Abstract
The temperate Salmonella enterica bacteriophage L is a close relative of the very well studied bacteriophage P22. In this study we show that the L procapsid assembly and DNA packaging genes, which encode terminase, portal, scaffold, and coat proteins, are extremely close relatives of the homologous P22 genes (96.3 to 99.1% identity in encoded amino acid sequence). However, we also identify an L gene, dec, which is not present in the P22 genome and which encodes a protein (Dec) that is present on the surface of L virions in about 150 to 180 molecules/virion. We also show that the Dec protein is a trimer in solution and that it binds to P22 virions in numbers similar to those for L virions. Its binding dramatically stabilizes P22 virions against disruption by a magnesium ion chelating agent. Dec protein binds to P22 coat protein shells that have expanded naturally in vivo or by sodium dodecyl sulfate treatment in vitro but does not bind to unexpanded procapsid shells. Finally, analysis of phage L restriction site locations and a number of patches of nucleotide sequence suggest that phages ST64T and L are extremely close relatives, perhaps the two closest relatives that have been independently isolated to date among the lambdoid phages.
Bacteriophage L was reported to have been isolated after it was induced by UV irradiation from Salmonella enterica strain LT2 by Bezdek and Amati (2) in Czechoslovakia in 1967. Curiously, currently used LT2 cultures do not carry this prophage, although they do carry up to four other intact prophages (7, 40), so either extant LT2 strains have lost their L prophage or the strain used by those authors had inadvertently become lysogenized by phage L from the environment. Whatever its exact origin, L is a temperate, short-tailed double-stranded-DNA (dsDNA) bacteriophage that infects Salmonella enterica serovar Typhimurium (2). It is a rather close relative of the well-studied phage P22, with which it can form viable hybrids (2, 3, 23). It has immunity (C2 and Cro repressors), C1 activator, and replication protein specificities different from those of P22, but P22 regulatory genes c3, 23, and 24, virion assembly genes 1, 2, 3, 5, 8, 9, 10, 16, and 20, and lysis genes 13, 15, and 19 have been shown to be interchangeable between the two phages (2-4, 23, 31, 36, 51). Unlike P22, L does not have an immunity I region (27, 57) or a sieB function (58). DNA heteroduplex analysis (62) as well as physical map (27) and genetic map (56) construction showed that related genes are present in the same order on the P22 and L chromosomes. L virions are very similar in appearance to P22 virions when viewed in the electron microscope with negative staining (2), the two virions contain generally similar structural proteins (2, 27, 39), and their virion DNAs are indistinguishable in length (27). However, L virions have a significantly lighter equilibrium banding density in CsCl gradients than those of P22 (2), and phage L virions contain a major structural protein with a molecular mass of approximately 15 kDa that appears to have no counterpart in P22 (27). We show here that phage L does indeed have head assembly genes that are in general highly similar to those of P22, but in addition, L carries a gene that encodes a 14-kDa protein, which is a decoration protein that binds to the outer surface of both L and P22 virions.
MATERIALS AND METHODS
Bacteria and phage strains and virion-related particle production.
Phage L was a gift from David Botstein, and nonsense mutant phage L strains L c− 3−am74 and L c− 13−am43 were the gift of Werner Bode; the L c− 3−am74 13−am43 double amber mutant phage was constructed for this study. Phage were propagated on Salmonella serovar Typhimurium strains DB7000 and DB7004 (63). Bacterial cultures were grown in Luria-Bertani broth at 37°. Phage L and P22 virions were purified, and DNA was isolated from them as previously described for phage P22 (12). All phages used in this study carried a clear-plaque mutation (c1−7 in P22 and c in L) to block lysogeny and ensure that all infected cells productively undergo lytic infection and a 13 mutation to block normal lysis and make handling of infected cells easier; the 2− and 3− mutations block DNA packaging and cause accumulation of procapsids, and the 10− mutation causes accumulation of heads that have packaged and subsequently lost their DNA (6). P22 and L procapsids were purified from P22 c1−7 2−amH200 13−amH101- and L c− 3−am74 13−am43-infected DB7000 cells as previously described (21). P22 empty heads were purified from P22 c1−7 10−amN107 13−amH101-infected DB7000 cells (6, 21). Expanded procapsid shells were prepared as follows: P22 procapsids were treated with 0.1% sodium dodecyl sulfate (SDS) for 30 min at room temperature, which releases minor proteins and scaffolding protein and causes expansion of the coat protein shell (14, 21); the coat protein shells were sedimented away from the released proteins by centrifugation for 2 h through a 10% sucrose cushion at 30,000 rpm in a Beckman SW50.1 ultracentrifuge rotor; the resulting pellet was resuspended in 1 mM MgCl2, 10 mM TrisCl (pH = 7.4)(TM).
Plasmid construction and Dec protein purification.
