Abstract
The effect of pH on atmospheric methane (CH4) consumption was studied with slurries of forest soils and with bacteria extracted from the same soils. Soil samples were collected from a mixed hardwood stand in New Hampshire, from jackpine and aspen stands at the BOREAS (Boreal Ecosystem Atmosphere Study) site near Thompson, northern Manitoba, from sites in southern Québec, including a beech stand and a meadow, and from a site in Ontario (cultivated humisol). Consumption of atmospheric CH4 (concentration, approximately 1.8 ppm) occurred at depths of >5 cm in both acidic (pH 4.5 to 5.2) and alkaline (pH 7.2 to 7.8) soils. In slurries of acidic soils, maximum activity occurred at different pH values (pH 4.0 to 6.5). Bacteria extracted from these soils by high-speed blending and density gradient centrifugation showed pH responses different from the pH responses of the slurries. In all cases, these bacteria had a methanotrophy pH optimum of 5.8 and exhibited no activity at pH 6.8 to 7.0, the pH optimum range for known methanotrophs. This difference in pH responses could be useful in modifying media currently used for isolation of these organisms. Methanotrophic activity was induced in previously non-CH4-consuming soils by preincubation with 5% (vol/vol) CH4 (50,000 μl of CH4 per liter) or by liquid enrichment with 20% CH4. The bacteria showed pH responses typical of known methanotrophs and not typical of preexisting consumers of ambient CH4. Furthermore, methanotrophs induced by high CH4 levels were more readily extracted from soil than preexisting ambient CH4 consumers were. In the alkaline soils, preexisting activity either was destroyed or resisted extraction by the procedure used. The results support the hypothesis that consumers of ambient CH4 in soils are physiologically distinct from the known methanotrophs.
Aerobic soils, such as forest soils, play an important role in the global methane (CH4) cycle by acting as a major sink for atmospheric CH4 (25). CH4 consumption is a biological process carried out primarily by aerobic CH4-oxidizing bacteria (21, 31, 42). This process occurs in a variety of environments, especially environments with anoxic zones where large amounts of methane are produced. Its occurrence in aerobic upland soils is interesting because, in general, the source of CH4 is the low ambient level in the atmosphere (1.7 to 1.8 ppm). Despite the low substrate concentration, consumption of atmospheric CH4 in these soils is a widespread phenomenon. Kinetic studies of CH4 consumption in soil suggest that there may be two groups of methanotrophs, those that utilize ambient (atmospheric) levels of CH4 and those that utilize higher levels, presumably due to a lower affinity (high Km) for CH4 (7). Although several methanotrophs that require high levels of CH4 (e.g., 1,000 ppm or higher) have been isolated and studied, organisms that consume atmospheric CH4 have yet to be cultured, and little is known about their physiology and ecology (25, 26, 39). It has been suggested that methanotrophs cannot grow at atmospheric levels of CH4 (14). However, it was recently shown by using radiolabelled CH4 that growth and survival at these levels is possible and that the pattern of lipid labelling in soil methanotrophs which consume ambient CH4 differs from the pattern of lipid labelling in known methanotrophs (34, 35). There is also the possibility that known methanotrophs, under certain conditions, may be responsible for atmospheric CH4 consumption (9). At present, it is not known what organisms consume atmospheric levels of CH4 in situ. Cultivation of these organisms would greatly aid our understanding of their ecology and allow workers to compare them to the known methanotrophs.
Here we report the effect of pH on atmospheric CH4 consumption in forest soils and on the bacteria responsible for this activity. We used bacteria extracted from soils by density gradient centrifugation to compare the pH responses of the organisms that consume atmospheric levels and the organisms that require higher concentrations of CH4.
MATERIALS AND METHODS
Sites and sampling.
Forest soil samples were obtained from jackpine (Pinus banksiana) and aspen (Populus tremuloides) stands at the BOREAS (Boreal Ecosystem Atmosphere Study) site in northern Manitoba; these samples were designated Pine and Aspen-1 and -2, respectively (3, 4, 36). Samples were also obtained from a mixed hardwood forest in New Hampshire, designated Mixed, where the dominant vegetation consisted of hemlock (Tsuga canadensis) and pin cherry (Prunus pensylvanica) (15), and from a beech (Fagus grandifolia) stand, designated Beech, in the Morgan Arboretum of McGill University, Ste. Anne-de-Bellevue, Québec, Canada. For comparison, two nonforested soils, a cultivated humisol (designated H) from Ottawa, Ontario, Canada, and a St. Bernard sandy loam (designated SB) from a meadow in Ste. Anne-de-Bellevue (19, 20), were also used.
The Pine and Aspen sites were on gneissic rock overlain by glacial deposits (37). The top 10 cm of Pine soil consisted of an organic layer (pH 4.0 to 4.50) that was underlain by a sandy mineral layer. The Aspen-1 and -2 soils consisted of organic litter (0 to 6 cm; pH 5.3 to 7.0) underlain by clay (pH 7.2 to 8.2) (4). The Mixed soil was from an area of inceptisols on acidic glacial till (15). The upper 7 cm was organic, and the lower layers sampled (to a depth of 12 cm) were unconsolidated mineral soil (pH 4.0 to 4.8 [both layers]) (4). The Beech soil consisted of a thick litter layer (thickness, 2 cm) underlain by a layer of mixed humus and sand (pH 4.1 to 4.3). The humisol and sandy loam (loss on ignition, about 60 and 7%, respectively) had pH values of 7.1 to 7.2 (19, 20).
