Abstract
One in ten of all new cancers diagnosed worldwide each year is a cancer of the female breast, and it is the most common cancer in women in both developing and developed areas. It is also the principal cause of death from cancer among women globally. We review the descriptive epidemiology of the disease, focusing on some of the key elements of the geographical and temporal variations in incidence and mortality in each world region. The observations are discussed in the context of the numerous aetiological factors, as well as the impact of screening and advances in treatment and disease management in high-resource settings.
Keywords: breast neoplasms, cancer registries, epidemiology, incidence, mortality, time trends
Introduction
Cancer of the breast in women is a major health burden worldwide. It is the most common cause of cancer among women in both high-resource and low-resource settings, and is responsible for over one million of the estimated 10 million neoplasms diagnosed worldwide each year in both sexes [1]. It is also the primary cause of cancer death among women globally, responsible for about 375,000 deaths in the year 2000 [1].
International comparisons of disease rates by area and time of diagnosis can provide important clues to the underlying causes of diseases and the effects of natural or planned interventions, and serve as indicators of the scope for preventive strategies. There is at least a 10-fold variation in breast cancer incidence rates worldwide [2], largely as a consequence of a range of socio-economically correlated differences in the population prevalence of several reproductive, hormonal and nutritional factors. In some high-resource countries, mammographic screening has considerably affected breast cancer diagnosis, registration and mortality.
Studies of migrants provided the first solid evidence that environmental (rather than genetic) determinants were responsible for most of the observed international and inter-ethnic differences in breast cancer incidence: comparisons of breast cancer risk in (low-risk) Asian populations migrating to the (high-risk) USA and their offspring revealed major increases in risk between successive generations [3], and increases in risk were observed in populations from European countries with relatively low incidence (Italy and Poland) after migration to Australia, particularly if the migration took place in childhood [4,5].
As a consequence of changing exposures to reproductive and nutrition-related determinants over time, women are at increasingly high risk of breast cancer, with incidence rates increasing in most countries and regions of the world in the past few decades. The most rapid rises are seen in developing countries, where breast cancer risk has historically been low relative to industrialised countries. Increasing trends in developing areas are often considered the result of the 'westernisation' of lifestyles, an ill-defined surrogate for changes in factors such as childbearing, dietary habits and exposure to exogenous oestrogen, towards a distribution closer in profile to that of women in industrialised countries.
The variations in mortality reflect, in part, variations in incidence (and its determinants), but mortality is also influenced by case-fatality. It is therefore affected by early diagnosis (another correlate of socio-economic status), either through screening or as a result of increasing individual awareness of the disease and its symptoms. In high-resource to medium-resource settings, advances in breast cancer therapy in recent years have made a considerable contribution to improved survival and the subsequent reduction or stabilisation of breast cancer death rates.
In this paper we review the descriptive epidemiology of the disease, focusing on some of the key elements of the geographical and temporal variations in incidence and mortality in each region of the world. The review includes published studies and some new analyses using incidence data from population-based cancer registries and mortality data from the WHO databank. We then discuss possible explanations for the results in the light of the changing prevalence of the known aetiological factors, the impact of screening and other preventive strategies, and progress in disease management; we conclude with some comments on future prospects for prevention.
Geographical variations worldwide
Worldwide, more than one million new cases of female breast cancer are diagnosed each year. It is the most commonly occurring neoplasm in women, accounting for over one-fifth of the estimated annual 4.7 million cancer diagnoses in females, and the second most common tumour, after lung cancer, in both sexes [1]. It is also the most common female cancer in both developing and developed countries, with most (55%) occurring in the latter regions, where age-standardised rates are three times higher than in developing areas [1].
The estimated number of deaths from female breast cancer in 2000 is considerably lower – about 375,000 deaths – reflecting the reasonably good overall survival. Prognosis is heavily dependant on stage of disease at presentation, however. In the Surveillance, Epidemiology, and End Results (SEER) registries in the USA, 5-year survival for localised cases in 1994 was about 97% but was only about 25% for cases with metastatic disease [6]. In developing countries, the differences in survival by stage at diagnosis are also very marked [7]. In Europe, 5-year relative survival rates vary from 83% in Sweden to 61% in Slovakia [8], and although trends show clear improvement over time, the utility of survival in monitoring patient outcome has been questioned in countries affected by artefactual increases in registrations due to screening [9].
Figure 1 shows the geographical variation in breast cancer incidence worldwide, as estimated for the year 2000. The highest incidence rates occur in northern and western Europe, northern America, Australia and New Zealand, and in southern countries of South America, notably Uruguay and Argentina. Clear geographical differences in risk are apparent within Europe, with elevated rates in northern and western Europe, whereas rates in most southern and eastern European countries are low to intermediate [10]. Incidence is low throughout Africa, Asia and most of Central and South America (Fig. 1). The corresponding map of breast cancer mortality conveys a similar picture. To highlight the international variability in risk of breast cancer incidence, Fig. 2 shows the age-standardised (world) incidence rates in selected populations based on cancer registry data from 1993 to 1997 compiled in the latest volume (volume VIII) of Cancer Incidence in Five Continents [2]. Rates of more than 100 per 100,000 are noted in several US states, whereas the highest rates are recorded in Montevideo in Uruguay (Fig. 2). Rates are elevated (50–100 per 100,000) in registries in geographically diverse areas of the world including most northern and western European countries, Canada, Israel and Argentina. US-born Filipinos and Hawaiian-born Chinese also have high rates (Fig. 2). In several Asian populations, including those in Hong Kong, Singapore (Chinese) and the Philippines (Manilla), rates are intermediate (30–50 per 100,000), as they are in Puerto Rico and Goiania (Brazil) in South America, and most eastern European populations (Fig. 2). The lowest rates are seen in several Chinese populations including the Quidong registry, with breast cancer incidence rates of about 10 per 100,000, whereas observed rates are also low (10–30 per 100,000) in eastern African populations in Zimbabwe and Uganda, Algiers in North Africa, several South-East Asian registries (Thailand and Vietnam), and several registries in India (Fig. 2). Koreans living in the USA have retained a relatively low breast cancer incidence rate (about 28 per 100,000) not dissimilar to that of Koreans living in Korea (21 per 100,000 in Seoul), in comparison with the high rates now seen in other US-born races of Asian descent, notably Filipinos (Fig. 2).
Figure 1.