The phage L dec gene was cloned into plasmid expression vectors pET-15b and pET-21b (Invitrogen) as follows. The dec gene was amplified by PCR, using primer pair 5′-GGGCCCCCCATATGGCAAACCCAAACTTCACG plus 5′-ATATATGGATCCTTAACTTCCTGATGTTGTTTCG or 5′-ATATATGGATCCCATGGCAAACCCAAACTTCACG plus 5′-GCGCGCCTCGAGACTTCCTGATGTTGTTTCG, respectively, and Elongase DNA polymerase (QIAGEN). The amplified products were cleaved with NdeI plus BamHI and XhoI plus BamHI, respectively, and cloned into similarly cleaved plasmid vector DNAs; DNA sequencing of the resulting plasmid DNA inserts confirmed that the expected L sequence had been cloned. The resulting Dec protein-encoding plasmids pDec-HisN and pDec-HisC (derived from pET-15b and pET-21b and expressing N- and C-terminally histidine-tagged Dec proteins, respectively) were moved into Escherichia coli strain BL21(DE3) pLysS (55), and cells were grown with shaking at 37° in 1,500 ml of Luria-Bertani broth in the presence of ampicillin and chloramphenicol. Dec expression was induced by addition of isopropyl-β-d-thiogalactopyranoside to a concentration of 0.4 mM when the culture reached 2 × 108 cells/ml. The induced culture was shaken for an additional four h at 37°C, at which time the cells were lysed as follows. Cells were pelleted by centrifugation at 7,000 rpm in a Beckman JA-10 rotor for 20 min, frozen at −70°, thawed, and resuspended in 50 ml of lysis buffer (10 mM imidazole, 300 mM NaCl, 50 mM NaPO4 [pH = 8.0]). Hen egg white lysozyme (Worthington) and DNase I (Sigma) were added (5 mg and 1 μg, respectively), and the suspension was incubated at room temperature for 10 min and sonicated briefly on ice. Cell debris was removed by centrifugation in a Beckman JA-10 rotor for 20 min at 10,000 rpm. The clarified lysate was passed over a nickel-nitrilotriacetic acid agarose column (QIAGEN) at room temperature, unbound proteins were washed out with lysis buffer (above), and the histidine-tagged protein was eluted with 250 mM imidazole-300 mM NaCl-50 mM NaPO4 buffer (pH = 8.0). Dec protein for sedimentation equilibrium analysis was further purified by passing it, at a starting concentration of about 5 mg/ml, over a fast-performance liquid chromatography (Pharmacia)-driven Superdex 200 (HiLoad 16/60; Amersham Biosciences) size exclusion column in 500 mM NaCl-10 mM TrisCl (pH = 7.4) at room temperature. The bulk of the Dec protein eluted at a position characteristic of globular proteins of about 90 kDa, and this was used for sedimentation equilibrium studies; however, about 20% was present as a peak of significantly larger material.
DNA and protein methods.
Nucleotide sequence determination for both strands from a whole-genome template was performed by dideoxy chain termination methods as previously described (16). The DNA sequence analysis software used was DNA Strider (19) and BLAST (1). Polyacrylamide gel electrophoresis of proteins in sodium lauryl sulfate (SDS), staining with Coomassie brilliant blue R-250 (Bio-Rad, Richmond, Calif.), and determination of the N-terminal amino acid sequence by the University of Utah Protein Facility were done as previously described (22).
Sedimentation equilibrium analysis.
Sedimentation equilibrium experiments were conducted in a Beckman Optima XL-A analytical ultracentrifuge equipped with UV optics, at 20°C, using a six-channel ANTi60 rotor with 12-mm-thick, charcoal-epon centerpieces. The three sample channels in each cell contained three different loading concentrations of Dec protein in 1 mM MgCl2-100 mM NaCl-10 mM Tris-Cl buffer (pH = 7.4) (identical results were obtained with a 500 mM concentration of NaCl), while reference channels contained the corresponding buffer only. Samples were centrifuged at 15,000 rpm until sedimentation and chemical equilibrium were attained. Cells were scanned radially in continuous mode, with data resulting from 10 absorbance readings taken at 0.001-cm intervals. Equilibrium was confirmed by no change in scans taken at four hourly intervals. The partial specific volume and the extinction coefficient for each protein were calculated from the amino acid sequence by using previously described methods (37, 38). Curve fitting and calculation of molecular mass were done with nonlinear least-square techniques, using the computer software NONLIN (35).
Nucleotide sequence accession number.
The nucleotide sequence of 8,338 bp in the head gene region of phage L has been deposited in GenBank under accession no. AY795968.
RESULTS AND DISCUSSION
Bacteriophage L procapsid assembly genes.
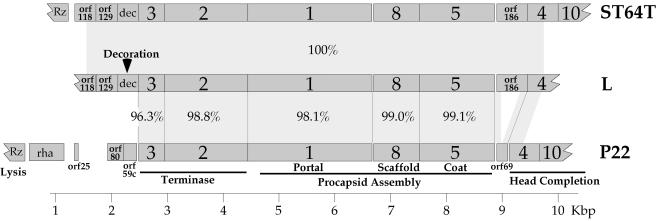
In order to begin the process of finding the gene that encodes the major virion protein that is unique in phage L when it is compared to its close relative, P22 (27), we determined the nucleotide sequence of 8,338 bp in the head gene region of phage L. While this work was in progress, the complete genome sequence of the Salmonella P22-like phage ST64T was reported (42), and the above-mentioned phage L sequence is identical to the parallel section of phage ST64T, from within orf118 to within gene 4. This phage L sequence was determined by using a primer walking strategy on whole wild-type L virion template DNA; primers with P22 sequence gave footholds in several locations in the large terminase subunit and scaffolding protein genes. The predicted genes in this region of the L genome and their relationships to those of phages P22 and ST64T are shown diagrammatically in Fig. 1. By homology to P22, they include all of the five genes necessary to build a procapsid and package DNA. These genes encode the small and large terminase, portal, scaffold, and coat proteins, and the five predicted proteins range in amino acid sequence identity from 96.3 to 99.1% between L and P22 (Fig. 1). The near-identity of these genes in phages L and P22 suggests strongly that they have the same functions in the two phages. There are also three predicted genes, orf118, orf129 and dec, present in this region of the L genome that have no P22 homolog and another gene, orf186, that has a more complex relationship to P22. Phage L orf186 (and the identical ST64T orf186) appears to be a longer version of the P22 gene orf69 which lies in the same position, immediately transcriptionally downstream of the coat protein gene, and whose function is unknown. The P22 orf69 protein has been shown to be nonessential to P22 growth in the laboratory (22), and comparison with L orf186 suggests that the P22 gene has suffered a substantial internal deletion. Thus, it is quite possible that orf69 is not a functional gene in P22. The roles of orf118, orf129, and orf186 in phages L and ST64T are unknown, as are the roles of P22 orf80 and orf59c (Fig. 1); to date there is no evidence that any of these five proteins is present in virions or procapsids, and we have shown that orf80 and orf59c are not essential for P22 growth (9).
FIG. 1.
Procapsid assembly region of the phage L genome. Maps of the phage L and P22 procapsid assembly regions are shown, where dark-gray rectangles represent genes. Gene names are shown on each gene, percent amino acid sequence identity is given between the encoded amino acid sequences for homologous genes, and light-gray areas between the maps highlight homologous regions.
