Abstract
The ability of phytopathogenic fungi to overcome the chemical defense barriers of their host plants is of great importance for fungal pathogenicity. We studied the role of cyclic hydroxamic acids and their related benzoxazolinones in plant interactions with pathogenic fungi. We identified species-dependent differences in the abilities of Gaeumannomyces graminis var. tritici, Gaeumannomyces graminis var. graminis, Gaeumannomyces graminis var. avenae, and Fusarium culmorum to detoxify these allelochemicals of gramineous plants. The G. graminis var. graminis isolate degraded benzoxazolin-2(3H)-one (BOA) and 6-methoxy-benzoxazolin-2(3H)-one (MBOA) more efficiently than did G. graminis var. tritici and G. graminis var. avenae. F. culmorum degraded BOA but not MBOA. N-(2-Hydroxyphenyl)-malonamic acid and N-(2-hydroxy-4-methoxyphenyl)-malonamic acid were the primary G. graminis var. graminis and G. graminis var. tritici metabolites of BOA and MBOA, respectively, as well as of the related cyclic hydroxamic acids. 2-Amino-3H-phenoxazin-3-one was identified as an additional G. graminis var. tritici metabolite of BOA. No metabolite accumulation was detected for G. graminis var. avenae and F. culmorum by high-pressure liquid chromatography. The mycelial growth of the pathogenic fungi was inhibited more by BOA and MBOA than by their related fungal metabolites. The tolerance of Gaeumannomyces spp. for benzoxazolinone compounds is correlated with their detoxification ability. The ability of Gaeumannomyces isolates to cause root rot symptoms in wheat (cultivars Rektor and Astron) parallels their potential to degrade wheat allelochemicals to nontoxic compounds.
Biochemical defense mechanisms of higher plants for pathogenic fungi include the accumulation of antifungal metabolites in response to pathogen attack and the presence of constitutive antifungal compounds. Degradation of these substances to less toxic products is an important method used by pathogens to overcome host defenses (4, 21).
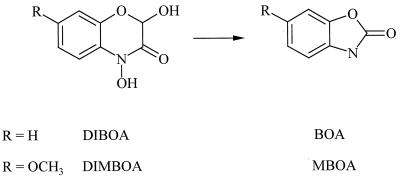
Cyclic hydroxamic acids and related benzoxazolinone compounds are an important group of allelochemicals in gramineous plants. They are found in corn, rye, and wheat but not in rice, barley, and oats (14). The maximum recorded level of hydroxamic acids in cultivated wheat is 11 mmol/kg (fresh weight) (6, 20). Root exudation of these compounds also has been observed (10, 16). In planta the hydroxamic acids 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA) and 2,4-dihydroxy-2H-1,4-benzoxazin-3(4H)-one (DIBOA) are sequestered and stabilized as (2R)-2-β-d-glucosides. The more toxic aglucones are produced in response to tissue damage or pathogen attack. In planta after cells are damaged, in aqueous solution, and in soil, cyclic hydroxamic acids decompose rapidly to form the respective ring-contracted compounds 6-methoxy-benzoxazolin-2(3H)-one (MBOA) and benzoxazolin-2(3H)-one (BOA) (Fig. 1) (1).
FIG. 1.
Decomposition of cyclic hydroxamic acids to respective benzoxazolinone derivatives.
Because of their inhibitory activity toward some fungi and bacteria, benzoxazinone allelochemicals and related derivatives are thought to be involved in plant disease resistance. For instance, antifungal effects on Helminthosporium turcicum (7), Septoria nodorum (2), and Microdochium nivale (22) have been observed. The accumulation of the antifungal compound 2-hydroxy-4,7-dimethoxy-2H-1,4-benzoxazin-3(4H)-one in wheat after infection with Puccinia graminis var. tritici was found by Bücker and Grambow (5). An increase in the DIMBOA content of resistant corn after infection with Exserohilum turcicum was observed by Zhu et al. (25).
Compared to phytoalexins, preformed antimicrobial compounds, such as benzoxazinone allelochemicals in wheat, rye, and corn or avenacines and avenacosides in oats (15), have received relatively little attention. Nevertheless, such compounds are the first to confront invading fungi and pests. The root-infecting fungi Gaeumannomyces graminis (Sacc.) Arx and D. Olivier var. tritici J. Walker, G. graminis (Sacc.) Arx and D. Olivier var. graminis, G. graminis (Sacc.) Arx and D. Olivier var. avenae (E. M. Turner), and Fusarium culmorum (Wm. G. Sm.) Sacc. are pathogens of cereals and grasses and have different host specificities. G. graminis var. tritici causes “take-all” (9) in wheat and barley and asymptomatically colonizes the surface of oat roots. G. graminis var. graminis is known to be both a biocontrol agent in wheat against G. graminis var. tritici and a pathogen of rice, wheat, and turfgrass (9, 19). G. graminis var. avenae is the main cause of oat take-all but is also pathogenic for wheat, barley, and turfgrass (8). In temperate regions, F. culmorum is a severe pathogen of cereals and grasses causing root rot and head blight.
Our objectives were to determine the role of benzoxazinone and benzoxazolinone allelochemicals from wheat in plant-pathogen interactions and to identify their main fungal degradation products.
MATERIALS AND METHODS
Fungal cultures.
Three G. graminis isolates were used: G. graminis var. tritici (field isolate; DSM 12044; Deutsche Sammlung von Mikroorganismen, Braunschweig, Germany), G. graminis var. graminis (CBS 541.86; Centraalbureau voor Schimmelcultures, Baarn, The Netherlands), and G. graminis var. avenae (CBS 870.73). G. graminis var. tritici was isolated from diseased wheat and identified by PCR (Zentrum für Agrarlandschaftsnutzung und Landnutzungsforschung E. V., Müncheberg, Germany). F. culmorum (field isolate; DSM 12043) was isolated from wheat. Isolates were maintained as mycelial colonies on potato dextrose agar (PDA) (potato dextrose broth [PDB; Difco], 24 g/liter; agar [Gibco BRL], 15 g/liter) after storage at −80°C in a cryoprotectant. For experimentation, mycelial plugs were transferred to 0.1× PDA and incubated at 23°C for approximately 10 days.
