Abstract
Background: Clinical pharmacists’ interventions (PIs) on drug-related problems (DRPs) in Vietnamese hypertensive outpatients are limited. Objectives: The objective was to investigate the prevalence and nature of DRPs, and factors which are likely to have DRPs, types of PIs, and their acceptance rate in 3 Vietnamese hospitals. Method: A prospective interventional study was conducted over a period of 3 months in 3 hospitals (from October 2021 to March 2022). Clinical pharmacists conducted medication reviews after collecting patient information from prescriptions and patient interviewing, and then identified the DRPs and suggested PIs according to the Vi-Med tool. These DRPs and PIs were reviewed by other superior clinical pharmacists and a consensus meeting with 3 cardiologists. Results: Of 381 patients included, 344 (90.23%) experienced 1 or more DRPs. A total of 820 DRPs were identified with an average of 2.15 DRPs per patient and 415 (50.61%) were hypertension-related issues. The most common DRPs identified were “administration mode” (46.34%), “missing indication” (18.05%), “non-conformity indication” (17.80%), and “dosage” (11.95%). Comorbidity (adjusted odds ratio [AOR] = 3.985, 95% CI: 1.597-9.942, P = 0.003) was the predictor of DRPs. Clinical pharmacists provided 739 PIs and 94.45% were accepted by physicians. Conclusion: The results of this study showed that DRPs were very common in hypertensive outpatients and highlighted the role of clinical pharmacists to identify and resolve DRPs through prompt interventions.
Keywords: clinical pharmacist, drug-related problems, pharmacist’s intervention, hypertension, Vietnam
Impacts on Practice
Drug-related problems (DRPs) are very common in hypertensive outpatients and frequently include inappropriate administration drugs, missing indication, nonconformity indication, the use of an inappropriate dose, major drug-drug interactions, unnecessary indication, and drug side effects.
Clinical pharmacists should focus especially on performing medication reviews for detecting DRPs that could have a negative impact on the patient’s health and providing recommendations to solve the DRPs in hypertensive outpatients.
Introduction
Hypertension (HTN) is 1 of the biggest global public health concerns. Hypertension is a major cause of heart complications, stroke, kidney failure, and even death and disabilities. According to the estimation of the World Health Organization, worldwide prevalence of high blood pressure (BP) was 22% and BP could be monitored in less than 20% of hypertensive patients in 2015. 1 In Vietnam, the overall prevalence of HTN was rapidly increasing, from 25.1% in 2008 to 47.3% in 2015.2,3 In particular, only 31.0% of hypertensive patients controlled their BP. 3
A drug-related problem (DRP) is an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes. 4 Hypertensive patients are at high risk of having DRPs. The majority of patients will require combination therapy to achieve a BP. Furthermore, hypertensive patients with advanced age, high prevalence of comorbidities, and severity of the disease often received multiple medications. 5 Polypharmacy has been strongly associated with DRPs. 6 Globally, DRPs among hypertensive patients were reported in studies as an average of 1.26-6.3 DRPs per hypertensive patient.7 -12 Several studies in some Southeast Asian countries such as Malaysia and Indonesia about DRPs in hypertensive patients reported more than 56% of the patients had at least 1 DRP.11,13,14
DRPs may lead to elevating morbidity, mortality, treatment costs, poor adherence, and longer hospitalization.11,15 To prevent or minimize the DRP, an effective intervention strategy is required. Clinical pharmacists are well situated in the health care system to identify and resolve DRPs, and develop the therapeutic plans.15,16
The role of clinical pharmacists in Vietnam is spreading. Clinical pharmacy was first developed in Vietnam in the 1990s. 17 The most recent development in this area was Vietnam’s Ministry of Health’s release of a Guideline on clinical pharmacy practice for pharmacists in the number of noncommunicable diseases in 2019. 18 This is considered a national and professional guide with scientific and practical value that will help implement and evaluate clinical pharmacy activities at hospitals. Of those, there is a detailed guideline of clinical pharmacy practice for the management of HTN. However, there have been no studies that evaluate clinical pharmacists’ interventions (PIs) on DRPs in Vietnamese hypertensive outpatients.
Aim of the Study
The aim of this study was to investigate the prevalence and nature of DRPs, and factors which are likely to have DRPs, types of PIs, and their acceptance rate in 3 Vietnamese hospitals.
Methods
Study Design and Clinical Setting
A prospective multicenter interventional study was conducted over a period of 3 months in 3 hospitals, namely Nguyen Tri Phuong Hospital (from October to December 2022) and Gia Dinh People’s Hospital (from December 2022 to February 2023) located in the south, and Hoa Vang District Medical Center (from January to March 2023) located in the middle of the country. The average number of outpatients per day for 3 hospitals is about 2000, 3000, and 250 patients, respectively.
The pharmacy departments of the 3 hospitals have started staffing clinical pharmacists in “the pharmacist-led clinic” as a pilot practice model of the multidisciplinary team since 2021. These pharmacists performed clinical functions including (1) medication review for identifying, preventing, and addressing DRPs and making PIs to physicians to improve patient outcomes and (2) patient education about HTN, goals of treatment, lifestyle changes, the importance of medication adherence, and home BP measurement. These activities were comprehensively documented. The results of patient education are presented in another article. This article only reported the results of medication review activities.
