Abstract
Germline mutations in the DNA mismatch repair (MMR) genes cause Lynch syndrome (LS). In this study, we identified and characterized a novel SINE-VNTR-Alu (SVA) insertion in exon 12 of MSH2 in an individual with early-onset colorectal cancer and a very strong LS family history. RT-PCR analysis indicated a larger aberrant MSH2 transcript in one of the family members. MSK-IMPACT next-generation sequencing and long-range PCR analyses revealed an insertion in MSH2 exon 12 at the c.1972 position in an antisense orientation. The insertion was further characterized as an SVA element approximately 3 kb in length, belonging to the SVA_F1 family of retrotransposons. This variant also segregated with LS related cancers in four affected family members in this family. Based on this evidence, this MSH2 SVA insertion is considered pathogenic.
Keywords: germline, Lynch syndrome, MSH2, SVA
1 |. INTRODUCTION
Lynch syndrome (LS) is an autosomal dominant inherited disease and its prevalence is 1% to 3% in unselected colorectal or endometrial cancer patients.1 It is characterized by increased risks for early-onset tumor development, especially for colorectal cancer (CRC), endometrial cancer, ovarian cancer, and other extracolonic tumors such as hepatobiliary, urothelial, brain or central nervous system tumors, as well as sebaceous tumors.2 Lynch syndrome is caused by germline mutations in one of the mismatch repair (MMR) and EPCAM genes.3 Tumors from LS patients normally exhibit high microsatellite instability (MSI-H) and loss of expression of one or more MMR proteins.4 Substitutions, small insertion/deletions, large deletions/duplications, inversions,5–7 as well as insertions of retrotransposon have been reported in the MMR genes as causes of LS.8,9
Retrotransposons are DNA sequences that proliferate in the genome using an RNA intermediate and a “copy- and-paste” retrotransposition mechanism. Retrotransposons can be subdivided into two groups distinguished by the presence or absence of long terminal repeats (LTRs). Retrotransposons without LTRs include Long Interspersed Elements 1 (LINE-1, L1), Alu elements (Short Interspersed Elements, SINE) and SVA (SINE-VNTR-Alu) elements.10,11 Approximately 124 retrotransposon insertions associated with human disease have been previously reported.12 In this study, we report an insertion of an SVA element at c.1972 inexon 12 of MSH2 as a novel cause of Lynch syndrome.
2 |. MATERIALS AND METHODS
2.1 |. Subject
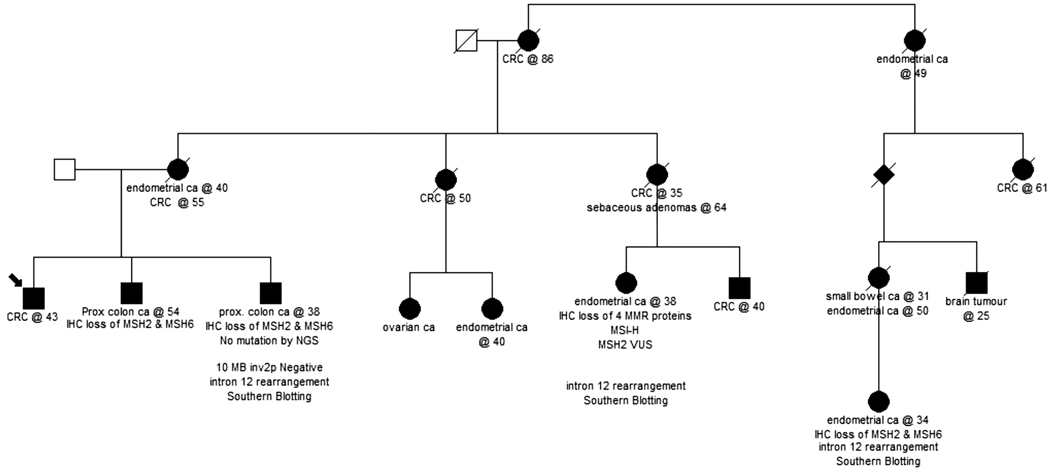
The patient, who is a 49-year-old man who was diagnosed with colon cancer at age 43, consented to an Institutional Review Board (IRB)-approved protocol at Memorial Sloan Kettering Cancer Center. A four-generation pedigree (Figure 1) indicated that other family members were affected with early-onset colorectal cancer (CRC) under age 50. The proband’s mother was diagnosed with metachronous endometrial and CRC and one maternal aunt was diagnosed with CRC at 50. Another maternal aunt of the index case was diagnosed with CRC at 35. Her daughter was diagnosed with endometrial cancer at age 38. One of the proband’s brothers had colon polyps and another brother was diagnosed with a screen detected colon cancer at age 38.
