Abstract
Background
Chronic cough (a cough lasting longer than four weeks) is a common problem internationally. Chronic cough has associated economic costs and is distressing to the child and to parents; ignoring cough may lead to delayed diagnosis and progression of serious underlying respiratory disease. Clinical guidelines have been shown to lead to efficient and effective patient care and can facilitate clinical decision making. Cough guidelines have been designed to facilitate the management of chronic cough. However, treatment recommendations vary, and specific clinical pathways for the treatment of chronic cough in children are important, as causes of and treatments for cough vary significantly from those in adults. Therefore, systematic evaluation of the use of evidence‐based clinical pathways for the management of chronic cough in children would be beneficial for clinical practice and for patient care. Use of a management algorithm can improve clinical outcomes; such management guidelines can be found in the guidelines for cough provided by the American College of Chest Physicians (ACCP) and the British Thoracic Society (BTS).
Objectives
To evaluate the effectiveness of using a clinical pathway in the management of children with chronic cough.
Search methods
The Cochrane Register of Controlled Trials (CENTRAL), the Cochrane Airways Group Specialised Register, MEDLINE, EMBASE, review articles and reference lists of relevant articles were searched. The latest search was conducted in January 2014.
Selection criteria
All randomised controlled trials of parallel‐group design comparing use versus non‐use of a clinical pathway for treatment of chronic cough in children (< 18 years of age).
Data collection and analysis
Results of searches were reviewed against predetermined criteria for inclusion. Two review authors independently selected studies and performed data extraction in duplicate.
Main results
One study was included in the review. This multi‐centre trial was based in five Australian hospitals and recruited 272 children with chronic cough. Children were randomly assigned to early (two weeks) or delayed (six weeks) referral to respiratory specialists who used a cough management pathway. When an intention‐to‐treat analysis was performed, clinical failure at six weeks post randomisation (defined as < 75% improvement in cough score, or total resolution for fewer than three consecutive days) was significantly less in the early pathway arm compared with the control arm (odds ratio (OR) 0.35, 95% confidence interval (CI) 0.21 to 0.58). These results indicate that one additional child will be cured for every five children treated via the cough pathway (number needed to treat for an additional beneficial outcome (NNTB) = 5, 95% CI 3 to 9) at six weeks. Cough‐specific parent‐reported quality of life scores were significantly better in the early‐pathway group; the mean difference (MD) between groups was 0.60 (95% CI 0.19 to 1.01). Duration of cough post randomisation was significantly shorter in the intervention group (early‐pathway arm) compared with the control group (delayed‐pathway arm) (MD ‐2.70 weeks, 95% CI ‐4.26 to ‐1.14).
Authors' conclusions
Current evidence suggests that using a clinical algorithm for the management of children with chronic cough in hospital outpatient settings is more effective than providing wait‐list care. Futher high‐quality randomised controlled trials are needed to perform ongoing evaluation of cough management pathways in general practitioner and other primary care settings.
Plain language summary
Clinical pathways for chronic cough in children
Background
Clinical pathways serve as a tool or algorithm (like a flow chart) that can be used in the treatment of patients with various chronic diseases. They provide a clear guide that assists doctors in diagnosing an illness and in making decisions with the patient about what treatment is needed or which specialists should be seen or tests ordered at each stage of progression of the disease. Overall the aim of clinical pathways is to provide efficient care for patients. Examples of patient decision aids are provided by the National Health Service (NHS) in the UK at http://www.rightcare.nhs.uk/index.php/shared‐decision‐making/about‐the‐pdas/.
Chronic cough in children is a significant medical problem that in some situations warrants thorough investigation. This review examined whether using clinical pathways was effective for evaluating and managing children with chronic cough (cough lasting longer than 4 weeks).
Study characteristics
Only a single multi‐centre study could be included in this review. Evidence is current to January 2014. This study was funded by the National Health and Medical Research Council of Australia.
Key results
This study of 272 children in five Australian hospitals reported that those randomly assigned to earlier treatment according to a clinical pathway showed improved clinical outcomes (cough resolved earlier and quality of life was better) compared with those who were randomly assigned to later use of the pathway. No adverse events were reported.
Quality of the evidence
The quality of evidence was graded as moderate. Evidence is limited, as only one study could be included in this review. This study was unable to completely blind participants to the clinical pathway.
Summary of findings
for the main comparison.
| Clinical pathway compared with usual care for treatment of children with chronic cough | ||||||
|
Patient or population: children with chronic cough of unknown origin lasting longer than 4 weeks Settings: paediatric hospital outpatient clinics Intervention: clinical pathway algorithm (clinical review within 2 weeks) Comparison: usual clinical care (clinical review at 6 weeks) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care group | Clincal pathway | |||||
|
Clinical failure—primary outcome (by intention‐to‐treat analysis) Follow‐up: 6 weeks |
71 per 100 |
47 per 100 (34 to 59) |
OR 0.35 (0.21 to 0.58) |
272 (1 study) | ⊕⊕⊕⊝ moderatea | |
|
PC‐QOL mean score at 6 weeks PC‐QOL is a 27‐item questionnaire. Each question has a 7‐point score ranging from 1 (worst quality of life) to 7 (best quality of life). Scores for each item were added and the average taken |
5.01 (SD 1.63) | 5.61 (5.2 to 6.02) |
Mean difference between groups 0.60 (0.19 to 1.01) weeks |
226 (1 study) | ⊕⊕⊕⊝ moderatea | |
| Duration of cough post randomisation | Mean duration of cough was 9.1 (SD 6.6) weeks | Mean duration of cough was 6.4 (4.84 to 7.96) weeks |
Mean difference between groups ‐2.70 (‐4.26 to ‐1.14) weeks |
226 (1 study) | ⊕⊕⊕⊝ moderatea | Cough resolution was defined as total resolution of cough or ≥ 75% improvement in cough scores for ≥ 3 days |
|
Proportion of adverse events experienced Follow‐up: 6 months |
0 per 1000 | 0 per 1000 | Not estimable | See comments | ⊕⊕⊕⊝ moderatea | No adverse events were reported |
|
Proportions of participants experiencing adverse events or complications Follow‐up: 6 months |
0 per 1000 | 0 per 1000 | Not estimable | See comments | ⊕⊕⊕⊝ moderatea | No adverse events were reported |
| *The basis for the assumed risk was the mean control group risk in the included study. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR; Odds ratio; PC‐QOL: Parent‐reported cough‐specific quality of life questionnaire; RR: Risk ratio; SD: Standard deviation. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aA single study was identified, and complete blinding was not possible for this type of intervention.