Allelochemicals.
DIMBOA was isolated from 7-day-old corn seedlings (Zea mays L. Marshall FAO 240, single hybrid) by the procedure of Woodward et al. (24). After purification by recrystallization from acetone-hexane, white crystals were obtained (mp, 161.5 to 163.5°C; mp reported elsewhere [13], 168 to 169°C). DIBOA was isolated from shoots of rye (Secale cereale L. cv. Marder) by an analogous procedure. After purification by recrystallization from acetone-hexane, white crystals were obtained (mp, 152 to 155°C; mp reported elsewhere [18], 155 to 157°C. BOA and MBOA were purchased from Aldrich (Deisenhofen, Germany) and Sigma (Deisenhofen, Germany), respectively.
Growth of fungal isolates in allelochemical-containing liquid media.
Experiments on the biodegradation of allelochemicals by pathogenic fungi were performed with 0.1× PDB (pH 5.8). The fungi were incubated with allelochemicals by placing a mycelial plug (0.8-cm diameter) from the margin of a freshly grown culture in 50 ml of liquid media containing allelochemicals at a final concentration of 1 mM. Cultures were incubated at 23°C on a rotary shaker at 98 rpm. Stock solutions of allelochemicals (0.1 M) were prepared in methanol, and aliquots were added to the flasks. The addition of methanol to PDB and fungi served as a control. The chemical stability of the allelochemicals was assayed with noninoculated flasks. Aliquot samples were taken after 0, 48, 96, and 168 h and frozen until analysis. Duplicate experiments with three replicates were performed. The degradation of allelochemicals and the formation of metabolites were monitored by high-pressure liquid chromatography (HPLC).
Isolation of degradation products.
After an incubation period of 168 h, the media were harvested by removal of the fungal mycelial plugs. The media were filtered and centrifuged (20 min, 4,500 × g), and the pH was adjusted to 3 with HCl (0.5 M). The solutions were extracted three times with an equal volume of freshly distilled diethyl ether. The combined organic phases were dried over anhydrous MgSO4. The solvent was removed in vacuo. The resulting crude products were separated by a preparative HPLC method. The collected HPLC fractions (retention times, 10.3, 10.4, and 12.6 min) were analyzed by means of 1H and 13C nuclear magnetic resonance (NMR) spectroscopy, electron impact mass spectrometry (EIMS), and UV-visible light (UV-VIS) absorption spectroscopy.
Synthesis of reference compounds 1 and 2.
For the synthesis of N-(2-hydroxyphenyl)-malonamic acid methyl ester (compound 1) and N-(2-hydroxy-4-methoxyphenyl)-malonamic acid methyl ester (compound 2), we added dropwise to a solution of 10 mmol of 2-aminophenol (for the synthesis of compound 1) or 2-amino-5-methoxyphenol (for the synthesis of compound 2, prepared by catalytic hydrogenation of 5-methoxy-2-nitrophenol in tetrahydrofuran over 5% platinium on activated charcoal) in 100 ml of tetrahydrofuran 5 mmol (6.83 g) of methyl chloroformyl acetate at 0°C. After being stirred for 1 h, the solution was filtered and the solvent was removed in vacuo. The remaining residue was dissolved in 100 ml of ethyl acetate and extracted three times with 50 ml of 10% aqueous HCl. The organic phase was separated and dried with anhydrous MgSO4, and the solvent was removed in vacuo. The remaining crystals were recrystallized from MeOH to yield 9.1 g (87%) of compound 1 (mp, 141 to 142°C) and 8.36 g (70%) of compound 2 (mp, 146 to 147°C) as colorless crystals.
(i) N-(2-Hydroxyphenyl)-malonamic acid methyl ester 1.
1H NMR (DMSO-d6) yielded the following: δ 3.60 (s, 2H, CH2), 3.63 (s, 3H, CH3O), 6.71 to 6.92 (m, 3H, aromatic), 7.88 (dd, 1H, 3J6,5 = 8 Hz, 4J6,4 = 1.2 Hz, H-6), 9.49 (s, 1H, OH), 9.84 (s, 1H, NH). 13C NMR (DMSO-d6) yielded the following: δ 43.7 (CH2), 52.8 (CO2CH3), 116.2 (C-3), 119.9 (C-5), 122.6 (C-6), 125.5 (C-4), 127.1 (C-1), 148.4 (C-2), 165.2 (CONH), 169.7 (CO2CH3). EIMS yielded the following m/z (percent relative intensity): 209 (M+, 15), 178 (5), 109 (100), 80 (25).
(ii) N-(2-Hydroxy-4-methoxyphenyl)-malonamic acid methyl ester 2.
1H NMR (DMSO-d6) yielded the following: δ 3.54 (s, 2H, CH2), 3.62 (s, 3H, CH3O), 3.66 (s, 3H, CO2CH3), 6.34 (dd, 3J5,6 = 8.6 Hz, 4J5,3 = 2.8 Hz, H-5), 6.44 (d, 1H, 4J3,5 = 2.8 Hz, H-3), 7.63 (d, 1H, 3J6,5 = 8.6 Hz, H-6), 9.39 (s, 1H, OH), 9.84 (s, 1H, NH). 13C NMR (DMSO-d6) yielded the following: δ 43.5 (CH2), 52.7 (CO2CH3), 55.9 (CH3O), 102.5 (C-3), 104.7 (C-5), 120.2 (C-1), 124.2 (C-6), 150.2 (C-2), 157.7 (C-4), 165.0 (CONH), 169.6 (CO2CH3). EIMS yielded the following m/z (percent relative intensity): 239 (M+, 31), 207 (28), 165 (34), 139 (100), 124 (95).
