Abstract
A novel category of variable tandem repeats (VNTR) called mycobacterial interspersed repetitive units (MIRUs) has been identified for Mycobacterium ulcerans (n = 39), M. marinum (n = 27), and one related organism. Fifteen MIRU loci were identified in the genome of M. marinum and were used to genotype M. ulcerans, M. marinum, and an M. marinum-like organism that is considered a possible missing link between M. marinum and M. ulcerans. Seven MIRU loci were polymorphic, and locus-specific PCRs for four of these loci differentiated seven M. ulcerans genotypes, four M. marinum genotypes, and a unique genotype for the missing link organism. The seven M. ulcerans genotypes were related to six different geographic origins of isolates. All isolates from West and Central Africa, including old and recent isolates, belonged to the same genotype, emphasizing the great spatiotemporal homogeneity among African isolates. Unlike the M. ulcerans genotypes, the four M. marinum genotypes could not be clearly related to the geographic origins of the isolates. According to MIRU-VNTR typing, all M. ulcerans and M. marinum isolates of American origin were closely related, suggesting a common American ancestor for these two pathogenic species on the American continents. MIRU typing has significant potential value for discriminating between reoccurrence and reinfection for M. ulcerans disease.
Mycobacterium ulcerans causes Buruli ulcer (BU), a disease that represents, after tuberculosis and leprosy, the third most common mycobacterial disease in humans (23, 27). BU has been observed in many tropical areas, but most patients have come from Central and West Africa and Australia (5, 11, 27). Epidemiologically, the disease is associated with riverine and swampy terrains (27). BU is a devastating disease characterized by necrotizing, ulcerative lesions of subcutaneous tissues and the overlying skin. The main specific genomic characteristics of M. ulcerans are the IS2404 element (34) and a 174-kb plasmid(s) that houses the genes for mycolactone, a polyketide toxin (39). M. marinum causes infections in humans and in fish (7, 31). Large outbreaks of infection due to M. marinum have been described in association with swimming pools (swimming pool granuloma) (42) and fish tanks (fish tank granuloma) (13, 14, 17, 19). M. ulcerans and M. marinum, once cultured, are readily identified by conventional mycobacterial characterization methods (46). Various DNA-based techniques have been used to type mycobacteria (6, 33, 35). Such studies have demonstrated a close taxonomic relationship between M. ulcerans and M. marinum, although M. ulcerans harbors IS2404 and M. marinum does not (34). Recently, however, IS2404 was found in an unusual mycobacterial isolate with phenotypic properties closely related to those of M. marinum (4).
Previous studies showed limited genotypic diversity in M. ulcerans, especially among isolates from a given geographic region. PCR-restriction profile analyses of 16S rRNAs showed four different genotypic profiles of M. ulcerans, i.e., the African, Southeast Asian, Mexican, and South American profiles (3). Amplified fragment length polymorphism clearly separates M. ulcerans from M. marinum but has limited use for intraspecies differentiation (3). Sequencing of the 3′ end of the 16S rRNA (30) categorized M. ulcerans into the following five types based on the continent of origin: one African type, one Australian type, one Mexican type, one Asian type, and one South American type. IS2404 restriction fragment length polymorphism (RFLP) fingerprint subtyping divided M. ulcerans isolates into six groups related to six geographical regions, including Africa, Australia, Mexico, South America, Asia, and Southeast Asia (2). Using 2426 PCR, Stinear et al. (36) recognized nine distinct profiles among M. ulcerans isolates that correlated with their geographic origins.
New molecular markers need to be explored for the development of more discriminatory typing methods. Tandem repeats (TRs) represent polymorphic structures in the genomes of highly monomorphic species such as Bacillus anthracis and Yersinia pestis (18). Recently, novel groups of TRs called mycobacterial interspersed repetitive units (MIRUs), discovered in M. tuberculosis, have been demonstrated to be excellent multilocus genotyping markers (40). MIRUs are 46- to 100-bp-long sequences that are dispersed in different copy numbers throughout the genome. MIRUs comprise small open reading frames (ORFs) whose extremities overlap those of the contiguous ORFs and are oriented in the same translational direction as that of the adjacent genes. Analyses of the sequences at the insertion sites suggested that MIRUs disseminate by transposition into DTGA sequence sites involved in translational coupling in polycistronic operons (40). Twelve polymorphic MIRU loci are currently used for the genotyping of M. tuberculosis. MIRUs have also been identified in the M. avium complex and can be used to differentiate M. avium subsp. paratuberculosis from other species of the M. avium complex (1).
For this study, we initially identified several MIRU loci in M. ulcerans and M. marinum. We then investigated whether or not MIRU-variable tandem repeat (MIRU-VNTR) typing was suitable for differentiating and subtyping M. ulcerans, M. marinum, and other closely related organisms.
MATERIALS AND METHODS
Mycobacterial isolates.
The majority of the isolates included in this study are part of the Institute of Tropical Medicine collection (Table 1). Based on conventional biochemical methods, a total of 39 isolates were assigned to the species M. ulcerans and 27 were assigned to the species M. marinum (45, 46). The isolates were also tested for the presence of IS2404 (34). The M. ulcerans isolates were all collected from patients, except for one (00-1441) which was cultivated from an aquatic insect (Hemiptera) in Benin (4). Eleven M. marinum isolates were of human origin and 16 were from animals. One IS2404-positive human isolate from France (00-1026), considered a possible “missing link” between M. marinum and M. ulcerans (4), was also included in this study. Fresh subcultures of all isolates were made in tubes of Löwenstein-Jensen medium.
TABLE 1.
