Abstract
The Escherichia coli yjhA (renamed nanC) gene encodes a protein of the KdgM family of outer membrane-specific channels. It is transcribed divergently from fimB, a gene involved in the site-specific inversion of the region controlling transcription of the fimbrial structural genes but is separated from it by one of the largest intergenic regions in E. coli. We show that nanC expression is induced by N-acetylneuraminic acid and modulated by N-acetylglucosamine. This regulation occurs via the NanR and NagC regulators, which also control fimB expression. nanC expression is also activated by the regulators cyclic AMP-catabolite activator protein, OmpR, and CpxR. When the NanC protein was reconstituted into liposomes, it formed channels with a conductance of 450 pS at positive potential and 300 to 400 pS at negative potential in 800 mM KCl. The channels had a weak anionic selectivity. In an ompR background, where the general porins OmpF and OmpC are absent, NanC is required for growth of E. coli on N-acetylneuraminic acid as the sole carbon source. All these results suggest that NanC is an N-acetylneuraminic acid outer membrane channel protein.
Porins are proteins that form water-filled channels across the outer membrane of gram-negative bacteria. These channels allow the diffusion of hydrophilic molecules (<600 Da for the general porins OmpF and OmpC) into the periplasm with no particular substrate specificity. In Escherichia coli this family of proteins is represented by the trimeric porins OmpC, OmpF, and PhoE. Elucidation of the structure of these proteins showed that each monomeric barrel is formed by 16 β-strands (5). Other outer membrane channels catalyze the specific diffusion of a particular class of nutrient and thus allow the uptake of compounds that would diffuse too slowly through the general porins (15).
The E. coli maltose-specific channel protein LamB and the sucrose channel protein ScrY are the best studied of this class of channel proteins. These proteins also form trimers, and each monomer consists of 18 transmembrane β-strands (24). A few other specific channels are known in E. coli: the aryl-β-d-glucoside channel protein BglH, the nucleoside transporter Tsx, and the long-chain fatty acid channel protein FadL (30, 13, 2, 28).
Recently a new class of specific channel proteins has been described in Erwinia chrysanthemi, the oligogalacturonate-specific channel protein KdgM (3). This small protein (216 amino acids) seems to be monomeric and forms a channel of 14 transmembrane β-strands (18). Proteins of the KdgM family are found in other gram-negative bacteria, including Erwinia carotovora, Yersinia pestis, Salmonella enterica serovar Typhimurium, Escherichia coli, Klebsiella pneumoniae, Vibrio halioticoli, and Pseudomonas species. A comparative genomic analysis has shown that most of the KdgM orthologue-encoding genes are clustered with genes involved in pectin degradation and that a binding site for KdgR, the regulator of pectin degradation pathways, can be detected in their upstream regulatory regions (22). Thus, most of these KdgM homologues could be involved in the transport of oligogalacturonates. The V. halioticoli gene is clustered with alginate lyase genes, and the channel could transport alginate degradation products. Two KdgM homologues exist in E. coli, YjhA and YshA. The genes yjhA and yshA are not controlled by KdgR, and the chromosomal location of these genes does not allow prediction of their substrates. The channel function of YshA (OmpL) has been proven, but its substrate was not characterized (6).
yjhA is the first gene of the presumed yjhATS operon. The function of YjhT and YjhS is not known, and they have no homology with proteins of known function. This operon is transcribed divergently from fimB, a gene whose product catalyzes the site-specific inversion of the region controlling transcription of the fimbrial structure genes (fimA to fimH) (7). The intergenic yjhA-fimB region is exceptionally large for E. coli (1.4 kb). Moreover, elements that regulate fimB and which could potentially regulate yjhA have been identified near the center of this region and include a NanR binding site. NanR is a regulator of N-acetylneuraminic acid (Neu5Ac or sialic acid) metabolism in E. coli (10). Sialic acids are a family of more that 40 nine-carbon monosaccharides present mostly in eukaryotes, where they play roles in cell-cell and cell-molecule interactions (29).
