Abstract
The Escherichia coli zupT (formerly ygiE) gene encodes a cytoplasmic membrane protein (ZupT) related to members of the eukaryotic ZIP family of divalent metal ion transporters. Previously, ZupT was shown to be responsible for uptake of zinc. In this study, we show that ZupT is a divalent metal cation transporter of broad substrate specificity. An E. coli strain with a disruption in all known iron uptake systems could grow in the presence of chelators only if zupT was expressed. Heterologous expression of Arabidopsis thaliana ZIP1 could also alleviate iron deficiency in this E. coli strain, as could expression of indigenous mntH or feoABC. Transport studies with intact cells showed that ZupT facilitates uptake of 55Fe2+ similarly to uptake of MntH or Feo. Other divalent cations were also taken up by ZupT, as shown using 57Co2+. Expression of zupT rendered E. coli cells hypersensitive to Co2+ and sensitive to Mn2+. ZupT did not appear to be metal regulated: expression of a Φ(zupT-lacZ) operon fusion indicated that zupT is expressed constitutively at a low level.
The ZIP family derived its name from the first identified members, ZRT, IRT-like protein (15). These proteins were initially identified as iron or zinc transporters in eukaryotes, but some members were subsequently shown to also transport other metals, such as manganese or cadmium. However, the specificity and affinity for different metals change with each ZIP transporter (10, 15). ZupT is the first characterized bacterial member of the ZIP family and was shown to be responsible for zinc uptake in Escherichia coli (13).
The transport mechanism for members of the ZIP family is still unknown. All of the functionally characterized ZIP proteins are predicted to have similar membrane topologies, with eight transmembrane domains and the amino- and carboxy-terminal ends of the protein located on the outside surface of the plasma membrane (12). Arabidopsis ZIP proteins range from 326 to 425 amino acids in length, the difference being largely due to the extension of a variable region probably located in the cytoplasm between transmembrane domains III and IV. In most cases, this variable region contains a potential metal-binding domain rich in His residues. For example, in ZIP1, this motif is HAGHVHIHTHASHGHTH. Although the function of this motif is unknown, its conservation in many of the ZIP proteins suggests it may have a role in metal transport or regulation (15).
In this report, we investigated the role and metal specificity of ZupT. Studies examining factors determining metal specificity of an individual transporter are often complicated by redundant transport systems. In E. coli, ferrous iron (Fe2+) is taken up with high affinity by the gene products of the feo locus (21). Ferrous iron may also be transported into the cytoplasm by the manganese permease MntH (24). The magnesium transporter CorA was also reported to be capable of ferrous iron uptake (4, 16), but recent work has demonstrated that Fe2+ is not transported by CorA and that Fe2+ does not significantly inhibit Mg2+ transport via CorA (27). Ferric iron (Fe3+) is taken up by the gene products of the fec locus as a complex with the chelator citrate (17) or by several other receptors for iron chelates (siderophores) in the outer membrane.
Therefore, E. coli strains deficient in all relevant iron and manganese uptake systems were created. We found that ZupT can transport iron and cobalt in addition to zinc and possibly manganese. The zupT gene was not induced by the presence or absence of metals and appears to be constitutively expressed.
MATERIALS AND METHODS
Bacterial strains and growth media.
Strains used are listed in Table 1. E. coli was grown in Luria-Bertani medium or Tris-buffered mineral salts medium (25) containing 2 g of glycerol and 3 g of Casamino Acids per liter. Antibiotics (chloramphenicol [15 to 20 μg/ml], kanamycin [25 μg/ml], ampicillin [100 μg/ml], and tetracycline [12.5 μg/ml]) and metals were added where appropriate.
TABLE 1.
E. coli strains and plasmids
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| W3110 | Wild type | |
| GG161 | W3110 ΔlacZYA::kan | 11 |
| SF1 | W3110 Φ(zupT-lacZ) ΔlacZYA::kan | This study |
| SF4 | W3110 Φ(zupT-lacZ) ΔznuABC::cat ΔlacZYA::kan | This study |
| SF14 | W3110 Φ(zupT-lacZ) ΔmntH ΔfeoABC ΔentC ΔlacZYA::kan | This study |
| GR417 | W3110 ΔentC::cat | This study |
| GR460 | W3110 ΔfeoABC::cat ΔentC | This study |
| GR489 | W3110 ΔmntH::cat ΔfeoABC ΔentC | This study |
| GR499 | W3110 ΔzupT::cat ΔmntH ΔfeoABC ΔentC | This study |
| GR507 | W3110 ΔzupT::cat ΔfeoABC ΔentC | This study |
| GR532 | W3110 ΔzupT::cat ΔmntH | This study |
| GR536 | W3110 ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentC ΔfeoABC | This study |
| GR537 | W3110 ΔfecABCDE::kan ΔmntH::cat ΔentC ΔfeoABC | This study |
| GR538 | W3110 ΔfecABCDE::kan ΔzupT::cat ΔentC ΔfeoABC | This study |
| GR539 | W3110 ΔfecABCDE::kan ΔentC::cat ΔfeoABC | This study |
| ECA281 | ΔzupT::cat ΔcorA | This study |
| Plasmids | ||
| pACYC184 | New England Biolabs | |
| pZUPT-low | zupT with appr. 300-bp upstream region EcoRI in pACYC184a | This study |
| pASK-IBA3 | IBA GmbH, Göttingen, Germany | |
| pZUPT | zupT from E. coli in pASK-IBA3 | 13 |
| pZITB | zitB from E. coli in pASK-IBA3 | 11 |
| pMntH | mntH from E. coli in pASK-IBA7 | This study |
| pFEO | feoABC from E. coli in pASK-IBA7 | This study |
| pYIIP | yiiP (fieF) from E. coli in pASK-IBA3 | 11 |
| pCZCD | czcD from Ralstonia metallidurans CH34 in pASK-IBA5 | 2 |
| pZIP1 | ZIP1 from Arabidopsis thaliana in pACYC184 | Natasha Grotz |
appr., approximately.
