Abstract
Laboratory conditions have been identified that cause the rapid death of cultures of cyanobacteria producing urease. Once the death phase had initiated in the stationary growth phase, cells were rapidly bleached of all pigmentation. Null mutations in the ureC gene, encoding the alpha subunit of urease, were constructed, and these mutants were no longer sensitive to growth in the presence of urea. High levels of peroxides, including lipid peroxides, were detected in the bleaching cells. Exogenously added polyunsaturated fatty acids triggered a similar death response. Vitamin E suppressed the formation of peroxides and delayed the onset of cell bleaching. The results suggest that these cyanobacterial cells undergo a metabolic imbalance that ultimately leads to oxidative stress and lipid peroxide formation. These observations may provide insights into the mechanism of sudden cyanobacterial bloom disappearance in nature.
Cyanobacteria are morphologically and physiologically diverse eubacterial phototrophs which perform oxygen-evolving photosynthesis. Certain cyanobacteria, such as species of Anabaena, Microcystis, Trichodesmium, and Synechococcus, are capable of forming massive blooms (26). In the spring and summer, when nutrients are abundant, blooms occur as the water temperature rises. Numerous field investigators have reported that cyanobacterial blooms sometimes disappear suddenly from lake or sea surfaces and that such disappearances can occur as rapidly as overnight (1, 21, 23). Three hypotheses have been proposed to explain the fates of such cyanobacterial blooms: (i) the cyanobacterial cells are lysed by virus infection; (ii) the cells are consumed by predators; and (iii) the bloom at the water surface is dispersed by mixing with bottom layers due to storms or tidal changes (21). However, no satisfying explanation for the large-scale disappearance or death of cyanobacterial blooms has yet been established. Considering the potential threat to both animals and humans posed by toxins produced by bloom-forming cyanobacteria (22), the ability to trigger such disappearances could have both economic and human health-related significance. In this study, we discovered growth conditions that promote catastrophic cell death during the stationary growth phase of both marine and freshwater cyanobacteria. On the basis of our laboratory observations, we propose a novel explanation for the massive, rapid die-off of cyanobacteria in natural blooms.
MATERIALS AND METHODS
Chemicals.
Oleic acid, linoleic acid, and α-linolenic acid were kindly provided by John H. Golbeck (The Pennsylvania State University). The purity of these free fatty acids, which were sealed under nitrogen in ampoules and had been analyzed at the Hormel Institute, University of Minnesota, was >99%. The sodium salt of oleic acid (O-7501), the sodium salt of linoleic acid (L-8134), ricinoleic acid (R-7257), cis-12-octadecenoic acid (O-8881), linoleladic acid (trans-9, trans-12-octadecadienoic acid; L-2126), (±)-α-tocopherol (vitamin E; T-3251), butylated hydroxytoluene (BHT; B-1378), methyl viologen (paraquat dichloride; M-2254), and xylenol orange (X-0127) were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Organisms and culture conditions.
A laboratory wild-type strain of Synechococcus sp. strain PCC 7002, designated strain PR6000, was originally obtained from S. E. Stevens, Jr., and has been maintained at The Pennsylvania State University. Mutant strain PR6080 of Synechococcus sp. strain PCC 7002, lacking polyunsaturated fatty acids, was constructed by interposon mutagenesis (20). A DNA fragment encoding the aminoglycoside 5′-phosphotransferase gene (aphII) of Tn5 was inserted into a unique restriction site within the cloned desA gene, encoding acyl-lipid Δ12 desaturase of Synechococcus sp. strain PCC 7000. Cells were grown photoautotrophically in medium A+ containing 1 mg of NaNO3 ml−1 (24) or medium A-U containing 50 mM urea (19) under constant illumination from cool-white fluorescent lamps (250 microeinsteins m−2 s−1) with aeration from 1% (vol/vol) CO2 in air at 38°C. For the selection of kanamycin-resistant mutants, kanamycin (50 μg ml−1) was added to the medium. When appropriate, 5 μM NiSO4 was added to the growth medium. Liquid cultures (25 ml) were incubated in glass culture tubes (22 by 175 mm). Cell growth was monitored by determining optical density at 550 nm (OD550) with a Spectronic 20 spectrophotometer (Milton Roy, Rochester, N.Y.). A cell suspension from an exponential-phase culture grown at 38°C in medium A+ at a light intensity of 250 microeinsteins m−2 s−1 with an OD550 of 1.0 contained 3.4 ± 0.3 μg of chlorophyll ml−1 and (1.0 ± 0.2) × 108 cells ml−1 as determined by microscopic count (19). Colony-forming activity was measured by plating cells on medium A+ solidified with 1.5% (wt/vol) Difco Bacto Agar. Colonies were counted after 10 days of growth under constant illumination.