Study Participants
Eligibility criteria were as follows: adult patients older than 18 years diagnosed with primary HTN, taking antihypertensive drugs for at least the past 1 month, came to the outpatient department for a refill, and voluntarily participated in this study. Patients who were pregnant, could not respond to or receive counseling by clinical pharmacists (Alzheimer’s disease, depression, bipolar disorder, etc), and were not accompanied by a family member were excluded.
Sample Size
Single proportion formula was used to calculate the minimum sample size needed. 19 We set α at 0.05 (Zα/2 = 1.96). A previous study showed that the prevalence of DRPs in hypertensive outpatients was 0.81, 20 95% confidence interval, 5% margin of error, the minimum sample size was calculated to be 236 participants.
Identification of DRPs, Suggestion of PIs, and Their Acceptance
Medication orders are generated by physicians, either electronically or manually, and then forwarded to the pharmacy for dispensing. Hypertensive outpatients were invited into a pharmacist-led clinic for a history medication review, and collecting information including patient general information, diagnosis, medical history, allergy status, adverse drug reactions (ADRs), lifestyle, drugs, laboratory data, and BP monitoring. Patients’ clinical characteristics and medication data were obtained from the prescriptions and direct interviews using a data collection form (see the Online Appendix A).
The 3 hospitals employed 2, 2, and 1 clinical pharmacist, respectively, to conduct medication reviews for detecting DRPs. These clinical pharmacists were trained for 8 hours 3 days, including (1) the purpose and process of the study; (2) skills to perform medication review; (3) knowledge of HTN and treatment; and (4) knowledge of antihypertensive drugs (indication, contraindication, dose, drug interaction, ADRs, principles of antihypertensive drug treatment, selection of antihypertensive drug in specific clinical condition). If any DRP was found, the pharmacist wrote a complete report that included all relevant patient information, the type and details of the DRP, and PIs for its management.
Identification of DRPs was based on guidelines of Vietnam’s Ministry of Health, 18 ,21 -23 the European Society of Cardiology/European Society of Hypertension (ESC/ESH) Guidelines for the management of arterial HTN, 24 and medication information leaflets. Vi-Med was used to categorize DRPs and PIs. 25 Vi-Med was validated as a tool to support the analysis of DRPs and PIs in Vietnamese hospitals. This form contains essential information on PIs that needs to be recorded, such as patient information, short descriptions and classifications of DRPs and PIs, methods by which a pharmacist and a physician communicate with respect to PI-related recommendations, and a physician’s acceptance of PIs. Vi-Med classified DRPs into 8 categories, including (1) unnecessary indication, (2) nonconformity indication, (3) missing indication, (4) dosage, (5) administration mode, (6) adverse drug effect, (7) drug interaction, and (8) drug monitoring. Vi-Med form and criteria for DRP identification are presented in Online Appendix B and Appendix C, respectively.
For content validation of DRPs and PIs, 3 superior clinical pharmacists reviewed them and followed by a consensus meeting with 3 cardiologists (1 from each hospital) 1 month later. If all cardiologists agree with the PI, this intervention is defined as “acceptance.” If 1 or more experts do not agree with the PI, this intervention is defined as “non-acceptance.”
Data Analysis
SPSS software version 20.0 was used to analyze the gathered data. Univariate binary logistic regression analysis was used to find association between patient’s characteristics and the presence of DRPs. To obtain the final variables associated with the presence of DRPs, all variables with a value of P < 0.2 in univariate analysis were entered in the multivariate logistic regression. A value of P < 0.05 was considered statistically significant.
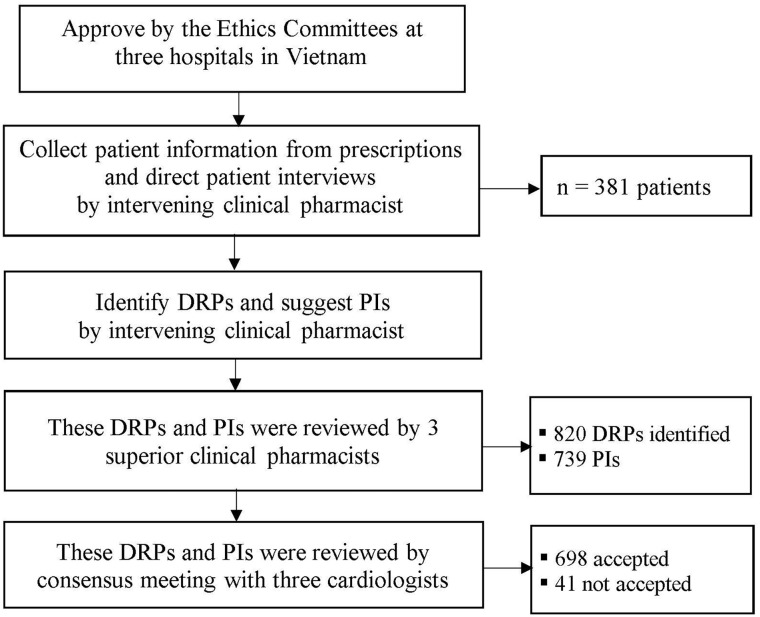
An overview of the study procedure and main results are shown in Figure 1.
Figure 1.
Overview of the study procedure and main results.
Abbreviations: DRPs, drug-related problems; PIs, pharmacists’ interventions.
Ethics Approval
This study protocol was approved by ethics committees at 3 hospitals in Vietnam (institutional review board [IRB] number: 01/TTYTHHV-HDK HKT; 1049/ NTP-CDT; 1050/NTP-CDT; 24/NDGD-HDDD) and by the IRB, Thailand (IRB number: 78.0319/EC.583). All patients provided written informed consent to participate in the study.
