Abstract
Photorefractive keratectomy (PRK) is a safe and popular corneal surgery performed worldwide. Nevertheless, there is potential risk of corneal haze development after surgery. Proper management of post PRK haze is important for good visual outcome. We performed a comprehensive review of the literature on the various risk factors and treatments for PRK haze, searching the PubMed, Google Scholar, SCOPUS, ScienceDirect, and Embase databases using relevant search terms. All articles in English from August 1989 through April 2023 were reviewed for this study, among which 102 articles were chosen to be included in the study. Depending on the characteristics of and examination findings on post PRK haze, different management options may be preferred. In the proposed framework, management of PRK haze should include a full workup that includes patient’s subjective complaints and loss of vision as well as visual acuity, biomicroscopy, anterior segment optical coherence tomography, epithelial mapping, and Scheimpflug densitometry. Topical steroid treatment for haze should be stratified based on early- or late-onset haze. Mechanical debridement or superficial phototherapeutic keratectomy (PTK) may be used to treat superficial corneal haze. Deep PTK and/or PRK can be used to treat deep corneal haze. Mitomycin-C and topical steroids are prophylactic post-surgery agents to prevent recurrence of haze.
Keywords: Corneal haze, Mitomycin-C, LASEK, PTK, PRK, EBMD, Mechanical corneal debridement, Epithelial corneal map, Corneal densitometry, LASIK
Key Summary Points
| Post photorefractive keratectomy (PRK) haze develops due to impaired healing mechanisms, including disruption of hemidesmosome contact, reduced epithelial cell proliferation, excessive inflammatory cytokines, lack of neurotrophic growth factors, and repeat traumas. |
| Different techniques, such as alcohol-assisted PRK, laser-assisted subepithelial keratectomy (LASEK), epithelial laser in situ keratomileusis (Epi-LASIK), and transepithelial PRK have been developed, each with its advantages and challenges in terms of tissue trauma, inflammation, and predictability of results. Alcohol-assisted and mechanical PRK are the most common approaches for surface ablation. |
| Primary risk factors for post PRK haze include spherical correction, astigmatism, and PRK enhancement after previous corneal refractive surgery. Secondary risk factors include dry eye disease, epithelial basement membrane dystrophy, delayed neurotrophic healing, vitamin deficiencies/supplements, ultraviolet (UV) radiation, and ethnic predilection. |
| Mitomycin C (MMC) is an effective preventative measure to reduce haze formation after PRK. The duration of MMC application may vary based on ablation depth, and studies have explored different concentrations. Preliminary evidence suggests that 0.01% MMC is effective in preventing post-PRK haze. |
| Early-onset haze (within the first 6 months) may respond better to intensive steroid therapy, while late-onset haze may require a shorter steroid regimen or surgical intervention. Surgical options include mechanical debridement or superficial phototherapeutic keratectomy (PTK) for superficial haze, and deep PTK or therapeutic myopic PRK ablation for deeper haze. |
Introduction
Refractive surgery, including, for example, photorefractive keratectomy (PRK), laser-assisted in situ keratomileusis (LASIK), and small incision lenticule extraction (SMILE), has become increasingly popular in the past three decades. Each type of surgery has its advantages and disadvantages. PRK can be performed in patients with thinner corneas and irregular topography who may not qualify for LASIK or SMILE; however, there is always a potential risk of corneal haze development, which may impair good visual outcome. Corneal haze is an abnormal, gradual onset, inflammatory response that causes opacification of the stroma. Its incidence was high in early PRK; for example, Gartry et al. reported a haze incidence of 92% in their study carried out in 1992 [1]. In this study, surface ablation was performed with broad beam excimer laser and without mitomycin C (MMC) or corticosteroids [1]. Newer laser technology, such as flying spot lasers and wavefront technology, have reduced the incidence of post PRK haze. Scanning lasers and wavefront technology create smoother ablation surfaces, have reduced inflammation, and promote healing [2–5]. A 1999 study of PRK eyes treated with scanning lasers found a haze incidence of 20% in 266 eyes [6]. With the addition of intraoperative MMC and postoperative topical steroids into the treatment regimen, the incidence of post PRK haze has decreased still further: MMC reduces myofibroblast proliferation and topical steroids further reduce postoperative inflammation. In a large, single-center study carried out in 2017, the authors reported an incidence of post PRK haze of 1.3% [5]. In this study, surface ablation was performed using excimer lasers with flying spot and wavefront technology, intraoperative MMC was used, and all patients received a course of postoperative topical steroids [5]. Despite these improvements, post PRK corneal haze requires prompt and careful management. The aim of this study is to propose a general framework that reflects the current literature.
Methods
A literature search was performed of articles in the PubMed, Google Scholar, SCOPUS, ScienceDirect, and Embase databases using the following search terms: “PRK haze,, “PRK Haze Management,” “PRK haze laser,” “PRK haze treatment,” “Corneal haze,” “PRK fibrosis,” “corneal fibrosis,” “surface ablation haze,” “PRK scar,” “Mitomycin C and corneal haze,” “Post PRK haze,” “Post refractive surgery haze,” “Late corneal haze PRK,” “PTK treatment haze,” “Radial keratectomy haze,” “mechanical debridement haze,” “SMILE enhancement haze,” “LASIK enhancement haze,” “PRK enhancement haze,” “vitamin D haze,” and “UV radiation haze.” This search identified 9542 articles. All articles in English published between August 1989 and April 2023 were reviewed for this study. As a first step, all non-English articles, articles without full texts, and redundant articles were eliminated, resulting in 7478 full texts remaining. These 7478 texts were then filtered for relevant titles. Titles which included words such as PRK haze, photorefractive keratectomy haze, surface ablation haze, and corneal refractive surgery haze were considered to be relevant and included for further review; this filtering eliminated all but 316 articles. The abstracts of these 316 articles were then assessed for their quality based on study design, study size, study date, journal source, and citation frequency by other authors, resulting in the elimination of a further 200 articles; the full text of each of the remaining 116 articles was carefully examined by two independent reviewers/authors; when these two reviewers disagreed, common consensus was reached after careful discussion and joint review of discrepant articles for its relevance to the topic, study design, and study significance. Care was taken to evaluate if the authors of the study answered the questions it originally set out to study and if there were any inherent biases. For example, reviewer 1 did not include a certain article on the incidence of post PRK corneal haze in a navy population due to the small sample size due to concern over the study’s rigor. However, reviewer 2 included this study because he believed it was relevant to the topic of ethnic predilection and should be included as one of the few studies in the literature on this topic. In this case, the article was included after joint discussion. Ultimately, 100 articles were chosen to be included in the study.
This review is based solely on previously conducted studies. It does not contain any new studies with human participants or animals performed by any of the authors.
Pathophysiology of Post PRK Haze
Post PRK haze develops as an inflammatory response to intraoperative epithelial insult. Immediately after disruption of the epithelium and basement membrane, a complex process of cytokine-mediated wound healing begins. Interleukin-1 (IL-1), IL-6, bone morpho-genic protein (BMP) 2 and 4, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), FAS ligand, tumor necrosis factor alpha (TNFα), platelet-activating factor (PAF), transforming growth factor beta (TGFβ), and insulin-like growth factor (IGF) 1 and 2 are released [7–9]. IL-1 and PDGF activate keratocytes, which then produce hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF), which in turn drive epithelial proliferation. Simultaneously, TGFβ transforms some keratocytes into myofibroblasts, which then secrete glycosaminoglycans (GAGs) and disarrayed fibrillar collagens [10, 11]. The molecules within this extracellular matrix do not have uniform alignment and block incoming light at different angles, thereby opacifying the corneal stroma.
The entire repair process is supported by neurotropic signal molecules, such as nerve growth factor (NGF), which extends corneal sensation to new epithelium. As the cornea heals and cytokine levels decrease, stromal myofibroblasts undergo apoptosis. Superficial epithelial defects may heal completely within the first 7–8 days. However, the process of corneal epithelial remodeling for more significant insults with basement membrane destruction may require up to 8 weeks [10–12].
PRK inherently involves disruption of the basement membrane and underlying stroma [12]. Therefore, post-PRK corneas will, on average, take longer to recover than typical epithelial defects. If re-epithelization is further delayed, prolonged myofibroblast proliferation and excessive extracellular matrix deposition can lead to subsequent haze development. This can occur when the underlying mechanisms of healing are disrupted, which include impairment of hemidesmosome contact between epithelium and basement membrane, limbal stem cell deficiencies that reduce epithelial cell proliferation, excessive inflammatory cytokines, lack of neurotrophic growth factors to stimulate healing, and repeat traumas [13].
Historical Trends of Post PRK Haze
Traditional broad beam lasers ablated the cornea with large, low-frequency, and fixed laser spots. This method had higher levels of corneal surface irregularity and caused increased thermal tissue damage, both of which are risk factors for the development of post PRK haze [14]. These issues are much reduced in procedures using modern, high-frequency flying spot lasers, which have lower raster energy and create a smoother ablation surface [15].
