Abstract
Mutacin II, elaborated by group II Streptococcus mutans, is a ribosomally synthesized and posttranslationally modified polypeptide antibiotic containing unusual thioether and didehydro amino acids. To ascertain the role of specific amino acid residues in mutacin II antimicrobial activity, we developed a streptococcal expression system that facilitates the replacement of the mutA gene with a single copy of a mutated variant gene. As a result, variants of mutacin II can be designed and expressed. The system was tested by constructing the following mutant peptides: ΔN1, V7A, P9A, T10A, T10S, C15A, C26A, and C27A. All of these mutacin II variants except ΔN1 and T10A, which were not secreted, were isolated, and their identities were verified by mass spectrometry. Variants P9A, C15A, C26A, and C27A failed to exert antimicrobial activity. Because the P9A and T10A variants comprise the “hinge” region of mutacin II, these observations suggest that in addition to the thioether and didehydro amino acids, the hinge region is essential for biological activity and biosynthesis or export of the peptide. Tandem mass spectrometry of the N-terminal part of the wild-type molecule and its C15A variant confirmed that the threonine at position 10 is dehydrated and present as a didehydrobutyrine residue. This analysis of the active T10S variant further suggested that a didehydro amino acid at this position is specific for antimicrobial activity and that the biosynthetic machinery does not discriminate between threonine and serine. In contrast, the lack of production of mutacin variants with alanine substituted for threonine at position 10, as well as the deletion of asparagine at the N terminus (ΔN1), indicates that specific residues in the propeptide may be crucial for certain steps in the biosynthetic pathway of this lantibiotic.
Mutacin II, produced by group II strains of Streptococcus mutans (16), is a 3,245-Da hydrophobic peptide comprised of two lanthionines, one β-methyllanthionine, and a didehydro amino acid (18). For this reason, mutacin II belongs to the lantibiotic family of ribosomally synthesized and posttranslationally modified peptide antibiotics (22, 23).
Based on their structures and the mechanisms that they use to kill their target cells, the lantibiotics described to date have been classified into two groups, type A and type B (22). Type A lantibiotics are elongated, screw-shaped peptides which act primarily by membrane perturbation. Type B peptides are globular and appear to inhibit specific enzyme functions. The prolantibiotic part of mutacin deduced from the recently cloned and sequenced structural gene mutA (28) shows similarities with sequences of lantibiotics classified as type A lantibiotics. A unique feature of mutacin II includes an N-terminal α-helix connected to the C-terminal part of the molecule via a rigid hinge region (17). Unlike type A lantibiotics, however, mutacin II exhibits a zero net charge, and there is a relatively large distance between intramolecular thioether-ring-forming residues. Like type B lantibiotics (10, 22), mutacin has at least one lanthionine ring originating from a dehydrated residue situated on the C-terminal side of its “cysteine” partner (17). Moreover, mutacin II exerts bactericidal activity by inhibiting an essential enzyme(s) involved in energy metabolism (7). Taken as a whole, the data show that mutacin II resembles type B lantibiotics more closely than it resembles type A lantibiotics despite the partial sequence similarity to type A lantibiotics.
Because lantibiotics are ribosomally synthesized peptides, their amino acid compositions can be modified via protein engineering to yield variants which can give important clues concerning structure-function relationships. So far, more than 40 different variants of lantibiotics have been reported and characterized (11); some of these exhibit improved properties, including increased activity, solubility, or stability.
