Keywords: congenital diaphragmatic hernia, pulmonary hypertension, spatial transcriptomics
Abstract
Abnormal pulmonary vascular development and function in congenital diaphragmatic hernia (CDH) is a significant factor leading to pulmonary hypertension. The lung is a very heterogenous organ and has marked cellular diversity that is differentially responsive to injury and therapeutic agents. Spatial transcriptomics provides the unmatched capability of discerning the differences in the transcriptional signature of these distinct cell subpopulations in the lung with regional specificity. We hypothesized that the distal lung parenchyma (selected as a region of interest) would show a distinct transcriptomic profile in the CDH lung compared with control (normal lung). We subjected lung sections obtained from male and female CDH and control neonates to spatial transcriptomics using the Nanostring GeoMx platform. Spatial transcriptomic analysis of the human CDH and control lung revealed key differences in the gene expression signature. Increased expression of alveolar epithelial-related genes (SFTPA1 and SFTPC) and angiogenesis-related genes (EPAS1 and FHL1) was seen in control lungs compared with CDH lungs. Response to vitamin A was enriched in the control lungs as opposed to abnormality of the coagulation cascade and TNF-alpha signaling via NF-kappa B in the CDH lung parenchyma. In male patients with CDH, higher expression of COL1A1 (ECM remodeling) and CD163 was seen. Increased type 2 alveolar epithelial cells (AT-2) and arterial and lung capillary endothelial cells were seen in control lung samples compared with CDH lung samples. To the best of our knowledge, this is the first use of spatial transcriptomics in patients with CDH that identifies the contribution of different lung cellular subpopulations in CDH pathophysiology and highlights sex-specific differences.
NEW & NOTEWORTHY This is the first use of spatial transcriptomics in patients with congenital diaphragmatic hernia (CDH) that identifies the contribution of different lung cellular subpopulations in CDH pathophysiology and highlights sex-specific differences.
INTRODUCTION
Congenital diaphragmatic hernia (CDH) is a complex disorder with a high mortality due to the associated pulmonary hypoplasia and pulmonary hypertension. Pulmonary hypoplasia and the associated pulmonary hypertension in CDH are a key factors in the 50% mortality seen in the early perinatal stage and often lead to the need for extracorporeal support (ECMO) use in surviving patients (1–3). Biological insights from human CDH-lung tissue that can elucidate the underlying mechanisms that drive CDH pathophysiology are an unmet knowledge gap.
Pulmonary hypoplasia in CDH is characterized by impaired branching morphogenesis, paucity of the number of alveoli, and abnormalities in the pulmonary epithelium and the mesenchyme (4). In addition, in the developing lung, there is a close interdependence and cross talk between the developing epithelium and endothelium. The developing pulmonary vasculature in the CDH lung is also underdeveloped and shows pathologic remodeling leading to endothelial dysfunction (5). As a result, neonates with CDH are often severely hypoxic due to poor gas exchange and exhibit marked pulmonary hypertension in the immediate postnatal period (6), which is often resistant to traditional therapies. The contribution of the different lung cell types to disease pathogenesis and the gene expression changes within these cells in the CDH lung could hold clues to future therapeutic strategies to improve outcomes.
The newborn lung exhibits many sex-specific differences during development, postnatal transition, and in the manifestation of neonatal lung diseases. Sex-based differences are evident during fetal lung development and have been recapitulated in animal models and in the human lung (7–9). Sex-related differences exist in many pediatric lung diseases, including respiratory distress syndrome, bronchopulmonary dysplasia, and asthma (7, 8, 10, 11). CDH has a higher incidence in male neonates (12). However, to our knowledge, no studies report on sex-specific differences in the gene expression in the human CDH lung.
The lung has tremendous cellular diversity, and the immune and structural microenvironment varies across different regions of the lung. Spatial biology studies can elucidate cellular identity in different tissue locations and by using unbiased-omics-based approaches, can measure their gene expression patterns. Our focus in this study was the distal lung parenchyma in the CDH lung, including the alveolar epithelial and the lung capillary endothelial cells, among other cell subpopulations. Our objective was to understand the lung cellular heterogeneity in the distal lung in human CDH with the goal of identifying gene targets and pathways playing a role in pathophysiology. We hypothesized that the distal lung parenchyma (selected as a region of interest) would show a distinct transcriptomic profile in the CDH lung compared with control (normal lung). We included both male and female lungs to discern the role of sex as a biological variable.
METHODS
Selection of Human Lung Sections for Spatial Transcriptomics
Patients with CDH and control patients (infants with congenital lung malformations who underwent resection from whom the normal surrounding lung is used as control sections) were carefully selected from an established patient repository at Texas Children’s Hospital. The samples were matched for sex and gestational age at birth. Detailed clinical information on the patient cohort including variables like specimen location, gestational age at birth, sex, race/ethnicity, and birthweight is included in Table 1. In addition, the type of congenital lung malformation and the type of procedure are detailed. Measures of CDH severity such as observed/expected total lung volume obtained by fetal MRI, % liver herniation, laterality of the CDH, need for a patch repair, survival days, ventilation days, need for, and duration of ECMO are included. Of note, all but one patient with CDH had a normal chromosomal microarray (CMA). One patient did not have the CMA results available. Most of the patients did not have any other associated anomalies apart from CDH. One patient had a bronchogenic cyst, and another had imperforate anus in addition to the CDH.
Table 1.
