Abstract
Background
Lymph node (LN) status is vital to evaluate the curative potential of relatively early gastric cancer (GC; T1–T2) treatment (endoscopic or surgery). Currently, there is a lack of robust and convenient methods to identify LN metastasis before therapeutic decision-making.
Methods
Genome-wide expression profiles of long noncoding RNA (lncRNA) in primary T1 gastric cancer data from The Cancer Genome Atlas (TCGA) was used to identify lncRNA expression signature capable of detecting LN metastasis of GC and establish a 10-lncRNA risk-prediction model based on deep learning. The performance of the lncRNA panel in diagnosing LN metastasis was evaluated both in silico and clinical validation methods. In silico validation was conducted using TCGA and Asian Cancer Research Group (ACRG) datasets. Clinical validation was performed on T1 and T2 patients, and the panel’s efficacy was compared with that of traditional tumor markers and computed tomography (CT) scans.
Results
Profiling of genome-wide RNA expression identified a panel of lncRNA to predict LN metastasis in T1 stage gastric cancer (AUC = 0.961). A 10-lncRNA risk-prediction model was then constructed, which was validated successfully in T1 and T2 datasets (TCGA, AUC = 0.852; ACRG, AUC = 0.834). Thereafter, the clinical performance of the lncRNA panel was validated in clinical cohorts (T1, AUC = 0.812; T2, AUC = 0.805; T1 + T2, AUC = 0.764). Notably, the panel demonstrated significantly better performance compared with CT and traditional tumor markers.
Conclusions
The novel 10-lncRNA could diagnose LN metastasis robustly in relatively early gastric cancer (T1–T2), with promising clinical potential.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10120-023-01428-8.
Keywords: Bioinformatics, Biomarkers, Gastric cancer, Lymph node metastasis, LncRNA signature
Background
Globally, gastric cancer (GC) is fifth in the list of most common cancers and ranks fourth as the most common cause of cancer death [1]. Lymph node (LN) metastasis is a major clinical feature of GC, which influences the poor prognosis of patients with GC [2]. Even for early GC, the 10-year survival rates of patients with or without LN metastasis are significantly different, at 72 and 92%, respectively [3]. Accurate evaluation of LN status in patients with GC before treatment is critical to evaluate the degree of disease and improve treatment strategies. Currently, the diagnosis of LN metastasis is carried out mainly using conventional tumor markers (carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19-9)) and computed imaging methods (computed tomography (CT) and positron emission tomography with CT (PET-CT)). Unfortunately, these methods show poor performance to clinically identify LN and frequently demonstrate poor correlation and high error rates [4, 5]. Thus, there is an urgent need for more accurate and reliable detection methods to identify LN metastasis in GC, which might be used to enhance the prognosis of patients with GC significantly.
Gastric cancer is still treated using surgery and endoscopic resection. Currently, Asian and European guidelines identify endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) as the first choice treatments for most cases of early GC (cT1a) and are considered to be safe and definitive treatments [6, 7]. However, patients who are considered to be at risk of LN metastasis after endoscopic surgery will undergo additional radical surgery, because of submucosal invasion (T1b), large tumor size, and poor differentiation [8]. Unfortunately, pathological examination of these post-gastrectomy tissues, especially from early GC, revealed that only about 20% of patients were identified as having LN metastasis [9, 10]. In past decades, the optimal extent of lymphadenectomy has also been discussed extensively in the field of surgery. With the development of precision medicine, for patients with GC with cT1-T2N0M0 status, laparoscopic sentinel node navigation surgery (LSNNS) was proposed for stomach preservation, which showed no difference in 3-year overall survival (3y-OS) and 3-year disease free survival (3y-DFS) compared with laparoscopic standard D2 gastrectomy, but resulted in better long-term quality of life and nutritional status [11, 12]. Prospective evaluation of sentinel lymph node navigation surgery for relatively early GC (T1–T2) is a current development trend of function-preserving, personalized, and minimized gastrectomy [12–14]. However, LSNNS is based on a comprehensive assessment of the LN status of patients, which is a challenge for its practical application. The lack of accurate and reliable detection of preoperative LN metastasis status means that many patients have experienced unnecessary overtreatment, which also limits the beneficial development of precision medicine.
It is highlighted by genome-wide association studies in cancer that single-nucleotide polymorphisms (SNPs) are related to cancer risk and more than 80% of cancer-associated SNPs occur in noncoding regions of the genome [15]. In addition, most somatic mutations, copy number alterations, and cancer-related SNPs are related to ncRNAs. Long noncoding RNAs (lncRNAs) account for the majority of human ncRNAs (approximately 76%) and maintain homogenous expression within and between tumor tissues [16, 17]. Functionally, long noncoding RNAs are found in sense or antisense orientation to protein-coding genes, in introns of protein-coding genes or in intergenic regions of the genome, and mediate positive or negative regulation [18]. Presently, the number of disease-related lncrnas identified by experiments is less than 1% of the identified sites, and its biological function needs to be further explored.
Herein, transcriptome-wide expression profiles of long noncoding RNA (lncRNA) were analyzed comprehensively and systematically, and a 10-lncRNA panel was established to identify GC LN metastasis (T1 and T2). We verified the effectiveness of the panel in independent databases and clinical tissue samples. The performance of the lncRNA panel was also compared with that of CEA, CA19-9, and CT, highlighting the value of this panel in predicting LN metastasis of T1 and T2 GC. The lncRNA panel could function as the basis for clinical decision-making for patients with GC.
