Key Points
Question
Can aspirin be safely avoided as part of an antithrombotic regimen including a vitamin K antagonist with a fully magnetically levitated left ventricular assist device (LVAD), and can such an approach reduce residual risk of nonsurgical bleeding complications?
Findings
In the ARIES-HM3 randomized trial of aspirin (100 mg/d) or placebo, avoidance of aspirin as part of an antithrombotic regimen that included a vitamin K antagonist in patients supported with an LVAD resulted in a significant decrease (34%) in major nonsurgical bleeding events and no significant increase in thromboembolic risk. The benefits of avoiding aspirin are consistent among those with prior vascular disease, including surgical or percutaneous coronary revascularization, obesity, or diabetes, which are characteristics associated with increased thrombosis risk.
Meaning
Aspirin is not required to maintain outcomes with a fully magnetically levitated LVAD in advanced heart failure, and exclusion from antithrombotic therapy is safe and associated with a reduction in bleeding events.
Abstract
IMPORTANCE
Left ventricular assist devices (LVADs) enhance quality and duration of life in advanced heart failure. The burden of nonsurgical bleeding events is a leading morbidity. Aspirin as an antiplatelet agent is mandated along with vitamin K antagonists (VKAs) with continuous-flow LVADs without conclusive evidence of efficacy and safety.
OBJECTIVE
To determine whether excluding aspirin as part of the antithrombotic regimen with a fully magnetically levitated LVAD is safe and decreases bleeding.
DESIGN, SETTING, and PARTICIPANTS
This international, randomized, double-blind, placebo-controlled study of aspirin (100 mg/d) vs placebo with VKA therapy in patients with advanced heart failure with an LVAD was conducted across 51 centers with expertise in treating patients with advanced heart failure across 9 countries. The randomized population included 628 patients with advanced heart failure implanted with a fully magnetically levitated LVAD (314 in the placebo group and 314 in the aspirin group), of whom 296 patients in the placebo group and 293 in the aspirin group were in the primary analysis population, which informed the primary end point analysis. The study enrolled patients from July 2020 to September 2022; median follow-up was 14 months.
Intervention
Patients were randomized in a 1:1 ratio to receive aspirin (100 mg/d) or placebo in addition to an antithrombotic regimen.
MAIN OUTCOMES AND MEASURES
The composite primary end point, assessed for noninferiority (−10% margin) of placebo, was survival free of a major nonsurgical (>14 days after implant) hemocompatibility-related adverse events (including stroke, pump thrombosis, major bleeding, or arterial peripheral thromboembolism) at 12 months. The principal secondary end point was nonsurgical bleeding events.
RESULTS
Of the 589 analyzed patients, 77% were men; one-third were Black and 61% were White. More patients were alive and free of hemocompatibility events at 12 months in the placebo group (74%) vs those taking aspirin (68%). Noninferiority of placebo was demonstrated (absolute between-group difference, 6.0% improvement in event-free survival with placebo [lower 1-sided 97.5% CI, −1.6%]; P < .001). Aspirin avoidance was associated with reduced nonsurgical bleeding events (relative risk, 0.66 [95% confidence limit, 0.51-0.85]; P = .002) with no increase in stroke or other thromboembolic events, a finding consistent among diverse subgroups of patient characteristics.
CONCLUSIONS AND RELEVANCE
In patients with advanced heart failure treated with a fully magnetically levitated LVAD, avoidance of aspirin as part of an antithrombotic regimen, which includes VKA, is not inferior to a regimen containing aspirin, does not increase thromboembolism risk, and is associated with a reduction in bleeding events.
TRIAL REGISTRATION
ClinicalTrials.gov Identifier: NCT04069156
This randomized clinical trial examines the safety of the exclusion of aspirin as part of an antithrombotic regimen including a vitamin K antagonist with a fully magnetically levitated left ventricular assist device.
Introduction
Left ventricular assist devices (LVADs) enhance quality and duration of life in patients with advanced heart failure, irrespective of eligibility for cardiac transplant.1 A fully magnetically levitated frictionless pump, engineered with wide blood flow pathways and a pulse in the pump due to fixed rotor speed changes, has demonstrated improved long-term survival by decreasing pump thrombosis and disabling strokes, but only modestly influenced bleeding complications.2,3 Mucosal surface bleeding, particularly in the gastrointestinal system, remains burdensome as a leading cause of hospitalization and health care resource use and has been increasing in frequency since the introduction of continuous-flow LVADs.4,5 Such frequent bleeding complications, which often recur, are attributable to high shear stress and low pulse pressure due to continuous flow, which promotes development of an acquired von Willebrand disease and arteriovenous malformations.6,7,8
Patients receiving LVAD support are treated with an antithrombotic regimen that includes aspirin and vitamin K antagonist (VKA) therapy, mandated on observations that platelet activation and inflammation predispose to device-related thromboembolic complications.9 The role of aspirin as part of the antithrombotic regimen with VKA in patients using LVADs is controversial and has not been adequately studied, and observational studies have found disparate results among different LVAD types. Clinical experience with the HeartWare Ventricular Assist Device (Medtronic), which has now been removed from distribution due to a high incidence of stroke and pump malfunction since 2021, suggested that 325 mg of daily aspirin was necessary to prevent pump malfunction.10 Exposure to lower-dose aspirin at 81 mg daily was associated with increased pump thrombosis and stroke with this older-generation LVAD.10,11 In contradistinction, an analysis with the fully magnetically levitated HeartMate 3 (HM3) LVAD, which is now the only widely approved and available LVAD, has suggested that low-dose aspirin is associated with similar hemocompatibility-related outcomes as with higher doses.12 Other observations of aspirin withdrawal after a bleeding event have shown a reduction in subsequent bleeds while preserving thrombosis-related outcomes with the HM3 LVAD.13,14 Thus, in an effort to address the ongoing burden of bleeding with LVADs, the hypothesis has been extended that aspirin may be avoided when VKAs are used for anticoagulation, particularly in the presence of a more hemocompatible fully magnetically levitated LVAD. Such an approach may also reduce the residual risk of nonsurgical bleeding complications. To evaluate the role of aspirin and its clinical impact, the Antiplatelet Removal and Hemocompatibility Events With the HeartMate 3 Pump (ARIES-HM3) trial was designed as a multinational, randomized, double-blind, placebo-controlled trial in patients with advanced heart failure with the HM3 LVAD.15
Methods
Study Design
We conducted a study comparing placebo or aspirin along with VKA therapy (target international normalized ratio of 2.0-3.0) in patients with advanced heart failure implanted with the HM3 LVAD. The trial rationale and design have been previously published.15 Patients were eligible for randomization provided they did not require additional postimplant mechanical circulatory support other than the HM3 LVAD. Eligibility criteria are provided in the Supplement 1.