Originally, epithelium was mechanically debrided before surface ablation. Concerns over tissue trauma and inflammatory healing led to the invention of alcohol-assisted PRK. In this technique, 18–20% ethyl-alcohol is used so the epithelium can be removed with minimal scraping, thereby reducing anterior basement membrane disruption [16]. In laser-assisted sub-epithelial keratectomy (LASEK), the epithelium is kept intact after it is separated from the stroma with alcohol and replaced back in its original position over the cornea after ablation [3]. The main concern was that stromal exposure to the tear film can increase inflammation, so the original epithelium was repositioned over the stroma after ablation. Instead of promoting healing, however, peripheral epithelial migration and keratocyte proliferation were delayed by dead epithelium tissue secondary to alcohol toxicity [3]. To avoid this issue, epithelial laser in situ keratomileusis (Epi-LASIK) uses an epithelial keratome to lift the epithelium from Bowman’s membrane, but this introduced the new problem of unpredictable cut depth. The Epi-LASIK shave would sometimes damage stroma or leave behind residual epithelium. Both created unpredictable PRK results. The most recent PRK technique is transepithelial PRK (t-PRK), which utilizes excimer laser epithelial removal [17]. Although t-PRK is becoming increasingly popular, only a few excimer lasers are approved for both epithelial and stromal ablation. More importantly, post PRK haze has been reported in t-PRK as well [18]. For these reasons, alcohol-assisted and mechanical PRK remain the most common approach for surface ablation in the USA and other parts of the world.
Risk Factors Concerning Post PRK Haze
Primary risk factors are directly linked to higher haze incidence. Secondary risk factors are indirectly linked to higher haze occurrence through increased delayed wound healing, which is strongly associated with increased incidence of post PRK haze development [19]. A summary of risk factors can be found in Table 1.
Table 1.
Risk factors associated with post photorefractive keratectomy haze development
| Primary risk | Criteria |
|---|---|
| Spherical correction | Individuals with > 5 D of myopic correction or hyperopia |
| Astigmatism correction | Individuals with > 3 D of astigmatism |
| Previous corneal refractive surgery | RK, PRK, LASIK, SMILE |
| Secondary risk | Treatment |
|---|---|
| Dry eye disease | Preservative-free eye drops, gel, punctal plug, immunomodulators, BCL, amniotic membrane, autologous blood serum |
| UV exposure | Eye protection, avoidance to excessive sun and UV exposure |
| Vitamin D deficiency | 6000–10,000 IU vitamin D daily for 8 weeks |
| EBMD | Patient counseling of risks and benefits; risk assessment by surgeon |
| Delayed neurotrophic healing | Review of history of delayed wound healing or corneal neuropathy; HbA1c check in patients with suspicion for undiagnosed diabetes mellitus |
BCL Bandage contact lens, D diopter, EBMD epithelial basement membrane dystrophy, HbA1c glycated hemoglobin, IU international units, LASIK laser assisted in situ keratomileusis, PRK photorefractive keratectomy, RK radial keratotomy, SMILE small incision lenticule extraction, UV ultraviolet radiation
Primary Risk Factors
Spherical Correction
Spherical correction appears to be positively correlated with the risk of post PRK haze based on several large studies. In Kaiserman et al.’s 2017 study of 5634 eyes, patients with high myopia developed post PRK corneal haze at twice the incidence of patients with moderate and low myopia (2.1% vs. 1.1%) [5]. Authors of other studies report similar results, although the specific cutoff for attempted spherical correction differs slightly among these studies [2, 4, 20]. This trend appears to be more strongly associated with hyperopic correction, with a reported 8.1-fold higher likelihood in the study of Kaiserman et al. [5]. These authors also noted a statistically significant increase in the risk for post PRK haze development and ablation depth [5]. This finding is in accordance with studies performed with older techniques as well as in other recent studies [2, 5, 18]. Although the underlying mechanism is not completely understood, greater ablation depths cause more keratocyte apoptosis and myofibroblast proliferation [21, 22]. The ablation profile may also play a key role in post PRK haze development, as evidenced by findings in hyperopia groups, with the haze in these groups reported to develop in a peripheral, bagel-like shape rather than the central haze seen in myopia groups [5].
Astigmatism
Individuals with astigmatism are at higher risks for post PRK haze development, independent of attempted spherical correction. In a study of patients with low to moderate myopia, preoperative astigmatism > 2 diopter (D) was a risk factor for developing post PRK haze [23]. Haze incidence increased for both early-onset, mild haze and late-onset, severe haze. In one study, the relative risk (RR) for > 3 D of astigmatism was 2.9 and 5 for early- and late-onset haze, respectively [5]. Astigmatic ablation appears to cause haze, as examinations show haze development along the steep axis of treatment [23]. One theory is the ablation shape and sharp angle, corneal curvature change induces an abnormal healing response.
PRK Enhancement After Previous Corneal Refractive Surgery
Radial keratotomy (RK). In a 1995 study of patients undergoing RK without the application of MMC, 18% of patients lost ≥ 1 lines of visual acuity secondary to post PRK haze [24]. In a study carried out much later with MMC application, the incidence decreased to between 8.3% and 19% at 6 months of follow-up [25]. Visually significant post PRK haze at 12 months was reported to be between 3.1% and 3.3%. Ghanem et al. [26] reported 4.9% of patients with central haze at 24 months post surgery. The number of radial incisions increased peripheral haze development post PRK. These studies reflect PRK procedures performed in the late 1990s and early 2000s, so the incidence for modern PRK enhancement after RK may differ.
PRK. In eyes that had prior PRK, PRK enhancements may increase the incidence of mild, clinically insignificant haze. In one study, 11 out of 188 eyes that underwent alcohol-assisted PRK enhancement developed haze, but the haze resolved in all eyes with no effect on visual outcome [27]. Similarly, Broderick et al. [28] reported clinically insignificant haze development in 7.9% of eyes and haze resolution without intervention. The incidence of clinically significant haze was much higher in older studies; however, the authors of these studies performed PRK using older lasers without MMC application [24].
LASIK. The literature is unclear on post PRK haze incidence after PRK enhancement in post-LASIK eyes. The incidence of post PRK haze was 2.7% in a 2022 study of 374 eyes; this occurrence rate is similar to haze incidence reported in virgin eyes [28]. However, the incidence is less favorable based on other studies (5.3–5.8%) [29–31]. These studies used alcohol-assisted PRK to perform the enhancement. Future, prospective studies may elucidate the true effects of PRK enhancement in post-LASIK eyes.
SMILE. In SMILE, one study reported that 25.4% of SMILE patients developed grade 1 haze and 6% developed grade 2 haze after t-PRK enhancement; however, all eyes achieved haze-free status by 3 months post surgery [32]. In two studies of 67 post-SMILE, alcohol-assisted PRK enhancement eyes, only one eye developed visually significant corneal haze; however, the follow-up was limited to 3 months [33, 34]. Larger, long-term studies are necessary to better understand the relationship between post PRK haze and PRK enhancement after SMILE.
Secondary Risk Factors
Dry Eye Disease
Dry eye disease (DED) is common in refractive surgery patients, with one study reporting preoperative dry eyes in 45.4% of patients [35]. DED can delay corneal wound healing by altering ocular surface microenvironment homeostasis. Increased tear film inflammation can increase the breakdown of corneal stromal extracellular matrixes through elevated levels of cytokines, chemokines, and enzymes [36]. Inflammation also reduces tear film instability and lubrication properties, leading to increased corneal abrasion in DED. It behooves the clinician to perform preoperative DED treatment to reduce post PRK haze incidence.
Epithelial Basement Membrane Dystrophy
Epithelial basement membrane dystrophy (EBMD) as a cause for post PRK haze has been reported in a case report [37]. Proper epithelial basement membrane adhesion is crucial to corneal wound closure. Underlying basement membrane disease, such as EBMD, predisposes patients to impaired wound healing [38, 39]. In EBMD, the basement membrane is irregular, and infiltrated with pseudocysts and “fingerprints” of parallel, redundant basement membrane [40]. Hemidesmosomal adhesion impairment between the epithelium and Bowman’s membrane is the pathophysiologic mechanism of EBMD. EMBD prevalence may be as high as 42% in the general population, and individuals with EBMD are at high risk of developing recurrent epithelial erosions [38, 41]. In one case report, a patient with EBMD had persistent epithelial defect 17 days post PRK. However, not all patients with EBMD develop post PRK haze [42]; sometimes, PRK can paradoxically treat EBMD as it removes abnormal epithelium and allows possible regrowth of normal epithelium [37, 43]. More studies are necessary to draw definitive conclusions on the association between EBMD and post PRK haze.
Vitamin Deficiencies/Supplements
Epidemiologic studies worldwide estimate vitamin D deficiency rates to be between 13% and 79% [44–49]. In the USA, 28.9–66% of individuals may be vitamin D deficient, with vitamin D insufficiency occurring at an even higher rate [44, 45]. Vitamin D plays an important role in DED. Vitamin D, by inducing IL-10 production, decreases the levels of tear film inflammatory cytokines, such as IL-1, IL-6, and TNFα [46]. Vitamin D also increases tear film stability by increasing tear osmolarity [49, 50]. Patients with vitamin D deficiency have increased Ocular Surface Disease Index (OSDI), decreased tear break up time (TBUT), and lower Schirmer levels [46, 51]. Vitamin D also promotes corneal wound healing through cathelicidin production, an epithelial antimicrobial. In vitamin D receptor knockout mice, corneas were thinner, with less epithelial cell diffusion [52]. In a prospective study, Kundu et al. [53] found reduced post PRK haze incidence after oral vitamin D supplementation.
Vitamin C is a known antioxidant found in tear films. Its role in promoting corneal wound healing, especially after alkali burns has been studied [54]. In corneal refractive surgeries, including PRK, vitamin C levels in the tear film are reduced, possibly due to epithelial loss [55]. A high concentration of ascorbic acid is retained in the corneal epithelium. Therefore, ascorbic acid supplementation has been studied. In one study of 96 eyes, 500 mg twice daily of vitamin C was prescribed for 1 week before LASEK and until 2 weeks after surgery: 33.3% and 37.5% of the treatment and placebo groups developed corneal haze, respectively [56]. Similarly, a double-masked, randomized controlled trial (RCT) in which patients received 250 mg daily vitamin C supplementation for 7 days did not show significant difference in subjective pain, corneal re-epithelization time, and corneal haze incidence compared to placebo [57]. Of note, studies investigating alkali burns utilized topical ascorbic acid, so perhaps topical delivery would have a more effective result in reducing oxidative stress and promoting wound healing after PRK.