Soils were obtained either as cores or in bulk as previously described (3, 4). Cores were sectioned into 2-cm-thick slices, which were placed into 1-liter Mason jars. Atmospheric CH4 consumption was measured for each section to determine the depth at which the greatest activity occurred. The lids of the jars were drilled and fitted with Suba-Seal serum stoppers (William Freeman and Co., Barnsley, England). Joints were sealed with silicone rubber. The headspace of each Mason jar contained room atmosphere with a CH4 level of 1.8 to 2.0 ppm. Changes in headspace CH4 concentrations over 48 h were measured by gas chromatography (see below). The following different depths of soil columns were used for experiments: Pine and Aspen soils, 0 to 5 and 5 to 10 cm; Mixed soil, 0 to 3, 3 to 7, and 7 to 12 cm; Beech soil, 6 to 8 cm; H soil, 0 to 10 cm; and SB soil, 6 to 8 cm.
Extraction of bacteria.
Bacteria were extracted from soils by a slight modification of the method of Priemé et al. (4, 33). Soil-distilled deionized water (1:3, wt/vol) slurries were blended with a homogenizer (Virtis Co., Inc., Gardiner, N.Y.) at 22,000 rpm for a total of 20 min. Blending was done in an ice bath in 5- to 10-min intervals with 5-min nonblending intervals to avoid overheating of the slurry. A portion of each blended slurry was then subjected to centrifugation with a Nycodenz (Life Technologies, Inc., Gaithersburg, Md.) solution having a density of 1.3 g ml−1 (30). The blended slurry (3 to 4 ml) was carefully added on top of 8 ml of the Nycodenz solution in a 26-ml polycarbonate centrifuge tube, which was then centrifuged (20,000 × g, 20 min, 4°C). The resulting layer of bacterial cells floating on the Nycodenz cushion in the tube was recovered with a syringe and needle, placed in a clean polycarbonate tube, and washed three times with 3 volumes of distilled water buffered with 10 mM phosphate buffer (pH 5.8 to 6.2) or with 10 mM 2-(N-morpholino)ethanesulfonate (MES) (13) buffer (pH 5.8 to 6.2). The washed cells were resuspended in a small volume (10 to 12 ml) of nitrate mineral salts solution (NMS) (42) buffered with MES (final concentration, 50 to 100 mM; pH 5.8) and were used immediately in experiments. It is not possible at present to know to what extent, if any, the extraction procedure injured the unknown methanotrophs of interest.
pH response.
The effect of pH on atmospheric CH4 consumption by extracted soil bacteria was determined in 58-ml serum bottles containing 4.5-ml portions of NMS having different pH values (pH 3.8 to 8.2) adjusted with 200 to 500 μl of 100 mM MES buffer. Suspensions (0.5 ml) of extracted bacteria were added to the bottles, and, if necessary, pH values were readjusted with free acid or sodium salt forms of MES at a concentration of 0.1 M. pH values were determined at the start and end of each incubation to detect possible changes, and the values reported below are the averages of the two measurements; the differences between the measurements were small (≤0.1 pH unit) (data not shown). The bottles were capped with gray butyl stoppers and aluminum crimps and incubated shaken (200 rpm, 25°C) under room atmosphere (1.8 to 2.0 ppm of CH4). The incubation periods varied from 2 to 5 days depending on the activity of the bacterial suspension. The rates of CH4 consumption were determined per gram (dry weight) of soil by using the weight of soil from which the bacteria were extracted.
The effect of pH on atmospheric CH4 consumption by soil slurries was determined in a similar way. Aliquots (5 ml) of soil-water slurries (1:3, wt/vol) were added to serum bottles, and pH values were adjusted with either 0.1 N HCl or 100 mM NaHCO3. The initial pH values for each incubation were determined 30 min after pH adjustment with acid or base, and final pH values were determined at the end of the incubation. The values reported below are the averages of these two pH measurements. The differences between the initial and final pH values were generally small (≤0.2 pH unit) (data not shown). Incubations were carried out as described above over a period of 12 to 48 h.
Occasionally, CH4 consumption was monitored by using high levels of headspace CH4 (2%, vol/vol) under the incubation conditions described above.
Unless otherwise stated, the values reported below are the means ± standard errors based on duplicate incubations.
Enrichments and pure cultures.
Enrichments for CH4-consuming organisms were carried out with field-moist soils, soil slurries, and extracted bacterial suspensions. Field-moist soils (100 g), which initially had undetectable or low rates of consumption (<1 nmol g [dry weight]−1 day−1) under both low and high CH4 concentrations, were incubated in 1-liter Mason jars with 5% (vol/vol) CH4 in air for 1 to 2 months. The headspace gases were renewed every 2 to 3 days, and CH4 consumption was monitored by gas chromatography. Slurries of soils that showed enrichment for CH4 consumption were prepared, and bacteria were extracted as described above for use in further experiments.
Bacterial extracts were also enriched with 20% (vol/vol) CH4 in air. A bacterial suspension (1 ml) was added to 10 ml of NMS (pH 5.8 to 6.0) in a 58-ml serum bottle and incubated at 25°C until it was turbid. Then 1 ml of the enrichment was transferred to a new serum bottle and used for experiments once the culture was grown. Soil slurries were enriched in the same way, but the initial inoculum was 1 ml of a 10−1 soil dilution in water.
