Abstract
Pyoverdine is the primary siderophore of the gram-negative bacterium Pseudomonas aeruginosa. The pyoverdine region was recently identified as the most divergent locus alignable between strains in the P. aeruginosa genome. Here we report the nucleotide sequence and analysis of more than 50 kb in the pyoverdine region from nine strains of P. aeruginosa. There are three divergent sequence types in the pyoverdine region, which correspond to the three structural types of pyoverdine. The pyoverdine outer membrane receptor fpvA may be driving diversity at the locus: it is the most divergent alignable gene in the region, is the only gene that showed substantial intratype variation that did not appear to be generated by recombination, and shows evidence of positive selection. The hypothetical membrane protein PA2403 also shows evidence of positive selection; residues on one side of the membrane after protein folding are under positive selection. R′, previously identified as a type IV strain, is clearly derived from a type III strain via a 3.4-kb deletion which removes one amino acid from the pyoverdine side chain peptide. This deletion represents a natural modification of the product of a nonribosomal peptide synthetase enzyme, whose consequences are predictive from the DNA sequence. There is also linkage disequilibrium between the pyoverdine region and pvdY, a pyoverdine gene separated by 30 kb from the pyoverdine region. The pyoverdine region shows evidence of horizontal transfer; we propose that some alleles in the region were introduced from other soil bacteria and have been subsequently maintained by diversifying selection.
Pseudomonas aeruginosa is a gram-negative bacterium commonly isolated from fresh water and soil. The opportunistic pathogen causes a variety of human infections, including infections of the ear canal, lung, eye, and urinary tract. P. aeruginosa is particularly dangerous for immunocompromised patients such as those with human immunodeficiency virus, cancer, or severe burn wounds. Most cystic fibrosis patients have pulmonary P. aeruginosa infections for the majority of their lives. Infection also occurs in many other organisms, including insects (9), nematodes (16), plants (48), and amoebas (52).
Iron is necessary for the growth and survival of almost all organisms, with the exception of Lactobacillus species and some intracellular parasites (3). Iron is incorporated into numerous protein moieties such as iron-sulfur clusters and participates in many cellular redox reactions. Although abundant on the surface of the Earth, iron is not readily accessible because it rapidly oxidizes to insoluble Fe3+ compounds under aerobic conditions. Bacteria obtain iron by a variety of mechanisms, including secretion and subsequent uptake of ferrisiderophores, direct uptake of ferrous iron, uptake of host iron-binding molecules, and proteolytic destruction of host proteins with subsequent iron scavenging (54). Mechanisms for iron acquisition via siderophores are diverse; at least 500 chemically distinct siderophores are known (69). Although almost all organisms require iron, excess iron is highly toxic in aerobic environments, particularly because of the production of hydroxyl radicals in the Fenton reaction.
Pyoverdines are siderophores made by members of the genus Pseudomonas (10, 37). Pyoverdine is the primary siderophore of P. aeruginosa, and pyoverdine mutants have attenuated virulence in multiple infection models (39, 66). Each strain of P. aeruginosa makes one of three pyoverdine types, each type having a distinct peptide side chain that is synthesized nonribosomally (40). The pyoverdine receptor is also type specific, transporting only its corresponding pyoverdine (14).
In a whole-genome-diversity study in which we compared two cystic fibrosis strains and one environmental strain of P. aeruginosa with the PAO1 reference strain, we found the pyoverdine region to be the most divergent alignable locus in the genome (64). Given the divergence in pyoverdine genes and the variety of different pyoverdine molecular structures, the pyoverdine locus appeared to be a possible target of diversifying selection. To determine the degree and patterns of diversity in pyoverdine genes, we sought to clone, sequence, and analyze the pyoverdine region from multiple strains.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are listed in Table 1. Strains were grown overnight on L plates at 37°C. Overnight growth at 37°C with solid or liquid Casamino Acids (Difco) medium was used to induce pyoverdine production. L plates containing 12.5 μg of chloramphenicol per ml were used to grow Escherichia coli containing fosmid clones. XL1-Blue MR E. coli cells were used as the host strain for fosmid clones (Stratagene, La Jolla, Calif.). Saccharomyces cerevisiae transformants carrying yeast capture clones were selected on uracil-deficient medium (63) with 2.5 μg of cycloheximide per ml. L plates containing 6 μg of chloramphenicol per ml were used to grow E. coli containing yeast capture clones. E. coli DH10B cells were used as the host cells for yeast capture cloning (24).
TABLE 1.
Strains used in this study
| Strain | Pyoverdine type | Origin | Description | Method used to clone pyoverdine region | Reference | GenBank accession no.(s) |
|---|---|---|---|---|---|---|
| PAO1 | I | Unknown | Wound isolate | 65 | NC_002516 | |
| 10-15 | I | Seattle, Wash. | Cystic fibrosis isolate | Fosmid library screen | 12 | AY765259, AY766167 |
| PA14 | I | Unknown | Wound isolate | 53 | NZ_AABQ00000000 | |
| 2-164 | II | Seattle, Wash. | Cystic fibrosis isolate | 64 | AF540993, AY766166 | |
| 1-60 | II | Seattle, Wash. | Cystic fibrosis isolate | 64 | AF540992, AY766165 | |
| MSH | II | Mt. St. Helens, Wash. | Environmental isolate | Fosmid library screen and yeast capture cloning | 64 | AY765263, AY766170 |
| 206-12 | III | Seattle, Wash. | Cystic fibrosis isolate | Fosmid library screen | 12 | AY765261, AY766168 |
| ATCC O13 | III | American Type Culture Collection | O-antigen serotype 13 | Yeast capture cloning | 56 | AY765262, AY766169 |
| R′ | IV | Thailand | Hospital isolate | Yeast capture cloning | 61 | AY765260, AY766171 |
Pyoverdine typing.
Pyoverdine strain typing was done via crude pyoverdine purification and isoelectric focusing gel electrophoresis as described (40). Once the nucleotide sequences of each pyoverdine sequence type became available, whole-cell PCR with pyoverdine type-specific primers was used to type strains (sequences available from the authors on request).
Fosmid library creation and screening.
P. aeruginosa genomic DNA was prepared by phenol-chloroform extraction and then further purified by ethanol precipitation. Genomic DNA was partially digested with restriction enzyme Sau3AI and size-separated by pulsed-field gel electrophoresis (Bio-Rad CHEF_DRII apparatus, 1% agarose gel, 14°C, 16 h, pulse ramp 1 to 6 s). DNA segments sized 35 to 52 kb were cut from the gel and recovered by electroelution in dialysis bags (SPETRA/POR molecular porous membrane tubing). Fosmid vector pFos1 was prepared as described (30) and genomic DNA fragments were ligated into the prepared vector with T4 DNA ligase (Roche, Basel, Switzerland).
The ligated vector was then packaged with Giga Pack III XL packaging extract (Stratagene), and E. coli cells were infected. Fosmid-containing colonies were picked into 384-well plates and also pooled into 96-well plates at eight colonies per pool. Fosmid-containing colonies were picked to 10-fold coverage of the genome, assuming average insert sizes of 40 kb.
