Abstract
Objective: To describe the mechanism of cyclin-dependent kinase (CDK) 4/6 inhibitors, mechanisms of resistance, and summarize various clinical trials used to determine the efficacy and safety of CDK4/6 inhibitor used for the treatment of hormone receptor-positive (HR+), human epidermal growth factor receptor 2 negative (HER2−), advanced or metastatic breast cancer. Data Sources: An extensive literature search using PubMed and notable sources was performed (2016 to February 2022) using the following search terms: CDK4/6 inhibitors, palbociclib, abemaciclib, ribociclib, CDK4/6 inhibitor resistance, FAT1 gene, luminal A breast cancer, luminal B breast cancer, HR+/HER2− breast cancer. Abstracts from conferences, national clinical trials, and drug monographs were reviewed. Study Selection and Data Extraction: Relevant clinical studies or those conducted in humans and updated clinical trials were considered. Data synthesis: The various clinical trials reviewed and results have led to numerous studies and expansions of U.S. Food and Drug Administration (FDA) approval. Although the use of CDK4/6 inhibitors has improved progression-free survival in patients with HR+, HER2− breast cancer, studies have shown that resistance pathways can cause cells to be insensitive to CDK4/6 inhibitors, leading to continued cell proliferation. Conclusions: CDK4/6 inhibitors are recommended as first-line therapy in combination with endocrine therapy for patients with HR+/HER2− advanced breast cancer. However, mutations and acquired resistance can occur that affect a patient’s response to treatment. Additional research needs to be conducted on strategies to overcome resistance and determine how ethnicity plays a role in resistance pathways.
Keywords: CDK4/6 inhibitors, HR+/HER2−, breast cancer, CDK4/6 inhibitor resistance pathways, clinical pharmacy, oncology, clinical trials
Overview of CDK4/6 Inhibitors
Cyclin-dependent kinases (CDKs) are involved in regulating the cell cycle. More specifically, the inhibitors act on the G1 and S phases, which involve cell growth, metabolism, and the DNA replication phase. 1 In the DNA replication cycle, CDK1 is the signaling factor that tells the DNA strand to move to the other phases. In most cancers, the CDKs are not functioning the way they should be, and cells are dividing uncontrollably. 2
The 2 major roles of CDKs are cell cycle regulation, which includes CDK1,2,4,6, and transcription regulation, which includes CDK7,8,9,11. 3 CDK4/6 is the main factor in regulating various tumors. Cyclin D-CDK4/6− retinoblastoma (cyclin D-CDK4/6-Rb) is the main signaling pathway that controls the G1 to S phase transition. When cyclin D binds to CDK4/6, it promotes retinoblastoma to be phosphorylated, which separates E2F from the Rb-E2F. 1 This signal causes the transition to the S phase and starts DNA replication. Any changes to this cyclin D-CDK4/6-Rb will cause many tumors. This process has been observed in many tumorigenesis processes. Retinoblastoma (Rb) is a tumor suppressor. 4 When inactivated, it will promote tumorigenesis and continue uncontrolled cell proliferation. Rb-E2F regulates the timing of cell cycle regulations specifically when cells move from G1 to S phase. 4 Targeting CDK4/6 can help prevent uncontrolled cell growth. CDK inhibitors work by inhibiting CDK4/6 from complexing with cyclin D, which in turn prevents subsequent phosphorylation of Rb, and then dephosphorylation of Rb to allow E2F to move the cell cycle from G1 to S phase.2,4
