Abstract
Curli fibers could be described as a virulence factor able to confer adherence properties to both abiotic and eukaryotic surfaces. The ability to adapt rapidly to changing environmental conditions through signal transduction pathways is crucial for the growth and pathogenicity of bacteria. OmpR was shown to activate csgD expression, resulting in curli production. The CpxR regulator was shown to negatively affect curli gene expression when binding to its recognition site that overlaps the csgD OmpR-binding site. This study was undertaken to clarify how the interplay between the two regulatory proteins, OmpR and CpxR, can affect the transcription of the curli gene in response to variation of the medium osmolarity. Band-shift assays with purified CpxR proteins indicate that CpxR binds to the csgD promoter region at multiple sites that are ideally positioned to explain the csg repression activity of CpxR. To understand the physiological meaning of this in vitro molecular phenomenon, we analyzed the effects of an osmolarity shift on the two-component pathway CpxA/CpxR. We establish here that the Cpx pathway is activated at both transcriptional and posttranscriptional levels in response to a high osmolarity medium and that CpxR represses csgD expression in high-salt-content medium, resulting in low curli production. However, csgD repression in response to high sucrose content is not mediated by CpxR but by the global regulatory protein H-NS. Therefore, multiple systems (EnvZ/OmpR, Cpx, Rcs, and H-NS) appear to be involved in sensing environmental osmolarity, leading to sophisticated regulation of the curli genes.
The ability of bacteria to recognize and adhere to specific surfaces is a fundamental aspect of microbial ecology and pathogenesis. Bacterial adhesins and fimbriae promote specific recognition and adhesion to diverse target molecules such as mammalian host tissue components or inorganic materials. Curli are highly adhesive proteinaceous fibers produced by Escherichia coli (37) and Salmonella spp. (15). Curli are involved in the colonization of inert surfaces and promote both initial adhesion and cell-cell interaction during biofilm development (3, 45, 57). Curli also mediate binding to a variety of host proteins (7, 37, 38, 54) and internalization of E. coli by eukaryotic cells (23). Production of these fibers has been shown to contribute to the symptoms seen during E. coli sepsis (8, 9, 27). Curli appear, therefore, as a virulence factor able to confer adherence properties to both abiotic and eukaryotic surfaces (29). The genes necessary for curli production are clustered in the csgBA and csgDEFG operons, which encode the curli subunits and regulate their transcription and transport, respectively. csgD encodes a key regulator of the FixJ family that positively regulates the production of curli and cellulose (11, 25, 49).
The ability to adapt rapidly to changing environmental conditions is crucial for the growth and pathogenicity of bacteria in their natural environments. Two-component systems that respond to particular stimuli by modifying the phosphorylated state of a cognate regulatory protein are the most prevalent form of signal transduction mediating bacterial response to environmental signals. Three two-component systems (Cpx, EnvZ/OmpR, and Rcs) are implicated in the regulation of curli biogenesis in E. coli and contribute to the modification of the bacterial surface in response to any change in osmolarity, among others factors (43, 44, 55). The role of osmolarity in biofilm has also been reported in Salmonella enterica serotype Typhimurium (50), Staphylococcus epidermidis (31), and Pseudomonas fluorescens (39). Genetic data indicate that the RcsC sensor kinase negatively regulates csgD (21) and that EnvZ/OmpR (47, 57) and CpxA/CpxR (19) are implicated in the regulation of curli biogenesis. In electrophoretic mobility shift assays (EMSAs), both OmpR and CpxR response regulators have been shown to bind immediately upstream of the csgD promoter (44). OmpR was shown to bind to a consensus sequence located −49.5 bp upstream of the transcriptional start site of csgD, and this subsequently activates the csgDEFG expression that, in turn, increases the expression of csgBA. The CpxA/CpxR pathway was shown to negatively affect curli gene expression (19) by the binding of CpxR to its recognition site overlapping the OmpR-binding site (44). However, whether CpxR competes with OmpR for binding to the DNA is an open question. In addition to these three two-component systems, global regulators such as H-NS (1, 28, 36) and IHF (22) control the expression of the curli genes. Moreover, Crl, a potential thermosensor accumulating at 30°C, was recently shown to interact directly with σS, and this interaction promotes binding of the σS-holoenzyme (E σS) to the csgBA promoter (10). Curli regulators also include MlrA (12). Hence, nine regulators have been involved in the regulation of curli expression through a complex network of interactions between transcription factors and the csg regulatory region. This allows a fine-tuning of curli expression depending on environmental conditions such as osmolarity, temperature, or starvation.
The present study was undertaken to gain a better understanding of the molecular mechanisms mediating the repression of the E. coli curli genes in a high-osmolarity medium. We studied how the interplay between the two regulatory proteins OmpR and CpxR affect the transcription of the csgD gene. In order to understand the physiological meaning of this in vitro molecular phenomenon, we analyzed the effects of an osmolarity shift on the CpxA/CpxR pathway at three levels: (i) the cpxR expression level, as estimated from gene fusion; (ii) the CpxR synthesis level, as estimated by immunodetection; and (iii) the degree of CpxR phosphorylation, as estimated from two-dimensional gel electrophoresis combined with immunodetection. We establish here that the Cpx pathway is activated by high osmolarity and that CpxR binds to the csgD promoter at multiple sites. CpxR repressor activity is, however, restricted to selective environmental conditions, such as a high salt concentration. Other regulators, such as H-NS and RcsB, are involved in csgD repression in response to closely related stimuli. The participation of multiple and sophisticated regulatory systems in the control of csg genes suggests a role for curli in a broad spectrum of complex environments.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The E. coli strains and plasmids used in the present study are listed in Table 1. The media used were Luria-Bertani broth and M63/2, a low-osmolarity minimal medium supplemented with glucose (0.2%). To obtain medium with high osmolarity, sucrose (8 and 20%) or NaCl (0.1 M) was added to M63/2-glucose. Kanamycin (50 μg/ml), ampicillin (50 μg/ml), tetracycline (10 μg/ml), chloramphenicol (20 μg/ml), and tryptophan (20 μg/ml) were added into the medium when necessary. Congo red indicator plates were prepared as described by Hammar et al. (25); on these plates, curli-producing bacteria form red colonies, whereas non-curli-producing cells remain white.
TABLE 1.