Fosmid pools were screened with PCR at multiple sites upstream and downstream of the pyoverdine region. Primers were designed to amplify approximately 500-bp fragments and amplicons were spaced 1 to 3 kb apart. Once PCR-positive pools were identified, individual fosmid-containing cells were screened with PCR. Positive individual cells were regrown in 3 ml of L broth at 37°C for 18 h, lysed with 0.2 N NaOH with 1% sodium dodecyl sulfate treatment, and DNA was precipitated with isopropanol. Purified fosmids were retested with PCR and by digestion with multiple restriction enzymes.
Yeast capture cloning.
Yeast capture cloning vectors were assembled via yeast recombination (45). PCR with tailed primers carrying homology to both vector and target DNA was used to build target segments. Primers used to amplify upstream of the pyoverdine region included sequences from genes pvdQ and pvdA, while primers used to amplify downstream of the pyoverdine region included sequences from genes PA2405 and PA2406. Targeting plasmid pEHS6 was assembled by transforming into S. cerevisiae the two target segments, a linearized yeast-E. coli shuttle vector, and a central fragment carrying the yeast wild-type CYH2 gene with the Amp/ori stuffer fragment (57).
Genomic DNA was prepared as described (34). After passing genomic DNA 20 times through a 26.5-gauge needle, sheared genomic DNA and the linearized yeast capture cloning vector were transformed into yeast spheroplasts (57). Plasmid DNA was prepared from pooled yeast colonies and transformed into E. coli cells (26). Yeast capture clones were screened by whole-cell PCR with primers specific to vector-insert junctions.
PCR was used to test for the presence of deletions in strains R′ and ATCC O13 by designing primers at the boundaries of each potential breakpoint. Primers located inside putative deletions were designed with sequence from type III strain 206-12.
Multiple complete digest gels and DNA sequencing of fosmid and yeast capture clones.
Multiple complete digest gels were used to verify the 35- to 52-kb size of each isolated fosmid insert or the size of each yeast capture clone (71). Restriction enzymes EcoRI, BstBI, and NcoI were used. When prior sequence data were available, fragment sizes from newly acquired fosmid or yeast capture clones were compared with those predicted from the sequence.
Shotgun libraries of fosmid and yeast capture clones were made by shearing and ligating prepared DNA into a pBluescript vector. Greater than eightfold sequence coverage was obtained for each clone by standard shotgun sequencing methods. Automated sequence finishing was done with Autofinish software (22), manual finishing was performed if necessary, and sequence assemblies were checked against multiple complete digest gel patterns. Sequences for strains PAO1, PA14, 1-60, and 2-164 were obtained from GenBank.
DNA sequence analysis.
All pairwise sequence alignments were performed with cross_match software, a banded implementation of the Smith Waterman algorithm (http://www.phrap.org/). Gene predictions were performed with Genemark (35). Protein domain predictions were performed with PFAM (6) and NCBI's Conserved Domain Database (36). The EMBOSS program lindna was used to generate windowed single nucleotide polymorphism (SNP) plots in Fig. 1, and the EMBOSS programs cusp and cai were used to estimate the codon adaptation index of a gene (58). The codon adaptation index of genes was calculated in reference to a codon table of all genes in the PAO1 genome. Nonribosomal peptide synthetase specificity was predicted with software at the website http://raynam.chm.jhu.edu/≈nrps/ (13). PCR primer design was performed with Primer3 software (60). Hydrophobicity plots were generated with the algorithm of Kyte and Doolittle (32) with a window size of 15 amino acids. The program PROF 1.0 was used to predict the secondary structure of proteins (46).
FIG. 1.
Gene structure and patterns of variation across nine pyoverdine region sequences. Blue and green arrows on the reference sequence line indicate the direction of transcription. In the top panel, the eight other sequences have been aligned to sequence from the reference strain, PAO1; bands of color indicate the local density of single-base-pair differences. In the bottom panel, central-region sequences (each approximately 33 kb) have been aligned with an arbitrarily chosen reference for each type since the central regions are not alignable between types. Protein domains are overlaid on the gene structure of the nonribosomal peptide synthetase genes and are colored as follows: activation domain-red, condensation domain-yellow, thiolation domain-orange, thioesterase domain-brown. Since there is ambiguity in the precise positions of the deletion breakpoints in R′ and ATCC O13, grayed regions show the range of possible breakpoints. While the deletion shown in ATCC O13 is a cloning artifact and that in R′ is a true deletion, both presumably arose by similar mechanisms. Letters A to K indicate regions divergent within pyoverdine sequence types (see Table 3). Two type III genes were predicted to be smaller than colinear genes in other types: pvdP was shorter by more than 250 codons and pvdM was shorter by approximately 50 codons. For simplicity, no effort was made to reflect this length variation in the figure. The scale (in base pairs) is shown in the bottom right of the figure and is consistent throughout the figure. Pyoverdine genes pvdL (PA2424), pvdG (PA2425), and pvdX (PA2428) are not shown in the figure; these genes are not divergent between type I and II strains (no type III sequence is currently available). pvdY has a 468-bp coding region in type I and III strains and a 912-bp coding region in type II strains.
To estimate deviations in tetranucleotide usage in a sequence, we used a Markov maximal-order model (27, 51, 59). This standard model produces an expected count of oligonucleotide usage that accounts for bias in smaller oligonucleotides contained within the oligonucleotide. Let W = (w1, w2, …wm) denote the word formed by m adjacent nucleotides and N(W) denote the observed number of occurrences of the word in a sequence of length n. The expected count E(W) of W is
E(W) = N(w1, w2, …wm−1) N(w2, w3, …wm)/N(w2, w3, …wm−1)
To measure how observed tetranucleotide usage deviated from the expectation, we calculated the frequency of divergence of a word F(W) as the ratio of the observed count N(W) to the expected count E(W). Linear regression analysis was then used to compare F(W) from two sequences, such as the pyoverdine region and the PAO1 genome, and produce an R2 value that estimated the similarity in tetranucleotide usage between the sequences.
Gene names were kept consistent with PAO1 gene naming conventions when predicted protein domains and synteny for genes from different types indicated obvious homologies. The nonribosomal peptide synthetase genes were given gene names consistent with the PAO1 nonribosomal peptide synthetase genes with a number added indicating their pyoverdine type. Type-specific genes were named based on predicted protein domains.
To search for additional type-specific genes outside the divergent pyoverdine region, nucleotide alignments were made between the genomes of type I strains PAO1 and PA14, the unfinished genome of a type II strain (data not shown), and the 0.5X shotgun sequence reads of three type II strains (64). Type III sequence data were not available for comparison outside of the pyoverdine region.
Tests for positive selection.
We used a maximum-likelihood method (73) to estimate whether a gene had experienced positive selection, and if so, we identified the specific codons that were under positive selection. Maximum-likelihood models allow one to test a great number of combinations of parameter values and give a score to each combination according to how well the parameters fit the data; the combination with the best score fits the given data best. Our data were a multiple alignment of nucleotide sequences obtained for each gene in the pyoverdine region. Also input to the models was a phylogenetic tree that was constructed for each gene. We used models whose parameters included the nonsynonymous-to-synonymous ratio ω, the transition-to-transversion ratio κ, and equilibrium codon frequencies π in each codon. ω is a measure of how frequently single-base-pair differences change the amino acid coded for in a codon (as opposed to silent, or synonymous changes): ω < 1 suggests purifying selection, ω = 1 suggests neutral evolution, and ω > 1 suggests positive selection. Values for ω and κ were estimated from the data, and codon frequencies were estimated from nucleotide base frequencies at each of the three codon positions. The genes with the highest scoring combination of parameter values that included a ω greater than 1 were evaluated as being under positive selection.
