Abstract
The potential of granular sludge from upflow anaerobic sludge blanket (UASB) reactors for bioremediation of chlorinated pollutants was evaluated by using carbon tetrachloride (CT) as a model compound. Granular sludges cultivated in UASB reactors on methanol, a volatile fatty acid mixture, or sucrose readily degraded CT supplied at a concentration of 1,500 nmol/batch (approximately 10 μM) without any prior exposure to organohalogens. The maximum degradation rate was 1.9 μmol of CT g of volatile suspended solids−1 day−1. The main end products of CT degradation were CO2 and Cl−, and the yields of these end products were 44 and 68%, respectively, of the initial amounts of [14C]CT and CT-Cl. Lower chlorinated methanes accumulated in minor amounts temporarily. Autoclaved (dead) sludges were capable of degrading CT at rates two- to threefold lower than those for living sludges, indicating that abiotic processes (mediated by cofactors or other sludge components) played an important role in the degradation observed. Reduced components in the autoclaved sludge were vital for CT degradation. A major part (51%) of the CT was converted abiotically to CS2. The amount of CO2 produced (23%) was lower and the amount of Cl− produced (86%) was slightly higher with autoclaved sludge than with living sludge. Both living and autoclaved sludges could degrade chloroform. However, only living sludge degraded dichloromethane and methylchloride. These results indicate that reductive dehalogenation, which was mediated better by living sludge than by autoclaved sludge, is only a minor pathway for CT degradation. The main pathway involves substitutive and oxidative dechlorination reactions that lead to the formation of CO2. Granular sludge, therefore, has outstanding potential for gratuitous dechlorination of CT to safe end products.
Chlorinated compounds are commonly found pollutants in the environment. Carbon tetrachloride (CT) is among the top 45 organic chemicals produced by the United States chemical industry, with 143,000 tons produced in 1991 (2). CT is used as a solvent in, for example, the chemical cleaning and metal industries. Like many other halogenated hydrocarbons, CT is a suspected carcinogen and therefore is a public health concern.
Higher chlorinated compounds are degraded more easily under anaerobic conditions than under aerobic conditions (44). The initial degradation of these compounds, often a dechlorination, can be carried out by specific halorespiring bacteria (10, 40, 43). However, acetogenic and methanogenic bacteria are able to transform chlorinated compounds via aspecific reactions. It has been suggested that the dechlorination reactions are mediated by cofactors like vitamin B12 and other corrinoids and by cofactor F430. These metalloporphyrins, which contain cobalt, nickel, or iron, are parts of enzymes that catalyze common pathways present in anaerobic bacteria, like the acetyl coenzyme A pathway and methane formation. Acetogenic and methanogenic bacteria contain elevated levels of such cofactors (11, 19, 26, 32). The concentrations of cofactors in the bacteria are strongly dependent on the substrate used for growth. Some microorganisms, like Methanosarcina barkeri grown on methanol, are known to excrete 40 to 70% of the corrinoids produced into the culture medium (32). On the other hand, acetogenic bacteria do not contain cofactor F430, whereas the cofactor levels in methanogens can be as high as 800 nmol/g (dry weight) (11). The dechlorination rates with the cofactors in vitro are lower than the rates of transformation via specific enzyme reactions.
A variety of dechlorination processes may be involved during the degradation of CT by unadapted sludge. Dechlorination can occur either chemically or by aspecific and specific biological reactions. Chemically, CT can be transformed in the presence of pyrite (FeS2), iron, or sulfide as a bulk electron donor (9, 22). Aspecific biological reactions are carried out without a lag phase and are catalyzed by cofactors which are either free or bound to enzymes in the cell. The specific biological reactions usually require a long adaptation period. This time span is often necessary to enrich for the appropriate bacteria in the consortium. Two strictly anaerobic acetogenic bacteria, which use methylchloride (MC) or dichloromethane (DCM) to support growth, have been isolated (31, 33). Although the dehalogenation of CT by unadapted (pure) cultures is largely attributed to the action of vitamin B12 and other corrinoids present in the cells, other unknown dechlorinating mechanisms may also play a role in the dechlorination of halogenated compounds (41).
In this research we evaluated the aspecific dechlorinating ability of unadapted acetogenic and methanogenic bacteria by using methanogenic granular sludge from upflow anaerobic sludge blanket (UASB) reactors and CT as a model compound. The sludge used had a high biomass content (27), which was enriched with acetogenic and methanogenic bacteria. By autoclaving the sludges and evaluating product formation, we distinguished between biological processes and abiotic processes (mediated by cofactors or reactions with sludge components) that occurred during the transformation of CT.
MATERIALS AND METHODS
Chemicals.
CT, chloroform (CF), and DCM (all pro analysis quality; E. Merck, Amsterdam, The Netherlands), as well as MC (purity, >99%; Hoekloos, Schiedam, The Netherlands), [14C]CT (specific activity, 0.15 GBq/mmol; NEN Life Science Products, Boston, Mass.), and [13C]CT (Isotec Inc., Miamisburg, Ohio), were used as received without further purification.
Granular sludge.
The granular sludge was grown in three UASB reactors which originally had been inoculated with granular sludge from a full-scale UASB reactor treating sugar beet refinery wastewater (CSM, Breda, The Netherlands). The reactors (volume, 10 liters) were fed with methanol, a mixture of volatile fatty acids (VFA), or sucrose, as well as a mineral medium containing (per liter) 1,040 mg of NH4Cl, 170 mg of KH2PO4, 170 mg of (NH4)2SO4, 150 mg of MgCl2 · 6H2O, 270 mg of KCl, and 18 mg of yeast extract. Trace elements were added by using a stock solution whose composition has been described previously (45). The hydraulic retention time in each of the reactors was 12 h, and the operating temperature was 30°C. The sludge content of each reactor was approximately 20 g of volatile suspended solids (VSS) liter−1. The methanogenic activity of the sludges was approximately 0.40 g of chemical oxygen demand (COD) of g of VVS−1 day−1. The loading rate of the sucrose-fed reactor was approximately 5 kg m−3 day−1 based on COD, which corresponded to 12 mM sucrose in the influent. Sodium bicarbonate (23 mM) was added to maintain pH stability. The VFA- and methanol-fed reactors were operated at a loading rate of 10 kg of COD m−3 day−1. The VFA (the acetate, propionate, and butyrate concentrations in the influent were 19, 14, and 13 mM, respectively) were neutralized with sodium hydroxide. Methanol was added at a concentration of 100 mM along with 30 mM NaHCO3. The COD removal efficiencies were at least 85%. No VFA were present in the effluents of the three reactors. The reactors were run for at least 6 months prior to sludge sampling.
