Abstract
Background
There is no immunization campaign that currently exist for respiratory syncytial virus (RSV). Seroprevalence studies are critical for assessing epidemiological dynamics before and during an immunization program. A systematic literature review was conducted to summarize the evidence from seroprevalence studies on RSV.
Methods
A systematic search of age-dependent RSV seroprevalence was conducted using the PubMed database and EMBASE. Age-dependent force of infections (FoI) and the decay rate of immunity were estimated. A mixture finite model was used, estimating the age-dependent disease state and the antibody concentrations in susceptible and infected or recovered populations.
Results
Twenty-one studies were identified from 15 countries, with studies using enzyme-linked immunosorbent assay being the most represented. Using a catalytic model, the age-dependent force of infection was estimated to be the lowest in infants aged 6 months to 1 year and increased in older age groups. The proportion ever-infected/recovered was estimated to be above 90% by 3 years of age.
Conclusions
The number of seroprevalence studies covering a broad range of ages are limited. The age-dependent FoI indicated that the risk of infection was greatest among those aged >5 years. Additional data using valid assays are required to describe the transmission dynamics of RSV infection.
Keywords: respiratory syncytial virus, risk of infection, RSV, seroepidemiology, statistical model
A systematic review was conducted to summarize the evidence from seroprevalence studies on RSV. Using a catalytic model, the age-dependent force of infection was estimated to be greatest among those aged >5 years.
Respiratory syncytial virus (RSV) is a single-stranded ribonucleic acid virus belonging to the family Pneumoviridae [1]. Manifestations of RSV infection range from mild upper respiratory symptoms to severe lower respiratory infections such as bronchiolitis and pneumonia, which can lead to death in high-risk populations such as infants or older individuals [2]. Because approved vaccines are lacking for any age group, the virus causes seasonal epidemics or local outbreaks, and it has a substantial public health impact globally [3, 4].
Seroprevalence studies are critical for assessing the epidemiological situation at the population level before and during the implementation of an immunization program [5, 6]. By sampling serum specimens randomly and cross-sectionally, seroprevalence data allow us to infer age-dependent seropositivity to specific antigens, which is representative of the infection status. Parameters governing transmission dynamics can also be estimated using data from seroprevalence studies, including the force of infection ([FoI] the rate at which susceptible individuals are infected), the incidence rate, and the basic reproduction number [7–9].
However, there are few national initiatives monitoring the seroprevalence of RSV. For example, among 29 European Union countries, only the Netherlands has implemented a population seroprevalence study for RSV, 23 countries have reported that they have neither implemented nor plan to implement such a study [10]. One potential technical difficulty with such studies is that serological markers determining susceptibility (or ever infected/recovered) to RSV infection have not been fully elucidated [11, 12]. Furthermore, the role of humoral immunity, such as RSV-specific immunoglobulin (Ig)G or neutralizing antibodies, has not been well established in adults, whereas the respiratory mucosal IgA level seems to reflect the history of infection in infants [13]. Another challenge is that RSV may infect several times during the lifetime of an individual, complicating epidemiological interpretation of seroprevalence data [14, 15]. Despite these issues, reviewing the current knowledge on the age-dependent serostatus of RSV would be significant in providing a deeper understanding of the epidemiology of RSV, and this may lead to future national or global scale surveys to understand the burden of RSV infection.
In this study, a systematic literature review was conducted to summarize the available evidence from seroprevalence studies on RSV at the population level. Using an age-dependent mathematical model (Bayesian estimation method) of reinfection, the age-dependent FoI and the decay rate of immunity in RSV infection were estimated, based on the identified seroprevalence data using a range of different assays: enzyme-linked immunosorbent assay (ELISA), neutralization, or complement fixation (CF). Motivated by recent work on the serological profile in a broad range of populations of anti-pre-F RSV protein, which is now considered as responsible for the majority of neutralizing antibodies against RSV [16, 17], a mixture finite model was also used, estimating the age-dependent disease state, as well as antibody concentration levels, in susceptible and infected or recovered populations.
METHODS
Systematic Literature Review
Search Strategy
This systematic review was conducted in accordance with the PRISMA statement [18]. We conducted searches of the PubMed database and EMBASE on May 2, 2022, using the search string “respiratory syncytial virus AND (antibody OR sero*)” in “Title/Abstract”. After submission of the first draft of the manuscript, additional relevant studies were searched using the same searching strategy from May 3, 2022 to March 6, 2023.
Study Selection
All titles and abstracts identified by the search strategy were independently screened by 2 authors (KN and HN). Studies on specific subgroups (eg, individuals infected with human immunodeficiency virus, patients with chronic obstructive pulmonary disease, healthcare workers, military workers) reporting the serostatus in symptomatic cases or maternal-cord pairs were excluded. Studies following specific cohort groups (eg, birth cohort) were also excluded. The same criteria were used for full-text eligibility. As an outcome, age-dependent seroprevalence data (ie, reports on the number of positive tests among samples) were sought, and such reports were collected manually and summarized descriptively.
