Abstract
Background
Dolutegravir (DTG), combined with a backbone of 2 nucleoside reverse transcriptase inhibitors, is currently the preferred first-line treatment for human immunodeficiency virus (HIV) in childhood. CHAPAS4 is an ongoing randomized controlled trial investigating second-line treatment options for children with HIV. We did a nested pharmacokinetic (PK) substudy within CHAPAS4 to evaluate the DTG exposure in children with HIV taking DTG with food as part of their second-line treatment.
Methods
Additional consent was required for children on DTG enrolled in the CHAPAS4 trial to participate in this PK substudy. Children weighing 14–19.9 kg took 25 mg DTG as dispersible tablets and children ≥20 kg took 50 mg film-coated tablets. Steady-state 24-hour DTG plasma concentration-time PK profiling was done at t = 0 and 1, 2, 4, 6, 8, 12, and 24 hours after observed DTG intake with food. Reference adult PK data and pediatric data from the ODYSSEY trial were used primarily for comparison. The individual target trough concentration (Ctrough) was defined as 0.32 mg/L.
Results
Thirty-nine children on DTG were included in this PK substudy. The geometric mean (GM) area under the concentration–time curve over the dosing interval (AUC0-24h) was 57.1 hours × mg/L (coefficient of variation [CV%], 38.4%), which was approximately 8% below the average AUC0-24h in children in the ODYSSEY trial with comparable dosages, but above the adult reference. The GM (CV%) Ctrough was 0.82 mg/L (63.8%), which was comparable to ODYSSEY and adult reference values.
Conclusions
This nested PK substudy shows that the exposure of DTG taken with food in children on second-line treatment is comparable with that of children in the ODYSSEY trial and adult references.
Clinical Trials Registration.ISRCTN22964075.
Keywords: HIV, DTG, pharmacokinetics, children, second-line
In a pharmacokinetic substudy of the CHAPAS4 second-line antiretroviral therapy trial, pharmacokinetic profiles of 39 children showed that the exposure of dolutegravir taken with food in this population is comparable with that of children and adult references in previous trials.
Currently there are 1.7 million children under the age of 15 with human immunodeficiency virus (HIV) worldwide. One of the main concerns in the treatment of HIV infections in children and adolescents is the increasing proportion of deaths and new infections due to HIV [1]. By treating these children long-term, the HIV viral replication can be suppressed; however, in 2020 only 53% of children were receiving antiretroviral treatment (ART) and still 30% of those treated had a detectable HIV viral load [2]. Consequently, the focus today is on increasing the proportion of children on effective ART.
Nowadays, the preferred drug for treatment-naive children with HIV is dolutegravir (DTG), combined with a backbone of 2 nucleoside reverse transcriptase inhibitors (NRTIs) [3]. DTG belongs to the class of integrase inhibitors and has a high genetic barrier to resistance, has low toxicity, can be dosed once daily, and has high potency at low milligram dosage. DTG for children is now rolled out globally, initially based on the results of the pediatric Once-daily DTG based ART in Young people vS. Standard thErapY (ODYSSEY) trial and the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1093 trial [4–7]. These studies investigated dolutegravir as a first-line and second-line treatment for children with HIV.
The Children with HIV in Africa – Pharmacokinetics and Acceptability of Simple second-line antiretroviral regimens (CHAPAS4) randomized controlled trial (ISRCTN22964075) is an ongoing pediatric trial also investigating DTG treatment in children with HIV. In this study, children are taking DTG as part of their second-line treatment. In the CHAPAS4 pharmacokinetic (PK) substudies, DTG was taken with a standardized food intake, whereas DTG was generally taken in a fasted state in the ODYSSEY and IMPAACT P1093 intensive PK substudies. In adults, a 30%–60% increased exposure of DTG is observed when taken with food, based on the fat content of the meal (low, moderate, or high) [8]. Because CHAPAS4, as well as ODYSSEY, had a nested PK substudy analyzed by the same laboratory at Radboud University Medical Center (Radboudumc), we had the opportunity to further deepen our understanding of DTG exposure in children. Moreover, because of the 2-factorial randomized study design of CHAPAS4, we were able to compare the PK of DTG in combination with the relatively newer NRTI backbone combination emtricitabine (FTC)/tenofovir alafenamide fumarate (TAF) versus other NRTI backbone combinations, consisting of either abacavir (ABC)/lamivudine (3TC) or zidovudine (ZDV)/3TC.
In this article, we present the results of an intensive DTG PK substudy nested within the pediatric CHAPAS4 trial to further evaluate DTG exposure in children with HIV.
