Abstract
Background
The burden of asthma on patients and healthcare systems is substantial. Interventions have been developed to overcome difficulties in asthma management. These include chronic disease management programmes, which are more than simple patient education, encompassing a set of coherent interventions that centre on the patients' needs, encouraging the co‐ordination and integration of health services provided by a variety of healthcare professionals, and emphasising patient self‐management as well as patient education.
Objectives
To evaluate the effectiveness of chronic disease management programmes for adults with asthma.
Search methods
Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Effective Practice and Organisation of Care (EPOC) Group Specialised Register, MEDLINE (MEDLINE In‐Process and Other Non‐Indexed Citations), EMBASE, CINAHL, and PsycINFO were searched up to June 2014. We also handsearched selected journals from 2000 to 2012 and scanned reference lists of relevant reviews.
Selection criteria
We included individual or cluster‐randomised controlled trials, non‐randomised controlled trials, and controlled before‐after studies comparing chronic disease management programmes with usual care in adults over 16 years of age with a diagnosis of asthma. The chronic disease management programmes had to satisfy at least the following five criteria: an organisational component targeting patients; an organisational component targeting healthcare professionals or the healthcare system, or both; patient education or self‐management support, or both; active involvement of two or more healthcare professionals in patient care; a minimum duration of three months.
Data collection and analysis
After an initial screen of the titles, two review authors working independently assessed the studies for eligibility and study quality; they also extracted the data. We contacted authors to obtain missing information and additional data, where necessary. We pooled results using the random‐effects model and reported the pooled mean or standardised mean differences (SMDs).
Main results
A total of 20 studies including 81,746 patients (median 129.5) were included in this review, with a follow‐up ranging from 3 to more than 12 months. Patients' mean age was 42.5 years, 60% were female, and their asthma was mostly rated as moderate to severe. Overall the studies were of moderate to low methodological quality, because of limitations in their design and the wide confidence intervals for certain results.
Compared with usual care, chronic disease management programmes resulted in improvements in asthma‐specific quality of life (SMD 0.22, 95% confidence interval (CI) 0.08 to 0.37), asthma severity scores (SMD 0.18, 95% CI 0.05 to 0.30), and lung function tests (SMD 0.19, 95% CI 0.09 to 0.30). The data for improvement in self‐efficacy scores were inconclusive (SMD 0.51, 95% CI ‐0.08 to 1.11). Results on hospitalisations and emergency department or unscheduled visits could not be combined in a meta‐analysis because the data were too heterogeneous; results from the individual studies were inconclusive overall. Only a few studies reported results on asthma exacerbations, days off work or school, use of an action plan, and patient satisfaction. Meta‐analyses could not be performed for these outcomes.
Authors' conclusions
There is moderate to low quality evidence that chronic disease management programmes for adults with asthma can improve asthma‐specific quality of life, asthma severity, and lung function tests. Overall, these results provide encouraging evidence of the potential effectiveness of these programmes in adults with asthma when compared with usual care. However, the optimal composition of asthma chronic disease management programmes and their added value, compared with education or self‐management alone that is usually offered to patients with asthma, need further investigation.
Plain language summary
Chronic disease management for asthma
Asthma is a chronic (long‐term) airway (breathing) disease affecting about 300 million people worldwide. People with asthma have many symptoms, such as wheezing, coughing and shortness of breath. The aim of a chronic disease management programme for asthma is to improve the quality and effectiveness of asthma care by creating a programme that is centred on patient's needs, encourages the co‐ordination of the health services provided by healthcare professionals such as doctors and nurses, who should work together, and focuses on helping the patients to manage their illness themselves as well as providing them with information to help them understand their illness.
This review found 20 studies that compared the effects of chronic disease management programmes in adults with asthma with the effects of usual care. The average age of the patients was 42.5 years, 60% were women, and they had moderate to severe asthma. Overall the evidence that was found was of moderate to low quality.
Chronic disease management programmes for adults with asthma probably improve patients' quality of life, reduce the severity of the asthma, and improve breathing as demonstrated by improved performance in lung function tests after 12 months. It is unclear whether chronic disease management programmes improve the patients' abilities to manage their own asthma or decrease the number of hospitalisations or emergency visits.
Summary of findings
Summary of findings for the main comparison. Chronic disease management compared with usual care for adults with asthma.
| Chronic disease management compared with usual care for adults with asthma | ||||||
| Patient or population: adults with asthma Settings: 7 studies in primary care practices, 3 in outpatient hospital departments, 3 in pharmacies, 2 in health maintenance organisations (HMOs), 5 in mixed settings Intervention: chronic disease management Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Chronic disease management | |||||
| Asthma‐specific quality of life score Measured on different scales in different studies. Higher scores indicate higher quality of life. Follow‐up: 3 to 12 months | The mean asthma‐specific quality of life score ranged across control groups from 3.8 to 5.31 | The mean asthma‐specific quality of life score in the intervention groups was 0.22 standard deviations higher (0.08 to 0.37 higher) | 1627 (8 studies) | ⊕⊕⊕⊝ moderate2 | A SMD of 0.22 represents a small improvement in quality of life. On the AQLQ scale, it represents a mean difference of 0.31 (0.11 to 0.53). MCID of AQLQ = 0.53,4 | |
| Number of hospitalisations per patient ‐ not reported | The mean number of hospitalisations per patient ranged across control groups from 0.06 to 1.23 | The mean number of hospitalisations per patient ranged across intervention groups from 0.02 to 0.4 | Not estimable | ‐ | Not assessed;see comment | Data too heterogeneous to perform meta‐analysis |
| Number of emergency room or unscheduled visits ‐ not reported | The mean number of emergency room or unscheduled visits per patient ranged across control groups from 0.02 to 1.4 | The mean number of emergency room or unscheduled visits per patient ranged across intervention groups from 0.02 to 1.9 | Not estimable | ‐ | Not assessed; see comment | Data too heterogeneous to perform meta‐analysis |
| Asthma exacerbations ‐ not measured | Not assessed | Not assessed | Not estimable | ‐ | Not assessed;see comment | No data available for meta‐analysis |
| Self‐efficacy score5 Measured on different scales in different studies. Higher scores indicate higher self‐efficacy Follow‐up: 3 to 12 months | 5 | The mean self‐efficacy score in the intervention groups was 0.51 standard deviations higher (0.08 lower to 1.11 higher) | 642 (5 studies) | ⊕⊕⊝⊝ low6,7 | A SMD of 0.51 represents a moderate improvement in self‐efficacy3,8 | |
| Asthma severity score5 Measured on different scales in different studies. Higher scores indicate lower severity Follow‐up: 6‐12 months | The mean asthma severity score in the intervention groups was 0.18 standard deviations higher (0.05 to 0.3 higher) | 1330 (6 studies) | ⊕⊕⊝⊝ low6,9 | A SMD of 0.18 represents a small improvement in asthma severity<BR/>3,8 | ||
| Days off work ‐ not measured | Not assessed | Not assessed | Not estimable | ‐ | Not assessed;see comment | No data available for meta‐analysis |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Values correspond to the AQLQ scores only 2 Downgraded because majority of studies were at high or unclear risk of bias 3 As a rule of thumb: SMD < 0.40 = small effect, SMD 0.40 to 0.70 = moderate effect, SMD > 0.70 = large effect 4 The score was estimated using a SD of 1.43 (control group of Gallbreath's study) 5 No assumed risk presented for usual groups because too much variation in the instruments or scales used 6 Downgraded because of clinical, statistical and measurement heterogeneity 7 Wide 95% CIs 8 Back‐transformation was not performed because all studies used another instrument or scale 9 Downgraded because three studies were at high risk of bias
Background
Description of the condition
Asthma is a chronic inflammatory disorder of the airways, affecting an estimated 300 million people worldwide (GINA 2012). The prevalence of asthma in adults is up to 10% in developed countries, and is currently rising (Asher 2006; Braman 2006; Masoli 2004). Despite being a common chronic disease, it does not rank among the 15 first projected causes of mortality or disability‐adjusted life years (Mathers 2006). Nevertheless, asthma places a substantial burden on affected people and healthcare systems, with morbidity, mortality, and economic burdens that have been increasing during the last 40 years (Braman 2006).
In order to achieve effective asthma control, the Global Initiative for Asthma (GINA) has been providing, since 2002, an evidence‐based global strategy for asthma management and prevention (GINA 2012). However, despite the existence of effective therapies and the development of evidence‐based guidelines, there are still significant practice variations and gaps between recommended care and current practice (Klomp 2008; Vermeire 2002). Asthma could be controlled, but its management remains suboptimal (Leuppi 2006; Vermeire 2002).
The difficulties in asthma management are multiple, including poor implementation of treatment guidelines, suboptimal patient education and self‐management, poor patient adherence to treatment and lifestyle modifications, neglect of preventive care, and lack of co‐ordination between healthcare providers, among others (Latry 2008; Mäkinen 1999; Pacheco 1999; Vermeire 2002). A variety of individual interventions have been used to address these issues and systematically assessed. For instance, a Cochrane review showed that self‐management programmes in adult asthmatics reduced healthcare utilisation, the number of days off work or school, nocturnal asthma, and improved quality of life (Gibson 2003). In contrast, limited patient education (information only) did not (Gibson 2002). Similarly, two other systematic reviews examining the effectiveness of written asthma action plans did not find evidence of benefit (Powell 2003; Toelle 2004). Nevertheless, given that the included studies were small and of low power, experts still recommend the use of written action plans (GINA 2012; NAEPP 2007). Finally, provider level interventions such as continuing medical education, reminder systems, or audit with feedback yielded inconsistent results across chronic diseases (Davis 1995; Davis 1999; Weingarten 2002). The combination of all these types of interventions is proposed in chronic disease management programmes.
Description of the intervention
Chronic disease management (CDM) was developed during the 1990s as a means of reorganising healthcare systems and medical treatment for chronic diseases such as heart failure, diabetes, depression, and chronic lung diseases. Its purpose is to enhance the quality and cost‐effectiveness of care for chronic diseases. CDM is centred on patients' needs, fosters the co‐ordination and integration of health services provided by various professionals who should work together (multidisciplinary care), and emphasizes patients’ self‐management as well as education and empowerment. CDM is also based on formal evidence of effectiveness and promotes continuous improvement processes through quality control (DMAA Definitions 2009; Ellrodt 1997; Epstein 1996; Faxon 2004; Hunter 1997; Kesteloot 1999; Pilnick 2001; Weingarten 2002).
Several definitions of CDM, which differ by the number and variety of elements that they integrate, have been published (DMAA Definitions 2009; Ellrodt 1997; Epstein 1996; Faxon 2004; Hunter 1997; Kesteloot 1999; Pilnick 2001; Weingarten 2002). In addition, the American Heart Association’s Disease Management Taxonomy Writing Group developed a system of classification intended to help categorise and compare disease management programmes (Krumholz 2006). Recently, to facilitate the understanding and communication about the concept of CDM, Schrijvers 2009 proposed a tentative definition of chronic disease management based on the elements found in the literature: "[CDM] consists of a group of coherent interventions designed to prevent or manage one or more chronic conditions using a systematic multidisciplinary approach potentially employing multiple treatment modalities. The goal of chronic disease management is to identify persons at risk for one or more chronic conditions, to promote self‐management by patients and to address the illness or conditions with maximum clinical outcome, effectiveness and efficiency regardless of treatment setting(s) or typical reimbursement patterns” (Schrijvers 2009). Because CDM programmes are adapted to the regional healthcare, social, and political contexts, they vary in terms of treatment modalities, frequency, intensity, and duration. Nevertheless, several systematic reviews have shown that CDM programmes are effective, at least for some outcomes and some chronic diseases such as diabetes (Egginton 2012; Elissen 2013; Knight 2005; Norris 2002; Pimouguet 2011), depression (Badamgarav 2003; Neumeyer‐Gromen 2004), chronic heart failure (Gohler 2006; Gonseth 2004; McAlister 2001; Roccaforte 2005), and chronic obstructive pulmonary disease (COPD) (Adams 2007; Kruis 2013; Lemmens 2013; Niesink 2007; Peytremann‐Bridevaux 2008), or across chronic conditions (de Bruin 2011; Ofman 2004; Ouwens 2005; Tsai 2005). As such, they are supported by an increasing number of healthcare systems (Busse 2004; Gogovor 2008; Montague 2007; Steuten 2007; Stock 2006) and have been implemented throughout Northern American and European countries during the past decade (NCSL DMP descriptions).
Why it is important to do this review
Asthma presents all characteristics described as mandatory for CDM suitability (Mechanic 2002; Velasco‐Garrido 2003). Indeed, asthma CDM programmes have yielded positive results in some studies and are considered a promising way to improve asthma management and reduce costs (Blaiss 2005; Durbin 1997; Steuten 2007a). Still, the effectiveness of CDM for adults with asthma has yet to be systematically and comprehensively assessed. One systematic review evaluating CDM programmes for patients with asthma found that these programmes reduced resource utilisation and improved some aspects of self‐management and organisation of care, but had almost no impact on asthma symptoms and lung function (Steuten 2007a). However, it only included studies published between December 2005 and December 2006 and considered both adults and children with asthma. The authors also showed that while process and outcome measures were more appropriately chosen than before, structure indicators were lacking. Two other systematic reviews published in 2009 (Lemmens 2009; Maciejewski 2009) provided further information. The Lemmens 2009 review showed that CDM programmes targeting adults with asthma or COPD improved quality of life and decreased the risk of hospitalisation, especially when the interventions included three components, but did not have any effect on emergency visits. The authors of the other review on CDM programmes, targeting only adults with asthma, decided not to conduct meta‐analyses because of heterogeneity and missing information; they concluded that the quality of studies was not optimal and that it was not possible to decide whether CDM would or would not be beneficial to patients with asthma (Maciejewski 2009).
Objectives
Primary objective
To assess the effectiveness of chronic disease management programmes for adults with asthma.
Secondary objectives
To assess the effectiveness of chronic disease management programmes for adults with asthma according to the intensity of the intervention (e.g., more intensive versus minimal interventions, in terms of number of intervention components and types of components, such as mainly centred on the patient versus on healthcare professionals).
Methods
Criteria for considering studies for this review
Types of studies
Eligible studies were randomised controlled trials (RCTs), non‐randomised controlled trials (NRCTs), controlled before‐after studies (CBAs), and interrupted time series studies (ITSs), allocating patients or clusters. According to the guidance from the Effective Practice and Organisation of Care (EPOC) Review group (EPOC 2013), CBA studies were eligible only if the pre‐ and post‐intervention periods for the study and control sites were the same; if the study and control sites were comparable with respect to the dominant reimbursement system, level of care, setting of care, and academic status; and if there were a minimum of two interventions and two control sites. ITS studies were eligible only if there was a clear, time‐defined beginning of the intervention and if there was a minimum of three measurement points available before and after the intervention.
The rationale for including study types other than RCTs was that it can be difficult to implement RCTs assessing complex disease management programmes. Additionally, this is a relatively new research area with few RCTs.
Types of participants
We included adult participants (over 16 years of age) with a diagnosis of asthma. We excluded studies in which patients with other significant pulmonary chronic disease (like moderate or severe COPD or bronchiectasis) represented a significant proportion of participants, unless subgroup analysis was available. In the same way, trials including both adults and children were included only if the majority of participants were over 16 years or if the adult subgroup was analysed independently.
Types of interventions
Based on several definitions of disease management (DMAA Definitions 2009; Ellrodt 1997; Epstein 1996; Faxon 2004; Hunter 1997; Kesteloot 1999; Pilnick 2001; Weingarten 2002), we considered the following five criteria for our operational definition of CDM:
at least one organisational component (i.e., elements that interfere with the care process or that aim to improve continuity of care) targeting patients (Steuten 2007a; Weingarten 2002);
at least one organisational component targeting healthcare professionals (e.g., physicians, nurses, etc.), the healthcare system, or both;
presence of a patient education or self‐management support component, or both;
active involvement of two or more healthcare professionals in patient care; and
minimum duration of three months (or 12 weeks) for at least one component.
Therefore, we only included CDM programmes that entirely met the above operational definition of chronic disease management (that is, all five criteria are compulsory). Below are listed the types of components that are usually proposed in CDM programmes, adapted to asthma patients. They directly relate to the above‐mentioned five criteria.
1. At least one organisational component targeting patients (each of the following was considered as an independent component):
case management (defined as explicit allocation of co‐ordination tasks to a case manager or a small team who takes responsibility for guiding the patient through the care process in the most efficient, effective, and acceptable way);
structured follow‐up (e.g., telephone calls, regular clinic visits, etc.) or encouragement for regular follow‐up;
home or outreach visits;
discharge planning in the case of hospitalisation;
advice or assistance, or both, if needed (e.g., a telephone hotline);
smoking cessation programmes recommended or proposed, or both; and
other (other components deemed compatible by all the review authors).
2. At least one organisational component targeting primarily healthcare professionals (for example physicians, nurses, etc.) or the healthcare system, or both, such as:
explicit teamwork and collaborative processes between healthcare providers;
physicians’ education and training (any format) or other healthcare professionals’ education and training, or both;
other quality improvement processes (e.g., reminder systems, clinical pathways, routine reporting, feedback loops, etc.);
integration of care (i.e., continuity of care between primary, secondary, and tertiary care);
financial incentives;
information technology (e.g., computerised medical records, reminders or prompts, etc.);
explicit use of evidence‐based medicine supports (e.g., use of evidence‐based clinical practice guidelines, etc.);
process and outcome measurements (at the patient level);
evaluation of CDM programmes (at the group level); and
other (other components deemed compatible by all the reviews authors).
3. Presence of a patient education or self‐management support component, or both. Patient education was defined as giving the patients information (materials, instructions, or both) regarding:
asthma;
management of the disease, its exacerbations, or both;
prevention of exacerbations (trigger recognition and reduction strategies);
smoking cessation;
exercise or physical activity.
The types of educational sessions included, for example:
distribution of published or printed material;
educational groups or meetings;
one‐on‐one educational sessions during visits (physician, nurses, etc.).
Self‐management support was defined as helping patients acquire the skills and knowledge to manage their own illnesses, providing self‐management tools, and routinely assessing their problems and accomplishments (Ouwens 2005). The types of self‐management support included, for example:
the availability of an action plan;
so‐called supervised reinforcement sessions;
regular checks of inhalation technique.
4. Two or more healthcare professionals actively involved in the patient care, such as:
general or family practitioners (GPs), primary care physicians, and general internists;
pulmonary care physicians;
respiratory care nurses (nurses with training in asthma management);
non‐specialised nurses;
physiotherapists;
pharmacists; and
other healthcare professionals (for example social workers).
5. Minimal duration of three months (12 weeks) for at least one component.
CDM programmes targeting chronic diseases require long lasting interventions, and should not be merely considered as another treatment modality but rather as a new way to organise care implemented from a long‐term perspective. Therefore, they needed to have at least one component from criteria one to three that lasted three months or more (arbitrary cut‐off point).
We compared CDM to standard care (varying from usual care to usual care including limited CDM components).
Types of outcome measures
Throughout the text, we use the term outcome in its broad sense to refer to the notion of dependent variable. Under that term, we considered clinically relevant effect measures (such as patient outcomes), process of care and intermediate measures, as well as structure indicators. These were based and adapted from a consensus of clinically relevant outcomes of an asthma patient management model (Clark 1994). Indicators relating to the implementation of CDM programmes, per se, were not considered. We divided our outcomes into two main groups: organisational and patient level outcomes. The list of possible outcomes, as well as the 10 outcomes selected as primary outcomes (specified in brackets) that were considered in the analyses, are shown below. We included 7 of these 10 primary outcomes in the Table 1, based on their clinical importance: quality of life, hospitalisation, emergency or unscheduled visits, asthma exacerbations, self‐efficacy, asthma severity, and days off school or work absences.
Organisational level outcomes
Organisation of care outcomes: participation rate for CDM programme; healthcare professionals’ satisfaction with programme.
Process outcomes: use of an action plan (primary); compliance with treatment schedule; prescription of inhaled corticosteroids; check of appropriate inhalation techniques; and smoking cessation advice or support, or both.
Healthcare utilisation outcomes: asthma‐related or all‐cause hospitalisation, or both, defined as any inpatient hospital stay (primary); asthma‐related or all‐cause unscheduled visits, or both, defined as urgent visits to hospital emergency departments (ED) or unscheduled physicians visits (primary); GP visits, defined as routine (scheduled) ambulatory care visits to a GP or family physician; and healthcare costs (direct and indirect, if available).
Patient level outcomes
Quality of life: an asthma‐specific quality of life instrument (primary) such as the St‐George Respiratory Questionnaire (Jones 1991), Living with Asthma Questionnaire (LWAQ) (Hyland 1991), Asthma Quality of Life Questionnaire (AQLQ) (Juniper 1992); a generic quality of life instrument such as the Short Form 36 (SF‐36) (Ware 1992), SF‐12 (Ware 1996), EQ‐5D (EuroQol Group 1990), or self reported subjective health.
Symptoms and activity level: asthma exacerbations (defined as prompting hospitalisation, ED visit, unscheduled medical visit, or rescue systemic glucocorticoids) (primary); asthma severity and symptoms (primary) (subjective measures that include asthma symptoms or severity scores, or both) (e.g., the Asthma Control Test (Nathan 2004), the Asthma Therapy Assessment Questionnaire (ATAQ) (Vollmer 1999)); days off school or work absences (due to asthma or other causes, or both) (primary); nights disturbed by asthma (sleep interruptions due to asthma or nights with asthma symptoms); days of restricted activity; use of rescue ß2‐agonists; and all‐cause mortality.
Self‐management: patients' asthma knowledge score; trigger recognition and reduction strategies; measures of self‐efficacy and self‐management (primary).
Pulmonary function tests: forced expiratory volume in 1 second (FEV1); peak expiratory flow rate (PEF); a combined measure of lung function, defined as either FEV1 or PEF (primary).
Patient satisfaction with care: measures of patient satisfaction (or experiences) with care (primary).
To define the timing of outcomes measurements, we grouped time points in three arbitrary intervals to represent short‐term, medium‐term, and long‐term outcomes (from 0 to 6 months, 6 to 12 months, and over 12 months).
Studies were excluded if none of the primary outcomes were reported.
Search methods for identification of studies
Electronic searches
M Fiander, Trials Search Co‐ordinator (TSC) for the EPOC review group, developed search strategies in consultation with the authors. The TSC searched the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects (DARE) for related systematic reviews and the databases listed below for primary studies. Searches were conducted to June 2014; exact search dates for each database are included in the search strategies in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5.
Cochrane Central Register of Controlled Trials (CENTRAL), OvidSP.
Cochrane EPOC Group Specialised Register (to 2012).
MEDLINE In‐Process and Other Non‐Indexed Citations (1946 on), OvidSP.
EMBASE (1947 on), OvidSP.
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1980 to 2012) , EBSCOhost.
PsycINFO (1806 on), OvidSP.
Search strategies were comprised of keywords, when available, and controlled vocabulary such as MeSH (Medical Subject Headings). Two methodological search filters were used to limit retrieval to appropriate study designs: the Cochrane highly sensitive search strategy (sensitivity‐ and precision‐maximizing version, 2008 revision) (Higgins 2011) to identify RCTs, and an EPOC methodology filter to identify non‐RCT designs. Language restrictions were not applied.
Searching other resources
We conducted handsearches of selected journals from 2000 to 2012. We also performed handsearching of reference lists of retrieved papers and relevant narrative or systematic reviews. To identify new and ongoing trials, we searched www.clinicaltrials.org and www.controlled‐trials.com/mrct.
Data collection and analysis
Selection of studies
We used a three‐step study screening procedure. First, based on titles only, one review author (CA, GG or IPB) excluded obviously non‐pertinent references. These excluded references were double‐checked by a second review author (CA, GG or IPB) to approve the exclusions. Then, based on abstracts, two review authors (CA, GG, POB or IPB) independently, and in duplicate, excluded previously retained articles if they represented a non‐original study, were obviously not focused on asthma, were obviously not on chronic disease management, or were clearly on another topic. Finally, articles deemed potentially relevant by any review author had their full texts assessed for eligibility by two review authors (CA, GG, POB or IPB). Reasons for excluding studies based on the full‐text assessment are described in the Characteristics of excluded studies table. Any disagreement about eligibility was resolved by discussion between the review authors and with the involvement of an arbitrator as necessary. Multiple published articles from a single study were treated as a single intervention evaluation. Because chronic disease management programmes were developed and first described in the early 1990s, studies from 1990 onwards were selected. In addition, since we were interested in the effectiveness of chronic disease management in adult asthmatic patients (16 years and over), we selected studies involving adults. The latter limit did not, however, exclude studies including both adult and non‐adult patients (< 16 years of age).
Data extraction and management
Two review authors (CA, GG or IPB) independently, and in duplicate, extracted data from selected studies using a tailored extraction form based on the generic Cochrane EPOC Review Group data collection checklist (EPOC 2013a). Any disagreement was resolved by discussion and if disagreement persisted an arbitrator was involved, as necessary. Where required, we sought additional information by contacting corresponding authors.
Asthma severity was determined by study self‐report, examination of FEV1 and PEF, or chronicity of asthma symptoms at baseline. Patients were categorised as having severe asthma if they had a mean FEV1 or PEF less than 0.6 of the predicted value, or if they reported daily asthma symptoms (Bateman 2008). Whenever possible, we categorised study populations as 'moderate to severe' if asthmatics with severe asthma were enrolled in the study population, and 'mild to moderate' otherwise.
Assessment of risk of bias in included studies
Two review authors (CA, GG or IPB) independently assessed the methodological quality of the included studies using the suggested risk of bias criteria for EPOC reviews (EPOC 2013b). Each individual component (sequence generation, allocation concealment, blinding of outcome assessment, completeness of outcome data, selective outcome reporting, baseline characteristics, baseline outcomes measurements, protection against contamination, and other sources of bias) was explicitly rated and categorised as being at low, unclear, or high risk of bias. Any disagreement was resolved by discussion or involvement of an arbitrator, or both. If necessary, we contacted study authors for additional information or clarification of the study methods. The same risk of bias table was used for all study designs considered in the review.
For sensitivity analyses, a summary assessment of the risk of bias of each study was done using one key domain of a study level entry (allocation concealment) and one key domain of an outcome level entry (incomplete outcome data) of the core Cochrane Collaboration tool. Studies were considered to be at: low risk of bias (high quality) if the two key domains were at low risk; at unclear risk of bias (moderate quality) if at least one of the key domains was at unclear risk and none at high risk; at high risk of bias (low quality) if at least one of the key domains was at high risk of bias.
Measures of treatment effect
In trials reporting score outcomes, we considered the results of the overall score if available. When not available, we selected one score or dimension of the scale as the representative outcome or calculated the average score if possible. If authors reported outcomes at more than one follow‐up period, we selected the period of follow‐up that matched the end of the intervention. The direction of the effect size was standardised so that a positive difference indicated improvement in the intervention group. Results of count data (that is, hospitalisations and ED or unscheduled visits) were treated as rate ratios.
For RCTs and NRCTs, we reported results of dichotomous outcomes as odd ratios (OR) and results of continuous outcomes as mean differences (MD) or standardised mean differences (SMD) if outcomes related to scores, using post‐intervention (follow‐up) values. We used the latter because there were more studies reporting these values and corresponding standard deviations (SD) compared to change from baseline values. In addition, because the number of patients at baseline and follow‐up were often not the same, change scores for individual studies could not be calculated by hand. Sensitivity analyses, using change from baseline values and change from baseline SDs, were conducted to assess the robustness of results according to the choice of MD estimates if data permitted.
Standardised effect sizes, which were calculated for continuous measures by dividing the difference in mean scores between the intervention and comparison group in each study by an estimate of the (pooled) SD, result in a 'scale free' estimate of the effect for each study. This can then be interpreted and pooled across studies regardless of the original scale of measurement used in each study (Laird 1990). We re‐expressed SMDs using rules of thumb (SMD < 0.4 = small effect, 0.4 to 0.7 = moderate effect , > 0.7 = large effect) or using the most commonly used instrument (back‐transformation of the effect size) to have measures that are clinically useful in daily practice, following the method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If available, we related the results to the minimal clinically important difference (MCID) of the instrument considered.
For CBA studies, we planned to report results of dichotomous outcomes as risk ratio (RR) derived from statistical analyses adjusting for baseline measures (such as logistic regressions) and results of continuous outcomes as MD or SMD derived from statistical analyses adjusting for baseline measures (such as linear regression models, mixed models, or hierarchical models). If adjusted results were not available, study data were excluded from the analyses.
Unit of analysis issues
Cluster‐randomised trials
Some cluster‐randomised trials might have a unit of analysis error, when the trial has not adjusted for data clustering. This error implies that confidence intervals and standard errors of effects are smaller (more precise) than they should be (Ukoumunne 1999). We noted the method of randomisation and unit of analysis for each included cluster trial and corrected the sample size by dividing it by the design effect, according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the intraclass correlation coefficient or the number of clusters was not reported and attempts to contact the authors were unsuccessful, study data were excluded from the analyses.
Cross‐over trials
In cross‐over trials, only data before cross‐over were considered to avoid any unit of analysis issues.
Studies with multiple treatment groups
In studies with one control group and two or more intervention groups that satisfied our CDM criteria, we combined the intervention groups to create a single pair‐wise comparison to avoid unit of analysis errors. For dichotomous outcomes, both the sample size and the number of patients with events were summed across groups. For continuous outcomes, means and SDs were combined using the formulae presented in table 7.7.a of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We contacted corresponding authors to request missing information whenever the published information did not allow us to decide whether to include or exclude a study. We also contacted them to get missing data (for example, SDs) in order to appropriately describe the study results or perform a meta‐analysis, or both.
In cases where SDs and change from baseline SDs were not reported by the authors, we computed them from reported standard errors, P values, or confidence intervals. If none of these values were reported, we imputed the SD (or change from baseline SD) by calculating the mean SD (or change from baseline SD) of the other studies included in the meta‐analysis using the same scale (following the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)). The method of imputation for each relevant study is described in the forest plot footnotes. The potential impact of missing data was addressed in the sensitivity analysis.
Assessment of heterogeneity
As suggested in Pigott 2013, we considered heterogeneity in terms of substantive features of complex interventions, methodological and procedural features of studies, as well as research characteristics and reporting context. These sources are included in what others categorise as clinical, methodological, and statistical heterogeneity (Gagnier 2012; Gagnier 2013). Statistical heterogeneity among trials was specifically examined with Cochran's Q test and by calculating the I2 statistic, which describe the proportion of variability in the summary estimate that is due to heterogeneity rather than by chance.
We conducted subgroup analyses to explore clinical heterogeneity in meta‐analyses including at least nine studies, according to the following planned study characteristics (unless specified as post hoc).
Comprehensiveness of the programme
We defined a comprehensive programme as including at least the median number of independent components of included studies (that is, eight components).
Dominant component of the programme
Two review authors (CA, IPB) independently, and in duplicate, selected a dominant component of the programme out of the following three main categories, which are linked to the first three criteria of the operational definition of CDM: organisational component targeting patients, organisational component targeting healthcare professionals or system, or educational component. It was done based on the number of various components present in each category, the main aim of the intervention, and the relative importance of the different components. If we could not determine one dominant component, we classified the CDM programme as mixed. Any disagreement was resolved by discussion.
Presence of limited CDM components in the control group (which were considered as usual care in the specific context of single studies) (post hoc)
We did not perform meta‐regression because there were less than 10 studies in our meta‐analyses.
Assessment of reporting biases
We assessed the presence of publication bias by means of funnel plots. This was done for exploratory purposes only, as the number of studies included in the meta‐analyses (less than 10) was insufficient to reach a conclusive result.
Data synthesis
Where possible, we conducted meta‐analyses using the Cochrane Review Manager software (Review Manager 2014) to calculate the overall effect size for all relevant primary outcomes. We pooled results of the RCTs and NRCTs separately using the random‐effects model (DerSimonian 1986) to incorporate some level of expected heterogeneity among pooled studies. All results were expressed with 95% confidence intervals. Baseline‐adjusted results for CBA studies were also combined separately, if available. For primary outcomes that could not be incorporated in a meta‐analysis, we provided a brief description of the results in the main text.
We presented the most important outcomes of the review in the Table 1, which includes an overall grading of the evidence using the GRADE approach, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This approach specifies four levels of quality (high, moderate, low, very low) for each outcome separately. The highest quality rating is for RCT evidence, but it can be downgraded depending on the presence of the following five factors: study limitations in the design and implementation suggesting high likelihood of bias; indirectness of evidence (indirect population, intervention, control, outcomes); unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses); imprecision of results (wide confidence intervals); and high probability of publication bias. Sound observational studies are generally rated as low quality but the following factors can increase the quality of evidence: large magnitude of effect; all plausible confounding would reduce a demonstrated effect; and a dose‐response gradient.
Sensitivity analysis
We explored the influence of the following characteristics on effect size: excluding studies at high risk of bias; excluding studies with imputed SDs; excluding studies using instruments of unknown validity; using change from baseline measures; and using the fixed‐effect model instead of random‐effects model.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
See: Figure 1.
1.
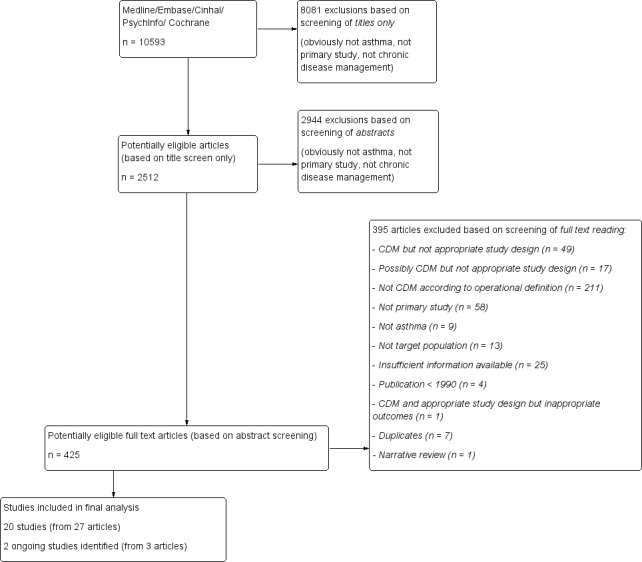
Flow diagram.
We identified a total of 10,593 records to June 2014. We screened the full texts of 425 potentially relevant articles. Of these, we excluded 395 articles, classified 3 articles (corresponding to 2 studies) under ongoing studies and retained 20 studies (from 27 articles) that met all our inclusion criteria.
Included studies
Design and setting
An overview of the characteristics of the included studies is provided in Table 2.