E. coli is able to use Neu5Ac as sole carbon source for growth. Catabolism of Neu5Ac requires the products of the nanATEK operon, yielding pyruvate and N-acetylglucosamine-6-phosphate (GlcNAc-6-P). GlcNAc-6-P is further metabolized to fructose-6-phosphate by NagA and NagB, two enzymes of the N-acetylglucosamine (GlcNAc) degradation pathway, whose synthesis is controlled by the nagC-encoded repressor (10, 20). Neu5Ac regulates transcription of fimB, and it has been suggested that it could also control the expression of the divergently expressed yjhATS operon (7, 29). We show in this work that yjhA is indeed regulated by NanR and several other regulators and that it encodes a Neu5Ac-specific channel.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains were grown in Luria-Bertani (LB) or M63 medium (14). M63 medium deprived of an ammonia source was used to test the effect of ammonia concentration, and M63 medium diluted twofold with various sucrose concentrations was used to test the effect of osmolarity. P1 transduction were carried out with P1vir (14). Strain NM522 was used for cloning experiments. Other E. coli strains used are 11814 (MG1655 Δlac) (A. Vianney), IBPC536 (thrA1 leuB6 hisG4 argE3 Δ(gpt-lac)62 thi-1 galK2 ara-14 xyl-5 mtl-1 tsx-33 kdgK51 rpsL31 supE44 recB21 recC22 sbcB15 sbcC201 nagC::tc) (19), TK821 (MC4100 ompR::Tn10) (8), MV679 (MG1655 ΔlacZYA ΔnanR) (I. Blomfield), PHL1242 (MG1655 ΔcpxR) (G. Jubelin), pop4129 (zhd::Tn10 Δcrp18) (J.-C. Lazzaroni), and IBPC1016 (JM101 mlc::tc nanR6) (20).
Molecular biology techniques.
Plasmid DNA preparation, DNA restriction and electrophoresis, and bacterial transformation and electroporation were all performed according to Sambrook et al. (23).
S1 nuclease protection and DNase footprint analysis.
Total RNA was purified by the hot phenol method, and S1 nuclease transcript mapping was carried out as previously described (10). The probes used were the PCR fragments Fim3-Fim2 and Fim1-Fim4, labeled at the Fim3 and Fim1 oligonucleotides prior to the PCR. Fim3 (5′-ACGTACGTCCAGTGTCGCAG) corresponds to amino acids 29 to 24 of the yjhA open reading frame. The relative positions of Fim1 (5′-CATCACCCGATGAGAAAGAACTG), Fim2 (5′-ACCAACATCAACAAGCCTCTCC), and Fim4 (5′-AGACCACCGCAAGTGTTCG) are indicated on Fig. 2A. DNase I footprint analysis was carried out as previously described with the Fim1-Fim2 PCR fragment as the probe labeled at the Fim1 site. NagC with a C-terminal His6 tag was the gift of Charles Bell and Mitchell Lewis, and chloramphenicol (CAP) was the gift of Annie Kolb.
FIG. 2.
NagC and CAP binding to the nanC regulatory region. The Fim1-Fim2 PCR fragment labeled at the Fim1 site was mixed with NagC and/or CAP at the concentration indicated. Cyclic AMP was included with CAP at 0.2 mM. The region protected by NagC and the three hypersensitive bands indicative of CAP binding are indicated. The marker is pBR322 digested with MspI.
YjhA expression and purification.
Production of porins in inclusion bodies followed by a renaturation step is now a well-established method to obtain active proteins (25, 3) To produce YjhA without its signal peptide, a DNA fragment was amplified by PCR with primers yjh-1 (5′-GAATTCCATGGCACTGGACGTACGTGGT) and yjh-2 (5′-GAATTTCTAGATTCATTGTGACTGTCTC). The NcoI and XbaI sites included in the primers (underlined) facilitated cloning of the fragment into expression vector pKSM717, yielding plasmid pYjh3, placing the coding sequence under the control of the T7 promoter. The mature form of YjhA was overproduced in inclusion bodies in E. coli strain BL21(DE3)/pLys. After 2 h of induction with isopropylthiogalactopyranoside (IPTG), cells were harvested and disrupted in a French cell in 50 mM Tris-HCl, pH 8.0-5 mM EDTA. Unbroken cells were eliminated by centrifugation for 5 min at 2,000 × g, and inclusion bodies were then pelleted by centrifugation 10 min at 8,000 × g. Inclusion bodies were resuspended in 10 mM Tris-HCl, pH 8.0, at a concentration of 10 mg/ml, and 6 M urea was added. After dissolution of the inclusion bodies, the solution was centrifuged at 20,0000 × g for 5 min, and the supernatant was dialyzed overnight in 10 mM Tris-HCl, pH 8.0-0.5% sodium dodecyl sulfate. YjhA was purified on preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by preparative Tris-Tricine-polyacrylamide gel electrophoresis.
