Abstract
Objectives
Prevention programs that can help adults improve the quality of their diets to reduce cancer risk are needed. This Phase IIa study prospectively tested a mHealth intervention designed to improve adherence to dietary quality guidelines for cancer prevention.
Methods
All participants (N = 62) received nutrition education and a self-regulation skills curriculum, with a primary target of changing grocery shopping behavior. Using a randomized, factorial design, the study varied whether each of the following 4 components were added to the 20-week intervention: (1) location-triggered app messaging, delivered when individuals arrived at grocery stores, (2) reflections on benefits of change, delivered with extra coaching time and tailored app messages, (3) coach monitoring, in which food purchases were digitally monitored by a coach, and (4) involvement of a household member in the intervention.
Results
Benchmarks were successfully met for recruitment, retention, and treatment acceptability. Across conditions, there were significant reductions in highly processed food intake (P < .001, η2 = .48), red and processed meat intake (P < .001, η2 = .20), and sugar-sweetened beverage intake (P = .008, η2 = .13) from pre-to post-treatment. Analyses examining whether each intervention component influenced change across time found that participants who received coach monitoring increased their intake of fruits, vegetables, and fiber, whereas those with no coach monitoring had less improvement (P = .01, η2 = .14). The improvement in red and processed meat was stronger among participants with household support ON, at a marginally significant level, than those with household support OFF (P = .056, η2 = .07).
Conclusion
This study showed feasibility, acceptability, and preliminary signals of efficacy of a remotely delivered intervention to facilitate adherence to dietary guidelines for cancer prevention and that coach monitoring and household support may be especially effective strategies. A fully powered clinical trial is warranted to test an optimized version of the intervention that includes nutrition education, self-regulation skills training, coach monitoring, and household member involvement.
Trial Registration
Keywords: cancer prevention, diet quality, dietary improvements, mHealth, self-regulation
Introduction
Quality of dietary intake (i.e., eating sufficient whole grains, vegetables, and fruit and limiting intake of processed foods, red and processed meat, and sugar-sweetened beverages) is a critical, modifiable risk factor for cancer.1,2 However, most Americans have low adherence to cancer prevention dietary guidelines.3-5 For example, over 90% of adults have inadequate fruit and vegetable intake (i.e., <5 servings per day) and nearly 70% consume too much processed meat (i.e., >two 50 g servings per week). 6 There is an urgent need for primary prevention resources that can help adults improve the quality of their diets to reduce cancer risk in the existing food environment, which makes self-regulation of eating highly challenging. 7 The current study integrated prominent psychosocial theories of motivation and self-regulation to develop and test a dietary quality intervention among adults with poor adherence to cancer prevention guidelines.
Achieving Stimulus Control Through Grocery Shopping Behavior
Humans have a biologically driven hedonic response to foods high in salt, sugar, and fat, 8 and these foods are ubiquitous in the US today. 9 When these highly palatable foods are brought into one’s home, self-regulation will be extremely challenging, regardless of intentions to eat these foods in moderation.10,11 By contrast, when healthy foods are available in the home and unhealthy foods are not, minimal self-control is needed to make healthy eating choices. Indeed, food cues have been shown to strongly influence eating behavior.12,13
As such, stimulus control, that is, modifying the home food environment to change the stimuli (foods) in the home, 14 is perhaps the most efficient way to improve adherence to dietary guidelines. The home food environment can only be modified by changing grocery shopping habits. Among adults, 60%–70% of intake comes from foods purchased in grocery stores.15,16 Therefore, grocery shopping habits have a powerful effect on dietary intake, making this behavior the most promising intervention target to promote dietary change. Previous interventions designed to change grocery shopping habits have primarily focused on providing education and produced only modest changes in food purchasing, with minimal evidence of long-term maintenance of change in dietary quality.17-19 These suboptimal results are unsurprising given the difficulty of self-regulation in the modern food environment.17,20,21 There are several tools for behavior change that can enhance self-regulation and motivation, that, when added to a foundation of nutrition education, might improve grocery shopping purchases, and ultimately, short and long-term adherence to dietary guidelines.
Motivation and Self-Regulation Strategies to Improve Grocery Shopping Purchases
Ample research shows that self-regulation is facilitated by teaching goal setting,22,23 implementation intentions (i.e., highly specific plans for enacting a goal, tied to a situational cue), 24 and self-monitoring. 25 Increasing the salience of dietary goals in key moments of decision making might also reduce the intention-behavior gap so often observed in dietary interventions. 26
Enhancing motivation is also critical for health behavior change. 27 Supportive accountability, in which a participant has a sense of being monitored by another person and reflects on progress with them, may be helpful for improving motivation to make healthy food choices.28,29 Motivation might also be enhanced by ensuring that participants perceive that their behavior change efforts will produce meaningful benefits.27,30 Finally, social factors within one’s household, including support for healthy eating and the perceived food preferences of family members, exert a strong influence on food purchases.31,32 Engaging household members in an intervention could provide an opportunity to elicit support for home food environment change and modify norms.
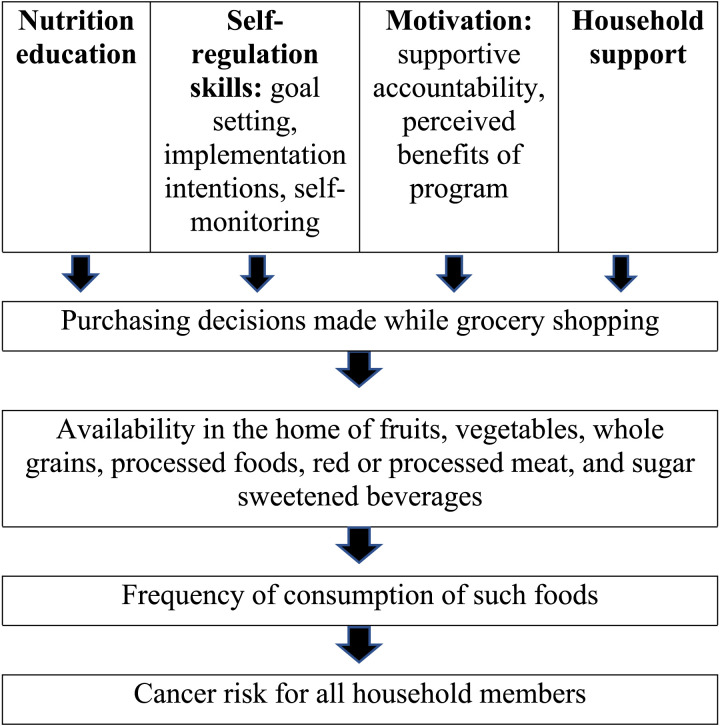
The conceptual model in Figure 1 integrates these theories of motivation and self-regulation, which was used to develop a mHealth intervention designed to improve adherence to dietary quality guidelines for cancer prevention. The remotely delivered intervention provided all participants with nutrition education and a skills curriculum that was informed by the science of self-regulation. Using a factorial design, the study varied whether each of the following 4 components were added to that core intervention package: (1) location-triggered messaging, in which educational messages are delivered via app just-in-time, when individuals arrived at grocery shopping locations, to enhance goal salience, (2) reflections on benefits of change, in which participants received extra coaching time and app messaging content devoted to identifying and reflecting on the personal benefits of dietary change, (3) coach monitoring, in which food purchases were digitally monitored by a coach through a system that passively collected participants’ item-level data from stores, in order to enhance supportive accountability, and (4) involvement of a household member in the intervention in order to harness support for home food environment change and provide an additional source of supportive accountability for the index participant. The primary aims of the study were to assess feasibility and acceptability of the intervention components, to assess the feasibility of recruitment and retention, and to examine whether participants had meaningful changes in dietary intake (primary outcome) and grocery store food purchases (exploratory outcome) from pre-to-post intervention. To identify the isolated effect of each intervention component, the study examined whether change across time depended on the presence of each component.
