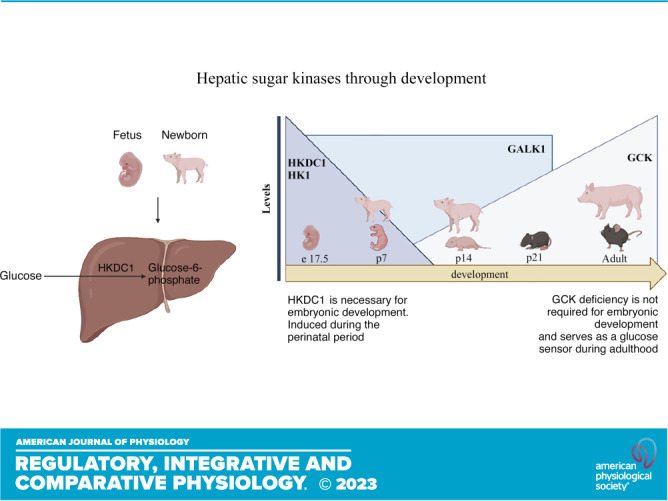
Keywords: glucose metabolism, hexokinase, HKDC1, lipid metabolism, perinatal metabolism
Abstract
During the perinatal period, unique metabolic adaptations support energetic requirements for rapid growth. To gain insight into perinatal adaptations, quantitative proteomics was performed comparing the livers of Yorkshire pigs at postnatal day 7 and adult. These data revealed differences in the metabolic control of liver function including significant changes in lipid and carbohydrate metabolic pathways. Newborn livers showed an enrichment of proteins in lipid catabolism and gluconeogenesis concomitant with elevated liver carnitine and acylcarnitines levels. Sugar kinases were some of the most dramatically differentially enriched proteins compared with neonatal and adult pigs including galactokinase 1 (Galk1), ketohexokinase (KHK), hexokinase 1 (HK1), and hexokinase 4 (GCK). Interestingly, hexokinase domain containing 1 (HKDC1), a newly identified fifth hexokinase associated with glucose disturbances in pregnant women, was highly enriched in the liver during the prenatal and perinatal periods and continuously declined throughout postnatal development in pigs and mice. These changes were confirmed via Western blot and mRNA expression. These data provide new insights into the developmental and metabolic adaptations in the liver during the transition from the perinatal period to adulthood in multiple mammalian species.
INTRODUCTION
Energy requirements in the perinatal period are high compared with other stages of life to enable optimal growth, tissue function, and other physiological requirements of the rapidly growing neonate (1). Glucose provided by maternal metabolism is the primary energetic substrate of the growing fetus during gestation (2). However, energy substrates for mammals change dramatically after birth, as newborns are fed exclusively with maternal milk or replacement formula, consisting mainly of complex proteins, high lipid content, and low carbohydrates coming from lactose (3). The metabolic adaptations to support the high energy and glucose demands during fetal and early life are regulated by multiple metabolic and hormonal signals that contribute to the metabolic differentiation of perinatal development (2).
During the postnatal period, the high glucose requirements to sustain growth are not entirely achieved through diet (4). In times of energy deficit, the liberation of fatty acids from triglycerides is mobilized from adipose stores and oxidized by mitochondria in the liver. Fatty acid oxidation sustains ATP, NADH, and acetyl-CoA production, enabling hepatic gluconeogenesis and ketogenesis (5). Gluconeogenesis is mainly active in the liver and the kidney; it aims to sustain glucose requirements for peripheral tissues while glucose levels are diminished using noncarbohydrate precursors to induce de novo glucose synthesis. This pathway is regulated in part by pyruvate carboxylase (PC) and glucose-6-phosphatase (G6P). In the liver, PC catalyzes the irreversible carboxylation of pyruvate to oxaloacetate, whereas G6P catalyzes the terminal step of glycogenolysis and gluconeogenesis. It has been described that gluconeogenesis capacity increases during late fetal development with a robust increase after birth due to hormonal signals (2). Transcription factors and genes encoding gluconeogenetic enzymes are expressed at a specific period during development to facilitate this transition (6).
In the fed state, for glucose to be utilized by cells, it needs to be transported across the cell membrane and then phosphorylated by sugar kinases; this step traps monosaccharides in cells and facilitates their metabolic engagement. Hexokinases (HK) catalyze phosphorylation of glucose to glucose-6-phosphate (7). There are four well-characterized mammalian hexokinases: HK1, HK2, HK3, and HK4, also known as glucokinase (GCK). HK1, HK2, and HK3 phosphorylate glucose and other related hexoses on their six carbon; these enzymes exhibit a low Km for glucose, and they are sensitive to feedback inhibition by glucose-6-phosphate (8). However, GCK phosphorylates glucose and has specific expression in the liver, some hypothalamic neurons, and β and α cells of the pancreas (9); it exhibits a higher Km for glucose and is not feedback inhibited by glucose-6-phosphate, which makes it a critical component of how these cells sense glucose (10). Once glucose is phosphorylated, glucose-6-phosphate could then be used by glycolysis, glycogen synthesis, the pentose phosphate pathway, etc. (11). After birth, galactokinase (Galk1) also contributes to energy homeostasis by utilizing galactose coming from milk (12). Galk1 is one of four enzymes of the Leloir pathway, which aims to convert galactose to galactose 1-phosphate (13, 14). Galactose results from intestinal hydrolysis of the disaccharide lactose and Galk1 catalyzes the phosphorylation of galactose to form galactose-1-phosphate, which can be converted to uridine diphosphate glucose and then synthesize glucose (15).
Recently, the gene encoding HKDC1 was identified as a novel fifth hexokinase expressed widely in human and mouse tissues, such as the small intestine, liver, lung, kidney, and brain (16). HKDC1 exhibits hexokinase activity and a lower Km than GCK (17, 18) and is expressed to a minimal degree in hepatocytes under normal conditions (19). Higher levels of HKDC1 have been detected in diverse metabolic diseases such as gestational diabetes mellitus, nonalcoholic steatohepatitis, and cancer (19–21). A genome-wide association study detected an association in the first intron of HKDC1 with gestational diabetes in 28-wk pregnant humans with glucose disturbances in a 2-h glucose tolerance test (20). Maternal hyperglycemia during pregnancy can adversely affect the growing fetus and increase the risk of type 2 diabetes and obesity in the offspring (17). In mice, interventions showed that HKDC1 overexpression during pregnancy (day 18) increased whole body glucose tolerance and insulin sensitivity (17). Also, HKDC1 liver-specific knockout mice showed a worse glucose tolerance and no changes in insulin sensitivity (22). Moreover, experiments in multiple liver cancer cell lines have demonstrated that HKDC1 ablation disturbs mitochondrial function and substrates flux into the TCA cycle (19). At the same time, acute in vivo overexpression of HKDC1 in the liver also produced mitochondrial dysfunction (21).
These data suggest the role of HKDC1 in regulating glucose metabolism and contribution to mitochondrial function in metabolic diseases; however, little is known about the metabolic effects of HKDC1 on perinatal development. Multiple genes and transcription factors modulate energetic pathways and offer flexibility under disparate physiological states (8). Thus, this work aims to understand the metabolic adaptations that enable postnatal liver growth and development.
EXPERIMENTAL PROCEDURES
Animals
Perinatal pig livers were kindly provided by Ray Koehler, Johns Hopkins University School of Medicine. Yorkshire pigs in postnatal 7-days old (p7; n = 6, 1 male, 5 females) and 14-days old (p14; n = 5, 5 females) were supplemented after birth with milk formula (Advanced birthright nutrition, Inc., Ralco). Adult Yorkshire pig livers (n = 6, 3 males, 3 females) were purchased from BioIVT (Hicksville, NY).
C57bl/6J male mice in prenatal 17.5 days (e17.5), 1-day-old postnatal (p1), 2-days-old (p2), 7-days-old (p7), 14-days-old (p14), 21-days-old (p21), and 9-wk-old mice (adults) were housed in ventilated racks with a 14-h/10-h light/dark cycle and fed a standard chow diet. All animals were euthanized in the fed state. We performed the procedures following the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the consent of the Johns Hopkins Medical School Animal Care and Use Committee.
Proteomics
Liver protein was extracted from the liver with equal masses using radioimmunoprecipitation assay buffer with protease inhibitors. Next, liver homogenates were submitted to the Johns Hopkins Center for Proteomics Core for complete proteome analysis using traditional mass spectrometry (MS)-based approaches to identify and quantify proteins.
TCA/acetone precipitation.
Proteins (50 μg) were reduced with 50 mM dithiothreitol in 10 mM triethylammonium bicarbonate (TEAB) at 60°C for 45 min. Then, 100 mM iodoacetamide in 10 mM TEAB was added to the samples and kept in the dark at room temperature for 15 min. Proteins (50 μg) were precipitated by adding eight volumes of 10% trichloroacetic acid in cold acetone at –20°C for 2 h to remove the MS interfering reagents. The pellet was centrifuged at 4°C at 16,000 g for 10 min, and the TCA/acetone supernatant was removed. Then, the pellet was washed with eight volumes of acetone for 10 min at −20°C previous to centrifuging at 16,000 g for 10 min at 4°C. The acetone supernatant was removed from the protein pellet.
Isobaric mass tag labeling.