Construction of a yjhA-lacZ fusion strain.
Primers yjh-3 (5′-GGTCCCTAGCGATTATTCCTGC) and yjh-4 (5′-CGACCTTGCGATAATTCACCCG) were used to amplify a DNA fragment extending 1 kb upstream and downstream of the yjhA coding sequence. The amplified fragment was cloned into plasmid pGEM-T (Promega, Madison, Wis.), and a lacZ-kanamycin resistance cassette was inserted into the unique BclI site of yjhA located at nucleotide 125. This construct was introduced into plasmid pKO3 and recombined into the E. coli chromosome according to the method described by Link et al. (12).
Reconstitution of YjhA in liposomes.
The purified protein YjhA (500 ng) was added to 2 ml of buffer (500 mM KCl, 10 mM HEPES-KOH, pH 7.4, 33 mM octylglucoside) containing 1 mg of sonicated lipids (asolectin from soybean, type IV-S). Detergent was removed with SM-2 Bio Beads (Bio-Rad, Hercules, Calif.) as previously described (3), and the proteoliposomes were recovered by ultracentrifugation. For planar bilayer experiments, the proteoliposomes were resuspended in 0.1 ml of 500 mM KCl-10 mM HEPES-KOH, pH 7.4. To obtain giant proteoliposomes amenable to patch-clamp recording, the proteoliposomes were subjected to a dehydration-rehydration cycle as previously described (1). Rehydration was performed in 100 mM KCl-10 mM HEPES-KOH, pH 7.4.
Electrophysiological recording.
Bilayers were formed from a solution of asolectin lipids dissolved in n-decane (30 mg/ml) across a 250-μm-diameter hole. Proteoliposomes (3 to 10 ng of protein/ml, final concentration) were added to the cis compartment. Fusion was induced by imposing a salt gradient between the two chambers: 800 mM KCl-10 mM HEPES-KOH, pH 7.4, in the cis compartment versus 100 mM KCl-10 mM HEPES-KOH, pH 7.4, in the trans compartment. The bilayer set-up was connected to the external circuit through salt bridges with Ag-AgCl electrodes. Unitary currents were recorded with an Axon 200B amplifier. The membrane potential refers to the potential of the cis side minus the potential of the trans side.
Patch-clamp recording on giant proteoliposomes was performed by standard patch-clamp methods. Patch electrodes were pulled from Pyrex capillaries (Corning code 7740) with a P-2000 laser pipette-puller (Sutter Instruments Co.); 2 μl of the giant proteoliposome suspension was deposited in a patch-clamp chamber and diluted by 2 ml of bath solution (100 mM KCl-10 mM HEPES-KOH, pH 7.4). Micropipettes were filled with a buffer similar to that of the patch-clamp chamber plus 5 mM MgCl2 and 2 mM CaCl2. Unitary currents were recorded with a Biologic RK-300 patch-clamp. The compounds whose effect was tested on channel activity were neutralized with KOH when necessary. Electrophysiological records were stored on digital audio tape (Biologic DTR 1200 DAT recorder) and subsequently filtered at 1 kHz (−3 dB point) through a four-pole Bessel low-pass filter, digitized off-line at a rate of 2 kHz, and analyzed on a personal computer.
RESULTS
Regulation of yjhA expression.
fimB expression is regulated by Neu5Ac through the binding of the NanR regulator at a specific site in the fimB-yjhA intergenic region (Fig. 1A) (7, 27). To verify whether NanR could also control yjhA expression, we measured expression of a yjhA-lacZ fusion in the absence and presence of Neu5Ac. In the absence of inducer, the expression of the fusion was very low and it was induced 20-fold by Neu5Ac (Table 1). When the fusion was introduced into a nanR background, its expression was identical to that in the Neu5Ac-induced strain and it was not further inducible by Neu5Ac, confirming that induction of yjhA by Neu5Ac occurs through NanR.
FIG. 1.
A. Intergenic fimB-nanC region. The promoters of fimB and nanC are indicated by bent arrows, and the location and orientation of oligonucleotides are indicated by arrows. The region from −520 to −420 from the nanC translation initiation codon is enlarged. The −35 and −10 regions of the promoter are underlined, the NanR binding site is boxed in gray, the NagC binding site is boxed in white, and the CAP binding site is underlined with a wavy line. B. S1 mapping of the nanC promoter. The probe used was the Fim1-4 PCR fragment with the Fim1 oligonucleotide labeled with [32P]ATP and polynucleotide kinase. It was hybridized with RNA (25 μg) isolated from the wild type or nanR mutant grown with the sugars indicated (lanes 1 to 6). Lane 7, tRNA control. Sequencing reactions with the Fim1 oligonucleotide are given, and the sequence of the −10 to +1 region on the nontemplate strand is given.