In practice, maximum likelihood does not test all possible parameters. One must provide a starting point for each parameter, and the software will then incrementally change the parameter values to find a local high score. For each test, we used starting values of 0.1, 1.0, and 3.0 for ω to ensure a broad sampling of likelihood space. When the likelihood scores from each of these starting values agreed, we were confident that we had obtained a true maximum and not a locally maximal score. Below are descriptions of two nested models that have detected positive selection in other systems and which we used to test pyoverdine genes for positive selection.
Model M0 assumed one class of ω for all sites (aligned codons). Model M3 assumed three classes of ω (ω0, ω1, and ω2) each with a respective proportion of sites p (p0, p1, and p2). To compare the models, we calculated twice the log difference between the highest likelihood estimates of models M0 and M3. This difference was compared to a chi square distribution with four degrees of freedom to account for the difference in the number of parameters between the models. Evidence for positive selection in a gene required two conditions: model M3 predict a proportion of codons in a gene to have ω > 1, and the likelihood score of model M3 to be significantly greater than the score of model M0.
To identify specific codons under positive selection, we compared two additional models. Model M7 assumed a beta distribution for values of ω in a gene. Model M8 assumed a beta distribution with an added class of ω for a proportion of sites p1. The beta distribution was used because it allowed for a range of ω values in a gene, but only values of ω between 0 and 1. If some codons in a gene were under positive selection, then model M8 would better fit the data because it allowed a fraction of codons to have ω > 1. Two parameters, p and q, described the shape of the beta distribution. Values for p and q as well as ω and p1 were estimated from the data. We compared twice the log likelihood difference between models M7 and M8 to a chi square distribution with two degrees of freedom. Evidence for positive selection in specific codons required two conditions: model M8 be significantly more likely than model M7, and model M8 predict a fraction of codons with ω > 1. When positive selection was predicted, we could predict specific codons under positive selection by calculating posterior probabilities at each codon with Bayes' theorem. In other words, since we knew that a fraction of codons had a ω value predicted by model M8, we could calculate the probability that each codon belonged to this class of ω.
The program codeml from the PAML software package was used to test genes for positive selection (72). Amino acid multiple alignments were created with ClustalW (67). Alignments were then back-translated to nucleotide sequences and a neighbor-joining tree was generated with the program Neighbor with the phylogenetic software package PHYLIP (18).
To identify the locations of positively selected amino acids in FpvA, we aligned the amino acid sequence of FpvA from PAO1 to sequences from three E. coli proteins of known structure: ferric citrate transporter FecA (19), ferric enterobactin transporter FepA (8), and ferrichrome-iron transporter FhuA (20). Each of these proteins is in the same protein family as FpvA, and contains a TonB-dependent receptor domain (PF00593).
Nucleotide sequence accession number.
Refer to Table 1 for sequence accession numbers.
RESULTS
Three sequence types at the pyoverdine locus correspond with the three structural types of pyoverdine.
In comparisons of three unfinished P. aeruginosa genomes to reference strain PAO1, we found the pyoverdine region to be the most divergent alignable region in the genome (64). This region of approximately 50 kb in PAO1 is one of the most highly diverged regions in the core P. aeruginosa genome. In the present study we used fosmid cloning and yeast capture cloning to isolate and sequence the pyoverdine region from multiple strains.
The three pyoverdine sequence types correspond to the three known structural types of pyoverdine (see Fig. 1). The sequences of the same pyoverdine type have background levels of nucleotide divergence (approximately 0.5%) across the entire region analyzed, while the sequences of different pyoverdine types are highly diverged. The amount of divergence between types is variable in different genes and is highest in the genes located in the center of the region (see Fig. 2). The nucleotide sequences from the central part of the region, including pvdE, fpvA, pvdD, pvdI, and pvdJ, are not alignable between pyoverdine types. Figure 1 shows a multiple alignment and windowed SNP plot for all sequences, Table 1 lists the strains used in this study, and Table 2 lists the functions of individual genes in the region.
FIG. 2.
Mismatch in pairwise amino acid alignments between genes of each pyoverdine type. The highest-scoring alignment was used for each gene, and although some predicted gene boundaries differed, alignments spanned the smaller of any two aligned genes. Since the nonribosomal peptide synthetase genes pvdD, pvdJ, and pvdI were highly repetitive and mosaic in structure, the alignments given represent the best match to any nonribosomal peptide synthetase gene in the reference strain for a given type. Strains PAO1, 2-164, and R′ were used as the reference strains for type I, II, and III pyoverdine, respectively.
TABLE 2.
Functions of genes in the pyoverdine region
| Gene(s) | Name(s) | Category | Divergence between types | Functiona |
|---|---|---|---|---|
| PA2385 | pvdQ | Unknown | No | Similarity to aculeacin A acylase |
| PA2386 | pvdA | Synthesis | Yes | l-Ornithine-N5-oxygenase |
| PA2387 | fpvI | Regulation | Yes | ECF sigma factor |
| PA2388 | fpvR | Regulation | Yes | Antisigma transmembrane sensor |
| PA2389-PA2391 | opmQ (PA2391) | Unknown | No | Similarity to RND/MFP/OMF efflux pumps |
| PA2392 | pvdP | Unknown | Yes | Unknown |
| PA2393 | pvdM | Unknown | Yes | Similarity to porcine dipeptidase |
| PA2394 | pvdN | Unknown | Yes | Similarity to isopenicillin N-epimerase |
| PA2395 | pvdO | Unknown | Yes | Unknown |
| PA2396 | pvdF | Synthesis | Yes | N5-hydroxyornithine transformylase |
| PA2397 | pvdE | Transport | Yes | ABC transporter |
| PA2398 | fpvA | Transport | Yes | Pyoverdine outer membrane receptor |
| PA2399-PA2402 | pvdD, pvdJ, pvdI | Synthesis | Yes | Nonribosomal peptide synthetases of the pyoverdine peptide side chain |
| PA2403 | Unknown | Yes | Unknown | |
| PA2404 | Unknown | Yes | Unknown | |
| PA2405-PA2410 | Unknown | No | Membrane proteins and ABC transporter | |
| PA2411 | Unknown | No | Similarity to thioesterase | |
| PA2412 | Unknown | No | Unknown | |
| PA2413 | pvdH | Unknown | No | Similarity to aminotransferase |
| PA2424 | pvdL | Synthesis | No | Nonribosomal peptide synthetase of the pyoverdine chromophore |
| PA2425 | pvdG | Unknown | No | Probable thioesterase |
| PA2426 | pvdS | Regulation | No | ECF sigma factor |
| PA2427 | pvdY | Unknown | Yes | Unknown |
| PA2428 | pvdX | Unknown | No | Unknown |
RND, resistance-nodulation-cell division; MFP, membrane fusion protein; OMF, outer membrane factor; ECF, extracytoplasmic-function.