TEAB (100 μL, 100 mM) with 5 μg trypsin/Lys-C was used to resuspend and digest the 10 pellets (50 μg each); these were kept at 37°C overnight. Tandem Mass Tag (TMT) 10-plex reagent (Thermo Fisher, Lot No. TJ268848) was used to label each sample, according to the manufacturer’s instructions. Afterward, the 10 TMT-labeled peptides were mixed and dried by vacuum centrifugation.
Peptide fractionation.
TEAB buffer (100 µL, 200 mM) was used to reconstitute the 500 μg of TMT-labeled peptides. The samples were then filtered through Pierce detergent removal columns (Fisher Scientific PN 87777) to eliminate the excess lipids, small molecules, and TMT label. Peptides in the flow-through were diluted in 2 mL in 10 mM TEAB in water and then loaded on an XBridge C18 guard column (5 µm, 2.1 × 10 mm, Waters) at 250 µL/min for 8 min. Peptides in the flow-through were then diluted to 2 mL in 10 mM TEAB in water and loaded on an XBridge C18 guard column (5 µm, 2.1 × 10 mm, Waters) at 250 µL/min for 8 min. Subsequently, a 0%–90% acetonitrile in 10 mM TEAB gradient was used for fractionation on an XBridge C18 column [5 µm, 2.1 × 100 mm column (Waters)] over 85 min at 250 µL/min on an Agilent 1200 series capillary HPLC with a microfraction collector. Twenty-four fractions were linked together from 84- to 250-μL fractions according to Wang et al. (23) and then dried.
Mass spectrometry analysis.
An Orbitrap-Fusion Lumos (Thermo Fisher Scientific) interfaced with an Easy-nLC1100 UPLC by reversed-phase chromatography was used to analyze the peptides in each of the 24 fractions. A 2%–90% acetonitrile in 0.1% formic acid gradient was used for 110 min at 300 nL/min on an in-house packed 75 µm × 150 mm ReproSIL-Pur-120-C18-AQ column 3 µm, 120 Å (Dr. Albin Maisch, Germany). Eluting peptides were sprayed through a 1-µm emitter tip (New Objective) at 2.4 kV into the mass spectrometer. Then, survey scans (MS) of precursor ions were obtained from 350 to 1,400 mass-to-charge ratio (m/z) at 120,000 resolution at 200 m/z. Data-dependent monitoring and 15 s dynamic exclusion were utilized to isolate precursor ions within 0.7 m/z. Afterward, the ions were fragmented using higher-energy collisional dissociation activation collision energy 35. The 1e5 automatic gain control (AGC), 250 ms maximum injection time (IT) at 50,000 resolution, was used to acquire the fragmentation spectra (MS/MS).
Data analysis.
The Proteome Discoverer v2.4 (PD2.4, Thermo Fisher Scientific) was used to process the fragmentation spectra, and Mascot v.2.8.0 (Matrix Science, London, UK) was utilized to search against the RefSeq Sus scrofa database. The search considered various criteria, such as 3 ppm precursor mass tolerance, one missed cleavage, trypsin enzyme, 0.01 Da fragment mass tolerance, with TMT 6Plex on NH2-terminus and carbamidomethylation on C as fixed and TMT 6Plex on K, oxidation on M, deamidation on N or Q as variable modifications. Mascot searches recognized peptides processed with PD2.4 utilizing percolator at a 5% false discovery rate confidence threshold, based on an auto-concatenated decoy database search. The isolation interference <30% filtered the peptide spectral matches (PSMs). Afterward, the relative protein abundances of recognized proteins were identified in PD2.4 from the normalized median ratio of TMT reporter ions, which have a signal-to-noise ratio > 1.5, in all PSMs from the same protein. In the mass spectrometry analysis, the technical variation in ratios is less than 10% (24).
Real-Time qPCR
Total liver RNA was isolated from frozen tissues using the RNeasy Mini Kit (QIAGEN). cDNA was synthesized from 1 to 2 μg of total RNA with the high-capacity cDNA reverse transcription kit (Applied Biosystems). The cDNA was diluted to 2 ng/μL and amplified by primers in a 20-μL reaction using SsoAdvanced SYBER green supermix (Bio-Rad). The analysis was made using the CFX Connect real-time system (Bio-Rad). B2M, B actin, and 18S were used as housekeeping genes. The sequences of primers utilized in the qPCR are shown in Table 1. Results were obtained by the relative standard curve method.
Table 1.
Mice and pigs primers for qPCR
| Gene | Species | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|---|
| G6p | Pig | TGTCCTCTTCCCCATCTGGT | CTTGAGGCTGGCGTTGTAGA |
| Pc | Pig | TACGTCGCCCACAACTTCAG | GAAGCGCATCGCAACATCAA |
| Pck1 | Pig | TTGAGGGCATCATCTTCGGA | GCGAAGGGGTCGTGCATAAT |
| Hk1 | Pig | CCTTGGATCTTGGCGGTTCT | CACTGCCATGCATGATGCTC |
| Gck | Pig | GAGATGAGCAGGTACGGAGC | GCAGTGCAGACATGCAACAA |
| Galk1 | Pig | GAGCACACGGACTACAACCA | GTCACAAGCTCCAGAGCCAT |
| Hkdc1 | Pig | GGTCCCTGAATCCTGGCAAA | AAATCTGCCCACGGAAGAGG |
| Pkl | Pig | CTGTGGCTGCTCGTAGTACC | CGTGGGAGAAGTTGAGTCGT |
| B2M | Pig | GTCACCAACTGGGACGACAT | GCAGGTCCCGAGAGAATGAG |
| 18S | Pig | GACGTGACTGCTCGGTGC | AACTCGACCGAGGGCACAA |
| Hk1 | Mouse | CACCGGCAGATTGAGGAAAC | CTCAGCCCCATTTCCATCTCT |
| Gck | Mouse | CTTGGCTGCCTGTCTTTTGC | AGTCCCACGATGTTGTTCCC |
| Galk1 | Mouse | GAGCACACGGACTACAACCAG | GCTCGTCTGCATCTTTGGAAG |
| Hkdc1 | Mouse | ATTCGTCAGGGCCATTCCAG | CACCACGTCTGTGTCCTGAA |
| B2M | Mouse | GACCGGCCTGTATGCTATCC | TCAGTCTCAGTGGGGGTGAA |
| 18S | Mouse | GCAATTATTCCCCATGAACG | GGCCTCACTAAACCATCCAA |
| B actin | Mouse | TATAAAACCCGGCGGCGCA | ATGGCTACGTACATGGCTGG |
| Mtco1 | Mouse | GTCTGATCCGTACTTATTACAG | GCTCATACTATTCCTATATAGCCG |
| Ndufb8 | Mouse | CATCTCTTCGGCTTTGTGGC | CAAAAAGCCCATCAAGCCTCC |
| Uqcrc2 | Mouse | GGCTTGTTCGTTAAAGCAGGCAGT | TGCCTTCTACAGTGTACGCCATGT |
| Atp5a | Mouse | GCTGAGGAATGTTCAAGCAGA | CCAAGTTCAGGGACATACCC |
Acylcarnitine Quantification
Standard methodology (25) was used to measure acylcarnitines in the liver of pigs. Sixty microliters of 3 mol·L−1 HCl in n-butanol were added to extracted samples; they were incubated for 15 min at 65°C, afterward dried under liquid nitrogen. Butylated acylcarnitines were reconstituted in 100 μL of mobile phase acetonitrile/water/formic acid (H2O/CH3CN:/HCOOH, 80:19.9:0.1 vol/vol%). Samples were vortexed, transferred to a centrifuge filter, spun, and transferred to an injection vial. Acylcarnitines were analyzed on an API 3200 (A B Sciex), operated in positive ion mode using a precursor ion scan for m/z 85, which was generated as a characteristic of a butyl ester of acylcarnitine species. The Chemoview (A B Sciex) application achieved the quantification of acylcarnitines. Metabolite concentrations were normalized to protein content.
Western Blot
Liver homogenates were extracted using RIPA buffer with protease inhibitors. The protein concentration was analyzed using the Pierce BCA protein assay kit (Thermo Scientific). Protein lysates (30 μg input) were used on an SDS-PAGE (10%–12% polyacrylamide gels) and then transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked in 5% nonfat milk-TBST (Tris-buffered saline with Tween 20) for 1 h at room temperature and incubated overnight with the primary antibodies at 1:1,000 in 3% BSA in TBST. The membranes were probed with anti-biotin-peroxidase antibody (Sigma, A4541); HK1, Invitrogen, Thermo Fisher Scientific, PA5-87969; GCK, Invitrogen, Thermo Fisher Scientific, PA5-95482; and HKDC1, Invitrogen, Thermo Fisher Scientific, PA5-35894. Heat shock chaperone 70 (7298, Santa Cruz Biotechnology) was utilized at 1:1,000 as a loading control. All the primary antibodies were used with the corresponding secondary antibodies conjugated to horseradish peroxidase (anti-rabbit or anti-mouse) or fluorescence-based (Cy3-conjugated anti-mouse or Cy5-conjugated anti-rabbit; Invitrogen, Thermo Fisher Scientific). All secondaries were incubated for 2 h at room temperature, utilized in 5% milk diluted at 1:1,000. Images were collected using the Amersham Prime enhanced chemiluminescent substrate (CYTIVA) with an Alpha Innotech FluorChem Q and presented with minimal image processing.
GO Term Analysis
Gene ontology (GO) term analysis was conducted to compare protein expression in 7-day-old and adult pigs using DAVID bioinformatics resources. Differentially expressed proteins (>1.5-fold change) that were significantly changed and enriched in both 7-day-old and adult pig livers were analyzed.