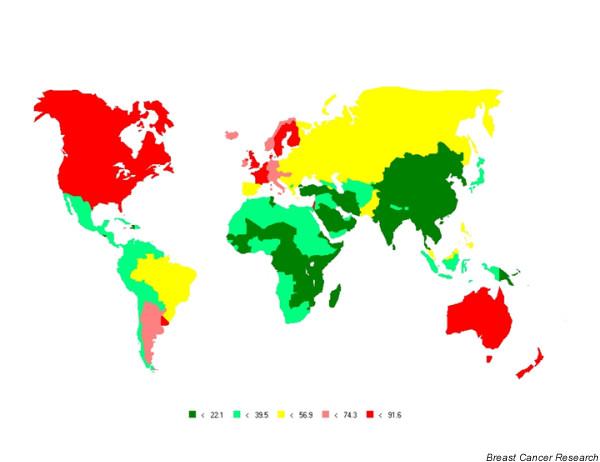
Breast cancer incidence worldwide: age-standardised rates (world population). Source: [1].
Figure 2.
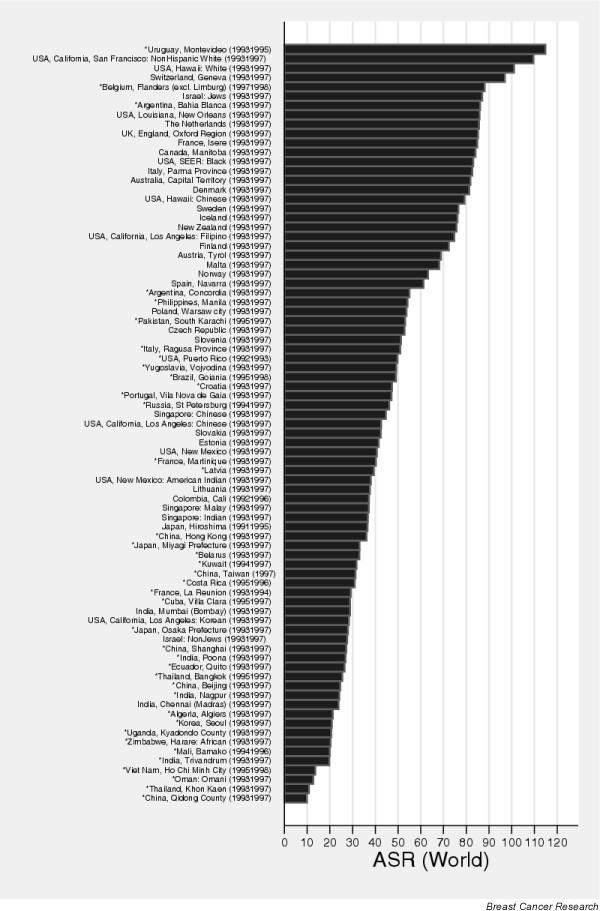
Variations in breast cancer incidence in selected cancer registries: age-standardised rates (world population). Source: [2].
Age-specific variations worldwide
Breast cancer incidence has a distinctive age-specific curve (Fig. 3). The rapid rate of increase before the menopause (ages 40–50) slows down after that, probably owing to diminishing levels of circulating oestrogens [11]. In low-incidence countries, the slope of the curve after the menopause may be flat, or even negative (for example in Khon Kaen in Fig. 3). This is a consequence of increasing risks of occurrence in consecutive generations of women rather than a real decline in risk with age [12]. The young age structure of populations in developing countries coupled with a rather flat age-incidence curve (Fig. 3) implies that the mean age at diagnosis in developing countries is lower than that of European and American populations.
Figure 3.
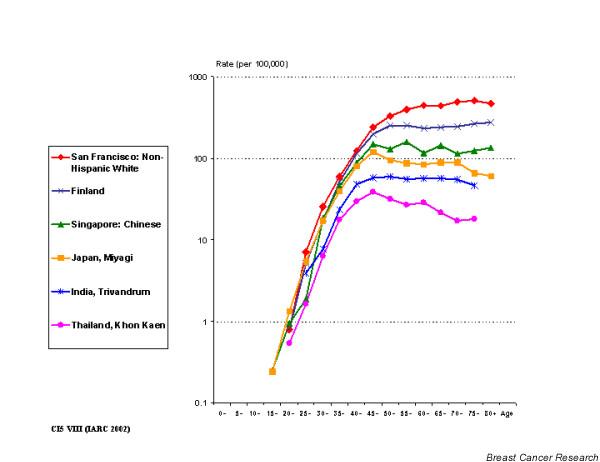
Age-specific breast cancer incidence rates in selected cancer registries. Source: [2].
Patterns of breast cancer by world region
The changing profile of breast cancer incidence and mortality between populations in each region, and within populations over time, is reviewed below, on the basis of the relevant published literature and with reference to Fig. 4, which shows time trends of overall incidence for selected countries in the Americas, in Asia/Oceania and in Europe, to Fig. 5, which contrasts the percentage change in mortality rates between 1985–87 and 1995–97 in selected countries worldwide, and to Figs 6,7,8, which show incidence and mortality trends in selected European populations based on data compiled in the EUROCIM database [13].
Figure 4.
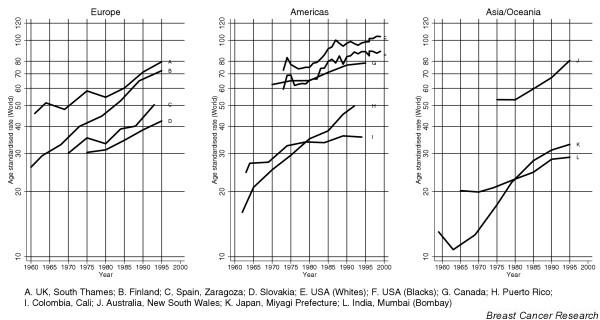
Breast cancer incidence trends over time in selected cancer registries in Europe, the Americas and Asia: age-standardised rates (world population). Source: [2].
Figure 5.
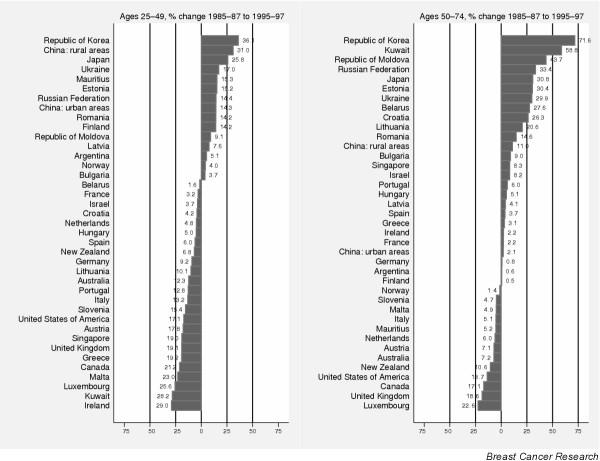
Percentage change in breast cancer mortality between 1985–87 and 1995–97 in women aged 50–74 and 25–49 years in selected countries worldwide, sorted by descending order of magnitude of the change (earlier period is 1988–90 for China, later period is 1994–96 for Argentina). Source: http://www-depdb.iarc.fr/who/menu.htm.
