Abstract
Four β-1,4-glucanases (cellulases) of the cellulolytic bacterium Cellulomonas fimi were purified from Escherichia coli cells transformed with recombinant plasmids. Previous analyses using soluble substrates had suggested that CenA and CenC were endoglucanases while CbhA and CbhB resembled the exo-acting cellobiohydrolases produced by cellulolytic fungi. Analysis of molecular size distributions during cellulose hydrolysis by the individual enzymes confirmed these preliminary findings and provided further evidence that endoglucanase CenC has a more processive hydrolytic activity than CenA. The significant differences between the size distributions obtained during hydrolysis of bacterial microcrystalline cellulose and acid-swollen cellulose can be explained in terms of the accessibility of β-1,4-glucan chains to enzyme attack. Endoglucanases and cellobiohydrolases were much more easily distinguished when the acid-swollen substrate was used.
Cellulose hydrolysis by aerobic fungi, such as Trichoderma reesei, is usually explained in terms of the synergistic activities of endo-β-1,4-glucanases and exocellobiohydrolases. Models that describe the attack of cellulose at susceptible regions by endoglucanases, followed by cellobiohydrolase attack at the newly formed chain ends, continue to form the basis of most discussions of enzymatic cellulose hydrolysis (2, 24).
Although the occurrence of endoglucanases and cellobiohydrolases in fungi is firmly established, the extent to which the cellulase systems of aerobic bacteria resemble those from fungi was unclear until recently, because there was little evidence for the presence of cellobiohydrolases in bacteria. However, it now appears that at least some cellulolytic bacteria produce enzymes similar to the fungal cellobiohydrolases. For example, Cellulomonas fimi produces at least six β-1,4-glucanases, of which four (CenA, CenB, CenC, and CenD) are endoglucanases and two (CbhA and CbhB) appear to be cellobiohydrolases that are the functional equivalents of T. reesei CBHI and CBHII (6, 15, 21, 22). Similar cellobiohydrolases have been described for the actinomycete Thermomonospora fusca (9).
C. fimi cellobiohydrolases.
The preferential attack of cellulose at the ends of glucan chains by C. fimi cellobiohydrolases CbhA and CbhB is strongly suggested by hydrolysis experiments using cellooligosaccharides or carboxymethylcellulose (CMC) (14, 15, 21, 22). However, we lack direct evidence for exohydrolytic activity on cellulose itself. Accordingly, we have examined the activities of CbhA and CbhB on cellulose by measurement of molecular size distributions during hydrolysis. Analysis of CenA was also included to allow comparison of cellobiohydrolase and endoglucanase activities.
C. fimi CenC.
Previous studies have indicated that CenA attacks susceptible linkages in soluble CMC in a relatively nonprocessive manner (7, 14); i.e., the enzyme dissociates from the substrate after each hydrolytic event. While CenB and CenD attack CMC in a similar way (14, 23), C. fimi CenC seems to act in a more processive fashion (16, 23). Therefore, CenC activity was analyzed in order to determine if the enzyme behaves in a similarly processive manner on cellulose.
Cellulose substrates.
Previous determinations of molecular size distribution during hydrolysis have shown that the choice of substrate is an important consideration (10). In this study we used two forms of cellulose: bacterial microcrystalline cellulose (BMCC) and phosphoric acid-swollen cellulose (PASC). These celluloses were chosen in order to simplify the interpretation of data by avoiding complications due to low surface/volume ratios and substrate heterogeneity, which are associated with the use of substrates like cotton or pulp fibers (24). Both BMCC and PASC have a high surface/volume ratio (17). BMCC is a highly crystalline form of cellulose I prepared from cellulose produced by Acetobacter xylinum. PASC is produced by swelling microcrystalline cellulose in concentrated phosphoric acid; although often described as amorphous, it is probably a low-crystallinity form of cellulose II (1). Recent data suggest that cellulose I and cellulose II contain glucan chains arranged in parallel orientation (12).
