Abstract
Erwinia chrysanthemi is a phytopathogenic bacterium that causes soft-rot diseases in a broad number of crops. The PhoP-PhoQ system is a key factor in pathogenicity of several bacteria and is involved in the bacterial resistance to different factors, including acid stress. Since E. chrysanthemi is confronted by acid pH during pathogenesis, we have studied the role of this system in the virulence of this bacterium. In this work, we have isolated and characterized the phoP and phoQ mutants of E. chrysanthemi strain 3937. It was found that: (i) they were not altered in their growth at acid pH; (ii) the phoQ mutant showed diminished ability to survive at acid pH; (iii) susceptibility to the antimicrobial peptide thionin was increased; (iv) the virulence of the phoQ mutant was diminished at low and high magnesium concentrations, whereas the virulence of the phoP was diminished only at low magnesium concentrations; (v) in planta Pel activity of both mutant strains was drastically reduced; and (vi) both mutants lagged behind the wild type in their capacity to change the apoplastic pH. These results suggest that the PhoP-PhoQ system plays a role in the virulence of this bacterium in plant tissues, although it does not contribute to bacterial growth at acid pH.
Bacteria of the family Enterobacteriaceae are able to colonize a wide range of natural habitats, and many species cause diseases in both animals and plants. Bacterial cells experience several types of stresses in natural situations, such as starvation, lack of iron, oxidative damage, heat, or acid pH (18). Most enteric bacteria, including Escherichia coli and Salmonella enterica serovar Typhimurium, are neutrophiles, they grow better at approximately neutral pH, but they are often exposed to acid stress in natural and pathogenic conditions (7, 42). This type of stress has been described as the combined biological effect of the low pH (inorganic component) and the weak organic acids (organic component) present in the milieu (7).
Plant pathogenic enterobacteria have to grow in the apoplast, the pH of which usually ranges between 5.0 and 6.5 but can be as low as 4.0. This acid pH is due to the abundance of organic acids, as citric or malic acid, and proton pumping from nearby cells (22). This is in contrast with animal pathogenic bacteria, which may be confronted with a more acidic pH in the animal's stomach.
Not surprisingly, some bacterial species have evolved mechanisms to withstand acidic stress. For example, for S. enterica serovar Typhimurium, an acid tolerance response has been described (17, 46), which enables the bacterium to survive to extremely low pH (3.0 to 4.0) if it became previously adapted to a mild acid pH (5.5 to 6.0). Moreover, acid resistance of E. coli has been described as the capacity of the bacterium to withstand an acidic pH of 2.5 or below if the cell is in stationary phase (10).
Erwinia chrysanthemi is a plant-pathogenic bacteria belonging to the Enterobacteriaceae. It causes soft-rot disease in a wide range of plant hosts, being able to infect essentially any nonlignified tissue (39). The typical maceration symptoms are mostly the result of secreted pectolytic enzymes that degrade the cell walls, ultimately leading to cell lysis and necrosis of the whole tissue (3, 4). In the initial stages of infection, the bacteria have to establish a population in the acidic plant apoplast. However, as the infection progresses, a concomitant alkalinization of the milieu occurs (37).
Most studies of E. chrysanthemi have been performed with two model strains: EC16, isolated from chrysanthemum plants (11), and 3937, isolated from saintpaulia plants (30). Although they produce similar symptoms and they attack a wide range of plants, the two strains have different virulences in different plant hosts. In addition, EC16 secretes four major pectic lyase isoenzymes in culture (PelABCE) (28), whereas 3937 secretes five isoenzymes (PelABCDE) (9). Little is known about the genetic relatedness of the strains.
Our previous work with strain EC16 has revealed that mutants in the phoP-phoQ operon are altered in their ability to grow at acid pH and also showed (i) decreased survival at acid pH in plant tissues, (ii) increased susceptibility to antimicrobial peptides, (iii) decreased virulence in several hosts, (iv) reduced production of pectolytic enzymes, and (vi) reduced ability to alkalinize plant tissues (31). The phoP-phoQ operon has been described as a key factor controlling virulence in S. enterica serovar Typhimurium (23, 35). PhoQ is a sensor histidine kinase that autophosphorylates in response to environmental conditions, and PhoP is a transcriptional regulator which controls the expression of genes that are essential for virulence, particularly involved in survival within macrophages, survival at acid pH, and resistance to antimicrobial peptides (16). The PhoP-PhoQ system also controls the expression of a set of around 40 proteins including proteases, phosphatases, and cation transporters (15).
It has been reported that the magnesium concentration in the bacterial environment is one of the signals that controls the PhoP-PhoQ system in S. enterica serovar Typhimurium (19). Also, we have previously reported that low magnesium concentrations induce expression of the phoQ gene in E. chrysanthemi EC16 (31). It is likely that this condition is commonly found by these pathogens in their respective hosts.