Batch experiments.
The sludges were washed two times with demineralized water and one time with basal medium to remove residual soluble substrates before the sludges were used in the batch experiments. Approximately 2-g (wet weight portions) of granular sludge were transferred to 120-ml serum flasks containing 20 ml of basal medium, as described previously (17). When chlorine balances were determined, the medium was slightly modified by replacing the chloride salts of calcium and magnesium with Ca(OH)2 and MgHPO4 · 2H2O. The pH values of the batches remained 7.2 to 7.3. The gas phase consisted of 80% N2 and 20% CO2. The flasks were sealed with Viton stoppers (Maag Technic AG, Dübendorf, Switzerland). The appropriate cosubstrate (1.5 g of COD/liter) and chlorinated methane were added to each batch as required. CT, CF, and DCM were added by using solutions prepared in anaerobic water, and MC was added as a gas with a gas-tight syringe (final concentration, approximately 1,500 nmol/batch). The batches were incubated statically at 30°C in the dark. The possible loss of the chlorinated compounds due to leakage through the stoppers was checked by using separate batches of medium to which no sludge was added.
Abiotic involvement of the cofactors.
The abiotic involvement of cofactors was tested by autoclaving the granular sludge, which inactivated all microbial activity. The granular sludge was autoclaved in basal medium for 90 min at 120°C three days prior to the start of the experiment and again for 30 min on the day that the experiment was started.
Corrinoid content of granular sludge.
The corrinoid content of the granular sludge was determined by previously described methods which were adjusted to facilitate the granular sludge determination (17, 20, 42). The absorption spectrum (λ, 200 to 800 nm) of a purified sample was determined with a Beckman spectrophotometer. Purity was calculated by using the ratio of absorbance at 361 nm (A361) to A548 (the A361/A548 ratios of calibration samples were 3.12 ± 0.12). The corrinoid concentration was quantified spectrophotometrically. A molar extinction coefficient of 7,316 M−1 cm−1 at 548 nm was determined with a calibration curve and was used in the calculations.
Experiments with [14C]CT.
The formation of CO2 from CT was investigated by following the degradation of [14C]CT. Experiments were carried out in 26-ml tubes containing 11 ml of medium and 2 ml of living or autoclaved crushed (to facilitate addition to the test tubes) sludge. The sludge was crushed by pressing a sludge suspension through sterile needles with decreasing diameters (the smallest needle was a Microlance 3 needle [25G5/8, 0.5 by 16 mm]). Labeled CT (total amount, around 2.5 × 105 dpm/tube) together with unlabeled CT was added dissolved in water to obtain the desired concentration. In case of the experiments with living sludge, the chlorinated compound was added in small portions (150 nmol of CT/tube). This low concentration was used to avoid the formation of CF at concentrations higher than the 50% inhibition concentration of CF for acetoclastic methanogens (1.7 mg/liter) (37). [14C]CT was added again after the previously added [14C]CT was completely transformed. A cosubstrate was not used in these experiments to avoid high pressures in the tube and to obtain low background methane concentrations. Prior experiments had shown that there were no major differences in CT degradation when a cosubstrate was not added. For each measurement six tubes were used. To dissolve all of the 14CO2 in the liquid phase, 1 ml of a 5 M NaOH solution was added to three tubes. The remaining three tubes were amended with 1.5 ml of 1 M HCl to remove all of the CO2 and bicarbonate from the liquid phase. To determine the amount of 14CO2 formed, the six tubes were treated identically. A 2-ml sample was taken from each tube and centrifuged at 15,000 × g for 5 min. The supernatant was stripped with air (50 ml/min) for 5 min. To 0.5 ml of the sample 4.5 ml of scintillation liquid was added (Ultima Gold; Packard Instrument BV, Groningen, The Netherlands), and the resulting mixture was counted for 3 min with a scintillation counter (model 1211 Rackbeta; LKB). The amount measured in the NaOH-treated tubes represented the total radioactivity (i.e., the activity of the nonvolatile compounds plus CO2). The amount measured in the HCl-amended tubes represented the total activity minus the CO2 activity. The amount of 14CO2 produced was calculated from the difference between the NaOH- and HCl-amended incubation preparations and was compared with a calibration curve prepared with NaH14CO3 and sludge (recovery rate, 93 to 99% of the H14CO3). The pellet (sludge) of the centrifuged samples was washed in 1 ml of demineralized water, centrifuged again, and dissolved in 1 ml of 5 M NaOH. Samples (0.25 ml) of the dissolved pellet were counted in scintillation liquid. The activities in these samples represented the amount of carbon incorporated into the biomass and the amount of [14C]CT adsorbed to the sludge. Chlorinated methane and CS2 concentrations were monitored in tubes which were simultaneously incubated with [12C]CT.
Experiments with [13C]CT.
The formation of acetate, formate, and methane from CT was measured by using [13C]CT that was added dissolved in water. The setup of the experiment was identical to that of the [14C]CT experiments described above. For each measurement six tubes were used. From each of the first three tubes a sample was taken and centrifuged at 15,000 × g for 5 min. Part of the supernatant was acidified with formic acid and stored at −20°C until the [13C]acetate analysis was conducted. Another part of the supernatant was used to determine the formate concentration after acidification with HCl. For the 13CH4 measurement another three tubes were acidified to pH 2 with 1 M HCl and stored at 4°C until further analysis.
Analytical methods.