The pyoverdine region has unusual codon usage and unusual oligonucleotide usage, both of which suggest a history of horizontal transfer. Codon usage and oligonucleotide usage are phylogenetic signals that reflect the relatedness of bacterial sequences (51, 62). Codon usage in the central type III pyoverdine region is unusual: the codon adaptation index, a measure of synonymous codon bias, of most type III pyoverdine genes is less than 0.55, whereas the genome average is 0.68 (standard deviation = 0.10). Tetranucleotide usage in the region is also unusual; types I and III have a poor fit to the average genome tetranucleotide usage, with R2 = 0.60 and R2 = 0.53, respectively. For comparison, similarly sized sequences from the genome fit the genome average tetranucleotide usage with mean R2 = 0.79 (standard deviation = 0.06). The G+C content of pyoverdine region genes from each type is not significantly different than the genome average of 66.6%. By protein BLAST analysis, the most divergent proteins from each type are similar to pyoverdine proteins from other Pseudomonas species. In PvdE, there is more similarity to a protein from another pseudomonad than to a different P. aeruginosa type. A similar trend occurs in FpvA, except that in two types the highest similarity is with a nonpseudomonal protein; type II FpvA is similar to a receptor from Agrobacterium tumefaciens, and type I FpvA is similar to a receptor from Azotobacter vinelandii.
Approximately 30 kb downstream of the divergent pyoverdine region, pvdY is also divergent between types (Iain Lamont, personal communication). There are two highly diverged alleles of pvdY: type II strains have a 912-bp coding region, and type I and III strains have a 468-bp coding region. The two alleles are not alignable by pairwise nucleotide alignment and have 40% identity at the amino acid level. pvdY from type II strains contains an acetyltransferase protein domain (COG1670, RimL domain, e-value = 7e-06), and both alleles are similar to ribosomal acetyltransferases from other Pseudomonas species. Both alleles of pvdY have unusual codon usage: each allele has a codon adaptation index of less than 0.54, compared to the genome average of 0.68.
Pyoverdine genes adjacent to pvdY, including pvdL (PA2424), pvdG (PA2425), pvdS (PA2426), and pvdX (PA2428), have background levels of divergence between type I and II strains. pvdX, a gene of unknown function that is the only known pyoverdine gene to be expressed in iron-replete conditions, appears to be present in all types and is not divergent between types (68). It is notable that pvdL, a nonribosomal peptide synthetase that synthesizes the pyoverdine chromophore, was not divergent, while the nonribosomal peptide synthetase genes pvdI, pvdJ, and pvdD, which synthesize the pyoverdine side chain, were highly divergent.
We searched for other type-specific genes over an interval from 60 kb upstream to 60 kb downstream of the divergent pyoverdine region. Comparing sequences from strains of types I and II, we found only two candidate genes. Type II strains are missing most or all of gene PA2416. Type II strains also have a predicted gene with a 798-bp coding region between PA2423 and pvdL (PA2424); the gene did not contain a predicted protein domain but encoded a product similar to a hypothetical protein from Chromobacterium violaceum.
Outer membrane receptor gene fpvA and nonribosomal peptide synthetase genes pvdI, pvdJ, and pvdD are the most divergent genes in the pyoverdine region.
Genes that function to synthesize and transport pyoverdine are the most divergent in the region. fpvA, the outer membrane pyoverdine receptor, is the most divergent gene by amino acid alignment (see Fig. 2). The next most divergent genes are immediately adjacent to fpvA and include the ABC transporter pvdE and the nonribosomal peptide synthetase genes pvdD, pvdJ, and pvdI. Other genes of moderate divergence between types include pvdP, pvdM, pvdN, pvdO, and PA2403. Type II strains also have moderate divergence in pvdA, fpvI, and fpvR and have a highly divergent downstream gene, pvdY.
Although genes in the central pyoverdine region are highly divergent by amino acid alignment (see Fig. 2), most colinear genes probably have related functions. Each type has genes colinear with fpvA, pvdE, and nonribosomal peptide synthetase genes, and the predicted secondary structures of the proteins encoded by both fpvA and pvdE are roughly conserved between types. These genes also share protein domains: fpvA genes have a TonB-dependent receptor domain (Pfam domain PF00593), pvdE genes have ABC transporter (PF00005) and ABC transporter transmembrane region (PF00664) domains, and nonribosomal peptide synthetase genes have activation (AMP-binding, PF00501), thiolation (phosphopantetheine attachment site, PF00550), condensation (PF00668), and thioesterase (PF00975) domains as well as putative epimerization domains. fpvA from each type and the nonribosomal peptide synthetase pvdD have been characterized in detail (1, 17, 50).
Pyoverdine type II and III sequences have type-specific genes.
Type II sequences have a gene with a 966-bp coding region that contains an esterase/lipase protein domain (AES domain, COG0657, e-value = 1e-45) and is located between fpvA and the nonribosomal peptide synthetase pvdJ (2). The AES gene has 69% amino acid similarity and 27% nucleotide divergence from the gene PP4218 in the P. putida genome, which also has an esterase/lipase protein domain and is colinear with esterase/lipase protein domain within the P. putida pyoverdine region (43). There is no significant similarity between the esterase/lipase protein domain and genes in the finished Pseudomonas syringae genome DC3000 (11) or the unfinished Pseudomonas fluorescens genome Pf0-1 (GenBank accession number AAAT03000000).
Type III sequences have a gene with a 954-bp coding region that contains a siderophore-interacting protein domain (SIP domain, COG2375, e-value = 2e-52), and is located between pvdI (3) and PA2403. There is no significant similarity between the siderophore-interacting protein domain and genes in the available Pseudomonas putida, P. syringae, and P. fluorescens genomes.
Most intratype divergence is a result of recombination between types.
There is approximately 0.5% nucleotide divergence between typical pairs of P. aeruginosa strains in most regions of the genome (28, 64). Although sequences of the same pyoverdine type primarily display background levels of divergence, there are 11 instances in the analyzed sequences of dramatically more pronounced divergence within a type (see Table 3). Most intratype differences are approximately 100 bp in length and are located within 400 bp of a region of intertype divergence. Since one allele is typically shared with another type, intratype divergence is the result of recombination between types. Recombination in P. aeruginosa is estimated to be very frequent (28, 47), so this evidence of recombination within the pyoverdine locus is not surprising. It is the swapping of divergent sequence between types that is notable. By shuffling sequences between types at the edges of divergent regions, recombination creates intratype divergence and may simultaneously erode the boundary regions of high intertype divergence.
TABLE 3.
Regions of intratype divergencea
| Location in Fig. 1 | Geneb | Length (bp) | Alleles observed in other typesc | Nearest intertype-divergent regiond (bp) |
|---|---|---|---|---|
| G | fpvA | 1,829 | No | >1,000 |
| A | pvdA | 959 | Yes | 0 |
| J | PA2403 | 839 | Yes | >1,000 |
| E | pvdN | 181 | Yes | 100 |
| I | Intergenic between pvdI and PA2403 | 131 | Yes | 133 |
| K | PA2404 | 106 | Yes | 350 |
| H | pvdD and pvdJ | 95 | Yes | 193 |
| F | pvdI and pvdJ | 91 | Yes | 392 |
| B | fpvR | 81 | Yes | 0 |
| C | pvdM | 75 | Yes | 324 |
| D | pvdN | 73 | Yes | 0 |
Intratype divergent regions contain more than three SNPs within each of two or more consecutive 30-bp windows (>10% divergence) when aligning sequences of the same type.