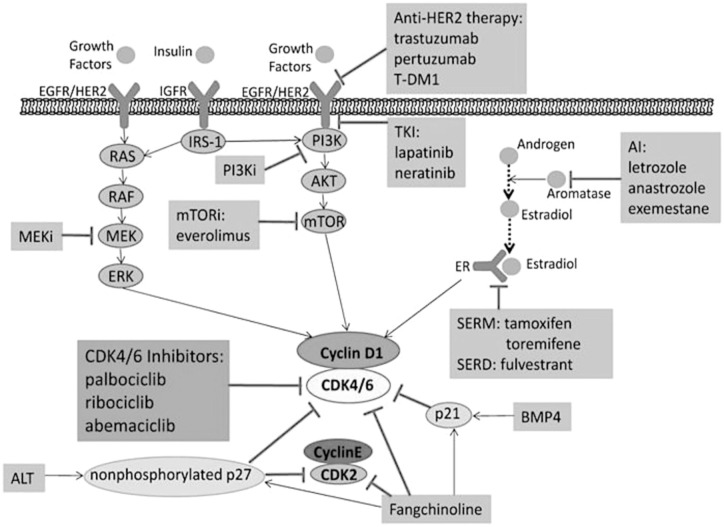
About 65% to 70% of breast cancers are hormone receptor-positive (HR+). The current pharmacological treatment for early-stage (stage I-IIIA) HR+/HER2-negative (HER2−) breast cancer is chemotherapy including dose-dense doxorubicin and cyclophosphamide for 4 cycles followed by weekly paclitaxel. HR+ HER2+ breast cancer treatment includes chemotherapy along with targeted therapy including trastuzumab and pertuzumab. 5 In the past, endocrine monotherapy was the preferred treatment for HR+/HER2− breast cancer. However, patients on endocrine monotherapy experience resistance to therapy and relapse.6,7 CDK4/6 inhibitors work synergistically with selective estrogen receptor blockers and selective estrogen receptor modulators (SERDs and SERMs), as shown in Figure 1. 8 Cyclin D1 expression is induced by estrogen. The mechanism in which estrogen regulates cyclin D1 is via transcription. 9
Figure 1.
CDK4/6 inhibitors work synergistically with selective estrogen receptor degraders (SERDs) and selective estrogen receptor modulators (SERMs) as shown above. Cyclin D1 expression is induced by estrogen. The mechanism in which estrogen regulates cyclin D1 is via transcription.8,9
Abbreviations: AI, aromatase inhibitor; ALT, alternative lengthening of telomeres; AKT, protein kinase B; BMP, bone morphogenetic protein; CDK, cyclin-dependent kinase; HER2, human epidermal growth factor receptor 2; EGFR, epidermal growth factor receptor; ERK, extracellular signal regulated kinase; IGFR, insulin- like growth factor; T- DM1, trastuzumab emstansine; MEK, mitogen activated kinase kinase; MEKi, mitogen activated kinase kinase inhibitor; RAS, rat sarcoma; RAF, rapidly accelerated fibrosarcoma; TKI, tyrosine kinase inhibitor.
Clinical trials such as the PALOMA-3 trial have revealed that CDK4/6 inhibitors with endocrine therapy (ET) have increased overall survival. 11 Currently, CDK4/6 inhibitors are the mainstay of treatment for HR+/HER2− advanced or metastatic breast cancer (MBC) (stage IIIB-IV). 6 The three FDA-approved CDK4/6 inhibitors include palbociclib, ribociclib, and abemaciclib. The various trials and results have led to numerous studies on the use of CDK4/6 inhibitors, along with expansions of FDA approval. Although the use of CDK4/6 inhibitors has improved progression-free survival (PFS) in patients with hormone receptor-positive, HER2− breast cancer, studies have shown that resistance pathways can cause cells to be insensitive to CDK4/6 inhibitors, leading to continued cell proliferation.6,7
Approved CDK4/6 Inhibitors and Clinical Trials
Palbociclib (Ibrance)
In 2016, the PALOMA-2 trial was the clinical trial that pioneered the FDA approval of CDK4/6 inhibitors, specifically palbociclib in postmenopausal women with HR+, HER2− breast cancer without prior cancer therapy. The study was an international, placebo-controlled, randomized, double-blind trial. The results revealed that patients who received palbociclib along with letrozole had a median PFS of 24.8 months compared to the PFS of 14.5 months in the group given placebo and letrozole. The most common side effects (SE) were neutropenia and leukopenia. 10 This study introduced CDK4/6 inhibitors to the market in pharmacotherapy. It allowed researchers to study the use of palbociclib in combination with other breast cancer therapies.