Total masses of the chlorinated methanes, carbon disulfide, H2, and methane were determined by headspace analysis. CT, CF, DCM, and carbon disulfide were analyzed by injecting 0.2 ml of headspace gas into a model 436 Chrompack gas chromatograph (GC) equipped with a flame ionization detector connected to a Sil 5CB column (25 m by 0.32 mm by 1.2 μm) and a splitter-injector (ratio, 1:50). The operating temperatures of the injector, column, and detector were 250, 50, and 300°C, respectively. The carrier gas was N2 with an inlet pressure of 50 kPa. The retention times were 5.3, 3.8, 2.5, and 2.7 min for CT, CF, DCM, and carbon disulfide, respectively. MC was analyzed by injecting 0.2 ml of headspace gas into a model 438A Chrompack GC equipped with a flame ionization detector connected to a Poraplot Q column (25 m by 0.32 mm by 10 μm) and a splitter-injector (ratio, 1:40). The operating temperatures of the injector, column, and detector were 225, 140, and 250°C, respectively. The retention time of MC was 2.3 min. The retention times and peak areas of all chlorinated methanes were determined with a Shimadzu model C-3A integrator. The lower detection limits of the chlorinated methanes were 30, 20, 38, and 30 nmol/batch for CT, CF, DCM, and MC, respectively. Hydrogen and methane were analyzed by injecting 0.4 ml of gas from the headspace into a Packard model 417 GC equipped with a thermal conductivity detector (100 mA) connected to a molecular sieve column (13×; 180 by 0.25 in.; 60 to 80 mesh). The temperatures of the column and detector were 100°C. Calibration curves were constructed by adding the required amount of the chlorinated methane, H2, or methane to a serum bottle containing 20 ml of basal medium without sludge. Sludge was omitted to avoid degradation. The bottles were allowed to equilibrate overnight at 30°C.
Chloride and formate concentrations were determined by high-performance liquid chromatography as described previously (38). The detection limits were 10 μM. Bromide and lactate were used as internal standards for chloride analysis and formate analysis, respectively. [13C]acetate and 13CH4 contents were determined by a GC-mass spectrometry analysis of liquid and gas phase samples as previously described (39). For the acetate analysis, m/z 62 acetate (100 μM) was added as an internal standard. The detection limit for [13C]acetate was 25 μM (375 nmol/tube) with a maximum background level of 2 mM [12C]acetate. The detection limit for 13CH4 was 10 μM (130 nmol/tube) with a maximum background level of 300 μM 12CH4. VFA and methanol concentrations were determined by GC as described previously (14). The COD for methanol, VFA, and sucrose solutions were determined by standard methods (1). The COD conversion factors utilized were 1.07, 1.50, 1.07, 1.52, and 1.82 g/g for sucrose, methanol, acetate, propionate, and butyrate, respectively. The VSS content of the sludge was determined by subtracting the ash content from the dry weight after the sludge was incubated overnight at 105°C. The ash content was determined after the dry sludge was heated at 600°C for 90 min.
RESULTS
Degradation of CT by unadapted sludges.
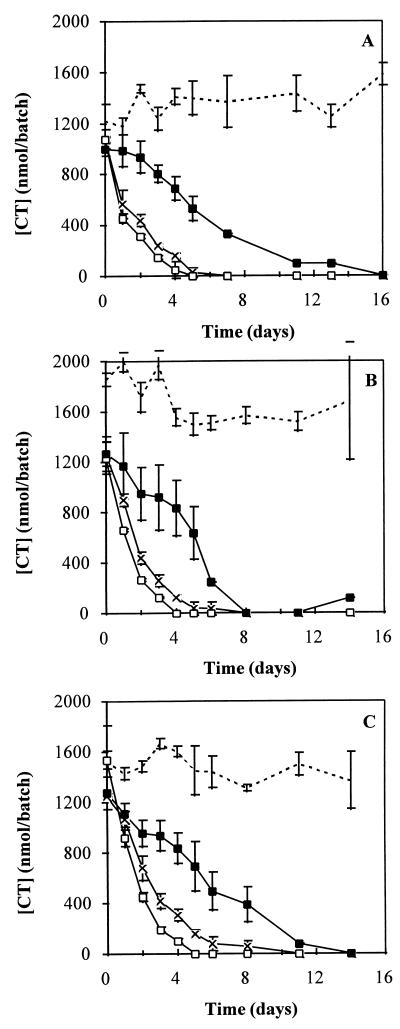
CT was rapidly degraded by unadapted methanogenic consortia (Fig. 1). The degradation occurred without any lag phase, irrespective of whether the preparations were supplemented with cosubstrate. The addition of cosubstrate, however, was associated with a slight enhancement of CT removal. The maximum rates of CT elimination were 1.3, 1.2, and 1.9 μmol of CT g of VSS−1 day−1 for methanol-, VFA-, and sucrose-fed sludges, respectively. The autoclaved sludge was also able to cause significant removal of CT, but the rate was generally only one-third to one-half the rate observed with living sludge. No significant removal of CT occurred when the compound was incubated in sterile medium in the absence of sludge.
FIG. 1.
Disappearance of CT in the presence of unadapted living sludge with (□) or without (×) cosubstrate, in the presence of autoclaved sludge (▪), or in sterile medium in the absence of sludge (dashed line). The sludge was grown in a methanol-fed (A), VFA-fed (B), or sucrose-fed (C) UASB reactor. The VSS contents of the batches for living sludge without cosubstrate, living sludge with cosubstrate, and autoclaved sludge were 128, 122, and 136 mg of VSS/batch, respectively, for methanol-fed sludge, 263, 259, and 268 mg of VSS/batch, respectively, for VFA-fed sludge, and 192, 197, and 228 mg of VSS/batch, respectively, for sucrose-fed sludge. The error bars indicate the standard deviations based on triplicate incubations.
Inhibition and stimulation of CT elimination by autoclaved sludge.