Background
Description of the condition
Cough is the most common symptom presenting to primary care internationally (Britt 1999; Cherry 2003; Irwin 2006). In Australia, 5.8 of every 100 visits to general practitioners are result of cough (Britt 2008). Chronic (prolonged) cough is also one of the most common symptoms presenting to respiratory physicians (Fitzgerald 2006). Thus, in Australia alone, these visits on a population level would equate to millions of dollars per year in Medicare rebates for general practitioner (GP) visits. Further, studies have shown that more than 80% of children who have seen specialists for chronic cough have had more than five medical visits, and over 20% had seen a doctor more than 20 times (Chang 2012; Marchant 2008). The burden of chronic cough (defined in children as cough lasting longer than four weeks) (Chang 2006b; Marchant 2006) is significant, both in terms of personal cost and impaired quality of life (Marchant 2008) and at a societal level when medication costs are substantial (Irwin 2006).
Chronic cough in children causes a significant burden of distress for parents (Marchant 2008). Furthermore, although cough may be seen as a merely troublesome symptom with no serious consequences, ignoring cough that may be the sole presenting symptom of an underlying respiratory disease may lead to delayed diagnosis and progression of serious illness or chronic respiratory morbidity (Barr 2005; Karakoc 2002). Thus for the management of chronic cough in children, it is important for clinicians to define which patients will benefit from which interventions and treatment approaches (including 'watchful waiting') (Gupta 2007).
Description of the intervention
The major aim of clinical pathways or guidelines is to improve diagnosis and/or management of the specific condition or symptom. They provide a step‐by‐step approach for the clinician that is based on preceding criteria. Currently, treatment recommendations for cough vary among published guidelines (Irwin 2006; Kohno 2006; Shields 2007), but none have been evaluated in a randomised controlled trial (RCT). We (Chang 2005; Chang 2006a; Marchant 2006) and others (Shields 2006) have argued that children with chronic cough should be evaluated and treated in accordance with guidelines specific to children, as both causative factors and treatment in children are significantly different from those in adults (Chang 2006b).
How the intervention might work
Clinical guidelines have been shown to provide more efficient and effective patient care (Fessler 2005) and, if well designed, can facilitate clinical decision making. This approach in turn should reduce variations in delivery of care and delays in diagnosis or treatment (Kwan 2004). Cough guidelines, which were first provided by Irwin (Irwin 1990), were designed to facilitate the management of chronic cough. Subsequent cough guidelines have been published by various societies.
Why it is important to do this review
Cohort studies suggest that use of cough clinical pathways or algorithms improves outcomes (resolution of cough and accurate diagnosis) in children (Asilsoy 2008; Karabel 2013; Rehman 2009) and adults (Irwin 1990). However, use of guidelines is not universally popular in medical circles (Preiser 2004) and may arguably result in negative outcomes (e.g. from missed or delayed diagnosis). Clinical guidelines are regarded by some as 'cook‐book medicine,' as bothersome and as negating critical thinking (Berg 1997). Examination, through a systematic review, of the effectiveness of using a clinical pathway in treating children with chronic cough would be useful for guiding clinical practice (Elbourne 2002). This is an update of a Cochrane review (Bailey 2004).
Objectives
To evaluate the effectiveness of using a clinical pathway in the management of children with chronic cough.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs of parallel‐group design comparing use versus non‐use of a clinical pathway for the treatment of children with chronic cough.
Types of participants
Inclusion criteria: children (< 18 years of age) with chronic (lasting longer than four weeks) cough of unknown origin.
Exclusion criteria: known preexisting respiratory illness causing cough.
Types of interventions
All randomised controlled comparisons of use of a clinical pathway. Review authors planned that trials examining use of other medications or interventions would be included if all participants were given equal access to such medications or interventions.
Types of outcome measures
Primary outcomes
Proportions of participants who were not cured or were not substantially improved at follow‐up (clinical failure).
Secondary outcomes
Proportions of participants who were not cured at follow‐up.
Proportions of participants who were not substantially improved at follow‐up.
Mean difference in cough indices (cough diary, cough frequency, cough‐specific quality of life scores, cough duration).
Proportions of participants experiencing adverse effects of the intervention (e.g. Cushing's syndrome from steroid overdose).
Proportions of participants experiencing complications (e.g. acute hospitalisations, chronic lung disease resulting from delayed diagnosis).
Proportions of participants who failed to improve while receiving treatment and mean clinical improvement were determined using the following hierarchy of assessment measures (Note: When two or more assessment measures were reported in the same study, the outcome measure listed first in the hierarchy was used).
Objective measurements of cough indices (cough frequency, cough receptor sensitivity).
Symptomatic assessment by participant (adult or child) (quality of life, Likert scale, visual analogue scale, level of interference of cough, cough diary).
Symptomatic assessment by parents/carers (quality of life, Likert scale, visual analogue scale, level of interference of cough, cough diary).
Symptomatic assessment by clinicians (Likert scale, visual analogue scale, level of interference of cough, cough diary).
Search methods for identification of studies
Electronic searches
Trials were identified from the following sources.
The Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 1).
The Cochrane Airways Group Specialised Register.
MEDLINE (Ovid) (1950 to January 2014).
EMBASE (Ovid) (1980 to January 2014).
Full search strategies are listed in Appendix 1. Conference abstracts were handsearched and grey literature was searched through the CENTRAL database.
Searching other resources
In addition to the electronic search, we checked the reference lists of relevant publications and contacted the authors of the included trial to ask for further information.
Data collection and analysis
Selection of studies
Retrieval of studies: Using article titles, abstracts or descriptors, two review authors (EJB and ABC in original review and search from 2009 to 2012; GBM and ABC in search from 2012 to 2014) independently reviewed literature searches to identify potentially relevant trials for full review. They conducted searches of bibliographies and texts to identify additional studies. From the full‐text articles obtained, the same two review authors independently assessed trials for inclusion on the basis of specific criteria. It was planned that disagreements would be resolved by third party adjudication (PM), but no disagreement was reported.
Data extraction and management
We had no disagreements but had planned to resolve disagreements through discussion with another review author (PSM). We extracted data using a standardised data collection form and managed them in Review Manager 5.2, in accordance with recommendations provided in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011). When required, we requested further information from trial authors.
Assessment of risk of bias in included studies
Two review authors (GBM and ABC) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). It was planned that disagreements would be resolved by discussion or by third party adjudication. We assessed risk of bias according to the following domains.
Allocation sequence generation (selection bias).
Concealment of allocation (selection bias).
Blinding of participants (performance bias).
Outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (reporting bias).
Measures of treatment effect
For the dichotomous outcome variables of each individual study, odds ratios were calculated using a modified intention‐to‐treat analysis. This analysis assumes that children not available for outcome assessment have not improved (and probably represents a conservative estimate of effect). Other indices were assumed to be normally distributed continuous variables, so the mean difference in outcomes could be estimated (weighted mean difference). It was planned that if studies reported outcomes using different measurement scales, the standardised mean difference would be used.
Unit of analysis issues
Cross‐over trials are not appropriate for this intervention and therefore were not planned for inclusion in any meta‐analysis performed. It was planned that cross‐over trials that met other review inclusion criteria would be described in the text.
Dealing with missing data
It was planned that Investigators or study sponsors would be contacted to verify key study characteristics and to provide missing numerical outcome data when necessary.
Assessment of heterogeneity
It was planned that heterogeneity between study results would be described and tested using the I2 statistic to ascertain whether it reached statistical significance (Higgins 2003). Heterogeneity is considered significant when the P value is less than 0.10 (Higgins 2011). As only one study was suitable for inclusion in the review, assessment of heterogeneity was not necessary.
Assessment of reporting biases
If reporting bias was suspected (see 'Selective reporting bias' in the 'Risk of bias' table below), we planned to contact study authors to ask them to provide missing outcome data. It was planned that if missing data were not provided, and if this was thought to introduce serious bias, the impact of including such studies in the overall assessment would be explored through a sensitivity analysis.
As a single study with complete outcome reporting was included in this review update, sensitivity analysis was not required.
Data synthesis
An initial qualitative comparison of all individually analysed studies was planned to examine whether pooling of results (meta‐analysis) was reasonable. This comparison would have taken into account differences in study populations, inclusion/exclusion criteria, interventions, outcome assessment and estimated effect size. Results from studies that met the inclusion criteria and reported any of the outcomes of interest would have been included in subsequent meta‐analyses. However, as only one study was suitable for inclusion (based on study characteristics and inclusion criteria of this review), a qualitative comparison of studies was not required. We created a 'Summary of findings' table (SoF) (Table 1) in accordance with methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011) and using GRADEpro software.
The summary weighted odds ratio (OR) and 95% confidence interval (95% CI) (fixed‐effect model) were calculated using RevMan. Numbers needed to treat for an additional beneficial outcome (NNTB) were calculated from the pooled OR, and its 95% CI was applied to a specified baseline risk with use of an online calculator (Cates 2003).
Subgroup analysis and investigation of heterogeneity
We had planned a priori subgroup analyses for children younger than seven years of age and for those seven years of age and older. As only a single study was identified for inclusion in the review, subgroup analyses and investigations of heterogeneity were not performed.
Sensitivity analysis
Sensitivity analyses were planned to assess the impact of potentially important factors on overall outcomes.
Analysis by type of clinical pathway (e.g. continent‐specific).
Analysis by setting, whereby frequency of causes of chronic cough may be different (e.g. general practitioners vs specialists, affluent vs non‐affluent countries, indigenous vs mainstream communities).
Analysis using a random‐effects model.
Analysis by "treatment received."
Analysis by "intention‐to‐treat."
As only a single study was included, subgroup (described above) and sensitivity analyses were not performed.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Combined searches (original and update reviews) performed by the Cochrane Airways Group identified 727 potentially relevant titles. After the abstracts were assessed, 10 studies were considered for inclusion in the review, and one study (Chang 2013) fulfilled the eligibility criteria of the review (Figure 1).
1.

Study flow diagram.
Included studies
The sole study included in the review was a multi‐centre study supported by a competitive, non‐commercial grant (National Health and Medical Research Council of Australia). The study protocol was published previously (Chang 2010). Study authors described the trial as a pragmatic RCT (Chang 2013) utilising a standardised clinical management pathway for management of chronic cough in children (i.e. two weeks (early) vs six weeks (delayed) of referral by their referring physician). Children were randomly assigned by their referring physician to an early management pathway (within three weeks of referral to the specialist practice) or to usual care (i.e. later management with the pathway around the six‐week waiting period required to obtain a regularly scheduled specialist appointment) (Chang 2013). The RCT did not strictly explore intervention versus standard care (i.e. use vs non‐use of a clinical pathway), as all participants received the intervention within the timing of the intervention (i.e. merely delayed). Study authors justified the study design by stating that a cluster‐blind RCT would not be feasible, as all centres involved in the study had similar standard clinical practices, in line with current recommendations (upon which the cough pathway was designed), and physicians were not comfortable withholding treatment for the purpose of a study. Similarly, the study authors acknowledged that strict time point adherence (rather than "early" and "delayed" use of the pathway) would introduce greater rigour to the study but stated that a pragmatic design was required for the real‐life clinical settings in which the study operated (Chang 2013). This study was conducted in paediatric hospital outpatient clinics at five centres in Australia, and investigators recruited children who were newly referred with chronic cough (lasting longer than four weeks). A total of 272 participants were included in the study; 152 were male. The mean age of study participants was 4.5 (standard deviation (SD) 3.7) years, and the median duration of cough at enrolment was 16 (interquartile range (IQR) 8 to 32) weeks (Chang 2013).
Nineteen children were not treated according to the clinical pathway, as they did not attend their first scheduled appointment with the respiratory physician (n = 8 from the intervention group; n = 11 from the control group). A further 22 children were withdrawn from the study (parents withdrew n = 3; lost to follow‐up n = 17; protocol violation n = 1; non‐adherence n = 1). Baseline data for 253 participants were therefore available, as were complete primary outcome data for 226 participants. Although the study was undertaken to evaluate outcome measures four weeks post use of the pathway in the early‐pathway arm and before use of the pathway in the delayed‐pathway arm, participating children were seen (hence the pathway was used) at 1.9 (SD 1) weeks and 5.1 (SD 1.8) weeks, respectively. Outcomes of the study were likely diluted.
The study used proportions of cough‐free (> 75% improvement in cough or total resolution of cough for three or more days according to cough diary) children and parent‐proxy quality of life score, both measured at week six, as the primary outcome measures. Excluded were children with a known chronic respiratory illness (previously diagnosed by a respiratory physician or confirmed on objective tests) such as cystic fibrosis or bronchiectasis.
For further details, see Characteristics of included studies.
Excluded studies
Nine studies were excluded (see Characteristics of excluded studies) because they used a non‐RCT design or did not use a specific management protocol for cough treatment.