The freshwater cyanobacteria Synechococcus sp. strain PCC 6301, Synechocystis sp. strain PCC 6803, and Anabaena sp. strain PCC 7120 were obtained from the Pasteur Culture Collection and grown under 120 microeinsteins m−2 s−1 in medium B-HEPES (3). When appropriate, sodium nitrate was replaced with urea as a nitrogen source.
Cloning and insertional mutagenesis of the ureC gene in Synechococcus sp. strain PCC 7002.
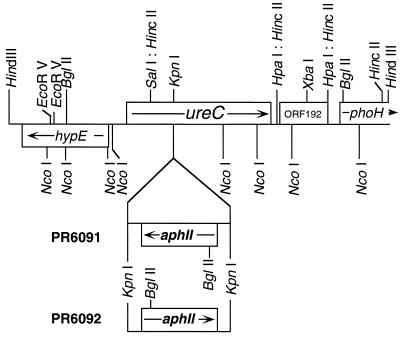
A 4.5-kb HindIII fragment was isolated from a genomic library of Synechococcus sp. strain PCC 7002 by cross-hybridization with a 1.0-kb portion of the ureC gene of Synechocystis sp. strain PCC 6803. The screening probe was amplified by PCR using genomic DNA from Synechocystis sp. strain PCC 6803 as the template and the following specific primers: forward, 5′-GGC AAT GGC AGT CAA CC; reverse, 5′-ATC CCG TTT GGT TAA CT (10). The ureC gene was insertionally inactivated by insertion of a 1.4-kb KpnI fragment derived from plasmid pRL170 (4) and containing the aphII gene of Tn5 into a unique KpnI site within the ureC coding region. Wild-type cells of Synechococcus sp. strain PCC 7002 (strain PR6000) were transformed with plasmid DNA containing the interrupted gene essentially described by Stevens and Porter (25). Kanamycin-resistant transformants were selected on plates with medium A+ supplemented with 50 μg of kanamycin ml−1. The ureC mutants were designated strain PR6091, in which the transcription orientation of the aphII gene is the opposite of that of ureC, and strain PR6092, in which the transcription orientation of the aphII gene is the same as that of the ureC gene (Fig. 1).
FIG. 1.
Physical map of the 4.5-kb HindIII fragment encoding the ureC gene of Synechococcus sp. strain PCC 7002. Arrows indicate the direction of transcription. The aphII gene, which encodes aminoglycoside 3′-phosphotransferase II and confers resistance to kanamycin, was inserted into the unique KpnI site of the ureC gene in both orientations. The ureC mutants were designated strain PR6091, in which the transcription orientation of the aphII gene is the opposite of that of ureC, and strain PR6092, in which the transcription orientation of the aphII gene is the same as that of the ureC gene.
Detection of ammonium.
The concentration of ammonium ions in the culture medium was determined by using the Nessler reagent (Aldrich, Milwaukee, Wis.). The culture medium was diluted 1,000-fold, and 1/20 volume of Nessler reagent was added. The A395 was immediately measured, and the ammonium concentration was determined from a standard curve of 25 to 200 μM ammonium chloride (12).
Detection of hydroperoxides.
The level of hydroperoxides in intact cyanobacterial cells was measured by the ferrous oxidation-xylenol orange method that was originally developed for detection of hydrogen peroxide (H2O2) in the nanomolar range and that has been applied for the detection of lipid hydroperoxides in yeast cells (2). Cells were collected by centrifugation, and the pellet was suspended in 0.8 ml of methanol containing 0.01% (wt/vol) BHT. After addition of 0.1 ml of reagent A [2.5 mM ammonium ferrous(II) sulfate; 0.25 M sulfuric acid] and 0.1 ml of reagent B (40 mM BHT; 1.25 mM xylenol orange in methanol), samples were quickly centrifuged to remove cell debris. The A560 of the supernatant was measured immediately. The amount of hydroperoxides was calculated from an apparent extinction coefficient (E560, 4.3 × 104 M−1 cm−1) (7, 8).
Polyunsaturated fatty acid sensitivity.
Cells of desA mutant strain PR6080 (20) were grown overnight in medium A-U containing 50 mM urea and 50 μg of kanamycin ml−1 under 250 microeinsteins of light m−2 s−1 and 1% CO2 conditions at 38°C. The cell suspension was diluted with fresh medium A-U to produce an OD550 of 1.0. Free fatty acids in methanol (25 μl) were added to the cell suspension (25 ml) to produce a final calculated concentration of 0.5 mM. Fatty acid sodium salts were dissolved in water and added to the cell suspensions to produce a final concentration of 0.5 mM. Changes in cell coloration were observed after overnight incubation under standard growth conditions.
Paraquat sensitivity.
Wild-type cells of Synechococcus sp. strain PCC 7002 were grown photoautotrophically in medium A+ under standard growth conditions at 38°C overnight. Freshwater strains Synechococcus sp. strain PCC 6301, Synechocystis sp. strain PCC 6803, and Anabaena sp. strain PCC 7120 were grown in medium B-HEPES. The cell suspension was diluted in the same medium to an OD550 of 0.5. Paraquat solutions in water (25 μl) were added to 25 ml of the cell suspension to final concentrations of 1 μM to 0.5 mM. After overnight incubation under standard growth conditions, visible changes in cell coloration were determined.