MATERIALS AND METHODS
Enzymes and cellulose preparations.
The genes encoding CenA (8), CenC (23), CbhA (15), and CbhB (21) were expressed in Escherichia coli, and the corresponding enzymes were purified by cellulose affinity chromatography and anion-exchange chromatography, as previously described. BMCC was prepared from A. xylinum (ATCC 23769) (5). PASC was prepared from Avicel PH101 by using 88% (wt/vol) phosphoric acid (27).
Enzymatic hydrolysis conditions.
Reaction mixtures contained 100 μg of bovine serum albumin per ml and 1 mg of BMCC per ml or 10 mg of PASC per ml in 50 mM citrate buffer (pH 7.0)–0.02% NaN3. The enzyme concentrations used for BMCC hydrolysis were 4 nmol of CenA, 4 nmol of CenC, 2 nmol of CbhA, and 2 nmol of CbhB per ml of reaction mix; for PASC hydrolysis the concentrations used were 10 nmol of CenA, 10 nmol of CenC, 40 nmol of CbhA, and 40 nmol of CbhB per ml of reaction mix. Control reactions contained no enzyme. Reaction mixtures were incubated at 37°C with gentle shaking for 96 h, and samples were withdrawn for analysis at appropriate intervals. Samples were centrifuged at 5,000 × g for 15 min, and supernatants were removed for analysis of soluble products. The cellulose pellet was washed three times with ice-cold distilled water and dried at 60°C prior to analysis of molecular size distribution.
Analysis of soluble products.
Glucose and soluble cellooligosaccharide in the supernatants were quantified by anion-exchange chromatography on a CarboPac PA-1 column using a DX-500 high-pressure liquid chromatography system equipped with a pulsed amperometric detector (Dionex, Sunnyvale, Calif.). Samples were run in triplicate. Glucose and cellooligosaccharide standards with a degree of polymerization (DP) of 2 to 5 were from Sigma Chemical Co., St. Louis, Mo., and Seikagaku America, Inc., Rockville, Md. The percent solubilization of cellulose was calculated from the total sugars released.
Analysis of size distribution of insoluble products.
The size distributions of cellulose molecules were determined by gel permeation chromatography of their tricarbanyl derivatives. Cellulose pellets were carbanilated as previously described (20). Derivatized samples were recovered by evaporation of the reactants (26), redissolved in iso-octane and evaporated to dryness. Samples were then redissolved in tetrahydrofuran to a concentration of about 0.2 mg/ml and filtered through a Teflon membrane (0.45-μm pore size) prior to analysis. A Waters 625 liquid chromatography system (Millipore Corp., Milford, Mass.) equipped with four TSK-GEL columns (Varian, Sunnyvale, Calif.) was used for gel permeation chromatography. The columns (G1000 HXL, G3000 HXL, G4000 HXL, and G6000 HXL) were connected in series and had nominal molecular weight cutoffs of 1 × 103, 6 × 104, 4 × 105, and 4 × 107, respectively. Samples were eluted with tetrahydrofuran at a flow rate of 1 ml/min. The column series was calibrated by using polystyrene standards (25). Cellulose tricarbanilates were quantified by their absorption at 254 nm by using a Waters 486 UV spectrophotometer. The DP of cellulose was calculated by dividing the molecular weight of the tricarbanilated derivative by the molecular weight of tricarbanilated anhydroglucose (i.e., DP = Mw/519). Number-average and weight-average DPs were calculated as previously described (28).
RESULTS
Cellulose preparations (BMCC or PASC) were incubated with C. fimi endoglucanase (CenA or CenC) or cellobiohydrolase (CbhA or CbhB) for up to 96 h. Aliquots of each reaction mixture were removed at various times, and the molecular size distributions of the insoluble cellulose fractions were analyzed by size-exclusion chromatography of their tricarbanilate derivatives. The hydrolysis of cellulose was assessed by quantitative analysis of soluble cellooligosaccharides released at corresponding incubation times (Table 1); this allowed the comparison of size distributions at equivalent levels of degradation by the four enzymes.