TABLE 1.
Effect of various inducing conditions and regulatory mutations on expression of the nanC-lacZ fusiona
| Mutation(s) | Inducer added | Sp actb (nmol/min/mg) |
|---|---|---|
| None | None | 13 ± 1 |
| None | Glucose | <1 |
| None | GlcNAc | 1 |
| None | Neu5Ac | 240 ± 18 |
| None | GlcNAc + Neu5Ac | 105 ± 12 |
| nanR | None | 248 ± 12 |
| nanR | Glucose | 21 ± 2 |
| nanR | GlcNAc | 223 ± 63 |
| nanR | Neu5Ac | 251 ± 21 |
| nanR crp | Glucose | 1 |
| nanR nagC | None | 360 ± 19 |
| nanR nagC | Glucose | 281 ± 42 |
| nanR nagC | GlcNAc | 344 ± 36 |
| nanR | M63 + glycerol | 322 ± 45 |
| nanR ompR | M63 + glycerol | 128 ± 15 |
| nanR cpxR | M63 + glycerol | 153 ± 34 |
| nanR cpxR ompR | M63 + glycerol | 62 ± 12 |
Bacteria were grown in LB medium at 30°C or in M63 medium where indicated. Inducing carbon sources were added at 0.1%.
Specific activities are expressed as nanomoles of o-nitrophenol formed per minute per milligram of bacteria (dry weight).
Since yjhA is regulated by nanR, we renamed it nanC (for nanR-regulated channel). Neu5Ac metabolism converges with that of GlcNAc. We tested the effect of GlcNAc and of a mutation in the GlcNAc metabolic pathway regulator, nagC, on nanC expression. In the wild-type background glucose and GlcNAc produced a 10-fold decrease in nanC expression which could be due to catabolite repression. However, in the nanR background only glucose or introduction of a crp mutation produced a strong decrease in expression, while the introduction of a nagC mutation produced a 50% increase in expression (Table 1), suggesting that GlcNAc and NagC have additional functions in nanC expression (see below).
Expression of porins and channels is often regulated in response to variations in environmental conditions (15). Neu5Ac and GlcNAc can be used by the bacteria as sole ammonia sources. However, ammonia deprivation did not induce expression of nanC (data not shown). Similarly, nanC expression was not modified by oxygen limitation. Expression of the general porins OmpC and OmpF is regulated by osmolarity via the two-component regulatory system EnvZ-OmpR (8). Expression of nanC was reduced 2.5-fold in an ompR background (Table 1). nanC expression was predicted to be regulated by the two-component regulatory system CpxR-CpxA by transcriptome analysis (16). Indeed, nanC expression was reduced twofold in a cpxR background. The effect of the cpxR and ompR mutations was cumulative (Table 1).
NagC and CAP binding to the nanC promoter.
The transcriptional start for nanC was mapped on mRNA prepared from a wild-type and nanR mutant (JM101 and IBPC 1016) strain during growth on glycerol, glucose, or sialic acid. A single start site was located 428 bp upstream of the nanC open reading frame using a probe starting within the nanC open reading frame. This transcript was mapped precisely using a shorter probe (Fim 1-Fim 4, Fig. 1B). The transcript was detected in the wild-type strain grown on sialic acid or in the nanR mutant in all three media, although it was low in RNA from the strain grown on glucose, confirming the strong catabolite repression. The start site corresponds to a −10 sequence of TATAAC and lies within the predicted NanR binding site containing two repeats of the GGTATA hexamer (10). NanR binds to this sequence (27). It can be noted that the nanC promoter is located in exactly the same position relative to the NanR binding site as the nanA promoter is within the NanR operator. There is no obvious −35 promoter sequence, but a potential CAP site is detectable on the sequence (Fig. 1A), centered at position −61.5, which is consistent with the strong catabolite repression observed with the nanC fusion. A NagC binding site is centered 65 nucleotides upstream of the transcription start site, so that it overlaps the CAP site.