Vitamin A plays a role in promoting limbal stem cell differentiation and wound healing [58]. In rabbits, 0.1% retinol palmitate has been shown to increase cataract incision wound healing [59]. In a 2013 study, Chelala et al. [60] performed a RCT in which eyes were treated with topical retinol palmitate ointment (250 IU/g per eye) after PRK. In terms of visual and refractive outcomes and subepithelial post PRK haze incidence at 3 months, the treatment group was not statistically different from the placebo group. However, a different study in which patietns with PRK received both retinol palmitate and vitamin E (alpha tocopheryl nicotinate) showed faster re-epithelialization time and lower post PRK haze incidence [61]. Vitamin E is an antioxidant found in all tissues and reported to reduce keratocyte apoptosis after traditional PRK [62]. In rabbits, topical 1% vitamin E ointment has been shown to have a weak but statistically significant effect in reducing post PRK haze [63]. Therefore, topical vitamin A and E may be beneficial for preventing post PRK haze. Further studies are necessary to elucidate the optimal dosing and exact benefit.
Ultraviolet Radiation
Exposure to utlraviolet (UV) radiation increases the rates of post PRK haze. In a long-term post PRK follow-up, 11 out of 404 eyes developed late haze in the high-UV group compared to none in the low-UV group [64], consistent with results from an animal study in which post-PRK rabbit eyes were irradiated with UV-B light [65]. To our knowledge, no studies have directly investigated the effects of UV radiation on the incidence of post PRK haze in clinical trials. We also did not find any studies reporting the effects of UV-A on the development of post PRK haze. However, it is well known that UV radiation can damage ocular structures [66, 67]. Post PRK patients are often advised to protect their eyes from UV radiation and avoid activities with excessive sun exposure [68]. Oral vitamin D supplementation can account for the loss of natural vitamin D production through sunlight. In a 2021 meta-analysis, oral vitamin D supplementation was found to be superior to UV radiation in raising serum vitamin D levels [69].
Ethnic Predilection
Racial ethnicities and pigmented irises have historically been considered to be risk factors for post PRK haze. However, the literature on this topic is conflicting. One study in a Middle East population showed higher post PRK haze development in eyes with brown irises compared to those with blue irises. Among 100 post PRK eyes with blue irises and 166 post PRK eyes with brown irises, corneal haze incidence was 5% and 28.9%, respectively (p < 0.001) [6], with two brown eyes and no blue eyes developing severe haze. Since the study was carried out in one ethnic group, the findings may suggest that dark pigmentation—and not ethnicity itself—is the risk factor for the development of post PRK haze. Similarly, at one time it was believed that African Americans were at a higher risk of post PRK haze due to the higher prevalence of keloid formation in this population. However, a study by Tanzer et al. did not find an increased incidence of post PRK haze in African Americans with a history of keloids [70]. In that study, among 19 African American eyes who underwent PRK, eight eyes developed grade 1+ corneal haze, but only one eye was from an individual with a history of keloid formation. Two other individuals in the PRK group had a history of keloids yet did not develop haze. All eyes in the study achieved 20/20 or better corrected distance visual acuity at 13 months of follow-up [70]. Studies comparing ethnicity or eye color are rare, so it is unclear if brown irises truly increase the risk of post PRK haze. However, the most recent study on this topic, performed in 2021, found a comparable incidence of post PRK haze among various ethnic populations if brown-eyed patients were treated with MMC application and topical steroids [71]. It is possible that the ethnic predilection of post PRK haze in this population is reduced with the use of current surgical techniques and post-operative care.
PRK Haze Prevention
Mitomycin-C
The lower incidence of haze in modern PRK is achieved primarily through prevention. MMC is an antimetabolite that prevents fibroblast proliferation and extracellular matrix deposition. No consensus exists on the duration of MMC application for post PRK haze prevention, but it is generally increased for greater ablation depths. In one study of 1011 eyes, Lee et al. stratified the application time to 30 s, 1 min, or 2 min for ablation depths of 100 µm, 100–120 µm, and > 120 µm, respectively [2]; the overall incidence of post PRK haze in this study was 3.2%. In a more recent study (2017), 0.02% MMC was applied for 20 s up to 60 s depending on the ablation depth; the incidence of post PRK haze was 2.1% [5]. Unfortunately, the authors of this study did not disclose the specific duration for each ablation depth . A much smaller study carried out in 2022 included 120 eyes that underwent t-PRK and then treated with 0.02% MMC for 30–50 s without specification of ablation depth [72]. In this study, one eye developed post PRK haze [72]. The concentration of MMC application has also been explored, with one RTC showing the effectiveness of 0.01% MMC for the prevention of post PRK haze; however, this study was limited by sample size [73]. Although in vivo studies have demonstrated that MMC can decrease keratocyte density, the intraoperative application of a single dose of 0.02% MMC has not been shown to cause toxicity [43, 74]. Overall, strong evidence supports the use of MMC as an effective preventative of post PRK haze.
Chilled Balanced Saline Solution
In the late 1990s, several studies demonstrated the effectiveness of cold balanced saline solution (BSS) corneal irrigation post surface ablation for the prevention of post PRK haze [75, 76]. Chilled fluids were understood to reduce excimer laser-related, thermal energy-induced inflammation. This inflammatory mechanism is of less concern in modern low-energy lasers. In a recent prospective study comparing irrigation with chilled versus room temperature BSS, the authors did not find a difference in outcome [77]. Nevertheless, chilled BSS remains a common practice as a final step upon the conclusion of the ablation treatment.
Corticosteroids
Topical corticosteroids prevent inflammation and keratocyte-to-fibroblast transformation; therefore, they are routinely prescribed to patients post PRK [74]. However, clinical evidence on the effectiveness of corticosteroids is less clear. Several large studies show that steroid treatment plays no role in reducing post PRK haze [1, 78]. The regimens tested included 0.1% dexamethasone for 3 months, prednisolone 1% for 3 months, and fluorometholone 0.1% for 3 months, but none showed superiority over placebo [79, 80], although a weak benefit was reported in high-myopia eyes [81]. Questions have also been raised regarding whether routine MMC use had confounded the effectiveness of topical corticosteroids in the prevention of post PRK haze. In a RCT by Gambato et al. [80], 20% of the eyes in the steroid group developed haze compared to 0% in the MMC group; corrected distance visual acuity was found to be significantly better in the MMC group, even at 5 years of follow-up [82]. One possibility is that the duration of steroid treatment is reduced post PRK. This finding corresponds with the results of a recent 2022 clinical trial in which the outcomes of 252 eyes in patients on 3-, 2-, and 1-month fluorometholone regimens were compared; the authors found no difference in post PRK haze development or visual outcomes [83]. Yet, the potential side effects of steroids are well known; in one study, elevated intraocular pressure (IOP) was detected in up to 7% of patients post PRK [84]. Steroids have the additional risk of increasing infection and cataract formation, delaying healing, and decreasing keratocyte density.
Other Factors
Post PRK patients are prone to DED due to corneal nerve compromise, especially 3–6 months post surgery [85, 86]. In one study, Hovanesian et al. [87] reported an incidence of 43% DED. Aggressive lubrication with preservative-free eye drops helps to maximize post PRK epithelial recovery. In patients who require additional management, a treatment ladder of gel, punctal plug, immunomodulators, bandage contact lens (BCL), amniotic membrane, and autologous blood serum drops can be implemented [36].
Clinicians may opt to test vitamin D levels before refractive surgery due to the high prevalence of vitamin D deficiency post PRK. 25-hydroxyvitamin D [25(OH)D] levels < 50 nmol/L are considered to indicate vitamin D deficiency. The standard adult treatment is 6000 IU daily for 8 weeks, while obese patients or those with malabsorption syndromes may require up to 10,000 IU per day [88]. Vitamin A and E supplementation can also be prescribed. Based on prior studies, a combination of 25,000 IU retinal palmitate and 230 mg α-tocopheryl nicotinate daily for 3 months can reduce the incidence of post PRK haze [60]. The exact dosage for vitamin C supplementation is less clear, as studies utilizing 500 mg twice daily for 3 weeks and 250 mg daily for 1 week did not show a benefit [56, 57].
Patients should avoid prolonged UV exposure and wear ocular UV protection. Other sources of excessive UV radiation, such as tanning, salons should also be avoided [66, 67]. Clinicians may want to inform their patients to be particularly careful about UV eye protection for the first two summers after PRK.
Promising Future Therapeutics for the Prevention of PRK Haze
There are numerous therapeutic medications that are being studied in animals and early human trials. The oral administration of the antioxidant cysteine at 200 mg daily was found to improve epithelial healing in 50 patients who underwent refractive surgery. Topical basic fibroblast growth factor appears to further augment this benefit [89].
Suberoylanilide hydroxamic acid (SAHA) is a derivative of trichostatin A, a signaling molecule involved in the inhibition of myofibroblast activation. SAHA in combination with 0.01% MMC has shown equivalency in reducing post PRK haze compared to 0.02% MMC alone in a rat model [90, 91]. SAHA has the benefit of decreased cellular toxicity compared to MMC based on preliminary results. Suppressor of mothers against decapentaplegic (SMAD7) protein is a key signaling molecule in activation of the TGFβ pathway. Its application was reported to reduce post PRK inflammation in rabbit corneas [92]. Targeted therapy likely has a better safety profile than antimetabolites such as MMC, which are broadly toxic to rapidly dividing cells. However, human trials are necessary to determine their effectiveness.