Mathematical Model
Estimation of the Force of Infection and the Rate of Decay in Each Assay
To estimate the age-dependent FoI and decay rate of seropositivity, we analyzed each set of seroprevalence data independently. Any studies reporting the number of positive tests among the samples for 2 or more age-groups (including ages <5 years) were included as a subject. The so-called “catalytic model” was used to capture the age-dependent infection dynamics of RSV, and considering the importance of maternal immunity during infancy, an MSIS (Maternal - Susceptible - Infected/Recovered - Susceptible) model was selected for our analysis [15, 19]. The MSIS model assumes that any individual can become seropositive either due to maternal antibody or natural infection, and the movement between states are governed by transition rates. The FoI, that is, hazard of seroconversion due to infection, was assumed to be age dependent. We modeled FoIs as piecewise constant, that is, FoI takes constant values , , and in the following ages, that is, 0.5–1 year, 1–5 years, and 5 years and over, respectively. The decay rate of seropositivity, ω, was assumed to be a constant across all ages. A conventional qualitative approximation of maternal immunity was used, that is, all individuals aged <0.5 years are immune due to maternal antibodies, and they become susceptible once they reach 6 months of age [8]. We denote the age at which maternal antibodies are lost by , and the expressions of seroprevalence in each age category, , are as follows:
| (1) |
where , , and are 6 months, 1 year, and 5 years, respectively. The observed age-dependent number of seropositive results was assumed to follow a binomial distribution. Supposing that there were seropositive and seronegative individuals observed in each age a from the seroprevalence study i, the likelihood function is proportional to the following:
| (2) |
where , and are the FoI for each age band and data from seroprevalence study i, respectively. The rate of decay, ω, was assumed to be identical among studies that share the same assay method.
Estimation of the Age-Dependent Disease Status for Respiratory Syncytial Virus Infection
To estimate the age-dependent proportion of ever-infected/recovered individuals for RSV infection, data on the anti-pre-F protein antibody concentration from Andeweg et al [16] and Berbers et al [17] were analyzed. The term “ever-infected/recovered for RSV infection” is specifically used here, because this immunological marker is considered responsible for the majority of neutralizing antibodies against RSV and therefore reflects the recovered status from RSV more accurately than other serological assays [20]. The finite mixture model was used to jointly quantify the age-dependent disease status and the anti-pre-F protein antibody concentration in the susceptible and ever-infected groups [21, 22]. For children aged <5 years, the mixture model was formulated using a hierarchical model that involves a latent variable indicating the unknown true disease status, that is, susceptible or ever-infected/recovered. Using the antibody concentration in each individual child aged <5 years [16], the likelihood of the antibody concentration (log scale) in each subject i, , and the latent classification of disease status, , are presented as follows:
| (3) |
| (4) |
where , , , are the means and variances for the susceptible group and the ever-infected/recovered group, respectively. is each individual's probability of being ever-infected/recovered as a function of his/her age. For the population aged >5 years for which individual antibody data were not available, we used the sample mean values for prespecified age groups [17]; the likelihood of the sample mean of the antibody concentration in each age category a, , is presented as follows:
| (5) |
where is the variance for each age category. Sensitivity analysis was conducted to test an alternative catalytic model that incorporated a boosting effect due to cellular immunity, which has been suggested by recent studies [23–25] (see Supplementary Data for the model and the analytical solutions).
Statistical Analysis
Bayesian estimation was applied throughout the following analyses [9]. For selection of the priors in the first part, we referred to 2 previous reports: using the best fitted MSIS model, Nyiro et al [19] estimated the FoI for 0- to 1-year-olds and for 1- to 12-year-olds as 0.78 (95% confidence interval [CI], .65–.97) and 1.69 (95% CI, 1.27–2.04) per year, respectively, and Nakajo and Nishiura [15] estimated the FoI for primary and secondary infection as 0.12 per month (95% CI, .07–.18) and 0.05 per month (95% CI, 0.04–0.06), respectively. Consequently, a gamma prior distribution with a mean of 0.8 per year and a standard deviation (SD) of 0.1 (shape and rate parameters: 64 and 80), and a gamma prior distribution with a mean of 1.7 per year and an SD of 0.2 (shape and rate parameters: 64 and 40), were chosen to estimate the FoI for those aged 0.5 to 1 year and 1 to 5 years, respectively. The prior distribution for those >5 years was set the same as that for children aged 1 to 5 years. A uniform distribution was also assessed as a noninformative prior distribution of FoI (Supplementary Data). For the rate of decay, a uniform distribution from 0 to 5 decay per year was chosen, given that there was little information on this particular parameter.
For the estimation of age-dependent disease status using a mixture model, the results of the posterior distribution for the FoI and the rate of decay in the first part were fully utilized. For the priors for the distribution of the 3 variances in the model, the half-Student-t-distribution (ν=4, μ = 0, σ = 10) was specifically selected. Utilizing estimates with an age-independent catalytic model, a gamma distribution with a mean of 0.8 per year and an SD of 0.1 was chosen as the prior for the FoI for those aged 0.5 to 1 year. For those aged 1 to 5 years and >5 years, a gamma distribution with a mean of 1.5 per year and an SD of 0.2 was adopted. For the rate of decay, a gamma distribution with a mean of 0.1 and an SD of 0.02 was specifically chosen.
The Bayesian inference was implemented in Stan Markov and Monte Carlo with the No-U-Turn sampler algorithm being used to estimate model parameters. The GR statistic (R-hat) was used to judge Markov chain Monte Carlo convergence with a threshold of <1.1. For model selection, widely applicable information criterion (WAIC) and leave-one-out (LOO) cross-validation using Pareto-smoothed importance sampling were used [26]. All analyses were conducted using R version 4.0.3 [27]. This study used publicly available data, and thus ethical approval was not required.
