Abstract
Bjerkandera sp. strain BOS55 is a white rot fungus that can bleach EDTA-extracted eucalyptus oxygen-delignified kraft pulp (OKP) without any requirement for manganese. Under manganese-free conditions, additions of simple physiological organic acids (e.g., glycolate, glyoxylate, oxalate, and others) at 1 to 5 mM stimulated brightness gains and pulp delignification two- to threefold compared to results for control cultures not receiving acids. The role of the organic acids in improving the manganese-independent biobleaching was shown not to be due to pH-buffering effects. Instead, the stimulation was attributed to enhanced production of manganese peroxidase (MnP) and lignin peroxidase (LiP) as well as increased physiological concentrations of veratryl alcohol and oxalate. These factors contributed to greatly improved production of superoxide anion radicals, which may have accounted for the more extensive biobleaching. Optimum biobleaching corresponded most to the production of MnP. These results suggest that MnP from Bjerkandera is purposefully produced in the absence of manganese and can possibly function independently of manganese in OKP delignification. LiP probably also contributed to OKP delignification when it was present.
White rot fungi produce extracellular oxidative enzymes which initiate the oxidation of lignin (10, 27). Due to their lignin-degrading capacity, whole cultures of various white rot fungi cause extensive brightness gains and delignification of kraft pulp (21, 29, 40, 42). Of all the extracellular oxidative enzymes produced by white rot fungi, manganese peroxidase (MnP), lignin peroxidase (LiP), manganese-independent peroxidase (MIP), and laccase, MnP is considered to be the most important enzyme involved in kraft biobleaching. The hypothesis is supported by several observations. A partial correlation between MnP activity and biobleaching has been found in several screening programs (2, 21, 40). In time course experiments, the occurrence of MnP coincides with the period of maximum brightness gains and delignification (23, 43). MnP-deficient mutants of the best-characterized biobleaching species, Trametes versicolor, are not able to cause bleaching, and the bleaching activity can be restored by the exogenous addition of MnP (3). Semipurified preparations of MnP have been shown to cause kraft pulp delignification in vitro (19, 24, 28, 43, 45). Furthermore, prior extraction of manganese (Mn) from kraft pulp results in the complete loss of biobleaching activity by white rot fungi (22, 44).
Recently, we demonstrated that the biobleaching activity of the white rot fungus Bjerkandera sp. strain BOS55 toward oxygen-delignified kraft pulp (OKP) was not dependent on the presence of Mn (39). Even when kraft pulp was extracted free of Mn by EDTA, it was bleached as extensively as pulp supplemented with Mn. In spite of the lack of Mn, MnP was found to be the major oxidative enzyme present in fast protein liquid chromatograms; LiP and MIP were also present. The production of MnP by Bjerkandera sp. strain BOS55 in the absence of Mn nutrients is remarkable because most white rot fungi require Mn for mnp gene expression and protein production (7, 17). The results suggested that under Mn-deficient conditions, MnP from Bjerkandera may have roles in pulp biobleaching.
The addition of physiological organic acids to cultures of Bjerkandera sp. strain BOS55 was shown to stimulate the production of MnP (37). Here we report on the stimulatory effects of simple organic acids on the Mn-independent biobleaching of eucalyptus OKP. The role of the organic acids in oxidative enzyme production and secondary metabolite concentrations was evaluated and compared to pulp brightness gains and decreases in kappa number.
MATERIALS AND METHODS
Microorganisms.
Bjerkandera sp. strain BOS55 (ATCC 90940) was maintained and an inoculum was prepared as described before (36).
Culture conditions.
The standard medium used contained 10 g of glucose per liter, 2.2 mM NH4+-N as ammonium tartrate, and BIII mineral medium (including a trace element solution) (54). The trace element solution was modified to provide an initial added Mn concentration of 0, 33, or 330 μM Mn(II) in the medium as MnSO4. The addition of 2,2-dimethylsuccinate (DMS) buffer to the medium was omitted, unless otherwise stated. In some experiments, organic acids were added to the medium at 1 to 50 mM. The pH of the organic acid-supplemented medium was adjusted to 4.5. Control cultures not receiving organic acids were adjusted to either pH 4.5 or pH 6.0 with NaOH. The medium was autoclaved (120°C for 20 min), and then a filter-sterilized thiamine solution (400 mg liter−1) was added (5 ml liter−1). Aliquots of 25 ml of medium were placed in a presterilized cultivation system as described below and were incubated statically in air at 27°C for 14 days.
The glassware for experiments conducted under Mn-free culture conditions was washed thoroughly with HNO3 (5 M) and then with double-distilled demineralized water to prevent Mn contamination.
The maximal soluble Mn concentration of the Mn-free trace element solution was 0.423 μM and, when diluted into the final biobleaching assay medium, accounted for approximately 0.004 μM. Direct measurements of soluble Mn in Mn-free assay medium containing up to 5 mM organic acid supplementation showed a maximal concentration of 0.010 μM. The concentrations of soluble Mn in assay medium supplied with 50 mM organic acid ranged from 0.041 to 0.072 μM.
Biobleaching assays.
OKP from Eucalyptus globulus was obtained as handsheets (60 g m−2) from a kraft mill in Pontevedra, Spain (ENCE). The OKP had a kappa number of 8.6 and a pulp brightness of 62 ISO (International Organization for Standardization) units. In experiments conducted with Mn-free medium, the handsheets were extracted with EDTA prior to fungal treatment to remove Mn present in the pulp (39). The residual Mn contamination in the pulp after EDTA extraction was determined previously to be 0.38 mg kg−1 (39), which would contribute 0.024 μM Mn in the biobleaching medium.
Fungal biobleaching of 3.8- by 3.8-cm2 pulp handsheets was assayed as described previously (39). All assays were carried out in quadruplicate, with the exception of the time course experiment, which was conducted with six replicates. Enzyme activities were monitored on days 6, 9, and 14 of incubation, unless otherwise indicated. Day-zero abiotic controls were obtained after 12 h of incubation of the handsheets in sterile medium. Before pulp brightness and kappa numbers were measured, the handsheets were extracted with oxalic acid for 1 h in order to remove precipitated MnO2 (38).
Enzyme assays.
MnP and MIP activities were measured by the oxidation of 2,6-dimethoxyphenol to coerulignone (E469, 49,600 M−1 cm−1) as described by De Jong et al. (11). LiP activity was measured by the oxidation of veratryl alcohol to veratraldehyde (E310, 9,300 M−1 cm−1) (54) and was corrected for veratryl alcohol oxidase activity. Aryl alcohol oxidase (AAO) activity was monitored spectrophotometrically as described by Muheim et al. (41) by monitoring the oxidation of p-anisyl alcohol to p-anisaldehyde (E290, 15,000 M−1 cm−1).