Statistical Analysis
Graphpad Prism version 9 was used to perform the statistical analyses. Significance was determined using one-way ANOVA with Bartlett’s post hoc correction. In all cases, P value < 0.05 was considered statistically significant.
RESULTS
Comparative Proteomics Reveals Metabolic Adaptations in Newborn Pigs
The perinatal period requires unique metabolic adaptations to enable the utilization of new substrates while maintaining rapid growth. To gain a better understanding of unique newborn adaptations, we performed quantitative tandem mass tag mass-spectrometry proteomics on the liver of postnatal day 7 piglets and adult pigs. A volcano plot shows differentially expressed proteins between the neonatal and adult period (Fig. 1A). Our analysis demonstrated 2,470 significantly enriched proteins in the newborn pig liver and 641 significantly enriched proteins in the adult pig liver. To gain further insight in the newborn and adult liver proteome, we analyzed the significantly changed proteins (>1.5-fold) to determine the enriched gene ontology (GO) terms. Notably, the GO terms “Ribosome” and “mRNA processing” were highly enriched in the newborn piglet along with other metabolic terms (Fig. 1B). Perhaps not surprisingly, the piglet enriches for genes associated with rapid growth and tissue expansion. In the adult pig liver, enriched GO terms related with lipid and cholesterol synthesis and metabolism were identified (Fig. 1C), such as “Lipid/Fatty metabolism.” These data suggest that the adult pig is relying more on fatty acid metabolism that progressively develops in the liver to adapt to the higher oxygen tension after birth (26). Altogether, this rich data set demonstrates that the proteomic adaptations in the liver at these developmental stages are dominated by their unique metabolic demands.
Figure 1.
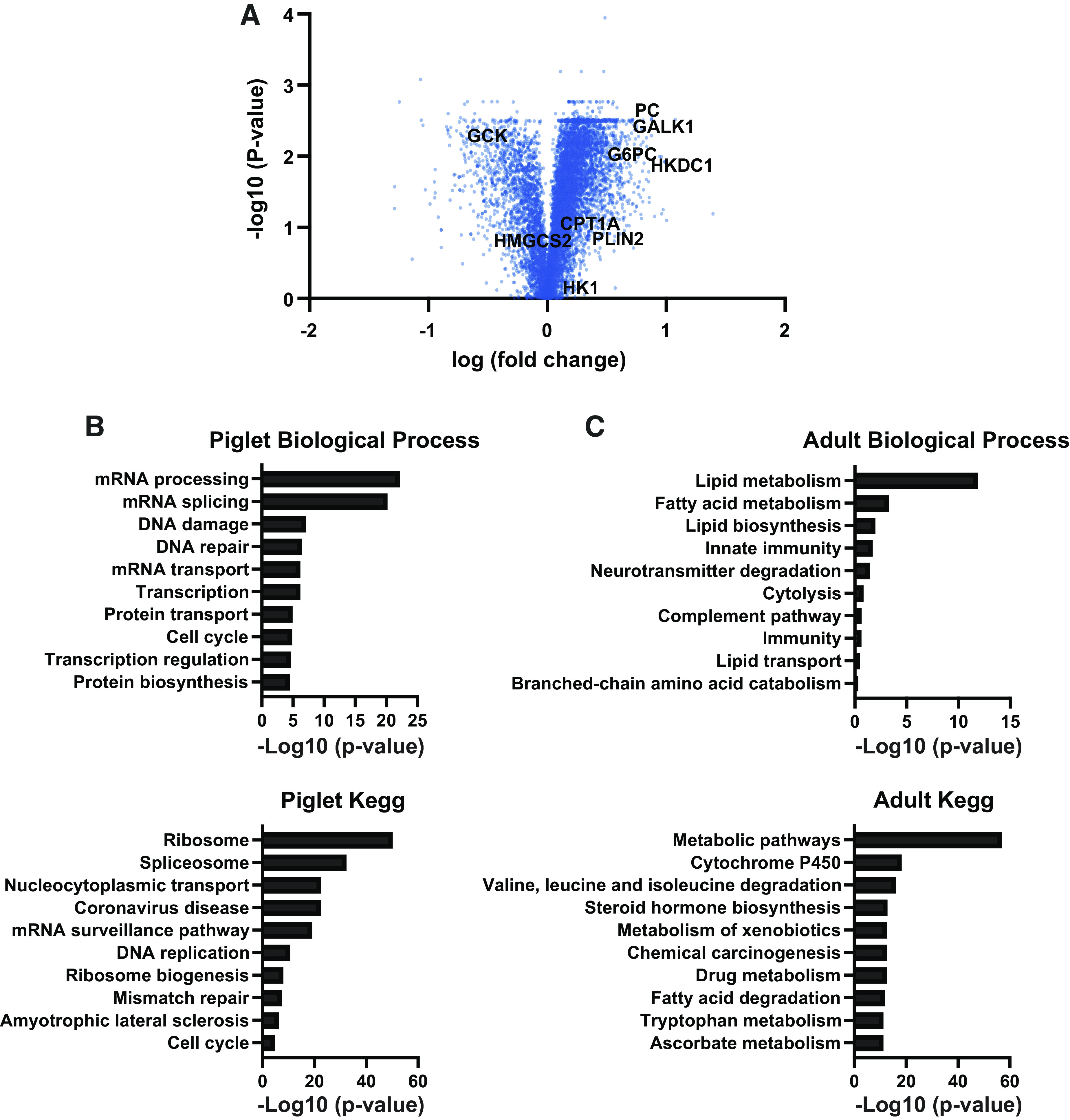
Quantitative proteomics for piglet and adult pig liver. A: volcano plot showing magnitude and significance for p7 pigs and adult liver proteome, measured by TMT-based quantitative spectrometry (n = 5). B: gene ontology for genes enriched in piglets as determined by quantitative proteomics. C: gene ontology for genes enriched in adult pigs as determined by quantitative proteomics.
Differential Lipid and Carbohydrate Metabolism in the Newborn and Adult Pig Liver
Newborns consume a high lipid and low/unique carbohydrate diet provided by maternal milk. In this period of life, the energetic requirements are significantly higher to support growth, and the utilization of lipid substrates coming from milk meets this demand. To better understand the unique requirements for fatty acid utilization during the perinatal period, we mined the proteomics data for changes in lipid-related proteins. The rate-limiting step in fatty acid oxidation, carnitine palmitoyltransferase 1a, was not significantly changed, but the peroxisomal carnitine octanoyltransferase (2.98-fold) was highly induced in the piglet liver (Table 2). The acyl-CoA dehydrogenase family were all significantly changed (acyl-CoA dehydrogenase very long chain 1.46-fold; acyl-CoA dehydrogenase long chain 0.38-fold; acyl-CoA dehydrogenase medium chain 0.49-fold; acyl-CoA dehydrogenase short chain 0.5-fold). Perilipin 4 (8.9-fold) is one of the most highly enriched proteins in the adult liver, whereas perilipin 5 (1.65-fold) was significantly induced in the piglet liver. Perilipin 5 has been shown to bridge lipid droplets and mitochondria to promote lipid oxidation (27). These data reveal a complex regulation of critical lipid oxidation mediators to facilitate lipid catabolism during the perinatal period.
Table 2.
Lipid metabolism proteins detected in proteomics analysis in the liver of p7 and adults pigs
| Proteomics Sus scrofa |
||||||
|---|---|---|---|---|---|---|
| Description | Gene symbol | Accession | Abundance ratio: (piglet)/(pig) | Abundance ratio adj. P value: (piglet)/(pig) | Log | Log10 |
| Carnitine O-palmitoyltransferase 1, liver isoform | CPT1A | NP_001123277.1 | 1.13 | 0.11 | 0.05 | 0.96 |
| Carnitine O-palmitoyltransferase 2, mitochondrial precursor | CPT2 | NP_001233172.1 | 0.61 | 0.07 | −0.21 | 1.18 |
| Perilipin-2 | PLIN2 | NP_999365.1 | 2.09 | 0.14 | 0.32 | 0.87 |
| Acyl-coenzyme A thioesterase 12 isoform X1 | ACOT12 | XP_003480920.2 | 0.90 | 0.77 | −0.04 | 0.12 |
| Pyruvate dehydrogenase kinase, isozyme 4 | PDK4 | NP_001152778.1 | 1.86 | 0.01 | 0.27 | 1.93 |
| Fatty acid-binding protein, heart | FABP3 | NP_001093401.1 | 0.34 | 0.02 | −0.46 | 1.71 |
| Hydroxymethylglutaryl-CoA synthase, mitochondrial precursor | HMGCS2 | NP_999545.1 | 0.31 | 0.14 | −0.51 | 0.84 |
| Hydroxymethylglutaryl-CoA synthase, cytoplasmic isoform X2 | HMGCS1 | XP_005657669.2 | 0.57 | 0.81 | −0.25 | 0.09 |
| Perilipin-1 | PLIN1 | NP_001033727.1 | 0.32 | 0.01 | −0.50 | 2.26 |
| Perilipin-3 isoform X1 | PLIN3 | XP_020937666.1 | 1.18 | 0.40 | 0.07 | 0.39 |
| Perilipin-4 isoform X3 | PLIN4 | XP_020939716.1 | 0.11 | 0.02 | −0.95 | 1.64 |
| Perilipin-5 | PLIN5 | NP_001116607.1 | 1.66 | 0.01 | 0.22 | 2.24 |
| Peroxisomal carnitine O-octanoyltransferase | CROT | NP_001230146.1 | 2.98 | 0.01 | 0.47 | 1.91 |
| Short-chain specific acyl-CoA dehydrogenase, mitochondrial isoform X1 | ACADS | XP_005670765.1 | 0.66 | 0.02 | −0.18 | 1.75 |
| Medium-chain specific acyl-CoA dehydrogenase, mitochondrial precursor | ACADM | NP_999204.1 | 0.49 | 0.03 | −0.31 | 1.51 |
| Long-chain specific acyl-CoA dehydrogenase, mitochondrial precursor | ACADL | NP_999062.1 | 0.38 | 0.005 | −0.42 | 2.31 |
| Very long-chain specific acyl-CoA dehydrogenase, mitochondrial isoform X1 | ACADVL | XP_020923466.1 | 1.46 | 0.02 | 0.16 | 1.65 |
To gain insight into how fatty acid substrates are utilized in the postnatal period in these animals, we measured short, medium, and long chain acylcarnitines in the livers of p7 piglets and adult pigs (Fig. 2). Piglet livers showed high concentrations of carnitine (Fig. 2A) and short-chain (Fig. 2B), medium-chain (Fig. 2C), and long-chain acylcarnitines (Fig. 2D) compared with the adult liver, suggesting that piglets rely heavily on mitochondrial fatty acid β-oxidation during this period of life. High acylcarnitine concentrations are consistent with the increased demand for gluconeogenesis and ketogenesis during this period, as hepatic fatty acid β-oxidation is required to facilitate gluconeogenesis and ketogenesis during the perinatal period.
