (performance bias) overall survival and one‐year survival rate | Low risk | No blinding. However, the results on the two outcomes were mainly determined by the biological, objective effect of treatments and unlikely to be affected by the participants' and personnels' knowledge of the assignment status |
| Blinding of participants and personnel (performance bias) progression‐free survival, objective response rate, quality of life, and serious adverse events | High risk | No blinding. Progression‐free survival, objective response rate, and serious adverse events are not objective outcomes and could be affected by participants' and/or personnels' knowledge of the assignment status. The study did not use quality of life as an outcome |
| Blinding of outcome assessment (detection bias) overall survival and one‐year survival rate | Low risk | No blinding. However, overall survival and one‐year survival rate were objective, "hard" outcomes and unlikely to have been affected by the subjective judgement of assessors |
| Blinding of outcome assessment (detection bias) progression‐free survival, objective response rate, quality of life, and serious adverse events | High risk | No blinding. The assessments of these outcomes involved subjective judgements and were vulnerable to the performance of assessors who were aware of the assignment status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The efficacy analysis was based on the intent to treat population defined as all randomized patients. The safety analysis was based on all patients who had received any dose of study treatment." Almost all (85, 98.8%) patients were available for safety analysis |
| Selective reporting (reporting bias) | Unclear risk | Data on quality of life was not reported in the paper. As no protocol or registration can be found for this trial, it is difficult to say whether quality of life was a pre‐specified outcome. Thus, the risk for selective reporting bias was considered unclear |
| Other bias | Low risk | No evidence about other bias was found |
ECOG PS ‐ Eastern Cooperative Oncology Group performance status EGFR ‐ epidermal growth factor receptor
NA ‐ not available
NSCLC ‐ non‐small cell lung cancer
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baselga 2000 | Not comparing cetuximab plus chemotherapy with chemotherapy alone |
| Belani 2008 | Single‐arm, non‐randomised study |
| Borghaei 2008 | Single‐arm, non‐randomised study |
| Gridelli 2008 | Not comparing cetuximab plus chemotherapy with chemotherapy alone |
| Gridelli 2010 | Not comparing cetuximab plus chemotherapy with chemotherapy alone |
| NCT00085501 | The drugs used in different arms were the same (treatment schedules were different) |
| NCT00097214 | Single‐arm, non‐randomised study |
| NCT00103207 | Single‐arm, non‐randomised study |
| NCT00112294 | Duplicate of an included study (Lynch 2010) |
| NCT00118118 | Single‐arm, non‐randomised study |
| NCT00148798 | Duplicate of an included study (Pirker 2009) |
| NCT00165334 | Single‐arm, non‐randomised study |
| NCT00193453 | Single‐arm, non‐randomised study |
| NCT00216203 | Single‐arm, non‐randomised study |
| NCT00828841 | Not comparing cetuximab plus chemotherapy with chemotherapy alone |
| NCT01004731 | Single‐arm, non‐randomised study |
| Pirker 2008 | Duplicate of an included study (Pirker 2009) |
| Robert 2005 | Single‐arm, non‐randomised study |
| Rosell 2003 | Duplicate of an included study (Rosell 2008) |
| Spigel 2010 | Single‐arm, non‐randomised study |
| Stinchcombe 2010 | Single‐arm, non‐randomised study |
| Thienelt 2005 | Single‐arm, non‐randomised study |
| Von Pawel 2006 | Duplicate of an included study (Pirker 2009) |
Characteristics of ongoing studies [ordered by study ID]
NCT00946712.