Isolates used for this study
| Species (n) | Strain | Geographical origin | Origin | Sourcea | MIRU profile | Yr of isolation |
|---|---|---|---|---|---|---|
| M. ulcerans (39) | 03-524 | Papua New Guinea | Human | Jo | A | 2003 |
| 04-1292 | Papua New Guinea | Human | Jo | A | 2004 | |
| 9537 | Papua New Guinea | Human | DD 11878 | B | 1971 | |
| 8756 | Japan | Human | ATCC 33728 | C | 1980 | |
| 94-1324 | Queensland, Australia | Human | LS 176862 | C | 1994 | |
| 94-1328 | Malaysia | Human | ITM | C | 1994 | |
| 94-1331 | Papua New Guinea | Human | ITM | C | 1994 | |
| 9540 | Queensland, Australia | Human | D.D. 11098 | C | 1978 | |
| 98-912 | China | Human | ITM | C | 1998 | |
| 5142 | Victoria, Australia | Human | JS ATCC 19423 | D | 1967 | |
| 5147 | Victoria, Australia | Human | JS | D | 1961 | |
| 94-339 | Victoria, Australia | Human | ITM | D | 1994 | |
| 9550 | Victoria, Australia | Human | DD 17679 | D | 1983 | |
| 5150 | Zaire | Human | LS | E | 1962 | |
| 5155 | Zaire | Human | LS | E | 1976 | |
| 94-511 | Ivory Coast | Human | ITM | E | 1994 | |
| 94-662 | Ivory Coast | Human | ITM | E | 1994 | |
| 96-658 | Angola | Human | ITM | E | 1996 | |
| 97-483 | Ghana | Human | ITM | E | 1997 | |
| 97-104 | Benin | Human | ITM | E | 1997 | |
| 98-239 | Benin | Human | ITM | E | 1998 | |
| 99-826 | Benin | Human | ITM | E | 1999 | |
| 99-831 | Benin | Human | ITM | E | 1999 | |
| 99-1567 | Benin | Human | ITM | E | 1999 | |
| 99-1642 | Benin | Human | ITM | E | 1999 | |
| 99-1768 | Benin | Human | ITM | E | 1999 | |
| 00-040 | Benin | Human | ITM | E | 2000 | |
| 00-358 | Benin | Human | ITM | E | 2000 | |
| 00-945 | Benin | Human | ITM | E | 2000 | |
| 00-1213 | Benin | Human | ITM | E | 2000 | |
| 00-1240 | Benin | Human | ITM | E | 2000 | |
| 00-1441 | Benin | Aquatic insect (hemiptera) | ITM | E | 2000 | |
| 01-076 | Benin | Human | ITM | E | 2001 | |
| 01-449 | Benin | Human | ITM | E | 2001 | |
| 842 | Surinam | Human | PVK 701357 | F | 1984 | |
| 7922 | French Guiana | Human | VVCIPT141090018 | F | 1990 | |
| 5114 | Mexico | Human | PL | G | 1953 | |
| 5143 | Mexico | Human | PL | G | 1961 | |
| M. marinum (27) | 1548 | United States | Armadillo | ITM | H | 1986 |
| 1717 | United States | Armadillo | ITM | H | 1986 | |
| 1725 | United States | Armadillo | ITM | H | 1986 | |
| 1726 | United States | Armadillo | ITM | H | 1986 | |
| 2318 | United States | Armadillo | ITM | H | 1986 | |
| 94-056 | Belgium | Human | ITM | I | 1994 | |
| 97-1321 | Belgium | Axolotl | ITM | I | 1997 | |
| 98-852 | Italy | Human | ITM | I | 1998 | |
| 99-822 | Belgium | Human | ITM | I | 1999 | |
| 99-3021 | Belgium | Human | ITM | I | 1999 | |
| 99-8022 | Belgium | Human | ITM | I | 1999 | |
| 00-1020 | France | Fish | ITM | I | 2000 | |
| 00-1021 | France | Fish | ITM | I | 2000 | |
| 00-1022 | France | Fish | ITM | I | 2000 | |
| 00-1023 | France | Fish | ITM | I | 2000 | |
| 00-1024 | France | Fish | ITM | I | 2000 | |
| 00-1028 | France | Fish | ITM | I | 2000 | |
| 01-2240 | Belgium | Human | ITM | I | 2001 | |
| 01-2562 | Belgium | Human | ITM | I | 2001 | |
| 02-308 | Belgium | Human | ITM | I | 2002 | |
| 02-716 | Belgium | Human | ITM | I | 2002 | |
| 02-2511 | Belgium | Human | ITM | I | 2002 | |
| 03-205 | Belgium | Human | ITM | I | 2003 | |
| 01-935 | Portugal | Fish | MTS | J | 2001 | |
| 94-979 | South Africa | Fish | KH | K | 1994 | |
| 94-996 | South Africa | Fish | KH | K | 1994 | |
| 97-1320 | South Africa | Fish | ITM | K | 1997 | |
| Missing link (1) | 00-1026 | France | Human | ITM | L | 2000 |
ITM, Institute of Tropical Medicine, Antwerp, Belgium; JS, J. Stanford, School of Pathology, London, United Kingdom; KH, K. Huchzermeyer, Veterinary Research Institute, Onderstepoort, South Africa; PL, Pedro Lavalle Aguilar; DD, D. Dawson, Laboratory of Microbiology and Pathology, Queensland Health, Brisbane, Australia; VV, V. Vincent, Institut Pasteur de Paris, Paris, France; PVK, P. Van Keulen, Academic Medical Center, Amsterdam, The Netherlands; MTS, M. T. Silva, IBMC, Porto, Portugal; Jo, Joseph Taylor, Wewak General Hospital, Papua New Guinea.
DNA extraction.
Briefly, 2 loopfuls of bacteria harvested from Löwenstein-Jensen medium were suspended in a sterile tube with glass beads containing disruption buffer (10 mg of beads/ml, 40 ml of 5 M guanidinium isothiocyanate, 1.25 ml of 1 M sodium citrate, 2.5 ml of 10% sarcosyl solution, 0.4 ml of β-mercaptoethanol, 2 ml of 0.5 M EDTA, 3.85 ml of mQ), shaken well, and kept at 7°C overnight. One volume of phenol-chloroform-isoamyl alcohol (24/24/1) was added to 600 μl of sample, vortexed briefly, and centrifuged for 5 min at 4°C. The supernatant was mixed with 0.1 volume of sodium acetate and 1 volume of isopropanol and kept at 4°C overnight. After centrifugation for 15 min at 4°C, the pellet was washed with 70% alcohol, and after drying, the DNA was resuspended in 1 ml of TE (10 mM Tris, 1 mM EDTA, pH 8.0).