In this work, we have constructed phoP and phoQ mutants in strain 3937 and studied the phenotypes of these mutants with respect to (i) virulence, (ii) survival at acid pH, (iii) sensitivity to antimicrobial peptides, and (iv) pectic enzyme production. This regulatory system appears to have an important role in the pathogenicity of this strain, as in strain EC16. However, the phenotypes of the phoP and phoQ mutants differed in both strains with respect to the above-mentioned features.
MATERIALS AND METHODS
Microbiological methods.
The bacterial strains and plasmids used in this study are described in Table 1. Strains of E. coli were cultivated at 37°C in Luria-Bertani medium. Strains of E. chrysanthemi were cultivated at 28°C in nutrient broth (NB; Difco, Detroit, Mich.), King's B medium (29), or modified basal medium A (MBMA) (44) (citric acid monohydrate was added instead of sodium citrate to buffer in the lower pH ranges) (17, 45) supplemented with 0.2% glycerol and 250 μM potassium phosphate (pH 7.0) (40). Antibiotics were added to the media at the following concentrations (micrograms per milliliter): ampicillin, 100; and kanamycin, 20.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli DH5α | supE44 Δlac U169 (φ80 lacZM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 24 |
| E. chrysanthemi | ||
| 3937 | Wild-type strain | 30 |
| BT123 | Δ(phoP)::Tn7 Camr, derivative of 3937 | This work |
| BT124 | Δ(phoO)::Tn7 Camr, derivative of 3937 | This work |
| Plasmids | ||
| pGEM T-easy | Ampr | Promega |
| pBluescript II SK(−) | Ampr | Stratagene |
| pB113 | pGEM carrying 3937 phoP and phoQ genes | This work |
| pB114 | pBluescript II carrying 3937 phoP mutant | This work |
| pB115 | pBluescript II carrying 3937 phoQ mutant | This work |
Short-chain organic acid inhibition and enzymatic assays.
Short-chain organic acid inhibition assays were performed by using the method described by López-Solanilla et al. (33). Organic acids were added to the basal medium A cultures without citric acid monohydrate at pH 5.5 and 7.0, as follows: acetic acid, benzoic acid, butyric acid, citric acid, formic acid, lactic acid, and propionic acid at 50, 100, and 200 μg/ml.
Culture filtrates containing a mixture of extracellular pectic enzymes were obtained as previously described (34). For the determination in planta of the enzymatic activities, chicory disks of 1 cm in diameter were inoculated with a suspension containing 107 bacterial cells and incubated at 28°C. After 4 h, the tissues were treated as indicated for the culture filtrates. The extracellular pectic lyase (Pel) activity was determined by monitoring the increase of absorbance at 232 nm as a result of the 4,5-unsaturated reaction products. This assay was performed as described by Collmer et al. (13).
DNA manipulation and sequencing.
The E. chrysanthemi 3937 phoP-phoQ operon was amplified by PCR with the oligonucleotides 5′-GAGAAACTGAAAGAACTGACCCGCG-3′ and 5′-CTTAATTCACGGACGAGGCGG-3′ (based on the phoP-phoQ operon sequence of E. chrysanthemi EC16) (31), cloned in pGEMT-easy (Promega, Madison, Wis.), and designated pB113 (Table 1). Plasmid pBluescript SK(−) (Stratagene, La Jolla, Calif.) was used for subcloning. Tn7 in vitro mutagenesis was performed with the genome priming system kit (GPS-1; New England Biolabs, Beverly, Mass.). Two mutagenized constructions (pB114 and pB115) (Table 1) bearing the Tn7 transposon within the phoP and phoQ genes, respectively, were selected and marker exchanged into the chromosome as previously described (26). The marker exchange was verified by DNA blot hybridization (data not shown). The corresponding mutant strains (BT123 and BT124) (Table 1) were selected for further analysis. Standard molecular cloning techniques employed in this study were performed as described previously (41). DNA sequencing of both strands was done by the chain termination method on double-stranded DNA templates with an ABI Prism dye terminator cycle sequencing kit (Perkin-Elmer, Norwalk, Conn.) in a 3100 DNA sequencer (Perkin-Elmer). Sequence alignments were performed at the National Center for Biotechnology Information (online) with the BLAST network service (1) and with The Institute for Genomic Research BLAST (http://www.tigr.org).
Susceptibility, lethality, and virulence assays.