TNM reduction.
The formation of superoxide anion radical was measured by a modified method based on the reduction of tetranitromethane (TNM) to trinitromethane (E350, 14,600 M−1 cm−1) by extracellular fluids (56). The extracellular fluids were recovered from biobleaching cultures at selected times over 14 days and were centrifuged (12,000 × g, 10 min). Aliquots (1 ml) of extracellular fluids were incubated at 30°C in a quartz cuvette together with 1 mM TNM. The increase in absorbance at 350 nm was monitored for several minutes and is reported as nanomoles of superoxide formed per milliliter per minute (e.g., units liter−1). No absorbance increase was detected in boiled extracellular fluids.
Determination of H2O2 concentration.
Centrifuged extracellular fluids were incubated for 20 min at 80°C in order to inactivate the enzymes present. The H2O2 concentration was determined by a modification of the method described by Pick and Keisari (46). The reaction mixture (1 ml) contained 150 μl of culture fluid, 200 μl of H2O, 200 μl of 0.5 M sodium phosphate buffer (pH 6), and 200 μl of 1.41 mM diammonium 2,2′-azinobis(3-ethyl-6-benzothiazoline sulfonate) (ABTS) as a substrate. The reaction was started by the addition of 200 μl of 5 U of horseradish peroxidase (Boehringer GmbH, Mannheim, Germany) ml−1. The maximal absorbance at 420 nm was measured after the peroxidase addition. As a blank, the same reaction mixture was used after preincubation of the sample with 6 U of catalase from Aspergillus niger (Sigma, St. Louis, Mo.). A calibration curve was established with known concentrations of H2O2.
FPLC.
Concentration of the extracellular culture fluid and fast protein liquid chromatography (FPLC) of the concentrated filtrate were done as described previously (39), with the exception that the proteins were eluted with a linear gradient of sodium acetate (pH 6.0) up to 450 mM over 45 min.
Analytical techniques.
Handsheet brightness was determined with a CM-508i spectrophotometer (Minolta Camera Benelux, Maarssenbroek, The Netherlands) by method T452 om-92 of the Technical Association of the Pulp and Paper Industry, Atlanta, Ga. The brightness is reported as the biologically mediated brightness gain (BMBG), which is the brightness of the fungus-treated OKP handsheet minus the brightness of the OKP handsheet incubated in parallel in sterile culture medium of the same composition. The change in pulp brightness with the sterile incubation was less than 2% (ISO units).
The kappa number of pulp samples was determined with potassium permanganate according to the micro method (6).
The Mn concentration was determined with membrane-filtered (0.2-μm-pore-size filter; Millipore) liquid samples that were acidified to pH 2 with HNO3 by inductively coupled plasma mass spectrometry at 257.6 nm (Elan 6000; The Perkin-Elmer Corp.–Meyvis en Co., Bergen op Zoom, The Netherlands). The detection limit of this technique for Mn was 0.002 μM.
Organic acids were analyzed by high-performance liquid chromatography with an Aminex HPX-87H column (Bio-Rad, Veenendaal, The Netherlands) at 40°C (thermostat controlled). The eluent was H2SO4 (5 mM) at a flow rate of 0.6 ml min−1. Organic acids were monitored in a UV detector at 210 nm. Compound identification was carried out by matching retention times of the observed products with their standards. The identity of oxalate was confirmed by the disappearance of the corresponding peak in the high-performance liquid chromatograms of selected samples preincubated with oxalate oxidase (Sigma).
Veratryl alcohol was analyzed by high-performance liquid chromatography by use of a previously described method (35).
Chemicals.
All chemicals were commercially available and were used without further purification.
RESULTS
Role of organic acid supplementation.
Biobleaching of OKP and MnP production by Bjerkandera sp. strain BOS55 were compared by use of culture medium without and with supplementation with glycolate (5 mM) and with four different Mn nutrient regimens. These included two Mn-free media with EDTA-extracted OKP, normal OKP, and OKP supplemented with 33 and 330 μM MnSO4. The measured soluble Mn concentration in the Mn-free media with EDTA-extracted OKP and normal OKP was less than 0.010 μM. Theoretically, the insoluble Mn content of the pulp contributed 0.024 and 1.480 μM Mn when expressed as a concentration in the media, respectively. The results in Table 1 demonstrate that the Mn-free media lacking glycolate supported limited biobleaching and only traces of MnP activity. On the other hand, with glycolate supplementation, the brightness gains were comparable to those in media containing Mn nutrients. Also, the titers of MnP were highly stimulated both in the presence and in the absence of Mn. The presence of Mn supported limited oxalate production, whereas oxalate was not detectable at all in the basal media without Mn or organic acid supplementation (Table 1). The inclusion of glycolate in the culture medium greatly stimulated oxalate production in both the absence and the presence of Mn nutrients. However, the oxalate concentration was higher when Mn was present.
TABLE 1.
Effect of glycolate on the manganese dependency of biobleaching of eucalyptus OKP by the white rot fungus Bjerkandera sp. strain BOS55a
| Medium and sample | BMBG (ISO units) in:
|
Peak MnP concn (U liter−1) in:
|
Oxalate concn (μM) on day 12 in expt 1 | ||
|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 1 | Expt 2 | ||
| No acid | |||||
| EDTA-extracted OKP | 3.8 ± 1.0 | 5.9 ± 1.2 | 1.9 ± 1.9 | 2.3 ± 0.5 | 0 |
| OKP | 6.3 ± 1.5 | NM | 0.5 ± 0.1 | NM | 0 |
| OKP + 33 μM Mn | 15.4 ± 0.6 | 11.2 ± 1.9 | 30.0 ± 9.5 | 34.9 ± 5.4 | 141 ± 31 |
| OKP + 330 μM Mn | NM | 10.2 ± 0.4 | NM | 74.5 ± 0.9 | NM |
| 5 mM Glycolate | |||||
| EDTA-extracted OKP | 11.9 ± 1.0 | 12.6 ± 1.1 | 21.0 ± 1.7 | 20.7 ± 2.9 | 587 ± 110 |
| OKP | 11.5 ± 1.3 | NM | 12.3 ± 2.4 | NM | 622 ± 62 |
| OKP + 33 μM Mn | 13.0 ± 2.0 | 15.9 ± 0.7 | 67.8 ± 13.9 | 73.6 ± 4.9 | 1070 ± 28 |
| OKP + 330 μM Mn | NM | 12.8 ± 0.9 | NM | 116.3 ± 28.2 | NM |
Data are averages for triplicate cultures ± standard deviations. NM, not measured.
