Abstract
Background:
Management of orthostatic hypotension (OH) prioritizes prevention of standing hypotension, sometimes at the expense of supine hypertension. It is unclear whether supine hypertension is associated with adverse outcomes relative to standing hypotension.
Objectives:
To compare the long-term clinical consequences of supine hypertension and standing hypotension among middle-aged adults with and without OH.
Methods:
The ARIC study (Atherosclerosis Risk in Communities) measured supine and standing blood pressure (BP) in adults aged 45 to 64 years, without neurogenic OH, between 1987 and 1989. We defined OH as a positional drop in systolic BP ≥20 mm Hg or diastolic BP ≥10 mm Hg, supine hypertension as supine BP≥140/≥90 mm Hg, and standing hypotension as standing BP≤105/≤65 mm Hg. Participants were followed for >30 years. We used Cox regression models to examine associations with cardiovascular disease events, all-cause mortality, falls, and syncope.
Results:
Of 12 489 participants (55% female, 26% Black, mean age 54 years, SD 6), 4.4% had OH. Among those without OH (N=11 943), 19% had supine hypertension and 21% had standing hypotension, while among those with OH (N=546), 58% had supine hypertension and 38% had standing hypotension. Associations with outcomes did not differ by OH status (P-interactions >0.25). Supine hypertension was associated with heart failure (hazard ratio, 1.83 [95% CI, 1.68–1.99]), falls (hazard ratio, 1.12 [95% CI, 1.02–1.22]), and all-cause mortality (hazard ratio, 1.45 [95% CI, 1.37–1.54]), while standing hypotension was only significantly associated with mortality (hazard ratio, 1.06 [95% CI, 1.00–1.14]).
Conclusions:
Supine hypertension was associated with higher risk of adverse events than standing hypotension, regardless of OH status. This challenges conventional OH management, which prioritizes standing hypotension over supine hypertension.
Keywords: atherosclerosis, cardiovascular diseases, hypertension, orthostatic hypotension, syncope
Orthostatic hypotension (OH), a drop from supine-to-standing systolic blood pressure (SBP) of 20 mm Hg or diastolic blood pressure (DBP) of 10 mm Hg,1,2 is a predictor of cardiovascular disease (CVD) events, stroke, falls, syncope, and premature death.1,3 While the exact pathophysiology is unknown, it is presumed that the drop in blood pressure (BP) results in organ hypoperfusion and progressive injury. As a result, clinical management of OH focuses on preventing standing hypotension through increased consumption of salt and fluids, de-escalation of antihypertensive therapies, or the introduction of mineralocorticoids or vasopressors.4 However, because OH is derived from both supine and standing BP measurements, it is conceivable that either an elevated supine BP (ie, supine hypertension) or a low standing BP (ie, standing hypotension) may drive the association of OH with adverse events. This has implications for the clinical management of OH, as supine hypertension would ideally be addressed by intensifying BP lowering treatment, which contrasts with traditional approaches to OH that increase BP. Adding to the uncertainty about optimal OH management, a recent meta-analysis demonstrated that more aggressive BP reduction lowered the incidence of OH in 9 trials of hypertension treatment.5
The ARIC study (Atherosclerosis Risk in Communities) has followed community-dwelling, middle-aged adults for over 30 years for the development of CVD events. While OH was a predictor of CVD (specifically coronary heart disease [CHD], stroke, and heart failure [HF]), falls, and syncope independent of other related risk factors in prior studies of ARIC,6–8 the associations of specific phenotypes of OH (ie, supine hypertension or standing hypotension) with adverse events have not been reported.
Our objectives in this study were to determine the association between supine hypertension and standing hypotension with CVD and adverse events according to OH status. We hypothesized that regardless of OH status (1) supine hypertension would be associated with CVD events due to high BP, while (2) standing hypotension would be associated with falls and syncope (due to cerebral hypoperfusion) and (3) both would be associated with all-cause mortality.
Methods
Data Availability
The data that support the findings of this study are available from the ARIC coordinating center with an approved proposal.
Study Population
The ARIC study is a prospective cohort of 15 792 adults. Participants aged 45 to 64 years were enrolled between 1987 and 1989 (visit 1) from 4 US communities (Forsyth County, North Carolina, Jackson, Mississippi, suburbs of Minneapolis, Minnesota, and Washington County, Maryland) and then followed to the present day. The ARIC protocol involved physical examinations, medical interviews, laboratory tests, and orthostatic BP measurements.9–11 Standing and supine BP measurements were introduced as an ancillary to the cohort and were measured among 13 157 participants. Our analytic sample was restricted to participants who attended baseline (visit 1) and were not missing covariates of interest (N=668), resulting in an analytic study population of 12 489 participants.
All participants provided written informed consent. The study protocol was approved by Institutional Review Boards at all study sites. The Beth Israel Deaconess Medical Center Institutional Review Board designated the present project as human subjects exempt research.
Exposures: Supine Hypertension, Standing Hypotension, and OH
During the baseline visit, BP was measured with an automatic office BP cuff (Dinamap 1846 SX oscillometric device) in the supine position after 20 minutes of rest approximately every 20 to 30 seconds over 2 minutes for up to 5 measurements.12,13 Then participants were asked to stand and immediately after standing, measurements were repeated approximately every 20 to 30 seconds over 2 minutes for up to 5 measurements. Supine hypertension was defined as mean supine SBP ≥140 or DBP ≥90 mm Hg. Standing hypotension was defined as a mean standing SBP ≤105 or DBP ≤65 mm Hg. OH was defined using the consensus definition thresholds as a decrease in either SBP or DBP of at least 20 or 10 mm Hg, respectively.1,2 OH phenotypes were based on the presence or absence of the following variables: OH, supine hypertension, or standing hypotension, resulting in 8 distinct phenotypes. Note that while rare, it was possible to not have OH and have both supine hypertension and standing hypotension, based on a qualifying SBP or DBP for either condition.
Outcomes: CVD and Mortality
Participants were followed up to December 31, 2019 for CVD events and mortality (follow-up was not available for the Jackson site after December 31, 2017). These events were ascertained through active surveillance of surrounding hospital records, regular (yearly before 2012; twice-yearly thereafter) telephone calls with participants, and linkage with state and national death indexes. Further details regarding ascertainment and adjudication of events have been described elsewhere.11 Incident myocardial infarction was defined as probable or definite myocardial infarction based on adjudicator review. Incident HF was defined by first hospitalization or death related to HF using an International Classification of Diseases, Ninth Revision (ICD-9) code of 428.x or International Classification of Diseases, Tenth Revision (ICD-10) code I50 in any position on the hospital discharge list or on a death certificate.14 Incident stroke events (including ischemic and hemorrhagic stroke) were ascertained through active surveillance of hospitalizations, cohort follow-up, and linkage with death registries. Both definite and probable stroke events were included and all events were adjudicated by committee review. Fatal CHD was defined by death related to CHD. CHD was based on a composite definition of probable or definite myocardial infarction, fatal CHD, cardiac procedure, or silent myocardial infarction based on ECG. An expert panel reviewed and adjudicated hospital records related to possible CVD (see Supplemental Methods for relevant ICD codes).
