Abstract
Flow cytometry and epifluorescence microscopy results for the euryarchaeon Methanothermobacter thermautotrophicus were consistent with filaments containing multiple cells. Filaments of one to four cells contained two to eight nucleoids. Single chromosome-containing cells were not observed. Filaments containing multiple genome copies displayed synchronous DNA replication initiation. Chromosome segregation occurred during replication or rapidly after replication termination.
Methanothermobacter thermautotrophicus has become an important model system for biochemical characterization of the archaeal chromosome replication machinery, providing the first functional evidence of helicase activity from an archaeal MCM complex (8, 12, 19) as well as the first structural information concerning these proteins (9, 17). Information concerning the activity and site-specific binding of the two Cdc6 homologues, which are known to play a key role in the control of eukaryotic DNA replication initiation, is also available for this species (7, 10). Insights into the organization of the cell cycle of M. thermautotrophicus will therefore have significant implications for understanding the mechanisms by which the eukaryote-like replication proteins can interface with the bacterial-type cell division proteins found in the euryarchaea (2) and will allow investigations of the cell cycle control mechanisms of these organisms.
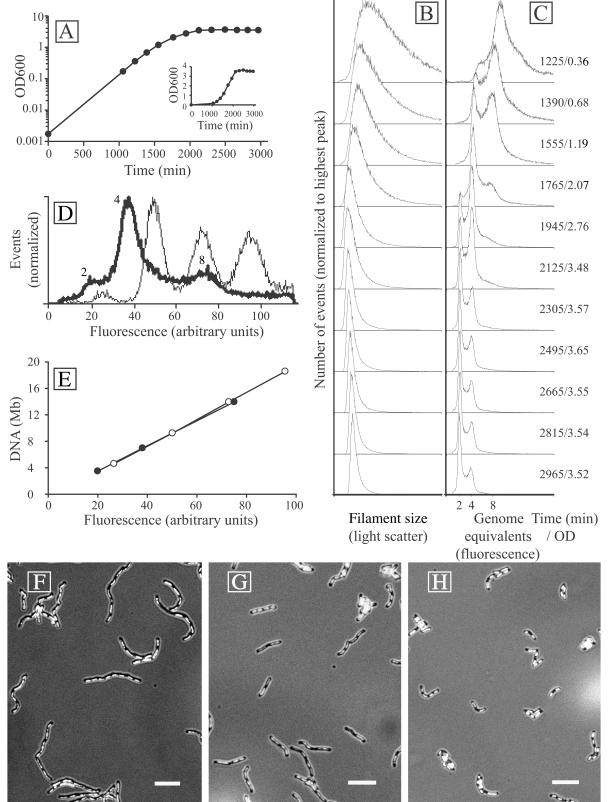
M. thermautotrophicus (DSMZ 1053) cultures were grown in a 3-liter bioreactor under chemoautotrophic conditions (16), with H2 and CO2 as the sole energy and carbon sources. Growth from an initial estimated optical density at 600 nm (OD600) of 0.002 resulted in at least five doublings before sampling and analysis commenced. The culture showed exponential growth to an OD600 of about 1 (1,500 min), a transition phase between 1,500 and 2,100 min, and then a stationary-phase plateau (Fig. 1A). Flow cytometry (5) was used to determine the DNA content of M. thermautotrophicus filaments at different growth stages (Fig. 1B and C). A correlation with the positions of peaks from flow cytometry of Escherichia coli MG1655 seqA::Tn10 cells, grown in M9 glucose medium to an OD of 0.1 and treated with 200 μg of rifampin/ml for 3 h to induce replication runout (20), indicated that the M. thermautotrophicus peaks shown in Fig. 1C could only correspond to two, four, and eight genomes (Fig. 1D). This result was confirmed by a plot of fluorescence versus the inferred chromosome content (Fig. 1E) (3), which yielded superimposed straight lines for both M. thermautotrophicus (genome size, 1.75 Mb; 49.5% GC) (22) and E. coli (genome size, 4.64 Mb; 50.8% GC) (6). The peaks in the DNA content distribution for M. thermautotrophicus at the 1,225-min time point therefore corresponded to 4 and 8 chromosomes, with a shoulder in the graph around a DNA content of 12 chromosomes (Fig. 1C). The DNA content gradually decreased with increasing ODs, and the eight-chromosome peak could no longer be detected after the 2,125-min time point. The four-chromosome peak dominated the DNA content distributions between the 1,765- and 2,125-min time points, followed by a dominant two-chromosome peak after the 2,125-min time point. Complete replication runout was not observed for the entire population in stationary phase, despite a clear plateau in the OD curve.