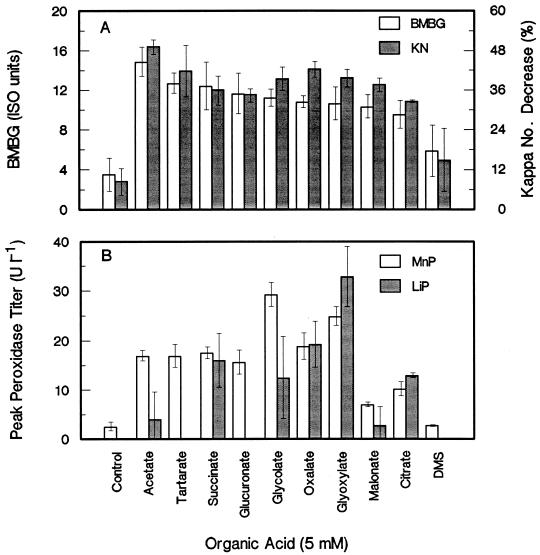
Aside from glycolate, a large number of other physiological organic acids supplied at 5 mM supported the stimulation of brightness gains and decreases in kappa number (Fig. 1A) as well as the stimulation of MnP activity (Fig. 1B) in the absence of Mn. Some of the acids, such as succinate, glycolate, oxalate, glyoxylate, and citrate, also markedly induced LiP activity. A nonphysiological acid, DMS, had only a marginal stimulatory effect on biobleaching and had no effect on MnP titers. However, DMS at 20 mM supported limited biobleaching stimulation, accounting for a BMBG of approximately 8.5 ISO units in various experiments (results not shown).
FIG. 1.
Effect of different organic acids supplied at 5 mM on BMBG and percent kappa number (KN) decrease (A) and on the peak titers of MnP and LiP (B) in manganese-free biobleaching cultures with EDTA-extracted pulp. Error bars indicate standard deviations.
Many of the physiological organic acids were metabolized during the course of the experiment (Table 2). Acetate, glycolate, glyoxylate, citrate, and succinate supplementation resulted in the formation of 0.10 to 0.76 mM oxalate. Media with acetate, glyoxylate, malonate, and succinate also resulted in the formation of 0.25 to 0.42 mM citrate. Furthermore, it was observed that succinate was converted stoichiometrically to acetate prior to being metabolized to citrate and oxalate.
TABLE 2.
Effect of adding different organic acids to biobleaching cultures of Bjerkandera sp. strain BOS55 on the formation of other organic acid metabolites under culture conditions lacking Mna
| Organic acid treatment (5 mM) | Eliminationb (%) | Concn (mM)c of:
|
||
|---|---|---|---|---|
| Oxalate | Citrate | Acetate | ||
| Acetate | 100 | 0.12 ± 0.06 | 0.40 ± 0.01 | |
| Glycolate | 100 | 0.76 ± 0.04 | ||
| Glyoxylate | 100 | 0.67 ± 0.00 | 0.42 ± 0.01 | |
| Citrate | 25 | 0.10 ± 0.02 | ||
| Oxalate | 30 | |||
| Malonate | 36 | 0.26 ± 0.14 | ||
| Tartrate | 0 | |||
| Glucuronate | 49 | |||
| Succinate | 100 | 0.36 ± 0.09 | 0.25 ± 0.15 | 9.7 ± 0.4 |
| DMS | 0 | |||
Data are averages for triplicate cultures ± standard deviations.
Percent decrease in the exogenously added organic acid level after 14 days.
Peak concentration of organic acid metabolite formed during 14 days of incubation (measured on days 6, 9, and 14).
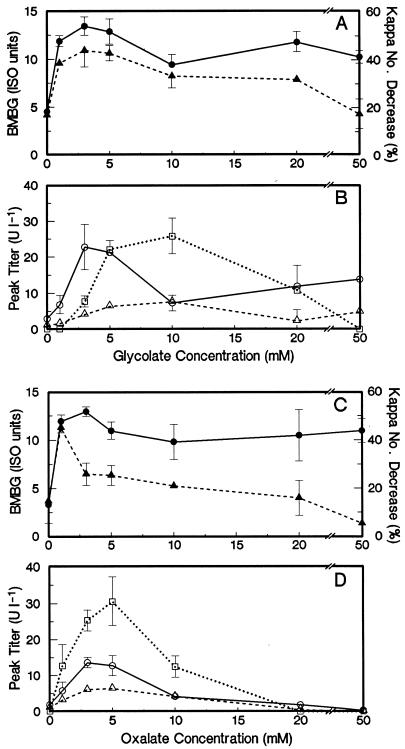
Effect of organic acid concentration.
The effect of organic acid concentration on brightness gains and decreases in kappa number in the absence of Mn was studied for glycolate and oxalate. The results are shown in Fig. 2. The optimum glycolate concentration range for brightness gains and decreases in kappa number was 1 to 5 mM. At higher glycolate concentrations, the percent decrease in kappa number (delignification) was reduced, while the brightness gains were affected less. The trend for MnP activity followed that for biobleaching. LiP activity was present in media supplied with 3 to 20 mM glycolate. However, there was no measurable LiP activity at 1 mM glycolate, which supported extensive brightness gains and delignification. MIP was produced to a lesser extent than the other enzymes, with optimal activities in media with 5 to 10 mM glycolate added.
FIG. 2.
Effect of the initial glycolate concentration (A and B) and oxalate concentration (C and D) on biobleaching and oxidative enzyme activities in manganese-free biobleaching cultures with EDTA-extracted pulp. (A and C) BMBG (•) and percent kappa number decrease (▴). (B and D) Peak titers of MnP (○), LiP (□), and MIP (▵). Error bars indicate standard deviations.
Oxalic acid supplementation from 1 to 50 mM supported extensive brightness gains; however, there was a sharp optimum at 1 mM oxalate for delignification. Beyond 1 mM, delignification steadily decreased with increasing oxalate concentrations. MnP, LiP, and MIP activities were present in media supplemented with 1 mM oxalate; however, optimal activities were observed with 3 to 5 mM oxalate. These enzymes were present only at trace levels or were nondetectable with 20 to 50 mM oxalate. At these high oxalate concentrations, brightness gains were clearly not due to delignification.
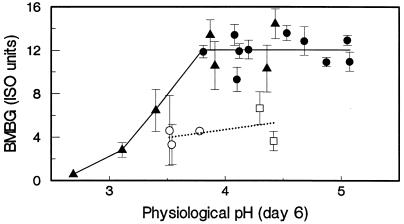
Optimal physiological pH for biobleaching.