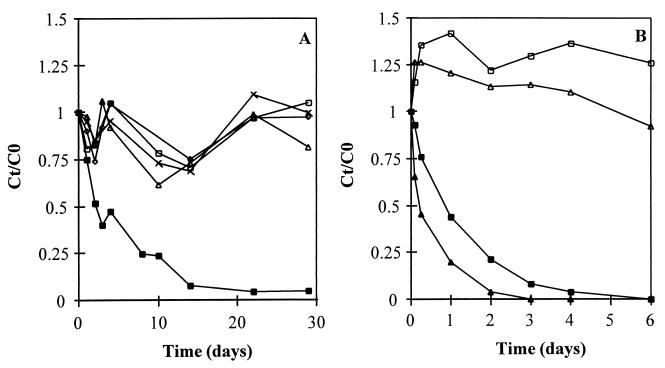
Autoclaved sludge from the VFA-fed reactor was incubated with different concentrations of H2O2 (1 to 5%) to determine whether it inhibited the CT-degrading capacity of the autoclaved sludge (Fig. 2A). The H2O2 treatments resulted in bleaching of the normally black sludge granules, resulting in white granules. All concentrations of H2O2 tested were sufficient to eliminate all of the CT removal capacity of the autoclaved sludge. Addition of reducing equivalents in the form of Ti(III) citrate (300 μM) to autoclaved sludge led to an increase in the CT degradation rate in the first 6 h of incubation, showing the importance of electron availability for the conversion of CT by autoclaved sludge (Fig. 2B). To examine the involvement of vitamin B12 in the dechlorination of CT by autoclaved sludge, 1-iodopropane was tested at concentrations of 0 to 100 mM (results not shown). 1-Iodopropane is a known inhibitor of reductive dehalogenation mediated by vitamin B12 because of its covalent binding to the cofactor (6). No significant effect on CT removal by autoclaved sludge cultivated in the VFA-fed reactor was found at 1-iodopropane concentrations up to 50 mM. Limited inhibition (60% of the CT removal rate) was observed at a 1-iodopropane concentration of 100 mM.
FIG. 2.
(A) CT elimination by autoclaved sludge (243 mg of VSS/batch) from the VFA-fed reactor in the presence of H2O2, given as the concentration at time t divided by the concentration at time zero (Ct/C0). Symbols: ▪, no H2O2; □, 1% (vol/vol) H2O2; ▵, 2% (vol/vol) H2O2; ×, ± 3.5% (vol/vol) H2O2; ◊, 5% (vol/vol) H2O2. The initial CT concentration was 3,900 nmol/batch. (B) CT elimination by autoclaved sludge (296 mg of VSS/batch) from the methanol-fed reactor with (▴) and without (▪) 300 μM Ti(III) citrate. Also shown are the concentrations in blanks with no sludge added with (▵) and without (□) 300 μM Ti(III)citrate. The initial CT concentration was 1,500 nmol/batch.
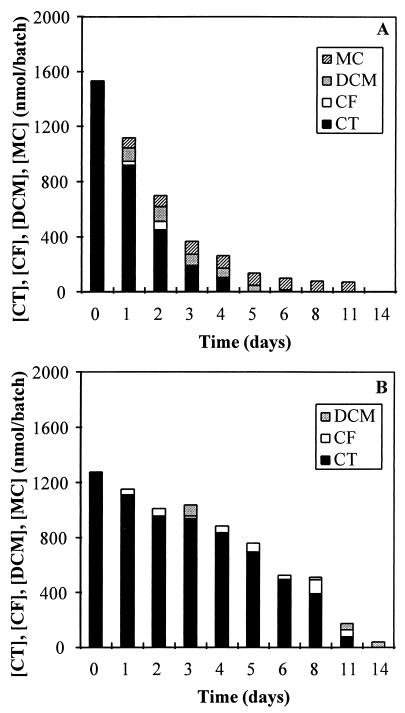
Identification of lower chlorinated methanes during CT degradation.
Lower chlorinated methanes were detected as intermediates during incubation of CT with living and autoclaved sucrose-fed anaerobic granular sludges. Similar results were obtained for the VFA- and methanol-fed sludges. CF, DCM, and MC were identified as transient intermediates during incubation with living sludge (Fig. 3A). The recovery of the different intermediates was never more than 10% of the initial amount of CT. When the cosubstrate was omitted, the lower chlorinated methanes were generally detectable for longer periods of time in the system. When CT was incubated with autoclaved sludge, CF and DCM could be detected as products, but no MC was formed (Fig. 3B). The intermediates were more stable in the presence of autoclaved sludge. The maximum molar yield of the intermediates was less than 8% of the CT initially present.
FIG. 3.
Degradation of CT and subsequent formation of intermediates by living (A) and autoclaved (B) granular sludges from the sucrose-fed reactor.
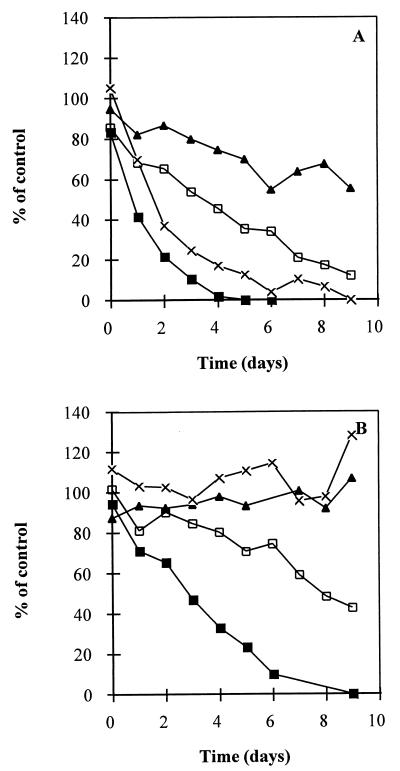
Degradability of lower chlorinated methanes by unadapted sludge.
Degradation of CT, degradation of CF, degradation of DCM, and degradation of MC were examined individually with living and autoclaved sludges from the methanol-fed reactor (Fig. 4). Living sludge was able to degrade all of the halomethanes (Fig. 4A). CT, CF, and DCM were transformed to lower chlorinated methanes. During degradation of CT by living sludge, the molar yield of intermediates was similar to the molar yield obtained with the sucrose sludge (Fig. 3A). The maximum yields of DCM and MC during the degradation of CF were 24 and 6%, respectively. Only 14% of the DCM degraded was recovered as MC. The autoclaved sludge rapidly eliminated CT, whereas CF was only partially removed (52%) within 9 days. The maximum yield of DCM was 5% of the initial CF concentration. DCM and MC were not degraded by autoclaved sludge (Fig. 4B).
FIG. 4.
Degradation of CT (▪) and the lower chlorinated methanes CF (□), DCM (▴), and MC (×) by living (A) and autoclaved (B) sludges from the methanol-fed reactor. The concentrations were normalized against the concentrations found in parallel incubated sterile blanks lacking sludge. The VSS contents of the batches for living and autoclaved sludges were 142 and 148 mg of VSS/batch, respectively, in CT incubations, 136 and 134 mg of VSS/batch, respectively, in CF incubations, 129 and 142 mg of VSS/batch, respectively, in DCM incubations, and 134 and 144 mg of VSS/batch, respectively, in MC incubations.