FIGURE 1.
Patient pedigree. The patient described here is a 49-year-old man who was diagnosed with colon cancer at 43. Nine family members were affected with colorectal cancer and six members were diagnosed with endometrial cancer in this family
2.2 |. Short term lymphocyte culture, RNA isolation, and RT-PCR
Peripheral blood lymphocytes were cultured for 5 days in PB-MAX (GIBCO/Invitrogen LifeTechnologies) and treated with or without Cycloheximide (Sigma-Aldrich) 4 to 6 hours prior to cell harvest.13 Total RNA was isolated using NucleoSpin RNA II (Machery-Nagel). Reverse transcription on 1 μg total RNA was performed using Omniscript RT Kit (Qiagen) with oligo-dT (15) primers (Promega). cDNA was amplified with a forward primer in MSH2 exon 6 (ATGGATAAGAACAGAATAGAGGAG) and a reverse primer in exon 16 (TCACGTAGTAACTTTTATTCGTG) using ExpandTM Long Template PCR system in combination with buffer 3 (Roche Diagnostics) with conditions as described by the manufacturer.
2.3 |. Southern blot analysis
Southern blot analysis was performed with BclI, BglII, HindIII and HincII genomic DNA digests, followed by hybridization with a cDNA probe encompassing MSH2 exon 10–16. Restriction fragment analysis was performed using in silico restriction maps of the region of interest derived from WebCutter 2.0 (http://rna.lundberg.gu.se/cutter2).
2.4 |. NGS and PCR analysis
Peripheral blood sample was collected from the patient using the EDTA Blood tubeandsubmitted tothe Diagnostics foracolorectal multi-gene panel test using the Memorial Sloan Kettering Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) Germline sequencing. Variant calling was performed as described previously.14,15 Germline variants were reviewed by board-certified molecular pathologist orclinical molecular genetics andclassified based onthe American College of Medical Genetics (ACMG) criteria.16
Long-range (LR) PCR, was performed on genomic DNA from the patient using the TaKaRa LA PCR Kit (TaKaRa, Clontech) according to the manufacturer’s protocol, an M13-tagged forward primer located in intron 11 (5′-GTAAAACGACGGCCAGT GGGTTTTGAATTCCCAAATG-3′) and an M13-tagged reverse primer in intron 12 (5′-CAGGAAACAGCTATGAC AAAACGTTACCCCCACAAAG-3′). The following thermal cycling conditions were used to perform LR-PCR: initial denaturation at 94°C for 2 minutes, 30 cycles at 98°C for 10 seconds, 58°C for 30 seconds, 68°C for 6 minutes and a final elongation at 68°C for 10 minutes. Sequencing reactions were performed with the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems) on an ABI 3730XL sequencer according to the manufacturer’s instructions.