Study enrollment occurred at 51 sites in North America (US, Canada), Europe (Czech Republic, Austria, Italy, France, UK), Kazakhstan, and Australia (Supplement 1). All patients provided written informed consent and approval by the institutional review board or ethics committees at individual centers, and country-specific regulatory authority approval was obtained as required. The steering committee provided trial oversight and vouched for the completeness and accuracy of the data and analyses and for fidelity of this report to the protocol. An independent statistician reviewed the data and verified the primary analyses. A clinical events committee adjudicated the causes of death and all primary end point adverse events, while an independent data and safety monitoring board ensured patient safety throughout the study.
Randomization and Blinding
Randomization to the placebo or aspirin groups occurred between postimplant days 2 and 7 in a 1:1 ratio in blocks of 4 stratified by study center. Randomization, medication requisition, and inventory management were implemented through a third-party vendor (WebEZ, Almac Group). The investigators, patients, and study sponsor were blinded to treatment assignment throughout the study.
Data Collection
Study data were collected at baseline; time of implant; time of randomization; week 1; discharge; and 1, 3, 6, 9, and 12 months for all patients. Race and ethnicity were self-reported. In patients continuing to receive the treatment group medication (aspirin or placebo) at 12 months, follow-up continued until study closure.
Primary End Point
The primary end point was a composite of survival free of nonsurgical major hemocompatibility-related adverse events (specifically stroke, pump thrombosis, major nonsurgical bleeding, and arterial peripheral thromboembolism) at 12 months, analyzed in the primary analysis population, which excluded patients with events occurring within 14 days after LVAD implantation (to avoid counting surgical complications not attributed to the treatment). Major nonsurgical bleeding included both moderate and severe bleeding events occurring more than 14 days after implantation. Moderate and severe bleeding events included those that occurred while the patient was hospitalized or led to hospitalization or urgent care and required a medical intervention. Details on classification as moderate or severe bleeding are available in Supplement 2. A sensitivity analysis to include all randomized patients was prespecified. Generalizability of the primary end point was assessed among prespecified subgroups including age, sex, race, region, surgical method, and disease severity, along with additional post hoc subgroups defined by etiology of heart failure, obesity, history of diabetes, coronary stenting, prior thrombotic history and stroke, bleeding history, atrial fibrillation, and prior coronary artery bypass surgery. Individual primary end point components, including nonsurgical bleeding, stroke, pump thrombosis, and arterial peripheral thromboembolism, were also analyzed. Definitions are provided in Supplement 2.
Principal Secondary End Point
The principal secondary end point was a comparison of nonsurgical bleeding rates in the primary analysis population assessed as first and recurrent events and described in events per 100 patient-years.
Safety End Points
To assess the safety of excluding antiplatelet therapy from the antithrombotic regimen, survival, stroke, and pump thrombosis rates were compared between the 2 study groups.
Descriptive End Points
We assessed the hemocompatibility score (an aggregate score that accounts for the occurrence of thromboembolic and bleeding events weighted based on clinical severity of events) between the groups by examining the proportion of patients with a score of 0 (no such events of even a mild nature) or greater.3 Postindex discharge hospitalizations were analyzed as event rates by reason for hospitalization and the number of hospital days averted was calculated. The cost of care was determined by comparing hospitalizations due to bleeding in US-based patients within the first year after implant, calculated as cost per hospitalization day using a previously published economic model analyzed in the US federal payer context (adjusted for inflation using the medical consumer price index from the US Bureau of Labor Statistics).16 Patient quality of life was measured using the EuroQol 5-Dimension 5-Level Questionnaire visual analog scale.
Medication Adherence, Aspirin Effect, and VKA Use
Adherence was determined by pill counts in returned bottles. An independent laboratory (Corgenix) measured serum thromboxane B2 (decreased levels indicate aspirin response) in all patients enrolled from the US in a blinded manner.17 VKA therapy was monitored in all patients using international normalized ratio measurements by calculating the time in therapeutic range through 12 months (defined as the number of person-days within a predetermined therapeutic range divided by the total number of person-days receiving VKA therapy).18
Sample Size Assessment
To demonstrate noninferiority of the placebo antithrombotic regimen with the Farrington-Manning test using a noninferiority margin of −10% for the difference of proportions at a 1-sided α of .025 with 80% power, 440 analyzable patients were required at 12 months in the primary analysis population, assuming a 71% primary end point rate in the aspirin group. The noninferiority margin of −10% for the 97.5% confidence limit (CL) was established based on the clinical interpretation of an assumed success rate in the aspirin group and a −1% minimum acceptable point estimate for the difference of proportions (if the placebo group had a success rate lower than the aspirin group) balanced by sample size and enrollment rate considerations. To account for postrandomization surgical outcomes (death or transplant within 14 days of implant) and clinical events impacting the administration of the treatment group medication, an additional 190 patients were randomized. The final sample size was 628 in the randomized population and 589 in the primary analysis population, which provided more than 90% power to assess the primary end point.
Statistical Analysis
Categorical variables are presented as number and percent, whereas continuous variables are presented as median (IQR), with between-group comparisons evaluated using Fisher exact test and Wilcoxon rank sum test, respectively. The primary end point assessing noninferiority of placebo with aspirin was considered to be met if the lower boundary of the 1-sided 97.5% CL of the difference in the composite success rate between treatment groups (placebo minus aspirin) was greater than the noninferiority margin (−10%) by the Farrington-Manning test at 12 months in the primary analysis population. Patients who withdrew from the study without a prior primary end point event were not included in the primary end point set. Transplant, explant due to recovery, or transition to open-label aspirin use (based on investigator preference) without nonsurgical major hemocompatibility-related adverse events was considered treatment success. Sensitivity analyses of the primary end point included the entire randomized population; worst-case scenario analysis, which categorized patients who withdrew without a primary end point event as treatment success in the aspirin group and treatment failure in the placebo group; and analysis of the impact of transition to open-label aspirin use in the primary analysis population to include posttransition events. Adjustment for randomization stratified by study center was completed using a generalized linear model with the inclusion of treatment group and study center as covariates in the model. Interaction testing of primary end point subgroups was completed by generalized linear modeling with terms for treatment group, subgroup, and their interaction. To evaluate events over longer follow-up duration, the primary end point and major adverse events were analyzed as time to first event by the Kaplan-Meier method comparing treatment groups at 24 months. Major adverse events were compared as rate of events per 100 patient-years to account for recurrent events in the primary analysis population and tested using Poisson regression. Cox proportional hazard modeling was performed with group as a single effect, shown in Kaplan-Meier analyses, and combined with clinically important baseline characteristics of age, diabetes, sex, race, severity of illness, and destination therapy. All P values are 2-tailed, with values less than .05 considered to be statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Study Patients
From July 2020 to September 2022, a total of 628 patients were randomized (314 patients per group; 29% Black; 22% women) (Figure 1; eFigure 1 in Supplement 2). Baseline characteristics were similar in the 2 treatment groups, except for more patients with diabetes in the placebo group vs the aspirin group (45% vs 36%) (Table 1).