| Trial name or title | |
| Methods | Randomised, phase III study |
| Participants | 1546 patients with advanced non‐small cell lung cancer |
| Interventions | Active Comparator: Arm I Patients receive carboplatin IV over 30 minutes and paclitaxel IV over 3 hours with or without bevacizumab IV over 30‐90 minutes on day 1. Treatment repeats every 21 days for up to 6 courses in the absence of disease progression or unacceptable toxicity. After completion of 6 courses, patients receiving bevacizumab may continue to receive bevacizumab (as above) in the absence of disease progression or unacceptable toxicity Experimental: Arm II Patients receive carboplatin and paclitaxel with or without bevacizumab as in arm I. Patients also receive cetuximab IV over 1‐2 hours on days 1, 8, and 15. Treatment repeats every 21 days for up to 6 courses in the absence of disease progression or unacceptable toxicity. After completion of 6 courses, patients may continue to receive cetuximab with or without bevacizumab (as above) in the absence of disease progression or unacceptable toxicity |
| Outcomes | Primary Outcome Measures: overall survival; progression‐free survival of EGFR FISH‐positive patients by institutional review Secondary Outcome Measures: overall survival and progression‐free survival of EGFR FISH‐positive patients by centralised review; progression‐free survival of the entire study population by centralised review and by institutional review; response; toxicity as assessed by NCI CTCAE version 4.0; comparison of other purported EGFR‐related biomarkers with EGFR IHC, EGFR FISH, and patient outcomes; Correlation of KRAS mutations with response and outcome |
| Starting date | |
| Contact information | |
| Notes |
EGFR ‐ epidermal growth factor receptor
Differences between protocol and review
1. LI Liu was newly added to the review as the second author.
2. Different from the protocol, we considered subgroup and sensitivity analyses only for the primary outcome (i.e. overall survival) in the full review. This is because there are multiple secondary outcomes, and subgroup and sensitivity analyses would probably produce false positive results due to multiple testing.
Contributions of authors
YZY and MC drafted the protocol; YZY, LL, and WXY performed the literature search; LL, WXY, HYF, HXF, and TJL conducted the data extraction and assessed the risk of bias; LL and YZY did the data analysis and drafted the initial manuscript; MC and TJL critically revised the manuscript; TJL supervised the progress and was responsible for the quality control of the whole project.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
None known.
New
References
References to studies included in this review
Butts 2007 {published data only}
- Butts CA, Bodkin D, Middleman EL, Englund CW, Ellison D, Alam Y, et al. Randomized phase II study of gemcitabine plus cisplatin or carboplatin [corrected], with or without cetuximab, as first‐line therapy for patients with advanced or metastatic non small‐cell lung cancer. Journal of Clinical Oncology 2007;25:5777‐84. [DOI] [PubMed] [Google Scholar]
Lynch 2010 {published data only}
- Lynch TJ, Patel T, Dreisbach L, McCleod M, Heim WJ, Hermann RC, et al. Cetuximab and first‐line taxane/carboplatin chemotherapy in advanced non‐small‐cell lung cancer: results of the randomized multicenter phase III trial BMS099. Journal of Clinical Oncology 2010;28(6):911‐7. [DOI] [PubMed] [Google Scholar]
Pirker 2009 {published data only}
- Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non‐small‐cell lung cancer (FLEX): an open‐label randomised phase III trial. The Lancet 2009;373(9674):1525‐31. [DOI] [PubMed] [Google Scholar]
Rosell 2008 {published data only}
- Rosell R, Robinet G, Szczesna A, Ramlau R, Constenla M, Mennecier BC, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first‐line therapy in EGFR‐expressing advanced non‐small‐cell lung cancer. Annals of Oncology 2008;19(2):362‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baselga 2000 {published data only}
- Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, et al. Phase I studies of anti‐epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. Journal of Clinical Oncology 2000;18(4):904‐14. [DOI] [PubMed] [Google Scholar]
Belani 2008 {published data only}
- Belani CP, Schreeder MT, Steis RG, Guidice RA, Marsland TA, Butler EH, et al. Cetuximab in combination with carboplatin and docetaxel for patients with metastatic or advanced‐stage nonsmall cell lung cancer: a multicenter phase 2 study. Cancer 2008;113(9):2512‐7. [DOI] [PubMed] [Google Scholar]
Borghaei 2008 {published data only}
- Borghaei H, Langer CJ, Millenson M, Ruth KJ, Litwin S, Tuttle H, et al. Phase II study of paclitaxel, carboplatin, and cetuximab as first line treatment, for patients with advanced non‐small cell lung cancer (NSCLC): results of OPN‐017. Journal of Thoracic Oncology 2008;3(11):1286‐92. [DOI] [PubMed] [Google Scholar]
Gridelli 2008 {published data only}
- Maione P, Mencoboni M, Carrozza F, Vigano MG, Gebbia V, Verusio C, et al. Addition of cetuximab (C) to gemcitabine (G) in elderly or performance status 2 (PS) patients (pts) with advanced non small‐cell lung cancer (NSCLC): The calc1 randomised phase 2 trials. Annals of Oncology. 2008; Vol. 19:ix24‐ix32.