Synthesis of reference compounds 3 and 4.
For the synthesis of N-(2-hydroxyphenyl)-malonamic acid (compound 3) and N-(2-hydroxy-4-methoxyphenyl)-malonamic acid (compound 4), we added to a solution of 10 mmol of compound 1 (for the synthesis of compound 3) or compound 2 (for the synthesis of compound 4) in 50 ml of ethanol 20 mmol (1.3 g) of 85% KOH in 10 ml of H2O. After being stirred for 1 h, the solution was acidified with concentrated HCl. The organic solvent was removed in vacuo, and 20 ml of H2O was added. The aqueous solution was extracted three times with 50 ml of ethyl acetate. The combined organic extracts were dried with anhydrous MgSO4, and the solvent was removed in vacuo. The remaining crystals were washed with diethyl ether and recrystallized to yield 1.5 g (77%) of compound 3 (mp, 148 to 150°C) (toluene) and 1.46 g (65%) of compound 4 (mp, 156 to 158°C) (ethyl alcohol).
(i) N-(2-Hydroxyphenyl)-malonamic acid.
1H NMR (DMSO-d6) yielded the following: δ 3.48 (s, 2H, CH2), 6.70 to 6.96 (m, 3H, aromatic), 7.89 (dd, 1H, 3J6,5 = 8.3 Hz, 4J6,4 = 1.2 Hz, H-6), 9.52 (s, 1H, OH), 9.82 (s, 1H, NH), 12.63 (s, 1H, COOH). 13C NMR (DMSO-d6) yielded the following: δ 44.1 (CH2), 116.2 (C-3), 119.9 (C-5), 122.4 (C-6), 125.4 (C-4), 127.2 (C-1), 148.3 (C-2), 165.8 (CONH), 170.9 (COOH). Infrared (IR) νmaxKBr cm−1: 1701, 1650, 1616, 1561, 1454. EIMS yielded the following m/z (percent relative intensity): 195 (M+, 3), 151 (8), 109 (100), 80 (23), 44 (75). High-resolution mass spectrometry (HRMS) m/z: found, 195.0530 (C9H9NO4, M+); calculated, 195.0532.
(ii) N-(2-Hydroxy-4-methoxyphenyl)-malonamic acid.
1H NMR (DMSO-d6) yielded the following: δ 3.41 (s, 2H, CH2), 3.65 (s, 3H, CH3O), 6.34 (dd, 3J5,6 = 8.8 Hz, 4J5,3 = 2.8 Hz, H-5), 6.42 (d, 1H, 4J3,5 = 2.8 Hz, H-3), 7.63 (d, 1H, 3J6,5 = 8.8 Hz, H-6), 9.40 (s, 1H, OH), 9.86 (s, 1H, NH), 12.58 (s, 1H, COOH). 13C NMR (DMSO-d6) yielded the following: δ 43.8 (CH2), 55.9 (CH3O), 102.6 (C-3), 104.8 (C-5), 120.4 (C-1), 124.0 (C-6), 150.0 (C-2), 157.6 (C-4), 165.6 (CONH), 170.8 (COOH). IR νmaxKBr cm−1: 1698, 1644, 1609, 1561, 1528. EIMS yielded the following m/z (percent relative intensity): 225 (M+, 5), 207 (4), 181 (20), 139 (72), 125 (100), 110 (23). HRMS m/z: found, 225.0633 (C10H11NO5, M+); calculated, 225.0637.
2-Amino-3H-phenoxazin-3-one.
2-Amino-3H-phenoxazin-3-one was synthesized by oxidation of 2-aminophenol by the procedure described by Friebe et al. (11). Analytical data: mp, 255 to 261°C; mp reported elsewhere [3], 256 to 257°C). UV (MeOH) λmax (log e): 231 nm (4.40), 432 nm (4.28); values reported elsewhere (12): 237 nm (4.34), 432 nm (4.29). EIMS yielded the following m/z (percent relative intensity): 212 (M+, 100), 185 (50), 184 (21). HRMS m/z: found, 212.0587 (C12H8N2O2, M+); calculated, 212.0586.
HPLC analysis.
HPLC separations of medium samples and synthetic reference compounds were performed on a Beckman model 126 chromatograph equipped with a model 186 diode-array detector and a 4.6- by 250-mm Ultrasphere ODS RP 18 column. Chromatographic conditions consisted of a mobile phase of H2O (containing 0.1% [vol/vol] trifluoroacetic acid) and methanol run at a flow rate of 1 ml/min. The column was eluted with the following solvent gradient profile: 0 to 1 min, 100% H2O; 1 to 11 min, 0 to 100% methanol (linear gradient); 11 to 14 min, 100% methanol. The chromatograms were detected at 275 and 430 nm.
Analytical devices.
Mass spectra were acquired by EIMS at 70 eV. HRMS was carried out with a Kratos model MS 50 apparatus. UV-VIS analysis was performed on a Beckman spectrophotometer. Melting points were determined with a Leitz microscope or a Boetius micro hot-stage apparatus and were corrected. NMR spectra were measured on a Varian Gemini 2000 spectrometer at 200.041 MHz (1H) or 50.305 MHz (13C). IR spectra were recorded in KBr with an ATI Mattson Spektrometer.
Bioassays. (i) Determination of fungal radial growth.