The L gene 3 through 5 region nucleotide sequence is 95.1% identical to the parallel region of phage P22. This is significantly less similar than the encoded amino acid sequences (Fig. 1), and most of the nucleotide differences reflect synonymous codons in the two phages. For example, the two 1 genes, whose encoded proteins are 98.1% identical, are 95.0% identical in nucleotide sequence; there are 14 predicted amino acid differences and 108 nucleotide sequence differences between these two genes. Of the 14 amino acid differences, 12 are similar amino acids in the two proteins. These features indicate that these genes have been under fairly strong selection against amino acid changes since their divergence. We also note that here, as well as in the other genes in this cluster, the sequence differences are notably patchy, with short regions containing more-numerous differences interspersed with longer regions of near-identity. For example, in the 34-codon region of gene 1 between codons 407 and 441, the two nucleotide sequences are only 76.5% identical and contain 12 identical codons and 22 different codons; comparison of the codons that are different between the two phages shows that this region has 18 synonymous codons and 4 nonsynonymous codons. On the other hand, the 653 bp at the 3′ end of gene 1 in these two sequences is 99.4% identical. Since the differences in the above 34-codon “divergent” region are mostly synonymous changes which should not be under selection and so should occur randomly across the genome, this sort of pattern suggests that the patches with higher levels of difference were introduced into L or P22 since their divergence by recombination with other more distant, but still fairly close, relatives (50, 53). This supports the notion that recombination among both distantly (30) and closely related (16) dsDNA tailed phages is an ongoing process.
In addition to phage L's region of 8,338 bp of identity to sequence of ST64T (described above), Schicklmaier and Schmieger (51) have reported a sequence of 2,188 bp that includes the phage L immunity C region, and it is 99.5% identical to that of ST64T. The ST64T sequence accurately predicts the locations of all but three of the 80 restriction sites that have previously been mapped across the phage L genome (27, 51), and this, in turn, if the three polymorphisms are due to single-nucleotide changes, translates to >99% nucleotide sequence identity in the recognition sites. The reported L genome size of 40,650 bp is probably within experimental error of the 41,679-bp size of the ST64T genome (27, 42). We also used P22 sequence-based primers to determine sequences from six different locations to the right of gene 4 in the L late operon. These sequences, which included parts of genes 7, 9, 10, 14, 20, and 26 and the gap between genes 16 and 9 (P22 gene names) and which covered 2,860 bp, had only one difference from the parallel ST64T sequence (in phage L, a C replaces a T at ST64T position 35086). In addition, the differences in sizes of the P22 and L gene 16 and gene 20 proteins (27) are explained if the ST64T and L sequences of these genes are very similar. All these findings combine to suggest that L and ST64T are very closely related indeed, and the possibility exists that although there appear to be a few single-base-pair polymorphisms, there may be no mosaic relationships between these two phages. We know of no other case of completely independent isolation of two lambdoid phages which are this similar to one another; in fact, independent isolation of such similar phages might be considered surprising considering their huge diversity (summarized in references 28 and 30).
Identification of the dec gene.
We isolated the approximately 15-kDa phage L major virion protein that has no P22 analogue (see above) (called “pX” by Hayden et al. [27]) from SDS polyacrylamide electrophoresis gels of L virion proteins (Fig. 2) and determined its N-terminal amino acid sequence to be H2N-ANPNFTPS. This sequence corresponds exactly to that predicted for the protein encoded by the L dec gene with its N-terminal methionine removed (methionine removal is common in enteric bacteria, especially when alanine is the second amino acid [24]). Our previous analysis of a P22-L hybrid phage showed that the dec gene must lie either in one of the two early operons or between the late promoter and gene 10 (27), and the dec gene lies within the latter of these intervals, immediately to the left of gene 3 (Fig. 1). This marks the first identification of a virion structural protein gene that is transcriptionally upstream of the terminase genes in the lambdoid phage group, which typically have a very stereotyped late operon gene order in which the small terminase subunit gene is the most promoter-proximal virion assembly gene (11, 13, 28). The two other types of lambdoid phage decoration protein genes, the lambda D gene (61, 66) and the ES18 8 gene (10), lie immediately to the left of the coat protein gene. The unusual location of dec might indicate that it has had an evolutionarily recent arrival into the L genome, but on the other hand the stop codon of dec is separated by only 3 bp from the initiation codon of gene 3, suggesting that there has been sufficient time for presumably optimal juxtaposition of these two genes in the L genome. We named this protein Dec protein (and its gene dec) because it “decorates” the coat protein shell (see below).
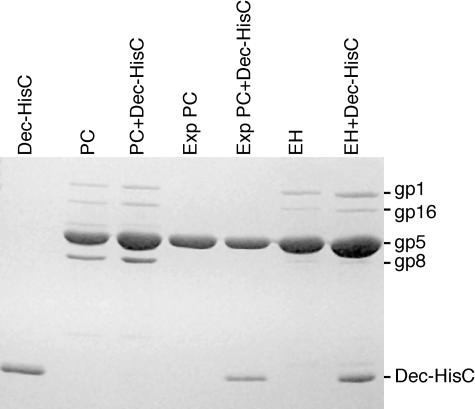
FIG. 2.
Phage L Dec protein binds to P22 virions. The indicated proteins were separated by 12.5% polyacrylamide-SDS gel electrophoresis and stained with Coomassie brilliant blue R-250. Phage L N-terminally histidine-tagged Dec protein (lane Dec-HisN) was purified as described in Materials and Methods. Purified P22 virions (lane P22) were mixed with this Dec protein (25.0 μg of Dec per μg [1010] of virions) and incubated at room temperature for 30 min in 1 mM MgCl2-10 mM TrisCl (pH = 7.4) (TM). The mixture was then sedimented onto a CsCl (density = 1.6 g/cc) cushion, and the virion-sized material was dialyzed against 1 mM MgCl2-10 mM TrisCl (pH = 7.4) (lane P22+Dec-HisN); a parallel experiment with multiple rounds of CsCl purification or with more than 10 times as much Dec protein showed an identically intense Dec band relative to coat protein (data not shown), showing that the particles used for the figure were saturated with Dec protein. Identical results were obtained in a similar experiment with C-terminally histidine-tagged Dec. Purified L virions are shown in lane L for comparison. The oligonucleotide-histidine tag retards the Dec protein's migration somewhat in SDS-polyacrylamide electrophoresis gels. P22 gp1 is portal protein (gp nomenclature refers to the fact that this is the gene product of gene 1); gp16 is an injection protein; gp5 is coat protein.