Gridelli 2010 {published data only}
- Gridelli C, Morabito A, Gebbia V, Mencoboni M, Carrozza F, Viganò MG, et al. Cetuximab and gemcitabine in elderly or adult PS2 patients with advanced non‐small‐cell lung cancer: The cetuximab in advanced lung cancer (CALC1‐E and CALC1‐PS2) randomized phase II trials. Lung Cancer 2010;67(1):86‐92. [DOI] [PubMed] [Google Scholar]
NCT00085501 {unpublished data only}
- NCT00085501. S0342: Paclitaxel, carboplatin, and cetuximab in treating patients with stage IIIB or stage IV non‐small cell lung cancer. www.clinicaltrials.gov/show/NCT00085501.
NCT00097214 {unpublished data only}
- NCT00097214. Carboplatin plus cetuximab for treatment of stage IIIB/IV non‐small cell lung cancer (NSCLC). www.clinicaltrials.gov/show/NCT00097214.
NCT00103207 {unpublished data only}
- NCT00103207. Cetuximab in treating patients with recurrent or stage IIIB or stage IV lung cancer. www.clinicaltrials.gov/show/NCT00103207.
NCT00112294 {unpublished data only}
- NCT00112294. Study of taxane/carboplatin +/‐ cetuximab as first‐line treatment for patients with advanced/metastatic non‐small cell lung cancer. www.clinicaltrials.gov/show/NCT00112294.
NCT00118118 {unpublished data only}
- NCT00118118. Cetuximab in treating patients with recurrent or progressive metastatic non‐small cell lung cancer. www.clinicaltrials.gov/show/NCT00118118.
NCT00148798 {unpublished data only}
- NCT00148798. Study of cisplatin/vinorelbine +/‐ cetuximab as first‐line treatment of advanced non small cell lung cancer (FLEX). www.clinicaltrials.gov/show/NCT00148798.
NCT00165334 {unpublished data only}
- NCT00165334. Cetuximab and vinorelbine in elderly subjects with lung cancer. www.clinicaltrials.gov/show/NCT00165334.
NCT00193453 {unpublished data only}
- NCT00193453. Gemcitabine, docetaxel, and cetuximab in patients with unresectable advanced non‐small cell lung cancer. www.clinicaltrials.gov/show/NCT00193453.
NCT00216203 {unpublished data only}
- NCT00216203. Pemetrexed plus cetuximab in patients with recurrent non small cell lung cancer. www.clinicaltrials.gov/show/NCT00216203.
NCT00828841 {unpublished data only}
- NCT00828841. Phase 2b study of cetuximab with platinum‐based chemo as first line treatment of recurrent or advanced NSCLC. www.clinicaltrials.gov/show/NCT00828841.
NCT01004731 {unpublished data only}
- NCT01004731. Study of anti‐epidermal growth factor receptor (EGFr) antibody, cetuximab, in combination with gemcitabine/carboplatin in patients with stage IV lung cancer. www.clinicaltrials.gov/show/NCT01004731.
Pirker 2008 {published data only}
- Pirker R, Szczesna A, Pawel J, Krzakowski M, Ramlau R, Park K, et al. FLEX: A randomized, multicenter, phase III study of cetuximab in combination with cisplatin/vinorelbine (CV) versus CV alone in the first‐line treatment of patients with advanced non‐small cell lung cancer (NSCLC) [Abstract No. 3]. Journal of Clinical Oncology: ASCO annual meeting proceedings. 2008.