RESULTS
Selected Studies
The flow chart of the literature search is shown in Figure 1. We identified 22 seroprevalence studies [16, 19, 28–47]. One study was excluded because age-specific data were not available [33]. The remaining 21 studies reporting age-dependent seroprevalence from 15 countries are summarized in Table 1.
Figure 1.
Flow diagram of the study selection.
Table 1.
Summary of the Studies Included in the Systematic Review
| Authors (Year Published) | Country | Year Sampled | Age | Sample Size | Assay | Antigen |
|---|---|---|---|---|---|---|
| Amaku (2009); Cox (1998) | Brazil | 1990–1991 | 0 to 40 years | 549 | ELISA IgG | Viral components |
| Andeweg (2021) | Netherlands | 2006–2007 and 2016–2017 | 0 to < 5 years | 682 | MIA | IgG and IgA for Pre-F, Post-F and N |
| Arankalle (2019) | India | 2016–2018 | 0 to 85 years | 695 | ELISA IgG and IgM | Viral components |
| Bhattarakosol (2003) | Thailand | Not specified | 0 to 5 years | 119 | ELISA IgG | Viral components |
| Brüssow (1991) | Equador, Germany | Not specified | 0 to 5 years | 1397 (E); 340 (G) | ELISA IgG and IgM | Viral components |
| Dunn (2013) | US | 1989–2001 | 0 to >5 years | 282 | ELISA IgG | F protein |
| Ebihara (2004) | Japan | 2001–2002 | 0 to 5 years | 100 | Neutralization | Whole virus |
| Golubjatnikov (1975) | Mexico | 1968 | 0 to 18 years | 642 | CF | Whole virus |
| Jennings (1972) | Jamaica | Not specified | 0 to >40 years | 558 | CF | Whole virus |
| Leogrande (1992) | Italy | 1989–1990 | 0 to 15 years | 2514 | Neutralization | Whole virus |
| Liu (2022) | China | 2017–2018 | Adult | 720 | MNA | Whole virus |
| Lu (2011) | China | 2008 | 0 to >60 years | 1156 | ELISA IgG | N protein |
| Madhavan (1974) | India | 1973 | 0 to >40 years | 316 | CF | Whole virus |
| Moss (1963) | UK | 1961–1962 | 0 to >15 years | 149 | CF | Whole virus |
| Njoku-Obi (1966) | Nigeria | 1962–64 | 0 to >45 years | 309 | CF | Whole virus |
| Nyiro (2017) | Kenya | 2007–2010 | 0 to <12 years | 960 | ELISA IgG | Viral components |
| Sastre (2012) | Germany | Not specified | 0 to 89 years | 1811 | ELISA IgG | F protein |
| Suto (1965) | Japan | 1963–1964 | 0 to 18 years | 514 | Neutralization | Whole virus |
| Terrosi (2009) | Italy | 2008 | 20 to over 80 years | 197 | IFA and MNA | Whole virus |
| Zhang (2008) | China | Not specified | 0 to 6 years | 324 | ELISA IgG | Viral components |
Abbreviations: CF, complement fixation; ELISA, enzyme-linked immunosorbent assay; IFA, indirect immunofluorescence assay; Ig, immunoglobulin; MIA, multiplex immunoassay; MNA, microneutralization assay; UK, United Kingdom; US, United States.
Seroprevalence
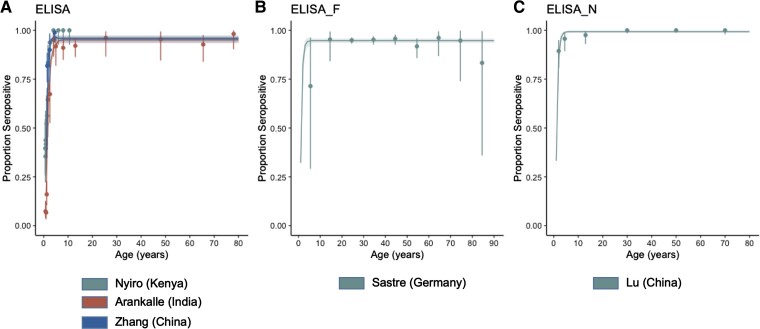
The patterns of age-dependent seroprevalence were similar regardless of the methodology, with the exception of CF. The observed age-dependent seroprevalence for each assay is shown for studies with digitalized data of 2 or more age groups including those aged <5 years (Figure 2).
Figure 2.
Age-specific seroprevalence of respiratory syncytial virus as determined by (A) enzyme-linked immunosorbent assay (ELISA), (B) ELISA for F protein, and (C) ELISA for N protein, respectively (0.5 year). Points and whiskers represent the observed proportions with their confidence intervals. The lines represent predictive seroprevalence curves with 95% credible intervals sampled from the fitted model.
For ELISA, 7 studies were identified from China, India, Kenya, Brazil, Thailand, Germany, and Ecuador (with 2 studies using the same data [28, 32] and 1 study using data from 2 countries [31]). Seropositivity of 100% was observed at 0 months [19, 31, 32]. Seropositivity was lowest approximately 6 to 12 months [19, 30–32, 46] then increased, reaching almost 100% approximately 3 years [19, 46] (Figure 2A). Among adults and older individuals, seropositivity was almost 100% [29, 32]. For ELISA of F protein and N protein, 2 studies (United States and Germany) [34, 43] and 1 study [39] were identified, respectively. The patterns of seropositivity for both assays were similar to those for ELISA, except that for F protein a decrease was observed in those aged 80 years and over [43] (Figure 2B and C).