The basal medium used in these studies did not contain buffer. Consequently, the pH in control cultures dropped during the experiments to values ranging from 3.5 to 3.7. On the other hand, the pH in organic acid-supplemented cultures ranged from 3.8 to 5.0 during the experiments. Consequently, the stimulatory effect of the organic acids on biobleaching and oxidative enzyme production could have been due to their pH-buffering effect. To exclude this possibility, control cultures were set at an initial pH of 6.0, which resulted in a drop to pH 4.4 to 4.5. The results shown in Fig. 3 indicate that the stimulatory effect of the organic acids was not due to their pH-buffering effect. The control cultures set at pH 6.0 did not support the stimulation of brightness gains, even though the physiological pH was comparable to that of the organic acid treatments. The physiological pH was defined as the pH on day 6, which was usually the lowest pH observed during the time period of biobleaching. The pH optima of biobleaching were studied by supplementing cultures with 5 mM tartrate and adjusting the initial pH from 3.0 to 6.0. The brightness gains were plotted as a function of the physiological pH together with data from other experiments with and without organic acid supplementation (Fig. 3). The data showed a broad optimal pH range (3.8 to 5.0) for biobleaching by Bjerkandera sp. strain BOS55 in the Mn-free, organic acid-supplemented cultures.
FIG. 3.
Effect of physiological pH on BMBG. Results are shown for control cultures not receiving organic acids and initially set at pH 4.5 (○) and at pH 6.0 (□). Results are also shown for cultures receiving from 1 to 5 mM organic acids and initially set at pH 4.5 (•) and for cultures receiving 5 mM tartrate and initially set at pHs ranging from 3.0 to 6.0 (▴). All data are from biobleaching experiments with manganese-free medium and EDTA-extracted OKP. The trend for control cultures is indicated by the dotted line, and the trend for organic acid-supplemented cultures is indicated by the solid line. Error bars indicate standard deviations.
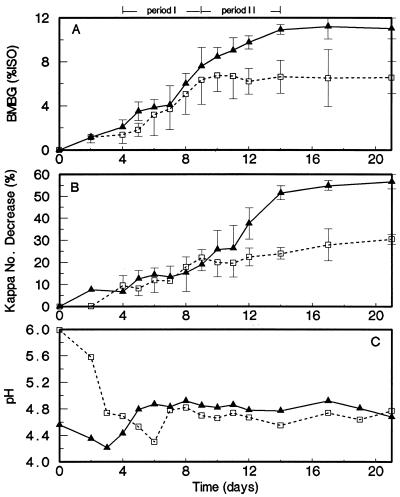
Time course of biobleaching.
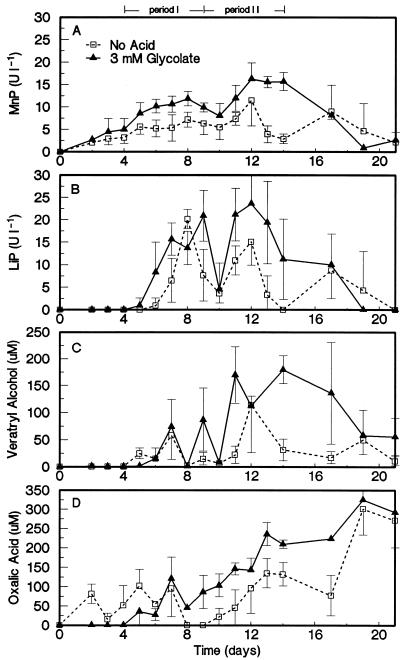
The time course of biobleaching was studied in a control culture set at an initial pH of 6.0 and in a culture treated with 3 mM glycolate and set at an initial pH of 4.5. The brightness gains and percent decrease in kappa numbers during 21 days of incubation are shown in Fig. 4. The pHs of both cultures were almost equal (pH 4.6 to 4.8) from day 7 onward. The biobleaching occurred in two distinct periods. In the first period, from days 4 to 9, brightness gains and delignification were observed in both the control and the glycolate-supplemented cultures. However, in the second period, from days 9 to 14, brightness gains and delignification continued in the glycolate-supplemented culture but not in the control culture. Moreover, the delignification was more extensive in the latter period.
FIG. 4.
BMBG (A), percent kappa number decrease (B), and pH (C) during the time course experiment in manganese-free biobleaching cultures with EDTA-extracted pulp. The control culture not receiving organic acids was set at an initial pH of 6.0 (□). The glycolate (3 mM)-amended culture was set at an initial pH of 4.5 (▴). Error bars indicate standard deviations.
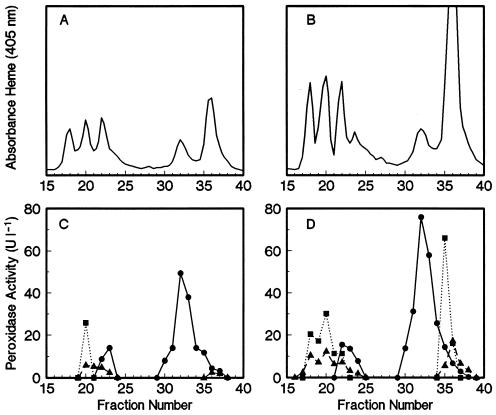
Oxidative enzyme activities of the ligninolytic system of Bjerkandera sp. strain BOS55 were measured for comparison as a function of biobleaching time (Fig. 5). MnP and LiP were the major oxidative enzymes present during the two periods of biobleaching. To a lesser extent, MIP activities were also detectable (Table 3). All of these enzyme activities were approximately two- to threefold higher in the glycolate-supplemented culture than in the control culture. Fast protein liquid chromatograms of extracellular heme proteins in 9-day-old cultures (Fig. 6) clearly illustrated the stimulated production of active MnP, LiP, and MIP. Similar chromatograms of 6-day-old cultures revealed that at that time, LiP was not detectable and an even greater stimulatory effect of the organic acids on MnP production was noted (results not shown). In all chromatograms, most of the MnP activity was associated with the heme protein in fractions 31 to 34.
FIG. 5.
Extracellular activities of MnP (A) and LiP (B) as well as physiological concentrations of veratryl alcohol (C) and oxalic acid (D) during the time course experiment in manganese-free biobleaching cultures with EDTA-extracted pulp. The control culture not receiving organic acids was set at an initial pH of 6.0 (□). The glycolate (3 mM)-amended culture was set at an initial pH of 4.5 (▴). Error bars indicate standard deviations.
TABLE 3.