Nucleotide sequence accession number.
The complete nucleotide sequence of the 4.5-kb HindIII fragment encoding the ureC gene of strain PCC 7002 was determined and has been submitted to GenBank (accession no. AF035751).
RESULTS
Death and peroxidation of cells grown with urea as the N source.
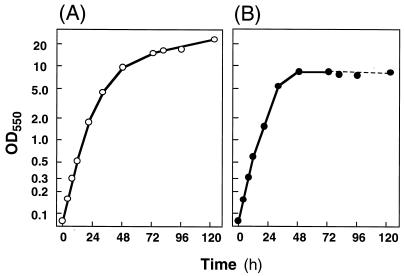
Under our standard laboratory growth conditions, the wild-type PR6000 strain of the unicellular marine cyanobacterium Synechococcus sp. strain PCC 7002 is grown in medium A+ containing nitrate as the nitrogen source. Figure 2A shows a typical growth curve for this organism under these conditions. When grown at 38°C at 250 microeinsteins m−2 s−1, wild-type strain PR6000 had a doubling time of about 4 h. During the mid- to late-exponential growth phase (OD550, 4 to 16; Fig. 2A), the colony-forming activity of cultures was constant at (4.4 ± 0.4) × 107 colonies ml−1 (OD550)−1 (n = 6). After 5 days of incubation, the OD550 typically reached 21 ± 2 (n = 11) and cell growth stopped. Although little or no change in the OD550 was observed, the colony-forming activity of cultures in stationary phase decreased but was still greater than 6 × 106 colonies ml−1 (OD550)−1 (n = 4).
FIG. 2.
Growth curves of Synechococcus sp. strain PCC 7002 in medium A+ (A) and in medium A-U (B). In medium A-U, cells entered the death phase after growth in culture for 72 h.
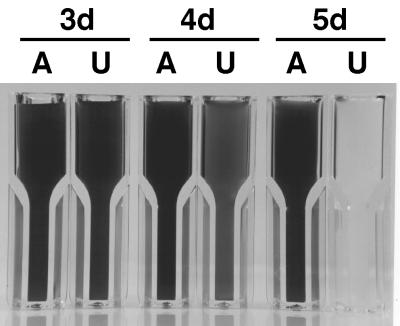
When strain PR6000 cells were grown in medium A-U containing 50 mM urea as the N source, the doubling time decreased to 3.5 h under otherwise standard growth conditions (Fig. 2B). The colony-forming activity of such cultures during the mid- to late-exponential growth phase (OD550, 3 to 6) was (6.5 ± 0.7) × 107 colonies ml−1 (OD550)−1 (n = 4). After the OD550 reached 10 ± 2 (n = 18), cell growth ceased. In stationary phase, the color of the culture changed from the normal blue-green to yellowish green, and this change consistently occurred 1 to 3 days after the culture reached stationary phase (Fig. 3). This chlorosis only occurred at OD550 values of greater than ∼7.5 after cultures had entered stationary phase. Once the bleaching process became visibly detectable, no colony-forming activity could be detected and the cells were rapidly (within 18 h) bleached of all pigmentation.
FIG. 3.
Bleaching of pigmentation of Synechococcus sp. strain PCC 7002 during stationary phase. Wild-type cells of Synechococcus sp. strain PCC 7002 (strain PR6000) were grown in medium A+ (lane A, control) and in medium A-U (lane U). 3d, cells 3 days after initial inoculation, in the stationary growth phase. 4d, 4 days after initial inoculation, in the initial stages of bleaching or the death phase. 5d, completely bleached cells 5 days after initial inoculation, at the end of the death phase.
When the concentration of urea in medium A-U was increased to 100 to 200 mM, the chlorosis and death phase occurred more quickly as the urea concentration increased. For Synechococcus sp. strain PCC 7002, the death phase in stationary phase was not initiated when the concentration of urea in medium A-U was reduced to 25 mM or less. At 5 mM urea, the cell color also changed from the normal blue-green to a chlorotic yellowish green. However, this change of cell color was not due to the initiation of cell death but rather was induced by nitrogen limitation. The chlorotic cells obtained in stationary phase after growth on medium containing 5 mM urea could recover normal cell coloration and growth when the cells were transferred into fresh A+ medium (nitrate as the N source). No ammonium was detected in the culture medium in stationary phase when the initial concentration of urea was 5 mM. When the initial concentration of urea in medium A-U was more than 5 mM, ammonium ions (≥1 mM) could be detected in the growth medium in the stationary growth phase (data not shown). However, the death phase was not triggered by the presence of ammonium ions in the medium during stationary phase, since addition of up to 100 mM ammonium chloride to stationary-phase cells grown in medium A+ did not cause cell bleaching or entrance into the death phase. Interestingly, the death phase for Synechococcus sp. strain PCC 7002 also required elevated CO2 conditions (1% [vol/vol] CO2 in air). Neither bleaching nor cell death occurred when cells were bubbled with air levels of CO2 (0.03%) and grown in medium A-U (50 mM urea). When 5 μM NiSO4 was added to medium A-U, the death phase occurred more quickly. Since nickel and carbon dioxide are components of the active site of urease (15), the elevated CO2 and Ni2+ could enhance the urease activity in cyanobacterial cells and thereby trigger the death phase.