Two studies from Japan [35, 44], 2 studies from Italy [38, 45], and 1 from China [47] were identified as using a neutralization assay; 3 studies on children or adolescents [35, 38, 44] and 1 on adults and older individuals [45]. One study targeting an adult group did not provide detailed information on age [47]. Seropositivity was high at birth (50%–89%). Once reaching its nadir at approximately 4 months to 1 year, it gradually increased with age to >80% in the oldest age category in all 3 studies investigating children/adolescents (Supplementary Figure 2A). Among adults and the older population, seroprevalence was highest at 93% in the youngest age group (ie, 20 to 60 years) and decreased with age to 36% in the >80 years group [45].
A more recent study in the Netherlands [16] examined age-dependent seroprevalence using a multiplex immunoassay for pre-F protein. In that study, “previously infected” was defined using IgA and IgG cutoffs among children aged <5 years. The proportion increased and reached 100% at approximately 20 months of age. As for indirect immunofluorescence assays, a single study from Italy was identified, clarifying the seroprevalence among adults including the older generation [45]. Seropositivity among adults was estimated to be >80%.
Five studies using a CF assay were performed in the 1960s−1970s [36, 37, 40–42]. Seroprevalence varied depending on the cutoffs that were independently determined. The proportion of seropositivity was highest in early infancy with a prevalence of approximately 40%–50% (Supplementary Figure 2B).
The Force of Infection and the Rate of Decay
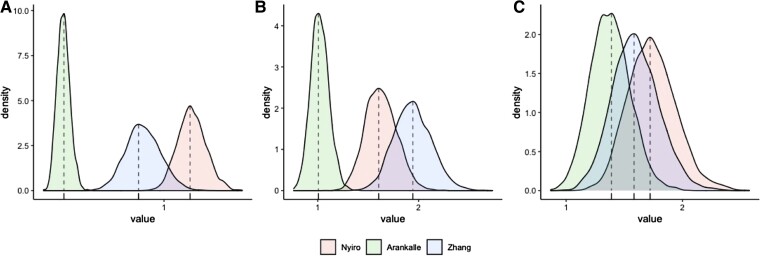
Our catalytic model allowed for estimation of the FoI and the rate of antibody decay by fitting to digitally available data from 10 studies, which involved at least 2 age groups (3 for ELISA, 1 for ELISA of F protein, 1 for ELISA of N protein, 1 for a neutralization assay, and 4 for CF). The FoI values across the 3 studies utilizing ELISA were 0.38 per year (95% credible interval [CrI], 0.30–0.46) to 1.17 per year (95% CrI, 1.01–1.36), 1.02 per year (95% CrI, 0.85–1.21) to 1.94 per year (95% CrI, 1.59–2.32), and 1.38 per year (95% CrI, 1.06–1.73) to 1.74 per year (95% CrI, 1.37–2.18) for those aged 0.5 to 1 year, 1 to 5 years, and 5 years and over, respectively (Table 2 and Figure 3). The rate of decay was estimated to be 0.07 per year (95% CrI, 0.05–0.10). The predicted age-dependent seropositivity captured the observed pattern accurately (Figure 2A). The estimated FoI for ELISA-F protein and ELISA-N protein were similar, whereas the rates of decay were 0.09 per year (95% CrI, 0.06–0.12) and 0.01 per year (95% CrI, 0.00–0.02), respectively (Table 2 and Figure 2B and C). The results of the neutralization assay and CF are shown in the Supplementary Material (Supplementary Table 1 and Figure 2A and B).
Table 2.
Estimated Parameters From the Catalytic Model (Median and 95% Credible Interval)
| Assay | Study | FoI (/Year) (Aged 0.5 to 1 Years) | FoI (/Year) (Aged 1 to 5 Years) | FoI (/Year) (Aged 5 Years and More) | Decay (/Year) |
|---|---|---|---|---|---|
| ELISA | Arankalle | 0.38 (0.30–0.46) | 1.02 (0.85–1.21) | 1.38 (1.06–1.73) | 0.07 (0.05–0.10) |
| Nyiro | 1.17 (1.01–1.36) | 1.63 (1.34–1.94) | 1.74 (1.37–2.18) | ||
| Zhang | 0.87 (0.67–1.09) | 1.94 (1.59–2.32) | 1.60 (1.23–2.01) | ||
| ELISA F | Sastre | 0.80 (0.61–1.02) | 1.58 (1.22–1.98) | 1.64 (1.27–2.05) | 0.09 (0.06–0.12) |
| ELISA N | Lu | 0.82 (0.63–1.02) | 1.56 (1.24–1.91) | 1.67 (1.30–2.09) | 0.01 (0.00–0.02) |
Abbreviation: ELISA, enzyme-linked immunosorbent assay; FoI, force of infections.
Figure 3.
Posterior distribution of force of infections for the age groups: (A) 0.5 to 1 year, (B) 1 to 5 years, and (C) 5 years and over. The posterior means are represented by vertical dashed lines.