Gene disruptions and deletions.
Genes were disrupted by the insertion of Kanr or Camr cassettes by employing a protocol developed in the laboratory of B. Wanner, based on the λ Red-recombinase system, as described previously (7). Multiple deletions were constructed by elimination of the respective resistance cassette and subsequent phage P1 transduction.
Deletion of mntH or zupT, respectively, in strain GR460 (ΔfeoABC::cat ΔentC) led to strains GR489 (ΔmntH::cat ΔfeoABC ΔentC) and GR507 (ΔzupT::cat ΔfeoABC ΔentC). A quadruple deletion mutant, GR499 (ΔzupT::cat ΔmntH ΔfeoABC ΔentC) was constructed from strain GR489. To delete all known iron uptake systems from E. coli necessary for growth in minimal medium, an additional deletion in the ferric-citrate uptake determinant, ΔfecABCDE, was introduced into the strains mentioned above, resulting in strains GR536 (ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentC ΔfeoABC), GR537 (ΔfecABCDE::kan ΔmntH::cat ΔentC ΔfeoABC), GR538 (ΔfecABCDE::kan ΔzupT::cat ΔentC ΔfeoABC), and GR539 (ΔfecABCDE::kan ΔentC::cat ΔfeoABC).
Construction of a zupT-lacZ operon fusion.
Expression of zupT was analyzed using a transcriptional fusion with lacZ as a reporter gene. To construct the chromosomal Φ(zupT-lacZ) transcriptional fusion in strain E. coli SF1 [W3110 Φ(zupT-lacZ) ΔlacZYA::kan], the 400 bp upstream and downstream of the zupT stop codon were separately amplified by PCR from chromosomal DNA of E. coli W3110. These fragments were digested with BamHI, and both fragments were joined and cloned into vector plasmid pGEM T-Easy (Promega, Madison, Wis.) in one step. As confirmed by sequencing, this led to a plasmid harboring an 800-bp zupT fragment with a BamHI site and an XbaI site located directly downstream of the stop codon of zupT, mutating the sequence CATTAATGGGACAGC (the TAA stop codon of zupT is in boldface) to CATTAAGGATCCGGGTCTAGAGGCCATTCACATCATCACCATTAATGGGACAGC (underlining indicates restriction sites for BamHI and XbaI). A promoterless lacZ gene was inserted into the BamHI/XbaI sites of this plasmid, and the fragment containing zupT-lacZ was cloned as a NotI fragment into plasmid pKO3 (23). Finally, the pKO3 hybrid plasmid with Φ(zupT-lacZ) was used in a double-recombination event to insert the lacZ gene downstream of zupT on the chromosome of E. coli GG161 (W3110 ΔlacZYA::kan) as described previously (11), resulting in strain SF1. The correct insertion and orientation of lacZ in E. coli strain SF1 were verified by PCR.
Cloning of zupT, mntH, and feo.
The open reading frame of zupT with its upstream region was PCR amplified from chromosomal DNA of E. coli strain W3110 and cloned into plasmid pGEM T-Easy (Promega). Inserts were sequenced and subcloned into the EcoRI site of low-copy-number vector pACYC184. The mntH or feoABC gene was PCR amplified and cloned into expression vector pASK-IBA7 (IBA GmbH, Göttingen, Germany).
CAS agar plates.
E. coli strains were grown overnight in Luria-Bertani medium with shaking at 37°C, diluted 1:500 into Tris-buffered minimal medium (25) supplemented with 2 ml of glycerol and 3 g of Casamino Acids per liter. Cultures were grown overnight and spread on Chrome Azurol S (CAS) agar plates. CAS agar plates were prepared as described previously (34).
Metal uptake.
Uptake experiments were performed by filtration. Stationary-phase cultures were diluted to 30 Klett units in minimal medium. The cells were then grown to an optical density of 60 Klett units, and gene expression was initiated with the addition of 200 μg of anhydrotetracycline (AHT) per liter. After growth for 35 min, cells were washed with Tris-buffered mineral salt medium without Casamino Acids and iron or with 10 mM Tris-HCl, pH 7.0. Metal uptake was started by addition of a mixture of ascorbate (final concentration, 1 mM) and FeSO4, labeled with 55FeCl3, (final iron concentration, 5 μM), or CoCl2 labeled with 57CoCl2, (final cobalt concentration, 5 μM). The cells were incubated with shaking, and 0.4- or 0.5-ml aliquots were filtered through nitrocellulose membranes (0.45 μm) at various times and immediately washed with 6 ml of 0.1 mM LiCl (for iron) or buffer (10 mM Tris-HCl [pH 7.0], 10 mM MgCl2) (for cobalt). The membranes were dried, and radioactivity was measured using a liquid scintillation counter (LS6500; Beckman, München, Germany). The dry weight (d.w.) was determined from the optical density using a calibration curve. 55FeCl3 and 57CoCl2 were from Perkin-Elmer (Boston, Mass.).
ZupT overexpression and purification.