The recent determination of the sequence of the structural gene of mutacin II (mutA) (28) from the mutacin II biosynthetic operon enabled us to explore ways to modify the biological and structural properties of mutacin II. Here, we report the development of a host-vector expression system for genetic manipulation of the mutacin II structural gene, mutA, and its application in a structure-activity study of mutacin II analogs generated by site-directed mutagenesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. The Escherichia coli strains used for subcloning and plasmid isolation were grown in Luria-Bertani medium (15) in the presence of the appropriate antibiotics. S. mutans strains and Streptococcus sobrinus OMZ176 were stored frozen at −70°C until they were needed, and they were grown as described previously in Todd-Hewitt broth (Difco Laboratories) (4) or in mixed Trypticase soy broth-yeast extract chemically defined medium (16) for mutacin production. Antibiotics were added to the media when needed (400 μg of kanamycin per ml and 5 μg of tetracycline per ml for streptococci; 50 μg of kanamycin per ml, 12.5 μg of tetracycline per ml, and 50 μg of ampicillin per ml for E. coli).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| S. mutans strains | ||
| T8 | Wild type, Mut+ | 21 |
| CBM0 | Tcr ΔmutA Mut−, expression host for mutacin II variants | This study |
| S. sobrinus OMZ176 | Mutacin-sensitive indicator | 4 |
| Plasmids | ||
| pUC19 | AprlacZα | 29 |
| pCRII | Apr Kmr, cloning vector | Invitrogen |
| pNoTA/T7 | Apr, cloning vector | 5′→3′ Inc. |
| pCBM1 | Apr Kmr, pCRII with 0.75-kb PCR fragment containing mutA downstream region | This study |
| pCBM2 | Apr Kmr, pCRII with 1.3-kb mut3 SSP-PCR fragment containing mutA upstream region | This study |
| pCBM3 | Apr Kmr, pCRII with both mutA upstream and downstream regions | This study |
| pCBM4 | Apr Kmr Tcr, pCBM3 with tetM gene inserted at HpaI site in the mutA upstream region | This study |
| pCBM5 | Apr, pNoTA/T7 with 2.2-kb mut2 SSP-PCR fragment containing complete mutA coding region | This study |
| pCBM6 | Apr Kmr, pCBM5 with aphIII gene inserted at HpaI site in the mutA upstream region, used for site-directed mutagenesis | This study |
| V7A | Apr Kmr, analog of pCBM6, confers production of V7A mutacin | This study |
| P9A | Apr Kmr, analog of pCBM6, confers production of P9A mutacin | This study |
| T10A | Apr Kmr, analog of pCBM6, confers production of T10A mutacin | This study |
| T10S | Apr Kmr, analog of pCBM6, confers production of T10S mutacin | This study |
| C15A | Apr Kmr, analog of pCBM6, confers production of C15A mutacin | This study |
| C26A | Apr Kmr, analog of pCBM6, confers production of C26A mutacin | This study |
| C27A | Apr Kmr, analog of pCBM6, confers production of C27A mutacin | This study |
| ΔN1 | Apr Kmr, analog of pCBM6, confers production of ΔN1 mutacin | This study |
Apr, ampicillin resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance; Mut−, mutacin negative; Mut+, mutacin positive.
DNA manipulation, transformation, and molecular cloning techniques.
S. mutans T8 chromosomal DNA was prepared as described previously (4). E. coli transformation and supercoiled plasmid DNA isolation were carried out by previously described methods (15). S. mutans transformation was performed as previously described (24).
Construction of the host expression system and mutA gene replacement vectors.
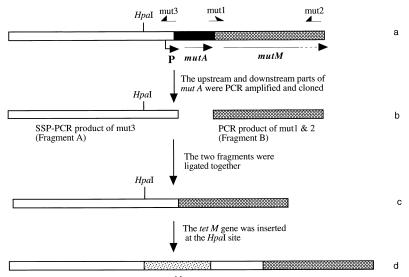
The strategy for constructing a mutacin-deficient strain, CBM0, is illustrated in Fig. 1. First, we PCR amplified a fragment downstream of mutA (fragment B) from wild-type T8 chromosomal DNA by using primers mut1 (GAGATCTTATCAAAAAGGAGAAAT) and mut2 (AATGTAAATCGGTCATATTGAGAG) (a BglII restriction site [underlined] was added to the 5′ end of mut1). The resulting amplicon was then cloned into vector pCRII with a TA cloning kit (Invitrogen) to create plasmid pCBM1. To amplify the region upstream of mutA (fragment A), primer mut3 (AGATCTAATATTTTACATTTACAG), with a BglII site (underlined), was used to initiate a single specific primer PCR (SSP-PCR) under the conditions described previously (19, 25). Chromosomal DNA from T8 was digested with restriction enzyme HaeIII. Vector pUC19 was digested with HincII. pUC19 and chromosomal HaeIII fragments were ligated with DNA ligase; the ligation mixture served as the template for SSP-PCR (19, 25). The amplified fragment A was also cloned into the pCRII vector to form plasmid pCBM2. After plasmids pCBM1 and pCBM2 were treated with restriction enzyme BglII, the upstream and downstream fragments (fragments A and B) were ligated into the pCRII vector to form the up- and downstream regions flanking the deleted mutA. The resultant construct was designated pCBM3.
FIG. 1.
Construction of strain CBM0 (ΔmutA). (a) Region of S. mutans T8 chromosome encoding mutA and start of mutM. The position of the mutacin promoter (P) is indicated by an arrow. (b) Up- and downstream portions of mutA were PCR amplified and cloned into the pCRII vector. (c) Two fragments were ligated into the pCRII vector. (d) tetM gene was inserted at the HpaI site. The final plasmid was treated with restriction enzyme XbaI and then used to transform wild-type S. mutans T8. A transformant which was tetracycline resistant and mutacin negative was designated S. mutans CBM0.