Methods
Public datasets and the identification of the gene-expression signature
To identify an lncRNA expression signature for the detection of lymph node (LN) metastasis in gastric cancer (GC), the study used genome-wide expression profiles of lncRNAs from primary tumors with and without LN metastasis, which were obtained from The Cancer Genome Atlas (TCGA) database. Only pathological T1 and T2 RNA-sequencing (RNA-Seq) data were used for further analysis. The T1 data were from 15 LN metastasis negative (LNN) and 5 LN metastasis positive (LNP) samples, and the T2 data were from 34 LNN and 48 LNP samples. The processed TCGA level 3 RNA-Seq data for GC were obtained from the Firehose Broad GDAC portal [19]. Independent validation data were downloaded from the Asian Cancer Research Group (ACRG). In the gene-level RNA-Seq by Expectation–Maximization (RSEM files), we converted the scaled estimates to transcripts per million (TPM) by multiplying them by 106, and then carrying out log2-transformation. We filtered all lncRNA expression levels from the TCGA and ACRG processed data according to the human gene annotation file [20] (https://ftp.ensembl.org/pub/release97/gtf/homo_sapiens/Homo_sapiens.GRCh38.97.chr.gtf.gz). Then, logistic regression analysis was performed using the Logistic Regression (LR) function from Pytorch [21] (citation https://arxiv.org/abs/1912.01703). Feature importance was estimated using coefficients from the LR model. To assess the lncRNA panel's diagnostic accuracy, the selected lncRNA features were used to construct a multivariate LR model, followed by calculation of the area under the receiver operator characteristic (ROC) curve (AUC) values. Ultimately, the probability of each patient being identified as LNP was used as the basis to calculate the risk scores. The flowchart of this study is shown in Supplementary Fig. 1.
Clinical cohort evaluation
To validate the identified lncRNA markers and for clinical training, we enrolled three independent patient cohorts comprising 245 cases in total. Cohort 1 consisted of 20 surgically resected GC specimens from 8 LNP patients and 12 LNN patients. Cohort 2 included 98 patients (LNP = 19, LNN = 79). Cohort 3 included 127 patients (LNP = 38, LNN = 89). Patients in the clinical cohorts were treated at the Lihuili Hospital affiliated to Ningbo University (China). These patients had biopsy-proven primary GC and underwent curative surgery between December 2017 and January 2022. During surgery, we obtained tissues samples from a representative malignant lesion located in the surgically excised stomach specimen. The tissue samples were added with RNAstore (CWBIO, Shanghai, China), frozen rapidly in liquid nitrogen, and stored at − 80 ºC. The summarized characteristics of the patients in the clinical cohorts are shown in Table 1 and Fig. 2A.
Table 1.
Clinical characteristics of the patients in cohorts 2 and 3
| Characteristics | Clinical cohort 2 (n = 98) | Clinical cohort 3 (n = 127) | ||
|---|---|---|---|---|
| LN positive (n = 19) | LN negative (n = 79) | LN positive (n = 38) | LN negative (n = 89) | |
| Age (years) | 69.53 ± 6.141 | 68.62 ± 7.550 | 69.42 ± 8.465 | 69.00 ± 0.769 |
| Sex | ||||
| Male | 11 | 40 | 23 | 49 |
| Female | 8 | 39 | 15 | 40 |
| CEA (ng/ml) | 4.226 ± 3.046 | 2.890 ± 1.597 | 4.750 ± 3.307 | 3.794 ± 2.098 |
| Positive | 7 | 8 | 10 | 21 |
| Negative | 12 | 71 | 28 | 68 |
| CA19-9 (U/ml) | ||||
| Positive | 8 | 8 | 9 | 14 |
| Negative | 11 | 71 | 29 | 75 |
| CT | ||||
| Positive | 5 | 9 | 12 | 10 |
| Negative | 14 | 70 | 26 | 79 |
| T stage | ||||
| T1 | 19 | 79 | 0 | 0 |
| T2 | 0 | 0 | 38 | 89 |
| N stage | ||||
| N0 | 0 | 79 | 0 | 89 |
| N1 | 16 | 0 | 25 | 0 |
| N2 | 3 | 0 | 10 | 0 |
| N3 | 0 | 0 | 3 | 0 |
| LV invasion | ||||
| Positive | 15 | 8 | 30 | 35 |
| Negative | 4 | 71 | 8 | 54 |
| Venous invasion | ||||
| Positive | 12 | 15 | 26 | 34 |
| Negative | 7 | 64 | 12 | 55 |
The CEA cutoff value is 5 ng/ml; the CA19-9 cutoff value is 37 U/ml
LN lymph node, CEA carcinoembryonic antigen, CA19-9 carbohydrate antigen 19–9, CT computed tomography, T stage tumor stage, N stage node stage, LV lymphovascular
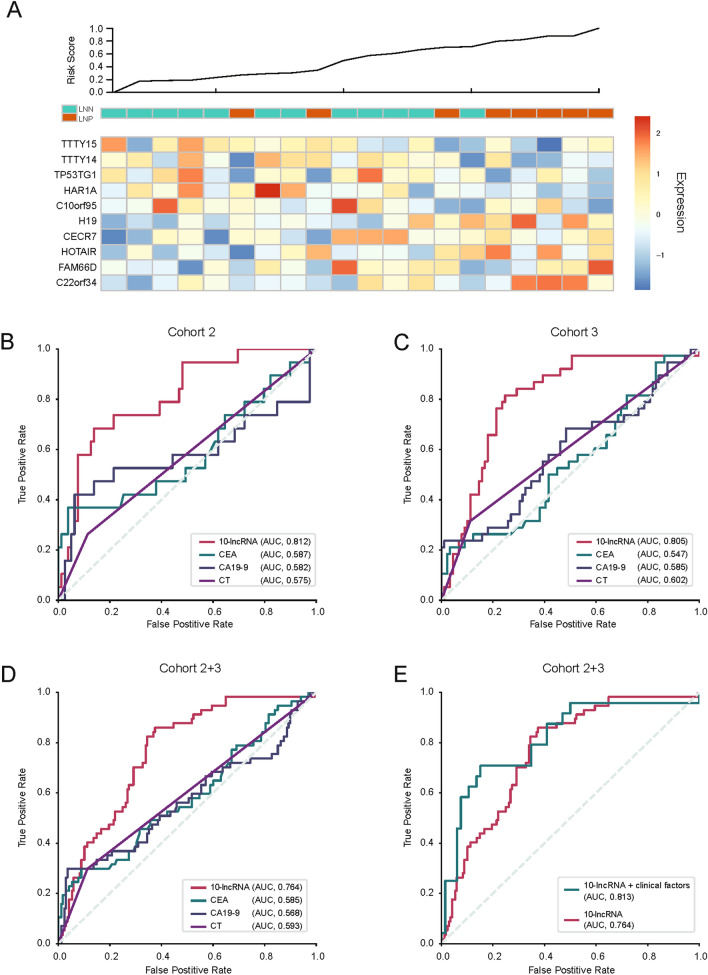
Fig. 2.