Methylosinus trichosporium OB3b and Methylobacter luteus (gifts from R. S. Hanson, University of Minnesota) were grown in 50 ml of NMS medium (pH 6.8) in 125-ml Erlenmeyer flasks with 20% (vol/vol) CH4 and air and shaken (200 rpm, 25°C). Mid-log-phase cells were recovered and washed twice with fresh NMS by centrifugation (8,000 × g, 10 min, 4°C), and then they were resuspended in 20 ml of NMS. The resuspended cells were used to determine the effect of pH on atmospheric CH4 consumption, as described above.
Analyses.
Headspace gas samples were obtained with a syringe four to six times during the incubation period and analyzed by gas chromatography (2). The bottle pressure was maintained by replacing the samples removed with equal volumes of air having a similar CH4 content. Appropriate corrections were made when CH4 consumption was calculated. A gas chromatograph equipped with a column (length, 2 m; diameter, 3 mm) packed with Porapak Q was used. Low levels of CH4 were determined by flame ionization detection, and higher levels of CH4 were determined by thermal conductivity detection.
Atmospheric CH4 consumption followed first-order kinetics. Rate constants were obtained from log-transformed time course data and rates calculated at 1.8 ppm. The CH4 consumption in experiments with high levels of methane (≥2% methane) was estimated from the difference between the CH4 concentrations at the start and end of incubation (2 days).
Soil pH was determined with soil-water slurries (1:3, wt/vol) by using a Fisher Accumet pH meter and electrode. Slurries were mixed, open to the atmosphere, with a magnetic stirrer for 20 min before the pH was measured. A pH value was recorded when the meter reading changed less than 0.1 pH unit per min.
RESULTS
pH response of atmospheric CH4 consumption by forest soils.
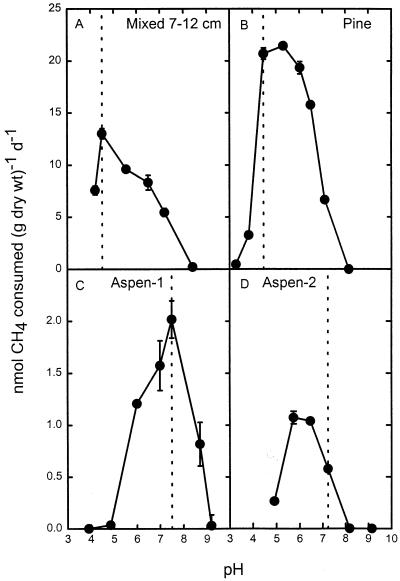
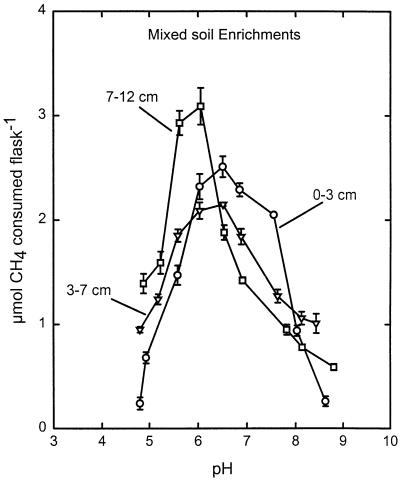
Atmospheric CH4 consumption in most of the forest soils studied occurred largely in subsurface soil layers (depth, >5 cm) (5), as previously observed (1, 3, 28). These active layers were used to test the effect of pH on atmospheric CH4 consumption by soil slurries. Activity was observed over a pH range of 3.5 to 9, with alkaline soils (Fig. 1C and D) showing little activity at pH values below 5. Despite large differences in soil pH, three of the soils tested (Fig. 1A through C) had pH optima at or near the natural pH, suggesting that the methanotrophs in these soils are at least partially adapted to their environmental pH values. Except for Aspen-1 (Fig. 1C), which had maximal activity at its natural pH, pH 7.6, the forest soils had acidic pH optima with values as low as pH 4.7 (Fig. 1A).
FIG. 1.
Atmospheric CH4 consumption at different pH values in slurries of four forest soils. (A) Mixed soil (depth, 7 to 12 cm). (B) Pine soil. (C) Aspen-1 soil. (D) Aspen-2 soil. The dotted lines indicate the natural pH of each soil. d, day.
Preincubation of field-moist soil with CH4.
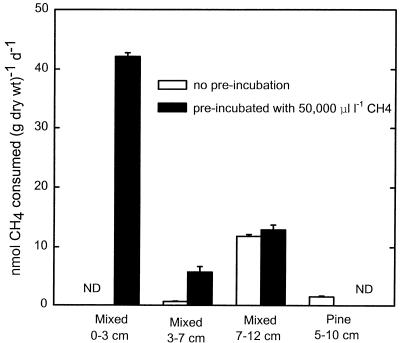
Several soils were enriched for methanotrophs by preincubation with a high level of CH4 (5%) to compare the pH responses of organisms favored by this treatment with the pH responses of preexisting CH4 consumers. Atmospheric CH4 consumption was induced by preincubation only in the Mixed soil layers at depths of 0 to 3 and 3 to 7 cm, which had little or no preexisting activity (Fig. 2). This enriched activity, however, was transient and became undetectable after 2 weeks of continuous incubation with 1.8 to 2.0 ppm of CH4 (5), as commonly occurs with known, high-Km methanotrophs (9, 26). Activity was not enhanced in the 7- to 12-cm horizon, the only layer of this soil with significant preexisting activity. The pH response of these enriched methanotrophs was compared to the pH response of the native consumers of atmospheric CH4 (see below).