1. Overview of characteristics of included studies.
| Study ID | Design (allocation) | Country and setting | Intervention name, duration, number of components, dominant component | Patients in intervention group | Patients in control group | Number of reported outcomes |
| RCTs | ||||||
| Armour 2007 | C‐RCT (pharmacy) |
Australia rural and urban pharmacies |
Pharmacy Asthma Care Program 6 months components: 8 dominant component: EDU |
N = 160 women: 67.5% mean age: 47.5 moderate‐severe asthma |
N = 186 women: 60.5% mean age: 50.4 moderate‐severe asthma |
Org: 1 Process: 6 QOL: 1 Symptoms/activity: 2 Self‐care: 5 Lung function: 2 |
| Cambach 1997 | RCT | Netherlands local physiotherapy practices |
Rehabilitation programme 3 months components: 6 dominant component: ORG_PT |
N = 22 women: 81.8% mean age: 40 mild‐moderate asthma |
N = 21 women: 66.7% mean age: 53 mild‐moderate asthma |
QOL: 1 Symptoms/activity: 3 |
| Castro 2003 | RCT | USA inpatients and outpatients in a hospital |
Use of an asthma nurse specialist to provide a multifaceted approach to asthma care for “high‐risk” inpatients 6 months components: 10 dominant component: mixed |
N = 50 women: 80% mean age: 35 moderate‐severe asthma |
N = 46 women: 85% mean age: 38 mod‐severe asthma |
HC use: 8 QOL: 1 |
| Charrois 2006 | RCT | Canada community rural pharmacies and PCPs |
Better Respiratory Education and Asthma Treatment in Hinton and Edson (BREATHE) 6 months components: 11 dominant component: mixed |
N = 36 women: 53% mean age: 35.7 moderate‐severe asthma |
N = 34 women: 53% mean age: 38.7 moderate‐severe asthma |
Org: 1 Process: 3 HC use: 1 Symptoms/activity: 2 Lung function: 1 |
| Couturaud 2002 | RCT | France outpatient clinic of two university hospitals |
Educational programme in asthmatic patients following treatment readjustment 12 months components: 7 dominant component: EDU |
N = 26 women: 69.4% mean age: 37.8 moderate‐severe asthma |
N = 28 women: 66.7% mean age: 38.1 moderate‐severe asthma |
HC use: 1 QOL:1 Symptoms/activity: 3 Self‐care: 3 Lung function: 1 |
| Galbreath 2008 | RCT | USA University Medical Center and PCP |
The South Texas Asthma Management Project (STAMP) 6 months components: 9 dominant component: mixed |
N = 262 women: 77.6% mean age: 42.3 moderate‐severe asthma |
N = 124 women: 77.6% mean age: 43.7 moderate‐severe asthma |
Org: 1 Process: 2 HC use: 4 QOL: 1 Symptoms/activity: 2 Lung function: 3 |
| Huang 2009 | RCT | Taiwan outpatient chest department of hospital |
Individualised self‐care education programme 6 months components: 6 dominant component: EDU |
N = 98 women: 29.5% mean age: na moderate‐severe asthma |
N = 50 women: 22% mean age: na moderate‐severe asthma |
Process: 1 HC use: 1 Symptoms/activity: 2 Self‐care: 3 Lung function: 4 |
| Kokubu 2000 | RCT | Japan hospital and patients' home |
Asthma telemedicine system 6 months components: 8 dominant component: ORG_HC |
N = 32 women: 62% mean age: 49.9 asthma severity na |
N = 34 women: 56% mean age: 47.3 asthma severity na |
HC use: 4 Pt satis: 1 QOL: 1 Symptoms/activity: 3 Self‐care: 3 Lung function: 1 |
| Martin 2009 | RCT | USA PCPs |
A community‐based intervention to improve asthma self‐efficacy in African American adults designed by the Chicago Initiative to Raise Asthma Health Equity (CHIRAH) 3 months components: 7 dominant component: EDU |
N = 19 women: 60% mean age: 33 asthma severity na |
N = 18 women: 77% mean age: 37 asthma severity na |
Org: 1 Process: 2 QOL: 1 Symptoms/activity: 3 Self‐care: 3 |
| Mayo 1990 | RCT | USA outpatient chest clinic of a hospital |
Outpatient programme designed to reduce readmissions for asthma exacerbations 8 months components: 6 dominant component: EDU |
N = 47 women: 70.2% mean age: 42 moderate‐severe asthma |
N = 57 women: 57.9% mean age: 42 moderate‐severe asthma |
HC use: 1 Symptoms/activity: 1 |
| McLean 2003 | RCT | Canada community pharmacies |
The British Columbia pharmacy asthma study incorporating an asthma care protocol provided by specially trained community pharmacists 12 months components: 7 dominant component: EDU |
N = 119 women: 63% mean age: na asthma severity na |
N = 105 women: 62.9% mean age: na asthma severity na |
HC use: 4 Pt satis: 1 QOL: 1 Symptoms/activity: 4 Self‐care: 1 Lung function: 1 |
| Petro 2005 | C‐RCT (provider) |
Germany PCPs | A disease management programme with a case manager 12 months components: 7 dominant component: ORG_HC |
N = 56 women: 54.2% mean age: 57.3 moderate‐severe asthma |
N = 55 women: 44% mean age: 55 moderate‐severe asthma |
HC use: 2 QOL: 3 Symptoms/activity: 1 Lung function: 2 |
| Schatz 2006 | RCT | USA Kaiser Permanente Medical Care program |
Regular care manager and intensive individualised educational visit 12 months components: 11 dominant component: mixed |
N = 30 women: 32.3% mean age: 45 moderate‐severe asthma |
N = 15 women: 54.8% mean age: 45.4 moderate‐severe asthma |
Process: 1 HC use: 1 QOL: 1 Symptoms/activity: 2 Self‐care: 1 |
| Smith 2005 | RCT | UK outpatient asthma clinics of a hospital and PCPs |
The Coping with Asthma Study (a home based, nurse led psychoeducational intervention for adults at risk of adverse asthma outcomes) 6 months components: 15 dominant component: EDU |
N = 42 women: 62% mean age: 38.2 moderate‐severe asthma |
N = 42 women: 84% mean age: 34.7 moderate‐severe asthma |
QOL: 6 Symptoms/activity: 1 Self‐care: 6 |
| Wilson 2010 | RCT | USA Kaiser Permanente clinics |
The Better Outcomes of Asthma Treatment (BOAT) study 9 months components: 9 dominant component: mixed |
N = 362 women: 56.2% mean age: 46.3 moderate‐severe asthma |
N = 189 women: 57.4% mean age: 45.1 moderate‐severe asthma |
Process: 1 HC use: 2 QOL: 1 Symptoms/activity: 5 Lung function: 2 |
| NRCT | ||||||
| Herborg 2001 | C‐NRCT (pharmacy) |
Denmark community pharmacies |
Therapeutic outcomes monitoring (TOM) programme 12 months components: 9 dominant component: ORG_PT |
N = 209 women: 57.6% mean age: 38.8 moderate‐severe asthma |
N = 204 women: 54.7% mean age: 42.4 moderate‐severe asthma |
Org : 3 HC use: 7 Pt satis: 1 QOL: 2 Symptoms/activity: 4 Self‐care: 1 Lung function: 1 |
| CBAs | ||||||
| Feifer 2004 | CBA | USA PCPs in a region covered by a health insurance company |
Population‐based asthma disease management programme using broad‐based educational interventions 12 months components: 7 dominant component: mixed |
N = 35,450 women: 56% mean age: na asthma severity na |
N = 35,450 women: 56% mean age: na asthma severity na |
Process: 3 HC use: 3 QOL: 1 Symptoms/activity: 4 Self‐care: 3 |
| Landon 2000 | CBA | USA community health centres |
Health Disparities Collaboratives disseminating quality improvement techniques 54 months components: ≥ 11 dominant component: ORG_HC |
N = 1696 (total/2) women: 63.5% mean age: 28.4 asthma severity na |
N = 1696 (total/2) women: 67.6% mean age: 34.4 asthma severity na |
Process: 7 HC use: 1 Symptoms/activity: 1 |
| Weng 2005 | CBA | Taiwan outpatient clinics of a hospital and PCPs |
A government‐sponsored outpatient‐based disease management programme 12 months components: 8 dominant component: EDU |
N = 854 women: 44.5% mean age: na asthma severity na |
N = 3188 women: 43% mean age: na asthma severity na |
Pro satis: 1 HC use: 4 |
| Windt 2010 | CBA | Germany PCPs |
Nationwide asthma disease management programme > 12 months components: ≥ 5 dominant component: ORG_HC |
N=317 women: 48.6% mean age: 36.5 asthma severity na |
N=317 women: 44.2% mean age: 36.5 asthma severity na |
Process: 8 HC use: 2 |
RCT: randomised controlled trial, NRCT: non‐randomised controlled trial, CBA: controlled before‐after study, C‐: cluster, PCP: primary care practice, EDU: educational and self‐management support component, ORG_PAT: organisational component targeting patients; ORG_HC organisational component targeting healthcare professionals or the healthcare system, na: not available, HC: healthcare, QOL: quality of life, Org: organisational, Pt satis: patient satisfaction, Pro satis: healthcare professionals' satisfaction
Fifteen studies were RCTs. Out of these 15 studies, one study was a cross‐over trial (Cambach 1997) and two studies were cluster‐RCTs with the unit of allocation being the provider in Petro 2005 and the pharmacy in Armour 2007. The other studies included were one NRCT (Herborg 2001) with a cluster design (unit of allocation: pharmacy) and four CBAs (Feifer 2004; Landon 2007; Weng 2005; Windt 2010) with at least two sites in both the control and intervention groups.
Nine studies recruited patients from primary care clinics or pharmacies (Armour 2007; Charrois 2006; Couturaud 2002; Herborg 2001; Landon 2007; Martin 2009; McLean 2003; Petro 2005; Schatz 2006). Two studies enrolled patients from respiratory care clinics (Cambach 1997; Huang 2009), three other studies recruited hospital inpatients (Castro 2003; Kokubu 2000; Mayo 1990), and four studies enrolled patients from the general population (Feifer 2004; Weng 2005; Wilson 2010; Windt 2010). The remaining two studies enrolled patients from more than one pool: Smith 2005 enrolled patients from both primary care and respiratory care clinics; and Galbreath 2008 recruited patients from the general population, primary care clinics and respiratory care clinics.
Three studies took place in pharmacies (Armour 2007; Herborg 2001; McLean 2003), seven in primary care practices (Cambach 1997; Feifer 2004; Galbreath 2008; Landon 2007; Martin 2009; Petro 2005; Windt 2010), three in outpatient hospital departments (Couturaud 2002; Huang 2009; Mayo 1990), and two in health management organisations (HMOs) (Schatz 2006; Wilson 2010). The remaining studies took place in mixed settings: inpatient and outpatient hospital departments (Castro 2003), inpatient hospital department and patients' home (Kokubu 2000), pharmacies and primary care practices (Charrois 2006), and outpatient hospital departments and primary care clinics (Smith 2005; Weng 2005).
Ten studies were carried out in North America, six in Europe, three in Asia, and one in Australia.
Study population
A total of 10,846 patients were included in 19 studies (between 37 and 4042 patients per study, median 111) when the CBA study reporting data from 70,900 patients from a health insurance company database was excluded (Feifer 2004). The mean age in the intervention and control groups varied between 28.0 and 57.3 years old (median 42.0) and the percentage of women between 22% and 85% (median 59%). Asthma severity in the 13 studies reporting it was rated as moderate‐severe in all except one study, where it was mild‐moderate (Cambach 1997). The baseline predicted FEV1 varied between 22.5% and 89% (median 69%) in eight studies where it was reported. The percentage of patients using inhaled corticosteroids was reported to be between 13.3% and 100% (median 78%) in six studies.
Interventions
See: Figure 2.
2.
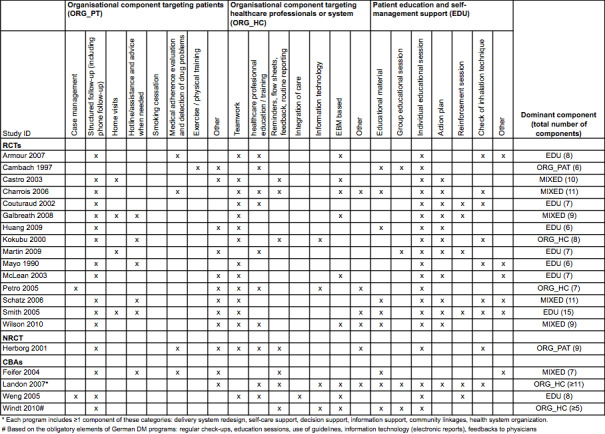
Description of intervention components by study.
All the programmes met the predefined five CDM criteria: at least one organisational component targeting patients, at least one organisational component targeting healthcare professionals or the healthcare system, patient education or self‐management support or both, the active involvement of two or more healthcare professionals in patient care, and a minimum duration of three months for at least one component. The number of independent components per programme ranged from 6 to 15 (mean 8.4; median 8). Eleven programmes comprising eight or more components (that is, including at least the median number of components) were defined as comprehensive programmes (Armour 2007; Castro 2003; Charrois 2006; Galbreath 2008; Herborg 2001; Kokubu 2000; Landon 2007; Schatz 2006; Smith 2005; Weng 2005; Wilson 2010), and the remaining nine, comprising seven or fewer components, were defined as less comprehensive.
The dominant component was 'educational' in eight studies, 'organisational targeting healthcare professionals or the healthcare system' in four studies, and 'organisational targeting patients' in two studies. We could not determine the dominant component in the remaining six studies, which were classified as mixed.
The most frequently assessed educational component was individual educational sessions (n = 19), followed by providing an action plan for self‐management support (n = 12), and verification of inhalation technique (n = 9). The most frequently assessed organisational component targeting patients involved structured follow‐up (n = 16), followed by having assistance and advice on demand via, for example, a hotline (n = 6). The most frequently assessed organisational component targeting healthcare professionals or the healthcare system involved explicit teamwork and collaborative processes between the healthcare providers (n = 15), followed by education and training of providers (n = 10), and explicit use of evidence‐based medicine supports (n = 9).
The duration of the programmes ranged from 3 months to more than 12 months (median 8.5 months).
Three studies assessed two intervention groups that fulfilled our CDM inclusion criteria (Galbreath 2008; Huang 2009; Wilson 2010). In these studies, we combined the two intervention arms and analysed them as a single intervention group.
Outcome (dependent variable) measures
A wide variety of outcomes were reported in the included studies (see Characteristics of included studies for all available outcomes). Here we describe briefly the outcomes reported in at least three studies. The a priori primary outcomes we defined in the protocol are the only ones we analysed. They are described in more detail in the section presenting the effects of the interventions.
Five studies reported patient participation rates in the programme and four reported the percentage of patients who received the intervention or components, or both. Five studies reported the percentage of patients with an action plan. Six studies reported prescription rates of inhaled corticosteroids and nine reported rates for prescription of other types of medication.
Fifteen studies reported healthcare utilisation outcomes: four reported on any healthcare use (hospitalisation or unscheduled visit, or both), and seven reported asthma‐related or all‐cause hospitalisations and asthma‐related or all‐cause unscheduled visits separately. Five studies reported cost data.
Fourteen studies reported asthma‐specific quality of life scores. Asthma severity scores were reported in nine studies and the number of symptomatic days in four studies. Three studies reported the number of days off work or school due to asthma. Ten studies reported the patients' actual use of medication. The reported self‐management outcomes included patients' asthma knowledge scores in seven studies, self‐efficacy scores in six studies, and compliance with treatment in four studies.
Pulmonary function tests such as FEV1, FEV1/FVC and PEF rate were reported in seven, four, and six studies, respectively.
Missing data
We attempted to contact the authors of 15 of the included studies to request additional data or information. We sent e‐mails to 10 authors as we were unable to identify the correct e‐mail address for the authors of the other five studies. Nine authors responded and five provided additional data. We imputed missing SDs for seven studies (Couturaud 2002; Galbreath 2008; Herborg 2001; Huang 2009; Kokubu 2000; Mayo 1990; McLean 2003).
Excluded studies
We excluded 395 studies after having assessed the full article (see Figure 1). We excluded 211 studies because the intervention did not meet the inclusion criteria of our CDM operational definition. We also excluded studies that used a design not included in our predefined list, for example a before‐after study with only one site for the intervention and control groups, even if they met or possibly met the inclusion criteria for our operational definition of CDM (n = 66). We also excluded studies for the following reasons: inappropriate target population (for example, only children included; n = 13); insufficient information to determine eligibility (n = 25); publication date before 1990, as the first CDM programmes were implemented after that date (n = 4); not primary studies (for example, editorials, comments, reviews; n = 58); and patients without asthma or from a mix of chronic diseases (n = 9). One study fulfilled our eligibility criteria but did not report appropriate outcomes. The primary reason for excluding studies that seemed to meet the eligibility criteria and could be considered relevant by some readers, but were not eligible after further inspection, are listed under Characteristics of excluded studies.
Risk of bias in included studies
The full details of risk of bias judgements by study are described in the Characteristics of included studies table. Figure 3 and Figure 4 summarise these. Using GRADE (see Table 1) the quality of the evidence was rated as moderate or low depending on the outcome.
3.
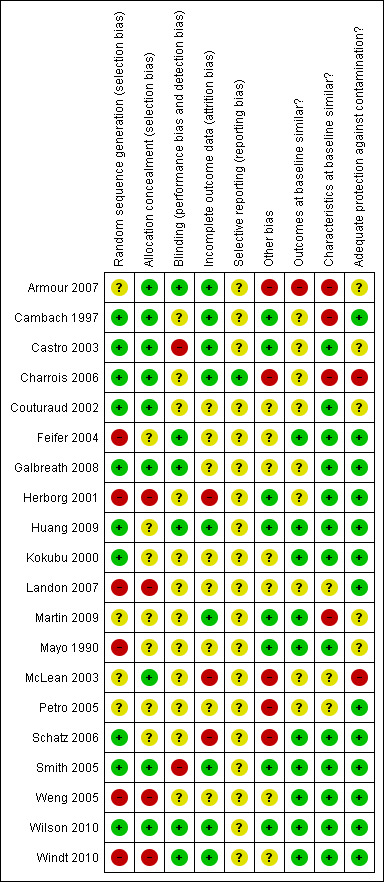
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
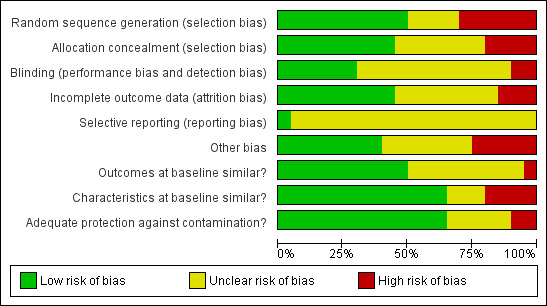
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Ten of the 15 RCTs reported the use of a computerised randomisation programme or a random number table to generate the allocation sequence and were thus considered to be at low risk of bias (Cambach 1997; Castro 2003; Charrois 2006; Couturaud 2002; Galbreath 2008; Huang 2009; Kokubu 2000; Schatz 2006; Smith 2005; Wilson 2010). The process of sequence generation was unclear for four studies, which stated that the study groups were randomly allocated (Armour 2007; Martin 2009; McLean 2003; Petro 2005). The remaining RCT was judged to have a high risk of bias because a quasi‐random method of allocation (last digit of hospital number) was used (Mayo 1990). In the NRCT and CBAs, allocation was judged to be at high risk of bias because of absence of randomisation (Herborg 2001; Landon 2007) and retrospective allocation (Feifer 2004; Weng 2005; Windt 2010).
Allocation concealment was reported in nine of the randomised studies but was unclear in the other six (Huang 2009; Kokubu 2000; Martin 2009; Mayo 1990; Petro 2005; Schatz 2006). Allocation was judged as not having been done in the other studies included (NRCT and CBAs).
Unit of allocation issues
Two of the three studies with a cluster design analysed the data taking into account the clustering effect (Armour 2007; Herborg 2001) and were included in our analyses. The third study (Petro 2005) analysed the data at the patient level, which artificially increases the precision of the statistical tests and can lead to inappropriate conclusions. The results of this study were excluded from all analyses because we were unable to determine the number of clusters in the study and therefore could not adjust the results.
Blinding
Six studies were at low risk of performance and detection bias because claims data were used or the assessors were blinded (Armour 2007; Feifer 2004; Galbreath 2008; Huang 2009; Wilson 2010; Windt 2010). Two studies were judged to be at high risk (Castro 2003; Smith 2005) and the risk for the remaining 12 studies was unclear.
Incomplete outcome data
Outcome data were considered complete when 80% or more of randomised patients were included in the analyses, when reasons for attrition were similar across groups, and when dropouts did not differ from the patients analysed. These were reported in nine studies (Armour 2007; Cambach 1997; Castro 2003; Charrois 2006; Huang 2009; Martin 2009; Smith 2005; Wilson 2010; Windt 2010). Outcome data were considered incomplete in three studies because less than 80% of randomised patients were analysed and no reasons were given for the missing data (Herborg 2001; McLean 2003; Schatz 2006). In the remaining eight studies, the number of patients or clusters lost to follow‐up was unclear or information was missing for us to fully assessed attrition bias (Couturaud 2002; Feifer 2004; Galbreath 2008; Kokubu 2000; Landon 2007; Mayo 1990; Petro 2005; Weng 2005).
Selective reporting
Only one study published an article on the design of the trial, reporting the outcomes to be measured in the trial (Charrois 2006), and was considered at low risk of reporting bias. All other studies were categorised as having an unclear risk of reporting bias because of missing information.
None of the exploratory funnel plots appeared asymmetrical.
Other potential sources of bias
Baseline measurement of the outcome of interest was reported in all studies except three (Castro 2003; Couturaud 2002; Galbreath 2008). In 10 of the studies reporting baseline measures, study groups were comparable at baseline for the outcomes (Feifer 2004; Huang 2009; Kokubu 2000; Martin 2009; Mayo 1990; Schatz 2006; Smith 2005; Weng 2005; Wilson 2010; Windt 2010); while in one study important differences were reported (Armour 2007), it was unclear if the differences in baseline measurements of the outcomes between groups were important in five studies. Finally, in one study (Petro 2005) there were important differences at baseline for the secondary outcomes but not the primary outcome (marked as unclear risk of bias).
All studies except two (McLean 2003; Petro 2005) reported patients' characteristics at baseline allowing an assessment of baseline heterogeneity between study groups. Four studies reported important differences between groups (Armour 2007; Cambach 1997; Charrois 2006; Martin 2009) (at high risk of bias), 13 studies reported no important differences (at low risk of bias), and one study (Landon 2007) reported important differences for some characteristics (at unclear risk).
Two studies (Charrois 2006; McLean 2003) were considered at high risk of contamination: trained pharmacists saw both the control and intervention patients. In five studies (Armour 2007; Castro 2003; Couturaud 2002; Martin 2009; Mayo 1990) it was unclear whether patients in the control groups had received more than usual care, which could have improved the care they had received and their outcomes. The other 13 studies were considered at low risk of contamination.
While no further bias was detected in 13 studies, five studies were considered at high risk and two at unclear risk for other bias. In four studies (Armour 2007; Couturaud 2002; McLean 2003; Schatz 2006) there was a risk of recruitment bias due to the design of the study (for example, selection of patients by pharmacist after allocation, low recruitment rate). In two other studies (Charrois 2006; Galbreath 2008) the intervention was poorly implemented with patients allocated to the intervention group completing only parts of the intervention, resulting in potential bias. Finally, in two studies (McLean 2003; Petro 2005) there was a high risk of bias due to analysis errors (unit of analysis error, cluster randomisation but analyses performed with patient level data).
Effects of interventions
See: Table 1
We reported the results using the 10 primary outcomes as predefined in our protocol, followed by the results of subgroup and additional sensitivity analyses. Data from one RCT (Petro 2005) could not be included in the meta‐analyses because we were unable to calculate the design effect due to missing information on the number of clusters (unit of analysis error). Also, we were unable to include the four CBA studies in the meta‐analyses in this report because data provided by authors were either insufficient or unadjusted.
Asthma‐specific quality of life
Fourteen of the 20 studies selected for inclusion in this review measured asthma‐specific quality of life using three validated instruments: the Asthma Quality of Life Questionnaire (AQLQ) or mini‐AQLQ in nine studies; the Living with Asthma Questionnaire (LWAQ) in four studies; and the Chronic Respiratory Disease Questionnaire (CRDQ) in one study. However, only nine of these studies provided data at follow‐up and could be included in the main meta‐analysis. Of these, two had missing SDs, which were estimated from the study data using the same instrument.
One study using the mini‐AQLQ was excluded from the meta‐analysis because follow‐up values were not available, due to copyright issues according to the corresponding author (Martin 2009). In this study, the intervention group had improved asthma quality of life compared with the control group after six months of follow‐up. Another study using the LWAQ (Petro 2005) was excluded from the meta‐analysis as data could not be adjusted for unit of analysis error. The study using the CRDQ (Cambach 1997) and one study using the LWAQ (Kokubu 2000) only provided data on change from baseline. They were excluded from the main meta‐analysis but we included them in the sensitivity analysis using change from baseline data. Feifer 2004, using the mini‐AQLQ, was excluded from the meta‐analysis because it was a CBA study and data were available only for the intervention group.
The main meta‐analysis included eight RCTs (Armour 2007; Castro 2003; Couturaud 2002; Galbreath 2008; McLean 2003; Schatz 2006; Smith 2005; Wilson 2010) with a total population of 1627 patients with a follow‐up of 3 to 12 months (see Figure 5; Analysis 1.1). The pooled SMD was 0.22 in favour of CDM (95% confidence interval (CI) 0.08 to 0.37), with a moderate degree of heterogeneity (I2 = 43%). The clinical significance of this SMD was low since, as a rule of thumb, a SMD lower than 0.4 indicates a small effect. In addition, the corresponding difference on the AQLQ scale after back‐transformation (0.30) was lower than the minimal clinically important difference (MCID) of the AQLQ or mini‐AQLQ, which is 0.5 according to the developers of the instrument (http://www.qoltech.co.uk/miniaqlq.html). The SMD for the NRCT (Herborg 2001), including 413 patients, was larger than the pooled SMD of RCTs (SMD 0.46, 95% CI 0.27 to 0.66) (see Figure 5; Analysis 1.1).
5.
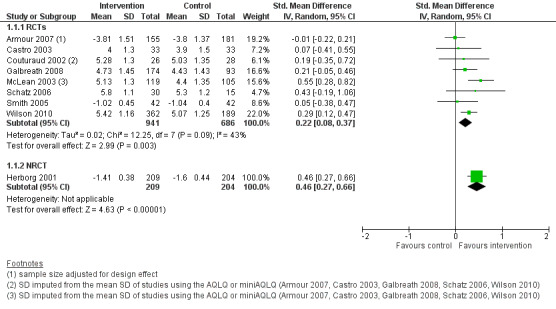
Forest plot of comparison: 1 Chronic disease management programme versus usual care, outcome: 1.1 Asthma‐specific quality of life score (post‐intervention measurements).
1.1. Analysis.
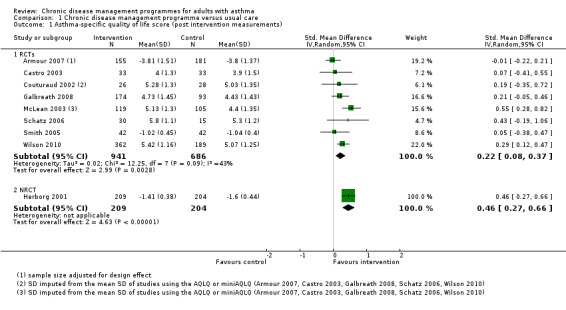
Comparison 1 Chronic disease management programme versus usual care, Outcome 1 Asthma‐specific quality of life score (post intervention measurements).
Excluding the two RCTs at high risk of bias (McLean 2003; Schatz 2006) from the meta‐analysis reduced the SMD (SMD 0.17, 95% CI 0.05 to 0.28) and the heterogeneity (I2 = 1%).
Subgroup analysis by quality of life instrument used
To determine if the heterogeneity of the results was due to the use of different instruments, we analysed the results from each instrument separately. This allowed us: i) to assess the effect of using a single instrument with its specific properties, and ii) to analyse the MD instead of the SMD. Seven studies including 1543 patients used the AQLQ, and one study including 84 patients used the LWAQ (Analysis 1.5). Subgroup analysis of the studies using the AQLQ scale showed a non‐clinically significant MD of 0.32 (clinical significance 0.5 or more) in favour of CDM (95% CI 0.12 to 0.52), while results of the study using the LWAQ scale were inconclusive (MD 0.02, 95% CI ‐0.16 to 0.20). Heterogeneity was not improved by restricting the analysis to studies using the same instrument (I2 = 42%).
1.5. Analysis.
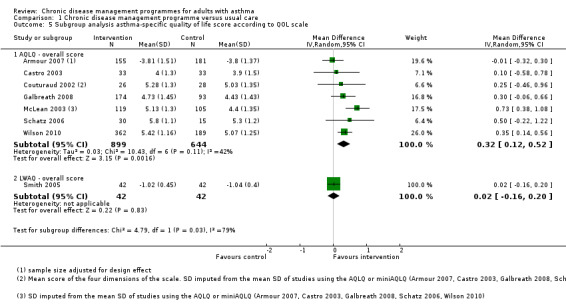
Comparison 1 Chronic disease management programme versus usual care, Outcome 5 Subgroup analysis asthma‐specific quality of life score according to QOL scale.
Hospitalisations
Nine studies reported hospitalisation data specifically. However, we could not perform a meta‐analysis because the data were skewed and heterogeneous, with wide variability in terms of length of measurement (hospitalisations within the last 1, 6, 8, or 12 months) and reasons for hospitalisation (due to asthma or any cause).
Three RCTs reported a reduction in hospitalisation for asthma in the intervention group compared with the control group. While Castro 2003 reported a 56% reduction in readmissions for asthma in the intervention group compared with the control group over 12 months (MD ‐0.5, 95% CI ‐1.0 to 0.0), Kokubu 2000 reported an 83% reduction in hospitalisations among patients at high risk for hospitalisations in the intervention group after 6 months compared with the control group (MD ‐0.29, 95% CI ‐0.49 to ‐0.09), and Mayo 1990 reported a 67% reduction in hospital readmissions for acute exacerbation in the intervention group after 8 months compared with the control group (MD ‐0.83, 95% CI ‐1.10 to ‐0.56).
In contrast, two RCTs (Galbreath 2008; McLean 2003) and one NRCT (Herborg 2001) did not report any differences between groups. However, the number of hospitalisations per patient during follow‐up was lower in these studies than in the RCTs reporting a reduction: the mean number of hospitalisations per patient was 0.12 during the 12 months of follow‐up in Galbreath 2008, 0.12 during one month of follow‐up in McLean 2003, and 0.04 during the 12 months of follow‐up in Herborg 2001; compared with 0.64, 0.21, and 0.85 in Castro 2003, Kokubu 2000, and Mayo 1990. In Petro 2005 there were no hospitalisations in the intervention group during the 12 months of follow‐up compared with 10% in the control group.
In the CBA study that assessed the impact on hospitalisation in both the intervention and control groups, the number of hospitalisations per patient after 12 months did not differ between the groups (Weng 2005).
Two RCTs (Charrois 2006; Schatz 2006) and two CBA studies (Landon 2007; Windt 2010) that reported the number of hospitalisations and ED visits as one outcome did not report any important differences between groups in the number or percentage of hospitalisations or ED visits during the study follow‐up.
Emergency department (ED) or unscheduled visits
Nine studies reported the number of ED or unscheduled visits. We could not perform a meta‐analysis because the data were skewed and heterogeneous, with wide variability in means and rates at baseline; length of follow‐up from 1 to 12 months; data treated as continuous data, rate or count; and studies including ED or unscheduled visits for asthma only versus for any reason.
Only one RCT (Kokubu 2000) showed a reduction in daytime ED visits per patient in the intervention group compared with the control group during the six month follow‐up, but no difference in night ED visits was observed.
The results from four RCTs and one NRCT did not show any difference between groups for the number of ED or unscheduled visits for asthma per patient during 12 months of follow‐up (Castro 2003; Couturaud 2002; Galbreath 2008; Herborg 2001) and 1 month of follow‐up (McLean 2003). Another RCT showed no difference in the percentage of patients with at least one unscheduled visit after six months of follow‐up (Huang 2009).
In the CBA study that assessed the impact on ED or unscheduled visits in both the intervention and control groups, there was no important reduction between groups in the number of ED visits per patient after 12 months (Weng 2005).
Asthma exacerbations
Asthma exacerbations, which we defined as prompting hospitalisation, an ED or unscheduled medical visit, or systemic rescue glucocorticoids, were not often reported as such in the included studies. We were therefore unable to perform a meta‐analysis due to the lack of data.
Couturaud 2002 and Mayo 1990 reported the number of unscheduled visits for asthma exacerbation and the number of hospitalisations for asthma exacerbation, respectively. In Couturaud 2002 the number of unscheduled visits for asthma exacerbation were comparable between groups, and in Mayo 1990 the number of readmissions for asthma exacerbation per patient for the intervention group was less than for the control group. We could not consider the other studies reporting healthcare use as they did not specify whether the use was for asthma exacerbations.
Finally, five studies reported oral corticosteroids use (Charrois 2006; Couturaud 2002; Herborg 2001; Kokubu 2000; Schatz 2006) but did not specify whether the use was for asthma exacerbation and data were too diverse and heterogenous to be combined. In all studies except one (Couturaud 2002), no important differences between the intervention and control groups were observed. In Couturaud 2002 the percentage of days of oral steroid intake was higher in the intervention group at follow‐up (P = 0.01).
Asthma self‐efficacy
Six studies reported on asthma self‐efficacy, using five different instruments: the Perceived Control of Asthma Questionnaire (PCAQ) in Armour 2007 and Smith 2005, the Asthma Self‐efficacy Scale in Huang 2009, the Chicago Initiative to Raise Asthma Health Equity Asthma Self‐Efficacy Scale in Martin 2009, open‐ended questions measuring self‐management ability in Couturaud 2002, and specific questions measuring self‐management skills in Feifer 2004. The first three instruments have been formerly validated but the questions used in Couturaud 2002 and Feifer 2004 have not. Data from Feifer 2004 were excluded from this meta‐analysis because the study was a CBA and data were only available for the intervention group.
The five studies (Armour 2007; Couturaud 2002; Huang 2009; Martin 2009; Smith 2005) in the meta‐analysis shown in Figure 6 and Analysis 1.7 included a total population of 642 patients, with a follow‐up of 3 to 12 months. The pooled SMD was 0.51 (95% CI ‐0.08 to 1.11) but this difference could not be established, as a negative effect or no difference, could not be ruled out. Pooling indicated a high degree of heterogeneity (I2 = 91%).
6.
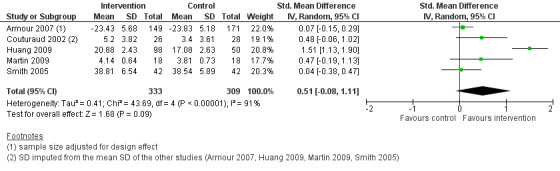
Forest plot of comparison: 1 Chronic disease management programme versus usual care, outcome: 1.7 Self‐efficacy score (post‐intervention measurements).
1.7. Analysis.
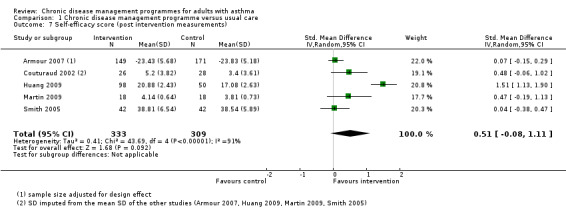
Comparison 1 Chronic disease management programme versus usual care, Outcome 7 Self‐efficacy score (post intervention measurements).
Removing the study that used a non‐validated instrument (Couturaud 2002) did not modify the overall result (no difference between groups) or reduce the heterogeneity (pooled SMD 0.52, 95% CI ‐0.21 to 1.26; I2 = 93%). No studies were at high risk of bias in this meta‐analysis. Removing the study with the most positive results (Huang 2009) decreased the pooled result (SMD 0.14, 95% CI ‐0.04 to 0.32) and reduced the heterogeneity (I2 = 0%).
Days off school or work absences
Three studies reported the impact of the intervention on days off school or work absences. Couturaud 2002 reported comparable percentages of days off work in the control and intervention groups after 12 months of follow‐up. In McLean 2003 the mean change from baseline in number of days off school or work did not differ between the intervention and control groups after 12 months of follow‐up. Feifer 2004 reported the number of productivity‐loss days among employed and unemployed patients in the intervention group only. These data were not pooled because of their heterogeneous formats.
Asthma severity
Seven studies reported asthma severity scores, using the Asthma Control Questionnaire (ACQ) (Charrois 2006), the Lara Asthma Symptom Scores (LASS) (Galbreath 2008), the asthma morbidity index (Herborg 2001), the Asthma Control Test (Huang 2009), the Asthma Therapy Assessment Questionnaire (ATAQ) (Wilson 2010), and asthma symptom scores based on different questionnaires (McLean 2003; Smith 2005). All instruments except those used in McLean 2003 and Smith 2005 had undergone validation. We adapted the instruments so that higher scores corresponded to less severe asthma for all measures.
The main meta‐analysis included six RCTs (Charrois 2006; Galbreath 2008; Huang 2009; McLean 2003; Smith 2005; Wilson 2010) with a total population of 1330 patients and a follow‐up of 6 to 12 months (see Figure 7; Analysis 1.8). The pooled SMD was 0.18 in favour of CDM (95% CI 0.05 to 0.30) representing a small effect clinically (SMD < 0.4). Pooling showed a low level of heterogeneity (I2 = 13%). The SMD for the NRCT (Herborg 2001), including 409 patients, was higher than the pooled SMD of the RCTs (SMD 0.47, 95% CI 0.27 to 0.66) (see Figure 7; Analysis 1.8).
7.
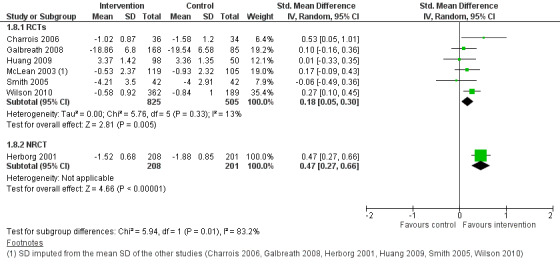
Forest plot of comparison: 1 Chronic disease management programme versus usual care, outcome: 1.8 Asthma severity score (post‐intervention measurements).
1.8. Analysis.
Comparison 1 Chronic disease management programme versus usual care, Outcome 8 Asthma severity score (post intervention measurements).
Removing the two studies that used instruments that had not been formally validated (McLean 2003; Smith 2005) or the study at high risk of bias (McLean 2003) from the meta‐analysis had little impact on the point estimate (pooled SMD 0.20, 95% CI 0.04 to 0.37; and SMD 0.17, 95% CI 0.01 to 0.33, respectively).
Two other studies measured the percentage of patients having severe asthma (Armour 2007) and the percentage of patients without various respiratory symptoms (Petro 2005). In Armour 2007 the multilevel logistic regression model found that the odds ratios (OR) for patients to change from the 'severe' category to the 'not severe' category ('moderate' or 'mild') were almost three times higher in the intervention group than in the control group (OR 2.68, 95% CI 1.64 to 4.37). In Petro 2005, after 12 months of follow‐up, the median percentage of patients not presenting severe symptoms remained similar in the control group (from 44% to 46%) but increased from 46% to 81% in the intervention group.
Use of an action plan
Five studies reported the percentage of patients with an action plan, but only two studies (Landon 2007; Martin 2009) provided data for both the intervention and control groups. In the first study, the percentage of patients with an asthma management plan was higher in the intervention group (27%) than in the control group (12%) at follow‐up (P < 0.001) (Landon 2007). In the second study, a greater percentage of patients in the intervention group had received an asthma action plan after 3 months of follow‐up (control group 18%; intervention group 45%) but this difference did not remain after 6 months of follow‐up (control group 23%; intervention group 20%; P = 0.17) (Martin 2009).
Patient satisfaction
Three studies reported outcomes on patient satisfaction. In Herborg 2001 and McLean 2003, patients in the intervention and control groups had similar high satisfaction scores at the end of the study. Only patients in the intervention group completed the satisfaction survey in Kokubu 2000, therefore the impact of the intervention could not be assessed.
Lung function
Nine studies reported outcomes on lung function: six reported the mean per cent of predicted FEV1 value (% of predicted value) (Armour 2007; Charrois 2006; Couturaud 2002; Galbreath 2008; Huang 2009; Wilson 2010) and five reported the PEF rate, reported as L/min in three studies (Herborg 2001; Huang 2009; McLean 2003) and as per cent of predicted value in two studies (Galbreath 2008; Kokubu 2000).
We combined data from the eight RCTs in one meta‐analysis using the SMD, including a population of 1559 patients, with a follow‐up of 6 to 12 months (Analysis 1.9). Overall, the pooled SMD for lung function was 0.19 in favour of CDM (95% CI 0.09 to 0.30). This SMD (small effect size if SMD < 0.4) corresponded to a difference, on the predicted FEV1 % scale, of 5.0%. There was no heterogeneity (I2 = 0%). In the FEV1 subgroup, the SMD was 0.16 (95% CI 0.05 to 0.27). In the PEF (L/min) and PEF (% predicted) subgroups, which only included one study each, the SMD was 0.30 (95% CI 0.03 to 0.56) and 0.53 (95% CI ‐0.01 to 1.06), respectively.
1.9. Analysis.
Comparison 1 Chronic disease management programme versus usual care, Outcome 9 Lung function (FEV1 and PEF) (post intervention measurements).
Removing the study at high risk of bias (McLean 2003) from the meta‐analysis did not affect the SMD (SMD 0.18, 95% CI 0.06 to 0.29).