Susceptibility to antimicrobial peptides was assayed as previously described (32). To perform lethality assays, 10 ml of MBMA at pH 3.0, 3.5, 4.0, 4.5, 5.5, and 7.0 was inoculated with 100 μl of a suspension containing 106 bacterial cells of E. chrysanthemi 3937 wild-type or phoP or phoQ mutant strains. Cells were incubated for 4 h at 28°C with shaking, and then a portion of each sample was diluted and plated on nutrient broth agar plates for assessment of bacterial viability. Three replicates were performed in each case.
Witloof chicory leaves were purchased from a local supermarket. The bacterial cells from an overnight NB liquid medium culture were washed with 10 mM or 10 μM MgCl2 and then resuspended in an appropriate volume of buffer to obtain the desired inoculum concentration. Virulence assays on chicory leaves were performed as previously described (5). Briefly, each chicory leaf was inoculated at two locations with E. chrysanthemi 3937 wild-type and phoP or phoQ mutant strains. Forty leaves were pair-inoculated with 10 μl of a suspension containing 103 bacterial cells in 10 mM MgCl2 or 10 μl of a suspension containing 104 bacterial cells in 10 μM MgCl2. The reason for using a different level of inoculum was to achieve a 100% frequency of infection for the wild-type strain in both experiments. Chicory leaves were incubated for 32 h in a moist chamber at 28°C. The virulence in this assay is measured as the macerated area. The differences between wild-type and mutant strains were statistically assessed with a paired Student's t test. To estimate the bacterial populations attained in plant tissues, 10 μl of a suspension containing 104 bacterial cells in 10 μM MgCl2 was inoculated in chicory leaves. The tissue was incubated in a moist chamber at 28°C for 24 h and ground, and the bacterial populations were estimated by dilution plating. To monitor pH variations in chicory leaves upon infection, the following pH indicator solutions (0.1%) were prepared: (i) bromocresol purple (Merck, KGaA, Darmstadt, Germany), which is yellow at a pH value below 5.2 and purple at a pH value above 6.8, and (ii) phenol red (Merck), which is yellow at a pH value below 6.4 and red-violet at a pH value above 8.2. The chicory leaves were inoculated with 10 μl of a suspension containing 104 bacterial cells of E. chrysanthemi 3937 and phoP and phoQ mutant strains. After different times of incubation at 28°C, the changes in the pH were monitored by adding the pH indicator solutions at the inoculation point.
RESULTS
Sensitivity to short-chain fatty acids in E. chrysanthemi 3937.
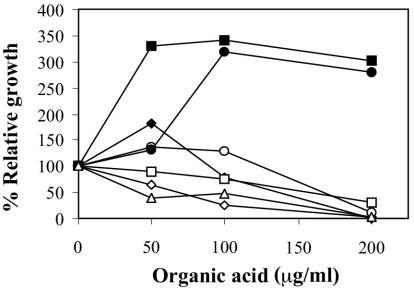
It has been known for a long time that short-chain organic acids present in the intestine can exert antimicrobial activity (2, 12). Furthermore, the addition of a mixture of these acids has been used to prevent Salmonella infections in chicken farms (25). Therefore, we decided to analyze the sensitivity of E. chrysanthemi 3937 to several weak organic acids, such as acetic, benzoic, butyric, citric, formic, lactic, and propionic acids. The sensitivity was measured as described in Material and Methods. At pH 7.0, it was found that the bacteria were inhibited by all of the acids assayed except citric and lactic acids, as shown in Fig. 1.
FIG. 1.
Susceptibility of E. chrysanthemi strain 3937 to several short-chain organic acids at pH 7.0: ♦, acetic acid; ⋄, benzoic acid; □, butyric acid; ▪, citric acid; ○, formic acid; •, lactic acid; ▵, propionic acid. E. chrysanthemi 3937 at pH 5.5 did not grow at this incubation time. Relative growth is expressed as the percentage of optical density attained by the cultures after 24 h in the presence of the indicated organic acid concentration with respect to the optical density attained in the absence of the organic acid. Results show the means of three replicates. Standard error bars are too small to be represented.
Growth and survival of strain 3937 and phoP and phoQ mutants in acid medium.
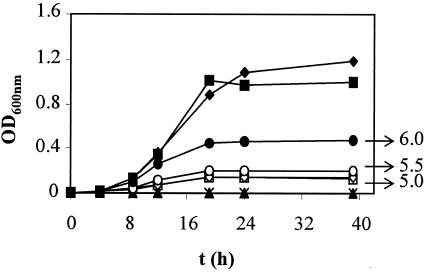
To study the behavior of strain 3937 in acid medium, growth curves at different pHs in MBMA were done. We observed that 3937 did not grow at pH 5.0 and 5.5 and grew poorly at pH 6.0 (Fig. 2). The growth curves at different pHs of the phoP and phoQ mutants were compared with that of the wild type (data not shown). Interestingly, these mutants did not show diminished ability to grow at acid pH, in contrast with behavior reported for the same mutations in strain EC16 (31).