A PCR-amplified tetM gene from transposon Tn916 (9) was inserted into the HpaI site in the upstream fragment of pCBM3 to form plasmid pCBM4. Plasmid pCBM4 was then linearized with restriction enzyme XbaI and used to transform S. mutans T8. Several tetracycline-resistant transformants were randomly picked, tested for the mutacin phenotype, and analyzed by PCR for evidence of deletion of the mutA gene in the chromosome. A strain which was tetracycline resistant and mutacin negative (due to deletion of the mutA gene) was designated S. mutans CBM0 and was used as the host strain to express the mutacin variants.
To construct the mutA gene replacement vectors, the entire mutA region was amplified via SSP-PCR with Pfu DNA polymerase and primer mut2 by using the T8 chromosomal DNA-pUC19 ligation mixture described above as the template. The resulting amplicon was cloned into vector pNOTA/T7 (5′→3′ Inc., Boulder, Colo.) to generate plasmid pCBM5. A PCR-amplified kanamycin resistance gene (aphIII) from Enterococcus faecalis (26) was inserted into pCBM5 at the HpaI site. The resultant construct was designated pCBM6 and was used directly for site-directed mutagenesis.
Site-directed mutagenesis and construction of S. mutans producing mutacin variants.
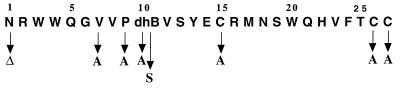
The positions of exchanged amino acids in the mutacin II molecule are shown in Fig. 2. Mutagenesis was accomplished by using a QuikChange site-directed mutagenesis kit (Stratagene Cloning Systems, La Jolla, Calif.) and different primer sets (Table 2). Each PCR mixture contained 100 ng of plasmid pCBM6 DNA template, 125 ng of each primer, nucleoside triphosphates, reaction buffer, and Pfu DNA polymerase as supplied by the manufacturer. Samples were placed in a thermal cycler, denatured for 30 s at 95°C, and then subjected to 18 cycles consisting of 95°C for 30 s, 55°C for 1 min, and 68°C for 13 min. The reaction mixtures were then cut with DpnI to digest the parental DNA template. A 4-μl aliquot of the DpnI-treated DNA was used to transform E. coli XL-1 Blue competent cells (Stratagene). Transformed cells were selected on Luria-Bertani agar containing kanamycin, and after overnight growth, colonies were picked. The DNA sequences of the putative mutA variants were confirmed by automated sequencing with an Applied Biosystems model 373 DNA sequencer. Once confirmed, each mutated plasmid was digested with XbaI and then transformed into S. mutans CBM0. Kanamycin-resistant and tetracycline-sensitive transformants were analyzed for the mutacin phenotype by the deferred antagonism assay described below. Detection of the appropriately sized mutA gene replacement expected from a reciprocal, double crossover event was accomplished as follows. DNA was extracted from S. mutans transformants by a Chelex 100 extraction method (27), as modified in our laboratory (12a). Briefly, cells from a 1.5-ml overnight culture were resuspended in 100 μl of sterile distilled water and mixed with 200 μl of Chelex DNA extraction reagent (Perkin-Elmer Corp., Alameda, Calif.). After incubation at 56°C for 30 min, the suspension was vortexed for 10 s and then incubated at 100°C for 10 min. The cell mixture was then centrifuged at the maximum speed (14,000 rpm) in a microcentrifuge for 5 min. Five microliters of the supernatant containing chromosomal DNA was used as the template DNA. Primers mut4 (TAGGATCCCCAACCTCCTTCATAC) and mut5 (CCGGTAAGTACATAGTGC), located up- and downstream of mutA, respectively, were added to a PCR mixture as described above. The appropriately sized PCR product was ascertained on a 0.8% agarose gel.
FIG. 2.
Primary structure of the unmodified propeptide of mutacin II and mutations that were introduced. T in position 10 is shown as dehydrated residue.
TABLE 2.