Results
Demographic Details of Patients With HTN
Of 381 patients, the mean age was 62.6 ± 8.6 years and 52.0% were female. About two-fifths of patients were retired and only 9.4% had a bachelor’s degree or more. The most common comorbidities were dyslipidemia (66.1%), diabetes (34.9%), and ischemic heart disease (18.9%). Regarding their lifestyle, current drinkers, current smokers, and individuals who exercise were 26.8%, 18.1%, and 58.8%, respectively. Duration of HTN and treatment was 8.2 ± 6.1 and 8.1 ± 6.1 years, respectively. The mean systolic and diastolic BP values were 139.4 ± 16.7 and 81.8 ± 10.5, respectively. About two-thirds of patients monitored their BP at home. Details of the patient’s baseline characteristics are summarized in Table 1.
Table 1.
Demographic Details of Patients With Hypertension.
| Characteristics | Number (%) |
|---|---|
| Age (mean ± SD, year) | 62.6 ± 8.6 |
| <65 years | 217 (57.0) |
| ≥65 years | 164 (43.0) |
| Gender, female | 198 (52.0) |
| Occupation | |
| Retirement | 152 (39.9) |
| Housewife | 94 (24.7) |
| Private servant | 47 (12.3) |
| Farmer | 44 (11.5) |
| Other (teacher, worker, businessman, security guard, jobless, trader, civil servant) | 44 (11.5) |
| Educational level | |
| Primary school or not graduated from primary school | 61 (16.0) |
| Junior high school | 132 (34.6) |
| Senior high school | 152 (39.9) |
| Bachelor’s degree or more | 36 (9.4) |
| Comorbidities (mean ± SD) | 2.1 ± 1.5 |
| Yes | 338 (88.7) |
| No | 43 (11.3) |
| Dyslipidemia | 252 (66.1) |
| Diabetes | 133 (34.9) |
| Ischemic heart disease | 74 (19.4) |
| Stomach disorder (GERD, stomach ulcer, gastritis) | 54 (14.2) |
| Angina | 33 (8.7) |
| Varicose vein | 24 (6.3) |
| Gout | 16 (4.2) |
| Heart failure | 8 (2.1) |
| Lifestyle | |
| Alcohol status, currently | 102 (26.8) |
| Smoking status, currently | 69 (18.1) |
| Doing exercise | 224 (58.8) |
| BMI (mean ± SD) | 24.5 ± 3.2 |
| Blood pressure | |
| Duration of HTN (mean ± SD, year) | 8.2 ± 6.1 |
| Duration of treatment for HTN (mean ± SD, year) | 8.1 ± 6.1 |
| Systolic BP (mean ± SD, mm Hg) | 139.4 ± 16.7 |
| Diastolic BP (mean ± SD, mm Hg) | 81.8 ± 10.5 |
| Monitoring BP at home | 265 (69.6) |
Abbreviations: BMI, body mass index; BP, blood pressure; GERD, gastroesophageal reflux disease; HTN, hypertension.
Drug-Related Problems
Prescribing pattern in antihypertensive medications
The mean number of drugs prescribed per patient was 5.0 ± 2.5, while the average of antihypertensive drugs per patient was 2.3 ± 0.8. About 40% of antihypertensive drugs were in the fixed-dose combination form. Regarding antihypertensive drug regimens, 2 combined drugs were most common, accounting for 53.6%, out of which angiotensin-converting enzyme inhibitors (ACEis) + thiazide diuretic (TD) (21.3%) were the most common pattern, followed by angiotensin II receptor blocker (ARB) + calcium channel blocker (CCB) (13.6%) and ARB + beta-blocker (BB) (12.9%). The fixed-dose combination of antihypertensive drugs was quite common, as 39.1% of patients had dual fixed-dose combination and only 1.0% of patients had the triple fixed-dose combination. The prescribing patterns in hypertensive patients are listed in Online Appendix D.
Types of DRPs
Among 381 patients, 344 (90.3%) experienced 1 or more DRPs. A total of 820 DRPs were identified with a mean (±SD) number of DRPs per patient of 2.2 ± 1.4. Overall, 50.6% of DRPs were due to HTN medications with a mean number of 1.1 ± 1.0 DRPs per patient. The most common DRPs were administration mode (46.3%). The reasons for these DRPs were inappropriate timing of administration, incomplete information on the drug regimen, inappropriate frequency of administration, and inappropriate selection of drug form. The other frequently identified DRPs were missing indication (18.0%), nonconformity indication (17.8), dosage (12.0%), major drug-drug interactions (2.6%), and ADRs (1.1%) of the total DRPs. Details of types of DRPs are shown in Table 2.
Table 2.