ZupT was purified by using Strep-TagII technology (IBA GmbH, Göttingen, Germany). The zupT gene was expressed from plasmid pZUPT in E. coli strain BL21 cells (Stratagene Europe, Amsterdam, The Netherlands). Cells were cultivated overnight at 37°C in Luria-Bertani broth, diluted 1:50 into 2 liters of fresh medium, and cultivated with shaking at 30°C up to an optical density at 600 nm of 1.0. Expression of zupT was induced by addition of 200 μg of anhydrotetracycline/liter, and incubation continued for 3 h. Cells were harvested by centrifugation (7,650 × g, 4°C, 15 min), suspended in 20 ml of buffer W (100 mM Tris-HCl [pH 8.0]), and broken twice via French press (SLM Aminco, Urbana, Ill., at 138 kPa) in the presence of protease inhibitor cocktail (Sigma-Aldrich, Deisenhofen, Germany) and DNaseI (10 g/liter). Debris was removed by centrifugation (23,400 × g, 15 min, 4°C), and the membrane fraction was isolated by ultracentrifugation (100,000 × g, 2 h, 4°C). The membrane pellet was suspended in buffer W to a final protein concentration of 10 g/liter. ZupT was solubilized with 1% (wt/vol) n-lauroyl sarcosine for 45 min on ice with stirring, and residual membrane fragments were removed by ultracentrifugation (100,000 × g, 30 min, 4°C). The resulting solubilized protein fraction was applied to a Strep-Tactin-Sepharose affinity chromatography column (bed volume, 2 ml), which was washed subsequently with 30 and 20 ml of buffer W containing 0.1% (wt/vol) n-lauroyl sarcosine with or without 1 M NaCl. Finally, ZupT was eluted with 100 mM Tris-HCl buffer (pH 8.0) containing 2.5 mM desthiobiotin and 0.05% (wt/vol) n-lauroyl sarcosine. Total ZupT protein yield was approximately 75 μg/liter of culture.
Immunoblotting.
ZupT protein samples were separated on sodium dodecyl sulfate-polyacrylamide gels, blotted (SemiDry-Blot; Biometra, Göttingen, Germany) onto a polyvinylidene difluoride membrane, and incubated with a Strep-Tactin horseradish peroxidase conjugate. Blots were developed with a chromogenic substrate as described previously (22).
Miscellaneous.
Standard molecular genetic techniques were used (33). Chromosomal DNA of E. coli strain W3110 was isolated by using Genomic-Tips (QIAGEN). PCR was performed with Pwo or Taq DNA polymerase (Roche, Fermentas). DNA sequencing was performed at the DNA Sequencing Service facility of the University of Arizona. The β-galactosidase activity in permeabilized cells was determined as published previously (11, 26).
RESULTS
ZupT is involved in iron uptake in E. coli.
There are multiple pathways of iron uptake in E. coli. In an effort to elucidate the role of ZupT in iron transport in E. coli, all known systems required for iron uptake in defined minimal medium were deleted. Deletion of entC, coding for isochorismate synthase 2, results in an inability to synthesize enterobactin, the indigenous catechol siderophore of E. coli (29). Deletion of feo reduces the ability of high-affinity ferrous iron uptake (21). The manganese permease MntH was also deleted because it can transport ferrous iron at high iron concentrations (24).
Single deletions of feoABC, mntH, or zupT in E. coli strain W3110 did not result in an iron-dependent phenotype with growth in mineral salt medium (data not shown). Deletion of entC rendered E. coli unable to produce enterobactin, and growth was slightly impaired under iron-depleted conditions (data not shown). A double deletion of feoABC and entC in strain GR460 did not exhibit a phenotype towards iron depletion significantly different from that of strain GR417 (ΔentC::cat) (data not shown).
When multiple-deletion strains were streaked on CAS agar plates, triple-deletion strains, GR507 (ΔzupT::cat ΔfeoABC ΔentC) or GR489 (ΔmntH::cat ΔfeoABC ΔentC), grew after 2 to 3 days, while the quadruple-deletion strain GR499 (ΔzupT::cat ΔmntH ΔfeoABC ΔentC) did not (Table 2). Cells lacking only enterobactin grew overnight, as did cells that expressed the siderophore but lacked both MntH and ZupT. The presence or absence of the high-affinity ferrous uptake system Feo in strains GR460 and GR417 had no effect on growth under this condition. These data suggested that for growth under conditions of iron deficiency on CAS agar plates, either production of enterobactin (EntC dependent) or any one of the other iron uptake systems allowed growth.
TABLE 2.
Growth of several E. coli strains on Chrome Azurol S agar plates
| Strain | Genotype | Characteristics |
|---|---|---|
| GR499 | ΔzupT::cat ΔmntH ΔfeoABC ΔentC | No growth |
| GR507 | ΔzupT::cat ΔfeoABC ΔentC | Growth after 2 to 3 days, no halos |
| GR489 | ΔmntH::cat ΔfeoABC ΔentC | Growth after 2 to 3 days, no halos |
| GR460 | ΔfeoABC::cat ΔentC | Growth overnight, no halos |
| GR417 | ΔentC::cat | Growth overnight, no halos |
| GR532 | ΔzupT::cat ΔmntH | Growth overnight, halos |
| W3110 | Wild type | Growth overnight, halos |
In liquid mineral salt medium with an added chelator, 2,2′-dipyridyl (DIP), both triple-deletion strains, GR489 and GR507, were slightly less iron dependent than their parental double-deletion strain, GR460 (ΔfeoABC::cat ΔentC) (Table 3). The quadruple-deletion mutant GR499 (ΔzupT::cat ΔmntH ΔfeoABC ΔentC) was much more iron dependent in minimal medium with DIP than the triple-deletion mutants GR489 and GR507 (Table 3). However, all strains grew equally well on Luria-Bertani agar (data not shown). This suggested that ZupT could mediate iron uptake. Growth of both triple- and quadruple-deletion mutants was restored to the level of growth of the double-deletion mutant GR460 (ΔfeoABC::cat ΔentC) when iron or manganese was added at a concentration equimolar to that of DIP (Table 3).