Losartan is an antihypertensive that decreases post PRK haze and myofibroblast generation in rabbit corneas [93]. One case of successful post-LASIK haze treatment with off-label topical losartan has been reported in the literature [94]. The patient developed striae at 4 days post surgery and required retreatment with flap lift. A dense subepithelial haze developed 48 days after retreatment and uncorrected distance visual acuity (UDVA) decreased to 20/200. The patient received 0.8 mg/mL losartan 6 times daily and vision improved to 20/30 at 4.5 months of follow-up [94].
Discussion
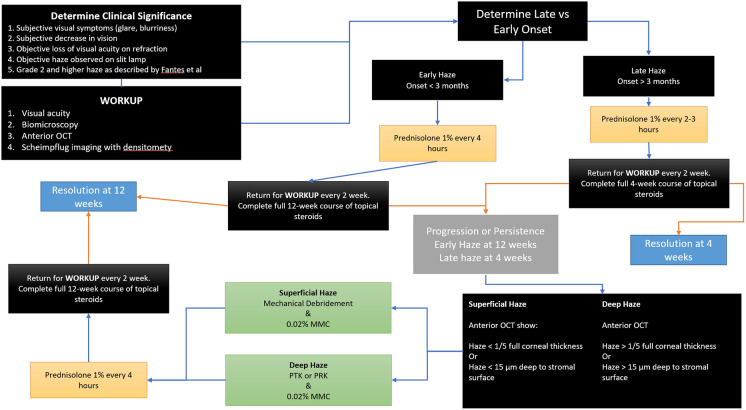
Inevitably, despite all precautions and suggested recommendations, clinicians may still encounter post PRK haze in 2–3.6% of patients [17, 35]. A recent French review discussed the various risk factors and preventatives for post PRK haze [85]. In this section, we discuss the management of PRK haze within the generalized framework of the current literature (Fig. 1).
Fig. 1.
A proposed framework for the management of post PRK haze. PRK Photorefractive keratectomy, OCT optical coherence tomography, MMC mytomycin
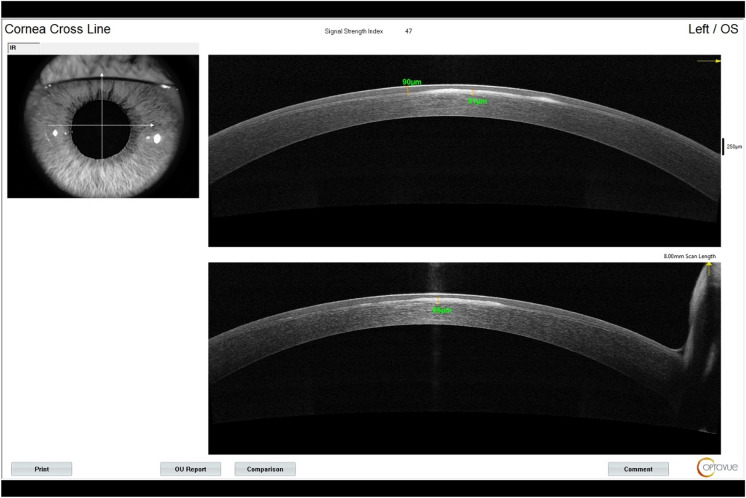
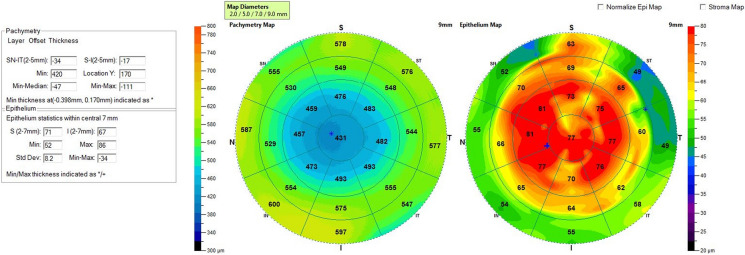
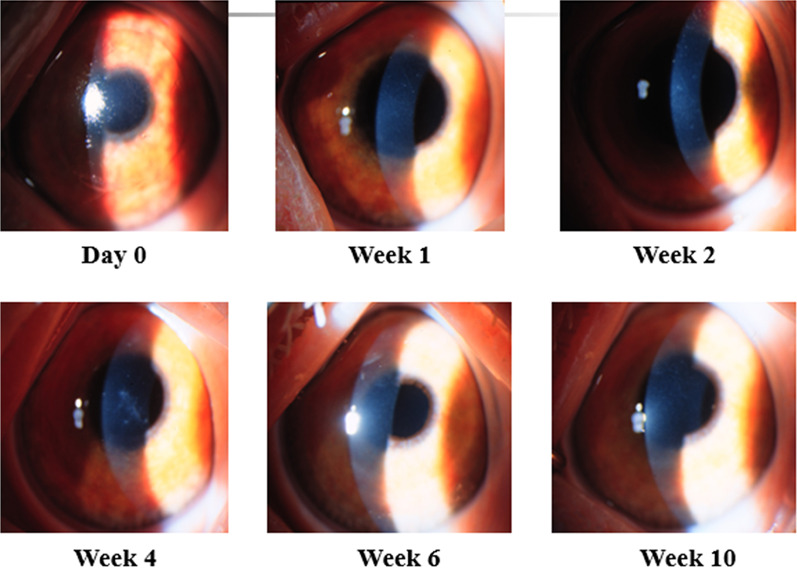
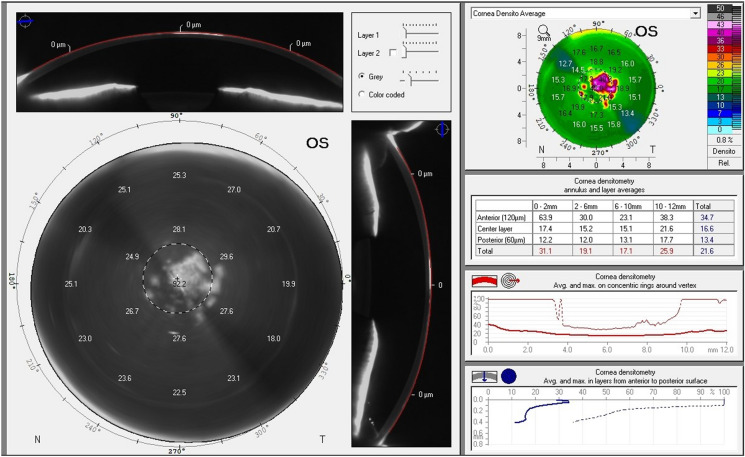
For patients’ initial post PRK haze presentation, we recommend a workup with imaging, including anterior OCT with two views of the corneal cross section (Fig. 2), 9-mm epithelial map (Fig. 3), and corneal densitometry using Scheimpflug technology. Almost all patients develop some level of mild corneal opacification after PRK, but this is a normal response to stromal damage by corneal fibroblasts [96, 97]. This haze is clinically insignificant and resolves naturally within 6–12 months, and it differs from the post PRK corneal haze that develops from stromal fibrosis, which is an abnormal healing response [96]. Significant haze will be defined by the following: (1) patient has subjective visual disturbance; (2) findings are visually significant; (3) patient has loss of visual acuity on refraction; (4) signs of haze on biomicroscopy; and (5) haze is assessed at grade 2 or higher, as described by Fantes et al. [98]. If all these criteria are not met, the haze may simply be monitored, and exploration of alternative explanations of visual disturbance are needed. Obscuration of anterior segment structures on biomicroscopy can be used to grade post PRK haze (Figs. 4, 5, 6, 7). Anterior segment OCT with two views of the corneal cross section and 9-mm epithelial map can help assess the depth of haze invasion and differentiate epithelial hyperplasia from stromal haze. Corneal densitometry by Scheimpflug imaging calculates the grayscale values (a proxy for corneal opacification) of the anterior, central, and posterior cornea, and further divides the cornea into expanding concentric rings, which enables assessment of the amount of haze relative to central cornea (Fig. 8). These imaging modalities are excellent toofs for tracking progress throughout the management course (Fig. 9).
Fig. 2.
Anterior OCT of the left eye with two views of the corneal cross section. Measurements are the epithelial and stromal haze depth (left and right measurements, respectively). OCT Optical coherence tomography
Fig. 3.