In solution, hydrolysis of urea results in a net increase in pH. However, the external pH of cultures after cell death had occurred in the urea-containing medium was essentially unchanged at pHs 7.8 to 8.3. Thus, a shift of pH to one that would promote cell death in the external medium is not the cause of cell death during growth on urea. On the other hand, cells were killed during growth on NH4Cl if adequate buffering was not provided because of acidification of the external medium resulting from ammonia utilization (data not shown). When cells were grown on 5 mM NH4Cl in medium A, the pH of the medium decreased to less than 5.0 and cells were killed and bleached. This acidification of the medium during growth on NH4Cl was easily remedied by increasing the buffering capacity of the growth medium by addition of HEPES-NaOH to the medium. When growth medium containing 5 mM NH4Cl was buffered with 12.5 to 200 mM HEPES-NaOH (pH 7.5), the pH of the growth medium was maintained above neutrality and no cell death occurred.
To test for possible lethal changes in the composition of medium A-U after cell death, fresh cells grown in medium A+ were inoculated into the supernatant of culture medium A-U after the debris from dead cells had been removed by centrifugation. Wild-type cells grew in this conditioned medium under standard growth conditions, and rapid cell death was not observed. When cell debris was collected by centrifugation and added to fresh, mid-exponential-phase cultures in medium A+, cell death did not occur. These results demonstrate that nutrients were not limiting in conditioned medium A-U and that no toxic compound(s) capable of causing rapid cell death remained in the culture medium or cell debris after cell death had occurred. These observations are consistent with the notion that the death phase is initiated by an intracellular event or process.
Similar results were obtained with the freshwater cyanobacteria Synechocystis sp. strain PCC 6803 and Anabaena sp. strain PCC 7120, two strains that can also synthesize urease and utilize this compound as the sole nitrogen source for growth (10, 11, 13). Anabaena sp. strain PCC 7120 was much more sensitive to urea and was killed when 5 mM urea was added to the growth medium, irrespective of the CO2 level supplied. Synechocystis sp. strain PCC 6803 exhibited intermediate sensitivity to urea and was killed when at least 25 mM urea was added to the growth medium. Interestingly, Synechococcus sp. strain PCC 6301, which cannot utilize urea as the sole nitrogen source and does not appear to synthesize urease (11), was not killed by urea concentrations of up to 200 mM, and no growth inhibition was detectable when growth media were supplemented with urea at concentrations of up to 100 mM. These results suggested that a direct or indirect product of urease activity causes cell death.
Urease deletion mutant.
To test directly the involvement of urease in the causation of cell death during growth on urea, urease null mutants were constructed by insertional inactivation of the ureC gene, encoding the α subunit of urease, in Synechococcus sp. strain PCC 7002. Both ureC disruption mutants, strains PR6091 and PR6092 (Fig. 1), did not grow at all on urea-containing medium. This indicates that the ureC gene product is essential for cell growth on urea as the sole nitrogen source and that these mutants had lost all urease activity. When these ureC disruption mutants were grown in medium A+ supplemented with 100 mM urea and 5 μM NiSO4, the mutant cells did not die, even after prolonged incubation in stationary phase, although wild-type cells were killed by this growth medium (Fig. 4). These results clearly demonstrate that hydrolysis of urea by urease is required for the urea-mediated cell death phenomenon.
FIG. 4.
Resistance of ureC mutants to urea in stationary phase. Wild-type cells of Synechococcus sp. strain PCC 7002 (strain PR6000) (lane A, control) and cells of ureC mutant strains PR6091 (lane B) and PR6092 (lane C) were grown in medium A+ supplemented with 100 mM urea and 5 μM NiSO4 for 5 days. The mutant cells did not die and were not bleached, even after prolonged incubation (up to 11 days), although wild-type cells were killed and bleached after 3 days when grown in this medium.
Lipid peroxide formation during the death phase.
