Abstract
Background
Although fecal microbiota transplant has been used to prevent recurrent Clostridioides difficile infection (rCDI), documented pathogen transmissions highlight inherent safety risks of minimally processed stool. We describe manufacturing processes for fecal microbiota spores, live (VOWST; VOS, formerly SER-109), a microbiota-based oral therapeutic of Firmicutes spores.
Methods
Bacterial inactivation kill curves were obtained after ethanol exposure for 4 model organisms spiked into process intermediates.
Results
Bacterial log reduction factors ranged from 6.5 log10 to 7.4 log10 and lysis of spiked organisms occurred rapidly within 30 seconds.
Conclusions
These experiments demonstrate substantial and rapid inactivation of representative organisms, supporting the potential benefit of VOS manufacturing processes to mitigate risk.
Keywords: Clostridioides difficile infection, microbiome, microbiome therapeutic
The pathogenesis of Clostridioides difficile infection (CDI) requires a “2-hit” process: exposure to spores and antibiotic-mediated disruption of the gastrointestinal microbiome, which plays a key role in preventing spore germination into toxin-producing vegetative bacteria. Toxin production can cause symptoms ranging from mild diarrhea to life-threatening colitis. C. difficile-targeted antibiotics resolve symptoms through rapid killing of vegetative bacteria but have no effect on spores, which germinate within a low-diversity microbiome, facilitating recurrent CDI (rCDI) [1]. Fecal microbiota transplantation (FMT) is used to prevent rCDI, as microbiome repair is an essential therapeutic goal when antibiotics fail. Efficacy rates range from 67% in controlled trials to 82% in observational case series, leading to guideline recommendations for FMT in rCDI, based on moderate-quality evidence [2, 3].
Recent reports have concluded that FMT has a favorable safety profile without transmission of infectious agents [4–6]. However, the safety follow-up in 1 report from a stool bank [4] ended in 2018 prior to transmission events in early 2019, which prompted Food and Drug Administration safety alerts [7]. One alert highlighted transmissions of Shiga-toxin producing Escherichia coli to 5 recipients, leading to hospitalizations and 1 cardiorenal death in a patient with preexisting cardiac disease; whether this pathogen played a role in the patient's demise was unclear [8]. In addition, transmission of extended-spectrum, β-lactamase–producing Escherichia coli from a hospital-based FMT program was identified with prospective adverse event (AE) monitoring within 2 FMT trials [9]. One immunocompromised patient died, another was hospitalized with bacteremia, and 5 became colonized. These transmission events highlight inherent safety risks of FMT [10].
Potential risk mitigation includes continuous review for new pathogens with fecal shedding, screening every donation, quarantining donations with bookend screening, and comprehensive, standardized donor testing. These methodologies may increase costs and safety concerns remain. Most assays that assess for pathogens are validated in patients with active infection rather than in asymptomatic carriers who may have lower levels of fecal shedding. Furthermore, donor screening algorithms are focused on known organisms while emerging pathogens require de novo assay development with inherent delays. Finally, retrospective AE reporting can preclude timely recognition of a donor as a transmission source [1, 11]. A strategic therapeutic approach that provides efficacy while mitigating risk is needed.
Fecal microbiota spores, live VOWST (formerly SER-109 and hereafter referred to as VOS for VOWST oral spores), a microbiota-based oral therapeutic comprised of Firmicutes bacterial spores, was superior to placebo in reducing risk of rCDI in a randomized controlled phase 3 trial in patients with history of recurrence [12]. The manufacturing process was designed to achieve a purified Firmicutes bacterial spore product through exposure to high ethanol concentrations to selectively inactivate nonproduct, vegetative bacteria, including potential undetected pathogenic bacteria. We evaluated the effect of ethanol-based inactivation operations on representative vegetative bacteria.
METHODS
The inactivation step was validated using spiking studies whereby specific model organisms were incorporated into manufacturing process intermediates and bacterial kill curves were measured after ethanol exposure. Four species were used to model the range of pathogenic or drug-resistant bacteria: Salmonella enterica for rod-shaped, gram-negative pathogens such as Escherichia coli and representative of carbapenem-resistant Enterobacteraceae; Listeria innocua for rod-shaped, gram-positive pathogens such as Listeria or Corynebacterium; Enterococcus faecalis for Enterococcus and Streptococcus including vancomycin-resistant enterococci; and Staphylococcus aureus for gram-positive, clustering cocci such as methicillin-resistant S. aureus (see Table 1 for characteristics of each strain). Strain sources, study execution, and methods to assess relative impact to risk mitigation are described in the Supplementary Materials.
Table 1.