TABLE 3.
Effect of different metals on iron depletion of E. coli strains harboring multiple gene deletionsa
| Strain | Genotype | Systems still functional | Growth yield in the presence of DIP andb:
|
||||
|---|---|---|---|---|---|---|---|
| No metal | MgCl2 | ZnCl2 | MnCl2 | FeCl3 | |||
| GR499 | ΔzupT::cat ΔmntH ΔfeoABC ΔentC | Fec | 0.070 ± 0.01 | 0.09 ± 0.04 | 0.14 ± 0.02 | 0.64 ± 0.02 | 0.66 ± 0.08 |
| GR507 | ΔzupT::cat ΔfeoABC ΔentC | Fec, MntH | 0.39 ± 0.01 | 0.31 ± 0.01 | 0.51 ± 0.09 | 0.69 ± 0.03 | 0.65 ± 0.01 |
| GR489 | ΔmntH::cat ΔfeoABC ΔentC | Fec, ZupT | 0.45 ± 0.03 | 0.42 ± 0.01 | 0.42 ± 0.01 | 0.64 ± 0.01 | 0.69 ± 0.08 |
| GR460 | ΔfeoABC::cat ΔentC | Fec, ZupT, MntH | 0.29 ± 0.02 | 0.29 ± 0.02 | 0.35 ± 0.04 | 0.52 ± 0.02 | 0.65 ± 0.09 |
| GR536 | ΔfecA-E::kan ΔzupT::cat | None | 0.09 ± 0.01 | 0.08 ± 0.02 | 0.17 ± 0.02 | 0.71 ± 0.01 | 0.22 ± 0.03 |
| ΔmntH ΔfeoABC ΔentC | |||||||
| GR538 | ΔfecA-E::kan ΔzupT::cat ΔfeoABC ΔentC | MntH | 0.52 ± 0 | 0.51 ± 0.01 | 0.51 ± 0.04 | 0.71 ± 0.01 | 0.81 ± 0 |
| GR537 | ΔfecA-E::kan ΔmntH::cat ΔfeoABC ΔentC | ZupT | 0.40 ± 0.2 | 0.42 ± 0.02 | 0.64 ± 0.01 | 0.76 ± 0.01 | 0.79 ± 0.02 |
| GR539 | ΔfecA-E::kan ΔfeoABC::cat ΔentC | ZupT, MntH | 0.50 ± 0.02 | 0.51 ± 0.05 | 0.68 ± 0.04 | 0.75 ± 0.02 | 0.83 ± 0.01 |
Overnight cultures grown in Luria-Bertani broth were diluted 1:500 in minimal medium and grown overnight. Cells were diluted 1:500 in fresh minimal medium, and after 2 h, cells were diluted 1:500 into fresh medium with the indicated additives. The dry weight was determined after 16 h of incubation at 37°C with shaking. Experiments were performed independently in duplicate, and the average with deviation was calculated.
All at a final concentration of 50 μM.
In dose-response experiments with iron deficiency induced by DIP, the quintuple-deletion strain GR536 (ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentC ΔfeoABC) was affected most (Table 3). Growth of the quintuple-deletion mutant GR536 was only slightly restored by addition of iron, suggesting that all important iron uptake systems were deleted in this strain, and thus, the cells were no longer able to take up sufficient iron even when iron was replete. Interestingly, growth of this mutant could be restored when manganese was added to the growth medium in equimolar concentrations with the chelator DIP.
If ZupT takes up iron, expression of zupT in E. coli should lead to enhanced growth of an iron uptake-deficient mutant. In the E. coli quadruple-deletion strain GR499 (ΔzupT::cat ΔmntH ΔfeoABC ΔentC), expression of zupT in trans from the low-copy-number plasmid pACYC184 (pZUPT-low) resulted in growth in complex medium containing DIP (data not shown), as opposed to the control strain that harbored only the vector plasmid.
ZupT mediates uptake of 55Fe and 57Co.
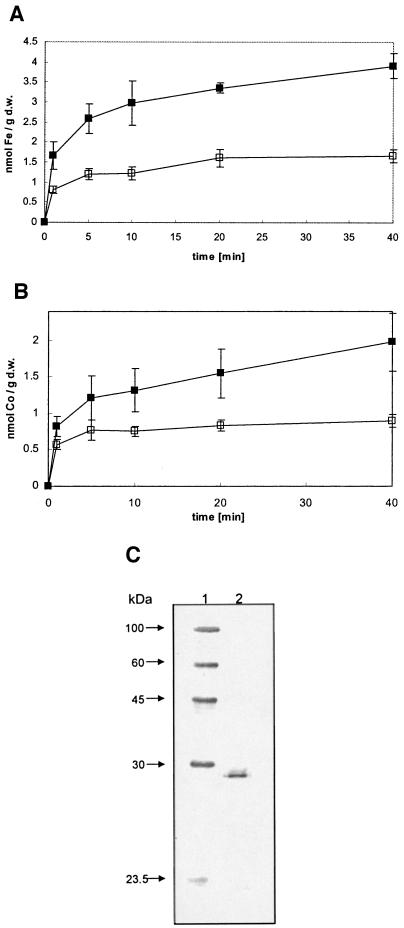
E. coli strain GR536 (ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentC ΔfeoABC) was transformed with plasmid pZUPT in order to measure uptake of 55Fe by ZupT, and expression of zupT was induced with AHT. Cells containing pZUPT showed a significant increase in 55Fe2+ uptake compared to the E. coli strain GR536 pASK-IBA3 vector control (Fig. 1A). This suggested that ZupT is responsible for iron uptake under these conditions. Since the experiments were performed in the presence of excess ascorbate, and thus, the iron would be present mainly in the ferrous state, the transported species is probably ferrous iron.