FIG. 2.
Atmospheric methane consumption in forest soils before and after preincubation with 5% CH4. ND, activities were undetectable; d, day; l, liter.
Effect of the extraction procedure on CH4 consumption by soil bacteria.
To determine the effect of pH on atmospheric CH4-consuming bacteria independent of the soil matrix and in the absence of effective culturing methods, we extracted mixed bacterial populations from several soils. Successful removal of active CH4-consuming bacteria depended on the soil type and treatment. For example, soil with activity induced by preincubation with 5% CH4 (Mixed soil at a depth of 0 to 3 cm) (Fig. 2) responded to the extraction procedure differently than soils with preexisting activity (Table 1). Blending of the soil slurries before centrifugation with Nycodenz resulted in a loss of activity of up to 50% in soils that exhibited native atmospheric CH4 consumption but not in the induced soil. Furthermore, bacteria extracted from the induced soil accounted for more than one-half of the activity in the original unblended soil. For soils with preexisting activity, bacterial extracts accounted for less than 10% of the activity originally present, possibly due to cell damage caused by the extraction procedure. In the case of Aspen-2 soil, blending reduced activity by less than 20%, but no activity was detectable in Nycodenz-separated cells. However, the nonforested humisol and sandy loam soils lost all activity after blending, and no activity was detected in bacterial extracts (Table 1). The different tolerances to the extraction procedure observed indicated that there were differences between the CH4 oxidizers in the forest and nonforested soils used and also between native consumers of ambient CH4 and methanotrophs induced by preincubation with high levels of CH4.
TABLE 1.
Atmospheric CH4 consumption in soil slurries before and after blending and by extracted bacteria
| Soil | Depth (cm) | CH4 consumption in un- blended slurry (nmol g [dry wt]−1 day−1) | CH4 consumption as % of unblended activity
|
|
|---|---|---|---|---|
| Blended slurry | Extracted bacteriaa | |||
| Mixedb | 0–3 | 56.1 ± 1.3 | 105 | 55 |
| Mixed | 7–12 | 6.24 ± 0.10 | 61 | 2 |
| Pine | 5–10 | 8.40 ± 0.20 | 47 | 7 |
| Aspen-2 | 5–10 | 0.15 ± 0.004 | 82 | NDc |
| Beech | 6–8 | 7.01 ± 0.49 | 49 | ndd |
| H | 0–10 | 0.50 ± 0.01 | ND | ND |
| SB | 6–8 | 0.62 ± 0.01 | ND | ND |
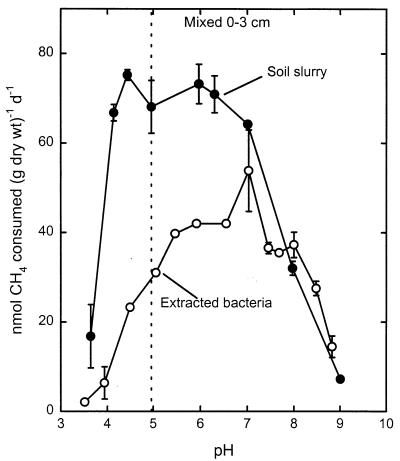
pH response of native atmospheric CH4 consumers extracted from soil.
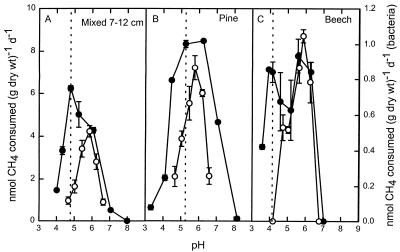
The pH response of CH4 consumption by extracted bacteria was evaluated for three forest soils from which sufficiently active preparations were obtained. The activity by extracted bacteria had a narrower pH range (pH 4.6 to 6.6) than the activity by the soil slurries had (Fig. 3). Despite differences in the pH responses between soil slurries, the responses of bacteria extracted from the three soils were nearly identical. All three preparations of extracted bacteria had a pH optimum of 5.8 to 5.9. In Mixed soil at a depth of 7 to 12 cm, the bacterial pH optimum was 1 pH unit higher than the pH optimum of the soil slurry. Soil slurries of the Pine soil and especially the Beech soil did not have clear and sharp pH optima but had significant activities at their natural pH values, unlike the respective extracted bacteria (Fig. 3). Thus, it appears that the soil matrix may protect CH4 consumers from detrimental pH values. These three soils all had acidic natural pH values (Fig. 3), but it is not known if they differed in buffering capacity. All attempts to extract active bacteria from soils with nearly neutral or basic pH values (Aspen-1 and -2, H, and SB soils) were unsuccessful (Table 1) (5).
FIG. 3.
pH response of atmospheric CH4 consumption by slurries of three forest soils (•) and by bacteria (○) extracted from the same soils. (A) Mixed soil (depth, 7 to 12 cm). (B) Pine soil. (C) Beech soil. The dotted lines indicate the natural pH of each soil. d, day.