E. coli K-12 strains and plasmids used in this study
| E. coli strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| E. coli strains | ||
| MG1655 (PHL565) | λ− F−rph-1 | Lab collection |
| PHL1242 | MG1655 ΔcpxR::kanfrt | This study |
| PHL1258 | MG1655 ΔcpxR, flipase of the kanfrt cassette from PHL1242 | This study |
| PHL1259 | PHL1258 csgD::uidA-kan | This study |
| PHL1265 | MG1655 csgD::uidA-kan | This study |
| PHL1089 | PHL1265 ompR331::Tn10 | 44 |
| PHL1311 | PHL1265 rcsB::Tn10 | This study |
| PHL395 | F− λ− IN(rrnD-rrnE)1 rph-1 Δ(lacZ169) Δhns-118 zch-Tn10 | 32 |
| PHL1318 | PHL1265 Δhns-118 zch-Tn10 | This study |
| PHL1251 | MG1655 trp::(cpxR::lacZ-kan) | This study |
| BL21(DE3) | F−dcm ompT hsdS(rB−mB) gal λ(DE3) | Stratagene |
| PHL906 | BL21(DE3) pCal-n-CpxR | 44 |
| PHL1227 | BL21(DE3) pET20-OmpR | This study |
| TE2680 | F−λ−IN(rrnD-rrnE)1 galK2 Δ(lac)X74 rpsL galK2 recD1903::Tn10d-Tet trpDC700::putPA1303::[Kans-Cmr-lac] | 2 |
| Plasmids | ||
| pET20-OmpR | pET-20b(+) with 760-bp fragment containing ompR ORF | This study |
| pCal-n-CpxR | pCal-n (Stratagene) with 700-bp fragment containing cpxR ORF | 44 |
| pCSG4 | pUC19 with a 3.5-kb HindIII fragment containing intergenic region between csgDEFG and csgBA | 36 |
| pRS551 | pBR322 with lacZYA genes; Ampr Kanr | 53 |
| pKOBEG | pSC101 ts, araC arabinose-inducible λ redγβα operon (Cmr) | 13 |
| pKD4 | Source of the excisable kanamycin-frt cassette | 16 |
| pCP20 | ts (replicate at 30°C) plasmid bearing the flp recombinase gene; Cmr Ampr | 14 |
Kans, Kanamycin sensitive; Ampr, ampicillin resistant; Kanr, kanamycin resistant; Cmr, chlorophenicol resistant; ORF, open reading frame.
Genetics methods.
Phage P1 transductions were carried out as described by Miller (34). Transduction of the cpxR::lacZ-kan fusion was obtained by selection on kanamycin plates and verification of tryptophan auxotrophy. Transduction of csgD::uidA-kan was obtained by selection on kanamycin plates and verification of the white color of transductants on Congo red indicator plates.
Construction of the ΔcpxR mutant.
The cpxR mutant of strain MG1655 was constructed by the use of the lambda-red recombination system as described in Chaveroche et al. (13) and Derbise et al. (17) and detailed at http://www.pasteur.fr/recherche/unites/Ggb/methodes.ang.html with the use of the thermosensitive pKOBEG plasmid that carries the λ phage redγβα operon under the control of the pBAD promoter. The cpxR gene (ATG to Stop) was first replaced by a kanamycin cassette flanked by two frt recombination sites amplified from the pKD4 plasmid (16). The ΔcpxR::kanfrt deletion was then transduced to MG1655, yielding the strain PHL1242. PHL1242 was then transformed with the thermosensitive pCP20 plasmid expressing the Flp flipase (14), thus leading to the excision of the kanamycin-frt cassette and leaving the natural cpxRA promoter taking the control of the cpxA gene (strain PHL1258). The deletion of cpxR in both PHL1242 and PHL1258 was verified by PCR.
Construction of a cpxR::lacZ fusion.
To obtain a cpxR::lacZ chromosomal fusion, a 718-bp DNA fragment corresponding to the cpxR promoter region and the beginning of the coding sequence, was amplified by PCR from MG1655 chromosomal DNA as the template and by using primers WB1 (5′-ATTAACAGGAGGGAATTCGTGCCGGCCTG-3′) and WB2 (5′-GCGCAGGATCCCGCGAATACGTG-3′), which contain, respectively, EcoRI and BamHI restriction sites (underscored sequences). The PCR fragment digested with EcoRI and BamHI was cloned into the corresponding sites of pRS551 (53) to give a transcriptional fusion to lacZ. Integration of the cpxR::lacZ fusion into the chromosome tryptophan locus was obtained as described by Elliott (20). Then, the chromosomal trp::(cpxR::lacZ-kan) fusion was transferred into a MG1655 strain by P1 transduction, resulting in the strain PHL1251.
Enzyme assays.
β-Glucuronidase activity in toluene-treated samples was measured by spectrophotometrically monitoring the hydrolysis of p-nitrophenyl-β-d-glucuronide into p-nitrophenol at 405 nm (5). Specific activity was expressed as units per milligram of protein, where 1 U corresponds to 1 nmol of product liberated per mg of total protein. β-Galactosidase activity was measured by monitoring the degradation of o-nitrophenyl-β-d-galactoside into o-nitrophenol, which absorbs at 420 nm (34). Specific activity was expressed as units per milligram of protein.
Overproduction and purification of OmpR-His6 protein, preparation of OmpR antibodies.
The ompR coding region was amplified by PCR from chromosomal DNA of the MG1655 strain by using the primers ompRXbaI (5′-GCTCTAGAGTTGCGAACCTTTGGGAGTAC-3′) and ompRXhoI (5′-CCGCTCGAGTGCTTTAGAGCCGTCCGGTAC-3′) carrying an XbaI and an XhoI site (underscored sequences), respectively. The 760-bp XbaI-XhoI fragment was then subcloned into the XbaI and XhoI unique sites of pET-20b(+). The resulting plasmid, pET20-OmpR, contains ompR under the control of the T7 promoter and with its own translational regulatory signals, and it expresses an OmpR protein with a His6 motif at its C terminus. pET20-OmpR was introduced into strain BL21(DE3), yielding strain PHL1227. Three hours after IPTG (isopropyl-β-d-thiogalactopyranoside) induction, cells were harvested and suspended in 50 mM NaH2PO4 (pH 8), 300 mM NaCl, and 1 mM imidazole. Crude protein extracts were obtained by disrupting bacteria by sonication at 20 MHz or by vortexing bacteria with glass beads (Sigma). They were incubated overnight with nickel-nitrilotriacetic acid affinity resin. Washes and elution were performed as recommended by the manufacturer (Qiagen). The purification of the OmpR-His6 protein yielded protein of 85% purity, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Antibodies to OmpR were obtained by immunization of a rabbit with purified OmpR-His6.
Purification of the CBP-CpxR protein and preparation of CpxR antibodies.
Construction of the calmodulin-binding protein (CBP)-CpxR fusion is described in a previous study (44). IPTG induction was carried out with a culture of strain PHL906. Crude protein extracts, obtained as described above, were incubated overnight with calmodulin affinity resin in CaCl2 binding buffer. Washes and elution were performed as recommended by the manufacturer (Stratagene). The purification of the CBP-CpxR protein yielded protein of 90% purity, as judged by SDS-PAGE. Antibodies to CpxR were obtained by immunization of a rabbit with purified CBP-CpxR.
EMSA.
The DNA probe D1D2, containing the csgDEFG promoter region (−128 to +12), was obtained by PCR amplification from pCSG4 with primers D1 (5′-CCAAATGTACAAGCTTTCTATCATTTC-3′) and D2 (5′-GGATTACATCTGATTTCAATCTAGCC-3′) (Fig. 3). The DNA probe was digested with HindIII and then 32P labeled by using the Klenow fragment of DNA polymerase. Binding reactions were carried out in 20 μl of 10 mM Tris-HCl (pH 7.4), 50 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol (DTT), 1 mM EDTA, 5% glycerol, 6 μg of bovine serum albumin, 1 μg of calf thymus DNA, and 20 mM acetyl phosphate as a phosphodonor molecule for OmpR and CpxR (30). After addition of the DNA probe (60,000 cpm), various amounts of purified OmpR-His6 and/or CBP-CpxR proteins were added simultaneously. For the supershift assays (Fig. 4B), either 1 μl of antiserum against OmpR or a nonspecific antiserum was added when necessary. The reaction mixtures were incubated for 30 min at 30°C prior to loading onto a 4% nondenaturing polyacrylamide gel (ratio of acrylamide to bisacrylamide, 80:1). Electrophoresis was carried out in 44 mM Tris-borate (pH 8)-0.5 mM EDTA. The ratio of the phosphorylated form of OmpR and CpxR in the binding reactions was determined by using bidimensional electrophoresis combined with immunodetection: 30% of each protein was phosphorylated under the conditions described here (data not shown).