FIG. 2.
Growth curves of E. chrysanthemi strain 3937 in MBMA at different pHs: ♦, pH 7.0; ▪, pH 6.5; •, pH 6.0; ○, pH 5.5; □, pH 5.0; ⋄, pH 4.5; ▴, pH 4.0; ▵, pH 3.5; *, pH 3.0. Results show the means of three replicates. Standard error bars are too small to be represented.
Also, we investigated the survival of wild-type and phoP and phoQ mutant strains after acid treatment, performing lethality assays as indicated in Material and Methods. Differences between the wild type and phoP and phoQ mutants were found at several pHs (Fig. 3). At pH 3.5, the difference between the wild type and phoQ mutant was 4 orders of magnitude.
FIG. 3.
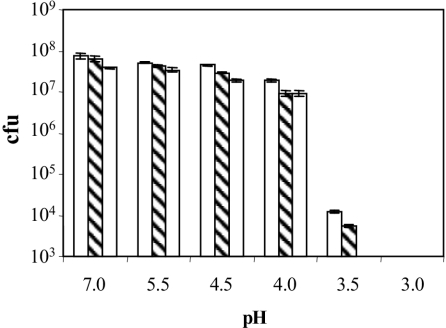
Survival at different pHs of E. chrysanthemi 3937 wild-type (empty bars), phoP (diagonally striped bars), and phoQ (dotted bars) strains. Each strain (104 CFU) was incubated for 4 h at the indicated pH at 28°C, and the appropriate dilutions were plated onto NB agar plates. Results show the means and standard errors of three replicates.
Virulence and enzymatic assays.
To investigate the possible effect on virulence of the phoP and phoQ mutations, chicory leaves were inoculated with E. chrysanthemi 3937 and phoP and phoQ strains. Since it is known that magnesium levels affect the PhoP-PhoQ system, we decided to perform the inoculations at high and low magnesium concentrations. Necrotic areas of the developed lesions were measured after 32 h. At high magnesium concentrations, statistically significant differences were found only between the wild-type and phoQ mutant strains (Table 2). In contrast, the virulence tests performed at low magnesium concentrations showed that both mutants were significantly less virulent than the wild-type strain. The necrotic areas produced by the phoP and phoQ mutants were 33 and 41%, respectively, of that of the wild type.
TABLE 2.
Effects of Δ(phoP)::Tn7 and Δ(phoO)::Tn7 mutations on the virulence of E. chrysanthemi 3937 on witloof chicory leaves at high and low Mg2+ concentrations
| Strain | MgCl2 concn | Size of lesion (cm2) (mean ± SE)a |
|---|---|---|
| 3937 (wild type) | 10 mM | 1.75 ± 0.03 |
| BT123 (phoP) | 10 mM | 1.20 ± 0.11b |
| 3937 (wild type) | 10 mM | 2.64 ± 0.17 |
| BT124 (phoQ) | 10 mM | 1.71 ± 0.10c |
| 3937 (wild type) | 10 μM | 1.16 ± 0.17 |
| BT123 (phoP) | 10 μM | 0.39 ± 0.03c |
| 3937 (wild type) | 10 μM | 1.23 ± 0.16 |
| BT124 (phoQ) | 10 μM | 0.50 ± 0.04c |
Values are the products of the length and width of the necrotic area.
Differences between wild-type and mutant strains are not significant according to the Student t test (P < 0.05).
Differences between wild-type and mutant strains are significant according to the Student t test (P < 0.05).
The bacterial populations attained in plant tissues by the wild-type and mutant strains were estimated. In a high magnesium (10 mM) concentration, the bacterial population of phoP was 78.5% of that of the wild type and that of phoQ was 86.6%. In a low magnesium (10 μM) concentration, the populations of phoP and phoQ were 35.3 and 56.3%, respectively. In general, there is a good agreement between the level of growth in planta and the virulence of the different strains (Table 2).
To study the possible role of the phoP-phoQ operon in the production of extracellular pectate lyase, this activity was analyzed in planta. The pectate lyase activity was reduced in the phoP and phoQ mutants. It was found that relative enzymatic activity of the mutants, expressed as the percentage of activity attained in the spectrophotometric assay with respect to that of the wild type, was 4.65 and 8.95% for phoP and phoQ, respectively. These results were the means of three replicates, and the standard error was less than 10% of the mean value.
Sensitivity to antimicrobial peptides.