Figure 1.
Conceptual model.
Methods
Study Design
In this paper, we report the results of a proof-of-concept test of the mHealth intervention’s feasibility, acceptability, and preliminary effectiveness, following Phase IIa of the ORBIT (Obesity-Related Behavioral Intervention Trials) model for intervention development, which is currently considered best practice for developing behavioral interventions to treat obesity and related diseases. 33 In a 20-week study, all participants completed a core intervention and were also randomly assigned through a factorial design to test the effects of 4 additional intervention components on dietary intake and grocery store purchases. The core intervention, which consisted of 3 nutrition education workshops, was delivered weekly over the first 3 weeks of the intervention period. The additional, randomized intervention components were delivered across the remainder of the 20-week intervention period with variable frequency (see “Intervention” for details). Participants were independently randomized to ON vs OFF each of the 4 experimental intervention components (i.e., the 4 experimental intervention components were not “bundled” together), yielding 16 different combinations of intervention components (see Appendix A). Random assignment was conducted with participants stratified by biological sex, body mass index (BMI), age, household size, and dietary adherence score. The statistician who generated the randomization sequence was not involved in any aspect of participant enrollment. The study was approved by the Institutional Review Board at Drexel University. All index participants provided written informed consent for participation. Adult household members of the index participants who were randomized to receive the household support component of the program also provided written informed consent. The reporting of this study conforms to CONSORT statements, 34 and the trial was registered at ClinicalTrials.gov (NCT04947150).
Participants
Index participants (N = 62) were recruited in 2 cohorts (cohort 1 n = 34 and cohort 2 n = 28) from the Philadelphia area in 2021 using mailings, social media outreach, and Craigslist listings. The intervention was advertised as one that could help participants learn healthier eating habits. Thomas Jefferson University’s Sidney Kimmel Cancer Center also supported recruitment efforts via their “honest broker” system, which identified and contacted potential participants using community resources. This method included identifying and contacting participants using community contacts and internal communication resources via the Jefferson Regional Liaison Office (e.g., individuals in the Jefferson community who met eligibility criteria were emailed about their interest in participating in the study). Interested individuals completed 3 eligibility steps: (1) completed a screening survey to assess preliminary eligibility, (2) attended an information session via videoconferencing, and (3) attended a baseline assessment, in which their final eligibility was determined. Participants were eligible if they were 18 years or older, fluent in English, the primary grocery shopper in their household, owned a smartphone with an iOS or Android-operating system that was compatible with program applications, and reported regularly shopping at stores that could passively stream item-level data from a store loyalty card to the project portal (Walmart, Target, ShopRite, or Wegmans). Additionally, all index participants were required to live in a household with another adult who agreed to serve in a support role during the study. Exclusion criteria for the study were as follows: active substance abuse, eating disorder or inability to comply with program dietary recommendations, plans to enroll in another lifestyle modification program within 6 months of program start, bariatric surgery history, pregnancy, breastfeeding, or plans to become pregnant in the next 6 months. The sample size was selected based on guidance for clinical trial planning provided by Lewis et al. 35
Intervention
The study included a core intervention and additional intervention components that utilized specific behavior change techniques targeting mechanisms of action 36 to improve adherence for dietary guidelines for cancer prevention.
Core Intervention
All index participants attended 3 nutritional education workshops via videoconferencing (90 minutes each; 10–15 participants each) delivered weekly on weekdays over the first 3 weeks of the 20-week intervention period. Content in each session focused on the 4 dietary quality recommendations for cancer prevention from the World Cancer Research Fund/American Institute for Cancer Research: (1) eat a diet rich in whole grains, vegetables, and fruit; (2) limit consumption of highly processed foods (defined as those high in fat, starches, or sugars); (3) limit consumption of red and processed meat; and (4) eliminate consumption of sugar-sweetened beverages.2,37 Each session consisted of education about the key dietary guidelines and behavioral skills training (e.g., stimulus control and use of implementation intentions). Each participant set guideline-related goals, engaged in meal planning, and created a grocery list at the end of each session. These behavior change techniques were used to foster knowledge of dietary guidelines and skills to regulate behavior. Workshops were delivered by master’s or PhD-level coaches (e.g., psychology, nutrition, or a related field) with previous experience conducting lifestyle modification. Coach continuity was maintained for participants for all intervention contact. All participants also received standardized, weekly educational messages in a study-designed smartphone app reminding them of nutritional recommendations and behavioral skills (e.g., healthy substitutions and meal planning) to promote adherence throughout the 20-week intervention. The program app also included graphs that displayed how well the participant’s grocery purchases from the previous 4 weeks aligned with the program dietary recommendations.
Additional, Randomized Intervention Components
Four experimental intervention components were either added or not added to the uniform intervention elements (i.e., the 3 nutrition workshop sessions and weekly messages). The 4 experimental components were location triggered messages, benefits of change, coach monitoring, and household support. The study was designed such that the program contact time and frequency varied by condition to evaluate the benefit of, among other things, the added contacts.
Participants randomized to the location-triggered messages received their weekly, standardized text messages in the program app when their smartphone was within a 50-meter geofence around the designated grocery stores that were self-reported by that participant at baseline. Messages were delivered only once per week across the 20-week intervention period, regardless of shopping frequency, and sent at the end of the week if the system did not detect the grocery store location within a given week. These messages served as prompts/cues that were designed to enhance mindfulness of program goals in the moment of decision-making at the grocery store to facilitate food purchasing consistent with program nutrition recommendations. Participants who were not randomized to location-triggered messages condition received their standardized, weekly messages at a uniform day and time.
Participants assigned to have the benefits of change component ON received additional intervention contact and content designed to help them reflect on the anticipated benefits of purchasing healthy food and changing dietary quality to increase motivation for eating behavior consistent with the dietary guidelines for cancer prevention. Additional components included the following: (1) 1 extra 60-minute workshop session (8 participants each), delivered via Zoom videoconferencing in week 5 by a master’s or PhD-level clinician (i.e., same staff who delivered the nutrition education workshops) to identify personally meaningful short- and long-term benefits of healthy eating (e.g., increased energy in the short-term and lower blood pressure in the long-term), (2) 3 individual coaching calls (20 minutes each; delivered in weeks 9, 13, and 17) to further illuminate personally meaningful benefits of change, and (3) customized benefits of change content added to weekly app messages after week 5. Participants assigned to have the benefits of change component OFF did not receive this extra intervention contact, and their weekly message did not highlight personal, anticipated benefits of change.
Participants randomized to coach monitoring ON received additional feedback from coaches, who viewed food purchasing information collected at the point of purchase in a web-based portal. This was made possible by an application programming interface, (API) that harvested data from store loyalty cards or linked credit cards and categorized items into dietary categories. Purchasing data were updated in the portal in real time throughout the 20-week intervention, and participants could self-report any purchases not captured there. Coaches, who were master’s or PhD-level clinicians (i.e., same staff who delivered nutrition education workshops), sent personalized weekly messages across the 20-week intervention period sharing observations and feedback on the participant’s objective food purchasing data; these messages were in addition to the standardized, weekly messages that all index participants received. Coaches also provided feedback in 3 phone calls with participants (20 minutes each; delivered in weeks 4, 10, and 15). The messages and calls were designed to improve motivation for dietary change by providing reinforcement for purchases consistent with the program’s dietary recommendations and enhancing supportive accountability. In the coach monitoring OFF condition, coaches did not have the ability to view purchasing data, and participants did not receive these extra coach calls or customized text messages.