We also looked at the impact of CDM on these three different measures of lung function in three separate meta‐analyses (Analysis 1.10; Analysis 1.11; Analysis 1.12), allowing us to use the MD. For FEV1, the MD for the predicted value was 2.81% in favour of CDM (95% CI 0.99 to 4.64). For PEF, the pooled MD was 33.52 L/min in favour of CDM (95% CI 11.38 to 55.65) for RCTs and the MD was 30.52 L/min (95% CI 7.46 to 53.58) for the NRCT. For PEF in predicted % values, the MD was 8.68% in favour of CDM (95% CI 3.73 to 13.63).
1.10. Analysis.
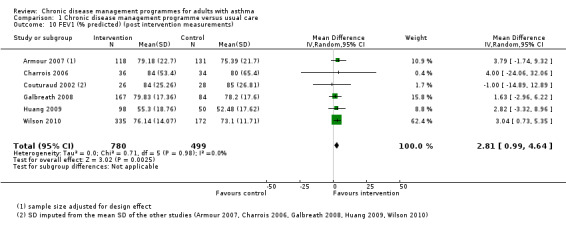
Comparison 1 Chronic disease management programme versus usual care, Outcome 10 FEV1 (% predicted) (post intervention measurements).
1.11. Analysis.
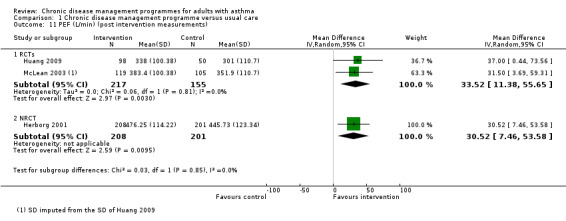
Comparison 1 Chronic disease management programme versus usual care, Outcome 11 PEF (L/min) (post intervention measurements).
1.12. Analysis.
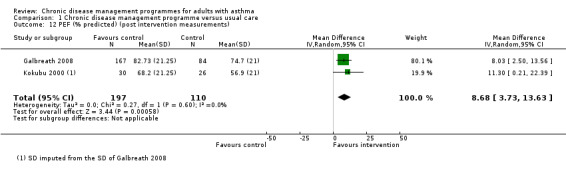
Comparison 1 Chronic disease management programme versus usual care, Outcome 12 PEF (% predicted) (post intervention measurements).
Subgroup analyses
We performed subgroup analyses for two outcomes with sufficient studies: asthma‐specific quality of life (Analysis 1.2; Analysis 1.3; Analysis 1.4) and lung function (Analysis 1.13; Analysis 1.14; Analysis 1.15). The results from these analyses did not show any differences in the impact of the intervention as a function of its comprehensiveness, the dominant component of the intervention, or the presence of limited CDM components in the control group.
1.2. Analysis.
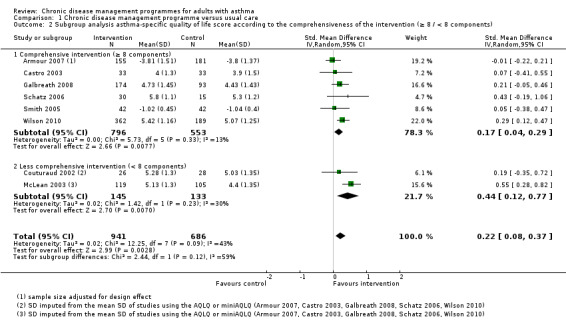
Comparison 1 Chronic disease management programme versus usual care, Outcome 2 Subgroup analysis asthma‐specific quality of life score according to the comprehensiveness of the intervention (≥ 8 / < 8 components).
1.3. Analysis.
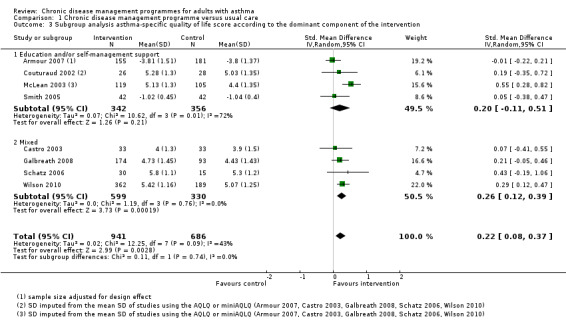
Comparison 1 Chronic disease management programme versus usual care, Outcome 3 Subgroup analysis asthma‐specific quality of life score according to the dominant component of the intervention.
1.4. Analysis.
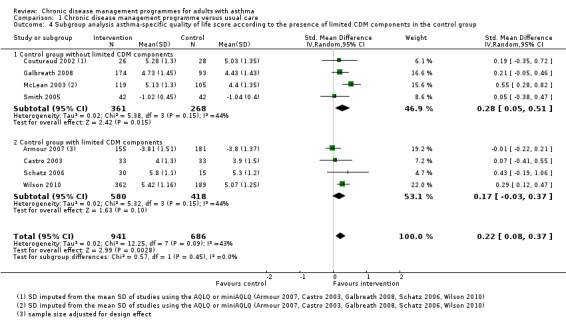
Comparison 1 Chronic disease management programme versus usual care, Outcome 4 Subgroup analysis asthma‐specific quality of life score according to the presence of limited CDM components in the control group.
1.13. Analysis.
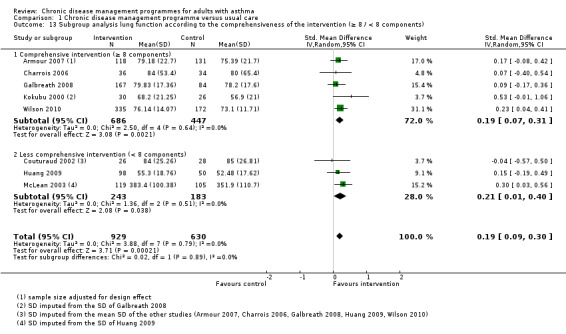
Comparison 1 Chronic disease management programme versus usual care, Outcome 13 Subgroup analysis lung function according to the comprehensiveness of the intervention (≥ 8 / < 8 components).
1.14. Analysis.
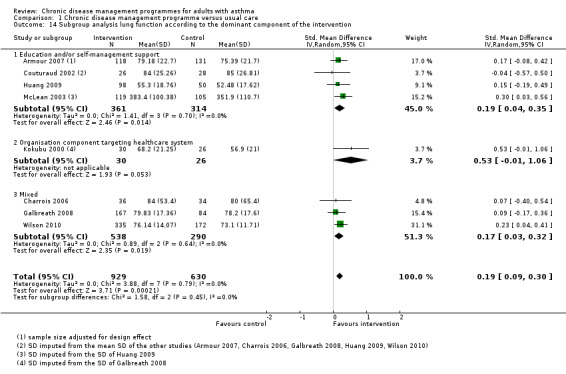
Comparison 1 Chronic disease management programme versus usual care, Outcome 14 Subgroup analysis lung function according to the dominant component of the intervention.
1.15. Analysis.
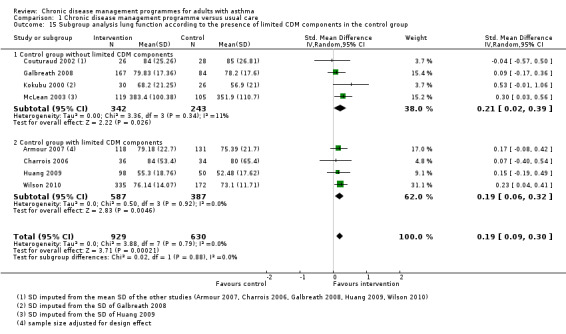
Comparison 1 Chronic disease management programme versus usual care, Outcome 15 Subgroup analysis lung function according to the presence of limited CDM components in the control group.
Additional sensitivity analyses
Similar results were observed when a fixed‐effect model rather than a random‐effects model was used, and when studies with imputed SDs (Couturaud 2002; Kokubu 2000; McLean 2003) or SDs estimated from a graph (Wilson 2010) were excluded.
The available data allowed us to analyse the change from baseline measurements instead of post‐intervention measurements for asthma‐specific quality of life (Analysis 1.6). The pooled SMD from the seven RCTs including 1547 patients (SMD 0.30, 95% CI 0.18 to 0.43) was higher than in the meta‐analysis with post‐intervention measures, although it did not reach clinical significance (SMD < 0.4), and heterogeneity (I2 = 25%) was lower. The SMD for the NRCT including 413 patients was similar to the pooled SMD of RCTs (SMD 0.37, 95% CI 0.18 to 0.57).
1.6. Analysis.
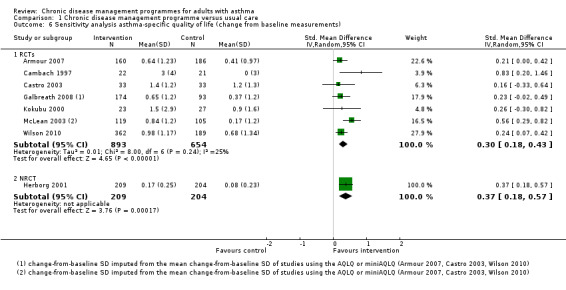
Comparison 1 Chronic disease management programme versus usual care, Outcome 6 Sensitivity analysis asthma‐specific quality of life (change from baseline measurements).
Discussion
Summary of main results
We reviewed the results from 20 studies that assessed the effectiveness of chronic disease management for adults with asthma. Results from the meta‐analyses showed that CDM programmes probably improve asthma‐specific quality of life (SMD 0.22, 95% CI 0.08 to 0.37), asthma severity scores (SMD 0.18, 95% CI 0.05 to 0.30), and lung function tests (SMD 0.19, 95% CI 0.09 to 0.30) but the results were inconclusive for self‐efficacy (SMD 0.51, 95% CI ‐0.08 to 1.11).
We could not combine data for hospitalisations and ED or unscheduled visits in a meta‐analysis because the data were skewed and too heterogenous; overall, the results from the individual studies were inconclusive. In addition, the data for the effectiveness on asthma exacerbations, days off work or school, use of an action plan, and patient satisfaction were sparse and meta‐analyses could not be performed. Although there were many different secondary outcomes in the included studies, only one study reported data on adverse events or mortality (Mayo 1990). In this study, during the eight months of follow‐up, there were no asthma‐related deaths in the intervention group but one patient died from asthma in the control group.
We did not observe any differences for the effectiveness of the intervention as a function of three pre‐specified features of the intervention: comprehensiveness of the intervention, the dominant component of the intervention, and presence of limited CDM components.
The seven clinically most important primary outcomes are summarised in the Table 1.
Overall completeness and applicability of evidence
We can reasonably consider that the results of this systematic review reflect what has been published on the effectiveness of CDM programmes in asthma. The 20 studies included in this review were identified after applying a comprehensive search strategy designed to identify interventions that met all five criteria of an operational definition of chronic disease management (that is, including at least one organisational component targeting patients, at least one organisational component targeting healthcare professionals or the healthcare system, patient education or self‐management support, active involvement of two or more healthcare professionals in patient care, and a minimum duration of three months).
We pre‐specified the 10 most relevant outcomes for people with asthma as primary outcomes for this systematic review. The studies all reported at least one of these 10 primary outcomes (average of seven primary and secondary outcomes per study) and a meta‐analysis could be performed for 4 out of these 10 outcomes, guaranteeing the relevance of our results. However, since the number of studies included in each meta‐analysis was rather low, except for the outcomes asthma‐specific quality of life and lung function (eight studies each), we were unable to conduct appropriate subgroup analyses or meta‐regression.
However, although we included study designs other than RCTs (that is, NRCTs, CBAs, and ITS), 49 studies had to be excluded because their design was considered to be at high risk of bias (for example, several CBA studies with only one control or intervention site rather than at least two control and two intervention sites as specified by the EPOC review group methodology). In addition, none of the four included CBAs with two control and two intervention groups could be included in the meta‐analyses because available data were incomplete or inappropriate. Although these excluded studies were not considered to be relevant in the assessment of the effectiveness of CDM programmes, it might be useful to investigate what data these studies could contribute to the development of CDM programmes, in terms of understanding which key components bring most benefits or in improving our knowledge about the contexts and settings where these programmes have been implemented.
The results of this review should be considered with caution since we observed statistical, clinical, and methodological heterogeneity. This heterogeneity was taken into account in the choice of statistical models used and in the assessment of the level of the evidence.
Quality of the evidence
We included 20 studies, 15 of which were RCTs, in this review, and included up to eight studies and 1627 patients in the meta‐analyses. Sensitivity analyses based on study quality (excluding studies at high risk of bias or with imputed SDs) did not change the direction, significance, or magnitude of the observed effectiveness.
Following the GRADE approach, we specified the levels of quality of the evidence (high, moderate, low, and very low) for the seven most important primary outcomes presented in our Table 1. This was done taking into account the study design, indirectness of the evidence, unexplained heterogeneity or inconsistency of the results, imprecision of the results, and high probability of publication bias.
Overall, the quality of the evidence was moderate to low despite the fact that studies for which a meta‐analysis could be performed were mostly RCTs. This was due mainly to study design limitations, which resulted in either unclear or high risk of bias in most cases, and in wide confidence intervals, therefore explaining why the level of evidence was downgraded by one or two levels depending on the outcomes.
Potential biases in the review process
We attempted to minimise biases in our review process by firstly using an explicit and detailed operational definition of what we considered as CDM to overcome the absence of a consensual definition of chronic disease management and help the reader understand which types of programmes were considered in this review. This definition included more than the traditional education and self‐management components evaluated previously in primary studies and a few systematic reviews. Secondly, we restricted study designs to those recommended by the EPOC Review Group methodology (EPOC 2013), which meant that CBA studies had to assess two intervention and two control groups. Finally, we performed a comprehensive search for primary studies.
The results of this systematic review should be interpreted considering the following limitations. As with most systematic reviews targeting complex interventions, such as CDM, several sources of heterogeneity must be acknowledged. In addition to methodological and statistical heterogeneity, the biggest source of heterogeneity was clinical heterogeneity due to context, settings, patients, and interventions, which differed across studies. We attempted to limit this clinical heterogeneity by having a clear operational definition and only including comprehensive interventions. In addition, heterogeneity was taken into consideration in the statistical analyses by using random‐effects models and in the quality evaluation of the studies, which resulted in downgrading in some cases. Despite this, a high level of unexplained statistical heterogeneity remained in the self‐efficacy meta‐analysis, which was mainly due to one study (Huang 2009) that included a higher proportion of men than the other studies. However, as other outcomes from this study did not stand out in the other meta‐analyses it is unlikely that the large positive results of this study were due to intervention or population characteristics. The atypical result for Huang 2009 could also be due to the self‐efficacy instrument used in this study, which was different from the other studies. Despite having included 20 studies in the review, only eight at most could be included in the meta‐analyses because of missing information and the wide range of outcomes reported in the different studies, which were too heterogeneous to be combined in some cases. Further, three of the outcomes (quality of life, self‐efficacy, asthma severity) were self‐reported by patients. However, patient reported outcome measures are increasingly being measured in evaluations of CDM programmes, because they are important to patients. Most instruments used in the included studies have been validated. Only two of the four studies that used an instrument that had not been validated contributed data to a meta‐analysis, and sensitivity analyses excluding these two studies did not modify the pooled estimate. Finally, there was often little information about the components of the interventions, their frequency and intensity for example, as well as the specific setting and context in which they were implemented, making their reproducibility in other settings difficult. However, this is inherent to CDM and, more generally, other quality improvement interventions, which are complex and context‐dependent (Davidoff 2009).
Agreements and disagreements with other studies or reviews
This review updates three previous systematic reviews assessing the effectiveness of CDM programmes in patients with asthma (Lemmens 2009; Maciejewski 2009; Steuten 2007a). The main differences between our review and the previous reviews are that i) our search strategy was more comprehensive and detailed, and did not have any restrictions for publication year, as did Steuten 2007a, thus giving a broader coverage of the intervention over time and more potential articles to screen, ii) the use of an a priori proposed operational definition of CDM, defined in the published protocol, which enabled the criteria for selecting studies to be clearer, and iii) the study designs considered in the current review included all those recommended by the EPOC group as being able to minimise potential bias (EPOC 2013). However, despite these differences, our results are consistent with previous reviews, that is small overall effects, improved quality of life (Lemmens 2009), no effect on emergency department (ED) visits (Lemmens 2009), and limited impact on lung function (Lemmens 2009; Steuten 2007a).
There are several explanations why we found little effect of CDM programmes in patients with asthma. First, the 'usual care' administered to the control group may have differed between studies and may have included some asthma education initiatives; since this has been considered as standard care of patients with asthma for a long time and reflects a good level of clinical management. Therefore, it may be difficult to demonstrate a difference of effect between a CDM programme and what is described as usual care. Second, the CDM programmes assessed in this systematic review were quite heterogeneous, therefore constituting a heterogeneous pooled intervention group that was compared with usual care, which was also heterogeneous, resulting in pooled odds ratios (ORs) tending towards a null effect. Third, the prevalence of asthma varies between countries, and heterogeneity in its diagnosis, severity, and phenotypes has been reported (Eder 2006). CDM could therefore be effective for only a subgroup of patients with asthma. Fourth, the length of key components and follow‐up periods may have been too short to allow the effects of long‐term interventions to be detected. Finally, demonstrating differences for outcomes that are not so frequent in the daily life of patients with asthma, such as hospitalisation and emergency or unscheduled visits, may be difficult.
The results of this review, showing trends towards some benefits for patients with asthma, are consistent with those from other systematic reviews assessing CDM programmes for different chronic diseases such as COPD (Kruis 2013; Lemmens 2013; Niesink 2007; Peytremann‐Bridevaux 2008), diabetes (Elissen 2013; Knight 2005; Norris 2002; Pimouguet 2011), heart failure (Gohler 2006; Gonseth 2004; McAlister 2001; Roccaforte 2005), and depression (Badamgarav 2003; Neumeyer‐Gromen 2004). CDM programmes seem to be less effective in asthma patients, however. In addition to the factors which may have led to underestimation of the effect of CDM in our analysis discussed above, other methodological issues could explain little effectiveness. As previously reported (Lemmens 2009), the quality of the studies assessing the effect of CDM for patient with asthma is suboptimal, and RCTs can be difficult to conduct in the community, a setting where patients with asthma often receive their care. Also, evaluation measures were often not pertinent and focused on outcomes rather than processes or structure measures (Steuten 2007a). Other explanations for a smaller effect in asthma, compared with other chronic diseases, may relate to the disease itself or its treatment since, compared with other common chronic diseases, asthma generally affects younger and otherwise healthy patients; and is observed as respiratory symptoms only. It could therefore be hypothesised that interventions limited to education or self‐management, or both, which primarily target the appropriate use of drugs and avoidance of triggers and which were frequently offered in the control groups of this review may be sufficient. However, there have been few published systematic reviews assessing the effectiveness of education or self‐management interventions despite there being a large number of primary studies. These interventions have been shown to greatly vary and be insufficiently documented (Sudre 1999). However, it is generally accepted that while patient education programmes limited to information only do not improve health outcomes (Gibson 2002), self‐management education programmes associated with regular practitioner review do (Gibson 2003). In our review, results of the exploratory subgroup analyses on the dominant component and the comprehensiveness of the implemented interventions were inconclusive. For patients with asthma, it is unknown if implementing interventions such as CDM programmes provides more benefit than education and self‐management support only.
The effects of CDM programmes for asthma can be contrasted to the effects of those programmes targeting patients with COPD, an obstructive disease characterised by respiratory symptoms and systemic consequences of chronic inflammation that affects older patients, where recent good quality evidence has confirmed the benefits of CDM programmes in terms of quality of life, hospitalisation, and exercise tolerance (Kruis 2013).
Authors' conclusions
Implications for practice.
Our systematic review provides moderate to low evidence that CDM programmes have a clinically small but positive effect on asthma‐specific quality of life and asthma severity, which are among the main objectives of asthma management and are the most crucial outcomes for the everyday life of patients with asthma. Our results also showed that lung function tests (FEV1 and PEF) were slightly improved with the CDM programmes.
Despite the moderate to low level of evidence, we can consider, overall, that the results of this systematic review represent encouraging evidence for the effectiveness of CDM programmes in adults with asthma. The development of CDM programmes must be with evidence of their benefits since the programmes are resource consuming and usually require organisational restructuring. Our data seem to be encouraging for further investment in the promotion, development, and evaluation of CDM programmes in asthma, a condition with a substantial burden for patients and healthcare systems. However, the optimal composition of asthma CDM programmes still needs further investigation, especially in terms of the specific components and the level of complexity. It seems very important to assess the benefits of CDM programmes with those from education or self‐management, or both, alone since the latter are usually offered to patients with asthma and represent usual care for a majority of healthcare professionals taking care of patients with asthma. This may be difficult since education, self‐management, and CDM programmes are not always readily distinguishable.
Implications for research.
We suggest that researchers planning future studies on the effectiveness of chronic disease management for patients with asthma consider the following issues.
Investigate the most responsive patients to determine whether a particular subpopulation of patients with asthma benefit more from CDM programmes than others. Future trials could categorise patients into subgroups according to disease severity or other criteria.
Describe the CDM programme in detail to identify which of the CDM components are more beneficial than others to patients with asthma, and assess if complex or intensive programmes are needed, and what components could be added to the current patient education and self‐management support provided. Hence, future trials should report and assess the components of the CDM programmes in detail, including their frequency and intensity. Future trials should also try to compare education and self‐management support with CDM programmes directly, or to compare different types of CDM programmes, in terms of comprehensiveness and intensity, for example rather than comparing with usual care.
Assess the impact of CDM on hospitalisations and emergency department or unscheduled visits more fully.
Consider not only outcome of care indicators but also structure and process of care indicators. Since outcome indicators do not seem to be greatly improved, emphasis should be put on the assessment of structure and process of care indicators to better interpret the outcome results.
Improve the quality and reporting of studies to increase the level of evidence and confidence in the results. It is crucial to improve both the quality of pragmatic studies assessing the effectiveness of these programmes and the quality of their reporting. Future trials describing methods and data collection more completely would increase the quality of the evidence for the results of systematic reviews.
Acknowledgements
We would like to thank Anne Pittet, medical librarian at the Centre Hospitalier Vaudois and University of Lausanne, for helping with the search strategies. We also thank the following reviewers who have helped to improve the content of the manuscript: Christopher Cates, Angela Coulter, Tamara Rader, Julia Worswick and Sasha Shepperd. We thank Margaret Haugh (MediCom Consult) for editorial assistance.
Appendices
Appendix 1. MEDLINE strategies 2014
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to Present>
RCT search
Search date: June 13, 2014
1 exp Asthma/ (105750) 2 asthma$.ti. or wheez$.ti,ab. (81128) 3 (asthmatic? or (asthma$ adj2 (chronic$ or patient?))).ab. (37283) 4 Bronchial Hyperreactivity/ (6788) 5 bronchial$ hyperreactivit$.ti,ab. (1957) 6 lung diseases, obstructive/ (17975) 7 (obstructive adj (lung disease or lung diseases)).ti. (1966) 8 or/1‐7 [Asthma] (139414) 9 chronic disease management.ti,ab. (1112) 10 (asthma adj3 (program or programs or programme or programme)).ti. (414) 11 exp Patient Care Team/ (54559) 12 (care adj2 team$).ti,ab. (8290) 13 or/11‐12 [Patient Care Team] (60111) 14 Disease management/ (11729) 15 ((disease adj2 management) or (chronic adj2 management)).ti,ab. (18495) 16 or/14‐15 [Disease Management] (27904) 17 Patient Care Management/ or Patient‐Centered Care/ or "Continuity of Patient Care"/ or Comprehensive Health Care/ (32517) 18 comprehensive health care.ti,ab. (679) 19 (care adj2 management).ti,ab. (8132) 20 (patient centred or patient centered or (continuity adj2 care)).ti,ab. (12492) 21 or/17‐20 [Care Management/continuity] (47989) 22 patient care planning/ or case management/ or critical pathways/ (45209) 23 ((care adj2 (algorithm? or pathway? or plan)) or CRITICAL pathway?).ti,ab. (7764) 24 (((written or action) adj3 plan?) or (planning adj2 care)).ti,ab. (9719) 25 or/22‐24 [Care Planning/Pathway] (59358) 26 "Delivery of Health Care, Integrated"/ (8391) 27 (integrat$ adj2 (care or healthcare)).ti,ab. (5974) 28 or/26‐27 [Integrated Care] (12868) 29 "length of stay"/ or patient readmission/ (64055) 30 ("length of stay" or readmission?).ti. (4613) 31 ((reduc$ or shorten or lower$) adj3 (hospitali?ation? or "length of stay" or readmiss$ or readmit$)).ab. (6951) 32 or/29‐31 [Length of Stay/Readmissions] (69373) 33 *hospitalization/ and (management or program? or programme or programmes or model? or reduc$ or impact or intervention or improving).ti. (2353) 34 patient discharge/ and ((chronic or plan? or planning or team? or collaborat$ or intervention?).ti. or (planning or team? or collaborat$ or (chronic adj3 (disease or model?))).ab.) (3383) 35 or/33‐34 [Discharge/reduce, manage hospitalizations] (5717) 36 Managed Care Programs/ (23447) 37 ((care or healthcare) adj3 (model? or program? or programme or programmes)).ti,ab. (27884) 38 or/36‐37 [Managed Care] (50315) 39 home care services/ or home care services, hospital‐based/ or home nursing/ (36216) 40 (home adj2 (service or services or care or healthcare or visit?)).ti,ab. (25334) 41 or/39‐40 [Home Care] (49205) 42 community health services/ or community health nursing/ or community networks/ or community pharmacy services/ or counseling/ (79125) 43 ((community adj3 (nursing or nurse or nurses or care or healthcare)) or community‐based).ti,ab. (52960) 44 or/42‐43 [Community Care] (121322) 45 Occupational Health Services/ and ((primary adj2 care) or disease management or specialist? or chronic disease? or chronic care or chronic condition?).ti,ab. (349) 46 (School Health Services/ not (child/ or child, preschool/ or exp infant/)) and (chronic or disease management).ti,ab. (48) 47 school health services/ and adolescent/ and (chronic or disease management).ti,ab. (116) 48 (((mobile or preventive or preventative or clinic?) adj2 (clinic? or service or services or health or health care or care or model?)) and (chronic or disease management)).ti,ab. (17833) 49 early medical intervention/ (712) 50 or/45‐49 [Misc Health Service] (19002) 51 exp Telemedicine/ or telenursing/ or remote consultation/ (15741) 52 ((telemedicine or telehealth$ or telenurs$ or tele‐medicin$ or tele‐health$ or tele‐nurs$ or ehealth or e‐health or remote consult$) adj10 chronic).ti,ab. or telephone.ti. (4984) 53 (PDA or hand‐held? or Iphone? or ipad? or i‐phone? or i‐pad? or blackberry or personal digital assistant? or webbased or web‐based web2$ or computeri?ed).ti,ab. (75884) 54 or/51‐53 [Telemed/Tech Terms] (95571) 55 Patient Education as Topic/ or health education/ or consumer health information/ or health literacy/ or health fairs/ (123583) 56 Patient Participation/ or Self care/ or Self administration/ or consumer participation/ (64052) 57 (patient? adj3 (participation or motivating)).ti,ab. (3762) 58 (patient? adj3 (education$ or educating or educate?)).ti,ab. (23517) 59 (self‐care or self‐manag$).ti,ab. (18430) 60 or/55‐59 [Patient Education/Self Care] (199836) 61 education, continuing/ or education, medical, continuing/ or education, nursing, continuing/ or education, pharmacy, continuing/ or education, professional, retraining/ or exp inservice training/ (69860) 62 ((continuing adj3 education$) or (CME adj3 (program$ or session? or meeting?)) or inservice? or workshop? or professional development).ti,ab. (46492) 63 ((physician? adj2 behavio?r?) or (upskill$ or up‐skill$)).ti,ab. (1818) 64 or/61‐63 [Continuing Education] (105728) 65 Nurse's Role/ or Physician's Role/ or Professional Role/ (66788) 66 ((role or roles) adj2 (chang$ or expand$ or extend$ or revision or revised or revising or nurse or nurse's or nursing or physician?)).ti,ab. (18920) 67 or/65‐66 [Professional Roles] (81662) 68 medical staff/ or exp medical staff, hospital/ or exp nurses/ or exp nursing staff/ or exp pharmacists/ or exp physicians/ (230078) 69 Primary Nursing/ or Nurse Clinicians/ or Nurse Practitioners/ or Community Health Nursing/ or Physician Assistants/ (44157) 70 nursing care/ or emergency nursing/ or holistic nursing/ or home nursing/ or nursing, practical/ or occupational health nursing/ or primary nursing/ or rehabilitation nursing/ (53149) 71 (nurse‐led or ((nurse or nurses or nursing) adj3 (primary adj2 (care or healthcare)))).ti,ab. (4079) 72 exp Allied Health Personnel/ (41633) 73 (allied health or physiotherapist? or physical therapist? or exercise therap$).ti,ab. (13910) 74 (nurse clinician? or nurse practitioner? or physician? Assistant?).ti,ab. (10377) 75 ("nurse‐led" or (nurse? adj2 (led or managed or coordinat$ or co‐ordinat$))).ti,ab. (3291) 76 or/68‐75 [General Medical Practitioners] (345435) 77 respiratory therapy department, hospital/ (382) 78 physical therapy department, hospital/ (310) 79 ((pulmonary or respiratory or respirolog$ or pneumology) adj2 (practitioner? or physician? or specialist? or doctor? or medicine or nurse or nurses)).ti,ab. (2911) 80 (pulmonologist? or respirologist? or pulmonology or respirology or pneumologist? or pneumology).ti,ab. (4427) 81 or/77‐80 [Specialist practitioners/discipline] (7799) 82 Decision Support Systems, Clinical/ or Decision Making, Computer‐Assisted/ or Medical Informatics Applications/ or Decision Support Techniques/ or decision making, organizational/ (30860) 83 (shared decision$ or decision aid? or (decision$ adj2 model$) or (decision$ adj support?) or (decision making adj2 computer$) or informatics).ti,ab. (23574) 84 ((clinical or clinician? or doctor? or medical or nurse or nurses or nursing or patient? or physician? or practitioner?) adj3 decision making).ti,ab. (17727) 85 or/82‐84 [Decision Support/Making] (62742) 86 "Referral and Consultation"/ or Gatekeeping/ (51835) 87 (Referral? adj3 (chronic or decreas$ or ((general or family) adj2 (doctor? or physician? or practitioner?)) or impact or improv$ or increas$ or intervention or plan or plans or primary care or primary health$ or program$ or reduc$ or specialist?)).ti,ab. (6917) 88 or/86‐87 [Referral] (55620) 89 Practice Guidelines as Topic/ or guidelines as topic/ or Guideline Adherence/ (122252) 90 (guideline? adj3 (implement$ or impact or adherence)).ti,ab. (8241) 91 Evidence‐Based Medicine/ and (change or changing or chronic or ((patient or care or disease) adj2 management) or impact or implement$ or influence or intervention? or model? or patient care or program? or programme or programmes or strategy or strategies or translation).ti,ab,hw. (23555) 92 or/89‐91 [Guidelines (topic/adherence)/ EBM] (144237) 93 Interdisciplinary Communication/ or Cooperative Behavior/ (36001) 94 (collaborat$ or "cross‐profession$" or interdisciplin$ or inter‐discipllin$ or intraprofession$ or intra‐profession$ or interprofession$ or inter‐profession$ or multidisciplin$ or multi‐disciplin$ or crossdisciplin$ or cross‐disciplin$ or team or teams or team‐based or (skill adj2 mix$)).ti,ab. (215885) 95 or/93‐94 [Interdisciplinary/Collaboration] (237175) 96 "Outcome Assessment (Health Care)"/ (48993) 97 outcome? Assessment?.ti,ab. (3731) 98 or/96‐97 [Outcome Assessment] (51815) 99 health services administration/ or "organization and administration"/ or efficiency, organizational/ or health facility administration/ or hospital administration/ (64124) 100 exp hospital restructuring/ or hospital shared services/ (9394) 101 centralized hospital services/ or pharmacy service, hospital/ or diagnostic services/ (12245) 102 models, organizational/ or multi‐institutional systems/ or organizational culture/ or exp organizational innovation/ or organizational objectives/ or institutional management teams/ (67611) 103 (organi?ational or restructuring or (organi?ation$ adj3 (change? or changing or initiat$ or structur$ or restrict$ or model?))).ti,ab. (56245) 104 or/99‐103 [Organisations/Org services/Org Admin] (186973) 105 total quality management/ or "quality of health care"/ or quality assurance, health care/ or benchmarking/ or quality improvement/ or Management Quality Circles/ or Quality Assurance, Health Care/ or "Quality of Health Care"/ or "United States Agency for Healthcare Research and Quality"/ (129088) 106 (quality adj2 (assessment? or assurance or circle? or implement$ or increase$ or improvement? or management or measure$ or outcome? or total)).ti,ab. (73012) 107 Peer Review, Health Care/ or Peer Review/ (7528) 108 or/105‐107 [Quality Improvement/Quality Care] (183213) 109 Physician Incentive Plans/ or reimbursement, incentive/ [ML] (4857) 110 ((physician? or practitioner? or doctor? or nurse or nurses) adj4 incentive? plan?).ti,ab. (33) 111 exp Health Personnel/ and (incentiv$ adj2 (economic or financial or monetar$ or payment? or reimburs$)).ti,ab. (561) 112 or/109‐111 [Incentives] (5278) 113 (insurance, health, reimbursement/ or reimbursement mechanisms/ or fee‐for‐service plans/ or "physician payment review commission"/ or medicare payment advisory commission/ or reimbursement, disproportionate share/ or relative value scales/) and chronic.ti,ab. (429) 114 (insurance, health, reimbursement/ or reimbursement mechanisms/ or fee‐for‐service plans/ or "physician payment review commission"/ or medicare payment advisory commission/ or reimbursement, disproportionate share/ or relative value scales/) and (change or changes or changing or chronic or effectiveness or impact or implement$ or intervention).ti,ab. (4959) 115 "fees and charges"/ or capitation fee/ or fee‐for‐service plans/ or fees, medical/ or fees, pharmaceutical/ or prescription fees/ or "rate setting and review"/ [ML] (22973) 116 (gainshar$ or payer‐provider? or payer‐patient?).ti,ab. (139) 117 ("pay for compliance" or "pay for participation" or "pay for performance" or "performance pay$" or P4P or "pay for quality improvement?" or P4QI or "fee‐for service?").ti,ab. (5057) 118 (payment? adj (blend$ or "blue cross" or bonus$ or capped or "episode of care" or fixed or government$ or insurance or insurer? or level? or linear or medicaid or medicare or non‐linear or per‐patient or per‐episode or per‐visit or performance or prospectiv$ or retroactiv$ or retrospectiv$ or reward$ or schedule? or system? or target$ or third‐part$ or threshold? or uncap$ or shared or variable or per‐visit?)).ti,ab. (3007) 119 or/113‐118 [Financial] (32314) 120 adult/ or exp aged/ or middle aged/ or young adult/ (5564077) 121 adult?.ti,ab,hw. or middle aged.ti,ab. (4481427) 122 or/120‐121 [Adults] (5977532) 123 exp child/ or adolescent/ (2408162) 124 (adolescent? or baby or babies or child$ or infant? or neonate? or neo‐nate? or p?ediatric$).ti. (886816) 125 *pediatrics/ or *neonatology/ or *perinatology/ (31200) 126 (infant? or toddler? or child$ or adolescent? or neonate? or neo‐nate? or (p?ediatric$ adj2 (patient? or inpatient?))).ab. (992901) 127 or/123‐126 [Child/Pediatrics] (2938570) 128 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti. (908159) 129 exp animals/ not humans.sh. (3949551) 130 128 not 129 [Cochrane RCT Filter 6.4.d Sens/Precision Maximizing] (838022) 131 (8 and 9 and 122) or ((8 and 9) not 127) [Screen all; do not add filter] (41) 132 ((10 and 122) or (10 not 127)) not 131 [Keyword results; do not add filter] (212) 133 ((8 and 13 and 122) or ((8 and 13) not 127)) and 130 [Set 1] (21) 134 ((8 and 16 and 122) or ((8 and 16) not 127)) and 130 [Set 1a] (120) 135 ((8 and 21 and 122) or ((8 and 21) not 127)) and 130 [Set 1b] (40) 136 ((8 and 25 and 122) or ((8 and 25) not 127)) and 130 [Set 1bc] (90) 137 ((8 and 28 and 122) or ((8 and 28) not 127)) and 130 [Set 2] (6) 138 ((8 and 32 and 122) or ((8 and 32) not 127)) and 130 [Set 2a] (107) 139 ((8 and 35 and 122) or ((8 and 35) not 127)) and 130 [Set 2c] (7) 140 ((8 and 38 and 122) or ((8 and 38) not 127)) and 130 [Set 3] (57) 141 ((8 and 41 and 122) or ((8 and 41) not 127)) and 130 [Set 4] (77) 142 ((8 and 44 and 122) or ((8 and 44) not 127)) and 130 [Set 5] (94) 143 ((8 and 50 and 122) or ((8 and 50) not 127)) and 130 [Set 6] (90) 144 ((8 and 54 and 122) or ((8 and 54) not 127)) and 130 [Set 7] (101) 145 ((8 and 60 and 122) or ((8 and 60) not 127)) and 130 [Set 8] (490) 146 ((8 and 64 and 122) or ((8 and 64) not 127)) and 130 [Set 9] (47) 147 ((8 and 67 and 122) or ((8 and 67) not 127)) and 130 [Set 10] (16) 148 ((8 and 76 and 122) or ((8 and 76) not 127)) and 130 [Set 11] (137) 149 ((8 and 81 and 122) or ((8 and 81) not 127)) and 130 [Set 12] (92) 150 ((8 and 85 and 122) or ((8 and 85) not 127)) and 130 [Set 13] (31) 151 ((8 and 88 and 122) or ((8 and 88) not 127)) and 130 [Set 14] (28) 152 ((8 and 92 and 122) or ((8 and 92) not 127)) and 130 [Set 15] (216) 153 ((8 and 95 and 122) or ((8 and 95) not 127)) and 130 [Set 16] (107) 154 ((8 and 98 and 122) or ((8 and 98) not 127)) and 130 [Set 17] (89) 155 ((8 and 104 and 122) or ((8 and 104) not 127)) and 130 [Set 18] (17) 156 ((8 and 108 and 122) or ((8 and 108) not 127)) and 130 [Set 19] (179) 157 ((8 and 112 and 122) or ((8 and 112) not 127)) and 130 [Set 20] (3) 158 ((8 and 119 and 122) or ((8 and 119) not 127)) and 130 [Set 21] (7) 159 13 or 16 or 21 or 25 or 28 or 32 or 35 or 38 or 41 or 44 or 50 or 54 or 60 or 64 or 67 or 76 or 81 or 85 or 88 or 92 or 95 or 98 or 104 or 108 or 112 or 119 (1661114) 160 (((8 and 159 and 122) or ((8 and 159) not 127)) and 130) not (or/131‐132) [RCT Results ] (1375) 161 (or/133‐158) not (or/131‐132) (1375) 162 (201211$ or 2013$ or 2014$).ep,ed,yr. [2012‐2014 Limits] (1965302) 163 161 and 162 [2014 RCT results] (103) 164 132 and 162 [2014 KW results] (21)
Non‐RCT search (using EPOC non‐RCT study designs filter)
Search date: June 18, 2014
