Abstract
The photochemical reaction center (RC) complex of Roseiflexus castenholzii, which belongs to the filamentous anoxygenic phototrophic bacteria (green filamentous bacteria) but lacks chlorosomes, was isolated and characterized. The genes coding for the subunits of the RC and the light-harvesting proteins were also cloned and sequenced. The RC complex was composed of L, M, and cytochrome subunits. The cytochrome subunit showed a molecular mass of approximately 35 kDa, contained hemes c, and functioned as the electron donor to the photo-oxidized special pair of bacteriochlorophylls in the RC. The RC complex appeared to contain three molecules of bacteriochlorophyll and three molecules of bacteriopheophytin, as in the RC preparation from Chloroflexus aurantiacus. Phylogenetic trees based on the deduced amino acid sequences of the RC subunits suggested that R. castenholzii had diverged from C. aurantiacus very early after the divergence of filamentous anoxygenic phototrophic bacteria from purple bacteria. Although R. castenholzii is phylogenetically related to C. aurantiacus, the arrangement of its puf genes, which code for the light-harvesting proteins and the RC subunits, was different from that in C. aurantiacus and similar to that in purple bacteria. The genes are found in the order pufB, -A, -L, -M, and -C, with the pufL and pufM genes forming one continuous open reading frame. Since the photosynthetic apparatus and genes of R. castenholzii have intermediate characteristics between those of purple bacteria and C. aurantiacus, it is likely that they retain many features of the common ancestor of purple bacteria and filamentous anoxygenic phototrophic bacteria.
Photosynthetic prokaryotes consist of anoxygenic photosynthetic bacteria and oxygenic cyanobacteria. Phylogenetic studies based on 16S rRNA gene sequences indicate that photosynthetic prokaryotes are widely distributed in the bacterial lineage, and one group of them, the green filamentous bacteria or filamentous anoxygenic phototrophic bacteria (16, 17), represented by Chloroflexus aurantiacus, is placed on the deepest branch among all photosynthetic bacteria (27). Filamentous anoxygenic phototrophs, therefore, have received considerable attention as an important group when considering the early evolution of photosynthetic organisms (4). The spectroscopic and biochemical properties of the photosynthetic apparatus in the group, however, have been studied only in C. aurantiacus, and the common properties of the photosynthetic apparatus in the filamentous anoxygenic phototrophic bacteria remain unclear.
The photochemical reaction center (RC) of C. aurantiacus consists of L, M, and cytochrome subunits and is closely related to those of purple bacteria (5, 30, 34, 35, 39). The genes coding for the RC subunits and peripheral light-harvesting (LH) polypeptides are located in two puf operons in C. aurantiacus. One operon includes pufLM, coding for the L and M subunits of the RC complex, respectively (35). The other operon contains pufBAC, coding for the β and α subunits of the light-harvesting complex and the cytochrome subunit of the RC complex (41). Crystallization and X-ray structure analyses of the RCs of two purple bacteria, Blastochloris viridis and Rhodobacter sphaeroides, have revealed that the core part of the RC consists of the L and M subunits, in which the special bacteriochlorophyll (BChl) dimer, the accessory BChl, bacteriopheophytin (BPhe), and quinones form an electron transport chain with twofold symmetry (8, 1, 2). Studies on the RC of C. aurantiacus have revealed that the accessory BChl binding to the M subunit, which is conserved in all purple bacteria, is replaced with a BPhe in this species (32, 35, 40). A membrane-bound cytochrome c that is closely associated with the L and M subunits works as the direct electron donor to the photo-oxidized special pair of BChls in most purple bacteria and C. aurantiacus. In purple bacteria, the rereduction of photo-oxidized hemes in the cytochrome subunit is attributed to small metalloproteins, such as a soluble c-type cytochrome (23) or a high-potential iron-sulfur protein (22). Neither a soluble cytochrome nor a high-potential iron-sulfur protein has been found in C. aurantiacus, and the rereduction of the cytochrome subunit has been attributed to a blue-copper protein, auracyanin (21). However, the photosynthetic electron transfer system in C. aurantiacus has not been completely defined, in part because the cytochrome subunits in C. aurantiacus easily detach from the L and M subunits during RC preparation (15, 25) and because of the difficulty in conducting spectroscopic analyses of electron transfer proteins in the membrane and the whole cells as a result of the large absorbance due to chlorosomes.
Two kinds of light-harvesting apparatuses are present in C. aurantiacus. The major antenna pigment, BChl c, is located in chlorosomes and has its in vitro absorption peak at around 740 nm (11). Chlorosomes are firmly attached to the inner side of the cytoplasmic membrane. The cytoplasmic membrane has a BChl a-containing light-harvesting complex, B806-866, with absorption peaks at 806 and 866 nm (11). The absorbance at 740 nm by chlorosomes is approximately 10-fold greater than that at 866 nm by the B806-866 complexes when cells are grown under low light intensity (incandescent lamp; 300 lx) and anaerobic conditions (29).
A filamentous photosynthetic bacterium, Roseiflexus castenholzii (DSM 13941, JCM 11240), described in 2002 belongs to the same phylogenetic and phenotypic group as C. aurantiacus (15). However, the organism lacks BChl c and chlorosomes typical of C. aurantiacus and the related filamentous anoxygenic phototrophs (15, 36). A similar chlorosomeless filamentous anoxygenic photosynthetic bacterium, Heliothrix oregonensis, was previously reported, but no pure culture has been available (31). R. castenholzii is therefore the first chlorosomeless filamentous anoxygenic phototrophic bacterium isolated as an axenic culture.
In the present study, we isolated and characterized the reaction center complex from R. castenholzii and also cloned and sequenced the genes for the RC proteins. Here, we report the structural and functional properties of the RC complexes in this organism and discuss the evolutionary relationships of the RC complexes of filamentous anoxygenic phototrophic bacteria and purple bacteria.