Clinical characteristics
| Patients with CDH | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specimen Location | Sex | Race | Death DOL, Days | GA at Birth, wk | Birth Weight, g | o/e TLV, % (Pre→Post FETO) | Liver Herniation, % (Pre→Post FETO) | ECMO | ECMO Duration, Days | FETO | Patch | CDH Side | Ventilation Days | Anomaly | CMA |
| RLL | Female | White | 14 | 40 1/7 | 3,079 | 19 →17 | 42 →39 | Yes | 14 | Yes | Yes | Right | 14 | None | Normal |
| L Lobe | Female | White | 0 | 36 4/7 | 3,101 | 14→9 | 26→31 | No | Yes | Not repaired | Left | Died within 24 h | None | Normal | |
| LUL | Female | White/Asian | 102 | 34 5/7 | 2,070 | 5→ “decreased” | 35→ “Grossly unchanged” | Yes | 18 | Yes | Yes | Bilateral | 102 | Bronchogenic cysts | Normal |
| LLL | Female | Black | 22 | 38 5/7 | 2,830 | 21→18 | 34→43 | Yes | 21 | Yes | Yes | Left | 22 | Imperforate anus | Normal |
| LLL | Male | White | 4 | 36 6/7 | 2,550 | 16 | 46 | Yes | 4 | No | Yes | Right | 4 | None | Normal |
| LUL | Male | White/Hispanic | 24 | 39 3/7 | 3,060 | 15 | 24 | Yes | 13 | No | Yes | Left | 24 | None | Normal |
| LLL | Male | White | 48 | 39 4/7 | 3,805 | 28 | 24 | Yes | 35 | No | Yes | Left | 48 | None | N/A |
| RLL | Male | White | 48 | 35 0/7 | 2,829 | 18→27 | 61→N/A | Yes | 25 | Yes | Yes | Left | 48 | None | Normal |
| Patients with CLM (controls) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specimen Location | Sex | Race | GA at Birth | Age at Procedure | Pathology | Procedure | ||||||||
| LLL | Female | White | 39 1/7 | 2 mo 27 days | Intralobar sequestration | Open | ||||||||
| LLL | Female | White | 36 0/7 | 6 mo 6 days | Intralobar sequestration | VATS | ||||||||
| LLL | Female | White | 38 2/7 | 5 mo 7 days | Intralobar sequestration | VATS | ||||||||
| LLL | Female | White | 40 0/7 | 6 mo 4 days | Intralobar sequestration | VATS | ||||||||
| LLL | Male | White | 39 1/7 | 8 mo | Bronchial atresia | VATS | ||||||||
| LLL | Male | White/Hispanic | 40 4/7 | 3 mo 20 days | Bronchial atresia | VATS | ||||||||
| LLL | Male | White/Hispanic | 36 3/7 | 3 mo | Intralobar sequestration | VATS | ||||||||
| LLL | Male | White/Hispanic | 40 5/7 | 3 mo 16 days | Bronchial atresia | VATS | ||||||||
CDH, congenital diaphragmatic hernia; CLM, congenital lung malformation; CMA, chromosomal microarray; DOL, day of life; ECMO, extracorporeal membrane oxygenation; FETO, fetoscopic endotracheal occlusion; GA, gestational age; LLL, left lower lobe; N/A, not available; RLL, right lower lobe; TLV, total lung volume; VATS, video-assisted thoracoscopic surgery.
Spatial Gene Profiling in the Control and CDH Lung
Formalin-fixed paraffin-embedded lung tissue sections from patients with CDH and control patients were analyzed using spatial transcriptomics on the Nanostring GeoMx platform. Lung sections were prepared for use with the GeoMX digital spatial profiler (DSP) and the GeoMX transcriptome (NanoString, WA). The lung sections were stained for nuclear marker (SYTO 13), PanCK (Pancytokeratin; epithelial marker), CD45 (immune marker), and CD31 (endothelial marker). Regions of interest (ROI) within the distal lung from each patient lung sample. The pathologist reviewed all samples to make sure that all the anatomical regions were adequately and accurately identified before ROI selection. For each lung section, we randomly ROIs from the distal lung parenchyma. Slides placed into the GeoMx, scanned at very high resolution (zoom-in up to 50 µm), and regions of interest (ROI) were selected using the “freehand” drawing tool on the GeoMx DSP software, which only allows a maximum diameter of 650 µm. The ROIs are measured in area sizes (µm2), with an average area of 387,590.5 ± 36,547.04 µm2. Each ROI was subjected to UV illumination, to collect the indexing oligonucleotides from the cells within the ROI, and counted using next-generation sequencing. Once all the samples were collected after ROI selection, the RNA library was constructed using manufacturer’s instructions [GeoMx DSP NGS Readout User Manual (nanostring.com)]. Libraries were submitted for sequencing on Illumina Platform using NovaSeq600, performed by the genomic and RNA profiling core at Baylor College of Medicine (Houston, TX). Samples were read with a sequencing depth of 100, at 300 M reads. The resulting FastQ files were converted into .DCC files using the Nanostring Pipeline algorithm, to “stitch” the FastQ file with corresponding spatial location (ROI). The resulting data set was analyzed by the spatial transcriptomics scripts for “R” package, supplied by Nanostring (GeoScript Hub | NanoString).
Data Analysis
Whole Transcriptome Atlas data collected from CDH and healthy lung samples from ROIs were spatially profiled from the tissues. After filtering regions for low reads, low saturation, and low detection rate (<1% genes detected), 69 ROIs were kept for downstream analysis. Biological differences as a function of disease status and structure at gene, cell type, and pathway levels were analyzed. Quality control, preprocessing, normalization, and differential expression were done using the recommended GeoMX Workflows (13). For spatial deconvolution, we used SpatialDecon (14) using the IPF Lung Cell Atlas (15) as the cell profile matrix, which contains 36 cell types (16) along with GeoMXTools (17), gene set variation analysis (GSVA) was used to detect biological pathways regulated by differentially expressed genes in our segments (18).
RESULTS
Histopathological Examination of CDH Lungs Showed Severe Pulmonary Hypoplasia
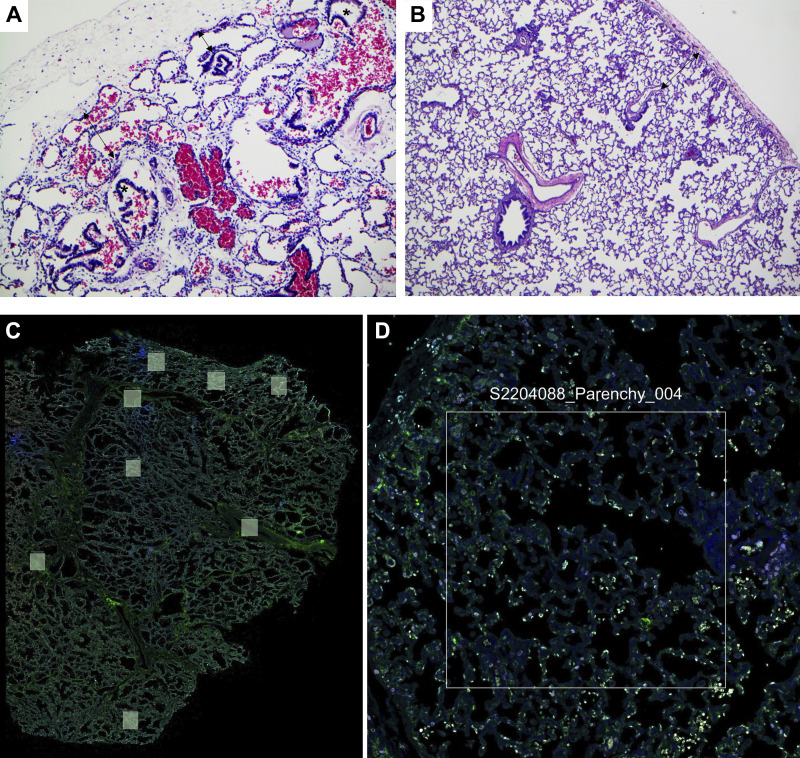
Representative hematoxylin-eosin (H&E) stained lung sections from a patient with CDH and a control patient at low and high magnification are shown in Fig. 1, A and B. The key histopathological features for each patient sample included in the spatial transcriptomics analysis are summarized in Supplemental Table S1. All CDH patient lungs showed evidence of severe pulmonary hypoplasia with decreased radial alveolar count and airways seen in close proximity to the pleural surface. Secondary changes related to mechanical ventilation (overexpanded airways and alveoli, pulmonary interstitial emphysema) were variably present. The stage of lung development was consistent with gestational and postnatal age in all cases. Each of the lung sections was then stained for Syto 13 (nuclear marker), PanCK (Pancytokeratin; epithelial marker), and CD45 (immune cell marker). A representative stained lung section in low and high magnification is shown in Fig. 1C. ROIs in the distal lung parenchyma without any airways or major vessels were selected randomly in each of the lung sections by a pathologist.