1 asthma/ (103892) 2 (asthma$ or wheez$).ti. (76459) 3 (asthma$ adj3 (sever$ or chronic$ or primary or major)).ab. (17035) 4 or/1‐3 [Asthma] (113631) 5 (chronic adj4 (model or management)).ti,ab. (23279) 6 (asthma adj3 (model? or program or programs or programme or programmes)).ti,ab. (4446) 7 (disease adj2 management adj5 (model? or program? or programme or programmes)).ti,ab. (1965) 8 (comprehensive health care or ((continuity or continu$) adj3 (care or healthcare))).ti,ab. (14246) 9 (care adj2 model?).ti,ab. (7996) 10 (patient centred or patient centered).ti,ab. (8330) 11 ((care adj2 (algorithm? or pathway? or plan)) or CRITICAL pathway?).ti,ab. (7779) 12 (((written or action) adj3 plan?) or (planning adj2 care)).ti,ab. (9739) 13 (integrat$ adj2 (care or healthcare)).ti,ab. (5980) 14 ((fewer or reduc$ or lower$ or shorten$) adj3 ("length of stay" or hospitali?ation? or readmission? or readmit$ or admission?)).ti,ab. (11818) 15 (hospitali?ation?.ti,hw. and (program? or programme or programmes or model? or reduc$ or impact or intervention or improving or reduc$ or lower$ or fewer).ti.) or (hospitali?ation? adj4 (reduc$ or fewer or lower)).ab. (11438) 16 (patient? discharg$ or discharge plan$).ti,hw. and ((impact or improv$ or initiativ$ or quality or chronic or plan?).ti. or (planning or team? or collaborat$ or intervention?).ti,ab.) (5689) 17 ((patient admission? or hospital$ admission? or readmission? or readmit$).ti,hw. and (fewer or reduc$ or lower$ or shorten$).ti.) or ((patient admission? or hospital$ admission? or readmission? or readmit$) adj4 (fewer or reduc$ or lower$)).ab. (3221) 18 ((community adj3 (nursing or nurse or nurses or care or healthcare)) or community‐based).ti,ab. (53018) 19 ((telephone? or telephoning or phone? or phoning or telemedicine or telehealth$ or telenurs$ or tele‐medicin$ or tele‐health$ or tele‐nurs$ or ehealth or e‐health or remote consult$) and chronic and (care or diseas$ or condition?)).ti,ab. (2822) 20 (PDA or Iphone? or ipad? or i‐phone? or i‐pad? or blackberry or personal digital assistant? or handheld or ((webbased or web‐based web2$ or computeri?ed) adj5 ((chronic or diseas$) adj2 (care or manag$ or diseas$ or condition$)))).ti,ab. (10507) 21 (patient? adj3 (participation or physician?)).ti,ab. (37380) 22 (patient? adj3 (education$ or educating or educate?) adj4 (part or intervention? or complex or program? or model or multifacet$ or multimod$ or combin$ or "in addition" or package or suite)).ti,ab. (2497) 23 ((self‐care or self‐manag$) adj4 (part or intervention? or complex or program? or model or multifacet$ or multimod$ or combin$ or "in addition" or package or suite)).ti,ab. (2831) 24 ((continuing adj3 education$) or (CME adj3 (program$ or session? or meeting?)) or inservice? or workshop? or professional development).ti,ab. (46525) 25 ((physician? adj2 behavio?r?) or (upskill$ or up‐skill$)).ti,ab. (1819) 26 ((role or roles) adj2 (chang$ or expand$ or extend$ or revision or revised or revising or nurse or nurse's or nursing or physician?)).ti,ab. (18937) 27 (((nurse or nurses or nursing) adj3 (primary adj2 (care or healthcare))) and specialist?).ti,ab. (162) 28 ((allied health or physiotherapist? or therapist?) adj7 (specialist? or partner$)).ti,ab. (575) 29 (nurse clinician? or nurse practitioner? or physician? Assistant?).ti,ab. (10382) 30 ("nurse‐led" or (nurse? adj2 (led or managed or coordinat$ or co‐ordinat$))).ti,ab. (3296) 31 (shared decision$ or decision aid? or (decision$ adj2 model$) or (decision$ adj support?) or (decision making adj2 computer$) or informatics).ti,ab. (23600) 32 ((clinical or clinician? or doctor? or medical or nurse or nurses or nursing or patient? or physician? or practitioner?) adj3 decision making).ti,ab. (17757) 33 (Referral? adj3 (chronic or decreas$ or ((general or family) adj2 (doctor? or physician? or practitioner?)) or impact or improv$ or increas$ or intervention or plan or plans or primary care or primary health$ or program$ or reduc$ or specialist?)).ti,ab. (6930) 34 (guideline? adj3 (implement$ or impact or ((improv$ or increas$) adj2 adherence))).ti,ab. (5788) 35 (collaborat$ or "cross‐profession$" or interdisciplin$ or inter‐discipllin$ or intraprofession$ or intra‐profession$ or interprofession$ or inter‐profession$ or multidisciplin$ or multi‐disciplin$ or crossdisciplin$ or cross‐disciplin$ or team or teams or team‐based or (skill adj2 mix$)).ti,ab. (216209) 36 (patient? adj2 outcome? adj3 (improv$ or increas$)).ti,ab. (13202) 37 ((organi?ation$ adj2 (change or changes or culture or intervention? or model?)) or multi‐institution$ or innovat$).ti,ab. (74359) 38 restructuring.ti,ab. (6341) 39 (quality adj2 (assessment? or assurance or circle? or implement$ or increase$ or improvement? or management or measure$ or outcome? or total)).ti,ab. (73102) 40 ((nurse or nurses or provider? or practitioner? or physician?) adj3 incentiv$).ti,ab. (1025) 41 (insurance or reimbursement or "fee‐for‐service?" or medicare or medicaid).ti,hw. and (change or changes or changing or chronic or effectiveness or impact or implement$ or intervention$).ti. (8394) 42 (gainshar$ or payer‐provider? or payer‐patient?).ti,ab. (139) 43 ("pay for compliance" or "pay for participation" or "pay for performance" or "performance pay$" or P4P or "pay for quality improvement?" or P4QI or "fee‐for service?").ti,ab. (5064) 44 (payment? adj (blend$ or "blue cross" or bonus$ or capped or "episode of care" or fixed or government$ or insurance or insurer? or level? or linear or medicaid or medicare or non‐linear or per‐patient or per‐episode or per‐visit or performance or prospectiv$ or retroactiv$ or retrospectiv$ or reward$ or schedule? or system? or target$ or third‐part$ or threshold? or uncap$ or shared or variable or per‐visit?)).ti,ab. (3010) 45 *"health care quality"/ and (improv$ or increas$ or decreas$ or reduc$ or outcome?).ti. (3701) 46 (practice pattern? or ((physician? or pharmacist?) adj2 led)).ti,ab. (5481) 47 ("cross‐profession$" or interdisciplin$ or inter‐discipllin$ or intraprofession$ or intra‐profession$ or interprofession$ or inter‐profession$ or multidisciplin$ or multi‐disciplin$ or crossdisciplin$ or cross‐disciplin$ or team‐based or (skill adj2 mix$)).ti,ab. (74309) 48 management.ti. (264199) 49 or/5‐48 [Intervention terms] (882410) 50 intervention?.ti. or (intervention? adj6 (clinician? or collaborat$ or community or complex or DESIGN$ or doctor? or educational or family doctor? or family physician? or family practitioner? or financial or GP or general practice? or hospital? or impact? or improv$ or individuali?e? or individuali?ing or interdisciplin$ or multicomponent or multi‐component or multidisciplin$ or multi‐disciplin$ or multifacet$ or multi‐facet$ or multimodal$ or multi‐modal$ or personali?e? or personali?ing or pharmacies or pharmacist? or pharmacy or physician? or practitioner? or prescrib$ or prescription? or primary care or professional$ or provider? or regulatory or regulatory or tailor$ or target$ or team$ or usual care)).ab. (163873) 51 (pre‐intervention? or preintervention? or "pre intervention?" or post‐intervention? or postintervention? or "post intervention?").ti,ab. [added 2.4] (10398) 52 (hospital$ or patient?).hw. and (study or studies or care or health$ or practitioner? or provider? or physician? or nurse? or nursing or doctor?).ti,hw. (721142) 53 demonstration project?.ti,ab. (1963) 54 (pre‐post or "pre test$" or pretest$ or posttest$ or "post test$" or (pre adj5 post)).ti,ab. (66412) 55 (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab. (621) 56 trial.ti. or ((study adj3 aim?) or "our study").ab. (637598) 57 (before adj10 (after or during)).ti,ab. (359155) 58 ("quasi‐experiment$" or quasiexperiment$ or "quasi random$" or quasirandom$ or "quasi control$" or quasicontrol$ or ((quasi$ or experimental) adj3 (method$ or study or trial or design$))).ti,ab,hw. (102099) 59 ("time series" adj2 interrupt$).ti,ab,hw. (1070) 60 (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month$ or hour? or day? or "more than")).ab. (9239) 61 pilot.ti. (40150) 62 Pilot projects/ (82634) 63 (clinical trial or controlled clinical trial or multicenter study).pt. (628551) 64 (multicentre or multicenter or multi‐centre or multi‐center).ti. (29237) 65 random$.ti,ab. or controlled.ti. (761933) 66 (control adj3 (area or cohort? or compare? or condition or design or group? or intervention? or participant? or study)).ab. not (controlled clinical trial or randomized controlled trial).pt. (413376) 67 "comment on".cm. or review.ti,pt. or randomized controlled trial.pt. (2934047) 68 (rat or rats or cow or cows or chicken? or horse or horses or mice or mouse or bovine or animal?).ti. (1345613) 69 exp animals/ not humans.sh. (3949551) 70 (or/50‐66) not (or/67‐69) [EPOC Methods Filter 2.4 Medline] (2157067) 71 exp adult/ or adult?.ti. or (adult? adj3 asthma$).ab. (5639003) 72 exp child/ or adolescent/ (2408162) 73 (child or children or infant or neonat$ or pre‐school or baby or babies or p?ediatric$ or perinat$ or teen$ or "school‐age?" or toddler?).ti,ab,hw. (2416343) 74 (adolescent? or adolescence).ti. (105354) 75 or/72‐74 [Child/Pediatrics] (3218406) 76 exp air pollution/ or dust/ or Antigens, Dermatophagoides/ or (dust? or mites or pollution or pollutant?).ti. (79218) 77 (and/4,49,71) not (or/73,76) (2136) 78 (and/4,49) not (or/75‐76) (6607) 79 70 and (or/77‐78) [Results EPOC Filter all years] (1442) 80 (201211$ or 2013$ or 2014$).ep,ed,yr. [2012‐2014 Limits] (1980315) 81 79 and 80 [EPOC 2014 results] (170)
Appendix 2. EMBASE strategy 2014
Embase <1947 to 2014 June 17> OVID Search date: June 18, 2014
1 exp *asthma/ (134484) 2 (asthma$ or wheez$).ti. (101147) 3 (asthma$ adj3 (sever$ or chronic$ or primary or major)).ab. (23848) 4 or/1‐3 [Asthma] (145506) 5 (exp asthma/ or bronchus hyperreactivity/) and chronic disease? management.ti,ab. (90) 6 4 and chronic disease? manag$.ti,ab. (58) 7 or/5‐6 [Focussed Key Terms] (92) 8 chronic disease management.ti,ab. (1409) 9 (asthma adj3 (model? or program or programs or programme or programmes)).ti,ab. (5828) 10 (care adj2 team$).ti,ab. (11602) 11 (disease adj2 management adj5 (model? or program? or programme or programmes)).ti,ab. (2840) 12 comprehensive health care.ti,ab. (903) 13 (care adj2 (model? or management)).ti,ab. (20461) 14 (patient centred or patient centered or (continuity adj2 care)).ti,ab. (15904) 15 ((care adj2 (algorithm? or pathway? or plan)) or CRITICAL pathway?).ti,ab. (11010) 16 (((written or action) adj3 plan?) or (planning adj2 care)).ti,ab. (12743) 17 (integrat$ adj2 (care or healthcare)).ti,ab. (7802) 18 ((fewer or reduc$ or lower$ or shorten$) adj3 ("length of stay" or hospitali?ation? or readmission? or readmit$ or admission?)).ti,ab. (17645) 19 hospitali?ation?.ti,hw. and (management or program? or programme or programmes or model? or reduc$ or impact or intervention or improving).ti. (22501) 20 (patient? discharg$ or discharge plan$).ti,hw. and ((improv$ or quality or chronic or plan?).ti. or (planning or team? or collaborat$ or intervention?).ti,ab.) (1165) 21 ((care or healthcare) adj3 (model? or program? or programme or programmes)).ti,ab. (35859) 22 (home adj2 (service or services or care or healthcare or visit?)).ti,ab. (30388) 23 ((community adj3 (nursing or nurse or nurses or care or healthcare)) or community‐based).ti,ab. (62459) 24 (((mobile or preventive or preventative or clinic?) and (clinic? or service or services or health or health care or care or model?) and chronic) or ((mobile or preventive or preventative or clinic?) adj3 (clinic? or service or services or health or health care or care or model?) adj5 chronic)).ti. (1555) 25 ((telephone? or telephoning or phone? or phoning or telemedicine or telehealth$ or telenurs$ or tele‐medicin$ or tele‐health$ or tele‐nurs$ or ehealth or e‐health or remote consult$) adj7 chronic).ti,ab. (453) 26 (PDA or Iphone? or ipad? or i‐phone? or i‐pad? or blackberry or personal digital assistant? or handheld or ((webbased or web‐based web2$ or computeri?ed) adj5 ((chronic or diseas$) adj2 (care or manag$ or diseas$ or condition$)))).ti,ab. (14824) 27 (patient? adj3 (participation or physician?)).ti,ab. (50452) 28 (patient? adj3 (education$ or educating or educate?) adj4 (part or intervention? or complex or program? or model or multifacet$ or multimod$ or combin$ or "in addition" or package or suite)).ti,ab. (3545) 29 ((self‐care or self‐manag$) adj4 (part or intervention? or complex or program? or model or multifacet$ or multimod$ or combin$ or "in addition" or package or suite)).ti,ab. (3604) 30 ((continuing adj3 education$) or (CME adj3 (program$ or session? or meeting?)) or inservice? or workshop? or professional development).ti,ab. (60692) 31 ((physician? adj2 behavio?r?) or (upskill$ or up‐skill$)).ti,ab. (2227) 32 ((role or roles) adj2 (chang$ or expand$ or extend$ or revision or revised or revising or nurse or nurse's or nursing or physician?)).ti,ab. (21418) 33 ((nurse or nurses or nursing) adj3 (primary adj2 (care or healthcare))).ti,ab. (2517) 34 ((allied health or physiotherapist? or physical therapist? or exercise therap$) adj4 (team? or team‐based or partner$ or collab$ or intervention?)).ti,ab. (833) 35 (nurse clinician? or nurse practitioner? or physician? Assistant?).ti,ab. (12375) 36 ("nurse‐led" or (nurse? adj2 (led or managed or coordinat$ or co‐ordinat$))).ti,ab. (4666) 37 (shared decision$ or decision aid? or (decision$ adj2 model$) or (decision$ adj support?) or (decision making adj2 computer$) or informatics).ti,ab. (29827) 38 ((clinical or clinician? or doctor? or medical or nurse or nurses or nursing or patient? or physician? or practitioner?) adj3 decision making).ti,ab. (22539) 39 (Referral? adj3 (chronic or decreas$ or ((general or family) adj2 (doctor? or physician? or practitioner?)) or impact or improv$ or increas$ or intervention or plan or plans or primary care or primary health$ or program$ or reduc$ or specialist?)).ti,ab. (9726) 40 (guideline? adj3 (implement$ or impact or ((improv$ or increas$) adj2 adherence))).ti,ab. (7943) 41 (collaborat$ or "cross‐profession$" or interdisciplin$ or inter‐discipllin$ or intraprofession$ or intra‐profession$ or interprofession$ or inter‐profession$ or multidisciplin$ or multi‐disciplin$ or crossdisciplin$ or cross‐disciplin$ or team or teams or team‐based or (skill adj2 mix$)).ti,ab. (302243) 42 (patient? adj2 outcome? adj3 (improv$ or increas$)).ti,ab. (18908) 43 ((organi?ation$ adj2 (change or changes or culture or intervention? or model?)) or multi‐institution$ or innovat$).ti,ab. (97271) 44 restructuring.ti,ab. (7406) 45 (quality adj2 (assessment? or assurance or circle? or implement$ or increase$ or improvement? or management or measure$ or outcome? or total)).ti,ab. (98681) 46 ((nurse or nurses or provider? or practitioner? or physician?) adj3 incentiv$).ti,ab. (1151) 47 (insurance or reimbursement or "fee‐for‐service?" or medicare or medicaid).ti,hw. and (change or changes or changing or chronic or effectiveness or impact or implement$ or intervention$).ti,ab. (48460) 48 (gainshar$ or payer‐provider? or payer‐patient?).ti,ab. (159) 49 ("pay for compliance" or "pay for participation" or "pay for performance" or "performance pay$" or P4P or "pay for quality improvement?" or P4QI or "fee‐for service?").ti,ab. (5970) 50 (payment? adj (blend$ or "blue cross" or bonus$ or capped or "episode of care" or fixed or government$ or insurance or insurer? or level? or linear or medicaid or medicare or non‐linear or per‐patient or per‐episode or per‐visit or performance or prospectiv$ or retroactiv$ or retrospectiv$ or reward$ or schedule? or system? or target$ or third‐part$ or threshold? or uncap$ or shared or variable or per‐visit?)).ti,ab. (3453) 51 (chronic adj3 manag$).ti,ab. (17404) 52 (collaborat$ or coordinat$ or co‐ordinat$ or (care team or (manag$ adj3 care) or interdisciplin$ or inter‐disciplin$ or multidisciplin$ or multi‐disciplin$ or (continuing adj2 education$) or eduational or action plan? or written plan? or quality improv$)).ti,ab. (461425) 53 *"health care quality"/ (61091) 54 (practice pattern? or ((physician? or pharmacist?) adj2 led)).ti,ab. (7501) 55 ((primary care or nurse or nurses or nursing or pulmonologist? or respirologist? or pulmonology or respirology or pneumologist? or pneumology) adj5 team?).ti,ab. (8454) 56 ("cross‐profession$" or interdisciplin$ or inter‐discipllin$ or intraprofession$ or intra‐profession$ or interprofession$ or inter‐profession$ or multidisciplin$ or multi‐disciplin$ or crossdisciplin$ or cross‐disciplin$ or team‐based or (skill adj2 mix$)).ti,ab. (106638) 57 (community or integrat$).ti. (178686) 58 management.ti. (341735) 59 or/8‐58 (1540362) 60 adult?.ti,hw. or (adult? adj3 asthma$).ab. (4694320) 61 (child or children or infant or neonat$ or pre‐school or baby or babies or p?ediatric$ or perinat$).ti,ab,hw. (2659040) 62 intervention?.ti. or (intervention? adj6 (clinician? or collaborat$ or community or complex or DESIGN$ or doctor? or educational or family doctor? or family physician? or family practitioner? or financial or GP or general practice? or hospital? or impact? or improv$ or individuali?e? or individuali?ing or interdisciplin$ or multicomponent or multi‐component or multidisciplin$ or multi‐disciplin$ or multifacet$ or multi‐facet$ or multimodal$ or multi‐modal$ or personali?e? or personali?ing or pharmacies or pharmacist? or pharmacy or physician? or practitioner? or prescrib$ or prescription? or primary care or professional$ or provider? or regulatory or regulatory or tailor$ or target$ or team$ or usual care)).ab. (211159) 63 (pre‐intervention? or preintervention? or "pre intervention?" or post‐intervention? or postintervention? or "post intervention?").ti,ab. [added 2.4] (13867) 64 (hospital$ or patient?).hw. and (study or studies or care or health$ or practitioner? or provider? or physician? or nurse? or nursing or doctor?).ti,hw. (1742418) 65 demonstration project?.ti,ab. (2399) 66 (pre‐post or "pre test$" or pretest$ or posttest$ or "post test$" or (pre adj5 post)).ti,ab. (100690) 67 (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab. (887) 68 trial.ti. or ((study adj3 aim?) or "our study").ab. (888019) 69 (before adj10 (after or during)).ti,ab. (479749) 70 (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month$ or hour? or day? or "more than")).ab. (12588) 71 pilot.ti. (51773) 72 (multicentre or multicenter or multi‐centre or multi‐center).ti. (40570) 73 random$.ti,ab. or controlled.ti. (955245) 74 review.ti. (317768) 75 (animal$ not human$).sh,hw. (3762097) 76 *experimental design/ or *pilot study/ or quasi experimental study/ (8561) 77 ("quasi‐experiment$" or quasiexperiment$ or "quasi random$" or quasirandom$ or "quasi control$" or quasicontrol$ or ((quasi$ or experimental) adj3 (method$ or study or trial or design$))).ti,ab. (128543) 78 ("time series" adj2 interrupt$).ti,ab. (1188) 79 (or/62‐73,76‐78) not (or/74‐75) [EPOC Methods Filter 2.4 EMBASE] (3478848) 80 controlled clinical trial/ or controlled study/ or randomized controlled trial/ [EM] (4410611) 81 (book or conference paper or editorial or letter or review).pt. not randomized controlled trial/ [Per BMJ Clinical Evidence filter] (3983119) 82 (random sampl$ or random digit$ or random effect$ or random survey or random regression).ti,ab. not randomized controlled trial/ [Per BMJ Clinical Evidence filter] (56912) 83 (animal$ not human$).sh,hw. (3762097) 84 80 not (or/81‐83) [Trial filter per BMJ CLinical Evidence] (2912054) 85 (2007$ or 2008$ or 2009$ or 201$).em. [Embase entry week] (8395693) 86 (2007$ or 2008$ or 2009$ or 201$).yr. (8212115) 87 ((and/4,59) not 61) or ((and/4,59) and 60) [Results before filters] (11993) 88 87 and 84 [RCT Results all years] (1895) 89 (and/79,87) not 88 [EPOC FIlter results all years] (3378) 90 88 and (or/85‐86) [RCT 2007‐Nov 22‐2012] (930) 91 89 and (or/85‐86) [EPOC 2007‐Nov‐22‐2012] (1922) 92 7 not (or/90‐91) [KW results all years] (74) 93 (201211$ or 2013$ or 2014$).em,yr,dp. (2361234) 94 88 and 93 [RCT 2012‐2014] (164) 95 89 and 93 [EPOC 2012‐2014] (585) 96 (92 and 93) not (94 or 95) [KW 2012‐2014]
Appendix 3. CINAHL strategy 2012
| CINAHL EBSCOhost (search date November 26, 2012) | |||
| # | Query | Limiters/Expanders | Results |
| S63 | (s27 and s52) not s62 [EPOC Filter Results] | Limiters ‐ Published Date from: 20070101‐20121131 Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 76 |
| S62 | s27 and s61 [Trial Filter Results] | Limiters ‐ Published Date from: 20070101‐20121131 Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 54 |
| S61 | S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 [Trial Filter] | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 137,122 |
| S60 | TI controlled AND TI ( trial or trials or study or experiment* or intervention ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 15,818 |
| S59 | AB ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) or AB ( (multi‐cent* n2 design*) or (multi‐cent* n2 study) or (multi‐cent* n2 studies) or (multi‐cent* n2 trial*) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 5,864 |
| S58 | TI multicentre or multicenter or multi‐centre or multi‐center | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 3,870 |
| S57 | TI ( cluster N2 trial* or cluster N2 study or cluster N2 group or cluster N2 groups or cluster N2 cohort or cluster N2 design or cluster N2 experiment* ) OR AB ( cluster N2 trial* or cluster N2 study or cluster N2 group or cluster N2 groups or cluster N2 cohort or cluster N2 design or cluster N2 experiment* ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 1,442 |
| S56 | TI ( control group or control groups OR control* experiment* or control* design or controlled study ) OR AB ( control group OR control groups or control* cohort* or controlled experiment* controlled design or controlled study) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 44,611 |
| S55 | TI random* or AB random* | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 97,503 |
| S54 | TI ( “clinical study” or “clinical studies” ) or AB ( “clinical study” or “clinical studies” ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 6,286 |
| S53 | (MM "Clinical Trials+") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 7,536 |
| S52 | S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 [EPOC Filter] | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 379,913 |
| S51 | TI ( (time points n3 over) or (time points n3 multiple) or (time points n3 three) or (time points n3 four) or (time points n3 five) or (time points n3 six) or (time points n3 seven) or (time points n3 eight) or (time points n3 nine) or (time points n3 ten) or (time points n3 eleven) or (time points n3 twelve) or (time points n3 month*) or (time points n3 hour*) or (time points n3 day*) or (time points n3 "more than") ) or AB ( (time points n3 over) or (time points n3 multiple) or (time points n3 three) or (time points n3 four) or (time points n3 five) or (time points n3 six) or (time points n3 seven) or (time points n3 eight) or (time points n3 nine) or (time points n3 ten) or (time points n3 eleven) or (time points n3 twelve) or (time points n3 month*) or (time points n3 hour*) or (time points n3 day*) or (time points n3 "more than") ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 1,348 |
| S50 | TI ( (control w3 area) or (control w3 cohort*) or (control w3 compar*) or (control w3 condition) or (control w3 group*) or (control w3 intervention*) or (control w3 participant*) or (control w3 study) ) or AB ( (control w3 area) or (control w3 cohort*) or (control w3 compar*) or (control w3 condition) or (control w3 group*) or (control w3 intervention*) or (control w3 participant*) or (control w3 study) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 41,291 |
| S49 | TI ( multicentre or multicenter or multi‐centre or multi‐center ) or AB random* | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 88,433 |
| S48 | TI random* OR controlled | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 30,082 |
| S47 | TI ( trial or (study n3 aim) or "our study" ) or AB ( (study n3 aim) or "our study" ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 73,597 |
| S46 | TI ( pre‐workshop or preworkshop or post‐workshop or postworkshop or (before n3 workshop) or (after n3 workshop) ) or AB ( pre‐workshop or preworkshop or post‐workshop or postworkshop or (before n3 workshop) or (after n3 workshop) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 285 |
| S45 | TI ( demonstration project OR demonstration projects OR preimplement* or pre‐implement* or post‐implement* or postimplement* ) or AB ( demonstration project OR demonstration projects OR preimplement* or pre‐implement* or post‐implement* or postimplement* ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 1,193 |
| S44 | (intervention? n6 clinician*) or (intervention? n6 community) or (intervention? n6 complex) or (intervention? n6 design*) or (intervention? n6 doctor*) or (intervention? n6 educational) or (intervention? n6 family doctor*) or (intervention? n6 family physician*) or (intervention? n6 family practitioner*) or (intervention? n6 financial) or (intervention? n6 GP) or (intervention? n6 general practice*) Or (intervention? n6 hospital*) or (intervention? n6 impact*) Or (intervention? n6 improv*) or (intervention? n6 individualize*) Or (intervention? n6 individualis*) or (intervention? n6 individualizi*) or (intervention? n6 interdisciplin*) or (intervention? n6 multicomponent) or (intervention? n6 multi‐component) or (intervention? n6 multidisciplin*) or (intervention? n6 multi‐disciplin*) or (intervention? n6 multifacet*) or (intervention? n6 multi‐facet*) or (intervention? n6 multimodal*) or (intervention? n6 multi‐modal*) or (intervention? n6 personalize*) or(intervention? n6 personalise*) or (intervention? n6 personalizing) or (intervention? n6 personalising) or (intervention? n6 pharmaci*) or (intervention? n6 pharmacist*) or (intervention? n6 pharmacy) or (intervention? n6 prescrib*) or (intervention? n6 prescription*) or (intervention? n6 primary care) or (intervention? n6 professional*) or (intervention? n6 provider*) or (intervention? n6 regulatory) or (intervention? n6 regulatory) or (intervention? n6 tailor*) or (intervention? n6 target*) or (intervention? n6 team*) or (intervention? n6 usual care) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 18,639 |
| S43 | TI ( collaborativ* or collaboration* or tailored or personalised or personalized ) or AB ( collaborativ* or collaboration* or tailored or personalised or personalized ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 33,831 |
| S42 | TI pilot | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 10,322 |
| S41 | (MH "Pilot Studies") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 26,636 |
| S40 | AB "before‐and‐after" | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 15,322 |
| S39 | AB time series | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 1,571 |
| S38 | TI time series | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 218 |
| S37 | AB ( before* n10 during or before n10 after ) or AU ( before* n10 during or before n10 after ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 29,065 |
| S36 | TI ( (time point*) or (period* n4 interrupted) or (period* n4 multiple) or (period* n4 time) or (period* n4 various) or (period* n4 varying) or (period* n4 week*) or (period* n4 month*) or (period* n4 year*) ) or AB ( (time point*) or (period* n4 interrupted) or (period* n4 multiple) or (period* n4 time) or (period* n4 various) or (period* n4 varying) or (period* n4 week*) or (period* n4 month*) or (period* n4 year*) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 44,227 |
| S35 | TI ( ( quasi‐experiment* or quasiexperiment* or quasi‐random* or quasirandom* or quasi control* or quasicontrol* or quasi* W3 method* or quasi* W3 study or quasi* W3 studies or quasi* W3 trial or quasi* W3 design* or experimental W3 method* or experimental W3 study or experimental W3 studies or experimental W3 trial or experimental W3 design* ) ) or AB ( ( quasi‐experiment* or quasiexperiment* or quasi‐random* or quasirandom* or quasi control* or quasicontrol* or quasi* W3 method* or quasi* W3 study or quasi* W3 studies or quasi* W3 trial or quasi* W3 design* or experimental W3 method* or experimental W3 study or experimental W3 studies or experimental W3 trial or experimental W3 design* ) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 10,908 |
| S34 | TI pre w7 post or AB pre w7 post | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 8,035 |
| S33 | MH "Multiple Time Series" or MH "Time Series" | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 1,205 |
| S32 | TI ( (comparative N2 study) or (comparative N2 studies) or evaluation study or evaluation studies ) or AB ( (comparative N2 study) or (comparative N2 studies) or evaluation study or evaluation studies ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | Display |
| S31 | MH Experimental Studies or Community Trials or Community Trials or Pretest‐Posttest Design + or Quasi‐Experimental Studies + Pilot Studies or Policy Studies + Multicenter Studies | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | Display |
| S30 | TI ( pre‐test* or pretest* or posttest* or post‐test* ) or AB ( pre‐test* or pretest* or posttest* or "post test* ) OR TI ( preimplement*" or pre‐implement* ) or AB ( pre‐implement* or preimplement* ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | Display |
| S29 | TI ( intervention* or multiintervention* or multi‐intervention* or postintervention* or post‐intervention* or preintervention* or pre‐intervention* ) or AB ( intervention* or multiintervention* or multi‐intervention* or postintervention* or post‐intervention* or preintervention* or pre‐intervention* ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | Display |
| S28 | (MH "Quasi‐Experimental Studies") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | Display |
| S27 | s25 or s26 | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 1,146 |
| S26 | s24 not (s17 or s18 or s19) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 932 |
| S25 | s24 and s21 | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 549 |
| S24 | s7 or s8 or s23 | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 1,676 |
| S23 | (s1 or s3) and (s9 or s10 or s11 or s12 or s13 or s14 or s15 or s16 ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 677 |
| S22 | MJ adult | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 1,356 |
| S21 | (MH "Adult+") OR TI ( adult or adults or adulthood ) OR AB (adult* n3 asthma*) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 643,434 |
| S20 | s17 or s18 or s19 | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 330,205 |
| S19 | TI ( pediatric* or paediatric* or child?? or children or children? or infant or infants or neonate or neonates or baby or babies or baby?? or neo‐nate or neo‐nates or adolescent or adolescents ) OR AB ( pediatric* or paediatric* or child?? or children or children? or infant or infants or neonate or neonates or baby or babies or baby?? or neo‐nate or neo‐nates ) OR MW ( pediatric* or paediatric* ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 207,448 |
| S18 | (MH "Hospitals, Pediatric") OR (MH "Pediatric Physical Therapy") OR (MH "Intensive Care Units, Pediatric") OR (MH "Pediatric Occupational Therapy") OR (MH "Association of Pediatric Oncology Nurses") OR (MH "Rehabilitation, Pediatric") OR (MH "National Association of Pediatric Nurse Associates and Practitioners") OR (MH "Pediatric Critical Care Nursing") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 9,442 |
| S17 | (MH "Child+") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 266,981 |
| S16 | TI ( "pay for compliance" or "pay for participation" or "pay for performance" or "perfomance pay*" or P4P or "pay for quality improvement*" or P4QI or "fee‐for‐service" or physician* incentiv* ) OR TI ( "pay for compliance" or "pay for participation" or "pay for performance" or "perfomance pay*" or P4P or "pay for quality improvement*" or P4QI or "fee‐for‐service" or physician* incentiv* ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 944 |
| S15 | (MH "Education, Medical, Continuing") OR (MH "Education, Nursing, Continuing") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 11,745 |
| S14 | TI ( continuing medical education* or professional development* or inservice or inservices ) OR AB ( continuing medical education* or professional development* or inservice or inservices ) OR (TI patient? n3 education*) or (AB patient? n3 education*) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 10,829 |
| S13 | TI ( (improv* AND patient* AND outcome*) ) OR AB (improv* patient* outcome*) or TI (chang* n3 (practice* or physician* or nurse or nurses or nursing)) or AB (chang* n3 (practice* or physician* or nurse or nurses or nursing) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 16,264 |
| S12 | TI ( (reduce* or reducing or decreas*) n3 ("length of stay" or "hospital stay" or hospitali*ation*) ) OR AB ( (reduce* or reducing or decreas*) n3 ("length of stay" or "hospital stay" or hospitali*ation*) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 2,319 |
| S11 | TI ( (reduce* or reducing or reduction or lower or fewer) n3 (admission* or readmission*) ) OR AB ( (reduce* or reducing or reduction or lower or fewer) n3 (admission* or readmission*) ) or TI (organi?ational n3 (change or changes or changing or structure or structures or model or models)) or AB (organi?ational n3 (change or changes or changing or structure or structures or model or models)) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 4,283 |
| S10 | TI ( care n3 (integrated or model or models or innovat* or pathway* or protocol* or guideline*) ) OR AB ( care n3 (integrated or model or models or innovat* or pathway* or protocol* or guideline*) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 14,895 |
| S9 | TI ( care n3 (team or teambased or teams or collaborat* or interdisciplin* or inter‐disciplin* or multidisciplin* or multi‐disciplin* or crossdisciplin* or cross disciplin* or community) ) OR AB ( care n3 (team or teambased or teams or collaborat* or interdisciplin* or inter‐disciplin* or multidisciplin* or multi‐disciplin* or crossdisciplin* or cross disciplin* or community) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 16,913 |
| S8 | (S1 or S3) AND (S4 or S5 or S6) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 451 |
| S7 | (S1 AND S2) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 744 |
| S6 | (MH "Disease Management") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 5,184 |
| S5 | TI chronic disease management OR AB chronic disease management | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 1,498 |
| S4 | TI ( care n2 (model or models) ) OR AB ( care n2 (model or models) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 6,074 |
| S3 | AB ( asthma* n3 (chronic or serious) ) OR TI ( asthma* or wheez* ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 12,793 |
| S2 | (MH "Chronic Disease") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 25,532 |
| S1 | (MH "Asthma") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase | 16,955 |
Appendix 4. PsycINFO strategy 2014
PsycINFO <1806 to June Week 2 2014> OVID Search date: June 18, 2014