Oligonucleotides used in site-directed mutagenesis
| Mutant peptide | Mutagenic oligonucleotidesa |
|---|---|
| V7A mutacin II | TGGTGGCAAGGTGCTGTGCCAACGGTCTCATATG, GACCGTTGGCACAGCACCTTGCCACCAACGAT |
| P9A mutacin II | CAAGGTGTTGTGGCTACGGTCTCATATGAGTGTC, CATATGAGACCGTAGCCACAACACCTTGCCACC |
| T10A mutacin II | GGTGTTGTGCCAGCTGTCTCATATGAGTGTCGC, CTCATATGAGACAGCTGGCACAACACCTTGCCAC |
| T10S mutacin II | GGTGTTGTGCCATCTGTCTCATATGAGTGTCGC, CTCATATGAGACAGATGGCACAACACCTTGCCAC |
| C15A mutacin II | GTCTCATATGAGGCTCGCATGAATTCATGGCAAC, TGAATTCATGCGAGCCTCATATGAGACCGTTGG |
| C26A mutacin II | CATGTTTTCACTGCTTGTTAAAAAATTAAAAATTATAACGG, TTTAATTTTTTAACAGCAGTGAAAACATGTTGCCATG |
| C27A mutacin II | GTTTTCACTTGCGCTTAAAAAATTAAAAATTAT, ATTTTTAATTTTTTAAGCGCAAGTGAAAACATGTTGCC |
| ΔN1 mutacin II | ACTATTTTGGGTGGTΔCGTTGGTGGCAAGGTG, ACCTTGCCACCAACGΔACCACCCAAAATAGTATC |
Mismatches are underlined.
Mutacin activity assay.
Mutacin activity was detected on Trypticase soy broth-yeast extract agar plates by the deferred antagonism technique (3, 21).
Purification and analysis of mutacin II and its variants.
Mutacin II and its variants were isolated by the ultrafiltration and selective precipitation method described previously (16). Purified mutacin variants were then analyzed by electrospray ionization (ESI)-mass spectrometry (MS) with a PE Sciex API III biomolecular mass analyzer (16, 18). Tandem MS (MS-MS) was accomplished by selecting the parent ion having a particular m/z value with the first quadrupole and allowing it to move into the second quadrupole, where it collided with argon atoms and fragmented. The fragments were then analyzed with the third quadrupole.
RESULTS
Construction of the host-vector expression system for mutagenesis of the mutacin II gene.
To produce an engineered mutacin, a host strain had to be constructed that carried all of the genetic information for modification, export, and processing of the mutated peptides in addition to the mutated mutA gene. To avoid producing a mixture of the mutant and wild-type peptide, we deleted the structural gene mutA from the wild-type strain S. mutans T8 to create strain CBM0 (see Materials and Methods). The resultant construct was tetracycline resistant and displayed a mutacin-negative phenotype (data not shown). PCR analysis of this mutacin-negative S. mutans strain confirmed that the mutA gene was not present (data not shown). S. mutans CBM0 was used as the recipient expression strain to receive the various mutA mutated sequences.
For genetic manipulation of the mutA gene, plasmid pCBM6 served as the target of site-directed mutagenesis and was used to deliver the mutated mutA copy to host strain CBM0. To determine if insertion of the aphIII gene into the upstream portion of the mutA gene interfered with mutacin production, the insert from plasmid pCBM6 was released with restriction enzyme XbaI and then used to transform strain CBM0. The resultant transformant (designated strain CBM kan) exhibited antimicrobial activity similar to the wild-type activity (data not shown). This control data indicated that the mutacin-negative phenotype exhibited by CBM0 was due solely to deletion of mutA and that, except for the structural gene mutA, strain CBM0 possessed all of the required components of the mutacin II biosynthetic apparatus.
The first mutation tested was the replacement of the valine at position 7 with alanine. As indicated by the PCR fragment analysis (data not shown), the mutated mutA variant correctly inserted into the CBM0 expression host. As anticipated, the V7A mutacin was produced and exhibited antimicrobial activity similar to that of the wild-type mutacin (Table 3). To verify that posttranslational processing similar to wild-type expression occurred, the V7A mutacin variant was purified and analyzed by using EIS-MS. The results confirmed that the substitution occurred, and the estimated molecular mass of the product was consistent with the expected value (Table 3).
TABLE 3.
Characterization of mutacin II variants
| Mutacin II | Calculated mass (Da) | Determined mass (Da)a | Biological activityb |
|---|---|---|---|
| Wild type | 3,245 | 3,245.4 ± 0.6 | +d |
| ΔN1 | 3,131 | NDc | − |
| V7A | 3,217 | 3,217.8 ± 0.1 | + |
| P9A | 3,219 | 3,218.4 ± 1.2 | ± |
| T10A | 3,233 | ND | − |
| T10S | 3,230 | 3,230.4 ± 1.1 | + |
| C15A | 3,213 | 3,212.4 ± 1.2 | − |
| C26A | 3,213 | 3,214.2 ± 0.7 | − |
| C27A | 3,213 | 3,212.8 ± 1.4 | ± |
Mass of purified extracellular product.