Figure 1.
Histopathological characterization of the CDH lungs: representative H&E stained lung tissue sections from CDH (A) and control lungs (B). A: lung section from patient with CDH showing severe pulmonary hypoplasia with airways (*) seen approaching pleural surface and markedly decreased radial alveolar count of 1–2 (double-headed arrows, H&E, ×100). B: lung section from control patient showing appropriately developed pulmonary parenchyma with radial alveolar count of 4–5 (double arrowheads; H&E, ×40). Representative low (C)- and high (D)-magnification lung section stained with a nuclear marker (SYTO 13), PanCK (Pancytokeratin; epithelial marker), and CD45 (immune cell marker) with demarcation of the region of interest for the selection of the distal lung parenchyma. CDH, congenital diaphragmatic hernia; H&E, hematoxylin-eosin.
Spatial Transcriptomic Analysis of the Human CDH and Control Lung Reveals Key Differences in the Gene Expression Signature
We used dimensional reduction techniques to identify broad patterns in the expression data. A clear separation was seen between the CDH and control samples as shown in the PCA plot in Supplemental Fig. S1A. Unsupervised hierarchical clustering also showed that genes with high coefficient of variation cluster by disease status (Supplemental Fig. S1B). The volcano plot in Fig. 2A shows the differentially expressed genes in the “parenchymal” regions between control and CDH lung samples. One hundred fifty-seven genes were significant at FDR <0.05 (false discovery rate). Fifty-two genes were significant using the dual thresholding of FDR < 0.05 and |log2 fold change| > 0.5. Violin plots of some key genes are shown in Fig. 2B. Decreased expression of alveolar epithelial-related genes (SFTPA1, SFTPA2, and SFTPC) and angiogenesis-related genes (EPAS1, FHL1, and CLDN5) was seen in CDH lungs compared with control lungs. Biological pathways enriched in CDH versus control lungs are shown in Fig. 2C. Pathways related to epithelial and endothelial cell differentiation, sprouting angiogenesis, and vasculogenesis were negatively enriched in the CDH lung, whereas neutrophil-mediated cytotoxicity markers were positively enriched.
Figure 2.
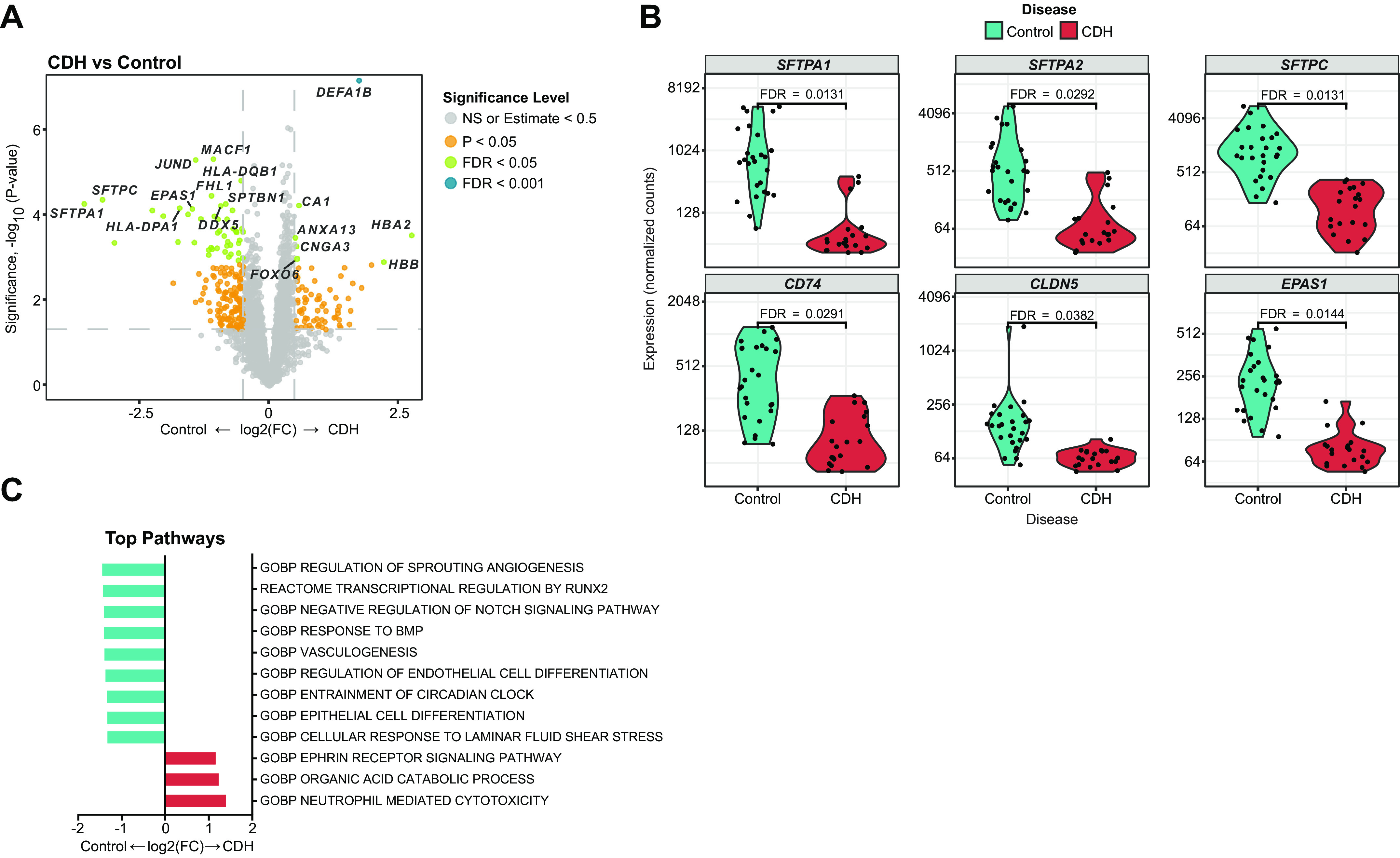
Spatial transcriptomic analysis of the human CDH and control lung reveals key differences in the gene expression signature. Spatial transcriptomic analysis of lung sections obtained from patients with CDH (n = 4/sex/group) were compared with age- and sex-matched controls. A: volcano plots showing differentially expressed genes (DEGs) in control vs. patients with CDH. DEGs upregulated in controls are shown on the left (negative) and genes upregulated in CDH are on the right (positive). Gray dot: NS, orange: P < 0.05, green: FDR < 0.05. B: violin plots showing normalized expression values (counts) of selected genes; epithelial (SFTPA1, SFTPA2, SFTPC, and CD74) and endothelial (EPAS1 and CLDN5) in CDH and control lungs. C: enriched biological pathways in the CDH vs. control lung. CDH, congenital diaphragmatic hernia; FDR, false discovery rate; NS, not significant.