Figure 6.
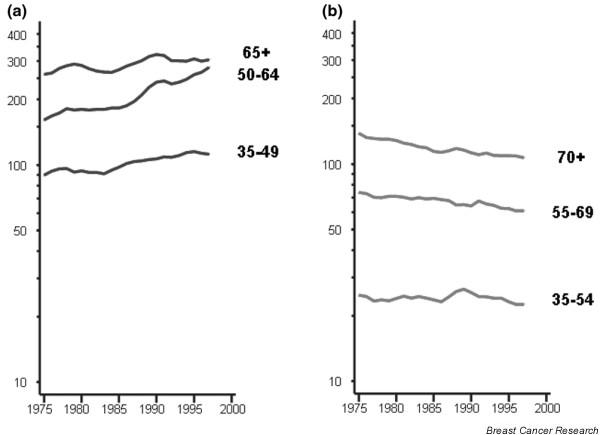
Incidence (a) and mortality trends (b) by age group in Sweden: truncated age-standardised rate (European population). Source: [13].
Figure 7.
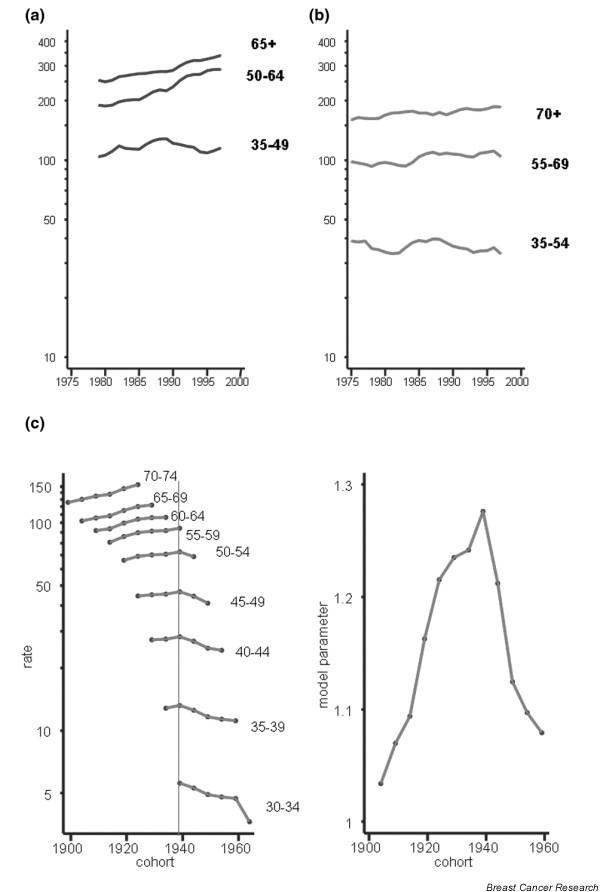
Incidence (a) and mortality trends (b) by age group in Denmark: truncated age-standardised rate (European population). (c) Breast cancer age-specific mortality by birth cohort, age-cohort model parameters for the same dataset. Source: [13].
Figure 8.
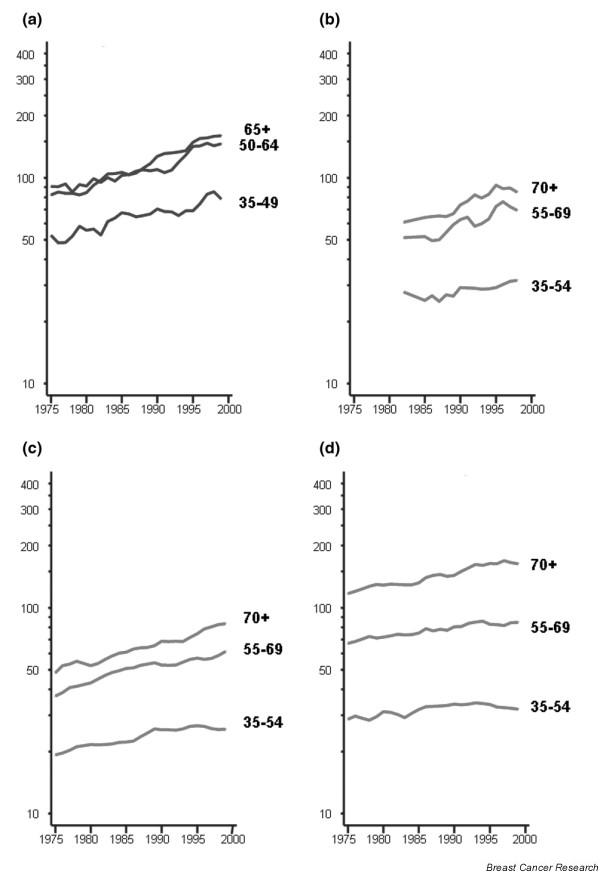
Incidence (a) and mortality trends (b) by age group in Estonia; mortality trends by age group in Romania (c); mortality trends by age group in Hungary (d): truncated age-standardised rate (European population). Source: [13].
Europe
Trends in incidence
In countries where national screening programmes started in the mid-to late 1980s (the Nordic countries, England and Wales and The Netherlands), incidence rates were increasing before organised screening activity [14] (for example A and B in Fig. 4). Mean annual increments of 1–3% were observed, with the largest increases being in Finland and The Netherlands (Eindhoven) [14]. Neither of these populations witnessed a screening-related increase [14,15] – a short-term upsurge in incidence within screened age groups due to the detection of prevalent cancers during the first screening round – as observed in England and Wales [16] and Sweden [17] (Fig. 6a). Quite substantial increases in incidence up to the mid-1990s – greater than 2% per year – were also seen in several countries that either had not introduced programmes, had implemented them very recently or had regional or pilot programmes under way (for example C and D in Fig. 4). In Norway, the annual increase was slightly less (1.6%) but the introduction of breast screening in 1996, covering 40% of the population [18], resulted in an upsurge in incidence [19]. Increases of between 2% and 4% in breast cancer incidence per year have been reported in six former-USSR regions between 1971 and 1987 [20].