MATERIALS AND METHODS
Growth conditions.
Cells were grown anaerobically at 50°C in 02YE medium (pH 7.2) in a glass fermentor vessel (MBF-500 M; EYELA, Tokyo, Japan) for 5 days (15). Sterilized N2 gas was bubbled in the medium, and illumination was provided with a tungsten lamp (100 W) placed 5 cm from the fermentor vessel.
Purification of RC complexes from R. castenholzii.
Harvested cells (about 15 g [wet weight]) were washed twice with 10 mM MOPS (morpholinepropanesulfonic acid)-KOH buffer (pH 8.0) and resuspended in the same buffer. Cells were disrupted with a precooled French pressure cell. The disrupted cell suspension was centrifuged at 7,000 × g for 20 min to remove the debris and then ultracentrifuged at 280,000 × g for 1.5 h to sediment the membrane fraction. The membrane fraction was suspended in MOPS-KOH buffer to give an A800 of 30 and then used for the following purification.
Membranes were solubilized in 10 mM Tris-HCl (pH 8.7) containing 2% n-octyl-β-d-glucopyranoside (Sigma, St. Louis, Mo.) to give an absorbance at 870 nm of about 5.0 under ice-cold temperature conditions. The solubilized membranes were centrifuged at 150,000 × g for 10 min, and the supernatant was applied to a DEAE Sepharose Fast Flow column (1.6 by 10 cm; Pharmacia Biotech Co., Uppsala, Sweden) equilibrated with 10 mM Tris-HCl (pH 8.0) containing 0.05% Triton X-100. Using a stepwise salt gradient, pigment proteins were eluted from the column with 100 mM NaCl. The eluted fraction was concentrated by ultrafiltration (Amicon Centriflo CF-25) and subjected to preparative polyacrylamide gel electrophoresis (4-mm-thick gel). The buffer system for electrophoresis was as described by Davis (7) with slight modifications: the gel buffer contained Triton X-100 at a final concentration of 0.1%, and the acrylamide concentration in the running gel was reduced to 5.7% (33). After electrophoresis, a blue-grayish band among three pigmented bands was extracted in 10 mM Tris-HCl (pH 8.7) containing 0.05% Triton X-100 and concentrated by ultrafiltration. The same electrophoresis (2-mm-thick gel) was repeated for further purification.
Spectroscopic analysis.
Absorption spectra were recorded with a double-beam spectrophotometer (UV-3000 or UV-160; Shimadzu, Kyoto, Japan) at room temperature. The oxidized-minus-reduced difference spectra were measured by adding K3Fe(CN)6 solution to give a final concentration of 1 μM or small amounts of solid Na2S2O4.
Flash-induced absorbance changes were measured at room temperature with a single-beam spectrophotometer assembled in our laboratory, as described by Matsuura and Shimada (20). Actinic illumination (>750 nm) was provided with a xenon flash (5-μs half-maximal duration). The RC preparation was suspended in 20 mM MOPS-KOH buffer (pH 7.0) supplemented with 2 mM sodium ascorbate, 10 mM vitamin K3, and 30 mM vitamin K1. Measurements were performed anaerobically under an Ar gas flow, and 60 measurements were averaged.
Chemical measurements and amino acid sequencing.
The protein content was determined with the Bio-Rad (Hercules, Calif.) protein assay kit with bovine serum albumin as the standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method of Laemmli (19). Samples containing 1% (wt/vol) SDS and 1% (vol/vol) β-mercaptoethanol were heated at 98°C for 7 min. High-range RPN756 (Amersham Pharmacia Biotech Co.) was used as the molecular weight marker. Heme staining was done by the methods of Thomas et al. (37). The concentration of RC was estimated by the absorbance change of heme c induced by a flash, using a difference extinction coefficient of 19.5 mM−1 cm−1 (13).
The N-terminal amino acid sequence of each protein contained in the purified complex was determined by Edman degradation (10) with a model 477A protein-sequencing system (Applied Biosystems, Foster City, Calif.).
Screening and sequencing of genes encoding the RC subunits.
The chromosomal DNA of R. castenholzii was extracted according to the method of Wilson (44). Chromosomal DNA was partially digested with Sau3AI. DNA fragments of 1 to 10 kb were fractionated by electrophoresis on a 1% agarose gel and then inserted into the BamHI site of the pUC18 vector. The ligated DNAs were used for the transformation of Escherichia coli.
Two oligonucleotides, 5′-TTCTGGGTRGGGCCGTTCTACGT-3′ and 5′-GAGAGCRYRTGGAASGGRT-3′, which contain sequences that are well conserved in purple bacteria and C. aurantiacus at the 5′ end region of the L subunit and the 3′ end region of the M subunit, respectively, were used as a PCR primer set. PCR products were labeled with digoxigenin by using a random labeling kit (Roche Diagnostics, Mannheim, Germany) for use as hybridization probes. The R. castenholzii DNA library was screened by four serial colony hybridization steps, and positive colonies were then selected. The plasmid DNA was purified with a FlexiPrep kit (Pharmacia Biotech Co.), and sequencing was carried out with a dye terminator cycle sequencing kit (Pharmacia Biotech Co.) and a 310 DNA sequencer (Applied Biosystems).
Sequencing of genes encoding the light-harvesting proteins.
The pufB and pufA genes of R. castenholzii were amplified by PCR. One of the primers has the sequence 5′-ATGASGGAYAARCCNCARAAYG-3′, designed based on the determined N-terminal amino acid sequence of the β subunit of the light-harvesting complex (MTDKPQND). The other primer has the sequence 5′-GCAGCAACCAACCGACAAA-3′, derived from the sequence at the region approximately 400 bp downstream from the 5′ region of the sequenced pufL. The PCR product was cloned in plasmid pUC119 and sequenced by M13 −20 and reverse primers.
Sequence data analysis.