Marked Sex-Specific Differences in Differentially Expressed Genes and Enriched Biological Pathways in Male and Female CDH in Lung Parenchymal Regions Analyzed by Spatial Transcriptomics
Volcano plots showing DEGs in the male and female CDH lung are shown in Fig. 3, A and B. Two hundred thirty-two genes were significant in the male CDH lung and 202 genes in the female CDH lung, having met the dual thresholding of FDR< 0.05 and |log2 fold change| > 0.5. Violin plots of key genes are shown in Fig. 3C (COL1A1 and CD163 in the male lung and CCN1 and SGK1 in the female lung). Overlap, or lack thereof, in DEGs and biological pathways, is shown in four-way Venn diagrams in Fig. 3D. Only a minority of genes (65 up- and 66 downregulated) were common between males and females. Key biological pathways enriched in the male and the female CDH lung compared with sex-matched control lungs are shown in Fig. 3E. Pathways related to leukocyte migration and glycolysis were enriched in the CDH male lung, whereas epithelial cell polarity and cell-ECM interactions were negatively enriched. In females with CDH, pathways including positive regulation of endothelial and epithelial cell differentiation, response to corticosteroids, and signaling by retinoic acid were negatively enriched. The majority of the enriched biological pathways were also distinct in the male and female CDH lung. The number of pathways in common and different in the male and female CDH lungs are shown in Fig. 3F.
Figure 3.
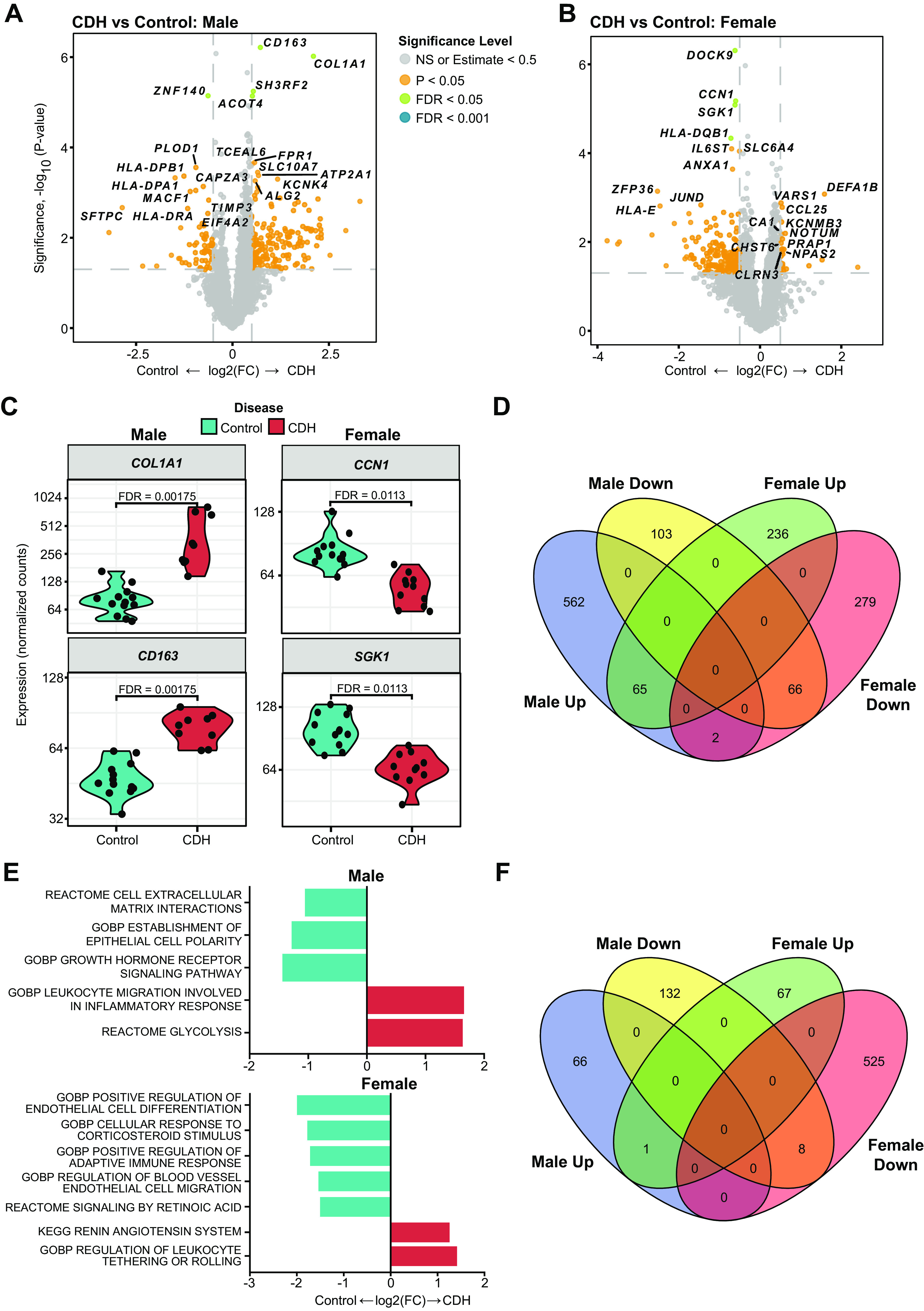
Marked sex-specific differences in differentially expressed genes and enriched biological pathways in male and female CDH in lung parenchymal regions analyzed by spatial transcriptomics. Spatial transcriptomic analysis of lung sections obtained from male and female patients with CDH (n = 4/sex/group) were compared with age- and sex-matched controls. Volcano plots showing differentially expressed genes (DEGs) in male control vs. patients with CDH (A) and female control vs. patients with CDH (B). DEGs upregulated in controls are shown on the left (negative) and genes upregulated in CDH are on the right (positive). Gray dot: NS, orange: P < 0.05, green: FDR < 0.05. C: violin plots showing normalized expression values (counts) of selected genes; in male and female CDH and control lungs. D: four-way Venn diagram showing minimal overlap between males and females in differentially expressed genes. E: enriched biological pathways in the male and female CDH lung compared with sex-matched control lungs. F: four-way Venn diagram showing very little overlap between males and females in enriched biological pathways. CDH, congenital diaphragmatic hernia; FDR, false discovery rate.