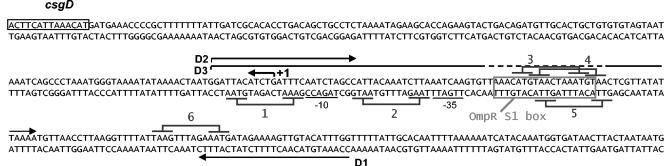
FIG. 3.
Representation of the csgD promoter region in E. coli strain MG1655 (AE000205). Primers used for amplification of the wild-type csgD promoter (D1D2) and the same promoter deleted of the OmpR/CpxR box (D1D3) are indicated by the large arrows. The position of the transcriptional start site of csgD is indicated by the small arrow, and the −10 and −35 regions are underlined. The OmpR binding site is boxed, and six putative CpxR binding sites are underlined.
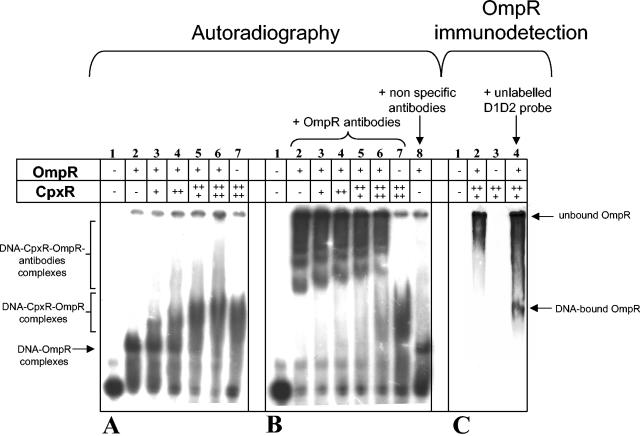
FIG. 4.
CpxR and OmpR bind simultaneously at the promoter region of csgD. Band-shift assays were performed with pure CBP-CpxR, pure OmpR-His6, and a 32P-labeled csgD promoter fragment in the absence (A) or in the presence (B) of antibodies raised to OmpR. D1D2 probe without protein (lanes 1), and D1D2 probe with 1.65 μM OmpR-His6 (lanes 2) are as shown. Increasing amounts of CBP-CpxR were added in the binding reaction containing 1.65 μM concentrations of OmpR-His6. D1D2 probe with 1.65 μM OmpR-His6 and 0.48 μM CBP-CpxR are shown in lanes 3; lanes 4 are the same as in lane 3 but with 0.96 μM CBP-CpxR; lanes 5 are the same as for lanes 3 but with 1.92 μM CBP-CpxR (lanes 5); lanes 6 are the same as for lanes 3 but with 2.88 μM CBP-CpxR; lanes 7 show D1D2 probe with 2.88 μM CBP-CpxR. A control lane contains D1D2 probe with 1.65 μM OmpR-His6 and nonspecific antibodies (panel B, lane 8). (C) Immunodetection of OmpR in DNA complexes after transfer of EMSA gel proteins onto a membrane as described in Materials and Methods. Lanes: 1, D1D2 probe without protein; 2, D1D2 probe with 1.65 μM OmpR-His6 and 1.92 μM CBP-CpxR; 3, D1D2 probe with 1.92 μM CBP-CpxR; 4, D1D2 probe with 1.65 μM OmpR-His6, 1.92 μM CBP-CpxR, and a fourfold excess of unlabeled D1D2 probe.
Identification of OmpR in DNA-protein complexes after blotting of EMSA gels.
The EMSA gel was electrotransferred onto a polyvinylidene difluoride membrane according to the method of Novak and Paradiso (35). Membranes were incubated with a 1:1,000 dilution of anti-OmpR antiserum. After extensive washing with Tris-buffered saline-0.1%Tween, the membrane was finally incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G. OmpR proteins were detected by chemiluminescence with an ECL Kit according to the manufacturer's instructions (Amersham).
Bidimensional electrophoresis.
Proteins were extracted as follows. The MG1655 strain was grown in M63/2-glucose or M63/2-glucose-20% sucrose at 30°C for 48 h, and cells were washed and resuspended in Tris 50 mM (pH 8). Cocktails of protease inhibitors and phosphatase inhibitors (Sigma) were added prior to sonication at 20 MHz. The protein concentration of lysis supernatants was estimated by the Bradford assay and 50 μg were precipitated with cold acetone at −20°C for at least 2 h. After centrifugation, the pellet was solubilized in 8 M urea-4%CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}-20 mM DTT-0.001% bromophenol blue-0.2% ampholytes 3-10. The passive rehydratation of the 7-cm ReadyStrip IPG(5-8) (Bio-Rad) was performed in a Protean immunoelectrofocusing (IEF) cell for 15 h at 20°C. IEF itself was carried out at 50 V for 15 min, followed by a first linear gradient of 50 to 150 V for 30 min and a second linear gradient of 150 to 250 V for 15 min. The IEF was achieved when 4,000 V and 16,000 V · h were reached. Prior to the second dimension, the IPG strips were equilibrated in 6 M urea-2.5% SDS-0.375 M Tris-HCl (pH 8.8)-30% glycerol-130 mM DTT for 15 min and then for 20 min in the same buffer with 135 mM iodoacetamide instead of DTT. SDS-PAGE was performed at 25 V for 30 min and was achieved at 200 V with 10% acrylamide gels.
Immunodetection and densitometric analysis.
After SDS-PAGE, proteins were electrotransferred onto a semidry transfer cell (Bio-Rad) according to the manufacturer's protocol and were detected after immunoblotting with the antiserum to CpxR and the second antibody, horseradish peroxidase-conjugated anti-rabbit immunoglobulin G. The protein bands were visualized by an enhanced chemiluminescence Western blotting kit (Amersham). The density of the protein bands or spots was quantified by using a densitometer and ImageMaster TotalLab software (Amersham).
RESULTS
OmpR binds to a unique site at the csgD promoter, whereas CpxR binds cooperatively to multiple sites.
In order to understand how OmpR interacts with CpxR to affect regulation at the csgD locus, we first assayed the binding of each purified regulator to a DNA fragment containing the transcriptional regulatory region of csgD. The specificity of the detected complexes was previously demonstrated by a competition experiment with an excess of specific or nonspecific DNA competitor (44).
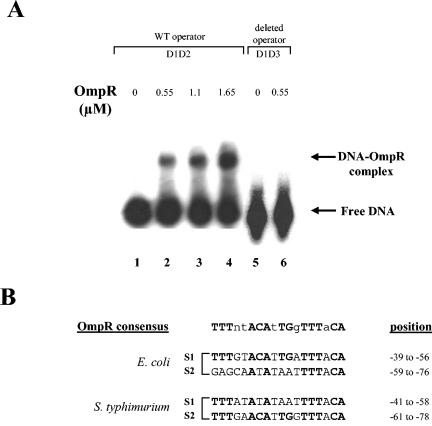
Increasing amounts of OmpR-His6 were added to the DNA fragment encompassing the csgD promoter and, unlike S. enterica serovar Typhimurium (22) but as previously shown in E. coli (44), only one complex was observed (Fig. 1A). According to the method described by Bading (4), the molecular weight of the protein participating in this protein-DNA complex was estimated to be 55 kDa, suggesting that the OmpR protein binds to the csgD promoter as a dimer. Indeed, Harrison-McMonagle et al. have shown that OmpR acts as a dimer, with each monomer interacting with the DNA helix in the major groove (26). Two close OmpR-binding sites have been observed in serovar Typhimurium (22). The sequence of the first OmpR binding site (S1) was highly conserved in both species, as described in Fig. 1B. However, unlike the site in serovar Typhimurium, only a degenerated OmpR consensus was observed within the E. coli second site S2 (Fig. 1B). This discrepancy between the two regulatory sequences could explain why OmpR was able to form two complexes with the csgD promoter region of serovar Typhimurium (22), whereas only one was observed with the homologous region of E. coli (Fig. 1A) (44). Indeed, no OmpR complex could be detected in E. coli when the first OmpR binding site S1 was deleted (Fig. 1A, lane 6).