Robert 2005 {published data only}
- Robert F, Blumenschein G, Herbst RS, Fossella FV, Tseng J, Saleh MN, et al. Phase I/IIa study of cetuximab with gemcitabine plus carboplatin in patients with chemotherapy‐naive advanced non‐small‐cell lung cancer. Journal of Clinical Oncology 2005;23(36):9089‐96. [DOI] [PubMed] [Google Scholar]
Rosell 2003 {published data only}
- Rosell R, Ramlau R, Szczesna A, Daniel C, Bertrand M, Garcia AR, et al. Randomized phase II clinical trial of cetuximab in combination with cisplatin (C) and cinorelbine (V) or CV alone in patients with advanced epidermal growth factor receptor (EGFR)‐expressing non‐small‐cell lung cancer (NSCLC) [abstract]. European Journal of Cancer: ECCO III European Cancer Conference; 2003 Sept 21‐25; Copenhagen, Denmark. 2003.
Spigel 2010 {published data only}
- Spigel DR, Greco FA, Thompson DS, Webb C, Rubinsak J, Inhorn RC, et al. Phase II study of cetuximab, docetaxel, and gemcitabine in patients with previously untreated advanced non‐small‐cell lung cancer. Clinical Lung Cancer 2010;11(3):198‐203. [DOI] [PubMed] [Google Scholar]
Stinchcombe 2010 {published data only}
- Stinchcombe TE, Bradford DS, Hensing TA, LaRocca RV, Saleh M, Evans T, et al. A multicenter phase II trial of carboplatin and cetuximab for treatment of advanced nonsmall cell lung cancer. Cancer Investigation 2010;28(2):208‐15. [DOI] [PubMed] [Google Scholar]
Thienelt 2005 {published data only}
- Thienelt CD, Bunn PA Jr, Hanna N, Rosenberg A, Needle MN, Long ME, et al. Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non‐small‐cell lung cancer. Journal of Clinical Oncology 2005;23(34):8786‐93. [DOI] [PubMed] [Google Scholar]
Von Pawel 2006 {published data only}
- Pawel J, Park K, et al. Phase III study comparing cisplatin/vinorelbine plus cetuximab versus cisplatin/vinorelbine as first‐line treatment for patients with epidermal growth factor (EGFR)‐expressing advanced non‐small cell lung cancer (NSCLC) (FLEX). Journal of Clinical Oncology: ASCO annual meeting proceedings. 42nd Annual Meeting of the American Society of Clinical Oncology; 2006 June 2‐6; Atlanta, GA. 2006.
References to ongoing studies
NCT00946712 {unpublished data only}
- NCT00946712. S0819: Carboplatin/paclitaxel with or without bevacizumab and/or cetuximab in stage IV or recurrent non‐small cell lung cancer. www.clinicaltrials.gov/show/NCT00946712.
Additional references
Blumenschein 2011
- Blumenschein GR Jr, Paulus R, Curran WJ, Robert F, Fossella F, Werner‐Wasik M, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non‐small‐cell lung cancer: RTOG 0324. Journal of Clinical Oncology 2011;29(17):2312‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butts 2005
- Butts. Study of Gemcitabine/Platinum +/‐ Cetuximab as First‐Line Treatment for Patients With Advanced/Metastatic Non‐Small Cell Lung Cancer. http://clinicaltrials.gov/ct2/show/study/NCT00112346 2005.
Dahabreh 2011
- Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA. Systematic review: anti‐epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Annals of Internal Medicine 2011;154(1):37‐49. [DOI] [PubMed] [Google Scholar]
Eberhard 2005
- Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non‐small‐cell lung cancer treated with chemotherapy alone and in combination with erlotinib. Journal of Clinical Oncology 2005;23(25):5900‐9. [DOI] [PubMed] [Google Scholar]
FDA 2004
- FDA. Final Labeling Text (bevacizumab). www.accessdata.fda.gov/drugsatfda_docs/label/2009/125085s0169lbl.pdf (accessed 19 May 2012).
FDA 2005
- FDA. Gefitinib (marketed as Iressa) Information. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm110473.htm (accessed 19 May 2012).
FDA 2010
- FDA 2010. Highlights for Prescribing Information (Tarceva). www.accessdata.fda.gov/drugsatfda_docs/label/2010/021743s14s16lbl.pdf (accessed 19 May 2012).
FDA 2011
- FDA. FDA Approves Erbitux to Treat Late‐Stage Head and Neck Cancer. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm278894.htm (accessed 23 May 2012).