Decoration proteins are semiessential or nonessential proteins which bind to the outside of the mature virus coat protein shell and sometimes stabilize those shells (reviewed in reference 13). Previously studied dsDNA tailed bacteriophage decoration proteins are the phage T4 Soc and Hoc proteins (25, 33, 34, 44, 47), the phage λ D protein (17, 18, 61, 66), and the φ29 head fiber protein (46, 59); the λ D protein is dispensable only under special low-DNA-content circumstances (54). In addition, adenovirus IX protein (26, 41, 48) and the herpesvirus VP26 (5, 43) and perhaps triplex (49, 52, 60) proteins can be classified as decoration proteins.
The L virions but not procapsids contain many Dec protein molecules.
The coat protein shell of phage L virion has the same T = 7 laevo structure as P22 (8, 45; L. Tang, J. Johnson and S. Casjens, unpublished data), and so has 420 molecules, minus the 5 or 10 molecules replaced by portal protein, of coat protein per virion. Several lines of experimental evidence support the notion that the phage L Dec protein decorates the exterior of the virion. First, we found the number of Dec molecules present per L virion to be 150 ± 30 by determining the intensity of its Coomassie brilliant blue R-250 staining relative to that of the coat protein in SDS polyacrylamide gels of purified virions (data not shown; see also reference 27). This agrees with the CsCl equilibrium banding density difference between L and P22 (2), which predicts that there are about 160 molecules of Dec per L virion. In addition, phage L procapsids were isolated by pelleting and resuspension, followed by sucrose gradient fractionation (21) after infection of the sup° host DB7000 by L 3−am74 13−am43, and no Dec protein was bound to them (data not shown). It seems unlikely that so many Dec molecules could be bound anywhere other than on the surface of the coat protein shell itself, especially if they are not present in the procapsid. Failure to bind to immature (unexpanded) coat protein shells is a feature of most other decoration proteins that have been studied (18, 25, 29, 43).
Dec binds to the exterior surface of expanded phage P22 coat protein shells.
Since P22 and L coat proteins are 99.1% identical (only 4 differences out of 430 amino acids) and since Bezdek and Amati (2) had shown that coinfection by P22 and L resulted in P22 DNA-containing virions with a lighter (phage L) density in CsCl equilibrium gradients, we wondered if L Dec protein might be able to decorate P22 virions. The phage L dec gene was cloned into expression vectors (details in Materials and Methods) to give plasmids pDec-HisN and pDec-HisC. In the presence of T7 RNA polymerase, these plasmids express Dec proteins which are oligonucleotide-histidine-tagged at their N- and C-terminal ends, respectively. Both tagged proteins are soluble and were purified to near-homogeneity by nickel affinity chromatography (Fig. 2) (see Materials and Methods). Mixing an excess of either of these proteins with purified P22 virions at room temperature for 30 min in TM resulted in Dec protein binding to the P22 virions as assayed by cosedimentation with virions through sucrose gradient and CsCl equilibrium density centrifugation (Fig. 2). Addition of SDS to a final concentration of 0.1% did not cause the release of Dec bound to P22 virions (data not shown), so its binding is tight and most likely specific. We note that addition of 8 or 20 amino acids (the oligonucleotide-histidine tags) to its termini does not disrupt Dec binding. Quantitation of the binding of saturating numbers of either N- or C-terminally histidine-tagged Dec protein molecules to P22 virions by measurement of Coomassie brilliant blue staining intensity indicated that 185 ± 20 molecules were bound, which is in reasonable agreement with the measured number of Dec molecules bound to native L virions (see above).
The particles that are precursors to virions have been studied in considerable detail for P22 (reviewed in reference 15). Coat protein first assembles into structures called procapsids which contain scaffolding protein in the interior and have a smaller diameter than mature virions. When DNA is packaged into procapsids, scaffolding protein leaves the structure, and the coat protein shell expands (with a concomitant poorly understood coat protein conformational change). Figure 3 shows the results of an experiment that tested whether Dec protein can bind to unexpanded shells and/or expanded procapsid shells. Purified Dec protein does not bind to procapsids (Fig. 3, lanes 2 and 3) but does bind to procapsids which have been expanded artificially in vitro by SDS treatment (lanes 4 and 5) (14) or which have packaged DNA and expanded in vivo but then lost their DNA (lanes 6 and 7). The fact that SDS-treated shells have lost their scaffold (gp8), gp16, and gp1 components (lanes 4 and 5) indicates that Dec does not bind virions through any of these proteins. The results clearly show that Dec protein binds expanded P22 coat protein shells but not unexpanded ones.
FIG. 3.
Dec protein binds only expanded P22 coat protein shells. Purified C-terminally histidine-tagged Dec protein (lane marked Dec-HisC) was mixed with purified P22 procapsids (PC), expanded P22 procapsids (Exp PC), or P22 “empty heads” (EH). These particles were produced as described in Materials and Methods. After incubation of an excess of Dec protein with the particles in TM for 30 min at room temperature, the particles were pelleted through a 10% sucrose cushion at 30,000 rpm in a Beckman SW50.1 ultracentrifuge rotor for 2 h and resuspended in fresh TM. Unassembled Dec protein does not pellet under these conditions. The component proteins of the particles, before and after Dec protein treatment, are displayed in a 12.5% polyacrylamide-SDS electrophoresis gel that was stained with Coomassie brilliant blue. The labels on the right are the same as in Fig. 2, and gp8 is scaffolding protein.