MIRU primer design and PCR.
Homologous MIRU sequences, together with corresponding flanking sequences, were identified in M. ulcerans and M. marinum by BLASTn searches against sequence databases for these species, available at http://genopole.pasteur.fr/Mulc/BuruList.html and http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi, respectively. The contiguous genes were checked on the Tuberculist web server (http://genolist.pasteur.fr/TubercuList). To investigate differences in the numbers of MIRU-TR motifs present in M. ulcerans and M. marinum, we designed MIRU locus-specific PCR primers (matching both species) from the MIRU flanking sequences by using Primer Premier 5 software (PremierBiosoft, Palo Alto, Calif.). The MIRU-specific primers used are listed in Table 2.
TABLE 2.
MIRU primers for the seven polymorphic MIRU loci
| MIRU locus | Forward primer (5′-3′) | Reverse primer (5′-3′) | Location in M. ulcerans | Location in M. marinum | Location in M. tuberculosis |
|---|---|---|---|---|---|
| 1 | GCTGGTTCATGCGTGGAAG | GCCCTCGGGAATGTGGTT | mu0115C04F 5528-6088 | mar5f11.p1ka 519538-519883 | 2818471-2819870 |
| 2 | ACGGTTGATCCTTGATGTGCT | ACGGTTGATCCTTGATGTGCT | mu0085H10R 173-621 | mar_ends-8b09.q1k 102968-103429 | 1624454-1626962 |
| 5 | CCCTGTCCATCCCTACCAGTT | GGCAAGGTGATCGCGTCA | mu0062H11F 627-1086 | mar949a06.p1n 34741-35472 | 579349-581492 |
| 7 | GAGGTCATCGACCGAGGGTT | GATTGGCTTCATACGGCTTG | mu0098H07R 674-1113 | mar755h11.p2k 1114 2463-2902 | 3191644-3193158 |
| 9 | GCCGAAGCCTTGTTGGACG | GGTTTCCCGCAGCATCTCG | mu0113D07F 1247-1688 | mar428a07.p1k 11576-12017 | 4004291-4006203 |
| 20 | CCACCAGTTCGCGTTTCCA | GACCGCCATCGATTCCACC | mu0228B02F 3044-3937 | mar434a05.p1k 194886-195779 | 960342-960151 |
| 33 | CAAGACTCCCACCGACAGGC | CGGATCGGCACGGTTCA | mu0043E11R 5568-6426 | mar257b11.s1c 45108-45908 | 3154654-3157521 |
PCR amplifications with all primers were performed in 30-μl mixtures containing 1.0 U of HotStarTaq polymerase (QIAGEN, Hilden, Germany), 3.0 μl of 10× PCR buffer, 6.0 μl of Qsolution, 1.5 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, a 0.6 pM concentration of each primer, and 4 ng of sample DNA. Reactions for all primer sets were preceded by 15 min of denaturation at 95°C and consisted of 40 cycles with 1 min of denaturation at 94°C, 1 min of annealing at 58°C, and 1 min of extension at 72°C, with a final extension of 10 min at 72°C.
Samples were visualized and analyzed with a bio-analyzer (Agilent Technologies, Waldbronn, Germany). Amplicon sizes were used to estimate the number of repeats at each locus. Sequencing revealed the actual number of copy repeats. A numerical tree was constructed by use of the unweighted-pair group method using average linkages (UPGMA) algorithm and the categorical coefficient (Fig. 1).
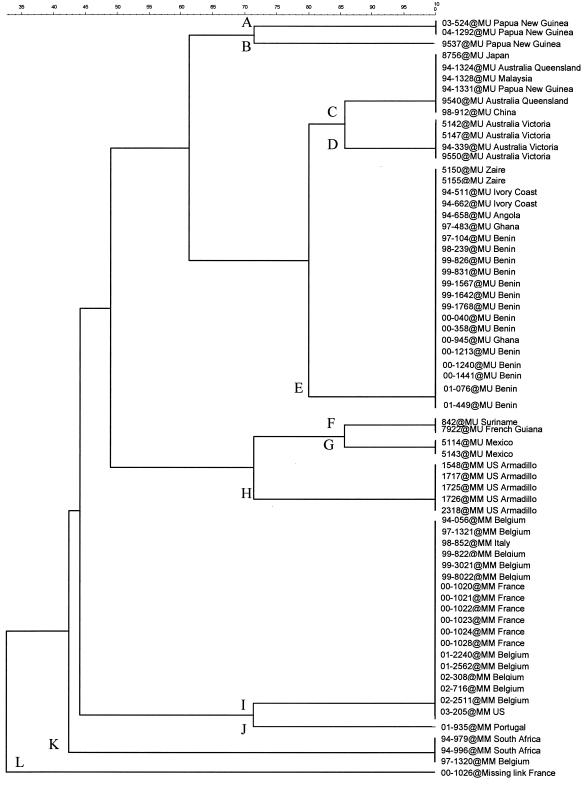
FIG. 1.
Categorical dendrogram created by UPGMA. In the top part, only M. ulcerans (MU) isolates are present, and in the bottom part, only M. marinum (MM) isolates are present. In the center, there is a mixture of M. ulcerans and M. marinum isolates with American origins.
RESULTS
Presence of MIRUs.
MIRUs were found in all isolates. A BLASTn search of the homologous MIRU sequences of the M. ulcerans and M. marinum genomes revealed 13 potential MIRU-VNTR loci in the partially completed M. ulcerans genome. Two other MIRU loci were identified by BLASTn searches with the contiguous genes of two highly polymorphic MIRU loci in M. tuberculosis.
MIRU characteristics.
Of the 15 potential MIRU-VNTR loci tested, 7 were polymorphic (Table 3). Amplification was robust for all loci tested for M. ulcerans, M. marinum, and the missing link, except for M. ulcerans isolates 842 from Surinam and 7922 from French Guiana at locus 1. At MIRU locus 2, tandem repeats were absent, except in M. marinum isolates from France, Italy, and Belgium. At MIRU locus 7, tandem repeats were absent, except in Papua New Guinea (PNG) isolates 9537, 03-524, and 04-1292. At MIRU locus 20, a tandem repeat was only present in the missing link isolate 00-1026.