Figure 2.
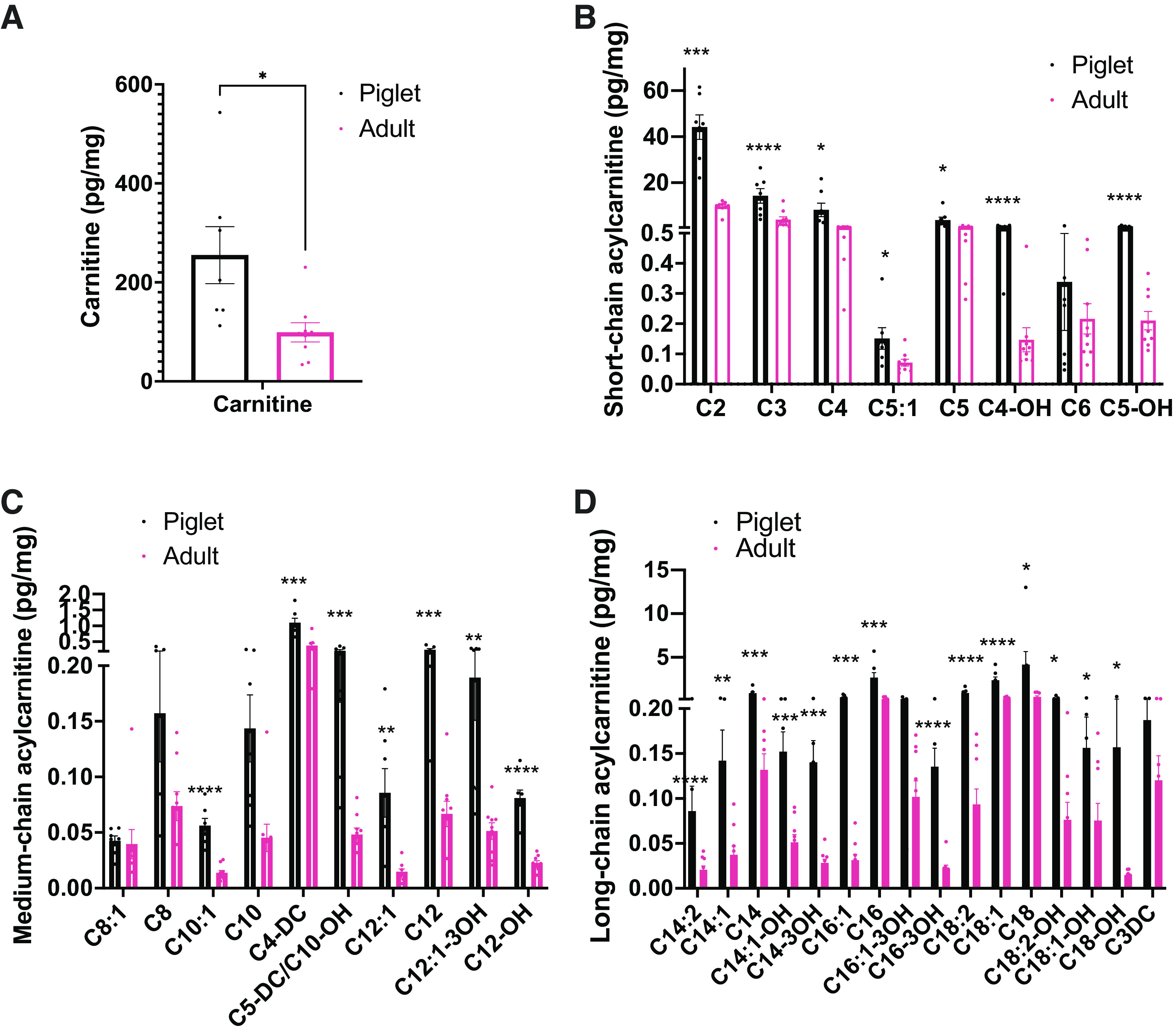
Free carnitine, short, medium, and long-chain acylcarnitines levels are higher in the piglet liver than the adult pig. Serum levels of free carnitine (A), short-chain acylcarnitines (B), medium-chain acylcarnitines (C), and long-chain acylcarnitines (D) from piglets and adult pigs determined by median-scaled untargeted metabolomics. (n = 6) All bars represent means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
As glucose requirements are not entirely accomplished by diet during the postnatal period, we decided to perform an analysis of the proteins that regulate endogenous glucose production through the gluconeogenesis pathway in neonate and adult pig livers. The proteomics data showed the rate-limiting gluconeogenic proteins pyruvate carboxylase (PC), cytosolic phosphoenolpyruvate carboxykinase (PCK1), and glucose-6-phosphatase (G6PC) significantly upregulated two- to fivefold in the 7-day-old pig liver (Table 3). To confirm this, we measured the expression of PC in the livers by Western blotting. This data demonstrated that biotinylated PC (Fig. 3A) in the liver was higher in piglets than in adult pigs. However, Pcx mRNA relative expression did not show differences between groups (Fig. 3B). Consistent with the proteomics data, mRNA relative expression of gluconeogenic genes G6pc and Pck1 exhibited higher levels in newborns (Fig. 3, C and D), indicating a greater requirement for the gluconeogenesis pathway in neonates. Pyruvate kinase (Pkl) mRNA relative levels appeared to be diminished in the newborn and induced in the p14 and adult groups (Fig. 3E), suggesting a differential role of this glycolytic enzyme during development.
Table 3.
Gluconeogenic proteins detected in proteomics analysis in the liver of p7 and adults pigs
| Proteomics Sus scrofa |
||||||
|---|---|---|---|---|---|---|
| Description | Gene symbol | Accession | Abundance ratio: (piglet)/(pig) | Abundance ratio adj. P value: (piglet)/(pig) | Log | Log10 |
| Pyruvate carboxylase, mitochondrial | PC | NP_999514.1 | 5.04 | 0.003 | 0.70 | 2.47 |
| Phosphoenolpyruvate carboxykinase, cytosolic (GTP) | PCK1 | NP_001116630.1 | 2.20 | 0.06 | 0.34 | 1.22 |
| Glucose-6-phosphatase | G6PC | NP_001106916.1 | 2.81 | 0.01 | 0.45 | 2.06 |
| Phosphoenolpyruvate carboxykinase (GTP), mitochondrial | PCK2 | NP_001155225.1 | 0.77 | 0.26 | −0.11 | 0.58 |
| Pyruvate kinase PKLR isoform X2 | PKLR | XP_020945381.1 | 0.77 | 0.08 | −0.11 | 1.11 |
Figure 3.