Participants randomized to household support ON nominated 1 adult household member to serve in a supporting role during the intervention. The household members received weekly messages in the program app throughout the 20-week intervention encouraging them to support the index participant’s dietary change efforts. The household member and index participant also jointly participated in 1 additional 60-minute workshop session (delivered in week 6) and 3 coaching calls (20 min each; delivered in weeks 7, 11, and 16) focused on household support. This additional contact was designed to improve self-regulation and motivation by engaging household support for home food environment changes and enhancing supportive accountability for dietary improvements. Household members of the index participants assigned to household support OFF received no text messages and attended no workshops or phone calls.
Measures
Feasibility and acceptability data were compared to benchmarks identified by the investigators at the time of project conception. Recruitment feasibility was operationalized with a benchmark of >5 participants enrolled per month of recruitment. Retention feasibility was operationalized as >70% of participants completing each follow-up assessment. A mean rating of >28 on the Treatment Acceptability Questionnaire (adapted) 38 at mid-treatment and post-treatment served as a benchmark for user-rater acceptability of the program. Eight items were rated on a 1 to 7 scale, with 7 being the most positive rating possible; the maximum total score possible was 56. The benchmark for feasibility of location-triggered messaging delivery was participant report that >95% of messages were delivered as intended. For food purchasing, a benchmark of >90% of participants having their food purchasing data captured by the program was established.
Dietary intake was measured at baseline and post-treatment (20 weeks). Participants recruited in Cohort 1 completed 3 full days of food recall at each time point using the NCI-developed Automated Self-Administered 24-hour Dietary Recall (ASA24). 39 Automated Self-Administered 24-hour Dietary Recall is based on the well-validated automated multiple pass method, which has been shown to be as or more accurate than nutritionist-administered 24-hour food recall when using doubly labeled water as the criterion.40,41 Due to poor acceptability of the ASA24 among Cohort 1 participants (i.e., reports of high perceived burden), Cohort 2 participants completed the Diet History Questionnaire (DHQ-III) 42 at baseline and 20 weeks, a comparable food frequency questionnaire developed by the NCI with reduced completion time. Both the ASA24 and DHQ-III provide item-level nutritional-level information for all food/drinks consumed and daily totals of dietary intake variables of interest (e.g., daily intake of vegetables).
The dietary guidelines provided by World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) specify limiting intake of processed foods high in fat, starches, or sugars including fast foods, readily available convenience foods, prepared dishes, snacks, baked goods, and desserts.2,27 In order to identify highly processed food items that meet these criteria, the NOVA classification system was used to flag and categorize foods. 43 According to this classification system, processed foods included salty snacks, frozen and prepared meals, baked goods, dessert, fried potatoes, candy, packaged bread and buns, refined grains, breakfast cereal, and processed cheese. Sugar-sweetened drinks included non-diet sodas, non-diet fruit drinks, energy drinks, and sugary coffee drinks. Any items that could be classified as highly processed or sugar-sweetened drinks were pulled from the ASA24 item-level output based on their Food and Nutrient Database for Dietary Studies (FNDDS) food code 44 and from the DHQ-III item-level output based on their coding in the NCI’s associated nutrient database and included in nutrient total calculations for processed foods or sugar-sweetened drinks. 45
The average daily intake of the following dietary variables was analyzed: fiber (grams), fruit (cups; intact whole or cut fruit not including fruit juices) and vegetables (cups; all vegetables except starches), added sugar from processed foods (grams), saturated fat from processed foods (grams), sodium from processed foods (grams), red meat (ounces of beef, veal, pork, lamb, or game), processed meat (grams of frankfurters, sausages, corned beef, and luncheon meat made from beef, pork, or poultry), and sugar-sweetened drinks (ounces). The average daily intake of red and processed meat was prorated over 7 days to account for the NCI dietary recommendation for these variables occurring at the weekly level (compared to daily). To calculate adherence to each of the 4 NCI dietary recommendations, the well-established WCRF/AICR scoring method supported by the NCI was used. 46 Participants received scores of 0 (failure to meet recommendation), .5 (partially meeting recommended levels), or 1 (fully meeting recommended level of intake) in each dietary domain based on pre-defined dietary intake cutoff values specified by the NCI expected to have clinical significance (see Appendix B for values). When the recommendation included sub-categories (e.g., NCI recommendation to eat a diet rich in wholegrains, vegetables, fruits, and beans includes adherence to daily fiber and fruit/vegetable intake), the adherence score consisted of the average of adherence on the sub-categories. Sub-scores for each dietary recommendation included the following: (1) average adherence scores for fiber and fruits and vegetables (Guideline 1); (2) average adherence scores for added sugar, saturated fat, and sodium in processed foods (Guideline 2); (3) average adherence scores for red and processed meats (Guideline 3); and (4) adherence score for sugar-sweetened drinks (Guideline 4).
To measure objective changes in grocery purchasing habits throughout the intervention, an API securely transferred participant’s item-level food purchases from designated grocery store loyalty program accounts (Wegmans, ShopRite, Target, and Walmart) to the study database. Summary nutrition variables (e.g., added sugar from processed foods) were created for each trip via linking item-level food purchases with nutrition databases (e.g., FNDDS). Given the novelty of this data collection method, the change in purchase amounts of foods categorized under the NCI guidelines was conceptualized as an exploratory outcome.
Statistical Analyses
To test improvements in dietary intake, we ran repeated measures ANOVAs that examined whether adherence to each guideline changed across time (i.e., from baseline to post-treatment). Models also included the interaction effects of each intervention component (location triggered messaging, benefits of change, coach monitoring, and household support) and time to determine whether these components could enhance change. Each condition variable was coded as 0 = OFF or 1 = ON, and all conditions were included in a single model for each outcome. We report the simple effects within these interactions, examining change over time when each condition was OFF and ON, respectively. We also report and interpret effect sizes (partial eta squared; η2) in addition to P-values. Effect sizes were interpreted as meaningful if they were .06 or higher, indicating a medium effect. Post hoc pairwise comparisons examined change in each guideline across time separately for each condition.
Data Availability
The data generated in this study are not publicly available due to participant privacy (given the small sample size and nature of the variables collected) but are available upon request from the corresponding author.
Results
Descriptive Statistics and Correlations
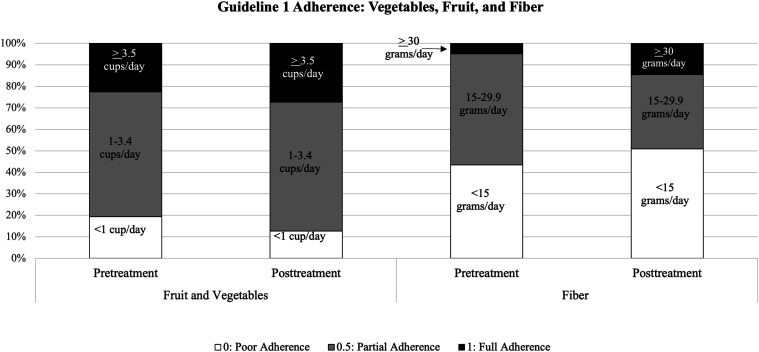
Participants (N = 62) were predominantly women (91.9%) with a mean age of 47.19 years (SD = 13.45) and mean BMI of 32.09 kg/m2 (SD = 8.03). Most participants had overweight (27.4%) or obesity (51.6%). Most participants were Non-Hispanic White (51.6%) or Non-Hispanic Black (30.6%), and 9.6% of participants were Hispanic. The majority of participants reported living with more 2 or more people in their home (61.3%), mean household size was 3.2 (SD = 1.4), and roughly half (48.4%) of participants reported living with 1 or more minor children in their home. Almost all participants (90.3%) reported shopping at grocery stores at least once per week. Information about baseline adherence to dietary guidelines can be seen in Figure 2 through 5. Data collection for all participants was completed by Summer 2022.