Type of DRPs.
| DRPs | Number (%), n = 820 | Example |
|---|---|---|
| Unnecessary indication | 18 (2.2) | |
| Drug use without indications | 15 (1.8) | Patient was not diagnosed with dyslipidemia, risk of MI, risk of angina, or risk of stroke and was prescribed atorvastatin |
| Duplicate prescription | 3 (0.4) | Patient taking 2 medications from the telmisartan in different medication brands |
| Nonconformity indication | 146 (17.8) | |
| Inappropriate drug choice compared with guidelines | 120 (14.6) | There is no specific indication for using bisoprolol, eg, HF, angina, post-MI, AF, or younger women with or planning pregnancy |
| Inappropriate drug combination | 11 (1.4) | Combination of an ACEi and ARB to treat hypertension |
| Contraindication | 15 (1.8) | Hydrochlorothiazide is contraindicated in hypertensive patient with gout |
| Missing indication | 148 (18.0) | |
| Valid indication without drug prescribed | 96 (11.7) | Hypertensive patient with heart failure without SGLT2 inhibitor prescribed |
| Missing drug combination | 52 (6.3) | Mono-antihypertensive drug therapy could not control BP |
| Dosage | 98 (12.0) | |
| Low dose | 97 (11.8) | Bisoprolol 2.5 mg daily to treat hypertension Losartan 25 mg daily to treat hypertension |
| Missing dose | 1 (0.2) | Missing dose of Novomix 30 Flexpen |
| Administration mode | 380 (46.3) | |
| Inappropriate drug form | 18 (2.2) | Amlodipine 5 mg was prescribed, 2 tablets once daily |
| Inappropriate timing of administration | 222 (27.1) | Metformin, ivabradine, etc are recommended to be used with meals, but the prescription is insufficiently instructed |
| Incomplete information on the drug regimen | 74 (9.0) | Nifedipine retard should be taken whole, do not chew or crush it, but the prescription is insufficiently instructed |
| Inappropriate frequency of administration | 66 (8.0) | Lisinopril 10 mg was prescribed twice daily Losartan 50 mg was prescribed twice daily |
| Drug side effect | 9 (1.1) | Dry cough (enalapril) Peripheral edema (amlodipine) |
| Major drug-drug interactions | 21 (2.6) | Spironolactone—lisinopril Enalapril—losartan |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BP, blood pressure; DRPs, drug-related problems; HF, heart failure; MI, myocardial infarction; SGLT2, sodium-glucose transporter 2.
Common drugs involved in DRPs
Regarding drugs commonly implicated in DRPs, BBs were the most frequently involved inappropriate drug choice and subtherapeutic dosage. There were 29 (7.6%) of the patients having HTN with specific conditions, for example, heart failure, angina, post-myocardial infarction, and atrial fibrillation which were in need of additional BBs. Meanwhile, 86 (22.6%) patients were prescribed BBs without having any specific conditions, and 53 (13.9%) of the patients were in need of increasing BB dose. Hypoglycemic medications were most commonly associated with inappropriate timing of administration (129 patients with 33.9%). Sodium-glucose transporter 2 (SGLT2) inhibitors were commonly not prescribed while patients had a valid indication. Common drugs involved in DRPs are presented in Table 3, while details of drugs implicated in DRPs are listed in Online Appendices E-K.
Table 3.
Common Drugs Involved in 820 DRPs.
| Drugs/Drug classes | Number |
|---|---|
| Antihypertensive drugs | |
| Beta-blockers | |
| Bisoprolol | 139 |
| Carvedilol | 12 |
| Metoprolol | 6 |
| Nebivolol | 4 |
| Angiotensin-converting enzyme inhibitors (ACEis) | |
| Enalapril | 12 |
| Lisinopril | 9 |
| Perindopril | 4 |
| Angiotensin II receptor blockers (ARBs) | |
| Losartan | 32 |
| Telmisartan | 11 |
| Candesartan | 8 |
| Valsartan | 6 |
| Irbesartan | 2 |
| Calcium channel blockers (CCBs) | |
| Amlodipine | 25 |
| Nifedipine | 25 |
| Lercanidipine | 13 |
| Felodipine | 13 |
| Diuretic | |
| Hydrochlorothiazide | 9 |
| Spironolactone | 9 |
| Indapamide | 3 |
| Other cardiovascular disease drugs | |
| Trimetazidine | 27 |
| Ivabradine | 18 |
| Atorvastatin | 21 |
| Aspirin | 11 |
| Fenofibrate | 3 |
| Rosuvastatin | 2 |
| Hypoglycemic drugs | |
| Metformin | 95 |
| Gliclazide | 45 |
| Insulin | 9 |
| Proton pump inhibitors (PPIs) | |
| Esomeprazole | 12 |
| Omeprazole | 8 |
| Pantoprazole | 7 |
| Others | |
| Tramadol | 17 |
Abbreviation: DRPs, drug-related problems.
Factors associated with DRPs
The multivariate logistic regression results revealed that comorbidity was the final factor having a statistically significant correlation with the presence of DRPs. This implies that the hypertensive patients with comorbidity were about 4 times more likely to have DRPs than hypertensive patients without comorbidity (adjusted odds ratio [AOR] = 3.985, 95% CI:1.597-9.942, P = 0.003). DRP determinants in prescribing are presented in Table 4.
Table 4.
Univariate and Multivariate Binary Logistic Regression Analysis of Predictors of DRPs.