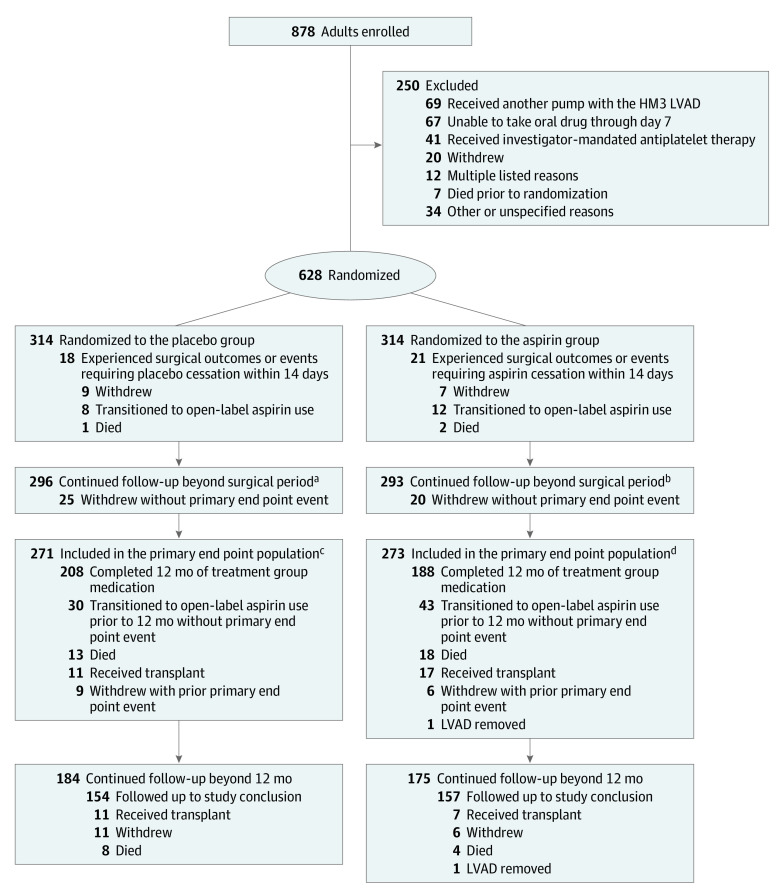
Figure 1. Flow of Patients in the Randomized Trial of Placebo Compared to Aspirin With a Vitamin K Antagonist in Patients With a Fully Magnetically Levitated Left Ventricular Assist Device.
Patients were followed up until 12 months for the evaluation of the primary end point in the primary end point set. Patients who continued receiving the treatment group medication (placebo or aspirin) at 12 months were eligible for continued follow-up beyond 12 months. Reasons for patient withdrawal are shown in eTable 1 in Supplement 2. Details on patient transition to open-label aspirin use can be found in eTables 2 and 3 in Supplement 2.
aMedian (IQR) of 14.2 (9.0-21.4) months.
bMedian (IQR) of 14.3 (7.2-20.6) months.
cConcluded follow-up at 12 months (n = 53).
dConcluded follow-up at 12 months (n = 55).
Table 1. Baseline Characteristics in the Primary Analysis Population.
| Characteristic | No. (%) | |
|---|---|---|
| Placebo (n = 296) | Aspirin (n = 293) | |
| Demographics | ||
| Age, y | ||
| Mean (SD) | 58 (13) | 57 (14) |
| Median (range) | 60 (20-79) | 59 (18-80) |
| Women | 72 (24) | 61 (21) |
| Men | 224 (76) | 232 (79) |
| Race and ethnicitya | ||
| Asian | 13 (4.4) | 9 (3.1) |
| Black | 93 (31.4) | 84 (28.7) |
| Native Hawaiian or Pacific Islander | 2 (0.7) | 3 (1.0) |
| White | 179 (60.5) | 181 (61.8) |
| Otherb | 3 (1.0) | 3 (1.0) |
| Did not provide or unspecified | 6 (2.0) | 13 (4.4) |
| Enrolled in North Americac | 251 (85) | 248 (85) |
| Medical history | ||
| Ischemic etiology of heart failure | 106 (35.8) | 101 (34.5) |
| History of atrial fibrillation | 137 (46.3) | 122 (41.6) |
| History of stroke | 44 (14.9) | 35 (11.9) |
| History of bleeding | 17 (5.7) | 12 (4.1) |
| History of diabetes | 134 (45.3) | 106 (36.2) |
| History of percutaneous coronary interventiond | 78 (26.4) | 72 (24.6) |
| Prior cardiac surgical procedures | ||
| Coronary artery bypass | 35 (11.8) | 32 (10.9) |
| Valve replacement or repair | 25 (8.4) | 25 (8.5) |
| Hemodynamics e | ||
| Left ventricular ejection fraction, % | 16 (12-21) [n = 252] | 17 (12-20) [n = 252] |
| Arterial blood pressure, mm Hg | ||
| Systolic | 106 (96-118) [n = 294] | 106 (95-117) [n = 291] |
| Diastolic | 69 (60-75) [n = 294] | 68 (61-75) [n = 291] |
| Mean arterial pressure, mm Hgf | 81 (74-88) [n = 281] | 80 (74-89) [n = 282] |
| Right atrial pressure, mm Hg | 10.0 (6.0-14.0) [n = 203] | 11.0 (6.0-15.0) [n = 200] |
| Pulmonary vascular resistance, Wood units | 3.03 (2.00-4.10) [n = 201] | 2.90 (1.90-4.40) [n = 187] |
| Cardiac index, L/min/m2 | 1.80 (1.52-2.28) [n = 231] | 1.82 (1.58-2.30) [n = 221] |
| Pulmonary-capillary wedge pressure, mm Hg | 25 (17-30) [n = 225] | 25 (19-30) [n = 216] |
| Laboratory values e , g | ||
| Hemoglobin, g/dL | 11.6 (10.1-13.1) | 11.7 (10.3-13.1) |
| Hematocrit, % | 36 (31-40) | 36 (32-40) |
| Platelets, ×103/μL | 200 (158-255) | 198 (150-253) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 60 (43-76) [n = 295] | 60 (44-75) [n = 291] |
| Total bilirubin, mg/dL | 0.90 (0.60-1.40) | 0.90 (0.60-1.40) [n = 290] |
| Baseline antithrombotic medicationh | ||
| Aspirin | 40 (13.5) | 47 (16.0) |
| VKAi | 7 (2.4) | 8 (2.7) |
| Direct oral anticoagulants (apixaban or rivaroxaban) | 11 (3.7) | 11 (3.8) |
| Intended goal of pump support | ||
| Bridge to transplant | 56 (18.9) | 58 (19.8) |
| Bridge to candidacy for transplant | 46 (15.5) | 50 (17.1) |
| Destination therapyj | 180 (60.8) | 174(59.4) |
| Bridge to recovery | 14 (4.7) | 11 (3.8) |
| INTERMACS profile (disease severity)k | ||
| 1 (most severe illness) | 12 (4.1) | 18 (6.1) |
| 2 | 76 (25.7) | 75 (25.6) |
| 3 | 133 (44.9) | 133 (45.4) |
| 4 | 66 (22.3) | 54 (18.4) |
| 5 | 6 (2.0) | 8 (2.7) |
| 6 | 3 (1.0) | 5 (1.7) |
| 7 (least severe illness) | 0 | 0 |
| LVAD surgical details | ||
| Procedures concurrent with LVAD implant | ||
| Patent foramen ovale closure | 15 (5.1) | 13 (4.4) |
| Left atrial appendage exclusion | 53 (17.9) | 44 (15.0) |
| Valve replacement or repair | 74 (25.0) | 82 (28.0) |
Race or ethnicity were self-reported.