Govindan 2006
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. Journal of Clinical Oncology 2006;24(28):4539‐44. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro 3.6. McMaster University, 2014.
Gridelli 2004
- Gridelli C, Ardizzoni A, Le Chevalier T, Manegold C, Perrone F, Thatcher N, et al. Treatment of advanced non‐small‐cell lung cancer patients with ECOG performance status 2: results of an European Experts Panel. Annals of Oncology 2004;15(3):419‐26. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, GRADE Working Group. Rating quality of evidence and strength of recommendations: What is "quality of evidence" and why is it important to clinicians?. British Medical Journal 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herbst 2010
- Herbst RS, Kelly K, Chansky K, Mack PC, Franklin WA, Hirsch FR, et al. Phase II selection design trial of concurrent chemotherapy and cetuximab versus chemotherapy followed by cetuximab in advanced‐stage non‐small‐cell lung cancer: Southwest Oncology Group study S0342. Journal of Clinical Oncology 2010;28(31):4747‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
IARC 2010
- International Agency for Research on Cancer. Fast stats: most frequent cancers. www.globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=900 (accessed 19 May 2012).
Jemal 2010
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: A Cancer Journal for Clinicians 2010;60(5):277‐300. [DOI] [PubMed] [Google Scholar]
Kim 2009
- Kim ES, Mauer AM, William WN Jr, Tran HT, Liu D, Lee JJ, et al. A phase 2 study of cetuximab in combination with docetaxel in chemotherapy‐refractory/resistant patients with advanced nonsmall cell lung cancer. Cancer 2009;115(8):1713‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2010
- Lin H, Jiang J, Liang X, Zhou X, Huang R. Chemotherapy with cetuximab or chemotherapy alone for untreated advanced non‐small‐cell lung cancer: a systematic review and meta‐analysis. Lung Cancer 2010;70(1):57‐62. [DOI] [PubMed] [Google Scholar]
Marino 1994
- Marino P, Pampallona S, Preatoni A, Cantoni A, Invernizzi F. Chemotherapy vs supportive care in advanced non‐small‐cell lung cancer. Results of a meta‐analysis of the literature. Chest 1994;106(3):861‐5. [DOI] [PubMed] [Google Scholar]
Miller 1981
- Miller AB HB, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47(1):207‐14. [DOI] [PubMed] [Google Scholar]
Mitsudomi 2011
- Mitsudomi T. Erlotinib, gefitinib, or chemotherapy for EGFR mutation‐positive lung cancer?. The Lancet Oncology 2011;12(8):710‐1. [DOI] [PubMed] [Google Scholar]
National Cancer Institute 2011
- National Cancer Institute. FDA Approval for Cetuximab. www.cancer.gov/cancertopics/druginfo/fda‐cetuximab (accessed 23 May 2012).
Nieder 2012
- Nieder C, Pawinski A, Dalhaug A, Andratschke N. A review of clinical trials of cetuximab combined with radiotherapy for non‐small cell lung cancer. Radiation Oncology 2012;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pirker 2012
- Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, et al. EGFR expression as a predictor of survival for first‐line chemotherapy plus cetuximab in patients with advanced non‐small‐cell lung cancer: analysis of data from the phase 3 FLEX study. The Lancet Oncology 2012;13(1):33‐42. [DOI] [PubMed] [Google Scholar]
Reeves 2011
- Reeves TD, Hill EG, Armeson KE, Gillespie MB. Cetuximab therapy for head and neck squamous cell carcinoma: a systematic review of the data. Otolaryngology‐Head and Neck Surgery 2011;144(5):676‐84. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schiller 2002
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. The New England Journal of Medicine 2002;346(2):92‐8. [DOI] [PubMed] [Google Scholar]
Stinchcombe 2009
- Stinchcombe TE, Socinski MA. Current treatments for advanced stage non‐small cell lung cancer. Proceedings of the American Thoracic Society 2009;6(2):233‐41. [DOI] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205‐16. [DOI] [PubMed] [Google Scholar]
Tol 2010
- Tol J, Punt CJ. Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clinical Therapeutics 2010;32(3):437‐53. [DOI] [PubMed] [Google Scholar]