Chlorine balance.
The chlorine balance after CT degradation was determined with both living and autoclaved sludges from the methanol-fed reactor (Table 1). The amounts of chlorine present in the products as chlorinated methanes or chloride (corrected for the background levels in the sludge) were measured after 6 days for living sludge and after 11 days for autoclaved sludge. After 6 days of incubation, 55 to 70% of the CT chlorine initially present in the incubation preparations was recovered as chloride with living sludge. After 6 days, the incubation preparations were spiked once more with CT, and the chlorine was released as chloride with similar yields (results not shown). Compared to living sludge, the autoclaved sludge released more chloride from CT. Up to 86% of the initial amount of CT chlorine was recovered as chloride after 11 days (Table 1). These results indicate that there was almost complete dechlorination of CT to nonchlorinated end products. Consequently, adsorption did not play a major role in the mechanism of CT removal by living or autoclaved sludge.
TABLE 1.
Chlorine balance for degradation of CT by living (methanol-grown) sludge with and without cosubstrate and by autoclaved sludge
| Prepn | Chlorine concn (nmol/batch)
|
% Recovery
|
||||||
|---|---|---|---|---|---|---|---|---|
| Zero time, CT | Day 6 or 11
|
Cl−a | Totalb | |||||
| CT | CF | DCM | MC | Cl−c | ||||
| Living, no cosubstrate | 5,049 ± 360d | BDLe | BDL | 351 ± 20 | 25 ± 35 | 2,806 ± 265 | 55.6 | 63.0 |
| Living, cosubstrate | 4,925 ± 113 | BDL | BDL | 244 ± 17 | 91 ± 5 | 3,359 ± 268 | 68.2 | 75.0 |
| Autoclaved | 5,566 ± 131 | BDL | BDL | 112 ± 25 | BDL | 4,771 ± 892 | 85.7 | 89.5 |
Efficiency of chloride release compared to CT at zero time.
Total amount of products (chlorinated methanes and chloride) compared to CT at zero time.
Amount of chlorine released corrected for the sludge background chloride level.
Mean ± standard deviation based on three determinations.
BDL, below detection limit.
Production of CO2, CS2, formate, acetate, and CH4 from CT.
[14C]CT was to a large extent (44%) degraded to 14CO2 by living sludge (Table 2). Addition of 50 mM 2-bromo-ethanesulfonic acid (BESA), a specific inhibitor of methanogenesis, resulted in the formation of more lower chlorinated methanes. Some (8 to 15%) of the [14C]CT added was found to be associated with the sludge. In the presence of autoclaved sludge, 51% of the [14C]CT could be recovered as CS2, and 23% was transformed to 14CO2. Approximately 20% of the [14C]CT was adsorbed to the sludge. Methane, formate, and acetate could not be detected as products of [13C]CT degradation by either living or autoclaved sludge, but the detection limits for these compounds were rather high. Altogether, a substantial amount of the CT added could be recovered as (labeled) products. Some possible products, like CO, could not be analyzed for, and it was not possible to measure the radioactivity in the gas phase. This resulted in an incomplete carbon balance.
TABLE 2.
Products formed after 30 days of incubation of CT with unadapted living methanol-grown granular sludge in the presence or absence of 50 mM BESA and with autoclaved methanol-grown sludge and in medium with no sludge added
| Prepn | BESA | Amt of CT and products from [13C]CT and [14C]CT (% of initial CT concn)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CT | LCMa | CS2 | 14CO2 | 14C-labeled biomassb | [13C]formate | 13CH4 | [13C]acetate | ||
| Medium | − | 98.3c | 0 | 0 | 0 | ||||
| Autoclaved | − | 2.9 | 5.1 | 50.6 | 23.3 | 18.8 | BDLd | BDL | BDL |
| Living | − | 0 | 6.8 | 0 | 44.2 | 15.1 | BDL | BDL | BDL |
| Living | + | 0 | 28.0 | 0 | 34.9 | 8.8 | BDL | BDL | BDL |
Lower chlorinated methanes (CF, CDM, and MC).
CT carbon incorporated into biomass and adsorbed to the biomass.
Mean based on at least three determinations.
BDL, below detection limit.
Determination of the corrinoid contents of granular sludges fed with different substrates.
The vitamin B12 contents of the sludges grown in the three reactors were 0.97, 0.45, and 0.60 mg g of VSS−1 for methanol-, VFA-, and sucrose-fed sludges, respectively.
DISCUSSION
This research shows that methanogenic consortia grown in anaerobic wastewater treatment systems like an UASB reactor are able to degrade CT without any prior adaptation. CT is extensively dechlorinated, and CO2 and Cl− are the main products formed by unadapted living sludge. The presence of BESA, a specific inhibitor of methanogenesis, led to accumulation of lower chlorinated methanes and less conversion to CO2, which showed the importance of methanogenesis in the dechlorination process. Autoclaved sludge was also able to degrade CT to an unusually high extent. The ability of the autoclaved sludge to degrade CT supports the hypothesis that cofactors, such as F430, or cobalamines, such as vitamin B12, are involved in the dechlorination of this compound. The main products of degradation of CT by autoclaved sludge were CS2, CO2, and Cl−. The rates of CT dechlorination by granular sludge observed in this study (1 to 2 μmol g of VSS−1 day−1) are comparable to the rates of dechlorination observed with adapted anaerobic sludge (>0.4 μmol g of VSS−1 day−1) (30) but lower than the rates of dechlorination of CT by pure cultures like Acetobacterium woodii (30 mmol g of protein−1 day−1), Methanobacterium thermoautotrophicum (0.8 mmol g of protein−1 day−1), and Desulfobacterium autotrophicum (15.4 mmol g of protein−1 day−1) (12, 13).