Age-Dependent Risk of Infection
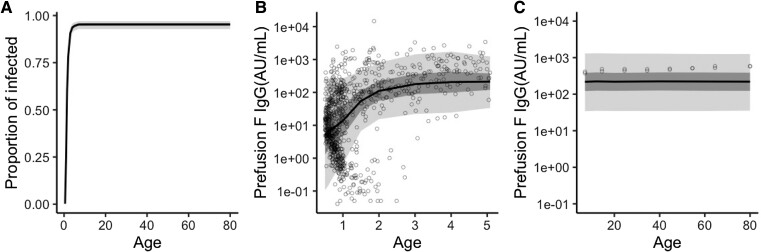
Using the finite mixture model fitted to the sampled anti-pre-F protein antibody concentration, the posterior means of the FoI were estimated to be 0.60 per year (95% CrI, 0.47–0.74), 1.36 per year (95% CrI, 1.12–1.62), and 1.56 per year (95% CrI, 1.20–1.97) for those aged 0.5 to 1 year, 1 to 5 years, and 5 years and over, respectively. The rate of decay was estimated to be 0.08 per year (95% CrI, 0.05–0.11). Other estimates, including the mean antibody concentration in each disease group, are listed in Table 3. The estimated age-dependent ever-infected/recovered proportions are shown in Figure 4A. The proportion of ever-infected/recovered was almost 80% by the age of 2 and had plateaued by the age of 7. The predicted antibody concentration for each subject (aged < 5 years) and the predicted mean of each age group (aged > 5 years) were plotted against the observed values (Figure 4B and C). Overall, the model captured the observed pattern accurately. The model that considers the boosting effect provided greater predictability than the SIS model in terms of both WAIC and LOO cross-validation (Table 3).
Table 3.
Estimated Parameters From the Mixture Model (Median and 95% Credible Interval)
| Model | SIS Model (No. of Parameters = 9) | SIW Model (No. of Parameters = 10) |
|---|---|---|
| Mean of IgG concentration against prefusion F for susceptible (log(AU/mL)) | 1.61 (1.40–1.82) | 1.61 (1.40–1.82) |
| Mean of IgG concentration against prefusion F for infected (log(AU/mL)) | 5.58 (5.41–5.75) | 5.60 (5.43–5.79) |
| Variance for susceptible for aged 0.5 to 5 years | 1.99 (1.86–2.14) | 1.99 (1.86–2.13) |
| Variance for infected for aged 0.5 to 5 years | 1.16 (1.05–1.27) | 1.16 (1.06–1.27) |
| Variance for aged >5 years | 0.88 (0.56–1.35) | 0.70 (0.42–1.09) |
| FoI for aged 0.5 to 1 years (/year) | 0.60 (0.47–0.74) | 0.59 (0.47–0.73) |
| FoI for aged 1 to 5 years (/year) | 1.36 (1.12–1.62) | 1.28 (1.05–1.54) |
| FoI for aged 5 years and more (/year) | 1.56 (1.20–1.97) | 1.50 (1.14–1.89) |
| Decay (/year) | 0.08 (0.05–0.11) | 0.10 (0.06–0.14) |
| boosting | NA | 6.60 (2.68–12.68) |
| WAIC | 5787.4 (SE = 192.7) | 5736.5 (SE = 190.7) |
| LOO | 5789.8 (SE = 193.1) | 5737.8 (SE = 190.9) |
Abbreviations: AU, arbitrary units; FoI, force of infection; Ig, immunoglobulin; LOO, leave-one-out cross validation; NA, not applicable; SE, standard error; WAIC, widely applicable information criterion.
Figure 4.
Age-dependent disease status for respiratory syncytial virus infection. (A) Predicted age-dependent proportion of ever-infected/recovered individuals, sampled from the fitted mixture model. The shaded area is the 95% credible interval. (B) Scatterplots of the observed serum immunoglobulin G (IgG) concentrations against prefusion F and the predicted concentrations for those aged <5 years. (C) Observed sampled means of the IgG concentrations against prefusion F and the predicted means for those aged >5 years. The dark and light shaded areas represent the 50% and 95% credible intervals (B and C), respectively.
DISCUSSION
We conducted a systematic literature review of studies on the age-dependent seroprevalence of RSV infection. Twenty-one studies were identified from 15 countries, with the number of studies using ELISA being the largest (7), followed by studies using CF or neutralization (5). Except for CF, most studies showed similar patterns of seropositivity: seroprevalence was lowest in those aged <0.5 to 1 year, increased in early childhood, reaching a plateau in older children and adults. Assuming an SIS model, the age-dependent FoI values were lowest in those aged 0.5 to 1 year and increased with age during early childhood, that is, aged 1 to 5 years and >5 years. We also estimated the age-dependent disease status using a finite mixture model. The proportion of ever-infected/recovered individuals was estimated to plateau at the age of 7.
To capture seroprevalence status at the population level, data on a broad range of age categories are needed. For age-dependent data in which the number of positive tests among serum samples from 2 or more age groups (including aged <5 years) are available, the largest number of studies (n = 4) used CF; however, all were published in the 1960s–1970s, and this method is now considered to be less sensitive than ELISA [48]. In addition, a broad range of childhood ages were covered by a single study using various methods, that is, ELISA-F, ELISA-N, and neutralization assays. Although neutralization is considered to be the gold standard to measure seroprotective status [20], there were no studies sampling seroprevalence in both children and adults. These findings highlight that more data based on valid assays are needed to fully describe the population-level transmission dynamics of RSV infection.