TABLE 3.
Sequence characterization of MIRU locia
| Locus | Sequence | Variant |
|---|---|---|
| Consensus | ATGANCCGCGCCGGCGACGAT----------GCAG------------------------------AGCGAAGCGATGAGGAGGAGCGGCGCC-G | |
| >1 | G...G..A-............----10----....--------------30--------------...........................A. | |
| >2 | G...T..C....A........----10----....--------------30--------------..............A...........A-. | |
| >3 | ........T...-.......C----10----....--------------30--------------.....G....................T-C | |
| >4 | .........A..A........----10----....--------------30--------------.............AC.....T......-C | |
| >5 | ...........TAA....C..----10----....--------------30--------------.............A......T......-. | |
| >6 | G...A..C.....A.......----10----..G.--------------30--------------G.............A...........T-. | |
| >7 | ....T......TT........----10----....--------------30--------------G...C.....................--A | |
| >9 | .......C.............----10----....--------------30--------------....C.........A..........G.-. | |
| >10 | C.CGC.TA.T....A......----10----...A--------------30--------------...C.........CA..........TA-C | |
| >12 | ....-....-.G-.....C.G----10----....--------------30--------------GC........C......A........A-. | |
| >14 | G...T.T..A...........----10----....--------------30--------------....CGG.....CT.....T...T...-A | |
| >11 | ....-.........T......----10----...C----TGGGGG-CACCGCCCGCTTGCGGGGGA-.C....A.....A...........TAA | |
| >13 | G...A......---.......AAGACGGGGC....CGATTGGGGGGCACCTCCCGCTTGCGGGGGA-------------............TT- | |
| >28 | ....T..A.........T.C.----10----....--------------30--------------CAG.....A.C..AAG..........T-C | |
| >20 | ....T..A.TT...T...C.C----10----C...AT------------28--------------G....T...G..-C.GCT...AC.CTGCT | |
| >33 | .....TT..A.ATC..CGCCG----10----CTGG--------------30--------------CGATCAGAAGGGGCAGACGGCGCTGAGC | |
| Consensus | NTGNNNNNCGCCGGCGACGATGCAGAGCGNAGCGATGAGGAGGAGCGGCGCNN | MTC |
| Consensus | CGCGCCGACGACGATGCAGAGCGCAGCGATGAGGAGGAGCGGCGC-AGATGAAT | MAC |
| Consensus | ATGACCCGCGCCGGCGACGATGCAGAGCGAAGCGATGAGGAGGAGCGGCGCC-G | MU |
| 1 | G...G..A-...........................................A. | A1 |
| 1 | A...G..A-...........................................A. | A2 |
| 2 | G...T..C....A..........................A...........A-. | B |
| 5 | ...........TAA....C...................A......T......-. | C |
| 7 | ....T......TT............G...C.....................--A | D |
| 9 | .......C.....................C.........A..........G.-. | E1 |
| 9 | .......C..............GGC....C.........A..........G.T | E2 |
The variant MIRU sequences of each locus were aligned, and similarities are indicated with dots. Gaps are indicated with dashes. The consensus sequences are given for the M. tuberculosis complex (MTC), the M. avium complex (MAC), and M. ulcerans (MU). A1 to E2 are variants of the consensus. We sequenced MIRU loci 1 and 9. The sequences of the other MIRU loci come from the Burulist on the Internet.
MIRUs are intergenic, and between the corresponding genes of M. tuberculosis and M. ulcerans, four MIRU-VNTR loci (loci 5, 7, 20, and 33) were found in M. ulcerans that corresponded to tuberculosis-MIRU loci (loci 4, 10, 30, and Qub15). The results are summarized in Table 4, in which the data report the numbers of repeat motifs amplified by each pair of locus-specific primers, resulting in MIRU profiles that are combined across these four loci.
TABLE 4.
MIRU profiles
| Species (n) | Origin | No. of isolates | No. of TRs at locusa
|
MIRU profileb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 9 | 33 | 2 | 7 | 20 | ||||
| M. ulcerans (39) | Papua New Guinea | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | A |
| Papua New Guinea (9537) | 1 | 2 | 1 | 1 | 3 | 0 | 2 | 0 | B | |
| Papua New Guinea (94-1331) | 1 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | C | |
| Japan, China, Malaysia | 3 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | C | |
| Australia (Queensland) | 2 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | C | |
| Australia (Victoria) | 4 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | D | |
| Africa | 22 | 3 | 1 | 1 | 3 | 0 | 1 | 0 | E | |
| Suriname, French Guiana | 2 | NA | 1 | 2 | 1 | 0 | 1 | 0 | F | |
| Mexico | 2 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | G | |
| M. marinum (27) | United States | 5 | 4 | 2 | 2 | 1 | 0 | 1 | 0 | H |
| France, Italy, Belgium | 18 | 1 | 4 | 2 | 3 | 2 | 1 | 0 | I | |
| Portugal | 1 | 1 | 4 | 2 | 5 | 0 | 1 | 5 | J | |
| South Africa, Belgium | 3 | 2 | 1 | 3 | 2 | 2 | 1 | 0 | K | |
| Missing link (1) | France | 1 | 1 | 2 | 6 | 2 | 0 | 1 | 1 | L |
NA, no amplification; NT, not tested. Allelic diversities were as follows: for locus 1, h = 0.621; for locus 2, h = 0.451; for locus 5, h = 0.534; for locus 7, h = 0.083; for locus 9, h = 0.552; for locus 20, h = 0.028; for locus 33, h = 0.888.
Profiles refer to those in Table 1.
A sequence analysis of PCR products obtained after the amplification of two polymorphic MIRU loci (1 and 9) from some of the isolates indicated that they contained at least one copy of an approximately 53-bp type II MIRU motif, as described by Supply et al. (40). MIRU locus 5 probably contains one 77-bp copy (type I according to Supply et al.). Type II differs from type I in that it has a 24-bp deletion. In all cases, the sequence of each individual 53-bp copy was homologous to those of the 53-bp MIRUs of M. tuberculosis 2296207 and M. bovis BCG 1173P2. A comparison of the MIRU consensus sequence of the M. tuberculosis complex and the M. avium complex (MAC) with the MIRU sequences found in M. ulcerans is presented in Table 3.