The antifungal activities of the allelochemicals were examined by measuring the radial mycelial growth of G. graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and F. culmorum on PDA containing 0.01, 0.1, and 1 mmol of BOA, MBOA, N-(2-hydroxyphenyl)-malonamic acid, and N-(2-hydroxy-4-methoxyphenyl)-malonamic acid per liter of methanol in petri dishes (35-mm diameter). PDA amended with methanol served as the control. Media were inoculated with a mycelial plug (0.3-mm diameter) from the margin of a freshly growing culture (0.1× PDA; 2.4 g of PDB per liter). Plates were incubated in the dark at 23°C for several days, depending on the growth of the fungi in the control plates. Colony size was expressed as the average of two replicate radii measured in opposite directions, and 50% effective doses (ED50) were calculated for each compound if inhibition exceeded 50%. Duplicate experiments with three replicates were performed with G. graminis var. graminis and G. graminis var. avenae, and five experiments with three replicates were performed with G. graminis var. tritici and F. culmorum.
(ii) Cress radicle elongation assay.
The phytotoxicities of BOA and MBOA and their degradation products were tested in a radicle elongation assay with cress (Lepidium sativum). Stock solutions of 7.5, 15, 30, 60, and 120 mM concentrations of these substances were prepared in methanol. Aliquots of 25 μl of stock solution were added to petri dishes (40-mm diameter) with filter paper, and 25 μl of methanol was added to the controls. The methanol was allowed to evaporate completely for at least 6 h. Ten seeds of L. sativum were placed in each dish and watered with 1.5 ml of tap water. After incubation for 3 days at 20°C in the dark, the radicle length was measured and expressed as a percentage of the control. Duplicate experiments with three replicates were performed.
(iii) Inoculation of wheat and oat seeds with fungal isolates.
Winter wheat (cultivars Astron and Rektor) and winter oats (cultivar Lowi) were inoculated with G. graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and F. culmorum to determine the ability of these strains to cause root rot and to affect plant growth. Control plants were not inoculated. Pots (7-cm diameter) were half filled with sterile sand, and four plugs from colonies growing on 0.1× PDA (0.8-cm diameter) were placed on the surface. The inoculum was covered with a 0.5-cm layer of sterile sand. Four germinated seeds of a cultivar were added per pot (four pots per cultivar), and a further layer (2 cm) of sand was placed on top. Pots were placed in water-filled trays (1-cm depth) and incubated for 7 weeks in a growth chamber at 15°C with an 8-h–16-h light-dark cycle. Roots were washed free of soil, air dried, and scored for symptoms (discoloration). In addition, fresh weights of plants were determined. Discoloration of the roots was estimated with a disease score: −, no symptoms; +, weak; ++, moderate; and +++, severe.
Statistical analysis.
The significance of differences in the degradation of allelochemicals (BOA and MBOA) and in cress radicle elongation was determined by a one-way analysis of variance followed by a multiple t test (least significant difference [LSD], P < 0.05). The significance of differences in fungal radial growth and seed inoculation tests was determined by a one-way analysis of variance followed by a Scheffé test procedure (P < 0.05) and a two-way analysis, respectively. Statistical analysis was performed with STATGRAPHICS Plus for Windows 1.4.
RESULTS
Metabolization of benzoxazolinone and 1,4-benzoxazin-3-one allelochemicals.
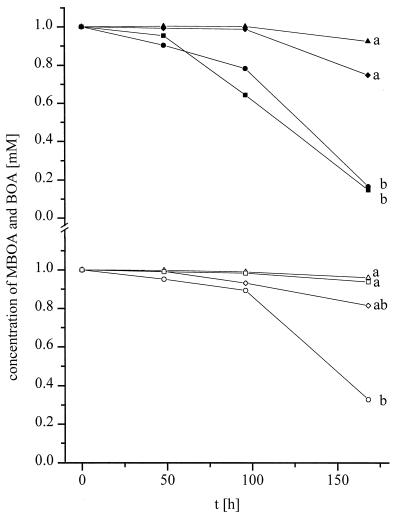
Differences in the degradation of BOA were observed following incubation of G. graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and F. culmorum in media containing 1 mM allelochemical (Fig. 2). Only a slight reduction in the initial concentration of BOA was found after 168 h of incubation of G. graminis var. avenae and G. graminis var. tritici. More extensive degradation occurred in the presence of the pathogens F. culmorum and G. graminis var. graminis. G. graminis var. tritici and G. graminis var. graminis differed in their ability to metabolize the allelochemicals. For MBOA (Fig. 2), no obvious degradation was found after 168 h of incubation of the oat pathogen G. graminis var. avenae or F. culmorum. Only G. graminis var. graminis and G. graminis var. tritici could degrade this allelochemical. G. graminis var. graminis degraded MBOA more effectively than did G. graminis var. tritici.
FIG. 2.
Degradation of 1 mM BOA (closed symbols) and 1 mM MBOA (open symbols) by Gaeumannomyces spp. G. graminis var. tritici [diamonds, G. graminis var. graminis [circles], and G. graminis var. avenae [triangles]) and F. culmorum (squares). Values are means of duplicate experiments with three replicates. Different letters indicate significant differences at 168 h (for BOA, LSD was ±0.48 mM; for MBOA, LSD was ±0.49 mM; P < 0.05). t, time.