METHODS
CHAPAS4 (ISRCTN22964075) is an ongoing open-label, multicenter, randomized trial enrolling 919 African children aged 3–15 years failing first-line ART. The enrolled children were randomized to DTG or a ritonavir-boosted protease inhibitor as third drug and to once-daily TAF and FTC versus standard of care (SOC: once-daily ABC/3TC or twice-daily ZDV/3TC) in a factorial design. Follow-up is for a minimum of 96 weeks. The parents/caretakers of the first children enrolled into CHAPAS4 who did not have prevalent tuberculosis at enrollment were asked to provide additional written consent to participate in the PK substudy; verbal consent was reconfirmed before initiating the PK sampling. Older children provided written assent as appropriate based on age, knowledge of HIV status, and local country guidelines. The main trial and substudies were approved by local and national ethics committees.
In accordance with World Health Organization (WHO) weight bands, children weighing 14–19.9 kg took 25 mg (5 dispersible tablets [DTs]) and children weighing ≥20 kg took 1 film-coated tablet containing 50 mg of DTG with a standardized breakfast (low fat [5%], ∼250 kcal), irrespective of the NRTI backbone. The children randomized to a TAF-containing backbone received a combination film-coated tablet of FTC and TAF (referred as FTC/TAF). Children weighing <25 kg took 120/15 mg FTC/TAF and children >25 kg took 200/25 mg FTC/TAF once daily. Children in the SOC group used an NRTI backbone containing ABC/3TC or ZDV/3TC, both as fixed-dose combination DTs, dosed according to the WHO weight bands. Breakfast (standardized, 250 kcal, 5% fat) was given 10 minutes before the morning dose. Intake of co-medications other than antiretroviral drugs was not allowed within the first 2 hours after DTG intake. The 24-hour PK profiles are taken 6 or 12 weeks after start of the treatment to achieve steady state for all drugs. We took blood samples at t = 0 and 1, 2, 4, 6, 8, 12, and 24 hours after observed DTG intake. Blood volumes taken were within blood draw limits for children established for research studies [9]. Blood samples were refrigerated immediately after the blood draw and processed within 1 hour of sampling. Plasma was separated and stored at −80°C until shipping to the laboratory of the Department of Pharmacy, Radboudumc, Nijmegen, the Netherlands, for quantification. DTG concentrations were measured using a validated liquid chromatography–tandem mass spectrometry bioanalytical quantification method with a 0.05 mg/L lower limit of quantification [10].
The goal was to have a minimum of 8 children per weight band with evaluable PK curves for DTG, leading to a total of at least 32 children with evaluable PK curves. We considered a PK curve nonevaluable if 2 or more of 8 blood samples hemolyzed or if a participant was considered to be nonadherent based on measured DTG concentrations. This is predefined as the baseline concentration (C0) being below the lower limit of quantification, if protocol violations had occurred or if concomitant medication was used that could interfere with DTG PK. Demographic information was presented as median with range. The primary PK parameter was the area under the concentration–time curve over the dosing interval (AUC0-24h), because this parameter provides the most complete information about the actual DTG exposure. Other PK parameters presented in this article were the trough concentration (Ctrough), the maximum plasma concentration (Cmax), the time to reach the maximum plasma concentration (Tmax), apparent oral clearance (CL/F), apparent oral clearance per kilogram of body weight (CL/F/kg), apparent volume of distribution, and the elimination half-life. PK parameters were calculated by noncompartmental analysis using Phoenix 64 WinNonlin and reported as geometric mean (GM) with geometric coefficient of variation (CV%) except for Tmax, reported as median with interquartile range. Based on the median plasma concentrations per nominal time point, median DTG plasma concentrations versus time profiles were created.
The aim was to achieve PK parameter results comparable to the values reported in children with comparable dosages (25 mg DT for 14 to <20 kg, and 50-mg film-coated tablet for ≥20 kg) in the pediatric ODYSSEY trial, and comparable to published values for approved adult DTG dosing. We primarily aimed to record comparable GM AUC0-24h to GM AUC0-24h of children in the pediatric ODYSSEY trial and to adult GM AUC0-24h on DTG 50-mg film-coated tablets given once daily under fasted conditions. Additionally, we aimed to achieve comparable GM Ctrough levels, measured 24 hours after observed DTG intake. The minimal target mean Ctrough was defined as 0.32 mg/L (reported 90% effective concentration [EC90] of DTG obtained in a 10-day adult monotherapy study [11]) and is used to calculate the proportion of children who fall below this target value. For the GM Cmax outcome, we also compared our results to published adult PK data on DTG 50 mg film-coated tablet given twice daily, because this is the highest approved daily dose that is still safe [12, 13]. Within the CHAPAS4 PK substudy, we also compared DTG GM AUC0-24h and GM Ctrough levels depending on the NRTI backbone. We used a 1-way analysis of variance (ANOVA) test to compare DTG PK parameters between the 3 different NRTI backbone groups, TAF/FTC, ABC/3TC, and ZDV/3TC. Additionally, we described the PK parameters by weight band.