Typing and genetic relationship of isolates.
Table 4 presents the results of MIRU-VNTR genotyping of 39 M. ulcerans isolates, 27 M. marinum isolates, and a missing link isolate from a temporally and geographically diverse collection (Table 1). The loci tested varied in their discriminatory abilities. Locus 33 produced five alleles and was the most polymorphic. The least polymorphic loci were loci 7 and 20.
As shown in Table 4, when MIRU loci 1, 5, 9, and 33 were used in combination, seven distinct profiles were identified for the 39 M. ulcerans isolates, four profiles were identified for the 27 M. marinum isolates, and a unique profile was identified for the missing link isolate. The seven M. ulcerans genotypes were largely grouped according to their geographical origins, with the exception of the Pacific genotype, which contained isolates from PNG, China, Japan, Malaysia, and Australia (Queensland) (profile C [1-1-1-3]). All of the African isolates (n = 22) produced a single profile (E [3-1-1-3]) and constituted the African genotype. For African isolates, we saw no difference between an isolate from an aquatic insect from Benin (00-1441) and isolates from humans. All African M. ulcerans isolates had three copies of MIRU motifs at locus 1 and differed from all other isolates tested. Two different genotypes were produced among the isolates from Australia, namely, the Victoria (D [1-1-1-2]) and Queensland (C [1-1-1-3]) genotypes, corresponding to the southern and northern parts of Australia, respectively. The two Queensland isolates formed part of the Pacific cluster, while the four Victorian isolates had a distinct genotype. Similarly, three different genotypes were distinguished for isolates from PNG, with one isolate having profile B (2-1-1-3), two isolates having profile A (2-1-1-1), and a fourth isolate having profile C and belonging to the Pacific cluster. A distinct profile (G [1-1-2-1]) was produced by the two Mexican isolates and formed the North American genotype. The isolates of Surinam and French Guiana produced a single profile (F [NA-1-2-1], with no amplification at locus 1) and constituted the South American genotype.
All M. marinum isolates were distinguished from M. ulcerans isolates. Within the M. marinum species, four groups could be distinguished. Of the 27 M. marinum isolates, which were from both human and animal origins, 18 isolates fell within the common profile I (1-4-2-3). Three animal isolates from Belgium and South Africa (94-979, 94-996, and 97-1320) shared a common genotype (K [2-1-3-2]). One isolate cultured from a fish in Portugal had a unique genotype (J [1-4-2-5]). The five American isolates were recovered from different organs of a newly captured wild armadillo in Louisiana. The three isolates from the liver and the two isolates from the spleen shared a common profile (H [4-2-2-1]). This armadillo was a wild armadillo captured in Louisiana and therefore did not belong to an armadillo colony. The missing link isolate from France (00-1026) had a unique profile (L [1-2-6-2]).
Allelic diversity and distribution of MIRU allele numbers.
Allelic diversity (h) is an indirect index of the heterogeneity of isolates and is a useful index of the discriminatory power provided by the loci under study if the isolates are representative of the population. Based on this study, four MIRU loci (MIRU loci 1, 5, 9, and 33) achieved a high diversity index (h > 0.5). These MIRU loci were the most discriminant loci. One MIRU locus (MIRU locus 2) had a medium diversity index (h < 0.5), and MIRU loci 7 and 20 were the least discriminant loci (h < 0.1).
DISCUSSION
Current typing methods for M. ulcerans are capable of resolving only geographical types and are consequently limited in their discriminatory power. The availability of M. ulcerans genome sequences presents an enormous resource for the identification of potential markers that are useful for indexing polymorphisms within the species. MIRUs are acceptable markers for indexing polymorphisms in M. tuberculosis and M. avium (1, 20). In this study, we described the first use of MIRU-VNTR typing of a set of M. ulcerans, M. marinum, and missing link isolates. The MIRU sequences found are homologous with those in M. tuberculosis and M. avium and may consequently have the same functional characteristics (1, 20, 40, 41). This includes the ATGA or GTGA start-stop codon and the putative ribosomal binding site in some MIRU motifs. The ribosomal binding site promotes the efficient and accurate translation of mRNA. Since the primers for amplification anneal in conserved contiguous genes, the MIRU loci in M. ulcerans and M. marinum containing tandem repeat MIRU motifs all occur at identical sites in the common portion of the DNA. Several loci containing MIRUs in M. tuberculosis are involved in biosynthesis pathways, such as those of amino acids, proteins, and cell wall constituents (see Table 2 for their locations in M. ulcerans and M. marinum). Other loci contain genes encoding proteins involved in oxidoreduction.
Unlike the case for M. tuberculosis, the lengths of PCR amplification products from M. ulcerans did not vary by multiples of precisely 53 bp, suggesting the presence of incomplete repeats. The fact that M. ulcerans has a low MIRU repeat copy number at each locus (Table 4) may explain the limited polymorphism observed for this species due to fewer chances for insertions or multiplication through misreadings. In M. tuberculosis, MIRU loci with larger numbers of repeat copies offer higher allelic diversities of up to eight distinct alleles (41). A MIRU locus with one extra MIRU motif repeat has one extra ORF start site. It is not known whether a variation in tandem repeat number results in a different function or change in the expression level of the contiguous downstream gene. Smaller numbers of MIRU motifs have, however, been correlated with a lower degree of immunogenicity of different BCG strains (20) and of the 316F MAP strain (1). MIRU locus 1 is positioned between two genes (scoA and scoB) that are responsible for fatty acid synthesis and degradation and are involved in cell wall synthesis. A shift in the open reading frames, causing them to start earlier or later in a MIRU, may result in a longer or a shorter protein with a slightly different function and a mutated cell wall protein as a result. Together with the presence of a different mycolactone variant, this might explain the difference in virulence between African isolates and isolates from elsewhere in the world. Mycolactone is a polyketide-derived macrolide toxin with cytotoxic properties (9). Recent studies demonstrated that isolates from different geographic origins produce a heterogeneous mixture of mycolactone variants, suggesting that there may be a correlation between mycolactone profiles and virulence (24). It also appeared that the genes encoding giant polyketide synthases are situated in a giant plasmid (39).