FIG. 1.
(A) DNA-binding assay with purified OmpR-His6 and the promoter region of csgD. 32P-end-labeled wild-type csgD promoter (D1D2 probe, see Fig. 3) was incubated without protein (lane 1) or with 0.55 μM (lane 2), 1.1 μM (lane 3), or 1.65 μM (lane 4) OmpR-His6. 32P-end-labeled deleted csgD promoter (D1D3 probe, see Fig. 3) was incubated without protein (lane 5) or with 0.55 μM (lane 6) of OmpR-His6. (B) Comparison of E. coli and S. enterica serotype Typhimurium OmpR binding sites. Site positions are relative to the transcriptional start site of csgD (agfD).
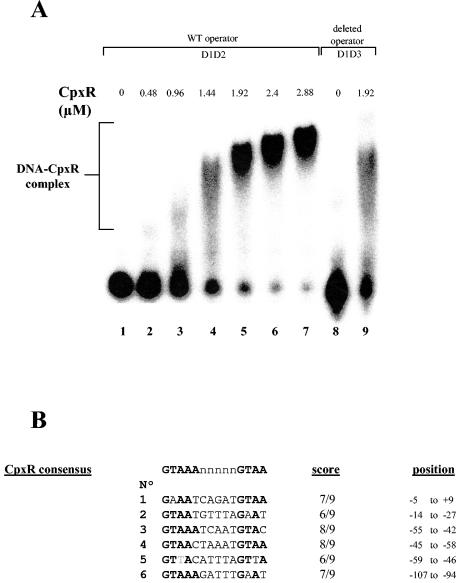
Purified CBP-CpxR was assayed for its specific DNA binding to the csgD promoter region. As opposed to results observed with OmpR, several protein-DNA complexes of decreasing mobility could be resolved with an increasing concentration of CpxR (Fig. 2A). This result suggests that CpxR interacts with several sites within the csgD control region. Indeed, fuzzy unstable complexes could still be detected when the main CpxR-binding site was deleted (Fig. 2A, lane 9). Evaluation of mobility shift data from Fig. 2, lanes 4 to 7, yielded a calculated molecular sizes of 275, 330, 385, and 440 kDa, corresponding to the binding of five, six, seven, and eight CpxR dimers, respectively. However, the calculated molecular weight of the protein responsible for the shift in gel electrophoretic mobility can be overestimated if the protein exhibits DNA-bending properties (4). Using the consensus sequence defined for CpxR by Pogliano et al. (42) and recently refined by De Wulf et al. (18), we found six potential CpxR binding sites within the csgD promoter region (Fig. 2B). Figure 3 shows that two of them (sites 1 and 2) are positioned between the −35 box and the transcriptional start site, which is an ideal position to exert a repressor activity. Moreover, three other sites overlap the OmpR activator binding site (sites 3, 4, and 5). These sites could probably not recruit three dimers of CpxR at the same time, but they could certainly disturb OmpR binding and, consequently, csgD transcription activation. Interestingly, the intensity of the CpxR complexes formed did not increase gradually as a function of the protein concentration. Rather, a 1.5- to 2-fold increase in protein concentration was sufficient to switch from unstable complexes (Fig. 2A, lanes 3 and 4) to a large amount of stable complex formation (Fig. 2A, lanes 5 to 7). This suggests that multiple CpxR molecules were bound in a cooperative fashion, like the global repressor protein H-NS binding pattern (24). All of these data together indicate a significant regulatory potential of CpxR on csgD transcription.
FIG. 2.
(A) DNA-binding assay with purified CBP-CpxR and the promoter region of csgD. 32P-end-labeled wild-type csgD promoter (D1D2 probe) was incubated without protein (lane 1) or with 0.48 μM (lane 2), 0.96 μM (lane 3), 1.44 μM (lane 4), 1.92 μM (lane 5), 2.4 μM (lane 6), or 2.88 μM (lane 7) CBP-CpxR. 32P-end-labeled deleted csgD promoter (D1D3 probe) was incubated without protein (lane 8) or with 1.92 μM (lane 9) CBP-CpxR. (B) Alignment of six putative CpxR binding sites. Scores are relative to the CpxR consensus defined by De Wulf (18). Site positions are relative to the transcriptional start site of csgD. CpxR binding sites are shown in Fig. 3.
OmpR and CpxR bind simultaneously at the csgD promoter.
Since OmpR and CpxR share a common binding site (Fig. 3) and CpxR seems to bind preferentially in a cooperative fashion, we directly examined the possibility of a competitive binding of the two proteins at the csgD promoter. When increasing amounts of CpxR were simultaneously added to binding reactions containing a constant quantity of OmpR, a major DNA-protein complex of slow mobility was observed. This complex comigrates with the DNA-CpxR complex (Fig. 4A, compare lanes 5 and 6 to lane 7). To determine whether OmpR is part of this slow mobility complex, antibodies raised to OmpR were added to the binding reactions. The addition of the anti-OmpR antibodies, but not of nonspecific antibodies, leads to a supershift of the OmpR containing-csgD promoter fragment (Fig. 4B, compare lanes 2, 7, and 8). The slow mobility complexes, observed when the two proteins are simultaneously added into the binding reactions (Fig. 4A, lanes 3 to 6), also upshift in the presence of anti-OmpR antibodies (Fig. 4B, lanes 3 to 6). These results indicate that, in addition to CpxR, the csgD promoter also binds OmpR. To confirm these results, anti-OmpR antibodies were used for the identification of the DNA-binding OmpR proteins after denaturing and Western blotting of the proteins of the EMSA gel onto a polyvinylidene difluoride membrane according to the method of Novak and Paradiso (35). Addition of a fourfold excess of unlabeled csgD promoter was required to increase the total amount of DNA-OmpR complexes and to allow for their immunodetection (Fig. 4C, compare lanes 2 and 4). This figure shows that the antibody-reactive band in the Western blot of the EMSA gel (Fig. 4C, lane 4) migrates at the same location as the slow mobility complex observed by autoradiography in the same conditions (Fig. 4A, lane 5). These in vitro experiments demonstrate that OmpR and CpxR bind simultaneously at the csgD promoter.
Response to high osmolarity: the Cpx activation pathway.