Figure 2.
Adherence to Guideline 1 (intake of vegetables, fruit, and fiber) pre-treatment vs post-treatment.
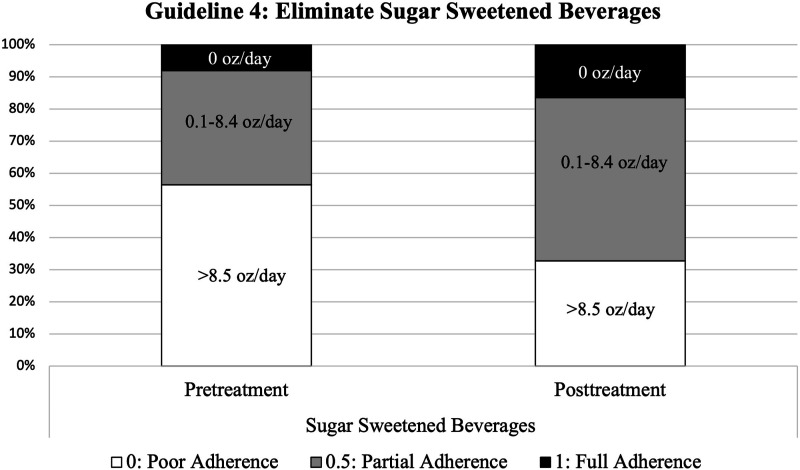
Figure 5.
Adherence to Guideline 4 (reduce intake of sugar-sweetened beverages) at pre-treatment and post-treatment.
Intervention Feasibility and Acceptability
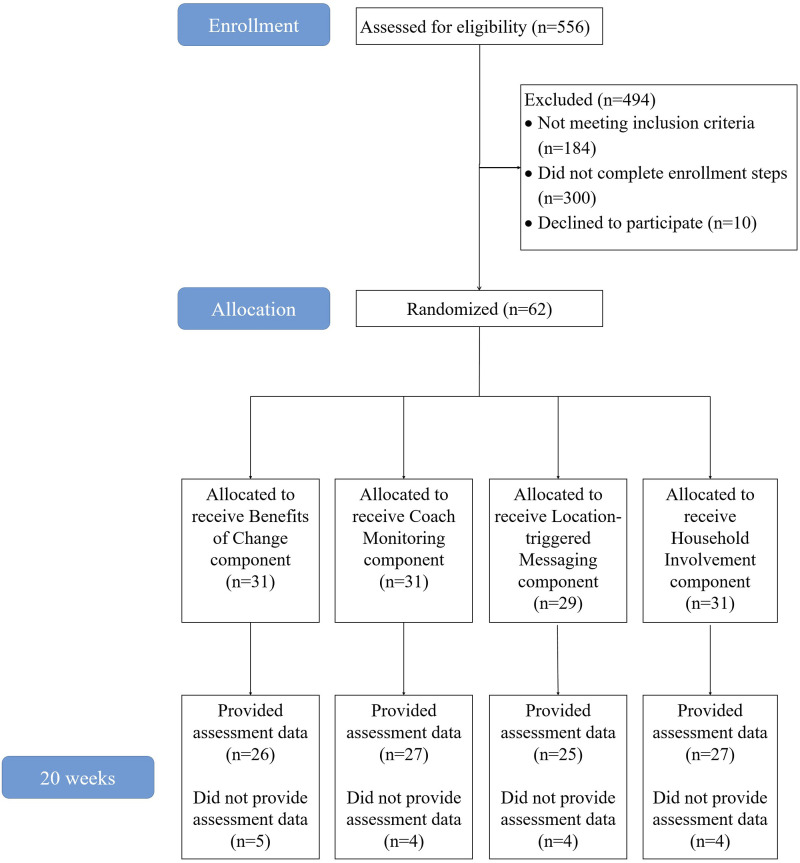
Benchmarks were successfully met for weekly rate of recruitment (which exceeded the benchmark of 5 participants/week) and retention (88.7% of participants completed the 20-week assessment, as shown in the CONSORT diagram in Figure 6). The mean score on the Treatment Acceptability Questionnaire was 45.76 out of 56 at the mid-treatment assessment and 46.81 at post-treatment, also exceeding the pre-specified benchmark. The 95% benchmark for location-triggered messaging receipt was not met. Zero participants assigned to location-triggered messaging ON agreed with the statement, “Every week, or all but one week, I received a message upon arrival at a designated grocery store.” Unfortunately, we were not able to identify the exact number of location-triggered messages that were successfully received by each participant in this condition.
Figure 6.
CONSORT diagram.
The study also did not meet the benchmark of having food purchasing data passively captured from store accounts for 90% of participants. At baseline, 59.6% of participants had available grocery purchasing data. At post-treatment, 53.2% of participants had available data. Only 24 (38.7%) participants had data at both timepoints. Because less than half of the sample provided data at both timepoints and because participants reported that their captured data often was not fully representative of their purchases for their week, analyses that examine change over time could be biased and thus were not conducted. Reasons for missing data included the following: (1) shopping at vendors where API data collection was not available (e.g., corner stores and farmers’ markets), (2) forgetting to use loyalty card or store-linked credit card, or (3) unknown technical failure in API data capture. After recognizing these challenges, we piloted a procedure with several participants in which they were asked to upload a photograph of their receipt or, if receipts with item detail were not available, to upload a photograph of items purchased. Purchase data capture rates improved markedly when providing these additional options.
Dietary Intake (Primary Outcome)
See Table 1 for mean adherence to each dietary guideline across time, for the full sample and separated by study component.
Table 1.
Guideline Adherence Scores at Baseline and Post-treatment for the Full Sample and Separated by Experimental Condition.
| N | Guideline 1: Fruit, Vegetables, and Fiber | Guideline 2: Highly Processed Foods | Guideline 3: Red and Processed Meat | Guideline 4: Sugar-Sweetened Beverages | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Treatment | P-Value | Baseline | Post-Treatment | P-Value | Baseline | Post-Treatment | P-Value | Baseline | Post-Treatment | P-Value | ||
| All participants | 62 | .43 | .45 | .58 | .53 | .74 | <.001 | .56 | .71 | <.001 | .26 | .41 | .008 |
| Coach sharing ON | 31 | .38 | .50 | .02 | .58 | .71 | <.001 | .56 | .72 | .01 | .26 | .40 | .09 |
| Coach sharing OFF | 31 | .48 | .39 | .11 | .58 | .77 | <.001 | .551 | .69 | .03 | .26 | .43 | .04 |
| BOC ON | 31 | .45 | .44 | .87 | .57 | .80 | <.001 | .61 | .72 | .20 | .21 | .38 | .05 |
| BOC OFF | 31 | .40 | .45 | .33 | .48 | .69 | <.001 | .50 | .70 | <.001 | .31 | .45 | .08 |
| Household ON | 31 | .44 | .44 | .92 | .48 | .79 | <.001 | .55 | .79 | <.001 | .30 | .43 | .103 |
| Household OFF | 31 | .41 | .45 | .49 | .58 | .70 | <.001 | .57 | .63 | .29 | .23 | .40 | .03 |
| Location ON | 32 | .47 | .44 | .56 | .54 | .73 | <.001 | .64 | .73 | .00 | .26 | .38 | .14 |
| Location OFF | 30 | .38 | .45 | .16 | .54 | .75 | <.001 | .48 | .68 | .13 | .26 | .45 | .02 |
Note. Higher scores indicate better adherence to the guideline. Bolded means indicate significant change during treatment within that condition.