As determined by a deferred antagonism assay (stabs).
ND, not detected.
+, activity; −, no activity; ±, low level of activity (less than 10% of the wild-type activity).
Mutations of cysteines involved in thioether bridge formation.
To explore the importance of the thioether bridges (16–18) in the antimicrobial activity, we replaced each of the three cysteines with alanine, one at a time, so that only one thioether bridge was disrupted each time. All mutated mutA copies were correctly integrated into the chromosome, as indicated by PCR analysis (data not shown), and the expected fully modified (dehydrated) mutacin II variants were secreted into the medium, as shown in Table 3. Two variants (C15A and C26A) failed to exhibit antimicrobial activity, suggesting that the thioether bridges are essential for antimicrobial activity. The C27A mutacin variant was secreted into the medium in noticeably lower amounts but exhibited residual antimicrobial activity (Table 3).
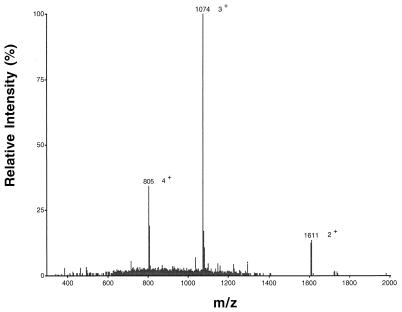
Data obtained previously indicated that the threonine residue at position 10 is likely to be modified to a didehydrobutyrine (Dhb) residue (16, 18). MS-MS of the mutacin C15A peptide (Fig. 3) showed that the threonine residue at position 10 is a dehydrated amino acid. ESI–MS-MS analysis indicated that a complete series of b ions corresponding to the N-terminal amino acid sequence (NRWWQGVVPdhBV−) were present; the m/z values were 271, 457, 643, 771, 828, 928, 1,027, 1,124, 1,207, and 1,306 for b2 to b11, respectively. These data, together with the rest of the spectra, indicate that the C15A variant has the same amino acid sequence as the wild-type molecule at the N terminus and provide direct proof that the threonine residue at position 10 is dehydrated.
FIG. 3.
MS-MS of the C15A variant of mutacin II. The doubly charged ion was allowed to move into the second quadrupole, where it collided with argon atoms. The fragments were then analyzed with the third quadrupole. The spectrum shows part of the N-terminal sequence with a series of b ions corresponding to the N-terminal amino acid sequence (NRWWQGVVPdhBV−); the m/z values were 271, 457, 643, 771, 828, 928, 1,027, 1,124, 1,207, and 1,306 for b2 to b11, respectively. The signals with m/z values of 1,027, 1,124, 1,207, and 1,306 represent fragments of the N termini ending with Val, Pro, Dhb, and Val, respectively.
Mutations of amino acids in the hinge region.
The N-terminal region of mutacin (residues one to eight) can form an amphipathic α-helix, as confirmed by nuclear magnetic resonance, followed by a rigid structure of the hinge region via a kink introduced by proline followed by Dhb (17). To determine the importance of this hinge region for mutacin biosynthesis and activity, we first replaced the proline at position 9 with alanine. The P9A mutacin analog was produced and processed correctly based on the ESI-MS data (Fig. 4), but its antimicrobial activity was markedly reduced (Table 3). When Dhb at position 10 was replaced by alanine, however, we could not detect any mutacin-like peptide variants, suggesting that the Dhb at position 10 is crucial in as-yet-undetermined steps in the biosynthetic processing of the lantibiotic. We then replaced the Dhb with didehydroalanine (Dha) (T10S) to address the question of whether the mutacin biosynthetic apparatus distinguishes Ser from Thr and thus produces Dha instead Dhb. The Dhb10Dha mutacin analog was correctly processed, as shown by ESI-MS, and exhibited antimicrobial activity similar to that of wild-type mutacin (Table 3).
FIG. 4.
EIS-MS analysis of purified P9A variant of mutacin II. The molecular mass calculated from multiply charged molecular ions was 3,218.4 ± 1.2 Da.
Deletion of the N-terminal amino acid.
Because each of the three cysteines appeared to be required for normal antimicrobial activity and because two of the cysteins were at the C terminus of the mutacin II molecule, we were unable to generate smaller functional mutacin analogs by deleting the C-terminal amino acids. Accordingly, we elected to delete the first N-terminal amino acid, asparagine. Although the mutated mutA copy was correctly integrated into the chromosome, as shown by the PCR fragment analysis (data not shown), no processed ΔN1 mutacin analog was detected.