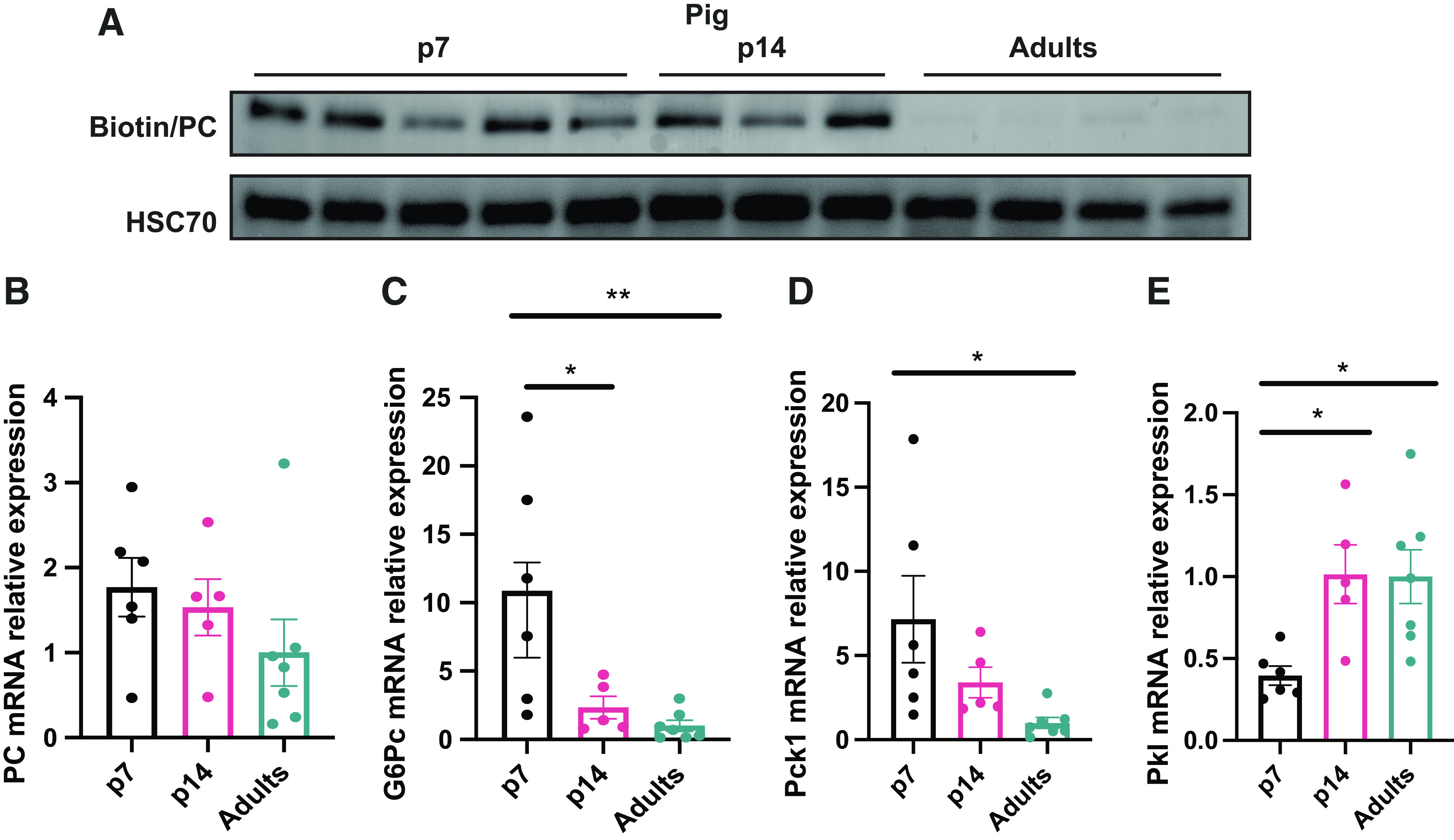
Piglets exhibit an enhanced gluconeogenesis program. A: antibiotin/HSC70 Western blot. B: relative mRNA levels in the liver of Pcx. G6Pc (C), Pck1 (D), Pkl (E) by quantitative RT-qPCR. (n = 6) All bars represent means ± SE. *P < 0.05, **P < 0.01 vs. p7.
GALK1 is Restricted to Early Postnatal Life in Mice and Pigs
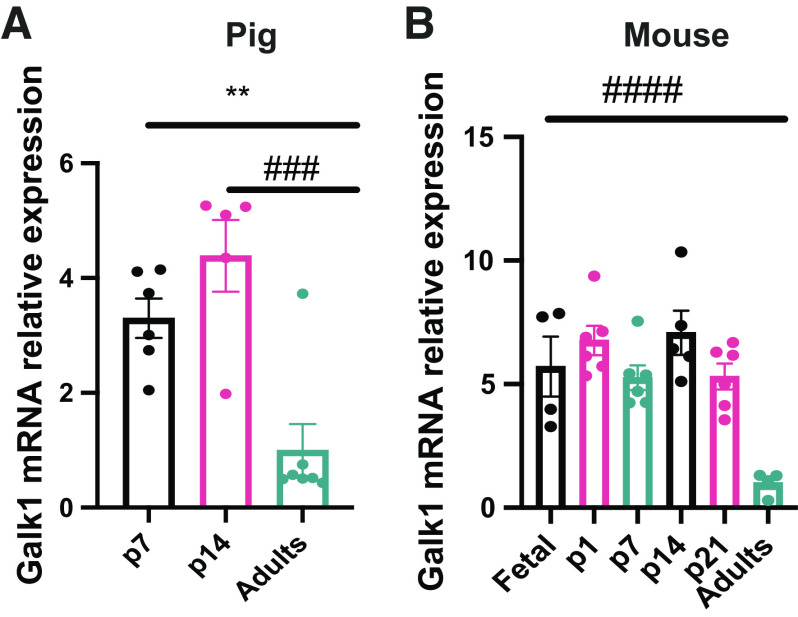
The proteomics data demonstrated that the sugar kinases are some of the most dramatically induced and suppressed proteins comparing neonatal and adult pigs (Table 4). Galactokinase (GALK1) was one of the most enriched proteins in the neonatal liver, whereas ketohexokinase was one of the most enriched proteins in the adult liver (Table 4). Galactose results from lactose hydrolysis and is one of the main carbohydrates coming from milk during early life (28). GALK1 is the main enzyme involved in the phosphorylation of galactose to galactose-1-phosphate and subsequent entry into glycolysis (13). GALK1 was highly differentially expressed in piglet liver compared with adult liver via proteomics analysis (Table 4). As milk is a primary energy source during the neonatal period, we decided to measure Galk1 mRNA expression in newborns and adult pigs. As expected, Galk1 expression is elevated in p7 and p14 piglets compared with adult liver, consistent with the proteomics data (Fig. 4A). This suggests that GALK1 is induced during the neonatal period to respond to lactose coming from diet and its activity is reduced during postnatal development as milk becomes relied upon less. As the data in pigs showed differential Galk1 expression through development, we decided to analyze its levels in mice to gain a better understanding of its expression through development. Interestingly, the results demonstrated that Galk1 expression is induced before birth. Galk1 is expressed in embryonic day 17.5 liver and that expression is preserved until at least p21 in mice (Fig. 4B). Galk1 expression was not found in adult mice. It has been shown that galactose provided by milk does not sustain glucose requirements entirely during the neonatal period (4); thus, other energetic substrates and metabolic pathways are required to maintain energy needs to sustain rapid growth.
Table 4.
Glucose kinases detected in proteomics analysis in the liver of p7 and adults pigs
| Proteomics Sus scrofa |
||||||
|---|---|---|---|---|---|---|
| Description | Gene symbol | Accession | Abundance ratio: (piglet)/(pig) | Abundance ratio adj. P value: (piglet)/(pig) | Log | Log10 |
| Galactokinase | GALK1 | XP_003131241.1 | 4.601 | 0.004 | 0.663 | 2.451 |
| Ketohexokinase isoform X4 | KHK | XP_003125350.1 | 0.146 | 0.003 | −0.836 | 2.599 |
| Hexokinase-1 | HK1 | NP_001230113.1 | 1.183 | 0.654 | 0.073 | 0.184 |
| Hexokinase-3 isoform X1 | HK3 | XP_003123703.1 | 1.844 | 0.052 | 0.266 | 1.282 |
| Glucokinase isoform X1 | GCK | XP_003134931.2 | 0.186 | 0.005 | −0.730 | 2.318 |
| Putative hexokinase HKDC1 | HKDC1 | XP_001928917.1 | 6.511 | 0.013 | 0.814 | 1.902 |
Figure 4.
GALK1 showed differential levels through development in pigs and mice. Galk1 mRNA relative levels in the liver by quantitative RT-qPCR in pigs (A) and in mice (B). (n = 4–6) All bars represent means ± SE. **P < 0.01 vs. p7. ###P < 0.001, ####P < 0.0001 vs. adults. GALK1, galactokinase.