Analyses of the DNA sequences were performed with the DNASIS program (Hitachi, Tokyo, Japan). The amino acid sequences deduced from the nucleotide sequences of puf genes were compared to references available in the databases (DDBJ, EMBL, and GenBank). The phylogenetic distances were calculated by the neighbor-joining method with CLUSTAL W version 1.7 (38), and the trees were constructed with the NJplot (28) program. Construction of the trees was performed by the neighbor-joining method, applying the Kimura two-parameter distance for RC subunits.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to DDBJ/EMBL/GenBank with accession number AB095768.
RESULTS
Isolation of the RC complexes.
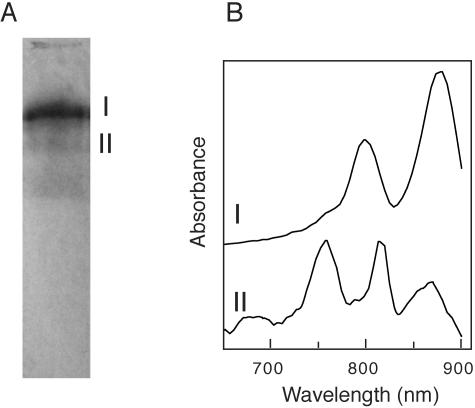
The RCs of R. castenholzii were isolated by use of an anion-exchange column and preparative PAGE. Figure 1 shows the result of preparative PAGE of the fraction eluted from the DEAE-Sepharose column with 100 mM NaCl. Among three pigmented bands, the band with the highest mobility contained carotenoids but no BChls. Two BChl-containing bands (bands I and II) were excised and extracted with 10 mM Tris-HCl (pH 8.7) containing 0.05% Triton X-100. The absorption spectrum of band II showed peaks at 867, 821, and 768 nm in the near-infrared region (Fig. 1B). This absorption spectrum was similar to that of the RC complex of C. aurantiacus (30, 34). On the other hand, the most pigmented band, band I, showed absorption peaks at 883 and 804 nm in the near-infrared region, similar to the case for the C. aurantiacus B806-866 LH complex. These absorption peaks are probably due to BChl a bound to the light-harvesting complex. In flash-excited measurements, both fractions showed fast oxidation of cytochromes. Thus, these fractions, bands II and I, seemed to contain the RC complex and RC-LH complex, respectively. Since band I appeared as a single band every time at the preparative native PAGE step, we regarded this as a stoichiometric RC-LH complex. Each fraction was further purified by another electrophoresis under the same conditions. Although some minor bands were still detected in the SDS-PAGE analysis in the RC fraction after the second preparative electrophoresis, the fraction contained three major polypeptides possibly corresponding to the L, M, and cytochrome subunits and was used as the RC preparation in further studies. No bands corresponding to the LH subunits were detected in the RC preparation (data not shown). SDS-PAGE analysis of the RC-LH complex showed five major bands, as described below, consisting of three RC subunit bands and two LH subunit bands (see Fig. 4).
FIG. 1.
Pigmented bands in preparative PAGE after anion-exchange chromatography (A) and near-infrared absorption spectra of two pigment bands containing bacteriochlorophylls (B). Two pigmented bands (I and II) were extracted in 10 mM Tris buffer (pH 8.7) containing 0.05% Triton X-100.
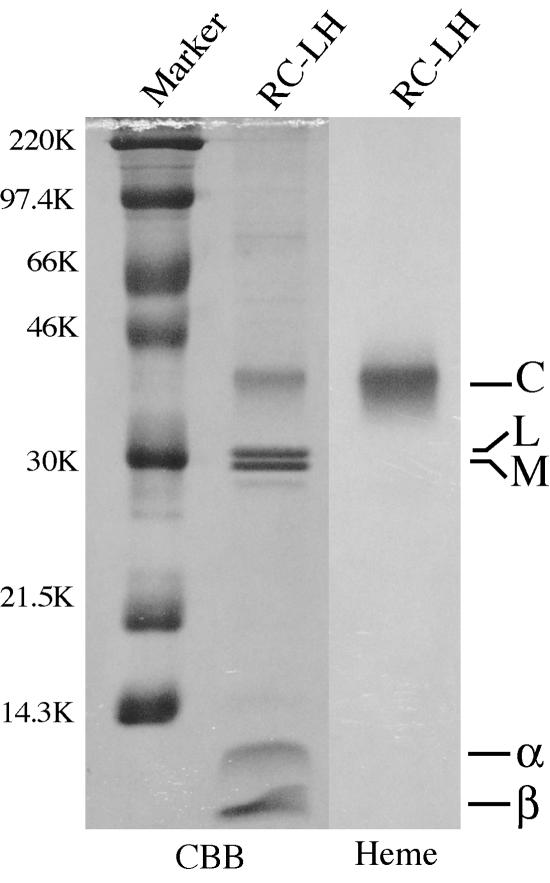
FIG. 4.
Polypeptide composition of the purified RC-LH complex. Seven micrograms of protein of the RC-LH complex was denatured by boiling for 7 min in the presence of 1% β-mercaptoethanol. After electrophoresis (15% acrylamide gel), the gel was stained with Coomassie brilliant blue (CBB) and a heme-staining reagent (Heme).
Spectroscopic and kinetic measurements of the RC complex.