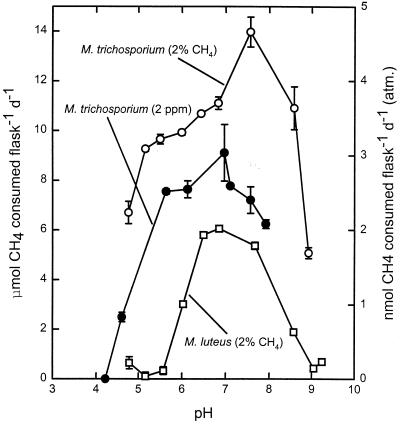
pH response of pure cultures of methanotrophs.
In contrast to the responses of extracted native bacteria, the pH responses of Methylosinus trichosporium OB3b and Methylobacter luteus occurred over a broader pH range (pH <5 to >9), and the optimal pH values were around pH 7 (Fig. 4). For Methylosinus trichosporium OB3b, incubation with atmospheric CH4 concentrations and incubation with higher CH4 concentrations gave essentially the same profile.
FIG. 4.
Methane consumption by Methylosinus trichosporium OB3b (○) and Methylobacter luteus (□) in the presence of 2% CH4 and by Methylosinus trichosporium OB3b in the presence of 2 ppm CH4 (•) at different pH values. Data are means from triplicate incubations. d, day.
pH response of experimentally CH4-enriched soils and extracted bacteria.
Liquid NMS medium enrichments resulted in growth of methanotrophs from all three Mixed soil layers (Fig. 5). With a high CH4 concentration (2%), these liquid enrichments had pH responses similar to those of the two known methanotrophs (Fig. 4), with activity over a broad pH range (pH <5 to 9) and pH optima near neutral pH. An acidic optimum (about pH 6) occurred in the 7- to 12-cm enrichment (Fig. 5), but this value was significantly higher than the pH optimum observed for the native activity of the same soil (pH 4.5) (Fig. 1). Similar responses were not observed in enrichments with Pine (Fig. 2) and Aspen (5) soils.
FIG. 5.
pH response of CH4 consumption by cultures enriched in the presence of 2% CH4 from the Mixed soil at depths of 0 to 3 cm (○), 3 to 7 cm (▵), and 7 to 12 cm (□). Measurements were carried out with atmospheres containing 2% CH4 in air. Data are means from triplicate incubations.
Because preincubated, field-moist Mixed soil from a depth of 0 to 3 cm showed the greatest enhancement of atmospheric CH4 consumption (Fig. 2), slurries and bacterial extracts from this enriched soil were tested for pH responses with a CH4 concentration of 1.8 ppm (Fig. 6). The range of the soil slurry response was similar to the range of the soil slurry responses of soils with preexisting activity (Fig. 1 and 3). However, the pH response of the extracted bacteria (Fig. 6) was similar to the pH response of known methanotrophs (Fig. 4), showing a wider pH range (pH 3.5 to 9) and higher optimum pH (pH 7) than bacteria extracted from soils with preexisting activity (Fig. 3). Once again, however, the soil slurry showed much higher activity at low pH values than the extracted bacteria did.
FIG. 6.
pH response of atmospheric CH4 consumption induced by preincubation with 5% CH4 in slurries of Mixed soil (depth, 0 to 3 cm) (•) and in bacteria extracted from this soil (○). The dotted line indicates the natural pH of the soil. d, day.
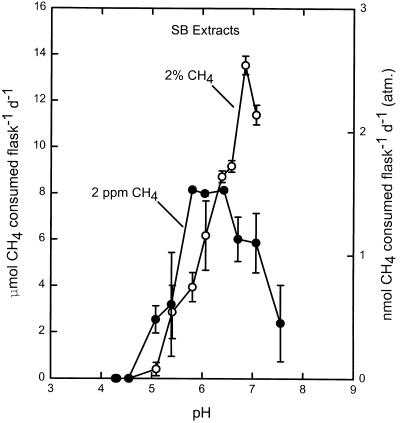
Although bacterial extracts from the meadow soil (SB soil) showed no activity (Table 1), both CH4 consumption by and presumably growth of methanotrophs were induced by incubation of the extracts with 20% CH4. The pH response of the resulting enrichment was similar with both 1.8 and 20,000 ppm (2%) (Fig. 7), and the pattern was the pattern exhibited by pure cultures of Methylosinus trichosporium OB3b under the same CH4 regime (Fig. 4). As observed for the Mixed soil enrichments (Fig. 5), activity by SB soil enrichments was undetectable after several days under atmospheric CH4 (5). This transient activity might coincide with the depletion of another, nonmethane substrate pool (e.g., methanol) in the cells (9). Similar results were obtained for bacterial extracts from Beech soil, but no enrichment occurred in extracts from Pine and Aspen soils (5).
FIG. 7.
pH response of CH4 consumption by washed SB soil bacterial extracts after enrichment by preincubation with 20% CH4, measured in the presence of 2 ppm of CH4 (•) and 2% CH4 (○). Prior to enrichment with CH4 no activity was detected in these extracts (Table 1). d, day.
DISCUSSION
Although methanotrophic enrichments can be recovered from some aerobic soils when they are incubated with high levels of CH4, as observed here, a methanotrophic culture showing stable, long-term consumption of atmospheric CH4 as a sole carbon source has yet to be isolated. Successful cultivation and characterization of such methanotrophs would be aided by a better understanding of how different ecological parameters affect them. The effects of temperature (27, 32), water activity (1, 26, 38), ammonium concentration (32, 40), and nutrient concentration (39) on atmospheric CH4 consumption in soils have been studied, but little is known yet about the ecology and physiology of the organisms responsible for this activity. Furthermore, because of potential matrix effects, it is difficult to deduce from soil experiments the actual physiological characteristics of the organisms. To overcome these problems, we compared the effects of pH on atmospheric CH4 consumption by soils and by extracted soil bacteria. We found that the pH response of atmospheric CH4 consumers differs from the pH response of known methanotrophs. This finding helps elucidate the physicochemical conditions required for activity.