MIRU loci might be hot spots for transcriptional errors, leading to a faster adaptation to environmental changes than that caused by random mutations. M. ulcerans is well adapted to a variety of natural reservoirs, such as aquatic insects (21, 29) and other aquatic organisms such as fish or snails (8, 28). This might be due to the protective mininiche that is created by mycolactone (22). M. ulcerans is a very slowly growing organism and probably remains dormant in its natural environment for long periods. The natural mutation rate should therefore be very low (15). All African isolates from West and Central Africa, including isolates from the 1960s and more recent isolates, belong to the same genotype. These results correspond to those from other studies using other markers such as IS2404-RFLP (2, 3), the 2426 PCR of Stinear et al. (36), and the 3′ end of the 16S RNA gene (30) and emphasize the marked spatiotemporal homogeneity among African isolates. It is an important observation that an isolate from an insect and isolates from humans share a common genotype in Africa.
Using a limited set of loci, we could differentiate two subtypes among Australian isolates (Queensland and Victoria) as well as three subtypes among PNG isolates, with one of the subtypes belonging to the same genotype as the North Australian subtype (Queensland). Similarly, Johnson et al. (16) and Stinear et al. (37) differentiated the Queensland isolates from the Victoria isolates and found two different subtypes for PNG isolates. Moreover, we have identified a third genotype for PNG (genotype A) that is shared by two recent isolates (03-524 and 04-1292). These isolates came from two patients originating from a region which is considered a new focus of BU in PNG (J. Taylor, personal communication). As determined by the 2426 PCR method (36), isolate 9537 (PNG I according to Stinear et al. and genotype B according to our study) is quite different from the other Asian strains included in our Pacific genotype C. Similarly, genotype A, which is represented by two isolates from PNG, is different from the PNG II group of Stinear et al. and from the Pacific genotype.
As with 2426 PCR results, our Pacific genotype C and Victorian genotype D are more related to the African genotype E than to the PNG genotypes A and B.
The discriminating capability of MIRU was somewhat lower than that of 2426 PCR (36), since 2426 PCR obtained different banding patterns for Malaysian isolates, a PNG II isolate (isolate 94-1331), and two isolates from China (98-912) and Japan (ATCC 33728). For these same isolates, we obtained the same MIRU profile.
Using MIRU-VNTR typing and an analysis of four loci, we could differentiate four subtypes among the M. marinum isolates (profiles H, I, J, and K). However, unlike the case for M. ulcerans, these subtypes could not be clearly related to the geographic origins of the isolates.
The striking phylogenetic closeness between M. ulcerans and M. marinum reported by Tønjum et al. (43) and IS2404-RFLP results (2, 3) further support the findings of Stinear et al. (38), whose comparative genetic analysis revealed a recent divergence of M. ulcerans from M. marinum. This hypothesis is supported by the following observations: (i) IS2404 elements are present in high copy numbers in geographical variants of M. ulcerans (35) but are absent from the closely related species M. marinum; (ii) similar to the occurrence of IS6110 in M. tuberculosis (10), the microaerophilic growth conditions required for M. ulcerans (25) may play a role in the stimulation of the transposition of IS2404 into the genome of this species; and (iii) MIRU locus amplification was positive for M. ulcerans and M. marinum by the use of identical primers.
Using MIRU-VNTR typing, we could differentiate M. ulcerans from M. marinum and the missing link. The dendrogram in Fig. 1 shows a close relationship between M. ulcerans isolates from North and South America and the five M. marinum isolates from a wild armadillo from Louisiana (26). All M. ulcerans and M. marinum isolates with American origins are closely related, suggesting that there is a common American ancestor for these two pathogenic species on the American continents.
The isolate 00-1026 (missing link) from France produced a unique profile (L) that differentiates it from M. ulcerans and M. marinum.
In this study, we identified MIRU sequences in M. ulcerans, M. marinum, and the missing link. We showed that MIRU-VNTR typing is capable of differentiating among M. ulcerans, M. marinum, and related species. Intraspecies differentiation, although limited, is thus possible with this method. Unlike 2426 PCR, MIRU-VNTR typing allowed us to compare M. ulcerans, M. marinum, and other closely related organisms, revealing for the first time the relationships between M. marinum and M. ulcerans isolates from the same general geographic areas (the southern part of North America and the northern part of South America).
Recently, mycobacteria that are closely related to M. ulcerans and M. marinum were isolated in the United States from striped bass (12, 32) and from frogs (44). These isolates, as well as additional M. ulcerans and M. marinum isolates from the United States and other parts of the world, should be investigated for a better understanding of the evolutionary relationships between M. ulcerans, M. marinum, and related organisms.
Besides intraspecies and interspecies differentiation among the M. ulcerans-M. marinum complex, MIRU-VNTR typing is a highly reproducible method that can also be applied directly to clinical specimens. More loci should be investigated to improve its discriminative power.
Acknowledgments
This work was partly supported by the Fund for Scientific Research of Flanders (Brussels, Belgium; grant no. G0301-01) and by the Damien Foundation (Brussels, Belgium).