RESULTS
Between January 2019 and March 2021, we enrolled 43 children with HIV from Uganda, Zambia, and Zimbabwe. Four children were excluded because of suspected nonadherence, samples not received, or unreliable AUC calculation (over 20% extrapolation of AUC0-24h sample due to missing samples), leaving 39 eligible PK profiles. Patient demographics of these 39 children are presented in Table 1.
Table 1.
Total Inclusions and Patient Demographics of the Children on Dolutegravir in the CHAPAS4 Pharmacokinetic Substudy
| Characteristic | All Children (N = 39) |
TAF/FTC Backbone (n = 19) |
ABC/3TC Backbone (n = 12) |
ZDV/3TC Backbone (n = 8) |
|---|---|---|---|---|
| Weight band, No. of children | ||||
| 14–19.9 kg | 9 | 4 | 3 | 2 |
| 20–24.9 kg | 10 | 5 | 2 | 3 |
| 25–34.9 kg | 8 | 4 | 3 | 1 |
| ≥35 kg | 12 | 6 | 4 | 2 |
| Sex, No. (%) | ||||
| Male | 21 (54) | 8 (42) | 10 (83) | 3 (47) |
| Female | 18 (46) | 11 (58) | 2 (17) | 5 (63) |
| Age, y, median (IQR; range) | 10.8 (7.8–13.3; 5.5–15.5) | 11.1 (7.8–13.2; 5.5–14.2) | 11.3 (7.9–14.3; 6.3–15.5) | 9.5 (6.9–13.9; 6.2–15.2) |
| Weight, kg, median (IQR; range) | 27.0 (20.0–35.8; 15.9–53.0) | 27.0 (20.0–35.2; 15.9–53.0) | 29.3 (19.6–37.5; 16.7–47.2) | 22.7 (19.3–34.6; 17.5–51.7) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; FTC, emtricitabine; IQR, interquartile range; TAF, tenofovir alafenamide fumarate; ZDV, zidovudine.
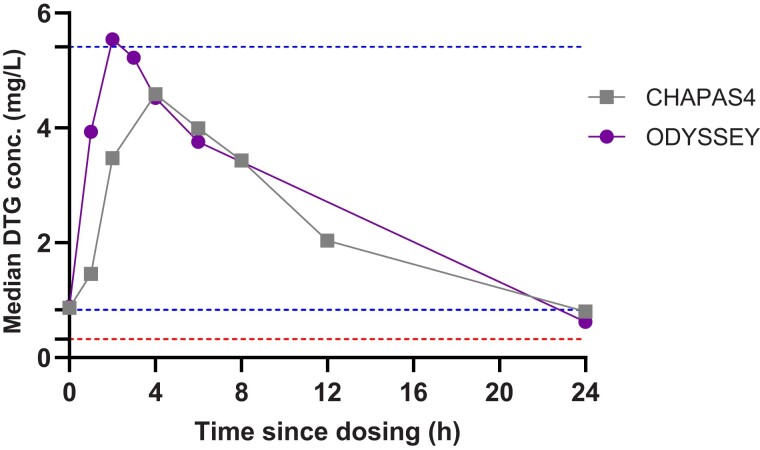
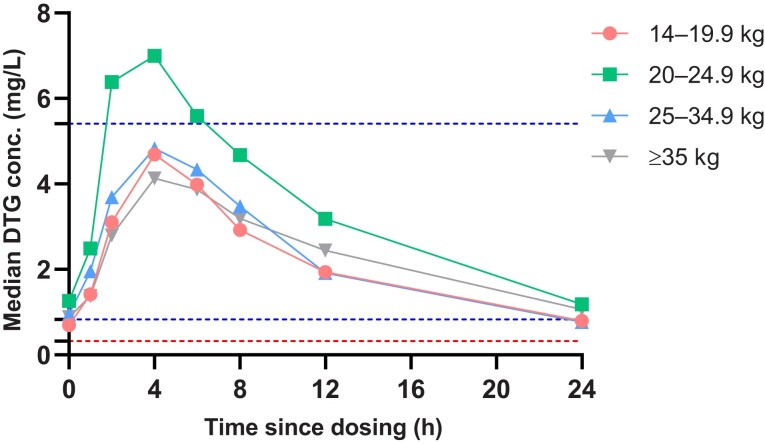
The descriptive statistics of the PK parameters of DTG for all children are summarized in Table 2. The GM (CV%) AUC0-24h was 57.1 (38.4%), approximately 8% below the AUC0-24h in children in the ODYSSEY trial with comparable dosages, but 32% higher than the reference of AUC0-24h in adults taking 50 mg once daily. We also compared our results in the 14 to <20 kg weight band group with data from the IMPAACT P1093 study (Supplementary Table 3) and demonstrated comparable GM AUC0-24h (GM: 50.4 hours × mg/L in our study vs 49.0 hours × mg/L in IMPAACT P1093) [5]. The GM Ctrough was 0.82 mg/L (63.8%), which was within ±5% of data observed in the ODYSSEY trial and in adults on 50 mg once daily. The observed GM Cmax was 4.97 mg/L (42.4%), which was 17% lower than in the ODYSSEY trial, but 49% higher than was reported in adults on 50 mg once daily. However, this value did not exceed the reference Cmax of 5.41 mg/L reported in adults on 50 mg twice daily. The median Tmax in children in CHAPAS4 was 2 hours delayed