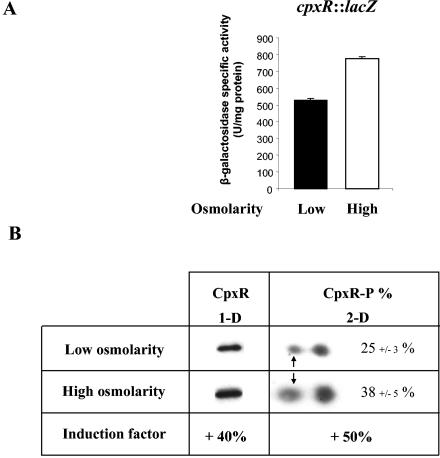
Since growth in a high-osmolarity medium turns off curli transcription (44), it was tempting to suggest that CpxR represses the transcription of csgD in response to high osmolarity. Moreover, a previous study has shown that a two- to fivefold induction of the cpxP::lacZ fusion was observed when bacteria encounter high-osmolarity conditions, suggesting that the Cpx pathway is likely to be activated by high osmolarity (44). We further analyzed the effects of an osmolarity shift on the Cpx pathway at three levels: (i) the cpxR expression level, as estimated from gene fusion; (ii) the CpxR synthesis level, as estimated by immunodetection; and (iii) the degree of CpxR phosphorylation, as estimated from two-dimensional gel electrophoresis combined with immunodetection. To determine whether osmolarity affects the transcription of the cpxR gene, a cpxR::lacZ fusion was constructed and introduced into the trp locus of MG1655 to obtain the merodiploid strain PHL1251. Figure 5A shows that transcription of the cpxR operon was positively affected at high osmolarity. We also examined the effects of osmolarity changes on the accumulation of CpxR by using an antiserum to CpxR. As shown in Fig. 5B, the cellular level of CpxR in the cells grown in high-osmolarity medium was 40% higher compared to the one in cells grown in low osmolarity. Moreover, the analysis of two-dimensional PAGE revealed two interesting points: 25% of total CpxR molecules were phosphorylated in the cells grown at low osmolarity (Fig. 5B), suggesting that the Cpx pathway was already partially activated in these conditions. In addition, the CpxR-P level was increased by >50% at high osmolarity (Fig. 5B), showing that an increase in activation could be obtained by increasing the osmolarity. Taken together, these results show that the Cpx pathway is induced by the osmolarity shift both at the transcriptional and protein levels. Adding-up of the protein amplification and the phosphorylation steps, the CpxR-P level at high osmolarity appears to be ∼2.1-fold higher than that at low osmolarity. This change in the CpxR-P concentration appears to account for the expression pattern of csgD observed at high and low osmolarity.
FIG. 5.
Effect of high osmolarity on CpxR pathway induction. (A) Transcriptional activation. The β-galactosidase activity of the cpxR::lacZ merodiploid strain (PHL1251) was measured in stationary-phase cells grown in low-osmolarity (M63/2-glucose and tryptophan) or high-osmolarity (M63/2-glucose, tryptophan, and 20% sucrose) medium. The results are representative of five independent β-galactosidase assays. (B) Phosphorylated CpxR accumulation. Proteins extracted from cultures of MG1655 grown in low-osmolarity (M63/2-glucose) or high-osmolarity (M63/2-glucose and 20% sucrose) medium were analyzed by one-dimensional gel electrophoresis combined with immunodetection of CpxR. This revealed that the cellular level of CpxR is 40% higher in the cells grown in high-osmolarity conditions. This induction factor was calculated with data resulting from four independent experiments. To determine the percentage of phosphorylated CpxR form (CpxR-P), equivalent protein extracts were subjected to three independent two-dimensional gel electrophoresis analyses combined with immunodetection of CpxR as described in Materials and Methods. The position of phosphorylated CpxR is indicated by small arrows. The density of the protein spots was determined by a densitometer to calculate the percentage of CpxR-P. Analysis of purified CpxR phosphorylation by acetyl phosphate confirmed that the acidic form observed in our assays is the phosphorylated form of CpxR (data not shown). The experimental isoelectric point of CpxR is ∼5.8.
CpxR is responsible for csgD repression in high-salt-concentration conditions.
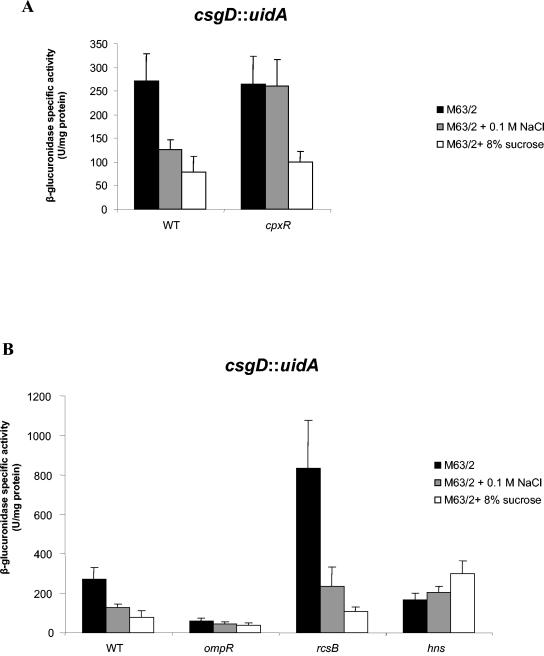
To further analyze the role of the Cpx pathway in the regulation of curli production, we compared the csgD expression level in the wild-type strain MG1655 and in its ΔcpxR derivative. In high-osmolarity medium, using NaCl or sucrose as the osmotic agent, lower csgD expression was detected in wild-type MG1655 strain (Fig. 6A). This result is consistent with previous results (44). Repression by a high salt concentration was not observed in the ΔcpxR derivative (Fig. 6A), and this demonstrates that CpxR mediates the csgD inhibition in a high-salt medium. Surprisingly, the csgD::uidA activity was still repressed in minimal medium supplemented with sucrose, even in the absence of the CpxR protein (Fig. 6A). Therefore, involvement of other regulatory proteins in the sucrose-dependent csgD repression was investigated. Römling and coworkers have found some evidence in serovar Typhimurium indicating that OmpR could act as a csgD repressor (22). To test this hypothesis in E. coli, we assayed the β-glucuronidase activity of an MG1655 csgD::uidA derivative carrying an ompR::Tn10 mutation. Despite weak csgD expression in the absence of the OmpR activator, repression was still observed in high-sucrose medium (Fig. 6B). Recent genetics data indicated that the Rcs two-component system negatively regulates csgD (21). In low-osmolarity medium, the csgD expression was indeed higher in a rcsB::Tn10 strain than in the wild-type strain (56) (Fig. 6B). This rcsB mutation did not relieve, however, the repression observed in the high-osmolarity medium (sucrose or NaCl). The global regulatory protein H-NS was shown to be involved in csg genes regulation, and it regulates other genes in response to osmolarity changes (2, 22, 36). Therefore, we tested the activity of csgD::uidA in an hns derivative. At low osmolarity the mutation of hns caused a reduction of csgD expression, thus indicating that H-NS acts as an activator of csgD in this condition. Addition of a high salt concentration to low-osmolarity medium slightly increased csgD expression in the hns mutant (Fig. 6B). However, in this hns mutant, the addition of a high sucrose concentration in the medium resulted in increased csgD expression, to the same level as the wild-type strain cultivated in low-osmolarity conditions (Fig. 6B). This result indicates that the high sucrose-mediated csgD repression observed in wild-type cells was actually H-NS dependent. These data demonstrate that CpxR and H-NS are involved in csgD regulation in response to medium osmolarity and that E. coli cells are able to discriminate between high osmolarity resulting from a high salt and that resulting from a high sucrose concentration.
FIG. 6.