3 |. RESULTS
Our proband is a 49-year-old man who was diagnosed with colon cancer at age 43. One of the proband’s brothers had colon polyps, and was subsequently diagnosed with a proximal colon cancer at age 54 which was MSH2 and MSH6 deficient on immunohistochemical (IHC) staining. Another brother was diagnosed with a screen detected colon cancer which also demonstrated loss of expression of the MSH2 and MSH6 proteins by IHC. However, no mutation was identified through next generation sequencing (NGS) and the 10 Mb inversion in MSH2 was not detected.5
Another maternal aunt of the index case was diagnosed with CRC at 35. Her daughter was diagnosed with endometrial cancer at age 38 which demonstrated MSI-H and loss of expression of MSH2 and MSH6 proteins by IHC. This family member was initially tested in 2007 for MLH1 and MSH2 sequencing and large rearrangement in a reference laboratory and was identified to have an MSH2 intron 12 rearrangement which was classified as variant of uncertain significance (VUS). Multiple family members affected with colon or endometrial cancer were tested and no mutation was identified, although tumor tissues of several individuals were tested and showed loss of MSH2 and MSH6 proteins with immunohistochemistry (IHC). A weak aberrant larger transcript was identified but not further characterized in lymphocyte RNA isolated from one of these family members who was affected with colorectal cancer (age 38) that showed loss of MSH2 and MSH6 expression (Figure S1a). Additional Southern blot analysis on genomic DNA of the same patient indicated the presence of a 3 kb insertion, possibly a large LINE-1 or SVA insertion (Figure S1b); restriction fragment analysis could narrow down the place of the insertion to a 1.45 kb region around MSH2 exon 12 (Figure S1c). The same rearrangement was shown with Southern blot analysis in the genomic DNA from another more distantly related family member; this individual presented with endometrial cancer at age 34, and showed loss of MSH2 and MSH6 in tumor tissue. However, the type of retrotransposon and the exact genomic location of the insertion were not determined.
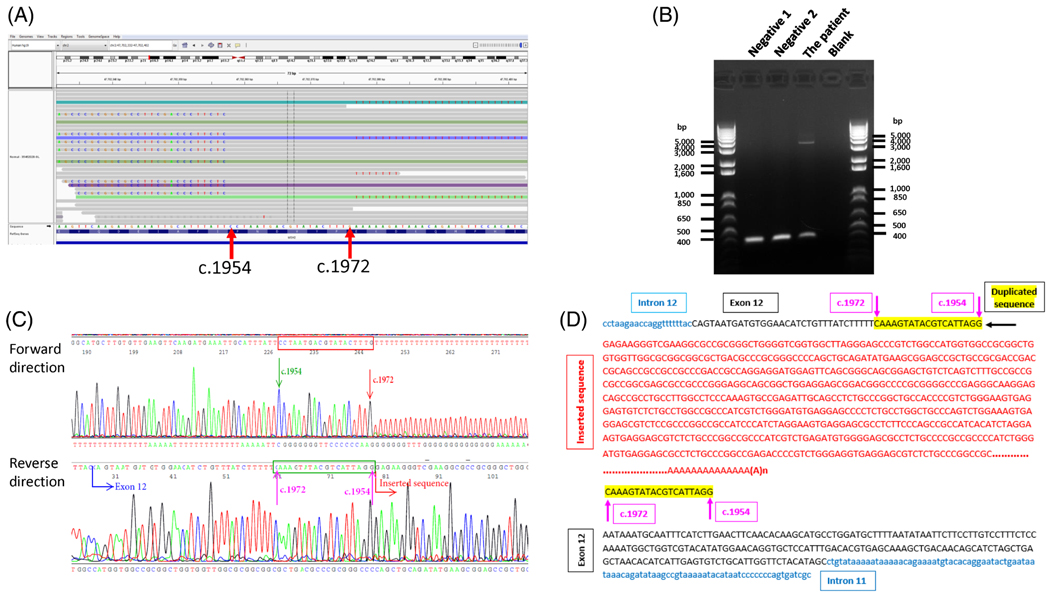
The proband was seen at the Clinical Genetics Service (CGS) at Memorial Sloan Kettering Cancer Center (MSKCC). Immunohistochemistry (IHC) analysis indicated loss of MSH2 and MSH6 proteins in the tumor. No MSH2 inversion was detected. Given the strong family history of colon cancer, a colorectal multi-gene panel test (sequencing and large rearrangement analysis of APC, EPCAM [large rearrangement only], MLH1, MSH2, MSH6, MUTYH, PMS2, POLD1, POLE, with add-on genes: PTEN, BRCA1, BRCA2) was performed at the Diagnostic Molecular Genetics laboratory at MSK. Testing identified aberrant sequences before c.1954 and after c.1972 positions in exon 12 of MSH2 (Figure 2A). No other mutations or VUSs were identified in the remaining 11 genes analyzed. The copy number of MSH2 exon 12 was normal based on our NGS analysis (Figure S2a) which was confirmed by multiplex ligation-dependent probe amplification (MLPA) (Figure S2b), indicating that the aberrant sequence was probably not due to a genomic deletion or duplication of the coding region of MSH2.