Other racial and ethnic groups included Hispanic (n = 2), Latino (n = 1), Lebanese (n = 1), and multiracial (n = 2).
North America includes US (primary analysis population enrollment: n = 491) and Canada (n = 8). The remaining patients were enrolled from the Czech Republic (n = 40), Austria (n = 16), Italy (n = 7), France (n = 2), UK (n = 3), Kazakhstan (n = 18), and Australia (n = 4).
Percutaneous coronary intervention includes stent, balloon angioplasty, and atherectomy.
In instances in which data may be missing for some subset of patients, total numbers are provided to describe the population in which the specific data are available.
Mean arterial pressure over study follow-up period is provided in eFigure 2 in Supplement 2.
Additional laboratory values are provided in eTable 4 in Supplement 2.
Baseline medications are any exposure within the preceding 30 days prior to implant, except aspirin, which was within the preceding 7 days.
Vitamin K antagonist (VKA) agents used in patients prior to left ventricular assist device (LVAD) implant include warfarin (n = 14) and fluindione (n = 1).
Destination therapy refers to those who are ineligible for cardiac transplant.
Disease severity defined by Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles, which range from 1 to 7; a profile of 1 represents the most severe illness and a profile of 7 represents the least severe illness.
Due to surgical events and outcomes, 39 patients were not included in the primary end point analysis, including 18 of 314 (5.7%) in the placebo group and 21 of 314 (6.7%) in the aspirin group. These patients were excluded due to surgical events that resulted in cessation of the treatment group medication (eg, bleeding events) or surgical outcomes, specifically death or withdrawal. Thus, the primary analysis population included 296 patients assigned to the placebo group and 293 patients assigned to the aspirin group. Follow-up was similar between the groups, with a median (IQR) follow-up of 14.2 (9.0-21.4) months in the placebo group and 14.3 (7.2-20.6) months in the aspirin group (Figure 1).
Primary End Point: Survival Free of Nonsurgical Major Hemocompatibility-Related Adverse Event
More patients reached 12 months alive and free of a nonsurgical major hemocompatibility-related adverse event in the placebo group than in the aspirin group (68.1% [186/273] vs 74.2% [201/271]; Table 2). The noninferiority criterion was met (absolute between-group difference, 6.0% [lower 1-sided 97.5% CL, −1.6%]; P < .001). The difference between the groups was driven by reduced bleeding events in the placebo group (22.5% vs 28.2%). When analyzed as a time to first event, fewer patients died or experienced a nonsurgical major hemocompatibility-related adverse event in the placebo group than the aspirin group through 24 months (36.9% vs 45.9%; hazard ratio [HR], 0.73 [95% CI, 0.55-0.97]; P = .03; Figure 2A). Sensitivity analyses of the primary end point concur with the primary analysis, including the randomized population (difference, 6.6% [lower 1-sided 97.5% CL, −1.3%]; P < .01) and the worst-case scenario (P = .02) analyses (eTable 6 in Supplement 2).
Table 2. Primary End Point Analysis of Survival Free of Nonsurgical Major Hemocompatibility-Related Adverse Events.
| Primary analysis population outcomes | No./total No. (%) | Difference (lower 97.5% confidence limit), % | P value | |
|---|---|---|---|---|
| Placebo (n = 296) | Aspirin (n = 293) | |||
| Noninferiority primary end point analysis (success)a | 201/271 (74.2) | 186/273 (68.1) | 6.0 (−1.6) | <.001 |
| Withdrawn patients without primary end point eventb | 25/296 (8.4) | 20/293 (6.8) | ||
| First event that resulted in treatment failurec | ||||
| Death | 4/271 (1.5) | 4/273 (1.5) | ||
| Bleeding | 61/271 (22.5) | 77/273 (28.2) | ||
| Stroke | 5/271 (1.8) | 6/273 (2.2) | ||
| Arterial peripheral thromboembolism | 0/271 | 0/273 | ||
| Pump thrombosis | 0/271 | 0/273 | ||
The primary end point is a composite of survival free of nonsurgical (>14 days after implant) major hemocompatibility-related adverse events (bleeding, stroke, pump thrombosis, or arterial peripheral thromboembolism) at 12 months after implant. Transplant, explant due to recovery, or transition to open-label aspirin use prior to 12 months without prior nonsurgical major hemocompatibility-related adverse events is considered a success. The rates of success in this category were 9.2% (25/271) in the placebo group vs 10.3% (28/273) in the aspirin group. Details of major surgical adverse events occurring less than or equal to 14 days after implant are provided in eTable 5 in Supplement 2.
The noninferiority end point was calculated excluding patients who were withdrawn without a primary end point event. Worst-case scenario allocation of withdrawn patients without primary end point event, specifically all patients in the placebo group considered as a treatment failure and all in the aspirin group considered a success, continued to demonstrate noninferiority (P = .02) as shown in eTable 6 in Supplement 2. Additional sensitivity analyses, specifically the evaluation of the primary end point as intent to treat and analysis of the primary analysis population as time to first event including follow-up after transition to open-label aspirin use, are also shown in eTable 6 and eFigures 3 and 4 in Supplement 2. Results of all sensitivity analyses agree with the primary end point analysis.
The event that occurred first was noted as the event resulting in treatment failure in the component analysis. Subsequent events were not accounted for in this analysis. Time-to-event analyses are shown in Figure 2 and eFigure 5 in Supplement 2. Death rates in the primary analysis population in the study were 7.1% (21/296) in the placebo group vs 7.5% (22/273) in the aspirin group (causes of death are shown in eTable 7 in Supplement 2). Death due to any cause (eFigure 7), competing outcomes (eFigure 8), and rate of bleeding events (eFigure 9) among the randomized population are shown in Supplement 2. Overall event rates, including recurrent events for bleeding, stroke, arterial peripheral thromboembolism, and pump thrombosis are depicted in Figure 3.
Figure 2. Estimates of the Probability of Death or Nonsurgical Major Hemocompatibility-Related Adverse Events and Nonsurgical Bleeding Events.