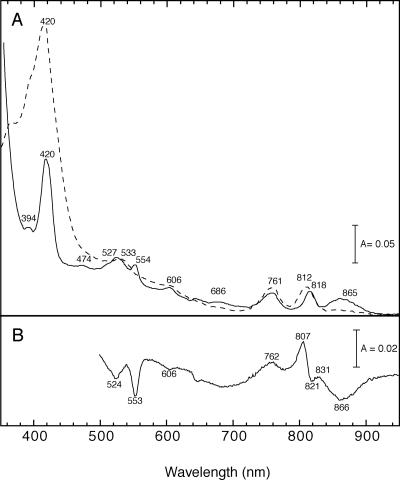
Figure 2 shows the absorption spectra of the RC preparation. The near-infrared absorption peaks in the reduced state were at 865, 818, and 761 nm. The absorption bands were assigned as the special pair of BChls (865 nm), the accessory BChl (818 nm), and BPhe (761 nm) based on the reported spectra of C. aurantiacus (30) and purple bacteria. The ratio of the peak height of accessory BChl to that of BPhe was almost 1:1, suggesting that the RC of R. castenholzii contains one molecule of the accessory BChl and three molecules of BPhe per special pair, as reported for C. aurantiacus (30). The presence of three molecules of BPhe was supported by sequence analysis of the RC proteins. An isoleucine residue is found in R. castenholzii in place of the histidine residue serving as a ligand to the Mg atom of the accessory BChl in the M subunits of purple bacteria (see Fig. 6).
FIG. 2.
Absorption spectra (A) and oxidized-minus-reduced difference spectra (B) of the RC preparation of R. castenholzii. The reduced form (solid line in panel A) was prepared by the addition of a small amount of Na2S2O4, and the oxidized form (dashed line in panel A) was prepared by addition of K3Fe(CN)6.
FIG. 6.
Alignment of the amino acid sequences of the M subunit in the RC complex. The upper two organisms belong to the filamentous anoxygenic phototrophic bacteria. The lower three organisms are purple bacteria. Consensus amino acid residues for pigment ligands are boxed. Identical amino acids are indicated by asterisks.
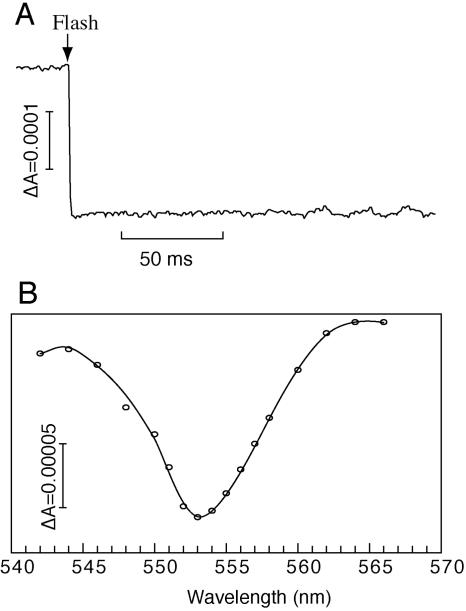
The K3Fe(CN)6 (oxidized)-minus-Na2S2O4 (reduced) difference spectra showed a distinct absorption decrease upon oxidation in the peaks at 866, 553, and 524 nm, corresponding to the bands of the special pair of BChls and the alpha and beta regions of cytochrome c, respectively (Fig. 2B). Fast photo-oxidation of the RC-bound cytochrome in the RC complex was observed as an absorbance decrease at 553 nm, with a reference at 540 nm, which is a characteristic feature of the RC-bound cytochrome subunit (Fig. 3A). Flash-induced difference spectra (Fig. 3B) showed a peak at 553 nm, which is similar to the oxidized-minus-reduced difference spectrum produced by chemical reagents (Fig. 2B). These results suggest that the cytochrome subunit is tightly bound to the RC and keeps its ability to donate an electron to the photo-oxidized special pair of BChls. A similar fast photo-oxidation of cytochromes was also observed in the RC-LH complex as well as in the membrane preparation (data not shown).
FIG. 3.
Flash-induced difference absorbance and spectral change of cytochrome c in the prepared photochemical reaction center of R. castenholzii. (A) A kinetic trace showing the flash-induced absorbance change at 553 to 540 nm. The trace is an average of 60 measurements at intervals of 45 s. (B) A corresponding transient spectrum at 20 ms after the actinic flash. The content of RC was adjusted to 0.003 μM. The buffer contained 20 mM MOPS-KOH (pH 7.0), 2 mM sodium ascorbate, and the redox mediators vitamin K3 (10 mM) and vitamin K1 (30 mM).
Protein composition of the reaction center complex.
The polypeptide composition of the RC-LH complex is shown in Fig. 4. Five major polypeptides were identified, with molecular weights of 38,000 (C), 30,600 (L), 29,000 (M), 11,500 (α), and 8,700 (β) in SDS-PAGE. The apparent molecular weight of the protein detected on the heme-stained gel was quite similar to the molecular weight calculated from the deduced amino acid sequence of pufC (37,242, including four molecules of heme). Heme-staining analysis revealed that the protein is a hemoprotein. Thus, the protein was identified as the cytochrome subunit in the reaction center complex.
The N-terminal amino acid sequences of the bands with molecular weights of 30,000 and 29,000 were APLPLPSGETLP and RGRETPGPID, respectively, and these bands were identified as the gene products of pufL and pufM, respectively. Two other bands that appeared in the low-molecular-weight region were identified as α and β polypeptides of the light-harvesting protein on the basis of the N-terminal amino acid sequences, MKDRPFEFRT and MTDKPQNDLVP, respectively, which coincided with the deduced amino acid sequences of pufA and pufB, respectively.
Sequence analyses of puf genes.
The nucleotide sequence contains four open reading frames (ORFs). These ORFs have considerably high sequence identities to those of pufB, pufA, pufL, pufM, and pufC genes of other photosynthetic bacteria, which encode the β and α subunits of the LH1 light-harvesting complex and the L, M, and cytochrome subunits of the RC complex, respectively. The tentative pufB and pufA genes of R. castenholzii encoded 55 and 42 amino acids, and their products showed the highest identity to those of pufB (56%) and pufA (56%) of C. aurantiacus. The molecular weights of these products were calculated to be 6,425 and 4,720, respectively.