Performance of the 10-long noncoding RNA (lncRNA) panel to identify lymph node metastasis status in the clinical validation cohorts
RNA extraction and quantitative real-time reverse transcription PCR (qRT-PCR) analysis
An RNeasy mini kit (QIAGEN, Hilden, Germany) was used to isolate total RNA from frozen surgical tissues, following the supplier's guidelines. The RT-PCR step of the qRT-PCR protocol was carried out using a SensiFAST probe Lo-ROX Kit (Bioline, London, UK) and the qPCR step used the QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Assay reproducibility was ensured via multiple techniques, such as including appropriate controls, excluding specimens with poor RNA quality, and the analysis of multiple replicates carried out at various time points. The QuantStudio 6 Flex Real-Time PCR System Software (Applied Biosystems) was used to assess gene expression. The expression level of ACTB (encoding beta actin) was used to determine and correct the relative expression of target genes, employing the 2–ΔCt method. In this method, ΔCt is the difference in cycle threshold (Ct) values between ACTB and the gene of interest. The data were then log2 transformed. Supplementary Table 1 details the PCR primers used.
Statistical evaluations
The method of DeLong [22] was used to assess the statistically significant differences among the ROC curves. Python (version 3.8, https://www.python.org/) was used to carry out the statistical analyses. Two-tailed t test-determined p values less than 0.05 indicated statistical significance.
LncRNA enrichment analysis
For lncRNA enrichment analysis, we used the website application constructed by Chen et al. [23] (https://doi.org/10.1093/nar/gkaa806). The data were visualized on a histogram and bubble chart using ggplot2 [24].
Results
Genome-wide lncRNA expression profiles identified a 10-lncRNA panel to predict LN metastasis in T1 and T2 stage GC
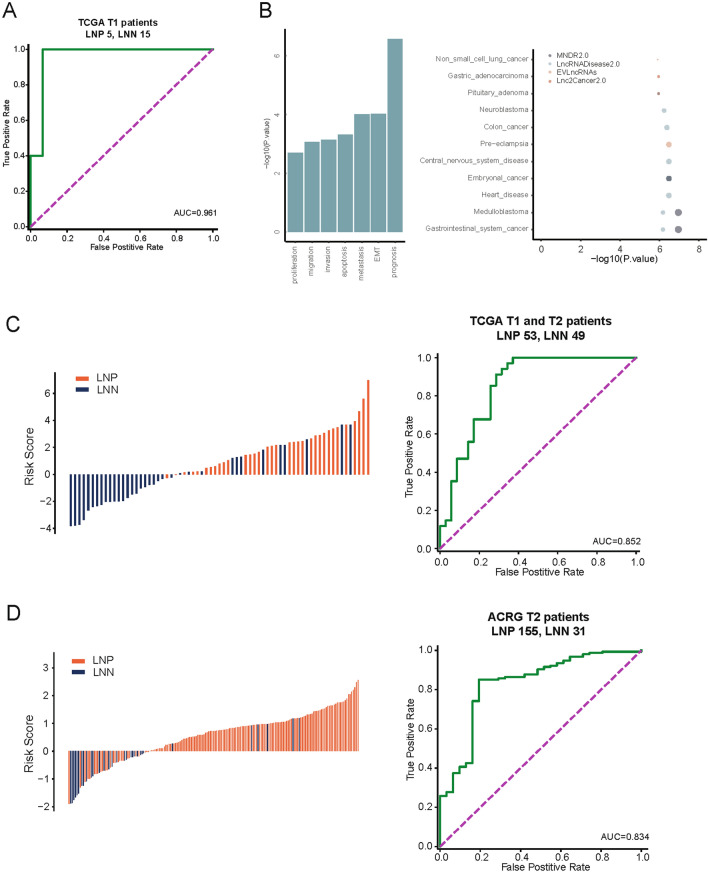
First, we systematically and comprehensively analyzed RNA-seq expression profiling data from patients with GC at T1 stage in the TCGA database, which included 5 LNP patients and 15 LNN patients, to identify an lncRNA expression signature to diagnose patients with T1 stage GC with LN metastasis using deep learning model. The validation of the holdout dataset demonstrated that the model could distinguish patients with LNP GC from those with LNN GC (AUC = 0.961, Fig. 1A). To make the lncRNA signature more practical and suitable for clinical use, we prioritized lncRNAs resulted in a 10-lncRNA signature for further validation based on the feature importance in logistic regression, which included five relatively highly expressed lncRNAs (H19, CECR7, HOTAIR, FAM66D, C22orf34) and five lncRNAs with relatively low expression (TTTY15, TTTY14, TP53TG1, HAR1A, C10orf95) in the LNP versus LNN comparison. In addition, tumor functional enrichment analysis was carried out for this panel. The results revealed that this 10-lncRNA panel was closely related to tumor prognosis, epithelial–mesenchyme transition (EMT), and metastasis, and was specific for gastrointestinal system cancer (Fig. 1B).