FIGURE 2.
MSK-IMPACT, long-range PCR and sequencing results after gel extraction. A, IGV shows aberrant sequence in exon 12 of MSH2. B, LR-PCR products run on 1% agarose gel. An extra band were observed in the patient, but not in controls. C, Electropherogram showing the site of insertion. D, Partial inserted sequences in red in anti-sense orientation with respect to the MSH2 gene transcription direction. Target site duplication is highlighted in yellow. DNA sequence in the first row matches with those in reverse direction in b
To investigate the nature and origin of the abnormal sequence, long-range (LR) PCR, was performed on genomic DNA from the patient. One band about 400 bp in length was present in negative controls (Figure 2B). Another band of a larger size (~3 to 4 kb) was observed in the patient but absent in the negative controls (Figure 2B). The larger aberrant fragment was extracted and sequenced with M13 forward and reverse primers. Sequence analysis of the extracted aberrant fragment revealed a targeted duplication of 19 bps, with the location of the insertion at c.1972, and part of the inserted sequence (660 bp) in an antisense orientation with respect to the MSH2 transcription direction (Figure 2C,D). The Repeat Masker (http://www.repeatmasker.org/cgi-bin/WEBRepeatMasker) indicated the inserted sequence belongs to an SVA element. To map the inserted sequence, we performed a BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi, human genome GRCh38.p12 reference, Annotation Release 109). A total of 27 hits covered almost all chromosomes, except chromosome 16, 18 and Y. The first alignment showed close to 100% homology between the 559 inserted sequence bp (except one nucleotide) and a region on chromosome 3 (Chr3: 48210600–48 211 258). The Repeat Masker indicated that an SVA repeat was present at this location.
The sequence from 48 210 600 to 48 220 000 in chromosome 3 was retrieved from the NCBI database and sequencing primers were designed based on the retrieved sequence. Apart from a short gap in the VNTR region that could not be sequenced due to the repetitive structure, we were able to decipher a total of 2937 bp inserted sequence without including the polyA tail in the antisense strand (-Figure S3a). The inserted sequence identified in our proband starts with a guanine nucleotide followed by a 295 bp exon 1 of the MAST2 gene, an Alu-like element (37 bp), an approximately 2.2 kb VNTR region with tandem repeats ranging from 37 to 54 bp long, a SINE-R region (492 bp), the putative polyadenylation signal AATAAA, and a long polyA tail. The inserted sequence is followed by a target site duplication (TSD) of 19 bps (MSH2 c.1954_1972) at the 3′end of the insertion (Figure 2D, Figure S3a). Interestingly, the inserted sequence is very close to the sequence from 48 210 600 to 48 213 111 in chromosome 3, except that the insertion has a longer VNTR (Figures S3a and S3b). Further analysis characterized it as a human specific SVA subfamily of retrotransposons termed SVA_F1 that contains a MAST2 5′ transduction group and is a fusion of MAST2 exon 1 containing CpG island and a 5′-truncated SVA17–19 (Figures S3c and S3d). Seventy-six members have been identified in the SVA_F1 subfamily in the human genome. In 96% of SVA_F1 members, the SVA element insert starts with a guanine residue19 and the SVA_F1 insertion in this case also starts with a “G”(Figure 2D, Figure S3a).