Both the L and M subunits of R. castenholzii appeared to be encoded by one of the four ORFs. The 5′ and 3′ halves of this ORF were shared by the tentative pufL and pufM genes, respectively (Fig. 5). The region including the 3′ half of pufL and the 5′ half of pufM was independently cloned and sequenced three times, and the results were the same every time. An ATG start codon was not found in close proximity to the region coding for the N-terminal amino acid sequence determined for the isolated M polypeptide. Moreover, a stop codon of the tentative pufL gene was not found in the expected region. Therefore, the C-terminal amino acid of the L subunit of R. castenholzii was not decided in this study, and the gene is referred to as pufLM in this report. The M subunit was estimated to contain 314 amino acids in the mature form, with a molecular weight of 35,944, assuming no processing at the C-terminal end. The deduced amino acid sequences of the L and M subunits of R. castenholzii showed relatively high identities to those of C. aurantiacus (52 and 44%, respectively). In this comparison, the valine residue at position 302 (nucleotides 1499 to 1501 [Fig. 5]) was regarded as the C-terminal amino acid of the L subunit on the basis of the sequence comparison with the C. aurantiacus L subunit. The percent identity between the L and M subunits of R. castenholzii (24%) was similar to those in C. aurantiacus (29%) and B. viridis (26%). The deduced amino acid sequence of the pufC product of R. castenholzii consisted of 319 amino acids and showed low identity to that of C. aurantiacus (27%).
FIG. 5.
Nucleotide and deduced amino acid sequences of the region of the pufLM gene encompassing the C-terminal region of the L subunit and the amino-terminal region of the M subunit of R. castenholzii. The putative ribosome-binding sites are shaded. Boldface indicates the amino acids identified by automated Edman sequencing of the purified polypeptides. The putative additional transmembrane helix is underlined. Numbers indicate the distance from the first nucleotide of the pufB gene.
Comparison of the primary structure with those of other RC proteins.
The amino acid sequences of the L and M subunits of R. castenholzii were compared with those of C. aurantiacus (35) and some purple bacteria, e.g., Blastochloris (formerly Rhodopseudomonas) viridis (24). The histidine residues coordinated to the Mg atoms of the special pair of BChls and to those of accessory BChl in the L subunit were conserved (data not shown), but the histidine residue in the M subunits of purple bacteria, which acts as a ligand to the accessory BChl, was replaced by isoleucine in R. castenholzii (Fig. 6). This change probably caused replacement of the accessory BChl of the M subunit with BPhe, as the corresponding site was replaced with leucine in the C. aurantiacus RC (35). An alignment of the amino acid sequences of the cytochrome subunits of R. castenholzii, C. aurantiacus, and B. viridis is shown in Fig. 7. Four heme-binding motifs (C-X-X-C-H) are also found in R. castenholzii. In R. castenholzii, the methionine residue functioning as the axial ligand to heme 4 in B. viridis is not conserved, as also reported for C. aurantiacus. The methionine residue M263, which was suggested as the alternative axial ligand to heme 4 in C. aurantiacus (9), is conserved in R. castenholzii (Fig. 7).
FIG. 7.
Comparison of the primary structures of the cytochrome subunits of R. castenholzii (R. cas), C. aurantiacus (C. aur), and B. viridis (B. vir). Consensus amino acid residues of heme-binding motifs are boxed, and axial ligands to the heme irons are shaded and indicated by the heme numbers. Identical amino acids are indicated by asterisks.
DISCUSSION
In this study, photochemically active RC complexes were isolated from R. castenholzii, and the nucleotide sequences of the genes coding for the RC subunits were determined. The R. castenholzii puf operon contained, starting from upstream, the pufB, -A, -L, -M, and -C genes. The alignment of the amino acid sequence of the M subunits of R. castenholzii, C. aurantiacus, and various purple bacteria indicates that the ligand to the Mg atom of the accessory BChl of the M subunit (typical of purple bacteria) is absent in R. castenholzii RC, as it is in C. aurantiacus. The absorption spectrum and oxidized-minus-reduced difference spectrum of the isolated RC from R. castenholzii showed almost the same characteristics as those observed in C. aurantiacus in the near-infrared region (30), suggesting that the RC of R. castenholzii contains one molecule of the accessory BChl and three molecules of the BPhe per special pair, as it does in the C. aurantiacus RC (32, 35, 40). Thus, it is likely that in photosynthetic bacteria belonging to the group of filamentous anoxygenic phototrophic bacteria, the accessory BChl in the M subunit of the RC, which is not involved in photosynthetic electron transfer even in purple bacterial RCs, is replaced by BPhe.
SDS-PAGE analysis revealed that the RC complex of R. castenholzii is composed of three subunits, namely, L, M, and cytochrome subunits. The H subunit, which is required for structural stability in the purple bacterial RC complexes, was not detected in the isolated RC complexes from R. castenholzii. C. aurantiacus also lacks the H subunit (25, 26, 34), suggesting that the absence of the H subunit is a common characteristic in the filamentous anoxygenic phototrophic bacteria. The lack of the H subunit in both bacteria indicates that its absence in C. aurantiacus is not due to the presence of chlorosomes near the cytoplasmic side of the RC. The cytochrome subunit bound to the isolated RC showed the capability of rapid electron donation to the photo-oxidized special pair of BChls. In C. aurantiacus, the cytochrome subunit was not present in the isolated complex, as it easily detaches from the RC core complex during the isolation procedure. Thus, kinetic measurements of the RC-associated cytochrome subunit have been carried out only in membrane preparations of C. aurantiacus (3, 45). However, the RC of R. castenholzii isolated in this study contained the cytochrome subunit, which showed a rapid photo-oxidation of c-type hemes. The isolated RC should be useful for further studies of photosynthetic electron transfer in filamentous anoxygenic phototrophic bacteria.