Different mechanisms may have played a role in the degradation of CT by granular sludge. Biological catalysts, as well as abiotic mechanisms mediated by enzyme cofactors as catalysts and chemical mechanisms (without mediation by a catalyst), can be responsible for CT degradation. Biologically, CT degradation has been observed under redox conditions varying from nitrate reducing to methanogenic. Both pure and mixed microbial cultures have been found to degrade CT, and the products formed are usually lower chlorinated methanes and nonchlorinated products like CO2. Sometimes CS2 and VFA are also formed (4, 5, 12, 16, 28, 36). It has been suggested that there are two major pathways that are used by these bacteria to degrade CT. First, there is a reductive route, in which lower chlorinated methanes are formed, which is catalyzed by corrinoids and cofactor F430. Second, CT is transformed via the oxidative or substitutive pathway to CO2 (13) (Fig. 5). Both pathways are heat stable, and there seems to be a shift toward the oxidative or substitutive route after cultures are autoclaved (similar to our results), probably due to the loss of protein-mediated electron transfer (13). The formation of CO2 by living cells may be attributed to CO or formate produced by vitamin B12 which is further transformed by CO dehydrogenase (25) (Fig. 5). CO or formate is formed via reductive dechlorination of CT by a two-electron transfer via dichlorocarbene, which is subsequently hydrolyzed (29). The pathway for the formation of CO2 by autoclaved cells has to our knowledge not been elucidated yet. It has been observed that the amount of methane formed via reductive dechlorination of MC never exceeds 5% of the total amount of CT or CF added in methanogenic mixed cultures (5) or pure cultures (34).
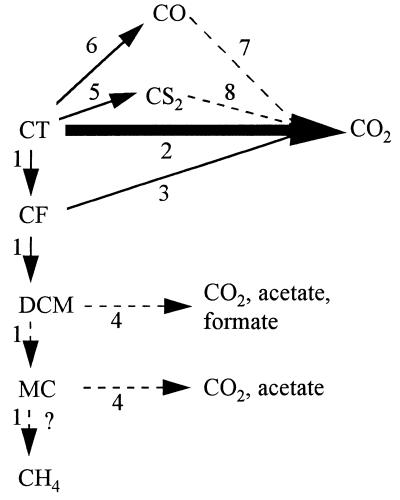
FIG. 5.
Possible pathways for CT degradation by unadapted granular sludge. The solid lines indicate transformations carried out by both living and autoclaved sludges. The dashed lines indicate transformations carried out only by living sludge. The numbers indicate the following processes which take place: 1, reduction; 2, substitution; 3, oxidation; 4, acetyl coenzyme A pathway; 5, chemical reaction with, for example, pyrite (FeS2) or cobalamines; 6, aspecific reactions with cobalamines or F430; 7, CO dehydrogenase; 8, chemical or biological reaction (?).
Chemical dechlorination of CT (without a catalyst) has been observed in the presence of Fe2+ (250 μM, pH 7.2) or sulfide (250 μM HS−, pH 7.8) at 50°C (9) and in the presence of pyrite (22). Among the products formed were CF, CS2, CO2, and formate. The formation of CO2 was ascribed to the hydrolysis of CS2. However, we did not observe this reaction in our experimental setup when CS2 was incubated in the presence of autoclaved sludge (data not shown). Nevertheless, organic molecules present in the (autoclaved) sludge could act as a mediator in the chemical conversion and thus increase reaction rates (9). Since CT was not degraded in blanks which contained 1 mM sulfide and no sludge, the results clearly indicate that a potential chemical catalyst originated from the sludge. The amount of iron in granular sludge from a reactor run under conditions similar to those used for growing the sludge in our experiments was around 10 mg g of total suspended solids−1 (15). This may result in a concentration as high as 1 mM in the medium.
Many in vitro studies have shown that metallocofactors like vitamin B12 and other cobalamines, as well as cofactor F430 (18, 24) and iron porphyrins (21), are capable of catalyzing degradation of CT and other chlorinated alkanes when a suitable electron donor is present. These reactions are abiotic, but the cofactors which are usually present as parts of enzymes may also function as mediators in biological systems. Vitamin B12 dechlorinates CT via reductive, oxidative, and substitutive pathways, depending on the electron donor used (3, 7, 8, 23, 25, 29). By comparing the products formed during degradation of CT by living and autoclaved cells of A. woodii and by vitamin B12, it was shown that vitamin B12 may be largely responsible for the dechlorination of CT by this bacterium (41).
The 13C experiments showed that CT is not transformed to CH4 as a major product by methanogenic consortia. Furthermore, neither formate nor acetate was a major product. This finding, together with the small amounts of lower chlorinated methane intermediates detected, indicates that reductive dehalogenation is only a minor pathway in the degradation of CT by unadapted granular sludge. CS2 was detected in incubations with autoclaved sludge, indicating that chemical (abiotic) transformations may be involved in the removal of CT. Apparently, living sludge maintains a redox potential low enough to prevent CS2 formation. Since the formation of CO2 from CT takes place immediately at the beginning of incubation (data not shown), it seems likely that the CO2 is formed by a direct substitution reaction from CT (Fig. 5). Whether the reaction takes place via CS2 or CO remains uncertain. Clearly, net oxidative and substitutive pathways are predominant in CT degradation by methanogenic granular sludge. The difference in product formation shows that the pathways used by living and autoclaved sludges are different.
There were no significant differences in dechlorination rate and product formation among the sludge grown on methanol, the sludge grown on VFA, and the sludge grown on sucrose. This was not expected because methylotrophic bacteria are known to have higher corrinoid contents than nonmethylotrophic bacteria (19, 26, 32). We assumed that autoclaving the sludge solubilized the intracellular vitamin B12. Our research showed that a 1.5- to 2-fold-higher corrinoid content in methanol-grown sludge did not lead to an increase in the CT degradation rate compared to sucrose- or VFA-fed sludge. Also, 1-iodopropane was found to be only a very weak inhibitor of dechlorination. We concluded that not the corrinoid content but the limited availability of electrons for dechlorination may have been the rate-limiting factor in the degradation of CT. This could be an explanation for the faster degradation in biological (living) systems than in autoclaved sludge. The fact that degradation was slightly stimulated by adding cosubstrate to living sludge also suggests that there was a shortage of reducing equivalents. Moreover, the addition of Ti(III) citrate enhanced the CT degradation by autoclaved sludge, and oxidation of all reducing equivalents with H2O2 led to complete inhibition of CT removal. However, the latter could also have been caused by a disruption of the cofactor structure (35). The diffusion rate of the chlorinated compound into the sludge and cells may also have influenced the reaction rate. We did indeed observe enhanced CT degradation when we used crushed sludge incubated in a rotary shaking incubator (which decreased the mass transport limitation) instead of granular sludge (unpublished results).