The gene containing the divergent sequence.
Yes indicates that one of the two alleles from the intratype divergence was observed in another type, presumably a result of recombination between types.
The distance from the intratype- divergent region to the nearest region of intertype divergence. This distance is significant because intratype divergence typically occurs adjacent to regions of large intertype divergence.
The outer membrane receptor gene fpvA contains the only example of extensive intratype divergence that cannot be explained in terms of recombination with other known pyoverdine types. Two different alleles exist in type II sequence types: strain 2-164 contains one allele, and strains 1-60 and MSH contain another allele that was recently characterized in detail (17). The alleles are 9% divergent at the nucleotide level and 4% divergent at the amino acid level.
Strain 1-60 contains two examples of an entire gene that is normally type specific that has recombined with one of the other pyoverdine types. Strain 1-60 makes a type II pyoverdine and has predominantly type II sequence in the pyoverdine region. However, the PA2403 and pvdA alleles in 1-60 more closely resemble those in a type I strain. Strain 1-60 appears to be a hybrid, harboring a mixture of alleles typically found in type I or type II strains.
fpvA and PA2403 show evidence of positive selection.
The extensive divergence in the pyoverdine region suggests an unusual evolutionary history. To discover whether positive selection contributed to the divergence, we tested each gene in the region for evidence of positive selection. These tests suggest that fpvA and PA2403, which accumulate nonsynonymous changes at an unusually high rate, are likely to have experienced positive selection (see Table 4).
TABLE 4.
Evidence for positive selectiona
| Gene | No. of sequences | No. of codons | Tree length | Model M0 estimate of ω | Model M8 parameter estimates
|
Positively selected sites predicted under model M8 | ||
|---|---|---|---|---|---|---|---|---|
| p1 | ω | Beta (p,q) | ||||||
| fpvA | 9 | 856 | 18.0 | 0.05 | 0.04 | 2.05 | 0.78, 20.20 | 281,316, 440, 485, 501,512, 513, 532,536, 546, 587, 588, 606, 610, 625, 630, 631,659,672, 691 |
| PA2403 | 8 | 403 | 1.2 | 0.09 | 0.07 | 2.49 | 0.13, 1.92 | 59, 64, 66, 68, 76, 77, 78, 82, 83,84, 88,95, 102,103, 105, 121, 207, 233, 250, 253, 255, 265, 273,274, 288, 312 |
In both fpvA and PA2403, twice the log likelihood ratio in comparing models M0 and M3 and models M7 and M8 indicated positive selection with significance to the 1% level. Model M3 predicted the presence of positive selection in each gene (parameter values not shown), and model M8 predicted positive selection in specific codons, where a proportion of codons p1 had a ω value significantly greater than 1. To identify specific codons under positive selection, Bayes' theorem was used to test whether each codon belonged to the subset of positively selected codons predicted by model M8. Positively selected sites under model M8 are shown with posterior probabilities from 95 to 100% underlined, 90 to 95% in bold, 70 to 90% in italics, and 50 to 70% in plain text. ω represents the nonsynonymous-to-synomymous ratio estimated under model M0, a model that assumes one ω for all codons in a gene. Also shown are parameters p and q for the beta distribution of model M8 and the tree length, measured as the number of nucleotide substitutions per codon. The tree length of fpvA is large because alleles of fpvA are highly diverged compared to alleles of PA2403. Refer to the text for a full description of the tests for positive selection.
fpvA contains a TonB-dependent receptor protein domain, consisting of a 22-bp antiparallel-strand β-barrel, a cork domain residing inside the β-barrel, and an N-terminal extension. Using crystal structures from other proteins with this domain, we find that all positively selected residues of FpvA are located in the β-barrel region. Although no proteins are yet known to interact with the β-barrel of FpvA, the β-barrel region of E. coli FhuA may interact with the TonB protein (29).
There is also evidence that positive selection has acted in PA2403. This gene, which is conserved in multiple Pseudomonas species, is of unknown function (55). PA2403 is not required for pyoverdine synthesis, but PA2403 mutants errantly produce pyoverdine in iron-replete conditions (44). With one exception, all of the positively selected amino acids reside outside the four transmembrane domains of the PA2403 protein (see Fig. 4). After protein folding, positively selected sites would reside on the same membrane face of the inner membrane, since two transmembrane domains separate the sites.
FIG. 4.
Hydrophobicity plot of PA2403 from PAO1 with codons predicted to be under positive selection superimposed. Four prominent hydrophobic regions (hydrophobicity > 2) likely represent transmembrane domains. Red asterisks represent individual codons predicted to be under positive selection, and asterisk size corresponds to posterior probability of positive selection. With one exception, all amino acids that are predicted to have been under positive selection fall between hydrophobic domains and likely reside on the same side of the membrane after protein folding. Hydrophobicity plots of PA2403 with sequence from pyoverdine type II and III genes give similar results.
The models used to test for positive selection assume that recombination or horizontal transfer did not introduce divergence from another allele (4). Thus, the results of these tests should be viewed with some caution, especially considering the similarity of FpvA to receptors in other species.
Nonribosomal peptide synthetase-predicted amino acid specificities are consistent with pyoverdine structural peptide sequences.
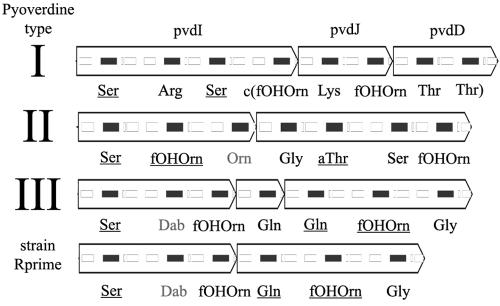
There is excellent correlation between the inferred amino acid sequence made by the nonribosomal peptide synthetase proteins and the primary structure of the pyoverdine that they produce. This correlation is possible because the nonribosomal peptide synthetase proteins are made of a series of cassettes, each of which catalyzes the addition of a particular amino acid to the pyoverdine. A cassette typically contains activation, thiolation, and condensation protein domains, which charge, bind, and connect, respectively, a specific amino acid to a growing peptide. The crystal structure of an activation domain allows homology-based association between cassettes and the amino acid they process during pyoverdine synthesis (13). For each pyoverdine type, the first activation domain in the nonribosomal peptide synthetase operon is specific for the first amino acid in the pyoverdine molecule, the residue closest to the pyoverdine chromophore. Continuing in the order of transcription through the nonribosomal peptide synthetase operon, activation domains are specific to the order of the amino acids in the pyoverdine, proceeding away from the chromophore (see Fig. 3 and 4). This order is consistent with the majority of other nonribosomal peptide synthetase enzymes.
FIG. 3.