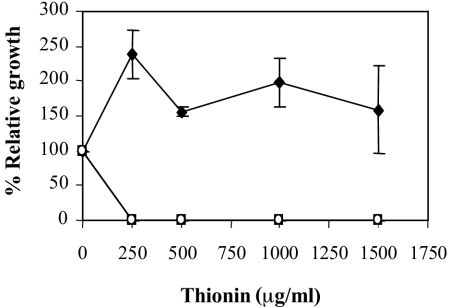
The sensitivity of phoP and phoQ mutant strains to antimicrobial peptides, derived from plants and animals, was compared with that of the wild type. Thionins are cysteine-rich antimicrobial peptides, found in many plant species, which show antimicrobial activity towards several fungal and bacterial pathogens at concentrations in the 50- to 500-μg/ml range (21). Protamine is a lineal antimicrobial peptide that it is frequently used as an antibiotic. Wheat thionin and salmon protamine were selected for in vitro inhibition tests. As shown in Fig. 4, the mutants were significantly more susceptible to thionin, although no differences were found with respect to protamine. Curiously, the wild-type strain appears to grow more in the presence of the antimicrobial peptide thionin. This effect has been previously reported (32), and it is probably due to peptide degradation and utilization by the bacteria. In contrast, other phytopathogenic bacteria are inhibited at concentrations in the 50- to 500-μg/ml range (21).
FIG. 4.
Susceptibility of the E. chrysanthemi 3937 wild type (♦) and the phoP (▪) and phoQ (○) mutants to thionin. Relative growth is expressed as the percentage of optical density attained by the cultures after 6 h in the presence of the indicated peptide concentration with respect to the optical density attained in the absence of the peptide. Results show the means and standard errors of three replicates.
Modification of apoplastic pH during infection.
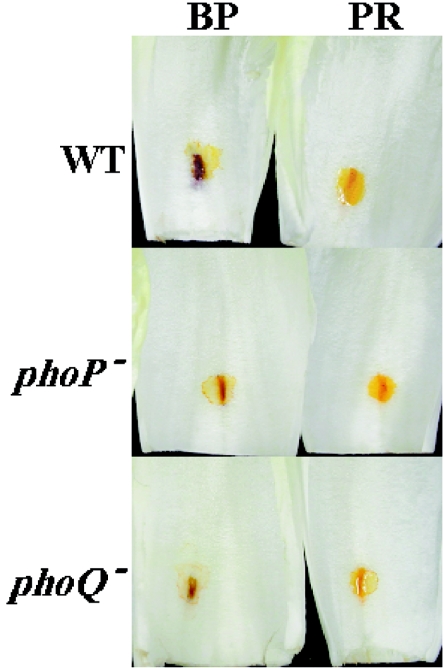
Since it has been reported that the pH value of the intercellular environment changes as a consequence of E. chrysanthemi colonization (37), we decided to carry out a comparative study during the course of the infection by using the wild-type and mutant strains with 10 μM magnesium chloride. Chicory leaves were inoculated as described in Materials and Methods with bacterial cells of the wild-type and phoP and phoQ mutant strains. pH indicator solutions were added at the inoculation site in a time-course experiment, and both mutants lagged behind the wild type in their ability to modify the apoplastic pH around the infection site (Fig. 5). In longer experiments, we have consistently observed that both wild-type and mutant strains eventually change the apoplastic pH to a pH value above 8.2 (data not shown).
FIG. 5.
pH modification of the chicory apoplast shown by color changes of pH indicator solutions after inoculation with a suspension of E. chrysanthemi 3937 wild-type (WT) and phoP and phoQ mutant strains in 10 μM MgCl2 at 6 h. BP, bromocresol purple (pH 5.2 to 6.8); PR, phenol red (pH 6.4 to 8.2).
DISCUSSION
The two-component regulatory system PhoP-PhoQ is a key feature for the pathogenicity of several gram-negative bacteria (20). Our previous work with E. chrysanthemi strain EC16 indicates that this system controls a set of characteristics that act coordinately to help the establishment and survival of the bacterial population in plant tissue, namely, the abilities to grow at acid pH, alkalinize the external pH, and withstand the action of antimicrobial peptides. These features seem to be far more important at the low inocula prevailing in natural infections and may play an important role in adapting the bacterium to the changing conditions found along its life cycle.
In this work, we have focused on the role of the PhoP-PhoQ system in E. chrysanthemi strain 3937. Surprisingly, these mutants were not affected in their ability to grow at acid pH, in contrast with the results reported for EC16 (31) and other bacteria in the Enterobacteriaceae, such as S. enterica serovar Typhimurium and Yersinia pestis (6, 38). Apparently, the PhoP-PhoQ system of strain 3937 is not involved in the control of growth at acid pH and probably is not a key feature of the pathogenicity of this strain. Actually, the data obtained in the survival in acid pH experiments suggest that this system is involved in the control of this feature. On the other hand, the PhoP-PhoQ system of strain 3937 had a higher effect in the total pectate lyase activity in planta than that of strain EC16. In 3937, the reduction in Pel activity in the mutants was 90 to 95%, whereas in EC16, the reduction was only about 60% (31). This is congruent with the idea that the basic strategy of strain 3937 relies on a rapid release of plant cell compounds.