To attempt to determine whether the virion-bound Dec protein is in fact on the surface of the phage particles, we examined whether P22 virions decorated with C-terminally histidine-tagged Dec (generated from pDec-HisC; see Materials and Methods) can bind to nickel agarose. The results summarized in Table 1 show that, under the conditions used, only ≤0.4% of P22 virions bound, while 30% of virions bound if they had been saturated with C-terminally histidine-tagged Dec (virions were separated from unbound Dec as described in the legend to Fig. 2). About 30 to 40% of both types of virions were lost during the procedure, and about 30% of the Dec-containing phage did not bind tightly (a reproducible result that we do not understand; longer incubation times or use of fewer virions also resulted in binding of only a fraction of the virions). Nonetheless, since a large fraction of virions bound only when the histidine-tagged Dec was present, and since the virion-bound histidine tag binds to nickel that is immobilized on agarose (and so should not be able to enter into the interior of the virion), these findings strongly suggest that the Dec protein is on the exterior of decorated P22 (and L) virions.
TABLE 1.
Histidine-tagged Dec protein on virions binds nickel agarose
| Fraction | P22 | P22 + Deca |
|---|---|---|
| Input phageb | 100 | 100 |
| Unbound phagec | 60 | 35 |
| Phage eluted by imidazoled | ≤0.4 | 33 |
C-terminally histidine tagged (see the text).
Defined as 10070. 1010 phage (about 2 μg) in a solution containing 50 mM NaPO4 buffer (pH = 8.0), 300 mM NaCl, 1 mM MgCl2, and 10 mM imidazole, shaken gently with 0.5 ml nickel-nitrilotriacetic acid agarose (Qiagen) for 3 h at 4°C.
Same buffer conditions as described in footnote b; sum of three washes by pelleting and resuspension in 1 ml of fresh buffer.
Same buffer conditions as described in footnote b with 250 mM imidazole; sum of three washes by pelleting and resuspension in 0.5 ml of fresh buffer.
Bound Dec protein stabilizes P22 virions.
To understand the function of Dec in more detail, we examined whether its presence stabilizes P22 virions. Purified P22 virions were treated with saturating amounts of the Dec protein; these particles were purified away from unbound Dec, and their stability in 83 mM EDTA at 50°C was compared to that of P22 virions without bound Dec. The presence of Dec was found to dramatically stabilize P22 virions; after 10 min, 85 to 90% of the P22 virions were destroyed by the treatment, but the same treatment had no measurable effect when the Dec protein was bound to the virions, even after 120 min (Fig. 4). The Mg2+ chelator EDTA is known to destabilize many dsDNA phages, and it is thought that loss of divalent cations increases the internal “DNA pressure” until a point is reached that the DNA is released from the particle. Thus, particles with less DNA are more EDTA resistant than particles with more tightly packed DNA (summarized by Earnshaw and Casjens [20]). The approximately 10% survivors among P22 virions without the Dec protein is likely due to particles that fortuitously contain a somewhat shorter DNA molecule, since not all P22 virions contain the same length of packaged DNA; i.e., individual virions have differing lengths of terminally redundant DNA (12). It is not known if the dec gene is essential to phage L propagation, but the fact that the extremely closely related P22 has no dec gene suggests that it may not be, at least in the laboratory. The above observations combine to show that the phage L Dec protein is bound to the outside of virions but not to procapsids and that it is likely not essential for phage L capsid assembly, and so we classify it as a decoration protein.
FIG. 4.
Bound Dec protein stabilizes P22 virions. P22 c1−7 13−amH101 virions with (circles) and without (squares) saturating amounts of N-terminally histidine-tagged Dec protein were diluted in 10 mM TrisCl (pH = 7.4)-83 mM EDTA to a final concentration of 108 virions/ml. After the indicated times at 50°C, aliquots were diluted in room-temperature TM, and titers were determined with Salmonella serovar Typhimurium strain DB7004. The two mutations were present in the phage genome to simplify growth and purification of the phage (6); they do not affect the virions produced in any way.
Dec is a trimer in solution.
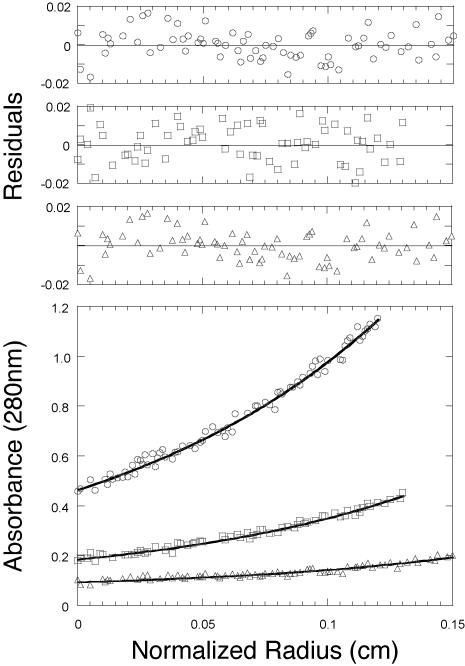
In order to determine the oligomeric state of the Dec protein in solution, we purified the C-terminally histidine-tagged protein to essential homogeneity by nickel column affinity and molecular sieve chromatography and performed sedimentation equilibrium analysis of its size (details in Materials and Methods). This method was chosen because it is independent of molecular shape (67). Figure 5 shows the measured concentration of the Dec protein as a function of position in the rotor cell after equilibrium was attained. Models describing the concentration distribution were fit to equilibrium absorbance versus radius data using nonlinear least-squares techniques and the analysis program NONLIN (35). We conclude from this analysis that the Dec protein exists as a trimer in solution. The data returned a value of 50.9 ± 8 kDa, in very good agreement with the sequence-derived molecular mass of 49.3 kDa for a trimer of the recombinant C-terminally histidine-tagged Dec protein (virtually identical results were obtained with N-terminally histidine-tagged Dec protein; data not shown). The final fit shown in Fig. 5 resulted from the simultaneous fitting of the three different concentration distributions. With longer centrifugation times, there was some evidence of further aggregation of these trimers. We also note that the Dec trimer (molecular mass, 49.3 kDa) elutes from a molecular sieving column where a globular protein of about 90 kDa is expected to elute (data not shown), suggesting that the trimer is rather far from spherical in shape.
FIG. 5.
Sedimentation equilibrium analysis of bacteriophage L Dec. The lower panel shows the experimental data points for three different loading concentrations of C-terminally histidine-tagged Dec, with the corresponding calculated curve fit (continuous line). The Dec protein fits a trimer model with an Mr of 50.9 ± 8. The upper panels show the residuals for this fit; they are small and random, indicating a good fit.