(A) CpxR represses csgD transcription in high-salt-concentration conditions. The β-glucuronidase activities were determined for strains PHL1265 (MG1655 csgD::uidA) and PHL1259 (MG1655 ΔcpxR csgD::uidA). The results are means and standard deviations from three independent β-glucuronidase assays. (B) H-NS mediates csgD repression in high-sucrose medium. The β-glucuronidase activities were determined for strains PHL1265 (MG1655 csgD::uidA), PHL1089 (MG1655 ompR331::Tn10 csgD::uidA), PHL1311 (MG1655 rcsB::Tn10 csgD::uidA), and PHL1318 (MG1655 Δhns118 csgD::uidA). The results are means and standard deviations from three independent β-glucuronidase assays. Cells were grown to early stationary phase at 30°C in M63/2-0.2% glucose supplemented or not with 0.1 M NaCl or 8% sucrose.
DISCUSSION
The curli intergenic region has the potential for a whole range of regulatory interactions. It is indeed one of the largest regions without coding capacity in E. coli and it displays unique features such as high curvature and low stability (40). The challenge of the present study was to analyze the network of regulators that regulate csgD promoter expression when cells encounter high-osmolarity conditions.
OmpR is required for transcriptional activation of the csgDEFG operon when CpxR acts as a repressor (19, 57). OmpR and CpxR recognition sites overlap immediately upstream of the RNA polymerase binding site (44). In order to characterize the mechanisms of curli gene repression and activation, the binding specificity of each purified regulator to the csgD promoter was carefully examined. Under our conditions, band-shift assays indicate that OmpR binds to a unique site (Fig. 1A). In contrast, six CpxR sites could be identified as closely related to the CpxR consensus sequence defined by De Wulf (18) (Fig. 2 and 3). Moreover, several putative CpxR sites can also be identified upstream of the csgBA operon (data not shown), and CpxR was previously shown to bind to a sequence centered at +10 relative to the transcriptional start site of csgBA operon (44). Hence, a total of 10 CpxR boxes could be identified in the csg intergenic region. This suggests that optimal csg repression depends on multiply bound CpxR molecules that interact in a complex way. From the csgD promoter sequence analysis (Fig. 3), we suggest that three different mechanisms of csgD repression are likely to occur: (i) CpxR sites 1 and 2 are in a good position to prevent RNA polymerase binding and consequently switch csgD expression off; (ii) binding of CpxR to sites 3, 4, or 5 is likely to disturb the activator OmpR binding to the csgD promoter (Fig. 3); and (iii) binding of CpxR to additional sites upstream csgBA promoter could affect both csgB and csgD transcription. Progressive occupancy of all of these CpxR sites could provide differential modulation of OmpR-mediated activation of csgD.
We have shown that OmpR is part of the stable complex formed when the two regulatory proteins OmpR and CpxR are present in binding reactions (Fig. 4). This result would rule out the second mechanism (ii) and suggest that the first mechanism (i) alone could explain the CpxR-mediated repression of csgD. However, the presence of several nearly perfect CpxR consensus inside the OmpR box strongly suggests the existence of a competition between OmpR and CpxR. We propose that this configuration could allow for a gradual curli gene expression. Depending on the respective activation of the EnvZ/OmpR and Cpx pathway, a certain competition could occur and would moderately disturb OmpR-dependent csgD activation. When bacteria encounter environmental conditions that fully activate the Cpx pathway, CpxR-P should bind at sites 1 and 2 and allow an extremely efficient csgD repression even if OmpR is still bound to the csgD promoter (as is the case in our in vitro experiments in Fig. 4). We suggest that CpxR, via its specific binding sites arranged over an extended region of DNA, functions as a modulator of OmpR activation. The occurrence of such multiple repressor binding sites has been described for CytR in the deoP2 promoter (41). The present study reveals the complexity of the control exerted on the csgD expression.
Growth of E. coli in a high osmolarity medium abolishes the expression of csg genes, resulting in low curli production and a planktonic mode of growth. We have shown here that the Cpx pathway is induced at both the transcriptional and the protein level in response to a high level of sucrose. These results indicate that, as previously suggested by using a target cpxP-lacZ fusion (44), the Cpx pathway responds to changes in osmolarity. With increasing salt or sucrose concentration, the activity of the csgD promoter decreases. However, when sucrose was used as an osmotic agent, CpxR had no effect on the csgD::uidA gene fusion expression (Fig. 6A). Although the Cpx pathway can sense both high ion and high sugar content, it seems that the CpxR repressor is only responsive to high salt. This suggests that very subtle regulatory interactions can take place between the csgD regulators. Interestingly, Römling and coworkers observed a slightly different situation in serovar Typhimurium. Whereas increasing the salt concentration led to a decrease in csgD promoter activity, no effect on csgD could be observed when sucrose was used as an osmotic agent (48). Sequence divergences between the two species in the csg intergenic region are likely to account for these differences in the regulatory process (Fig. 1B).
We observed that a strong repression of csgD occurs in a high-sucrose medium independently of CpxR. In order to determine which proteins mediate this sucrose repression, mutation in two regulatory genes, rcsB and hns, known to be involved in osmoregulation were introduced into the MG1655 csgD::uidA strain (2, 55). Recent genetic data indicate that the Rcs pathway negatively regulates csgD (21, 56). Indeed, we found that RcsB acts as a strong repressor of the csgD transcription in low-osmolarity medium. Nevertheless, the sucrose-mediated repression of the csgD operon was independent of RcsB (Fig. 6B). The hns118 mutation was then introduced in the MG1655 csgD::uidA background. In the absence of H-NS, the csgD expression level in high-sucrose medium rises to the level observed in the wild-type strain grown in a low-osmolarity medium (Fig. 6B). This result shows that H-NS mediates the csgD repression in high-sucrose medium. H-NS is a histone-like nucleoid structuring protein that has no identified binding sequence but prefers an AT-rich DNA region with curved structures (52). Using rich medium and different genetic backgrounds, H-NS has previously been shown to either activate or repress csgD transcription (22, 36). Although we show here that H-NS is the key repressor that switches off csgD transcription in a high-sucrose medium, our results also illustrate the fact that the role of H-NS in csg transcription is complex and depends on the strain background.
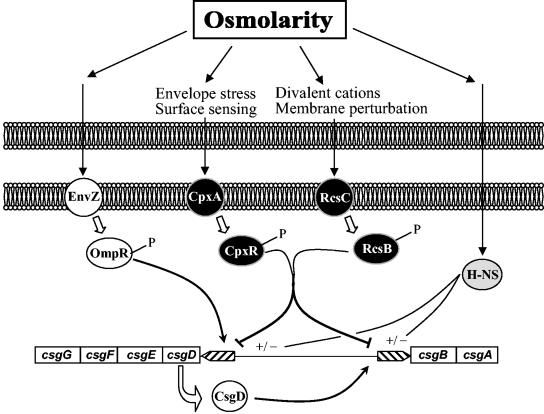
In response to osmolarity, a complex regulatory network controls initial adhesion and biofilm formation via the csgD gene. Such complexity could be justified by the challenge faced by bacteria: switching from a planktonic mode of growth to biofilm communities. We propose a step-by-step model of biofilm development integrating osmolarity microenvironmental changes. In medium of low to moderate osmolarity, csgD is activated by OmpR, which results in high curli production by swimming cells (G. Jubelin and P. Stewart, unpublished observations). Cells with curli then bind easily to the inert surface when approaching it. We suggest that the key problem in curli synthesis is the repression of csg transcription through multiple repressor systems responding to closely related stimuli (Fig. 7), such as (i) surface sensing, envelope stress, and high salt content for CpxR; (ii) osmolarity, desiccation, and membrane perturbation for RcsB; and (iii) high sucrose content for H-NS. In response to immobilization to a surface, cells encounter conditions of higher osmolarity (33, 46) and gene expression is largely modified (6). Under these conditions, the Cpx pathway is activated, and accumulation of CpxR-P allows the cooperative binding of these proteins to the csgD promoter region, resulting in the repression of curli synthesis. It can be hypothesized that transient escape from neighboring cells can occur, for example, upon cell division completion. In this case, the “free” cells can sense the surrounding liquid medium, allowing for OmpR-mediated activation of curli production. This may help to ensure the following anchoring to neighbor fixed cells through curli interactions, which will lead to new “stop” signals (contact and increasing osmolarity), turning the curli biogenesis back off.