DISCUSSION
Two different expression systems for lantibiotic protein engineering exist; the first involves a plasmid-borne, complementary copy of a structural gene in the host strain, and the second involves replacement of the wild-type gene with a mutated copy by using gene replacement procedures (11). Here, we describe a gene replacement strategy in which a streptococcal expression system for engineering mutacin II was used. In this system, the wild-type mutA gene in the expression host was deleted and then replaced with a mutagenized analog. This approach results in exclusive production of the engineered mutacin II while the normal gene dosage, regulatory responses, and balance between structural and biosynthetic genes are retained (11). A similar approach was employed previously to engineer analogs of the lantibiotics subtilin (14), nisin (8), gallidermin (20), and epidermin (1, 2).
Lantibiotics contain a variety of modified residues, including lanthionine and β-methyl-lanthionine, which are thought to contribute to stabilization of the biologically active conformation of these peptides (12). Our results support this contention. Two of the three mutacin variants in which cysteine residues were replaced by alanine (C15A and C26A) failed to exhibit antimicrobial activity, suggesting that the thioether bridges contribute to this attribute. The C27A mutacin variant, however, retained some residual antimicrobial activity. One possible explanation for the apparent reduction in the size of the inhibition zone was that less variant peptide was exported from the cells. Alternatively, the antimicrobial activity may have been reduced as a result of the absence of the thioether ring formed by the Cys-27 residue. Although the amount of the C27A peptide exported into liquid medium was reduced compared with wild-type expression, the reduced production level was not compatible with the decreased activity level. Therefore, it seems likely that the thioether bridge formed by Cys-27 is important for both antimicrobial activity and proper folding and recognition by, for example, the exporting system. Similar results were obtained for Pep5, in which two analogs (C27A and C33A) showed significantly lower antimicrobial activity than wild-type Pep5 (2). In epidermin, almost all C-terminal alterations of preepidermin, which affected thioether bridge formation of rings C or D, including deletion of the last two cysteine residues, resulted in a complete loss of epidermin production (20).
Bierbaum et al. (2) have shown that a novel thioether ring can be introduced into Pep5; therefore, it is possible that thioether rings other than the one that carries the mutated amino acid are affected in our Cys→Ala mutants. We do not think that this happened in our experiments, however, because MS analyses of the CNBr cleavage products of the wild-type molecule and its variant C15A indicated that the organization of the thioether bridges was site specific and that the free didehydro amino acids remain as such and are not recognized as possible alternative partners for cysteine bridging (unpublished data).
The replacement of Dhb-10 in mutacin II by alanine, which resulted in the absence of peptide product, clearly indicated that, at least in the present study, this dehydrated residue is required for production or stability of mature mutacin II. The antimicrobial activity of the Dhb10Dha analog, similar to the wild-type activity, further confirmed the importance of a dehydrated residue at this position for expression of active mutacin II. Other workers have shown that the dehydro residues in nisin contribute to structural instability, as indicated by the production of spontaneous degradation products resulting from cleavage of the peptide bond at Dha-5 and Dha-33 (5). The lack of production of Dhb10A mutacin II may be due to a block at some stage of its biosynthesis or export. Another possibility that could explain the absence of the correctly processed gene product in the medium could be the instability of the mutacin variant T10A, although this seems less probable. Generally, introduction or removal of dehydrated residues can either stimulate or reduce activity. In subtilin, the exchange of the Dha residue at position 5 for Ala led to the loss of sporicidal activity but not pore-forming antibacterial activity (13). Similarly, the replacement of Dha-5 by alanine has no effect on the activity of nisin as an inhibitor of bacterial growth but does drastically decrease its level of activity as an inhibitor of spore outgrowth (6). Dodd et al. (8) showed that in nisin both Dha-5 and Dha-33 are not critical for activity. On the other hand, Dha5Dhb nisin is about 2- to 10-fold less active than nisin (12), and both Pep16A Pep5 and Pep20A Pep5 are significantly less active than Pep5 (2).
A unique feature of mutacin II is the putative formation of an α-helix at the N terminus connected to the rest of the molecule by Pro-Dhb (17). Replacement of the proline residue by alanine dramatically decreased the activity (Table 3), although the mutacin variant was produced and secreted. It thus appears that both P-9 and T-10 (modified as Dhb) in the hinge region play important structural and biological roles. Until now, however, the term hinge region has been reserved for a flexible region between thioether rings. Moreover, hinge regions appear to be common to several type A lantibiotics and evidently are important for antimicrobial activity. For example, a Pep5 mutant (K18P) with a mutation involving the hinge region showed a dramatic decrease in activity against Staphylococcus simulans 22 (1). We suggest that the definition of hinge region be expanded to include any region, flexible or rigid, that connects the thioether ring domain to the other domains of a lantibiotic molecule. Under this expanded definition, our observations concerning the hinge region’s importance in mutacin II antimicrobial activity are consistent with the reported role of this region in type A lantibiotics.