1 asthma/ (3697) 2 asthma$.ti. or wheez$.ti,ab. (3309) 3 (asthma$ adj2 (chronic$ or sever$ or patient?)).ab. (1414) 4 or/1‐3 [Asthma] (4246) 5 chronic disease management.ti,ab,id. (312) 6 (asthma adj3 (program or programs or programme or programme)).ti. (60) 7 (care adj2 team$).ti,ab. (2252) 8 Disease management/ (4013) 9 ((disease adj2 management) or (chronic adj2 management)).ti,ab. (3018) 10 comprehensive health care.ti,ab. (147) 11 (care adj2 management).ti,ab. (1845) 12 (patient centred or patient centered or (continuity adj2 care)).ti,ab. (4083) 13 patient care planning/ or case management/ or critical pathways/ (6411) 14 ((care adj2 (algorithm? or pathway? or plan)) or CRITICAL pathway?).ti,ab. (1616) 15 (((written or action) adj3 plan?) or (planning adj2 care)).ti,ab. (3796) 16 (integrat$ adj2 (care or healthcare)).ti,ab. (1852) 17 ("length of stay" or readmission?).ti. (820) 18 ((reduc$ or shorten or lower$) adj3 (hospitali?ation? or "length of stay" or readmiss$ or readmit$)).ab. (929) 19 ((care or healthcare) adj3 (model? or program? or programme or programmes)).ti,ab. (10259) 20 (home adj2 (service or services or care or healthcare or visit?)).ti,ab. (8362) 21 ((community adj3 (nursing or nurse or nurses or care or healthcare)) or community‐based).ti,ab. (24785) 22 (((mobile or preventive or preventative or clinic?) adj2 (clinic? or service or services or health or health care or care or model?)) and (chronic or disease management)).ti,ab. (3325) 23 ((telemedicine or telehealth$ or telenurs$ or tele‐medicin$ or tele‐health$ or tele‐nurs$ or ehealth or e‐health or remote consult$) adj10 chronic).ti,ab. or telephone.ti. (2194) 24 (PDA or hand‐held? or Iphone? or ipad? or i‐phone? or i‐pad? or blackberry or personal digital assistant? or webbased or web‐based web2$ or computeri?ed).ti,ab. (15173) 25 (patient? adj3 (participation or motivating)).ti,ab. (1389) 26 (patient? adj3 (education$ or educating or educate?)).ti,ab. (4909) 27 (self‐care or self‐manag$).ti,ab. (10951) 28 ((continuing adj3 education$) or (CME adj3 (program$ or session? or meeting?)) or inservice? or workshop? or professional development).ti,ab. (26654) 29 ((physician? adj2 behavio?r?) or (upskill$ or up‐skill$)).ti,ab. (694) 30 ((role or roles) adj2 (chang$ or expand$ or extend$ or revision or revised or revising or nurse or nurse's or nursing or physician?)).ti,ab. (6779) 31 (nurse‐led or ((nurse or nurses or nursing) adj3 (primary adj2 (care or healthcare)))).ti,ab. (935) 32 (allied health or physiotherapist? or physical therapist? or exercise therap$).ti,ab. (3078) 33 (nurse clinician? or nurse practitioner? or physician? Assistant?).ti,ab. (1915) 34 ("nurse‐led" or (nurse? adj2 (led or managed or coordinat$ or co‐ordinat$))).ti,ab. (668) 35 (shared decision$ or decision aid? or (decision$ adj2 model$) or (decision$ adj support?) or (decision making adj2 computer$) or informatics).ti,ab. (7901) 36 ((clinical or clinician? or doctor? or medical or nurse or nurses or nursing or patient? or physician? or practitioner?) adj3 decision making).ti,ab. (4864) 37 (Referral? adj3 (chronic or decreas$ or ((general or family) adj2 (doctor? or physician? or practitioner?)) or impact or improv$ or increas$ or intervention or plan or plans or primary care or primary health$ or program$ or reduc$ or specialist?)).ti,ab. (2200) 38 (guideline? adj3 (implement$ or impact or adherence)).ti,ab. (1581) 39 Evidence‐Based Medicine/ and (change or changing or chronic or ((patient or care or disease) adj2 management) or impact or implement$ or influence or intervention? or model? or patient care or program? or programme or programmes or strategy or strategies or translation).ti,ab,hw. (7811) 40 (collaborat$ or "cross‐profession$" or interdisciplin$ or inter‐discipllin$ or intraprofession$ or intra‐profession$ or interprofession$ or inter‐profession$ or multidisciplin$ or multi‐disciplin$ or crossdisciplin$ or cross‐disciplin$ or team or teams or team‐based or (skill adj2 mix$)).ti,ab. (115493) 41 outcome? Assessment?.ti,ab. (1195) 42 (organi?ational or restructuring or (organi?ation$ adj3 (change? or changing or initiat$ or structur$ or restrict$ or model?))).ti,ab. (82564) 43 (quality adj2 (assessment? or assurance or circle? or implement$ or increase$ or improvement? or management or measure$ or outcome? or total)).ti,ab. (13754) 44 exp Health Personnel/ and (incentiv$ adj2 (economic or financial or monetar$ or payment? or reimburs$)).ti,ab. (181) 45 (gainshar$ or payer‐provider? or payer‐patient?).ti,ab. (61) 46 ("pay for compliance" or "pay for participation" or "pay for performance" or "performance pay$" or P4P or "pay for quality improvement?" or P4QI or "fee‐for service?").ti,ab. (1177) 47 (payment? adj (blend$ or "blue cross" or bonus$ or capped or "episode of care" or fixed or government$ or insurance or insurer? or level? or linear or medicaid or medicare or non‐linear or per‐patient or per‐episode or per‐visit or performance or prospectiv$ or retroactiv$ or retrospectiv$ or reward$ or schedule? or system? or target$ or third‐part$ or threshold? or uncap$ or shared or variable or per‐visit?)).ti,ab. (397) 48 telemedicine/ (2494) 49 client education/ (3023) 50 exp managed care/ or health care delivery/ or health maintenance organizations/ or exp case management/ or "cost containment"/ or disease management/ or fee for service/ or health care costs/ or exp health care services/ or exp health insurance/ or "quality of care"/ or exp treatment planning/ or utilization reviews/ (111636) 51 treatment duration/ (3265) 52 hospital discharge/ or facility discharge/ (1695) 53 discharge planning/ or hospital admission/ (2010) 54 community services/ or community welfare services/ or home visiting programs/ or public health services/ or exp community facilities/ or integrated services/ or outreach programs/ or exp self help techniques/ (38344) 55 Health Promotion/ or Self Care Skills/ or Self Management/ (22686) 56 client participation/ (1363) 57 continuing education/ or exp inservice training/ or professional development/ (15709) 58 inservice training/ or on the job training/ (955) 59 adult learning/ (994) 60 health care policy/ or policy making/ or health care reform/ or clinical governance/ or government policy making/ or exp health care administration/ (36306) 61 peer evaluation/ (2123) 62 (exp health personnel/ or exp allied health personnel/ or exp medical personnel/ or exp mental health personnel/ or counselors/ or exp social workers/ or exp therapists/) and (((chang$ or improv$) adj3 (care or patient outcome? or practice? or model?)) or incentive?).ti. (494) 63 (((chang$ or improv$) adj3 (care or patient outcome? or practice? or model?)) or incentive?).ti. (6090) 64 chronic illness/ (7996) 65 or/6‐63 [Intervetion terms] (469196) 66 limit 65 to (100 childhood <birth to age 12 yrs> or 120 neonatal <birth to age 1 mo> or 140 infancy <age 2 to 23 mo> or 160 preschool age <age 2 to 5 yrs> or 180 school age <age 6 to 12 yrs> or 200 adolescence <age 13 to 17 yrs>) [Limit not valid in PsycINFO; records were retained] (60936) 67 limit 65 to ("300 adulthood <age 18 yrs and older>" or 320 young adulthood <age 18 to 29 yrs> or 340 thirties <age 30 to 39 yrs> or 360 middle age <age 40 to 64 yrs> or "380 aged <age 65 yrs and older>" or "390 very old <age 85 yrs and older>") (219146) 68 adult?.ti,hw. or (asthma$ adj3 adult?).ab. (89638) 69 (adolescent? or baby or babies or child$ or infant? or neonate? or neo‐nate? or p?ediatric$ or toddler?).ti,ab,id. (675528) 70 pediatricians/ or pediatrics/ (16025) 71 3 and 5 [Asthma & Chronic Disease] (3) 72 3 and 65 [Asthma & CDM terms] (433) 73 72 and 68 [Asthma & Adult KW] (72) 74 72 not (or/69‐70) [Asthma not Child/Pediatrics] (266) 75 limit 72 to ("300 adulthood <age 18 yrs and older>" or 320 young adulthood <age 18 to 29 yrs> or 340 thirties <age 30 to 39 yrs> or 360 middle age <age 40 to 64 yrs> or "380 aged <age 65 yrs and older>" or "390 very old <age 85 yrs and older>") (264) 76 limit 72 to (100 childhood <birth to age 12 yrs> or 120 neonatal <birth to age 1 mo> or 140 infancy <age 2 to 23 mo> or 160 preschool age <age 2 to 5 yrs> or 180 school age <age 6 to 12 yrs> or 200 adolescence <age 13 to 17 yrs>) [Limit not valid in PsycINFO; records were retained] (172) 77 72 not 76 (261) 78 71 or 73 or 74 or 75 or 77 (361) 79 78 not child$.ti. (324) 80 79 not p?ediatric$.ti. (310) 81 78 and (child$ and adult?).ti. (4) 82 80 or 81 (314) 83 limit 82 to yr="2007 ‐ 2012" (141) 84 limit 82 to yr="2012‐2014" (46)
Appendix 5. Cochrane Library strategy 2014
EBM Reviews ‐ Cochrane Central Register of Controlled Trials <May 2014>, EBM Reviews ‐ Cochrane Database of Systematic Reviews <2005 to May 2014>, EBM Reviews ‐ Database of Abstracts of Reviews of Effects <2nd Quarter 2014> Search date: June 18, 2014 1 exp Asthma/ (8558) 2 asthma$.ti. or wheez$.ti,ab. (16581) 3 (asthma$ adj2 (chronic$ or sever$ or patient?)).ab. (5481) 4 or/1‐3 [Asthma] (18899) 5 chronic disease management.ti,ab. (94) 6 (asthma adj3 (program or programs or programme or programme)).ti. (236) 7 exp Patient Care Team/ (1121) 8 (care adj2 team$).ti,ab. (487) 9 Disease management/ (404) 10 ((disease adj2 management) or (chronic adj2 management)).ti,ab. (2033) 11 Patient Care Management/ or Patient‐Centered Care/ or "Continuity of Patient Care"/ or Comprehensive Health Care/ (748) 12 comprehensive health care.ti,ab. (6) 13 (care adj2 management).ti,ab. (984) 14 (patient centred or patient centered or (continuity adj2 care)).ti,ab. (627) 15 patient care planning/ or case management/ or critical pathways/ (946) 16 ((care adj2 (algorithm? or pathway? or plan)) or CRITICAL pathway?).ti,ab. (403) 17 (((written or action) adj3 plan?) or (planning adj2 care)).ti,ab. (465) 18 "Delivery of Health Care, Integrated"/ (155) 19 (integrat$ adj2 (care or healthcare)).ti,ab. (403) 20 "length of stay"/ or patient readmission/ (5103) 21 ("length of stay" or readmission?).ti. (305) 22 ((reduc$ or shorten or lower$) adj3 (hospitali?ation? or "length of stay" or readmiss$ or readmit$)).ab. (1562) 23 *hospitalization/ and (management or program? or programme or programmes or model? or reduc$ or impact or intervention or improving).ti. (0) 24 patient discharge/ and ((chronic or plan? or planning or team? or collaborat$ or intervention?).ti. or (planning or team? or collaborat$ or (chronic adj3 (disease or model?))).ab.) (225) 25 Managed Care Programs/ (198) 26 ((care or healthcare) adj3 (model? or program? or programme or programmes)).ti,ab. (2769) 27 home care services/ or home care services, hospital‐based/ or home nursing/ (1556) 28 (home adj2 (service or services or care or healthcare or visit?)).ti,ab. (2221) 29 community health services/ or community health nursing/ or community networks/ or community pharmacy services/ or counseling/ (3591) 30 ((community adj3 (nursing or nurse or nurses or care or healthcare)) or community‐based).ti,ab. (3947) 31 Occupational Health Services/ and ((primary adj2 care) or disease management or specialist? or chronic disease? or chronic care or chronic condition?).ti,ab. (19) 32 (School Health Services/ not (child/ or child, preschool/ or exp infant/)) and (chronic or disease management).ti,ab. (3) 33 school health services/ and adolescent/ and (chronic or disease management).ti,ab. (5) 34 (((mobile or preventive or preventative or clinic?) adj2 (clinic? or service or services or health or health care or care or model?)) and (chronic or disease management)).ti,ab. (1391) 35 early medical intervention/ (54) 36 exp Telemedicine/ or telenursing/ or remote consultation/ (880) 37 ((telemedicine or telehealth$ or telenurs$ or tele‐medicin$ or tele‐health$ or tele‐nurs$ or ehealth or e‐health or remote consult$) adj10 chronic).ti,ab. or telephone.ti. (1145) 38 (PDA or hand‐held? or Iphone? or ipad? or i‐phone? or i‐pad? or blackberry or personal digital assistant? or webbased or web‐based web2$ or computeri?ed).ti,ab. (4209) 39 Patient Education as Topic/ or health education/ or consumer health information/ or health literacy/ or health fairs/ (8363) 40 Patient Participation/ or Self care/ or Self administration/ or consumer participation/ (3730) 41 (patient? adj3 (participation or motivating)).ti,ab. (582) 42 (patient? adj3 (education$ or educating or educate?)).ti,ab. (2451) 43 (self‐care or self‐manag$).ti,ab. (2733) 44 education, continuing/ or education, medical, continuing/ or education, nursing, continuing/ or education, pharmacy, continuing/ or education, professional, retraining/ or exp inservice training/ (1275) 45 ((continuing adj3 education$) or (CME adj3 (program$ or session? or meeting?)) or inservice? or workshop? or professional development).ti,ab. (1344) 46 ((physician? adj2 behavio?r?) or (upskill$ or up‐skill$)).ti,ab. (8268) 47 Nurse's Role/ or Physician's Role/ or Professional Role/ (467) 48 ((role or roles) adj2 (chang$ or expand$ or extend$ or revision or revised or revising or nurse or nurse's or nursing or physician?)).ti,ab. (412) 49 medical staff/ or exp medical staff, hospital/ or exp nurses/ or exp nursing staff/ or exp pharmacists/ or exp physicians/ (2613) 50 Primary Nursing/ or Nurse Clinicians/ or Nurse Practitioners/ or Community Health Nursing/ or Physician Assistants/ (758) 51 nursing care/ or emergency nursing/ or holistic nursing/ or home nursing/ or nursing, practical/ or occupational health nursing/ or primary nursing/ or rehabilitation nursing/ (538) 52 (nurse‐led or ((nurse or nurses or nursing) adj3 (primary adj2 (care or healthcare)))).ti,ab. (688) 53 exp Allied Health Personnel/ (610) 54 (allied health or physiotherapist? or physical therapist? or exercise therap$).ti,ab. (1734) 55 (nurse clinician? or nurse practitioner? or physician? Assistant?).ti,ab. (383) 56 ("nurse‐led" or (nurse? adj2 (led or managed or coordinat$ or co‐ordinat$))).ti,ab. (656) 57 respiratory therapy department, hospital/ (3) 58 physical therapy department, hospital/ (17) 59 Decision Support Systems, Clinical/ or Decision Making, Computer‐Assisted/ or Medical Informatics Applications/ or Decision Support Techniques/ or decision making, organizational/ (668) 60 (shared decision$ or decision aid? or (decision$ adj2 model$) or (decision$ adj support?) or (decision making adj2 computer$) or informatics).ti,ab. (1151) 61 ((clinical or clinician? or doctor? or medical or nurse or nurses or nursing or patient? or physician? or practitioner?) adj3 decision making).ti,ab. (735) 62 "Referral and Consultation"/ or Gatekeeping/ (1185) 63 (Referral? adj3 (chronic or decreas$ or ((general or family) adj2 (doctor? or physician? or practitioner?)) or impact or improv$ or increas$ or intervention or plan or plans or primary care or primary health$ or program$ or reduc$ or specialist?)).ti,ab. (679) 64 Practice Guidelines as Topic/ or guidelines as topic/ or Guideline Adherence/ (1513) 65 (guideline? adj3 (implement$ or impact or adherence)).ti,ab. (728) 66 Evidence‐Based Medicine/ and (change or changing or chronic or ((patient or care or disease) adj2 management) or impact or implement$ or influence or intervention? or model? or patient care or program? or programme or programmes or strategy or strategies or translation).ti,ab,hw. (498) 67 Interdisciplinary Communication/ or Cooperative Behavior/ (653) 68 (collaborat$ or "cross‐profession$" or interdisciplin$ or inter‐discipllin$ or intraprofession$ or intra‐profession$ or interprofession$ or inter‐profession$ or multidisciplin$ or multi‐disciplin$ or crossdisciplin$ or cross‐disciplin$ or team or teams or team‐based or (skill adj2 mix$)).ti,ab. (7984) 69 "Outcome Assessment (Health Care)"/ (3808) 70 outcome? Assessment?.ti,ab. (630) 71 health services administration/ or "organization and administration"/ or efficiency, organizational/ or health facility administration/ or hospital administration/ (105) 72 exp hospital restructuring/ or hospital shared services/ (7) 73 centralized hospital services/ or pharmacy service, hospital/ or diagnostic services/ (104) 74 models, organizational/ or multi‐institutional systems/ or organizational culture/ or exp organizational innovation/ or organizational objectives/ or institutional management teams/ (277) 75 (organi?ational or restructuring or (organi?ation$ adj3 (change? or changing or initiat$ or structur$ or restrict$ or model?))).ti,ab. (1069) 76 total quality management/ or "quality of health care"/ or quality assurance, health care/ or benchmarking/ or quality improvement/ or Management Quality Circles/ or Quality Assurance, Health Care/ or "Quality of Health Care"/ or "United States Agency for Healthcare Research and Quality"/ (1373) 77 (quality adj2 (assessment? or assurance or circle? or implement$ or increase$ or improvement? or management or measure$ or outcome? or total)).ti,ab. (7952) 78 Peer Review, Health Care/ or Peer Review/ (60) 79 Physician Incentive Plans/ or reimbursement, incentive/ [ML] (45) 80 ((physician? or practitioner? or doctor? or nurse or nurses) adj4 incentive? plan?).ti,ab. (1) 81 exp Health Personnel/ and (incentiv$ adj2 (economic or financial or monetar$ or payment? or reimburs$)).ti,ab. (16) 82 (insurance, health, reimbursement/ or reimbursement mechanisms/ or fee‐for‐service plans/ or "physician payment review commission"/ or medicare payment advisory commission/ or reimbursement, disproportionate share/ or relative value scales/) and chronic.ti,ab. (11) 83 (insurance, health, reimbursement/ or reimbursement mechanisms/ or fee‐for‐service plans/ or "physician payment review commission"/ or medicare payment advisory commission/ or reimbursement, disproportionate share/ or relative value scales/) and (change or changes or changing or chronic or effectiveness or impact or implement$ or intervention).ti,ab. (61) 84 "fees and charges"/ or capitation fee/ or fee‐for‐service plans/ or fees, medical/ or fees, pharmaceutical/ or prescription fees/ or "rate setting and review"/ [ML] (138) 85 (gainshar$ or payer‐provider? or payer‐patient?).ti,ab. (7) 86 ("pay for compliance" or "pay for participation" or "pay for performance" or "performance pay$" or P4P or "pay for quality improvement?" or P4QI or "fee‐for service?").ti,ab. (195) 87 (payment? adj (blend$ or "blue cross" or bonus$ or capped or "episode of care" or fixed or government$ or insurance or insurer? or level? or linear or medicaid or medicare or non‐linear or per‐patient or per‐episode or per‐visit or performance or prospectiv$ or retroactiv$ or retrospectiv$ or reward$ or schedule? or system? or target$ or third‐part$ or threshold? or uncap$ or shared or variable or per‐visit?)).ti,ab. (71) 88 or/5‐87 [Interventions] (67311) 89 adult/ or exp aged/ or middle aged/ (333136) 90 adult?.ti,hw. or (asthma$ adj3 adult?).ab. (304183) 91 or/89‐90 [Adult] (388755) 92 exp child/ or adolescent/ (95058) 93 exp pediatrics/ or neonatology/ or perinatology/ (441) 94 (adolescent? or baby or babies or child$ or infant? or neonate? or neo‐nate? or p?ediatric$ or toddler?).ti,ab,hw,kw. (149042) 95 or/92‐94 [Child] (149048) 96 (and/4,88) not 95 [Asthma not child/pediatrics] (755) 97 (and/4,88) and 91 [Asthma & Adult] (784) 98 or/96‐97 (1121) 99 limit 98 to yr="2007 ‐ 2012" [Limit not valid in DARE; records were retained] (349) 100 limit 98 to yr="2012‐2014" [Limit not valid in DARE; records were retained] (128) 101 from 100 keep 1‐108 (108) [Cochrane Central Database of Controlled Trials] 102 from 100 keep 109‐118 (10) [Cochrane Database of Systematic Reviews] 103 from 100 keep 119‐128 (10) [Database of Abstracts of Reviews of Effects]
Data and analyses
Comparison 1. Chronic disease management programme versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Asthma‐specific quality of life score (post intervention measurements) | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 RCTs | 8 | 1627 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.08, 0.37] |
| 1.2 NRCT | 1 | 413 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.27, 0.66] |
| 2 Subgroup analysis asthma‐specific quality of life score according to the comprehensiveness of the intervention (≥ 8 / < 8 components) | 8 | 1627 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.08, 0.37] |
| 2.1 Comprehensive intervention (≥ 8 components) | 6 | 1349 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [0.04, 0.29] |
| 2.2 Less comprehensive intervention (< 8 components) | 2 | 278 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.12, 0.77] |
| 3 Subgroup analysis asthma‐specific quality of life score according to the dominant component of the intervention | 8 | 1627 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.08, 0.37] |
| 3.1 Education and/or self‐management support | 4 | 698 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.11, 0.51] |
| 3.2 Mixed | 4 | 929 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.12, 0.39] |
| 4 Subgroup analysis asthma‐specific quality of life score according to the presence of limited CDM components in the control group | 8 | 1627 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.08, 0.37] |
| 4.1 Control group without limited CDM components | 4 | 629 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [0.05, 0.51] |
| 4.2 Control group with limited CDM components | 4 | 998 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.03, 0.37] |
| 5 Subgroup analysis asthma‐specific quality of life score according to QOL scale | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 AQLQ ‐ overall score | 7 | 1543 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.12, 0.52] |
| 5.2 LWAQ ‐ overall score | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.16, 0.20] |
| 6 Sensitivity analysis asthma‐specific quality of life (change from baseline measurements) | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 RCTs | 7 | 1547 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.18, 0.43] |
| 6.2 NRCT | 1 | 413 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.18, 0.57] |
| 7 Self‐efficacy score (post intervention measurements) | 5 | 642 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.08, 1.11] |
| 8 Asthma severity score (post intervention measurements) | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 RCTs | 6 | 1330 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [0.05, 0.30] |
| 8.2 NRCT | 1 | 409 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.66] |
| 9 Lung function (FEV1 and PEF) (post intervention measurements) | 8 | 1559 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.09, 0.30] |
| 9.1 FEV1 (% predicted) | 6 | 1279 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [0.05, 0.27] |
| 9.2 PEF (L/min) | 1 | 224 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.03, 0.56] |
| 9.3 PEF (% predicted) | 1 | 56 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.01, 1.06] |
| 10 FEV1 (% predicted) (post intervention measurements) | 6 | 1279 | Mean Difference (IV, Random, 95% CI) | 2.81 [0.99, 4.64] |
| 11 PEF (L/min) (post intervention measurements) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 RCTs | 2 | 372 | Mean Difference (IV, Random, 95% CI) | 33.52 [11.38, 55.65] |
| 11.2 NRCT | 1 | 409 | Mean Difference (IV, Random, 95% CI) | 30.52 [7.46, 53.58] |
| 12 PEF (% predicted) (post intervention measurements) | 2 | 307 | Mean Difference (IV, Random, 95% CI) | 8.68 [3.73, 13.63] |
| 13 Subgroup analysis lung function according to the comprehensiveness of the intervention (≥ 8 / < 8 components) | 8 | 1559 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.09, 0.30] |
| 13.1 Comprehensive intervention (≥ 8 components) | 5 | 1133 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.07, 0.31] |
| 13.2 Less comprehensive intervention (< 8 components) | 3 | 426 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [0.01, 0.40] |
| 14 Subgroup analysis lung function according to the dominant component of the intervention | 8 | 1559 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.09, 0.30] |
| 14.1 Education and/or self‐management support | 4 | 675 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.04, 0.35] |
| 14.2 Organisation component targeting healthcare system | 1 | 56 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.01, 1.06] |
| 14.3 Mixed | 3 | 828 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [0.03, 0.32] |
| 15 Subgroup analysis lung function according to the presence of limited CDM components in the control group | 8 | 1559 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.09, 0.30] |
| 15.1 Control group without limited CDM components | 4 | 585 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [0.02, 0.39] |
| 15.2 Control group with limited CDM components | 4 | 974 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.06, 0.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Armour 2007.
| Methods | C‐RCT, unit of allocation: pharmacies (n = 57), patient recruitment: patients or clients of primary care clinic or pharmacy Setting: Rural and urban pharmacies, New South Wales, Victoria, Queensland Australia |
|
| Participants |
Control patients: n = 205 (186 at follow‐up (f/u)), women: 60.5%, mean age: 50.4, smoking: 23%, moderate‐severe asthma (according to symptoms), FEV1: 22.5%, ICS use: 81% Intervention patients: n = 191 (165 at f/u), women: 67.5%, mean age: 47.5, smoking: 20.9%, moderate‐severe asthma, FEV1: 22.8%, ICS use: 85.3% |
|
| Interventions |
Name and duration of programme: Pharmacy Asthma Care Program during 6 months Intervention group components Organisational ‐ patients: structured follow‐up; adherence assessment; detection of drug‐related problems Organisational ‐ healthcare professionals/system: pharmacist education and training; referral to a GP as appropriate (e.g. for a change of medication or dose); programme development based on national guidelines Patient education: one on one education on targeted counselling and education on the condition, medication and lifestyle issues (e.g. trigger factors) Self‐management support: review of inhaler technique; goal setting and review Frequency: baseline, 1 month, 3 months (optional), 6 months Healthcare professionals involved: GPs, pharmacists Control group components Usual care (which includes risk assessment and spirometry training for pharmacists) Number of components and dominant component: 8, education and self‐management |
|
| Outcomes |
Organisational level Organisation of care: participation rate; number of interventions per patient; % intervention patients receiving intervention components Process: % patients referred to GP; % patients with action plan; % patients with prescription of reliever; % patients with prescription of preventer + reliever Patient level Quality of life: AQLQ score Asthma symptoms and activity level: asthma severity (% patients with mild, moderate and severe asthma) (primary); mean daily dose of salbutamol Self‐management: CQ score; PCAQ score; BMQ score; % patients adherent to preventer medication; % patients with correct inhaler technique Pulmonary function: mean FEV1; mean FEV1/FVC Time of outcome measurement: at 6 months |
|
| Notes | Unit of analysis error (pharmacies randomised, patients analysed) taken into account in analyses presented in article (change from baseline). We also used the unadjusted data sent by authors for final values. We adjusted the sample size for the design effect (= 1.03) based on the study's ICC (0.006). CQ: consumer asthma knowledge score; PCAQ: perceived control of asthma questionnaire; AQLQ: asthma‐related quality of life questionnaire; BMQ: brief medication questionnaire |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pharmacists were not informed as to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT for primary outcome; for secondary outcomes, no significant differences between patients who were recruited and those who completed the study. Control patients: 205 ‐ 19 loss to follow‐up = 186 (90.7%) Intervention patients: 191 ‐ 26 loss to follow‐up = 165 (86.4%) Control pharmacies: 28 ‐ 4 with no patient recruitment = 24 (85.7%) Intervention pharmacies: 29 ‐ 3 with no patient recruitment = 26 (89.7%) |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Other bias | High risk | Potential recruitment bias, as pharmacies recruited patients after allocation. unclear when FEV1 is measured |
| Outcomes at baseline similar? | High risk | Higher proportion of patients with severe asthma in intervention group than in control group (88% versus 71%, P < 0.001) |
| Characteristics at baseline similar? | High risk | Higher proportion of previous smokers (P = 0.05) and patients with other lung disease (P < 0.001) in control patients than intervention patients |
| Adequate protection against contamination? | Unclear risk | Allocation by cluster but questionnaires at baseline could contribute to education |
Cambach 1997.
| Methods | RCT (cross‐over), patient recruitment: patients or clients of respiratory care clinic Setting: Local physiotherapy practices, Netherlands |
|
| Participants |
Control patients: n = 21, women: 66.7%, mean age: 53, mild‐moderate asthma (according to FEV1), FEV1: 84%, dyspnoea score (CRDQ): 18, ICS use: not reported Intervention patients: n = 22, women: 81.8%, mean age: 40, mild‐moderate asthma (according to FEV1), FEV1: 89%, dyspnoea score (CRDQ): 18, ICS use: not reported |
|
| Interventions |
Name and duration of programme: rehabilitation programme run in local physiotherapy practices during 3 mo before cross‐over Intervention group components Organisational ‐ patients: recreational activities Organisational ‐ healthcare professionals or system: course on pulmonary rehabilitation for physiotherapists Patient education: group sessions on normal or pathological respiration, medication treatment, inhalation technique and sanitation or resources; one on one education on techniques of breathing retraining and evacuation of mucus; exercise training; group sessions on relaxation techniques Frequency: 2 individual sessions of 45 min on breathing retraining and mucus evacuation; group sessions: 6 sessions of 45 min on education, exercise training 2 times/week for 90 min; recreational activities 1 time/week for 45 minutes; 6 relaxation sessions of 45 minutes Healthcare professionals involved: physiotherapists; nurses Control group components Usual care Number of components and dominant component: 6, organisational ‐ patients |
|
| Outcomes |
Patient level Quality of life: CRDQ score: fatigue, emotion, mastery, and dyspnoea scores Asthma symptoms and activity level: mean endurance time during cycling at 75% Wmax; mean cardiac frequency during cycling at 60% Wmax; mean walking distance Time of outcome measurement: at 3 months |
|
| Notes | First 3 months considered only (before cross‐over) CRDQ: Chronic Respiratory Disease Questionnaire |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation procedure with closed envelopes |
| Allocation concealment (selection bias) | Low risk | Block randomisation procedure with closed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No indication in text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total of 99 patients ‐ 10 dropouts = 89 randomised ‐ 23 loss to f/u (9 Iintervention and 14 control patients) = 66 patients included (74%) Baseline characteristics of 33 dropouts not significantly different from 66 completed. Similar rates of dropouts between groups. |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | No other bias detected |
| Outcomes at baseline similar? | Unclear risk | No statistical test for asthma subgroup |
| Characteristics at baseline similar? | High risk | Significant difference between groups for age and FEV1 |
| Adequate protection against contamination? | Low risk | Unlikely that control group received intervention before cross‐over |
Castro 2003.
| Methods | RCT, patient recruitment: hospital inpatients admitted for dyspnoea Setting: inpatient and outpatient setting, Barnes‐Jewish Hospital, Missouri, USA |
|
| Participants |
Control patients: n = 46, women: 85%, mean age: 38, moderate‐severe asthma (according to FEV1), FEV1: 58%, ICS use: not reported Intervention patients: n = 50, women: 80%, mean age: 35, moderate‐severe asthma (according to FEV1), FEV1: 57%, ICS use: not reported |
|
| Interventions |
Name and duration of programme: Use of an asthma nurse specialist to provide a multifaceted approach to asthma care for “high‐risk” inpatients, tailored to patients, during 6 months Intervention group components Organisational ‐ patients: psychosocial support and screening for professional counselling; consultation with social services to facilitate discharge planning; provision of outpatient follow‐up through phone contact and home visits as necessary; assessing need for allergy skin testing Organisational ‐ healthcare professionals/system: teamwork and collaborative processes between providers (suggestion by nurse to GP regarding current regimen, flow sheet as direct communication between nurse and GP); explicit use of EBM for care (regimen in accordance with National Asthma Education and Prevention Program II); daily 'asthma care' flow sheet Patient education: one on one education on management of the disease, prevention of exacerbation, smoking cessation, use of spacer, medication delivery technique, peak flow monitoring Self‐management support: asthma self‐management plan Frequency: tailored to patients Healthcare professionals involved: GPs; respiratory care nurses Control group components Usual care (which includes asthma education as well as inhaler technique and peak flow monitoring by respiratory therapist and nurse in hospital) Number of components and dominant component: 10, mixed (organisational ‐ patients, organisational ‐ healthcare professionals or system) |
|
| Outcomes |
Organisational level Healthcare utilisation: asthma‐related hospitalisations (absolute number, mean number per patient) (primary); non‐asthma‐related hospitalisations (absolute number, mean number per patient); GP visits (absolute number, mean number per patient); ED visits (absolute number, mean number per patient); asthma‐related hospital days (absolute number, mean number per patient); non‐asthma‐related hospital days (absolute number, mean number per patient); mean time to readmission; mean healthcare costs per patient Costs: total healthcare costs per patient Patient level Quality of life: AQLQ score: overall, activity, symptom, emotional, and environmental scores Time of outcome measurement: at 6 mo |
|
| Notes | AQLQ: Asthma Quality of Life Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "study patients were randomly assigned in a blind selection procedure using a pre‐randomised assignment in a sealed letter" |
| Allocation concealment (selection bias) | Low risk | See supra |
| Blinding (performance bias and detection bias) All outcomes | High risk | Data were collected by asthma nurses who knew allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all randomised patients |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | No other bias detected |
| Outcomes at baseline similar? | Unclear risk | No measurement of primary outcome at baseline. ED visits at baseline: 4.8 versus 5.6, but not significant |
| Characteristics at baseline similar? | Low risk | "both groups were well balanced with respect to all baseline characteristics, and there was no significant differences between the groups" |
| Adequate protection against contamination? | Unclear risk | Unclear if GP saw both intervention and control patients |
Charrois 2006.
| Methods | RCT, patient recruitment: patients or clients of primary care clinic or pharmacy Setting: community rural pharmacies and primary care practices, Alberta, Canada |
|
| Participants |
Control patients: n = 34, women: 53%, mean age: 38.7, moderate‐severe asthma (according to ACQ), ACQ score: 1.91, FEV1: not reported, ICS use: 76.5% Intervention patients: n = 36, women: 53%, mean age: 35.7, moderate‐severe asthma (according to ACQ), ACQ score: 1.45, FEV1: not reported, ICS use: 69.4% |
|
| Interventions |
Name and duration of programme: Better Respiratory Education and Asthma Treatment in Hinton and Edson (BREATHE), during 6 months Intervention group components Organisational ‐ patients: structured follow‐up; assessment of medication adherence; optimisation of drug therapy (assessment of medications by pharmacist) Organisational ‐ healthcare professionals or system: teamwork and collaborative processes between providers (referral to respiratory therapist or physician, or both, as needed); pharmacist training; quality improvement processes (routine reporting); explicit use of evidence‐based medicine for development of action plan and medication assessment (Canadian asthma guidelines) Patient education: distribution of printed material and one on one education on asthma, management of the disease (asthma medication) Self‐management support: action plan; inhaler technique assessment or education Frequency: reinforcement session at 1 week; phone call at 2 weeks; pharmacist visit at 1, 2, 4, 6 months; respiratory therapist visit at 2, 6 months Healthcare professionals involved: pharmacists; respiratory therapists Control group components Usual care (which includes provision of asthma education booklet, general advice as needed and assessment of inhaler technique; one referral to respiratory physiotherapist for FEV1 measurement, and two follow‐up visits to pharmacist Number of components and dominant component: 11, mixed (organisational ‐ healthcare professionals or system, education and self‐management) |
|
| Outcomes |
Organisational level Process: participation rate; % intervention patients with action plan; % intervention patients with education at each visit (no data); % intervention patients with treatment recommendation (no data); % patients with prescription of inhaled corticosteroids Healtcare utilisation: number of ED visits or hospitalisation Patient level Asthma symptoms and activity level: ACQ score (primary); number of courses of oral steroids Pulmonary function: mean FEV1 Time of outcome measurement: at 6 months |
|
| Notes | ACQ: Asthma Control Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patient was randomised by an Internet randomisation service trough an external centre. Sealed envelopes were provided for randomisation for sites without Internet access" |
| Allocation concealment (selection bias) | Low risk | Central allocation and sealed envelopes: compared with supra |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No indication in text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data for 32/34 patients in control group (94%) and 29/36 in intervention group (81%), but ITT analyses |
| Selective reporting (reporting bias) | Low risk | Reported outcomes match planned study outcomes published in design article |
| Other bias | High risk | Poor application of intervention: 2/3 with complete f/u, 3/4 with action plan, 1/2 with education at each visit, 1/2 with treatment recommendation |
| Outcomes at baseline similar? | Unclear risk | No statistical comparisons for primary outcome. For one of the secondary outcomes (unscheduled physician visit), statistically significant difference between the two study groups at baseline |
| Characteristics at baseline similar? | High risk | "Statistically significant differences between the two study groups with regards to the results of previous pulmonary function tests, inhaler technique use, use of peak flow meter" |
| Adequate protection against contamination? | High risk | All pharmacists received training and they saw both intervention and control patients |
Couturaud 2002.
| Methods | RCT, patient recruitment: patients or clients of primary care clinic or pharmacy Setting: outpatient clinic of two university hospitals, France |
|
| Participants |
Control patients: n = 36, women: 66.7%, mean age: 38.1, smokers: 8.3%, moderate‐severe asthma (according to GINA), FEV1: 85%, ICS use: 100% Intervention patients: n = 36, women: 69.4%, mean age: 37.8, smokers: 16.7%, moderate‐severe asthma (according to GINA), FEV1: 83%, ICS use: 100% |
|
| Interventions |
Name and duration of programme: Educational programme in asthmatic patients following treatment readjustment, during 12 months Intervention group components Organisational ‐ patients: structured follow‐up Organisational ‐ healthcare professionals or system: teamwork and collaborative processes between providers (self‐management plan sent to GP); nurse training Patient education: one on one education on asthma, management of the disease (effects and purpose of asthma drug), prevention of exacerbations Self‐management support: action plan; proper use of inhaler device; reinforcement sessions Frequency: 30‐60 min sessions at 1, 2, 6, 9, 12 months Healthcare professionals involved: respiratory care nurse; hospital physician; GP Control group components Usual care Number of components and dominant component: 7, education and self‐management |
|
| Outcomes |
Organisational level Healtcare utilisation: number of unscheduled visits (to GP, ED or MD, for asthma exacerbation) Patient level Quality of life: AQLQ score Asthma symptoms and activity level: absence of asthma symptoms (% symptom‐free days) (primary); % days of oral steroids intake; % days off work Self‐management: asthma knowledge score; self‐management ability score; compliance with medicine score (Morisky questionnaire) Pulmonary function: mean FEV1 Time of outcome measurement: at 12 months |
|
| Notes | AQLQ: Asthma Quality of Life Questionnaire Supplementary data sought, but author replied data were unavailable |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation using a table of permutations |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No indication in text |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 72 patients randomised ‐ 18 dropouts = 54 completed (75%). No statistical difference between dropouts and completed, but no information on difference between dropouts in control and intervention groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | Patients were randomised after run‐in period, possibly selecting more compliant patients |
| Outcomes at baseline similar? | Unclear risk | Not measured at baseline |
| Characteristics at baseline similar? | Low risk | No significant differences between groups for clinical and demographical characteristics |
| Adequate protection against contamination? | Unclear risk | Patients in control group had to monitor their PEF and record their daily symptoms, possibly providing help for self‐management |
Feifer 2004.