TABLE 1.
Solubilization and DPmax during hydrolysis of BMCC and PASC by C. fimi cellulasesa
| Enzyme and incubation time (h) | BMCC
|
PASC
|
||
|---|---|---|---|---|
| Percent Solubilization (total) | DPmax | Percent Solubilization (total) | DPmax | |
| Control | 0 | 238 | 0 | 257 |
| CenA | ||||
| 0.1 | 2 | 238 | 9 | 244 |
| 2 | NDb | ND | 28 | 44 |
| 6 | 7 | 238 | 32 | ND |
| 12 | 8 | 260 | 38 | ND |
| 24 | 12 | 260 | 43 | 39 |
| 48 | 17 | 260 | 48 | ND |
| 96 | 25 | 260 | 61 | 40 |
| CenC | ||||
| 0.1 | 1 | 323 | 9 | 191 |
| 1 | ND | ND | 15 | 92 |
| 2 | ND | ND | 17 | 93 |
| 6 | 2 | 295 | 19 | ND |
| 12 | 2 | 295 | 25 | 87 |
| 24 | 2 | 295 | 28 | ND |
| 48 | 3 | 323 | 34 | 45 |
| 96 | 3 | 295 | 45 | 42 |
| CbhA | ||||
| 0.1 | 8 | 280 | 6 | 217 |
| 6 | 31 | 335 | ND | ND |
| 12 | 45 | 335 | 29 | 166 |
| 24 | 52 | 403 | 37 | 156 |
| 48 | 55 | 403 | 38 | 156 |
| 96 | 67 | 403 | 50 | 137 |
| CbhB | ||||
| 0.1 | 3 | 280 | 2 | 257 |
| 6 | 11 | 335 | ND | ND |
| 12 | 12 | 306 | 12 | 209 |
| 24 | 14 | 306 | 15 | 224 |
| 48 | 16 | 306 | 19 | 224 |
| 96 | 18 | 335 | 27 | 196 |
Analysis of soluble products.
Incubation of BMCC with CbhA for 96 h resulted in 67% solubilization (Table 1). The soluble products consisted largely of cellobiose (0.69 mg/ml). Low levels of cellotriose (≤0.01 mg/ml) were detected after incubation for up to 48 h (Fig. 1).
FIG. 1.
Release of soluble sugars during hydrolysis of BMCC by CenA, CenC, CbhA, and CbhB for up to 96 h. Glucose (G1, ▪) and cellotriose (G3, ▴) (left-hand y axis) and cellobiose (G2, •) (right-hand y axis) were analyzed by anion-exchange chromatography, as described in Materials and Methods.
Significant solubilization of BMCC by CenA (25%) and CbhB (18%) was also observed (Table 1). The soluble products were largely cellobiose (0.24 and 0.16 mg/ml, respectively) (Fig. 1). A trace of cellotriose (<0.01 mg/ml) was seen after only 5 min of incubation with CenA, but this rapidly fell to undetectable levels. With CbhB, the cellotriose level reached 0.02 mg/ml and then remained relatively constant. CenC solubilized BMCC only to a very low degree (3%) (Table 1; Fig. 1).
All enzymes efficiently hydrolyzed PASC. The total extents of solubilization after incubation with CenA, CenC, CbhA, and CbhB were 61, 45, 50, and 27%, respectively (Table 1). Cellobiose was the major product for all enzymes (5.7, 4.3, 4.9, and 2.2 mg/ml, respectively) (Fig. 2). Cellotriose was detected following incubation with CenA, CbhA, and CbhB. Oligosaccharides with DPs higher than that of cellotriose were not observed.
FIG. 2.