In most countries, the upward trend in incidence occurred across the age groups 35–49, 50–64 and 65–74 years [14], and as a result, attributing the time trend to period and cohort influences is difficult, although in studies that have examined unscreened women, or have adjusted for screening effects, cohort effects are evident in Denmark [21-23], Norway [24] and Sweden [25].
The most recent data indicate some signs of a plateau in incident trends in several countries during the mid-1990s, particularly in The Netherlands, in Sweden and in England and Wales [14]. This has been speculated to be a result of a cohort-specific peak in incidence [15], although the observations are also consistent with what would be expected after the initial breast screening round: a decline after the post-screening increase to a level slightly higher than that before screening [26].
Trends in mortality
Mortality in most countries has increased from the 1950s until at least the 1980s, particularly in eastern and southern countries of Europe. A levelling off and subsequent decline in breast cancer mortality from the early 1990s became evident in England and Wales [9,27,28] and The Netherlands [27] and has now also been observed in several other European countries [14], although the declines are often confined to women aged less than 50 years (Fig. 5).
The pattern in Sweden is unusual because a rather stable or slowly declining trend, notably in middle-aged and elderly women, has been observed since the 1960s [29], and declines are evident in all age groups at least since the late 1980s (Fig. 6b). In Denmark, both incidence (Fig. 7a) and mortality (Fig. 7b) are declining in young women, and strong cohort effects are observed, with decreasing rates in women born in successive generations after 1940 (Fig. 7c). In Finland, such a reduction in mortality rates has not occurred (Fig. 5). Some recent decreases in mortality are observed in several countries without national screening programmes, although these tend to be confined mainly to younger age groups (Fig. 5). Mortality is increasing in several eastern European or former Soviet countries, characterised by relatively low rates in the past, such as the Russian Federation (Fig. 5), Estonia (Fig. 8b, showing similar trends to incidence in Fig. 8a), Romania (Fig. 8c) and Hungary (Fig. 8d).
North America
Trends in incidence
The pattern observed in the USA and Canada is broadly similar to that of Europe, with similar increases in incidence in both white and black women [30] (E–G in Fig. 4). Most of this increase occurred in the period between 1980 and 1987 [6] and is related to rises in mammography-detected incident cases as a result of the intensification of breast screening at this time [31]. The overall rate of increase has slowed to 0.6% per year since the late 1980s [32]. Early studies of Connecticut incidence data, before widespread mammography emerged, had commented on the importance of birth cohort effects [21]. A proposition that incidence rates of breast cancer were levelling off in women born after 1925 [33] has been almost impossible to follow up in the light of the effects of subsequent screening on incidence.
Trends in mortality
The levelling off in mortality and subsequent decline that took place in several northern European countries in the 1980s was also noted in both the USA [34] and Canada [35], and the extent of the decrease in both younger and older women is shown in Fig. 5. Although the trends were similar from the 1970s through to the mid-1980s in both US whites and blacks, they diverged thereafter, with white women experiencing a levelling off and subsequent decline in mortality from the early 1990s, whereas in contrast mortality increased slightly in black women throughout the period [30]. From 1992 to 2000, breast cancer death rates among white women declined in 38 US states, whereas among blacks increases were observed in several states [32].
There have been several studies examining calendar period and birth components of the mortality trends [36,37]. A decline in breast cancer rates among women born after about 1920 has been reported in Canada and in the USA among both blacks and whites. Birth cohort trends for all women were similar until about 1940, with a moderation of mortality risk beginning in about 1924. A marked moderation of risk by birth cohort was observed for US white women born after 1950, whereas stable or slightly decreasing trends were observed for US black women and Canadian women. The slope of the mortality trend by calendar period increased in the 1980s compared with the 1970s for all women. In the last calendar period, in the early 1990s, a trend of decreasing mortality rates was found for US white and Canadian women [37].
Australia and New Zealand
Incidence of breast cancer in New South Wales (representing about one-third of Australian women) increased steadily from the early to mid-1980s (J in Fig. 4), and by 1995 was nearly 50% higher than in 1983. The greatest increase was in the target age group for mammographic screening (50–69 years), which became available from 1984 on a limited basis and in 1992 was nationwide and accessible to all women aged at least 40 years [38]. In New Zealand there were steady increases in both Maori and non-Maori incidence rates from 1978–92 [39].
Breast cancer mortality in Australia rose steadily from the early 1970s to the late 1980s [40]. Between 1985–1989 and 1990–1994, breast cancer mortality fell by 3.2% in women 50–69 years of age and by 4.2% in 25–49-year-olds, with little change (-0.2%) in breast cancer mortality in older women in this period [40]. The proportion of women screened in all age groups increased substantially between 1988 and 1994, and by 1994 nearly 65% of women in the target age group had had at least one mammogram [40]. Cohort-specific mortality has been reported to be rather uniform [41], particularly in young women [40].
Japan
Although breast cancer remains relatively rare in Japan, incidence (K in Fig. 4) and mortality (Fig. 5) have been rising quite rapidly, which is consistent with increasing risk in successive generations of women [42]. The overall incidence has been increasing since the mid-1970s [42,43] although the increase has been much larger than for mortality, demonstrating improving prognosis over time [42]. In more recent epochs, accelerating increases in the incidence rates have been attributed to both period and cohort effects [43,44].
Developing countries
There is a paucity of sufficiently long time series of high-quality cancer data in many developing areas at the present time, but nonetheless, where they are available, increases in breast cancer incidence and mortality are seen, an observation often more apparent within recent birth cohorts [45], and a probable consequence of the adoption of western lifestyles [46].
Latin America
Most countries have intermediate rates of breast cancer occurrence. Incidence and mortality rates have been observed to be increasing in most countries [46]; incidence has at least doubled, for instance, in Cali, Colombia, and in Puerto Rico (H and I in Fig. 5) between the early 1970s and the mid-1990s. In Uruguay, Argentina and Chile, women are at high or intermediate risk, and mortality rates in younger women have been reported to be more or less constant over time [45].