Risk of bias in included studies
Risk of bias in the included study is summarised in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Assessed as low risk of bias. Study authors clearly described computer‐generated randomisation sequencing with concealed allocation.
Blinding
Assessed as low risk of bias. Although participants and research personnel collecting data were not specifically informed about the study arm to which they were allocated, the design of the study made complete blinding not feasible. At the time the study was conducted, the usual wait time to see a clinician in the public health setting was used as the time frame for the delayed‐pathway arm (control) (i.e. around six weeks) and usual wait time for private clinics was used as the time frame for the early‐pathway arm (intervention) (one to three weeks). Regarding the objective character of outcome measures, we did not expect high risk of bias with clinical failure. With regards to subjective outcome measures, we do not expect high risk of bias for parent‐reported cough‐specific quality of life score (PC‐QOL), as a standardised approach was implemented for all study participants.
Incomplete outcome data
Assessed as low risk of bias. Study authors (Chang 2013) stated that complete outcome data were obtained in more than 90% of participants.
Selective reporting
Assessed as low risk of bias, with study authors clearly describing in the published manuscript the progress of all randomly assigned participants. Limitations of the study were identified and discussed by the study authors.
Other potential sources of bias
The number of potentially eligible participants who were not enrolled (declined participation or were not approached for participation) is not stated by the study authors. This may introduce an unclear assessment of recruitment selection bias.
Effects of interventions
See: Table 1
As only one study met the criteria for inclusion in this review, no meta‐analysis could be performed. The effects of intervention presented below are reported by the single included study (Chang 2013).
Primary outcome
Proportions of participants who were not cured or were not substantially improved at follow‐up (clinical failure)
Intention‐to‐treat analysis revealed that clinical failure was significantly lower in the early‐pathway arm (intervention) compared with the delayed‐pathway arm (control) (OR 0.35, 95% CI 0.21 to 0.58; Analysis 1.1), as presented in Figure 4. The control event rate (i.e. the number of clinical failures reported from the control group) was 70.5% versus the intervention event rate of 46% (Chang 2013). These results indicate that one child will be cured for every five children treated by using the cough pathway at six weeks (NNTB = 5, 95% CI 3 to 9; Cates plot, Figure 5).
1.1. Analysis.
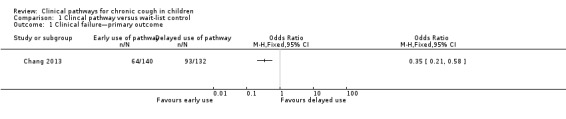
Comparison 1 Clincal pathway versus wait‐list control, Outcome 1 Clinical failure—primary outcome.
4.

Forest plot of comparison: 1. Primary outcome, outcome: 1.1. Clinical failure—primary outcome (by intention‐to‐treat analysis).
5.
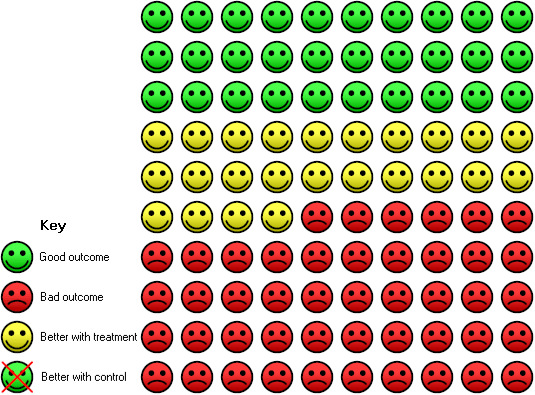
In the control group, 71 of 100 participants were not cured at follow‐up over 6 weeks compared with 46 of 100 (95% CI 33 to 58) for the active treatment group.
Secondary outcomes
Proportions of participants who were not cured at follow‐up
The proportion of participants not cured at follow‐up (secondary outcome) is the same as the proportion of participants with clinical failure (see primary outcome above) (Analysis 1.2).
1.2. Analysis.
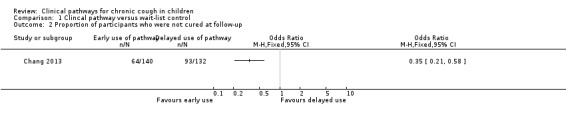
Comparison 1 Clincal pathway versus wait‐list control, Outcome 2 Proportion of participants who were not cured at follow‐up.
Proportions of participants who were not substantially improved at follow‐up
The study reported only on participants cured or not cured at follow‐up. Participants not substantially improved were considered not cured.
Mean differences in cough indices (cough diary, cough frequency, cough‐specific quality of life scores, cough duration)
The parent‐reported cough‐specific quality of life score (PC‐QOL) at week six was significantly better (i.e. higher) for those in the early‐pathway arm compared with those in the delayed‐pathway arm (MD 0.60 points, 95% CI 0.19 to 1.01; Analysis 1.3).
1.3. Analysis.
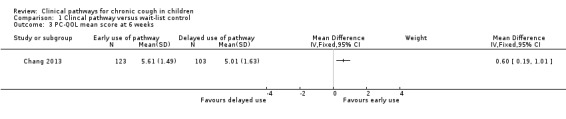
Comparison 1 Clincal pathway versus wait‐list control, Outcome 3 PC‐QOL mean score at 6 weeks.
Duration of cough post randomisation was significantly shorter in the intervention group (early‐pathway arm) compared with the control group (delayed‐pathway arm) (MD ‐2.70 weeks, 95% CI ‐4.26 to ‐1.14; Analysis 1.4).
1.4. Analysis.
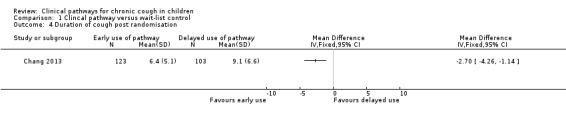
Comparison 1 Clincal pathway versus wait‐list control, Outcome 4 Duration of cough post randomisation.
Proportions of participants experiencing adverse effects of the intervention or complications
Study authors reported that none of the participants in the intervention (pathway) group and none in the control (standard care) group experienced adverse events.
Other outcomes
Once the cough algorithm was used, irrespective of whether it was applied early or was delayed, the duration of cough was similar. Also, in contrast to results reported for a cough‐specific quality of life, no differences were noted between groups in terms of generic health‐related quality of life (PedsQL) score at six weeks (early arm: median 92.5, IQR 81 to 96.5; delayed arm: median 87, IQR 76 to 96.3).