versus the median Tmax observed in children in ODYSSEY and adult reference studies where DTG was taken on an empty stomach. Median DTG plasma concentrations versus time profiles for the total group are shown in Figure 1 and compared with those reported in the ODYSSEY study. Median CHAPAS4 DTG plasma concentrations versus time profiles by weight bands are shown in Figure 2. PK parameters for each weight band are shown in SupplementaryTable 1. The exposure of DTG in the 20–24.9 kg weight band is higher compared to the other weight bands, similar to the results in ODYSSEY. PK parameters of DTG by formulation (DT vs film-coated tablet) in this study are shown in SupplementaryTable 2. No relevant formulation effects on the exposure of DTG were observed.
Table 2.
Summary of Dolutegravir Pharmacokinetic Parameters in Children Within CHAPASA4 and Reference Studies
| Parameter | Total | TAF/FTC Backbone | ABC/3TC Backbone | ZDV/3TC Backbone | ODYSSEY [4, 7] | Adults FCT 50 mg QD [11] |
Adults FCT 50 mg BD [12, 13] |
|---|---|---|---|---|---|---|---|
| AUC0-24h (h × mg/L) | 57.1 (38.4) | 49.3 (42.4) | 61.5 (30.1) | 72.2 (23.7) | 61.8 (29.7) | 43.4 (20) | 93.4 (50) |
| Ctrough (mg/L) | 0.82 (63.8) | 0.70 (76.4) | 0.84 (47.6) | 1.13 (42.7) | 0.8 (51.1) | 0.83 (26) | 2.72 (70) |
| Cmax (mg/L) | 4.97 (42.4) | 4.33 (48.1) | 5.43 (25.1) | 6.06 (41.3) | 6.0 (26.7) | 3.34 (16) | 5.41 (50) |
| Tmax (h) | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) | 4.0 (2.5–5.5) | 2.0 (2.0–3.0) | 2.0 (1.0–4.0) | 2.0 (0–7.9) |
| T1/2 (h) | 8.05 (26.9) | 8.07 (32.8) | 7.80 (20.0) | 8.40 (22.7) | 7.8 (22.9) | 12.0 (22) | … |
| CL/F (L/h) | 0.75 (45.0) | 0.88 (47.4) | 0.68 (32.2) | 0.58 (43.3) | … | … | … |
| Vd/F (L) | 8.67 (51.4) | 10.2 (57.0) | 7.70 (31.4) | 7.06 (54.1) | … | … | … |
Data are presented as geometric mean (coefficient of variation), except for Tmax, which is presented as median (interquartile range).
Abbreviations: 3TC, lamivudine; ABC, abacavir; AUC0-24h, area under the concentration–time curve over the dosing interval; BD, twice daily; CL/F, apparent oral clearance; Cmax, maximum plasma concentration; Ctrough, trough concentration; FCT, film-coated tablet; FTC, emtricitabine; IQR, interquartile range; ODYSSEY, Once-daily DTG based ART in Young people vS. Standard thErapY; QD, once daily; T1/2, elimination half-life; TAF, tenofovir alafenamide fumarate; Tmax, time to reach the maximum plasma concentration; Vd/F, apparent volume of distribution; ZDV, zidovudine.