Outcomes: Falls and Syncope
Falls and syncope were defined at the first occurrence of any related hospitalization or claim for inpatient or outpatient services after baseline visit. Outcomes were ascertained through active hospital surveillance and linkage to Centers for Medicare and Medicaid Services claims data from 1985 to 2018 using ICD-9 and ICD-10 codes (see Supplemental Methods).15 Note that for many participants, linkage with Centers for Medicare and Medicaid Services would have occurred after age 65 years. Participants lost to follow-up were administratively censored.
Covariates of Interest
Participants reported their age, sex, and race. Race was combined with research center (White, Washington County, Maryland; Black, Jackson, Mississippi; White, Minneapolis, Minnesota; Black, Forsyth, North Carolina; White, Forsyth, North Carolina). High-density lipoprotein and total cholesterol were measured in serum using traditional assays. Heart rate was extracted from electrocardiograms. Body mass index was derived from height and weight measurements. Seated BP was measured using a random zero sphygmomanometer and based on the average of the second 2 measurements. Estimated glomerular filtration rate was determined using the 2021 Chronic Kidney Disease Epidemiology Collaboration race-free creatinine definition.16 Prevalent CHD, prevalent HF, and prevalent stroke were based on self-report. Diabetes was defined by random serum glucose ≥200 mg/dL or fasting ≥126 mg/dL. Hypertension was defined based on a mean seated SBP ≥140 or DBP ≥90 or self-reported antihypertension medication use in the past 2 weeks. Antihypertension medication use in the past 2 weeks was also based on the review of active medications. Participants reported alcohol use (never, former, current), education level (less than high school, high school or vocational school, at least some college or professional school), physical activity (as defined by Baecke physical activity questionnaire),17 smoking status (never, former, current), and use of antidepressant, sedative, hypnotic, antipsychotic, anticholinergic agents and anti-parkinsonian medications (these included anticholinergic and dopaminergic agents, monoamine oxidase inhibitors, and carbidopa-levodopa.) Additional details related to the definitions of these covariates are located in the Covariates section of the Supplemental Methods.
Statistical Analysis
Baseline characteristics were compared using means and proportions. The association of supine hypertension or standing hypotension with events was determined using Cox proportional hazards models that included both supine hypertension and standing hypotension. We compared supine hypertension and standing hypotension coefficients, using Wald tests (via the post-estimation test command). These models were implemented overall and by OH strata. Outcomes included CHD, stroke, HF, falls, syncope, and all-cause mortality. OH-by-supine hypertension and OH-by-standing hypotension interaction terms were used to determine whether associations differed by OH status. We used log-log plots to assess the Cox proportionality assumption.
Models were unadjusted, minimally adjusted (age/sex/race-center adjusted), and fully adjusted for age, sex, race-center, high-density lipoprotein cholesterol, total cholesterol, heart rate, body mass index, estimated glomerular filtration rate, prevalent CHD, prevalent HF and prevalent stroke, diabetes, alcohol use, education level, physical activity, smoking status, and use of antidepressant, sedative, hypnotic, antipsychotic, and anticholinesterase agents. In a sensitivity analysis, we also adjusted for baseline hypertension and baseline antihypertensive medication use.
We also characterized the continuous relationship between supine SBP and standing SBP with outcomes in the fully adjusted model above using restricted cubic splines. Four knots were positioned, using Harrell’s method. Both models were expressed relative to the median value and were truncated at the 0.5th and 99.5th percentiles. These models were adjusted as described above. Sensitivity analyses were performed with the above model using supine and standing DBP. We compared models with and without interaction terms between splines and OH, using log-likelihood ratio tests.
We examined OH phenotypes with respect to the same 6 outcomes using Cox models adjusted for the covariates above. Phenotypes included OH participants without supine hypertension or standing hypotension, with either condition, and with both conditions. This was similarly implemented among participants without OH. The reference group was participants who did not have OH, did not have supine hypertension, and did not have standing hypotension. We similarly used log-log plots to assess the Cox proportionality assumption.
All analyses were conducted using Stata 15.1 (Stata Corp, College Station, TX).
Results
Participant Characteristics at Baseline Assessment
The study population (N=12 489) was 55% women and 26% Black with a mean (SD) age of 54.1 (5.8) years at baseline (Table 1), median follow-up time (24.0–28.0 years). Four percent of the study population (N=546) had OH. Among participants with OH, the mean (SD) seated SBP was 131.8 (22.4) mm Hg, while among those without OH, it was 120.6 (18.6) mm Hg. Among participants with OH, 58% had supine hypertension and 38% had standing hypotension, while among those without OH, 19% had supine hypertension and 21% had standing hypotension. Although the vast majority of the population did not have neurogenic OH, a small group of <1% were taking anti-parkinsonian medications.
Table 1.
Baseline Characteristics, Mean (SD) or %
| Overall, N=12 489 | No OH, N=11 943 | OH, N=546 | |
|---|---|---|---|
| Age, y | 54.1 (5.8) | 54.0 (5.7) | 57.5 (5.3) |
| Female, % | 55 | 55 | 56 |
| Race-study center, % | |||
| Washington County (White participants) | 25 | 25 | 26 |
| Jackson (Black participants) | 23 | 22 | 26 |
| Minneapolis (White participants) | 26 | 26 | 17 |
| Forsyth (Black participants) | 3 | 3 | 6 |
| Forsyth (White participants) | 23 | 23 | 25 |
| Seated SBP, mm Hg | 121.1 (18.9) | 120.6 (18.6) | 131.8 (22.4) |
| Seated DBP, mm Hg | 73.4 (11.2) | 73.3 (11.1) | 75.9 (12.8) |
| Supine SBP, mm Hg | 125.1 (20.0) | 124.1 (19.2) | 146.4 (23.6) |
| Supine DBP, mm Hg | 72.4 (9.8) | 72.1 (9.6) | 78.3 (11.2) |
| Standing SBP, mm Hg | 125.0 (20.3) | 125.2 (20.1) | 121.2 (23.8) |
| Standing DBP, mm Hg | 75.5 (10.5) | 75.7 (10.4) | 71.6 (11.8) |
| Heart rate, beats/min | 66.7 (10.2) | 66.6 (10.1) | 69.3 (12.6) |
| Body mass index, kg/m2 | 27.6 (5.3) | 27.6 (5.2) | 27.9 (6.4) |
| Estimated glomerular filtration rate, mL/min per 1.73 meter squared | 101.5 (13.2) | 101.8 (12.8) | 94.7 (18.7) |
| Diabetes, % | 12 | 11 | 25 |
| Hypertension, % | 34 | 33 | 61 |
| History of coronary disease, % | 5 | 5 | 10 |
| History of stroke, % | 2 | 2 | 3 |
| History of heart failure, % | 4 | 4 | 9 |
| Antihypertensive medication use*, % | 30 | 29 | 55 |
| Antidepressant use, % | 3 | 3 | 6 |
| Anti-parkinsonian medication use, % | 0 | 0 | 1 |
| Sedative use, % | 1 | 1 | 1 |
| Hypnotic use, % | 2 | 2 | 2 |
| Antipsychotic use, % | 1 | 1 | 2 |
| Anticholinergic use, % | 2 | 2 | 4 |
| Leisure index, U | 2.4 (0.6) | 2.4 (0.6) | 2.2 (0.6) |
| Alcohol use, % | |||
| Never | 25 | 25 | 29 |
| Former | 19 | 18 | 26 |
| Current | 57 | 57 | 45 |
| Education level, % | |||
| Less than high school | 23 | 22 | 38 |
| High school or vocational school | 41 | 41 | 35 |
| At least some college or professional school | 36 | 37 | 27 |
| Smoking status, % | |||
| Never | 41 | 41 | 36 |
| Former | 33 | 33 | 31 |
| Current | 26 | 26 | 33 |
| Supine hypertension, % | 21 | 19 | 58 |
| Standing hypotension, % | 22 | 21 | 38 |
Baseline characteristics. Supine hypertension was defined as mean supine SBP of ≥140 or DBP or ≥90 mm Hg. Standing hypotension was defined as a mean standing SBP of ≤105 or DBP ≤65 mm Hg. Participants’ leisure time and physical activity was assessed via the ARIC study/Baecke Physical Activity questionnaire. ARIC indicates Atherosclerosis Risk in Communities study; DBP, diastolic blood pressure; OH, orthostatic hypotension; and SBP, systolic blood pressure.