Fig. 1.
The long noncoding RNA (lncRNA) expression-based signature to identify lymph node metastasis in T1 and T2 stage gastric cancer
Considering that both T1N0M0 and T2N0M0 are stage I gastric cancer, and the significance of T2 lesions in the current precision medicine of GC, the predictive accuracy of the 10-lncRNA panel was also validated in the dataset containing patients with T2 GC. Notably, individual lncRNAs showed limited performance in external independent datasets, while the integration of all 10 lncRNAs demonstrated significant performance. Multivariate LR analysis was then used to obtain a 10-lncRNA risk-prediction model: risk score = − 0.141 * TTTY15 − 0.140 * TTTY14 − 0.117 * TP53TG1 − 0.100 * HAR1A − 0.074 * C10orf95 + 0.166 * H19 + 0.212 * CECR7 + 0.222 * HOTAIR + 0.226 * FAM66D + 0.236 * C220rf34. Using a larger TCGA cohort (including 20 T1 and 82 T2 patients) and a cohort of 186 T2 patients from the ACRG (Fig. 1C, D), the risk model could differentiate LNP from LNN patients (AUC = 0.85, Fig. 1C; AUC = 0.83, Fig. 1D). This 10-lncRNA panel exhibited a robust performance in two independent validated datasets, highlighting its potential for diagnostic prediction of LN metastasis in patients with T1 and T2 stage GC.
A. Receiver operating characteristic (ROC) curves revealing the diagnostic performance of the model to distinguish lymph node-positive (LNP) and lymph node-negative (LNN) patients in The Cancer Genome Atlas (TCGA) T1 samples. B. Histogram and bubble diagrams showing the enrichment analysis of cancer hallmark and disease in the 10-lncRNA panel. C. The lymph node (LN) risk scores divided by LN status in the T1 and T2 cohorts from the TCGA, shown as a waterfall diagram, and a receiver operating characteristic (ROC) curve showing how the 10-lncRNA risk-prediction model performed in diagnosing patients with T1 and T2 stage disease in the TCGA data. D. The LN risk scores divided by LN status in the T2 cohort from Asian Cancer Research Group (ACRG), shown as a waterfall diagram, and a ROC curve showing how the 10-lncRNA risk-prediction model performed in diagnosing patients T2 stage disease in the ACRG data.
Validation of the 10-lncRNA risk-prediction model to identify lymph node metastasis in independent clinical cohorts
The accuracy of diagnosis using the 10-lncRNA panel was assessed using RNA-seq in validation clinical cohort 1 and by qRT-PCR in validation clinical cohorts 2 and 3 (Table 1). All patients in cohort 1 were in T1 stage, which included 8 LNP and 12 LNN patients. The heatmap of the 10-lncRNA panel and the risk score curve are shown in Fig. 2A. As expected, there was a significant difference in the expression of corresponding lncRNAs between the LNN and LNP samples, revealing an effective diagnostic performance by our risk-prediction model (Fig. 2A).
The patients in cohort 2 and 3 had T1 (19 LNP and 79 LNN) stage and T2 (38 LNP and 89 LNN) stage GC, respectively. Multivariate LR analysis was used to assess the effectiveness of the 10-lncRNA panel in T1–T2 tumors. In cohort 2, the panel showed an AUC of 0.812 (Table 2 and Fig. 2B), and in cohort 3, the AUC was 0.805 (Table 2 and Fig. 2C). However, the predictive performance of our panel in cohort 2 + 3 (T1 + T2) was slightly reduced (AUC = 0.764, Fig. 2D) compared with verification using the individual cohorts, although there was still a good effect. This might be related to the high heterogeneity of GC and the difference in the overall expression of 10 lncRNAs in the T1 and T2 specimens. Overall, the validation results agreed with those obtained using the training cohort: the 10-lncRNA panel could robustly and effectively distinguish LNP from LNN in patients with T1 and T2 stage GC.
Table 2.