HK1 and GCK Showed Differential Expression through Liver Development in Mice and Pigs
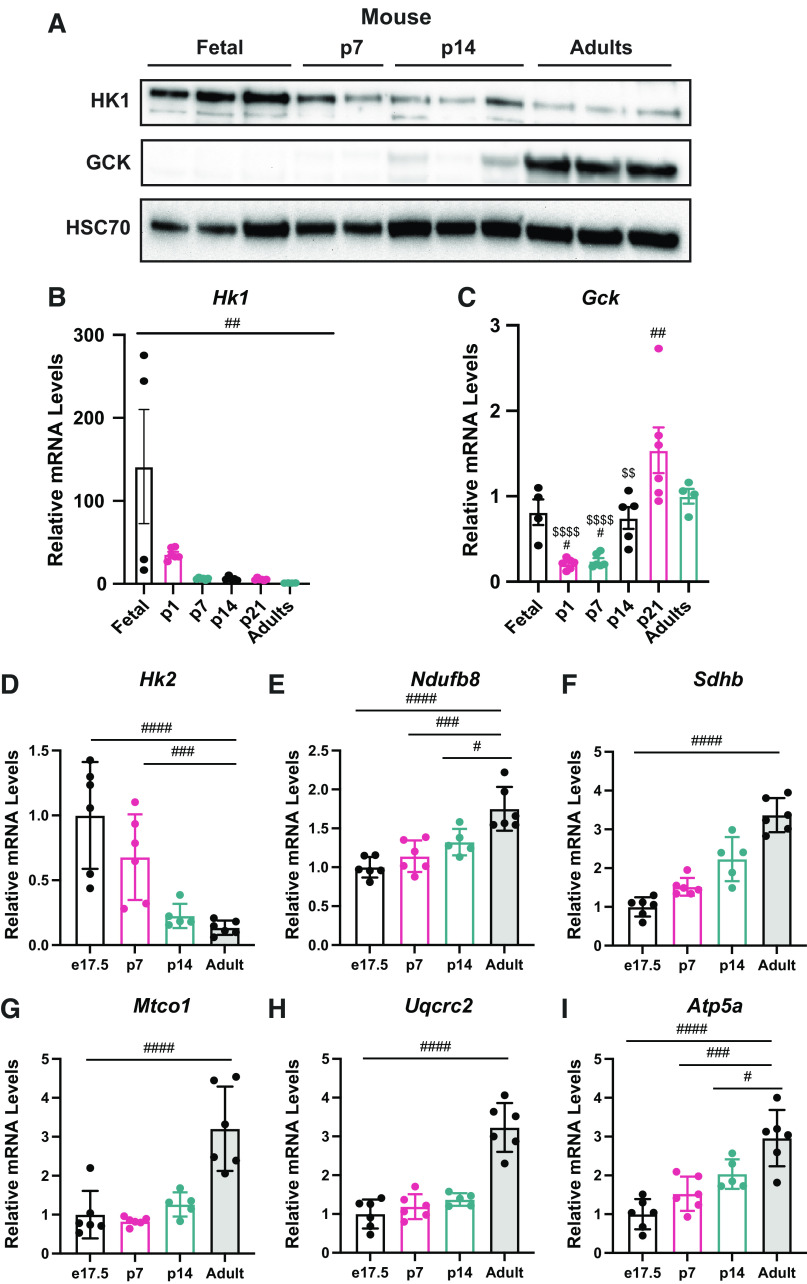
As glucose phosphorylation plays a critical role in glucose metabolism and the proteomic data showed differential levels of sugar kinases in the newborn liver, we analyzed HK1 and GCK expression to understand their contribution in different stages of life (Table 4). To gain understanding about HK1 and GCK levels in the fetal period and through development in other mammalian species, we analyzed the livers of e17.5, p7, p14, p21, and adult mice. Western blotting (Fig. 5A) and mRNA relative levels (Fig. 5B) showed that HK1 levels are highly induced in e17.5 fetus, and it drops considerably after birth in p7 mice. GCK showed an opposite pattern of expression, where GCK protein levels (Fig. 5A) and mRNA relative levels (Fig. 5C) appear diminished in the mouse fetal and neonatal period and showed a higher induction in the adult mice. HK2 showed a similar suppression until adulthood (Fig. 5D). In addition, expression of mitochondrial markers of Complex I, II, III, IV, and V were all increased throughout postnatal development (Fig. 5, E–I). These data indicate the changing role of the liver from a period of growth to an adult tissue that uses GCK to sense systemic glucose levels.
Figure 5.
Glucose kinases showed differential levels through development in mice. A: Western blots for HK1, GCK, and HSC70 in the liver of mice. B: Hk1 mRNA relative levels in the liver by quantitative RT-qPCR in mice. C: Gck mRNA relative levels in the liver by quantitative RT-qPCR in mice. Expression of Hk2 (D), Ndufb8 (E), Sdhb (F), Mtco1 (G), Uqcrc2 (H), and Atp5a (I) in mouse liver (n = 4–6). All bars represent means ± SE. #P < 0.05, ##P < 0.01, ### P < 0.001, ####P < 0.0001. GCK, glucokinase; HK1, hexokinase 1.
Hepatic HKDC1 Levels Are Enriched in the Perinatal Period in Pigs and Mice
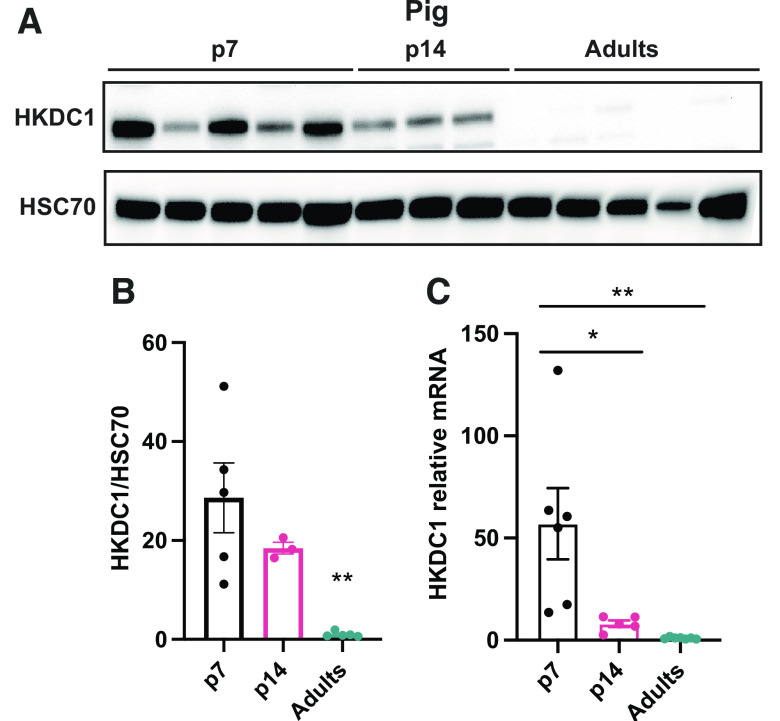
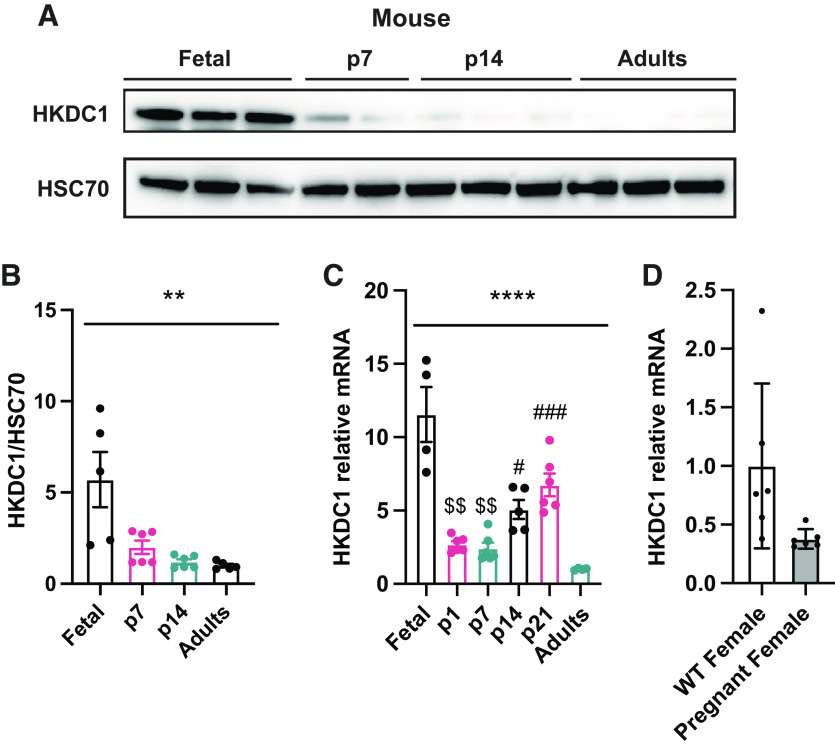
The proteomics data demonstrated that the sugar kinases are some of the most dramatically induced and suppressed proteins comparing neonatal and adult pigs (Table 4). A relatively newly discovered and somewhat enigmatic hexokinase, hexokinase domain containing 1 (HKDC1) was one of the most dramatically enriched proteins in the piglet liver. Recently published data has shown that HKDC1 is induced during pregnancy in adult female mice to regulate glucose metabolism and improve insulin sensibility (17). As mentioned, the fetal and neonatal periods are stages where metabolic adaptations change dramatically depending on the anabolic or catabolic demand. Proteomics analysis showed that HKDC1 is significantly induced in the p7 piglets in comparison with the adult group (Table 4); thus, we decided to confirm HKDC1 protein levels by Western blot and mRNA relative expression in the pig liver to corroborate this data. HKDC1 expression and protein levels are highly induced in p7 piglets, and their levels drop considerably during the second week of life to be almost absent during the adult period (Fig. 6, A–C). To understand if HKDC1 was induced after birth or before the perinatal period, and to verify if these data were replicable in other mammal species, we analyzed hepatic HKDC1 expression and protein levels in the fetus (e17.5), neonates (p1, p2, p7, p14), and adult mice. Our data demonstrate that HKDC1 protein levels (Fig. 7, A and B) and mRNA relative levels (Fig. 7C) were highly induced in mice during the fetal period (e17.5) and their levels descend progressively after birth. Furthermore, given the implication of HKDC1 during the perinatal period, we measured the relative mRNA expression between pregnant female and nonpregnant female livers and found that there was no difference between these groups (Fig. 7D). Therefore, pregnancy in mice did not induce HKDC1 expression in the mouse liver. These data suggest that HKDC1 may play a crucial role in glucose phosphorylation and utilization to preserve fetal and perinatal growth and development.
Figure 6.
HKDC1 is enriched in the newborn piglet liver. A: Western blots for HKDC1 and HSC70 in the liver of pigs. Western blot HKDC1/HSC70 fold change (B) and Hkdc1 mRNA relative (C) levels in the liver by quantitative RT-qPCR in pigs. All bars represent means ± SE. *P < 0.05, **P < 0.01 vs. p7.