This is based on medication review (not just self-report). Note only 12 482 participants underwent this review, 11 936 without orthostatic hypotension and 546 with orthostatic hypotension.
Association of Supine Hypertension or Standing Hypotension With Outcomes
In the overall population, supine hypertension was associated with increased risk for all outcomes of interest including CHD, HF, stroke, falls, syncope, and all-cause mortality (Table 2; Table S1). Standing hypotension was only significantly associated with all-cause mortality (hazard ratio [HR], 1.06 [95% CI, 1.00–1.14]). When directly compared, the coefficients for supine hypertension and standing hypotension were significantly different from each other for all outcomes of interest with the exception of falls (P=0.058). Findings were similar after adjustment for baseline hypertension and baseline anti-hypertensive medication use.
Table 2.
Association of Supine Hypertension or Standing Hypotension With Outcomes
| Supine Hypertension (≥140/≥90) | Standing Hypotension (≤105/≤65) | P Comparing Supine Hypertension and Standing Hypotension | |
|---|---|---|---|
| HR (95% CI) | H R (95% CI) | ||
| All, N=12 489 participants | |||
| Coronary heart disease, N=2981 events | 1.50 (1.37–1.64) | 0.95 (0.86–1.05) | <0.001 |
| Heart failure, N=2878 events | 1.83 (1.68–1.99) | 1.06 (0.96–1.18) | <0.001 |
| Ischemic and hemorrhagic stroke, N=1272 events | 1.80 (1.58–2.04) | 0.98 (0.84–1.15) | <0.001 |
| Fall, N=3532 events | 1.12 (1.02–1.22) | 1.01 (0.92–1.09) | 0.058 |
| Syncope, N=3165 events | 1.24 (1.13–1.35) | 1.02 (0.93–1.12) | 0.001 |
| All-cause mortality, N=6727 events | 1.45 (1.37–1.54) | 1.06 (1.00–1.14) | <0.001 |
| No OH, N=11 943 participants | |||
| Coronary heart disease, N=2771 events | 1.46 (1.33–1.60) | 0.91 (0.81–1.01) | <0.001 |
| Heart failure, N=2670 events | 1.76 (1.61–1.93) | 1.00 (0.90–1.12) | <0.001 |
| Ischemic and hemorrhagic stroke, N=1181 events | 1.76 (1.53–2.01) | 0.95 (0.80–1.13) | <0.001 |
| Fall, N=3389 events | 1.08 (0.98–1.19) | 1.00 (0.92–1.09) | 0.19 |
| Syncope, N=2999 events | 1.19 (1.09–1.31) | 0.99 (0.90–1.09) | 0.003 |
| All-cause mortality, N=6284 events | 1.40 (1.31–1.49) | 1.02 (0.95–1.09) | <0.001 |
| OH, N=546 participants | |||
| Coronary heart disease, N=210 events | 1.28 (0.90–1.80) | 0.87 (0.61–1.24) | 0.028 |
| Heart failure, N=208 events | 1.71 (1.20–2.43) | 1.06 (0.74–1.51) | <0.001 |
| Ischemic and hemorrhagic stroke, N=91 events | 1.27 (0.74–2.19) | 0.79 (0.44–1.41) | 0.093 |
| Fall, N=143 events | 1.55 (1.02–2.37) | 1.10 (0.71–1.70) | 0.11 |
| Syncope, N=166 events | 1.01 (0.69–1.48) | 0.87 (0.58–1.30) | 0.47 |
| All-cause mortality, N=443 events | 1.42 (1.12–1.81) | 1.07 (0.84–1.37) | 0.018 |
Cox proportional hazards models with both supine hypertension and standing hypotension. Models were adjusted for age, sex, race-center, eGFR, BMI, heart rate, HDLc, total cholesterol, prevalent CHD, prevalent HF, prevalent stroke, diabetes, alcohol status, education, physical activity, smoking status, antidepressant use, sedative use, hypnotic use, anti-psychotic use, and anticholinesterase use. Heart failure numbers are lower at 11 932 due to missing event ascertainment. Among those without OH there were 11 434 participants and among those with OH there were 498 participants. All interactions by OH status were >0.092. For tabulation of events by blood pressure phenotype see Table S1. BMI indicates body mass index; CHD, coronary heart disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDLc, high-density lipoprotein cholesterol; OH, orthostatic hypotension; and SBP, systolic blood pressure.
We did not observe statistically significant interactions between OH status and outcomes (P-interaction values were all >0.09). In stratified analyses, among participants with OH, supine hypertension was associated with HF, falls, and all-cause mortality. In contrast, in stratified analyses standing hypotension was not significantly associated with any of the outcomes of interest among the OH or non-OH participants. Among participants without OH, coefficients differed significantly between supine hypertension and standing hypotension for CHD, HF, stroke, and all-cause mortality, while for participants with OH, coefficients differed significantly only for HF and all-cause mortality.
The stronger associations between supine hypertension with adverse events compared with standing hypotension were corroborated by splines examining the continuous associations between standing SBP (Figure 1; Figure S1) and supine SBP (Figure 2; Figure S2) by OH status. Regardless of OH status, higher hazards for HF, stroke, and all-cause mortality were consistently observed at standing SBP values above 120 mm Hg without evidence of increased risk below 120 mm Hg. Similarly, a supine SBP below 120 mm Hg was consistently associated with a lower risk of adverse events. There was no significant differences between spline models with or without interaction terms by OH status.