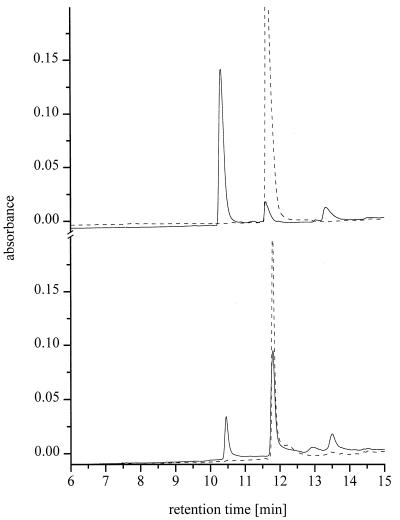
No obvious accumulation of metabolites was detected by HPLC when G. graminis var. avenae and F. culmorum were cultured in media containing BOA and MBOA. Different degradation products of BOA and MBOA were found for G. graminis var. tritici and G. graminis var. graminis. Figure 3 shows examples of the metabolic transformation of BOA and MBOA by the more active G. graminis var. graminis isolate. Following the consumption of the allelochemicals, the production of hydrophilic degradation products of BOA (retention time for compound A, 10.3 min) and MBOA (retention time for compound B, 10.4 min) was observed for both G. graminis var. tritici and G. graminis var. graminis by HPLC of the media. In addition, the formation of different hydrophobic by-products of BOA (retention times for G. graminis var. tritici and G. graminis var. graminis, 12.6 and 13.3 min, respectively) and MBOA (retention time for G. graminis var. graminis, 13.6 min) was observed after longer incubation times. The amounts of the main hydrophilic metabolites produced correlated well with the fungal ability to degrade benzoxazolinone allelochemicals in liquid media.
FIG. 3.
HPLC of incubation media containing 1 mM BOA (top) and 1 mM MBOA (bottom) at initial time (- - - ) and after 168 h of incubation of G. graminis var. graminis (—). Chromatograms were monitored at 270 nm. Retention times: BOA, 11.6 min; compound A, 10.3 min; MBOA, 11.8 min; compound B, 10.5 min.
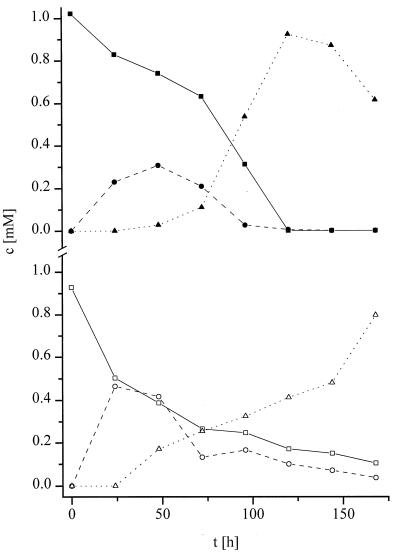
Incubation of G. graminis var. tritici and G. graminis var. graminis in 1 mM solutions of the cyclic hydroxamic acids DIBOA and DIMBOA also yielded the hydrophilic decomposition products A and B. The chemical decomposition products BOA and MBOA were also detected during the biotransformation of the cyclic hydroxamic acids. The time course of the biotransformation of DIBOA and DIMBOA by G. graminis var. graminis is shown in Fig. 4.
FIG. 4.
Degradation of DIBOA (top) and DIMBOA (bottom) by G. graminis var. graminis. Initial concentrations were 1 mM. Concentrations (c) were determined by HPLC of media (calibration was done with synthetic samples of compounds A and B). Symbols: ▪, DIBOA; •, BOA; ▴, compound A; □, DIMBOA; ○, MBOA; ▵, compound B. t, time.
Identification of fungal metabolites produced by G. graminis var. tritici and G. graminis var. graminis.
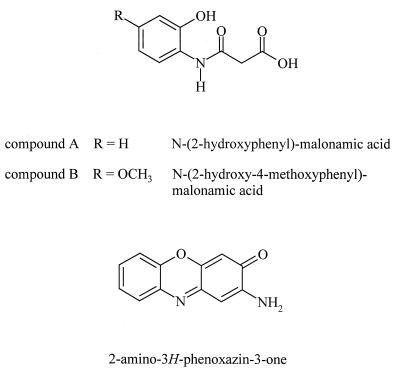
The main degradation products of the allelochemicals BOA and MBOA were obtained from the culture media of G. graminis var. graminis by either extraction and purification of the crude products by preparative HPLC. As described below, the degradation products compound A and compound B were subsequently identified as N-(2-hydroxyphenyl)-malonamic acid (compound 3) and N-(2-hydroxy-4-methoxyphenyl)-malonamic acid (compound 4) (Fig. 5), hitherto unreported compounds.
FIG. 5.
Chemical structures of N-(2-hydroxyphenyl)-malonamic acid, N-(2-hydroxy-4-methoxyphenyl)-malonamic acid, and 2-amino-3H-phenoxazin-3-one.
NMR spectral methods were used to characterize the chemical structures of the metabolites and the synthesized reference compounds. Both malonamic acids were fully characterized by 1H NMR, 13C NMR, distortionless enhancement by polarization transfer, and carbon-hydrogen correlative spectroscopy. Structures were confirmed by mass spectrometry and by UV absorption spectra. For N-(2-hydroxyphenyl)-malonamic acid and N-(2-hydroxy-4-methoxyphenyl)-malonamic acid, absorption maxima at 212, 246, and 286 nm and at 212, 250, and 288 nm, respectively, were determined with methanol. Final proof of the structures was obtained by complete identification of the spectra of both metabolites and their synthesized counterparts. The synthesis was accomplished by acylation of 2-aminophenol and 2-amino-5-methoxyphenol, respectively, with methyl chloroformyl acetate followed by alkaline cleavage of the esters obtained.
When BOA was metabolized by G. graminis var. tritici, an additional hydrophobic by-product accumulated after longer incubation times. Analysis of the UV spectra and mass spectrometry data identified this substance as 2-amino-3H-phenoxazin-3-one (Fig. 5). The mass spectrometry data and the chromatographic behavior of the substance were identical to those of the synthetic compound prepared by chemical oxidation of 2-aminophenol. HPLC of culture media of G. graminis var. graminis containing BOA or MBOA revealed degradation products with UV absorption spectra and retention times similar to those of 2-amino-3H-phenoxazin-3-one, but these have not been further characterized.