| Methods | CBA, patient recruitment: general population (i.e. clients of health insurance) Setting: practices in a US region covered by a specific health insurance company |
|
| Participants |
Control patients: n = 35,450, women: 56%, mean age: not reported, asthma severity: not reported, FEV1: not reported, ICS use: not reported Intervention patients: n = 35,450, women: 56%, mean age: not reported (5 to 17 yr: 27%; 18 to 44 yr: 27%; 45 to 64 yr: 24%; 65 plus yr: 22%), asthma severity: not reported, FEV1: not reported, ICS use: not reported |
|
| Interventions |
Name and duration of programme: population‐based asthma disease management programme using broad‐based educational interventions, during 12 months Intervention group components Organisational ‐ patients: telephone counselling centre, refill reminders, compliance reminders, pollen count alerts Organisational ‐ healthcare professionals/system: asthma management flow sheets Patient education: distribution of educational material (5 workbooks, 2 newsletters) on asthma therapy, self‐management techniques, and trigger avoidance Frequency: workbooks mailed at 2 month interval, newsletter at 6 month interval Healthcare professionals involved: GP, pharmacists Control group components Usual care Number of components and dominant component: 7, mixed (organisational ‐ healthcare professionals or system, education and self‐management) |
|
| Outcomes |
Organisational level Process: % patients who used one or more controllers; average number of controller prescriptions dispensed per patient; average number of reliever prescriptions dispensed per patient For intervention group only: % patients with an action plan; a peak flow meter; a plan for how to treat triggers Healthcare utilisation (for intervention group only): % patients reporting 4 or more outpatient visits; one or more emergency room (ER) visits; one or more hospitalisation Quality of life (for intervention group only): mini‐AQLQ score Asthma symptoms and activity level (for intervention group only): productivity loss in days Self‐management (for intervention group only): % patients who know how to use peak flow meter; aware of triggers for asthma; aware of how medications can manage allergies Time of outcome measurement: at 12 months |
|
| Notes | AQLQ: Asthma‐specific Quality of Life Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective allocation |
| Allocation concealment (selection bias) | Unclear risk | Retrospective allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Claims data |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No other bias detected |
| Outcomes at baseline similar? | Low risk | Matched control group |
| Characteristics at baseline similar? | Low risk | Matched control group |
| Adequate protection against contamination? | Low risk | Contamination unlikely |
Galbreath 2008.
| Methods | RCT, patient recruitment: general population, patients or clients of primary care clinic or pharmacy, patients or clients of respiratory care clinic Setting: University Medical Center and private primary practices, South Texas, USA |
|
| Participants |
Control patients: n = 143, women: 77.6%, mean age: 43.7, moderate‐severe asthma (according to GINA score), FEV1 (pre): 76.9 Leukotriene inhibitor use: 30.1%, inhaled corticosteroid (ICS) use (alone): 13.3%, ICS use (alone or in combination): 65% Intervention (a) patients: n = 143, women: 79.7%, mean age: 42.4, moderate‐severe asthma (according to GINA score), FEV1 (pre): 78.2 Leukotriene inhibitor use: 34.3%, ICS use (alone): 13.3%, ICS use (alone or in combination): 66.4% Intervention (b) patients: n = 143, women: 75.5%, mean age: 42.1, moderate‐severe asthma (according to GINA score), FEV1 (pre): 75.3 Leukotriene inhibitor use: 38.5%, ICS use (alone): 20.3%, ICS use (alone or in combination): 72.7% |
|
| Interventions |
Name and duration of programme: The South Texas Asthma Management Project (STAMP) comparing two national guideline–based asthma management strategies: telephonic DM (CDM group), or telephonic DM plus in‐home visits (augmented CDM group), during 6 months Intervention (a) group components (CDM) Organisational ‐ patients: structured follow‐up; hotline if needed Organisational ‐ healthcare professionals or system: explicit teamwork between healthcare providers; explicit use of EBM supports for programme Patient education: phone calls; topic of education: not clear Self‐management support: providing an action plan; supervised reinforcement sessions Frequency: 6 to 7 phone calls by nurse Healthcare professionals involved: GP; respiratory care nurse Intervention (b) group components (augmented CDM) Organisational ‐ patients: intervention (a); home visits with home environment evaluation Organisational ‐ healthcare professionals or system: intervention (a) Patient education: intervention (a) Self‐management support: intervention (a); instruction on use of equipment Frequency: intervention (a); 4 home visits at 1, 2, 3, and 6 months Healthcare professionals involved: intervention (a); respiratory therapist Control group components Usual care Number of components and dominant component: 9, mixed (organisational ‐ patients, education and self‐management) |
|
| Outcomes |
Organisational level Organisational: % patients completing ≥ 80% of CDM intervention Process: % patients who initiated controller therapy Healthcare utilisation: time to first ED visit or inpatient hospitalisation for asthma (primary); number of urgent office visits for asthma per patient per year (primary); number of ED visits for asthma per patient per year (primary); number of inpatient admissions for asthma per patient per year (primary) Patient level Quality of life: AQLQ score (primary) Asthma symptoms and activity level: number of corticosteroids burst (no data); LASS score Pulmonary function: mean FEV1, FEV1/FVC, PEF Time of outcome measurement: at 12 months |
|
| Notes | AQLQ: Asthma Quality of Life Questionnaire; LASS: Lara Asthma Symptom Scale The author identified 5 primary outcomes out of 7 outcomes. Only results of adult population included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a sequence of randomly permuted blocks generated with stata" |
| Allocation concealment (selection bias) | Low risk | "the randomisation sequence was transferred to a series of consecutively numbered, sealed cardboard randomisation boxes, packaged to ensure blindness from sound or weight of box" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded research staff at randomisation; blinded research staff administered study questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 99% of data for healthcare utilisation and event. Around 60% of self‐reported data (similar rates across groups). Difference in withdrawal (7 versus 2 versus 1) probably not relevant |
| Selective reporting (reporting bias) | Unclear risk | PFT measured at each study visit but not reported |
| Other bias | Unclear risk | 70% of study patients completed ≥ 80% of intervention |
| Outcomes at baseline similar? | Unclear risk | Baseline data only available for 1 outcome |
| Characteristics at baseline similar? | Low risk | No significant differences |
| Adequate protection against contamination? | Low risk | Unlikely that control patient received any components of intervention group |
Herborg 2001.
| Methods | C‐NRCT, unit of allocation: pharmacy (n = 31), patient recruitment: patients or clients of primary care clinic or pharmacy Setting: Community pharmacies throughout Denmark |
|
| Participants |
Control patients: n = 236 (204 at 12 month f/u), women: 54.7%, mean age: 42.4, moderate‐severe asthma (according to study), FEV1: not reported, ICS use: not reported Intervention patients: n = 264 (209 at 12 month f/u), women: 57.6%, mean age: 38.8, moderate‐severe asthma (according to study), FEV1: not reported, ICS use: not reported |
|
| Interventions |
Name and duration of programme: therapeutic outcomes monitoring (TOM) programme, during 12 months Intervention group components Organisational ‐ patients: structured follow‐up; process and outcome measurement at the patient's level (PEFR, symptoms); identify and analyse drug therapy problems Organisational ‐ healthcare professionals or system: teamwork and collaborative processes between providers (patient, physician, pharmacist partnership); pharmacist training; routine reporting; meetings to discuss changes Patient education: one on one education on asthma and management of the disease Self‐management support: regular checks of inhalation technique Frequency: monthly visit to pharmacy Healthcare professionals involved: GP; pharmacist Control group components Usual care Number of components and dominant component: 9, organisational ‐ patients |
|
| Outcomes |
Organisational level Organisation of care: GP, physician and patient participation rates Process outcomes: number of oral corticosteroid courses per patient; drug consumption (mean defined daily dose (DDD) per user per day) for short‐acting beta‐agonists, long‐acting beta‐agonists, total beta‐agonists, inhaled adrenergic agonists, ICS, inhaled anticholinergics, inhaled anti‐allergics, oral beta‐agonists and theophylline; drug therapy problems Healthcare utilisation: number of GP visits; number of GP phone contacts; number of specialist visits; number of physician on call visits; number of ED visits; number of hospital admissions; number of asthma clinic visits Patient level Patient satisfaction: DCPP score Quality of life: LWAQ score; NHP score Asthma symptoms and activity level: asthma morbidity index; number of days of sickness per patient Self‐management: asthma knowledge score; number of inhalation errors per patient Pulmonary function: mean PEF Time of outcome measurement: at 12 months |
|
| Notes | Unit of analysis error (pharmacies randomised, patients analysed) taken into account in analyses DCPP: Danish College of Pharmacy Practice; LWAQ: Living with Asthma Questionnaire; NHP: Notthingham Health Profile |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20.8% dropped out in intervention group and 13.6% in control group, but no reasons were provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Hierarchical structure of data taken into account in analyses |
| Outcomes at baseline similar? | Unclear risk | Differences at baseline, but no statistical test provided |
| Characteristics at baseline similar? | Low risk | Characteristics appear well balanced (age, sex) |
| Adequate protection against contamination? | Low risk | Intervention pharmacies worked solely with intervention patients |
Huang 2009.
| Methods | RCT, patient recruitment: patients or clients of respiratory care clinic Setting: outpatient chest department of teaching hospital, Taiwan |
|
| Participants |
Control patients: n = 58, women: 22%, age (45 to 64): 40%, moderate‐severe asthma (according to GINA score), FEV1 (pre): 51.8, ICS use: not reported Intervention (a) patients: n = 58, women: 35%, age (45 to 64): 43%, moderate‐severe asthma (according to GINA score), FEV1 (pre): 51.7, ICS use: not reported Intervention (b) patients: n = 57, women: 24%, age (45 to 64): 39%, moderate‐severe asthma (according to GINA score), FEV1 (pre): 50.9, ICS use: not reported |
|
| Interventions |
Name and duration of programme: Individualised self‐care education programmes (with and without peak‐flow monitoring) in older adults with moderate‐to‐severe asthma, during 6 months Intervention (a) group components (CDM) Organisational ‐ patients: structured follow‐up; outcome measurement (day and night‐time asthma symptoms recorded by patients); involvement of family members Organisational ‐ healthcare professionals or system: explicit teamwork between healthcare providers Patient education: distribution of material and one on one educational phone calls on asthma, management of the disease, prevention of exacerbation, and physical activity Self‐management support: providing an action plan Frequency: phone call once a week Healthcare professionals involved: GP; non‐specialised nurse Intervention (b) group components (augmented CDM) Organisational ‐ patients: intervention (a) Organisational ‐ healthcare professionals/system: intervention (a) Patient education: intervention (a); how to use a peak flow meter and manage asthma based on values Self‐management support: intervention (a); use of peak flow meter Frequency: intervention (a) Healthcare professionals involved: intervention (a) Control group components Usual care (which includes routine asthma education programme with computer‐aided, self‐learning video) Number of components and dominant component: 6, education and self‐management |
|
| Outcomes |
Organisational level Process: number of type of medications; % change of medication dose Healthcare utilisation: number of unscheduled ED visits (MD, hospital, ED) Patient level Asthma symptoms and activity level: asthma control test score Self‐management: asthma self‐care competence (knowledge and skills) score (primary); asthma self‐care behaviour score (primary); asthma self‐efficacy score (primary) Pulmonary function: mean FEV1, PEF, FVC, FEV1/FVC Time of outcome measurement: at 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "used a computer‐developed random table to assign patients to intervention groups" |
| Allocation concealment (selection bias) | Unclear risk | "allocation was concealed from recruiting RA" but no details provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "RA collecting data and author who assessed and analysed outcomes were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 173 randomised ‐ 25 losses to follow‐up = 148 patients (85.5%). Similar rates and reasons across groups (see figure 1) |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | No other bias detected |
| Outcomes at baseline similar? | Low risk | "groups were well‐balanced for lung function, asthma self‐care competence, behaviours, self‐efficacy" |
| Characteristics at baseline similar? | Low risk | "groups were well‐balanced for baseline demographic characteristics" |
| Adequate protection against contamination? | Low risk | Unlikely that nurse called control patients |
Kokubu 2000.
| Methods | RCT, patient recruitment: patients from hospital (n = 17) Setting: hospital and patients' home, Japan |
|
| Participants |
Control patients: n = 34, women: 56%, mean age: 47.3, asthma severity: not reported, FEV1: not reported, ICS use: not reported Intervention patients: n = 32, women: 62%, mean age: 49.9, asthma severity: not reported, FEV1: not reported, ICS use: not reported |
|
| Interventions |
Name and duration of programme: asthma telemedicine system, during 6 months Intervention group components Organisational ‐ patients: structured follow‐up; telephone hotline Organisational ‐ healthcare professionals or system: explicit teamwork between healthcare providers; fax sent to physician; information technology Patient education: one on one educational phone calls on asthma and management of the disease Self‐management support: providing an action plan; regular checks of inhalation technique Frequency: not clear Healthcare professionals involved: pulmonary care physicians; respiratory care nurses Control group components Usual care Number of components and dominant component: 8, organisational ‐ healthcare professionals or system |
|
| Outcomes |
Organisational level Process: mean inhaled corticosteroid dose (puff/day) Healthcare utilisation: hospitalisation rate (hospitalisation/patient/6 months); night ER visits rate; daytime ER visits rate Costs: direct and indirect cost savings Patient level Patient satisfaction: satisfaction survey Quality of life: improvement in QoL score Asthma symptoms and activity level: mean inhaled ß2‐agonists dose (puff/day); mean oral corticosteroid dose (tab/day) Self‐management: compliance with prescribed inhaled corticosteroids; compliance with oral corticosteroids; compliance with daily PEF measurements Pulmonary function: mean PEF Time of outcome measurement: at 6 months |
|
| Notes | We only used the data presented in the primary reference for the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Telephone registration randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear in the article |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | No other bias detected |
| Outcomes at baseline similar? | Low risk | No significant differences (see table 3 in the article) |
| Characteristics at baseline similar? | Low risk | No significant differences (see table 3 in the article) |
| Adequate protection against contamination? | Low risk | Unlikely |
Landon 2007.
| Methods | CBA, patient recruitment: patients/clients of primary care clinic or pharmacy Setting: Community health centres throughout USA (n = 48) |
|
| Participants |
Control patients: n = not clear, women: 67.6%, mean age: 34.4, asthma severity: not reported, FEV1: not reported, ICS use: not reported Intervention patients: n = not clear, women: 63.5%, mean age: 28.4, asthma severity: not reported, FEV1: not reported, ICS use: not reported Total patients with asthma: n = 3392 |
|
| Interventions |
Name and duration of programme: Health Disparities Collaboratives (each generally including 20 or more community health centres) disseminating quality improvement techniques developed by the Institute for Healthcare Improvement, during 4.5 years Intervention group components* Organisational ‐ patients: community linkages component (access to resources (e.g., donated medical services) in the community for the benefit of patients in community health centres; providing services to an entire community (e.g., “Diabetes Awareness Day”)) Organisational ‐ healthcare professionals or system: delivery system redesign components (improvement of care management, missed‐appointment follow‐up, organisation of the practice team; change of care delivery roles; patient visits planning); decision support component (guidelines, protocols, and prompts; providers education; facilitating specialty and expert consultation); information support components (patient registry systems; improving the collection or use of data for care management; providing performance data to individual providers or to the group or organisation); health system organisation component (increase administrators' motivation and ability to improve care for patients with chronic disease, increase providers’ motivation and ability to be involved in such improvements, or improve the overall ability of the system or institution to engage in co‐ordinated quality improvement efforts); physician training; explicit teamwork (creation of improvement teams) Patient education and self‐management support: self‐care support component (providing education or care guidelines to patients, increase patient motivation for self‐care, assessment of self‐care needs or abilities, providing support tools or resources to improve self‐care, collaborative decision making with patients) Frequency: variable in the centres Healthcare professionals involved: teams from community health centres Control group components Usual care Number of components and dominant component: ≥11, organisational ‐ healthcare professionals or system |
|
| Outcomes |
Organisational level Process outcomes: % patients with an action plan; % patients assessed for smoking status and cessation advice; % patients assessed for exposure to smoke; % patients with advice on smoking; % patients vaccinated for influenza; % patients assessed for asthma severity; overall quality of care provided score (prevention and screening, monitoring and treatment, outcomes) Healthcare utilisation: % patients with no urgent care, ER visit, hospitalisation for asthma Patient level Asthma symptoms and activity level: % patients treated with anti‐inflammatory medication Time of outcome measurement: at 2 to 3 years |
|
| Notes | *The study evaluated a range of interventions that took place in 48 community health centres. Each intervention had to include at least 1 component of the 6 major components described above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation |
| Allocation concealment (selection bias) | High risk | No randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear whether outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether all data were collected |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | No other bias detected |
| Outcomes at baseline similar? | Unclear risk | No P values provided for comparisons between groups |
| Characteristics at baseline similar? | Unclear risk | Significant differences between groups for Charlson morbidity index, age and insurance type |
| Adequate protection against contamination? | Low risk | External control centres |
Martin 2009.
| Methods | RCT, patient recruitment: patients or clients of primary care clinic or pharmacy Setting: primary care clinics, Chicago, USA |
|
| Participants |
Control patients: n = 22, women: 77%, mean age: 37, asthma severity: not clear, FEV1: not reported, ICS use: 77% Intervention patients: n = 20, women: 60%, mean age: 33, asthma severity: not clear, FEV1: not reported, ICS use: 70% |
|
| Interventions |
Name and duration of programme: A community‐based intervention to improve asthma self‐efficacy in African American adults designed by the Chicago Initiative to Raise Asthma Health Equity (CHIRAH), during 12 weeks Intervention group components Organisational ‐ patients: home visits; financial incentive Organisational ‐ healthcare professionals/system: healthcare professionals training Patient education: educational groups and outreach visits on asthma, management of the disease, prevention of exacerbation, smoking cessation, physical activity, use of spacer, inhalation techniques, symptom monitoring, and communicating with provider Self‐management support: providing an action plan; reinforcement sessions Frequency: 4 group sessions and 6 home visits Healthcare professionals involved: community health worker, social worker, member of study team Control group components Usual care (which includes 2 mailings with asthma education information) Number of components and dominant component: 7, education and self‐management |
|
| Outcomes |
Organisational level Process: participation rate; % patients with action plan; % patients using spacer Patient level Quality of life: mini‐AQLQ score Asthma symptoms and activity level: number of symptomatic days; number of symptomatic nights; number of times inhaled corticosteroids were used Self‐management: self‐efficacy score (primary); asthma knowledge score; coping skills score Time of outcome measurement: at 6 months |
|
| Notes | AQLQ: Asthma Specific Quality of Life Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomisation done in pairs": pairwise randomisation where each centre recruited 2 persons at a time (pair) and randomised one to the intervention and one to the control group. But no description of the randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up data missing for 2 intervention and 4 control patients (14%) at 3 months and 1 intervention and 3 control patients (10%) at 6 months |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | No other bias detected |
| Outcomes at baseline similar? | Low risk | No statistical difference for primary and secondary outcomes |
| Characteristics at baseline similar? | High risk | Statistical difference for educational level, household income, perceived general health |
| Adequate protection against contamination? | Unclear risk | Not clear |
Mayo 1990.
| Methods | RCT, patient recruitment: hospital inpatients admitted for dyspnoea Setting: hospital outpatient chest clinic, New York, USA |
|
| Participants |
Control patients: n = 57, women: 57.9%, mean age: 42, moderate‐severe asthma (according to study), FEV1: not reported, ICS use: not clear Intervention patients: n = 47, women: 70.2%, mean age: 42, moderate‐severe asthma (according to study), FEV1: not reported, ICS use: not clear |
|
| Interventions |
Name and duration of programme: outpatient programme designed to reduce readmissions for asthma exacerbations among adults with asthma, during 8 months before partial cross‐over Intervention group components Organisational ‐ patients: structured follow‐up; advice or assistance if needed Organisational ‐ healthcare professionals or system: teamwork and collaborative processes between providers (nurse practitioner shared responsibilities with physician) Patient education: one on one education on asthma and management of the disease Self‐management support: regular checks of inhalation technique; patients received spacer device and peak flow meter Frequency: 2 x 1 h visits, followed by ≥ 30 min visits, depending on patient's preferences and level of asthma activity Healthcare professionals involved: pulmonary care physician; respiratory care nurse Control group components Usual care Number of components and dominant component: 6, education and self‐management |
|
| Outcomes |
Organisational level Healthcare utilisation: number of hospital admissions (mean and mean admissions per patient); total hospitalisation days; hospitalisation days per patient Patient level Asthma symptoms and activity level: mortality rate Time of outcome measurement: at 8 months |
|
| Notes | We only considered results at 8 months, before part of the control patients were crossed to the intervention group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random allocation by last digit of hospital number |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Ten patients lost to follow‐up in intervention group, no information on control group |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | No other bias detected |
| Outcomes at baseline similar? | Low risk | No statistical difference |
| Characteristics at baseline similar? | Low risk | No statistical difference |
| Adequate protection against contamination? | Unclear risk | Not clear if physician or nurse practitioner, or both, saw both intervention and control patients |
McLean 2003.
| Methods | RCT, patient recruitment: patients or clients of primary care clinic or pharmacy Setting: community pharmacies, British Columbia, Canada |
|
| Participants |
Control patients: n = 214, women: 62.9%, mean age: not clear, asthma severity: not clear, FEV1: not reported, ICS use: % not reported Intervention patients: n = 191, women: 63.0%, mean age: not clear, asthma severity: not clear, FEV1: not reported, ICS use: % not reported |
|
| Interventions |
Name and duration of programme: the British Columbia pharmacy asthma study incorporating an asthma care protocol provided by specially trained community pharmacists, during 12 months Intervention group components Organisational ‐ patients:structured follow‐up; outcome measurements at the patient's level (PEF reading); patients participation in decisions Organisational ‐ healthcare professionals/system: teamwork and collaborative processes between providers (physicians informed or consulted regarding all results and interventions); explicit use of EBM supports Patient education: one on one education on asthma, management of the disease, prevention of exacerbations, and use of peak flow meter Self‐management support: providing of action plan; calendars/diaries provided to record PEF rate Frequency: every 2 to 3 weeks for first 3 appointments, then every 3 months Healthcare professionals involved: GP; pharmacists Control group components Usual care Number of components and dominant component: 7, education and self‐management |
|
| Outcomes |
Organisational level Healthcare utilisation: number of emergency visits per patient in previous month; number of hospital days per patient in previous month; number of medical visits per patient in previous month; majors costs per month per patient Patient level Patient satisfaction: score on survey Quality of life: Juniper score (+ 4 subscores) Asthma symptoms and activity levels: total asthma symptoms score (+ 8 subscores); number of days off school or work in previous month; dose/day of ß2‐agonists; dose/day of inhaled corticosteroids Self‐management: asthma knowledge score (+ 4 subscores) Pulmonary function: mean PEF Time of outcome measurement: at 12 months |
|
| Notes | Juniper: asthma‐specific quality of life questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Procedure to assign patients not described |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up of pharmacies, pharmacists and patients without reasons provided. Control patients not included in analyses |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | High risk | Possible recruitment bias by pharmacist; clusters not taken into account in analyses; not ITT because cross‐over taken into account; patients in usual care completed diary, were taught proper inhaler technique, which may have improved care received |
| Outcomes at baseline similar? | Unclear risk | No statistical test provided |
| Characteristics at baseline similar? | Unclear risk | Not described |
| Adequate protection against contamination? | High risk | Pharmacist sees control and intervention patients |
Petro 2005.
| Methods | C‐RCT, unit of allocation: providers (n = nc), patient recruitment: patients or clients of primary care clinic or pharmacy Setting: private primary practices, Germany |
|
| Participants |
Control patients: n = 55, women: 44.0%, mean age: 55.0, smokers: 28%, moderate‐severe asthma (according to study), FEV1: not reported, ICS use: not reported Intervention patients: n = 56, women: 54.2%, mean age: 57.3, smokers: 22.9%, moderate‐severe asthma (according to study), FEV1: not reported, ICS use: not reported |
|
| Interventions |
Name and duration of programme: a disease management programme involving a case manager who carries out patient instructions, evaluates symptoms and lung function values on a daily basis and supervises treatment goals with the aid of predetermined algorithms, during 12 months Intervention group components Organisational ‐ patients: case management; outcome measurements at the patient level Organisational ‐ healthcare professionals or system: teamwork and collaborative processes between providers (discussion between GP and case manager); case manager training; quality improvement process (PulmAssist Plus), information technology (data transmitted by modem) Patient education: one on one education on themes linked to asthma Frequency: daily monitoring of FEV and PEF Healthcare professionals involved: GP; case manager Control group components Usual care Number of components and dominant component: 7, organisational ‐ healthcare professionals or system |
|
| Outcomes |
Organisational level Healthcare utilisation: % patients with asthma‐related hospitalisations; cost difference Patient level Quality of life: FLA score (primary); EQ‐5D score and VAS Asthma symptoms and activity level: % patients without asthma symptoms Pulmonary function: FEV1 (no data provided in article), PEFR (no data provided in article) Time of outcome measurement: at 12 months |
|
| Notes | Unit of analysis error (provider randomised, patients analysed) were not taken into account in the published analyses. The design effect can not be computed as the number of clusters is unknown. Results were excluded from our meta‐analyses FLA: Fragebogen zür Lebensqualität bei Asthma, based on Living with Asthma Questionnaire; EQ‐5D: descriptive system of health‐related quality of life states consisting of five dimensions (mobility, self‐care, usual activities, pain and discomfort, anxiety and depression) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear for providers; for patients: 111 randomised ‐ 8 losses to follow‐up in intervention group ‐ 5 losses to follow‐up in control group = 98 (88%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | High risk | Incorrect analysis (unit of analysis error: cluster allocation but patient level analysis) |
| Outcomes at baseline similar? | Unclear risk | No statistical difference for primary outcome, but statistical difference for EQ‐5D |
| Characteristics at baseline similar? | Unclear risk | No statistical test provided (sex: 44% versus 54%) |
| Adequate protection against contamination? | Low risk | Randomisation by provider |
Schatz 2006.
| Methods | RCT, patient recruitment: patients or clients of primary care clinic or pharmacy Setting: Kaiser Permanente Medical Care programme, San Diego, USA |
|
| Participants |
Control patients: n = 31, women: 54.8%, mean age: 45.4, smokers: 16.7%, moderate‐severe asthma (according to FEV1), FEV1: 69.2%, ICS use: % not reported Intervention patients: n = 31, women: 32.3%, mean age: 45, smokers: 22.6%, moderate‐severe asthma (according to FEV1), FEV1: 66.7%, ICS use: % not reported |
|
| Interventions |
Name and duration of programme: A regular care manager follow‐up in addition to an initial intensive individualised educational visit and use of a potent controller medication, during 12 months Intervention group components Organisational ‐ patients: structured follow‐up; advice or assistance as needed; distribution of free inhalers; review of patient's healthcare utilisation Organisational ‐ healthcare professionals or system: teamwork and collaborative processes between providers (GP contacted if inadequate control) Patient education: distribution of material and one on one education on asthma, management of the disease and inhalation technique Self‐management support: action plan; peak flow meter given with instructions; symptom and peak flow diaries; review of inhalation technique Frequency: initial visit with follow‐up at 1, 6, and 12 months; phone calls 1/month Healthcare professionals involved: GP, care manager Control group components Usual care (which includes distribution of free inhalers, distribution of material on asthma and its management, action plan, peak flow meter given with instructions, and symptom and peak flow diaries) Number of components and dominant component: 11, mixed (organisational ‐ patients, education and self‐management) |
|
| Outcomes |
Organisational level Process: prescription of oral steroids Healthcare utilisation: % patients with any asthma‐related hospitalisation or ED visit Patient level Quality of life: mini‐AQLQ score (primary) Asthma symptoms and activity level: number of symptom‐free days; number of ß2‐agonists canisters Self‐management: asthma knowledge score Time of outcome measurement: at 12 months |
|
| Notes | Mini‐AQLQ: Asthma Quality of Life Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation using a computer‐generated list of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No description of concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Electronic records used for some data; no description if blinding for questionnaire data |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "follow‐up data were available on less than half of the control group patients at 12 months"; 72 patients randomised ‐ 17 losses to follow‐up (1 in intervention, 16 in usual care) = 45 patients (72.5%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Low enrolment rate (7%) and significant differences between enrolled and not enrolled for age, sex, inhaled steroids use, and oral steroids |
| Outcomes at baseline similar? | Low risk | No significant differences for all outcomes except one (inhaled steroids) |
| Characteristics at baseline similar? | Low risk | No significant differences |
| Adequate protection against contamination? | Low risk | No risk of contamination (care manager only for intervention patients) |
Smith 2005.
| Methods | RCT, patient recruitment: patients or clients of primary care clinic or pharmacy, patients or clients of respiratory care clinic Setting: hospital outpatient asthma clinics and general practices, Norfolk, Suffolk, UK |
|
| Participants |
Control patients: n = 45, women: 84%, mean age: 34.7, smokers: 17.4%, moderate‐severe asthma (according to study self‐report), FEV1: not reported, ICS use: 100% Intervention patients: n = 47, women: 62%, mean age: 38.2, smokers: 19.4%, moderate‐severe asthma (according to study self‐report), FEV1: not reported, ICS use: 100% |
|
| Interventions |
Name and duration of programme: The Coping with Asthma Study (a home‐based, nurse led psycho‐educational intervention for adults at risk of adverse asthma outcomes), during 6 months Intervention group components Organisational ‐ patients: structured follow‐up; advice and/or assistance as needed; involvement of family members; liaison with health and social care professionals; home visits Organisational ‐ healthcare professionals or system: teamwork and collaborative processes between providers (GP and health psychologist available to nurse as supervisors if needed; referral to specialist); manual to standardise delivery and general content of intervention Patient education: distribution of material and one on one education on asthma, management of the disease, prevention of exacerbations, smoking cessation, exercise Self‐management support: action plan; supervised reinforcement sessions; inhalation technique; use of peak flow device; collaborative problem solving approach; workbook with homework Frequency: visits every 2 weeks for 2 months (˜1 hour); phone calls every 2 weeks for 2 months then every month for 4 months Healthcare professionals involved: respiratory care nurse; GP; health psychologist Control group components Usual care Number of components and dominant component: 15, education and self‐management |
|
| Outcomes |
Patient level Quality of life: LAQ score; SF‐36 physical function score; SF‐36 mental health score; HADS anxiety score; HADS depression score; GHQ‐12 psychiatric morbidity score Asthma symptoms and activity level: asthma symptom control score (primary) Self‐management: % patients monitoring their peak flow; % patients using reliever inhaler > 4 times/day; % patients currently smoking; % patients identifying additional triggers; perceived control of asthma score; medication compliance score Time of outcome measurement: at 12 months |
|
| Notes | LAQ: Living with Asthma Questionnaire; SF‐36: general health status assessed by the Short Form 36; HADS: Hospital Anxiety and Depression Scale; GHQ‐12: General Health Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation by third party not involved in patient care using open computer generated block randomisation" |
| Allocation concealment (selection bias) | Low risk | By third party not involved in patient care |
| Blinding (performance bias and detection bias) All outcomes | High risk | "no attempts were made to blind assessment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "small numbers of individual missing questionnaire items were replaced with ample medians to allow calculation of total scores for each scale"; 92 patients randomised ‐ 8 losses to follow‐up ("no clear differences between these and patients completing the study") = 84 in ITT |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Random‐effects model used to adjust for hierarchical structure of data |
| Outcomes at baseline similar? | Low risk | Baseline imbalance adjusted for in analyses |
| Characteristics at baseline similar? | Low risk | Imbalance for sex, education, hospitalisation or ED visit but adjusted for in analyses |
| Adequate protection against contamination? | Low risk | No risk of contamination (home visits) |
Weng 2005.
| Methods | CBA, patient recruitment: general population (i.e. clients of the National Health Insurance) Setting: Hospital outpatient clinics and primary care clinics run by the National Health Insurance, Taiwan |
|
| Participants |
Control patients: n = 3188, women: 43%, mean age: not reported (18 plus yr: 72%), asthma severity: not reported, FEV1: not reported, ICS use: not reported Intervention patients: n = 854, women: 44.5%, mean age: not reported (18 plus yr: 71.4%), asthma severity: mild‐moderate, FEV1: not reported, ICS use: not reported |
|
| Interventions |
Name and duration of programme: A government‐sponsored outpatient‐based disease management programme for patients with asthma, during 12 months Intervention group components Organisational ‐ patients: case management; structure follow‐up Organisational ‐ healthcare professionals or system: explicit teamwork between primary care physician and case manager; physician education and training; integration of care (case manager assured communication between key departments); explicit use of guidelines Patient education: one on one educational sessions on recognition of asthma triggers, environmental control, symptoms and early warning signs, medication usage and side effects, use of spacer devices and peak flow meters, and self‐management of asthma exacerbations Self‐management support: supervised reinforcement sessions Frequency: reinforcement sessions every 3 months Healthcare professionals involved: general physicians, selected specialists, registered nurses, physician assistants Control group components Usual care Number of components and dominant component: 8, education and self‐management |
|
| Outcomes |
Organisational level Organisation of care: HC professional satisfaction Healthcare utilisation: number of outpatient department visits; number of emergency department visits; number of inpatient visits; length of stay Time of outcome measurement: at 12 months |
|
| Notes | We only considered patients already diagnosed with asthma for inclusion in this review, as patients newly diagnosed with asthma were very young and did not meet our inclusion criteria for age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective allocation |
| Allocation concealment (selection bias) | High risk | Retrospective allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Claims data |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No other bias detected |
| Outcomes at baseline similar? | Low risk | Matched control group (compared with table 3) |
| Characteristics at baseline similar? | Low risk | Matched control group |
| Adequate protection against contamination? | Low risk | Contamination unlikely |
Wilson 2010.
| Methods | RCT, patient recruitment: general population (i.e. clients of health insurance) Setting: Kaiser Permanente clinics, USA (n = 5) |
|
| Participants |
Control patients: n = 204, women: 57.4%, mean age: 45.1, smokers: 16.2%, moderate‐severe asthma (according to GINA), FEV1: ˜70%, ICS use: % not clear Intervention (a) patients: n = 204, women: 55.9%, mean age: 46.9, smokers: 16.2%, moderate‐severe asthma (according to GINA), FEV1: ˜70%, ICS use: % not clear Intervention (b) patients: n = 204, women: 56.4%, mean age: 45.7, smokers: 15.2%, moderate‐severe asthma (according to GINA), FEV1: ˜70%, ICS use: % not clear |
|
| Interventions |
Name and duration of programme: the Better Outcomes of Asthma Treatment (BOAT) study, involving asthma education and two in‐person and three brief phone encounters, with or without shared decision making (SDM), where non‐physician clinicians and patients negotiated a treatment regimen that accommodated patient goals and preferences, during 9 months Intervention (a) group components (CDM) Organisational ‐ patients: structured follow‐up Organisational ‐ healthcare professionals or system: teamwork and collaborative processes between providers (discussion of recommendations between care manager and physician); healthcare professional training; explicit use of guidelines; quality control (audio taping to ensure proper intervention delivery) Patient education: distribution of material and one on one education on asthma, management of the disease, and instruction on inhaler technique Self‐management support: action plan Frequency: session 1 at baseline (50 to 60 min), session 2 at 1 month (20 to 30 min), phone call at 3, 6, 9 months Healthcare professionals involved: GP, care manager (nurse, respiratory therapist, pharmacist, or physician assistant) Intervention (b) group components (augmented CDM) Organisational ‐ patients: intervention (a); shared decision making for treatment regimen Organisational ‐ healthcare professionals or system: intervention (a) Patient education: intervention (a) Self‐management support: intervention (a) Frequency: intervention (a) Healthcare professionals involved: intervention (a) Control group components Usual care (which includes referral to asthma care management programmes) Number of components and dominant component: 9, mixed (organisational ‐ patient, education and self‐management) |
|
| Outcomes |
Organisational level Process: continuous medication acquisition index for ICS only, all asthma controller (ICS, leukotriene modifiers, cromolyn sodium, theophylline), LABAs, and SABAs; % patients dispensed a LABA Healthcare utilisation: asthma‐related visits, costs Patient level Quality of life: mini‐AQLQ score Asthma symptoms and activity level: ATAQ score Pulmonary function: FEV1; FEV1/FEV6 Time of outcome measurement: at 24 months |
|
| Notes | Mini‐AQLQ: Asthma Quality of Life Questionnaire; ATAQ: Asthma Therapy Assessment Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐based adaptive randomisation algorithm was used |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from staff randomising patients |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All study personnel, except for care managers, were blinded to patient's study assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See figure 2: less than 20% loss to follow‐up, rate is similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Trial registered on www.clinicaltrials.gov but pre‐determined outcomes not mentioned |
| Other bias | Low risk | No other bias detected |
| Outcomes at baseline similar? | Low risk | See figures 3, 4, 5 |
| Characteristics at baseline similar? | Low risk | See table 1 |
| Adequate protection against contamination? | Low risk | Care managers of intervention group (a) and intervention group (b) were trained separately and worked independently |
Windt 2010.
