Dear Editor,
We here report on a 82-year-old patient with relapsed/refractory multiple myeloma (RRMM), who was successfully treated with the T cell engaging bispecific antibody teclistamab targeting B cell maturation antigen (BCMA) [1–4]. Our patient was newly diagnosed with lambda (λ) light chain (LC) MM in July 2021 at age 80 and initially presented with cervical pain. A whole-body computed tomography (WB-CT) scan and laboratory investigations revealed multiple osteolytic lesions, anemia (13.2g/dL) and impaired kidney function (serum creatinine 1.21mg/dL; GFR 58mL/min/1.73qm) representing symptomatic MM fulfilling 3/4 CRAB criteria (hypercalcemia, renal dysfunction, anemia, bone involvement). Bone marrow biopsy confirmed a substantial plasma cell infiltration of 80% and unfavorable cytogenetics (t(11;14); monosomy 13; CKS1B gene amplification in 1q21), the ISS/R-ISS were I and II, respectively, and his λ-serum free light chains (FSLCs) were elevated at 236mg/L. His revised myeloma comorbidity index (R-MCI) was 5/9 (=intermediate-fit), reflecting relevant comorbidities including NSTEMI, multivessel coronary artery disease, and severe aortic valve stenosis [5, 6].
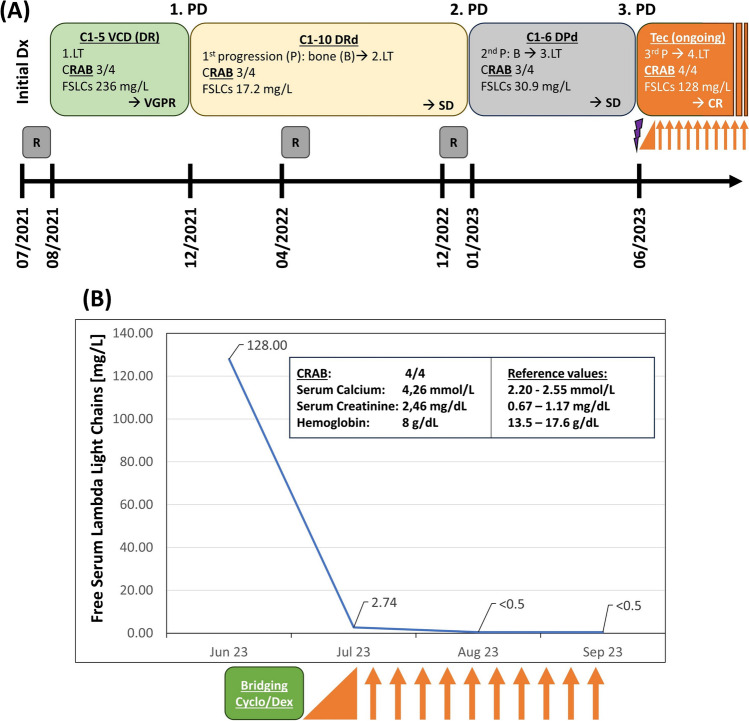
During first-line therapy (1.LT), the patient received radiation therapy of symptomatic bone lesions and five cycles of bortezomib-cyclophosphamide-dexamethasone (VCD; cyclophosphamide: 750mg absolute dose day (d) 1 i.v.; dexamethasone 20mg; bortezomib 1.3mg/m2 s.c. d1,8,15), achieving good 1.LT tolerance and very good partial response (VGPR; Fig. 1A). Due to bone progression in December 2021, a 2.LT with daratumumab-lenalidomide-dexamethasone (DRd) was administered for a total of 10 cycles, achieving serological stable disease (SD), painless, constant bone lesions, and good quality of life (QoL). Due to WB-CT confirmed bone progression with exacerbating pain in January 2023, daratumumab-pomalidomide-dexamethasone (DPd) was given as 3.LT for 6 cycles. However, 4/4 CRAB symptoms with hypercalcemia, impaired renal function, anemia, and bone pain reoccurred, verified by increased λ-SFLCs (128mg/L; Fig. 1A, B). After having received three prior lines of therapy, including a proteasome inhibitor (PI), two immunomodulatory drugs (IMiDs), and an anti-CD38-antibody, the patient presented with triple-refractory RRMM (Fig. 1B). His general condition had deteriorated (Eastern Cooperative Oncology Group [ECOG] 2; R-MCI: 7/9=frail), accompanied by weight loss and neutropenia. Since the patient, his family, and our treatment team remained supportive of further anti-myeloma treatment, the patient was enrolled in a compassionate use program with teclistamab for RRMM after PI, IMiD, and CD38-antibody-exposure.
Fig. 1.