Paloma-3
In 2016, the results of the PALOMA-3 clinical trial revealed that the overall survival of the palbociclib and fulvestrant group was 34.9 months compared to the 28 months overall survival of the placebo and fulvestrant group.11,12 Following the release of these results, the FDA approved palbociclib for treating patients with HR+, HER2-negative advanced breast cancer (ABC) who experienced disease progression after ET. Unlike the PALOMA-2 trial, the PALOMA-3 trial included women regardless of menopausal status (premenopausal, perimenopausal, or postmenopausal). Inclusion criteria also included women who failed previous ET, unlike the PALOMA-2 study, which included women without prior therapy. 10 An extended follow-up of 73.3 months of the PALOMA-3 trial revealed a continued improvement in the overall survival rate in patients who received palbociclib with fulvestrant (23.3%) compared to the placebo arm (16.8%).11,12 PALOMA-3 is included along with PALOMA-2 in the FDA drug monograph. The PALOMA trials are summarized in Table 1.
Table 1.
The Table Summarizes Phase 3 PALOMA, MONALEESA, and MONARCH Trials and the Studies’ Impact on FDA Approval.
| Trial and date of study | Patient population | Dosing scheme | Results | Food and Drug Administration (FDA) approval/special considerations |
|---|---|---|---|---|
| PALOMA 210 | 666 participants Postmenopausal women with (estrogen receptor) ER+ HER2− metastatic breast cancer No prior anticancer therapy for advanced disease |
Palbociclib 125 mg by mouth daily for 3 weeks and 1 week off every 28-day cycle plus letrozole 2.5 mg by mouth continuously Or Placebo 125 mg by mouth daily for 3 weeks and 1 week off every 28-day cycle plus letrozole 2.5 mg by mouth continuously |
PFS was 24.8 months in the palbociclib group vs. 14.5 months in the placebo group | FDA approved in 2015 in combination with an aromatase inhibitor for postmenopausal women with HR+/HER2− advanced breast cancer |
| PALOMA 311,12 | 521 participants Premenopausal or postmenopausal women with HR+/HER2− advanced breast cancer Progression of cancer on endocrine therapy One line of previous chemotherapy for ABC was allowed |
Palbociclib 125 mg by mouth daily for 3 weeks and 1 week off every 28-day cycle plus fulvestrant 500 mg IM on day 1 and 15 of cycle 1, then on day 1 of each cycle thereafter Or Placebo 125 mg by mouth daily for 3 weeks and 1 week off every 28-day cycle plus fulvestrant 500 mg IM on day 1 and 15 of cycle 1, then on day 1 of each cycle thereafter Patients who were perimenopausal or premenopausal received goserelin |
73.3-month median follow-up Median overall survival (OS) was 34.8 months in the palbociclib group and 28 months in the placebo group |
FDA approved in 2017 in combination with fulvestrant (a selective estrogen degrader [SERD]) for women with HR+/HER2− advanced breast cancer who progressed following endocrine therapy |
| MONALEESA 2 Study date: 2016-201713 |
213 women Postmenopausal women over the age of 18 with HR+/HER2-negative locoregionally recurrent or metastatic breast cancer who did not receive previous therapy for advanced disease and whose disease was not improved following therapy |
ribociclib 600 mg by mouth for 3 weeks on and 1 week off with letrozole 2.5 mg per day or placebo plus letrozole 2.5 mg | Median progression-free survival (PFS) rate was 27.6 months with ribociclib versus 15 months with placebo | Initial FDA approval in 2017 was based on the MONALEESA-2 trial. The FDA approved ribociclib with the indication to treat postmenopausal women with HR-positive, HER2-negative metastatic breast cancer along with aromatase inhibitor as first-line therapy |
| MONALEESA3 Study Date: 2015-201814 |
726 women Postmenopausal women over the age of 18 with histologically/cytologically confirmed HR+/HER2 ABC Patient could have received less than 1 line of endocrine therapy (ET) but not chemotherapy |