It is generally believed that membrane lipids are major targets for cellular damage induced by oxygen radicals (9). The photosynthetic or thylakoid membranes in many cyanobacteria contain high levels of polyunsaturated fatty acids (16) which are easily oxidized to form lipid peroxides (9). Since the bleached cultures of Synechococcus sp. strain PCC 7002 in medium A-U had a strong odor resembling that of mushrooms (determined empirically by questioning laboratory members), it was thus reasoned that the death conditions were causing the formation of volatile products, the so-called “green notes” (6), derived from oxidative degradation of polyunsaturated fatty acids. Therefore, the levels of lipid peroxides of whole cells grown in medium A-U during the death phase were measured. Peroxide levels increased dramatically as chlorosis increased. In cells for which the onset of chlorosis or bleaching was first detected (Fig. 3, 4d, U), peroxide levels were about 0.4 ± 0.1 nmol ml−1 (OD550)−1 (this level increased to 2.2 ± 0.7 nmol ml−1 [OD550]−1) in cells that had undergone 15 to 18 h of bleaching (Fig. 3, 5d, U). Peroxide levels were below the detection limit (0.1 nmol ml−1 [OD550]−1) in stationary-phase cells grown in medium A+ or in exponential-phase cells grown in medium A-U.
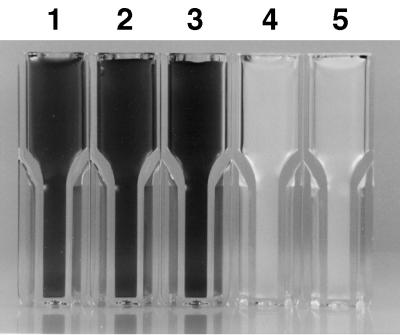
FIG. 5.
Effects of polyunsaturated fatty acids on cell death and pigment oxidation. Cells of desA mutant strain PR6080 containing no polyunsaturated fatty acids (14) were grown at 38°C in medium A-U with 1% (vol/vol) CO2 in air, and the culture (OD550, ∼1.0) was divided into five equal aliquots. The aliquots were incubated under the same growth conditions overnight with no addition (lane 1), with 0.1% (vol/vol) methanol (lane 2), with 18:1 (9) fatty acid (lane 3), with 18:2 (9, 12) fatty acid (lane 4), or with 18:3 (9, 12, 15) fatty acid (lane 5). The calculated final concentration of free fatty acids was 0.5 mM. Cells in lanes 4 and 5 were killed and completely bleached of all pigmentation.
When 0.5 mM vitamin E, an antioxidant and a scavenger of radicals, was added to wild-type cells in medium A-U at 24-h intervals after 3 days of growth, the onset of the death phase was delayed by 24 to 36 h. However, once the death phase had been initiated, addition of vitamin E did not stop the progressive cell bleaching, which led to complete death in a manner similar to that observed in the absence of vitamin E. Daily addition of vitamin E was required to observe a significant delay in the onset of the death phase.
Rapid cell death triggered by polyunsaturated fatty acids.
The results presented above suggest that growth in the presence of a high concentration of urea ultimately leads to the formation of toxic lipid peroxides and that all cellular pigmentation is rapidly destroyed by oxidative reactions under these conditions. To investigate the toxicity of fatty acids for cyanobacterial cells, we initially tested the effects of various fatty acids by using mutant strain PR6080, which contains no polyunsaturated fatty acids (20). Oleic acid [18:1 (9)], its sodium salt, cis-12-octadecenoic acid [18:1 (12)], and linoleladic acid [trans-9, trans-12-octadecenoic acid; 18:2 (trans-Δ9, trans-Δ12)] all had little or no effect on the cyanobacterial cells, and no obvious changes were observed in cultures after overnight incubation with these fatty acids (data not shown). Linoleic acid [18:2 (9, 12)], linoleic acid-sodium salt α-linolenic acid [18:3 (9, 12, 15)], and ricinoleic acid [18:1 (9, Δ12-OH)] triggered rapid cell death. Within 18 h of the addition of these fatty acids to cultures, no surviving cells could be detected and all pigmentation was completely bleached (Fig. 5). Hence, fatty acids with a high propensity to form lipid peroxides, linoleic acid and its derivatives (9), triggered cell death and peroxidation while fatty acids with a low propensity to form lipid peroxides did not. Cell death and bleaching occurred after addition of polyunsaturated fatty acids whether the cells were incubated under light or dark conditions. However, the cell suspension remained blue after overnight incubation in the dark with linoleic acid 18:2 (9, 12) (data not shown). This result suggests that phycobiliproteins were less susceptible to bleaching under these conditions than chlorophyll. Wild-type Synechococcus sp. strains PCC 7002 and PCC 6301, Synechocystis sp. strain PCC 6803, and Anabaena sp. strain PCC 7120 were also sensitive to 18:2 but not 18:1 fatty acids.