Biological activity of benzoxazolinone allelochemicals and their fungal metabolites. (i) Fungal radial growth.
The addition of BOA and MBOA to nutrient media inhibited fungal growth (Table 1). At concentrations of 0.01 and 0.1 mM BOA and MBOA, growth was not reduced. Fungal growth was poorest at 1 mM BOA and MBOA. All four fungi were more sensitive to MBOA than to BOA. G. graminis var. tritici was strongly inhibited by MBOA (ED50, 58.0 μg/ml). G. graminis var. avenae tolerated about a twofold higher concentration (ED50, 110.7 μg/ml) and G. graminis var. graminis tolerated about a threefold higher concentration (ED50, 152.8 μg/ml) of MBOA. The mycelial growth of F. culmorum was only slightly affected by 1 mM BOA and MBOA. In addition, a yellow-brown discoloration of the G. graminis var. tritici and G. graminis var. graminis culture media was visible at 1 mM BOA and MBOA, indicating a release of substances into the media.
TABLE 1.
Mycelial growth of G. graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and F. culmorum exposed to benzoxazolinones and respective detoxification products in four experiments
| Substance | Concn (mM) | % Mycelial growth, relative to control, of (SD)a:
|
|||
|---|---|---|---|---|---|
| G. graminis var. tritici | G. graminis var. graminis | G. graminis var. avenae | F. culmorum | ||
| BOA | 0.01 | 99 (4) | 102 (1) | 101 (5) | 94 (13) |
| 0.10 | 82 (15) | 101 (1) | 97 (3) | 91 (8) | |
| 1.00 | 35 (22) A | 53 (2) AB | 27 (38) A | 83 (9) B | |
| ED50 (μg/ml) | 101 | —b | 92 | — | |
| MBOA | 0.01 | 105 (12) | 101 (1) | 100 (10) | 89 (10) |
| 0.10 | 81 (7) | 99 (4) | 93 (2) | 87 (12) | |
| 1.00 | 13 (17) A | 46 (0) AB | 25 (35) AB | 72 (11) B | |
| ED50 (μg/ml) | 58 | 153 | 111 | — | |
| N-(2-Hydroxyphenyl)-malonamic acid | 0.01 | 93 (39) | 102 (2) | 96 (5) | 97 (5) |
| 0.10 | 101 (29) | 100 (1) | 98 (3) | 95 (8) | |
| 1.00 | 96 (15) | 100 (1) | 97 (1) | 94 (8) | |
| N-(2-Hydroxy-4-methoxyphenyl)-malonamic acid | 0.01 | 112 (20) | 101 (2) | 98 (4) | 92 (11) |
| 0.10 | 96 (17) | 98 (3) | 99 (2) | 95 (7) | |
| 1.00 | 96 (22) | 97 (1) | 89 (8) | 88 (14) | |
Different letters indicate significant differences in the fungal growth of different isolates at 1 mM; no significant differences were found at lower concentrations (Scheffé test; P < 0.05). Values for radial mycelial growth of controls were 12.1 ± 1.0, 13.1 ± 1.6, 12.2 ± 2.4, and 14.0 ± 1.4 mm for G. graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and F. culmorum, respectively.
—, ED50 exceeded 1 mM.
None of the fungal isolates was sensitive to 1 mM N-(2-hydroxyphenyl)-malonamic acid or 1 mM N-(2-hydroxy-4-methoxyphenyl)-malonamic acid (Table 1), confirming the expected detoxifying process. Growth was reduced by no more than 13% compared to the growth of untreated controls. N-(2-Hydroxy-4-methoxyphenyl)-malonamic acid was slightly more inhibitory than N-(2-hydroxyphenyl)-malonamic acid, mirroring the results obtained for MBOA and BOA.
(ii) Cress radicle elongation assay.
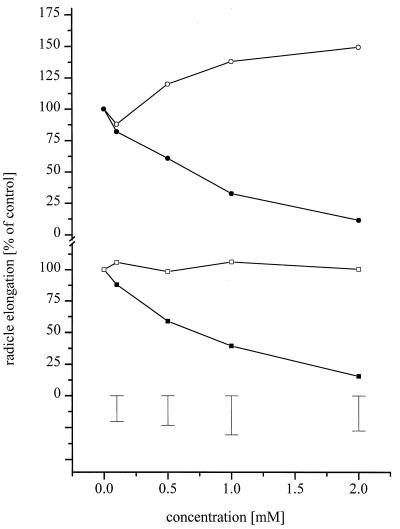
These tests showed that BOA and MBOA affected seedling development of cress (Fig. 6). In contrast, no inhibitory effects were observed with N-(2-hydroxyphenyl)-malonamic acid or N-(2-hydroxy-4-methoxyphenyl)-malonamic acid at concentrations of up to 2 mM. Growth stimulation was observed with N-(2-hydroxyphenyl)-malonamic acid at a 2 mM concentration.
FIG. 6.
Cress radicle elongation assay of BOA (•) and MBOA (▪) in comparison to their respective degradation products N-(2-hydroxyphenyl)-malonamic acid (○) and N-(2-hydroxy-4-methoxyphenyl)-malonamic acid (□). Error bars show LSD (P < 0.05).
(iii) Inoculation of wheat and oat seeds with fungal isolates.