Figure 1. Adjusted hazard ratios (solid line) for outcomes according to the standing systolic blood pressure (SBP), using a restricted cubic spline with 4 knots determined by Harrell’s method.
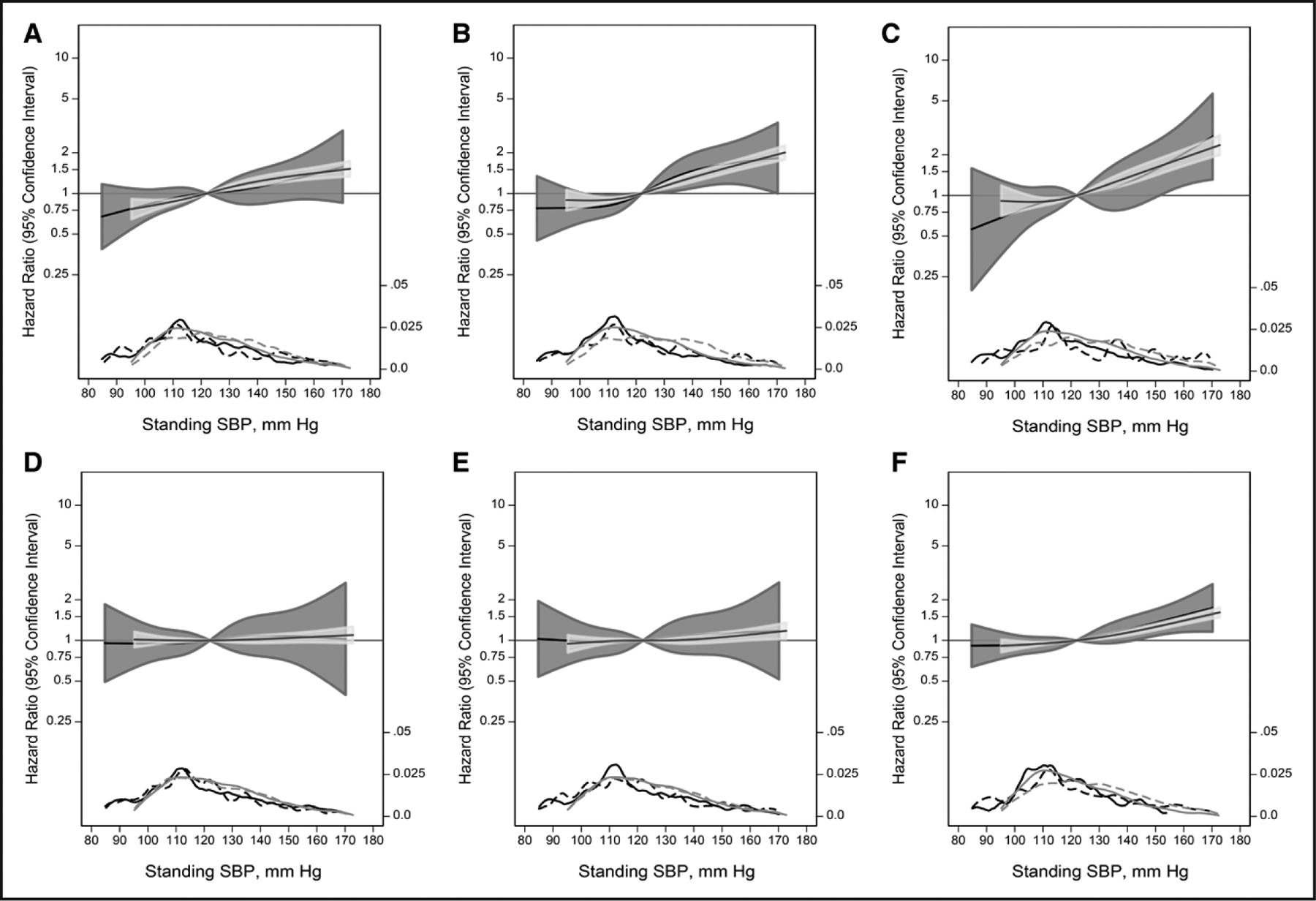
A, Coronary heart disease. B, Heart failure. C, Stroke. D, Fall. E, Syncope. F, All-cause mortality. Dark gray shade represents 95% CI for orthostatic hypotension (OH) participants, light gray represents 95% CI for participants without OH. Both models were expressed relative to the median value and were truncated at the 0.5th and 99.5th percentiles. Models were adjusted for age, sex, race-study center, estimated glomerular filtration rate, body mass index, diabetes status, drinking status, smoking status, diuretic use, antidepressant use, sedative use, hypnotic use, antipsychotic use, and anticholinergic use. The hazard ratios are shown on a natural log scale. Included are kernel density plots showing the distribution of standing SBP (solid lines; dark gray, OH participants; light gray, no OH participants) and distribution of events (dashed lines; dark gray, OH participants; light gray, no OH participants). Log-likelihood ratio tests did not identify any significant difference between models with or without interaction terms between splines and OH.
Figure 2. Adjusted hazard ratios (solid line) for outcomes according to the supine systolic blood pressure (SBP), using a restricted cubic spline with 4 knots determined by Harrell’s method.