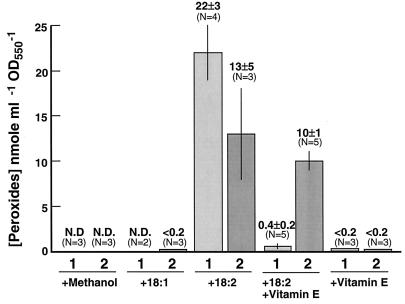
Figure 6 shows the levels of peroxides induced by addition of free fatty acids to cells of desA mutant strain PR6080. The formation of peroxides, especially lipid peroxides, correlates precisely with cell death and pigment bleaching. For example, oleic acid [18:1 (9)] did not cause cell death and did not induce peroxide formation, whereas linoleic acid [18:2 (9, 12)] triggered cell death and promoted formation of peroxides. Interestingly, vitamin E suppressed the formation of peroxides. These results strongly suggest that peroxidation of polyunsaturated fatty acids is involved in the killing phenomenon triggered by polyunsaturated fatty acids.
FIG. 6.
Effect of free fatty acids on cellular peroxide formation. Cells of desA mutant strain PR6080 containing no polyunsaturated fatty acids (6) were grown at 38°C in medium A-U on 1% (vol/vol) CO2 in air, and the culture (OD550, ∼1.0) was divided into five equal aliquots. Peroxide levels were determined as described in Materials and Methods after 20 h (column 1) or 40 h (column 2) of incubation with 0.1% (vol/vol) methanol (the solvent for the fatty acid additions), 18:1 (9), 18:2 (9, 12) with or without vitamin E, or vitamin E alone, as indicated. The calculated fatty acid concentration was 0.5 mM. N.D., none detected.
To investigate the possibility that toxic compounds were accumulating in the growth medium, linoleic acid [18:2 (9, 12)] was added to a culture of desA mutant strain PR6080, which was isolated by a dialysis bag having a pore size capable of passing compounds with molecular weights of 6,000 to 8,000. When linoleic acid was added to cells inside the bag, only the cells inside the bag were killed. Correspondingly, addition of linoleic acid to the cells outside the bag killed only the cells outside the dialysis bag (Fig. 7). These results demonstrate that direct contact between cells and the free fatty acids is required for initiation of cell death by linoleic acid. Any toxic compound, should one exist, must have a nominal molecular weight greater than approximately 8,000. Considering that the added fatty acids formed visible, floating droplets on the surface of the medium, these results suggest that direct contact with the fatty acids is required to initiate cell death.
FIG. 7.
Dialysis bag experiment. Cells of desA mutant strain PR6080 containing no polyunsaturated fatty acids (6) were grown at 38°C in medium A-U (150 ml in a glass tube [34 by 295 mm]) with 1% (vol/vol) CO2 in air (OD550, approximately 1), and 25 ml of the culture was placed into a dialysis bag (17.5-mm diameter; molecular weight cutoff, 6,000 to 8,000). Linoleic acid [18:2 (9, 12); 150 μl of a 0.5 M solution in methanol] was added to the cell culture inside the dialysis bag (A) or to that outside the bag (B). The calculated final concentration of free fatty acids was 0.5 mM, but lipid droplets could be seen floating on the surface of the culture medium. After 2 to 3 days of incubation under standard culture conditions, cells in contact with the fatty acid-containing solution were killed and bleached. The dialysis bags were taken from the culture tubes and photographed.
Paraquat sensitivity.
Paraquat, also called methyl viologen, is a herbicide that can be reduced through a one-electron transfer from reduced Fe-S centers associated with photosystem I, resulting in the formation of a stable cation radical. The rapid, toxic effect of paraquat on plants is due to subsequent reduction of oxygen, forming the superoxide radical anion (4a). To investigate the possible involvement of superoxide during the initiation of the death phase, the effect of paraquat on Synechococcus sp. strain PCC 7002 was tested. Addition of 0.5 mM paraquat to cultures led to complete cell death after overnight incubation under standard growth conditions. Cell growth was inhibited, but cells retained their blue-green coloration when the paraquat concentration was reduced to 100 to 200 μM. Little or no visible effect of paraquat was observed at concentrations below 10 μM. These results show that the elevation of superoxide anions, which is produced by addition of paraquat to cells, can also mimic the initiation of rapid cell death and the complete bleaching of cells which accompanies cell death during growth on urea or in the presence of polyunsaturated fatty acids. Similar results were also obtained with Synechocystis sp. strain PCC 6803, Synechococcus sp. strain PCC 6301, and Anabaena sp. strain PCC 7120, although each of these strains was much more sensitive to paraquat than was Synechococcus sp. strain PCC 7002. Synechocystis sp. strain PCC 6803 and Synechococcus sp. strain PCC 6301 were killed by 10 μM paraquat, while Anabaena sp. strain PCC 7120 was killed by 1 μM paraquat.