Methane consumption occurs in environments with pH values ranging from <4 to 9 (11, 17, 22, 24, 25). Furthermore, several methanotrophic bacteria can grow in pure culture at pH values below 5 and as high as 9 (12, 22). Methanotrophic yeasts capable of growth at pH 3.5 were isolated from lake water and soil (43), as well as ruminant dung (36), although their environmental significance is not clear (25). In general, the known, high-Km, methanotrophic bacteria are neutrophilic (pH 6.8 to 7.0) (21, 42), although moderately acidophilic enrichments from acid peats have been reported recently (16). We found that atmospheric CH4 consumption by extracted soil bacteria was optimal at pH 5.8, 1 pH unit below the pH optimum of other methanotrophs, and was negligible at neutral pH. Thus, the culture media currently used for methanotrophs, which usually have a circumneutral pH (7, 12, 42), are not appropriate for enrichment of these atmospheric CH4 consumers. However, attempts to isolate these organisms by using pH ≤5.8 media were unsuccessful (5, 21), and other factors may also be important (16).
The similarity of the pH profiles of bacteria extracted from three acidic soils with diverse types of vegetation cover (coniferous, deciduous, and mixed) from diverse geographical locations (north-central Canada, southeastern Canada, and northeastern United States) suggests that the pH response observed might be widespread for atmospheric CH4 consumers. Furthermore, the well-characterized methanotroph Methylosinus trichosporium OB3b showed the same pH response (circumneutral pH optimum) for CH4 consumption in the presence of both 2 ppm of CH4 and 2% CH4, supporting the hypothesis that consumers of ambient CH4 in soils are physiologically distinct from the known methanotrophs. The fact that the extracts showed no significant activity at pH values below 4 suggests that yeasts were not important members of the CH4-consuming population. Similarly, we did not detect filamentous organisms during microscopic examination of the extracts (5), indicating that the CH4 consumption was consumption by nonfilamentous bacteria. Of course, this does not necessarily preclude the involvement of fungi or actinomycetes in unblended, unextracted soil.
Actively CH4-oxidizing bacteria could not be extracted from the otherwise active circumneutral soils tested, possibly because of physical damage to the cells, so a direct comparison of these organisms with the organisms extracted from the acidic soils was not possible. Differences in pH responses between extracted bacteria and soil slurries may have been due to selective damage to a subset of the methanotroph population during the extraction procedure. Alternatively, effects of the physical and chemical soil environment may also have played a role. We found that slurries were active over broader pH ranges than extracted bacteria were, suggesting the possibility that the soil matrix shields methanotrophs to some extent from harmful chemical environments. Such shielding could be due to bacteria existing in soil aggregates (33). Components such as ammonium may also contribute to apparent soil pH optima unrelated to the physiological optimum of the methanotrophs present. Sitaula et al. (41) concluded that greater consumption of atmospheric CH4 in soils perfused with pH 3.3 water than in soils perfused with pH 4 and 5 water was due to a decrease in CH4 monooxygenase inhibition caused by conversion of ammonia to the ammonium form at the lower pH (6). However, the ratio of ammonia to ammonium is very small throughout the pH range from pH 3.3 to 5. Nevertheless, similar mechanisms may explain why we observed a pH optimum with the Mixed soil (depth, 7 to 12 cm) slurry that was more than 1 pH unit lower (pH 4.5) than the pH optimum of the extracted washed bacteria from the same soil. Dunfield et al. (18) found evidence that high-Km methanotrophs in acidic peat soils are only partially adapted to the native pH. This observation supports our findings obtained with atmospheric CH4 consumers in acidic forest soils that the native soil pH is not optimal for CH4 consumption by either extracted bacteria or some soil slurries. Another possible consideration is that nitrifying bacteria may be responsible for CH4 consumption in some soils (23), but autotrophic nitrifiers would likely be more active at less acidic pH values (10).
In our study, acidic soils had the greatest CH4 consumption rates, but this is not always the case (3, 23). However, it is clear that changes in pH can drastically affect the activity of soils. Slight acidification resulted in the cessation of CH4 consumption in some circumneutral soils (23) (see above). Liming of a forest soil also resulted in a decrease in CH4 consumption (45). These effects suggest that there has been more adaptation of methanotrophs to their environment than was observed in temperate and arctic peats (18), and they show how soil chemical changes related to land use can affect this important activity in the short or long term.
Our results also indicate that there may be basic differences between methanotrophic populations in different soils. Although bacteria extracted from the acidic forest soils showed similar responses to the treatments employed, the circumneutral soils lost all activity after high-speed blending. It is not clear why atmospheric CH4 consumption in the latter soils is more sensitive to blending and extraction. Possible reasons include greater fragility of the organisms involved or a stronger requirement for attachment of microbial cells to soil particles (33). Although the mechanism is not clear, the adsorption of bacterial cells to solid supports is known to be an important factor in increased metabolic activity (29). A similar difference in the efficiency of extraction of atmospheric CH4 consumers has also been described for Danish and Norwegian forest soils (33).