Asia
Age-adjusted incidence is low in most countries, although rates are more than 50 per 100,000 (world standardised rate) in Manila in the Philippines and South Karachi in Pakistan. Rates in Singapore, particularly among the Chinese, are also relatively high for the region. Studies comparing the risks in migrants to the USA and their offspring born there have revealed substantial increases in risk between first, second and third generations. Rising incidence has been observed in India [47] and also in Singapore, where there were average annual increases of 3.6% between 1968 and 1992, attributed mainly to birth cohort effects [48]. The extent of the mean annual rate of increase in Singapore, as reported in the latter study, ranged from 4.4% in Malays to 1.4% in Indians [48]. In China, mortality increased over the period 1987–99 in both rural and urban areas, the change being more evident in rural areas although the rates have remained lower than in urban females [49]. The twofold increase in mortality in Taiwan between the 1960s and 1990s has been attributed to both period and cohort influences [50], whereas in Hong Kong increases of the same order of magnitude were considered to be primarily the result of cohort effects [51].
Africa
Although breast cancer is the most common neoplasm among women in developing countries, if Africa is taken as a whole it ranks second most frequent to cervical cancer [1]. However, it is the most common malignancy in North Africa and in urban settings within the sub-Saharan region such as Abidjan (Côte d'Ivoire) [52], and until recently also in Harare, Zimbabwe, although changes in the evolution of the AIDS epidemic have led to its now having been overtaken by Kaposi's sarcoma [53]. In the few datasets available for the study of time trends in Africa, increases in incidence, for example, are apparent [45]. There have been twofold increases in breast cancer incidence in Ibadan, Nigeria [54], and in Kampala, Uganda [55], between the 1960s and the late 1990s. Steady increases in breast cancer mortality rates of the same order of magnitude have also been noted from the early 1960s in Mauritius [54].
Explaining geographical and temporal variations in breast cancer
The changes in breast cancer incidence and mortality over time seem complex, although broadly speaking the largest increases in risk have been seen in populations of women historically at lowest risk, often within developing countries, whereas relatively recent departures from the long-term trend have been observed in several, mainly western, countries.
Risk factors
In general, the high rates of breast cancer in developed countries are the consequence of a higher prevalence of the known risk factors for the disease, many of which – early age at menarche, nulliparity, late age at first birth, late age at any birth, low parity, and late menopause – relate to the hormonal (largely oestrogen) milieu to which the breast is exposed from menarche to the cessation of ovulation at menopause [56]. The higher parity and earlier age at first pregnancy of women seen in many developing countries might account for much of the lower incidence of breast cancer in these regions relative to developed countries. The long-standing hypothesis that breast-feeding of longer duration is protective [57] has been affirmed again recently [58].
The association between socio-economic status and risk of breast cancer is well established, with women in higher socio-economic groupings being at higher risk. When social class is measured by income or education level, the variations in risk largely accord with the differential distribution of known risk factors, as observed in the USA [59]. The greater risk for women from affluent backgrounds is, however, outweighed by their lower mortality, women from deprived backgrounds often presenting with more advanced disease [60].
Exposure to exogenous hormones as oral contraceptives [61] and hormone replacement therapy [58] result in an increase in the risk of breast cancer. The risk conferred by oral contraceptive use is, however, rather small and although it persists for up to 10 years after cessation, cancers in these women, as with women taking hormone replacement therapy, are usually not clinically advanced at presentation, thus rendering the impact on mortality rather modest.
Although there is a long-established correlation between the incidence of breast cancer and dietary fat intake in populations [62], the true relation between fat intake and breast cancer does not seem to be particularly strong or consistent [63,64]. The effect of large weight gains after the age of 18 has been shown to be a strong risk factor for breast cancer in postmenopausal women [65], with risk increasing by 2% per unit body mass index [66]. Excessive alcohol intake also seems to increase risk, with a recent re-analysis of 53 studies indicating that about 4% of breast cancers in developed countries might be attributable to its consumption [67].
The changing patterns of childbearing and breastfeeding, of exogenous hormonal intake and of dietary factors including obesity and reduced physical activity have certainly contributed to trends in incidence and mortality. Pinpointing the particular factors that have contributed in different populations worldwide has proved a major challenge, and the underlying reasons are certain to be multiple and interactive. The accompanying evidence on exposure to endogenous and exogenous oestrogen indicates that the lifetime length of exposure to endogenous oestrogen has been increasing, which is consistent with upward trends in incidence of breast cancer, particularly in developed countries.
Early detection and mammographic screening
Mammographic screening for women aged 50–69 years is effective in reducing breast cancer mortality, and reductions in mortality have been observed where screening has been introduced [68,69]. Evidence that at least part of this decline can be attributed to screening comes from the expected increase in incidence of early stage and in situ breast cancers, followed by a decline in advanced cancer and subsequent mortality in the UK, northern Europe and Australia [70-73]. It has been estimated that about one-third of the overall 21% reduction in breast cancer mortality in the UK by 1998 (10 years after screening began) was due directly to screening [74], although the time lag before any benefits from screening can be expected [75], together with the reduction in mortality resulting from notable advances in treatment (see below), makes quantification of the contribution of each problematic. One of the indirect beneficial effects of screening might have been a shift towards earlier diagnosis of breast cancer, as a result of the publicity surrounding the disease and its prevention.
The role of treatment and management
Reductions in mortality before the introduction of screening, and in those countries without screening activity, indicate that several improvements in disease management might explain many of the observed declines in mortality [75,76]. In the UK [9] and Finland [71], the rapid decline in mortality rates shortly after implementation of screening programmes was probably due in part to an increased use of tamoxifen among postmenopausal women with node-positive disease. The Early Breast Cancer Trialists' Cooperative Group reported in a meta-analysis of 55 randomised adjuvant trials that tamoxifen reduced the incidence of contralateral breast cancers by 47% at 5 years [77]. It is likely that the increasing use of this anti-oestrogen has contributed to decreases in mortality from breast cancer in women who are positive for oestrogen receptor in developed countries during the 1990s [78]. However, it has been suggested that the absolute benefit is more modest [79], because most trials reported on oestrogen receptor-positive women with early disease, whereas about one-third of women are negative for the oestrogen receptor, and many women with breast cancer do not present with early stage disease.
A likely contributory factor to the decline, as noted in the UK, has been the establishment of treatment protocols, improved chemotherapeutic options and better therapeutic guidelines [74]. Specific structural changes that have embraced specialisation of breast cancer care (such as centralised treatment, adjustments in clinician workload, and use of multidisciplinary teams) have been shown to improve outcome [80].
Preventive strategies in the future
The primary risk factors for breast cancer are not easily modifiable because they stem from prolonged endogenous hormonal exposures. The beneficial impact on breast cancer mortality from wider implementation of screening and continuing improvements in treatment are likely to accrue. Primary prevention strategies aimed at promoting breastfeeding, particularly in relation to duration, might also be beneficial [81].