| Methods | CBA, patient recruitment: general population (i.e. clients of health insurance) Setting: primary care practices throughout Germany (region covered by one health insurance company) |
|
| Participants |
Control patients: n = 317, women: 44.2%, mean age: 36.5, asthma severity: not reported, FEV1: not reported, ICS use: not reported Intervention patients: n = 317, women: 48.6%, mean age: 36.5, asthma severity: not reported, FEV1: not reported, ICS use: not reported |
|
| Interventions |
Name and duration of programme: nationwide asthma disease management programme (duration varies according to specific programme) Intervention group components* Organisational ‐ patients: structured follow‐up Organisational ‐ healthcare professionals or system: use of guidelines; information technology (electronic reports); feedback to physicians Patient education: education sessions Frequency: not clear Healthcare professionals involved: not clear Control group components Usual care Number of components and dominant component: ≥ 5, organisational ‐ healthcare professionals or system |
|
| Outcomes |
Organisational level Process: % patients with prescription of: ICS, ICS as single agent, ICS/LABA in a single inhaler, controller to total medication ratio ≥ 0.5, oral corticosteroids, theophylline, leukotriene receptor antagonists, cromolyn combined with LABA, LABAs without ICSs Healthcare utilisation: % patients with emergency care (hospitalisations or ED visits), % patients doctor hopping (with an anti‐asthmatic drug prescription from at least 3 different providers) Time of outcome measurement: at 12 months |
|
| Notes | *All patients in the intervention group were enrolled in a German disease management programme, with the following obligatory elements; regular check‐ups, education sessions, use of guidelines, information technology (electronic reports), feedback to physicians | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective allocation |
| Allocation concealment (selection bias) | High risk | Retrospective allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Claims data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all patients |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No other bias detected |
| Outcomes at baseline similar? | Low risk | Matched control group (compared with table 1) |
| Characteristics at baseline similar? | Low risk | Matched control group (compared with table 1) |
| Adequate protection against contamination? | Low risk | Contamination unlikely |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| [Advocate's disease] 2003 | CDM but inappropriate study design |
| [Asthma DM] 2005 | CDM but inappropriate study design |
| [Asthma patients] 1998 | Insufficient information |
| [Asthma project] 1999 | CDM but inappropriate study design |
| [Integrated care] 1994 | Insufficient information |
| [Population‐based] 1998 | Insufficient information |
| Afifi 2007 | Not CDM according to operational definition |
| Allen‐Ramey 2002 | CDM but inappropriate study design |
| Bailey 1990 | Not CDM according to operational definition |
| Bailey 1999 | Not CDM according to operational definition |
| Baker 2003 | Possibly CDM but inappropriate study design |
| Barbanel 2003 | Not CDM according to operational definition |
| Bolin 2005 | Possibly CDM but inappropriate study design |
| Bolton 1991 | Not CDM according to operational definition |
| Brandao 2009 | Possibly CDM but inappropriate study design |
| Buchner 1998 | Not CDM according to operational definition |
| Burton 2001 | Not CDM according to operational definition |
| Burton 2001a | Not CDM according to operational definition |
| Carmo 2011 | CDM but inappropriate study design |
| Chamnan 2010 | Not CDM according to operational definition |
| Charlton 1990 | Not CDM according to operational definition |
| Charlton 1992 | Possibly CDM but inappropriate study design |
| Choy 1999 | Not CDM according to operational definition |
| Clark 2007 | Not CDM according to operational definition |
| Clark 2010 | Not CDM according to operational definition |
| Cordina 2001 | Not CDM according to operational definition |
| Cote 1997 | Not CDM according to operational definition |
| Cote 2000 | Not CDM according to operational definition |
| Cote 2001 | Not CDM according to operational definition |
| Cruz 2010 | CDM but inappropriate study design |
| Curtin 1998 | CDM but inappropriate study design |
| D'Souza 2000 | CDM but inappropriate study design |
| Dall 2010 | Not CDM according to operational definition |
| De Oliveira 1999 | Not CDM according to operational definition |
| Delaronde 2002 | Possibly CDM but inappropriate study design |
| Delaronde 2005 | Not CDM according to operational definition |
| Donald 2008 | Not CDM according to operational definition |
| Dozor 2011 | Not target population |
| Dzyngel 1994 | Not CDM according to operational definition |
| Emmerton 2003 | CDM but inappropriate study design |
| Erhola 2003 | CDM but inappropriate study design |
| Fardy 1999 | Not CDM according to operational definition |
| Fireman 2004 | CDM but inappropriate study design |
| Ford 1996 | Not CDM according to operational definition |
| Gallefoss 1999 | Not CDM according to operational definition |
| Gallefoss 1999a | Not CDM according to operational definition |
| Gallefoss 2000 | Not CDM according to operational definition |
| Gallefoss 2000a | Not CDM according to operational definition |
| Gallefoss 2001 | Not CDM according to operational definition |
| Gallefoss 2002 | Not CDM according to operational definition |
| Gallefoss 2003 | Not CDM according to operational definition |
| Garrett 1994 | Not CDM according to operational definition |
| Groban 1998 | CDM but inappropriate study design |
| Haahtela 2006 | CDM but inappropriate study design |
| Hartmann 2005 | Possibly CDM but inappropriate study design |
| Heard 1999 | Not CDM according to operational definition |
| Hertzman 2005 | CDM but inappropriate study design |
| Hesselink 2004 | Not CDM according to operational definition |
| Holton 2010 | Not CDM according to operational definition |
| Hopman 1999 | Not CDM according to operational definition |
| Horswell 2008 | Insufficient information |
| Ignacio‐Garcia 1995 | Not CDM according to operational definition |
| Ignacio‐Garcia 2002 | CDM but inappropriate study design |
| Janson 2009 | Not CDM according to operational definition |
| Johnson 2003 | CDM but inappropriate study design |
| Jones 1995 | Not CDM according to operational definition |
| Jounieaux 2003 | Not CDM according to operational definition |
| Jowers 2000 | CDM but inappropriate study design |
| Kelso 1996 | CDM but inappropriate study design |
| Kligler 2011 | Not CDM according to operational definition |
| Knoell 1998 | Not CDM according to operational definition |
| Kotses 1996 | Not CDM according to operational definition |
| Lahdensuo 1996 | Not CDM according to operational definition |
| Legorreta 2000 | Not CDM according to operational definition |
| Lemaigre 2010 | Not CDM according to operational definition |
| Licskai 2012 | CDM but inappropriate study design |
| Lind 2006 | CDM but inappropriate study design |
| Lindberg 1999 | CDM but inappropriate study design |
| Lindberg 2002 | CDM but inappropriate study design |
| Linden 2007 | Not CDM according to operational definition |
| Lo 2006 | CDM but inappropriate study design |
| Ludwig‐Beymer 1998 | Not CDM according to operational definition |
| Magar 2005 | Not CDM according to operational definition |
| Maljanian 1999 | CDM but inappropriate study design |
| Mangiapane 2005 | CDM but inappropriate study design |
| Mehuys 2008 | Not CDM according to operational definition |
| Mildenhall 1998 | Not CDM according to operational definition |
| Morisky 2009 | Not CDM according to operational definition |
| Moudgil 2000 | Not CDM according to operational definition |
| Mu 2006 | CDM but inappropriate study design |
| Mu 2008 | CDM but inappropriate study design |
| Munroe 1997 | CDM and study design alright, but inappropriate outcomes |
| Narhi 2001 | CDM but inappropriate study design |
| Narhi 2002 | CDM but inappropriate study design |
| Park 2010 | Not CDM according to operational definition |
| Patel 2004 | CDM but inappropriate study design |
| Pauley 1995 | CDM but inappropriate study design |
| Peretz 2012 | Not target population |
| Pilotto 2004 | Not CDM according to operational definition |
| Premaratne 1999 | Not CDM according to operational definition |
| Rossiter 2000 | Not CDM according to operational definition |
| Saini 2004 | CDM but inappropriate study design |
| Saini 2008 | CDM but inappropriate study design |
| Saini 2011 | Not CDM according to operational definition |
| Schonlau 2005 | Not CDM according to operational definition |
| Schott‐Baer 1999 | Not CDM according to operational definition |
| Schulz 2001 | Not CDM according to operational definition |
| Scott 2009 | Not target population |
| Shelledy 2009 | Not CDM according to operational definition |
| Smith 2007 | Not CDM according to operational definition |
| Sommaruga 1995 | Not CDM according to operational definition |
| Souza‐Machado 2010a | CDM but inappropriate study design |
| Steuten 2006 | CDM but inappropriate study design |
| Swanson 2000 | Not CDM according to operational definition |
| Tatis 2005 | CDM but inappropriate study design |
| Thoonen 2003 | Not CDM according to operational definition |
| Tinkelman 2004 | CDM but inappropriate study design |
| To 2008 | CDM but inappropriate study design |
| Treadwell 2009 | CDM but inappropriate study design |
| Tschopp 2002 | CDM but inappropriate study design |
| Tschopp 2005 | CDM but inappropriate study design |
| Van Damme 1994 | Possibly CDM but inappropriate study design |
| van der Meer 2009 | Not CDM according to operational definition |
| van der Palen 2001 | Not CDM according to operational definition |
| Wang 2011 | Insufficient information |
| Weinberger 2002 | Not CDM according to operational definition |
| Williams 2007 | Possibly CDM but inappropriate study design |
| Yang 2010 | Not CDM according to operational definition |
| Yawn 2008 | Not CDM according to operational definition |
Characteristics of ongoing studies [ordered by study ID]
Ahmed 2011.
| Trial name or title | My asthma portal: a web‐based self management intervention |
| Methods | Design: Parallel multicentred 2‐arm randomised controlled trial Setting: pulmonary clinics in two tertiary care hospitals in Montreal, Canada |
| Participants | Males and females, aged 18 to 69 years, with a confirmed asthma diagnosis, and classified as having poor asthma control by their doctor |
| Interventions | Intervention group: personalised web‐based application that provides self‐management support by combining personal asthma health information with opportunities to self‐monitor and receive feedback from the care team using a web‐based system. It includes tailored asthma education and aims to modify health behaviours related to medication adherence, action plan use, and physical activity Control group: usual care (including an asthma nurse who provides education and follow‐up as needed and follow‐up phone calls between visits by the asthma nurse) |
| Outcomes | Organisation of care: asthma‐related ED visits or hospitalisations, costs, and other resource utilisation Asthma control: % patients overusing rescue fast acting bronchodilators (beta2‐agonists) (primary) Asthma quality of life: score on the mini‐AQLQ (primary) Self‐management: score on the Chronic Disease Self‐Efficacy Scale, adherence to controller asthma medications Acceptability and attitudes toward the web portal: score on the Technology Acceptance Model (TAM) questionnaire, the number of minutes patients spent logged into the system/week, the number of days/week and times that patients logged in, and features of the system used including number of messages sent to the asthma nurse |
| Starting date | March 2010 |
| Contact information | Sara Ahmed, School of Physical and Occupational Therapy, McGill University, Montreal, Canada sara.ahmed@mcgill.ca |
| Notes | controlled‐trials.com identifier: ISRCTN34326236 AQLQ: Asthma Quality of Life Questionnaire |
Arguel 2013.
| Trial name or title | Internet Intervention called Healthy.me to Improve Asthma Management |
| Methods | Design: randomised controlled trial with a 2‐group parallel design Setting: Australia |
| Participants | Adults (aged 18 years or above) with a doctor diagnosis of asthma, living in Australia at the time of the study and with easy access to the Internet and e‐mail on a regular basis |
| Interventions | Intervention group: a web‐based personally controlled health management system (PCHMS) called Healthy.me supports consumers with asthma to encourage the uptake and use of a personal written asthma action plan, and to proactively seek self‐management advice and schedule planned general practitioner visits before experiencing an asthma exacerbation. It features a Personal Health Record (PHR) and pillbox allowing for self‐recording of medical test results, health measurements, current medications and medication adherence, a schedule or to‐do lists or reminders, consumer‐specific care pathways, social communication spaces which supports interaction across the continuum of care between participants and clinicians, and an online appointment booking service Control group: usual care (with access to a static webpage, without PCHMS features or any interactive component, with links to Australian information websites about asthma) Duration: 12 months |
| Outcomes | Organisation of care: number of planned and unplanned visits to healthcare providers for asthma issues Process: % patients with an asthma action plan (new or revised) (primary), usage patterns of Healthy.me and attrition rates Quality of life: competing demands on health and asthma Asthma symptoms and activity levels: score on ACQ, score on the Asthma Exacerbation Questionnaire, days lost from work Self‐management: adherence to the asthma action plan |
| Starting date | March 1, 2013 |
| Contact information | Amaël Arguel, Centre for Health Informatics, Australian Institute of Health Innovation, University of New South Wales, Sydney, Australia a.arguel@unsw.edu.au |
| Notes | Australian New Zealand Clinical Trials Registry CTRN12612000716864 ACQ: Asthma Control Questionnaire |
Differences between protocol and review
We planned in the protocol to perform subgroup analysis on the duration of the intervention and the disease severity. This was not possible due to the lack of relevant data. We added one subgroup analysis on the presence of limited CDM components in the control group to further explore clinical heterogeneity.
The search strategies published in the protocol were revised to improve the sensitivity of the search terms and to comply with EPOC and Cochrane Collaboration search methodologies.
We did not include ITS studies. If this was the case, results and analyses would have been expressed and run separately from other designs, according to guidance found on the EPOC Review Group website (EPOC‐specific resources for review authors/ITS analyses).
Contributions of authors
IPB took the initiative to conduct this review and was the leading author. IPB, GG, POB and BB wrote the protocol and approved its final version. IPB, CA, GG, POB, in pairs, selected trials; CA, IPB and GG extracted data and assessed risk of bias. IPB and CA wrote the review, and GG, POB and BB contributed to its final version. All authors provided general advice on the review and approved the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Bourse de la Commission pour la Promotion Académique des Femmes, Faculté de Biologie et Médecine de lUniversité de Lausanne, Switzerland.
Dr Isabelle Peytremann‐Bridevaux was supported by a grant from this bursary in 2008
-
Swiss National Foundation for Science (PROSPER 32333B‐123817), Switzerland.
Dr Isabelle Peytremann‐Bridevaux is currently supported by a grant from this Foundation
Declarations of interest
No potential conflict of interest.
New
References
References to studies included in this review
Armour 2007 {published and unpublished data}
- Armour C, Bosnic‐Anticevich S, Brillant M, Burton D, Emmerton L, Krass I, et al. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax 2007;62(6):496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordois A, Armour C, Brillant M, Bosnic‐Anticevich S, Burton D, Emmerton L, et al. Cost‐effectiveness analysis of a pharmacy asthma care program in Australia. Disease Management and Health Outcomes 2007;15(6):387‐96. [Google Scholar]
Cambach 1997 {published data only}
- Cambach W, Chadwick‐Straver RV, Wagenaar RC, Keimpema AR, Kemper HC. The effects of a community‐based pulmonary rehabilitation programme on exercise tolerance and quality of life: a randomized controlled trial. European Respiratory Journal 1997;10(1):104‐13. [DOI] [PubMed] [Google Scholar]
- Cambach W, Chadwick‐Straver RV, Wagenaar RC, Keimpema AR, Kemper HC, Rijswijk H. Feasibility of a pulmonary rehabilitation programme in community based physiotherapy practices. Journal of Rehabilitation Sciences 1994;7(4):104‐12. [Google Scholar]
Castro 2003 {published data only}
- Castro M, Zimmermann NA, Crocker S, Bradley J, Leven C, Schechtman KB. Asthma intervention program prevents readmissions in high healthcare users. American Journal of Respiratory and Critical Care Medicine 2003;168(9):1095‐9. [DOI] [PubMed] [Google Scholar]
Charrois 2006 {published and unpublished data}
- Charrois T, Newman S, Sin D, Senthilselvan A, Tsuyuki RT. Improving asthma symptom control in rural communities: the design of the Better Respiratory Education and Asthma Treatment in Hinton and Edson study. Controlled Clinical Trials 2004;25(5):502‐14. [PUBMED: 15465619] [DOI] [PubMed] [Google Scholar]
- Charrois TL, Newman SC, Senthilselvan A, Tsuyuki RT. Improving asthma control in the rural setting: The BREATHE (Better Respiratory Education and Asthma Treatment in Hinton and Edson) study. Canadian Pharmacists Journal 2006;139(4):44‐50. [Google Scholar]
Couturaud 2002 {published data only}
- Couturaud F, Proust A, Frachon I, Dewitte JD, Oger E, Quiot JJ, et al. Education and self‐management: a one‐year randomized trial in stable adult asthmatic patients. Journal of Asthma 2002;39(6):493‐500. [DOI] [PubMed] [Google Scholar]
Feifer 2004 {published data only}
- Feifer RA, Verbrugge RR, Khalid M, Levin R, O'Keefe GB, Aubert RE. Improvements in asthma pharmacotherapy and self‐management: an example of a population‐based disease management program. Disease Management & Health Outcomes 2004;12(2):93‐102. [Google Scholar]
Galbreath 2008 {published and unpublished data}
- Galbreath AD, Smith B, Wood PR, Inscore S, Forkner E, Vazquez M, et al. Assessing the value of disease management: Impact of 2 disease management strategies in an underserved asthma population. Annals of Allergy, Asthma and Immunology 2008;101(6):599‐607. [DOI] [PubMed] [Google Scholar]
- Peters JI, McKinney JM, Smith B, Wood P, Forkner E, Galbreath AD. Impact of obesity in asthma: evidence from a large prospective disease management study. Annals of Allergy, Asthma and Immunology 2011;106(1):30‐5. [DOI] [PubMed] [Google Scholar]
Herborg 2001 {published data only}
- Herborg H, Soendergaard B, Froekjaer B, Fonnesbaek L, Jorgensen T, Hepler CD, et al. Improving drug therapy for patients with asthma‐‐part 1: Patient outcomes [see comments]. Journal of the American Pharmaceutical Association 2001;41(4):539‐50. [DOI] [PubMed] [Google Scholar]
- Herborg H, Soendergaard B, Jorgensen T, Fonnesbaek L, Hepler CD, Holst H, et al. Improving drug therapy for patients with asthma‐part 2: Use of antiasthma medications. Journal of the American Pharmaceutical Association 2001;41(4):551‐9. [PUBMED: 11486981] [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang TT, Li YT, Wang CH. Individualized programme to promote self‐care among older adults with asthma: randomized controlled trial. Journal of Advanced Nursing 2009;65(2):348‐58. [DOI] [PubMed] [Google Scholar]
Kokubu 2000 {published data only}
- Kokubu F, Nakajima S, Ito K, Makino S, Kitamura S, Fukuchi Y, et al. Hospitalization reduction by an asthma tele‐medicine system. Arerugi ‐ Japanese Journal of Allergology 2000;49(1):19‐31. [PubMed] [Google Scholar]
- Kokubu F, Suzuki H, Sano Y, Kihara N, Adachi M. Tele‐medicine system for high‐risk asthmatic patients. Arerugi ‐ Japanese Journal of Allergology 1999;48(7):700‐12. [PubMed] [Google Scholar]
Landon 2007 {published data only}
- Landon B E, Hicks L S, O'Malley A J, Lieu T A, Keegan T, McNeil B J, et al. Improving the management of chronic disease at community health centers. New England Journal of Medicine 2007;356(9):921‐34. [DOI] [PubMed] [Google Scholar]
Martin 2009 {published and unpublished data}
- Martin M A, Catrambone C D, Sharp L, Evans A, Kee R, Rucker Whitaker C, et al. The efficacy of a community asthma team intervention to improve asthma self efficacy for urban African American adults. American Thoracic Society International Conference, May 16‐21. 2008.
- Martin MA, Catrambone CD, Kee RA, Evans AT, Sharp LK, Lyttle C, et al. Improving asthma self‐efficacy: Developing and testing a pilot community‐based asthma intervention for African American adults. Journal of Allergy and Clinical Immunology 2009;123(1):153‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayo 1990 {published data only}
- Mayo PH, Richman J, Harris HW. Results of a program to reduce admissions for adult asthma [see comment]. Annals of Internal Medicine 1990;112(11):864‐71. [DOI] [PubMed] [Google Scholar]
McLean 2003 {published data only}
- McLean W, Gillis J, Waller R. The BC Community Pharmacy Asthma Study: A study of clinical, economic and holistic outcomes influenced by an asthma care protocol provided by specially trained community pharmacists in British Columbia. Canadian Respiratory Journal 2003;10(4):195. [DOI] [PubMed] [Google Scholar]
Petro 2005 {published data only}
- Petro W, Schulenburg JM, Greiner W, Weithase J, Schulke A, Metzdorf N. [Efficacy of a disease management programme in asthma] [German]. Pneumologie 2005;59(2):101‐7. [DOI] [PubMed] [Google Scholar]
Schatz 2006 {published data only}
- Schatz M, Gibbons C, Nelle C, Harden K, Zeiger RS. Impact of a care manager on the outcomes of higher risk asthmatic patients: a randomized controlled trial. Journal of Asthma 2006;43(3):225‐9. [DOI] [PubMed] [Google Scholar]
Smith 2005 {published data only}
- Smith JR, Mildenhall S, Noble MJ, Shepstone L, Koutantji M, Mugford M, et al. The Coping with Asthma Study: a randomised controlled trial of a home based, nurse led psychoeducational intervention for adults at risk of adverse asthma outcomes. Thorax 2005;60(12):1003‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weng 2005 {published data only}
- Weng HC. Impacts of a government‐sponsored outpatient‐based disease management program for patients with asthma: a preliminary analysis of national data from Taiwan. Disease Management 2005;8(1):48‐58. [DOI] [PubMed] [Google Scholar]
Wilson 2010 {published and unpublished data}
- Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. American Journal of Respiratory and Critical Care Medicine 2010;181(6):566‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Windt 2010 {published data only}
- Windt R, Glaeske G. Effects of a German asthma disease management program using sickness fund claims data. Journal of Asthma 2010;47(6):674‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
[Advocate's disease] 2003 {published data only}
- [no author]. Advocate's disease management program reduces readmissions for CHF and asthma. Performance Improvement Advisor 2003;7(3):44‐7. [PubMed] [Google Scholar]
[Asthma DM] 2005 {published data only}
- [no author]. Asthma DM program demonstrates substantial savings in CO pilot study. Disease Management Advisor 2005;11(4):46‐7. [PubMed] [Google Scholar]
[Asthma patients] 1998 {published data only}
- [no author]. Asthma patients get assertive about helping themselves in innovative DM program. Healthcare Demand & Disease Management 1998;4(8):126‐8. [PubMed] [Google Scholar]
[Asthma project] 1999 {published data only}
- [no author]. Asthma project cuts hospitalizations by 95%. Healthcare Benchmarks 1999;6(1):10‐2. [PubMed] [Google Scholar]
[Integrated care] 1994 {published data only}
- Osman LM, Abdalla MI, Russell IT, Fiddes J, Friend JAR, Legge JS, et al. Integrated care for asthma: Matching care to the patient. European Respiratory Journal 1996;9(3):444. [DOI] [PubMed] [Google Scholar]
- [no author]. Integrated care for asthma: a clinical, social, and economic evaluation. Grampian Asthma Study of Integrated Care (GRASSIC). BMJ (Clinical research ed.) 1994;308(6928):559‐64. [PUBMED: 8148678] [PMC free article] [PubMed] [Google Scholar]
[Population‐based] 1998 {published data only}
- [no author]. Population‐based asthma management produces dramatic cuts in ER visits, hospitalization. Healthcare Demand & Disease Management 1998;4(5):77‐9. [PubMed] [Google Scholar]
Afifi 2007 {published data only}
- Afifi AA, Morisky DE, Kominski GF, Kotlerman JB. Impact of disease management on health care utilization: evidence from the "Florida: A Healthy State (FAHS)" Medicaid Program. Preventive Medicine 2007;44(6):547‐53. [DOI] [PubMed] [Google Scholar]
Allen‐Ramey 2002 {published data only}
- Allen‐Ramey FC, Diette GB, McDonald RC, Skinner EA, Steinwachs DM, Wu AW. Methods aimed at improving asthma care and outcomes management: a case study. Disease Management & Health Outcomes 2002;10(8):495‐503. [Google Scholar]
Bailey 1990 {published data only}
- Bailey WC, Richards JM Jr, Brooks CM, Soong SJ, Windsor RA, Manzella BA. A randomized trial to improve self‐management practices of adults with asthma. Archives of Internal Medicine 1990;150(8):1664‐8. [PubMed] [Google Scholar]
Bailey 1999 {published data only}
- Bailey WC, Kohler CL, Richards JM Jr, Windsor RA, Brooks CM, Gerald LB, et al. Asthma self‐management: do patient education programs always have an impact?. Archives of Internal Medicine 1999;159(20):2422‐8. [DOI] [PubMed] [Google Scholar]
Baker 2003 {published data only}
- Baker D, Middleton E, Campbell S. The impact of chronic disease management in primary care on inequality in asthma severity. Journal of Public Health Medicine 2003;25(3):258‐60. [DOI] [PubMed] [Google Scholar]
Barbanel 2003 {published data only}
- Barbanel D, Eldridge S, Griffiths C. Can a self‐management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax 2003;58(10):851‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolin 2005 {published data only}
- Bolin J, Gamm L, Kash B, Peck B M. Chronic disease management in rural and underserved populations: Innovation and system improvement help lead to success. Managed Care Interface 2005;18(3):37‐41+55. [PubMed] [Google Scholar]
Bolton 1991 {published data only}
- Bolton MB, Tilley BC, Kuder J, Reeves T, Schultz LR. The cost and effectiveness of an education program for adults who have asthma. Journal of General Internal Medicine 1991;6(5):401‐7. [DOI] [PubMed] [Google Scholar]
Brandao 2009 {published data only}
- Brandao HV, Cruz CM, Santos Jr Ponte EV, Guimaraes A, Augusto Filho A. Hospitalizations for asthma: impact of a program for the control of asthma and allergic rhinitis in Feira de Santana, Brazil. Jornal Brasileiro de Pneumologia 2009;35(8):723‐9. [DOI] [PubMed] [Google Scholar]
Buchner 1998 {published data only}
- Buchner DA, Butt LT, Stefano A, Edgren B, Suarez A, Evans RM. Effects of an asthma management program on the asthmatic member: patient‐centered results of a 2‐year study in a managed care organization. American Journal of Managed Care 1998;4(9):1288‐97. [PubMed] [Google Scholar]
Burton 2001 {published data only}
- Burton WN, Connerty CM, Schultz AB, Chen CY, Edington DW. Bank One's worksite‐based asthma disease management program. Journal of Occupational and Environmental Medicine 2001;43(2):75‐82. [DOI] [PubMed] [Google Scholar]
Burton 2001a {published data only}
- Burton WN, Schultz AB, Connerty CM, Chen C, Edington DW. Asthma disease management: a worksite‐based asthma education program. Disease Management 2001;4(1):3‐13. [DOI] [PubMed] [Google Scholar]
Carmo 2011 {published data only}
- Carmo T A, Andrade S M, Cerci Neto A. Evaluation of an asthma control program in family health units. Cadernos de Saude Publica 2011;27(1):162‐72. [DOI] [PubMed] [Google Scholar]
Chamnan 2010 {published data only}
- Chamnan P, Boonlert K, Pasi W, Yodsiri S, Pong‐on S, Khansa B, et al. Implementation of a 12‐week disease management program improved clinical outcomes and quality of life in adults with asthma in a rural district hospital: pre‐ and post‐intervention study. Asian Pacific Journal of Allergy and Immunology 2010;28(1):15‐21. [PUBMED: 20527511] [PubMed] [Google Scholar]
Charlton 1990 {published data only}
- Charlton I, Charlton G, Broomfield J, Mullee MA. Evaluation of peak flow and symptoms only self management plans for control of asthma in general practice [see comment]. BMJ 1990;301(6765):1355‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charlton 1992 {published data only}
- Charlton I, Charlton G, Broomfield J, Campbell M. An evaluation of a nurse‐run asthma clinic in general practice using an attitudes and morbidity questionnaire. Family Practice 1992;9(2):154‐60. [DOI] [PubMed] [Google Scholar]
Choy 1999 {published data only}
- Choy DK, Tong M, Ko F, Li ST, Ho A, Chan J, et al. Evaluation of the efficacy of a hospital‐based asthma education programme in patients of low socioeconomic status in Hong Kong. Clinical & Experimental Allergy 1999;29(1):84‐90. [DOI] [PubMed] [Google Scholar]
Clark 2007 {published data only}
- Clark NM, Gong ZM, Wang SJ, Lin X, Bria WF, Johnson TR. A randomized trial of a self‐regulation intervention for women with asthma. Chest 2007;132(1):88‐97. [DOI] [PubMed] [Google Scholar]
Clark 2010 {published data only}
- Clark NM, Gong ZM, Wang SJ, Valerio MA, Bria WF, Johnson TR. From the female perspective: Long‐term effects on quality of life of a program for women with asthma. Gender Medicine 2010;7(2):125‐36. [PUBMED: 20435275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cordina 2001 {published data only}
- Cordina M, McElnay JC, Hughes CM. Assessment of a community pharmacy‐based program for patients with asthma. Pharmacotherapy 2001;21(10):1196‐203. [DOI] [PubMed] [Google Scholar]
Cote 1997 {published data only}
- Cote J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, et al. Influence on asthma morbidity of asthma education programs based on self‐management plans following treatment optimization. American Journal of Respiratory and Critical Care Medicine 1997;155(5):1509‐14. [DOI] [PubMed] [Google Scholar]
Cote 2000 {published data only}
- Cote J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, et al. Influence of asthma education on asthma severity, quality of life and environmental control. Canadian Respiratory Journal 2000;7(5):395‐400. [DOI] [PubMed] [Google Scholar]
Cote 2001 {published data only}
- Cote J, Bowie DM, Robichaud P, Parent JG, Battisti L, Boulet LP. Evaluation of two different educational interventions for adult patients consulting with an acute asthma exacerbation. American Journal of Respiratory and Critical Care Medicine 2001;163(6):1415‐9. [DOI] [PubMed] [Google Scholar]
Cruz 2010 {published data only}
- Cruz AA, Souza‐Machado A, Franco R, Souza‐Machado C, Ponte EV, Santos PM, et al. The impact of a program for control of asthma in a low‐income setting. World Allergy Organization Journal 2010;3(4):167‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtin 1998 {published data only}
- Curtin K, Hayes BD, Holland CL, Katz LA. Computer‐generated intervention for asthma population care management. Effective Clinical Practice 1998;1(1):43‐6. [PubMed] [Google Scholar]
D'Souza 2000 {published data only}
- D'Souza W J, Slater T, Fox C, Fox B, Karu H, Gemmell T, et al. Asthma morbidity 6 yrs after an effective asthma self‐management programme in a Maori community. European Respiratory Journal 2000;15(3):464‐9. [DOI] [PubMed] [Google Scholar]
Dall 2010 {published data only}
- Dall TM, Askarinam Wagner RC, Zhang Y, Yang W, Arday DR, Gantt CJ. Outcomes and lessons learned from evaluating TRICARE's disease management programs. American Journal of Managed Care 2010;16(6):438‐46. [PUBMED: 20560687] [PubMed] [Google Scholar]
Delaronde 2002 {published data only}
- Delaronde S. Using case management to increase antiinflammatory medication use among a managed care population with asthma. Journal of Asthma 2002;39(1):55‐63. [DOI] [PubMed] [Google Scholar]
Delaronde 2005 {published data only}
- Delaronde S, Peruccio DL, Bauer BJ. Improving asthma treatment in a managed care population. American Journal of Managed Care 2005;11(6):361‐8. [PubMed] [Google Scholar]
De Oliveira 1999 {published data only}
- Oliveira MA, Faresin SM, Bruno VF, Bittencourt AR, Fernandes AL. Evaluation of an educational programme for socially deprived asthma patients. European Respiratory Journal 1999;14(4):908‐14. [DOI] [PubMed] [Google Scholar]
Donald 2008 {published data only}
- Donald KJ, McBurney H, Teichtahl H, Irving L. A pilot study of telephone based asthma management. Australian Family Physician 2008;37(3):170‐3. [PubMed] [Google Scholar]
Dozor 2011 {published data only}
- Dozor AJ, Stout JW, Smith K, Zhou C, Solomon C, Mangione‐Smith R. Learning From A Distance: a cluster randomized trial of a program to improve asthma care through interactive, online spirometry training and feedback. American Journal of Respiratory and Critical Care Medicine 2011;183 Meeting Abstracts:A5473. [Google Scholar]
Dzyngel 1994 {published data only}
- Dzyngel B, Kesten S, Chapman KR. Assessment of an ambulatory care asthma program. Journal of Asthma 1994;31(4):291‐300. [DOI] [PubMed] [Google Scholar]
Emmerton 2003 {published data only}
- Emmerton L, Shaw J, Kheir N. Asthma management by New Zealand pharmacists: a pharmaceutical care demonstration project. Journal of Clinical Pharmacy and Therapeutics 2003;28(5):395‐402. [DOI] [PubMed] [Google Scholar]
Erhola 2003 {published data only}
- Erhola M, Makinen R, Koskela K, Bergman V, Klaukka T, Makela M, et al. The asthma programme of Finland: an evaluation survey in primary health care. International Journal of Tuberculosis and Lung Disease 2003;7(6):592‐8. [PubMed] [Google Scholar]
Fardy 1999 {published data only}
- Fardy HJ. The 3+ plan for asthma management. Australian Family Physician 1999;28(2):95. [PubMed] [Google Scholar]
Fireman 2004 {published data only}
- Fireman B, Bartlett J, Selby J. Can disease management reduce health care costs by improving quality?. Health Affairs 2004;23(6):63‐75. [DOI] [PubMed] [Google Scholar]
Ford 1996 {published data only}
- Ford ME, Edwards G, Rodriguez JL, Gibson RC, Tilley BC. An empowerment‐centered, church‐based asthma education program for African American adults. Health & Social Work 1996;21(1):70‐5. [DOI] [PubMed] [Google Scholar]
Gallefoss 1999 {published data only}
- Gallefoss F, Bakke PS, Rsgaard PK. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1999;159(3):812‐7. [DOI] [PubMed] [Google Scholar]
Gallefoss 1999a {published data only}
- Gallefoss F, Bakke PS. How does patient education and self‐management among asthmatics and patients with chronic obstructive pulmonary disease affect medication?. American Journal of Respiratory and Critical Care Medicine 1999;160(6):2000‐5. [DOI] [PubMed] [Google Scholar]
Gallefoss 2000 {published data only}
- Gallefoss F, Bakke PS. Impact of patient education and self‐management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease. Respiratory Medicine 2000;94(3):279‐87. [DOI] [PubMed] [Google Scholar]
Gallefoss 2000a {published data only}
- Gallefoss F, Bakke PS. Patient satisfaction with healthcare in asthmatics and patients with COPD before and after patient education. Respiratory Medicine 2000;94(11):1057‐64. [DOI] [PubMed] [Google Scholar]
Gallefoss 2001 {published data only}
- Gallefoss F, Bakke PS. Cost‐effectiveness of self‐management in asthmatics: a 1‐yr follow‐up randomized, controlled trial. European Respiratory Journal 2001;17(2):206‐13. [DOI] [PubMed] [Google Scholar]
Gallefoss 2002 {published data only}
- Gallefoss F, Bakke PS. [The effect of patient education in asthma, a randomized controlled trial] [Norwegian]. Tidsskrift for den Norske laegeforening 2002;122(28):2702‐6. [PubMed] [Google Scholar]
Gallefoss 2003 {published data only}
- Gallefoss F, Bakke PS. Does smoking affect the outcome of patient education and self‐management in asthmatics? [References]. Patient Education and Counseling 2003;49(1):91‐7. [DOI] [PubMed] [Google Scholar]
Garrett 1994 {published data only}
- Garrett J, Fenwick JM, Taylor G, Mitchell E, Stewart J, Rea H. Prospective controlled evaluation of the effect of a community based asthma education centre in a multiracial working class neighbourhood. Thorax 1994;49(10):976‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groban 1998 {published data only}
- Groban MD, Evans RM, Edgren B, Butt LT, Stefano A, Fernandes DJ, et al. Clinical benefits and cost reduction associated with a comprehensive asthma management programme at a managed care organisation. Disease Management & Health Outcomes 1998;4(2):93‐100. [Google Scholar]
Haahtela 2006 {published data only}
- Haahtela T, Tuomisto L E, Pietinalho A, Klaukka T, Erhola M, Kaila M, et al. A 10 year asthma programme in Finland: major change for the better. Thorax 2006;61(8):663‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann 2005 {published data only}
- Hartmann C W, Maio V, Goldfarb N I, Cobb N, Nash D B. Asthma management programs in managed care organizations. Disease Management 2005;8(6):339‐45. [DOI] [PubMed] [Google Scholar]
Heard 1999 {published data only}
- Heard AR, Richards IJ, Alpers JH, Pilotto LS, Smith BJ, Black JA. Randomised controlled trial of general practice based asthma clinics [see comment]. Medical Journal of Australia 1999;171(2):68‐71. [DOI] [PubMed] [Google Scholar]
Hertzman 2005 {published data only}
- Hertzman PA, Kelly HW, Coultas D. Chronic illness care in Russia: a pilot project to improve asthma care in a "closed city". Chest 2005;127(3):861‐5. [DOI] [PubMed] [Google Scholar]
Hesselink 2004 {published data only}
- Hesselink AE, Penninx BW, Windt DA, Duin BJ, Vries P, Twisk JW, et al. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: results from a randomised controlled trial. Patient Education & Counseling 2004;55(1):121‐8. [DOI] [PubMed] [Google Scholar]
Holton 2010 {published data only}