Summary of the individual lncRNA performance to predict lymph node metastasis in clinical cohorts 2 and 3
| lncRNA | Clinical cohort 2 | Clinical cohort 3 | ||||
|---|---|---|---|---|---|---|
| AUC | Specificity | Sensitivity | AUC | Specificity | Sensitivity | |
| TTTY15 | 0.659 | 0.947 | 0.557 | 0.586 | 0.658 | 0.584 |
| TTTY14 | 0.526 | 0.368 | 0.949 | 0.656 | 0.579 | 0.753 |
| TP53TG1 | 0.610 | 0.895 | 0.443 | 0.644 | 0.447 | 0.865 |
| HAR1A | 0.562 | 0.684 | 0.544 | 0.670 | 0.526 | 0.798 |
| C10orf95 | 0.672 | 0.737 | 0.595 | 0.690 | 0.526 | 0.888 |
| H19 | 0.676 | 0.772 | 0.526 | 0.676 | 0.629 | 0.684 |
| CECR7 | 0.709 | 0.709 | 0.789 | 0.669 | 0.742 | 0.737 |
| HOTAIR | 0.710 | 0.975 | 0.421 | 0.689 | 0.921 | 0.500 |
| FAM66D | 0.701 | 0.709 | 0.842 | 0.707 | 0.843 | 0.632 |
| C22orf34 | 0.702 | 0.570 | 0.947 | 0.695 | 0.730 | 0.711 |
| Risk score | 0.812 | 0.861 | 0.684 | 0.805 | 0.753 | 0.816 |
AUC area under the curve
A. The risk score curve and heatmap of the lncRNAs expressed between lymph node-positive (LNP) and lymph node-negative (LNN) patients in clinical cohort 1. B and C. Receiver operating characteristic (ROC) curves showing how the 10-lncRNA risk-prediction model performed in identifying lymph node (LN) metastasis compared with that of the carcinoembryonic antigen (CEA), carbohydrate antigen 19–9 (CA19-9), and computed tomography (CT) in clinical cohort 2 (B) (p < 0.05) and clinical cohort 3 (C) (p < 0.05), respectively. D. ROC curves illustrating the diagnostic value for identification of LN metastasis of the 10-lncRNA panel compared with that of CEA, CA19-9, and CT in clinical cohort 2 and cohort 3 (p < 0.05). E. ROC curves illustrating the diagnostic accuracy of the combinatorial model integrating the 10-lncRNA panel and clinicopathological features in clinical cohort 2 + 3 compared with that of the 10-lncRNA panel alone (p < 0.05).
The 10-lncRNA panel showed better diagnostic power compared with conventional tumor markers and CT in LN metastasis
For the surgical management of patients with GC, generally, enhanced CT imaging is employed to determine clinical N stage before surgery. Typically, CT features such as an LN diameter ≥ 1 cm, ring or heterogeneous enhancement, are employed to diagnose LN metastasis. However, CT imaging cannot successfully diagnose most cases of LN metastasis, or there may be misdiagnosis; therefore, only the pathological examination of surgically excised tissue can confirm LM metastasis in such cases. As shown in Table 3, we demonstrated that our 10-lncRNA risk-prediction model could effectively identify LN metastasis using univariate and multivariate analyses, independent of preoperative clinical characteristics such as sex, age, conventional tumor markers, and CT.
Table 3.
Univariate and multivariate logistic regression analyses of the statistical significance of the 10-lncRNA risk score to diagnose LN metastasis status in clinical cohorts 2 and 3
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| Age | 1.012 | 0.971–1.054 | 0.578 | 1.004 | 0.955–1.057 | 0.888 |
| Sex | 1.312 | 0.713–2.414 | 0.383 | 1.421 | 0.687–2.939 | 0.342 |
| CEA | 2.037 | 1.017–4.079 | 0.045 | 1.528 | 0.654–3.572 | 0.327 |
| CA19-9 | 2.820 | 1.369–5.813 | 0.005 | 4.104 | 1.677–10.046 | 0.002 |
| CT | 3.333 | 1.588–6.997 | 0.001 | 4.073 | 1.661–9.991 | 0.002 |
| Risk score | 8.889 | 4.085–19.341 | < 0.0001 | 11.072 | 4.706–26.046 | < 0.0001 |
LN lymph node, CI confidence interval, CEA carcinoembryonic antigen, CA19-9 carbohydrate antigen 19–9, CT computed tomography
To assess the diagnostic efficiency of the panel, its performance was compared with that of conventional tumor markers (CEA and CA19-9) and CT in clinical cohort. Our 10-lncRNA panel showed significant superiority over preoperative clinical factors, CEA, CA19-9, and CT (Fig. 2B, C and D, comparison of the AUC values were compared using the DeLong test). In addition, we combined the 10-lncRNA panel with clinicopathological features (CEA, CA19-9, and CT) in cohorts 2 + 3. The results were also encouraging: this combination further improved the diagnostic accuracy of our panel (AUC = 0.813) compared with the 10-lncRNA panel alone (Fig. 2E). In conclusion, we constructed and validated a 10-lncRNA panel that demonstrated robust discriminative power compared with current preoperative management approaches to identify cases of LNP gastric cancer.
Discussion
Currently, minimally invasive or non-invasive, stomach-preserving, function-preserving, and individualized treatment has become a trend in global GC treatment. Clinically, determining LN status is crucial to indicate and evaluate the curative potential of GC endoscopic treatment and surgery, especially in patients with relatively early GC (T1–T2). Pathological diagnosis following radical gastrectomy remains the optimal way to evaluate a patient's GC's LN status, considering our lack of effective molecular markers that can robustly detect LN metastasis before therapeutic decision-making. Moreover, only patients with GC in situ (Tis stage) and T1a GC without LN metastasis can be treated successfully using endoscopic mucosal or submucosal resection. However, the actual LN metastasis rate of early GC (T1) is only around 20%. In addition, the incidence of regional LN metastasis is limited in patients with T2 GC, in which D2 gastrectomy might be an excessively invasive surgery, involving in a significant waste of medical resources [9, 10, 12]. Currently, the development of sentinel node navigation surgery (SNNS) and laparoscopic surgery in GC provides a direction for minimally invasive gastric surgery. The study group of the Japan Society of SNNS has already formulated the standard procedure for SNNS, which uses a dual tracer comprising technetium 99 m–labeled tin colloid and 1% isosulfan blue dye [25]. Although several single institutions have reported the successful use of SNNS, because GC has a somewhat complex lymphatic flow, there still are controversial aspects regarding the application of SNNS [12, 26, 27].