BMBG and kappa number (KN) decrease rates, average enzyme titers, and average secondary metabolite concentrations for two different time periods of the biobleaching time course experiment
| Period and culture | BMBG rate (change in ISO units day−1) | KN decrease rate (% change in KN day−1) | pH | Level of:
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme (U liter−1)
|
Metabolite (μM)
|
|||||||||
| MnP | LiP | MIP | AAO | H2O2 | Veratryl alcohol | Oxalate | ||||
| I (days 4–9) | ||||||||||
| Control | 1.0 | 2.6 | 4.6 | 5.4 | 5.9 | 2.3 | 0.7 | 1.1 | 19 | 51 |
| Glycolate (3 mM) | 1.0 | 2.0 | 4.8 | 9.3 | 9.9 | 3.3 | 0.5 | 1.5 | 30 | 52 |
| II (days 9–14) | ||||||||||
| Control | 0.0 | 0.6 | 4.7 | 6.2 | 6.8 | 2.4 | 0.1 | 2.6 | 38 | 72 |
| Glycolate (3 mM) | 0.7 | 6.0 | 4.8 | 12.9 | 16.8 | 5.6 | 0.2 | 1.4 | 111 | 153 |
FIG. 6.
(A and B) FPLC profiles of heme proteins of 9-day-old extracellular fluids from the control culture (A) and the 3 mM glycolate-amended culture (B) from the time course experiment. (C and D) Peroxidase activities in collected fractions for the control culture (C) and the glycolate-amended culture (D). Symbols: •, MnP; ▪, LiP; ▴, MIP.
The concentrations of two important secondary metabolites implicated in the ligninolytic system, veratryl alcohol and oxalate, were also monitored (Fig. 5). These metabolites were found at higher concentrations in the glycolate-supplemented culture than in the control culture. Their average concentration was two- to threefold higher during the second period of biobleaching in the glycolate-supplemented culture, which contained 111 μM veratryl alcohol and 153 μM oxalate (Table 3). The physiological concentration of H2O2 during biobleaching ranged from 1.1 to 2.6 μM and was not affected much by the organic acid supplementation (Table 3). During the periods when biobleaching occurred, only low levels of AAO activity were detected (Table 3).
Role of organic acids in superoxide production.
The extracellular fluids of selected biobleaching cultures were monitored periodically for the production of superoxide anion by measuring rates of TNM reduction. The results shown in Table 4 indicate that organic acids dramatically stimulated the production of superoxide. Rates of up to approximately 0.5 U liter−1 were detected in oxalate-supplemented cultures. On the other hand, no superoxide production or otherwise comparatively low rates of superoxide production were detected in control cultures set at an initial pH of 4.5 or 6.0, respectively.
TABLE 4.
Effect of adding different organic acids to Mn-free biobleaching cultures of Bjerkandera sp. strain BOS55 on the peak rate of superoxide production measured on the basis of TNM reductiona
| Organic acid treatment (mM) | Initial pH | Superoxide produced (U liter−1) |
|---|---|---|
| None | 4.5 | 0.00 |
| None | 6.0 | 0.08 ± 0.06 |
| Glycolate (3) | 4.5 | 0.30 ± 0.21 |
| Glycolate (5) | 4.5 | 0.39 ± 0.06 |
| Oxalate (5) | 4.5 | 0.46 ± 0.06 |
| Acetate (5) | 4.5 | 0.36 ± 0.09 |
| Tartrate (5) | 4.5 | 0.21 ± 0.16 |
Data are averages for triplicate cultures ± standard deviations.
DISCUSSION
The results of this work demonstrate that simple physiological organic acids are important components in the Mn-independent biobleaching system of Bjerkandera. The addition of organic acids to Mn-free biobleaching cultures at concentrations of as low as 1 to 3 mM permitted extensive delignification (50%) and brightness gains (13 ISO units) for eucalyptus OKP. The brightness gains and delignification obtained by adding organic acids were comparable to those obtained by adding Mn to the culture medium (39, 40).
Four possible stimulatory roles of the organic acids in the Mn-independent pulp biobleaching system can be considered. These include pH-buffering effects of the organic acids and the stimulated production of extracellular peroxidases, secondary metabolites, and reduced oxygen radicals by the organic acids.
pH-buffering effects of organic acids.
Organic acids provided better pH buffering of the culture medium than what was found in control cultures. However, the stimulatory effect of the acids was not due solely to pH regulation. Biobleaching and the production of all the vital components of the ligninolytic system (peroxidases and secondary metabolites) were stimulated in organic acid-supplemented cultures with an initial pH of 4.5 compared to control cultures with an initial pH of 6.0 (to provide a similar physiological pH). Furthermore, 5 mM DMS, a nonphysiological acid, had no noteworthy stimulatory effect on biobleaching or enzyme production.
Role of organic acids in extracellular peroxidase production.
In parallel with biobleaching, the activities and production of isozymes from three families of extracellular peroxidases (MnP, LiP, and MIP) were also highly stimulated by the exogenous organic acid supplements. Although Mn stimulates MnP production by Bjerkandera sp. strain BOS55, MnP is still produced at appreciable levels in the absence of Mn (35, 37). This behavior is distinct from that of many other white rot fungi having an absolute requirement for Mn for the expression of active MnP production (7, 17).
Previous work showed that simple organic acids added to Mn-containing medium stimulated MnP production in Bjerkandera sp. strain BOS55 (37). In this study, a similar pattern of stimulation was observed under the Mn-free biobleaching conditions used. The highest MnP activity (30 U liter−1) was obtained with 5 mM glycolate. The mechanism of stimulation does not appear to be unique to MnP, since LiP production was also induced by oxalate or oxalate precursors. A plausible mechanism is that gene expression occurs in response to reduced oxygen radicals derived from organic acids. Oxidative stress caused by O2 and H2O2 has been shown to stimulate mnp gene transcription in Phanerochaete chrysosporium (32).
Effect of organic acids on secondary metabolite concentration.
The physiological concentrations of oxalate and veratryl alcohol were also stimulated by the organic acids. These secondary metabolites have several roles in lignin degradation (50, 52). Veratryl alcohol not only protects LiP from H2O2 inactivation (8) but also has cofactor and mediator roles in LiP catalysis as well (50). Veratryl alcohol was a requirement for LiP to depolymerize soluble radiolabelled synthetic lignin during in vitro experiments (18). Also, veratryl alcohol was required for the biobleaching of OKP by a semipurified preparation of LiP from P. chrysosporium (4). The unbound veratryl alcohol cation radical is too short-lived (0.5 ms) to behave as a diffusible oxidant and thus cannot account for the oxidation of poorly accessible lignin in pulp fibers (26). Nonetheless, there is good evidence that lignin in OKP is more highly exposed and accessible to LiP than is lignin in unbleached kraft pulp (4).
The roles of veratryl alcohol in LiP catalysis can be extended to include certain types of MnP isozymes capable of veratryl alcohol oxidation. MnP isozymes with this property have been described for two white rot fungal genera, Lentinus edodes and Pleurotus spp. (9, 34, 49). While the veratryl alcohol-oxidizing capacity of L. edodes was found to be dependent on the presence of Mn, MnP from Pleurotus spp. directly oxidizes veratryl alcohol in the absence of Mn.