Predicted amino acid specificity of nonribosomal peptide synthetase genes from the pyoverdine region. Protein domains are shown superimposed on genes, solid black rectangles representing activation domains and outlined rectangles representing other nonribosomal peptide synthetase domains. Amino acids in gray were not predicted due to lack of similarity to known sequences. Strain R′ contained an in-frame deletion compared to type III strains that removed one amino acid from the pyoverdine side chain and fused two nonribosomal peptide synthetase genes. Only gene names for pyoverdine type I strains are listed. Underlined residues indicate d-isomers, but specific isomers were not predicted from structural data. fOHOrn, formyl-N5-hydroxyornithine; Dab, diaminobutryic acid; aThr, allo-threonine; c, cyclic.
Previous work showed that R′ synthesizes a rare fourth type of pyoverdine, a type III pyoverdine with a missing glutamine residue (61). Consistent with these findings, R′ has a type III sequence type with a deletion of one nonribosomal peptide synthetase cassette, whose predicted specificity is glutamine.
Nonribosomal peptide synthetase enzymes are studied for their ability to generate many small molecules in a combinatorial fashion (2, 42), although attempts to manipulate the order and specificity of the nonribosomal peptide synthetase enzymes have encountered mixed success. The deletion in R′ represents a natural example of nonribosomal peptide synthetase modification. This event also highlights the arbitrariness of the boundaries between nonribosomal peptide synthetase genes, since the in-frame fusion of two predicted nonribosomal peptide synthetase genes produced a functional product. It is unclear whether the novel peptide sequence in the R′ pyoverdine confers any selective advantage; it is clear that deletion or duplication can rapidly change nonribosomal peptide synthetase genes because of their internally repetitive and highly modular structures.
DISCUSSION
Major genomic differences between strains of P. aeruginosa fall into two broad categories. Some strains contain an insertion of one or more genes that are not present in other strains. Examples include the mobile genetic islands pKLK106 and pKLC102 (31) and pathogenicity islands PAG1 (34), PAG2(C), PAG3(SG) (33), and PAPI-1 and PAPI-2 (25). These insertion-deletion polymorphisms do not occur randomly throughout the genome, but often in hotspots such as specific tRNA genes. Plasmids are similar in that they are present or absent in different strains. In contrast, the pyoverdine region is like the O-antigen biosynthetic region (56), the pilA locus (28), and the flagellar glycosylation region (5): each is present in all strains, but the genes in each locus are highly divergent between strains. This replacement island phenomenon presumably results from diversifying selection, a type of selection that maintains multiple alleles in a population.
It is interesting that alleles of pvdE or fpvA are more similar to genes from other soil bacteria than to other P. aeruginosa alleles. This situation is typical of a region under diversifying selection, where divergent alleles predating a speciation event can be inherited in both new species. However, since there is unusual codon usage and tetranucleotide usage in some pyoverdine types, horizontal transfer seems a more probable explanation for the trans-species polymorphism observed at this locus. Since the pyoverdine region does not show unusual G+C content, perhaps some pyoverdine genes originated in another organism with high G+C content. Other Pseudomonas species are primary candidates for the source of divergent alleles, but high similarity to fpvA was also seen in the high-G+C soil bacteria Agrobacterium tumefaciens and Azotobacter vinelandii.
The pyoverdine outer membrane receptor gene fpvA may drive diversity at the pyoverdine locus. To evolve a new pyoverdine type, both the nonribosomal peptide synthetase and the receptor must coevolve, maintaining their mutual specificity. Receptors are typically specific to a particular pyoverdine structure; however, multiple specificity has been reported, such as in the putative uptake of type II pyoverdine by the type III FpvA receptor (17, 38, 41). Our analysis identifies fpvA as the most divergent gene alignable between types in the region and under positive selection. fpvA is chromosomally located among the most divergent genes in the region and has the only intratype variation that is not a result of recombination between types.
Type II fpvA is an entry site for pyocin S3, a molecule made by some P. aeruginosa strains that is taken up by and lethal to some other strains (7). An alternative type I receptor, fpvB, was recently identified; the receptor is present in strains of each pyoverdine type and is regulated differently than fpvA, suggesting that there is selection to maintain alternative modes of regulation in the receptor (21). Viewed together, these aspects of fpvA function suggest a dynamic evolutionary history, where change in the receptor gene leads to further changes in the system of pyoverdine genes. Adjacent to fpvA, the nonribosomal peptide synthetase genes pvdI, pvdJ, and pvdD and the putative ABC transporter pvdE are also highly divergent and probably coevolve with fpvA. This coevolution would select against recombination events between types that separate these genes, particularly selecting against separation of the receptor gene and the nonribosomal peptide synthetase genes. Repeated rounds of receptor change followed by compensatory mutations elsewhere could result in rapid divergence between pyoverdine sequence types.
Diversifying selection seems to be acting on type-specific genes regardless of their chromosomal location. pvdY is separated by 30 kb from the divergent pyoverdine region but has type-specific divergence. This gene, whose function is currently unknown, presumably has a type-specific function in the pyoverdine system even though it is distantly located from other type-specific genes. It remains possible that there are still other type-specific pyoverdine genes elsewhere in the genome.
One of the most puzzling results of this study is the mixture of alleles in strain 1-60. Two genes in the type II strain have type I alleles, which are significantly divergent from and are present in place of type II alleles. To have become divergent, each allele must have had a selective advantage and must have evolved separately from alleles of other types for a relatively long period of time. Yet we now observe this mixture of alleles functioning together. If the alleles of different types can function together, how have the alleles been able to diverge from one another?
Lastly, we considered possible sources of diversifying selection at the pyoverdine locus of P. aeruginosa. Outer membrane protein genes such as the pyoverdine receptor fpvA are common targets for entry by phage or pyocins, and siderophore diversity may be a resistance mechanism. Siderophore diversity may also be a defense against ferrisiderophore stealing. Siderophore production is a cooperative behavior, since diffusion makes the ferrisiderophore available to any cell with an appropriate receptor. However, all cooperative activities invite cheating (23, 70). Many bacterial genome sequences, including the P. aeruginosa genome, contain numerous putative siderophore receptor genes without the corresponding siderophore synthesis genes (15, 49, 69). In iron-poor environments, it may be beneficial for a bacterial strain to make a siderophore that is distinct in structure from the major varieties present in the environment. This evolutionary dynamic could lead to continual generation of new siderophores whose selective advantage to a particular strain is constantly compromised when other strains acquire a compatible receptor.
Acknowledgments
This work was supported by grant MILLEROOVO (Samuel I. Miller, Principal Investigator) from the Cystic Fibrosis Foundation as well as a Center for Excellence in Genome Sciences grant (P50 HG02351) to Maynard V. Olson.
We thank the staff of the University of Washington Genome Center. We are also grateful to Willie Swanson for advice on testing for positive selection, to Jean-Marie Meyer and Jane Burns for providing strains, to Ruolan Qiu for assistance with fosmid library construction, and to Arnold Kas for software assistance.