REFERENCES
- 1.Bull, T. J., K. Sidi-Boumedine, E. J. McMinn, K. Stevenson, R. Pickup, and J. Hermon-Taylor. 2003. Mycobacterial interspersed repetitive units (MIRU) differentiate Mycobacterium avium subspecies paratuberculosis from other species of the Mycobacterium avium complex. Mol. Cell. Probes 17:157-164. [DOI] [PubMed] [Google Scholar]
- 2.Chemlal, K., K. De Ridder, P. A. Fonteyne, W. M. Meyers, J. Swings, and F. Portaels. 2001. The use of IS2404 restriction fragment length polymorphisms suggests the diversity of Mycobacterium ulcerans from different geographical areas. Am. J. Trop. Med. Hyg. 64:270-273. [DOI] [PubMed] [Google Scholar]
- 3.Chemlal, K., G. Huys, P. A. Fonteyne, V. Vincent, A. G. Lopez, L. Rigouts, J. Swings, W. M. Meyers, and F. Portaels. 2001. Evaluation of PCR-restriction profile analysis and IS2404 restriction fragment length polymorphism and amplified fragment length polymorphism fingerprinting for identification and typing of Mycobacterium ulcerans and M. marinum. J. Clin. Microbiol. 39:3272-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemlal, K., G. Huys, F. Laval, V. Vincent, C. Savage, C. Gutierrez, M. A. Lanéelle, J. Swings, W. M. Meyers, M. Daffé, and F. Portaels. 2002. Characterization of an unusual mycobacterium: a possible missing link between Mycobacterium marinum and Mycobacterium ulcerans. J. Clin. Microbiol. 40:2370-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debacker, M., J. Aguiar, C. Steunou, C. Zinsou, W. M. Meyers, A. Guédénon, J. T. Scott, M. Dramaix, and F. Portaels. 2004. Mycobacterium ulcerans disease (Buruli ulcer) in rural hospital, southern Benin, 1997-2001. Emerg. Infect. Dis. 10:1391-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Beenhouwer, H., L. Zhong, P. de Rijk, C. van Eekeren, and F. Portaels. 1995. Detection and identification of mycobacteria by DNA amplification and oligo-specific capture plate hybridization. J. Clin. Microbiol. 33:2994-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decostere, A., K. Hermans, and F. Heasebrouck. 2004. Piscine mycobacteriosis: a literature review covering the agent and the disease it causes in fish and humans. Vet. Microbiol. 99:159-166. [DOI] [PubMed] [Google Scholar]
- 8.Eddyani, M., D. Ofori-Adjei, G. Teugels, D. De Weirdt, D. Boakye, W. M. Meyers, and F. Portaels. 2004. Potential role for fish in transmission of Mycobacterium ulcerans disease (Buruli ulcer): an environmental study. Appl. Environ. Microbiol. 70:5679-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. L. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854-857. [DOI] [PubMed] [Google Scholar]
- 10.Ghanekar, K., A. McBride, O. Dellagostin, S. Thorne, R. Mooney, and J. McFadden. 1999. Stimulation of transposition of the Mycobacterium tuberculosis insertion sequence IS6110 by exposure to a microaerobic environment. Mol. Microbiol. 33:982-993. [DOI] [PubMed] [Google Scholar]
- 11.Hayman, J. A., H. B. Fleming, D. A. Monash, and I. M. Miller. 1991. Mycobacterium ulcerans infection in paradise. Med. J. Aust. 155:130. [DOI] [PubMed] [Google Scholar]
- 12.Heckert, R. A., S. Elankumaran, A. Milani, and A. Baya. 2001. Detection of a new Mycobacterium species in wild striped bass in the Chesapeake Bay. J. Clin. Microbiol. 39:710-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huminer, D., S. D. Pitlik, C. Block, L. Kaufman, S. Amit, and J. B. Rosenfeld. 1986. Aquarium-borne Mycobacterium marinum skin infection. Report of a case and review of the literature. Arch. Dermatol. 122:698-703. [PubMed] [Google Scholar]
- 14.Hurst, L. C., P. C. Amadio, M. A. Badalamente, J. L. Ellstein, and R. J. Dattwyler. 1987. Mycobacterium marinum infections of the hand. J. Hand. Surg. 12:428-435. [DOI] [PubMed] [Google Scholar]
- 15.Jackson, K. M., R. Edwards, D. E. Leslie, and J. A. Hayman. 1995. Molecular method for typing Mycobacterium ulcerans. J. Clin. Microbiol. 33:2250-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, P. D., M. G. Veitch, P. E. Flood, and J. A. Hayman. 1995. Mycobacterium ulcerans infection on Phillip Island, Victoria. Med. J. Aust. 162:221-222. [DOI] [PubMed] [Google Scholar]
- 17.King, A. J., J. A. Fairley, and J. E. Rasmussen. 1983. Disseminated cutaneous Mycobacterium marinum infection. Arch. Dermatol. 119:268-269. [PubMed] [Google Scholar]
- 18.Le Flèche, P., Y. Hauck, L. Onteniente, A. Prieur, F. Denoeud, V. Ramisse, V. Sylvestre, G. Benson, F. Ramisse, and G. Vergnaud. 2001. A tandem repeats database for bacterial genomes: application to the genotyping of Yersinia pestis and Bacillus anthracis. BMC Microbiol. 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linell, L., and A. Norden. 1954. Mycobacterium balnei: a new acid-fast bacillus occurring in swimming pools and capable of producing skin lesions in humans. Acta Tuberc. Pneumol. Scand. 33(Suppl.):1-84. [PubMed] [Google Scholar]
- 20.Magdalena, J., A. Vachée, P. Supply, and C. Locht. 1998. Identification of a new DNA region specific for members of Mycobacterium tuberculosis complex. J. Clin. Microbiol. 36:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsollier, L., R. Robert, J. Aubry, J. P. Saint André, H. Kouakou, P. Legras, A. L. Manceau, C. Mahaza, and B. Carbonnelle. 2002. Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol. 68:4623-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsollier, L., T. Stinear, J. Aubry, J. P. Saint André, R. Robert, P. Legras, A. L. Manceau, C. Audrain, S. Bourdon, H. Kouakou, and B. Carbonnelle. 2004. Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl. Environ. Microbiol. 70:1097-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyers, W. M., and F. Portaels. 2003. Buruli ulcer: Mycobacterium ulcerans infection, p. 583-585. In D. A. Warrell, T. M. Cox, J. D. Firth, and E. J. Benz, Jr. (ed.), Oxford textbook of medicine, 4th ed. Oxford University Press, Oxford, England.