Another difference found between the phoQ mutants of both strains is related to the sensitivity to antimicrobial peptides. In EC16, the mutant showed an increased sensitivity to antimicrobial peptides from both animal and plant origin (protamine and thionin) (31), whereas in the 3937 mutant, only the sensitivity to thionin was altered (Fig. 4). A possible explanation of the differential effect between antimicrobial peptides could be based in the particular set of genes activated by PhoP-PhoQ; this set of genes is not known at present (neither in strain 3937 nor in strain EC16). For example, it cannot be ruled out that the PhoP-PhoQ system of strain EC16 activates a mechanism effective against protamine, which is not regulated by this system in the 3937 strain.
Several works with other Enterobacteriaceae have shown that magnesium is an important factor in the regulation of the PhoP-PhoQ system (19, 27, 36). It has been reported that PhoQ acts as a magnesium sensor of bacteria in animal tissues (19, 43). Our results clearly indicate that the magnesium concentration plays an important role in the altered pathogenic features of the phoP and phoQ mutants of strain 3937. As shown in Table 2, the mutants had a noticeably diminished virulence at low magnesium concentrations. There are very few data regarding the availability of magnesium for bacteria during infection in the plant apoplast. It is generally true that magnesium is abundant in plants (8, 22), but it could be tightly linked to other components to the plant cell wall. The model resulting from these data is that E. chrysanthemi cells encountered low magnesium levels in the apoplast before the infection; this magnesium level induces the PhoP-PhoQ system, which helps the cell to tolerate several types of stresses. A prediction of this model is that phoP and phoQ mutants survive poorly in the apoplast, and the reduced number of bacterial cells could explain the reduced virulence and reduced pectate lyase activity. Our finding of a good agreement between the virulence of the strains and the bacterial populations attained in plant tissues is congruent with this model.
The fact that the mutation of phoQ, coding a sensor kinase, had a larger effect on virulence at high magnesium concentrations than the mutation of phoP, encoding a transcriptional regulator, could look puzzling at first sight. Although we do not have a satisfactory explanation, this result suggests that alternative sensory proteins could phosphorylate PhoP or/and alternative regulator proteins could be activated by PhoQ.
Considering the published data (31) and those presented in this work, it is possible to compare the behavior of the two strains with respect to growth curves in minimal medium at different pHs, sensitivity to organic acids, survival at acid pH, and the modification of apoplastic pH along the infection process. It was shown that EC16 grew better at low pH (31) than 3937 (Fig. 2). In contrast, the survival at low pH of EC16 (31) was lower than that of 3937 (Fig. 3). Moreover, the ability to change the apoplastic pH of EC16 was lower than that of the strain 3937 (data not shown). Considering these data altogether, we can hypothesize that both strains are using different strategies to cope with acid stress. Strain 3937 relies on survival at low pH, together with a higher ability to modify the pH of the milieu, whereas the EC16 strategy is likely based on the capacity to grow under the initial conditions. The fact that strain 3937 possesses an additional major isozyme of pectate lyase is congruent with this hypothesis because it enables strain 3937 to more rapidly change the apoplastic pH due to a faster release of plant cell contents.
Plant tissues are extraordinarily rich in weak organic acids, for example, the titratable acidity of lemon fruit is as high as 6 M; in most vegetative tissues, the content of free titratable acids is 0.2 to 0.4 g per 100 g of fresh tissue (8). We have found that strain 3937 was inhibited at a 200-μg/ml concentration of acetic, benzoic, butyric, formic, and propionic acids (Fig. 1). These inhibitory concentrations are well bellow the total titratable acidity found in plant tissues, which suggests that these compounds could constitute a barrier for the proliferation of bacterial cells. The strains 3937 and EC16 showed differences with respect to their ability to grow in the presence of these organic acids: EC16 showed a higher ability for growing in the presence of all of the organic acids assayed, except citric and lactic acids (data not shown). These results are in line with the fact that EC16 grows better in acidic conditions.
Although E. chrysanthemi is considered a broad-host-range pathogen, it is known that different strains have different virulences according to the host plant (39). Surprisingly, the natural variability of strains in soft-rot erwinias has received little attention, in spite of the pioneering work of Dickey (14), which showed that strains of E. chrysanthemi isolated in the same host usually show similar characteristics, independent of geographic origin. Since plant tissues differ with respect to apoplastic pH and the type and amount of organic acids and antimicrobial peptides, among over features, we can expect that different strains have evolved finely tuned regulatory mechanisms to improve the colonization of particular host plants. Clearly, the genetic basis of strain specificity in this bacterium poses an interesting question, which merits further investigation.