DNase I footprinting confirmed that both proteins bind to the same region (Fig. 2). Although the CAP site does not show very high affinity, when both proteins were added simultaneously, CAP (50 nM) seems to bind preferentially, as shown by the formation of the classic pattern of three hypersensitive bands (Fig. 2, lanes 9 to 11). The competition between NagC and CAP for binding to the same site explains the complex catabolite repression pattern of nanC expression (Table 1). The intracellular formation of GlcNAc releases NagC from its site and in consequence allows the binding of CAP to its overlapping site. Thus, in the absence of NagC binding, even in the presence of the lowered cyclic AMP (cAMP)/CAP concentration produced by growth on GlcNAc or glucose, cAMP/CAP can bind and activate nanC expression. In support of this interpretation, in the nanR strain, expression was low on glucose but high on GlcNAc, whereas in the nanR nagC strain it was high on both sugars.
Sialic acid is degraded via GlcNAc-6-P and so it should produce inducing signals for both regulators. This dual regulation by Neu5Ac and GlcNAc means that it is probably only when the flux through the combined nan-nag pathways is high enough to generate both inducing signals (a condition seen when both compounds are present in the growth medium) that the operon is fully induced. However, when both Neu5Ac and GlcNAc were added simultaneously to the growth medium, the level of nanC expression was lower than when Neu5Ac alone was added (Table 1), probably because of the catabolite repression provoked by addition of a high concentration of GlcNAc.
Physiological role of NanC.
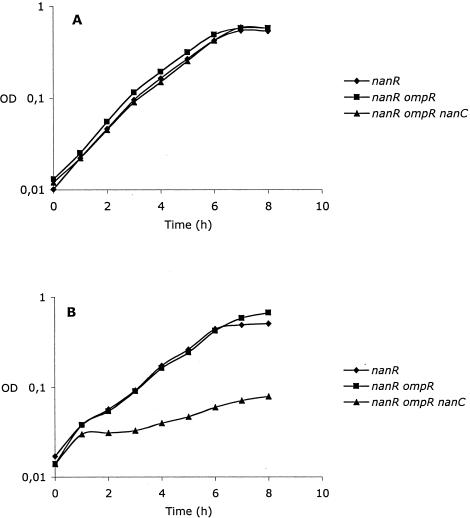
The induction of nanC expression by Neu5Ac and GlcNAc let us suppose that it could be a channel allowing entry of these compounds into the bacteria. To test this hypothesis, growth of a nanR strain, a nanR ompR strain, and its nanC derivative was tested with these compounds as the sole carbon source. The presence of the nanR mutation prevents induction problems that could arise if the substrates cannot efficiently induce their transport systems, and the ompR mutation prevents expression of the general porins OmpC and OmpF through which these compounds could enter. While no difference was observed for growth with glucosamine, glucose (used as a control, data not shown), or GlcNAc as the sole carbon source for the three strains (Fig. 3A), the nanC mutant was unable to grow with Neu5Ac (Fig. 3B). In contrast, a nanR nanC mutant (which is OmpF+ OmpC+) could grow with this substrate (data not shown). This indicates that, in the absence of the porins OmpF and OmpC, the channel formed by nanC is the only way for Neu5Ac to enter the bacteria. Glucose and GlcNAc probably enter by other weakly expressed general porins.
FIG. 3.
Growth of strains MV679 (nanR, diamonds), I3065 (nanR ompR, squares), and I3040 (nanR ompR nanC, triangles) with (A) GlcNAc or (B) Neu5Ac as the sole carbon source.
Porin activity of NanC.
Purified NanC proteins were reconstituted into liposomes. Addition of these liposomes to the cis compartment of a bilayer chamber, at low protein concentration, resulted in insertion in the planar lipid bilayer of channels that were open at 0 mV and at low, positive, or negative voltage (Fig. 4). At high voltages, positive or negative, the channels closed in a manner characterized by large, slow transitions. Sporadic fast closures were often superimposed on the slow kinetic closure. The tendency to close was higher at positive than at negative potential. Similar characteristics were observed in patch-clamp experiments performed on giant proteoliposomes reconstituted with NanC. The main conductance of the channel in symmetrical 800 mM KCl medium was 450 pS at positive potential and 300 to 400 pS at negative potential. Lower subconductances were also observed. The channels were weakly selective for anions with a reversal potential of around 20 mV under asymmetrical conditions (800 mM KCl versus 10 mM KCl).
FIG. 4.