The ability to enrich for high-Km methanotrophs (by using 5 to 20% CH4) in some of our soils and extracted bacterial mixtures indicates that these organisms are already present in the soils. However, they are likely to be inactive, as observed with the Mixed soil at a depth of 0 to 3 cm, since the CH4 concentrations in the gas spaces of these aerobic soils are usually too low (≤1.8 ppm) (15, 33) to support long-term activity by these organisms (9). It has been suggested that CH4 produced deep in forest soils, particularly under wet conditions, may help support methanotrophy in the upper aerobic layers (44) and may explain why some of these soils may harbor high-Km methanotrophs. However, our results suggest that these methanotrophs are distinct from those responsible for native atmospheric CH4 consumption in soils. For example, the enrichment cultures obtained with high CH4 concentrations of soil and extracted bacterial mixtures generally showed pH responses typical of the known, cultured methanotrophs (in the presence of both high and atmospheric CH4 levels) and not of the extracted, native methanotrophs. Bender and Conrad (8) also found that induction of methanotrophy in several soils was optimal at circumneutral pH values, even though the range of native pH values in the soils used was 4.5 to 8.1. Furthermore, unlike native activity, the methanotrophic activity induced in Mixed soil at a depth of 0 to 3 m by preincubation with high CH4 levels was not affected by blending, and the active bacteria were removed from the soil with relative ease. The tight association of native methanotrophs with the soil has been described by Priemé et al. (33). The same authors also described these organisms as very slow growing, which partially explains the lack of success in obtaining them in pure culture.
In summary, we observed different pH responses with native and laboratory cultures and enrichments of methanotrophs, supporting the contention that atmospheric CH4 consumption is carried out by organisms distinct from the known methane oxidizers. The pH parameters for activity of atmospheric CH4 consumers that we determined should aid in attempts to successfully cultivate these organisms.
ACKNOWLEDGMENTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and NSERC-BOREAS.
We thank P. Crill, K. Savage, and J. Paquin for collecting some of the soil samples.
REFERENCES
- 1.Adamsen A P S, King G M. Methane consumption in temperate and subarctic forest soils: rates, vertical zonation, and responses to water and nitrogen. Appl Environ Microbiol. 1993;59:485–490. doi: 10.1128/aem.59.2.485-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaral J A, Archambault C, Richards S R, Knowles R. Denitrification associated with Groups I and II methanotrophs in a gradient enrichment system. FEMS Microbiol Ecol. 1995;18:289–298. [Google Scholar]
- 3.Amaral J A, Knowles R. Localization of methane consumption and nitrification activities in some boreal forest soils and the stability of methane consumption on storage and disturbance. J Geophys Res Atmos. 1997;102:29,255–29,260. [Google Scholar]
- 4.Amaral J A, Knowles R. Inhibition of methane consumption in forest soils and pure cultures of methanotrophs by aqueous forest soil extracts. Soil Biol Biochem. 1997;29:1713–1720. [Google Scholar]
- 5.Amaral, J. A., and R. Knowles. Unpublished data.
- 6.Bédard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender M, Conrad R. Kinetics of CH4 oxidation in oxic soils exposed to ambient air or high CH4 mixing ratios. FEMS Microbiol Ecol. 1992;101:261–270. [Google Scholar]
- 8.Bender M, Conrad R. Effect of methane concentrations and soil conditions on the induction of methane oxidation activity. Soil Biol Biochem. 1995;27:1517–1527. [Google Scholar]
- 9.Benstead J B, King G M, Williams H G. Methanol promotes atmospheric methane oxidation by methanotrophic cultures and soils. Appl Environ Microbiol. 1998;64:1091–1098. doi: 10.1128/aem.64.3.1091-1098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bock E, Koops H-P, Harms H. Cell biology of nitrifying bacteria. In: Prosser J I, editor. Nitrification. Oxford, United Kingdom: IRL Press; 1986. pp. 17–38. [Google Scholar]
- 11.Born M, Dörr H, Levin I. Methane consumption in aerated soils of the temperate zone. Tellus. 1990;42:2–8. [Google Scholar]
- 12.Bowman J P, Sly L I, Nichols P D, Hayward A C. Revised taxonomy of the methanotrophs: description of Methylobacter gen. nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes only the group I methanotrophs. Int J Syst Bacteriol. 1993;43:735–753. [Google Scholar]
- 13.Calbiochem-Behring. A guide for the preparation and use of buffers in biological systems. La Jolla, Calif: Calbiochem-Behring, Division of American Hoechst Corp.; 1975. [Google Scholar]
- 14.Conrad R. Capacity of aerobic microorganisms to utilize and grow on atmospheric trace gases (H2, CO, CH4) In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Washington, D.C: American Society for Microbiology; 1984. pp. 461–467. [Google Scholar]
- 15.Crill P M. Seasonal patterns of methane uptake and carbon dioxide release by a temperate woodland soil. Global Biogeochem Cycles. 1991;5:319–334. [Google Scholar]