Release of soluble sugars during hydrolysis of PASC by CenA, CenC, CbhA, and CbhB. Glucose (G1, ▪) and cellotriose (G3, ▴) (left-hand y axis) and cellobiose (G2, •) (right-hand y axis) were analyzed by anion-exchange chromatography, as described in Materials and Methods.
Analysis of molecular size distributions of insoluble cellulose during hydrolysis.
The size distribution curves for untreated BMCC and PASC showed two maxima (Fig. 3). Major and minor peaks occurred at DPs of ∼250 and ∼18 in both cellulose preparations. The size distribution for BMCC was broader than that for PASC, as reflected in the calculated polydispersity values (weight-average DP/number-average DP) of 4.3 and 2.9, respectively. The BMCC curve also shows the presence of a small fraction of material with a DP of <7. Cellooligosaccharides of this size are normally water soluble (18). Their occurrence in the insoluble fraction suggests that they are either adsorbed to the surface of BMCC particles or occluded within them.
FIG. 3.
Molecular size distributions of untreated BMCC and PASC. DP is presented on a logarithmic scale.
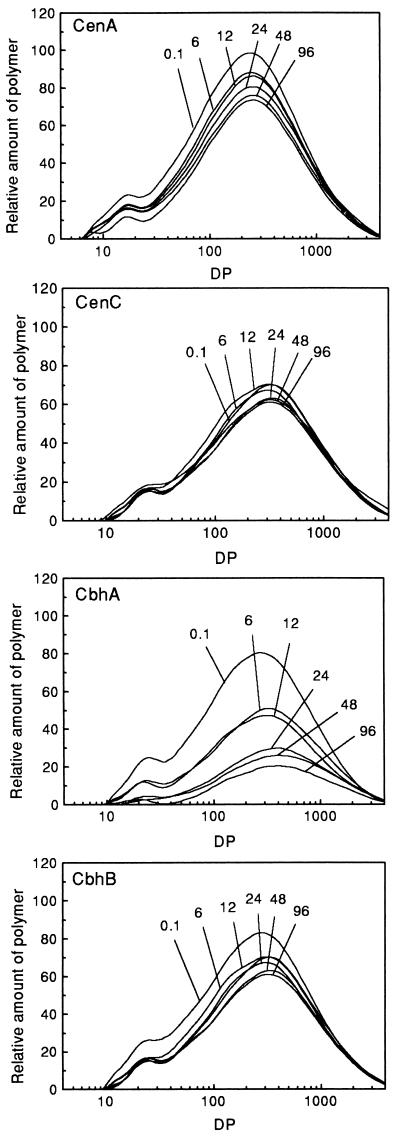
The size distribution curves for BMCC during hydrolysis are shown in Fig. 4. For all enzymes, hydrolysis was accompanied by a slight increase in the DP of the major peak maximum (Table 1). For CenA, this occurred between 6 and 12 h. More substantial shifts in the positions of the major peak maximum were seen with CenC and CbhB. The increase occurred after only a few minutes of incubation with CenC, although the total extent of substrate solubilization at this time was very low. The largest shift in the position of the major peak was seen with CbhA. No significant shift in the position of the minor peak in BMCC was observed with any of the C. fimi enzymes (Fig. 4).
FIG. 4.
Molecular size distributions of BMCC during hydrolysis by CenA, CenC, CbhA, and CbhB for up to 96 h. The incubation time (hours) corresponding to each curve is indicated. DP is presented on a logarithmic scale.
Corresponding size distribution curves during the hydrolysis of PASC are shown in Fig. 5. In contrast to the patterns observed with BMCC, a major decrease in the position of the major peak maximum occurred during incubation with CenA or CenC (Fig. 5; Table 1). A decrease in the position of the major peak maximum was also seen with CbhA and CbhB, but these shifts were significantly smaller than those with the endoglucanases. The minor peak maximum remained visible after prolonged incubation with CbhA or CbhB but was no longer evident in the CenA and CenB incubations, probably as a result of the accumulation of large quantities of low-DP material. Similar peaks of low-DP material have been observed in cellulose preparations from various sources, but their significance remains unclear (10, 11).