FIG. 1.
(A) Growth curve of M. thermautotrophicus in batch fermentor culture. The log(OD600) values of bioreactor samples were plotted against time. The insert shows a linear plot of the same data. (B and C) Flow cytometry filament size (light scatter) and DNA content (fluorescence after staining with a combination of ethidium bromide and mithramycin A) distributions in the same culture at different time points. The time (in minutes) after inoculation of the culture and the OD600 (average of four independent samples) are indicated to the right. (D) Superimposed DNA fluorescence peak positions from M. thermautotrophicus (thick line) and E. coli MG1655 seqA::Tn10 treated with rifampin (thin line). The numbers next to the M. thermautotrophicus peaks indicate the deduced numbers of genome equivalents. (E) Peak positions plotted versus chromosome sizes of E. coli (open circles) and M. thermautotrophicus (closed circles) indicate a comparable linear relationship between fluorescence and DNA content for both organisms. (F to H) Epifluorescence micrographs of DAPI-stained M. thermautotrophicus filaments demonstrating that filamentation decreases in a fermentor batch culture over time. Bars, 5 μm. (F) Exponentially growing cells from the 1,390-min time point. (G) Cells from the 1,945-min time point. (H) Stationary-phase cells from the 2,665-min time point.
The filament size distributions also displayed changes over the course of cultivation. Samples from exponential-phase cultures produced a broad light-scatter peak (Fig. 1B), consistent with M. thermautotrophicus cells forming filaments of different lengths (23, 24). The distribution was significantly extended to the right, towards high light-scatter values, indicating extensive filamentation in part of the population. At higher OD values, the light scatter decreased and a sharper peak was obtained, indicating that the range of filament lengths and the average filament length decreased. In stationary phase, after the 2,305-min time point, no further changes in either the light scatter or fluorescence distribution occurred. The flow cytometry data were confirmed by examining DAPI (4′,6-diamidino-2-phenylindole)-stained samples by epifluorescence microscopy (Fig. 1F to H). The average number of nucleoids in the filaments gradually decreased during culture growth, in agreement with the decrease in cellular DNA content observed by flow cytometry analysis.
The nucleoids were regularly distributed inside the filaments (Fig. 1F to H), and the number of nucleoids (243 filaments counted) (data not shown) correlated well with the number of genome equivalents determined by flow cytometry. This indicates that a majority of the nucleoids consisted of only a single chromosome and that genome segregation therefore must have occurred rapidly after the termination of chromosome replication or in parallel with replication elongation. This in turn implies a short, or no, G2 phase, in contrast to the case for crenarchaea from the genus Sulfolobus, in which the G2 phase is the dominating cell cycle period (4, 18). In E. coli, genome segregation occurs concomitantly with chromosome replication, and in this respect, the M. thermautotrophicus cell cycle displays a bacterial type of organization. Exponentially growing M. thermautotrophicus filaments contained between two and eight copies of the chromosome. These were distributed in a 2n fashion (Fig. 1C), again similar to the situation in E. coli (21), implying a synchronous initiation of replication when multiple origins are present in the same cell. This is in contrast to the apparently random copy number distribution observed for the euryarchaeon Methanocaldococcus jannaschii (15) and indicates significant differences in cell cycle regulation between these two methanogenic species.