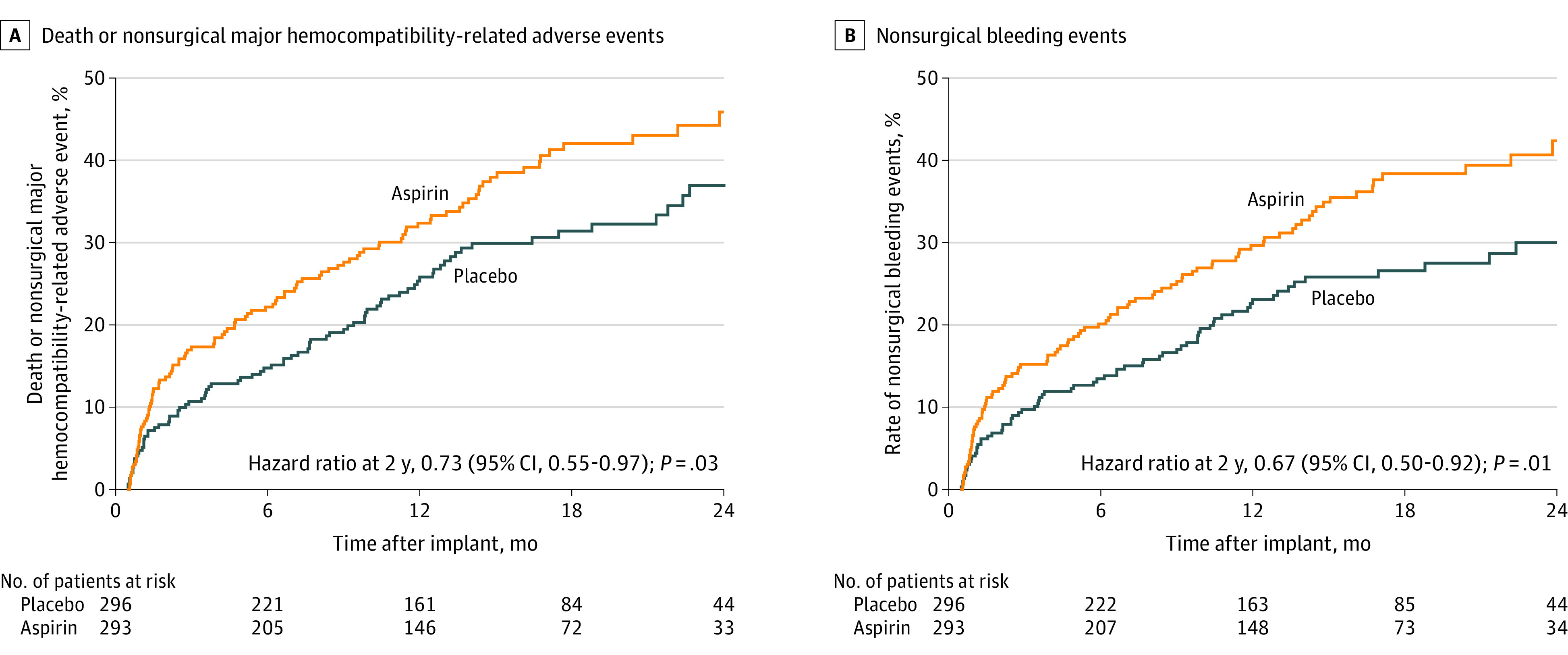
The probability of death or nonsurgical major hemocompatibility-related adverse event reflects the failure of the primary end point. In this 24-month time-to-event analysis, patients in the placebo group experienced decreased probability of primary end point failure at 12 months (25.8 for placebo vs 32.4 for aspirin) and 24 months (36.9 for placebo vs 45.9 for aspirin; log-rank P = .03). Decreased primary end point failure was driven by decreased nonsurgical bleeding at 12 months (23.1 for placebo vs 29.7 for aspirin) and at 24 months (30.0 for placebo vs 42.4 for aspirin; log-rank P = .01). Hazard ratios were calculated by Cox proportional hazard modeling with treatment group as the only effect. Adjusted hazards models are provided in eFigure 10 in Supplement 2. Median (IQR) follow-up was 14.2 (9.0-21.4) months in the placebo group and 14.3 (7.2-20.6) months in the aspirin group.
Principal Secondary End Point: Nonsurgical Bleeding Events
Fewer first nonsurgical bleeding events were seen in the placebo group vs the aspirin group at 24 months (30.0 vs 42.4; P = .01; Figure 2B). In the placebo group, there were reductions in cumulative (first and recurrent) nonsurgical bleeding events (relative risk [RR], 0.66 [95% CI, 0.51-0.85]; P = .002; Figure 3A). The difference in bleeding was for both moderate and severe bleeding events, and included gastrointestinal bleeding (RR, 0.61 [95% CI, 0.42-0.87]; P = .007).
Figure 3. Principal Secondary End Point Details and Safety End Points .
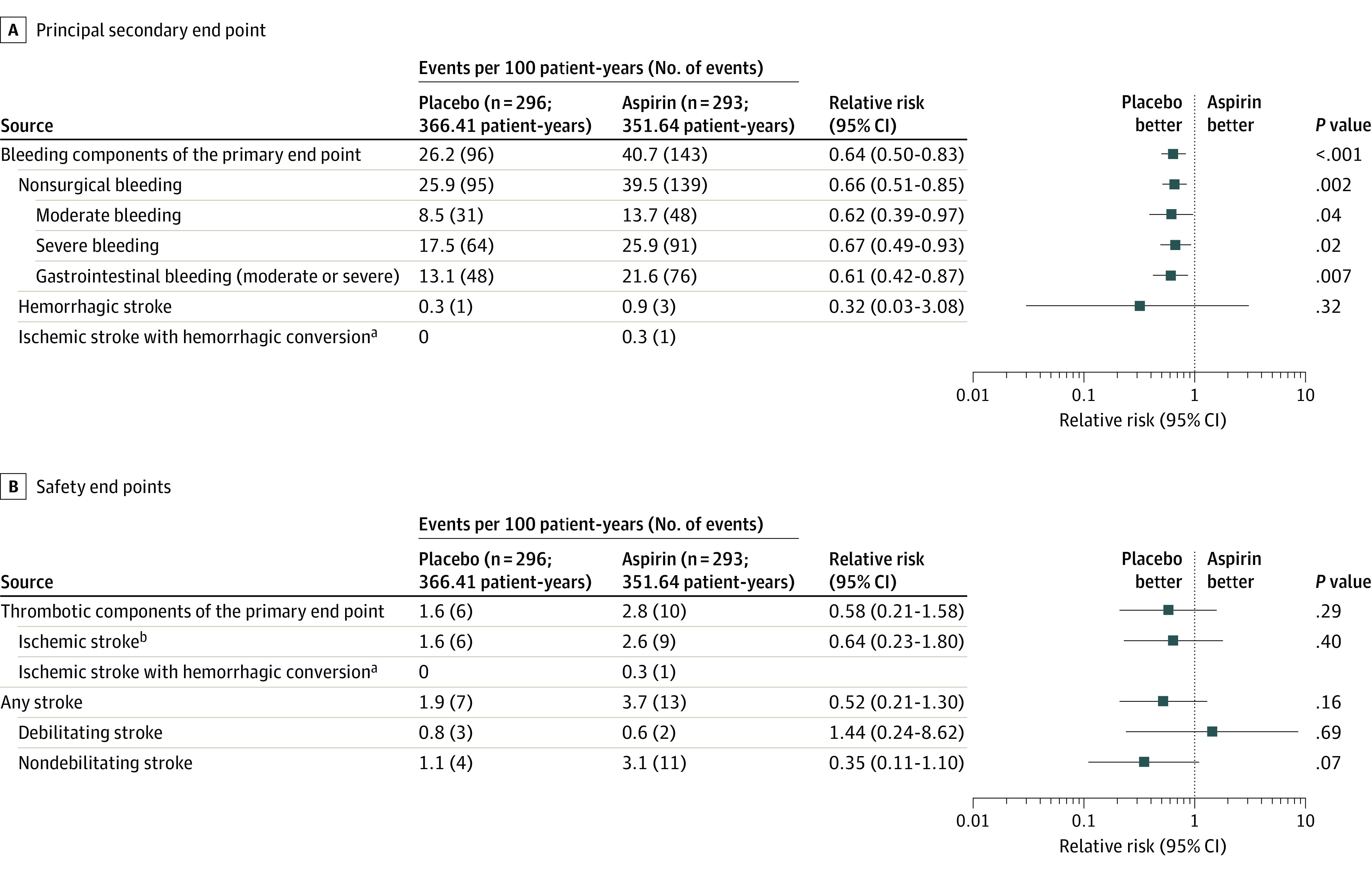
With the avoidance of aspirin, the relative risk of bleeding (the principal secondary end point, including recurrent bleeding) was reduced with no change in safety end points. Bleeding event reduction is observed in both moderate and severe bleeding categories as well as gastrointestinal bleeding. Bleeding events included those that occur while hospitalized or that led to hospitalization or urgent care and required medical intervention. Details on classification as moderate or severe are available in Supplement 2. Events are shown as events per 100 patient-years (to account for first and recurrent events). The relative risk is depicted as a forest plot with 95% CIs and P values. Time-to-event analyses of stroke (eFigure 5 in Supplement 2), overall survival (eFigure 6 in Supplement 2), thrombotic components (eFigure 11 in Supplement 2), and bleeding components of the primary end point (eFigure 12 in Supplement 2) are provided in the Supplement 2. Adjusted hazard models of major adverse events are shown in eFigure 10 in Supplement 2.