Most of the organic acids tested in this study are de novo metabolites detected in white rot fungi or have been detected in kraft pulp biobleaching cultures (13, 31, 48, 53, 58). Many of the organic acids, e.g., acetate, glycolate, glyoxylate, citrate, and succinate, served as precursors for the formation of oxalate in Bjerkandera sp. strain BOS55. Oxalate is a common secondary metabolite of many white rot fungi (13, 53). Two enzymes in basidiomycetes are responsible for the formation of oxalate: oxalacetase or glyoxylate oxidase (12, 53). Oxalacetase is an Mn-dependent enzyme, which would account for the stimulated production of oxalate under culture conditions with 33 μM Mn (Table 1). In media lacking Mn, the formation of oxalate most likely can be accounted for by glyoxylate oxidase. Consistent with these hypotheses was the finding that glyoxylate and the structurally related glycolate were the best precursors for oxalate formation (Table 2).
Effect of organic acids on reduced oxygen radicals.
Reduced oxygen species can be formed from the oxidation of oxalate and other physiological organic acids (12, 52). Mn(III) formed from the oxidation of Mn(II) by MnP can oxidize oxalate and glyoxylate to free radicals, ultimately leading to the formation of superoxide (25, 30). Veratryl alcohol cation radical formed by LiP mediates the oxidation of oxalate in a similar fashion (5, 47, 52). In this study, the occurrence of superoxide anion radical was demonstrated in cultures receiving organic acid supplements by measuring the reduction of a well-known superoxide scavenger, TNM (56).
The superoxide anion radical may have roles in lignin degradation which could account for the stimulated delignification of kraft pulp in cultures supplemented with organic acids. The superoxide anion radical has been suggested to account for the direct oxidation of recalcitrant lignin model compounds by Mn(III) oxalate (20). The importance of the superoxide anion radical for kraft pulp delignification by laccase mediator systems has already been emphasized in experiments in which superoxide dismutase was used to inhibit biobleaching (51). The superoxide anion radical has also been implicated in the mechanisms of oxygen delignification and peroxide bleaching (15). Although superoxide itself shows no reactivity toward kraft pulp lignin, it can behave as a strong oxidant of radicals formed in the lignin molecule, causing ring opening and cleavage between Cα—Cβ bonds (15). Therefore, the stimulating effect of the superoxide anion radical on delignification during white rot fungal biobleaching can only be rationalized if the lignin is first oxidized to cation radicals by the oxidative enzymes.
Correspondence of biobleaching to ligninolytic enzymes.
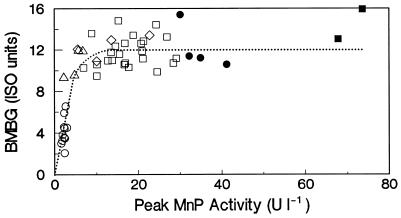
MnP and LiP were the most predominant oxidative enzymes produced during biobleaching. MnP was consistently present under all culture conditions which supported extensive biobleaching. The correspondence of brightness gains with the maximal titer of MnP under variable culture conditions, with and without Mn as well as with and without physiological organic acids, is plotted in Fig. 7. From 1 to 10 U liter−1, there was a strong linear correlation between brightness gains and the MnP titer (R2 = 0.786, P < 0.01); thereafter, an increased MnP titer showed little ability to improve brightness gains. This trend suggests that MnP from Bjerkandera might be involved in biobleaching in the absence of Mn. This trend also indicates that once a minimum level of biocatalyst has been exceded, other factors, such as poor lignin bioavailability, appear to become rate limiting.
FIG. 7.
Correlation between peak MnP activity and extent of BMBG after 14 days of incubation of EDTA-extracted OKP with Bjerkandera sp. strain BOS55. The open symbols indicate cultures with manganese-free media. The closed symbols correspond to cultures with 33 μM manganese. Circles represent no organic acids, triangles represent 1 mM organic acids, diamonds represent 3 mM organic acids, and squares represent 5 mM organic acids.
Provided that Mn is present, MnP is known to delignify kraft pulp in vitro (19, 24, 28, 43, 45) as well as to depolymerize synthetic lignins (18, 57). Mn was found to be an obligate mediator substrate of MnP from P. chrysosporium for the oxidation of other substrates (16). However, it is not yet fully clear how MnP can function in the absence of Mn.
Alternative mediators or cofactors besides Mn have to be considered. Several precedents from the literature give insights into possible alternatives. MnP from Ceriporiopsis subvermispora is known to oxidize several N-substituted aromatics in the absence of Mn (55). Furthermore, the mnp gene from C. subvermispora was shown to contain an aromatic binding site (33). Naturally occurring N-substituted aromatics (3-hydroxyanthranillate and 4-hydroxymethylquinoline) have also been identified as important secondary metabolites in some white rot fungi (1, 14). MnP isozymes purified from Pleurotus spp. were shown to be capable of directly oxidizing 2,6-dimethoxyphenol and veratryl alcohol in the absence of Mn (34, 49). These MnP isozymes might be able to directly oxidize phenolic moieties in OKP lignin or otherwise utilize veratryl alcohol as a nondiffusible mediator.
Aside from MnP, LiP was detected in biobleaching cultures stimulated by oxalate or oxalate precursors. When present, LiP could possibly have been involved in the biobleaching, since veratryl alcohol was available. LiP from P. chrysosporium in combination with veratryl alcohol was previously shown to be effective in the in vitro depolymerization of radiolabelled synthetic lignin (18) and in causing brightness gains and delignification of OKP (4).
ACKNOWLEDGMENTS
This study was carried out with financial support from the Commission of the European Communities Agriculture and Fisheries (FAIR) specific RTD program CT95-0805, “Oxidative Enzymes for the Pulp and Paper Industry.” Support given to M.T.M. and G.F. from the Wageningen Agricultural University fellowship program is also greatly appreciated.