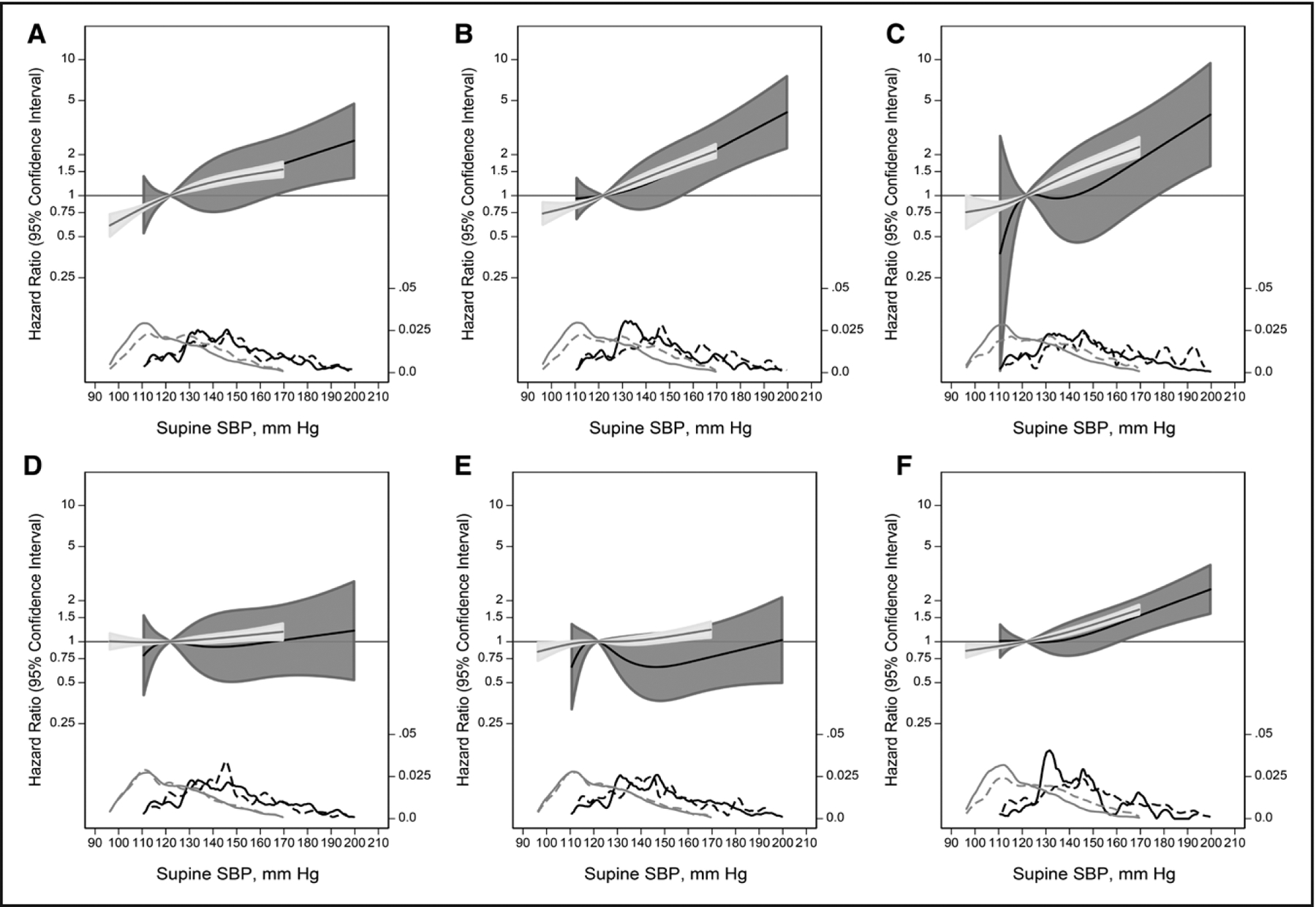
A, Coronary heart disease. B, Heart failure. C, Stroke. D, Fall. E, Syncope. F, All-cause mortality. Dark gray shade represents 95% CI for orthostatic hypotension (OH) participants, light gray represents 95% CI for participants without OH. Both models were expressed relative to the median value and were truncated at the 0.5th and 99.5th percentiles. Models were adjusted for age, sex, race-study center, estimated glomerular filtration rate, body mass index, diabetes status, drinking status, smoking status use in last 2 weeks, diuretic use, antidepressant use, sedative use, hypnotic use, antipsychotic use, and anticholinergic use. The hazard ratios are shown on a natural log scale. Included are kernel density plots showing the distribution of supine SBP (solid lines; dark gray, OH participants; light gray, no OH participants) and distribution of events (dashed lines; dark gray, OH participants; light gray, no OH participants). Log-likelihood ratio tests did not identify any significant difference between models with or without interaction terms between splines and OH.
OH Phenotypes and CVD Outcomes
Of the phenotypes examined, the most common was the group without OH, without supine hypertension, and without standing hypotension (N=7237) and the least common was the group without OH, with supine hypertension, and with standing hypotension (which occurred in N=15 individuals with wide pulse pressure; Table 3). Among participants with OH, those with supine hypertension, but no standing hypotension, were observed to have the greatest magnitude of risk for CHD (HR, 2.09 [95% CI, 1.71–2.56]), HF (HR, 2.74 [95% CI, 2.25–3.34]), and stroke (HR, 2.65 [95% CI, 1.99–3.53]). In contrast, OH without supine hypertension, but with standing hypotension, was only associated with CHD (HR, 1.49 [95% CI,1.15–1.93]) and HF (HR, 1.93 [95% CI, 1.49–2.51]), but not stroke (HR, 1.37 [95% CI, 0.86–2.17]).
Table 3.
OH Phenotypes and Cardiovascular Disease Outcomes, N=12 489
| CHD, N=2981 | HR (95% CI) | P Value |
|---|---|---|
| No OH with supine hypertension without standing hypotension | 1.43 (1.30–1.57) | <0.001 |
| No OH without supine hypertension and with standing hypotension | 0.91 (0.82–1.01) | 0.089 |
| No OH with both supine hypertension and standing hypotension | 2.05 (0.92–4.59) | 0.081 |
| OH without supine hypertension or standing hypotension | 1.66 (1.14–2.41) | 0.008 |
| OH with supine hypertension without standing hypotension | 2.09 (1.71–2.56) | <0.001 |
| OH without supine hypertension and with standing hypotension | 1.49 (1.15–1.93) | 0.002 |
| OH with both supine hypertension and standing hypotension | 1.65 (0.97–2.82) | 0.066 |
| Heart failure, N=2878 (of 11 932) | ||
| No OH with supine hypertension without standing hypotension | 1.76 (1.60–1.93) | <0.001 |
| No OH without supine hypertension and with standing hypotension | 1.01 (0.90–1.13) | 0.87 |
| No OH with both supine hypertension and standing hypotension | 2.32 (1.04–5.21) | 0.041 |
| OH without supine hypertension or standing hypotension | 1.45 (0.96–2.19) | 0.080 |
| OH with supine hypertension without standing hypotension | 2.74 (2.25–3.34) | <0.001 |
| OH without supine hypertension and with standing hypotension | 1.93 (1.49–2.51) | <0.001 |
| OH with both supine hypertension and standing hypotension | 2.40 (1.41–4.07) | 0.001 |
| Stroke, N=1272 | ||
| No OH with supine hypertension without standing hypotension | 1.74 (1.52–1.99) | <0.001 |
| No OH without supine hypertension and with standing hypotension | 0.97 (0.82–1.15) | 0.70 |
| No OH with both supine hypertension and standing hypotension | 1.63 (0.40–6.57) | 0.49 |
| OH without supine hypertension or standing hypotension | 1.93 (1.09–3.41) | 0.025 |
| OH with supine hypertension without standing hypotension | 2.65 (1.99–3.53) | <0.001 |
| OH without supine hypertension and with standing hypotension | 1.37 (0.86–2.17) | 0.19 |
| OH with both supine hypertension and standing hypotension | 2.28 (1.07–4.83) | 0.032 |
OH phenotypes and cardiovascular outcomes, N=12 489. Reference group (no OH no supine hypertension, no standing hypotension) n=7237. Phenotype 1 (no OH, with supine hypertension, no standing hypotension) N=2132. Phenotype 2 (no OH, no supine hypertension, with standing hypotension) N=2465. Phenotype 3 (no OH, with supine hypertension, with standing hypotension) N=15. Phenotype 4 (OH, no supine hypertension, no standing hypotension) N=115. Phenotype 5 (OH, with supine hypertension, no standing hypotension) N=305. Phenotype 6 (OH, no supine hypertension, with standing hypotension) N=179. Phenotype 7 (OH, with supine hypertension, with standing hypotension) N=41. Cox proportional hazard models adjusted for age, sex, race-center, eGFR, BMI, heart rate, HDLc, total cholesterol, prevalent CHD, prevalent CHF, prevalent stroke, diabetes, alcohol status, education, physical activity, smoking status, antidepressant use, sedative use, hypnotic use, antipsychotic use, and anticholinesterase use. Phenotype 3 (no OH, with supine hypertension, with standing hypotension) was rare, but occurred when either SBP or DBP met criterion for supine hypertension or standing hypotension, but the difference in SBP or DBP upon standing to not meet criterion for OH. BMI indicates body mass index; CHD, coronary heart disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDLc, high-density lipoprotein cholesterol; OH, orthostatic hypotension; and SBP, systolic blood pressure.