FIG. 7.
Model of the regulatory network controlling curli production and biofilm formation in E. coli. Curli production at low osmolarity results from transcriptional activation of the csgD promoter by OmpR. When cells encounter high-salt medium, the Cpx pathway is activated. CpxR-P binds to the csgD promoter and consequently switches csgD expression off. The involvement of the regulatory systems EnvZ/OmpR, CpxA/CpxR, RcsC/RcsB, and H-NS in curli production is illustrated. Activation of all of these regulatory proteins is dependent on medium osmolarity. The different regulations are indicated by arrows for positive controls or via a line with a bar for negative controls. H-NS can exert either activation or repression, depending on the environmental conditions.
The determination of the molecular consequences of complex “osmoswitches,” such as the one analyzed here, will require further investigations. It is likely however, owing to the wide range of external solute concentration encountered by enterobacteria within normal gastrointestinal tract environment or during gut or urinary tract infections (51), that the ability to respond to osmotic conditions play a role in enterobacterial colonization of specific niches.
Acknowledgments
We thank Sylvie Reverchon and William Nasser for their interest in our work, helpful discussions, and the gift of strains; Géraldine Effantin for technical help; and Valerie James and Sandra Da Re for critical reading of the manuscript.
This study was supported by grants from the Centre National de la Recherche Scientifique (Réseau “Infections Nosocomiales”). C.B. and J.-M.G. are supported by the Institut Pasteur, Paris, France, and CNRS URA2172 grants.
REFERENCES
- 1.Arnqvist, A., A. Olsen, and S. Normark. 1994. Sigma S-dependent growth-phase induction of the csgBA promoter in Escherichia coli can be achieved in vivo by sigma 70 in the absence of the nucleoid-associated protein H-NS. Mol. Microbiol. 13:1021-1032. [DOI] [PubMed] [Google Scholar]
- 2.Atlung, T., and H. Ingmer. 1997. H-NS: a modulator of environmentally regulated gene expression. Mol. Microbiol. 24:7-17. [DOI] [PubMed] [Google Scholar]
- 3.Austin, J. W., G. Sanders, W. W. Kay, and S. K. Collinson. 1998. Thin aggregative fimbriae enhance Salmonella enteritidis biofilm formation. FEMS Microbiol. Lett. 162:295-301. [DOI] [PubMed] [Google Scholar]
- 4.Bading, H. 1988. Determination of the molecular weight of DNA-bound protein(s) responsible for gel electrophoretic mobility shift of linear DNA fragments exemplified with purified viral myb protein. Nucleic Acids Res. 16:5241-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardonnet, N., and C. Blanco. 1992. ′uidA-antibiotic-resistance cassettes for insertion mutagenesis, gene fusions and genetic constructions. FEMS Microbiol. Lett. 72:243-247. [DOI] [PubMed] [Google Scholar]
- 6.Beloin, C., J. Valle, P. Latour-Lambert, P. Faure, M. Kzreminski, D. Balestrino, J. A. Haagensen, S. Molin, G. Prensier, B. Arbeille, and J. M. Ghigo. 2004. Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Mol. Microbiol. 51:659-674. [DOI] [PubMed] [Google Scholar]
- 7.Ben Nasr, A., A. Olsen, U. Sjobring, W. Muller-Esterl, and L. Bjorck. 1996. Assembly of human contact phase proteins and release of bradykinin at the surface of curli-expressing Escherichia coli. Mol. Microbiol. 20:927-935. [DOI] [PubMed] [Google Scholar]
- 8.Bian, Z., A. Brauner, Y. Li, and S. Normark. 2000. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J. Infect. Dis. 181:602-612. [DOI] [PubMed] [Google Scholar]
- 9.Bian, Z., Z. Q. Yan, G. K. Hansson, P. Thoren, and S. Normark. 2001. Activation of inducible nitric oxide synthase/nitric oxide by curli fibers leads to a fall in blood pressure during systemic Escherichia coli infection in mice. J. Infect. Dis. 183:612-619. [DOI] [PubMed] [Google Scholar]
- 10.Bougdour, A., C. Lelong, and J. Geiselmann. 2004. Crl, a low temperature induced protein in Escherichia coli that binds directly to the stationary phase sigma subunit of RNA polymerase. J. Biol. Chem. 279:19540-19550. [DOI] [PubMed] [Google Scholar]
- 11.Brombacher, E., C. Dorel, A. J. Zehnder, and P. Landini. 2003. The curli biosynthesis regulator CsgD co-ordinates the expression of both positive and negative determinants for biofilm formation in Escherichia coli. Microbiology 149:2847-2857. [DOI] [PubMed] [Google Scholar]
- 12.Brown, P. K., C. M. Dozois, C. A. Nickerson, A. Zuppardo, J. Terlonge, and R. Curtiss III. 2001. MlrA, a novel regulator of curli (AgF) and extracellular matrix synthesis by Escherichia coli and Salmonella enterica serovar Typhimurium. Mol. Microbiol. 41:349-363. [DOI] [PubMed] [Google Scholar]
- 13.Chaveroche, M. K., J. M. Ghigo, and C. d'Enfert. 2000. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 28:E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 15.Collinson, S. K., L. Emody, K. H. Muller, T. J. Trust, and W. W. Kay. 1991. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J. Bacteriol. 173:4773-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derbise, A., B. Lesic, D. Dacheux, J. M. Ghigo, and E. Carniel. 2003. A rapid and simple method for inactivating chromosomal genes in Yersinia. FEMS Immunol. Med. Microbiol. 38:113-116. [DOI] [PubMed] [Google Scholar]
- 18.De Wulf, P., A. M. McGuire, X. Liu, and E. C. Lin. 2002. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277:26652-26661. [DOI] [PubMed] [Google Scholar]
- 19.Dorel, C., O. Vidal, C. Prigent-Combaret, I. Vallet, and P. Lejeune. 1999. Involvement of the Cpx signal transduction pathway of Escherichia coli in biofilm formation. FEMS Microbiol. Lett. 178:169-175. [DOI] [PubMed] [Google Scholar]
- 20.Elliott, T. 1992. A method for constructing single-copy lac fusions in Salmonella typhimurium and its application to the hemA-prfA operon. J. Bacteriol. 174:245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrieres, L., and D. J. Clarke. 2003. The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K-12 and controls the expression of a regulon in response to growth on a solid surface. Mol. Microbiol. 50:1665-1682. [DOI] [PubMed] [Google Scholar]
- 22.Gerstel, U., C. Park, and U. Romling. 2003. Complex regulation of csgD promoter activity by global regulatory proteins. Mol. Microbiol. 49:639-654. [DOI] [PubMed] [Google Scholar]
- 23.Gophna, U., M. Barlev, R. Seijffers, T. A. Oelschlager, J. Hacker, and E. Z. Ron. 2001. Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect. Immun. 69:2659-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govantes, F., A. V. Orjalo, and R. P. Gunsalus. 2000. Interplay between three global regulatory proteins mediates oxygen regulation of the Escherichia coli cytochrome d oxidase (cydAB) operon. Mol. Microbiol. 38:1061-1073. [DOI] [PubMed] [Google Scholar]
- 25.Hammar, M., A. Arnqvist, Z. Bian, A. Olsen, and S. Normark. 1995. Expression of two csg operons is required for production of fibronectin- and Congo red-binding curli polymers in Escherichia coli K-12. Mol. Microbiol. 18:661-670. [DOI] [PubMed] [Google Scholar]
- 26.Harrison-McMonagle, P., N. Denissova, E. Martinez-Hackert, R. H. Ebright, and A. M. Stock. 1999. Orientation of OmpR monomers within an OmpR:DNA complex determined by DNA affinity cleaving. J. Mol. Biol. 285:555-566. [DOI] [PubMed] [Google Scholar]
- 27.Herwald, H., M. Morgelin, A. Olsen, M. Rhen, B. Dahlback, W. Muller-Esterl, and L. Bjorck. 1998. Activation of the contact-phase system on bacterial surfaces: a clue to serious complications in infectious diseases. Nat. Med. 4:298-302. [DOI] [PubMed] [Google Scholar]
- 28.Hommais, F., E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertuy, J. P. Le Caer, A. Danchin, and P. Bertin. 2001. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol. Microbiol. 40:20-36. [DOI] [PubMed] [Google Scholar]
- 29.Jubelin, G., C. Dorel, and P. Lejeune. Role of biofilms in infections caused by Escherichia coli. In J. Pace, M. Rupp, and R. Finsh (ed.), Biofilm infections: roles of antibiotic tolerance and resistance, in press. Marcel Dekker, New York, N.Y.