Summary.
We have shown that bacteriophage L encodes a capsid decoration protein, although no homologue of it is encoded by the extremely closely related phage P22. We also note that the two phage T4 decoration proteins, Soc and Hoc, are not encoded by the very closely related phage T2L (64, 65). The absence of decoration proteins in one member of a pair of very closely related phages in two such different contexts as the extremely distantly related P22/L and T2/T4 phage groups suggests that these are truly “accessory” proteins that may be advantageous only in certain niches and whose genes may be gained or lost by phages frequently over evolutionary time. We have found no recognizable amino acid similarity among the different decoration proteins.
About 50 to 70 trimers of Dec bind tightly to the surface of phage L and phage P22 virions, where they dramatically stabilize virions against a magnesium-chelating agent. In this stabilizing function, it is similar to the decoration (or “cement”) proteins of other dsDNA viruses. In some of the cases where its stabilizing influence has been studied, the decoration protein is present in the same or nearly the same number of molecules as the major structural coat protein of the icosahedral shell. For example, one phage lambda gene D protein (54) is bound to each coat protein, and one phage T4 Soc protein (32) binds to each nonvertex coat protein that is not adjacent to a vertex (810 Soc proteins and 930 gp23* coat proteins per T4 virion). On the other hand, the adenovirus XI protein is present in only 240 copies in a virion that has 750 molecules of coat protein (26), one phage T4 Hoc protein molecule binds to the center of each coat hexamer (25), and oligomers of the φ29 head fiber protein bind only to the quasi-threefold axes between hexamers and pentamers of the caps of its elongated icosahedral head (59); of these, only the adenovirus XI protein is known to exert a stabilizing influence. The phage L Dec protein is present in somewhat less than one molecule per two molecules of coat protein and is a trimer in solution. Such a decoration protein trimer might be expected to bind to the surface of an icosahedral virus like the P22 coat T = 7 protein shell at points of local threefold or local sixfold rotational symmetry. For example, if Dec trimers were to bind coat hexamers, there would be 180 Dec molecules bound. Although this value is consistent with the 140 to 200 molecules of Dec that bind to L and P22 virions, there are other possible models for Dec binding that are equally consistent with its oligomeric state and the virion's structure; for example, binding to one of the alternative icosahedrally symmetric subsets of the seven local threefold symmetric sites on each face could also give 180 molecules bound. A true understanding of the locations of its binding sites must await direct structural determination of virions with bound Dec protein.
Acknowledgments
We thank David Botstein for wild-type phage L, Werner Bode for phage L amber mutant strains, and Wai Mun Huang for help with fast-performance liquid chromatography protein purification.
This work was supported by NSF grant MCB-990526 to S.R.C.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bezdek, M., and P. Amati. 1967. Properties of P22 and a related Salmonella typhimurium phage. I. General features and host specificity. Virology 31:272-278. [DOI] [PubMed] [Google Scholar]
- 3.Bezdek, M., J. Soska, and P. Amati. 1970. Properties of P22 and a related Salmonella typhimurium phage. 3. Studies on clear-plaque mutants of phage L. Virology 40:505-513. [DOI] [PubMed] [Google Scholar]
- 4.Bode, W. 1979. Regulation of late functions in Salmonella bacteriophages P22 and L studied by assaying endolysin synthesis. J. Virol. 32:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booy, F. P., B. L. Trus, W. W. Newcomb, J. C. Brown, J. F. Conway, and A. C. Steven. 1994. Finding a needle in a haystack: detection of a small protein (12-kDa VP26) in a large complex (the 200-MDa capsid of herpes simplex virus). Proc. Natl. Acad. Sci. USA 91:5652-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botstein, D., C. H. Waddell, and J. King. 1973. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. I. Genes, proteins, structures and DNA maturation. J. Mol. Biol. 80:669-695. [DOI] [PubMed] [Google Scholar]
- 7.Bunny, K., J. Liu, and J. Roth. 2002. Phenotypes of lexA mutations in Salmonella enterica: evidence for a lethal lexA null phenotype due to the Fels-2 prophage. J. Bacteriol. 184:6235-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casjens, S. 1979. Molecular organization of the bacteriophage P22 coat protein shell. J. Mol. Biol. 131:1-14. [DOI] [PubMed] [Google Scholar]
- 9.Casjens, S., K. Eppler, R. Parr, and A. R. Poteete. 1989. Nucleotide sequence of the bacteriophage P22 gene 19 to 3 region: identification of a new gene required for lysis. Virology 171:588-598. [DOI] [PubMed] [Google Scholar]
- 10.Casjens, S., E. B. Gilcrease, D. A. Winn-Stapley, P. Schicklmaier, H. Schmieger, M. L. Pedulla, M. E. Ford, J. M. Houtz, G. Hatfull, and R. Hendrix. 2005. The generalized transducing Salmonella bacteriophage ES18: complete genome sequence and DNA packaging strategy. J. Bacteriol. 187:1091-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casjens, S., G. Hatfull, and R. Hendrix. 1992. Evolution of dsDNA tailed-bacteriophage genomes. Semin. Virol. 3:383-397. [Google Scholar]
- 12.Casjens, S., and M. Hayden. 1988. Analysis in vivo of the bacteriophage P22 headful nuclease. J. Mol. Biol. 199:467-474. [DOI] [PubMed] [Google Scholar]
- 13.Casjens, S., and R. Hendrix. 1988. Control mechanisms in dsDNA bacteriophage assembly, p. 15-91. In R. Calendar (ed.), The bacteriophages, vol. 1. Plenum Press, New York, N.Y. [Google Scholar]
- 14.Casjens, S., and J. King. 1974. P22 morphogenesis. I. Catalytic scaffolding protein in capsid assembly. J. Supramol. Struct. 2:202-224. [DOI] [PubMed] [Google Scholar]
- 15.Casjens, S., and P. Weigele. Headful packaging by bacteriophage P22. In C. Catalano (ed.), Viral genome packaging, in press. Landes Publishing, Georgetown, Tex.