ribociclib 600 mg by mouth daily 3 weeks on and 1 week off plus fulvestrant 500 mg IM on days 1 and 15 for the first 28 day cycle, then on day 1 every 28 days | PFS of ribociclib and fulvestrant was 20.5 compared to 12.8 months in the placebo plus fulvestrant arm | FDA-approved indication for treatment of HR-positive, HER2-negative advanced breast cancer (ABC) or metastatic breast cancer (MBC) in postmenopausal women after failure of treatment with endocrine therapy |
| MONALEESA 7 Study Date: 2014-201715 |
672 women Premenopausal or perimenopausal women over the age of 18 with histologically/cytologically confirmed HR+/HER2 ABC Patient could have received less than 1 line of endocrine therapy (ET) but not chemotherapy for ABC Locoregionally recurrent or metastatic breast cancer who had not received previous therapy for advance disease and who disease was not amendable to curative therapy |
ribociclib 600 mg by mouth daily or placebo with tamoxifen 20 mg daily or letrozole 2.5 mg or anastrozole 1 mg, all with goserelin |
Median PFS in the group given tamoxifen or letrozole and ribociclib was 22. 1 months and 11 months in placebo group Median PFS in nonsteroidal aromatase inhibitor and ribociclib group was 27. 5 months vs. 13.8 months in the placebo group |
FDA expanded indication in 2018 approval for HR-positive, HER2-negative ABC or MBC in peri or premenopausal women as initial therapy |
| MONARCH 1 Study dates: 2016-201816 |
132 women Women over the age of 18 with HR+ for ER or PgR Patients with metastatic breast cancer Patient must have progressed on or after prior endocrine therapy and had prior treatment with at least 2 chemo chemotherapy regimen and at least one but no more than 2 of which has been administered in a metastatic setting one regimen must have included a taxane either in the adjuvant setting or metastatic setting |
200 mg by mouth continuously every 12 hours | Objective response rate 19.7%. PFS of abemaciclib group was 6 months, and OS was 17.7 months. Diarrhea, fatigue, and nausea were the most common side effects (SE), with an increase in Cr as the most common lab value |
FDA approved in 2017 for monotherapy after previous treatment with endocrine therapy and 1-2 chemotherapy regimens. |
| MONARCH 2 Study dates: 2014-201717 |
669 women Women over the age of 18 with any menopausal status Patients were required to have a disease that progressed while receiving neoadjuvant or adjuvant ET 12 months or less after adjuvant ET or while receiving ET for ABC Patient must not have received more than one ET or any prior chemotherapy for ABC |
150 mg by mouth twice daily continuously with 500 mg fulvestrant | In patients with measurable disease, the objective response rate was 48.1% in the abemaciclib group PFS of 16.4 for abemaciclib and fulvestrant group compared to 9.4 months in fulvestrant alone group Diarrhea and neutropenia are common SE |
FDA approved in 2017 in combination with fulvestrant following disease progression during or after endocrine therapy |
| MONARCH 3 Study dates: 2014-201818 |
493 patients Postmenopausal women greater than the age of 18 with locoregionally recurrent HR+/HER2− breast cancer or metastatic breast cancer without prior therapy for disease state |
150 mg by mouth twice daily continuously with either 1 mg anastrozole or 2.5 mg letrozole | Objective response rate is 59% in patients with measurable diseases in the abemaciclib group Median PFS was not reached in the abemaciclib arm Common SE diarrhea and mainly grade 1 Most common SE: 3-4 grade neutropenia and leukopenia |
FDA approved in 2018 in combination therapy with letrozole or anastrozole as initial therapy |
Abbreviations: ABC, advanced breast cancer; ER, estrogen receptor; ET, endocrine therapy; FDA, Food and Drug Administration; HR+, hormone receptor-positive; HER2, human epidermal growth factor receptor 2; MBC, metastatic breast cancer; OS, overall survival; PFS, progression-free survival; SE, side effects; SERD, selective estrogen receptor degraders.