- 30.Kenney, L. J., M. D. Bauer, and T. J. Silhavy. 1995. Phosphorylation-dependent conformational changes in OmpR, an osmoregulatory DNA-binding protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 92:8866-8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knobloch, J. K., K. Bartscht, A. Sabottke, H. Rohde, H. H. Feucht, and D. Mack. 2001. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183:2624-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurent-Winter, C., P. Lejeune, and A. Danchin. 1995. The Escherichia coli DNA-binding protein H-NS is one of the first proteins to be synthesized after a nutritional upshift. Res. Microbiol. 146:5-16. [DOI] [PubMed] [Google Scholar]
- 33.Lejeune, P. 2003. Contamination of abiotic surfaces: what a colonizing bacterium sees and how to blur it. Trends Microbiol. 11:179-184. [DOI] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Novak, U., and L. Paradiso. 1995. Identification of proteins in DNA-protein complexes after blotting of EMSA gels. BioTechniques 19:54-55. [PubMed] [Google Scholar]
- 36.Olsen, A., A. Arnqvist, M. Hammar, S. Sukupolvi, and S. Normark. 1993. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol. Microbiol. 7:523-536. [DOI] [PubMed] [Google Scholar]
- 37.Olsen, A., A. Jonsson, and S. Normark. 1989. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature 338:652-655. [DOI] [PubMed] [Google Scholar]
- 38.Olsen, A., M. J. Wick, M. Morgelin, and L. Bjorck. 1998. Curli, fibrous surface proteins of Escherichia coli, interact with major histocompatibility complex class I molecules. Infect. Immun. 66:944-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen, A. G., L. J. Jensen, S. Brunak, H. H. Staerfeldt, and D. W. Ussery. 2000. A DNA structural atlas for Escherichia coli. J. Mol. Biol. 299:907-930. [DOI] [PubMed] [Google Scholar]
- 41.Perini, L. T., E. A. Doherty, E. Werner, and D. F. Senear. 1996. Multiple specific CytR binding sites at the Escherichia coli deoP2 promoter mediate both cooperative and competitive interactions between CytR and cAMP receptor protein. J. Biol. Chem. 271:33242-33255. [DOI] [PubMed] [Google Scholar]
- 42.Pogliano, J., A. S. Lynch, D. Belin, E. C. Lin, and J. Beckwith. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11:1169-1182. [DOI] [PubMed] [Google Scholar]
- 43.Pratt, L. A., and T. J. Silhavy. 1995. Porin regulon of Escherichia coli, p. 105-126. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, D.C.
- 44.Prigent-Combaret, C., E. Brombacher, O. Vidal, A. Ambert, P. Lejeune, P. Landini, and C. Dorel. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 183:7213-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prigent-Combaret, C., G. Prensier, T. T. Le Thi, O. Vidal, P. Lejeune, and C. Dorel. 2000. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli, and colanic acid. Environ. Microbiol. 2:450-464. [DOI] [PubMed] [Google Scholar]
- 46.Prigent-Combaret, C., O. Vidal, C. Dorel, and P. Lejeune. 1999. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J. Bacteriol. 181:5993-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romling, U., Z. Bian, M. Hammar, W. D. Sierralta, and S. Normark. 1998. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J. Bacteriol. 180:722-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romling, U., W. Bokranz, U. Gerstel, H. Lunsdorf, M. Nimtz, W. Rabsch, H. Tschape, and X. Zogaj. 2003. Dissection of the genetic pathway leading to multicellular behavior in Salmonella enterica serotype thyphimurium and other Enterobacteriaceae, p. 231-261. In M. Wilson and D. Devine (ed.), Medical implications of biofilms. Cambridge University Press, Cambridge, United Kingdom.
- 49.Romling, U., M. Rohde, A. Olsen, S. Normark, and J. Reinkoster. 2000. AgfD, the checkpoint of multicellular and aggregative behavior in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 36:10-23. [DOI] [PubMed] [Google Scholar]
- 50.Romling, U., W. D. Sierralta, K. Eriksson, and S. Normark. 1998. Multicellular and aggregative behavior of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249-264. [DOI] [PubMed] [Google Scholar]
- 51.Ross, D. L. 1983. Textbook of urinalysis and bodily fluids. Appleton-Century-Crofts, Norwalk, Conn.
- 52.Schroder, O., and R. Wagner. 2002. The bacterial regulatory protein H-NS-a versatile modulator of nucleic acid structures. Biol. Chem. 383:945-960. [DOI] [PubMed] [Google Scholar]
- 53.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 54.Sjobring, U., G. Pohl, and A. Olsen. 1994. Plasminogen, absorbed by Escherichia coli expressing curli or by Salmonella enteritidis expressing thin aggregative fimbriae, can be activated by simultaneously captured tissue-type plasminogen activator (t-PA). Mol. Microbiol. 14:443-452. [DOI] [PubMed] [Google Scholar]
- 55.Sledjeski, D. D., and S. Gottesman. 1996. Osmotic shock induction of capsule synthesis in Escherichia coli K-12. J. Bacteriol. 178:1204-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vianney, A., G. Jubelin, S. Renault, C. Dorel, P. Lejeune, and J. C. Lazzaroni. Escherichia coli tol-pal and rcs genes affect curli synthesis. Microbiology, submitted for publication. [DOI] [PubMed]
- 57.Vidal, O., R. Longin, C. Prigent-Combaret, C. Dorel, M. Hooreman, and P. Lejeune. 1998. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 180:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]