LncRNAs are mRNA-like transcripts of > 200 nucleotides with no capacity to encode proteins [25]. A variety of cancers show abnormal expression of lncRNAs, which have diverse functions in gene regulation, cell biological behavior, and tumor initiation and progression [28, 29]. To date, there have been a considerable number of studies on lymph node metastasis of GC; however, most of them explored the regulatory mechanism of a single lncRNA [30, 31]. Although these studies are meaningful and significant, the lack of a comprehensive and dynamic understanding of lymph node metastasis limits the clinical application value of these findings. The recent development and popularization of high-throughput sequencing technologies have increased our understanding of the molecular characteristics of GC [32, 33]. Notably, the different T stages of GC have strong histological heterogeneity, and the correlation between lncRNAs and LN metastasis in relatively early GC (T1–T2) remains unexplored.
In this article, we used RNA-sequencing to gain insights into the molecular biology of tumor heterogeneity and disease processes to identify LN metastasis. A systematic and comprehensive analysis of transcriptome-wide expression profiles of patients with T1–T2 GC, with and without LN metastasis, was used to establish an optimized 10-lncRNA panel to identify LN metastasis using logistic regression analysis. Subsequently, the panel was validated in three independent validation cohorts based on RNA-seq and qRT-PCR, achieving encouraging results. Our study is based on the concept of minimally invasive and non-invasive, devoted to the prediction of lymph node metastasis in early gastric cancer and clinical decision support. At the initial stage of the study, we also verified the predictive value of our panel in patients with T3 and T4 stage GC by TCGA and ACRG databases, but the results were not as expected (Supplementary Fig. 2), which may related to the heterogeneity of GC with different T stages.
Carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19-9) are the most commonly used clinical monitoring serum indicators of digestive system tumors. It has been widely reported that elevated serum CEA and CA19-9 levels correlated well with lymph node metastasis, lymphatic invasion, stage grouping, and depth of invasion [4, 34–36]. Specifically, the thresholds of protein biomarkers were set according to clinical instruction, with 5 ng/mL for CEA and 37 U/mL for CA19-9. Fan et al. also reported that elevated CEA and CA19-9 level was correlated with the presence of lymph node metastasis in early GC, but the diagnostic sensitivity of CEA and CA19-9 was not satisfactory [4]. Our further analysis demonstrated the superiority of the 10-lncRNA panel over current clinicopathological factors, including CEA, CA19-9, and CT-based imaging, to diagnose LN metastasis in patients with GC. Although the accuracy of 10-lncRNA panel in combined cohort 2 + 3 was slightly decreased, its diagnostic accuracy improved again after combining it with clinicopathological features. We also performed functional and expression enrichment analysis of the 10 lncRNAs, several of which are related to metastasis and prognosis. LncRNA H19 is considered a carcinogenic factor in GC, and its upregulation is related to tumor cell proliferation, invasion, migration, and EMT [37]. HOTAIR has been reported to be related to the expression of HER2 (encoding human epidermal growth factor receptor 2) and facilitates GC lymph node metastasis [38]. A study using TCGA-based bioinformatics analysis and microarray analysis revealed that HAR1A is a tumor suppressor involved in tumor progression via EMT regulation and is negatively associated with prognosis [39, 40]. In our panel, HAR1A also acted as a negative factor for early lymph node metastasis in GC. Similarly, TP53TG1 and TTTY15 have been confirmed to be differentially expressed in GC tissues compared with that in normal gastric mucosa [28, 41]. Finally, as biomarkers, each lncRNA in our panel was endowed with an additional diagnostic coefficient and made a significant contribution to the identification of LN metastasis.
This study has certain limitations. First, this was a retrospective study, and its design means that although we validated our findings in multiple clinical cohorts, prospective studies are still required. Second, the main aim of this study was to find early-stage GC biomarkers; therefore, the samples were concentrated in the T1 and T2 GC stages, which limited the sample size to discover biomarkers and had a certain impact on obtaining the panel with maximum efficiency. To overcome these limitations, larger cohorts comprising patients with GC and T1 and T2 LN metastasis are required, which might involve the participation of multiple medical institutions.
Conclusion
Our panel provided and validated a class of biomarkers that could robustly categorize patients with relatively early GC according to their LN status prior to therapeutic decision-making, thus permitting individualized treatment. Our panel offers promising diagnostic potential to identify patients with GC with or without LN metastasis; however, our findings should be validated prospectively using clinical cohorts.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the National Natural Science Funds of Young Scientists of China (No. 81802944), the Key Project of Ningbo Natural Science Foundation (No. 2021J279), Natural Science Foundation of Zhejiang Province (LQ21H160013), the Medical Science and Technology Project of Zhejiang Province (2023KY1033, and 2023KY236), the Natural Science Foundation of Ningbo (No. 2022J264), Ningbo Digestive System Tumor Clinical Medicine Research Center (No. 2019A21003), Project of Zhejiang Medical and Health Platform Plan (No. 2022KY1079), and Ningbo Public Welfare Science & Technology Major Project (No. 2021S106).
Abbreviations
- LN
Lymph node
- GC
Gastric cancer
- lncRNA
Long noncoding RNA
- TCGA
The cancer genome atlas
- ACRG
Asian cancer research group
- CT
Computed tomography
- AUC
Area under the curve
- CEA
Carcinoembryonic antigen
- CA19-9
Carbohydrate antigen 19-9
- PET-CT
Positron emission tomography with CT
- ESD
Endoscopic submucosal dissection
- EMR
Endoscopic mucosal resection
- LSNNS
Laparoscopic sentinel node navigation surgery
- OS
Overall survival
- DFS
Disease free survival
- RNA-Seq
RNA-sequencing
- LNN
Lymph node metastasis negative
- LNP
Lymph node metastasis positive
- TPM
Transcripts per million
- LR
Logistic regression
- ROC
Receiver operator characteristic
- qRT-PCR
Quantitative real-time reverse transcription PCR
- EMT
Epithelial–mesenchyme transition
- SNNS
Sentinel node navigation surgery
Author contributions
ZD: data curation; project administration; writing—original draft. HX: data curation; formal analysis; project administration. HW: data curation; project administration. XC, ZC, and SC: investigation. YH: writing; formal analysis. HL: conceptualization; methodology; writing—review and editing. WY: conceptualization; methodology; writing—review and editing. CL: conceptualization; methodology; writing—review and editing.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
All the authors have no conflict of interest related to the manuscript.