FIG. 5.
Molecular size distributions of PASC during hydrolysis by CenA, CenC, CbhA, and CbhB for up to 96 h. The incubation time (hours) corresponding to each curve is indicated. DP is presented on a logarithmic scale.
Comparisons of the cellulose molecular size distributions at approximately equivalent levels of enzymatic solubilization were made (Fig. 6). The data are the same as those shown in the corresponding curves in Fig. 4 and 5, but the DP axis is scaled linearly in Fig. 6. Data for hydrolysis of BMCC by CenC is excluded because the extent of solubilization after 96 h was only 3%.
FIG. 6.
Molecular size distributions of BMCC and PASC after approximately equal levels of solubilization. For BMCC, the extents of solubilization by CenA, CbhA, and CbhB were 25, 31, and 18%, respectively. For PASC, the extents of solubilization by CenA, CenC, CbhA, and CbhB were 28, 25, 29, and 27%, respectively. Further details are contained in Table 1. DP is presented on a linear scale.
The data in Fig. 6 correspond to 25, 31, and 18% solubilization of BMCC by CenA, CbhA, and CbhB, respectively. The overall shapes of DP distribution profiles were similar before and after solubilization, although hydrolysis was accompanied by a slight increase in the DP of the major peak maximum with all enzymes.
Solubilization of PASC by CenA, CenC, CbhA, and CbhB (28, 25, 29, and 27%, respectively) resulted in the loss of high-DP material and the accumulation of lower-DP material in all cases. However, the DP distribution profiles were not similar. The downward shift in the distribution profiles was much more pronounced for the endoglucanases CenA and CenC than for CbhA or CbhB (Fig. 6).
DISCUSSION
The cellulose-degrading system of C. fimi contains at least six cellulases. Four of these enzymes (CenA, CenB, CenC, and CenD) were designated as endoglucanases and two (CbhA and CbhB) were designated as cellobiohydrolases, based largely on analysis of CMC hydrolysis (6, 15, 21, 22, 24). CenC appeared to show a more processive action, relative to the other endoglucanases (23). In the present investigation we have compared the activities of CenA, CenC, CbhA, and CbhB by determining the molecular size distribution products obtained by hydrolysis of two types of insoluble cellulose preparations.
The analysis of soluble products showed that all enzymes gave cellobiose as the major product (Fig. 1 and 2), as expected for cellobiohydrolases but not necessarily for endoglucanases. In addition, low cellotriose concentrations were observed during PASC hydrolysis by CbhA and CbhB. Cellotriose is not hydrolyzed by CbhB (21); consequently, it accumulates during the incubation period. The endoglucanase CenA, which hydrolyzes cellotriose (4), produces initially higher levels of cellotriose which subsequently decline, most likely due to further hydrolysis. The results emphasize the difficulty of distinguishing the activities of the various enzymes from analysis of soluble products only.
The DP distribution of the insoluble products after BMCC hydrolysis showed a moderate shift towards higher DP for all four enzyme treatments (Fig. 4; Table 1). A similarly small upward shift in the position of the major peak maximum was also observed during hydrolysis of BMCC by T. reesei CBHII (11). The DP distribution profiles for BMCC during separate treatment by the four enzymes in the present work show a striking similarity, and consequently the modes of action of the different enzymes were not distinguished when BMCC was used as the substrate. In the PASC incubations, however, a different pattern was seen (Fig. 5; Table 1). During the incubation with endoglucanases, a major decrease of the DP position of the major peak maximum was observed. A significantly smaller downward shift was observed for CbhA and CbhB.