Deletion of the codon for the first amino acid, asparagine, resulted in the loss of production. Thus, it does not seem probable that minimization of the molecule at the N terminus is feasible. This suggests that this amino acid is required for some step(s) of mutacin biosynthesis. Because it borders the G-G cleavage site, it seems more likely that N-1 plays a role in correct recognition by the protease for cleavage of the signal peptide. Multiple sequence alignment (28) of the lantibiotics with G-G type leader peptides revealed strong conservation of aliphatic amino acid residues at positions −3 and −4 and the very frequent presence of a charged residue at position +1. It thus seems plausible that the ΔN mutation did not provide sufficient substrate recognition for the protease, resulting in a lack of production and export of the mature mutacin. Similarly, the G-2A mutation is known to result in a lack of production in other peptides with G-G type leader peptides.
In summary, we successfully constructed a streptococcal expression system for protein engineering of the lantibiotic mutacin II. Application of the system to an initial structure-function study identified determinants required for antibacterial activity and thus yielded important information that may allow workers to modulate the peptide properties and eventually target specific human pathogens.
ACKNOWLEDGMENTS
This work was supported by NIH grant DE09082. The mass spectrometer was purchased by funds from NIH Instrumentation Grant S10RR06487 and from UAB. Operation of the Mass Spectrometer Shared Facility was supported in part by NCI Core Research Support Grant P30 CA13148 to the UAB Comprehensive Cancer Center.
REFERENCES
- 1.Bierbaum G, Reis M, Szekat C, Sahl H-G. Construction of an expression system for engineering of the lantibiotic Pep 5. Appl Environ Microbiol. 1994;60:4332–4338. doi: 10.1128/aem.60.12.4332-4338.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bierbaum G, Szekat C, Josten M, Heidrich C, Kempter C, Jung G, Sahl H-G. Engineering of a novel thiether bridge and role of modified residues in the lantibiotic Pep 5. Appl Environ Microbiol. 1996;62:385–392. doi: 10.1128/aem.62.2.385-392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caufield P W, Childers N K, Allen D N, Hansen J B. Distinct bacteriocin groups correlate with different groups of Streptococcus mutans plasmids. Infect Immun. 1985;48:51–56. doi: 10.1128/iai.48.1.51-56.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caufield P W, Shah G R, Hollingshead S K. Use of transposon Tn916 to inactivate and isolate a mutacin-associated gene from Streptococcus mutans. Infect Immun. 1990;58:4126–4135. doi: 10.1128/iai.58.12.4126-4135.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan W C, Bycroft B W, Lian L-Y, Roberts G C K. Isolation and characterization of two degradation products derived from the peptide antibiotic nisin. FEBS Lett. 1989;252:29–36. [Google Scholar]
- 6.Chan W C, Dodd H M, Horn N, Maclean K, Lian L-Y, Bycroft B W, Gasson M J, Roberts G C K. Structure-activity relationships in the peptide antibiotic nisin: role of dehydroalanine. Appl Environ Microbiol. 1996;62:2966–2969. doi: 10.1128/aem.62.8.2966-2969.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chikindas M L, Novak J, Driessen A J M, Konings W N, Schilling K M, Caufield P W. Mutacin II, a bactericidal lantibiotic from Streptococcus mutans. Antimicrob Agents Chemother. 1995;39:2656–2660. doi: 10.1128/aac.39.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd H M, Horn N, Giffard C J, Gasson M J. A gene replacement strategy for engineering nisin. Microbiology. 1996;142:47–55. doi: 10.1099/13500872-142-1-47. [DOI] [PubMed] [Google Scholar]
- 9.Flannagan S E, Zitzow L A, Su Y A, Clewell D B. Nucleotide sequence of the 18-kb conjugative transposon Tn916 from Enterococcus faecalis. Plasmid. 1994;32:350–354. doi: 10.1006/plas.1994.1077. [DOI] [PubMed] [Google Scholar]
- 10.Jung G. Lantibiotics: a survey. In: Jung G, Sahl H G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM Science Publishers; 1991. pp. 1–34. [Google Scholar]
- 11.Kuipers O P, Bierbaum G, Ottenwalder B, Dodd H M, Horn N, Metzger J, Kupke T, Gnau V, Bongers R, van den Bogaard P, Kosters H, Rollema H S, de Vos W M, Siezen R J, Jung G, Götz F, Sahl H G, Gasson M J. Protein engineering of lantibiotics. Antonie Leeuwenhoek. 1996;69:161–170. doi: 10.1007/BF00399421. [DOI] [PubMed] [Google Scholar]
- 12.Kuipers O P, Rollema H S, Yap W M G J, Boot H J, Siezen R J, de Vos W M. Engineering dehydrated amino acid residues in the antimicrobial peptide nisin. J Biol Chem. 1992;267:24340–24346. [PubMed] [Google Scholar]
- 12a.Li, Y. Unpublished data.