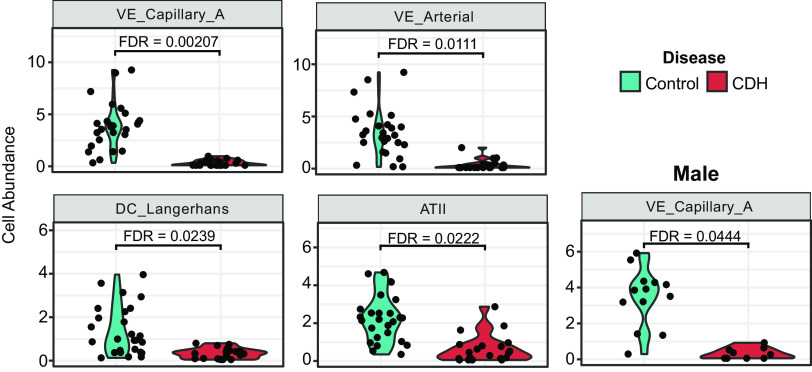
Quantitative Differences in Cell Type Abundance Using Spatial Deconvolution in the Human CDH Lung Reveals Involvement of Alveolar Epithelial and Pulmonary Capillary Endothelial Cells
Spatial deconvolution is an analysis technique that estimates the relative abundance of cell types for each region of interest segment. The algorithm utilizes a prespecified cell profile matrix generated from scRNA-seq data to deconvolute GeoMx data (19). For this analysis, we used IPF Lung Cell Atlas data, which contains 36 cell types (16). We analyzed deconvolution results for a given cell type within an ROI segment as an abundance beta estimate. The abundance beta can be thought of as a relative abundance score; the higher the beta for a given cell type, the more abundant that cell type is in that ROI segment. Spatial deconvolution findings are shown in Fig. 4. Compared with controls, decreased AT-2 cells, arterial, capillary endothelial cells, and Langerhans cells were seen in the CDH lungs (Fig. 4A). When stratified by biological sex, male lungs showed a more significant (FDR < 0.05) reduction in lung capillary endothelial cells compared with females.
Figure 4.
Quantitative differences in cell type abundance using spatial deconvolution in the human CDH lung reveals involvement of alveolar epithelial and pulmonary capillary endothelial cells. Spatial deconvolution analysis of lung sections obtained from patients with CDH (n = 4/sex/group) were compared with age- and sex-matched controls. Violin plots showing cell type abundance in control vs. patients with CDH and male control vs. patients with CDH. CDH, congenital diaphragmatic hernia.
DISCUSSION
The lung comprises a great diversity of cell types. The relative proportions of different types of cells and their relative microanatomical location are critical for normal lung development as well as proper postnatal transition. CDH is characterized by severe fetal lung hypoplasia, but an unbiased examination of the human CDH lung and the gene expression changes compared with the normal lung have not been reported before. Pulmonary hypertension in patients with CDH is often the most pressing clinical problem after birth (20), often necessitating anti-PH therapies, the need for aggressive mechanical ventilation, and ECMO (21). Most of the research in pulmonary hypertension in CDH has rightfully focused on the pulmonary artery endothelial cells, which are very distinct from the lung capillary endothelial cells. Whether the relative proportions of other endothelial cell populations are altered, and the expression of angiogenesis-related genes in the distal lung has not been investigated. In this study, spatial transcriptomics provides the capability of discerning the differences in transcriptional signature of these distinct vascular beds and the lung epithelial cells in the distal lung. To the best of our knowledge, this is the first use of spatial transcriptomics in patients with CDH that identifies the contribution of different lung cellular subpopulations in CDH pathophysiology with regional specificity. Importantly, this approach reveals the possible involvement of the lung capillary endothelial cells in endothelial dysfunction seen in patients with CDH.
We noticed decreased AT-2 cells in our deconvolution data and decreased expression of surfactant-related genes in the distal lung ROIs. In one study, in the murine nitrofen model, a significant reduction in the proportion of AT-1 cells and an increase in the proportion of AT-2 cells in the hypoplastic lung was reported, supporting impaired alveolar epithelial cell differentiation in the CDH lung (22). In another study, the nitrofen-exposed rat lung had decreased SP-C expression that further decreased when the nitrofen-exposed lung was cultured ex vivo in compression microdevices (23). Tracheal aspirate samples from infants with CDH showed lower SP-B, disaturated phosphatidylcholine, and SP-A levels, as well as lower rates of SP-B synthesis (24, 25). Our studies thus support the relative paucity and expression of surfactant-related genes and AT-2 cells. We also show decreased expression of CD74 in the CDH lung. CD74 is the receptor for macrophage migration inhibitory factor (MIF). AT-2 cells in the lung express CD74 (26). In the lung, MIF-CD74 signaling promotes epithelial cell proliferation and restores the epithelial barrier after injury (27). On the other hand, in pulmonary hypertension studies, increased expression of CD74 was observed and thought to contribute to the proinflammatory endothelial milieu (28, 29). The epithelial and/or endothelial-specific role of CD74 in CDH needs to be elucidated.
VE_Capillary_A subset (15) that was decreased in the CDH lungs corresponds to the aerocyte population of lung capillary endothelial cells (Car4+, Endrb+, Apln+) (30). Disruption of this population of endothelial cells adversely impacts alveolarization (31). Our finding of decreased numbers of this endothelial cell subpopulation probably points to a developmental impact on lung angiogenesis and alveolarization in patients with CDH that may or may not be secondary to the intestinal herniation and lung compression.