| Variables | Category | DRPs | COR (95% CI) | P value | AOR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| Yes (n = 344) | No (n = 37) | ||||||
| Gender | Male | 167 (48.5%) | 16 (43.2%) | 1 | |||
| Female | 177 (51.5%) | 21 (56.8%) | 0.808 (0.408-1.600) | 0.540 | |||
| Age, years | <65 | 195 (56.7%) | 22 (59.5%) | 1 | |||
| ≥65 | 149 (43.3%) | 15 (40.5%) | 1.121 (0.562-2.234) | 0.746 | |||
| Occupation | Farmer | 37 (10.8%) | 7 (18.9%) | 1 | 1 | ||
| Retirement | 134 (39.0%) | 18 (48.6%) | 1.408 (1.408-3.627) | 0.478 | 0.670 (0.232-1.935) | 0.459 | |
| Housewife | 88 (25.6%) | 6 (16.2%) | 2.775 (0.873-8.817) | 0.084 | 1.305 (0.377-4.514) | 0.674 | |
| Private servant | 45 (13.1%) | 2 (5.4%) | 4.257 (0.834-21.738) | 0.082 | 1.581 (0.282-8.880) | 0.603 | |
| Other | 40 (11.6%) | 4 (10.8%) | 1.892 (0.512-6.993) | 0.339 | 0.992 (0.246-4.000) | 0.991 | |
| Education | Primary school or not graduated | 53 (15.4%) | 8 (21.6%) | 1 | |||
| Junior high school | 119 (34.6%) | 13 (35.1%) | 1.382 (0.541-3.531) | 0.499 | |||
| Senior high school | 140 (40.7%) | 12 (32.4%) | 1.761 (0.682-4.548) | 0.242 | |||
| Bachelor degree or more | 32 (9.3%) | 4 (10.8%) | 1.208 (0.336-4.334) | 0.772 | |||
| Comorbidities | Yes | 314 (91.3%) | 24 (64.9%) | 5.669 (2.620-12.268) | <0.001 | 3.985 (1.597-9.942) | 0.003 |
| No | 30 (8.7%) | 13 (35.1%) | 1 | 1 | |||
| Smoking | Yes | 65 (18.9%) | 4 (10.8%) | 1 | |||
| No | 279 (81.1%) | 33 (89.2%) | 0.520 (0.178-1.520) | 0.232 | |||
| Drink | Yes | 93 (27.0%) | 9 (24.3%) | 1 | |||
| No | 251 (73.0%) | 28 (75.7%) | 0.868 (0.395-1.907) | 0.724 | |||
| Exercising | Yes | 197 (57.3%) | 27 (73.0%) | 1 | 1 | ||
| No | 147 (42.7%) | 10 (27.0%) | 2.015 (0.946-4.292) | 0.069 | 2.035 (0.901-4.594) | 0.087 | |
| HTN duration in years | <5 | 113 (32.8%) | 21 (56.8%) | 1 | 1 | ||
| ≥5 | 231 (67.2%) | 16 (43.2%) | 2.683 (1.348-5.340) | 0.005 | 1.986 (0.904-4.360) | 0.087 | |
| Polypharmacy | Yes | 169 (49.1%) | 26 (70.3%) | 2.448 (1.172-5.109) | 0.017 | 1.367 (0.590-3.166) | 0.466 |
| No | 175 (50.9%) | 11 (29.7%) | 1 | 1 | |||
| Number of antihypertensive medication | 1 | 46 (13.4%) | 3 (8.1%) | 1 | 0.369 | ||
| ≥2 | 298 (86.6%) | 34 (91.9%) | 0.572 (0.169-1.937) | ||||
Abbreviations: AOR, adjusted odds ratio; COR, crude odds ratio; DRPs, drug-related problems; HTN, hypertension. P-values set in boldface indicate statistical significance (p<0.05).
Pharmacists’ Interventions to Solve the DRPs
A total of 739 PIs were suggested, an average of 1.9 PIs per patient. The most common PIs were administration mode optimization (50.7%), out of which change in timing of administration (29.8%) for the drugs recommended to be used before meals, during meals, or after meals was the most common. The second common intervention was the addition of drugs (20.4%) which were most commonly associated with the addition of antihypertensive drugs for better control of BP and the addition of SGLT2 inhibitors for preventing cardiovascular events in patients with type 2 diabetes mellitus and high cardiovascular disease. The third common intervention was drug switch (17.1%), followed by dose increasing (5.8%). Detailed information on PIs is indicated in Table 5. A total of 698 (94.5%) interventions were accepted.
Table 5.
Suggestions Provided by Intervening Pharmacists.
| Suggestions provided | Number (%), n = 739 | Example |
|---|---|---|
| Drug addition | 151 (20.4) | Addition of dapagliflozin in hypertensive patients with heart failure |
| Drug switch | 126 (17.1) | Switch from bisoprolol to enalapril in patients with no specific indication for using beta-blocker, eg, heart failure, angina, post-myocardial infarction, atrial fibrillation |
| Dose increasing | 43 (5.8) | Increase of atorvastatin dose from 5 to 10 mg for better reduction of low-density lipoprotein cholesterol |
| Drug discontinuation | 27 (3.7) | Discontinuation of ivabradine where there were unclear indications of ivabradine |
| Drug monitoring optimization | 17 (2.3) | Suggestions to monitor serum potassium in patients receiving spironolactone and lisinopril (major drug-drug interaction) |
| Administration mode optimization | 375 (50.7%) | |
| Change in timing of administration | 220 (29.8%) | Suggestions to take gliclazide 30 minutes before meal time |
| Add instructions to the prescription for special dosage forms (long-acting, modified release, etc) | 74 (10.0%) | Suggestions or instructions to take drugs in patients receiving trimetazidine MR, nifedipine retard, alendronate, etc |
| Change in frequency of administration | 63 (8.5%) | Switch from taking losartan twice daily to taking it once daily |
| Change in the selection of drug form | 18 (2.4%) | Switch from amlodipine 5 mg to amlodipine 10 mg in patients who are taking 2 tablets of amlodipine 5 mg once daily |
| Total | 739 (100) |
Discussion
This is the first prospective study conducted in Vietnam to implement medication reviews by clinical pharmacists to detect DRPs and to identify factors associated with the occurrence of DRPs in 3 tertiary care hospitals in Vietnam.