Discussion
Summary of main results
Only a single study fulfilled the inclusion criteria. This multi‐centre study involved 272 children enrolled from hospital outpatient departments in Australia. This body of evidence was graded as moderate quality through the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. An ITT analysis revealed that clinical failure was significantly lower in the early‐pathway arm (clinical review within two weeks) compared with the control arm (delayed use of pathway; clinical review at six weeks) (OR 0.35, 95% CI 0.21 to 0.58). For the secondary outcome of mean score for cough‐specific parent‐reported quality of life, the score was significantly better in the early‐pathway group (0.60 units, 95% CI 0.19 to 1.01) compared with the control group. This is seen just at the minimum important difference (MID) (using the distribution method for calculating MID) (Newcombe 2010; Newcombe 2010b). The intervention group also had significantly shorter duration of cough post randomisation compared with the control group (MD ‐2.70, 95% CI ‐4.26 to ‐1.14). No adverse events were reported.
Overall completeness and applicability of evidence
Clinical pathways are used for various chronic diseases to facilitate diagnosis; aid decision making; and provide efficient care to patients. Chronic cough in children is a significant medical problem that in some situations warrants thorough investigation. This review is limited, as only data from a single study are available. Nevertheless, data support the use of clinical management pathways for chronic cough in children in a tertiary care setting.
Although the RCT in this review planned to compare outcomes four weeks post use of the pathway in the early‐pathway arm and before use of the pathway in the delayed‐pathway arm (i.e. within six weeks of referral), pragmatically this was not feasible, and children entered the protocol at times that were not strictly adhered to, resulting in treatment of children by respiratory paediatricians in accordance with the pathway at 1.9 (SD 1) weeks and 5.1 (SD 1.8) weeks, respectively. This flexibility in treatment time means that study results are likely to be diluted, as children in the delayed‐pathway arm received treatment before measurements were undertaken.
Limitations
The algorithm applied in the included study was used by respiratory physicians (all but one person was a respiratory physician); therefore, any effect that might be attributed to expertise required to use the algorithm cannot be identified. However, steps within the algorithm are simple and explicit, and most (85%) of the children had diagnoses that could be made easily in primary care. For example, key steps such as distinguishing between wet and dry cough (Chang 2005b) and categorising specific versus non‐specific cough (Marchant 2006b) are both feasible and reliable.
Thus, although the same pathway could be used in general practice, treatment outcomes may be different, as the pathway is dependent on thorough history taking and examination (including identifying the presence of crepitations). In general practice, agreement of items in preschool children (most children with chronic cough are of preschool age) such as wheeze and chest examination findings has been shown to be poor (kappa values range from 0.12 to 0.39) (Hay 2004). Thus, applicability of the pathway (without concurrent education) in general practice cannot be ascertained. Further education for primary care providers on how to use the algorithm is likely required for the algorithm to be as successful as was reported in the included study.
A wait‐list RCT pragmatic approach was used in the included study. The design of this study is similar to the wait‐list approach used for some RCTs, such as those examining psychological interventions or paediatric surgery (e.g. tonsil‐adenoidectomy for obstructive sleep apnoea), for which primary outcomes are selected before the intervention is decided. Arguably, early versus delayed use of the algorithm represents an alternative valid approach (c.f. use vs non‐use of an algorithm) that can be used to determine whether the algorithm is efficacious, as effectively timing the primary outcome (at week six) tests use versus non‐use of the algorithm.
Quality of the evidence
Given that only one study could be included in this review, the extent of the evidence is limited. Other than the unclear risk of bias associated with blinding of participants, the risk of bias for other criteria was low. This multi‐centre study involved a relatively large number of participants (i.e. for cough‐related studies). The consistency of favourable outcomes in the intervention arm (including duration of cough post randomisation) supports the unlikely presence of bias. Also, the generic quality of life measure used (PedsQL), which is a less sensitive measure for cough, was not significantly different between groups, but the cough‐specific quality of life score (PC‐QOL) was significantly better in the early‐pathway arm. Arguably, if quality of life was subject to clinically important bias, PedsQL score would also be significantly better in the intervention group (early‐pathway arm) compared with the control group (delayed‐pathway arm).
In addition to significant differences between groups in primary outcomes (PC‐QOL and proportion 'cough‐free'), the duration of cough post randomisation was significantly different between groups (early‐pathway vs delayed‐pathway groups). Cough duration at baseline (Table 1 in the included study) and post use of the algorithm was similar in the two groups. Thus, it is most likely that use of the algorithm accounted for differences between groups.
Potential biases in the review process
Two of the authors of this review are co‐authors of the sole RCT that was included in this review. However, we took steps to reduce bias by double‐entering data, and the primary author of this review was not involved in the included RCT.
Agreements and disagreements with other studies or reviews
Data from cohort studies (Asilsoy 2008; Karabel 2013; Rehman 2009) are concordant with results of this review, which included only RCTs. These cohort studies used a cough algorithm that is similar to the one described in the included study. We are not aware of any other systematic reviews with which these results can be compared.
Authors' conclusions
Implications for practice.
The limited available evidence presented here suggests that use of management protocols in the diagnosis and treatment of children with chronic cough (lasting longer than four weeks) is effective in improving clinical outcomes (cough‐free, shorter duration of cough and improved parent‐proxy cough‐specific quality of life).
Implications for research.
Further high‐quality randomised controlled trials are needed for ongoing evaluation of the use of clinical pathways for the management of chronic cough in children. In these trials, settings should include general practitioner and other primary care settings and use of the cough algorithm should be compared with non‐use of the algorithm. A cluster‐randomised trial design is likely the most feasible study design in general practice. Use of validated cough outcome measures is essential. The ascribed diagnostic criteria and the definition of cough resolution should be decided a priori. Ideally, an objective cough outcome (such as cough counts) should also be included as an outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 8 January 2014 | New search has been performed | Literature search updated |
| 8 January 2014 | New citation required and conclusions have changed | One new study included; conclusions updated |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 22 June 2009 | New search has been performed | Literature search rerun; no new studies found |
| 22 July 2008 | Amended | Converted to new review format |
| 1 February 2008 | New citation required and conclusions have changed | Substantive amendments made |
Acknowledgements
We thank Chris Cates, Toby Lasserson and Emma Welsh for their advice and for providing support and comments on the protocol and the review. We also thank Susan Hansen and Elizabeth Stovold for performing the searches and for obtaining the relevant articles.