Activity of NanC channels at different electrical potentials. Fusion to the planar bilayer of proteoliposomes reconstituted with purified NanC was induced by imposing a salt gradient between the two chambers. After insertion of the channels in the bilayer, symmetrical medium (800 mM KCl, 10 mM HEPES-KOH, pH 7.4) was established in the two chambers, and the activity was recorded. The bilayer was subjected to various positive and negative potentials as indicated. Between pulses, the bilayer was held at 0 mV. The beginning of each trace corresponds to the onset of each pulse. The dashed line corresponds to zero current.
The effect of different compounds on channel conduction through the NanC channel was examined in patch-clamp experiments. The tested compound was either superfused in the bath on the excised patch or present in the pipette and tested at different voltages at opposite polarities. Neu5Ac up to 50 mM did not induce any blockade of the channels when it was perfused in the bath (n = 9) or present in the pipette (n = 3). We also tested a possible effect of colominic acid, a mixture of polymers of Neu5Ac. Up to 50 mM (final concentration of the monomer), no effect was observed in the bath (n = 5) or in the pipette (n = 4). Finally, neither 70 mM GlcNAc (n = 3) nor 50 mM trigalacturonate (n = 3), which blocks KdgM porin channels, had any clear effect.
DISCUSSION
The results presented here suggest that NanC is an outer membrane channel protein allowing the entry of Neu5Ac into E. coli. In electrophysiological experiments, purified NanC reconstituted in pure lipid bilayers exhibited the classical properties of voltage-dependent porins. NanC proteins form high-conductance channels which are open at low membrane potentials. The conductance and the weak anion selectivity of NanC are similar to that of KdgM, which belongs to the same protein family (3). Unlike KdgM, which can be closed only at positive potential, NanC exhibited voltage-dependent closure at both polarities. In the case of KdgM, which is involved in oligogalacturonide transport, we showed that trigalacturonate was able to induce fast blockade of the channel (3). No effect of Neu5Ac was observed in the experiments performed with NanC.
Since the ability to detect blockade is dependent on the residence time of the blocker in the channel and hence on its size, we tested colominic acid, a mixture of polymers of Neu5Ac. The exact composition of this commercial mixture, which cannot be used as a carbon source for growth by E. coli, is not known, and thus its lack of effect is no proof that NanC channels cannot be blocked by long polymers of Neu5Ac. Clearly, our electrophysiological experiments cannot document a specificity of NanC porin channels for sialic acid.
However, strong indications that Neu5Ac could be a NanC substrate come from the facts that Neu5Ac induces NanC synthesis and that NanC is required for the entry of Neu5Ac in the absence of the general porins OmpC and OmpF. Neu5Ac has a molecular mass (309 Da) that allows its entry into bacteria through these nonspecific porins (exclusion size, 600 Da). Why should E. coli possess a specific channel for this compound? The concentration of free sialic acid is generally low in most animal tissues (29), and a specific channel would allow efficient uptake of this good carbon and nitrogen source that would otherwise diffuse too slowly by the general porins because of their low concentration. Neu5Ac can also be found as a homopolymer (polysialic acid) at the surface of E. coli K1 strains or as the terminal sugar of polysaccharidic chains at the surface of eukaryotic cells. Intestinal mucus is rich in these sialic acid-containing carbohydrates (4). A specific channel could be useful for the uptake of these oligomeric sugars.
nanC forms a putative operon with two genes, yjhS and yjhT. These genes encode proteins with a possible sequence signal that could be involved in the periplasmic degradation of Neu5Ac-containing oligomers. The fact that a nanC mutant is still able to grow on Neu5Ac indicates that the products of these two genes are not required for the metabolism of this compound.
The identified or predicted substrates of the KdgM family members are oligogalacturonate, oligorhamnogalacturonate, alginate, and Neu5Ac (3, 22). The common feature of these compounds is the presence on the sugars of an acidic function. The marine bacterium Vibrio furnissii is able to metabolize chitin, a polymer of GlcNAc, a nonacidic derivative of Neu5Ac. The chitooligosaccharides enter the bacteria not through a KdgM family channel protein but through a general porin belonging to the PhoE family (11). Determination of the three-dimensional structure of KdgM or another member of the family should clarify why a specific family of channel proteins is required for acid sugars and how these compounds cross the channel.
E. coli yshA (ompL), the closest nanC homologue, belongs to group of eight genes that probably form an operon. All these genes present homologies with genes involved in sugar metabolism: two genes of the family of sodium:galactoside symporters (yihP and yihO), a glycoside hydrolase (yihQ), an aldose epimerase (yihR), a sugar epimerase (yihS), an aldolase (yihT), and an oxidoreductase (yihU). Thus, yshA is also probably involved in the transport of a sugar, probably acidic.