Prescribing Pattern in Antihypertensive Medications
In our study, the number of antihypertensive drugs per patient was 2.3 ± 0.8. This finding was higher than the outcome of a study done in Northern Ethiopia which was 1.41 ± 0.53 antihypertensive drugs per patient. 26 Previous studies showed that fixed-dose combination provided better BP control than either free combination or monotherapy.24,27 In this study, 39.1% of patients had the dual fixed-dose combination and 1.0% of patients had the triple fixed-dose combination. The use of 2-drug combinations was the most common pattern of pharmacotherapy (53.6%) which was consistent with current guidelines; preferred 2-drug combinations are an RAS blocker with a CCB or a diuretic.21,24
Identified DRPs
The study found that DRPs were extremely common among hypertensive patients, as 90.23% of hypertensive patients experienced 1 or more DRPs. The average number of DRPs per patient was 2.2 ± 1.4. This result is slightly higher than the finding done in Malaysia 6 and in Ethiopia 10 which were 1.9 ± 1.5 and 1.86 ± 0.53, respectively. On the contrary, it is lower than the finding done in Jordan (6.3 ± 2.6). 7 There are several explanations for these differences: variability in DRP classification, study participants, prescriber’s qualifications, how and when DRPs were identified, and references to identify DRPs.
In this study, inappropriate administration was the most common DRP, accounted for 46.3% of total DRPs. The primary reason for this DRP was the inappropriate timing of administration (27.1%), which was mostly related to hypoglycemic agents and proton pump inhibitor drugs. The second reason was incomplete information on the drug regimen (9.0%), which was common with nifedipine retard, felodipine modified release, and trimetazidine modified release. These special dosage forms should be swallowed whole, not chewed or crushed, but there were no instructions provided. The third reason was the inappropriate frequency of administration (8.0%); it was observed frequently among users of ARBs, BBs, or CCBs. These drugs can be taken once daily but were prescribed 2 times daily.
Missing indication (18.0%) was the second most common DRP in this study. The first reason for this DRP was a valid indication without drug prescribed (11.7%), lower than the finding reported by Kusumawardani et al 13 (25.6%) and by Weldegebreal et al 26 (17.1%). The second reason for this DRP was the missing drug combination (6.3%).
The third most common DRP was nonconformity indication (17.8%). The first explanation for this DRP was inappropriate drug choice compared with guidelines (14.6%) which was lower than the finding reported by Ukoha-Kalu et al 28 (18.9%), but higher than the finding presented by Zaman Huri et al 6 (8.8%). The second explanation was contraindication (1.8%) which was lower than the figure published by Zaman Huri et al 6 (7.5%), but higher than the figure published by Kefale et al 10 (0.8%). The third explanation was inappropriate drug combination (1.4%).
Inappropriate dose selection was quite common in our study (12.0%), including low dose (11.8%) and missing dose (0.2%). This finding was in line with the previous study. 10 Low doses may reduce the effectiveness of treatment, while missing dose might cause problems in taking drugs by patients.
Major drug interactions accounted for 2.6% of the total DRPs identified in our study, which was lower than the finding reported by Zaman Huri et al 6 (16.3%). The difference might be due to our study only using major interactions for DRPs and using different interaction checkers.
Unnecessary indications were found in a small proportion (2.2%). These DRPs occurred when patients were prescribed by medications which were not indicated, such as ivabradine, atorvastatin, or therapeutic duplication. The studies about DRPs done in Malaysia and Indonesia also showed that unnecessary indications accounted for a small proportion of the total DRPs identified, 0.3% and 13.5%, respectively.6,13
During the conduct of our study, we found that ADRs were uncommon, and represented only 1.1% of the total DRPs identified. When interviewing patients, we recorded 8 patients with dry cough due to enalapril or lisinopril and 1 patient with peripheral edema due to amlodipine. These ADRs were only detected when clinical pharmacists interviewed patients. In previous studies, cough is 1 of the common adverse effects in patients taking ACEis, approximately 1.5%-11% of patients treated with ACEis. 29
Factors Associated With DRPs
Participants with comorbidity were about 4 times more likely to develop DRPs than participants without comorbidity. Our outcome was consistent with the studies conducted by Kefale et al 10 and Weldegebreal et al. 26 In our study, 88.7% of hypertensive patients had at least 1 comorbidity. Hence, more attention should be paid to current treatment guidelines to limit unnecessary prescribing.
Pharmacists’ Interventions to Solve the DRPs
The study reported a very high rate of physician’s acceptance (94.5%) which is higher than 52.4% in India as well as 71.6% in Ghana.15,30 The main reason is the research team has developed a comprehensive clinical pharmacy practice procedure for hypertensive outpatients. All clinical pharmacists who conducted clinical pharmacy activities received a significant amount of training and were supervised by hospital pharmacotherapy specialists, and comprehensive patient information was collected by direct interview.
Strengths and Limitations
The study has several strengths. First, the study was conducted in 3 large hospitals in Vietnam. As a result, the findings were relatively robust and other hospitals can use our study as a reference to replicate pharmacy services in the care of hypertensive patients. Second, medication review was conducted based on fully completed patient’s clinical characteristics, and medication data were obtained from the prescriptions and direct patient interviews. Third, PIs were evaluated by experts including 3 cardiologists with a high acceptance rate which may reflect the high quality of PIs.