Guideline 1: Increase Fiber and Fruit/Vegetable Intake
Repeated measures ANOVAs showed no significant change in adherence to guideline 1 from baseline to post-treatment across all participants (F(1, 50) = .32, P = .58, η2 = .01). Figure 2 illustrates change in adherence scores over time. However, there was a significant interaction with a large effect size between coaching monitoring condition and time, such that participants with coach monitoring ON had a significant increase in adherence, whereas those with coaching monitoring OFF did not (F(1, 50) = .27, P = .01, η2 = .14). There were no other significant interactions between time and study condition, and no other notable effect sizes.
Guideline 2: Reduce Highly Processed Foods
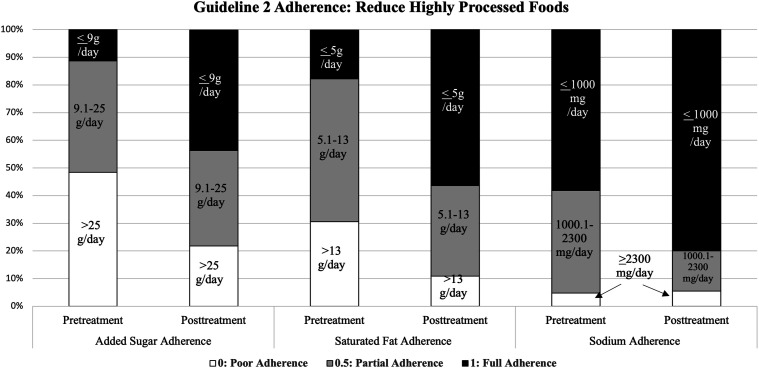
Across all participants, there was a large, significant improvement in adherence to highly processed food guidelines from baseline to post-treatment (F(1, 50) = 45.68, P < .001, η2 = .48), as can be seen in the decrease of intake of sugar, sodium, and fat associated with processed foods in Figure 3. There was also a significant interaction between household support condition and time, such that the improvement among participants with household support condition ON was greater than those with household support condition OFF (F(1, 50) = 8.72, P < .005, η2 = .15). There were no other significant interactions between time and study condition or other notable effect sizes.
Figure 3.
Adherence to Guideline 2 (reduce intake of highly processed foods) at pre-treatment vs post-treatment.
Guideline 3: Reduce Red and Processed Meat
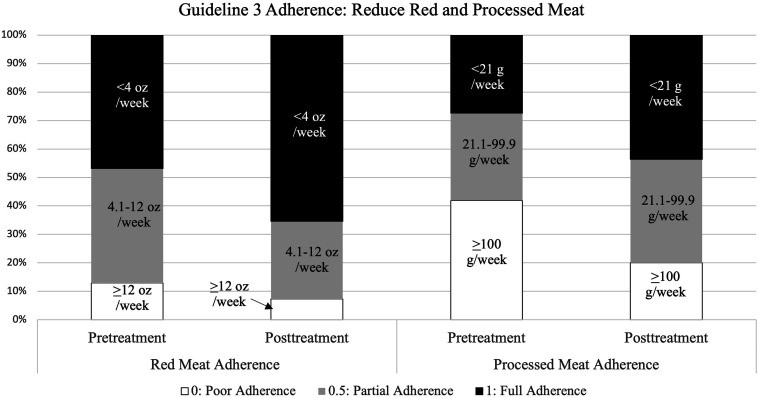
There was a significant improvement in adherence to red and processed meat guidelines from baseline to post-treatment across participants (F(1, 50) = 12.29, P < .001, η2 = .20) (Figure 4). There was also a marginal interaction between household support condition and time, such that the improvement among participants with household support ON was stronger than those with household support OFF (F(1, 50) = 3.82, P = .056, η2 = .07). There were no other significant interactions between time and study condition or other notable effect sizes.
Figure 4.
Adherence to Guideline 3 (reduced intake of red and processed meat) at pre-treatment vs post-treatment.
Guideline 4: Eliminate Sugar-Sweetened Beverages
There was a significant improvement in adherence to sugar-sweetened beverages from baseline to post-treatment across participants (F(1, 50) = 7.69, P = .008, η2 = .13) (Figure 5). There were no significant interactions between time and any experimental condition and no notable effect sizes.
Discussion
This Phase IIa pilot study demonstrated feasibility, acceptability, and preliminary signals of efficacy of a remotely delivered intervention designed to facilitate adherence to dietary guidelines for cancer prevention. The nutrition education and skills-training workshops based on self-regulation theory showed the potential for strong impact on the intake of processed foods, red and processed meats, and sugar-sweetened beverages. The study’s factorial design allowed testing of unique intervention components, which suggested that coach monitoring and involvement of household members may further facilitate improvements in dietary intake. The results indicate that the coach monitoring and household member intervention components should be retained for future testing in the optimized version of the intervention package, while the current forms of the location-triggered messaging and benefits of change components do not merit inclusion without further development. A fully powered clinical trial is warranted to test the potential of the optimized intervention to improve long-term adherence to cancer prevention dietary guidelines in comparison to a control group.
This phased approach to intervention development, where identification of active treatment components is tested prior to inclusion in a randomized-controlled trial (i.e., Multiphase Optimization Strategy [MOST]), 47 is becoming increasingly common in digital health research.48,49 It has been successfully used to refine digital, scalable treatments for smoking cessation,50-52 weight loss, 53 physical activity, 54 sleep, 54 substance use,55,56 and HIV prevention. 56 This study serves as an example of applying this model to the area of cancer prevention and dietary quality and shows the utility of the “screening” phase of the MOST design, to ensure that future intervention packages for dietary quality are streamlined and only including active treatment components.
The study met feasibility benchmarks for recruitment, retention, and treatment acceptability. The strong recruitment and retention rates are particularly notable given the challenges of engaging adults in prevention programs (often due to low perceived risk).57,58 Across conditions, large effect sizes were observed, consistent with clinically significant amounts of change, in reductions in intake of processed foods, red and processed meats, and sugar-sweetened beverages. For instance, at baseline only 11% of participants were consuming low amounts of sugar daily (<9 g/day) and 15% were consuming low amounts of saturated fat (<4 g/day), while at post-intervention it was 45% and 56%, respectively. While this study was not a fully powered trial, these results show promise that the core components may provide a sound foundation of nutrition education and self-regulation skills in a way that supports behavior change for several of the dietary guidelines for cancer prevention. This is noteworthy, given that many previous intervention approaches which have implemented behavioral strategies to modify the nutritional quality of supermarket purchases found no significant effects.59-61 Additionally, prior interventions with proven efficacy for improving adherence to cancer prevention dietary guidelines have been more intense, comprehensive, and longer in duration (i.e., 12 months, including sessions with a nutritionist).62,63 The significant effects and effect sizes observed for this pilot study are sufficient to warrant a fully powered trial to test efficacy.
In this pilot study, participants who received coach monitoring had significantly greater improvement in adherence to cancer prevention Guideline 1 (increasing fiber, whole grains, fruits, and vegetables), with a large effect size, than those that did not receive coach monitoring. This adds to the growing evidence supporting the importance of including direct communication with a professional in remote, digital health interventions. 64 Participants might have felt enhanced supportive accountability with the awareness that a coach was monitoring their food purchases, and this may have motivated them to make purchasing decisions that were consistent with program guidelines. Coaches who had access to participant’s food purchasing data might also have provided more tailored, high-quality feedback and goal-setting assistance than coaches who could not view purchasing data. Previous research has shown that tailored feedback can promote behavior change.65,66 Lastly, participants who had coach monitoring ON received more contact time (three, 20-minute calls), and thus it is possible that individual time with a coach would have the same benefit even without the data-sharing component. Future work can be designed to elucidate the mechanism of action for coach monitoring and confirm these results in a larger sample with a longer period of follow-up.