REFERENCES
- 1.Ackerley, D. F., T. T. Caradoc-Davies, and I. L. Lamont. 2003. Substrate specificity of the nonribosomal peptide synthetase PvdD from Pseudomonas aeruginosa. J. Bacteriol. 185:2848-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackerley, D. F., and I. L. Lamont. 2004. Characterization and genetic manipulation of peptide synthetases in Pseudomonas aeruginosa PAO1 in order to generate novel pyoverdines. Chem. Biol. 11:971-980. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 4.Anisimova, M., R. Nielsen, and Z. Yang. 2003. Effect of recombination on the accuracy of the likelihood method for detecting positive selection at amino acid sites. Genetics 164:1229-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora, S. K., M. C. Wolfgang, S. Lory, and R. Ramphal. 2004. Sequence polymorphism in the glycosylation island And flagellins of Pseudomonas aeruginosa. J. Bacteriol. 186:2115-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman, A., L. Coin, R. Durbin, R. D. Finn, V. Hollich, S. Griffiths-Jones, A. Khanna, M. Marshall, S. Moxon, E. L. Sonnhammer, D. J. Studholme, C. Yeats, and S. R. Eddy. 2004. The Pfam protein families database. Nucleic Acids Res. 32:D138-D141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baysse, C., J. M. Meyer, P. Plesiat, V. Geoffroy, Y. Michel-Briand, and P. Cornelis. 1999. Uptake of pyocin S3 occurs through the outer membrane ferripyoverdine type II receptor of Pseudomonas aeruginosa. J. Bacteriol. 181:3849-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56-63. [DOI] [PubMed] [Google Scholar]
- 9.Bucher, G. E., and J. M. Stephens. 1957. A disease of grasshoppers caused by the bacterium Pseudomonas aeruginosa (Schroeter) Migula. Can. J. Microbiol. 3:611-625. [DOI] [PubMed] [Google Scholar]
- 10.Budzikiewicz, H. 1993. Secondary metabolites from fluorescent pseudomonads. FEMS Microbiol. Rev. 10:209-228. [DOI] [PubMed] [Google Scholar]
- 11.Buell, C. R., V. Joardar, M. Lindeberg, J. Selengut, I. T. Paulsen, M. L. Gwinn, R. J. Dodson, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, S. Daugherty, L. Brinkac, M. J. Beanan, D. H. Haft, W. C. Nelson, T. Davidsen, N. Zafar, L. Zhou, J. Liu, Q. Yuan, H. Khouri, N. Fedorova, B. Tran, D. Russell, K. Berry, T. Utterback, S. E. Van Aken, T. V. Feldblyum, M. D'Ascenzo, W. L. Deng, A. R. Ramos, J. R. Alfano, S. Cartinhour, A. K. Chatterjee, T. P. Delaney, S. G. Lazarowitz, G. B. Martin, D. J. Schneider, X. Tang, C. L. Bender, O. White, C. M. Fraser, and A. Collmer. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 100:10181-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183:444-452. [DOI] [PubMed] [Google Scholar]
- 13.Challis, G. L., J. Ravel, and C. A. Townsend. 2000. Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem. Biol. 7:211-224. [DOI] [PubMed] [Google Scholar]
- 14.Cornelis, P., D. Hohnadel, and J. M. Meyer. 1989. Evidence for different pyoverdine-mediated iron uptake systems among Pseudomonas aeruginosa strains. Infect. Immun. 57:3491-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelis, P., and S. Matthijs. 2002. Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ. Microbiol.. 4:787-798. [DOI] [PubMed] [Google Scholar]
- 16.Darby, C., C. L. Cosma, J. H. Thomas, and C. Manoil. 1999. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:15202-15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Chial, M., B. Ghysels, S. A. Beatson, V. Geoffroy, J. M. Meyer, T. Pattery, C. Baysse, P. Chablain, Y. N. Parsons, C. Winstanley, S. J. Cordwell, and P. Cornelis. 2003. Identification of type II and type III pyoverdine receptors from Pseudomonas aeruginosa. Microbiology 149:821-831. [DOI] [PubMed] [Google Scholar]
- 18.Felsenstein, J. 1993. PHYLIP (phylogeny inference package) version 3.5c, distributed by the author. Department of Genome Sciences, University of Washington, Seattle, Wash..
- 19.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 21.Ghysels, B., B. T. Dieu, S. A. Beatson, J. P. Pirnay, U. A. Ochsner, M. L. Vasil, and P. Cornelis. 2004. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology 150:1671-1680. [DOI] [PubMed] [Google Scholar]
- 22.Gordon, D., C. Desmarais, and P. Green. 2001. Automated finishing with autofinish. Genome Res. 11:614-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin, A. S., S. A. West, and A. Buckling. 2004. Cooperation and competition in pathogenic bacteria. Nature 430:1024-1027. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan, D., J. Jessee, and F. R. Bloom. 1991. Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 204:63-113. [DOI] [PubMed] [Google Scholar]
- 25.He, J., R. L. Baldini, E. Deziel, M. Saucier, Q. Zhang, N. T. Liberati, D. Lee, J. Urbach, H. M. Goodman, and L. G. Rahme. 2004. The broad host range pathogen Pseudomonas aeruginosa strain PA14 carries two pathogenicity islands harboring plant and animal virulence genes. Proc. Natl. Acad. Sci. USA 101:2530-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman, C. S., and F. Winston. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267-272. [DOI] [PubMed] [Google Scholar]
- 27.Karlin, S., and L. R. Cardon. 1994. Computational DNA sequence analysis. Annu. Rev. Microbiol.. 48:619-654. [DOI] [PubMed] [Google Scholar]
- 28.Kiewitz, C., and B. Tummler. 2000. Sequence diversity of Pseudomonas aeruginosa: impact on population structure and genome evolution. J. Bacteriol. 182:3125-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killmann, H., C. Herrmann, A. Torun, G. Jung, and V. Braun. 2002. TonB of Escherichia coli activates FhuA through interaction with the beta-barrel. Microbiology 148:3497-3509. [DOI] [PubMed] [Google Scholar]
- 30.Kim, U. J., H. Shizuya, P. J. de Jong, B. Birren, and M. I. Simon. 1992. Stable propagation of cosmid sized human DNA inserts in an F factor based vector. Nucleic Acids Res. 20:1083-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klockgether, J., O. Reva, K. Larbig, and B. Tummler. 2004. Sequence analysis of the mobile genome island pKLC102 of Pseudomonas aeruginosa C. J. Bacteriol. 186:518-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 33.Larbig, K. D., A. Christmann, A. Johann, J. Klockgether, T. Hartsch, R. Merkl, L. Wiehlmann, H. J. Fritz, and B. Tummler. 2002. Gene islands integrated into tRNA(Gly) genes confer genome diversity on a Pseudomonas aeruginosa clone. J. Bacteriol. 184:6665-6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang, X., X. Q. Pham, M. V. Olson, and S. Lory. 2001. Identification of a genomic island present in the majority of pathogenic isolates of Pseudomonas aeruginosa. J. Bacteriol. 183:843-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukashin, A. V., and M. Borodovsky. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchler-Bauer, A., J. B. Anderson, C. DeWeese-Scott, N. D. Fedorova, L. Y. Geer, S. He, D. I. Hurwitz, J. D. Jackson, A. R. Jacobs, C. J. Lanczycki, C. A. Liebert, C. Liu, T. Madej, G. H. Marchler, R. Mazumder, A. N. Nikolskaya, A. R. Panchenko, B. S. Rao, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, S. Vasudevan, Y. Wang, R. A. Yamashita, J. J. Yin, and S. H. Bryant. 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer, J. M. 2000. Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch. Microbiol.. 174:135-142. [DOI] [PubMed] [Google Scholar]