The age-dependent FoI was estimated using the dataset from the Netherlands and indicated that the risk of infection was elevated during postinfancy and became even greater among those aged >5 years. Recent studies using serological data during the coronavirus disease 2019 pandemic suggest that asymptomatic infections could commonly occur in adults to maintain antibody levels [24,25], and our results using the catalytic model may support this hypothesis. Our model suggested that most of population would have been infected by the age of 3 years old (Figure 4A), and the higher FoI in older children would be required to transit a remaining (small) fraction of susceptibles to a state of infected. Age-dependent rates of contact that are associated with increasing contact behavior as a function of age may also contribute to higher FoI in older children. The decay rate was estimated to be 0.08 per year, corresponding to approximately 9 years as half-life. In a birth cohort that was followed up for 3 years, decay rate, measured by neutralization antibody titer, was reported as 0.33 per month (ie, 2 months as half-life) [49]. Our cohort included broader aged groups than the previous study, suggesting that a decay rate in aged group would be slower, although it must be noted that we used pre-F antibody levels to quantify the decay rate. We investigated the correlation between FoIs and decay rate and found no apparent correlations between the parameters (Supplementary Figure 3).
It must be emphasized that the transmission dynamics of RSV have not been fully clarified. For example, the basic reproduction number R0 has not been estimated (except for one poorly defined R0), and, thus, we have yet to understand the required vaccination coverage of the forthcoming immunization rollout. Considering the age dependency of FoIs that was suggested in our research, the impact of vaccination on the dynamic nature of RSV transmission at the population level requires further investigation, including the optimal age of vaccination and/or effective vaccination strategies (eg, targeting mothers). Transmission dynamics among adults and the older generation remain to be systematically investigated.
By considering single epidemiological factors, specific knowledge gaps are highlighted. We predominantly tracked seroepidemiological studies among infants, potentially excluding studies on other age groups. Furthermore, studies using recently emerged novel assay methods, such as pre-F antibody-based assays, are scarce and therefore poorly represented. Future studies that address these 2 points are needed. In addition to age-dependent FoI estimation, the mechanisms of transmission that govern age dependence among children have remained largely unknown. We should explore the site and opportunity for transmission (eg, the importance of household transmission, and the age at which the risk of transmission at school increases) and also the age and characteristics of the primary source case. Such social heterogeneities in transmission should be explored in future seroepidemiological studies, stratifying seroprevalence by social structures.
The limitations of this study were as follows: first, the catalytic model used may be an oversimplification of the real-life situation. Not only the estimated FoI and rate of decay, but also the epidemiological determinants of those estimates need to be further explored. Second, other undocumented aspects of transmission, including the seasonality of transmission, were ignored. Third, we examined 2 different types of prior distributions for estimating the age-dependent FoI. Although the variance of the posterior distribution with noninformative prior was only slightly greater than that of the informative prior, the validity of our catalytic-type mixture model should be subject to further validation using additional empirical data in the future.
CONCLUSIONS
In conclusion, a systematic literature review was conducted to summarize the evidence from seroprevalence studies on RSV and found that the number of studies covering a broad range of ages was very limited. Age-dependent FoI values were estimated, with the lowest FoI being identified in those aged 0.5 to 1 year, with increased values among older age groups, perhaps reflecting the increased contact rate. To understand the transmission dynamics, further epidemiological studies using valid assays including the pre-F antibody over a broader range of age groups are required.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
K Nakajo, Kyoto University School of Public Health, Yoshidakonoecho, Sakyoku, Kyoto, Japan; Sanofi K.K. Tokyo Opera City Tower, Shinjuku-ku, Tokyo, Japan.
H Nishiura, Kyoto University School of Public Health, Yoshidakonoecho, Sakyoku, Kyoto, Japan.
Notes
Acknowledgments. We thank Professor Niel Hens (Hasselt University) for providing the R code, and Dr. Kate Fox (Edanz Group; https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author contributions . KN contributed to concept, writing, analysis, and collecting data. HN contributed to planning, concept, writing, and analysis and guarantees the work.
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. HN received funding from a Health and Labor Sciences Research Grant (20CA2024, 20HA2007, 21HB1002, and 21HA2016), the Japan Agency for Medical Research and Development (JP20fk0108140, JP20fk0108535, and JP21fk0108612), the Japan Society for the Promotion of Science KAKENHI (21H03198), an Environment Restoration and Conservation Agency of Japan Grant (JPMEERF20S11804) of the Environmental Restoration and Conservation Agency of Japan, Kao Health Science Research, the Daikin GAP Fund Program of Kyoto University, the Japan Science and Technology Agency SICORP program (JPMJSC20U3 and JPMJSC2105), and the RISTEX Program for Science of Science, Technology and Innovation Policy (JPMJRS22B4). We thank local governments, public health centers, and institutes for surveillance, laboratory testing, epidemiological investigations, and data collection. Funding to pay the Open Access publication charges for this article was provided by Japan Society for the Promotion of Science (21H03198).