Acknowledgments
We acknowledge Ana Guío Carrión and Carlos Rojas for technical assistance.
This work was financed by the Comunidad de Madrid (Dirección General de Investigación) 07B/0003/2002.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baik, H. S., S. Bearson, S. Dunbar, and J. W. Foster. 1996. The acid tolerance response of Salmonella typhimurium provides protection against organic acids. Microbiology 142(Pt 11):3195-3200. [DOI] [PubMed] [Google Scholar]
- 3.Barras, F., F. Van Gijsegem, and A. K. Chatterjee. 1994. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu. Rev. Phytopathol. 32:201-234. [Google Scholar]
- 4.Bateman, D. F., and H. G. Basham. 1976. Degradation of plant cell walls and membranes by microbial enzymes, p. 316-335. In R. Heitefuss and P. H. Williams (ed.), Encyclopedia of plant physiology. Physiological plant pathology, vol. 4. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 5.Bauer, D. W., A. J. Bogdanove, S. V. Beer, and A. Collmer. 1994. Erwinia chrysanthemi hrp genes and their involvement in soft rot pathogenesis and elicitation of the hypersensitive response. Mol. Plant-Microbe Interact. 7:573-581. [DOI] [PubMed] [Google Scholar]
- 6.Bearson, B. L., L. Wilson, and J. W. Foster. 1998. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J. Bacteriol. 180:2409-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearson, S., B. Bearson, and J. W. Foster. 1997. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 147:173-180. [DOI] [PubMed] [Google Scholar]
- 8.Belitz, H. D., and W. Grosch. 1985. Química de los alimentos, 2nd ed. Springer-Verlag, Berlin, Germany.
- 9.Boccara, M., A. Diolez, M. Rouve, and A. Kotoujansky. 1988. The role of individual pectate lyases of Erwinia chrysanthemi strain 3937 in pathogenicity on saintpaulia plants. Physiol. Mol. Plant Pathol. 33:95-104. [Google Scholar]
- 10.Castanie-Cornet, M. P., T. A. Penfound, D. Smith, J. F. Elliott, and J. W. Foster. 1999. Control of acid resistance in Escherichia coli. J. Bacteriol. 181:3525-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee, A. K., K. K. Thurn, and D. A. Feese. 1983. Tn5-induced mutations in the enterobacterial phytopathogen Erwinia chrysanthemi. Appl. Environ. Microbiol. 45:644-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherrington, C. A., M. Hinton, G. R. Pearson, and I. Chopra. 1991. Short-chain organic acids at pH 5.0 kill Escherichia coli and Salmonella spp. without causing membrane perturbation. J. Appl. Bacteriol. 70:161-165. [DOI] [PubMed] [Google Scholar]
- 13.Collmer, A., J. L. Ried, and M. S. Mount. 1988. Assay methods for pectic enzymes. Methods Enzymol. 161:329-335. [Google Scholar]
- 14.Dickey, R. S. 1979. Erwinia chrysanthemi: a comparative study of phenotypic properties of strains from several hosts and other Erwinia species. Phytopathology 69:324-329. [Google Scholar]
- 15.Ernst, R. K., T. Guina, and S. I. Miller. 2001. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect. 3:1327-1334. [DOI] [PubMed] [Google Scholar]
- 16.Fields, P. I., E. A. Groisman, and F. Heffron. 1989. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science 243:1059-1062. [DOI] [PubMed] [Google Scholar]
- 17.Foster, J. W., and H. K. Hall. 1990. Adaptive acidification tolerance response of Salmonella typhimurium. J. Bacteriol. 172:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster, J. W., and M. P. Spector. 1995. How Salmonella survive against the odds. Annu. Rev. Microbiol. 49:145-174. [DOI] [PubMed] [Google Scholar]
- 19.Garcia, V. E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 20.Garcia, V. E., F. C. Soncini, and E. A. Groisman. 1994. The role of the PhoP/PhoQ regulon in Salmonella virulence. Res. Microbiol. 145:473-480. [DOI] [PubMed] [Google Scholar]
- 21.García-Olmedo, F., A. Molina, J. M. Alamillo, and P. Rodríguez-Palenzuela. 1998. Plant defense peptides. Biopolymers 47:479-491. [DOI] [PubMed] [Google Scholar]
- 22.Grignon, C., and H. Sentenac. 1991. pH and ionic conditions in the apoplast. Annu. Rev. Plant Physiol. 42:103-128. [Google Scholar]
- 23.Groisman, E. A., E. Chiao, C. J. Lipps, and F. Heffron. 1989. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl. Acad. Sci. USA 86:7077-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 25.Hinton, M., and A. H. Linton. 1988. The control of salmonella infections in broiler chicken by the acid treatment of their feed. Vet. Rec. 123:416-421. [DOI] [PubMed] [Google Scholar]