4 |. DISCUSSION
To date, 4015 unique mutations detected in the four MMR genes (MLH1, MSH2, MSH6, and PMS2) have been recorded in MMR genes in the Human Genome Mutation database (HGMD Professional 2020.4). Eleven (11) gross insertions larger than 20 base pairs (bp) have been reported, and five of these large insertions involved retrotransposons, four of which were Alu insertions with two in each of the MLH120,21 and MSH2 genes22,23 and one was an SVA insertion in PMS2.9 Up to now, no SVA insertion has been reported in MSH2. In this study, we identified an insertion of an SVA element at c.1972 in exon 12 of MSH2 in a patient with very strong family history of Lynch syndrome.
The SVA insertion in MSH2 exon 12 likely occurred through LINE-1-mediated retrotransposition as it exhibits several classical features of this process18,24 as shown in Figure 2D and Figure S3a: (a) insertion at consensus LINE-1 endonuclease cleavage site 5′-TTTT/AA-3′ (where “/”denotes the cleavage site); (b) the presence of a direct repeat TSD of 19 bp in length, within the size range of 4–20 bp that is typical for LINE-1 mediated retrotranspositions; (c) a long polyA tail preceded by the putative polyadenylation signal AATAAA; and (d) presence of 5′ transducing and truncation, a structural variation encompassing more than 8% of all SVA elements in the human genome.17,24,25
In silico splicing site prediction analysis using Splicing Site Prediction by Neural Network (https://www.fruitfly.org/seq_tools/splice.html) showed that this insertion introduces two donor sites. One is located at about 85 bp downstream of the poly T track with predicted splice score 0.69, and the other one is at approximately 313 bp downstream of the poly T track with score of 0.93. RT-PCR analysis indicated an aberrant MSH2 transcript with ~300 bp longer than the wild type transcript in one of the family members (Figure S1a). It is possible that the splice donor site ~313 bp downstream of poly T track is used to splice-out the remainder of the SVA, and DNA sequences downstream of the insertion, in conjunction with the canonical splice acceptor site of intron 12. Depending on the number of Ts in the polyT track, the splicing-in fragment may create a stop codon (TAA is located 24 bp downstream of polyT track) or trigger a frame-shift mutation introducing premature termination codons (PTC) located in the exonized SVA sequence. PTC may cause the degradation of the transcript by nonsense-mediated mRNA decay (NMD). This may explain the absence of the abnormal transcript in the cultured lymphocytes without cycloheximide treatment (Figure S1a).
According to ACMG variant classification criteria,16 this SVA insertion is classified as pathogenic (PVS1: predicted null variant, PM2: absent in population data, PP1: cosegregation with disease in multiple family members). However, it is difficult to detect such pathogenic SVA insertions by standard methods of genetic testing. Sanger sequencing of the coding region and flanking intronic sequences is normally used to detect point mutations, small deletions or small insertions, and MLPA is generally applied for the detection of large genomic deletions and duplications in diagnostic laboratories.26 The large SVA insertions in the MMR genes are most likely underreported because they are disproportionately overlooked with PCR-based mutation detection performed on patient genomic DNA27 or by the general NGS pipeline. Our CNV pipeline and MLPA analysis also showed there was no deletion or duplication present in MSH2 (Figures S2b and S2c). After the Alu pipeline was implicated in the bioinformatic analysis, it was detected with VAF of only 9% and LR PCR defined the identity of the insertion. From the clinical perspective, the Alu-pipeline should be implicated in the bioinformatic analysis to better detect similar variants.
It has been estimated that the contribution of retrotranspositional insertion to the total number of disease-causing mutations ranges from 0.17%28 to 0.4%. This would suggest that a total of 6 to 16 retrotranspositional events would have been detected according to the total number of 4015 variants identified in the four MMR genes (MLH1, MSH2, MSH6, and PMS2) in HGMD Professional 2020.4. To date, only 5 these elements have been identified. Therefore, some retrotranspositional insertions have been overlooked until now. For lynch syndrome suspected patients with clear indication of a mismatch repair defect (such as loss of MSH2 in IHC) and absence of detectable germline mutation, clinicians and diagnostic services may scrutinize them for this rather occult retrotranspotional insertion.