Fungal isolates showed large differences in their ability to cause root rot symptoms in wheat and oats (Table 2). Plant fresh weight varied considerably, but the differences due to pathogen inoculation were not significant. There was no obvious relationship between the discoloration of roots and plant fresh weight. G. graminis var. graminis caused more discoloration than G. graminis var. tritici in both cultivars Rektor and Astron but induced no symptoms in oats. G. graminis var. avenae induced only weak discoloration of oat roots and cultivar Rektor. F. culmorum caused severe root rot in wheat cultivar Astron but not cultivar Rektor. In contrast, cultivar Rektor was attacked more by the Gaeumannomyces isolates. F. culmorum caused symptoms in oats. The ability of the isolates to cause symptoms in cultivars Rektor and Astron parallels their potential to degrade hydroxamic acids to nearly nontoxic metabolites.
TABLE 2.
Root discoloration and reduction of plant fresh weight after inoculation of G. graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and F. culmorum (Fc) on wheat and oats in four experiments
| Pathogen | Wheat cv. Astron
|
Wheat cv. Rektor
|
Oats cv. Lowi
|
|||
|---|---|---|---|---|---|---|
| Discolorationa | Fresh wt, g (SD)b | Discolorationa | Fresh wt, g (SD)b | Discolorationa | Fresh wt, g (SD)b | |
| None (control) | − | 3.4 (0.2) | − | 3.6 (0.1) | − | 3.4 (0.2) |
| G. graminis var. tritici | + | 1.8 (0.1) | ++ | 1.8 (0.2) | + | 2.7 (0.1) |
| G. graminis var. graminis | ++ | 2.4 (1.1) | +++ | 2.2 (0.2) | − | 2.9 (0.5) |
| G. graminis var. avenae | − | 2.8 (1.3) | + | 2.2 (0.1) | + | 3.2 (0.2) |
| F. culmorum | +++ | 1.4 (0.7) | − | 1.8 (0.3) | + | 2.9 (1.7) |
Discoloration of the root system: −, no symptoms; +, weak; ++, moderate; +++, severe.
For total plants. No significant differences (P < 0.05).
DISCUSSION
There is increasing evidence that secondary plant compounds play an important role in soil ecosystems, especially in interactions between plants and microorganisms. We have shown that allelochemicals naturally produced by cereals such as wheat, corn, and rye interact with Gaeumannomyces spp. and F. culmorum.
Isolates of G. graminis and F. culmorum vary in their ability to degrade the benzoxazolinone allelochemicals BOA and MBOA (Fig. 2). We describe the fungal biotransformation of 1,4-benzoxazin-3(4H)-one and benzoxazolinone compounds to N-(2-hydroxyphenyl)-malonamic acid and N-(2-hydroxy-4-methoxyphenyl)-malonamic acid by G. graminis var. graminis and G. graminis var. tritici. Richardson and Bacon (17) observed complete catabolization of 2.5 mM BOA and MBOA by the endophytic corn pathogen Fusarium moniliforme. Although the isolated detoxification products have not been further characterized, the UV absorption spectra are similar to the spectra of isolated and synthetic N-(2-hydroxyphenyl)-malonamic acid and N-(2-hydroxy-4-methoxyphenyl)-malonamic acid. Therefore, we suggest that benzoxazolinone allelochemicals are detoxified by Gaeumannomyces spp. by the same pathway as by F. moniliforme.
Detoxification of benzoxazolinones to N-phenylmalonamic acids was nearly complete, since mycelial growth inhibition by the detoxification products was less than 13% at a 1 mM concentration. Fungal growth was reduced by the benzoxazinones BOA and MBOA (Table 1). ED50 ranged from 58 to 153 μg/ml, depending on the fungal species. Growth inhibition could be reversed by transferring inoculation plugs to untreated PDA, indicating a fungistatic mode of action. Richardson and Bacon (17) obtained complete growth inhibition of F. moniliforme at BOA concentrations between 500 and 1,000 μg/ml.
F. moniliforme isolated from rice catabolized neither BOA nor MBOA but was still able to infect corn seedlings (17). The authors concluded that the benzoxazolinones alone were not responsible for impeded infection. Our data suggest similar mechanisms because G. graminis var. avenae was not able to degrade BOA and MBOA but still was able to induce symptoms on the wheat cultivar Rektor. Nevertheless, the higher capability of G. graminis var. graminis to detoxify BOA and MBOA corresponds well with its higher tolerance for these allelochemicals in comparison to G. graminis var. avenae and G. graminis var. tritici. Furthermore, G. graminis var. graminis induced more severe root rot symptoms in both wheat cultivars than did G. graminis var. tritici and G. graminis var. avenae.
For G. graminis var. graminis and G. graminis var. tritici, the degradation of benzoxazolinones was accompanied by the release of a yellow pigment into the media. 2-Amino-3H-phenoxazin-3-one was identified as an additional degradation product of BOA after longer incubation times of G. graminis var. tritici. This substance was also isolated as a BOA metabolite of soil bacteria (12) and root-colonizing bacteria (11). Degradation products of BOA and MBOA with aminophenoxazinone-like UV spectra and chromatographic behavior were also observed for G. graminis var. graminis. On the basis of the present data, we cannot determine if aminophenoxazinone compounds are by-products of fungal degradation of benzoxazolinones or if they are produced at a later stage of metabolization of phenylmalonamic acids.
Weibull and Niemeyer (23) reported the decomposition of DIMBOA by an aqueous extract of Septoria tritici at a rate of 39%, but they did not find any corresponding decomposition product. In our experiments, the majority of BOA was also degraded by F. culmorum but, as in S. tritici, no accumulation of degradation products was observed. Obviously, benzoxazolinone allelochemicals are metabolized by fungi by different detoxification pathways. Pathogens such as F. culmorum, which have a wider host range, are more likely adapted to plant defense factors than are fungi that are involved with more specific host-parasite interactions. Nevertheless, to prove ecological relevance, in vivo investigation will be necessary.