Prevention trials have shown that tamoxifen lowers breast cancer incidence by 30–40% in high-risk women [82]. Currently, it is the only agent to have general approval for chemoprevention of breast carcinoma. However, as tamoxifen and raloxifene raise the risk of thromboembolic disease and endometrial cancer about twofold, different strategies are being pursued to improve the risk : benefit ratio of chemoprevention. Several ongoing trials are investigating a range of preventive regimes, with considerable interest in the aromatase inhibitors [83].
Further study is necessary to determine which genes consistently predict known breast cancer risk factors, to be able to screen for these and implement prevention [84]. Surgical intervention should largely be limited to those women who have a mutation in BRCA1 or BRCA2. Mastectomy has already been chosen by some women who have these mutations, and it can be taken that the number opting for this therapy will increase [85].
Of crucial importance is access to breast cancer care, its extent of coverage, and the particular modalities of such care. This includes interventions that target deprived societal groups and, critically in developing regions of the world, the provision and extension of these services to counter the increasing rates of breast cancer observed in most developing countries.
Conclusions
Despite much research directed at understanding and controlling breast cancer, it persists as a major health burden. The interpretation of breast cancer incidence and mortality patterns are complex in view of the numerous and interactive known and putative risk determinants, the introduction of screening and the substantial improvements in therapy. It is therefore likely that the descriptive epidemiology of breast cancer will continue to provide insights into the complex causation of this important disease and will allude to the role of primary prevention, early diagnosis and treatment.
Competing interests
None declared.
Acknowledgments
Acknowledgements
We gratefully acknowledge cancer registries that have contributed data to the "Cancer Incidence in Five Continents" volumes and to EUROCIM, thus enabling the above analysis of incidence trends. Peter McCarron is supported by a Department of Health (UK) public health career scientist award.
References
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide IARC Cancer Base No 5 [10] Lyon, France: IARC; 2001. [Google Scholar]
- Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J. Cancer Incidence in Five Continents. VIII. Lyon: IARC Press; 1997. [Google Scholar]
- Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- Geddes M, Parkin DM, Khlat M, Balzi D, Buiatti E. Cancer in Italian Migrants Populations. Lyon: IARC; 1993. IARC Scientific Publication No. 123. [Google Scholar]
- Tyczynski J, Tarkowski W, Parkin DM, Zatonski W. Cancer mortality among polish migrants to Australia. Eur J Cancer. 1994;30A:478–484. doi: 10.1016/0959-8049(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK, eds . SEER Cancer Statistics Review, 1975–2001. Bethesda: National Cancer Institute; 2004. [Google Scholar]
- Sankaranarayanan R, Parkin DM, Black RJ. Cancer Survival in Developing Countries. Lyon: IARC; 1998. [PubMed] [Google Scholar]
- Coleman MP, Gatta G, Verdecchia A, Esteve J, Sant M, Storm H, Allemani C, Ciccolallo L, Santaquilani M, Berrino F. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol. 2003;14(Suppl 5):V128–V149. doi: 10.1093/annonc/mdg756. [DOI] [PubMed] [Google Scholar]
- Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99–166. doi: 10.1016/S0959-8049(01)00350-1. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Ross R, Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1988;48:246–253. [PubMed] [Google Scholar]
- Moolgavkar SH, Stevens RG, Lee JA. Effect of age on incidence of breast cancer in females. J Natl Cancer Inst. 1979;62:493–501. doi: 10.1093/jnci/62.3.493. [DOI] [PubMed] [Google Scholar]
- European Network of Cancer Registries . EUROCIM Version 40. Lyon: European Network of Cancer Registries; 2001. [Google Scholar]
- Botha JL, Bray F, Sankila R, Parkin DM. Breast cancer incidence and mortality trends in 16 European countries. Eur J Cancer. 2003;39:1718–1729. doi: 10.1016/S0959-8049(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Nab HW, Mulder PG, Crommelin MA, van der Heijden LH, Coebergh JW. Is the peak in breast cancer incidence in sight? A study conducted in the southeastern Netherlands. Eur J Cancer. 1994;30A:50–52. doi: 10.1016/s0959-8049(05)80018-8. [DOI] [PubMed] [Google Scholar]
- Quinn M, Allen E. Changes in incidence of and mortality from breast cancer in England and Wales since introduction of screening. United Kingdom Association of Cancer Registries. BMJ. 1995;311:1391–1395. doi: 10.1136/bmj.311.7017.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson I, Bergstrom R, Barlow L, Adami HO. Recent trends in breast cancer incidence in Sweden. Br J Cancer. 1998;77:167–169. doi: 10.1038/bjc.1998.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Registry of Norway . Cancer in Norway; 2000. Oslo: Cancer Registry of Norway (Kreftregisteret); 2003. [Google Scholar]
- Zahl PH, Strand BH, Maehlen J. Incidence of breast cancer in Norway and Sweden during introduction of nationwide screening: prospective cohort study. BMJ. 2004. [DOI] [PMC free article] [PubMed]
- Zaridze DG, Basieva TH. Incidence of cancer of the lung, stomach, breast, and cervix in the USSR: pattern and trends. Cancer Causes Control. 1990;1:39–40. doi: 10.1007/BF00053182. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Moolgavkar SH, Lee JAH. Temporal trends in breast cancer. Am J Epidemiol. 1982;115:759–777. doi: 10.1093/oxfordjournals.aje.a113358. [DOI] [PubMed] [Google Scholar]
- Ewertz M, Carstensen B. Trends in breast cancer incidence and mortality in Denmark, 1943–1982. Int J Cancer. 1988;41:46–51. doi: 10.1002/ijc.2910410110. [DOI] [PubMed] [Google Scholar]
- Rostgaard K, Vaeth M, Holst H, Madsen M, Lynge E. Age-period-cohort modelling of breast cancer incidence in the Nordic countries. Stat Med. 2001;20:47–61. doi: 10.1002/1097-0258(20010115)20:1<47::AID-SIM613>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tretli S, Gaard M. Lifestyle changes during adolescence and risk of breast cancer: an ecologic study of the effect of World War II in Norway. Cancer Causes Control. 1996;7:507–512. doi: 10.1007/BF00051882. [DOI] [PubMed] [Google Scholar]
- Persson I, Bergstrom R, Sparen P, Thorn M, Adami HO. Trends in breast cancer incidence in Sweden 1958–1988 by time period and birth cohort. Br J Cancer. 1993;68:1247–1253. doi: 10.1038/bjc.1993.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter SD, Day NE. Estimation of the duration of a pre-clinical disease state using screening data. Am J Epidemiol. 1983;118:865–886. doi: 10.1093/oxfordjournals.aje.a113705. [DOI] [PubMed] [Google Scholar]
- Hermon C, Beral V. Breast cancer mortality rates are levelling off or beginning to decline in many western countries: analysis of time trends, age-cohort and age-period models of breast cancer mortality in 20 countries. Br J Cancer. 1996;73:955–960. doi: 10.1038/bjc.1996.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R. Mortality from breast cancer in UK has decreased suddenly. BMJ. 1998;317:476–477. doi: 10.1136/bmj.317.7156.476b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornefalk A, Persson I, Bergstrom R. Trends in breast cancer mortality among Swedish women 1953–92: analyses by age, period and birth cohort. Br J Cancer. 1995;72:493–497. doi: 10.1038/bjc.1995.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey JV, Jr, Devesa SS, Brinton LA. Recent trends in breast cancer incidence and mortality. Environ Mol Mutagen. 2002;39:82–88. doi: 10.1002/em.10062. [DOI] [PubMed] [Google Scholar]
- Wun LM, Feuer EJ, Miller BA. Are increases in mammographic screening still a valid explanation for trends in breast cancer incidence in the United States? Cancer Causes Control. 1995;6:135–144. doi: 10.1007/BF00052774. [DOI] [PubMed] [Google Scholar]
- Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- MacMahon B. Temporal trends in breast cancer incidence. Am J Epidemiol. 1982;116:867. doi: 10.1093/oxfordjournals.aje.a113477. [DOI] [PubMed] [Google Scholar]
- Smigel K. Breast cancer death rates decline for white women [news] J Natl Cancer Inst. 1995;87:173. doi: 10.1093/jnci/87.3.173. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute of Canada Canadian Cancer Statistics 1998 National Cancer Institute of Canada. 1998.