REFERENCES
- 1.Abraham W R, Spassov G. 4-Hydroxymethyl-quinoline from Polyporus species. Phytochemistry. 1991;30:371–372. [Google Scholar]
- 2.Addleman K, Archibald F. Kraft pulp bleaching and delignification by dikaryons and monokaryons of Trametes versicolor. Appl Environ Microbiol. 1993;59:266–273. doi: 10.1128/aem.59.1.266-273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addleman K, Dumonceaux T, Paice M G, Bourbonnais R, Archibald F S. Production and characterization of Trametes versicolor mutants unable to bleach hardwood kraft pulp. Appl Environ Microbiol. 1995;61:3687–3694. doi: 10.1128/aem.61.10.3687-3694.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbeloa M, de Leseleuc J, Goma G, Pommier J C. An evaluation of the potential of lignin peroxidases to improve pulps. Tappi J. 1992;75:215–221. [Google Scholar]
- 5.Barr D P, Shah M M, Grover T A, Aust S D. Production of hydroxyl radical by lignin peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1992;298:480–485. doi: 10.1016/0003-9861(92)90438-3. [DOI] [PubMed] [Google Scholar]
- 6.Berzins V. Micro kappa numbers. Pulp Pap Mag Can. 1966;1966:T206–T208. [Google Scholar]
- 7.Bonnarme P, Jeffries T W. Mn(II) regulation of lignin peroxidases and manganese-dependent peroxidases from lignin-degrading white-rot fungi. Appl Environ Microbiol. 1990;56:210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancel A M, Orth A B, Tien M. Lignin and veratryl alcohol are not inducers of the ligninolytic system of Phanerochaete chrysosporium. Appl Environ Microbiol. 1993;59:2909–2913. doi: 10.1128/aem.59.9.2909-2913.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dannibale A, Crestini C, Dimattia E, Giovannozzi-Sermanni G. Veratryl alcohol oxidation by manganese-dependent peroxidase from Lentinus edodes. J Biotechnol. 1996;48:231–239. [Google Scholar]
- 10.De Jong E, Field J A, de Bont J A M. Aryl alcohols in the physiology of ligninolytic fungi. FEMS Microbiol Rev. 1994;13:153–188. [Google Scholar]
- 11.De Jong E, Cazemier A E, Field J A, de Bont J A M. Physiological role of chlorinated aryl alcohols biosynthesized de novo by the white rot fungus Bjerkandera sp. strain BOS55. Appl Environ Microbiol. 1994;60:271–277. doi: 10.1128/aem.60.1.271-277.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutton M V, Evans C S. Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment. Can J Microbiol. 1996;42:881–895. [Google Scholar]
- 13.Dutton M V, Evans C S, Atkey P T, Wood D A. Oxalate production by Basidiomycetes, including the white-rot species Coriolus versicolor and Phanerochaete chrysosporium. Appl Microbiol Biotechnol. 1993;39:5–10. [Google Scholar]
- 14.Eggert C, Temp U, Dean J F D, Eriksson K E L. Laccase-mediated formation of the phenoxazinone derivative, cinnabarinic acid. FEBS Lett. 1995;376:202–206. doi: 10.1016/0014-5793(95)01274-9. [DOI] [PubMed] [Google Scholar]
- 15.Gierer J. Formation and involvement of superoxide (O2 · −/ HO2 · ) and hydroxyl (OH · ) radicals in TCF bleaching processes: a review. Holzforschung. 1997;51:34–46. [Google Scholar]
- 16.Glenn J K, Akileswaran L, Gold M H. Mn(II) oxidation is the principal function of the extracellular Mn-peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1986;251:688–696. doi: 10.1016/0003-9861(86)90378-4. [DOI] [PubMed] [Google Scholar]
- 17.Gold M H, Alic M. Molecular biology of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Microbiol Rev. 1993;57:605–622. doi: 10.1128/mr.57.3.605-622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammel K E, Jensen K A, Mozuch M D, Landucci L L, Tien M, Pease E A. Ligninolysis by a purified lignin peroxidase. J Biol Chem. 1993;268:12274–12281. [PubMed] [Google Scholar]
- 19.Harazono K, Kondo R, Sakai K. Bleaching of hardwood kraft pulp with manganese peroxidase from Phanerochaete sordida YK-624 without addition of MnSO4. Appl Environ Microbiol. 1996;62:913–917. doi: 10.1128/aem.62.3.913-917.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hattori T, Shimada M. Abstracts of the 6th International Conference Biotechnology in the Pulp and Paper Industry. 1995. MnP-mimetic breakdown of the recalcitrant nonphenolic β-O-4 lignin model substrates by use of Mn(III)/oxalate/O2 system, abstr. P-F1 53; p. 158. [Google Scholar]
- 21.Hirai H, Kondo R, Sakai K. Screening of lignin-degrading fungi and their ligninolytic enzyme activities during biological bleaching of kraft pulp. Mokuzai Gakkaishi. 1994;40:980–986. [Google Scholar]
- 22.Hirai H, Kondo R, Sakai K. Effect of metal ions on biological bleaching of kraft pulp with Phanerochaete sordida YK-624. Mokuzai Gakkaishi. 1995;41:69–75. [Google Scholar]
- 23.Kaneko R, Iimori T, Yoshikawa H, Machida M, Yoshioka H, Murakami K. A possible role of manganese peroxidase during biobleaching by the pulp bleaching fungus SKB-1152. Biosci Biotechnol Biochem. 1994;58:1517–1518. [Google Scholar]
- 24.Kaneko R, Iimori T, Miyawaki S, Machida M, Murakami K. Biobleaching with manganese peroxidase purified from the pulp bleaching fungus SKB-1152. Biosci Biotechnol Biochem. 1995;59:1584–1585. [Google Scholar]
- 25.Khindaria A, Grover T A, Aust S D. Oxalate-dependent reductive activity of manganese peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1994;314:301–306. doi: 10.1006/abbi.1994.1446. [DOI] [PubMed] [Google Scholar]
- 26.Khindaria A, Yamazaki I, Aust S D. Veratryl alcohol oxidation by lignin peroxidase. Biochemistry. 1995;34:16860–16869. doi: 10.1021/bi00051a037. [DOI] [PubMed] [Google Scholar]
- 27.Kirk T K, Farrell R L. Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 28.Kondo R, Harazono K, Sakai K. Bleaching of hardwood kraft pulp with manganese peroxidase secreted from Phanerochaete sordida YK-624. Appl Environ Microbiol. 1994;60:4359–4363. doi: 10.1128/aem.60.12.4359-4363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo R, Kurashiki K, Sakai K. In vitro bleaching of hardwood kraft pulp by extracellular enzymes excreted from white rot fungi in a cultivation system using a membrane filter. Appl Environ Microbiol. 1994;60:921–926. doi: 10.1128/aem.60.3.921-926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuan I C, Tien M. Glyoxylate-supported reactions catalyzed by Mn peroxidase of Phanerochaete chrysosporium: activity in the absence of added hydrogen peroxide. Arch Biochem Biophys. 1993;302:447–454. doi: 10.1006/abbi.1993.1238. [DOI] [PubMed] [Google Scholar]