Few studies have reported on whether sex as a biological variable influences outcomes in CDH. A European CDH registry (n = 219) reported greater mortality in females (32), whereas another meta-analysis (n = 347) reported no sex difference (33). Yet another recent study reported a higher incidence of tracheostomy in male infants with CDH, with tracheostomy correlated with severity of pulmonary hypertension (34). In this study, we report the differences in gene expression and biological pathways in the distal CDH lung with carefully selected sex-matched controls. COL1A1 and CD163 were increased in the male CDH lung. Increased COL1A1 expression contributes to pulmonary hypertension and is also a marker for endothelial-to-mesenchymal transition (35–38). CD163 is a marker of monocyte-derived macrophages in the lung, which are profibrotic and proinflammatory(39). SGK-1 (serum- and glucocorticoid-inducible kinase) was decreased in the female CDH lung, which is a stress-responsive kinase involved in alveolar fluid clearance (40, 41). SGK-1 expression is increased in pulmonary endothelial cells in PAH, and loss of Sgk-1 decreased hypoxia-induced PAH in mice (29, 42–44). Cellular communication network factor 1 (CCN1) is a matricellular protein that can modulate many biological processes, including inflammation and repair (45). CCN1 levels are indicative of the severity of lung injury(46, 47). Interestingly, CCN1 expression was increased in hypoxia-exposed human pulmonary artery endothelial cells and human pulmonary artery smooth muscle cells. Furthermore, CCN1 suppressed hypoxia-induced smooth muscle contraction and was vasodilatory (48, 49). CCN1 expression was decreased in the female CDH lung, but whether the epithelial or endothelial-specific role predominates needs to be investigated. Response to retinoic acid (Vitamin A) was decreased in the female CDH lungs. Antenatal vitamin A administration attenuates lung hypoplasia in a murine CDH model (50) and connections between clinical, environmental, and genetic factors point to the essential role of vitamin A signaling in CDH pathophysiology (51). The spatial deconvolution revealed the relative paucity of distal lung epithelial and capillary endothelial cells in CDH. In addition, we also show decreased enrichment of dendritic cells in the CDH lung. Prior studies have pointed to the importance of these cells for the resolution of lung injury (52, 53).
The limitations of this study include the lack of information on cell-population-specific interrogation of gene expression in the distal lung of patients with CDH. Also, the transcriptional state of other microanatomical regions in the lung, such as the proximal arteries, needs further study and would be of great interest from the CDH-PH perspective. The results of spatial deconvolution do not confirm the relative proportions of different lung cell subpopulations. To conclude, this study highlights the first use of spatial transcriptomics in patients with CDH that identifies the contribution of different distal lung cellular subpopulations in CDH pathophysiology and highlights sex-specific differences.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Fig. S1 and Supplemental Table S1: https://doi.org/10.6084/m9.figshare.22822355.v1.
GRANTS
This work was supported in part by National Institutes of Health Grants R01 HL144775, R01 HL146395, and R21 HD100862 (to K.L).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.L. and J.P.G. conceived and designed research; K.L., O.O.O., J.D.H., J.R.Q., H.L., F.P., and S.G.K. performed experiments; K.L., A.C., M.E.C.G., and N.C.-S. analyzed data; K.L., A.C., and M.E.C.G. interpreted results of experiments; K.L., O.O.O., A.C., M.E.C.G., N.C.-S., and J.R.Q. prepared figures; K.L., O.O.O., A.C., and M.E.C.G. drafted manuscript; K.L., O.O.O., A.C., M.E.C.G., N.C.-S., J.D.H., J.G., H.L., F.P., J.P.G., and S.G.K. edited and revised manuscript; K.L., O.O.O., A.C., M.E.C.G., N.C.-S., J.D.H., J.G., J.R.Q., H.L., F.P., and J.P.G. approved final version of manuscript.
REFERENCES
- 1. Harrison MR, Bjordal RI, Langmark F, Knutrud O. Congenital diaphragmatic hernia: the hidden mortality. J Pediatr Surg 13: 227–230, 1978. doi: 10.1016/S0022-3468(78)80391-1. [DOI] [PubMed] [Google Scholar]
- 2. Brownlee EM, Howatson AG, Davis CF, Sabharwal AJ. The hidden mortality of congenital diaphragmatic hernia: a 20-year review. J Pediatr Surg 44: 317–320, 2009. doi: 10.1016/j.jpedsurg.2008.10.076. [DOI] [PubMed] [Google Scholar]
- 3. Guner YS, Harting MT, Jancelewicz T, Yu PT, Di Nardo M, Nguyen DV. Variation across centers in standardized mortality ratios for congenital diaphragmatic hernia receiving extracorporeal life support. J Pediatr Surg 57: 606–613, 2022. doi: 10.1016/j.jpedsurg.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 4. Olutoye OO, Short WD, Gilley J, Hammond JD, Belfort MA, Lee TC, King A, Espinoza J, Joyeux L, Lingappan K, Gleghorn JP, Keswani SG. The cellular and molecular effects of fetoscopic endoluminal tracheal occlusion in congenital diaphragmatic hernia. Front Pediatr 10: 925106, 2022. doi: 10.3389/FPED.2022.925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donahoe PK, Longoni M, High FA. Polygenic causes of congenital diaphragmatic hernia produce common lung pathologies. Am J Pathol 186: 2532–2543, 2016. doi: 10.1016/J.AJPATH.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zani A, Chung WK, Deprest J, Harting MT, Jancelewicz T, Kunisaki SM, Patel N, Antounians L, Puligandla PS, Keijzer R. Congenital diaphragmatic hernia. Nat Rev Dis Primers 8: 37, 2022. doi: 10.1038/S41572-022-00362-W. [DOI] [PubMed] [Google Scholar]
- 7. Lingappan K, Alur P, Eichenwald E. The need to address sex as a biological variable in neonatal clinical studies. J Pediatr 255: 17–21, 2023. doi: 10.1016/j.jpeds.2022.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 311: L481–L493, 2016. doi: 10.1152/AJPLUNG.00047.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayward-Piatkovskyi B, Gonyea CR, Pyle SC, Lingappan K, Gleghorn JP. Sex-related external factors influence pulmonary vascular angiogenesis in a sex-dependent manner. Am J Physiol Heart Circ Physiol 324: H26–H32, 2023. doi: 10.1152/AJPHEART.00552.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liptzin DR, Landau LI, Taussig LM. Sex and the lung: observations, hypotheses, and future directions. Pediatr Pulmonol 50: 1159–1169, 2015. doi: 10.1002/PPUL.23178. [DOI] [PubMed] [Google Scholar]
- 11. Carey MA, Card JW, Voltz JW, Arbes SJ, Germolec DR, Korach KS, Zeldin DC. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 18: 308–313, 2007. doi: 10.1016/J.TEM.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connell MG, Corbett HJ, Purvis A, Losty PD, Jesudason EC. Sex and congenital diaphragmatic hernia. Pediatr Surg Int 22: 95–98, 2006. doi: 10.1007/S00383-005-1579-2. [DOI] [PubMed] [Google Scholar]
- 13. Reeves J, Divakar P, Ortogero N, Griswold M, Yang Z, Zimmerman S, Vitancol R, David H. GeoMxWorkflows: GeoMx Digital Spatial Profiler (DSP) Data Analysis Workflows. R Package Version 1.4.0 (Online). https://www.bioconductor.org/packages/release/workflows/html/GeoMxWorkflows.html [2023 Apr 22].