aAn ischemic stroke with hemorrhagic conversion event is counted within both the composite “bleeding components of the primary end point” and “thrombotic components of the primary end point.”
bExcludes ischemic stroke with hemorrhagic conversion.
Safety End Points
Aspirin avoidance resulted in a reduction in major bleeding events without an increased risk for major thrombotic events (Figure 3). There was no difference in thrombotic event components of the primary end point between the study groups (RR, 0.58 [95% CI, 0.21-1.58]; P = .29; Figure 3B), including no difference between groups for ischemic and hemorrhagic stroke (RR, 0.52 [95% CI, 0.21-1.30]; P = .16). There were no instances of pump thrombosis. There were no differences in survival (eFigure 6 in Supplement 2) or causes of mortality between the groups (eTable 7 in Supplement 2). Rates of other serious adverse events were similar between the groups (eTable 8 and eFigures 5 and 11 in Supplement 2)
Subgroup Analyses
There were no significant interactions on the primary end point for subgroups of age, sex, race, obesity, disease severity, history of diabetes, ischemic etiology of heart failure, history of coronary stenting, thrombosis history, history of atrial fibrillation, coronary artery bypass procedure, or LVAD surgical implant method (Figure 4). Patients with a history of bleeding or stroke were noted to experience more benefit from aspirin avoidance.
Figure 4. Subgroup Analysis of the Primary End Point (Primary Analysis Population).
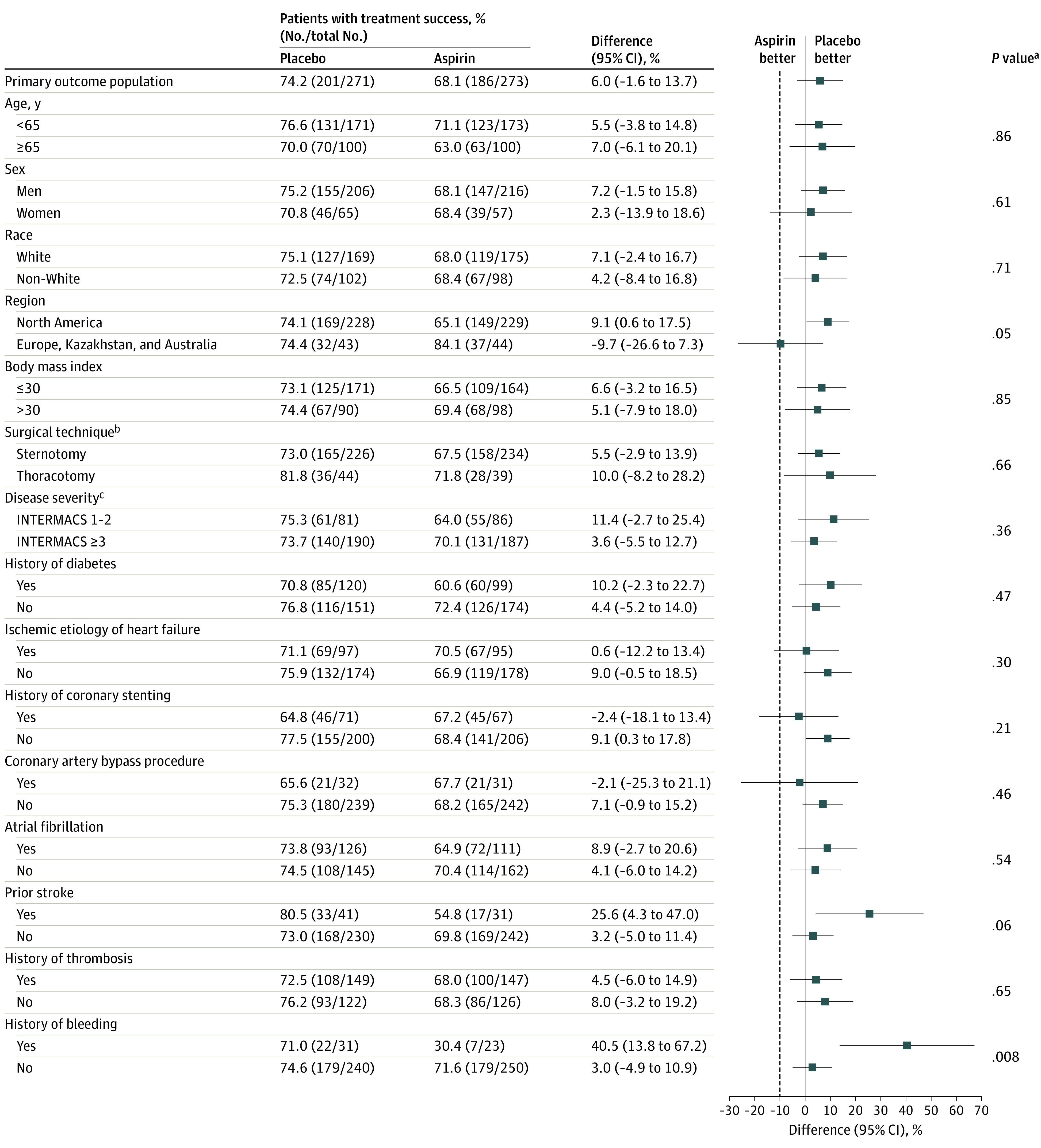
There were no significant interactions on the primary end point for most subgroups, including age, sex, race and ethnicity, obesity, disease severity, history of diabetes, ischemic etiology of heart failure, history of coronary stenting, thrombosis history, history of atrial fibrillation, coronary artery bypass surgery, or left ventricular assist device (LVAD) surgical implant method. Patients with a history of bleeding or stroke were noted to experience more benefit from aspirin avoidance. The dashed line represents the noninferiority margin of −10% for the success difference.
aInteraction testing of primary end point subgroups was completed by general linearized modeling with terms for treatment group, subgroup, and their interaction.
bSurgical techniques include sternotomy, referring to full median sternotomy, and thoracotomy, referring to left thoracotomy combined with right thoracotomy or hemisternotomy.
cDisease severity defined by Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles, which range from 1 to 7; a profile of 1 represents the most severe illness and a profile of 7 the least severe illness.