FIG. 1.
Metal uptake by E. coli expressing zupT. Overnight cultures grown in Luria-Bertani broth were diluted 1:500 in Tris-buffered mineral salt medium, grown overnight, inoculated at 30 Klett units into fresh medium at 37°C, and grown to 60 Klett units. Expression of zupT was induced with of 200 ng of AHT/ml for 35 min. Uptake was started by addition of (A) a reaction mix of 55Fe (1 μCi), FeSO4 (final concentration, 5 μM), and 1 mM ascorbate or (B) 57CoCl2 (1 μCi of 57Co) (final concentration, 5 μM). At defined time points, cellular metal accumulation was determined by the filtration method. Shown are (A) E. coli strain GR536 (ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentCΔfeoABC) pZupT (▪) or pASK-IBA3 (□) and (B) E. coli strain ECA281 (ΔzupT::cat ΔcorA) pZupT (▪) or pASK-IBA3 (□). Averages with standard deviations for three independent experiments are shown. (C) Western blot of Strep-TagII-labeled ZupT protein expressed from plasmid pZUPT in E. coli: lane 1, Strep-Tag protein ladder (IBA GmbH, Göttingen, Germany); lane 2, ZupT (0.6 μg of ZupT protein). d.w., dry weight.
Likewise, the presence of pZupT in E. coli strain ECA281 (ΔzupT::cat ΔcorA), which also contains a deletion in the gene of the cobalt transporter CorA, resulted in increased cobalt accumulation, which was not observed for the vector-only control (Fig. 1B). These results indicated that ZupT is able to transport iron and cobalt in addition to zinc.
Iron transport by different transporters of E. coli.
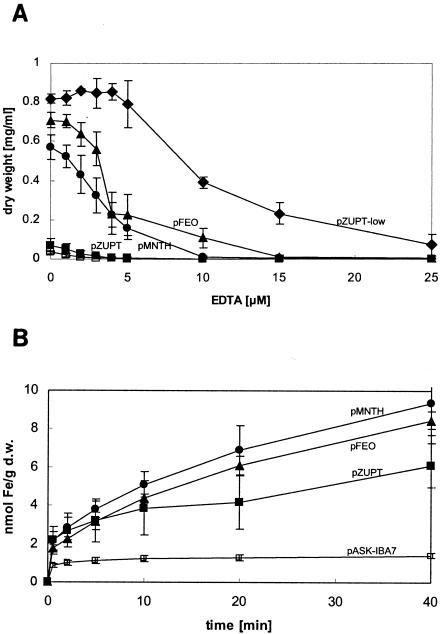
To compare the ability of ZupT to transport iron with that of other E. coli iron transporters, time course experiments were performed under iron depletion conditions. The transporter gene zupT, mntH, or feoABC, respectively, was expressed from the inducible tet promoter of high-copy-number plasmid pASK-IBA3 in E. coli strain GR536 (ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentC ΔfeoABC) with increasing concentrations of EDTA. Additionally, zupT was expressed from low-copy-number plasmid pACYC184 (pZUPT-low) under its native promoter. Figure 2A shows that MntH and Feo enabled strain GR536 to grow in the presence of EDTA, with Feo being a little more efficient than MntH. The presence of plasmid pZUPT-low resulted in the best growth in the presence of EDTA of all strains tested. However, zupT expressed from pZUPT exhibited a phenotype very similar to that of the negative control. This suggested that the expression level of zupT has to be low.
FIG. 2.
Growth and iron uptake in E. coli expressing different iron transporters. Dose response experiments (panel A) or iron uptake (panel B) for E. coli strain GR536 (ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentC ΔfeoABC) harboring plasmids pASK-IBA7 (□), pMNTH (•), pFEO (▴), pZUPT (▪), or pZUPT-low (♦) from at least triplicate experiments with standard deviations are shown. Overnight cultures of E. coli strain GR536 (ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentC ΔfeoABC) grown in Luria-Bertani broth were diluted 1:500 in Tris-buffered mineral salt medium and grown overnight. (A) Cells were diluted 1:500 in fresh medium, and after 2 h of growth at 37°C, cells were diluted 1:500 in fresh medium with iron or different concentrations of EDTA. Cell growth was monitored as the optical density at 600 nm after 16 h of incubation at 37°C with shaking, and the final growth yield is given, or (B) 30 Klett units of cultures were inoculated into fresh medium at 37°C and grown to 60 Klett units. Expression of genes under control of the tet promoter from plasmid pASK-IBA7 was induced with of 200 ng of AHT/ml for 35 min. Uptake was started by addition of a reaction mix of 55Fe (1 μCi), FeSO4 (final concentration, 5 μM), and 1 mM ascorbate.
Iron uptake experiments with E. coli strain GR538, expressing only zupT, mntH, or feoABC, were performed (Fig. 2B). While the presence of the vector control did not lead to increased iron accumulation, the expression of any iron transporter resulted in increased uptake of iron. Under the conditions tested, MntH was the most effective, followed by Feo and ZupT. In contrast to the dose-response curves (Fig. 2A), expression of pZUPT-low did not lead to significantly increased iron accumulation compared to results with the vector control (data not shown). A possible explanation is that increased and unregulated iron accumulation caused by high-level expression of zupT resulted in disadvantageous iron overload of the cells and thus diminished growth.