Figure 7.
HKDC1 is enriched in the fetus and newborn in mouse liver. A: Western blots for HKDC1 and HSC70 in the liver of mice. Western blot HKDC1/HSC70 fold change (B) and Hkdc1 mRNA relative (C) levels in the liver by quantitative RT-qPCR. D: Hkdc1 mRNA relative levels in the liver of pregnant (day 17.5) and nonpregnant female mice by quantitative RT-qPCR. All bars represent means ± SE. **P < 0.01, ****P < 0.0001 vs. p7. $$P < 0.01 vs. p14. #P < 0.05, ###P < 0.001 vs. adults.
DISCUSSION
In the perinatal period, there is a high energy demand for thermoregulation, physical activity, and growth (29). Tissue-specific metabolic pathways change through development to maintain energetic homeostasis (30). During high glucose availability, hexokinase activity is essential for glucose phosphorylation to glucose-6-phosphate in the cell and further incorporation into metabolic processes determined by cellular requirements (7). In this work, via global unbiased proteomics, we identified the induction of the novel fifth hexokinase that is elevated during the fetal period and declines precipitously during postnatal development. This regulation is consistent transcriptionally and across mice and pigs.
HKDC1 has hexokinase activity (17) and is required for embryonic development. Deleting both HKDC1 alleles causes in-utero fetal lethality (16), but its role during fetal and perinatal development is not well understood. HKDC1 has been recognized as a regulator of glucose metabolism and regulator of cell hexokinase activity in cells, as reducing HKDC1 expression resulted in a dose-dependent decreases in cellular hexokinase activity in HepG2 cells (17). Its contribution to glucose metabolism has also been shown in an HKDC1 knockdown mouse model (HKDC1+/−; 28 wk age), where mice exhibited an impaired glucose regulation in a glucose tolerance test likely due to reduced whole body glucose utilization, indicated by diminished hepatic energy storage and reduced peripheral tissue uptake (16). Likewise, its role in mitochondrial function has been reported in three liver cancer cell lines (HepG2, Hep3b, and SNU475 cell lines), where HKDC1 suppression diminished mitochondrial basal and maximal respiration (19), as well as an enhanced glucose consumption fueling the pentose phosphate and hexosamine biosynthetic pathways but diminishing TCA cycle flux, thus suggesting it is an essential role for mitochondrial function (19). However, a prolonged HKDC1 overexpression can dysregulate glucose homeostasis and induce nucleotide synthesis in male mice (21). Also, mice with a HKDC1 overexpression showed a bigger cell size and higher hepatocyte proliferative potential causing a larger liver (21).
Interestingly, hepatic levels of hexokinase I, II, IV, and V are not induced widely through development and seem to be conditioned by the metabolic demands. Our results showed that GCK levels are low in the mouse fetus and the newborn pig and mice, and its levels appeared induced in adult mice and pigs, serving as a glucose sensor for regulation and insulin secretion (31). However, it has been shown that animals globally deficient in GCK do not die during embryonic development but within a few days after birth from severe diabetes (32), whereas the heterozygous inactivating mutations of GCK can cause hyperglycemia and hypoglycemia in mice (33). Similar data was found in an in vitro functional assay of cultured fetal mouse liver in a circumfusion system, a technique used for preserving the development of differentiation in mammalian embryo and adult human tissue over prolonged periods (34). In fetal embryos, HK1 activity gradually increased and reached maximum levels at 6 days in culture, and its activity was not influenced by insulin treatment, suggesting that HK1 in fetal mouse liver cultured in a system for 2 wk showed the same response to insulin as adult mouse liver. Although GCK activity was not detected in the fetal mouse liver, it was the only hexokinase found in the mammalian adult liver (34).
After birth energetic substrates change dramatically as lipids and galactose coming from milk are the main dietary source of energy for the newborn. The liver is the main organ for galactose metabolism and it can participate in the glycolytic pathway. Mice and pig data showed that hepatic Galk1 expression was induced since the fetal period and maintained after birth during the postnatal period, suggesting that Galk1 expression is detectable and active since late embryonic development as possible preparation for galactose utilization after birth. Similar results were found in the human fetal liver, red blood cells, the lung, the spleen, and cardiac muscle at 10 wk of gestation, and the activity of Galk1 was higher in red blood cells and liver in the second and third semester than any time postnatally (35). Interestingly, the activity of Galk1 from a child was slightly inferior and with a lower Km value for galactose than the embryonic levels in 20- to 28-wk-old fetuses (36). These data provide new insight into understanding glucose regulation by sugar kinases and its differential activity in response to specific metabolic requirements through development.
Energy requirements during perinatal growth are not entirely sustained by diet, thus alternative metabolic pathways are induced to sustain glucose levels. In this work, we identified the induction of the hepatic gluconeogenesis pathway as a mechanism involved in endogenous glucose production. Gluconeogenesis is not active in the fetus, and its activity develops in postnatal life, reaching maximum levels of activity in the fifth day of life in rats (37). Similar data was shown in the human newborn, where gluconeogenesis appears soon after birth and supply up to 70% to the total glucose produced (6). PC activity, quantified in the mitochondrial fractions of the liver by a 14CO2 fixation method, showed three major activity peaks in fetal and newborn rats. PC first peak was found 1 day before birth, but its activity was undetected at birth; the second peak was reported 6 h after delivery with a maximum level at 12 h, although this peak is just sustained for a few hours. The last one reaches maximum levels on the sixth day after birth and decreases slowly to increase again after weaning (38). It is known that gluconeogenesis is induced in response to changes in insulin, cortisol, glucagon, and catecholamines (39); substrates such as aspartate, glutamate, and alanine were recognized as significant substrates contributing to this pathway in the postnatal rat liver (37).
Other substrates such as lipids are utilized to sustain energy requirements during perinatal development. Lipids stored as triglycerides in adipocytes are liberated by TG lipase to be consequently oxidized in mitochondria (5). Our work suggests that triglyceride content in the liver is similar in newborns and adults, however, the postnatal liver presented a higher amount of carnitine and acylcarnitines, suggesting that fatty acid oxidation is higher in the newborn to produce energy from breast milk lipids and provide the hepatocytes with ATP and NADH to ensure gluconeogenesis (5) and generate acetyl-CoA for ketogenesis. In the postnatal liver, fatty acid oxidation and ketogenesis are regulated by pancreatic hormones, as cultured hepatocytes from rabbit fetuses treated with glucagon or cAMP showed induction of long-chain fatty acid oxidation by regulating carnitine palmitoyltransferase I (CPT I; 40).
Perspectives and Significance
The acceleration of technological advancements has enabled the addition of nonclassical animal models to fundamental biologic questions. Here we utilized the domestic pig to identify liver-specific adaptations in postnatal development. We have characterized a wide array of differentially expressed proteins between newborn and adult liver, many of these proteins being involved the unique metabolic adaptations during the perinatal period. We further confirmed a subset of these differences by orthogonal methods and in an entirely different species, the laboratory mouse. The shared regulation over a large evolutionary distance further corroborates these changes and lends credence to their importance.
DATA AVAILABILITY
Proteome data are available through ProteomeXchange PXD037264 (https://www.ebi.ac.uk/pride/archive/projects/PXD037264).
SUPPLEMENTAL DATA
Supplemental Material: https://doi.org/10.6084/m9.figshare.23816514.v1.
GRANTS
This work was supported in part by a National Institutes of Health Grant R01DK120530, R01DK116746 (to M.J.W.), and R01NS111230 (to S.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S. and M.J.W. conceived and designed research; U.M.-G., J.C. and S.S. performed experiments; U.M.-G. and S.S. analyzed data; U.M.-G. and S.S. interpreted results of experiments; U.M.-G. prepared figures; U.M.-G. drafted manuscript; U.M.-G., S.S., and M.J.W. edited and revised manuscript; U.M.-G., J.C., S.S., and M.J.W. approved final version of manuscript.