Participants assigned to involve a household member in the intervention had significantly greater reductions in intake of processed foods, with a large effect size, than those who did not receive this component. A marginally significant difference with a medium effect size was also observed in red and processed meat intake, favoring the household support intervention component. These results must be interpreted with caution given the pilot nature of this work, but they are consistent with the notion that adult household members play a large role in household food decisions, including grocery shopping.31,32 The presence of a discouraging household member who is not supportive of changes could serve as a large barrier to successfully modifying dietary intake (e.g., stocking the house with fewer processed foods). Direct involvement of household members may have worked to reduce this barrier. The household members involved in the intervention also received direct education on cancer prevention guidelines so that they were aware of the index participant’s goals, and they were taught how to provide effective support. The joint calls with coaches provided an opportunity to identify household dynamics that may have impeded change and facilitate effective problem solving. We are not aware of any prior intervention studies that have attempted to include household members in cancer prevention dietary efforts. These findings suggest multi-level interventions are promising for promoting dietary change.
The benefits of change intervention component did not produce effects large enough to warrant inclusion in the optimized intervention package. Reflecting on the benefits of healthy eating has been shown to facilitate dietary adherence in some other studies.67,68 However, many of the benefits of improving dietary quality tend to be long-term (e.g., decrease disease risk and increased longevity). It can be challenging for distal health outcomes to motivate dietary adherence. In the moment of food choice, there is a strong pull towards good tasting, rewarding food that serves as a motivational magnet 69 dominating attention toward shorter-term reward (e.g., enjoyment of taste). Thus, bringing long-term benefits to mind may be insufficient to override this strong reward drive and resist temptation. 69 Additional methods of motivation enhancement may need to be developed.
Across conditions, participants significantly decreased intake of sugar-sweetened beverages, but none of the experimental intervention components further boosted this effect. The uniform components of the intervention, which included 3 core workshop sessions and core content in weekly messages that taught self-regulation skills and provided nutrition education, appear to have been sufficient to promote changes in intake with a large effect size. The significant change observed across conditions is especially notable given that behavior change interventions often do not have a significant impact on sugar-sweetened beverage intake among adults. 70 It is possible that a ceiling effect limited the amount of additional change that could be produced by the experimental intervention components. It could also be that the focus on grocery shopping limited additional change that could be observed, given that research suggests adults drink 48% of calories from sugar-sweetened beverages at locations outside of the home. 71 Therefore, although participants may have reduced the amount of sugary drinks purchased at the grocery store, they may have needed additional intervention tools to change their intake patterns outside of the home.
This study had several strengths, including the testing of a remote, scalable intervention that has the potential for widespread impact on cancer prevention via increased adherence to WCRF/AICR dietary recommendations. 2 To our knowledge, there are very few primary prevention interventions for improving dietary quality for cancer prevention, and so this study fills an important need for public health. The conceptual model was innovative in that it moved beyond basic applications of stimulus control theory to create a more nuanced approach for behavior change that better appreciates how challenging it is to modify food purchasing behavior in the modern food environment. The study’s factorial design also allowed for unique testing of the 4 specific intervention components, informing efforts to optimize the intervention package. Finally, the intervention targeted household-level food purchasing decisions and involved enrollment of a household member in addition to the index participant. Therefore, multiple individuals throughout the household may benefit from changes made to the home food environment. Future research should provide a more rigorous evaluation of how changes made to the home food environment via this multi-level intervention impact dietary quality and cancer risk for other household members. Future research should also measure at baseline the frequency with which participants eat foods prepared at home and determine if the benefit of this intervention may be especially strong for those with a particular high or low frequency.
This study also had several limitations. Location-triggered messages that were intended to be triggered with geofencing technology often did not function as intended, limiting the ability to conduct a valid test of the efficacy of this intervention component. Due to the lack of reliability of the passive, digital collection of food purchasing data, future research is also needed to determine whether the intervention significantly changed purchasing behavior. To minimize missing purchasing data in the future, when participants purchase food from outlets that are not included in the automated data collection system, they should have an option of submitting pictures of receipts or of items purchased (which is necessary when no itemized receipt is given, such as at a farmer’s market or corner store, or if a receipt is lost). The sample size was small and predominantly women which limits generalizability. Although we did not have specific hypotheses about how the interaction between 2 or more intervention components could influence outcomes, we were also not powered for exploratory analyses of these interactions. The high participant burden of the ASA24 also dictated a different method of assessing dietary intake in the second cohort of participants. Factors outside the scope of the current study, including specific participant location (e.g., suburb or metropolitan Philadelphia) and participant mode of transportation to grocery stores, were not examined but could affect grocery shopping behaviors. Finally, this study did not include a control condition, which is necessary in the next stage of intervention development. The factorial design allowed for a full examination of the potential interactive effects of each intervention component (e.g., coaching call on, benefits of change on, location triggered off, and household on); however; we did not test these interactions, as we had no a priori hypotheses that the potential benefits of each intervention component would be anything other than additive (e.g., we had no a priori hypotheses that the effectiveness of the coach sharing ON would depend on which other components were ON).
Conclusion
Quality of dietary intake is a critical, modifiable target for cancer prevention, as well as prevention of other diseases. The preliminary data from this study show feasibility and acceptability of a remotely delivered intervention to improve adherence to cancer prevention dietary guidelines. Findings can inform the creation of an optimized intervention package that retains only the intervention components that showed adequate feasibility, acceptability, and effect sizes (coach monitoring and household member involvement). That optimized intervention package can then be tested in a Phase III efficacy trial that is fully powered, includes a usual care comparison group, and conducts comprehensive, long-term assessment of outcomes. Continuing this line of research is critical for dietary-focused primary prevention of cancer.
Appendix.
Abbreviations
- AICR
American Institute for Cancer Research
- ANOVA
Analysis of variance
- API
Application programming interface
- ASA24
Automated Self-Administered 24-hour Dietary Recall
- BMI
Body mass index
- BOC
Benefits of change condition
- CM
Coach monitoring condition
- DHQ-III
Diet History Questionnaire-III
- FNDDS
Food and Nutrient Database for Dietary Studies
- HH
Household involvement condition
- mHealth
Mobile health
- NCI
National Cancer Institute
- WCRF
World Cancer Research Fund
Appendix A. Experimental Treatment Conditions, Determined by Which Factors Were Added to the Core Intervention Components.
| Condition | Core Intervention (Education + Behavioral Skills) | Location-Triggered Messages | Benefits of Change | Coach Monitoring | Household Support |
|---|---|---|---|---|---|
| 1 | ON | ON | ON | ON | ON |
| 2 | ON | ON | ON | ON | OFF |
| 3 | ON | ON | ON | OFF | ON |
| 4 | ON | ON | OFF | ON | ON |
| 5 | ON | OFF | ON | ON | ON |
| 6 | ON | OFF | OFF | ON | ON |
| 7 | ON | OFF | ON | OFF | ON |
| 8 | ON | OFF | ON | ON | OFF |
| 9 | ON | ON | OFF | OFF | ON |
| 10 | ON | ON | OFF | ON | OFF |
| 11 | ON | ON | ON | OFF | OFF |
| 12 | ON | ON | OFF | OFF | OFF |
| 13 | ON | OFF | ON | OFF | OFF |
| 14 | ON | OFF | OFF | ON | OFF |
| 15 | ON | OFF | OFF | OFF | ON |
| 16 | ON | OFF | OFF | OFF | OFF |
Appendix B. Dietary Intake Values Associated With NCI Scoring Criteria for Each Dietary Recommendation.