- 16.Casjens, S., D. Winn-Stapley, E. B. Gilcrease, R. Morona, C. Kühlewein, J. E. Chua, P. A. Manning, W. Inwood, and A. J. Clark. 2004. The chromosome of Shigella flexneri bacteriophage Sf6: complete nucleotide sequence, genetic mosaicism, and DNA packaging. J. Mol. Biol. 339:379-394. [DOI] [PubMed] [Google Scholar]
- 17.Casjens, S. R., and R. W. Hendrix. 1974. Locations and amounts of major structural proteins in bacteriophage lambda. J. Mol. Biol. 88:535-545. [DOI] [PubMed] [Google Scholar]
- 18.Dokland, T., and H. Murialdo. 1993. Structural transitions during maturation of bacteriophage lambda capsids. J. Mol. Biol. 233:682-694. [DOI] [PubMed] [Google Scholar]
- 19.Douglas, S. E. 1994. DNA Strider. A Macintosh program for handling protein and nucleic acid sequences. Methods Mol. Biol. 25:181-194. [DOI] [PubMed] [Google Scholar]
- 20.Earnshaw, W., and S. Casjens. 1980. DNA packaging by the double-stranded DNA bacteriophages. Cell 21:319-331. [DOI] [PubMed] [Google Scholar]
- 21.Earnshaw, W., S. Casjens, and S. Harrison. 1976. Assembly of the head of bacteriophage P22, X-ray diffraction from heads, proheads and related structures. J. Mol. Biol. 104:387-410. [DOI] [PubMed] [Google Scholar]
- 22.Eppler, K., E. Wyckoff, J. Goates, R. Parr, and S. Casjens. 1991. Nucleotide sequence of the bacteriophage P22 genes required for DNA packaging. Virology 183:519-538. [DOI] [PubMed] [Google Scholar]
- 23.Favre, R., P. Amati, and M. Bezdek. 1968. Properties of P22 and a related Salmonella typhimurium phage. II. Effect of H markers on infection of strain 1559. Virology 35:238-247. [DOI] [PubMed] [Google Scholar]
- 24.Flinta, C., B. Persson, H. Jornvall, and G. von Heijne. 1986. Sequence determinants of cytosolic N-terminal protein processing. Eur. J. Biochem. 154:193-196. [DOI] [PubMed] [Google Scholar]
- 25.Fokine, A., P. R. Chipman, P. G. Leiman, V. V. Mesyanzhinov, V. B. Rao, and M. G. Rossmann. 2004. Molecular architecture of the prolate head of bacteriophage T4. Proc. Natl. Acad. Sci. USA 101:6003-6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furcinitti, P. S., J. van Oostrum, and R. M. Burnett. 1989. Adenovirus polypeptide IX revealed as capsid cement by difference images from electron microscopy and crystallography. EMBO J. 8:3563-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayden, M., M. B. Adams, and S. Casjens. 1985. Bacteriophage L: chromosome physical map and structural proteins. Virology 147:431-440. [DOI] [PubMed] [Google Scholar]
- 28.Hendrix, R., and S. Casjens. Bacteriophage lambda and its genetic neighborhood. In R. Calendar (ed.), Bacteriophages II, in press. Oxford Press, New York, N.Y.
- 29.Hendrix, R. W., and S. R. Casjens. 1975. Assembly of bacteriophage lambda heads: protein processing and its genetic control in petit lambda assembly. J. Mol. Biol. 91:187-199. [DOI] [PubMed] [Google Scholar]
- 30.Hendrix, R. W., M. C. Smith, R. N. Burns, M. E. Ford, and G. F. Hatfull. 1999. Evolutionary relationships among diverse bacteriophages and prophages: all the world's a phage. Proc. Natl. Acad. Sci. USA 96:2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilliker, S. 1981. Characterization of the Salmonella phage L early genes using lambda immL hybrid phages. Virology 114:161-174. [DOI] [PubMed] [Google Scholar]
- 32.Ishii, T., Y. Yamaguchi, and M. Yanagida. 1978. Binding of the structural protein soc to the head shell of bacteriophage T4. J. Mol. Biol. 120:533-544. [DOI] [PubMed] [Google Scholar]
- 33.Ishii, T., and M. Yanagida. 1977. The two dispensable structural proteins (soc and hoc) of the T4 phage capsid; their purification and properties, isolation and characterization of the defective mutants, and their binding with the defective heads in vitro. J. Mol. Biol. 109:487-514. [DOI] [PubMed] [Google Scholar]
- 34.Iwasaki, K., B. L. Trus, P. T. Wingfield, N. Cheng, G. Campusano, V. B. Rao, and A. C. Steven. 2000. Molecular architecture of bacteriophage T4 capsid: vertex structure and bimodal binding of the stabilizing accessory protein. Soc. Virol. 271:321-333. [DOI] [PubMed] [Google Scholar]
- 35.Johnson, M. L., J. J. Correia, and D. A. Yphantis. 1981. Analysis of data from analytical ultracentrifuge by non-linear least squares techniques. Biophys. J. 36:575-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahmann, R., and H. Prell. 1971. Complementation between P22 amber mutants and phage L. Mol. Gen. Genet. 113:363-366. [DOI] [PubMed] [Google Scholar]
- 37.Laue, T. M. 1995. Sedimentation equilibrium as thermodynamic tool. Methods Enzymol. 259:427-452. [DOI] [PubMed] [Google Scholar]
- 38.Laue, T. M., B. D. Shah, T. M. Ridgeway, and S. L. Pelletier. 1992. Computer aided interpretation of analytical sedimentation data for proteins, p. 90-125. In S. E. Harding, A. J. Rowe, and J. C. Horton (ed.), Ultracentrifugation in biochemistry and polymer science. Cambridge University Press, Cambridge, United Kingdom.