OH Phenotypes and Noncardiovascular Disease Outcomes
Participants with OH and supine hypertension, but no standing hypotension, were the only group associated with falls (HR, 1.57 [95% CI, 1.24–1.99]; Table 4). Moreover, this group was most strongly associated with syncope (HR, 1.89 [95% CI, 1.51–2.38]) and was also associated with all-cause mortality. In contrast, OH without supine hypertension, but with standing hypotension, was only associated with syncope (HR, 1.43 [95% CI, 1.08–1.89]) and all-cause mortality, but not falls (HR, 1.14 [95% CI,0.87–1.49]). However, having both supine hypertension and standing hypotension was the strongest predictor of death both for those with OH (HR, 2.12 [95% CI, 1.51–2.97]) and without OH (HR, 2.19 [95% CI, 1.29–3.71]).
Table 4.
OH Phenotypes and Noncardiovascular Disease Outcomes, N=12 489
| Fall, N=3532 | HR (95% CI) | P Value |
|---|---|---|
| No OH with supine hypertension without standing hypotension | 1.07 (0.98–1.18) | 0.14 |
| No OH without supine hypertension and with standing hypotension | 1.00 (0.92–1.09) | 0.99 |
| No OH with both supine hypertension and standing hypotension | 1.88 (0.84–4.21) | 0.12 |
| OH without supine hypertension or standing hypotension | 0.82 (0.49–1.37) | 0.45 |
| OH with supine hypertension without standing hypotension | 1.57 (1.24–1.99) | <0.001 |
| OH without supine hypertension and with standing hypotension | 1.10 (0.83–1.47) | 0.51 |
| OH with both supine hypertension and standing hypotension | 0.75 (0.34–1.68) | 0.49 |
| Syncope, N=3165 | ||
| No OH with supine hypertension without standing hypotension | 1.17 (1.06–1.29) | 0.001 |
| No OH without supine hypertension and with standing hypotension | 0.99 (0.90–1.09) | 0.89 |
| No OH with both supine hypertension and standing hypotension | 1.65 (0.62–4.41) | 0.32 |
| OH without supine hypertension or standing hypotension | 1.66 (1.12–2.46) | 0.012 |
| OH with supine hypertension without standing hypotension | 1.89 (1.51–2.38) | <0.001 |
| OH without supine hypertension and with standing hypotension | 1.43 (1.08–1.89) | 0.013 |
| OH with both supine hypertension and standing hypotension | 1.59 (0.85–2.96) | 0.15 |
| All-cause mortality, N=6727 | ||
| No OH with supine hypertension without standing hypotension | 1.38 (1.30–1.47) | <0.001 |
| No OH without supine hypertension and with standing hypotension | 1.02 (0.95–1.09) | 0.65 |
| No OH with both supine hypertension and standing hypotension | 2.19 (1.29–3.71) | 0.004 |
| OH without supine hypertension or standing hypotension | 1.48 (1.13–1.94) | 0.004 |
| OH with supine hypertension without standing hypotension | 2.08 (1.81–2.39) | <0.001 |
| OH without supine hypertension and with standing hypotension | 1.62 (1.36–1.93) | <0.001 |
| OH with both supine hypertension and standing hypotension | 2.12 (1.51–2.97) | <0.001 |
Orthostatic hypotension phenotypes and noncardiovascular outcomes, N=12 489. Reference group (no OH no supine hypertension, no standing hypotension) n=7237. Phenotype 1 (no OH, with supine hypertension, no standing hypotension) N=2132. Phenotype 2 (no OH, no supine hypertension, with standing hypotension) N=2465. Phenotype 3 (no OH, with supine hypertension, with standing hypotension) N=15. Phenotype 4 (OH, no supine hypertension, no standing hypotension) N=115. Phenotype 5 (OH, with supine hypertension, no standing hypotension) N=305. Phenotype 6 (OH, no supine hypertension, with standing hypotension) N=179. Phenotype 7 (OH, with supine hypertension, with standing hypotension) N=41. Cox proportional hazard models adjusted for age, sex, race-center, eGFR, BMI, heart rate, HDLc, total cholesterol, prevalent CHD, prevalent HF, prevalent stroke, diabetes, alcohol status, education, physical activity, smoking status, antidepressant use, sedative use, hypnotic use, antipsychotic use, and anticholinesterase use. Phenotype 3 (no OH, with supine hypertension, with standing hypotension) was rare, but occurred when either SBP or DBP met criterion for supine hypertension or standing hypotension, but the difference in SBP or DBP upon standing to not meet criterion for OH. BMI indicates body mass index; CHD, coronary heart disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDLc, high-density lipoprotein cholesterol; OH, orthostatic hypotension; and SBP, systolic blood pressure.
DISCUSSION
In this middle-aged, community-based population, supine hypertension was associated with adverse clinical outcomes, and was more strongly associated with adverse events than standing hypotension even among adults with OH. Our study also identified differences in OH phenotypes with supine hypertension in absence of standing hypotension being an important predictor of both CVD and non-CVD events, including falls. These findings challenge traditional views about the role of hypoperfusion as a driver of end-organ injury among adults with OH, and place a greater focus on hypertension as a potential cause of adverse events. Future research should evaluate BP reduction as a potential intervention to prevent adverse outcomes among adults with OH.
OH is prevalent among as many as 10% of adults with hypertension,18–20 an association often attributed to antihypertensive medication use.21 However, many treatments for OH can also be a cause of supine hypertension, which is often given lower priority than preventing adverse hypotensive events (eg, falls and syncope).4,22As far as we know, our study is among the first to directly evaluate the relative risks associated with these 2 conditions. Contrary to typical clinical priorities in OH, we found that supine hypertension was a more important predictor of both cardiovascular and noncardiovascular events compared with standing hypotension regardless of OH status.