- Holford TR, Roush GC, McKay LA. Trends in female breast cancer in Connecticut and the United States. J Clin Epidemiol. 1991;44:29–39. doi: 10.1016/0895-4356(91)90198-I. [DOI] [PubMed] [Google Scholar]
- Tarone RE, Chu KC, Gaudette LA. Birth cohort and calendar period trends in breast cancer mortality in the United States and Canada. J Natl Cancer Inst. 1997;89:251–256. doi: 10.1093/jnci/89.3.251. [DOI] [PubMed] [Google Scholar]
- Giles GG, Amos A. Evaluation of the organised mammographic screening programme in Australia. Ann Oncol. 2003;14:1209–1211. doi: 10.1093/annonc/mdg326. [DOI] [PubMed] [Google Scholar]
- Armstrong W, Borman B. Breast cancer in New Zealand: trends, patterns, and data quality. N Z Med J. 1996;109:221–224. [PubMed] [Google Scholar]
- Smith CL, Kricker A, Armstrong BK. Breast cancer mortality trends in Australia: 1921 to 1994. Med J Aust. 1998;168:11–14. doi: 10.5694/j.1326-5377.1998.tb123335.x. [DOI] [PubMed] [Google Scholar]
- Hermon C, Beral V. Breast cancer mortality rates are levelling off or beginning to decline in many western countries: analysis of time trends, age-cohort and age-period models of breast cancer mortality in 20 countries. Br J Cancer. 1996;73:955–960. doi: 10.1038/bjc.1996.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai K, Suzuki S, Ohno Y, Kawamura T, Tamakoshi A, Aoki R. Epidemiology of breast cancer in Japan. Int J Epidemiol. 1995;24:285–291. doi: 10.1093/ije/24.2.285. [DOI] [PubMed] [Google Scholar]
- Nagata C, Kawakami N, Shimizu H. Trends in the incidence rate and risk factors for breast cancer in Japan. Breast Cancer Res Treat. 1997;44:75–82. doi: 10.1023/A:1005726110649. [DOI] [PubMed] [Google Scholar]
- Minami Y, Tsubono Y, Nishino Y, Ohuchi N, Shibuya D, Hisamichi S. The increase of female breast cancer incidence in Japan: emergence of birth cohort effect. Int J Cancer. 2004;108:901–906. doi: 10.1002/ijc.11661. [DOI] [PubMed] [Google Scholar]
- Parkin DM. Cancer in developing countries. Cancer Surveys. 1994;19/20:519–561. [PubMed] [Google Scholar]
- Coleman MP, Esteve J, Damiecki P, Arslan A, Renard H. Trends in Cancer Incidence and Mortality. Lyon: IARC; 1993. [DOI] [PubMed] [Google Scholar]
- Yeole BB, Kurkure AP. An epidemiological assessment of increasing incidence and trends in breast cancer in Mumbai and other sites in India, during the last two decades. Asian Pac J Cancer Prev. 2003;4:51–56. [PubMed] [Google Scholar]
- Seow A, Duffy SW, McGee MA, Lee J, Lee HP. Breast cancer in Singapore: trends in incidence 1968–1992. Int J Epidemiol. 1996;25:40–45. doi: 10.1093/ije/25.1.40. [DOI] [PubMed] [Google Scholar]
- Yang L, Parkin DM, Li L, Chen Y. Time trends in cancer mortality in China: 1987–1999. Int J Cancer. 2003;106:771–783. doi: 10.1002/ijc.11300. [DOI] [PubMed] [Google Scholar]
- Chie WC, Chen CF, Lee WC, Chen CJ, Lin RS. Age-period-cohort analysis of breast cancer mortality. Anticancer Res. 1995;15:511–515. [PubMed] [Google Scholar]
- Leung GM, Thach TQ, Lam TH, Hedley AJ, Foo W, Fielding R, Yip PS, Lau EM, Wong CM. Trends in breast cancer incidence in Hong Kong between 1973 and 1999: an age-period-cohort analysis. Br J Cancer. 2002;87:982–988. doi: 10.1038/sj.bjc.6600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echimane AK, Ahnoux AA, Adoubi I, Hien S, M'Bra K, D'Horpock A, Diomande M, Anongba D, Mensah-Adoh I, Parkin DM. Cancer incidence in Abidjan, Ivory Coast: first results from the cancer registry, 1995–1997. Cancer. 2000;89:653–663. doi: 10.1002/1097-0142(20000801)89:3<653::AID-CNCR22>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Chokunonga E, Levy LM, Bassett MT, Mauchaza BG, Thomas DB, Parkin DM. Cancer incidence in the African population of Harare, Zimbabwe: second results from the cancer registry 1993–1995. Int J Cancer. 2000;85:54–59. doi: 10.1002/(sici)1097-0215(20000101)85:1<54::aid-ijc10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Ferlay J, Hamdi-Cherif M, Sitas F, Thomas JO, Wabinga H, Whelan SL. Cancer in Africa: Epidemiology and Prevention. Lyon: IARC; 2003. [Google Scholar]
- Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer. 2000;82:1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. 'Hormonal' risk factors, 'breast tissue age' and the age-incidence of breast cancer. Nature. 1983;303:767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- Lane-Claypon JE. A Further Report on Cancer of the Breasts, with Special Reference to its Associated Antecedent Conditions. London: HMSO; 1926. Report on Public Health and Medical Subjects No. 32. [Google Scholar]
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/S0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Heck KE, Pamuk ER. Explaining the relation between education and postmenopausal breast cancer. Am J Epidemiol. 1997;145:366–372. doi: 10.1093/oxfordjournals.aje.a009114. [DOI] [PubMed] [Google Scholar]