Ethical approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Affiliated Lihuili Hospital, Ningbo University (no. KY2022SL436-01) on 13 December 2022, with an exemption from informed consent. No specific consent is needed for statistical analyses of aggregated de-identified data. For this study, the raw data were first extracted from HIS, and patients’ identities, including names, screening IDs, patient IDs, and mobile phone numbers, were de-identified.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhe-bin Dong, Han-ting Xiang and Heng-miao Wu have equally contributed to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-a Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Brown M, Assen FP, Leithner A, Abe J, Schachner H, Asfour G, Bago-Horvath Z, Stein JV, Uhrin P, Sixt M, et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359(6382):1408-+. doi: 10.1126/science.aal3662. [DOI] [PubMed] [Google Scholar]
- 3.Ren G, Cai R, Zhang W-J, Ou J-M, Jin Y-N, Li W-H. Prediction of risk factors for lymph node metastasis in early gastric cancer. World J Gastroenterol. 2013;19(20):3096–3107. doi: 10.3748/wjg.v19.i20.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, Guo M, Lian X, Fan D, Zhang H. Diagnostic and prognostic value of CEA, CA19–9, AFP and CA125 for early gastric cancer. Bmc Cancer. 2017 doi: 10.1186/s12885-017-3738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito T, Kurokawa Y, Takiguchi S, Miyazaki Y, Takahashi T, Yamasaki M, Miyata H, Nakajima K, Mori M, Doki Y. Accuracy of multidetector-row CT in diagnosing lymph node metastasis in patients with gastric cancer. Eur Radiol. 2015;25(2):368–374. doi: 10.1007/s00330-014-3373-9. [DOI] [PubMed] [Google Scholar]
- 6.Japanese Gastric Canc A. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20(1):1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pimentel-Nunes P, Libanio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, Esposito G, Lemmers A, Maselli R, Messmann H, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European society of gastrointestinal endoscopy (ESGE) guideline—update 2022. Endoscopy. 2022;54(06):591–622. doi: 10.1055/a-1811-7025. [DOI] [PubMed] [Google Scholar]
- 8.Hatta W, Gotoda T, Koike T, Masamune A. History and future perspectives in Japanese guidelines for endoscopic resection of early gastric cancer. Dig Endosc. 2020;32(2):180–190. doi: 10.1111/den.13531. [DOI] [PubMed] [Google Scholar]
- 9.Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12(3):148–152. doi: 10.1007/s10120-009-0515-x. [DOI] [PubMed] [Google Scholar]
- 10.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3(4):219–225. doi: 10.1007/PL00011720. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y-W, Min J-S, Yoon HM, An JY, Eom BW, Hur H, Lee YJ, Cho GS, Park Y-K, Jung MR, et al. Laparoscopic sentinel node navigation surgery for stomach preservation in patients with early gastric cancer: a randomized clinical trial. J Clin Oncol. 2022;40(21):2342-+. doi: 10.1200/JCO.21.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31(29):3704-+. doi: 10.1200/JCO.2013.50.3789. [DOI] [PubMed] [Google Scholar]
- 13.Eom BW, Yoon HM, Min JS, Cho I, Park J-H, Jung MR, Hur H, Kim Y-W, Park YK, Nam B-H, et al. Prospective multicenter feasibility study of laparoscopic sentinel basin dissection after endoscopic submucosal dissection for early gastric cancer: SENORITA 2 trial protocol. J Gastric Cancer. 2019;19(2):157–164. doi: 10.5230/jgc.2019.19.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoji Y, Kumagai K, Kamiya S, Ida S, Nunobe S, Ohashi M, Yoshimizu S, Horiuchi Y, Yoshio T, Ishiyama A, et al. Prospective feasibility study for single-tracer sentinel node mapping by ICG (indocyanine green) fluorescence and OSNA (one-step nucleic acid amplification) assay in laparoscopic gastric cancer surgery. Gastric Cancer. 2019;22(4):873–880. doi: 10.1007/s10120-018-00919-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Qiu C, Zhang Q, Liu B, Cui Q. Genome-wide analysis of human SNPs at long intergenic noncoding RNAs. Hum Mutat. 2013;34(2):338–344. doi: 10.1002/humu.22239. [DOI] [PubMed] [Google Scholar]
- 16.Khurana E, Fu Y, Chakravarty D, Demichelis F, Rubin M, Gerstein M. Role of non-coding sequence variants in cancer. Nat Rev Genet. 2016;17(2):93–108. doi: 10.1038/nrg.2015.17. [DOI] [PubMed] [Google Scholar]
- 17.Lin C, Yang L. Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol. 2018;28(4):287–301. doi: 10.1016/j.tcb.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477(7364):295–U260. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Firehose Broad GDAC portal. http://gdac.broadinstitute.org. Accessed 1 July 2022
- 20.The human gene annotation file. https://ftp.ensembl.org/pub/release97/gtf/homo_sasapie/Homo_sapiens.GRCh38.97.chr.gtf.gz. Accessed 3 July 2022
- 21.Pytorch. https://arxiv.org/abs/1912.01703. Accessed 2 July 2022