Epithelial map of the left eye showing thickened epithelium > 50 µm
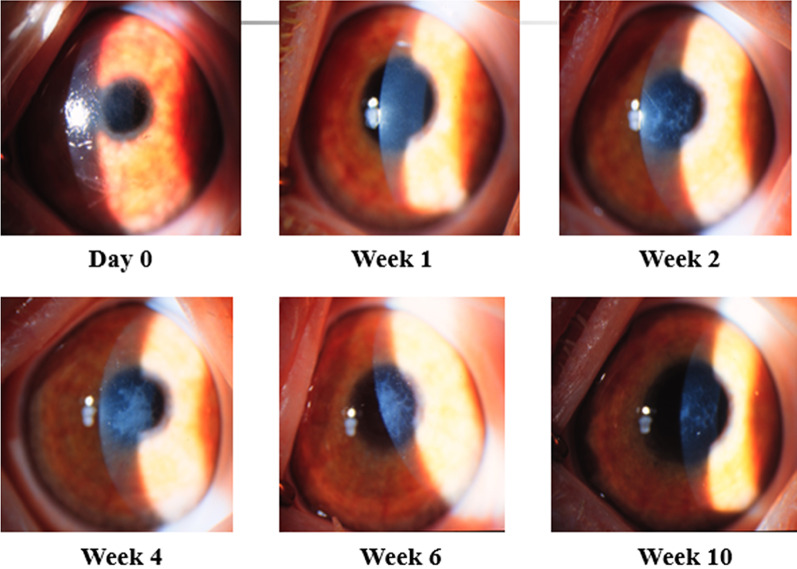
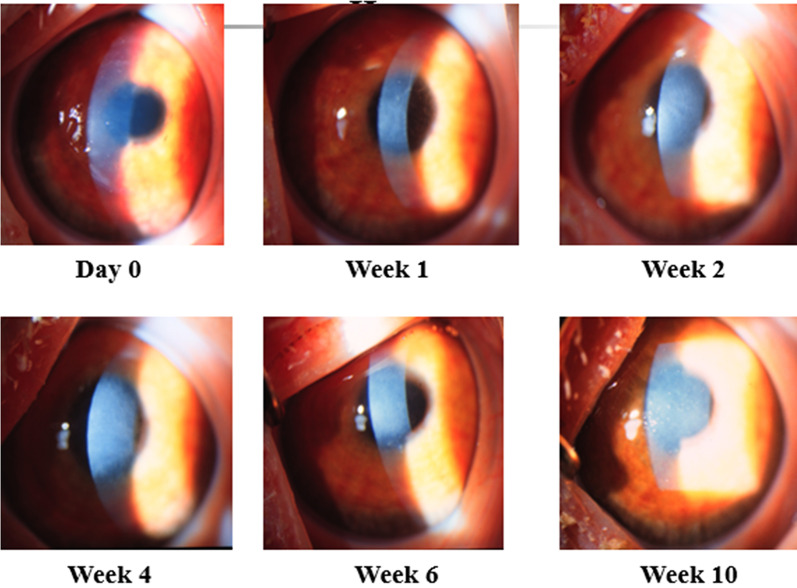
Fig. 4.
Mild corneal haze without obscuration of anterior segment structures
Fig. 5.
Moderate corneal haze partially obscuring anterior segment details
Fig. 6.
Severe corneal haze with marked obscuration of anterior segment structures
Fig. 7.
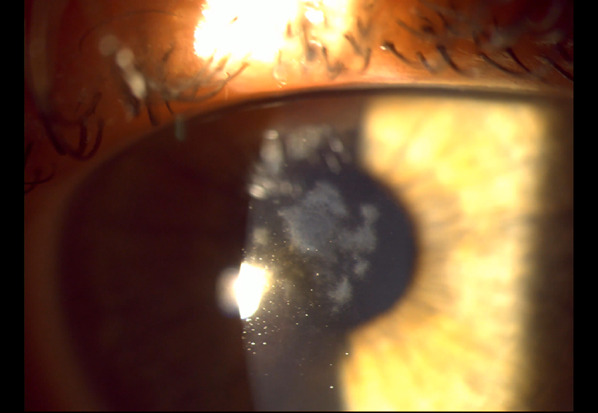
Biomicroscopy of the left eye demonstrating central stromal haze affecting the visual axis
Fig. 8.
Scheimpflug densitometry of the left eye revealing central corneal post PRK haze. PRK Photorefractive keratectomy
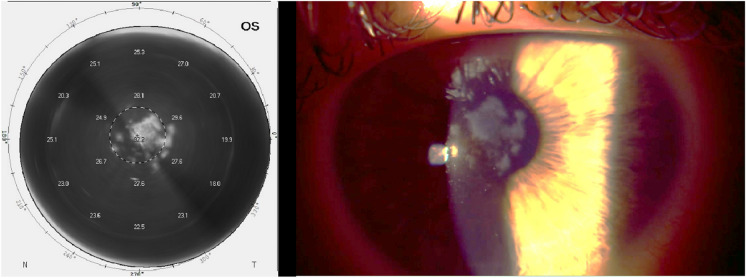
Fig. 9.
Side-by-side view of biomicroscopy and Scheimpflug densitometry of the left eye demonstrating densitometry’s remarkable similarity to haze observed on biomicroscopy
Once post PRK haze is determined to be visually significant, clinicians may desire to differentiate early- from late-onset haze. Early-onset post PRK haze develops within the first 3 months of surgery and potentially responds better to topical steroids [97]. Steroid resistance is common in the late-onset haze that develops up to 1 year after PRK [5, 96]. For early-onset haze, we recommend an initial 12-week trial of intensive steroid therapy [5, 99]. Patients can start prednisolone 1% every 4 h and return for workup every 2 weeks. Repeated anterior OCTs, epithelial maps, and densitometry are excellent tools to monitor treatment response. If post PRK haze persists up to 12 weeks, it has likely progressed to late-onset haze and further intervention is necessary [5, 99]. For late-onset haze, a shorter steroid regimen could be trialed. We recommend prednisolone 1% every 2–3 h, with bi-weekly workups and imaging. Instead of 12 weeks, surgical management may be initiated if there is no response or progression within 4 weeks. If the patient improves on steroid monotherapy, treatment can be slowly tapered until symptoms completely resolve.
Surgical intervention for haze persistence could be discussed with the patient [7]. Corneal OCT, epithelial mapping, and corneal densitometry can guide the clinician toward the best specific approach for the patient in terms of post PRK haze management. If haze involvement is less than one fifth of the full corneal thickness or is < 15 μm deep into the stroma on anterior OCT, the refractive changes secondary to stromal removal are to be likely small. Mechanical debridement or superficial PTK are both valid management options in superficial haze treatment [100]. After epithelial debridement, the surgeon can, based on their preference, use a beaver or crescent blade or a diamond burr to remove the underlying haze.
The stromal scar may be strongly adherent to the underlying healthy tissue; therefore, many firm, uniform strokes may be necessary for complete removal of the scar tissue. Care should be taken to debride the entire optical treatment zone. During the procedure, multiple slit lamp examinations of the cornea may be necessary until the surgeon feels comfortable that they have removed enough of the scar. Afterwards, a sponge can be used to apply 0.02% MMC to the surface for 2 min [5, 7, 53, 72], following which the cornea will be irrigated continuously with 20–30 cc of cold or room temperature saline solution. The application of MMC and the appropriate concentration are still controversial topics, especially for low myopia eyes [96], due to MMC-related tissue toxicity. However, studies performed to date show no evidence of epithelial cell and keratocyte toxicity [97]. As for endothelial cell toxicity, only two studies have found evidence of endothelial cell loss with MMC application whereas numerous studies have not found such evidence [2, 73, 101]. Across > 20 years of studies, MMC application has been demonstrated to be safe. Alternatively, a superficial PTK of < 15 μm can be performed to remove superficial post PRK haze. If haze involvement is more than one fifth of the corneal thickness or deeper than 15 μm into the stroma based on anterior OCT, a deep PTK or therapeutic myopic PRK ablation can be performed [100]. For deep PTK and/or therapeutic PRK, the laser ablation depth is set to the haze depth, after accounting for the thickness of the epithelial hyperplasia. A hyperopic PRK of the myopic ablation zone periphery is an appropriate option for myopic changes secondary to stromal tissue loss because of treatment. Post-treatment MMC and saline irrigation remain the same for deep PTK and/or therapeutic PRK as it was for other treatments. Topical corticosteroids should be prescribed to patients regardless of treatment option. Although 1% prednisolone is routinely prescribed, dexamethasone, loteprednol, or fluorometholone may be equally efficacious [102]. Such an approach would potentially resolve haze; however, there is always a risk of recurrence despite treatment [103].
Strengths, Weaknesses, and Future Research
The strength of this research is the comprehensive review of the literature by two independent reviewers and the graphic summation of current literature and clinical knowledge, presented within the generalized framework, for the management of post PRK haze. Weaknesses of this research include the lack of inclusion of non-English literature, which may create bias in the included evidence. Additionally, as this is not a meta-analysis, the studies in this article are not weighted using any particular methodology and may be influenced by the inherent bias by each author. Future research investigating the effectiveness of the proposed general framework will help further refine the management of post PRK haze. Additional research on the usefulness of specific imaging techniques (anterior OCT, Scheimpflug densitometry) in monitoring post PRK haze may be helpful.
Conclusion
Photorefractive keratectomy is a common corneal refractive surgery that is likely to remain popular in the near future. Therefore, research on the management of post operative complications needs to continue. Post PRK haze has declined in incidence with the implementation of newer laser technology and adjunctive therapy. However, a small population of patients still develop long-term complications of post PRK haze that ultimately impact good visual outcome. The proposed general framework is a culmination of the current literature and clinical experience for the management of post PRK haze. With continuous advancements in corneal refractive surgery and emerging therapies, the incidence of post PRK haze will hopefully continue to decline in the future.
Acknowledgements
We thank Daniel Durrie (Durrie Vision) for providing the images for Figs. 4-6.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work, and have given final approval to the version to be published.
Author Contribution
Each of the authors listed contributed substantially to the work. Conceptualization: Majid Moshirfar. Methodology: Josh S. Theis, Qiancheng Wang, and Majid Moshirfar. Validation: Majid Moshirfar. Investigation: Josh S. Theis, Qiancheng Wang, and Majid Moshirfar. Resources: Majid Moshirfar. Writing—original draft preparation: Qiancheng Wang, Josh S. Theis, Kaiden B. Porter, Isabella M. Stoakes, and Carter J. Payne. Writing—review and editing: Qiancheng Wang, Josh S. Theis, Isabella M. Stoakes, Carter J. Payne, and Majid Moshirfar. Visualization: Qiancheng Wang, Josh S. Theis, and Majid Moshirfar. Supervision: Majid Moshirfar and Phillip C. Hoopes. Project administration: Majid Moshirfar and Qiancheng Wang. All authors have read and agreed to the published version of the manuscript.