- 14. Griswold M, Danaher P. SpatialDecon: Deconvolution of Mixed Cells from Spatial and/or Bulk Gene Expression Data. R Package Version 1.8.0. (Online). https://bioconductor.org/packages/release/bioc/html/SpatialDecon.html [2023 Apr 22].
- 15. Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, Chu SG, Raby BA, DeIuliis G, Januszyk M, Duan Q, Arnett HA, Siddiqui A, Washko GR, Homer R, Yan X, Rosas IO, Kaminski N. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv 6: eaba1983, 2020. doi: 10.1126/SCIADV.ABA1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neumark N, Cosme C, Rose KA, Kaminski N. The idiopathic pulmonary fibrosis cell atlas. Am J Physiol Lung Cell Mol Physiol 319: L887–L893, 2020. doi: 10.1152/AJPLUNG.00451.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ortogero N, Yang Z, Vitancol R, Griswold M, Henderson D. GeomxTools: NanoString GeoMx Tools. R Package Version 3.2.0. (Online). https://www.bioconductor.org/packages/release/bioc/html/GeomxTools.html [2023 Apr 22].
- 18. Hänzelmann S, Castelo R, Guinney J. GSVA: Gene set variation analysis for microarray and RNA-Seq data. BMC Bioinformatics 14: 7–15, 2013. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Danaher P, Kim Y, Nelson B, Griswold M, Yang Z, Piazza E, Beechem JM. Advances in mixed cell deconvolution enable quantification of cell types in spatial transcriptomic data. Nat Commun 13: 385, 2022. doi: 10.1038/S41467-022-28020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta VS, Harting MT. Congenital diaphragmatic hernia-associated pulmonary hypertension. Semin Perinatol 44: 151167, 2020. doi: 10.1053/j.semperi.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 21. Gien J, Kinsella JP. Management of pulmonary hypertension in infants with congenital diaphragmatic hernia. J Perinatol 36: S28–S31, 2016. doi: 10.1038/jp.2016.46. [DOI] [PubMed] [Google Scholar]
- 22. Takayasu H, Nakazawa N, Montedonico S, Sugimoto K, Sato H, Puri P. Impaired alveolar epithelial cell differentiation in the hypoplastic lung in nitrofen-induced congenital diaphragmatic hernia. Pediatr Surg Int 23: 405–410, 2007. doi: 10.1007/S00383-006-1853-Y. [DOI] [PubMed] [Google Scholar]
- 23. Fox ZD, Jiang G, Ho KKY, Walker KA, Liu AP, Kunisaki SM. Fetal lung transcriptome patterns in an ex vivo compression model of diaphragmatic hernia. J Surg Res 231: 411–420, 2018. doi: 10.1016/J.JSS.2018.06.064. [DOI] [PubMed] [Google Scholar]
- 24. Cogo PE, Zimmermann LJI, Rosso F, Tormena F, Gamba P, Verlato G, Baritussio A, Carnielli VP. Surfactant synthesis and kinetics in infants with congenital diaphragmatic hernia. Am J Respir Crit Care Med 166: 154–158, 2002. doi: 10.1164/RCCM.2108028. [DOI] [PubMed] [Google Scholar]
- 25. Cogo PE, Simonato M, Danhaive O, Verlato G, Cobellis G, Savignoni F, Peca D, Baritussio A, Carnielli VP. Impaired surfactant protein B synthesis in infants with congenital diaphragmatic hernia. Eur Respir J 41: 677–682, 2013. doi: 10.1183/09031936.00032212. [DOI] [PubMed] [Google Scholar]
- 26. Farr L, Ghosh S, Moonah S. Role of MIF cytokine/CD74 receptor pathway in protecting against injury and promoting repair. Front Immunol 11: 1273, 2020. doi: 10.3389/FIMMU.2020.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marsh LM, Cakarova L, Kwapiszewska G, Von Wulffen W, Herold S, Seeger W, Lohmeyer J. Surface expression of CD74 by type II alveolar epithelial cells: a potential mechanism for macrophage migration inhibitory factor-induced epithelial repair. Am J Physiol Lung Cell Mol Physiol 296: L442–L452, 2009. doi: 10.1152/AJPLUNG.00525.2007. [DOI] [PubMed] [Google Scholar]
- 28. Le Hiress M, Tu L, Ricard N, Phan C, Thuillet R, Fadel E, Dorfmüller P, Montani D, De Man F, Humbert M, Huertas A, Guignabert C. Proinflammatory signature of the dysfunctional endothelium in pulmonary hypertension. Role of the macrophage migration inhibitory factor/CD74 complex. Am J Respir Crit Care Med 192: 983–997, 2015. doi: 10.1164/RCCM.201402-0322OC. [DOI] [PubMed] [Google Scholar]
- 29. Rodor J, Chen SH, Scanlon JP, Monteiro JP, Caudrillier A, Sweta S, Stewart KR, Shmakova A, Dobie R, Henderson BEP, Stewart K, Hadoke PWF, Southwood M, Moore SD, Upton PD, Morrell NW, Li Z, Chan SY, Handen A, Lafyatis R, de Rooij LPMH, Henderson NC, Carmeliet P, Spiroski AM, Brittan M, Baker AH. Single-cell RNA sequencing profiling of mouse endothelial cells in response to pulmonary arterial hypertension. Cardiovasc Res 118: 2519–2534, 2022. doi: 10.1093/CVR/CVAB296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gillich A, Zhang F, Farmer CG, Travaglini KJ, Tan SY, Gu M, Zhou B, Feinstein JA, Krasnow MA, Metzger RJ. Capillary cell-type specialization in the alveolus. Nature 586: 785–789, 2020. doi: 10.1038/s41586-020-2822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vila Ellis L, Cain MP, Hutchison V, Flodby P, Crandall ED, Borok Z, Zhou B, Ostrin EJ, Wythe JD, Chen J. Epithelial Vegfa specifies a distinct endothelial population in the mouse lung. Dev Cell 52: 617–630.e6, 2020. doi: 10.1016/j.devcel.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Long AM, Bunch KJ, Knight M, Kurinczuk JJ, Losty PD; BAPS-CASS. One-year outcomes of infants born with congenital diaphragmatic hernia: a national population cohort study. Arch Dis Child Fetal Neonatal Ed 104: F643–F647, 2019. doi: 10.1136/ARCHDISCHILD-2018-316396. [DOI] [PubMed] [Google Scholar]
- 33. Vieira R, Pearse R, Rankin J. Mortality factors in infants with congenital diaphragmatic hernia: a systematic review. Birth Defects Res 110: 1241–1249, 2018. doi: 10.1002/BDR2.1376. [DOI] [PubMed] [Google Scholar]