REFERENCES
- 1.Atkinson J, Morand P, Arnason J T, Niemeyer H M, Bravo H R. Analogues of the cyclic hydroxamic acid 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one: decomposition to benzoxazolinones and reaction with mercaptoethanol. J Org Chem. 1991;56:1788–1800. [Google Scholar]
- 2.Baker E A, Smith I M. Antifungal compounds in winter wheat resistant and susceptible to Septoria nodorum. Ann Appl Biol. 1977;87:67–73. [Google Scholar]
- 3.Bolognese A, Scherrilo G, Schäfer W. Reaction of o-aminophenol and p-benzoquinone in acetic acid. J Heterocycl Chem. 1986;23:1003–1006. [Google Scholar]
- 4.Bowyer P, Clarke B R, Lunness P, Daniels M J, Osbourne A E. Host range of a plant pathogenic fungus determined by a saponin detoxifying enzyme. Science. 1995;267:371–374. doi: 10.1126/science.7824933. [DOI] [PubMed] [Google Scholar]
- 5.Bücker C, Grambow H J. Alterations in 1,4-benzoxazinone levels following inoculation with stem rust in wheat leaves carrying various alleles for resistance and their possible role as phytoalexins in moderately resistant leaves. Z Naturforsch Teil C. 1990;45:1151–1155. [Google Scholar]
- 6.Copaja S V, Barria B N, Niemeyer H M. Hydroxamic acid content of perennial Triticeae. Phytochemistry. 1991;30:1531–1534. [Google Scholar]
- 7.Couture R M, Routley D, Dunn G M. Role of cyclic hydroxamic acids in monogenic resistance of maize to Helminthosporium turcicum. Physiol Plant Pathol. 1971;1:515–521. [Google Scholar]
- 8.Crombie L. Natural resistance and susceptibility of plant hosts to fungal attack: the chemical base. In: Casida J E, editor. Pesticides and alternatives. Amsterdam, The Netherlands: Elsevier Science Publishers B. V. (Biomedical Division); 1990. pp. 497–512. [Google Scholar]
- 9.Duffy B K, Weller D M. Use of Gaeumannomyces graminis var. graminis alone and in combination with fluorescent Pseudomonas spp. to suppress take-all of wheat. Plant Dis. 1995;79:907–911. [Google Scholar]
- 10.Friebe A, Schulz M, Kück P, Schnabl H. Phytotoxins from shoot extracts and root exudates of Agropyron repens seedlings. Phytochemistry. 1995;38:1157–1159. [Google Scholar]
- 11.Friebe A, Wieland I, Schulz M. Tolerance of Avena sativa to the allelochemical benzoxazolinone. Degradation of BOA by root-colonizing bacteria. Appl Bot. 1996;70:150–154. [Google Scholar]
- 12.Gagliardo R W, Chilton W S. Soil transformation of 2(3H)-benzoxazolone of rye into phytotoxic 2-amino-3H-phenoxazin-3-one. J Chem Ecol. 1992;18:1683–1691. doi: 10.1007/BF02751095. [DOI] [PubMed] [Google Scholar]
- 13.Hartenstein H, Lippmann T, Sicker D. An efficient procedure for the isolation of pure 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA) from maize. Indian J Heterocycl Chem. 1992;2:75–76. [Google Scholar]
- 14.Niemeyer H M. Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defence chemicals in the Gramineae. Phytochemistry. 1988;27:3349–3358. [Google Scholar]
- 15.Osbourne A E. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell. 1996;8:1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petho M. Occurrence and physiological role of benzoxazinones and their derivatives. VI. Isolation of hydroxamic acids from wheat and rye root secretions. Acta Agron Hung. 1992;41:167–175. [Google Scholar]
- 17.Richardson M D, Bacon C W. Catabolism of 6-methoxy-benzoxazolinone and benzoxazolinone by Fusarium moniliforme. Mycologia. 1995;87:510–517. [Google Scholar]
- 18.Sicker D, Prätorius B, Mann G, Meyer L. A convenient synthesis of 2,4-dihydroxy-2H-1,4-benzoxazin-3(4H)-one. Synthesis. 1989;1989:211–212. [Google Scholar]
- 19.Smiley R W, Dernoeden P H, Clarke B B. Compendium of turfgrass diseases. St. Paul, Minn: APS Press; 1993. [Google Scholar]
- 20.Thackray D J, Wratten S D, Edwards P J, Niemeyer H M. Resistance to the aphids Sitobion avenae and Rhopalosiphum padi in Gramineae in relation to hydroxamic levels. Ann Appl Biol. 1990;116:573–582. [Google Scholar]
- 21.VanEtten H D, Matthews D E, Matthews P S. Phytoalexin detoxification: importance for pathogenicity and practical implications. Annu Rev Phytopathol. 1989;27:143–164. doi: 10.1146/annurev.py.27.090189.001043. [DOI] [PubMed] [Google Scholar]
- 22.Wahlroos Ö, Virtanen A I. On the antifungal effect of benzoxazolinone and 6-methoxybenzoxazolinone, respectively on Fusarium nivale. Acta Chem Scand. 1958;12:124–128. [Google Scholar]
- 23.Weibull J, Niemeyer H M. Changes in dihydroxymethoxybenzoxazinone glycoside content in wheat plants infected by three plant pathogenic fungi. Physiol Mol Plant Pathol. 1995;47:201–212. [Google Scholar]
- 24.Woodward D, Corcuera L J, Helgeson J P, Kelman A, Upper C D. Decomposition of 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one in aqueous solution. Plant Physiol. 1978;61:796–802. doi: 10.1104/pp.61.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y L, He R, Liu J L. Changes of DIMBOA contents in resistant and susceptible near-isolines of maize during infection with Exserohicum turcicum. Acta Agron Sinica. 1994;20:653–657. [Google Scholar]