Characteristics of Model Organisms
| Bacterial species | Shape | Cell Wall | Model Organism |
|---|---|---|---|
| Salmonella enterica | Rod | Gram-negative | Rod-shaped gram-negative pathogenic bacteria such as enteropathogenic Escherichia coli and carbapenem-resistant Enterobacteriaceae |
| Listeria innocua | Rod | Gram-positive | Rod-shaped gram-positive pathogenic bacteria such as Listeria or Corynebacterium |
| Enterococcus faecalis | Cocci in chains | Gram-positive | Gram-positive diplococcus, representative of Enterococcus and Streptococcus, including vancomycin-resistant enterococci |
| Staphylococcus aureus | Cocci in clusters | Gram-positive | Gram-positive clustering cocci including methicillin-resistant Staphylococcus aureus |
RESULTS
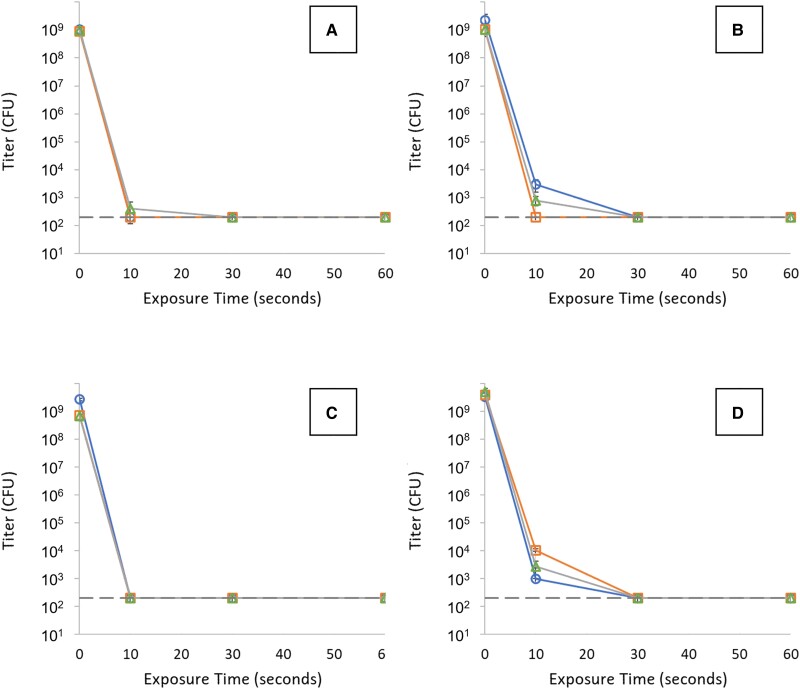
Bacterial log reduction factors ranged from 6.5 log10 to 7.4 log10 (Figure 1). The full range of estimates is limited by the maximum bacterial titers that could be achieved in culture and the lower limits of assay detection. In all cases, lysis of the spiked organisms in the process matrix was rapid with titers reaching the assay limit of detection within 30 seconds for every replicate (Figure 1). The minimum observed inactivation rate was 13.4 log10/minute corresponding to an inactivation D value (time required to achieve 1 log10 reduction) of 4.5 seconds, consistent with rapid bacterial inactivation. For perspective, the reported D value for steam autoclaving is 20 times slower at 1.5 minutes. The VOS manufacturing process maintains an ethanol contact for a minimum of 5 minutes, which is capable of bacterial inactivation well in excess of the total vegetative bacterial content of donor stool.
Figure 1.
Inactivation kill curves (titer vs exposure time) obtained for 4 model organisms spiked into VOWST manufacturing process intermediates: (A) Salmonella enterica, (B) Listeria inoccua, (C) Enterococcus faecalis, and (D) Staphylococcus aureus. Each graph contains 3 replicate studies for each organism. Horizontal dashed lines represent LOD (200 CFU/mL) for the plating assay. Error bars are presented for each sample with a titer above LOD and represent 1 standard deviation of the measured value based on triplicate measurements. Abbreviations: CFU, colony-forming unit; LOD, limit of detection.
DISCUSSION
These experiments demonstrate substantial and rapid inactivation of representative model organisms, supporting the potential benefit of VOS manufacturing processes to mitigate risk to patients while providing beneficial Firmicutes needed for efficacy in reducing risk of rCDI. Firmicutes spores possess several inherent attributes that enable selective purification and efficient drug delivery. Resistance of spores to ethanol enables selective inactivation of vegetative microbes, including drug-resistant bacteria. Spore dormancy, which includes resistance to aerobic conditions, enables the full processing time required for spore purification from fibers and fecal solids, leading to removal of 99% of the total mass of donor materials. Spore resistance to gastric acid and oxygen enables oral delivery. Germination of spores into replicating vegetative bacteria also supports a low pill burden.
Although the reported risk is low, FMT-related transmissions of pathogenic bacteria are arguably predictable outcomes and may be underreported due to few controlled trials with rigorous AE reporting [10]. Additionally, emerging bacterial pathogens are continually being recognized and cannot always be anticipated. Drug-resistant bacteria can lead to hard-to-treat infections with high rates of morbidity and mortality, highlighting the implications of undetected bacterial pathogens, which have been identified in healthy community residents without demographic risk factors [13]. Finally, there remains no safety net for FMT products when screening failures occur due to false-negative testing, asymptomatic carriage below the limit of assay detection, or unrecognized novel bacterial pathogens. As demonstrated here, validated processes for inactivation reduce bacterial pathogen transmission risk beyond donor screening alone. In addition, we reported that the VOS manufacturing steps inactivate fungi, parasites, and viruses, including a model coronavirus [14, 15].