Expression of zupT from a plasmid renders E. coli W3110 more sensitive to cobalt and manganese.
In plants, members of the ZIP family are responsible for iron, zinc, cadmium, and manganese transport (15). In E. coli, expression of zupT from a medium-copy-number plasmid rendered cells hypersensitive to zinc and cadmium (13). To study ZupT function, zupT including its putative promoter region was expressed from the low-copy-number plasmid pACYC184 (pZUPT-low). pZUPT-low expressed in wild-type E. coli W3110 resulted in a moderately hypersensitive phenotype against zinc (data not shown). However, in medium without added metals, growth was identical to that of a plasmid-only control. Thus, this strain could be used as a tool for studying sensitivity to other divalent metal cations mediated by ZupT.
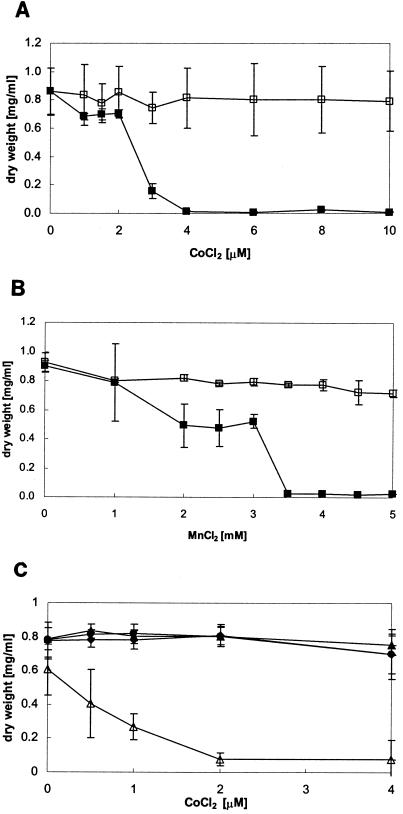
Studies of radioactive 57Co2+ uptake showed that ZupT can mediate Co2+ accumulation, and this was corroborated by growth experiments. Figure 3A shows that E. coli strain W3110 was rendered Co2+ hypersensitive when zupT was expressed in trans from the low-copy-number plasmid pACYC184. In addition to ZupT, cobalt might be taken up nonspecifically by the magnesium uptake system CorA in E. coli but probably with a much lower affinity (30). There was a limited range of tolerance against this cation between 1 and 2 μM Co2+. Expression of zupT also rendered cells more sensitive to manganese. However, the inhibitory concentration for Co2+ (Fig. 3A) was lower by a factor of 10−3 than that for Mn2+ (Fig. 3B). Thus, ZupT likely mediates manganese uptake but with poor affinity.
FIG. 3.
Effect of cobalt or manganese on growth of E. coli W3110 expressing zupT and alleviation of cobalt toxicity by CDF transporters. Dose-response curves with different CoCl2 concentrations are shown. Cultures were grown as described in the legend to Fig. 2 and challenged with the indicated concentrations of CoCl2. Cell growth was monitored as the optical density at 600 nm after 16 h of incubation at 37°C with shaking, and the dry weight was determined. E. coli strains were (A, B) W3110 pZUPT-low (▪) and E. coli W3110 pACYC184 (□) and (C) W3110 pZUPT-low pASK-IBA3 (▵), pZUPT-low pZITB (▴), pZUPT-low pYIIP, (•), and pZUPT-low pCZCD (♦). Experiments were performed in duplicate (A) or triplicate (B), and the averages and standard deviations were calculated.
Cation diffusion facilitators (CDF) comprise a family of metal permeases whose members are found in all kingdoms of life (32). We reasoned that if pZUPT-low rendered E. coli hypersensitive to Co2+, coexpression of a CDF permease that catalyzes efflux of Co2+ should result in alleviation of this hypersensitivity if Co2+ were a substrate of these CDF proteins. When zitB, yiiP (fieF), or czcD was expressed in strain E. coli W3110 pZUPT-low on pASK-IBA vectors, increasing concentrations of cobalt no longer led to growth inhibition in minimal medium, in contrast to results with strain W3110 pZUPT-low pASK-IBA3 (Fig. 3C). In bacteria, the first characterized member of this family, CzcD, was shown to confer resistance to Co2+, Zn2+, and Cd2+ (1, 28). Recently we showed that ZitB, one of the two intrinsic CDF proteins in E. coli, is responsible for Zn2+ resistance (11). The second CDF permease, YiiP (now named FieF), was recently shown to be involved in iron detoxification (12). Growth restoration clearly indicated that the three CDF transporters tested are capable of also transporting Co2+ across the cytoplasmic membrane and thereby probably counteract Co2+ uptake by ZupT.
The ZIP1 transporter from Arabidopsis thaliana functions as an iron uptake system in E. coli.
Growth of the quintuple deletion mutant GR536 (ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentC ΔfeoABC) was severely affected by iron limitation (Table 3). This strain appeared ideally suited for heterologous expression of genes coding for iron uptake transporters and to study their function without interference by other systems. ZIP1 was chosen because it is an important iron uptake system in roots of higher plants (14). ZIP1 from A. thaliana was functionally expressed in E. coli strain GR536. Growth of the mutant strain could be slightly restored by ZIP1 in iron-depleted medium (Fig. 4). Expression of zupT in trans in this strain resulted in a much higher level of tolerance to EDTA than expression of ZIP1 (Fig. 4). This difference could be due to a much higher level of expression of zupT, since we were able to obtain a strong signal for ZupT but not for ZIP1 in a Western blot. Nevertheless, E. coli strain GR536 could be successfully employed for functional expression of a eukaryotic ZIP transporter in bacteria.