- 38.Meyer, J. M., V. A. Geoffroy, C. Baysse, P. Cornelis, I. Barelmann, K. Taraz, and H. Budzikiewicz. 2002. Siderophore-mediated iron uptake in fluorescent Pseudomonas: characterization of the pyoverdine-receptor binding site of three cross-reacting pyoverdines. Arch. Biochem. Biophys. 397:179-183. [DOI] [PubMed] [Google Scholar]
- 39.Meyer, J. M., A. Neely, A. Stintzi, C. Georges, and I. A. Holder. 1996. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 64:518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer, J. M., A. Stintzi, D. De Vos, P. Cornelis, R. Tappe, K. Taraz, and H. Budzikiewicz. 1997. Use of siderophores to type pseudomonads: the three Pseudomonas aeruginosa pyoverdine systems. Microbiology 143:35-43. [DOI] [PubMed] [Google Scholar]
- 41.Meyer, J. M., A. Stintzi, and K. Poole. 1999. The ferripyoverdine receptor FpvA of Pseudomonas aeruginosa PAO1 recognizes the ferripyoverdines of P. aeruginosa PAO1 and P. fluorescens ATCC 13525. FEMS Microbiol. Lett. 170:145-150. [DOI] [PubMed] [Google Scholar]
- 42.Mootz, H. D., D. Schwarzer, and M. A. Marahiel. 2002. Ways of assembling complex natural products on modular nonribosomal peptide synthetases. Chembiochem 3:490-504. [DOI] [PubMed] [Google Scholar]
- 43.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. Chris Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. A. Eisen, K. N. Timmis, A. Dusterhoft, B. Tummler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol.. 4:799-808. [DOI] [PubMed] [Google Scholar]
- 44.Ochsner, U. A., P. J. Wilderman, A. I. Vasil, and M. L. Vasil. 2002. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol. Microbiol.. 45:1277-1287. [DOI] [PubMed] [Google Scholar]
- 45.Oldenburg, K. R., K. T. Vo, S. Michaelis, and C. Paddon. 1997. Recombination-mediated PCR-directed plasmid construction in vivo in yeast. Nucleic Acids Res. 25:451-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouali, M., and R. D. King. 2000. Cascaded multiple classifiers for secondary structure prediction. Protein Sci. 9:1162-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pirnay, J. P., D. De Vos, C. Cochez, F. Bilocq, A. Vanderkelen, M. Zizi, B. Ghysels, and P. Cornelis. 2002. Pseudomonas aeruginosa displays an epidemic population structure. Environ. Microbiol.. 4:898-911. [DOI] [PubMed] [Google Scholar]
- 48.Plotnikova, J. M., L. G. Rahme, and F. M. Ausubel. 2000. Pathogenesis of the human opportunistic pathogen Pseudomonas aeruginosa PA14 in Arabidopsis. Plant Physiol. 124:1766-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poole, K., and G. A. McKay. 2003. Iron acquisition and its control in Pseudomonas aeruginosa: many roads lead to Rome. Front. Biosci. 8:d661-d686. [DOI] [PubMed] [Google Scholar]
- 50.Poole, K., S. Neshat, K. Krebes, and D. E. Heinrichs. 1993. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J. Bacteriol. 175:4597-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pride, D. T., R. J. Meinersmann, T. M. Wassenaar, and M. J. Blaser. 2003. Evolutionary implications of microbial genome tetranucleotide frequency biases. Genome Res. 13:145-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pukatzki, S., R. H. Kessin, and J. J. Mekalanos. 2002. The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 99:3159-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahme, L. G., E. J. Stevens, S. F. Wolfort, J. Shao, R. G. Tompkins, and F. M. Ausubel. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899-1902. [DOI] [PubMed] [Google Scholar]
- 54.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol.. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 55.Ravel, J., and P. Cornelis. 2003. Genomics of pyoverdine-mediated iron uptake in pseudomonads. Trends Microbiol.. 11:195-200. [DOI] [PubMed] [Google Scholar]
- 56.Raymond, C. K., E. H. Sims, A. Kas, D. H. Spencer, T. V. Kutyavin, R. G. Ivey, Y. Zhou, R. Kaul, J. B. Clendenning, and M. V. Olson. 2002. Genetic variation at the O-antigen biosynthetic locus in Pseudomonas aeruginosa. J. Bacteriol. 184:3614-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raymond, C. K., E. H. Sims, and M. V. Olson. 2002. Linker-mediated recombinational subcloning of large DNA fragments using yeast. Genome Res. 12:190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rice, P., I. Longden, and A. Bleasby. 2000. EMBOSS: the European Molecular Biology open software suite. Trends Genet. 16:276-277. [DOI] [PubMed] [Google Scholar]
- 59.Rocha, E. P., A. Viari, and A. Danchin. 1998. Oligonucleotide bias in Bacillus subtilis: general trends and taxonomic comparisons. Nucleic Acids Res. 26:2971-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365-386. [DOI] [PubMed] [Google Scholar]
- 61.Ruangviriyachai, C., D. U. Fernandez, R. Fuchs, J. M. Meyer, and H. Budzikiewicz. 2001. A new pyoverdin from Pseudomonas aeruginosa R'. Z. Naturforsch. C 56:933-938. [DOI] [PubMed] [Google Scholar]
- 62.Sharp, P. M., and W. H. Li. 1987. The codon Adaptation Index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 15:1281-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 64.Spencer, D. H., A. Kas, E. E. Smith, C. K. Raymond, E. H. Sims, M. Hastings, J. L. Burns, R. Kaul, and M. V. Olson. 2003. Whole-genome sequence variation among multiple isolates of Pseudomonas aeruginosa. J. Bacteriol. 185:1316-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 66.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 68:1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vasil, M. L., and U. A. Ochsner. 1999. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol. Microbiol.. 34:399-413. [DOI] [PubMed] [Google Scholar]
- 69.Visca, P., L. Leoni, M. J. Wilson, and I. L. Lamont. 2002. Iron transport and regulation, cell signalling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol.. 45:1177-1190. [DOI] [PubMed] [Google Scholar]
- 70.West, S. A., and A. Buckling. 2003. Cooperation, virulence and siderophore production in bacterial parasites. Proc. R. Soc. Lond. B Biol. Sci. 270:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong, G. K., J. Yu, E. C. Thayer, and M. V. Olson. 1997. Multiple-complete-digest restriction fragment mapping: generating sequence-ready maps for large-scale DNA sequencing. Proc. Natl. Acad. Sci. USA 94:5225-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang, Z. 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13:555-556. [DOI] [PubMed] [Google Scholar]
- 73.Yang, Z., R. Nielsen, N. Goldman, and A. M. Pedersen. 2000. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 155:431-449. [DOI] [PMC free article] [PubMed] [Google Scholar]