References
- 1. Karron RA. Respiratory syncytial virus vaccines, eds. Plotkin's vaccines. 7th ed. Amsterdam: Elsevier, 2017: pp 943–9. [Google Scholar]
- 2. Hall CB, Kopelman AE, Douglas RG Jr, et al. Neonatal respiratory syncytial virus infection. N Engl J Med 1979; 300:393–6. [DOI] [PubMed] [Google Scholar]
- 3. Li Y, Johnson EK, Shi T, et al. National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. Lancet Respir Med 2021; 9:175–85. [DOI] [PubMed] [Google Scholar]
- 4. Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222(Suppl 7):S577–83. [DOI] [PubMed] [Google Scholar]
- 5. Metcalf CJE, Farrar J, Cutts FT, et al. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet 2016; 388:728–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Winter AK, Martinez ME, Cutts FT, et al. Benefits and challenges in using seroprevalence data to inform models for measles and rubella elimination. J Infect Dis 2018; 218:355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bollaerts K, Riera-Montes M, Heininger U, et al. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: deriving incidence from seroprevalence data. Epidemiol Infect 2017; 145:2666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goeyvaerts N, Hens N, Aerts M, Beutels P. Model structure analysis to estimate basic immunological processes and maternal risk for parvovirus B19. Biostatistics 2011; 12:283–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rees EM, Waterlow NR, Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group , Lowe R, Kucharski AJ. Estimating the duration of seropositivity of human seasonal coronaviruses using seroprevalence studies. Wellcome Open Res 2021; 6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. European Centre for Disease Prevention and Control . Survey on the implementation of integrated surveillance of respiratory viruses with pandemic potential. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/Integrated_respiratory_surveillance_survey_results-2022.pdf. Accessed 4 Nov 2022.
- 11. Russell CD, Ungerb SA, Waltona M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev 2017; 30:481–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiseman DJ, Thwaites RS, Drysdale SB, et al. Immunological and inflammatory biomarkers of susceptibility and severity in adult respiratory syncytial virus infections. J Infect Dis 2020; 222(Suppl 7):S584–91. [DOI] [PubMed] [Google Scholar]
- 13. Tsutsumi H, Matsuda K, Yamazaki H, Ogra PL, Chiba S. Different kinetics of antibody responses between IgA and IgG classes in nasopharyngeal secretion in infants and children during primary respiratory syncytial virus infection. Acta Paediatr Jpn 1995; 37:464–8. [DOI] [PubMed] [Google Scholar]
- 14. Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas GR Jr. Respiratory syncytial virus infections within families. N Engl J Med 1976; 294:414–9. [DOI] [PubMed] [Google Scholar]
- 15. Nakajo K, Nishiura H. Age-specific hospitalization risk of primary and secondary respiratory syncytial virus infection among young children. Int J Infect Dis 2022; 124:14–20. [DOI] [PubMed] [Google Scholar]
- 16. Andeweg SP, Schepp RM, van de Kassteele J, Mollema L, Berbers GAM, van Boven M. Population-based serology reveals risk factors for RSV infection in children younger than 5 years. Sci Rep 2021; 11:8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berbers G, Mollema L, van der Klis F, den Hartog G, Schepp R. Antibody responses to respiratory syncytial virus: a cross-sectional serosurveillance study in the Dutch population focusing on infants younger than 2 years. J Infect Dis 2021; 224:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nyiro JU, Kombe IK, Sande CJ, et al. Defining the vaccination window for respiratory syncytial virus using age-seroprevalence data for children in Kilifi, Kenya. PLoS One 2017; 12:e0177803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Magro M, Mas V, Chappell K, et al. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci USA 2012; 109:3089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hens N, Shkedy Z, Aerts M, Faes C, van Damme P, Beutels P. Modelling infectious disease parameters based on serological and social contact data. New York: Springer Science + Business Media, 2012. [Google Scholar]
- 22. Zylbersztejn A, Pembrey L, Goldstein H, et al. Respiratory syncytial virus in young children: community cohort study integrating serological surveys, questionnaire and electronic health records, born in Bradford cohort, England, 2008 to 2013. Euro Surveill 2021; 26:2000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Green CA, Sande CJ, de Lara C, et al. Humoral and cellular immunity to RSV in infants, children and adults. Vaccine 2018; 36:6183–90. [DOI] [PubMed] [Google Scholar]
- 24. den Hartog G, van Kasteren PB, Schepp RM, Teirlinck AC, van der Klis FRM, van Binnendijk RS. Decline of RSV-specific antibodies during the COVID-19 pandemic. Lancet Infect Dis 2023; 23:23–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reicherz F, Xu RY, Abu-Raya B, et al. Waning immunity against respiratory syncytial virus during the COVID-19 pandemic. J Infect Dis 2022; 226:2064–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vehtari A, Gabry J, Magnusson M, et al. loo: Efficient Leave-One-Out Cross-Validation and WAIC for Bayesian Models. Available at: https://cran.r-project.org/web/packages/loo/loo.pdf. Accessed 25 Nov 2022.
- 27. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available at: https://www.R-project.org/. Accessed 28 March 2022.