- Adams J, White M, Forman D. Are there socioeconomic gradients in stage and grade of breast cancer at diagnosis? Cross sectional analysis of UK cancer registry data. BMJ. doi:10.1136/bmj.38114.679387.AE (published 2 June 2004) [DOI] [PMC free article] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–1727. doi: 10.1016/S0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15:617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Spiegelman D, Adami HO, Beeson L, van den Brandt PA, Folsom AR, Fraser GE, Goldbohm RA, Graham S, Howe GR, et al. Cohort studies of fat intake and the risk of breast cancer – a pooled analysis. N Engl J Med. 1996;334:356–361. doi: 10.1056/NEJM199602083340603. [DOI] [PubMed] [Google Scholar]
- Smith-Warner SA, Spiegelman D, Adami HO, Beeson WL, van den Brandt PA, Folsom AR, Fraser GE, Freudenheim JL, Goldbohm RA, Graham S, et al. Types of dietary fat and breast cancer: a pooled analysis of cohort studies. Int J Cancer. 2001;92:767–774. doi: 10.1002/1097-0215(20010601)92:5<767::AID-IJC1247>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Magnusson C, Baron J, Persson I, Wolk A, Bergstrom R, Trichopoulos D, Adami HO. Body size in different periods of life and breast cancer risk in post-menopausal women. Int J Cancer. 1998;76:29–34. doi: 10.1002/(SICI)1097-0215(19980330)76:1<29::AID-IJC6>3.3.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91:421–430. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1053>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Jr, Coates RJ, Liff JM, Talamini R, Chantarakul N, et al. Alcohol, tobacco and breast cancer – collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S, Coleman EA, Broeders M, Codd M, de Koning H, Fracheboud J, Moss S, Paci E, Stachenko S, Ballard-Barbash R. Breast cancer screening programmes in 22 countries: current policies, administration and guidelines. International Breast Cancer Screening Network (IBSN) and the European Network of Pilot Projects for Breast Cancer Screening. Int J Epidemiol. 1998;27:735–742. doi: 10.1093/ije/27.5.735. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer . Breast Cancer Screening. Lyon: IARC Press; 2002. [Google Scholar]
- McCann J, Stockton D, Day N. Breast cancer in East Anglia: the impact of the breast screening programme on stage at diagnosis. J Med Screen. 1998;5:42–48. doi: 10.1136/jms.5.1.42. [DOI] [PubMed] [Google Scholar]
- Hakama M, Pukkala E, Heikkila M, Kallio M. Effectiveness of the public health policy for breast cancer screening in Finland: population based cohort study. BMJ. 1997;314:864–867. doi: 10.1136/bmj.314.7084.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar L, Fagerberg G, Duffy SW, Day NE, Gad A, Grontoft O. Update of the Swedish two-county program of mammographic screening for breast cancer. Radiol Clin North Am. 1992;30:187–210. [PubMed] [Google Scholar]
- Kricker A, Farac K, Smith D, Sweeny A, McCredie M, Armstrong BK. Breast cancer in New South Wales in 1972–1995: tumor size and the impact of mammographic screening. Int J Cancer. 1999;81:877–880. doi: 10.1002/(SICI)1097-0215(19990611)81:6<877::AID-IJC7>3.3.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Blanks RG, Moss SM, McGahan CE, Quinn MJ, Babb PJ. Effect of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990–8: comparison of observed with predicted mortality. BMJ. 2000;321:665–669. doi: 10.1136/bmj.321.7262.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatoi I, Miller AB. Why is breast-cancer mortality declining? Lancet Oncol. 2003;4:251–254. doi: 10.1016/S1470-2045(03)01037-4. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Collyar DE, Somerfield MR, Pfister DG. American Society of Clinical Oncology technology assessment on breast cancer risk reduction strategies: tamoxifen and raloxifene. J Clin Oncol. 1999;17:1939–1955. doi: 10.1200/JCO.1999.17.6.1939. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. doi: 10.1016/S0140-6736(97)11423-4. [DOI] [PubMed] [Google Scholar]
- Chu KC, Tarone RE, Kessler LG, Ries LA, Hankey BF, Miller BA, Edwards BK. Recent trends in US breast cancer incidence, survival, and mortality rates. J Natl Cancer Inst. 1996;88:1571–1579. doi: 10.1093/jnci/88.21.1571. [DOI] [PubMed] [Google Scholar]
- Peto R. Five years of tamoxifen – or more? J Natl Cancer Inst. 1996;88:1791–1793. doi: 10.1093/jnci/88.24.1791. [DOI] [PubMed] [Google Scholar]
- Selby P, Gillis C, Haward R. Benefits from specialised cancer care. Lancet. 1996;348:313–318. doi: 10.1016/S0140-6736(96)02482-8. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, Boyle P. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- Cuzick J. Aromatase inhibitors in prevention – data from the ATAC (arimidex, tamoxifen alone or in combination) trial and the design of IBIS-II (the second International Breast Cancer Intervention Study) Recent Results Cancer Res. 2003;163:96–103. doi: 10.1007/978-3-642-55647-0_9. [DOI] [PubMed] [Google Scholar]
- Feigelson HS, Henderson BE. Future possibilities in the prevention of breast cancer: role of genetic variation in breast cancer prevention. Breast Cancer Res. 2000;2:277–282. doi: 10.1186/bcr69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geel AN. Prophylactic mastectomy: the Rotterdam experience. Breast. 2003;12:357–361. doi: 10.1016/S0960-9776(03)00136-X. [DOI] [PubMed] [Google Scholar]