The chromosome and nucleoid distributions suggest that wild-type M. thermautotrophicus cells contain two identical chromosomes (four after replication). A previous examination of M. thermautotrophicus filaments by electron microscopy revealed the presence of cross walls (23), suggesting the possibility that the chromosomes may in fact be contained within discrete compartments. The digestion of the pseudomurein cell walls of filaments from a related species, Methanothermobacter marburgensis, has been reported to result in the release of multiple protoplasts, although the DNA content of these units was not determined (13).
It is likely that the decrease in the number of genome equivalents per filament as the cultures approached stationary phase was due to a reduced replication initiation frequency in combination with successive cell divisions (1). Part of the population continued to display intermediate DNA contents in stationary phase, which may have been due to residual growth but would require an exact match between proliferating and resting cells at each time point since the distributions were invariant. A more likely possibility is that this result was due to stalled replication forks in these cells, e.g., due to medium component limitation. The extensive filamentation and consequent high chromosome content in cultures at low ODs indicate a significantly reduced cell division frequency relative to chromosomal replication when cultures are diluted in fresh medium.
Our results demonstrate several differences in DNA content and nucleoid distribution between M. thermautotrophicus and M. jannaschii, the other methanogen for which these parameters have been investigated (15). There are also significant differences between M. thermautotrophicus and the euryarchaeon Archaeoglobus fulgidus (14) as well as crenarchaeal Sulfolobus species (4), most notably the complete lack of cells containing a single chromosome for M. thermautotrophicus. This is also different from the situation in E. coli, which otherwise resembles M. thermautotrophicus in that the chromosomes are distributed in a 2n fashion and that genome segregation occurs rapidly upon the completion of DNA replication. Despite these differences, it is interesting that both Sulfolobus and Methanothermobacter maintain a majority of the cell population with at least two chromosomes during exponential growth, possibly as a means of maintaining a backup copy of the chromosome at high temperatures, which are known to result in elevated levels of DNA damage (11).
Acknowledgments
We thank Jan Olsson and Santanu Dasgupta for E. coli samples and Setareh Chong and Dawn Coverley for critically reading the manuscript.
This work was supported by a Swedish Research Council Biodiversity grant, the Swedish Graduate Research School in Genomics and Bioinformatics, the Leverhulme Trust, and the Biotechnology and Biological Sciences Research Council. J.P.J.C. is a BBSRC David Phillips Research Fellow.
REFERENCES
- 1.Åkerlund, T., K. Nordström, and R. Bernander. 1995. Analysis of cell size and DNA content in exponentially growing and stationary-phase batch cultures of Escherichia coli. J. Bacteriol. 177:6791-6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernander, R. 2003. The archaeal cell cycle: current issues. Mol. Microbiol. 48:599-604. [DOI] [PubMed] [Google Scholar]
- 3.Bernander, R., J. E. D. Palm, and S. G. Svärd. 2001. Genome ploidy in different stages of the Giardia lamblia life cycle. Cell Microbiol. 3:55-62. [DOI] [PubMed] [Google Scholar]
- 4.Bernander, R., and A. Poplawski. 1997. Cell cycle characteristics of thermophilic archaea. J. Bacteriol. 179:4963-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernander, R., T. Stokke, and E. Boye. 1998. Flow cytometry of bacterial cells: comparison between different flow cytometers and different DNA stains. Cytometry 31:29-36. [DOI] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Capaldi, S. A., and J. M. Berger. 2004. Biochemical characterization of Cdc6/Orc1 binding to the replication origin of the euryarchaeon Methanothermobacter thermoautotrophicus. Nucleic Acids Res. 32:4821-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong, J. P. J., M. K. Hayashi, M. N. Simon, R.-M. Xu, and B. Stillman. 2000. A double-hexamer archaeal minichromosome maintenance protein is an ATP-dependent DNA helicase. Proc. Natl. Acad. Sci. USA 97:1530-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher, R. J., B. E. Bishop, R. P. Leon, R. A. Sclafani, C. M. Ogata, and X. S. Chen. 2003. The structure and function of MCM from archaeal M. thermoautotrophicum. Nat. Struct. Biol. 10:160-167. [DOI] [PubMed] [Google Scholar]