FIG. 4.
Activity of A. thaliana ZIP1 in E. coli. Overnight cultures of E. coli strain GR536 (ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentC ΔfeoABC) grown in Luria-Bertani broth were diluted 1:500 into Tris-buffered mineral salt medium and grown overnight. Cells were diluted 1:500 into fresh medium, and after 2 h of growth at 37°C, cells were diluted 1:500 into fresh medium with iron or different concentrations of EDTA. Cell growth was monitored as the optical density at 600 nm after 16 h of incubation at 37°C with shaking, and the dry weight was determined. E. coli strain GR536 (ΔfecABCDE::kan ΔzupT::cat ΔmntH ΔentC ΔfeoABC) pACYC184 (white), pZUPT-low (black), and pZIP1 (grey) are shown. Experiments were performed in triplicate, and the averages with standard deviations were calculated.
The gene encoding the divalent metal permease ZupT is expressed constitutively.
In plants, expression of the ZIP transporters ZIP1 and ZIP3 or IRT1 are induced in roots in response to zinc or iron deficiency (5, 8, 14). To elucidate regulation of the gene for the sole ZIP transporter ZupT from E. coli, its expression was investigated. Analysis in silico of the coding region of zupT suggested that the gene was expressed as a monocistronic transcript. No potential regulatory gene could be identified immediately up- or downstream of its open reading frame. Regulator-binding sites for known metal-responsive transcription factors also could not be detected. To study transcriptional regulation of zupT, a zupT-lacZ operon fusion was constructed in E. coli. Thereby, the zupT open reading frame was not altered, resulting in a functional ZupT protein.
E. coli strain SF1 Φ(zupT-lacZ) was challenged with the metal chelators EDTA or DIP to induce metal depletion. The addition of chelators did not lead to a significant change in Φ(zupT-lacZ) expression. While unchallenged cells exhibited 50.3 ± 4.2 Miller units of β-galactosidase activity, the presence of DIP resulted in 46.9 ± 3.9 Miller units, and the presence of EDTA resulted in 55.4 ± 1.0 Miller units. Moreover, addition of metals (Zn2+, Co2+, Mn2+, or Fe2+) to strain SF1 Φ(zupT-lacZ) did not lead to altered expression of Φ(zupT-lacZ) (activities ranging from 42.2 ± 5.4 to 50.2 ± 5.2 Miller units). This indicated that in a wild-type background, expression of zupT was constitutive and the level of expression was rather low.
E. coli harbors several divalent cation uptake mechanisms, and we have shown that ZupT functions as a zinc uptake permease (13). To examine zupT expression without the interference of other metal uptake systems, the Φ(zupT-lacZ) fusion was constructed in E. coli strain SF4 [Φ(zupT-lacZ) ΔznuABC::cat ΔlacZYA::kan], lacking the high-affinity zinc uptake system (31). The reporter Φ(zupT-lacZ) was also introduced into E. coli strain GR489 (ΔmntH::cat ΔfeoABC ΔentC), lacking ferrous iron and manganese uptake systems and unable to synthesize the siderophore enterobactin, leading to E. coli strain SF14 [Φ(zupT-lacZ) ΔmntH ΔfeoABC ΔentC ΔlacZYA::kan]. Addition of chelators or metals did not significantly alter Φ(zupT-lacZ) expression in those reporter strains (data not shown) compared to results with the reporter in the wild-type strain. This suggested that expression of zupT in E. coli might always be constitutive at a low level, even when other high-affinity uptake systems were deleted.
DISCUSSION
In this report, we establish that ZupT is a broad-range metal ion transporter in E. coli. ZupT appears to be constitutively expressed at a low level and able to take up the divalent cations Zn2+, Fe2+, Co2+, and possibly Mn2+. These results are in agreement with the broad substrate specificity of some members of the ZIP family, such as IRT1 (15). ZIP proteins have not previously been shown to transport Co2+. The transport mechanism for the ZIP family is at present unknown. The transporters of the ZIP family could work as a channel, since the cations would move along their concentration gradient and membrane potential. A symport is also possible; hZIP2 is reported to cotransport zinc and bicarbonate (9).
E. coli strain GR536, which is devoid of all iron uptake systems relevant for growth in mineral salt medium, was used for studies of single iron transport systems and could be highly useful in future studies of the physiological and biochemical parameters of diverse transporters. Interestingly, many of our mutants were deficient not only in iron uptake but also in manganese uptake. Addition of manganese to E. coli strain GR536 fully restored growth. A similar phenomenon was also observed with metal uptake mutants of Salmonella enterica serovar Typhimurium and Streptococcus pyogenes (3, 19). Thus, some organisms apparently can live without iron but then have an absolute requirement for manganese (18). For E. coli, excess cytoplasmic levels of iron can lead to growth inhibition, but under physiological conditions this is countered by at least two iron efflux pumps (12). Iron appears to exert its toxic effect by production of superoxide in Streptococcus pneumoniae (20). In addition, manganese appears to counter the effect of iron toxicity, as recently observed with S. pneumoniae and Deinococcus radiodurans (20, 6). In E. coli the iron and manganese interplay needs to be further elucidated.
Acknowledgments
This work was supported by NIEHS grant ESO4940 with funds from EPA to C.R., by grants Ni262/4-1 and GR2061/1-1 of the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie to D.H.N. and to G.G., and funding from the Deutsche Forschungsgemenschaft (FR1724/2-1) and DAAD to S.F.