- Holton CH, Beilby JJ, Harris MF, Harper CE, Proudfoot JG, Ramsay EN, et al. Systematic care for asthma in Australian general practice: a randomised controlled trial. The Medical Journal of Australia 2010;193(6):332‐7. [PUBMED: 20854237] [DOI] [PubMed] [Google Scholar]
Hopman 1999 {published data only}
- Hopman WM, Owen JG, Gagne E. Disease management. Assessment of the effect of asthma education on outcomes. Managed Care Interface 1999;12(5):89‐93. [PubMed] [Google Scholar]
Horswell 2008 {published data only}
- Horswell R, Butler MK, Kaiser M, Moody‐Thomas S, McNabb S, Besse J, et al. Disease management programs for the underserved. Disease Management 2008;11(3):145‐52. [DOI] [PubMed] [Google Scholar]
Ignacio‐Garcia 1995 {published data only}
- Ignacio‐Garcia JM, Gonzalez‐Santos P. Asthma self‐management education program by home monitoring of peak expiratory flow. American Journal of Respiratory and Critical Care Medicine 1995;151(2:Pt 1):t‐9. [DOI] [PubMed] [Google Scholar]
Ignacio‐Garcia 2002 {published data only}
- Ignacio‐Garcia JM, Pinto‐Tenorio M, Chocron‐Giraldez MJ, Cabello‐Rueda F, Lopez‐Cozar Gil AI, Ignacio‐Garcia JM, et al. Benefits at 3 yrs of an asthma education programme coupled with regular reinforcement. European Respiratory Journal 2002;20(5):1095‐101. [DOI] [PubMed] [Google Scholar]
Janson 2009 {published data only}
- Janson SL, McGrath KW, Covington JK, Cheng SC, Boushey HA, Janson Susan L, et al. Individualized asthma self‐management improves medication adherence and markers of asthma control. Journal of Allergy and Clinical Immunology 2009;123(4):840‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2003 {published data only}
- Johnson AE, Yin M, Berg G. Utilization and financial outcomes of an asthma disease management program delivered to Medicaid members: results of a three‐group comparison study. Disease Management & Health Outcomes 2003;11(7):455‐65. [Google Scholar]
Jones 1995 {published data only}
- Jones KP, Mullee MA, Middleton M, Chapman E, Holgate ST. Peak flow based asthma self‐management: a randomised controlled study in general practice. British Thoracic Society Research Committee. Thorax 1995;50(8):851‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jounieaux 2003 {published data only}
- Jounieaux V, Guillaume C, Malka M, Wursthorn M, Girod I, Baron‐Papillon F. [Medico‐economic evaluation of a care management program for asthmatic patients] [French]. Sante Publique (Vandoeuvre‐Les‐Nancey) 2003;15(4):449‐64. [PubMed] [Google Scholar]
Jowers 2000 {published data only}
- Jowers JR, Schwartz AL, Tinkelman DG, Reed KE, Corsello PR, Mazzei AA, et al. Disease management program improves asthma outcomes. American Journal of Managed Care 2000;6(5):585‐92. [PubMed] [Google Scholar]
Kelso 1996 {published data only}
- Kelso TM, Abou‐Shala N, Heilker GM, Arheart KL, Portner TS, Self TH. Comprehensive long‐term management program for asthma: effect on outcomes in adult African‐Americans. American Journal of the Medical Sciences 1996;311(6):272‐80. [DOI] [PubMed] [Google Scholar]
Kligler 2011 {published data only}
- Kligler B, Homel P, Blank A E, Kenney J, Levenson H, Merrell W. Randomized trial of the effect of an integrative medicine approach to the management of asthma in adults on disease‐related quality of life and pulmonary function. Alternative Therapies in Health & Medicine 2011;17(1):10‐5. [PubMed] [Google Scholar]
Knoell 1998 {published data only}
- Knoell DL, Pierson JF, Marsh CB, Allen JN, Pathak DS. Measurement of outcomes in adults receiving pharmaceutical care in a comprehensive asthma outpatient clinic. Pharmacotherapy 1998;18(6 I):1365‐74. [PubMed] [Google Scholar]
Kotses 1996 {published data only}
- Kotses H, Stout C, McConnaughy K, Winder JA, Creer TL. Evaluation of individualized asthma self‐management programs. Journal of Asthma 1996;33(2):113‐8. [DOI] [PubMed] [Google Scholar]
Lahdensuo 1996 {published data only}
- Lahdensuo A, Haahtela T, Herrala J, Kava T, Kiviranta K, Kuusisto P, et al. Randomised comparison of guided self management and traditional treatment of asthma over one year. BMJ 1996;312(7033):748‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legorreta 2000 {published data only}
- Legorreta AP, Leung K, Berkbigler D, Evans R, Liu X. Outcomes of a population‐based asthma management program: quality of life, absenteeism, and utilization. Annals of Allergy, Asthma and Immunology 2000;85(1):28‐34. [DOI] [PubMed] [Google Scholar]
Lemaigre 2010 {published data only}
- Lemaigre V, Bergh O, Victoir A, Peuter S, Verleden GM. Effects of a shortened asthma self‐management group program. Acta Clinica Belgica 2010;65(1):29‐36. [DOI] [PubMed] [Google Scholar]
Licskai 2012 {published data only}
- Licskai C, Sands T, Ong M, Paolatto L, Nicoletti I. Using a knowledge translation framework to implement asthma clinical practice guidelines in primary care. International Journal for Quality in Health Care 2012;24(5):538‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lind 2006 {published data only}
- Lind A, Kaplan L, Berg GD. Evaluation of an asthma disease management program in a Medicaid population. Disease Management & Health Outcomes 2006;14(3):151‐61. [Google Scholar]
Lindberg 1999 {published data only}
- Lindberg M, Ahlner J, Moller M, Ekstrom T. Asthma nurse practice‐‐a resource‐effective approach in asthma management. Respiratory Medicine 1999;93(8):584‐8. [DOI] [PubMed] [Google Scholar]
Lindberg 2002 {published data only}
- Lindberg M, Ahlner J, Ekstrom T, Jonsson D, Moller M. Asthma nurse practice improves outcomes and reduces costs in primary health care. Scandinavian Journal of Caring Sciences 2002;16(1):73‐8. [DOI] [PubMed] [Google Scholar]
Linden 2007 {published data only}
- Linden A, Berg GD, Wadhwa S. Evaluation of a medicaid asthma disease management program. Disease Management 2007;10(5):266‐72. [DOI] [PubMed] [Google Scholar]
Lo 2006 {published data only}
- Lo CT, Weng HC. Building explanatory model of asthma‐specific quality of life ‐ An analysis of patients enrolled in an asthma disease management program. Taiwan Journal of Public Health 2006;25(2):125‐34. [Google Scholar]
Ludwig‐Beymer 1998 {published data only}
- Ludwig‐Beymer P, Peterson S, Gorman C. Improving care for adults with asthma across the continuum. Journal of Nursing Care Quality 1998;12(6):48‐55. [DOI] [PubMed] [Google Scholar]
Magar 2005 {published data only}
- Magar Y, Vervloet D, Steenhouwer F, Smaga S, Mechin H, Rocca Serra JP, et al. Assessment of a therapeutic education programme for asthma patients: "un souffle nouveau". Patient Education & Counseling 2005;58(1):41‐6. [DOI] [PubMed] [Google Scholar]
Maljanian 1999 {published data only}
- Maljanian R, Wolf S, Goethe J, Hernandez P, Horowitz S. An inner‐city asthma disease management initiative: Results of an outcomes evaluation. Disease Management & Health Outcomes 1999;5(5):285‐93. [Google Scholar]
Mangiapane 2005 {published data only}
- Mangiapane S, Schulz M, Muhlig S, Ihle P, Schubert I, Waldmann HC. Community pharmacy‐based pharmaceutical care for asthma patients. Annals of Pharmacotherapy 2005;39(11):1817‐22. [DOI] [PubMed] [Google Scholar]
Mehuys 2008 {published data only}
- Mehuys E, Bortel L, Bolle L, Tongelen I, Annemans L, Remon JP, et al. Effectiveness of pharmacist intervention for asthma control improvement. The European Respiratory Journal 2008;31(4):790‐9. [DOI] [PubMed] [Google Scholar]
Mildenhall 1998 {published data only}
- Mildenhall S. A home‐based coping skills programme for high‐risk asthma sufferers. Asthma in General Practice 1998;6(2):23‐4. [Google Scholar]
Morisky 2009 {published data only}
- Morisky DE, Kominski GF, Afifi AA, Kotlerman JB, Morisky Donald E, Kominski Gerald F, et al. The effects of a disease management program on self‐reported health behaviors and health outcomes: Evidence from the "Florida: A Healthy State (FAHS)" Medicaid Program. Health Education & Behavior 2009;36(3):505‐17. [DOI] [PubMed] [Google Scholar]
Moudgil 2000 {published data only}
- Moudgil H, Marshall T, Honeybourne D. Asthma education and quality of life in the community: a randomised controlled study to evaluate the impact on white European and Indian subcontinent ethnic groups from socioeconomically deprived areas in Birmingham, UK [see comment]. Thorax 2000;55(3):177‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mu 2006 {published data only}
- Mu S, He QY, Yu B, Cui YL, Wang YZ. [The impact of an asthmatic patient education program on asthma control and quality of life]. [Chinese Journal of Tuberculosis and Respiratory Diseases] 2006;29(11):731‐4. [PUBMED: 17327051] [PubMed] [Google Scholar]
Mu 2008 {published data only}
- Mu S, He QY, Lin JT. [Impact of "three‐in‐one" asthma education and management model on asthma control in adult patients]. [Chinese Journal of Internal Medicine] 2008;47(8):630‐3. [PubMed] [Google Scholar]
Munroe 1997 {published data only}
- Munroe WP, Kunz K, Dalmady‐Israel C, Potter L, Schonfeld WH. Economic evaluation of pharmacist involvement in disease management in a community pharmacy setting. Clinical Therapeutics 1997;19(1):113‐23. [DOI] [PubMed] [Google Scholar]
Narhi 2001 {published data only}
- Narhi U, Airaksinen M, Tanskanen P, Enlund H. The effects of a pharmacy‐based intervention on the knowledge and attitudes of asthma patients. Patient Education and Counseling 2001;43(2):171‐7. [DOI] [PubMed] [Google Scholar]
Narhi 2002 {published data only}
- Narhi U, Airaksinen M, Enlund H. Pharmacists solving problems in asthma management ‐ Experiences from a one‐year intervention programme in Finland. International Journal of Pharmacy Practice 2002;10(1):55. [Google Scholar]
Park 2010 {published data only}
- Park J, Jackson J, Skinner E, Ranghell K, Saiers J, Cherney B. Impact of an adherence intervention program on medication adherence barriers, asthma control, and productivity daily activities in patients with asthma. Journal of Asthma 2010;47(10):1072‐7. [DOI] [PubMed] [Google Scholar]
Patel 2004 {published data only}
- Patel PH, Welsh C, Foggs MB. Improved asthma outcomes using a coordinated care approach in a large medical group [Review] [18 refs]. Disease Management 2004;7(2):102‐11. [DOI] [PubMed] [Google Scholar]
Pauley 1995 {published data only}
- Pauley TR, Magee MJ, Cury JD. Pharmacist‐managed, physician‐directed asthma management program reduces emergency department visits. Annals of Pharmacotherapy 1995;29(1):5. [DOI] [PubMed] [Google Scholar]
Peretz 2012 {published data only}
- Peretz P J, Matiz L A, Findley S, Lizardo M, Evans D, McCord M. Community health workers as drivers of a successful community‐based disease management initiative. American Journal of Public Health 2012;102(8):1443‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilotto 2004 {published data only}
- Pilotto LS, Smith BJ, Heard AR, McElroy HJ, Weekley J, Bennett P. Trial of nurse‐run asthma clinics based in general practice versus usual medical care. Respirology 2004;9(3):356‐62. [DOI] [PubMed] [Google Scholar]
Premaratne 1999 {published data only}
- Premaratne UN, Sterne JA, Marks GB, Webb JR, Azima H, Burney PG. Clustered randomised trial of an intervention to improve the management of asthma: Greenwich asthma study [see comment]. BMJ 1999;318(7193):1251‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossiter 2000 {published data only}
- Rossiter LF, Whitehurst‐Cook MY, Small RE, Shasky C, Bovbjerg VE, Penberthy L, et al. The impact of disease management on outcomes and cost of care: a study of low‐income asthma patients. Inquiry 2000;37(2):188‐202. [PubMed] [Google Scholar]
Saini 2004 {published data only}
- Saini B, Krass I, Armour C. Development, implementation, and evaluation of a community pharmacy‐based asthma care model. Annals of Pharmacotherapy 2004;38(11):1954‐60. [DOI] [PubMed] [Google Scholar]
Saini 2008 {published data only}
- Saini B, Filipovska J, Bosnic‐Anticevich S, Taylor S, Krass I, Armour C. An evaluation of a community pharmacy‐based rural asthma management service. Australian Journal of Rural Health 2008;16(2):100‐8. [DOI] [PubMed] [Google Scholar]
Saini 2011 {published data only}
- Saini B, LeMay K, Emmerton L, Krass I, Smith L, Bosnic‐Anticevich S, et al. Asthma disease management‐Australian pharmacists' interventions improve patients' asthma knowledge and this is sustained. Patient Education and Counseling 2011;83(3):295‐302. [PUBMED: 21621947] [DOI] [PubMed] [Google Scholar]
Schonlau 2005 {published data only}
- Schonlau M, Mangione‐Smith R, Chan KS, Keesey J, Rosen M, Louis TA, et al. Evaluation of a quality improvement collaborative in asthma care: does it improve processes and outcomes of care? [see comment]. Annals of Family Medicine 2005;3(3):200‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schott‐Baer 1999 {published data only}
- Schott‐Baer D, Christensen M. Research for practice. A pilot program to increase self‐care of adult asthma patients. MEDSURG Nursing 1999;8(3):178‐83. [PubMed] [Google Scholar]
Schulz 2001 {published data only}
- Schulz M, Verheyen F, Muhlig S, Muller JM, Muhlbauer K, Knop‐Schneickert E, et al. Pharmaceutical care services for asthma patients: a controlled intervention study. Journal of Clinical Pharmacology 2001;41(6):668‐76. [DOI] [PubMed] [Google Scholar]
Scott 2009 {published data only}
- Scott A. Impact of disease management program interventions for Medicaid patients with asthma. Dissertation. Arizona State University, 2009. [Google Scholar]
Shelledy 2009 {published data only}
- Shelledy DC, Legrand TS, Gardner DD, Peters JI, Shelledy David C, Legrand Terry S, et al. A randomized, controlled study to evaluate the role of an in‐home asthma disease management program provided by respiratory therapists in improving outcomes and reducing the cost of care. Journal of Asthma 2009;46(2):194‐201. [DOI] [PubMed] [Google Scholar]
Smith 2007 {published data only}
- Smith L, Bosnic‐Anticevich SZ, Mitchell B, Saini B, Krass I, Armour C. Treating asthma with a self‐management model of illness behaviour in an Australian community pharmacy setting. Social Science & Medicine 2007;64(7):1501‐11. [DOI] [PubMed] [Google Scholar]
Sommaruga 1995 {published data only}
- Sommaruga M, Spanevello A, Migliori GB, Neri M, Callegari S, Majani G. The effects of a cognitive behavioural intervention in asthmatic patients. Monaldi Archives for Chest Disease 1995;50(5):398‐402. [PubMed] [Google Scholar]
Souza‐Machado 2010a {published data only}
- Souza‐Machado C, Souza‐Machado A, Franco R, Ponte EV, Barreto ML, Rodrigues LC, et al. Rapid reduction in hospitalisations after an intervention to manage severe asthma. The European Respiratory Journal 2010;35(3):515‐21. [PUBMED: 19643941] [DOI] [PubMed] [Google Scholar]
Steuten 2006 {published data only}
- Steuten L, Vrijhoef B, Merode F, Wesseling GJ, Spreeuwenberg C. Evaluation of a regional disease management programme for patients with asthma or chronic obstructive pulmonary disease. International Journal for Quality in Health Care 2006;18(6):429‐36. [DOI] [PubMed] [Google Scholar]
Swanson 2000 {published data only}
- Swanson V, Wright S, Power KG, Duncan B, Morgan J, Turner E, et al. The impact of a structured programme of asthma care in general practice. International Journal of Clinical Practice 2000;54(9):573‐80. [PubMed] [Google Scholar]
Tatis 2005 {published data only}
- Tatis V, Remache D, DiMango E. Results of a culturally directed asthma intervention program in an inner‐city Latino community. Chest 2005;128(3):1163‐7. [DOI] [PubMed] [Google Scholar]
Thoonen 2003 {published data only}
- Thoonen BP, Schermer TR, Boom G, Molema J, Folgering H, Akkermans RP, et al. Self‐management of asthma in general practice, asthma control and quality of life: a randomised controlled trial [see comment]. Thorax 2003;58(1):30‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tinkelman 2004 {published data only}
- Tinkelman D, Wilson S. Asthma disease management: regression to the mean or better? [see comment]. American Journal of Managed Care 2004;10(12):948‐54. [PubMed] [Google Scholar]
To 2008 {published data only}
- To T, Cicutto L, Degani N, McLimont S, Beyene J. Can a community evidence‐based asthma care program improve clinical outcomes? A longitudinal study. Medical Care 2008;46(12):1257‐66. [DOI] [PubMed] [Google Scholar]
Treadwell 2009 {published data only}
- Treadwell J, Bean G, Warner W. Supporting disease management through intervention in the medical home. Professional Case Management 2009;14(4):192‐7. [PUBMED: 19625938] [DOI] [PubMed] [Google Scholar]
Tschopp 2002 {published data only}
- Tschopp J M, Frey J G, Pernet R, Burrus C, Jordan B, Morin A, et al. Bronchial asthma and self‐management education: implementation of guidelines by an interdisciplinary programme in health network. Swiss Medical Weekly 2002;132(7‐8):92‐7. [DOI] [PubMed] [Google Scholar]
Tschopp 2005 {published data only}
- Tschopp JM, Frey JG, Janssens JP, Burrus C, Garrone S, Pernet R, et al. Asthma outpatient education by multiple implementation strategy. Outcome of a programme using a personal notebook. Respiratory Medicine 2005;99(3):355‐62. [DOI] [PubMed] [Google Scholar]
Van Damme 1994 {published data only}
- Damme R, Drummond N, Beattie J, Douglas G. Integrated care for patients with asthma: Views of general practitioners. British Journal of General Practice 1994;44(378):9‐13. [PMC free article] [PubMed] [Google Scholar]
van der Meer 2009 {published data only}
- Meer V, Bakker MJ, Hout WB, Rabe KF, Sterk PJ, Kievit J, et al. Internet‐based self‐management plus education compared with usual care in asthma: a randomized trial. Annals of Internal Medicine 2009;151(2):110‐20. [DOI] [PubMed] [Google Scholar]
van der Palen 2001 {published data only}
- Palen J, Klein JJ, Zielhuis GA, Herwaarden CL, Seydel ER. Behavioural effect of self‐treatment guidelines in a self‐management program for adults with asthma. Patient Education and Counseling 2001;43(2):161‐9. [PUBMED: 11369149] [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang W, Huang K, Liu Q, Zhu Y, Wang C. Evaluating the effectiveness of systematic asthma patient education programs and self‐management. Respirology Conference: 16th Congress of the Asian Pacific Society of Respirology Shanghai China.Conference Start: 20111103 Conference End: 20111106. Conference Publication. November 2011; Vol. 16:200.
Weinberger 2002 {published data only}
- Weinberger M, Murray MD, Marrero DG, Brewer N, Lykens M, Harris LE, et al. Effectiveness of pharmacist care for patients with reactive airways disease: a randomized controlled trial [see comment]. JAMA 2002;288(13):1594‐602. [DOI] [PubMed] [Google Scholar]
Williams 2007 {published data only}
- Williams A, Harris M, Daffurn K, Davies G P, Pascoe S, Zwar N. Sustaining chronic disease management in primary care: Lessons from a demonstration project. Australian Journal of Primary Health 2007;2:121‐8. [Google Scholar]
Yang 2010 {published data only}
- Yang W, Dall TM, Zhang Y, Hogan PF, Arday DR, Gantt CJ. Disease management 360 degrees: a scorecard approach to evaluating TRICARE's programs for asthma, congestive heart failure, and diabetes. Medical Care 2010;48(8):683‐93. [PUBMED: 20613658] [DOI] [PubMed] [Google Scholar]
Yawn 2008 {published data only}
- Yawn B P, Bertram S, Wollan P. Introduction of asthma APGAR tools improve asthma management in primary care practices. Journal of Asthma and Allergy 2008;1:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Ahmed 2011 {published data only}
- Ahmed S, Bartlett S J, Ernst P, Lin C J, Pare G, Perreault R, et al. My asthma portal: Preliminary results of a web‐based self management intervention. American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS 2011 Denver, CO United States.Conference Start: 20110513 Conference End: 20110518. 2011; Vol. 183.
- Ahmed S, Bartlett SJ, Ernst P, Pare G, Kanter M, Perreault R, et al. Effect of a web‐based chronic disease management system on asthma control and health‐related quality of life: study protocol for a randomized controlled trial. Trials 2011;12:260. [PUBMED: 22168530] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arguel 2013 {published data only}
- Arguel A, Lau AY, Dennis S, Liaw ST, Coiera E. An internet intervention to improve asthma management: rationale and protocol of a randomized controlled trial. JMIR Research Protocols 2013;2(2):e28. [PUBMED: 23942523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adams 2007
- Adams SG, Smith PK, Allan PF, Anzueto A, Pugh JA, Cornell JE. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Archives of Internal Medicine 2007;167(6):551‐61. [PUBMED: 17389286] [DOI] [PubMed] [Google Scholar]
Asher 2006
- Asher MI, Montefort S, Björkstén B, Lai CKW, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross‐sectional surveys. Lancet 2006;368(9537):733‐43. [DOI] [PubMed] [Google Scholar]
Badamgarav 2003
- Badamgarav E, Weingarten SR, Henning JM, Knight K, Hasselblad V, Gano A, et al. Effectiveness of disease management programs in depression: a systematic review. The American Journal of Psychiatry 2003;160(12):2080‐90. [DOI] [PubMed] [Google Scholar]
Bateman 2008
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. The European Respiratory Journal 2008;31(1):143‐78. [DOI] [PubMed] [Google Scholar]
Blaiss 2005
- Blaiss Michael S. Asthma disease management: a critical analysis. Annals of Allergy, Asthma & Immunology 2005;95(5 Suppl 1):S10‐6. [DOI] [PubMed] [Google Scholar]
Braman 2006
- Braman SS. The global burden of asthma. Chest 2006;130(1 Suppl):4S‐12S. [DOI] [PubMed] [Google Scholar]
Busse 2004
- Busse R. Disease management programs In Germany's statutory health insurance system. Health Affairs 2004;23(3):56‐67. [DOI] [PubMed] [Google Scholar]
Clark 1994
- Clark NM, Starr‐Schneidkraut NJ. Management of asthma by patients and families. American Journal of Respiratory and Critical Care Medicine 1994;149(2 Pt 2):S54‐66; discussion S67‐8. [DOI] [PubMed] [Google Scholar]
Davidoff 2009
- Davidoff F, Batalden P, Stevens D, Ogrinc G, Mooney SE. Publication guidelines for quality improvement studies in health care: evolution of the SQUIRE project. BMJ (Clinical research ed.) 2009;338:a3152. [PUBMED: 19153129] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 1995
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 1995;274(9):700‐5. [DOI] [PubMed] [Google Scholar]
Davis 1999
- Davis D, O'Brien MA, Freemantle N, Wolf FM, Mazmanian P, Taylor‐Vaisey A. Impact of formal continuing medical education: do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?. JAMA 1999;282(9):867‐74. [DOI] [PubMed] [Google Scholar]
de Bruin 2011
- Bruin SR, Heijink R, Lemmens LC, Struijs JN, Baan CA. Impact of disease management programs on healthcare expenditures for patients with diabetes, depression, heart failure or chronic obstructive pulmonary disease: a systematic review of the literature. Health Policy 2011;101(2):105‐21. [PUBMED: 21592607] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
DMAA Definitions 2009
- Definition of disease management. Disease Management Association of America. Available at http://www.dmaa.org/phi_definition.asp. Accessed on 20 December 2009.
Durbin 1997
- Durbin CG Jr. The role of the respiratory care practitioner in the continuum of disease management. Respiratory Care 1997;42:159‐68. [Google Scholar]
Eder 2006
- Eder W, Ege MJ, Mutius E. The asthma epidemic. The New England Journal of Medicine 2006;355(21):2226‐35. [PUBMED: 17124020] [DOI] [PubMed] [Google Scholar]
Egginton 2012
- Egginton JS, Ridgeway JL, Shah ND, Balasubramaniam S, Emmanuel JR, Prokop LJ, et al. Care management for Type 2 diabetes in the United States: a systematic review and meta‐analysis. BMC Health Services Research 2012;12:72. [PUBMED: 22439920] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elissen 2013
- Elissen AM, Steuten LM, Lemmens LC, Drewes HW, Lemmens KM, Meeuwissen JA, et al. Meta‐analysis of the effectiveness of chronic care management for diabetes: investigating heterogeneity in outcomes. Journal of Evaluation in Clinical Practice 2013;19(5):753‐62. [PUBMED: 22372830] [DOI] [PubMed] [Google Scholar]
Ellrodt 1997
- Ellrodt G, Cook DJ, Lee J, Cho M, Hunt D, Weingarten S. Evidence‐based disease management. JAMA 1997;278(20):1687‐92. [PubMed] [Google Scholar]
EPOC 2013
- Effective Practice, Organisation of Care (EPOC). What study designs should be included in an EPOC review and what should they be called? EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services 2013. Available at: http://epocoslo.cochrane.org/epoc‐specific‐resources‐review‐authors. Accessed on 18 May 2014.
EPOC 2013a
- Effective Practice, Organisation of Care (EPOC). Data extraction and management. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services 2013. Available at: http://epocoslo.cochrane.org/epoc‐specific‐resources‐review‐authors.
EPOC 2013b
- Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Oslo Norwegian Knowledge Centre for the Health Services 2013. Available at: epocoslo.cochrane.org/epoc‐specific‐resources‐review‐authors.
Epstein 1996
- Epstein RS, Sherwood LM. From outcomes research to disease management: a guide for the perplexed. Annals of Internal Medicine 1996;124(9):832‐7. [DOI] [PubMed] [Google Scholar]
EuroQol Group 1990
- The EuroQol Group. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16(3):199‐208. [DOI] [PubMed] [Google Scholar]
Faxon 2004
- Faxon DP, Schwamm LH, Pasternak RC, Peterson ED, McNeil BJ, Bufalino Vincent, et al. Improving quality of care through disease management: principles and recommendations from the American Heart Association's Expert Panel on Disease Management. Circulation 2004;109(21):2651‐4. [DOI] [PubMed] [Google Scholar]
Gagnier 2012
- Gagnier JJ, Moher D, Boon H, Beyene J, Bombardier C. Investigating clinical heterogeneity in systematic reviews: a methodologic review of guidance in the literature. BMC Medical Research Methodology 2012;12:111. [PUBMED: 22846171] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gagnier 2013
- Gagnier JJ, Morgenstern H, Altman DG, Berlin J, Chang S, McCulloch P, et al. Consensus‐based recommendations for investigating clinical heterogeneity in systematic reviews. BMC Medical Research Methodology 2013;13:106. [PUBMED: 24004523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2002
- Gibson PG, Powell H, Coughlan J, Wilson A, Hensley MJ, Abramson MJ, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD001005] [DOI] [PubMed] [Google Scholar]
Gibson 2003
- Gibson PG, Powell H, Coughlan J, Wilson A, Abramson MJ, Haywood P, et al. Self‐management education and regular practitioner review for adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001117] [DOI] [PubMed] [Google Scholar]
GINA 2012
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention: 2012 Update. http://www.ginasthma.org/ 2012.
Gogovor 2008
- Gogovor A, Savoie M, Moride Y, Krelenbaum M, Montague T. Contemporary disease management in Quebec. Healthcare Quarterly 2008;11(1):30‐7. [DOI] [PubMed] [Google Scholar]
Gohler 2006
- Gohler A, Januzzi JL, Worrell SS, Osterziel KJ, Gazelle GS, Dietz R, et al. A systematic meta‐analysis of the efficacy and heterogeneity of disease management programs in congestive heart failure. Journal of Cardiac Failure 2006;12(7):554‐67. [PUBMED: 16952790] [DOI] [PubMed] [Google Scholar]
Gonseth 2004
- Gonseth J, Guallar‐Castillón P, Banegas JR, Rodríguez‐Artalejo F. The effectiveness of disease management programmes in reducing hospital re‐admission in older patients with heart failure: a systematic review and meta‐analysis of published reports. European Heart Journal 2004;25(18):1570‐95. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunter 1997
- Hunter DJ, Fairfield G. Disease management. BMJ (Clinical research ed.) 1997;315(7099):50‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyland 1991
- Hyland ME, Finnis S, Irvine SH. A scale for assessing quality of life in adult asthma sufferers. Journal of Psychosomatic Research 1991;35(1):99‐110. [DOI] [PubMed] [Google Scholar]
Jones 1991
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respiratory Medicine 1991;85 Suppl B:25‐31; discussion 33‐7. [DOI] [PubMed] [Google Scholar]
Juniper 1992
- Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax 1992;47(2):76‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kesteloot 1999
- Kesteloot K. Disease management. A new technology in need of critical assessment. International Journal of Technology Assessment in Health Care 1999;15(3):506‐19. [PubMed] [Google Scholar]
Klomp 2008
- Klomp H, Lawson JA, Cockcroft DW, Chan BT, Cascagnette P, Gander L, et al. Examining asthma quality of care using a population‐based approach. CMAJ: Canadian Medical Association Journal = Journal De l'Association Medicale Canadienne 2008;178(8):1013‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 2005
- Knight K, Badamgarav E, Henning JM, Hasselblad V, Gano AD, Ofman JJ, et al. A systematic review of diabetes disease management programs. The American Journal of Managed Care 2005;11(4):242‐50. [PubMed] [Google Scholar]
Kruis 2013
- Kruis AL, Smidt N, Assendelft WJ, Gussekloo J, Boland MR, Rutten‐van Molken M, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. The Cochrane Database of Systematic Reviews 2013;10:CD009437. [PUBMED: 24108523] [DOI] [PubMed] [Google Scholar]
Krumholz 2006
- Krumholz HM, Currie PM, Riegel B, Phillips CO, Peterson ED, Smith R, et al. A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation 2006;114(13):1432‐45. [DOI] [PubMed] [Google Scholar]
Laird 1990
- Laird NM, Mosteller F. Some statistical methods for combining experimental results. International Journal of Technology Assessment in Health Care 1990;6(1):5‐30. [DOI] [PubMed] [Google Scholar]
Latry 2008
- Latry P, Pinet M, Labat A, Magand JP, Peter C, Robinson P, et al. Adherence to anti‐inflammatory treatment for asthma in clinical practice in France. Clinical Therapeutics 2008;30(6):1058‐68. [DOI] [PubMed] [Google Scholar]
Lemmens 2009
- Lemmens KM, Nieboer AP, Huijsman R. A systematic review of integrated use of disease‐management interventions in asthma and COPD. Respiratory Medicine 2009;103(5):670‐91. [PUBMED: 19155168] [DOI] [PubMed] [Google Scholar]
Lemmens 2013
- Lemmens KM, Lemmens LC, Boom JH, Drewes HW, Meeuwissen JA, Steuten LM, et al. Chronic care management for patients with COPD: a critical review of available evidence. Journal of Evaluation in Clinical Practice 2013;19(5):734‐52. [PUBMED: 22133473] [DOI] [PubMed] [Google Scholar]
Leuppi 2006
- Leuppi JD, Steurer‐Stey C, Peter M, Chhajed PN, Wildhaber JH, Spertini F. Asthma control in Switzerland: a general practitioner based survey. Current Medical Research and Opinion 2006;22(11):2159‐66. [DOI] [PubMed] [Google Scholar]
Maciejewski 2009
- Maciejewski ML, Chen SY, Au DH. Adult asthma disease management: an analysis of studies, approaches, outcomes, and methods. Respiratory Care 2009;54(7):878‐86. [PUBMED: 19558739] [DOI] [PubMed] [Google Scholar]
Masoli 2004
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59(5):469‐78. [DOI] [PubMed] [Google Scholar]
Mathers 2006
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine 2006;3(11):e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
McAlister 2001
- McAlister FA, Lawson FM, Teo KK, Armstrong PW. A systematic review of randomized trials of disease management programs in heart failure. The American Journal of Medicine 2001;110(5):378‐84. [DOI] [PubMed] [Google Scholar]
Mechanic 2002
- Mechanic RE. Disease Management: A Promising Approach for Health Care Purchasers. Washington, DC: National Health Care Purchasing Institute 2002. Available at http://www.futurevision.com.ua/nhcpi/files/3df220809e247.pdf. Accessed on 10 May 2009.
Montague 2007
- Montague TJ, Gogovor A, Krelenbaum M. Time for chronic disease care and management. The Canadian Journal of Cardiology 2007;23(12):971‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mäkinen 1999
- Mäkinen S, Suominen T, Lauri S. What asthma patients know about their illness and its treatment. Clinical Nurse Specialist 1999;13(6):277‐82. [DOI] [PubMed] [Google Scholar]
NAEPP 2007
- [no author]. National Asthma Education and Prevention Program Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma‐Summary Report. Journal of Allergy and Clinical Immunology 2007;120(5 Suppl):S94‐S138. [DOI] [PubMed] [Google Scholar]
Nathan 2004
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. The Journal of Allergy and Clinical Immunology 2004;113(1):59‐65. [DOI] [PubMed] [Google Scholar]
NCSL DMP descriptions
- State Disease Management Program Descriptions. National Conference of State Legislature. Available at http://www.ncsl.org/programs/health/StateDiseasemgmt1.htm.
Neumeyer‐Gromen 2004
- Neumeyer‐Gromen A, Lampert T, Stark K, Kallischnigg G. Disease management programs for depression: a systematic review and meta‐analysis of randomized controlled trials. Medical Care 2004;42(12):1211‐21. [DOI] [PubMed] [Google Scholar]
Niesink 2007
- Niesink A, Trappenburg JCA, Weert‐van OGH, Lammers JWJ, Verheij TJM, Schrijvers AJP. Systematic review of the effects of chronic disease management on quality‐of‐life in people with chronic obstructive pulmonary disease. Respiratory Medicine 2007;101(11):2233‐9. [DOI] [PubMed] [Google Scholar]
Norris 2002
- Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, et al. The effectiveness of disease and case management for people with diabetes. A systematic review. American Journal of Preventive Medicine 2002;22(4 Suppl):15‐38. [DOI] [PubMed] [Google Scholar]
Ofman 2004
- Ofman JJ, Badamgarav E, Henning JM, Knight K, Gano AD, Levan RK, et al. Does disease management improve clinical and economic outcomes in patients with chronic diseases? A systematic review. The American Journal of Medicine 2004;117(3):182‐92. [DOI] [PubMed] [Google Scholar]
Ouwens 2005
- Ouwens M, Wollersheim H, Hermens R, Hulscher M, Grol R. Integrated care programmes for chronically ill patients: a review of systematic reviews. International Journal for Quality in Health Care 2005;17(2):141‐6. [DOI] [PubMed] [Google Scholar]
Pacheco 1999
- Pacheco Y, Zureik M, Dussopt C, Thiriet C. [Patient knowledge of asthma: results of a national survey in pneumology]. Revue De Pneumologie Clinique 1999;55(6):353‐63. [PubMed] [Google Scholar]
Peytremann‐Bridevaux 2008
- Peytremann‐Bridevaux I, Staeger P, Bridevaux PO, Ghali WA, Burnand B. Effectiveness of chronic obstructive pulmonary disease‐management programs: systematic review and meta‐analysis. The American Journal of Medicine 2008;121(5):433‐43.e4. [DOI] [PubMed] [Google Scholar]
Pigott 2013
- Pigott T, Shepperd S. Identifying, documenting, and examining heterogeneity in systematic reviews of complex interventions. Journal of Clinical Epidemiology 2013;66(11):1244‐50. [PUBMED: 23953079] [DOI] [PubMed] [Google Scholar]
Pilnick 2001
- Pilnick A, Dingwall R, Starkey K. Disease management: definitions, difficulties and future directions. Bulletin of the World Health Organization 2001;79(8):755‐63. [PMC free article] [PubMed] [Google Scholar]
Pimouguet 2011
- Pimouguet C, Goff M, Thiebaut R, Dartigues JF, Helmer C. Effectiveness of disease‐management programs for improving diabetes care: a meta‐analysis. CMAJ: Canadian Medical Association Journal = Journal de l'Association Medicale Canadienne 2011;183(2):E115‐27. [PUBMED: 21149524] [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell 2003
- Powell H, Gibson PG. Options for self‐management education for adults with asthma. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roccaforte 2005
- Roccaforte R, Demers C, Baldassarre F, Teo KK, Yusuf S. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta‐analysis. European Journal of Heart Failure 2005;7(7):1133‐44. [DOI] [PubMed] [Google Scholar]
Schrijvers 2009
- Schrijvers G. Disease management: a proposal for a new definition. International Journal of Integrated Care 2009;9:e06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steuten 2007
- Steuten L, Palmer S, Vrijhoef B, Merode F, Spreeuwenberg C, Severens H. Cost‐utility of a disease management program for patients with asthma. International Journal of Technology Assessment in Health Care 2007;23(2):184‐91. [DOI] [PubMed] [Google Scholar]
Steuten 2007a
- Steuten L, Lemmens K, Vrijhoef B. Health technology assessment of asthma disease management programs. Current Opinion in Allergy and Clinical Immunology 2007;7(3):242‐8. [DOI] [PubMed] [Google Scholar]
Stock 2006
- Stock SAK, Redaelli M, Lauterbach KW. Population‐based disease management in the German statutory health insurance: implementation and preliminary results. Disease Management and Health Outcomes 2006;14(1):5‐12. [Google Scholar]
Sudre 1999
- Sudre P, Jacquemet S, Uldry C, Perneger TV. Objectives, methods and content of patient education programmes for adults with asthma: systematic review of studies published between 1979 and 1998. Thorax 1999;54(8):681‐7. [PUBMED: 10413719] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toelle 2004
- Toelle BG, Ram FSF. Written individualised management plans for asthma in children and adults. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002171] [DOI] [PubMed] [Google Scholar]
Tsai 2005
- Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta‐analysis of interventions to improve care for chronic illnesses. The American Journal of Managed Care 2005;11(8):478‐88. [PUBMED: 16095434] [PMC free article] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PubMed] [Google Scholar]
Velasco‐Garrido 2003
- Velasco‐Garrido M, Busse R, Hisashige A. Are disease management programmes (DMPs) effective in improving quality of care for people with chronic conditions?. Copenhagen, WHO Regional Office for Europe (Health Evidence Network report) Available at http://www.euro.who.int/document/e82974.pdf. Accessed 10 May 2009 2003.
Vermeire 2002
- Vermeire PA, Rabe KF, Soriano JB, Maier WC. Asthma control and differences in management practices across seven European countries. Respiratory Medicine 2002;96(3):142‐9. [DOI] [PubMed] [Google Scholar]
Vollmer 1999
- Vollmer WM, Markson LE, O'Connor E, Sanocki LL, Fitterman L, Berger M, et al. Association of asthma control with health care utilization and quality of life. American Journal of Respiratory and Critical Care Medicine 1999;160(5 Pt 1):1647‐52. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Ware 1996
- Ware J, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34(3):220‐33. [DOI] [PubMed] [Google Scholar]
Weingarten 2002
- Weingarten SR, Henning JM, Badamgarav E, Knight K, Hasselblad V, Gano A, et al. Interventions used in disease management programmes for patients with chronic illness‐‐‐which ones work? Meta‐analysis of published reports. BMJ 2002;325(7370):925. [DOI] [PMC free article] [PubMed] [Google Scholar]