Christopher Cates was the editor for this review and commented critically on the review.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor Cough explode all trees #2 MeSH descriptor Bronchitis explode all trees #3 cough* or bronchit* #4 (#1 OR #2 OR #3) #5 MeSH descriptor Critical Pathways, this term only #6 MeSH descriptor Clinical Protocols, this term only #7 MeSH descriptor Guidelines, this term only #8 MeSH descriptor Practice Guidelines, this term only #9 (clinical path* or clinical guide* or critical path* or care map* or care path* or pathway*) #10 (#5 OR #6 OR #7 OR #8 OR #9) #11 MeSH descriptor Pediatrics explode all trees #12 MeSH descriptor Child explode all trees #13 MeSH descriptor Infant explode all trees #14 MeSH descriptor Adolescent explode all trees #15 child* or paediat* or pediat* or adolesc* or infan* or toddler* or bab* or young* or preschool* or "pre school*" or pre‐school* or newborn* or "new born*" or new‐born* or neo‐nat* or neonat* #16 (#11 OR #12 OR #13 OR #14 OR #15) #17 (#4 AND #10 AND #16)
MEDLINE (Ovid)
1 exp COUGH/ 2 exp BRONCHITIS/ 3 (cough$ or bronchit$). 4 or/1‐3 5 clinical pathways/ or Clinical Protocols/ 6 guidelines/ or practice guidelines/ 7 exp "guideline [publication type]"/ 8 (clinical path$ or clinical guide$ or critical path$ or care map$ or care path$ or pathway$).mp. 9 or/5‐8 10 adolescent/ or exp child/ or exp infant/ 11 exp pediatrics/ 12 (child$ or paediat$ or pediat$ or adolesc$ or infan$ or toddler$ or bab$ or young$ or preschool$ or pre school$ or pre‐school$ or newborn$ or new born$ or new‐born$ or neo‐nat$ or neonat$).mp. 13 or/10‐12 14 4 and 9 and 13
RCT filter
1. (controlled clinical trial or randomised controlled trial).pt. 2. (randomised or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. Animals/ 10. Humans/ 11. 9 not (9 and 10) 12. 8 not 11
EMBASE (Ovid)
1 exp COUGHING/ 2 exp BRONCHITIS/ 3 (cough$ or bronchit$).tw. 4 or/1‐3 5 clinical pathway/ 6 practice guideline/ or clinical pathway/ or clinical protocol/ or consensus development/ or good clinical practice/ or nursing care plan/ or nursing protocol/ 7 (clinical path$ or clinical guide$ or critical path$ or care map$ or care path$ or pathway$).mp. 8 or/5‐7 9 exp adolescent/ or exp child/ or exp infant/ or exp newborn/ 10 (child$ or paediat$ or pediat$ or adolesc$ or infan$ or toddler$ or bab$ or young$ or preschool$ or pre school$ or pre‐school$ or newborn$ or new born$ or new‐born$ or neo‐nat$ or neonat$).tw. 11 exp pediatrics/ 12 or/9‐11 13 4 and 8 and 12
RCT filter
1. Randomized Controlled Trial/ 2. randomisation/ 3. controlled clinical trial/ 4. Double Blind Procedure/ 5. Single Blind Procedure/ 6. Crossover Procedure/ 7. (clinica$ adj3 trial$).tw. 8. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 9. exp Placebo/ 10. placebo$.ti,ab. 11. random$.ti,ab. 12. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 13. (crossover$ or cross‐over$).ti,ab. 14. or/1‐13 15. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 16. human/ or normal human/ or human cell/ 17. 15 and 16 18. 15 not 17 19. 14 not 18
Cochrane Airways Group Register of Trials (CAGR)
#1 MeSH DESCRIPTOR cough
#2 (cough*) AND (INREGISTER)
#3 COUGH:MISC1
#4 #1 OR #2 OR #3
#5 guideline* or pathway* or protocol*
#6 (care NEXT map*) or (care NEXT path*)
#7 #5 or #6
#8 #4 and #7
Data and analyses
Comparison 1. Clincal pathway versus wait‐list control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure—primary outcome | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Proportion of participants who were not cured at follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 PC‐QOL mean score at 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Duration of cough post randomisation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Proportion of adverse events experienced | 1 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Proportions of participants experiencing adverse events or complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.5. Analysis.
Comparison 1 Clincal pathway versus wait‐list control, Outcome 5 Proportion of adverse events experienced.
1.6. Analysis.

Comparison 1 Clincal pathway versus wait‐list control, Outcome 6 Proportions of participants experiencing adverse events or complications.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chang 2013.
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: children (< 18 years of age) with chronic cough (> 4 weeks) newly referred to specialist paediatric respiratory clinics at 5 Australian sites (Brisbane, Darwin, Melbourne, Sydney, Canberra). Exclusion criteria: children with known respiratory illness previously diagnosed by a respiratory physician or confirmed on objective testing (e.g. cystic fibrosis, bronchiectasis) before the time of referral Children assessed: n = 346 (n = 30 did not meet inclusion criteria, n = 44 declined participation) Children randomised: n = 272 (early use n = 140, delayed use n = 132) |
|
| Interventions |
|
|
| Outcomes | Primary outcomes:
|
|
| Notes | Because of the nature of the study, data collected up until the week 6 time point have been selected for inclusion in this review, as this represents use (early arm) vs non‐use (delayed arm) of the algorithm. Information beyond week 6 of the study has not been included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors refer to previously published trial protocol (Chang 2010): Study authors clearly describe computer‐generated randomisation sequence using permutated blocks of 4 or 6, stratified according to participant age and study site location |
| Allocation concealment (selection bias) | Low risk | Study authors state concealed allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Complete blinding was not possible. Participants and research personnel collecting data were not specifically informed about the arm to which they were allocated. However, the allocated arm could easily be determined if these individuals made the effort to look at time between random assignment and clinical review |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study authors clearly describe (in previously published protocol (Chang 2010)) outcomes measured by blinded assessor and provide a description of how this was achieved |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data were measured in > 90% of participants |
| Selective reporting (reporting bias) | Low risk | Progress of all randomly assigned participants was clearly described |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asilsoy 2008 | Evaluation of chronic cough using American College of Chest Physicians (ACCP) guidelines. Excluded from review, as not a randomised controlled trial (RCT) |
| Dettmar 2009 | Study excluded, as not an RCT and not examining use of a management pathway. Study was a prospective cohort study of an online diagnostic website for adult patients with chronic cough. The diagnostic website allowed participants to provide information about their likely diagnosis that was based on a predetermined algorithm, which differentiated among 3 common causes of chronic cough (reflux, asthma and rhinitis) according to European Respiratory Society guidelines for chronic cough |
| English 2006 | Study excluded, as not RCT and not examining use of a pathway for chronic cough. Study was a cross‐sectional evaluation of the accuracy of guidelines for screening patients for tuberculosis. Study found that with implementation of clinical guidelines for nurse practitioner screening of patients for suspected tuberculosis infection, a 68% increase in the rate of tuberculosis case detection was reported |
| Flores‐Hernandez 1999 | Study excluded, as not RCT and not examining use of a clinical pathway for chronic cough in children. Before and after study of clinical guidelines for the management of acute respiratory infection, findings show that after implementation of management guidelines, inappropriate prescribing of antibiotics and cough syrups was decreased |