| Guideline | Adherence Scoring | |
|---|---|---|
| Guideline 1: Eat a diet rich in wholegrains, vegetables, fruits, and beans | Total fiber (g/day) | ≥30 g = 1 15–<30 g = 0.5 <15 g = 0 |
| Fruits/vegetables (cups/day) | ≥3.5 cups = 1 1–< 3.5 cups = 0.5 <1 cup = 0 |
|
| Guideline 2: Limit consumption of “fast foods” and other processed foods high in fat, starches, or sugars | Total added sugar from ultra-processed foods per day (g/day). | <9 g = 1 9–≤25g = 0.5 >25 g = 0 |
| Total saturated fat from ultra-processed food per day (g/day). | ≤5 g = 1 5–≤13 g = 0.5 >13 g = 0 |
|
| Total sodium from ultra-processed food per day (mg/day). | ≤1000 mg = 1 1000–<2300 mg = 0.5 ≥2300 mg = 0 |
|
| Guideline 3: Limit consumption of red and processed meats | Red meat (oz/week) | <4 oz cooked = 1 4–<12 oz cooked = 0.5 ≥12 oz cooked = 0 |
| Processed meat (g/week) | <21 g = 1 21–<100 g = 0.5 ≥100 g = 0 |
|
| Guideline 4: Limit consumption of sugar-sweetened drinks | Total sugar-sweetened drinks (oz/day) | 0 oz = 1 >0–≤8.5 oz = 0.5 >8.5 oz = 0 |
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Cancer Institute (R21CA252933) awarded to Dr Butryn as Principal Investigator.
Ethical Statement
Ethical Approval
This study was approved by the Institutional Review Board at Drexel University (study ID 2003007695).
Statement of Human and Animal Rights
All procedures in this study were conducted in accordance with the approved protocols of Drexel University’s Institutional Review Board.
Statement of Informed Consent
Written informed consent was obtained from the participants for their anonymized information to be published in this article.
ORCID iDs
Nicole T. Crane https://orcid.org/0000-0003-0378-670X
Marny M. Ehmann https://orcid.org/0000-0003-2834-8095
Brandy-Joe Milliron https://orcid.org/0000-0003-1113-9043
References
- 1.Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA A Cancer J Clin. 2020;70(4):245-271. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund/American Institute for Cancer Research . Diet, Nutrition, Physical Activity, and Cancer: A Global Perspective. Continuous Update Project Expert Report; 2018. Available at dietandcancerreport.org [Google Scholar]
- 3.Dietary Guidelines Advisory Committee . Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service; 2020. doi: 10.52570/DGAC2020 [DOI] [Google Scholar]
- 4.Food and Nutrition Service . Healthy Eating Index Scores for Americans; 2022. U.S. Department of Agriculture. Available at https://www.fns.usda.gov/hei-scores-americans [Google Scholar]
- 5.Martin C, Hoy MK, Clemens J, Moshfegh A. Demographic characteristics associated with variety of fruit and vegetable intake: What we eat in America, NHANES 2013–2016 (FS02-06-19). Curr Dev Nutr. 2019;3(Supplement_1):nzz051. [Google Scholar]
- 6.Rehm CD, Penalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999-2012. JAMA. 2016;315(23):2542-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kremers SPJ, de Bruijn G-J, Visscher TLS, van Mechelen W, de Vries NK, Brug J. Environmental influences on energy balance-related behaviors: A dual-process view. Int J Behav Nutr Phys Act. 2006;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gearhardt AN, Schulte EM. Is food addictive? A review of the science. Annu Rev Nutr. 2021;41(1):387-410. [DOI] [PubMed] [Google Scholar]
- 9.Eurídice Martínez S, Larissa Galastri B, Maria Laura da Costa L, Jean-Claude M, Dariush M, Carlos Augusto M. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ Open. 2016;6(3):e009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: Reward-driven brain overrides repletion signals. Int J Obes (Lond). 2009;33(Suppl 2):S8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen DA. Neurophysiological pathways to obesity: Below awareness and beyond individual control. Diabetes. 2008;57(7):1768-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen DA, Babey SH. Contextual influences on eating behaviours: Huristic processing and dietary choices. Obes Rev. 2012;13(9):766-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kegler MC, Alcantara I, Haardorfer R, Gazmararian JA, Ballard D, Sabbs D. The influence of home food environments on eating behaviors of overweight and obese women. J Nutr Educ Behav. 2014;46(3):188-196. [DOI] [PubMed] [Google Scholar]
- 14.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139(3):629-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drewnowski A, Rehm CD. Energy intakes of US children and adults by food purchase location and by specific food source. Nutr J. 2013;12:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spees CK, Clark JE, Hooker NH, Watowicz RP, Taylor CA. Dietary intake contributions of food and beverages by source and food security status in US adults. J Nutr Educ Behav. 2017;49(8):667-673.e1. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann-Boyce J, Bianchi F, Piernas C, et al. Grocery store interventions to change food purchasing behaviors: A systematic review of randomized controlled trials. Am J Clin Nutr. 2018;107(6):1004-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ball K, McNaughton SA, Le HN, Abbott G, Stephens LD, Crawford DA. ShopSmart 4 Health: Results of a randomized controlled trial of a behavioral intervention promoting fruit and vegetable consumption among socioeconomically disadvantaged women. Am J Clin Nutr. 2016;104(2):436-445. [DOI] [PubMed] [Google Scholar]
- 19.Anzman-Frasca S, McGovern L, Ferrante MJ, et al. Effects of a grocery shopping intervention designed to improve diet adherence in diabetes: A randomized trial. Obesity. 2023;31(1):62-73. [DOI] [PubMed] [Google Scholar]
- 20.Bangia D, Palmer-Keenan DM. Grocery store podcast about omega-3 fatty acids influences shopping behaviors: A pilot study. J Nutr Educ Behav. 2014;46(6):616-620. [DOI] [PubMed] [Google Scholar]
- 21.Bangia D, Shaffner DW, Palmer-Keenan DM. A point-of-purchase intervention using grocery store tour podcasts about omega-3s increases long-term purchases of omega-3-rich food items. J Nutr Educ Behav. 2017;49(6):475-480.e1. [DOI] [PubMed] [Google Scholar]
- 22.Latham EALGP. Goal setting theory. In: Drillings HFON, eds. Motivation: Theory and Research. New York: Routledge; 2012. [Google Scholar]
- 23.Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: A systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32-42. [DOI] [PubMed] [Google Scholar]
- 24.Gollwitzer PM, Oettingen G. Implementation intentions. In: Encyclopedia of Behavioral Medicine. New York: Cham: Springer International Publishing; 2020:1159-1164. [Google Scholar]
- 25.Harkin B, Webb TL, Chang BP, et al. Does monitoring goal progress promote goal attainment? A meta-analysis of the experimental evidence. Psychol Bull. 2016;142(2):198-229. [DOI] [PubMed] [Google Scholar]
- 26.Sheeran P, Webb TL. The intention–behavior gap. Soc pers psychol compass. 2016;10(9):503-518. [Google Scholar]
- 27.Michaelsen MM, Esch T. Motivation and reward mechanisms in health behavior change processes. Brain Res. 2021;1757:147309. [DOI] [PubMed] [Google Scholar]
- 28.Nyer PU, Dellande S. Public commitment as a motivator for weight loss. Psychol Mark. 2010;27(1):1-12. [Google Scholar]
- 29.Mohr DC, Cuijpers P, Lehman K. Supportive accountability: A model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res. 2011;13(1):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullan B, Norman P, Boer H, Seydel E. Protection Motivation Theory. Predicting and changing health behaviour: Research and practice with social cognition models. Berkshire: Open University Press; 2015. [Google Scholar]
- 31.Raskind IG, Woodruff RC, Ballard D, et al. Decision-making processes shaping the home food environments of young adult women with and without children. Appetite. 2017;113:124-133. [DOI] [PubMed] [Google Scholar]
- 32.Hollywood LE, Cuskelly GJ, O'Brien M, et al. Healthful grocery shopping. Perceptions and barriers. Appetite. 2013;70:119-126. [DOI] [PubMed] [Google Scholar]