The N-terminal 20 to 30 amino acid residues in the precursor form of the cytochrome subunit of B. viridis function as a signal peptide and are not found in the mature protein which has been secreted into the periplasmic space (42). The cytochrome is a lipoprotein, since the N-terminal amino acid residue of the mature form of the protein is a cysteine modified by two fatty acid molecules. These fatty acids are thought to be an anchor of the cytochrome in the photosynthetic membrane (43). Comparison of the amino acid sequence with that of the C. aurantiacus reaction center cytochrome subunit suggests that the cleavage site is situated after the alanine residue 32 in the precursor form of the cytochrome subunit of R. castenholzii (9). Also, a well-conserved sequence, V-X-A (12), was found at positions −3 and −1 of this predicted signal sequence, as it is in the cytochrome subunit of C. aurantiacus (9). However, a cysteine residue is absent in the respective part of the predicted mature form of the cytochrome subunit of R. castenholzii, as had been shown for the cytochrome subunit of C. aurantiacus (9), suggesting that the R. castenholzii cytochrome subunit does not contain a fatty acid membrane anchor. However, a very hydrophobic region found at amino acid residues 19 to 41 from the N terminus of the pufC product may function as a hydrophobic membrane anchor in R. castenholzii cytochrome subunit, as has been described by Hucke et al. (18).
An interesting feature was found in the connecting region of the sequence coding for the L and M subunits in this organism. The start codon, ATG, was not found in the expected region for the pufM gene, and no stop codons for the pufL gene were found in the expected 3′ end region. Possible start codons other than ATG, such as GTG, TTG, or ATT, have been known in prokaryotes (14). The TTG codon was present as the 8th upper codon (Fig. 5) from the N-terminal amino acid determined in the direct polypeptide sequence, and two GTG codons were also present as the 3rd and 14th codons, respectively. Since the putative Shine-Dalgarno sequence (GGCG or GGAG) was found for the 3rd GTG as well as for the 14th GTG codon, one of these codons may be the start codon for pufM. However, it seems quite rare for such unusual stop and start codons to be present in pairs in continuous genes. In general, the L and M subunits of purple bacteria each have five transmembrane helixes (8), with the N-terminal region located on the cytoplasmic side of the inner membrane (6). Hydrophobicity analysis of the pufLM gene product suggests the presence of 11 membrane-spanning regions, with the additional transmembrane helix in the region of the sequence between the expected end of the L subunit and the beginning of the M subunit (Fig. 5). It is possible that the pufLM product is inserted as one polypeptide in the membrane. The additional membrane-spanning region would result in the same topological position of the residues that bind the cofactors as in other RCs. No cleavage site for signal peptidase II (12) was found in the region spanning the L and M subunits. Further studies are needed to clarify the steps in protein maturation.
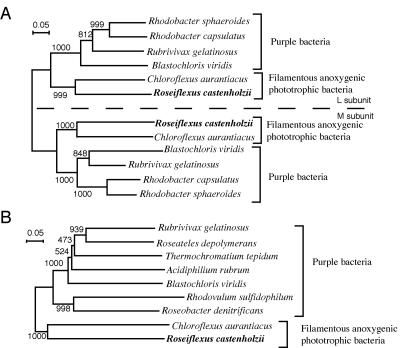
The sequential arrangement of genes (pufB, -A, -L, -M, and -C) found in R. castenholzii is the same as that generally observed in many purple bacteria, e.g., B. viridis. On the other hand, these genes are split into two operons, puf1 and puf2, in C. aurantiacus; puf1 contains two genes for the RC (puf1L and -M), and puf2 contains the gene for the cytochrome subunit (puf2C) together with puf2B and puf2A (41). Although R. castenholzii belonged to the same phylum as C. aurantiacus, this organism had the same puf gene arrangement as purple bacteria. Figure 8 shows the phylogenetic relationships of PufL/M (Fig. 8A) and PufC (Fig. 8B) of R. castenholzii with those of C. aurantiacus and purple bacteria. The RC complex of R. castenholzii was phylogenetically closest to that of C. aurantiacus in all phototrophic bacteria, and the phylogenetic position is consistent with that based on 16S rRNA gene sequences (15). The similarity of the puf gene arrangements in R. castenholzii and purple bacteria indicates that the splitting of the puf operon occurred after the divergence of C. aurantiacus and R. castenholzii and that the puf genes of the common ancestor of filamentous anoxygenic phototrophic bacteria and purple bacteria probably had the sequential arrangement pufB, -A, -L, -M, and -C in the operon. Further analyses of the photosynthesis genes in R. castenholzii would be useful to elucidate the ancestral characteristics of the photosynthetic apparatus and genes of purple bacteria and filamentous anoxygenic phototrophic bacteria. The absence of chlorosomes and the conserved gene arrangement of the puf operon reported in this study may be examples of such interesting information.
FIG. 8.
Phylogenetic trees showing the relationships of RC subunits of purple bacteria and filamentous anoxygenic phototrophic bacteria. Trees were constructed from the deduced amino acid sequences of the L and M subunits (A) and cytochrome subunit (B). Bootstrap values from 1,000 replicates are indicated at the branching points. The accession numbers of the sequences used to construct the trees are as follows: C. aurantiacus, X14979 and AF288462; R. sphaeroides, AJ010302; Rhodobacter capsulatus, Z11165; Rubrivivax gelatinosus, AB034704; B. viridis, X03915 and X05768; Roseateles depolymerans, AB028938; Roseobacter denitrificans, X83392; Rhodovulum sulfidophilum, AB020784; Acidiphilium rubrum, AB005218.
Acknowledgments
We thank Mamoru Mimuro, Kyoto University, for stimulating discussions and Nahomi Nakayama, Tokyo Metropolitan University, for the culture of R. castenholzii cells. We are grateful to Kiyonobu Honma, State University of New York at Buffalo, for useful advice on revision of the manuscript.