Descriptive End Points
More patients in the placebo group vs the aspirin group maintained a hemocompatibility score of 0 throughout the median 14 months of follow-up (71.7 vs 63.1 among randomized population; P = .03; eTable 9 in Supplement 2). Rates of hospitalization due to nonsurgical bleeding were lower in patients randomized to the placebo group than those in the aspirin group (13.6 vs 23.9 events per 100 patient-years; P = .002; eTable 10 in Supplement 2). A 41% reduction was observed in the cost of bleeding episodes (cost saving of $380 092) in the placebo group compared with the aspirin group (eTable 11 in Supplement 2). Additionally, patients assigned to the placebo group were hospitalized 293 (47%) fewer days for bleeding (eTable 12 in Supplement 2). Quality of life was improved after implant for both groups, with no difference between study groups (eFigure 13 in Supplement 2).
Medication Adherence, Aspirin Effect, and VKA Use
Medication adherence was high and not different between the placebo vs aspirin group (median [IQR] adherence, 98.3% [92.3%-100%] vs 97.9% [91.7%-100%]; P = .43). Levels of thromboxane B2 were reduced in the aspirin group, but remained unchanged from baseline in the placebo group (eFigure 14 in Supplement 2). Median (IQR) VKA use, as assessed by time in therapeutic range, was similar between placebo (55.1% [39.5%-73.9%]) and aspirin (58.9% [39.5%-69.0%]) groups (eFigure 15 in Supplement 2).
Discussion
This study found that excluding aspirin from an antithrombotic regimen that includes a VKA in patients with advanced heart failure who received a fully magnetically levitated LVAD is noninferior to an aspirin-containing regimen and associated with a decrease in bleeding events, while not increasing the risk of thromboembolic events. Benefits are due to aspirin avoidance, as was confirmed by differential thromboxane B2 levels between the 2 treatment groups. A similar exposure to VKA therapy was determined based on rates of time in therapeutic range between the groups. Based on these results, it was estimated that for every 100 patients implanted with the study LVAD, aspirin avoidance prevented 14.5 major bleeding events in the first year. This translates to a 47% decrease in days spent in the hospital and a 41% reduction in cost of care for bleeding encounters. These findings suggest that avoidance of aspirin provides meaningful improvement in clinical outcomes in patients with advanced heart failure who receive a study LVAD.
Shear stress induced by continuous-flow LVADs stimulates platelet activation and increases proinflammatory cytokines, integrins, and nuclear factor-κB.7,19 Aspirin, even in low doses, inhibits platelet activation pathways by irreversibly inhibiting cyclooxygenase-1 and exerting anti-inflammatory effects by inhibiting cyclooxygenase-2.20,21 This knowledge has prompted widespread use of aspirin as a mandatory part of antithrombotic regimens that accompany LVAD therapy. Results of this trial were unable to confirm any benefit of low-dose aspirin and have challenged clinical assumptions that attribute a benign nature to this drug. This trial is also the first to conclusively test medical therapy in patients receiving LVAD support and provides evidence that clinical assumptions must be challenged. The magnitude of benefit observed by simply avoiding the potentially toxic influence of low-dose aspirin further endorses the need for well-curated evidence in this field, which has, to date, been largely driven by observational data.
Importantly, this study also demonstrates that the benefits of avoiding aspirin are consistent among those with prior vascular disease, including surgical or percutaneous coronary revascularization, obesity, or diabetes, which are characteristics historically associated with increased thrombosis risk. Hematological alterations, such as the development of acquired von Willebrand disease, may decrease thrombosis predisposition and VKA therapy may provide sufficient antithrombotic effects without the need for concomitant aspirin use. Although the placebo group had numerically more patients with older age, female sex, or Black race (characteristics associated with increased bleeding), the benefits remained consistent among these risk groups.22,23,24
Limitations
This study has several limitations. First, patients with early surgical complications and those who required mechanical support devices in addition to the implanted LVAD were excluded, as well as patients for whom investigators deemed aspirin therapy necessary. However, the randomized population analysis, which included patients experiencing early surgical complications and those who transitioned to open-label aspirin use, provided similar findings to the primary analysis and supports avoidance of aspirin. The findings that patients with underlying conditions with increased vascular risk also benefited from aspirin avoidance should provide reassurance to those clinicians who consider such therapy mandatory.
Second, most patients (85%) in the study were recruited from North America, and there is wide variation in patient enrollment across the remaining geographic territories. Therefore, observed geographical interactions are limited by the differential in sample size.25,26 Further detailed analyses in this regard are planned because other factors related to health care system differences, which may require scrutiny, cannot be excluded. One issue pertains to the proportionately lower rate of inclusion of women in this study. In prior analyses, this lower rate of use of LVADs in women has not only been limited to clinical trials, but also within real-world practice.24 The reasons for this are unclear, but this lower rate may represent a referral gap, underappreciation of advanced heart failure in women, or anatomical constraints of body size or left ventricular cavity size.24 It has also been observed that women have a greater burden of hemocompatibility-related events compared with men, and this study should provide greater comfort to clinicians in this regard because bleeding events were reduced in a similar manner among the 2 sexes.
Third, aspirin avoidance in patients was only studied at the outset after implant of a LVAD, so it is not certain that the effect of withdrawing aspirin in patients who were prescribed this drug and receiving chronic LVAD support would be similar to the observed findings. Fourth, these findings may be device-specific and not applicable across the spectrum of any LVAD.
Conclusions
In patients with advanced heart failure receiving support from a fully magnetically levitated LVAD, avoidance of aspirin as part of an antithrombotic regimen that includes a VKA was noninferior to an aspirin-containing regimen and associated with a decrease in bleeding events while not increasing risk of thromboembolic events. The benefits of aspirin avoidance are associated with a decrease in hospitalization rates and cost of care due to bleeding complications.