- 24.Mve-Obiang, A., R. E. Lee, F. Portaels, and P. L. C. Small. 2003. Heterogeneity of mycolactones produced by clinical isolates of Mycobacterium ulcerans: implications for virulence. Infect. Immun. 71:774-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palomino, J. C., A. M. Obiang, L. Realini, W. M. Meyers, and F. Portaels. 1998. Effect of oxygen on growth of Mycobacterium ulcerans in the BACTEC system. J. Clin. Microbiol. 36:3420-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portaels, F., G. P. Walsh, K. De Ridder, R. Malaty, M. T. Silva, C. H. Binford, and W. M. Meyers. 1987. Cultivable mycobacteria isolated from 32 newly captured wild armadillos (Dasypus novemcinctus) from Louisiana. Int. J. Lepr. 55:788. [Google Scholar]
- 27.Portaels, F. 1995. Epidemiology of mycobacterial diseases, p. 207-222. In M. Schuster (ed.), Mycobacterial diseases of the skin. Elsevier Science Inc., New York, N.Y.
- 28.Portaels, F., K. Chemlal, P. Elsen, P. D. R. Johnson, J. A. Hayman, J. Hibble, R. Kirkwood, and W. M. Meyers. 2001. Mycobacterium ulcerans in wild animals. Rev. Sci. Tech. 20:252-264. [DOI] [PubMed] [Google Scholar]
- 29.Portaels, F., P. Elsen, A. Guimaraes-Peres, P. A. Fonteyne, and W. M. Meyers. 1999. Insects in the transmission of Mycobacterium ulcerans infection. Lancet 353:986. [DOI] [PubMed] [Google Scholar]
- 30.Portaels, F., P. A. Fonteyne, H. De Beenhouwer, P. de Rijk, A. Guédénon, J. A. Hayman, and W. M. Meyers. 1996. Variability in 3′ end of 16S rRNA sequence of Mycobacterium ulcerans is related to geographic origin of isolates. J. Clin. Microbiol. 34:962-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pradinaud, R. 2003. Mycobactérioses cutanées environnementales dont l'infection à Mycobacterium ulcerans (ulcère de Buruli). Encycl. Méd. Chir. Dermatol. (Paris) 8-038-F-15:1-9. [Google Scholar]
- 32.Rhodes, M. W., H. Kator, S. Kotob, P. van Berkum, I. Kaattari, W. Vogelbein, F. Quinn, M. M. Floyd, W. R. Butler, and C. A. Ottinger. 2002. Mycobacterium shottsii sp. nov., a slowly growing species isolated from Chesapeake Bay striped bass (Morone saxatilis). Int. J. Syst. Evol. Microbiol. 53:421-424. [DOI] [PubMed] [Google Scholar]
- 33.Rogall, T., T. Flohr, and E. C. Böttger. 1990. Differentiation of Mycobacterium species by direct sequencing of amplified DNA. J. Gen. Microbiol. 136:1915-1920. [DOI] [PubMed] [Google Scholar]
- 34.Ross, B. C., L. Marino, F. Oppedisano, R. Edwards, R. M. Robins-Browne, and D. R. Johnson. 1997. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 35:1696-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth, A., M. Fischer, M. E. Hamid, S. Michalke, W. Ludwig, and H. Mauch. 1998. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J. Clin. Microbiol. 36:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stinear, T., J. K. Davies, G. A. Jenkin, J. A. Hayman, F. Portaels, B. C. Ross, F. Oppedisano, M. Purcell, J. A. Hayman, and P. D. R. Johnson. 2000. A simple PCR method for rapid genotype analysis of Mycobacterium ulcerans. J. Clin. Microbiol. 38:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stinear, T., J. K. Davies, G. A. Jenkin, J. A. Hayman, F. Oppedisano, and P. D. Johnson. 2000. Identification of Mycobacterium ulcerans in the environment from regions in Southeast Australia in which it is endemic with sequence capture-PCR. Appl. Environ. Microbiol. 66:3206-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stinear, T., B. C. Ross, J. K. Davies, L. Marino, R. M. Robins-Brown, F. Oppedisano, A. Sievers, and P. D. R. Johnson. 2004. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J. Clin. Microbiol. 37:1018-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stinear, T. P., A. Mve-Obiang, P. L. C. Small, W. Frigui, M. J. Pryor, R. Brosch, G. A. Jenkin, P. D. R. Johnson, J. K. Davies, R. E. Lee, S. Adusumilli, T. Garnier, S. F. Haydock, P. F. Leadlay, and S. T. Cole. 2004. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc. Natl. Acad. Sci. USA 101:1345-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Supply, P., J. Magdalena, S. Himpens, and C. Locht. 1997. Identification of novel intergenic repetitive units in a mycobacterial two-component system operon. Mol. Microbiol. 26:991-1003. [DOI] [PubMed] [Google Scholar]
- 41.Supply, P., E. Mazars, S. Lesjean, V. Vincent, B. Gicquel, and C. Locht. 2000. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol. Microbiol. 36:762-771. [DOI] [PubMed] [Google Scholar]
- 42.Tolmach, J. A., and S. B. Frank. 1953. Granuloma of skin with tubercle formation following swimming pool injury. JAMA 151:724-726. [DOI] [PubMed] [Google Scholar]
- 43.Tønjum, T., D. B. Welty, E. Jantzen, and P. L. Small. 1998. Differentiation of Mycobacterium ulcerans, M. marinum, and M. haemophilum: Mapping of their relationships to M. tuberculosis by fatty acid profile analysis, DNA-DNA hybridization, and 16S rRNA gene sequence analysis. J. Clin. Microbiol. 36:918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trott, K. A., B. A. Stacy, B. D. Lifland, H. E. Diggs, R. M. Harland, M. K. Khokha, T. C. Grammer, and J. M. Parker. 2004. Characterization of a Mycobacterium ulcerans-like infection in a colony of African tropical clawed frogs (Xenopus tropicalis). Comp. Med. 54:309-317. [PubMed] [Google Scholar]
- 45.Vincent Lévy-Frébault, V., and F. Portaels. 1992. Proposed minimal standards for the genus mycobacterium and for description of new slowly growing mycobacterium species. Int. J. Syst. Bacteriol. 42:315-323. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. 2001. Buruli ulcer. Diagnosis of Mycobacterium ulcerans disease, p. 92. In F. Portaels, P. Johnson, and W. M. Meyers (ed.), A manual for health care providers. W.H.O./CDS/CPE/GBUI/2001.4. World Health Organization, Geneva, Switzerland.