Funding
No funding or sponsorship was received for this study or the publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethical Approval
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
Majid Moshirfar, Qiancheng Wang, Josh S. Theis, Kaiden B. Porter, Isabella M. Stoakes, Carter J. Payne, Phillip C. Hoopes have any conflicts of interest to declare.
References
- 1.Gartry DS, Kerr Muir MG, Marshall J. Excimer laser photorefractive keratectomy. Ophthalmology. 1992;99(8):1209–1219. doi: 10.1016/S0161-6420(92)31821-4. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Chung HS, Jeon YC, Boo SD, Yoon YD, Kim JG. Photorefractive keratectomy with intraoperative mitomycin-C application. J Cataract Refract Surg. 2005;31(12):2293–2298. doi: 10.1016/j.jcrs.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Reilly CD, Panday V, Lazos V, Mittelstaedt BR. PRK vs LASEK vs Epi-LASIK: a comparison of corneal haze, postoperative pain and visual recovery in moderate to high myopia. Nepal J Ophthalmol. 2010;2(2):97–104. doi: 10.3126/nepjoph.v2i2.3715. [DOI] [PubMed] [Google Scholar]
- 4.Kuo IC, Lee SM, Hwang DG. Late-onset corneal haze and myopic regression after photorefractive keratectomy (PRK) Cornea. 2004;23(4):350–355. doi: 10.1097/00003226-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Kaiserman I, Sadi N, Mimouni M, Sela T, Munzer G, Levartovsky S. Corneal breakthrough haze after photorefractive keratectomy with mitomycin C: incidence and risk factors. Cornea. 2017;36(8):961–966. doi: 10.1097/ICO.0000000000001231. [DOI] [PubMed] [Google Scholar]
- 6.Tabbara KF, El-Sheikh HF, Sharara NA, Aabed B. Corneal haze among blue eyes and brown eyes after photorefractive keratectomy. Ophthalmology. 1999;106(11):2210–2215. doi: 10.1016/S0161-6420(99)90507-9. [DOI] [PubMed] [Google Scholar]
- 7.Spadea L, Giammaria D, Trabucco P. Corneal wound healing after laser vision correction. Br J Ophthalmol. 2016;100(1):28–33. doi: 10.1136/bjophthalmol-2015-306770. [DOI] [PubMed] [Google Scholar]
- 8.Tuft SJ, Gartry DS, Rawe IM, Meek KM. Photorefractive keratectomy: Implications of corneal wound Healing. Br J Ophthalmol. 1993;77(4):243–247. doi: 10.1136/bjo.77.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moshirfar M, Desautels JD, Walker BD, Murri MS, Birdsong OC, Hoopes PCS. Mechanisms of optical regression following corneal laser refractive surgery: epithelial and stromal responses. Med Hypothesis Discov Innov Ophthalmol. 2018;7(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Tomás-Juan J, Murueta-Goyena Larrañaga A, Hanneken L. Corneal regeneration after photorefractive keratectomy: a review. J Optom. 2015;8(3):149–169. doi: 10.1016/j.optom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaidyanathan U, Hopping GC, Liu HY, et al. Persistent corneal epithelial defects: a review article. Med Hypothesis Discov Innov Ophthalmol. 2019;8(3):163–176. [PMC free article] [PubMed] [Google Scholar]
- 12.Clinch TE, Moshirfar M, Weis JR, Ahn CS, Hutchinson CB, Jeffrey JH. Comparison of mechanical and transepithelial deb-ridement during photorefractive keratectomy. Ophthalmology. 1999;106(3):483–489. doi: 10.1016/S0161-6420(99)90135-5. [DOI] [PubMed] [Google Scholar]
- 13.Miller DD, Hasan SA, Simmons NL, Stewart MW. Recurrent corneal erosion: a comprehensive review. Clin Ophthalmol. 2019;13:325–335. doi: 10.2147/OPTH.S157430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh YT, Wang IJ, Hu FR. Anterior corneal optical irregularity measured by higher-order aberrations induced by a broad beam excimer laser. Clin Exp Optom. 2012;95(5):522–530. doi: 10.1111/j.1444-0938.2012.00718.x. [DOI] [PubMed] [Google Scholar]
- 15.Gokul KC, Kandel H, Valiño L, et al. Computational study for temperature distribution in ArF excimer laser corneal refractive surgeries using different beam delivery techniques. Lasers Med Sci. 2022;37(3):1709–1716. doi: 10.1007/s10103-021-03420-z. [DOI] [PubMed] [Google Scholar]
- 16.Ghoreishi M, Attarzadeh H, Tavakoli M, et al. Alcohol-assisted versus mechanical epithelium removal in photorefractive keratectomy. J Ophthalmic Vis Res. 2010;5(4):223–227. [PMC free article] [PubMed] [Google Scholar]
- 17.Gadde AK, Srirampur A, Katta KR, Mansoori T, Armah SM. Comparison of single-step transepithelial photorefractive keratectomy and conventional photorefractive keratectomy in low to high myopic eyes. Indian J Ophthalmol. 2020;68(5):755. doi: 10.4103/IJO.IJO_1126_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Radi M, Shehata M, Mostafa MM, Aly MOM. Transepithelial photorefractive keratectomy: a prospective randomized comparative study between the two-step and the single-step techniques. Eye (Lond). 2023;37(8):1545-52. 10.1038/s41433-022-02174-4. [DOI] [PMC free article] [PubMed]
- 19.Talamo JH, Hatch KM, Woodcock EC. Delayed epithelial closure after PRK associated with topical besifloxacin use. Cornea. 2013;32(10):1365–1368. doi: 10.1097/ICO.0b013e31829e1e8c. [DOI] [PubMed] [Google Scholar]
- 20.Lin N, Yee SB, Mitra S, Chuang AZ, Yea RW. Prediction of corneal haze using an ablation depth/corneal thickness ratio after laser epithelial keratomileusis. J Refract Surg. 2004;20(6):797–802. doi: 10.3928/1081-597X-20041101-07. [DOI] [PubMed] [Google Scholar]
- 21.Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82(5):788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohan RR, Hutcheon AEK, Choi R, et al. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76(1):71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 23.Thomas KE, Brunstetter T, Rogers S, Sheridan MV. Astigmatism: Risk factor for postoperative corneal haze in conventional myopic photorefractive keratectomy. J Cataract Refract Surg. 2008;34(12):2068–2072. doi: 10.1016/j.jcrs.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Maloney RK, Chan WK, Steinert R, Hersh P, OÇonnell M. A multicenter trial of photorefractive keratectomy for residual myopia after previous ocular surgery. Summit therapeutic refractive study group. Ophthalmology. 1995;102(7):1042–1052. doi: 10.1016/s0161-6420(95)30913-x. [DOI] [PubMed] [Google Scholar]
- 25.Koch DD, Maloney R, Hardten DR, Dell S, Sweeney AD, Wang L. Wavefront-guided photorefractive keratectomy in eyes with prior radial keratotomy. A multicenter study. Ophthalmology. 2009;116:9. doi: 10.1016/j.ophtha.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Ghanem RC, Ghanem VC, Ghanem EA, Kara-José N. Corneal wavefront-guided photorefractive keratectomy with mito-mycin-C for hyperopia after radial keratotomy: two-year follow-up. J Cataract Refract Surg. 2012;38(4):595–606. doi: 10.1016/j.jcrs.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 27.Moshirfar M, Villarreal A, Thomson AC, et al. PRK enhancement for residual refractive error after primary PRK: a ret-rospective study. Ophthalmol Ther. 2021;10(1):175–185. doi: 10.1007/s40123-021-00331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broderick KM, Sia RK, Ryan DS, et al. Wavefront-optimized surface retreatments of refractive error following previous laser refractive surgery: a retrospective study. Eye Vis. 2016;3:1. doi: 10.1186/s40662-016-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moshirfar M, Basharat NF, Kelkar N, Bundogji N, Ronquillo YC, Hoopes PC. Visual outcomes of photorefractive keratectomy enhancement after primary LASIK. J Refract Surg. 2022;38(11):733–740. doi: 10.3928/1081597X-20221019-01. [DOI] [PubMed] [Google Scholar]
- 30.Schallhorn SC, Venter JA, Hannan SJ, Hettinger KA, Teenan D. Flap lift and photorefractive keratectomy enhancements after primary laser in situ keratomileusis using a wavefront-guided ablation profile: refractive and visual outcomes. J Cataract Refract Surg. 2015;41(11):2501–2512. doi: 10.1016/j.jcrs.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Hecht I, Mimouni M, Rabina G, Kaiserman I. Re-treatment by flap relift versus surface ablation after myopic laser in situ keratomileusis. Cornea. 2020;39(4):443–450. doi: 10.1097/ICO.0000000000002189. [DOI] [PubMed] [Google Scholar]
- 32.Gab-Alla AA. SmartSurfACE transepithelial photorefractive keratectomy with mitomycin C enhancement after small incision lenticule extraction. Eye Vis. 2021;8:1. doi: 10.1186/s40662-021-00254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siedlecki J, Siedlecki M, Luft N, et al. Surface ablation versus circle for myopic enhancement after SMILE: a matched com-parative study. J Refract Surg. 2019;35(5):294–300. doi: 10.3928/1081597X-20190416-02. [DOI] [PubMed] [Google Scholar]
- 34.Siedlecki J, Luft N, Kook D, et al. Enhancement after myopic small incision lenticule extraction (SMILE) using surface ablation. J Refract Surg. 2017;33(8):513–518. doi: 10.3928/1081597X-20170602-01. [DOI] [PubMed] [Google Scholar]