REFERENCES
- 1. Butte NF. Energy requirements of infants. Public Health Nutr 8: 953–967, 2005. doi: 10.1079/PHN2005790. [DOI] [PubMed] [Google Scholar]
- 2. Bowman CE, Arany Z, Wolfgang MJ. Regulation of maternal–fetal metabolic communication. Cell Mol Life Sci 78: 1455–1486, 2021. doi: 10.1007/s00018-020-03674-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: a review on its composition and bioactivity. Early Hum Dev 91: 629–635, 2015. doi: 10.1016/j.earlhumdev.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 4. Böhme H-J, Sparmann G, Hofmann E. Biochemistry of liver development in the perinatal period. Experientia 39: 473–483, 1983. doi: 10.1007/BF01965164. [DOI] [PubMed] [Google Scholar]
- 5. Selen ES, Choi J, Wolfgang MJ. Discordant hepatic fatty acid oxidation and triglyceride hydrolysis leads to liver disease. JCI Insight 6: e135626, 2021. doi: 10.1172/jci.insight.135626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalhan S, Parimi P. Gluconeogenesis in the fetus and neonate. Semin Perinatol 24: 94–106, 2000. doi: 10.1053/sp.2000.6360. [DOI] [PubMed] [Google Scholar]
- 7. Wilson JE. Hexokinases. Rev Physiol Biochem Pharmacol 126: 65–198, 1995. doi: 10.1007/BFb0049776. [DOI] [PubMed] [Google Scholar]
- 8. Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 206: 2049–2057, 2003. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 9. Van Schaftingen E. Energy metabolism|hexokinase/glucokinase. In: Encyclopedia of Biological Chemistry III, edited by Jez, J. Amsterdam, The Netherlands: Elsevier, 2021, p. 149–161. [Google Scholar]
- 10. Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep 5: 171–176, 2005. doi: 10.1007/s11892-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 11. Lenzen S. A fresh view of glycolysis and glucokinase regulation: history and current status. J Biol Chem 289: 12189–12194, 2014. doi: 10.1074/jbc.R114.557314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kliegman RM, Sparks JW. Perinatal galactose metabolism. J Pediatr 107: 831–841, 1985. doi: 10.1016/S0022-3476(85)80173-6. [DOI] [PubMed] [Google Scholar]
- 13. Holden HM, Rayment I, Thoden JB. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem 278: 43885–43888, 2003. doi: 10.1074/jbc.R300025200. [DOI] [PubMed] [Google Scholar]
- 14. Caputto R, Leloir LR. The enzymatic transformation of galactose into glucose derivatives. J Biol Chem 179: 497, 1949. doi: 10.1016/S0021-9258(18)56863-0. [DOI] [PubMed] [Google Scholar]
- 15. Holden HM, Thoden JB, Timson DJ, Reece RJ. Galactokinase: structure, function and role in type II galactosemia. Cell Mol Life Sci 61: 2471–2484, 2004. doi: 10.1007/s00018-004-4160-6. [DOI] [PubMed] [Google Scholar]
- 16. Ludvik AE, Pusec CM, Priyadarshini M, Angueira AR, Guo C, Lo A, Hershenhouse KS, Yang G-Y, Ding X, Reddy TE, Lowe WL, Layden BT. HKDC1 is a novel hexokinase involved in whole-body glucose use. Endocrinology 157: 3452–3461, 2016. doi: 10.1210/en.2016-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo C, Ludvik AE, Arlotto ME, Hayes MG, Armstrong LL, Scholtens DM, Brown CD, Newgard CB, Becker TC, Layden BT, Lowe WL, Reddy TE. Coordinated regulatory variation associated with gestational hyperglycaemia regulates expression of the novel hexokinase HKDC1. Nat Commun 6: 6069, 2015. doi: 10.1038/ncomms7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zapater JL, Lednovich KR, Khan MW, Pusec CM, Layden BT. Hexokinase domain-containing protein-1 in metabolic diseases and beyond. Trends Endocrinol Metab 33: 72–84, 2022. doi: 10.1016/j.tem.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khan MW, Terry AR, Priyadarshini M, Ilievski V, Farooq Z, Guzman G, Cordoba-Chacon J, Ben-Sahra I, Wicksteed B, Layden BT. The hexokinase “HKDC1” interaction with the mitochondria is essential for liver cancer progression. Cell Death Dis 13: 660, 2022. doi: 10.1038/s41419-022-04999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayes MG, Urbanek M, Hivert M-F, Armstrong LL, Morrison J, Guo C, Lowe LP, Scheftner DA, Pluzhnikov A, Levine DM, McHugh CP, Ackerman CM, Bouchard L, Brisson D, Layden BT, Mirel D, Doheny KF, Leya MV, Lown-Hecht RN, Dyer AR, Metzger BE, Reddy TE, Cox NJ, Lowe WL; HAPO Study Cooperative Research Group. Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes 62: 3282–3291, 2013. [Erratum in Diabetes 62: 3641, 2013]. doi: 10.2337/db12-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pusec CM, De Jesus A, Khan MW, Terry AR, Ludvik AE, Xu K, Giancola N, Pervaiz H, Daviau Smith E, Ding X, Harrison S, Chandel NS, Becker TC, Hay N, Ardehali H, Cordoba-Chacon J, Layden BT. Hepatic HKDC1 expression contributes to liver metabolism. Endocrinology 160: 313–330, 2019. doi: 10.1210/en.2018-00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khan MW, Priyadarshini M, Cordoba-Chacon J, Becker TC, Layden BT. Hepatic hexokinase domain containing 1 (HKDC1) improves whole body glucose tolerance and insulin sensitivity in pregnant mice. Biochim Biophys Acta Mol Basis Dis 1865: 678–687, 2019. doi: 10.1016/j.bbadis.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Yang F, Gritsenko MA, Wang Y, Clauss T, Liu T, Shen Y, Monroe ME, Lopez-Ferrer D, Reno T, Moore RJ, Klemke RL, Camp DG, 2nd, Smith RD. Reversed-phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells. Proteomics 11: 2019–2026, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herbrich SM, Cole RN, West KP, Jr., Schulze K, Yager JD, Groopman JD, Christian P, Wu L, O’Meally RN, May DH, McIntosh MW, Ruczinski I. Statistical inference from multiple iTRAQ experiments without using common reference standards. J Proteome Res 12: 594–604, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee J, Choi J, Scafidi S, Wolfgang MJ. Hepatic fatty acid oxidation restrains systemic catabolism during starvation. Cell Rep 16: 201–212, 2016. doi: 10.1016/j.celrep.2016.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rando G, Tan CK, Khaled N, Montagner A, Leuenberger N, Bertrand-Michel J, Paramalingam E, Guillou H, Wahli W. Glucocorticoid receptor-PPARα axis in fetal mouse liver prepares neonates for milk lipid catabolism. eLife 5: e11853, 2016. doi: 10.7554/eLife.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bosma M, Sparks LM, Hooiveld GJ, Jorgensen JA, Houten SM, Schrauwen P, Kersten S, Hesselink MKC. Overexpression of PLIN5 in skeletal muscle promotes oxidative gene expression and intramyocellular lipid content without compromising insulin sensitivity. Biochim Biophys Acta 1831: 844–852, 2013. doi: 10.1016/j.bbalip.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 28. Demirbas D, Coelho AI, Rubio-Gozalbo ME, Berry GT. Hereditary galactosemia. Metabolism 83: 188–196, 2018. doi: 10.1016/j.metabol.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 29. Theil PK, Lauridsen C, Quesnel H. Neonatal piglet survival: impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 8: 1021–1030, 2014. doi: 10.1017/S1751731114000950. [DOI] [PubMed] [Google Scholar]
- 30. Solmonson A, Faubert B, Gu W, Rao A, Cowdin MA, Menendez-Montes I, Kelekar S, Rogers TJ, Pan C, Guevara G, Tarangelo A, Zacharias LG, Martin-Sandoval MS, Do D, Pachnis P, Dumesnil D, Mathews TP, Tasdogan A, Pham A, Cai L, Zhao Z, Ni M, Cleaver O, Sadek HA, Morrison SJ, DeBerardinis RJ. Compartmentalized metabolism supports midgestation mammalian development. Nature 604: 349–353, 2022. doi: 10.1038/s41586-022-04557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bali D, Svetlanov A, Lee H-W, Fusco-DeMane D, Leiser M, Li B, Barzilai N, Surana M, Hou H, Fleischer N, DePinho R, Rossetti L, Efrat S. Animal model for maturity-onset diabetes of the young generated by disruption of the mouse glucokinase gene. J Biol Chem 270: 21464–21467, 1995. doi: 10.1074/jbc.270.37.21464. [DOI] [PubMed] [Google Scholar]
- 32. Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using cre recombinase. J Biol Chem 274: 305–315, 1999. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 33. Gloyn AL. Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum Mutat 22: 353–362, 2003. doi: 10.1002/humu.10277. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura T, Kumegawa M. Functional differentiation of mouse fetal liver in circumfusion system cultures. J Biochem 79: 723–730, 1976. doi: 10.1093/oxfordjournals.jbchem.a131124. [DOI] [PubMed] [Google Scholar]
- 35. Abi-Hanna A, Saavedra JM. Galactose. In: Encyclopedia of Human Nutrition, edited by Caballero, B. Elsevier, 1998, p. 377–383. [Google Scholar]
- 36. Shin-Buehring YS, Beier T, Tan A, Osang M, Schaub J. The activity of galactose-1-phosphate uridyltransferase and galactokinase in human fetal organs. Pediatr Res 11: 1045–1051, 1977. doi: 10.1203/00006450-197710000-00004. [DOI] [PubMed] [Google Scholar]
- 37. Yeung D, Oliver I. Gluconeogenesis from amino acids in neonatal rat liver. Biochem J 103: 744–748, 1967. doi: 10.1042/bj1030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salto R, Girón MD, del Mar Sola M, Vargas AM. Evolution of pyruvate carboxylase and other biotin containing enzymes in developing rat liver and kidney. Mol Cell Biochem 200: 111–117, 1999. doi: 10.1023/a:1007091116952. [DOI] [PubMed] [Google Scholar]
- 39. Hume R, Burchell A, Williams FLR, Koh DKM. Glucose homeostasis in the newborn. Early Hum Dev 81: 95–101, 2005. doi: 10.1016/j.earlhumdev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 40. Pegorier JP, Prip-Buus C, Duee PH, Girard J. Hormonal control of fatty acid oxidation during the neonatal period. Diabete Metab 18: 156–160, 1992. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material: https://doi.org/10.6084/m9.figshare.23816514.v1.
Data Availability Statement
Proteome data are available through ProteomeXchange PXD037264 (https://www.ebi.ac.uk/pride/archive/projects/PXD037264).