DISCUSSION
The studies presented here demonstrate that rapid cell death of urease-producing cyanobacteria occurs under specific growth conditions in the laboratory. The cell death and oxidation of pigmentation observed during the stationary growth phase in the presence of urea could be mimicked by addition of either polyunsaturated fatty acids or paraquat to the growth medium—treatments that induce oxidative stress. Vitamin E suppressed the bleaching and delayed the onset of the death phase. These results strongly implicate the peroxidation of lipids in the cell death and pigment oxidation phenomenon that we have observed. Paraquat causes an increase of superoxide radicals by short-circuiting photosynthetic electron transport by capturing electrons from the reduced Fe-S centers of photosystem I, thereby forming superoxide; superoxide radicals, in turn, can attack membrane lipids to form lipid peroxides (4a, 9). It remains to be determined in further studies what the primary event is that triggers the catastrophic cell death phenomenon in cyanobacterial cultures grown with urea as the N source. However, once the increase in cellular levels of superoxide radical or hydrogen peroxide has commenced and the formation of lipid peroxides is detectable, cells lose all pigmentation within 18 h. This can be explained by a peroxidative chain reaction of all intracellular materials, including membrane lipids. These reactions must occur inside the cyanobacterial cells in the postexponential growth phase, because the medium in which cells had been killed did not contain compounds capable of initiating the death phase.
Although they did not characterize it in detail, Gorham et al. (5) noted that urea promoted the death of the toxic, bloom-forming cyanobacterium Anabaena flos-aquae. It was also previously reported that growth of Synechococcus sp. strain PCC 7002 began to decline before depletion of the urea in the medium, although this observation was not characterized further (18). In our studies, the concentration of ammonium ions in the growth medium increased to significant levels (≥1 mM) in the midexponential phase when cells were cultured in medium A-U containing 50 mM urea. However, high concentrations of ammonium ions alone did not induce the peroxidative death phenomenon because when stationary-phase cells grown in medium A+ (the nitrogen source was nitrate) were transferred to a medium containing 100 mM NH4Cl at the same cell density, they retained their blue-green color. Since Synechococcus sp. strain PCC 6301, a strain that cannot use urea as the sole nitrogen source and apparently does not synthesize urease, was never observed to undergo urea-induced death (at concentrations of up to 200 mM) and Synechococcus sp. strain PCC 7002 ureC mutants were not killed when grown in the presence of urea, we conclude that urease plays the critical role in potentiating this phenomenon.
Urea is initially hydrolyzed by urease to form ammonia and carbamate, which subsequently decomposes to carbonic acid and ammonia, resulting in an increase in intracellular pH (14, 15). The increased level of ammonium ions (≥1 mM) in the medium in the midexponential phase of growth on urea suggested that hydrolysis of urea exceeded the consumption of ammonia by cells. Hydrolysis of urea presumably continues in the postexponential phase. Although the internal pH could increase as urea passively diffuses into the cells and is hydrolyzed, passive loss of NH3 from the cells could actually cause the intracellular pH to decrease significantly. In either case, a pH imbalance inside the postexponential-phase cells could either lead to cell death directly or impair the oxidative protection mechanism(s). Alternatively, such an imbalance might affect electron transport components that would lead to an increase in active oxygen species in the cells. However, it remains to be discovered precisely what causes cell death and leads to the formation of the oxidizing radicals and peroxides.
The phenomenon reported here may provide novel insights into the mechanism of the sudden disappearance of cyanobacterial blooms in nature (1, 21, 23). Considering the rapidity of bloom disappearance in the field, the majority of the research community seemingly believes that viruses cause lysis of the cells, and thus, viral infections have been extensively studied (17, 21). Our studies suggest that peroxidative chain reactions could also account for rapid bloom disappearance phenomena and that such chain reactions might be triggered by nutrient stress conditions in stationary-phase cells within the bloom. A metabolic imbalance could lead to a change in intracellular pH or the formation of active oxygen species; additionally, suppression or inactivation of the normal oxidative protection mechanism(s) might occur and thus increase intracellular active oxygen species. Alternatively, cell lysis in blooms could release polyunsaturated fatty acids that could, in turn, promote the formation of peroxides. In either case, catastrophic cell death would occur and the algal bloom would be bleached by an increase in intracellular peroxides due to a peroxidative chain reaction. The ability to promote such processes artificially could lead to better mechanisms for the control of toxic, bloom-forming cyanobacteria.
ACKNOWLEDGMENTS
This work was supported by USPHS grant GM-31625 to D.A.B. T.S. was the recipient of a postdoctoral fellowship from the Yamada Foundation (Osaka, Japan) in 1995.
We thank Tanja Gruber, Kaori Inoue, Elena Vassilieva (Penn State University), and Masayuki Ohmori (Tokyo University) for their helpful suggestions and Veronica L. Stirewalt for technical assistance.