However, some limitations include PIs being evaluated retrospectively by a consensus group of 3 cardiologists without transferring to treated physicians for patients and lack of evaluation of PI’s impact. Further research work, most preferably, a randomized controlled trial, should be performed to confirm the benefit of the service.
Conclusion
This study showed that the addition of a clinical pharmacist in the pharmacist-led clinic was effective to detect DRPs and suggests PIs to optimize drug use for hypertensive outpatients. It is needed that training and daily teamwork procedures based on these results should be implemented. These activities should be maintained and developed in these 3 hospitals as well as replicated in other hospitals for hypertensive outpatients.
Supplemental Material
Supplemental material, sj-docx-1-pmt-10.1177_87551225231199358 for Drug-Related Problems and Pharmacists’ Interventions in Hypertensive Outpatients: A Multicenter Prospective Study in 3 Vietnamese Hospitals by Bon Huu Huynh, Surakit Nathisuwan, Pramote Tragulpiankit, Vinh Dai Nguyen, Nam Huu Huynh, Thu Le Anh Vu, Loc Thi Hong Huynh and Ha Thi Vo in Journal of Pharmacy Technology
Acknowledgments
The authors would like to express their deep gratitude to the directors and ethical committee members from 3 hospitals who reviewed their protocol and gave them permission to conduct this study. They would like to acknowledge the clinical pharmacists (Ha Thi Thuy, Dang Thi Nga, and Nguyen Thi Lien) and Nguyen Lien Nhut, MD, at Nguyen Tri Phuong Hospital; Pham Hong Tham, PhD, and Pham Truong My Dung, MD, at Gia Dinh People’s Hospital; and Tran Van Hai, MD, at Hoa Vang District Medical Center for supporting data collection and reviewing pharmacist interventions.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Bon Huu Huynh  https://orcid.org/0000-0002-3253-861X
https://orcid.org/0000-0002-3253-861X
Nam Huu Huynh  https://orcid.org/0000-0002-9562-3926
https://orcid.org/0000-0002-9562-3926
Thu Vu Le Anh  https://orcid.org/0009-0006-6079-5708
https://orcid.org/0009-0006-6079-5708
Loc Thi Hong Huynh  https://orcid.org/0009-0002-5912-0675
https://orcid.org/0009-0002-5912-0675
Ha Thi Vo  https://orcid.org/0000-0001-6170-0957
https://orcid.org/0000-0001-6170-0957
Supplemental Material: Supplemental material for this article is available online.
References
- 1. World Health Organization. Hypertension. Accessed February 10, 2022. https://www.who.int/health-topics/hypertension
- 2. Son PT, Quang NN, Viet NL, et al. Prevalence, awareness, treatment and control of hypertension in Vietnam-results from a national survey. J Hum Hypertens. 2012;26(4):268-280. doi: 10.1038/jhh.2011.18 [DOI] [PubMed] [Google Scholar]
- 3. The 2nd Vietnam hypertension conference. Published 2016. https://tanghuyetap.vn/tai-nguyen/hoi-nghi-tang-huyet-ap-viet-nam-lan-2 (In Vietnamese)
- 4. Pharmaceutical Care Network Europe. PCNE DRP classification now 8.03. Published 2019. Accessed August 29, 2023. https://www.pcne.org/upload/files/318_PCNE_classification_V8-03.pdf
- 5. Munger MA. Polypharmacy and combination therapy in the management of hypertension in elderly patients with co-morbid diabetes mellitus. Drugs Aging. 2010;27(11):871-883. doi: 10.2165/11538650-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 6. Zaman Huri H, Fun Wee H. Drug related problems in type 2 diabetes patients with hypertension: a cross-sectional retrospective study. BMC Endocr Disord. 2013;13:2. doi: 10.1186/1472-6823-13-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abu Farha R, Basheti I, Abu Al Ruz H, Alsaleh A, AbuRuz S. Assessment of drug-related problems and their impact on blood pressure control in patients with hypertension. Eur J Hosp Pharm. 2016;23(3):126-130. doi: 10.1136/ejhpharm-2015-000712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farha RA, Saleh A, Aburuz S. The impact of drug related problems on health-related quality of life among hypertensive patients in Jordan. Pharm Pract (Granada). 2017;15(3):995. doi: 10.18549/PharmPract.2017.03.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ayele Y, Melaku K, Dechasa M, Ayalew MB, Horsa BA. Assessment of drug related problems among type 2 diabetes mellitus patients with hypertension in Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia. BMC Res Notes. 2018;11(1):728. doi: 10.1186/s13104-018-3838-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kefale B, Tegegne GT, Kefale Y, Molla M, Ewunetei A, Degu A. Magnitude and determinants of drug therapy problems among type 2 diabetes mellitus patients with hypertension in Ethiopia. SAGE Open Med. 2020;8:2050312120954695. doi: 10.1177/2050312120954695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Redzuan AM, Ramli AR, Pheng MTH. Drug-related problems in hypertensive patients with multiple comorbidities. J Pharm Res. 2017;1(3):000113. [Google Scholar]