NanC regulation seems to be quite complex. NanR and NagC are two regulators that control nanC expression by binding to or in the vicinity of its promoter. They also regulate fimB expression in a still unexplained way, since their binding sites are located more than 500 bp upstream of the fimB promoters (27). However, it is clear that they allow coordination of nanC and fimB expression, since NanR and NagC repress one and activate the other of these divergently arranged genes. The presence of sialic acid, an indicator of the presence of an animal host, induces the sialic acid degradation pathway (the first step of which is NanC) and inhibits fimB, which regulates E. coli adhesion by type I fimbriae. Two other regulators, OmpR and CpxR, activate nanC expression, although a direct control has not been proven.
Regulation of porin gene expression by osmolarity via OmpR is a well-studied phenomenon (8). Control of fimB expression by osmolarity has recently been described, and OmpR has the role of repressor (26). Thus, it has opposite actions on nanC (activation) and fimB (repression). Such differential actions are also observed for NanR and NagC. CpxR and OmpR control the formation of adhesion structures such as curli and Pap pili (9, 17, 21). It would not be surprising if, like NanR, NagC, and OmpR, CpxR plays a role in the control of type 1 fimbriation.
Additional regulatory proteins controlling nanC probably exist: introduction in a strain bearing a nanC-lacZ fusion of a plasmid containing the regulatory region of nanC led to a level of expression of the fusion 18-fold higher than in the nanR mutant (data not shown). This suggests the existence of an additional repressor(s) controlling nanC expression that would be titrated by the presence of the plasmid. A role of these regulators in the control of fimB expression remains to be proven, but its complex regulation by far upstream sequences that control nanC transcription shows that nanC and fimB expression is highly intertwined. These intricate regulations may be the reason for the exceptional length of the nanC-fimB intergenic region. Through these common regulatory mechanisms, sialic acid appears to be not just a carbon source, but also a complex environmental signal controlling the way of life of E. coli.
Acknowledgments
We thank Ian Blomfield and Corinne Dorel for strains and helpful discussions, Charles Bell and Mitchell Lewis for the gift of NagC, Annie Kolb for CAP, Anne Bibonne for technical assistance, and Nicole Cotte-Pattat for reading the manuscript.
This work was supported by grants from the Centre National de la Recherche Scientifique and from the Ministère de l'Education et de la Recherche.
REFERENCES
- 1.Berrier, C., A. Coulombe, C. Houssin, and A. Ghazi. 1989. A patch-clamp study of ion channels of inner and outer membranes and of contact zones of E. coli, fused into giant liposomes. Pressure-activated channels are localized in the inner membrane. FEBS Lett. 259:27-32. [DOI] [PubMed] [Google Scholar]
- 2.Black, P. N., B. Said, C. R. Ghosn, J. V. Beach, and W. D. Nunn. 1987. Purification and characterization of an outer membrane-bound protein required for long-chain fatty acid transport in Escherichia coli. J. Biol. Chem. 310:389-394. [PubMed] [Google Scholar]
- 3.Blot, N., C. Berrier, N. Hugouvieux-Cotte-Pattat, A. Ghazi, and G. Condemine. 2002. The oligogalacturonate-specific porin KdgM of Erwinia chrysanthemi belongs to a new porin family. J. Biol. Chem. 277:7936-7944. [DOI] [PubMed] [Google Scholar]
- 4.Chang, D.-E., D. J. Smalley, D. L. Tucker, M. L. Leatham, W. E. Norris, S. J. Stevenson, A. B. Anderson, J. E. Grissom, D. C. Laux, P. S. Cohen, and T. Conway. 2004. Carbon nutrition in the mouse intestine. Proc. Natl. Acad. Sci. USA 101:7427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan, S. W., T. Schirmer, G. Rummel, M. Steiert, R. Ghosh, R. A. Pauptit, J. N. Jansonius, and J. P. Rosenbusch. 1992. Crystal structures explain functional properties of two Escherichia coli porins. Nature 358:727-733. [DOI] [PubMed] [Google Scholar]
- 6.Dartigalongue, C., H. Nikaido, and S. Raina. 2000. Protein folding in the periplasm in the absence of primary oxidant DsbA: modulation of redox potential in periplasmic space via OmpL porin. EMBO J. 19:5980-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Labany, S., B. K. Sohanpal, M. Lahooti, R. Akerman, and I. C. Blomfield. 2003. Distant cis-active sequences and sialic acid control the expression of fimB in Escherichia coli K-12. Mol. Microbiol. 49:1109-1118. [DOI] [PubMed] [Google Scholar]