- 31.Kuan I C, Tien M. Stimulation of Mn peroxidase activity: a possible role for oxalate in lignin biodegradation. Proc Natl Acad Sci USA. 1993;90:1242–1246. doi: 10.1073/pnas.90.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, Alic M, Brown J A, Gold M H. Regulation of manganese peroxidase gene transcription by hydrogen peroxide, chemical stress, and molecular oxygen. Appl Environ Microbiol. 1995;61:341–345. doi: 10.1128/aem.61.1.341-345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lobos S, Larrondo L, Salas L, Karahanian E, Vicuña R. Cloning and molecular analysis of a cDNA and the Cs-mnp1 gene encoding a manganese peroxidase isoenzyme from the lignin-degrading basidiomycete Ceriporiopsis subvermispora. Gene. 1998;206:185–193. doi: 10.1016/s0378-1119(97)00583-0. [DOI] [PubMed] [Google Scholar]
- 34.Martinez M J, Ruizduenas F J, Guillen F, Martinez A T. Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. Eur J Biochem. 1996;237:424–432. doi: 10.1111/j.1432-1033.1996.0424k.x. [DOI] [PubMed] [Google Scholar]
- 35.Mester T, de Jong E, Field J A. Manganese regulation of veratryl alcohol in white rot fungi and its indirect effect on lignin peroxidase. Appl Environ Microbiol. 1995;61:1881–1887. doi: 10.1128/aem.61.5.1881-1887.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mester T, Pena M, Field J A. Nutrient regulation of extracellular peroxidases in the white rot fungus, Bjerkandera sp. strain BOS55. Appl Microbiol Biotechnol. 1996;44:778–784. [Google Scholar]
- 37.Mester T, Field J A. Optimization of manganese peroxidase production by the white rot fungus Bjerkandera sp. strain BOS55. FEMS Microbiol Lett. 1997;155:161–168. [Google Scholar]
- 38.Moreira M T, Feijoo G, Sierra-Alvarez R, Lema J, Field J A. Oxalic acid treatment as a posttreatment to increase the brightness of kraft pulps bleached by white-rot fungi. Biotechnol Techniques. 1996;10:559–564. [Google Scholar]
- 39.Moreira M T, Feijoo G, Sierra-Alvarez R, Lema J, Field J A. Manganese is not required for biobleaching of oxygen-delignified kraft pulp by the white rot fungus Bjerkandera sp. strain BOS55. Appl Environ Microbiol. 1997;63:1749–1755. doi: 10.1128/aem.63.5.1749-1755.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreira M T, Feijoo G, Sierra-Alvarez R, Lema J, Field J A. Biobleaching of oxygen delignified kraft pulp by several white rot fungal strains. J Biotechnol. 1997;53:237–251. doi: 10.1128/aem.63.5.1749-1755.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muheim A, Waldner R, Leisola M S A, Feichter A. An extracellular aryl-alcohol oxidase from the white rot fungus, Bjerkandera adusta. Enzyme Microb Technol. 1990;12:204–209. [Google Scholar]
- 42.Paice M G, Jurasek L, Ho C, Bourbonnais R, Archibald F. Direct biological bleaching of hardwood pulp with the fungus Coriolus versicolor. Tappi J. 1989;75:217–221. [Google Scholar]
- 43.Paice M G, Reid I D, Bourbonnais R, Archibald F S, Jurasek L. Manganese peroxidase, produced by Trametes versicolor during pulp bleaching, demethylates and delignifies kraft pulp. Appl Environ Microbiol. 1993;59:260–265. doi: 10.1128/aem.59.1.260-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paice M G. Abstracts of the European Conference on Pulp and Paper Research. Stockholm, Sweden: Swedish Pulp and Paper Institute; 1996. Manganese peroxidase—a key enzyme for pulp bleaching; pp. 87–88. [Google Scholar]
- 45.Paice M G, Archibald F S, Bourbonnais R, Reid I D, Renuad S. Proceedings of the Biological Sciences Symposium. Atlanta, Ga: Tappi Press; 1997. Manganese peroxidase catalyzed bleaching of kraft pulps; pp. 343–345. [Google Scholar]
- 46.Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38:161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 47.Popp J L, Kalyanaraman B, Kirk T K. Lignin peroxidase oxidation of Mn2+ in the presence of veratryl alcohol, malonic or oxalic acid and oxygen. Biochemistry. 1990;29:10475–10480. doi: 10.1021/bi00498a008. [DOI] [PubMed] [Google Scholar]
- 48.Roy B P, Archibald F. Effects of kraft pulp and lignin on Trametes versicolor carbon metabolism. Appl Environ Microbiol. 1993;59:1855–1863. doi: 10.1128/aem.59.6.1855-1863.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarkar S, Martinez A T, Martinez M J. Biochemical and molecular characterization of a manganese peroxidase isozyme from Pleurotus ostreatus. Biochim Biophys Acta. 1997;1339:23–30. doi: 10.1016/s0167-4838(96)00201-4. [DOI] [PubMed] [Google Scholar]
- 50.Schick-Zapanta L, Tien M. The roles of veratryl alcohol and oxalate in fungal lignin degradation. J Biotechnol. 1997;53:93–102. [Google Scholar]
- 51.Sealey J E, Runge T M, Ragauskas A J. Proceedings of the Biological Sciences Symposium. Atlanta, Ga: Tappi Press; 1997. Biobleaching of kraft pulps with laccase and hydroxybenzotriazole; pp. 339–342. [Google Scholar]
- 52.Shimada M, Ma D B, Akamatsu Y, Hattori T. A proposed role of oxalic acid in wood decay systems of wood-rotting basidiomycetes. FEMS Microbiol Rev. 1994;13:285–296. [Google Scholar]
- 53.Shimada M, Akamatsu Y, Tokimatsu T, Mii K, Hattori T. Possible biochemical roles of oxalic acid as a low molecular weight compound involved in brown-rot and white-rot wood decays. J Biotechnol. 1997;53:103–113. [Google Scholar]
- 54.Tien M, Kirk T K. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 1988;161B:238–248. [Google Scholar]
- 55.Urzua U, Larrondo L F, Lobos S, Larrain J, Vicuña R. Oxidation reactions catalyzed by manganese peroxidase isoenzymes from Ceriporiopsis subvermispora. FEBS Lett. 1995;371:132–136. doi: 10.1016/0014-5793(95)00874-9. [DOI] [PubMed] [Google Scholar]
- 56.Wariishi H, Gold M H. Lignin peroxidase compound III. Mechanism of formation. J Biol Chem. 1990;265:2070–2077. [PubMed] [Google Scholar]
- 57.Wariishi H, Valli K, Gold M H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991;176:269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]
- 58.Wariishi H, Valli K, Gold M H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium—kinetic mechanism and role of chelators. J Biol Chem. 1992;267:23688–23695. [PubMed] [Google Scholar]