- 33.Czajkowski SM, Powell LH, Adler N, et al. From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. 2015;34(10):971-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis M, Bromley K, Sutton CJ, McCray G, Myers HL, Lancaster GA. Determining sample size for progression criteria for pragmatic pilot RCTs: The hypothesis test strikes back. Pilot Feasibility Stud. 2021;7(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey RN, Connell LE, Johnston M, et al. Behavior change techniques and their mechanisms of action: A synthesis of links described in published intervention literature. Ann Behav Med. 2019;53(8):693-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer research third expert report on diet, nutrition, physical activity, and cancer: Impact and future directions. J Nutr. 2020;150(4):663-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunsley J. Treatment acceptability of symptom prescription techniques. J Counsel Psychol. 1993;40:139-143. [Google Scholar]
- 39.Automated Self-Administered 24-Hour (ASA24) Dietary Assessment Tool . Division of Cancer Control & Population Sciences, National Cancer Institute. Available at https://epi.grants.cancer.gov/asa24/ [Google Scholar]
- 40.Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324-332. [DOI] [PubMed] [Google Scholar]
- 41.ASA24 Evaluation and Validation . Division of Cancer Control & Population Sciences, National Cancer Institute; 2017. Available at https://epi.grants.cancer.gov/asa24/respondent/validation.html [Google Scholar]
- 42.Diet History Questionnaire III (DHQ III) . Division of Cancer Control & Population Sciences, National Cancer Institute; 2021. Available at https://epi.grants.cancer.gov/dhq3/ [Google Scholar]
- 43.Monteiro CA, Levy RB, Claro RM, Castro IR, Cannon G. A new classification of foods based on the extent and purpose of their processing. Cad Saúde Pública. 2010;26(11):2039-2049. [DOI] [PubMed] [Google Scholar]
- 44.Food and Nutrient Database for Dietary Studies (FNDDS) . U.S. Department of Agriculture, Agricultural Research Service. Washington, DC; 2014. Available at https://data.nal.usda.gov/dataset/food-and-nutrient-database-dietary-studies-fndds [Google Scholar]
- 45.Diet History Questionnaire III (DHQ III) . DHQ III Database Methods. Division of Cancer Control & Population Sciences, National Cancer Institute; 2021. Available at https://epi.grants.cancer.gov/dhq3/databse-methods.html [Google Scholar]
- 46.Turati F, Dalmartello M, Bravi F, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer research recommendations and the risk of breast cancer. Nutrients. 2020;12(3):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): New methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5 Suppl):S112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs MA, Graham AL. Iterative development and evaluation methods of mHealth behavior change interventions. Curr Opin Psychol. 2016;9:33-37. [Google Scholar]
- 49.Tomlinson M, Rotheram-Borus MJ, Swartz L, Tsai AC. Scaling up mHealth: Where is the evidence? PLoS Med. 2013;10(2):e1001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strecher VJ, McClure JB, Alexander GL, et al. Web-based smoking-cessation programs: Results of a randomized trial. Am J Prev Med. 2008;34(5):373-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McClure JB, Derry H, Riggs KR, et al. Questions about quitting (Q2): Design and methods of a multiphase optimization strategy (MOST) randomized screening experiment for an online, motivational smoking cessation intervention. Contemp Clin Trials. 2012;33(5):1094-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nathan KC, Megan AJ, Jessie S, Wileyto EP, Amanda LG. Diffusion of an evidence-based smoking cessation intervention through Facebook: A randomised controlled trial study protocol. BMJ Open. 2014;4(1):e004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfammatter AF, Marchese SH, Pellegrini C, Daly E, Davidson M, Spring B. Using the preparation phase of the multiphase optimization strategy to develop a messaging component for weight loss: Formative and pilot research. JMIR Form Res. 2020;4(5):e16297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buman MP, Epstein DR, Gutierrez M, et al. BeWell24: Development and process evaluation of a smartphone “app” to improve sleep, sedentary, and active behaviors in US Veterans with increased metabolic risk. Transl Behav Med. 2016;6(3):438-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garnett C, Crane D, Michie S, West R, Brown J. Evaluating the effectiveness of a smartphone app to reduce excessive alcohol consumption: Protocol for a factorial randomised control trial. BMC Publ Health. 2016;16(1):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyrick DL, Rulison KL, Fearnow-Kenney M, Milroy JJ, Collins LM. Moving beyond the treatment package approach to developing behavioral interventions: Addressing questions that arose during an application of the multiphase optimization strategy (MOST). Transl Behav Med. 2014;4(3):252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: A meta-analytic review. Prev Med. 2004;38(4):388-402. [DOI] [PubMed] [Google Scholar]
- 58.Dillard AJ, Couper MP, Zikmund-Fisher BJ. Perceived risk of cancer and patient reports of participation in decisions about screening: The DECISIONS study. Med Decis Making. 2010;30(5 Suppl):96s-105s. [DOI] [PubMed] [Google Scholar]
- 59.Gustafson A, Gillespie R, DeWitt E, et al. Online pilot grocery intervention among rural and urban residents aimed to improve purchasing habits. Int J Environ Res Public Health. 2022;19(2):871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moran AJ, Khandpur N, Polacsek M, et al. Make it fresh, for less! A supermarket meal bundling and electronic reminder intervention to promote healthy purchases among families with children. J Nutr Educ Behav. 2019;51(4):400-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piernas C, Aveyard P, Lee C, et al. Evaluation of an intervention to provide brief support and personalized feedback on food shopping to reduce saturated fat intake (PC-SHOP): A randomized controlled trial. PLoS Med. 2020;17(11):e1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coumans JMJ, Bolman CAW, Oenema A, Lechner L. The effects of a web-based computer-tailored diet and physical activity intervention based on self-determination theory and motivational interviewing: A randomized controlled trial. Internet Interv. 2022;28:100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masala G, Palli D, Ermini I, et al. The DAMA25 study: Feasibility of a lifestyle intervention programme for cancer risk reduction in young Italian women with breast cancer family history. Int J Environ Res Public Health. 2021;18(23):12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mair JL, Salamanca-Sanabria A, Frese B, Jakob R, Kowatsch T, Haug S. Effective behavior change techniques in digital health interventions targeting non-communicable diseases: An umbrella review. OSF Preprints. 2023. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryan K, Dockray S, Linehan C. A systematic review of tailored eHealth interventions for weight loss. Digit Health. 2019;5:2055207619826685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: Five key components. Telemed J E Health. 2010;16(9):931-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Neill J, Daniel TO, Epstein LH. Episodic future thinking reduces eating in a food court. Eat Behav. 2016;20:9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forman EM, Hoffman KL, Juarascio AS, Butryn ML, Herbert JD. Comparison of acceptance-based and standard cognitive-based coping strategies for craving sweets in overweight and obese women. Eat Behav. 2013;14(1):64-68. [DOI] [PubMed] [Google Scholar]
- 69.Appelhans BM, French SA, Pagoto SL, Sherwood NE. Managing temptation in obesity treatment: A neurobehavioral model of intervention strategies. Appetite. 2016;96:268-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vargas-Garcia EJ, Evans CEL, Prestwich A, Sykes-Muskett BJ, Hooson J, Cade JE. Interventions to reduce consumption of sugar-sweetened beverages or increase water intake: Evidence from a systematic review and meta-analysis. Obes Rev. 2017;18(11):1350-1363. [DOI] [PubMed] [Google Scholar]
- 71.Kit BK, Fakhouri TH, Park S, Nielsen SJ, Ogden CL. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999-2010. Am J Clin Nutr. 2013;98(1):180-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are not publicly available due to participant privacy (given the small sample size and nature of the variables collected) but are available upon request from the corresponding author.