- 28. Amaku M, Azevedo RS, de Castro RM, Massad E, Coutinho FA. Relationship among epidemiological parameters of six childhood infections in a non-immunized Brazilian community. Mem Inst Oswaldo Cruz 2009; 104:897–900. [DOI] [PubMed] [Google Scholar]
- 29. Arankalle VA, Kulkarni R, Malshe N, Palkar S, Lalwani S, Mishra AC. Seroepidemiology of respiratory syncytial virus in western India with special reference to appropriate age for infant vaccination. J Med Virol 2019; 91:1566–70. [DOI] [PubMed] [Google Scholar]
- 30. Bhattarakosol P, Pancharoen C, Mungmee V, Thammaborvorn R, Semboonlor L. Seroprevalence of anti-RSV IgG in Thai children aged 6 months to 5 years. Asian Pac J Allergy Immunol 2003; 21:269–71. [PubMed] [Google Scholar]
- 31. Brüssow H, Werchau H, Sidoti J, et al. Age-related prevalence of serum antibody to respiratory syncytial virus in Ecuadorian and German children. J Infect Dis 1991; 163:679–80. [DOI] [PubMed] [Google Scholar]
- 32. Cox MJ, Azevedo RS, Cane PA, Massad E, Medley GF. Seroepidemiological study of respiratory syncytial virus in São Paulo state, Brazil. J Med Virol 1998; 55:234–9. [DOI] [PubMed] [Google Scholar]
- 33. Doggett JE. Antibodies to respiratory syncytial virus in human sera from different regions of the world. Bull World Health Organ 1965; 32:849–53. [PMC free article] [PubMed] [Google Scholar]
- 34. Dunn SR, Ryder AB, Tollefson SJ, Xu M, Saville BR, Williams JV. Seroepidemiologies of human metapneumovirus and respiratory syncytial virus in young children, determined with a new recombinant fusion protein enzyme-linked immunosorbent assay. Clin Vaccine Immunol 2013; 20:1654–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ebihara T, Endo R, Kikuta H, Ishiguro N, Ishiko H, Kobayashi K. Comparison of the seroprevalence of human metapneumovirus and human respiratory syncytial virus. J Med Virol 2004; 72:304–6. [DOI] [PubMed] [Google Scholar]
- 36. Golubjatnikov R, Allen VD, Olmos-Blancarte MP, Inhorn SL. Serologic profile of children in a Mexican highland community: prevalence of complement-fixing antibodies to Mycoplasma pneumoniae, respiratory syncytial virus and parainfluenza viruses. Am J Epidemiol 1975; 101:458–64. [DOI] [PubMed] [Google Scholar]
- 37. Jennings R. Adenovirus, parainfluenza virus and respiratory syncytial virus antibodies in the sera of Jamaicans. J Hyg (Lond) 1972; 70:523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leogrande G. Studies on the epidemiology of child infections. 3. Parainfluenza viruses (types 1–4) and respiratory syncytial virus infections. Microbios 1992; 72:55–63. [PubMed] [Google Scholar]
- 39. Lu G, Gonzalez R, Guo L, et al. Large-scale seroprevalence analysis of human metapneumovirus and human respiratory syncytial virus infections in Beijing, China. Virol J 2011; 8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Madhavan HN, Badrinath S, Agarwal SC. Sero-epidemiological survey of respiratory syncytial virus infection in Pondicherry area–South India. Indian J Med Res 1974; 62:1804–7. [PubMed] [Google Scholar]
- 41. Moss PD, Adams MO, Tobin JO. Serological studies with respiratory syncytial virus. Lancet 1963; 1:298–300. [DOI] [PubMed] [Google Scholar]
- 42. Njoku-Obi AN, Ogunbi O. Viral respiratory diseases in Nigeria: a serological survey, II. complement fixing antobody levels of adenoviruses, respiratory syncytial virus, psittacosis virus. J Trop Med Hyg 1966; 69:147–9. [PubMed] [Google Scholar]
- 43. Sastre P, Ruiz T, Schildgen O, Schildgen V, Vela C, Rueda P. Seroprevalence of human respiratory syncytial virus and human metapneumovirus in healthy population analyzed by recombinant fusion protein-based enzyme linked immunosorbent assay. Virol J 2012; 9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suto T, Yano N, Ikeda M, et al. Respiratory syncytial virus infection and its serologic epidemiology. Am J Epidemiol 1965; 82:211–24. [DOI] [PubMed] [Google Scholar]
- 45. Terrosi C, Di Genova G, Martorelli B, Valentini M, Cusi MG. Humoral immunity to respiratory syncytial virus in young and elderly adults. Epidemiol Infect 2009; 137:1684–6. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Q, Yang XQ, Zhao Y, Zhao XD. High seroprevalence of human metapneumovirus infection in children in Chongqing. China. Chin Med J (Engl) 2008; 121:2162–6. [PubMed] [Google Scholar]
- 47. Liu Z, Wu S, Xian Y, et al. Seroprevalence of neutralizing antibodies against the respiratory syncytial virus in healthy adults in Guangzhou, southern China. J Med Virol 2022; 94:4378–82. [DOI] [PubMed] [Google Scholar]
- 48. Taggart EW, Hill HR, Martins TB, Litwin CM. Comparison of complement fixation with two enzyme-linked immunosorbent assays for the detection of antibodies to respiratory viral antigens. Am J Clin Pathol 2006; 125:460–6. [PubMed] [Google Scholar]
- 49. Sande CJ, Mutunga MN, Okiro EA, Medley GF, Cane PA, Nokes DJ. Kinetics of the neutralizing antibody response to respiratory syncytial virus infections in a birth cohort. J Med Virol 2013; 85:2020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.