- 35.Zhao PF, Zhou YH, Hu YB, et al. Evaluation of preoperative dry eye in people undergoing corneal refractive surgery to correct myopia. Int J Ophthalmol. 2021;14(7):1047–1051. doi: 10.18240/ijo.2021.07.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Vimalin JM, Qu Y, et al. Dry eye management: targeting the ocular surface microenvironment. Int J Mol Sci. 2017;18:7. doi: 10.3390/ijms18071398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocha-de-Lossada C, Rachwani-Anil R, Colmenero-Reina E, Borroni D, Sánchez-González JM. Laser refractive surgery in corneal dystrophies. J Cataract Refract Surg. 2021;47(5):662–670. doi: 10.1097/j.jcrs.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg ME, Tervo TMT, Petroll WM, Vesaluoma MH. In vivo confocal microscopy of patients with corneal recurrent erosion syndrome or epithelial basement membrane dystrophy. Ophthalmology. 2000;107(3):565–573. doi: 10.1016/S0161-6420(99)00086-X. [DOI] [PubMed] [Google Scholar]
- 39.Dastgheib KA, Clinch TE, Manche EE, Hersh P, Ramsey J. Sloughing of corneal epithelium and wound healing complications associated with laser in situ keratomileusis in patients with epithelial basement membrane dystrophy. Am J Ophthalmol. 2000;130(3):297–303. doi: 10.1016/s0002-9394(00)00504-3. [DOI] [PubMed] [Google Scholar]
- 40.Werblin TP, Hirst LW, Stark WJ, Maumenee IH. Prevalence of map-dot-fingerprint changes in the cornea. Br J Ophthalmol. 1981;65(6):401–409. doi: 10.1136/bjo.65.6.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pérez-Santonja JJ, Galal A, Cardona C, Artola A, Ruíz-Moreno JM, Alió JL. Severe corneal epithelial sloughing during laser in situ keratomileusis as a presenting sign for silent epithelial basement membrane dystrophy. J Cataract Refract Surg. 2005;31(10):1932–1937. doi: 10.1016/j.jcrs.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 42.Wu PY, Tsui MC, Chang CK, Chang HW, Chen WL. Epithelial basement membrane dystrophy after femtosecond laser–assisted LASIK successfully treated with in vivo confocal microscopy–assisted photorefractive keratectomy. J Cataract Refract Surg. 2020;46(12):e17–e19. doi: 10.1097/j.jcrs.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 43.Kymionis GD, Diakonis VF, Bouzoukis DI, Yoo SH, Pallikaris IG. Photorefractive keratectomy in a patient with epithelial basement membrane dystrophy. Semin Ophthalmol. 2007;22(1):59–61. doi: 10.1080/08820530601162768. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Baylin A, Levy PD. Vitamin D deficiency and insufficiency among US adults: prevalence, predictors and clinical im-plications. Br J Nutr. 2018;119(8):928–936. doi: 10.1017/S0007114518000491. [DOI] [PubMed] [Google Scholar]
- 45.Mirfakhraee S, Ayers CR, McGuire DK, Maalouf NM. Longitudinal changes in serum 25-hydroxyvitamin D in the Dallas Heart Study. Clin Endocrinol (Oxf) 2017;87(3):242–248. doi: 10.1111/cen.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain N, Sharma P, Chouhan JK. A study of the association between vitamin D deficiency and dry eye syndrome (DES) in the Indian population. Indian J Ophthalmol. 2022;70(2):500–504. doi: 10.4103/ijo.IJO_1921_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cashman KD, Dowling KG, Škrabáková Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daly RM, Gagnon C, Lu ZX, et al. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: a national, population-based study. Clin Endocrinol (Oxf) 2012;77(1):26–35. doi: 10.1111/j.1365-2265.2011.04320.x. [DOI] [PubMed] [Google Scholar]
- 49.Ritu G, Gupta A. Vitamin D deficiency in India: prevalence, causalities and interventions. Nutrients. 2014;6(2):729–775. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichols KK, Mousavi M. Clinical assessments of dry eye disease: tear film and ocular surface health. In: Galor A, editor. Dry eye disease. Amsterdam: Elsevier; 2023. p. 15–23. 10.1016/B978-0-323-82753-9.00002-3.
- 51.Kurtul BE, Özer PA, Aydinli MS. The association of vitamin D deficiency with tear break-up time and Schirmer testing in non-Sjögren dry eye. Eye (Lond) 2015;29(8):1081–1084. doi: 10.1038/eye.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu X, Watsky MA. Effects of vitamin D receptor knockout on cornea epithelium gap junctions. Invest Ophthalmol Vis Sci. 2014;55(5):2975–2982. doi: 10.1167/iovs.13-13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kundu G, D’Souza S, Lalgudi VG, et al. Photorefractive keratectomy (PRK) Prediction, Examination, tReatment, Follow-up, Evaluation, Chronic Treatment (PERFECT) protocol—a new algorithmic approach for managing post PRK haze. Indian J Ophthalmol. 2020;68(12):2950. doi: 10.4103/IJO.IJO_2623_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aika SS, Uenoyama K, Hiroi K, Tanioka H, Takase K, Hikita M. Laboratory investigations Graefe’s archive for clinical and experimental ascorbic acid phosphate ester and wound healing in rabbit corneal alkali burns: epithelial basement membrane and stroma. Ophthalmology. 1993;231:221. doi: 10.1007/BF00918845. [DOI] [PubMed] [Google Scholar]
- 55.Bilgihan A, Bilgihan K, Toklu Y, Konuk O, Yis O, Hasanreisoğlu B. Ascorbic acid levels in human tears after photorefractive keratectomy, transepithelial photorefractive keratectomy, and laser in situ keratomileusis. J Cataract Refract Surg. 2001;27(4):585–588. doi: 10.1016/s0886-3350(00)00877-4. [DOI] [PubMed] [Google Scholar]
- 56.Yulish M, Beiran I, Miller B, Pikkel J. Ascorbate prophylaxis with mitomycin-C for corneal haze after laser-assisted sub-epithelial keratectomy. Isr Med Assoc J. 2012;14(6):382–385. [PubMed] [Google Scholar]
- 57.Alishiri A, Mosavi SA. Ascorbic acid versus placebo in postoperative lid edema postphotorefractive keratectomy: A dou-ble-masked, randomized, prospective study. Oman J Ophthalmol. 2019;12(1):4–9. doi: 10.4103/ojo.OJO_187_2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ubels JL, Edelhauser HF, Austin KH. Healing of experimental corneal wounds treated with topically applied retinoids. Am J Ophthalmol. 1983;95(3):353–358. doi: 10.1016/s0002-9394(14)78305-9. [DOI] [PubMed] [Google Scholar]
- 59.Kastl PR, Rosenthal WN, Batlle I, Karcioglu Z. Topical vitamin A ointment increases healing of cataract incisions. Ann Ophthalmol. 1987;19(5):175–177. [PubMed] [Google Scholar]
- 60.Chelala E, Dirani A, Fadlallah A, Fahd S. The role of topical vitamin A in promoting healing in surface refractive procedures: a prospective randomized controlled study. Clin Ophthalmol. 2013;7:1913–1918. doi: 10.2147/OPTH.S52280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vetrugno M, Maino A, Cardia G, Quaranta GM, Cardia L. A randomised, double masked, clinical trial of high dose vitamin A and vitamin E supplementation after photorefractive keratectomy. Br J Ophthalmol. 2001;85(5):537. doi: 10.1136/BJO.85.5.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bilgihan K, Adiguzel U, Sezer C, Akyol G, Hasanreisoglu B. Effects of topical vitamin E on keratocyte apoptosis after tradi-tional photorefractive keratectomy. Ophthalmologica. 2001;215(3):192–196. doi: 10.1159/000050857. [DOI] [PubMed] [Google Scholar]
- 63.Bilgihan K, Ozdek S, Ozoğul C, Gurelik G, Bilgihan A, Hasanreisoğlu B. Topical vitamin E and hydrocortisone acetate treatment after photorefractive keratectomy. Eye (Lond) 2000;14(Pt 2):231–237. doi: 10.1038/eye.2000.60. [DOI] [PubMed] [Google Scholar]
- 64.Stojanovic A, Nitter TA. Correlation between ultraviolet radiation level and the incidence of late-onset corneal haze after photorefractive keratectomy. J Cataract Refract Surg. 2001;27(3):404–410. doi: 10.1016/s0886-3350(00)00742-2. [DOI] [PubMed] [Google Scholar]
- 65.Nagy ZZ, Hiscott P, Seitz B, Schlötzer-Schrehardt U, Süveges I, Naumann GO. Clinical and morphological response to UV-B irradiation after excimer laser photorefractive keratectomy. Surv Ophthalmol. 1997;42(Suppl 1):S64–76. doi: 10.1016/s0039-6257(97)80028-8. [DOI] [PubMed] [Google Scholar]
- 66.Sliney DH. Photoprotection of the eye—UV radiation and sunglasses. J Photochem Photobiol B. 2001;64(2–3):166–175. doi: 10.1016/s1011-1344(01)00229-9. [DOI] [PubMed] [Google Scholar]
- 67.Oliva MS, Taylor H. Ultraviolet radiation and the eye. Int Ophthalmol Clin. 2005;45(1):1–17. [PubMed] [Google Scholar]
- 68.Al-Sharif EM, Stone DU. Correlation between practice location as a surrogate for UV exposure and practice patterns to prevent corneal haze after photorefractive keratectomy (PRK) Saudi J Ophthalmol. 2016;30(4):213. doi: 10.1016/J.SJOPT.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moradi S, Shahdadian F, Mohammadi H, Rouhani MH. A comparison of the effect of supplementation and sunlight exposure on serum vitamin D and parathyroid hormone: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;60(11):1881–1889. doi: 10.1080/10408398.2019.1611538. [DOI] [PubMed] [Google Scholar]