Unadapted methanogenic granular sludge was shown to be a suitable source of dechlorinating activity. Although the degradation rates are low, dechlorination of CT is carried out without prior adaptation, and the degradation of CT is extensive and leads to nonhazardous products like CO2. The presence of living sludge is essential to maintain sufficient reducing conditions. The dechlorination rate can potentially be increased by crushing the sludge or by incubating the preparation with shaking, thus decreasing the mass transport limitation.
ACKNOWLEDGMENTS
We thank Wim Roelofsen for technical assistance and Serve Kengen and Hans Scholten for valuable discussions.
This research was supported by a grant (project 92014) from the Innovative Research Program on Environmental Biotechnology (IOP-Milieubiotechnologie) of the Ministries of Economic Affairs and Housing, Physical Planning, and Environment.
REFERENCES
- 1.American Public Health Association. Standard methods for examination of water and wastewater. 16th ed. Washington, D.C: American Public Health Association; 1985. [Google Scholar]
- 2.Anonymous. Facts and figures for the chemical industry. Chem Eng News. 1993;1993:40–45. [Google Scholar]
- 3.Assaf-Anid N, Hayes K F, Vogel T M. Reductive dechlorination of carbon tetrachloride by cobalamin(II) in the presence of dithiothreitol—mechanistic study, effect of redox potential and pH. Environ Sci Technol. 1994;28:246–252. doi: 10.1021/es00051a010. [DOI] [PubMed] [Google Scholar]
- 4.Becker J G, Freedman D L. Use of cyanocobalamin to enhance anaerobic biodegradation of chloroform. Environ Sci Technol. 1994;28:1942–1949. doi: 10.1021/es00060a027. [DOI] [PubMed] [Google Scholar]
- 5.Bouwer E J, McCarty P L. Transformations of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983;45:1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brot N, Weissbach H. Enzymatic synthesis of methionine. Chemical alkylation of the enzyme-bound cobamide. J Biol Chem. 1965;240:3064–3070. [PubMed] [Google Scholar]
- 7.Chiu P, Reinhard M. Metallocoenzyme-mediated reductive transformation of carbon tetrachloride in titanium(III) citrate aqueous solution. Environ Sci Technol. 1995;29:595–603. doi: 10.1021/es00003a006. [DOI] [PubMed] [Google Scholar]
- 8.Chiu P, Reinhard M. Transformation of carbon tetrachloride by reduced vitamin B12 in aqueous cysteine solution. Environ Sci Technol. 1996;30:1882–1889. [Google Scholar]
- 9.Curtis G P, Reinhard M. Reductive dehalogenation of hexachloroethane, carbon tetrachloride, and bromoform by anthrahydroquinone disulfonate and humic acid. Environ Sci Technol. 1994;28:2393–2401. doi: 10.1021/es00062a026. [DOI] [PubMed] [Google Scholar]
- 10.DeWeerd K A, Mandelco L, Tanner S, Woese C R, Sulfita J M. Desulfomonile tiedjei gen. nov. and sp. nov., a novel anaerobic, dehalogenating, sulfate-reducing bacterium. Arch Microbiol. 1990;154:23–30. [Google Scholar]
- 11.Diekert G, Konheiser U, Piechulla K, Thauer R K. Nickel requirement and factor F430 content of methanogenic bacteria. J Bacteriol. 1981;148:459–464. doi: 10.1128/jb.148.2.459-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egli C, Tschan T, Scholtz R, Cook A M, Leisinger T. Transformation of tetrachloromethane to dichloromethane and carbon dioxide by Acetobacterium woodii. Appl Environ Microbiol. 1988;54:2819–2824. doi: 10.1128/aem.54.11.2819-2824.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egli C, Stromeyer S, Cook A M, Leisinger T. Transformation of tetra- and trichloromethane to CO2 by anaerobic bacteria is a non-enzymic process. FEMS Microbiol Lett. 1990;68:207–212. [Google Scholar]
- 14.Florencio L, Noznevnikova A, van Langerak A, Stams A J M, Field J A, Lettinga G. Acidophilic degradation of methanol by a methanogenic enrichment culture. FEMS Microbiol Lett. 1993;109:1–6. [Google Scholar]
- 15.Florencio L, Jenicek P, Field J A, Lettinga G. Effect of cobalt on the anaerobic degradation of methanol. J Ferment Bioeng. 1993;75:368–374. [Google Scholar]
- 16.Hashham S A, Scholze R, Freedman D L. Cobalamin-enhanced anaerobic biotransformation of carbon tetrachloride. Environ Sci Technol. 1995;29:2856–2863. doi: 10.1021/es00011a023. [DOI] [PubMed] [Google Scholar]
- 17.Holliger C. Reductive dehalogenation by anaerobic bacteria. Ph.D. thesis. Wageningen, The Netherlands: Wageningen Agricultural University; 1992. [Google Scholar]
- 18.Holliger C, Schraa G, Stupperich E, Stams A J M, Zehnder A J. Evidence for the involvement of corrinoids and factor F430 in the reductive dechlorination of 1,2-dichloroethane by Methanosarcina barkeri. J Bacteriol. 1992;174:4427–4434. doi: 10.1128/jb.174.13.4427-4434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue K, Kageyama S, Miki K, Morinaga T, Kamagata Y, Nakamura K, Mikami E. Vitamin B12 production by Acetobacterium sp. and its tetrachloromethane-resistant mutants. J Ferment Bioeng. 1992;73:76–78. [Google Scholar]
- 20.Kengen S W, Daas P J, Duits E F, Keltjens J T, van der Drift C, Vogels G D. Isolation of a 5-hydroxybenzimidazolyl cobamide-containing enzyme involved in the methyltetrahydromethanopterin:coenzyme M methyltransferase reaction in Methanobacterium thermoautotrophicum. Biochim Biophys Acta. 1992;1118:249–260. doi: 10.1016/0167-4838(92)90282-i. [DOI] [PubMed] [Google Scholar]
- 21.Klecka G M, Gonsior S J. Reductive dechlorination of chlorinated methanes and ethanes by reduced iron(II) porphyrins. Chemosphere. 1984;13:391–402. [Google Scholar]