In summary, we describe here for the first time an SVA insertion into the coding sequence of MSH2 mediated by LINE-1 protein machinery. Precise location of SVA insertion and determination of the specific SVA sequence in the MSH2 gene are important for the interpretation of genetic variants, establishment of the diagnosis of Lynch Syndrome and cancer management to guide genetic testing of family members and potentially preimplantation genetic testing. Furthermore, cancer affected family members identified to have Lynch Syndrome may further benefit from immune checkpoint inhibitors which are FDA-approved for MMR deficient and MSI-H tumors, the hallmark of Lynch Syndrome associated tumors. Therefore, identification and characterization of the SVA elements and their roles in cancer predisposition genes can not only lead to a higher diagnostic yield of genetic testing, but also pave the path for genomic precision medicine and cancer prevention and therapy.
Supplementary Material
ACKNOWLEDGMENT
This study was funded by Department of Pathology, Memorial Sloan Kettering Cancer Center.
Funding information
Department of Pathology, Memorial Sloan Kettering Cancer Center
Footnotes
CONFLICT OF INTEREST
Z. K. S. reports that an immediate family member serves as a consultant in the field of ophthalmology for Adverum Biotechnologies, Genentech/Roche, Novartis, Neurogene, Gyroscope Tx, Optos Plc, Regeneron, RegenexBio, and Spark Therapeutics. Dr. Zhang: Honoraria (Future Technology Research LLC, BGI, Illumina); Honoraria and Travel and accommodation expenses (Roche Diagnostics Asia Pacific). Family members hold leadership position and ownership interests of Scipher Health.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
We have shared our data with the scientific community and deposited this variant into the ClinVar database. Here is the link: https://submit.ncbi.nlm.nih.gov/subs/clinvar_wizard/SUB8577511/overviewID:SUB8577511.
REFERENCES
- 1.de la Chapelle A. The incidence of Lynch syndrome. Fam Cancer. 2005;4(3):233–237. 10.1007/s10689-004-5811-3. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SA, Leininger A. The genetic basis of Lynch syndrome and its implications for clinical practice and risk management. Appl Clin Genet. 2014;7:147–158. 10.2147/TACG.S51483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Da Silva F, Wernhoff P, Dominguez-Barrera C, Dominguez-Valentin M. Update on hereditary colorectal cancer. Anticancer Res. 2016;36(9):4399–4405. 10.21873/anticanres.10983. [DOI] [PubMed] [Google Scholar]
- 4.Boland CR, Koi M, Chang DK, Carethers JM. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: from bench to bedside. Fam Cancer. 2008;7(1):41–52. 10.1007/s10689-007-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhees J, Arnold M, Boland CR. Inversion of exons 1–7 of the MSH2 gene is a frequent cause of unexplained Lynch syndrome in one local population. Fam Cancer. 2014;13(2):219–225. 10.1007/s10689-013-9688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16(3):357–361. 10.1007/s10689-016-9960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q, Hesson LB, Nunez AC, et al. A cryptic paracentric inversion of MSH2 exons 2–6 causes Lynch syndrome. Carcinogenesis. 2016;37(1):10–17. 10.1093/carcin/bgv154. [DOI] [PubMed] [Google Scholar]
- 8.Peltomaki P, Vasen H. Mutations associated with HNPCC predisposition – update of ICG-HNPCC/INSiGHT mutation database. Dis Markers. 2004;20(4–5):269–276. 10.1155/2004/305058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Klift HM, Tops CM, Hes FJ, Devilee P, Wijnen JT. Insertion of an SVA element, a nonautonomous retrotransposon, in PMS2 intron 7 as a novel cause of Lynch syndrome. Hum Mutat. 2012;33(7):1051–1055. 10.1002/humu.22092. [DOI] [PubMed] [Google Scholar]
- 10.Cordaux R, Batzer MA. The impact of retrotransposons on human genome evolution. Nat Rev Genet. 2009;10(10):691–703. 10.1038/nrg2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rebollo R, Romanish MT, Mager DL. Transposable elements: an abundant and natural source of regulatory sequences for host genes. Annu Rev Genet. 2012;46:21–42. 10.1146/annurev-genet-110711-155621. [DOI] [PubMed] [Google Scholar]