| Hover 2000 | Study excluded, as not RCT and not examining chronic cough in children. Study was an evaluation performed via pretreatment and post‐treatment analysis and randomised chart review of implementation of principles of the American Academy of Pediatrics for the management of common office infections. Study did not utilise a clinical pathway and did not treat children with chronic cough |
| Nagel 2009 | Review article presenting diagnostic pathway and treatment options for chronic (> 4 weeks) cough in children (published in German). Excluded, as not a research study. Paper presented a diagnostic and management pathway similar to those presented by Chang 2013 and Rehman 2009, and reiterated that cough lasting longer than 4 weeks in a child warrants thorough investigation for underlying pathology |
| Norton 2007 | Prospective cohort study examining the effectiveness of a clinical pathway in reducing hospitalisation for acute asthma episodes in children presenting to the emergency department of a children's hospital. Study showed that after the clinical pathway had been implemented, hospital admissions in children with moderate to severe asthma were reduced by > 50% with no increase in re‐presentations. Excluded, as not RCT and pathway designed for acute asthma care, not chronic cough |
| Rehman 2009 | This study was excluded, as it was not an RCT. This prospective cohort study of a management algorithm for diagnosis of causes of chronic cough in children 6 to 59 months of age was specifically designed for developing countries. Investigators aimed to establish the positive predictive value of the algorithm. This study found that the positive predictive value of the algorithm in predicting clinical diagnosis was 0.921 |
| Rutten 1991 | RCT examining use of an educational programme (participant handout) on cough and effects on the consulting behaviour of participants after they had received the intervention. Study excluded, as reported participant numbers did not specify numbers of children included in the study. We contacted the study author to obtain the numbers relevant for children; these data were not available. Study also excluded, as the intervention used was not a clinical pathway, and the intervention was used for participants presenting for acute cough episodes, not for chronic cough |
| Spelman 1991 | Prospective cohort study examining the hypothesis that children with chronic cough will develop asthma. 106 participants with chronic cough, younger than 10 years of age, from Irish general practitioners, were treated according to an asthma protocol for 16 weeks. Follow‐up 2 years later showed that 71 children had been subsequently diagnosed with asthma. Study excluded, as not RCT, and the protocol used was not specific to the treatment of children with chronic cough |
Differences between protocol and review
Differences between the protocol and the review are described throughout the text. We updated the risk of bias tool and other methods to bring the review in line with current recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions and added a summary of findings table. We removed many of the sensitivity analyses specified in methods used previously for this review.
Contributions of authors
The first review was written primarily by EJB and ABC, who also reviewed the abstracts and articles. In this updated review, EJB and ABC reviewed the searches from 2008 to 2012; GBM and ABC reviewed search data from 2012 to 2014. GBM and ABC extracted and entered data for the 2014 update and drafted the review. All review authors approved the review before submission.
Sources of support
Internal sources
Royal Children's Hospital Foundation, Brisbane, Australia.
External sources
National Health and Medical Research Council, Australia.
Declarations of interest
ABC and PSM are authors of the included trial of a management protocol for the treatment of children with chronic cough. EJB was involved in the early conduct of this trial (for the first year of the trial) but was not involved in development of the study protocol, data analysis or manuscript production.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Chang 2013 {published and unpublished data}
- Chang AB, Robertson CF, Asperen PP, Glasgow NJ, Masters IB, Teoh L, et al. Effect of a cough algorithm on chronic cough in children: a multicentre randomised controlled trial. Pediatrics 2013;131:e1576‐83. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asilsoy 2008 {published data only}
- Asilsoy S, Bayram E, Agin H, Apa H, Can D, Gulle S. Evaluation of chronic cough in children. Chest 2008;134(6):1122‐8. [DOI] [PubMed] [Google Scholar]
Dettmar 2009 {published data only}
English 2006 {published data only}
- English RG, Bachmann MO, Bateman ED, Zwarenstein MF, Fariall LR, Bheekie A, et al. Diagnositc accuracy of an integrated respiratory guideline in identifying patients with respiratory symptoms requiring screening for pulmonary tuberculosis: a cross‐sectional study. BMC Pulmonary Medicine 2006;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flores‐Hernandez 1999 {published data only}
- Flores‐Hernandez S, Treyo Y, Perez JA, Reyes‐Morales H, Perez‐Cuevas R, Loera‐Romo G, et al. Design and applicability of a clinical guide for appropriate attention in acute respiratory infections [Diseno y aplicabilidad de una guia clinica para la atencion apropiada en las infecciones respiratorias agudas]. Gaceta Medica De Mexico 1999;135(2):121‐37. [PubMed] [Google Scholar]
Hover 2000 {published data only}
- Hover AR, Cornwell V, Stevenson S, Sponenberg D. Evaluations of the American Academy of Pediatrics principles on management of common office infections in a managed care setting. Missouri Medicine 2000;97(12):541‐4. [PubMed] [Google Scholar]
Nagel 2009 {published data only}
- Nagel F, Griese M. What to do if children cough for more than 4 weeks: diagnostic pathways and therapeutic options [Infektion, Allergie order Fehlbildung? Wenn Kinder chronisch husten]. MMW Fortschritte Der Medizin 2009;151:48‐52. [PubMed] [Google Scholar]
Norton 2007 {published data only}
- Norton SP, Pusic MV, Taha F, Heathcote S, Carleton BC. Effect of a clinical pathway on the hospitalisation rates of children with asthma: a prospective study. Archives of Disease in Childhood 2007;92:60‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rehman 2009 {published data only}
- Rehman A, Waraich MM, Ullah I. Algorithm for the diagnosis of chronic cough in children 6 to 59‐Months. Pakistan Paediatric Journal 2009;33(1):30‐8. [Google Scholar]
Rutten 1991 {published and unpublished data}
- Rutten G, Eijk J, Beek M, Velden H. Patient education about cough: effect on the consulting behaviour of general practice patients. British Journal of General Practice 1991;41:289‐92. [PMC free article] [PubMed] [Google Scholar]
Spelman 1991 {published data only}
- Spelman R. Two‐year follow up of the management of chronic or recurrent cough in children according to an asthma protocol. British Journal of General Practice 1991;41:406‐9. [PMC free article] [PubMed] [Google Scholar]
Additional references
Barr 2005
- Barr RL, McCrystal DJ, Perry CF, Chang AB. A rare cause of specific cough in a child: the importance of following‐up children with chronic cough. Cough 2005;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berg 1997
- Berg M. Problems and promises of the protocol. Social Science and Medicine 1997;44(8):1081. [DOI] [PubMed] [Google Scholar]
Britt 1999
- Britt H, Sayer GP, Miller GC, Charles J, Scahill S, Horn F. Bettering the evaluation and care of health ‐ a study of general practice activity. General Practice Series. Cat. no. GEP 1. Canberra: Australian Institute of Health and Welfare, 1999. [Google Scholar]
Britt 2008
- Britt H, Miller GC, Bayram C, Pan Y, Henderson J, Valenti L, et al. General practice activity in Australia 2006‐07. http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=6442456174 (accessed 11 March 2014).