Figure 1.
Median dolutegravir (DTG) plasma concentrations versus time profiles of the Children with HIV in Africa – Pharmacokinetics and Acceptability of Simple second-line antiretroviral regimens (CHAPAS4) pharmacokinetic substudy compared to Once-daily DTG based ART in Young people vS. Standard thErapY (ODYSSEY) outcomes. The lowest horizontal dotted line indicates DTG in vivo 90% effective concentration. The center horizontal dotted line indicates the geometric mean (GM) adult reference trough level for 50 mg given once daily, whereas the upper horizontal dotted line indicates the GM adult reference maximum level for 50 mg given twice daily.
Figure 2.
Median dolutegravir (DTG) plasma concentrations versus time profiles of Children with HIV in Africa – Pharmacokinetics and Acceptability of Simple second-line antiretroviral regimens (CHAPAS4) pharmacokinetic substudy by weight band. The lowest horizontal dotted line indicates dolutegravir in vivo 90% effective concentration. The center horizontal dotted line indicates the geometric mean (GM) adult reference trough level for 50 mg given once daily, whereas the upper horizontal dotted line indicates the GM adult reference maximum level for 50 mg given twice daily.
NRTI backbone randomization in CHAPAS4 allowed us to compare DTG PK parameters between different NRTI backbones: FTC/TAF, ABC/3TC, and ZDV/3TC. Ninety (49%) children were on TAF/FTC, 12 (31%) on ABC/3TC NRTI backbone, and 8 (20%) on ZDV/3TC. Descriptive summaries of these different NRTI backbone groups and the corresponding PK parameters are also shown in Table 1 and Table 2, respectively. No notable differences were observed between the demographics of the backbone randomization groups.
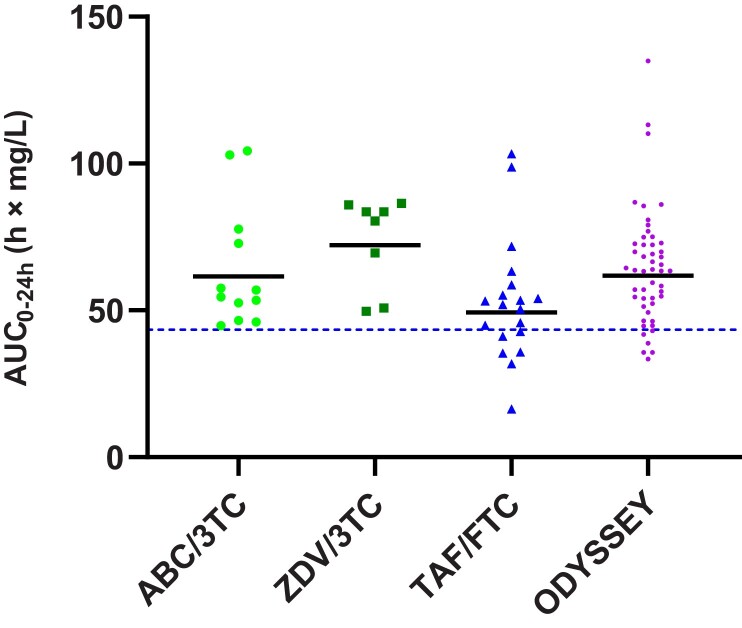
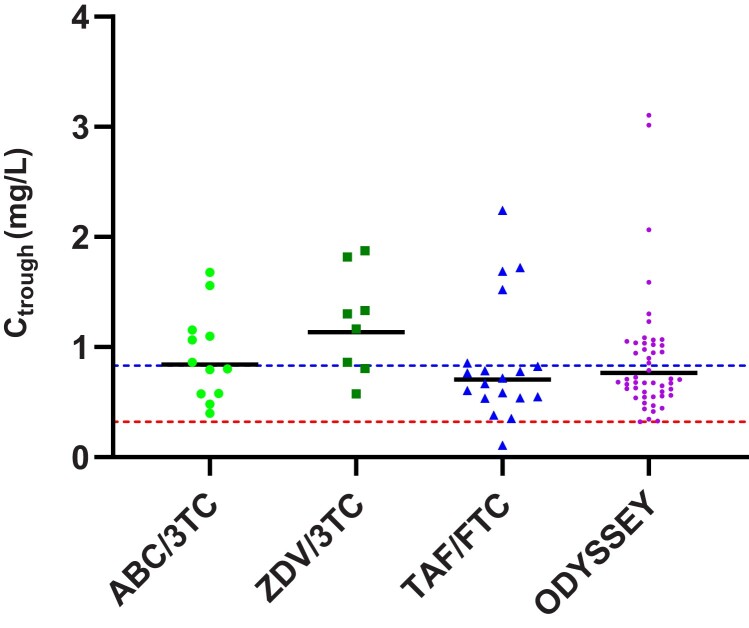
The individual AUC0-24h and Ctrough values in the different NRTI backbone groups are shown in Figures 3 and 4, respectively, with ODYSSEY values for reference. Using the 1-way ANOVA test showed a statistically significant difference between NRTI backbone groups for AUC0-24h values (P = .031) and a not statistically significant difference for Ctrough values (P = .151). There was a trend toward lower values for the TAF/FTC NRTI backbone group compared to the SOC NRTI backbone groups. The GM DTG AUC0-24h in the TAF/FTC NRTI backbone group was 49.3 hour × mg/L (42.4%), which was 20% lower than with ABC/3TC and 32% lower than with ZDV/3TC, but above the reference value of adults on once-daily 50 mg film-coated tablet. The GM DTG Ctrough in the TAF/FTC NRTI backbone group was 0.70 mg/L (76.4%), 17% lower than with ABC/3TC and 38% lower than with ZDV/3TC. Despite the 20%–40% lower values for GM AUC0-24h and GM Ctrough in the FTC/TAF NRTI backbone group versus the SOC NRTI backbones, the individual Ctrough levels of DTG in children in CHAPAS4 were comparable with children in the ODYSSEY trial, as shown in Figure 4.