- 70.Tanzer DJ, Isfahani A, Schallhorn SC, LaBree LD, McDonnell PJ. Photorefractive keratectomy in African Americans including those with known dermatologic keloid formation. Am J Ophthalmol. 1998;126(5):625–629. doi: 10.1016/s0002-9394(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 71.Alfawaz AM. Haze prevention following photorefractive keratectomy in brown eyes. J Fr Ophtalmol. 2021;44(6):835–841. doi: 10.1016/j.jfo.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Al-Mohaimeed MM. Effect of prophylactic mitomycin C on corneal endothelium following transepithelial photorefractive keratectomy in myopic patients. Clin Ophthalmol. 2022;16:2813–2822. doi: 10.2147/OPTH.S375587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hofmeister EM, Bishop FM, Kaupp SE, Schallhorn SC. Randomized dose-response analysis of mitomycin-C to prevent haze after photorefractive keratectomy for high myopia. J Cataract Refract Surg. 2013;39(9):1358–1365. doi: 10.1016/j.jcrs.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 74.Talamo JH, Gollamudi S, Green WR, De La Cruz Z, Filatov V, Stark WJ. Modulation of corneal wound healing after excimer laser keratomileusis using topical mitomycin C and steroids. Arch Ophthalmol. 1991;109(8):1141–1146. doi: 10.1001/archopht.1991.01080080101040. [DOI] [PubMed] [Google Scholar]
- 75.Niizuma T, Ito S, Hayashi M, Futemma M, Utsumi T, Ohashi K. Cooling the cornea to prevent side effects of photorefractive keratectomy. J Refract Corneal Surg. 1994;10(2 Suppl):S262–S266. [PubMed] [Google Scholar]
- 76.Kitazawa Y, Maekawa E, Sasaki S, Tokoro T, Mochizuki M, Ito S. Cooling effect on excimer laser photorefractive keratectomy. J Cataract Refract Surg. 1999;25(10):1349–1355. doi: 10.1016/s0886-3350(99)00207-2. [DOI] [PubMed] [Google Scholar]
- 77.Neuffer MC, Khalifa YM, Moshirfar M, Miffin MD. Prospective comparison of chilled versus room temperature saline irri-gation in alcohol-assisted photorefractive keratectomy. Nepal J Ophthalmol. 2013;5(2):154–160. doi: 10.3126/nepjoph.v5i2.8706. [DOI] [PubMed] [Google Scholar]
- 78.Aras C, Ozdamar A, Aktunç R, Erçikan C. The effects of topical steroids on refractive outcome and corneal haze, thickness, and curvature after photorefractive keratectomy with a 6.0-mm ablation diameter. Ophthalmic Surg Lasers. 1998;29(8):621–627. doi: 10.3928/1542-8877-19980801-03. [DOI] [PubMed] [Google Scholar]
- 79.Vetrugno M, Maino A, Quaranta GM, Cardia L. The effect of early steroid treatment after PRK on clinical and refractive outcomes. Acta Ophthalmol Scand. 2001;79(1):23–27. doi: 10.1034/J.1600-0420.2001.079001023.X. [DOI] [PubMed] [Google Scholar]
- 80.Gambato C, Ghirlando A, Moretto E, Busato F, Midena E. Mitomycin C modulation of corneal wound healing after photo-refractive keratectomy in highly myopic eyes. Ophthalmology. 2005;112(2):208–218. doi: 10.1016/j.ophtha.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 81.Lipshitz I, Loewenstein A, Varssano D, Lazar M. Late onset corneal haze after photorefractive keratectomy for moderate and high myopia. Ophthalmology. 1997;104(3):369–373. doi: 10.1016/s0161-6420(97)30306-6. [DOI] [PubMed] [Google Scholar]
- 82.Gambato C, Miotto S, Cortese M, Ghirlando A, Lazzarini D, Midena E. Mitomycin C-assisted photorefractive keratectomy in high myopia: a long-term safety study. Cornea. 2011;30(6):641–645. doi: 10.1097/ICO.0b013e31820123c8. [DOI] [PubMed] [Google Scholar]
- 83.Hashemi H, Pakbin M, Pakravan M, et al. Effect of short versus long-term steroid on corneal haze after photorefractive keratectomy: a randomized, double-masked clinical trial. Am J Ophthalmol. 2022;235:211–220. doi: 10.1016/j.ajo.2021.09.028. [DOI] [PubMed] [Google Scholar]
- 84.Busool Y, Mimouni M, Vainer I, et al. Risk factors predicting steroid-induced ocular hypertension after photorefractive keratectomy. J Cataract Refract Surg. 2017;43(3):389–393. doi: 10.1016/j.jcrs.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 85.Charpentier S, Keilani C, Maréchal M, et al. Corneal haze post photorefractive keratectomy. J Fr Ophtalmol. 2021;44(9):1425–1438. doi: 10.1016/j.jfo.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 86.Sambhi RDS, Sambhi GDS, Mather R, Malvankar-Mehta MS. Dry eye after refractive surgery: a meta-analysis. Can J Ophthalmol. 2020;55(2):99–106. doi: 10.1016/j.jcjo.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 87.Murakami Y, Manche EE. Prospective, randomized comparison of self-reported postoperative dry eye and visual fluctuation in LASIK and photorefractive keratectomy. Ophthalmology. 2012;119(11):2220–2224. doi: 10.1016/j.ophtha.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 88.Hovanesian JA, Shah SS, Maloney RK. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J Cataract Refract Surg. 2001;27(4):577–584. doi: 10.1016/s0886-3350(00)00835-x. [DOI] [PubMed] [Google Scholar]
- 89.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an en-docrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 90.Meduri A, Bergandi L, Perroni P, Silvagno F, Aragona P. Oral l-cysteine supplementation enhances the long term-effect of topical basic fibroblast growth factor (bFGF) in reducing the corneal haze after photorefractive keratectomy in myopic patients. Pharmaceuticals. 2020;13:4. doi: 10.3390/PH13040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tandon A, Tovey JCK, Waggoner MR, et al. Vorinostat: a potent agent to prevent and treat laser-induced corneal haze. J Refract Surg. 2012;28(4):285–290. doi: 10.3928/1081597X-20120210-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Woo JE, Park WC, Yoo YH, Kim SW. The efficacy of co-treatment with suberoylanilide hydroxamic acid and mitomycin c on corneal scarring after therapeutic keratectomy: an animal study. Curr Eye Res. 2014;39(4):348–358. doi: 10.3109/02713683.2013.859272. [DOI] [PubMed] [Google Scholar]
- 93.Gupta S, Rodier JT, Sharma A, et al. Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo. PLoS ONE. 2017;12:3. doi: 10.1371/JOURNAL.PONE.0172928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sampaio LP, Hilgert GSL, Shiju TM, Santhiago MR, Wilson SE. Losartan inhibition of myofibroblast generation and late haze (scarring fibrosis) after PRK in rabbits. J Refract Surg. 2022;38(12):820–829. doi: 10.3928/1081597X-20221026-03. [DOI] [PubMed] [Google Scholar]
- 95.Pereira-Souza AL, Ambrósio R, Bandeira F, Salomão MQ, Lima AS, Wilson SE. Topical losartan for treating corneal fibrosis (haze): first clinical experience. J Refract Surg. 2022;38(11):741–746. doi: 10.3928/1081597X-20221018-02. [DOI] [PubMed] [Google Scholar]
- 96.Tananuvat N, Winaikosol P, Niparugs M, Chaidaroon W, Tangmonkongvoragul C, Ausayakhun S. Twelve-month out-comes of the wavefront-optimized photorefractive keratectomy for high myopic correction compared with low-to-moderate myopia. Clin Ophthalmol. 2021;22(15):4775–4785. doi: 10.2147/OPTH.S346992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carlos-de-Oliveira R, Wilson SE. Biological effects of mitomycin C on late corneal haze stromal fibrosis following PRK. Exp Eye Res. 2020;200:108218. doi: 10.1016/j.exer.2020.108218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fantes FE. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108(5):665. doi: 10.1001/archopht.1990.01070070051034. [DOI] [PubMed] [Google Scholar]
- 99.Salomao MQ, Wilson SE. Corneal molecular and cellular biology update for the refractive surgeon. J Refract Surg. 2009;25(5):459–466. doi: 10.3928/1081597X-20090422-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sudanaboina P, Murthy SI, Rathi VM. Excimer laser phototherapeutic keratectomy with mitomycin C application to treat haze after myopic photorefractive keratectomy. Indian J Ophthalmol. 2020;68(12):3030. doi: 10.4103/IJO.IJO_1845_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gharaee H, Zarei-Ghanavati S, Alizadeh R, Abrishami M. Endothelial cell changes after photorefractive keratectomy with graded usage of mitomycin C. Int Ophthalmol. 2018;38(3):1211–1217. doi: 10.1007/s10792-017-0584-5. [DOI] [PubMed] [Google Scholar]
- 102.Karimian F, Faramarzi A, Fekri S, et al. Comparison of loteprednol with fluorometholone after myopic photorefractive keratectomy. J Ophthalmic Vis Res. 2017;12(1):11–16. doi: 10.4103/2008-322X.200161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Porges Y, Ben-Haim O, Hirsh A, Levinger S. Phototherapeutic keratectomy with mitomycin C for corneal haze following photorefractive keratectomy for myopia. J Refract Surg. 2003;19(1):40–43. doi: 10.3928/1081-597X-20030101-08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.