- 26.Hugouvieux-Cotte-Pattat, N., and J. Robert-Baudouy. 1992. Analysis of the regulation of the pelBC genes in Erwinia chrysanthemi 3937. Mol. Microbiol. 6:2363-2376. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, C. R., J. Newcombe, S. Thorne, H. A. Borde, L. J. Eales-Reynolds, A. R. Gorringe, S. G. P. Funnell, and J. J. McFadden. 2001. Generation and characterization of a PhoP homologue mutant of Neisseria meningitidis. Mol. Microbiol. 39:1345-1355. [DOI] [PubMed] [Google Scholar]
- 28.Keen, N. T., D. Dahlbeck, B. Staskawicz, and W. Belser. 1984. Molecular cloning of pectate lyase genes from Erwinia chrysanthemi and their expression in Escherichia coli. J. Bacteriol. 159:825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King, E. O., M. K. Ward, and O. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 30.Kotoujansky, A., M. Lemattre, and P. Boistard. 1982. Utilization of a thermosensitive episome bearing transposon Tn10 to isolate Hfr donor strains of Erwinia carotovora subsp. chrysanthemi. J. Bacteriol. 150:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llama-Palacios, A., E. López-Solanilla, C. Poza-Carrión, F. García-Olmedo, and P. Rodríguez-Palenzuela. 2003. The Erwinia chrysanthemi phoP-phoQ operon plays an important role in growth at low pH, virulence and bacterial survival in plant tissue. Mol. Microbiol. 49:347-357. [DOI] [PubMed] [Google Scholar]
- 32.López-Solanilla, E., F. García-Olmedo, and P. Rodríguez-Palenzuela. 1998. Inactivation of the sapA to sapF locus of Erwinia chrysanthemi reveals common features in plant and animal bacterial pathogenesis. Plant Cell 10:917-924. [PMC free article] [PubMed] [Google Scholar]
- 33.López-Solanilla, E., M. Pernas, R. Sánchez-Monge, G. Salcedo, and P. Rodríguez-Palenzuela. 1999. Antifungal activity of a plant cystatin. Mol. Plant-Microbe Interact. 12:624-627. [Google Scholar]
- 34.Miguel, E., C. Poza-Carrión, E. López-Solanilla, I. Aguilar, A. Llama-Palacios, F. García-Olmedo, and P. Rodríguez-Palenzuela. 2000. Evidence against a direct antimicrobial role of H2O2 in the infection of plants by Erwinia chrysanthemi. Mol. Plant-Microbe Interact. 13:421-429. [DOI] [PubMed] [Google Scholar]
- 35.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minagawa, S., H. Ogasawara, A. Kato, K. Yamamoto, Y. Eguchi, T. Oshima, H. Mori, A. Ishihama, and R. Utsumi. 2003. Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli. J. Bacteriol. 185:3696-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nachin, L., and F. Barras. 2000. External pH: an environmental signal that helps to rationalize pel gene duplication in Erwinia chrysanthemi. Mol. Plant-Microbe Interact. 13:882-886. [DOI] [PubMed] [Google Scholar]
- 38.Oyston, P. C. F., N. Dorrell, K. Williams, S. R. Li, M. Green, R. W. Titball, and B. W. Wren. 2000. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect. Immun. 68:3419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perombelon, M. C., and A. Kelman. 1980. Ecology of the soft-rot Erwinias. Annu. Rev. Phytopathol. 18:361-387. [Google Scholar]
- 40.Roeder, D. L., and A. Collmer. 1985. Marker-exchange mutagenesis of a pectate lyase isozyme gene in Erwinia chrysanthemi. J. Bacteriol. 164:51-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. A. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 42.Slonczewski, J. L., and J. W. Foster. 1987. pH-regulated genes and survival at extreme pH, p. 1539-1549. In R. Curtiss III, J. L. Ingraham, E. C. C. Lin, B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 43.Smith, R. L., and M. E. Maguire. 1998. Microbial magnesium transport: unusual transporters searching for identity. Mol. Microbiol. 28:217-226. [DOI] [PubMed] [Google Scholar]
- 44.Torriani, A. 1960. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim. Biophys. Acta 38:460-479. [DOI] [PubMed] [Google Scholar]
- 45.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 93:273-284. [PubMed] [Google Scholar]
- 46.Wilmes-Riesenberg, M. R., B. Bearson, J. W. Foster, and R. Curtiss III. 1996. Role of the acid tolerance response in virulence of Salmonella typhimurium. Infect. Immun. 64:1085-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]