Figure 3.
Individual area under the concentration–time curve over the dosing interval (AUC0-24h) values of dolutegravir with different nucleoside reverse transcriptase inhibitor backbones in CHAPAS4 in comparison with ODYSSEY data. The dotted line indicates the geometric mean adult reference AUC0-24h for 50 mg given once daily. Abbreviations: 3TC, lamivudine; ABC, abacavir; AUC0-24h, area under the concentration–time curve over the dosing interval; CHAPAS4, Children with HIV in Africa – Pharmacokinetics and Acceptability of Simple second-line antiretroviral regimens; FTC, emtricitabine; ODYSSEY, Once-daily DTG based ART in Young people vS. Standard thErapY; TAF, tenofovir alafenamide fumarate; ZDV, zidovudine.
Figure 4.
Individual trough levels of dolutegravir (DTG) concentrations with different nucleoside reverse transcriptase inhibitor backbones in CHAPAS4 in comparison with ODYSSEY data. The lowest horizontal dotted line indicates DTG in vivo 90% effective concentration. The upper horizontal dotted line indicates the geometric mean adult reference trough level for 50 mg given once daily. Abbreviations: 3TC, lamivudine; ABC, abacavir; Ctrough, trough concentration; CHAPAS4, Children with HIV in Africa – Pharmacokinetics and Acceptability of Simple second-line antiretroviral regimens; FTC, emtricitabine; ODYSSEY, Once-daily DTG based ART in Young people vS. Standard thErapY; TAF, tenofovir alafenamide fumarate; ZDV, zidovudine.
Figure 4 also shows that 1 child on DTG in this CHAPAS4 PK substudy, who was part of the FTC/TAF backbone group, did not meet the Ctrough target of 0.32 mg/L. This child had a Ctrough of 0.11 mg/L, was 13 years old, weighed 35 kg, and was on the 50-mg (adult) DTG dose.
DISCUSSION
To our knowledge, this CHAPAS4 PK substudy is the first study to show appropriate drug exposures in children taking once-daily DTG with food as second-line treatment. We compare our PK results with the pediatric ODYSSEY and IMPAACT P1093 trials and published reference values from adult studies where DTG is generally taken without food [4, 5, 11–13]. The main PK parameter to compare these PK results was the AUC0-24h, because this PK parameter considers the entire PK curve, including the absorption and the elimination phase. The efficacy of DTG, based on Ctrough levels, has been determined in previous studies.