We thank Grit Schleuder for skillful technical assistance. Thanks are due Natasha Grotz and Mary Lou Guerinot (Dartmouth College) for the gift of ZIP1.
REFERENCES
- 1.Anton, A., C. Grosse, J. Reissmann, T. Pribyl, and D. H. Nies. 1999. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J. Bacteriol. 181:6876-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton, A., A. Weltrowski, C. J. Haney, S. Franke, G. Grass, C. Rensing, and D. H. Nies. 2004. Characteristics of zinc transport by two bacterial cation diffusion facilitators from Ralstonia metallidurans and Escherichia coli. J. Bacteriol. 186:7499-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer, E., I. Bergevin, D. Malo, P. Gros, and M. F. Cellier. 2002. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 70:6032-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamnongpol, S., and E. A. Groisman. 2002. Mg2+ homeostasis and avoidance of metal toxicity. Mol. Microbiol. 44:561-571. [DOI] [PubMed] [Google Scholar]
- 5.Connolly, E. L., J. P. Fett, and M. L. Guerinot. 2002. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14:1347-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly, M. J., E. K. Gaidamakova, V. Y. Matrosova, A. Vasilenko, M. Zhai, A. Venkateswaran, M. Hess, M. V. Omelchenko, H. M. Kostandarithes, K. S. Makarova, L. P. Wackett, J. K. Fredrickson, and D. Ghosal. 2004. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025-1028. [DOI] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eide, D., M. Broderius, J. Fett, and M. L. Guerinot. 1996. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. USA 93:5624-5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaither, L. A., and D. J. Eide. 2000. Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 275:5560-5564. [DOI] [PubMed] [Google Scholar]
- 10.Gaither, L. A., and D. J. Eide. 2001. The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. J. Biol. Chem. 276:22258-22264. [DOI] [PubMed] [Google Scholar]
- 11.Grass, G., B. Fan, B. P. Rosen, S. Franke, D. H. Nies, and C. Rensing. 2001. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 183:4664-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grass, G., M. Otto, B. Fricke, C. J. Haney, C. Rensing, D. H. Nies, and D. Munkelt. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol. 2005. 183:9-18. [DOI] [PubMed]
- 13.Grass, G., M. D. Wong, B. P. Rosen, R. L. Smith, and C. Rensing. 2002. ZupT is a Zn(II) uptake system in Escherichia coli. J. Bacteriol. 184:864-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grotz, N., T. Fox, E. Connolly, W. Park, M. L. Guerinot, and D. Eide. 1998. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad Sci. USA 95:7220-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerinot, M. L. 2000. The ZIP family of metal transporters. Biochim. Biophys. Acta 1465:190-198. [DOI] [PubMed] [Google Scholar]
- 16.Hantke, K. 1997. Ferrous iron uptake by a magnesium transport system is toxic for Escherichia coli and Salmonella typhimurium. J. Bacteriol. 179:6201-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussein, S., K. Hantke, and V. Braun. 1981. Citrate-dependent iron transport system in Escherichia coli K-12. Eur. J. Biochem. 117:431-437. [DOI] [PubMed] [Google Scholar]
- 18.Jakubovics, N. S., and H. F. Jenkinson. 2001. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147:1709-1718. [DOI] [PubMed] [Google Scholar]
- 19.Janulczyk, R., S. Ricci, and L. Bjorck. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 71:2656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston, J. W., L. E. Myers, M. M. Ochs, W. H. Benjamin, Jr., D. E. Briles, and S. K. Hollingshead. 2004. Lipoprotein PsaA in virulence of Streptococcus pneumoniae: surface accessibility and role in protection from superoxide. Infect. Immun. 72:5858-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kammler, M., C. Schon, and K. Hantke. 1993. Characterization of the ferrous iron uptake system of Escherichia coli. J. Bacteriol. 175:6212-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, S. M., G. Grass, C. J. Haney, B. Fan, B. P. Rosen, A. Anton, D. H. Nies, and C. Rensing. 2002. Functional analysis of the Escherichia coli zinc transporter ZitB. FEMS Microbiol. Lett. 215:273-278. [DOI] [PubMed] [Google Scholar]
- 23.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makui, H., E. Roig, S. T. Cole, J. D. Helmann, P. Gros, and M. F. Cellier. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol. Microbiol. 35:1065-1078. [DOI] [PubMed] [Google Scholar]
- 25.Mergeay, M., D. Nies, H. G. Schlegel, J. Gerits, P. Charles, and F. Van Gijsegem. 1985. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 162:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Moncrief, M. B., and M. E. Maguire. 1999. Magnesium transport in prokaryotes. J. Biol. Inorg. Chem. 4:523-527. [DOI] [PubMed] [Google Scholar]
- 28.Nies, D. H. 1992. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J. Bacteriol. 174:8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozenberger, B. A., T. J. Brickman, and M. A. McIntosh. 1989. Nucleotide sequence of Escherichia coli isochorismate synthetase gene entC and evolutionary relationship of isochorismate synthetase and other chorismate-utilizing enzymes. J. Bacteriol. 171:775-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park, M. H., B. B. Wong, and J. E. Lusk. 1976. Mutants in three genes affecting transport of magnesium in Escherichia coli: genetics and physiology. J. Bacteriol. 126:1096-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patzer, S. I., and K. Hantke. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28:1199-1210. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen, I. T., and M. H. Saier, Jr. 1997. A novel family of ubiquitous heavy metal ion transport proteins. J. Membr. Biol. 156:99-103. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]