In conclusion, clinical development of microbiome therapeutics needs to consider efficacy in addition to risk mitigation. VOS manufacturing processes lead to a purified Firmicutes product providing efficacy, as observed in a randomized controlled trial, while mitigating pathogen transmission risk, a highly desired outcome for vulnerable patients at risk for rCDI.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Christopher W J McChalicher, Seres Therapeutics, Cambridge, Massachusetts, USA.
Mary-Jane Lombardo, Seres Therapeutics, Cambridge, Massachusetts, USA.
Sahil Khanna, Mayo Clinic, Rochester, Minnesota, USA.
Gregory J McKenzie, Seres Therapeutics, Cambridge, Massachusetts, USA.
Elizabeth M Halvorsen, Seres Therapeutics, Cambridge, Massachusetts, USA.
Sanabel Almomani, Seres Therapeutics, Cambridge, Massachusetts, USA.
Brian Schuster, Seres Therapeutics, Cambridge, Massachusetts, USA.
Brooke R Hasson, Seres Therapeutics, Cambridge, Massachusetts, USA.
Barbara H McGovern, Seres Therapeutics, Cambridge, Massachusetts, USA.
David S Ege, Seres Therapeutics, Cambridge, Massachusetts, USA.
John G Auniņš, Seres Therapeutics, Cambridge, Massachusetts, USA.
Notes
Author contributions. C. W. J. M. contributed overall study supervision; study concept and design; validation, analysis and interpretation of the data; drafting of the manuscript; and critical revision of the manuscript. M.-J. L. contributed study design and review and critical revision of the manuscript. S. K. and B. R. H. performed review and critical revision of the manuscript. G. J. M. contributed study concept and design, and validation and data curation. B. H. M. performed drafting of the manuscript, and review and critical revision of the manuscript. J. G. A. contributed study concept and design, overall study supervision, and review and critical revision of the manuscript. E. H. performed validation and data curation. S. A. and B. S. performed acquisition of data. D. S. E. contributed overall study supervision, and review and critical revision of the manuscript. All authors approved the final draft submitted.
Financial support. This work was supported by Seres Therapeutics.
References
- 1. Wilcox MH, McGovern BH, Hecht GA. The efficacy and safety of fecal microbiota transplant for recurrent Clostridium difficile infection: current understanding and gap analysis. Open Forum Infect Dis 2020; 7:ofaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73:e1029–44. [DOI] [PubMed] [Google Scholar]
- 3. Tariq R, Pardi DS, Bartlett MG, Khanna S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis 2018; 68:1351–8. [DOI] [PubMed] [Google Scholar]
- 4. Osman M, Budree S, Kelly CR, et al. Effectiveness and safety of fecal microbiota transplantation for Clostridioides difficile infection: results from a 5,344 patient cohort study. Gastroenterology 2022; 163:319–22. [DOI] [PubMed] [Google Scholar]
- 5. Saha S, Mara K, Pardi DS, Khanna S. Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Gastroenterology 2021; 160:1961–9.e3. [DOI] [PubMed] [Google Scholar]
- 6. Kelly CR, Yen EF, Grinspan AM, et al. Fecal microbiota transplantation is highly effective in real-world practice: initial results from the FMT national registry. Gastroenterology 2021; 160:183–92.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Food and Drug Administration . Important safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse reactions due to transmission of multi-drug resistant organisms, 2019. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse. Accessed 20 July 2022.
- 8. Food and Drug Administration . Safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse events likely due to transmission of pathogenic organisms, 2020. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse-events-likely. Accessed 20 July 2022.
- 9. DeFilipp Z, Bloom PP, Soto MT, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. New Engl J Med 2019; 381:2043–50. [DOI] [PubMed] [Google Scholar]
- 10. Blaser MJ. Fecal microbiota transplantation for dysbiosis—predictable risks. New Engl J Med 2019; 381:2064–6. [DOI] [PubMed] [Google Scholar]
- 11. Zellmer C, Sater MRA, Huntley MH, Osman M, Olesen SW, Ramakrishna B. Shiga toxin-producing Escherichia coli transmission via fecal microbiota transplant. Clin Infect Dis 2020; 72:e876–80. [DOI] [PubMed] [Google Scholar]
- 12. Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. New Engl J Med 2022; 386:220–9. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention . Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.
- 14. McChalicher C, Abdulaziz A, Zhou SS, et al. Manufacturing process of SER-109, a purified investigational microbiome therapeutic, reduces risk of coronavirus transmission from donor stool. Open Forum Infect Dis 2022; 9:ofac448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGovern BH, Ford CB, Henn MR, et al. SER-109, an investigational microbiome drug to reduce recurrence after Clostridioides difficile infection: lessons learned from a phase 2 trial. Clin Infect Dis 2021; 72:2132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.