Trial protocol and statistical analysis plan
eMethods and eResults
Nonauthor collaborators
Data sharing statement
References
- 1.Mehra MR, Nayak A, Desai AS. Life-prolonging benefits of LVAD therapy in advanced heart failure: a clinician’s action and communication aid. JACC Heart Fail. 2023;11(8 pt 1):1011-1017. doi: 10.1016/j.jchf.2023.05.013 [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Goldstein DJ, Cleveland JC, et al. Five-year outcomes in patients with fully magnetically levitated vs axial-flow left ventricular assist devices in the MOMENTUM 3 randomized trial. JAMA. 2022;328(12):1233-1242. doi: 10.1001/jama.2022.16197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uriel N, Colombo PC, Cleveland JC, et al. Hemocompatibility-related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation. 2017;135(21):2003-2012. doi: 10.1161/CIRCULATIONAHA.117.028303 [DOI] [PubMed] [Google Scholar]
- 4.Hammer Y, Bitar A, Aaronson KD. Gastrointestinal bleeding on continuous-flow left ventricular assist device therapy. ESC Heart Fail. 2023;10(4):2214-2224. doi: 10.1002/ehf2.14433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidula H, Takeda K, Estep JD, et al. Hospitalization patterns and impact of a magnetically-levitated left ventricular assist device in the MOMENTUM 3 trial. JACC Heart Fail. 2022;10(7):470-481. doi: 10.1016/j.jchf.2022.03.007 [DOI] [PubMed] [Google Scholar]
- 6.Bansal A, Uriel N, Colombo PC, et al. Effects of a fully magnetically levitated centrifugal-flow or axial-flow left ventricular assist device on von Willebrand factor: a prospective multicenter clinical trial. J Heart Lung Transplant. 2019;38(8):806-816. doi: 10.1016/j.healun.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 7.Shah P, Tantry US, Bliden KP, Gurbel PA. Bleeding and thrombosis associated with ventricular assist device therapy. J Heart Lung Transplant. 2017;36(11):1164-1173. doi: 10.1016/j.healun.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 8.Del Rio-Pertuz G, Nair N. Gastrointestinal bleeding in patients with continuous-flow left ventricular assist devices: a comprehensive review. Artif Organs. 2023;47(1):12-23. doi: 10.1111/aor.14432 [DOI] [PubMed] [Google Scholar]
- 9.Consolo F, Sferrazza G, Motolone G, et al. Platelet activation is a preoperative risk factor for the development of thromboembolic complications in patients with continuous-flow left ventricular assist device. Eur J Heart Fail. 2018;20(4):792-800. doi: 10.1002/ejhf.1113 [DOI] [PubMed] [Google Scholar]
- 10.Slaughter MS, Pagani FD, McGee EC, et al. ; HeartWare Bridge to Transplant ADVANCE Trial Investigators . HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;32(7):675-683. doi: 10.1016/j.healun.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 11.Najjar SS, Slaughter MS, Pagani FD, et al. ; HVAD Bridge to Transplant ADVANCE Trial Investigators . An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33(1):23-34. doi: 10.1016/j.healun.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 12.Saeed O, Colombo PC, Mehra MR, et al. Effect of aspirin dose on hemocompatibility-related outcomes with a magnetically levitated left ventricular assist device: an analysis from the MOMENTUM 3 study. J Heart Lung Transplant. 2020;39(6):518-525. doi: 10.1016/j.healun.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consolo F, Raimondi Lucchetti M, Tramontin C, Lapenna E, Pappalardo F. Do we need aspirin in HeartMate 3 patients? Eur J Heart Fail. 2019;21(6):815-817. doi: 10.1002/ejhf.1468 [DOI] [PubMed] [Google Scholar]
- 14.Lim HS, Ranasinghe A, Chue C, Mascaro J. Two-year outcome of warfarin monotherapy in HeartMate 3 left ventricular assist device: a single-center experience. J Heart Lung Transplant. 2020;39(10):1149-1151. doi: 10.1016/j.healun.2020.06.012 [DOI] [PubMed] [Google Scholar]
- 15.Mehra MR, Crandall DL, Gustafsson F, et al. Aspirin and left ventricular assist devices: rationale and design for the international randomized, placebo-controlled, non-inferiority ARIES HM3 trial. Eur J Heart Fail. 2021;23(7):1226-1237. doi: 10.1002/ejhf.2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehra MR, Salerno C, Cleveland JC, et al. Healthcare resource use and cost implications in the MOMENTUM 3 long-term outcome study. Circulation. 2018;138(18):1923-1934. doi: 10.1161/CIRCULATIONAHA.118.035722 [DOI] [PubMed] [Google Scholar]
- 17.Kidson-Gerber G, Weaver J, Gemmell R, Prasan AM, Chong BH. Serum thromboxane B2 compared to five other platelet function tests for the evaluation of aspirin effect in stable cardiovascular disease. Heart Lung Circ. 2010;19(4):234-242. doi: 10.1016/j.hlc.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 18.Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: comparative evaluation of measures of time-in-therapeutic range. J Thromb Thrombolysis. 2003;15(3):213-216. doi: 10.1023/B:THRO.0000011377.78585.63 [DOI] [PubMed] [Google Scholar]
- 19.Apostoli A, Bianchi V, Bono N, et al. Prothrombotic activity of cytokine-activated endothelial cells and shear-activated platelets in the setting of ventricular assist device support. J Heart Lung Transplant. 2019;38(6):658-667. doi: 10.1016/j.healun.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16(11):675-686. doi: 10.1038/s41569-019-0225-y [DOI] [PubMed] [Google Scholar]
- 21.Morris T, Stables M, Hobbs A, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183(3):2089-2096. doi: 10.4049/jimmunol.0900477 [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DJ, Naka Y, Horstmanshof D, et al. Association of clinical outcomes with left ventricular assist device use by bridge to transplant or destination therapy intent: the multicenter study of MagLev technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) randomized clinical trial. JAMA Cardiol. 2020;5(4):411-419. doi: 10.1001/jamacardio.2019.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheikh FH, Ravichandran AK, Goldstein DJ, et al. Impact of race on clinical outcomes after implantation with a fully magnetically levitated left ventricular assist device: an analysis from the MOMENTUM 3 trial. Circ Heart Fail. 2021;14(10):e008360. doi: 10.1161/CIRCHEARTFAILURE.120.008360 [DOI] [PubMed] [Google Scholar]
- 24.Ramu B, Cogswell R, Ravichandran AK, et al. Clinical outcomes with a fully magnetically levitated left ventricular assist device among women and men. J Am Coll Cardiol HF. Published online October 11, 2023. doi: 10.1016/j.jchf.2023.08.020 [DOI] [PubMed] [Google Scholar]
- 25.Mirza KK, Xie R, Cowger J, et al. Comparative analysis of regional outcomes and adverse events after continuous-flow left ventricular assist device implantation: an IMACS analysis. J Heart Lung Transplant. 2020;39(9):904-914. doi: 10.1016/j.healun.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 26.Nayak A, Mehra MR. Global challenges in left ventricular assist device therapy: a tale across two continents. Eur J Heart Fail. 2022;24(7):1316-1318. doi: 10.1002/ejhf.2570 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eMethods and eResults
Nonauthor collaborators
Data sharing statement