This work was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
REFERENCES
- 1.Allen, J. P., G. Feher, T. O. Yeates, H. Komiya, and D. C. Rees. 1987. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc. Natl. Acad. Sci. USA 84:5730-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, J. P., G. Feher, T. O. Yeates, H. Komiya, and D. C. Rees. 1987. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc. Natl. Acad. Sci. USA 84:6162-6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blankenship, R. E., R. Feick, B. D. Bruce, C. Kirmaier, D. Holten, and R. C. Fuller. 1983. Primary photochemistry in the facultative green photosynthetic bacterium Chloroflexus aurantiacus. J. Cell Biochem. 22:251-261. [DOI] [PubMed] [Google Scholar]
- 4.Blankenship, R. E. 1992. Origin and evolution of photosynthesis. Photosynth. Res. 33:91-111. [PubMed] [Google Scholar]
- 5.Bruce, B. D., R. C. Fuller, and R. E. Blankenship. 1982. Primary photochemistry in the facultatively aerobic green photosynthetic bacterium Chloroflexus aurantiacus. Proc. Natl. Acad. Sci. USA 79:6532-6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunisholz, R. A., V. Wiemken, F. Suter, R. Bachofen, and H. Zuber. 1984. The light-harvesting polypeptides of Rhodospirillum rubrum. II. Localisation of the amino-terminal regions of the light-harvesting polypeptides B 870-alpha and B 870-beta and the reaction-centre subunit L at the cytoplasmic side of the photosynthetic membrane of Rhodospirillum rubrum G-9+. Hoppe-Seyler's Z. Physiol. Chem. 365:689-701. [DOI] [PubMed] [Google Scholar]
- 7.Davis, B. J. 1964. Disk electrophoresis. II. Method and application to human serum proteins. Ann. N. Y. Acad. Sci. 121:404-427. [DOI] [PubMed] [Google Scholar]
- 8.Deisenhofer, J., O. Epp, K. Miki, R. Huber, and H. Michel. 1985. Structure of the protein subunit in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3Å resolution. Nature 318:618-624. [DOI] [PubMed] [Google Scholar]
- 9.Dracheva, S., J. C. Williams, G. Van Driessche, J. J. Van Beeumen, and R. E. Blankenship. 1991. The primary structure of cytochrome c-554 from green photosynthetic bacterium Chloroflexus aurantiacus. Biochemistry 30:11451-11458. [DOI] [PubMed] [Google Scholar]
- 10.Edman, P., and A. Henschen. 1975. Sequence determination, p. 232-279. In S. B. Needleman (ed.), Protein sequence determination, 2nd ed. Springer Verlag, Berlin, Germany.
- 11.Feick, R. G., M. Fitzpatrick, and R. C. Fuller. 1982. Isolation and characterization of cytoplasmic membrane from the green bacterium Chloroflexus aurantiacus. J. Bacteriol. 150:905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franzen, L. G., J. D. Rochaix, and G. V. Heijne. 1990. Chloroplast transit peptides from the green alga Chlamydomonas reinhardtii share features with both mitochondrial and higher plant chloroplast presequences. FEBS Lett. 260:165-168. [DOI] [PubMed] [Google Scholar]
- 13.Ganago. A. O., V. S. Gubanov., A. V. Klevanik, A. N. Melkozernov, A. Y. Shkuropatov, and V. A. Shuvalov. 1988. Comparative study of special and kinetic properties of electron transfer in purple and green photosynthetic bacteria, p. 109-117. In J. M. Olson, J. G. Ormerod, J. Amesz, E. Stackebrandt, and H. G. Trüper (ed.), Green photosynthetic bacteria. Plenum Publishing Corporation, New York, N.Y.
- 14.Gualerzi, C. O., and C. L. Pon. 1990. Initiation of mRNA translation in prokaryotes. Biochemistry 29:5881-5889. [DOI] [PubMed] [Google Scholar]
- 15.Hanada, S., S. Takaichi, K. Matsuura, and K. Nakamura. 2002. Roseiflexus castenholzii gen. nov., sp. nov., a thermophilic, filamentous, photosynthetic bacterium which lacks chlorosomes. Int. J. Syst. Evol. Microbiol. 52:187-193. [DOI] [PubMed] [Google Scholar]
- 16.Hanada, S., and B. K. Pierson. 2002. The family Chloroflexaceae. In M. Dworkin et al. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed. (release 3.11, 22 November 2002). Springer-Verlag, New York, N.Y.
- 17.Hanada, S. 2003. Filamentous anoxygenic phototrophs in hot springs. Microbes Environ. 18:51-61. [Google Scholar]
- 18.Hucke, O., E. Schiltz, G. Drews, and A. Labahn. 2003. Sequence analysis reveals new membrane anchor of reaction centre-bound cytochromes possibly related to PufX. FEBS Lett. 535:166-170. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Matsuura, K., and K. Shimada. 1986. Cytochrome functionally associated to photochemical reaction centers in Rhodopseudomonas palustris and Rhodopseudomonas acidophila. Biochim. Biophys. Acta 852:9-18. [Google Scholar]
- 21.McManus, J. D., D. C. Brune, J. Han, J. Sanders-Loehr, T. E. Meyer, M. A. Cusanovich, G. Tollin, and R. E. Blankenship. 1992. Isolation, characterization, and amino acid sequence of auracyanins, blue copper proteins from the green photosynthetic bacterium Chloroflexus aurantiacus. J. Biol. Chem. 267:6531-6540. [PubMed] [Google Scholar]
- 22.Menin, L., J. Gaillard, P. Parot, B. Schoepp, W. Nitschke, and A. Vermeglio. 1998. Role of HiPIP as electron donor to the RC-bound cytochrome in photosynthetic purple bacteria. Photosynth. Res. 55:343-348. [Google Scholar]
- 23.Meyer, T. E., and T. J. Donohue. 1995. Cytochromes, iron sulfur, and copper proteins mediating electron transfer from the cyt bc1 complex to photosynthetic reaction center complexes, p. 725-745. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, London, United Kingdom.