- 10.Grabowski, B., and Z. Kelman. 2001. Autophosphorylation of archaeal Cdc6 homologues is regulated by DNA. J. Bacteriol. 183:5459-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grogan, D. W. 2000. The question of DNA repair in hyperthermophilic archaea. Trends Microbiol. 8:180-185. [DOI] [PubMed] [Google Scholar]
- 12.Kelman, Z., J.-K. Lee, and J. Hurwitz. 1999. The single minichromosome maintenance protein of Methanobacterium thermoautotrophicum ΔH contains DNA helicase activity. Proc. Natl. Acad. Sci. USA 96:14783-14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiener, A., H. König, J. Winter, and T. Leisinger. 1987. Purification and use of Methanobacterium wolfei pseudomurein endopeptidase for lysis of Methanobacterium thermoautotrophicum. J. Bacteriol. 169:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maisnier-Patin, S., L. Malandrin, N.-K. Birkeland, and R. Bernander. 2002. Chromosome replication patterns in the hyperthermophilic euryarchaea Archaeoglobus fulgidus and Methanocaldococcus (Methanococcus) jannaschii. Mol. Microbiol. 45:1443-1450. [DOI] [PubMed] [Google Scholar]
- 15.Malandrin, L., H. Huber, and R. Bernander. 1999. Nucleoid structure and partition in Methanococcus jannaschii: an archaeon with multiple copies of the chromosome. Genetics 152:1315-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nölling, J., M. Frijlink, and W. M. de Vos. 1991. Isolation and characterisation of plasmids from different strains of Methanobacterium thermoformicicum. J. Gen. Microbiol. 137:1981-1986. [Google Scholar]
- 17.Pape, T., H. Meka, S. Chen, G. Vicentini, M. van Heel, and S. Onesti. 2003. Hexameric ring structure of the full-length archaeal MCM protein complex. EMBO Rep. 4:1079-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poplawski, A., and R. Bernander. 1997. Nucleoid structure and distribution in thermophilic Archaea. J. Bacteriol. 179:7625-7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shechter, D. F., C. Y. Ying, and J. Gautier. 2000. The intrinsic DNA helicase activity of Methanobacterium thermoautotrophicum ΔH minichromosome maintenance protein. J. Biol. Chem. 275:15049-15059. [DOI] [PubMed] [Google Scholar]
- 20.Skarstad, K., and E. Boye. 1988. Perturbed chromosomal replication in recA mutants of Escherichia coli. J. Bacteriol. 170:2549-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skarstad, K., E. Boye, and H. B. Steen. 1986. Timing of initiation of chromosome replication in individual Escherichia coli cells. EMBO J. 5:1711-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, D. R., L. A. Doucette-Stamm, C. Deloughery, H. Lee, J. Dubois, T. Aldredge, R. Bashirzadeh, D. Blakely, R. Cook, K. Gilbert, D. Harrison, L. Hoang, P. Keagle, W. Lumm, B. Pothier, D. Qiu, R. Spadafora, R. Vicaire, Y. Wang, J. Wierzbowski, R. Gibson, N. Jiwani, A. Caruso, D. Bush, H. Safer, D. Patwell, S. Prabhakar, S. McDougall, G. Shimer, A. Goyal, S. Pietrokovski, G. M. Church, C. J. Daniels, J.-I. Mao, P. Rice, J. Nölling and J. N. Reeve. 1997. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J. Bacteriol. 179:7135-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeikus, J. G., and R. S. Wolfe. 1973. Fine structure of Methanobacterium thermoautotrophicum: effect of growth temperature on morphology and ultrastructure. J. Bacteriol. 113:461-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeikus, J. G., and R. S. Wolfe. 1972. Methanobacterium thermoautotrophicus sp. nov., an anaerobic, autotrophic, extreme thermophile. J. Bacteriol. 109:707-715. [DOI] [PMC free article] [PubMed] [Google Scholar]