- 34. Al Baroudi S, Collaco JM, Lally PA, Harting MT, Jelin EB. Clinical features and outcomes associated with tracheostomy in congenital diaphragmatic hernia. Pediatr Pulmonol 55: 90–101, 2020. doi: 10.1002/PPUL.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Z, Wermuth PJ, Benn BS, Lisanti MP, Jimenez SA. Caveolin-1 deficiency induces spontaneous endothelial-to-mesenchymal transition in murine pulmonary endothelial cells in vitro. Am J Pathol 182: 325–331, 2013. doi: 10.1016/J.AJPATH.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Golob MJ, Wang Z, Prostrollo AJ, Hacker TA, Chesler NC. Limiting collagen turnover via collagenase-resistance attenuates right ventricular dysfunction and fibrosis in pulmonary arterial hypertension. Physiol Rep 4: e12815, 2016. doi: 10.14814/phy2.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Z, Schreier DA, Abid H, Hacker TA, Chesler NC. Pulmonary vascular collagen content, not cross-linking, contributes to right ventricular pulsatile afterload and overload in early pulmonary hypertension. J Appl Physiol (1985) 122: 253–263, 2017. doi: 10.1152/JAPPLPHYSIOL.00325.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang B, Niu W, Dong HY, Liu ML, Luo Y, Li ZC. Hypoxia induces endothelial-mesenchymal transition in pulmonary vascular remodeling. Int J Mol Med 42: 270–278, 2018. doi: 10.3892/IJMM.2018.3584/DOWNLOAD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhattacharya M. Insights from Transcriptomics: CD163+ Profibrotic Lung Macrophages in COVID-19. Am J Respir Cell Mol Biol 67: 520–527, 2022. doi: 10.1165/RCMB.2022-0107TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. BelAiba RS, Djordjevic T, Bonello S, Artunc F, Lang F, Hess J, Görlach A. The serum- and glucocorticoid-inducible kinase Sgk-1 is involved in pulmonary vascular remodeling: role in redox-sensitive regulation of tissue factor by thrombin. Circ Res 98: 828–836, 2006. doi: 10.1161/01.RES.0000210539.54861.27. [DOI] [PubMed] [Google Scholar]
- 41. Süvari L, Janér C, Helve O, Kaskinen A, Turpeinen U, Pitkänen-Argillander O, Andersson S. Postnatal gene expression of airway epithelial sodium transporters associated with birth stress in humans. Pediatr Pulmonol 54: 797–803, 2019. doi: 10.1002/PPUL.24288. [DOI] [PubMed] [Google Scholar]
- 42. Xi X, Zhang J, Wang J, Chen Y, Zhang W, Zhang X, Du J, Zhu G. SGK1 mediates hypoxic pulmonary hypertension through promoting macrophage infiltration and activation. Anal Cell Pathol (Amst) 2019: 3013765, 2019. doi: 10.1155/2019/3013765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zarrinpashneh E, Poggioli T, Sarathchandra P, Lexow J, Monassier L, Terracciano C, Lang F, Damilano F, Zhou JQ, Rosenzweig A, Rosenthal N, Santini MP. Ablation of SGK1 impairs endothelial cell migration and tube formation leading to decreased neo-angiogenesis following myocardial infarction. PLoS One 8: e80268, 2013. doi: 10.1371/JOURNAL.PONE.0080268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ranchoux B, Harvey LD, Ayon RJ, Babicheva A, Bonnet S, Chan SY, Yuan JXJ, Perez VJ. Endothelial dysfunction in pulmonary arterial hypertension: an evolving landscape (2017 Grover Conference Series). Pulm Circ 8: 2045893217752912, 2018. doi: 10.1177/2045893217752912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu Y, Almuntashiri S, Han Y, Wang X, Somanath PR, Zhang D. The roles of CCN1/CYR61 in pulmonary diseases. Int J Mol Sci 21: 7810, 2020. doi: 10.3390/IJMS21217810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morrell ED, Grazioli S, Hung C, Kajikawa O, Kosamo S, Stapleton RD, Gharib SA, Amado-Rodríguez L, Albaiceta G, Wurfel MM, Matute-Bello G. Alveolar CCN1 is associated with mechanical stretch and acute respiratory distress syndrome severity. Am J Physiol Lung Cell Mol Physiol 319: L825–L832, 2020. doi: 10.1152/AJPLUNG.00073.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grazioli S, Gil S, An D, Kajikawa O, Farnand AW, Hanson JF, Birkland T, Chen P, Duffield J, Schnapp LM, Altemeier WA, Matute-Bello G. CYR61 (CCN1) overexpression induces lung injury in mice. Am J Physiol Lung Cell Mol Physiol 308: L759–L765, 2015. doi: 10.1152/ajplung.00190.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee SJ, Zhang M, Hu K, Lin L, Zhang D, Jin Y. CCN1 suppresses pulmonary vascular smooth muscle contraction in response to hypoxia. Pulm Circ 5: 716–722, 2015. doi: 10.1086/683812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu Y, Tang BL, Lu ML, Wang HX. Astragaloside IV improves pulmonary arterial hypertension by increasing the expression of CCN1 and activating the ERK1/2 pathway. J Cell Mol Med 27: 622–633, 2023. doi: 10.1111/JCMM.17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baptista MJ, Melo-Rocha G, Pedrosa C, Gonzaga S, Teles A, Estevão-Costa J, Areias JC, Flake AW, Leite-Moreira AF, Correia-Pinto J. Antenatal vitamin A administration attenuates lung hypoplasia by interfering with early instead of late determinants of lung underdevelopment in congenital diaphragmatic hernia. J Pediatr Surg 40: 658–665, 2005. doi: 10.1016/j.jpedsurg.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 51. Gilbert RM, Gleghorn JP. Connecting clinical, environmental, and genetic factors point to an essential role for vitamin A signaling in the pathogenesis of congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol 324: L456–L467, 2023. doi: 10.1152/AJPLUNG.00349.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manicone AM, Huizar I, Mcguire JK. Matrilysin (Matrix Metalloproteinase-7) regulates anti-inflammatory and antifibrotic pulmonary dendritic cells that express CD103 (αEβ7-integrin). Am J Pathol 175: 2319–2331, 2009. doi: 10.2353/ajpath.2009.090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Unkel B, Hoegner K, Clausen BE, Lewe-Schlosser P, Bodner J, Gattenloehner S, Janßen H, Seeger W, Lohmeyer J, Herold S. Alveolar epithelial cells orchestrate DC function in murine viral pneumonia. J Clin Invest 122: 3652–3664, 2012. doi: 10.1172/JCI62139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1 and Supplemental Table S1: https://doi.org/10.6084/m9.figshare.22822355.v1.
Data Availability Statement
Data will be made available upon reasonable request.