Our results showed that children on second-line treatment taking DTG with food and dosed according to the WHO weight bands reached slightly lower drug exposures to children with similar dosages in the ODYSSEY trial, but above the adult reference for 50 mg once daily and therefore considered to be appropriate. The overall higher pediatric DTG AUC0-24h in children versus adults is not expected to affect toxicity as it is lower than observed in adults taking twice-daily 50-mg film-coated tablet, the highest licensed daily dose for DTG [11–13]. The GM Cmax in the ODYSSEY trial exceeded the reference parameters in adults on twice-daily DTG, but the safety data in the randomized ODYSSEY trial were reassuring. Moreover, the higher exposure by means of higher AUC0-24h and Cmax was in line with our expectation, because of the relatively higher milligram per kilogram body weight dosing in children compared to the fixed-dose 50-mg dosage in adults. DTG clinical studies showed that the Ctrough concentrations of DTG correlates with reduction in HIV-1 viral load, and therefore we also compared this parameter with reference studies and the in vivo EC90 target of 0.32 mg/L [11]. Our results showed similar GM Ctrough levels as seen to be effective in the ODYSSEY trial, which are on average lower than in adults due to relatively higher clearance per kilogram of body weight. Importantly, 95% of the children in our study had a Ctrough value above the minimum target of 0.32 mg/L. If we divide our group children into weight bands, we see the same trend as was seen in the ODYSSEY trial, with higher exposure in the 20–24.9 kg weight band. This can be explained by the relatively higher milligram per kilogram dose, as this is the first weight band to receive the 50-mg adult dose.
Because CHAPAS4 is still an ongoing study and the viral load and safety data are not yet available, comparison of PK results is limited to the ODYSSEY trial and other available pediatric and adult data for clinical interpretation. With our results, we expect that we can extrapolate the efficacy and safety results from the ODYSSEY trial with children on first- and second-line treatment to the children taking DTG only as second-line treatment, as were included in CHAPAS4. Efficacy, safety, and tolerability data for the main study will be reported separately.
The median DTG plasma time curve of both trials is comparable, except for the later Tmax in the CHAPAS4 study relative to ODYSSEY, possibly explained by the food effect. However, contrary to what we expected, the influence of food on DTG AUC0-24h appears to be limited. This differs from adult studies, where a 30%–60% higher AUC has been observed when DTG is taken with food, depending on the amount of fat in the meal [8]. Our data suggest that the influence of food on DTG exposure in children might be less pronounced than in adults, suggesting that DTG can be taken safely with or without food by children. However, it must be noted that here we used a cross-study comparison of PK data from different children participating in 2 clinical trials whereas in adults a well-controlled intrasubject comparison of the food effect was performed. In addition, in CHAPAS4 the children only received a low-fat (5%) standardized breakfast before dosing, and we have no PK data of children taking the DTG tablets with a higher fat percentage meal.
Comparison of the DTG concentrations of the children in the PK substudy receiving a TAF-containing backbone, compared to those receiving SOC NRTI backbones, demonstrated a trend of 20%–40% lower GM AUC0-24h and GM Ctrough of DTG in children on FTC/TAF (Table 2 and Figures 3 and 4). Moreover, we see similar trough levels for the FTC/TAF backbone group versus ODYSSEY if we compare individual DTG Ctrough levels, suggesting that the clinical relevance of the lower exposure relative to the SOC backbones is likely to be negligible. The GM AUC0-24h in the FTC/TAF NRTI backbone group is also above the adult reference for 50 mg DTG once daily. The PK mechanism that may account for the lower exposure of DTG in combination with an FTC/TAF NRTI backbone in children remains unknown. Our observations are in contrast with results seen in adults, where a slightly higher exposure of DTG in combination with TAF has been reported [14]. One child in our PK substudy did not meet the Ctrough target and was taking DTG in combination with FTC/TAF, but as a limitation of this study, we were unable to relate this lower exposure to DTG to virological efficacy as the CHAPAS4 trial is still ongoing at the time of writing this article.
In conclusion, this nested PK substudy within the CHAPAS4 trial shows that the exposure of DTG taken with food in children on second-line treatment is comparable with the DTG exposure in children and adults taking DTG when fasting. This helps further support the recommendation that there are clinically no restrictions around administering DTG with or without food in children and will therefore not change current practice. The main efficacy and safety results of the CHAPAS4 trial of >900 children followed for 96 weeks will be available later in 2023.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Lisanne A H Bevers, Department of Pharmacy, Research Institute for Medical Innovation, Radboud University Medical Center, Nijmegen, The Netherlands.
Hylke Waalewijn, Department of Pharmacy, Research Institute for Medical Innovation, Radboud University Medical Center, Nijmegen, The Netherlands; Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, South Africa.
Alexander J Szubert, Medical Research Council Clinical Trials Unit, University College London, London, United Kingdom.
Chishala Chabala, Children’s Hospital, University Teaching Hospital, Lusaka, Zambia.
Mutsa Bwakura-Dangarembizi, University of Zimbabwe Clinical Research Centre, Harare.
Shafic Makumbi, Joint Clinical Research Centre, Mbarara Regional Centre of Excellence, Mbarara, Uganda.