- 13.Liu W, Hansen J N. The antimicrobial effect of a structural variant of subtilin against outgrowing Bacillus cereus T spores and vegetative cells occurs by different mechanisms. Appl Environ Microbiol. 1993;59:648–651. doi: 10.1128/aem.59.2.648-651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Hansen J N. Enhancement of the chemical and antimicrobial properties of subtilin by site-directed mutagenesis. J Biol Chem. 1992;267:25078–25085. [PubMed] [Google Scholar]
- 15.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 16.Novak J, Caufield P W, Miller E J. Isolation and biochemical characterization of a novel lantibiotic mutacin from Streptococcus mutans. J Bacteriol. 1994;176:4316–4320. doi: 10.1128/jb.176.14.4316-4320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak J, Chen P, Kirk M, Barnes S, Jablonsky M J, Holaday S K, Krishna N R, Baker J, Caufield P W. Structure and activity of the lantibiotic mutacin II. Evidence for two domains? Nat Biotechnol Short Rep. 1997;8:87. [Google Scholar]
- 18.Novak J, Kirk M, Caufield P W, Barnes S, Morrison K, Baker J. Detection of modified amino acids in lantibiotic peptide mutacin II by chemical derivatization followed by electrospray ionization mass spectroscopic analysis. Anal Biochem. 1996;236:358–360. doi: 10.1006/abio.1996.0181. [DOI] [PubMed] [Google Scholar]
- 19.Novak J, Novak L, Shah G R, Woodruff W, Caufield P W. Transposon mutagenesis: cloning of chromosomal DNA from the site of Tn916 insertion using polymerase chain reaction. Biotechnol Tech. 1997;11:51–54. [Google Scholar]
- 20.Ottenwalder B, Kupke T, Brecht T, Gnau V, Metzger J, Jung G, Götz F. Isolation and characterization of genetically engineered gallidermin and epidermin analogs. Appl Environ Microbiol. 1995;61:3894–3903. doi: 10.1128/aem.61.11.3894-3903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrot M, Caufield P W, Lavoie M C. Preliminary characterization of four bacteriocins from Streptococcus mutans. Can J Microbiol. 1990;36:123–130. doi: 10.1139/m90-022. [DOI] [PubMed] [Google Scholar]
- 22.Sahl H-G, Jack R W, Bierbaum G. Lantibiotics: biosynthesis and biological activities of peptides with unique post-translational modifications. Eur J Biochem. 1995;230:827–853. doi: 10.1111/j.1432-1033.1995.tb20627.x. [DOI] [PubMed] [Google Scholar]
- 23.Schnell N, Entian K-D, Schneider U, Götz F, Zähner H, Kellner R, Jung G. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulfide-rings. Nature. 1988;333:276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- 24.Shah G R, Caufield P W. Enhanced transformation of Streptococcus mutans by modifications in culture conditions. Anal Biochem. 1993;214:343–346. doi: 10.1006/abio.1993.1503. [DOI] [PubMed] [Google Scholar]
- 25.Shyamala V, Ames G F-L. Genome walking by single specific primer-polymerase chain reaction. Methods Enzymol. 1993;217:436–446. doi: 10.1016/0076-6879(93)17082-g. [DOI] [PubMed] [Google Scholar]
- 26.Trieu-Cuot P, Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′5"-amino glycoside phosphotransferase type III. Gene. 1983;23:331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- 27.Walsh P S, Metzger D A, Higuchi R. Chelex® 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques. 1991;10:506–513. [PubMed] [Google Scholar]
- 28.Woodruff W A, Novak J, Caufield P W. Sequence analysis of mutA and mutM genes involved in the biosynthesis of the lantibiotic mutacin II in Streptococcus mutans. Gene. 1998;206:37–43. doi: 10.1016/s0378-1119(97)00578-7. [DOI] [PubMed] [Google Scholar]
- 29.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]