Cates 2003 [Computer program]
- Cates C. Visual Rx. Online NNT Calculator. http://www.nntonline.net/, 2003.
Chang 2010
- Chang AB, Robertson CF, Asperen PP, Glasgow NJ, Masters IB, Mellis CM. Can a management pathway for chronic cough in children improve clinical outcomes: protocol for a multicentre evaluation. Trials 2010;11:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2005
- Chang AB. Cough: are children really different to adults?. Cough 2005;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2005b
- Chang AB, Eastburn MM, Gaffney J, et al. Cough quality in children: a comparison of subjective vs. bronchoscopic findings. Respiratory Research 2005;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2006a
- Chang AB, Landau LI, Asperen P, Glasgow NJ, Robertson CF, Marchant JM, et al. Cough in children: definitions and clinical evaluation. position statement of the Thoracic Society of Australia and New Zealand. Medical Journal of Australia 2006;184(8):398‐403. [DOI] [PubMed] [Google Scholar]
Chang 2006b
- Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence‐based clinical practice guidelines. Chest 2006;129(1):260S‐83S. [DOI] [PubMed] [Google Scholar]
Chang 2012
- Chang AB, Robertson CF, Asperen PP, et al. A multi‐centre study on chronic cough in children: burden and etiologies based on a standardized management pathway. Chest 2012;142:943‐50. [DOI] [PubMed] [Google Scholar]
Cherry 2003
- Cherry DK, Burt CW, Woodwell DA. National ambulatory medical care survey: 2001 summary. Advance Data 2003;11(337):1‐44. [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fessler 2005
- Fessler HE, Brower RG. Protocols for lung protective ventilation. Critical Care Medicine 2005;33(3 Suppl):S223‐7. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2006
- Fitzgerald DA. Guest editorial. Paediatric Respiratory Reviews 2006;7(1):1. [DOI] [PubMed] [Google Scholar]
Gupta 2007
- Gupta A, McKean M, Chang AB. Management of chronic non‐specific cough in childhood: an evidence based review. Archives of Diseases in Childhood 2007;92:33‐9. [DOI] [PubMed] [Google Scholar]
Hay 2004
- Hay AD, Wilson A, Fahey T, Peters TJ. The inter‐observer agreement of examining pre‐school children with acute cough: a nested study. BMC Family Practice 2004;5:4. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S [editors]. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.cochrane‐handbook.org.
Irwin 1990
- Irwin RS, Curely FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. American Review of Respiratory Disease 1990;141(3):640‐7. [DOI] [PubMed] [Google Scholar]
Irwin 2006
- Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, et al. Diagnosis and management of cough executive summary: ACCP evidence‐based clinical practice guidelines. Chest 2006;129(1 Suppl):1S‐23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karabel 2013
Karakoc 2002
- Karakoç F, Karadağ B, Akbenlioğlu C, Ersu R, Yildizeli B, Yüksel M, et al. Foreign body aspiration: what is the outcome?. Pediatric Pulmonology 2002;34(1):30‐6. [DOI] [PubMed] [Google Scholar]
Kohno 2006
- Committee for the Japanese Respiratory Society Guidelines for Management of Cough, Kohno S, Ishida T, Uchida Y, Kishimoto H, Sasaki H, et al. The Japanese Respiratory Society guidelines for management of cough. Respirology 2006;11(Suppl 4):S135‐S186. [DOI] [PubMed] [Google Scholar]
Kwan 2004
- Kwan J, Sandercock P. In‐hospital care pathways for stroke. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD002924.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchant 2006
- Marchant JA, Masters IB, Taylor S, Cox NC, Seymour GJ, Chang AB. Evaluation and outcome of young children with chronic cough. Chest 2006;129(5):1132‐41. [DOI] [PubMed] [Google Scholar]
Marchant 2006b
- Marchant JM, Masters IB, Taylor SM, Chang AB. Utility of signs and symptoms of chronic cough in predicting specific cause in children. Thorax 2006;61(8):694‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchant 2008
- Marchant JM, Newcombe PA, Juniper EF, Sheffield JK, Stathis SL, Chang AB. Chronic cough in children and its burden on parents. Chest 2008;134:303‐9. [DOI] [PubMed] [Google Scholar]
Newcombe 2010
- Newcombe PA, Sheffield JK, Chang AB. Minimally important change in a parent‐proxy quality of life questionnaire for pediatric chronic cough (PC‐QOL). Chest 2010;139:576‐80. [DOI] [PubMed] [Google Scholar]
Newcombe 2010b
- Newcombe PA, Sheffield JK, Juniper EF, Petsky HL, Willis C, Chang AB. Validation of a parent‐proxy quality‐of‐life questionnaire (PC‐QOL) for paediatric chronic cough. Thorax 2010;65(9):819‐23. [DOI] [PubMed] [Google Scholar]
Preiser 2004
- Preiser JC, Ledoux D. The use of protocols for nutritional support is definitely needed in the intensive care unit. Critical Care Medicine 2004;32(11):2354‐5. [DOI] [PubMed] [Google Scholar]
Shields 2006
- Shields MD. Diagnosing chronic cough in children. Thorax 2006;61(8):647‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 2007
- Shields MD, Bush A, Everard ML, McKenzie SA, Primhak R. British Thoracic Society Guidelines: recommendations for the assessment and management of cough in children. Thorax 2007;63(Suppl 3):iii1‐iii15. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bailey 2004
- Bailey EJ, Morris PS, Kruske SG, Chang AB. Clinical pathways for chronic cough in children. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006595.pub2] [DOI] [PubMed] [Google Scholar]