REFERENCES
- 1.Borstad G A. The influence of the meandering Guiana Current on surface conditions near Barbados—temporal variations of Trichodesmium (Cyanophyta) and other plankton. J Mar Res. 1982;40:435–452. [Google Scholar]
- 2.Do T Q, Schultz J R, Clarke C F. Enhanced sensitivity of ubiquinone-deficient mutants of Saccharomyces cerevisiae to products of autoxidized polyunsaturated fatty acids. Proc Natl Acad Sci USA. 1996;93:7534–7539. doi: 10.1073/pnas.93.15.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubbs J M, Bryant D A. Molecular cloning and transcriptional analysis of the cpeBA operon of the cyanobacterium Pseudanabaena sp. PCC 7409. Mol Microbiol. 1991;5:3073–3085. doi: 10.1111/j.1365-2958.1991.tb01867.x. [DOI] [PubMed] [Google Scholar]
- 4.Elhai J, Wolk C P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allow cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 4a.Fridovich I, Hassan H M. Paraquat and the exacerbation of oxygen toxicity. Trends Biochem Sci. 1979;4:113–115. [Google Scholar]
- 5.Gorham P R, McLachlan J, Hammer U T, Kim W K. Isolation and culture of toxic strains of Anabaena flos-aquae (Lyngb.) de Bréb. Verh Int Verein Limnol. 1964;15:796–804. [Google Scholar]
- 6.Häusler A, Münch T. Microbial production of natural flavors. By degrading fatty acids from plants and other natural sources, microorganisms generate an array of aromas and flavors. ASM News. 1997;63:551–559. [Google Scholar]
- 7.Jiang Z-Y, Woollard A C S, Wolff S P. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids. 1991;26:853–856. doi: 10.1007/BF02536169. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Z-Y, Hunt J V, Wolff S P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 9.Kagan V E. Lipid peroxidation in biomembranes. Boca Raton, Fla: CRC Press, Inc.; 1988. [Google Scholar]
- 10.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 11.Kratz W A, Myers J. Nutrition and growth of several blue-green algae. Am J Bot. 1955;42:282–287. [Google Scholar]
- 12.Liotenberg S, Campbell D, Rippka R, Houmard J, Tandeau de Marsac N. Effect of the nitrogen source on phycobiliprotein synthesis and cell reserves in a chromatically adapting filamentous cyanobacterium. Microbiology. 1996;142:611–622. doi: 10.1099/13500872-142-3-611. [DOI] [PubMed] [Google Scholar]
- 13.Mackerras A H, Smith G D. Urease activity of the cyanobacterium Anabaena cylindrica. J Gen Microbiol. 1986;132:2749–2752. [Google Scholar]
- 14.Mobley H L T, Hausinger R P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989;53:85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mobley H L T, Island M D, Hausinger R P. Molecular biology of microbial ureases. Microbiol Rev. 1995;59:451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murata N, Nishida I. Lipids of blue-green algae (cyanobacteria) In: Stumpf P K, editor. The biochemistry of plants. Vol. 9. San Diego, Calif: Academic Press; 1987. pp. 315–347. [Google Scholar]
- 17.Ohki K, Fujita Y. Isolation of a lysogenic virus from the marine cyanophyte Phormidium persicinum. Plant Cell Physiol. 1995;36S:360. [Google Scholar]
- 18.Paone D A M, Stevens S E., Jr Nitrogen starvation and the regulation of glutamine synthetase in Agmenellum quadruplicatum. Plant Physiol. 1981;67:1097–1100. doi: 10.1104/pp.67.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakamoto T, Bryant D A. Growth at low temperature causes nitrogen limitation in the cyanobacterium Synechococcus sp. strain PCC 7002. Arch Microbiol. 1998;169:10–19. doi: 10.1007/s002030050535. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto T, Shen G, Higashi S, Murata N, Bryant D A. Alternation of low-temperature susceptibility of the cyanobacterium Synechococcus sp. PCC 7002 by genetic manipulation of membrane lipid unsaturation. Arch Microbiol. 1998;169:20–28. doi: 10.1007/s002030050536. [DOI] [PubMed] [Google Scholar]
- 21.Sellner K G. Trophodynamics of marine cyanobacteria blooms. In: Carpenter E J, Capone D G, Rueter J G, editors. Marine pelagic cyanobacteria: Trichodesmium and other diazotrophs. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 75–94. [Google Scholar]
- 22.Shimizu Y. Microalgal metabolites: a new perspective. Annu Rev Microbiol. 1996;50:431–465. doi: 10.1146/annurev.micro.50.1.431. [DOI] [PubMed] [Google Scholar]
- 23.Steven D M, Glombitza R. Oscillatory variation of a phytoplankton population in a tropical ocean. Nature (London) 1972;237:105–107. [Google Scholar]
- 24.Stevens S E, Jr, Patterson C O P, Myers J. The production of hydrogen peroxide by blue-green algae: a survey. J Phycol. 1973;9:427–430. [Google Scholar]
- 25.Stevens S E, Jr, Porter R D. Transformation in Agmenellum qudruplicatum. Proc Natl Acad Sci USA. 1980;77:6052–6056. doi: 10.1073/pnas.77.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterbury J B, Watson S W, Valois F W, Franks D G. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can Bull Fish Aquat Sci. 1986;214:71–120. [Google Scholar]