Ribociclib (Kisqali)
In 2017, ribociclib was approved by the FDA following the MONALEESA-2 study. The randomized, double-blind, placebo-controlled trial included postmenopausal women with HR-positive, HER2-negative MBC without previous treatment. The most prevalent adverse effects were neutropenia in the ribociclib group (72%) and nausea (69%). These SE, along with fatigue, were the most across all MONALEESA trials. Median PFS of the ribociclib and letrozole group was 25.3 months compared to 16 months PFS of the placebo-letrozole group. 13 Table 1 summarizes the MONALEESA trials.13 -15
CompLEEment study
CompLEEment-1 is a multicenter, phase 3b trial that assessed 3246 patients to determine the safety, efficacy, and tolerability of ribociclib and letrozole in pre or postmenopausal women and men with HR+ HER2− ABC without previous ET. The study began in 2016 and was completed in 2019. Unlike the previous MONALEESA studies, the primary outcome of CompLEEment was the number of participants with adverse events. Secondary outcomes were overall response rate and time to progression. At the end of the study, 98.7% of patients had an adverse effect. The most prevalent adverse effect was neutropenia, which was seen in 74.5% of patients, and nausea, which was seen in 35.9% of patients. The results regarding the efficacy of the total population showed that the clinical benefit rate was 70.7%, the overall response rate was 64%, and the median time to progression was 26.7 months. 19
EarLEE
The EarLEE-1 trial took place from 2017 to 2020. The double-blind, placebo-controlled, phase 3 trial included men and women with high-risk HR+/HER2− high-risk early breast cancer. The study was done to compare the safety and tolerability of ribociclib with ET compared to placebo and ET. The experimental group was given ribociclib 600 mg by mouth daily, 3 weeks on and 1 week off for 28 days for 26 cycles and letrozole 2.5 mg, anastrazole 1 mg, exemestane 25 mg by mouth daily or tamoxifen 20 mg daily for 60 months. Patients in the placebo group took the placebo in combination with standard adjuvant ET. The primary outcome of the study was the number of participants with adverse and serious adverse events. The results revealed that 15.38% of the ribociclib group experienced serious adverse events including cardiac disorders and blood system disorders, compared to 8.3% in the placebo group. The most common side effect in the ribociclib group was neutropenia (53.85%). 20
Coralleen
CORALLEEN is a parallel arm, multicenter phase 2 trial that took place in Spain from 2017 to 2019. Recruited patients were postmenopausal women with stage I-IIIA HR+/HER2− breast cancer. Patients either received ribociclib plus letrozole or 4 cycles of chemotherapy (doxorubicin and cyclophosphamide), followed by a weekly dose of taxol for 12 weeks. Results of the study showed that 46.9% of patients in the ribociclib plus letrozole group versus 46.1% of the chemotherapy group were at low risk of relapse. The most common adverse effects in the CDK4/6 plus letrozole group were neutropenia and high alanine transaminase concentrations, which were grade 3 to 4 SE. This trial included more participants compared to EARLEE and compared the risk of relapse in postmenopausal early-stage breast cancer patients who received chemotherapy versus ribociclib and an aromatase inhibitor. 21
Pace
The PACE trial was performed from August 2017 to December 2022. The objective of the phase 2 clinical trial was to evaluate the PFS of fulvestrant (ET) alone, fulvestrant and palbociclib or fulvestrant, palbociclib, and avelumab. The study included 220 participants with metastatic HR+/HER2− breast cancer. Participants were premenopausal women aged 18 taking monthly gonadotropin-releasing hormone agonist therapy and postmenopausal men/women with progression or relapse during or 12 months after completing endocrine and CDK4/6 inhibitor adjuvant treatment. Randomization for each treatment group was 1:2:1. The first group was administered 2 injections of fulvestrant intramuscularly