- 24.Michel, H., K. A. Weyer, H. Gruenberg, I. Dunger, D. Oesterhelt, and F. Lottspeich. 1986. The ‘light’ and ‘medium’ subunits of photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of genes, nucleotide and amino acid sequence. EMBO J. 5:1149-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ovchinnikov, Y. A., N. G. Abdulaev, A. S. Zolotarev, B. E. Shmukler, A. A. Zargarov, M. A. Kutuzov, I. N. Telezhinskaya, and N. B. Levina. 1988. Photosynthetic reaction center of Chloroflexus aurantiacus. I. Primary structure of the L-subunit. FEBS Lett. 231:237-242. [DOI] [PubMed] [Google Scholar]
- 26.Ovchinnikov, Y. A., N. G. Abdulaev, B. E. Shmukler, A. A. Zargarov, M. A. Kutuzov, I. N. Telezhinskaya, N. B. Levina, and A. S. Zolotarev. 1988. Photosynthetic reaction center of Chloroflexus aurantiacus: primary structure of the M-subunit. FEBS Lett. 232:364-368. [DOI] [PubMed] [Google Scholar]
- 27.Oyaizu, H., B. Devrunner-Vossbrinck, L. Mandelko, J. A. Studier, and C. R. Woese. 1987. The green non-sulfur bacteria: a deep branching in the eubacterial line of descent. Syst. Appl. Microbiol. 9:47-53. [DOI] [PubMed] [Google Scholar]
- 28.Perriére, G., and M. Gouy. 1996. WWW-Query: an on-line retrieval system for biological sequence banks. Biochimie 78:364-369. [DOI] [PubMed] [Google Scholar]
- 29.Pierson, B. K., and R. W. Castenholz. 1974. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch. Microbiol. 100:5-24. [DOI] [PubMed] [Google Scholar]
- 30.Pierson, B. K., and J. P. Thornber. 1983. Isolation and spectral characterization of photochemical reaction centers from the thermophilic green bacterium Chloroflexus aurantiacus strain J-10-fl. Proc. Natl. Acad. Sci. USA 80:80-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierson, B. K., S. J. Giovannoni, D. A. Stahl, and R. W. Castenholz. 1985. Heliothrix oregonensis, gen. nov., sp. nov., a phototrophic filamentous gliding bacterium containing bacteriochlorophyll a. Arch. Microbiol. 142:164-167. [DOI] [PubMed] [Google Scholar]
- 32.Scherer, P. O. J., and S. F. Fischer. 1987. Model studies to low-temperature optical transitions of photosynthetic reaction centers. II. Rhodobacter sphaeroides and Chloroflexus aurantiacus. Biochim. Biophys. Acta 891:157-164. [Google Scholar]
- 33.Shimada, K. 1985. Light-harvesting pigment-protein complexes of Rhodopseudomonas sphaeroides forma sp. denitrificans. J. Biochem. 98:211-217. [DOI] [PubMed] [Google Scholar]
- 34.Shiozawa, J. A., F. Lottspeich, and R. Feick. 1987. The photochemical reaction center of Chloroflexus aurantiacus is composed of two structurally similar polypeptides. Eur. J. Biochem. 16:595-600. [DOI] [PubMed] [Google Scholar]
- 35.Shiozawa, J. A., F. Lottspeich, D. Oesterhelt, and R. Feick. 1989. The primary structure of Chloroflexus aurantiacus reaction-center polypeptides. Eur. J. Biochem. 180:75-84. [DOI] [PubMed] [Google Scholar]
- 36.Takaichi, S., S. Maoka, M. Yamada, K. Matsuura, Y. Haikawa, and S. Hanada. 2001. Absence of carotenes and presence of a tertiary methoxy group in a carotenoid from a thermophilic filamentous photosynthetic bacterium, Roseiflexus castenholzii. Plant Cell Physiol. 42:1355-1362. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, P. E., D. Ryan, and W. Levin. 1976. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 75:168-176. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Vliet, P., D. Zannoni, W. Nitschke, and A. W. Rutherford. 1991. Membrane-bound cytochromes in Chloroflexus aurantiacus studied by EPR. Eur. J. Biochem. 199:317-323. [DOI] [PubMed] [Google Scholar]
- 40.Vasmel, H., J. Amesz, and A. J. Hoff. 1986. Analysis by excitation theory of the optical properties of the Chloroflexus aurantiacus reaction center. Biochim. Biophys. Acta 852:159-168. [Google Scholar]
- 41.Watanabe, Y., R. G. Feick, and J. A. Shiozawa. 1995. Cloning and sequencing of the genes encoding the light-harvesting B806-866 polypeptides and initial studies on the transcriptional organization of puf2B, puf2A and puf2C in Chloroflexus aurantiacus. Arch. Microbiol. 163:124-130. [DOI] [PubMed] [Google Scholar]
- 42.Weyer, K. A., F. Lottspeich, H. Gruenberg, F. Lang, D. Oesterhelt, and H. Michel. 1987. Amino acid sequence of the cytochrome subunit of the photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 6:2197-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weyer, K. A., W. Schafer, F. Lottspeich, and H. Michel. 1987. The cytochrome subunit of the photosynthetic reaction center from Rhodopseudomonas viridis is a lipoprotein. Biochemistry 26:2909-2914. [Google Scholar]
- 44.Wilson, K. 1994. Preparation of genomic DNA from bacteria, p. 2.4.1-2.4.5. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 45.Zannoni, D., and G. Venturoli. 1987. The mechanism of photosynthetic electron transport and energy transduction by membrane fragment from Chloroflexus aurantiacus, p. 135-143. In J. M. Olson, J. G. Ormerod, J. Amesz, E. Stackebrandt, and H. G. Trüper (ed.), Green photosynthetic bacteria. Plenum Publishing Corp., New York, N.Y.