Prior work suggests that end-organ injury from OH is caused by profound drops in BP while standing.23 However, our study demonstrates that, elevated (not low) standing SBP was associated with higher risk of CVD events and mortality, even among adults with OH. Moreover, lower supine BP was consistently associated with lower risk of adverse events, while higher supine BP was associated with higher risk, even among adults with OH. This is consistent with prior work associating OH with carotid intimal thickness, a marker for subclinical CVD, which could contribute to BP dysregulation.7 Whether adults with OH might benefit from lowering standing BP is beyond the scope of the present study, but should be a focus of subsequent research.
OH with supine hypertension and no standing hypotension was strongly associated with both CVD and non-CVD outcomes, and was the only phenotype associated with falls. A similar observation was made among 1500 participants of the TILDA study (The Irish Longitudinal Study on Aging), where supine or seated hypertension were important risk factors for falls among adults with OH.24 Nevertheless, this finding is somewhat contradictory to prevailing beliefs that BP treatment causes falls.22,25 While mechanisms of this association are unclear, our findings are consistent with studies showing that poorer BP control is associated with more OH and higher risk of fall events.26 We speculate that the pathogenesis of this finding may be related to greater degrees of BP dysregulation among adults with supine hypertension and OH,27 and pressure natriuresis, causing nocturia and exacerbating OH in the morning.28–30 However, these pathways cannot be established in the present analysis.
This study has a number of limitations. First, the total number of study participants with OH was relatively small (N=546), so precision was reduced for some comparisons. Stratification into different OH phenotypes further reduced these numbers and as a result findings for the nonsignificant OH phenotypes should be interpreted with caution. Nevertheless, we observed no evidence of an OH interaction with outcomes, suggesting that stratification between OH and no OH populations was unnecessary. Second, the study population was middle-aged and ambulatory, so results may not be generalizable to populations with neurogenic OH, older adults, adults with symptomatic hypotension, or to adults taking antihypotensive medications. Third, falls and syncope were derived from ICD-9 and ICD-10 codes, and these events were not adjudicated. Prior studies have shown that while these codes are specific, they may have reduced sensitivity for less severe falls,31 leading to underascertainment of adverse events. Fourth, the study lacked follow-up OH measurements, which prevented assessment of OH change over time. Such information would be informative for evaluating how changes in hypertension diagnoses and treatment might affect OH, but was not available in the ARIC cohort. Fifth, despite our adjustment for seated hypertension and antihypertensive medication use, it is possible that supine hypertension does not predict adverse events independently of seated BP. This topic should be evaluated further in subsequent work. Finally, residual confounding is always a concern with observational studies.
Our study has several strengths. First, ARIC included a large sample of Black and White middle-aged adults; as a result, our findings are generalizable to a broad ambulatory population. Second, ARIC staff underwent rigorous training to execute the study’s standardized OH protocol along with other covariates. Third, CVD events represent the primary outcomes of the ARIC study and thus these were monitored closely and adjudicated.
Our study has important implications. OH is known to be associated with CVD events, falls, and syncope independent of other related risk factors2,7 While current OH treatment focuses on BP augmentation and stabilization, this indiscriminate treatment approach to all patients with OH could have adverse health implications especially if hypertension is confirmed to be a driving mechanism of organ injury and adverse events. Our data indicates that adults with supine hypertension are at higher risk of adverse events and death than those without supine hypertension. Whether individuals with supine hypertension might benefit from more aggressive hypertension treatment, particularly those with elevated standing BP, constitutes an important area for future research.
Conclusions
In conclusion, in this population of middle-aged, community-dwelling adults, supine hypertension was more strongly associated with adverse events than standing hypotension even among adults with OH. Our data suggest that when making clinical management decisions in patients with OH, it may be important to differentiate between those with or without supine hypertension and standing hypotension, rather than taking a uniform approach, as is currently recommended. Future research should focus on whether BP reduction would reduce risk among adults with OH and supine hypertension in absence of standing hypotension.
Supplementary Material
Novelty and relevance.
What Is New?
We compared the long-term clinical consequences of supine hypertension and standing hypotension among middle-aged adults and found that supine hypertension was more strongly associated with adverse events than standing hypotension even among adults with orthostatic hypotension (OH).
This challenges conventional OH management, which prioritizes standing hypotension over supine hypertension.
What Is Relevant?
Management of ambulatory OH is often a clinical challenge, interfering with the management of hypertension due to concerns about worsening symptomatic standing hypotension and falls.
Clinical/Pathophysiological Implications?
Our findings suggest that reducing supine hypertension may be an important focus to prevent long-term cardiovascular outcomes and potentially falls.
Perspectives.
Current clinical management of OH prioritizes prevention of standing hypotension, sometimes at the expense of supine hypertension. It is not known whether supine hypertension is associated with adverse outcomes relative to standing hypotension. In this study, we compared the long-term clinical consequences of supine hypertension and standing hypotension among middle-aged adults with and without OH and found that supine hypertension was more strongly associated with adverse events than standing hypotension even among adults with OH. Our findings challenge conventional OH management, which prioritizes standing hypotension over supine hypertension. Instead our data suggests that when making clinical management decisions in patients with OH, it may be important to differentiate between those with or without supine hypertension and standing hypotension, rather than taking a uniform approach, as is currently recommended. Future research should focus on whether BP reduction would reduce risk among adults with OH and supine hypertension in absence of standing hypotension.
Acknowledgments
The authors thank the staff and participants of the ARIC study (Atherosclerosis Risk in Communities) for their important contributions.
Sources of Funding
S.P. Juraschek was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grant R01HL153191. E. Selvin was supported by NIH/NHLBI grant K24 HL152440. P.L. Lutsey was supported by NIH/NHLBI grant K24 HL159246. The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract nos. (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005).
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- BP
blood pressure
- CHD
coronary heart disease
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HF
heart failure
- HR
hazard ratio
- OH
orthostatic hypotension
- SBP
systolic blood pressure
Footnotes
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.123.21215. For Sources of Funding and Disclosures, see page 2445 & 2446.