- 8.Garret, S., R. K. Taylor, and T. J. Silhavy. 1983. Isolation and characterization of chain-terminating nonsense mutations in a porin regulator gene, envZ. J. Bacteriol. 156:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2001. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 20:1508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalivoda, K. A., S. M. Steenbergen, E. R. Vimr, and J. Plumbridge. 2003. Regulation of sialic acid catabolism by the DNA binding protein NanR in Escherichia coli. J. Bacteriol. 185:4806-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keyhani, N. O., X.-B. Li, and S. Roseman. 2000. Chitin catabolism in the marine bacterium Vibrio furnissii. Identification and molecular cloning of a chitoporin. J. Biol. Chem. 275:33068-33076. [DOI] [PubMed] [Google Scholar]
- 12.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maier, C., E. Bremer, A. Schmid, and R. Benz. 1988. Pore forming activity of the Tsx protein from the outer membrane of Escherichia coli. Demonstration of a nucleoside-specific binding site. J. Biol. Chem. 263:2493-2499. [PubMed] [Google Scholar]
- 14.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshima, T., H. Aiba, Y. Masuda, S. Kanaya, M. Sugiura, B. L. Wanner, H. Mori, and T. Mizuno. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46:281-291. [DOI] [PubMed] [Google Scholar]
- 17.Otto, K., and T. J. Silhavy. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 99:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellinen, T., H. Ahlfors, N. Blot, and G. Condemine. 2003. Topology of the Erwinia chrysanthemi oligogalacturonate porin KdgM. Biochem. J. 372:329-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plumbridge, J. A. 1991. Repression and induction of the nag regulon of Escherichia coli K-12: the roles of nagC and nagA in maintenance of the uninduced state. Mol. Microbiol. 5:2053-2062. [DOI] [PubMed] [Google Scholar]
- 20.Plumbridge, J., and E. Vimr. 1999. Convergent pathways for utilization of the aminosugars N-acetylglucosamine, N-acetylmannosamine, and N-acetylneuraminic acid by Escherichia coli. J. Bacteriol. 181:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prigent-Combaret, C., E. Brombacher, O. Vidal, A. Ambert, P. Lejeune, P. Landini, and C. Dorel. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 183:7213-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodionov, D. A., M. S. Gelfand, and N. Hugouvieux-Cotte-Pattat. 2004. Comparative genomics of the KdgR regulon in Erwinia chrysanthemi 3937 and other gamma-proteobacteria. Microbiology 150:3571-3590. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Schirmer, T., T. A. Keller, Y.-F. Wang, and J. P. Rosenbusch. 1995. Structural basis for sugar translocation through maltoporin channels at 3.1 A. Science 267:512-514. [DOI] [PubMed] [Google Scholar]
- 25.Schmid, B., M. Krömer, and G. E. Schulz. 1996. Expression of porin from Rhodopseudomonas blastica in Escherichia coli inclusion bodies and folding into exact native structure. FEBS Lett. 381:111-114. [DOI] [PubMed] [Google Scholar]
- 26.Schwan, W. R., J. L. Lee, F. A. Lenard, B. T. Matthews, and M. T. Beck. 2002. Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli. Infect. Immun. 70:1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohanpal, B. K., S. El-Labany, M. Lahooti, J. A. Plumbridge, and I. C. Blomfield. 2004. Integrated regulatory responses of fimB to N-acetylneuraminic (sialic) acid and GlcNAc in Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 101:16322-16327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Berg, B., P. N. Black, W. M. Clemons Jr., and T. A. Rapoport. 2004. Crystal structure of the long-chain fatty acid transporter FadL. Science 304:1506-1509. [DOI] [PubMed] [Google Scholar]
- 29.Vimr, E. R., K. A. Kalivoda, E. L. Deszo, and S. M. Steenbergen. 2004. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 68:132-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, J., J. M. Betton, V. Michel, M. Hoffnung, and A. Charbit. 1997. Identification of a cryptic porin gene in the Escherichia coli genome: expression and insertion of a monomeric form of the protein into the outer membrane. Mol. Microbiol. 26:1141-1143. [PubMed] [Google Scholar]