Differences in the behaviors of the four enzymes on BMCC and PASC are particularly evident in Fig. 6, where the cellulose DP distributions are compared at approximately equal levels of solubilization. For all enzymes, it is evident that substantial solubilization of BMCC occurred without major changes in the overall shapes of distribution profiles. Loss of high-DP cellulose was slightly more pronounced for CenA than for CbhA and CbhB, as expected for a randomly acting endoglucanase, but the activity of CenA is not easily distinguished from the activities of the two cellobiohydrolases when BMCC is used as the substrate.
In contrast, differences between the activities of the various enzymes were clearly seen when PASC was used as the substrate (Fig. 6). Solubilization of PASC by CenA, CenC, CbhA, and CbhB to equal levels resulted in the loss of high-DP material and the accumulation of lower-DP material in all cases, but the shifts in the distribution profiles were much more pronounced for the endoglucanases CenA and CenC than for CbhA or CbhB. The relatively small decreases in DP observed with CbhA and CbhB on PASC provide evidence that both are predominantly exo-acting enzymes, confirming a similar conclusion based on analyses of CMC hydrolysis using viscometric measurement of hydrolysis (14, 15, 21, 22). The data support our earlier suggestion that C. fimi CbhA and CbhB correspond to similar pairs of cellobiohydrolases seen in fungal cellulase systems and, more generally, that aerobic fungi and bacteria have similar types of cellulase systems (6, 22).
The differences between the molecular size distribution data obtained with BMCC and PASC can be explained by the model presented in Fig. 7. BMCC is shown as a highly crystalline substrate in which only cellulose chains on the surface are susceptible to enzyme attack. Internal glucan chains become accessible to endoglucanases and cellobiohydrolases only after extensive solubilization of overlying molecules. Consequently, differences in mode of attack by the two types of enzyme are not easily observed. However, a much larger proportion of the substrate is accessible to simultaneous attack in the case of PASC; as a result, differences in the modes of attack by the various enzymes are much more evident in size distribution analyses. PASC is clearly a better substrate than BMCC to distinguish between endo- and exo-acting cellulases, and the data show that designation of exoglucanase activity on the basis of molecular size distribution data obtained with BMCC alone (11) should be viewed with caution.
FIG. 7.
Models of PASC and BMCC, before and after 25% solubilization by C. fimi CenA and CbhB, based on molecular size distribution data. Solid lines represent individual β-1,4-glucan chains with a DP of ∼250, equal to the major peak maximum of untreated substrates. Broken or shorter lines represent shorter chains. In both forms of cellulose, the chains are shown in parallel orientation, with reducing ends towards the right. CenA and CbhB molecules are represented as filled and unfilled ovals, respectively. CenA is shown as a nonprocessive endoglucanase that attacks accessible glucan chains randomly (4); CbhB is shown as a processive exoglucanase that attacks accessible glucan chains from the reducing end (6). In PASC, most glucan chains are accessible as result of swelling and disruption of crystallinity; in contrast, only those chains on the surface of BMCC are accessible to enzymes.
In addition, the data in Fig. 6 also provide support for the previous suggestion that CenC is a relatively processive endoglucanase, showing a tendency to make several successive attacks before dissociating from the substrate to renew its attack elsewhere (23). It is anticipated that the molecular size distribution profiles for a processive endoglucanase would show characteristics that are intermediate between those of a nonprocessive endoglucanase and those of an exo-acting cellobiohydrolase. Examination of the PASC data in Fig. 6 shows that this is indeed the case for CenC. Evidence for two other relatively processive β-1,4-glucanases in bacteria was reported recently (3, 9). The role of these enzymes in the attack of cellulosic substrates is currently unknown, but it is noteworthy that starch-degrading systems include α-amylases with a high degree of processivity (13, 19).
ACKNOWLEDGMENTS
We thank Emily Kwan for expert technical assistance.
H.S. acknowledges the Svenska Institutet for travel support.