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Zhang J, Gao Y, Li Y, Feng C, Song C, Ning Z, Zhou X, Zhao J, Feng M, Zhang Y, Wei L, Pan Q, Jiang Y, Qian F, Han J, Yang Y, Wang Q, Li C. LncSEA: a platform for long non-coding RNA related sets and enrichment analysis. Nucleic Acids Res. 2021;49(D1):D969–D980. doi: 10.1093/nar/gkaa806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2009. [Google Scholar]
- 25.Uenosono Y, Natsugoe S, Ehi K, Arigami T, Hokita S, Aikou T. Detection of sentinel nodes and micrometastases using radioisotope navigation and immunohistochemistry in patients with gastric cancer. Br J Surg. 2005;92(7):886–889. doi: 10.1002/bjs.4953. [DOI] [PubMed] [Google Scholar]
- 26.Ichikura T, Sugasawa H, Sakamoto N, Yaguchi Y, Tsujimoto H, Ono S. Limited gastrectomy with dissection of sentinel node stations for early gastric cancer with negative sentinel node biopsy. Ann Surg. 2009;249(6):942–947. doi: 10.1097/SLA.0b013e3181a77e7e. [DOI] [PubMed] [Google Scholar]
- 27.Yano K, Nimura H, Mitsumori N, Takahashi N, Kashiwagi H, Yanaga K. The efficiency of micrometastasis by sentinel node navigation surgery using indocyanine green and infrared ray laparoscopy system for gastric cancer. Gastric Cancer. 2012;15(3):287–291. doi: 10.1007/s10120-011-0105-6. [DOI] [PubMed] [Google Scholar]
- 28.Wen X, Han W, Liu C. Long non-coding RNA TTTY15 silencing inhibits gastric cancer progression by sponging microRNA-98-5p to down-regulate cyclin D2 expression. Bioengineered. 2022;13(3):7380–7391. doi: 10.1080/21655979.2022.2047398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021 doi: 10.1083/jcb.202009045.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia Y, Yan Q, Zheng Y, Li L, Zhang B, Chang Z, Wang Z, Tang H, Qin Y, Guan X-Y. Long non-coding RNA NEAT1 mediated RPRD1B stability facilitates fatty acid metabolism and lymph node metastasis via c-Jun/c-Fos/SREBP1 axis in gastric cancer. J Exp Clin Cancer Res. 2022 doi: 10.1186/s13046-022-02449-4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z, Zhi Q, Wang D, Zhang L, Preston B, Brandon C, Kuang Y, Miao R, Shi Y, Guo X. Long noncoding RNA C21orF96 promotes the migration, invasion and lymph node metastasis in gastric cancer. Anticancer Agents Med Chem. 2016;16(9):1101–1108. doi: 10.2174/1871520616666151116122921. [DOI] [PubMed] [Google Scholar]
- 32.Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C, Shen H, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suh Y-S, Na D, Lee J-S, Chae J, Kim E, Jang G, Lee J, Min J, Ock C-Y, Kong S-H, et al. Comprehensive molecular characterization of adenocarcinoma of the gastroesophageal junction between esophageal and gastric adenocarcinomas. Ann Surg. 2022;275(4):706–717. doi: 10.1097/SLA.0000000000004303. [DOI] [PubMed] [Google Scholar]
- 34.Kochi M, Fujii M, Kanamori N, Kaiga T, Kawakami T, Aizaki K, Kasahara M, Mochizuki F, Kasakura Y, Yamagata M. Evaluation of serum CEA and CA19-9 levels as prognostic factors in patients with gastric cancer. Gastric Cancer. 2000;3(4):177–186. doi: 10.1007/PL00011715. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu N, Wakatsuki T, Murakami A, Yoshioka H, Hamazoe R, Kanayama H, Maeta M, Koga S. Carcinoembryonic antigen in gastric cancer patients. Oncology. 1987;44(4):240–244. doi: 10.1159/000226486. [DOI] [PubMed] [Google Scholar]
- 36.Kodera Y, Yamamura Y, Torii A, Uesaka K, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. The prognostic value of preoperative serum levels of CEA and CA19-9 in patients with gastric cancer. Am J Gastroenterol. 1996;91(1):49–53. [PubMed] [Google Scholar]
- 37.Gan L, Lv L, Liao S. Long non-coding RNA H19 regulates cell growth and metastasis via the miR-22-3p/Snail1 axis in gastric cancer. Int J Oncol. 2019;54(6):2157–2168. doi: 10.3892/ijo.2019.4773. [DOI] [PubMed] [Google Scholar]
- 38.Liu X-h, Sun M, Nie F-q, Ge Y-b, Zhang E-b, Yin D-d, Kong R, Xia R, Lu K-h, Li J-h, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331–3p in gastric cancer. Mol Cancer. 2014 doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee C-P, Ko AM-S, Nithiyanantham S, Lai C-H, Ko Y-C. Long noncoding RNA HAR1A regulates oral cancer progression through the alpha-kinase 1, bromodomain 7, and myosin IIA axis. J Mol Med Jmm. 2021;99(9):1323–1334. doi: 10.1007/s00109-021-02095-x. [DOI] [PubMed] [Google Scholar]
- 40.Ma J, Cao K, Ling X, Zhang P, Zhu J. LncRNA HAR1A suppresses the development of non-small cell lung cancer by inactivating the STAT3 pathway. Cancers. 2022 doi: 10.3390/cancers14122845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang D, Ou X, Sun K, Zhou X, Li Y, Shi P, Zhao Z, He Y, Peng J, Xu J. m6A modification-mediated lncRNA TP53TG1 inhibits gastric cancer progression by regulating CIP2A stability. Cancer Sci. 2022;113(12):4135–4150. doi: 10.1111/cas.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.