References
- 1.The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470–1470. doi: 10.1212/WNL.46.5.1470 [DOI] [PubMed] [Google Scholar]
- 2.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5 [DOI] [PubMed] [Google Scholar]
- 3.Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER, Windham BG, Griswold ME, Heiss G, Selvin E. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med. 2017;177:1316–1323. doi: 10.1001/jamainternmed.2017.2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figueroa JJ, Basford JR, Low PA. Preventing and treating orthostatic hypotension: as easy as A, B, C. Cleve Clin J Med. 2010;77:298–306. doi: 10.3949/ccjm.77a.09118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juraschek SP, Hu J-R, Cluett JL, Ishak A, Mita C, Lipsitz LA, Appel LJ, Beckett NS, Coleman RL, Cushman WC, et al. Effects of intensive blood pressure treatment on orthostatic hypotension: a systematic review and individual participant–based meta-analysis. Ann Intern Med. 2021;174:58–68. doi: 10.7326/M20-4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose KM, Tyroler HA, Nardo CJ, Arnett DK, Light KC, Rosamond W, Sharrett AR, Szklo M. Orthostatic hypotension and the incidence of coronary heart disease: the atherosclerosis risk in communities study. Am J Hypertens. 2000;13:571–578. doi: 10.1016/s0895-7061(99)00257-5 [DOI] [PubMed] [Google Scholar]
- 7.Juraschek SP, Daya N, Appel LJ, Miller ER, McEvoy JW, Matsushita K, Ballantyne CM, Selvin E. Orthostatic hypotension and risk of clinical and subclinical cardiovascular disease in middle-aged adults. J Am Heart Assoc. 2018;7:e008884. doi: 10.1161/JAHA.118.008884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juraschek SP, Taylor AA, Wright JT, Evans GW, Miller ER, Plante TB, Cushman WC, Gure TR, Haley WE, Moinuddin I, et al. ; SPRINT Research Group. Orthostatic hypotension, cardiovascular outcomes, and adverse events: results from SPRINT. Hypertension. 2020;75:660–667. doi: 10.1161/HYPERTENSIONAHA.119.14309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JD, Folsom AR, Coresh J, Sharrett AR, Couper D, Wagenknecht LE, Mosley TH, Ballantyne CM, Boerwinkle EA, Rosamond WD, et al. The ARIC (Atherosclerosis Risk in Communities) Study. J Am Coll Cardiol. 2021;77:2939–2959. doi: 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, Shahar E, Kalsbeek W. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender and ethnicity. J Clin Epidemiol. 1996;49:1441–1446. doi: 10.1016/0895-4356(95)00047-x [DOI] [PubMed] [Google Scholar]
- 11.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0 [DOI] [PubMed] [Google Scholar]
- 12.Mundt KA, Chambless LE, Burnham CB, Heiss G. Measuring ankle systolic blood pressure: validation of the Dinamap 1846 SX. Angiology. 1992;43:555–566. doi: 10.1177/000331979204300703 [DOI] [PubMed] [Google Scholar]
- 13.The National Heart, Lung, and Blood Institute. ARIC Manual 11: Sitting Blood Pressure. Accessed August 31, 2022. https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/Sitting_Blood_Pressure_and_Postural_Changes_in_Blood_Pressure_and_Heart_Rate.1_11.pdf [Google Scholar]
- 14.Kucharska-Newton AM, Heiss G, Ni H, Stearns SC, Puccinelli-Ortega N, Wruck LM, Chambless L. Identification of heart failure events in medicare claims: the atherosclerosis risk in communities (ARIC) study. J Card Fail. 2016;22:48–55. doi: 10.1016/j.cardfail.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juraschek SP, Daya N, Appel LJ, Miller ER, Windham BG, Pompeii L, Griswold ME, Kucharska-Newton A, Selvin E. Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens. 2017;30:188–195. doi: 10.1093/ajh/hpw108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et al. ; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson MT, Ainsworth BE, Wu H-C, Jacobs DR, Leon AS. Ability of the atherosclerosis risk in communities (ARIC)/Baecke questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–693. doi: 10.1093/ije/24.4.685 [DOI] [PubMed] [Google Scholar]
- 18.Fedorowski A, Burri P, Melander O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J Hypertens. 2009;27:976–982. doi: 10.1097/hjh.0b013e3283279860 [DOI] [PubMed] [Google Scholar]
- 19.Di Stefano C, Milazzo V, Totaro S, Sobrero G, Ravera A, Milan A, Maule S, Veglio F. Orthostatic hypotension in a cohort of hypertensive patients referring to a hypertension clinic. J Hum Hypertens. 2015;29:599–603. doi: 10.1038/jhh.2014.130 [DOI] [PubMed] [Google Scholar]
- 20.Applegate WB, Davis BR, Black HR, Smith WM, Miller ST, Burlando AJ. Prevalence of postural hypotension at baseline in the systolic hypertension in the Elderly Program (SHEP) Cohort. J Am Geriatr Soc. 1991;39:1057–1064. doi: 10.1111/j.1532-5415.1991.tb02869.x [DOI] [PubMed] [Google Scholar]
- 21.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 22.Chen T, Shao F, Chen K, Wang Y, Wu Z, Wang Y, Gao Y, Cornelius V, Li C, Jiang Z. Time to clinical benefit of intensive blood pressure lowering in patients 60 years and older with hypertension: a secondary analysis of randomized clinical trials. JAMA Intern Med. 2022;182:660–667. doi: 10.1001/jamainternmed.2022.1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagro J, Laurenssen NCW, Schalk BWM, Schoon Y, Claassen JAHR, Rikkert MGMO. Diastolic blood pressure drop after standing as a clinical sign for increased mortality in older falls clinic patients. J Hypertens. 2012;30:1195–1202. doi: 10.1097/HJH.0b013e328352b9fd [DOI] [PubMed] [Google Scholar]
- 24.Donoghue OA, O’Connell MDL, Bourke R, Kenny RA. Is orthostatic hypotension and co-existing supine and seated hypertension associated with future falls in community-dwelling older adults? Results from The Irish Longitudinal Study on Ageing (TILDA). PLoS One. 2021;16:e0252212. doi: 10.1371/journal.pone.0252212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margolis KL. Antihypertensive medication use in older adults at risk for hip fracture. JAMA. 2019;322:1608–1609. doi: 10.1001/jama.2019.13951 [DOI] [PubMed] [Google Scholar]
- 26.Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383–389. doi: 10.1111/j.1532-5415.2011.03317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain SM, Ernst ME, Barker AL, Margolis KL, Reid CM, Neumann JT, Tonkin AM, Phuong TLT, Beilin LJ, Pham T, et al. Variation in mean arterial pressure increases falls risk in elderly physically frail and prefrail individuals treated with antihypertensive medication. Hypertension. 2022;79:2051–2061. doi: 10.1161/HYPERTENSIONAHA.122.19356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omboni S, Smit AA, van Lieshout JJ, Settels JJ, Langewouters GJ, Wieling W. Mechanisms underlying the impairment in orthostatic tolerance after nocturnal recumbency in patients with autonomic failure. Clin Sci Lond. 2001;101:609–618. [PubMed] [Google Scholar]
- 29.Ejaz AA, Haley WE, Wasiluk A, Meschia JF, Fitzpatrick PM. Characteristics of 100 consecutive patients presenting with orthostatic hypotension. Mayo Clin Proc. 2004;79:890–894. doi: 10.4065/79.7.890 [DOI] [PubMed] [Google Scholar]
- 30.Uzu T, Takeji M, Yamauchi A, Kimura G. Circadian rhythm and postural change in natriuresis in non-dipper type of essential hypertension. J Hum Hypertens. 2001;15:323–327. doi: 10.1038/sj.jhh.1001185 [DOI] [PubMed] [Google Scholar]
- 31.Eriksson H, Caidaul K, Larsson B, Ohlson L-O, Welin L, Wilhelmsen L, Svärdsudd K. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the ARIC coordinating center with an approved proposal.