Joan Nangiya, Joint Clinical Research Centre, Research Department, Kampala, Uganda.
Vivian Mumbiro, University of Zimbabwe Clinical Research Centre, Harare.
Veronica Mulenga, Children’s Hospital, University Teaching Hospital, Lusaka, Zambia.
Victor Musiime, Joint Clinical Research Centre, Research Department, Kampala, Uganda.
David M Burger, Department of Pharmacy, Research Institute for Medical Innovation, Radboud University Medical Center, Nijmegen, The Netherlands.
Diana M Gibb, Medical Research Council Clinical Trials Unit, University College London, London, United Kingdom.
Angela Colbers, Department of Pharmacy, Research Institute for Medical Innovation, Radboud University Medical Center, Nijmegen, The Netherlands.
Notes
Author contributions. L. B., A. C., and D. B. conceived the study and drafted the manuscript. All authors made crucial revisions to and approved the final manuscript.
Acknowledgments . The authors thank the participants of the CHAPAS4 trial and their families, the principal investigators and their staff at all the centers participating in the CHAPAS4 trial, and the technicians of the Department of Pharmacy of Radboudumc.
Financial support . The CHAPAS4 trial is sponsored by University College London (UCL), with central management by the Medical Research Council (MRC) Clinical Trials Unit at UCL, supported by MRC core funding (MC_UU_00004/03). The main funding for this study is provided by the European and Developing Countries Clinical Trials Partnership (EDCTP; TRIA2015-1078). Additional funding is received from ViiV Healthcare, Janssen Pharmaceuticals, and Gilead Sciences, Inc.
References
- 1. Joint United Nations Programme on HIV/AIDS . Young people and HIV.2021. Available at: https://www.unaids.org/sites/default/files/media_asset/young-people-and-hiv_en.pdf. Accessed 20 January 2023.
- 2. Joint United Nations Programme on HIV/AIDS . Fact sheet 2022.2022. Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed 20 January 2023.
- 3. World Health Organization (WHO) . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring. Geneva, Switzerland: WHO, 2021.
- 4. Bollen PDJ, Moore CL, Mujuru HA, et al. . Simplified dolutegravir dosing for children with HIV weighing 20 kg or more: pharmacokinetic and safety substudies of the multicentre, randomised ODYSSEY trial. Lancet HIV 2020; 7:e533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruel TD, Acosta EP, Liu JP, et al. . Pharmacokinetics, safety, tolerability, and antiviral activity of dolutegravir dispersible tablets in infants and children with HIV-1 (IMPAACT P1093): results of an open-label, phase 1–2 trial. Lancet HIV 2022; 9:e332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turkova A, Waalewijn H, Chan MK, et al. . Dolutegravir twice-daily dosing in children with HIV-associated tuberculosis: a pharmacokinetic and safety study within the open-label, multicentre, randomised, non-inferiority ODYSSEY trial. Lancet HIV 2022; 9:e627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waalewijn H, Chan MK, Bollen PDJ, et al. . Dolutegravir dosing for children with HIV weighing less than 20 kg: pharmacokinetic and safety substudies nested in the open-label, multicentre, randomised, non-inferiority ODYSSEY trial. Lancet HIV 2022; 9:e341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song I, Borland J, Chen S, et al. . Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob Agents Chemother 2012; 56:1627–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howie SR. Blood sample volumes in child health research: review of safe limits. Bull World Health Organ 2011; 89:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bollen P, Freriksen J, Konopnicki D, et al. . The effect of pregnancy on the pharmacokinetics of total and unbound dolutegravir and its main metabolite in women living with human immunodeficiency virus. Clin Infect Dis 2021;72:121–7. [DOI] [PubMed] [Google Scholar]
- 11. Min S, Sloan L, DeJesus E, et al. . Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS 2011; 25:1737–45. [DOI] [PubMed] [Google Scholar]
- 12. Eron JJ, Clotet B, Durant J, et al. . Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING study. J Infect Dis 2013; 207:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. ViiV Healthcare . A pilot study assessing the integrase inhibitor GSK1349572 in HIV-infected persons with virus resistant to raltegravir.2013. Available at: https://clinicaltrials.gov/ct2/show/results/NCT00950859?term=dolutegravir&recrs=e&cond=Hiv&outc=AUC&age=1&draw=4&rank=30. Accessed 20 October 2022.
- 14. US Food and Drug Administration . Clinical pharmacology review addendum: F/TAF or FTC/TAF.2015. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208215Orig1s000ClinPharmR.pdf. Accessed 22 December 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.