REFERENCES
- 1.Atalla R H. The structures of native celluloses. In: Suominen P, Reinikainen T, editors. Trichoderma reesei cellulases and other hydrolases. Helsinki, Finland: Foundation for Biotechnical and Industrial Fermentation; 1993. pp. 25–39. [Google Scholar]
- 2.Béguin P, Aubert J-P. The biological degradation of cellulose. FEMS Microbiol Rev. 1994;13:25–58. doi: 10.1111/j.1574-6976.1994.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 3.Christakopoulos P, Kekos D, Marcis B J, Claeyssens M, Bhat M K. Purification and characterization of a less randomly acting endo-β-d-glucanase from the culture filtrate of Fusarium oxysporium. Arch Biochem Biophys. 1995;316:428–433. doi: 10.1006/abbi.1995.1057. [DOI] [PubMed] [Google Scholar]
- 4.Damude H G, Ferro V, Withers S G, Warren R A J. Substrate specificity of endoglucanase A from Cellulomonas fimi: fundamental differences between endoglucanases and exoglucanases from family 6. Biochem J. 1996;315:467–472. doi: 10.1042/bj3150467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilkes N R, Jervis E, Henrissat B, Tekant B, Miller R C, Jr, Warren R A J, Kilburn D G. The adsorption of a bacterial cellulase and its two isolated domains to crystalline cellulose. J Biol Chem. 1992;267:6743–6749. [PubMed] [Google Scholar]
- 6.Gilkes N R, Kwan E, Kilburn D G, Miller R C, Warren R A J. Attack of carboxymethylcellulose at opposite ends by two cellobiohydrolases from Cellulomonas fimi. J Biotechnol. 1997;57:83–90. [Google Scholar]
- 7.Gilkes N R, Langsford M L, Kilburn D G, Miller R C, Jr, Warren R A J. Mode of action and substrate specificities of cellulases from cloned bacterial genes. J Biol Chem. 1984;259:10455–10459. [PubMed] [Google Scholar]
- 8.Gilkes N R, Warren R A J, Miller R C, Jr, Kilburn D G. Precise excision of the cellulose binding domains from two Cellulomonas fimi cellulases by a homologous protease and the effect on catalysis. J Biol Chem. 1988;263:10401–10407. [PubMed] [Google Scholar]
- 9.Irwin D C, Spezio M, Walker L P, Wilson D B. Activity studies of eight purified cellulases: specificity, synergism and binding domain effects. Biotechnol Bioeng. 1993;42:1002–1013. doi: 10.1002/bit.260420811. [DOI] [PubMed] [Google Scholar]
- 10.Kleman-Leyer K M, Gilkes N R, Miller R C, Jr, Kirk T K. Changes in the molecular size distribution of insoluble celluloses by the action of recombinant Cellulomonas fimi cellulases. Biochem J. 1994;302:463–469. doi: 10.1042/bj3020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleman-Leyer K M, Siika-Aho M, Teeri T T, Kirk T K. The cellulases endoglucanase I and cellobiohydrolase II of Trichoderma reesei act synergistically to solubilize native cotton cellulose but not to decrease its molecular size. Appl Environ Microbiol. 1996;62:2883–2887. doi: 10.1128/aem.62.8.2883-2887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurer A, Fengel D. Parallel orientation of the molecular chains in cellulose I and cellulose II deriving from higher plants. Holz Roh- Werkstoff. 1992;50:493. [Google Scholar]
- 13.Mazur A K, Nakatani H. Multiple attack mechanism in the porcine pancreatic alpha-amylase hydrolysis of amylose and amylopectin. Arch Biochem Biophys. 1993;306:29–38. doi: 10.1006/abbi.1993.1476. [DOI] [PubMed] [Google Scholar]