- 22.Kriegman-King M R, Reinhard M. Transformation of carbon tetrachloride by pyrite in aqueous solution. Environ Sci Technol. 1994;28:692–700. doi: 10.1021/es00053a025. [DOI] [PubMed] [Google Scholar]
- 23.Krone U E, Thauer R K, Hogenkamp H P C. Reductive dehalogenation of chlorinated C1-hydrocarbons mediated by corrinoids. Biochemistry. 1989;28:4908–4914. doi: 10.1021/bi00452a027. [DOI] [PubMed] [Google Scholar]
- 24.Krone U E, Laufer K, Thauer R K, Hogenkamp H P C. Coenzyme F430 as a possible catalyst for the reductive dehalogenation of chlorinated C1 hydrocarbons in methanogenic bacteria. Biochemistry. 1989;28:10061–10065. doi: 10.1021/bi00452a027. [DOI] [PubMed] [Google Scholar]
- 25.Krone U E, Thauer R K, Hogenkamp H P C, Steinbach K. Reductive formation of carbon monoxide from CCl4 and freons 11, 12, and 13 catalyzed by corrinoids. Biochemistry. 1991;30:2713–2719. doi: 10.1021/bi00224a020. [DOI] [PubMed] [Google Scholar]
- 26.Krzycki J, Zeikus J G. Quantification of corrinoids in methanogenic bacteria. Curr Microbiol. 1980;3:243–245. doi: 10.1007/BF02602456. [DOI] [PubMed] [Google Scholar]
- 27.Lettinga G, van Velsen A F M, Hobma S W, de Zeeuw W, Klapwijk A. Use of upflow sludge blanket (USB) reactor concept for biological wastewater treatment, especially for anaerobic treatment. Biotechnol Bioeng. 1980;22:699–734. [Google Scholar]
- 28.Lewis T A, Crawford R L. Physiological factors affecting carbon tetrachloride dehalogenation by the denitrifying bacterium Pseudomonas sp. strain KC. Appl Environ Microbiol. 1993;59:1635–1641. doi: 10.1128/aem.59.5.1635-1641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis T A, Morra M J, Brown P D. Comparative product analysis of carbon tetrachloride dehalogenation catalyzed by cobalt corrins in the presence of thiol or titanium(III) reducing agents. Environ Sci Technol. 1996;30:292–300. [Google Scholar]
- 30.Long J L, Stensel H D, Ferguson J F, Strand S E, Ongerth J E. Anaerobic and aerobic treatment of chlorinated aliphatic compounds. J Environ Eng. 1993;119:300–320. [Google Scholar]
- 31.Mägli A, Wendt M, Leisinger T. Isolation and characterization of Dehalobacterium formicoaceticum gen. nov. sp. nov., a strictly anaerobic bacterium utilizing dichloromethane as source of carbon and energy. Arch Microbiol. 1996;166:101–108. [Google Scholar]
- 32.Mazumder T P, Nishio N, Fukuzaki S, Nagai S. Production of extracellular vitamin B12 compounds from methanol by Methanosarcina barkeri. Appl Microbiol Biotechnol. 1987;26:511–516. [Google Scholar]
- 33.Meßmer M, Wolhfarth G, Diekert G. Methyl chloride metabolism of the strictly anaerobic, methyl chloride-utilizing homoacetogen strain MC. Arch Microbiol. 1993;160:383–387. [Google Scholar]
- 34.Mikesell M D, Boyd S A. Dechlorination of chloroform by Methanosarcina strains. Appl Environ Microbiol. 1990;56:1198–1201. doi: 10.1128/aem.56.4.1198-1201.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nazhat N B, Golding B T, Johnson G R A, Jones P. Destruction of vitamin B12 by reaction with ascorbate: the role of hydrogen peroxide and the oxidation state of cobalt. J Inorg Biochem. 1989;36:75–81. [Google Scholar]
- 36.Picardal F, Arnold R G, Huey B B. Effects of electron donor and acceptor conditions on reductive dehalogenation of tetrachloromethane by Shewanella putrefaciens 200. Appl Environ Microbiol. 1995;61:8–12. doi: 10.1128/aem.61.1.8-12.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanz J L, Rodriguez N, Amils R. Effect of chlorinated aliphatic hydrocarbons on the acetoclastic methanogenic activity of granular sludge. Appl Microbiol Biotechnol. 1997;47:324–328. [Google Scholar]
- 38.Scholten J C M, Stams A J M. The effect of sulfate and nitrate on methane formation in a freshwater sediment. Antonie van Leeuwenhoek. 1995;68:309–315. doi: 10.1007/BF00874141. [DOI] [PubMed] [Google Scholar]
- 39.Scholten, J. C. M., J. Vogelaar, A. van Ittersum, K. Hordijk, W. Roelofsen, and A. J. M. Stams. Kinetics of 13C-acetate transformation in a freshwater sediment under different redox conditions. Submitted for publication.
- 40.Scholtz-Muramatsu H, Neumann A, Meßmer M, Moore E, Diekert G. Isolation and characterization of Dehalospirillum multivorans gen. nov. sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol. 1995;163:48–56. [Google Scholar]
- 41.Stromeyer S A, Strumpf K, Cook A M, Leisinger T. Anaerobic degradation of tetrachloromethane by Acetobacterium woodii: separation of dechlorinative activities in cell extracts and roles for vitamin B12 and other factors. Biodegradation. 1992;3:113–123. [Google Scholar]
- 42.Stupperich E, Steiner L, Rühlemann M. Isolation and analysis of bacterial cobamides by high-performance liquid chromatography. Anal Biochem. 1986;155:365–170. doi: 10.1016/0003-2697(86)90447-1. [DOI] [PubMed] [Google Scholar]
- 43.Utkin I, Dalton D D, Wiegel J. Specificity of reductive dehalogenation of substituted ortho-chlorophenols by Desulfitobacterium dehalogenans JW/IU-DC1. Appl Environ Microbiol. 1995;61:1677. doi: 10.1128/aem.61.4.1677-1677c.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogel T M, Criddle C S, McCarty P L. Transformation of halogenated compounds. Environ Sci Technol. 1987;21:722–736. doi: 10.1021/es00162a001. [DOI] [PubMed] [Google Scholar]
- 45.Zehnder A J B, Huser B A, Brock T D, Wuhrmann K. Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1980;124:1–11. doi: 10.1007/BF00407022. [DOI] [PubMed] [Google Scholar]