- 39.Lew, K., and S. Casjens. 1975. Identification of early proteins coded by bacteriophage P22. Virology 68:525-533. [DOI] [PubMed] [Google Scholar]
- 40.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 41.Meulenbroek, R. A., K. L. Sargent, J. Lunde, B. J. Jasmin, and R. J. Parks. 2004. Use of adenovirus protein IX (pIX) to display large polypeptides on the virion-generation of fluorescent virus through the incorporation of pIX-GFP. Mol. Ther. 9:617-624. [DOI] [PubMed] [Google Scholar]
- 42.Mmolawa, P. T., H. Schmieger, C. P. Tucker, and M. W. Heuzenroeder. 2003. Genomic structure of the Salmonella enterica serovar Typhimurium DT 64 bacteriophage ST64T: evidence for modular genetic architecture. J. Bacteriol. 185:3473-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newcomb, W. W., B. L. Trus, N. Cheng, A. C. Steven, A. K. Schaefer, D. J. Tenney, S. K. Weller, and J. C. Brown. 2000. Isolation of herpes simplex procapsids from cells infected with protease-deficient mutant virus. J. Virol. 74:1663-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olson, N. H., M. Gingery, F. A. Eiserling, and T. S. Baker. 2001. The structure of isometric capsids of bacteriophage T4. Virology 279:385-391. [DOI] [PubMed] [Google Scholar]
- 45.Prasad, B. V., P. E. Prevelige, E. Marietta, R. O. Chen, D. Thomas, J. King, and W. Chiu. 1993. Three-dimensional transformation of capsids associated with genome packaging in a bacterial virus. J. Mol. Biol. 231:65-74. [DOI] [PubMed] [Google Scholar]
- 46.Reilly, B. E., R. A. Nelson, and D. L. Anderson. 1977. Morphogenesis of bacteriophage φ29 of Bacillus subtilis: mapping and functional analysis of the head fiber gene. J. Virol. 24:363-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren, Z., and L. W. Black. 1998. Phage T4 SOC and HOC display of biologically active, full-length proteins on the viral capsid. Gene 215:439-444. [DOI] [PubMed] [Google Scholar]
- 48.Rosa-Calatrava, M., L. Grave, F. Puvion-Dutilleul, B. Chatton, and C. Kedinger. 2001. Functional analysis of adenovirus protein IX identifies domains involved in capsid stability, transcriptional activity, and nuclear reorganization. J. Virol. 75:7131-7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saad, A., Z. H. Zhou, J. Jakana, W. Chiu, and F. J. Rixon. 1999. Roles of triplex and scaffolding proteins in herpes simplex virus type 1 capsid formation suggested by structures of recombinant particles. J. Virol. 73:6821-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sawyer, S. 1989. Statistical tests for detecting gene conversion. Mol. Biol. Evol. 6:526-538. [DOI] [PubMed] [Google Scholar]
- 51.Schicklmaier, P., and H. Schmieger. 1997. Sequence comparison of the genes for immunity, DNA replication, and cell lysis of the P22-related Salmonella phages ES18 and L. Gene 195:93-100. [DOI] [PubMed] [Google Scholar]
- 52.Spencer, J. V., W. W. Newcomb, D. R. Thomsen, F. L. Homa, and J. C. Brown. 1998. Assembly of the herpes simplex virus capsid: preformed triplexes bind to the nascent capsid. J. Virol. 72:3944-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephens, J. C. 1985. Statistical methods of DNA sequence analysis: detection of intragenic recombination or gene conversion. Mol. Biol. Evol. 2:539-556. [DOI] [PubMed] [Google Scholar]
- 54.Sternberg, N., and R. Weisberg. 1977. Packaging of coliphage lambda DNA. II. The role of the gene D protein. J. Mol. Biol. 117:733-759. [DOI] [PubMed] [Google Scholar]
- 55.Studier, W., A. Rosenberg, J. Dunn, and J. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 56.Susskind, M. M., and D. Botstein. 1978. Molecular genetics of bacteriophage P22. Microbiol. Rev. 42:385-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Susskind, M. M., and D. Botstein. 1978. Repression and immunity in Salmonella phages P22 and L: phage L lacks a functional secondary immunity system. Virology 89:618-622. [DOI] [PubMed] [Google Scholar]
- 58.Susskind, M. M., A. Wright, and D. Botstein. 1971. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. II. Genetic evidence for two exclusion systems. Virology 45:638-652. [DOI] [PubMed] [Google Scholar]
- 59.Tao, Y., N. H. Olson, W. Xu, D. L. Anderson, M. G. Rossmann, and T. S. Baker. 1998. Assembly of a tailed bacterial virus and its genome release studied in three dimensions. Cell 95:431-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trus, B. L., F. P. Booy, W. W. Newcomb, J. C. Brown, F. L. Homa, D. R. Thomsen, and A. C. Steven. 1996. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly. J. Mol. Biol. 263:447-462. [DOI] [PubMed] [Google Scholar]
- 61.Wendt, J. L., and M. Feiss. 2004. A fragile lattice: replacing bacteriophage λ's head stability gene D with the shp gene of phage 21 generates the Mg2+-dependent virus, λ shp. Virology 326:41-46. [DOI] [PubMed] [Google Scholar]
- 62.Wiggins, B. A., and S. Hilliker. 1985. Map of DNA homology between the genomes of Salmonella bacteriophages P22 and L. J. Virol. 56:1034-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winston, F., D. Botstein, and J. H. Miller. 1979. Characterization of amber and ochre suppressors in Salmonella typhimurium. J. Bacteriol. 137:433-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yanagida, M. 1977. Molecular organization of the shell of T-even bacteriophage. II. Arrangement of subunits in the head shells of giant phages. J. Mol. Biol. 109:515-537. [DOI] [PubMed] [Google Scholar]
- 65.Yanagida, M., Y. Suzuki, and T. Toda. 1984. Molecular organization of the head of bacteriophage T-even: underlying design principles. Adv. Biophys. 17:97-146. [DOI] [PubMed] [Google Scholar]
- 66.Yang, F., P. Forrer, Z. Dauter, J. F. Conway, N. Cheng, M. E. Cerritelli, A. C. Steven, A. Pluckthun, and A. Wlodawer. 2000. Novel fold and capsid-binding properties of the lambda-phage display platform protein gpD. Nat. Struct. Biol. 7:230-237. [DOI] [PubMed] [Google Scholar]
- 67.Yphantis, T. 1964. Equilibrium ultracentrifugation of dilute solutions. Biochemistry 3:297-317. [DOI] [PubMed] [Google Scholar]