- 12. Babirye M, Yadesa TM, Tamukong R, Obwoya PS. Prevalence and factors associated with drug therapy problems among hypertensive patients at hypertension clinic of Mbarara Regional Referral Hospital, Uganda: a cross-sectional study. Ther Adv Cardiovasc Dis. 2023;17:17539447231160319. doi: 10.1177/17539447231160319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kusumawardani LA, Andrajati R, Nusaibah A. Drug-related problems in hypertensive patients: a cross-sectional study from Indonesia. J Res Pharm Pract. 2020;9(3):140-145. doi: 10.4103/jrpp.JRPP_20_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Julaeha J, Fudjiati E, Eff ARY. Assessment of drug therapy problems among type 2 diabetes patients with hypertension comorbidity in Indonesia. Borneo J Pharm. 2020;3(3):190-198. [Google Scholar]
- 15. Amankwa Harrison M, Marfo AFA, Buabeng KO, Nkansah FA, Boateng DP, Ankrah DNA. Drug-related problems among hospitalized hypertensive and heart failure patients and physician acceptance of pharmacists’ interventions at a teaching hospital in Ghana. Health Sci Rep. 2022;5(5):e786. doi: 10.1002/hsr2.786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deawjaroen K, Sillabutra J, Poolsup N, Stewart D, Suksomboon N. Characteristics of drug-related problems and pharmacist’s interventions in hospitalized patients in Thailand: a prospective observational study. Sci Rep. 2022;12(1):17107. doi: 10.1038/s41598-022-21515-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vo TH, Bedouch P, Nguyen TH, et al. Pharmacy education in Vietnam. Am J Pharm Educ. 2013;77(6):114. doi: 10.5688/ajpe776114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vietnam’s Ministry of Health. [Guidance on clinical pharmacy practice for pharmacists in a number of non-communicable diseases]. Published 2019. https://tanghuyetap.vn/tai-nguyen/hoi-nghi-tang-huyet-ap-viet-nam-lan-2 (In Vietnamese)
- 19. Eng J. Sample size estimation: how many individuals should be studied. Radiology. 2003;227(2):309-313. doi: 10.1148/radiol.2272012051 [DOI] [PubMed] [Google Scholar]
- 20. Hussein M, Lenjisa JL, Woldu MA, et al. Assessment of drug related problems among hypertensive patients on follow up in Adama Hospital Medical College, East Ethiopia. Clinic Pharmacol Biopharmaceut. 2014;3:122. doi: 10.4172/2167-065X.1000122 [DOI] [Google Scholar]
- 21. Vietnam National Heart Association. [2018 VNHA/VSH guidelines for diagnosis and treatment of hypertension in adults]. Published 2018. http://vnha.org.vn/data/Khuyen-Cao-THA-2018.pdf (In Vietnamese)
- 22. Vietnam National Heart Association. [Recommendations for the diagnosis and treatment of acute and chronic heart failure]. Published 2022. https://kcb.vn/phac-do/quyet-dinh-1857-qd-byt-ngay-05-07-2022-ve-viec-ban-hanh-tai-lieu-chuyen-mon-huong-dan-chan-doan-va-dieu-tri-suy-tim-cap-.html (In Vietnamese)
- 23. Vietnam’s Ministry of Health. [Guidelines for the diagnosis and treatment of type 2 diabetes]. Published 2020. http://canhgiacduoc.org.vn/Hotro/daotao/1857/H%C6%B0%E1%BB%9Bng-d%E1%BA%ABn-ch%E1%BA%A9n-%C4%91o%C3%A1n-v%C3%A0-%C4%91i%E1%BB%81u-tr%E1%BB%8B-%C4%91%C3%A1i-th%C3%A1o-%C4%91%C6%B0%E1%BB%9Dng-t%C3%ADp-2.htm (In Vietnamese)
- 24. Williams B, Mancia G, Spiering W, et al. 2018. ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 25. Vo TH, Hoang TL, Faller EM, Nguyen DT. Development and validation of the Vi-Med® tool for medication review. J Appl Pharm Sci. 2020;10(02):086-096. [Google Scholar]
- 26. Weldegebreal AS, Tezeta F, Mehari AT, Gashaw W, Dessale KT, Legesse NY. Assessment of drug therapy problem and associated factors among adult hypertensive patients at Ayder comprehensive specialized hospital, Northern Ethiopia. Afr Health Sci. 2019;19(3):2571-2579. doi: 10.4314/ahs.v19i3.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mancia G, Rea F, Corrao G, Grassi G. Two-drug combinations as first-step antihypertensive treatment. Circ Res. 2019;124(7):1113-1123. doi: 10.1161/CIRCRESAHA.118.313294 [DOI] [PubMed] [Google Scholar]
- 28. Ukoha-Kalu BO, Adibe MO, Ukwe CV. Identification and resolution of drug therapy problems among hypertensive patients receiving care in a Nigerian Hospital—a pilot study. Ann Clin Hypertens. 2020;4:020-023. doi: 10.29328/journal.ach.1001024 [DOI] [Google Scholar]
- 29. Pinto B, Jadhav U, Singhai P, Sadhanandham S, Shah N. ACEI-induced cough: a review of current evidence and its practical implications for optimal CV risk reduction. Indian Heart J. 2020;72(5):345-350. doi: 10.1016/j.ihj.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shareef J, Fernandes J, Samaga LN. Clinical pharmacist interventions in drug therapy in patients with diabetes mellitus and hypertension in a University Teaching Hospital. Int J Pharm Sci Res. 2015;6(10):4424-4432. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pmt-10.1177_87551225231199358 for Drug-Related Problems and Pharmacists’ Interventions in Hypertensive Outpatients: A Multicenter Prospective Study in 3 Vietnamese Hospitals by Bon Huu Huynh, Surakit Nathisuwan, Pramote Tragulpiankit, Vinh Dai Nguyen, Nam Huu Huynh, Thu Le Anh Vu, Loc Thi Hong Huynh and Ha Thi Vo in Journal of Pharmacy Technology