A Treatment regimens in our patient over a course of 2 years and B free serum lambda light chain (FSLC) decline upon response to teclistamab treatment. A At initial diagnosis (Dx) in 07/2023, the 80-year-old patient presented with λ-serum free light chains (FSLCs) of 236mg/L and fulfilled 3 out of 4 CRAB criteria (fulfilled ones depicted bold and underlined). After initial radiation therapy (R), he received five cycles (C) of VCD (bortezomib/cyclophosphamide/ dexamethasone) as a first line therapy (LT) leading to a very good partial response (VGPR). Upon bone (B) progression (P) (=first progressive disease, 1.PD), 10 C of DRd (Daratumumab/lenalidomide/dexamethasone) were administered. Stable disease (SD) was achieved. Due to again progressive bone disease (2.PD), the therapy was switched to DPd (Daratumumab/pomalidomide/dexamethasone) for six C. This led to SD again. B In June 2023, the patient presented with 4/4 CRAB criteria and FSLCs of 128mg/L (=3.PD). Due to his substantial disease burden, a bridging therapy with 200mg/day cyclophosphamide (Cyclo) and dexamethasone (Dex) 20mg/day (purple arrow in A/green box in B) was applied for 5 days until teclistamab (Tec) was followed. Tec was given as part of a compassionate use program. Ramp-up doses of teclistamab (orange triangle; corresponds to C1 with the following three doses: day 1: 0.06mg/kg; day 3: 0.3mg/kg; day 5: 1.5mg/kg) were administered. Subsequently, the bispecific antibody was administered subcutaneously once weekly (1.5 mg/kg) in the outpatient setting (orange arrows; representing ongoing Cs) and is well tolerated. Upon Tec response, FSLCs impressively dropped to <0.5mg/L. Serum and urine became immunofixation negative (CR; see further details in Supplementary Table 1)
The patient was hospitalized and received multimodal supportive therapy for his general stabilization. Due to persisting bone pain, hypercalcemia, and renal impairment, a short-term bridging therapy with 200mg/day cyclophosphamide and dexamethasone 20mg/day for 5 days was given to limit disease progression until teclistamab initiation. Thereafter, step-up dosing of teclistamab was performed according to the recommended dosing schedule, and thereupon given at 1.5mg/kg/week in the outpatient setting (Fig. 1B). No signs of cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS) occurred during initiation or thereafter [7, 8]. Upon hospital discharge, the general condition was substantially improved (ECOG 1, R-MCI 5/9). The patient achieved a complete remission (CR) with normalized λ-SFLCs of <0.5mg/L 1 and 2 months after teclistamab initiation (Fig. 1B, Suppl. Table 1). Mild hematologic adverse events, without need for supportive treatment, occurred (Common Terminology Criteria of Adverse Events [CTCAE] grade I pancytopenia) [9].
This case impressively demonstrates the feasibility, safety and efficacy of teclistamab treatment, also in very elderly patients (>80 years=octogenarians) suffering from symptomatic RRMM after PI, IMiD, and 38-antibody-exposure, suggesting that difficult-to-treat, elderly and/or frail patients may benefit from BCMA- or GPRC5D-bispecifics and others to come [10]. In line with this, we have now treated two other octogenarians with teclistamab in the same program, also achieving a rewarding response, good tolerance after as yet median follow-up of 7 weeks (range: 5–12) and all three patients continuing outpatient teclistamab treatment very successfully (Suppl. Table 1). With much longer follow-up, we thrive to report again on these very challenging cases as representatives of a difficult-to-treat patient cohort, that also greatly benefits from the fascinating innovations in modern oncology.
Supplementary information
(DOCX 16.5 kb)
Author contribution
MD and ME wrote the paper; ME, RW, GM, JK, and MB initiated the analysis and detailed discussions on the bispecific use in very elderly MM patients; all authors read, corrected, and approved the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interest
Profs. Drs. M. Engelhardt, R.Wäsch, and C.Miething have received study support, honoraria and traveling support from Amgen, BMS, GSK, Janssen, Sanofi, Takeda. Prof. Dr. M. Engelhardt serves as the Multiple Myeloma Editor for Annals of Hematology. All other authors declare no conflicts of interest. All procedures performed were in accordance with the ethical standards of the institution UKF and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Footnotes
The original version of this article was revised: This article was originally published with the suppl file still has track changes.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shrivastava T, Van Rhee F, Al Hadidi S. Targeting B cell maturation antigen in patients with multiple myeloma: current perspectives. Onco Targets Ther. 2023;16:441–464. doi: 10.2147/ott.s370880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreau P, van de Donk NWCJ, Delforge M, et al. Comparative Efficacy of teclistamab versus current treatments in real-world clinical practice in the prospective LocoMMotion study in patients with triple-class-exposed relapsed and/or refractory multiple myeloma. Adv Ther. 2023;40:2412–2425. doi: 10.1007/s12325-023-02480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Usmani SZ, Garfall AL, van de Donk NWCJ, et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet. 2021;398:665–674. doi: 10.1016/S0140-6736(21)01338-6. [DOI] [PubMed] [Google Scholar]
- 4.Mateos MV, Chari A, Usmani SZ, et al. Comparative efficacy of teclistamab versus physician’s choice of therapy in the long-term follow-up of APOLLO, POLLUX, CASTOR, and EQUULEUS clinical trials in patients with triple-class exposed relapsed or refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2023;23:385–393. doi: 10.1016/J.CLML.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt M, Dold SM, Ihorst G, et al. Geriatric assessment in multiple myeloma patients: Validation of the international Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica. 2016;101:1110–1119. doi: 10.3324/haematol.2016.148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelhardt M, Domm AS, Dold SM, et al. A concise revised myeloma comorbidity index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102:910–921. doi: 10.3324/haematol.2016.162693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin TG, Mateos MV, Nooka A, et al. Detailed overview of incidence and management of cytokine release syndrome observed with teclistamab in the MajesTEC-1 study of patients with relapsed/refractory multiple myeloma. Cancer. 2023;129:2035–2046. doi: 10.1002/cncr.34756. [DOI] [PubMed] [Google Scholar]
- 8.Qi Y, Liu H, Li X, et al. Blinatumomab as salvage therapy in patients with relapsed/refractory B-ALL who have failed/progressed after anti-CD19-CAR T therapy. Ann Med. 2023;55:2230888. doi: 10.1080/07853890.2023.2230888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National USD of H and HS-NI of H (2017) Common Terminology Criteria for Adverse Events (CTCAE). 10.1039/DT9930001947
- 10.Rasche L, Wäsch R, Munder M, et al. Novel immunotherapies in multiple myeloma – chances and challenges. Haematologica. 2021;106:2555–2565. doi: 10.3324/haematol.2020.266858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16.5 kb)