- 14.Meinke A, Gilkes N R, Kilburn D G, Warren R A J, Miller R C., Jr . Cellobiohydrolase A is a major Cellulomonas fimi β-1,4-glucanase analogous to Trichoderma reesei cellobiohydrolase II. In: Shimada K, Ohmiya K, Kobayashi Y, Hoshino S, Sakka K, Karita S, editors. Genetics, biochemistry and ecology of lignocellulose degradation. Tokyo, Japan: Uni Publishers; 1993. pp. 286–297. [Google Scholar]
- 15.Meinke A, Gilkes N R, Kwan E, Kilburn D G, Warren R A J, Miller R C., Jr Cellobiohydrolase A from the cellulolytic bacterium Cellulomonas fimi is a β-1,4-exocellobiohydrolase analogous to Trichoderma reesei CBH II. Mol Microbiol. 1994;12:413–422. doi: 10.1111/j.1365-2958.1994.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 16.Moser B, Gilkes N R, Kilburn D G, Warren R A J, Miller R C., Jr Purification and characterization of endoglucanase C of Cellulomonas fimi, the cloning of its gene, and analysis of in vivo transcripts of the gene. Appl Environ Microbiol. 1989;55:2480–2487. doi: 10.1128/aem.55.10.2480-2487.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong E, Gilkes N R, Miller R C, Jr, Warren R A J, Kilburn D G. The cellulose-binding (CBDCex) domain of an exoglucanase from Cellulomonas fimi: production in Escherichia coli and characterization of the polypeptide. Biotechnol Bioeng. 1993;42:401–409. doi: 10.1002/bit.260420402. [DOI] [PubMed] [Google Scholar]
- 18.Pereira A N, Mobedshahi M, Ladisch M R. Preparation of cellodextrins. Methods Enzymol. 1988;160:26–38. [Google Scholar]
- 19.Robyt J F, French D. Multiple attack and polarity of action of porcine pancreatic alpha-amylase. Arch Biochem Biophys. 1970;138:662–670. doi: 10.1016/0003-9861(70)90394-2. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder L R, Haigh F C. Cellulose and wood pulp polysaccharides. Gel permeation chromatographic analysis. TAPPI. 1979;62:103–105. [Google Scholar]
- 21.Shen H, Gilkes N R, Kilburn D G, Miller R C J, Warren R A J. Cellobiohydrolase B (CbhB), a second cellobiohydrolase from the cellulolytic bacterium Cellulomonas fimi. Biochem J. 1995;311:67–74. doi: 10.1042/bj3110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen H, Meinke A, Tomme P, Damude H, Kwan E, Kilburn D G, Miller R C, Jr, Warren R A J, Gilkes N R. Cellulomonas fimi cellobiohydrolases. ACS Symp. 1995;618:174–196. [Google Scholar]
- 23.Tomme P, Kwan E, Gilkes N R, Kilburn D G, Warren R A J. Characterization of CenC, an enzyme from Cellulomonas fimi with both endo- and exoglucanase activities. J Bacteriol. 1996;178:4216–4223. doi: 10.1128/jb.178.14.4216-4223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomme P, Warren R A J, Gilkes N R. Cellulose hydrolysis by bacteria and fungi. Adv Microb Physiol. 1995;37:1–81. doi: 10.1016/s0065-2911(08)60143-5. [DOI] [PubMed] [Google Scholar]
- 25.Valtasaari L, Saarela K. Determination of the chain length distribution of cellulose by gel permeation chromatography using the tricarbanilate derivative. Paperi Puu. 1975;57:5–10. [Google Scholar]
- 26.Wood B F, Conner A H, Hill C G J. The effects of precipitation on the molecular weight distribution of cellulose tricarbanylate. J Appl Polymer Sci. 1986;32:3703–3712. [Google Scholar]
- 27.Wood T M. Preparation of crystalline, amorphous and dyed cellulase substrates. Methods Enzymol. 1988;160:19–25. [Google Scholar]
- 28.Yau W W, Kirkland J J, Bly D D. Modern size-exclusion liquid chromatography. New York, N.Y: John Wiley; 1979. [Google Scholar]