- 16.Dedysh S N, Panikov N S, Tiedje J M. Acidophilic methanotrophic communities from Sphagnum peat bogs. Appl Environ Microbiol. 1998;64:922–929. doi: 10.1128/aem.64.3.922-929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dörr H, Katruff L, Levin I. Soil texture parameterization of the methane uptake in aerated soils. Chemosphere. 1993;26:697–713. [Google Scholar]
- 18.Dunfield P, Knowles R, Dumont R, Moore T. Methane production and consumption in temperate and subarctic peat soils: response to temperature and pH. Soil Biol Biochem. 1993;25:321–326. [Google Scholar]
- 19.Dunfield P F, Knowles R. Biological oxidation of nitric oxide in a humisol. Biol Fertil Soils. 1997;24:294–300. [Google Scholar]
- 20.Dunfield P F, Knowles R. Organic matter, heterotrophic activity, and NO consumption in soils. Global Change Biol. 1998;4:199–207. [Google Scholar]
- 21.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heyer J, Suckow R. Ökologische Untersuchungen der Methanoxydation in einem sauren Moorsee. Limnologica. 1985;16:247–266. [Google Scholar]
- 23.Hütsch B W, Webster C P, Powlson D S. Methane oxidation in soil as affected by land use, pH and fertilization. Soil Biol Biochem. 1994;26:1613–1622. [Google Scholar]
- 24.King G M. Dynamics and controls of methane oxidation in a Danish wetland sediment. FEMS Microbiol Ecol. 1990;74:309–324. [Google Scholar]
- 25.King G M. Ecological aspects of methane oxidation, a key determinant of global methane dynamics. Adv Microbiol Ecol. 1992;12:431–468. [Google Scholar]
- 26.King G M. Ecophysiological characteristics of obligate methanotrophic bacteria and methane oxidation in situ. In: Murrell J C, Kelly D P, editors. Microbial growth on C1 compounds. Andover, United Kingdom: Intercept Ltd.; 1994. pp. 303–313. [Google Scholar]
- 27.King G M, Ademsen A P S. Effects of temperature on methane consumption in a forest soil and in pure cultures of the methanotroph Methylomonas rubra. Appl Environ Microbiol. 1992;58:2758–2763. doi: 10.1128/aem.58.9.2758-2763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koschorreck M, Conrad R. Oxidation of atmospheric methane in soil: measurements in the field, in soil cores and in soil samples. Global Biogeochem Cycles. 1993;7:109–121. [Google Scholar]
- 29.Kurdish I K, Kigel N F. Effect of palygorskite, a clayey mineral, on physiological activity and adhesion of methanotrophic bacteria. Mikrobiol Zh (Kiev) 1992;54:73–78. [Google Scholar]
- 30.Lindahl V, Bakken L R. Evaluation of methods for extraction of bacteria from soil. FEMS Microbiol Ecol. 1995;16:135–142. [Google Scholar]
- 31.Mancinelli R L. The regulation of methane oxidation in soil. Annu Rev Microbiol. 1995;49:581–605. doi: 10.1146/annurev.mi.49.100195.003053. [DOI] [PubMed] [Google Scholar]
- 32.Nesbit S P, Breitenbeck G A. A laboratory study of factors influencing methane uptake by soils. Agric Ecosyst Environ. 1992;41:39–54. [Google Scholar]
- 33.Priemé A, Sitaula J I B, Klemedtsson A K, Bakken L R. Extraction of methane-oxidizing bacteria from soil particles. FEMS Microbiol Ecol. 1996;21:59–68. [Google Scholar]
- 34.Roslev P, Iversen N, Henriksen K. Oxidation and assimilation of atmospheric methane by soil methane oxidizers. Appl Environ Microbiol. 1997;63:874–880. doi: 10.1128/aem.63.3.874-880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roslev P, Iversen N, Henriksen K. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Metabolism of atmospheric methane in soils, abstr. H-249; p. 339. [Google Scholar]
- 36.Saha U, Sen M. Methane oxidation by Candida tropicalis. Natl Acad Sci Lett (India) 1989;12:373–376. [Google Scholar]
- 37.Savage K, Moore T R, Crill P M. Methane and carbon dioxide exchanges between the atmosphere and northern boreal forest soils. J Geophys Res. 1998;102:29,279–29,288. [Google Scholar]
- 38.Schnell S, King G M. Responses of methanotrophic activity in soils and cultures to water stress. Appl Environ Microbiol. 1996;62:3203–3209. doi: 10.1128/aem.62.9.3203-3209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnell S, King G M. Stability of methane oxidation capacity to variations in methane and nutrient concentrations. FEMS Microbiol Ecol. 1995;17:285–294. [Google Scholar]
- 40.Schnell S, King G M. Mechanistic analysis of ammonium inhibition of atmospheric methane consumption in forest soils. Appl Environ Microbiol. 1994;60:3514–3521. doi: 10.1128/aem.60.10.3514-3521.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sitaula B K, Bakken L R, Abrahamsen G. CH4 uptake by temperate forest soil: effect of N input and soil acidification. Soil Biol Biochem. 1995;27:871–880. [Google Scholar]
- 42.Whittenbury R, Phillips K C, Wilkinson J F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970;61:205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 43.Wolf H J, Hanson R S. Isolation and characterization of methane-utilizing yeasts. J Gen Microbiol. 1979;114:187–194. [Google Scholar]
- 44.Yavitt J B, Fahey T J, Simmons J A. Methane and carbon dioxide dynamics in a northern hardwood ecosystem. Soil Sci Soc Am J. 1995;59:796–804. [Google Scholar]
- 45.Yavitt J B, Simmons J A, Fahey T J. Methane fluxes in a northern hardwood ecosystem in relation to acid precipitation. Chemosphere. 1993;26:721–730. [Google Scholar]