- 12.Hancks DC, Kazazian HH Jr. Roles for retrotransposon insertions in human disease. Mob DNA. 2016;7:9. 10.1186/s13100-016-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Klift HM, Tops CM, Bik EC, et al. Quantification of sequence exchange events between PMS2 and PMS2CL provides a basis for improved mutation scanning of Lynch syndrome patients. Hum Mutat. 2010;31(5):578–587. 10.1002/humu.21229. [DOI] [PubMed] [Google Scholar]
- 14.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015; 17(3):251–264. 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng DT, Prasad M, Chekaluk Y, et al. Comprehensive detection of germline variants by MSK-IMPACT, a clinical diagnostic platform for solid tumor molecular oncology and concurrent cancer predisposition testing. BMC Med Genomics. 2017;10(1):33. 10.1186/s12920-017-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damert A, Raiz J, Horn AV, et al. 5′-transducing SVA retrotransposon groups spread efficiently throughout the human genome. Genome Res. 2009;19(11):1992–2008. 10.1101/gr.093435.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hancks DC, Ewing AD, Chen JE, Tokunaga K, Kazazian HH Jr. Exon-trapping mediated by the human retrotransposon SVA. Genome Res. 2009;19(11):1983–1991. 10.1101/gr.093153.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bantysh OB, Buzdin AA. Novel family of human transposable elements formed due to fusion of the first exon of gene MAST2 with retrotransposon SVA. Biochemistry (Mosc). 2009;74(12):1393–1399. 10.1134/s0006297909120153. [DOI] [PubMed] [Google Scholar]
- 20.Leclerc J, Flament C, Lovecchio T, et al. Diversity of genetic events associated with MLH1 promoter methylation in Lynch syndrome families with heritable constitutional epimutation. Genet Med. 2018;20 (12):1589–1599. 10.1038/gim.2018.47. [DOI] [PubMed] [Google Scholar]
- 21.Solassol J, Larrieux M, Leclerc J, et al. Alu element insertion in the MLH1 exon 6 coding sequence as a mutation predisposing to Lynch syndrome. Hum Mutat. 2019;40(6):716–720. 10.1002/humu.23725. [DOI] [PubMed] [Google Scholar]
- 22.Marshall B, Isidro G, Boavida MG. Insertion of a short Alu sequence into the hMSH2 gene following a double cross over next to sequences with chi homology. Gene. 1996;174(1):175–179. 10.1016/0378-1119(96)00515-x. [DOI] [PubMed] [Google Scholar]
- 23.Kloor M, Sutter C, Wentzensen N, et al. A large MSH2 Alu insertion mutation causes HNPCC in a German kindred. Hum Genet. 2004;115 (5):432–438. 10.1007/s00439-004-1176-9. [DOI] [PubMed] [Google Scholar]
- 24.Raiz J, Damert A, Chira S, et al. The non-autonomous retrotransposon SVA is trans-mobilized by the human LINE-1 protein machinery. Nucleic Acids Res. 2012;40(4):1666–1683. 10.1093/nar/gkr863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Xing J, Grover D, et al. SVA elements: a hominid-specific retroposon family. J Mol Biol. 2005;354(4):994–1007. 10.1016/j.jmb.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 26.Tops CM, Wijnen JT, Hes FJ. Introduction to molecular and clinical genetics of colorectal cancer syndromes. Best Pract Res Clin Gastroenterol. 2009;23(2):127–146. 10.1016/j.bpg.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Chen JM, Ferec C, Cooper DN. LINE-1 endonuclease-dependent retrotranspositional events causing human genetic disease: mutation detection bias and multiple mechanisms of target gene disruption. J Biomed Biotechnol. 2006;2006(1):56182–56189. 10.1155/JBB/2006/56182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazazian HH Jr. An estimated frequency of endogenous insertional mutations in humans. Nat Genet. 1999;22(2):130. 10.1038/9638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have shared our data with the scientific community and deposited this variant into the ClinVar database. Here is the link: https://submit.ncbi.nlm.nih.gov/subs/clinvar_wizard/SUB8577511/overviewID:SUB8577511.