on cycle 1 on days 1 and 15 every month and palbociclib daily for 21 days on and 7 days off on a 28-day cycle. The next group was administered 2 injections of fulvestrant intramuscularly on cycle 1 on days 1 and 15 every month alone. The last group was administered fulvestrant with the same dosing strategy as the previous groups plus palbociclib daily for 21 days on and 7 days off on a 28-day cycle, and avelumab was administered intravenously once every 2 weeks. Results of the trial revealed that PFS was 4.8 months for fulvestrant alone arm compared to PFS of 4.6 months in fulvestrant plus palbociclib arm. Therefore, the PFS was not superior to fulvestrant alone. In addition, PFS survival was 8.1 months for the triplet combination arm. Adverse side effect of grade 3 or 4 neutropenia was reported in 32.7% of patients in fulvestrant plus palbociclib and 49.1% in the triplet arm. The significance of this study provided the PFS effect with avelumab, a PDL1 inhibitor, which was double that of the other treatment arms. This result signified the need for more research on PDL1 in combination with CDK4/6 inhibitor and fulvestrant. In addition, the study provided the effect of PFS following disease progression and prior CDK4/6 inhibitor use with ET.22,23
RIGHT Choice Trial
Results of the RIGHT Choice trial were presented at the San Antonio Breast Cancer Symposium. The trial took place from 2019 to 2022. It was the first phase 2 randomized, open-label, multicenter trial that compared a CDK4/6 inhibitor plus ET versus combination chemotherapy in premenopausal or perimenopausal patients with aggressive, HR+/HER2− ABC. Advanced or MBC included life-threatening visceral crisis, rapid disease progression, or symptomatic disease. There were a total of 223 participants assigned in a 1:1 ratio to receive either chemotherapy combination regimen (docetaxel/capecitabine, paclitaxel/gemcitabine, or capecitabine/vinorelbine) or ribociclib plus aromatase inhibitor (AI) plus goserelin therapy. Results of the study revealed that patients treated with ribociclib and ET had a PFS of 24 months versus 12.3 months for patients treated with chemotherapy. Most of the SE included diarrhea and fatigue. The results of the trial proved that ribociclib plus ET is an effective and clinically significant option in patients with aggressive breast cancer.22,24
Abemaciclib (Verzenio)
In 2017, the FDA approved abemaciclib for the treatment of HR+, HER2− advanced, or MBC in combination with fulvestrant following disease progression after endocrine treatment. Abemaciclib was also approved as monotherapy following failed treatment with chemotherapy and ET for MBC. The studies that led to the initial FDA approval were the MONARCH 1 and MONARCH 2 studies.16,17
Monarch 3
A randomized, double-blind, placebo-controlled phase 3 study compared abemaciclib in Japanese patients with nonsteroidal aromatase inhibitor (NSAI). There were 493 patients. The objectives were to evaluate the objective response rate, PFS rate, and safety. Patients either received abemaciclib or a placebo. In addition, the physician decided if the patient received 1 mg anastrozole or 2.5 mg letrozole. This trial resulted in a PFS of 28.18 months in the abemaciclib arm vs. 14.76 months in the placebo arm. 18 The Japanese PK was comparable to the overall population. The SE was consistent with the other trials. The most prevalent SE included diarrhea and neutropenia. Compared to the MONARCH 2 trial, MONARCH 3 revealed that abemaciclib plus an NSAI was an effective treatment in postmenopausal women with HR+/HER2− breast cancer as initial therapy. 18 Table 1 summarizes the MONARCH clinical trials.
MONARCH Plus
The MONARCH plus study from 2016 to 2019 was a randomized, double-blind, phase 3 study. Inclusion criteria included postmenopausal women with HR+/HER2-negative ABC without prior therapy for a disease state or who had disease progression during previous ET but no previous chemotherapy. Cohort A included patients without prior therapy. Patients were given abemaciclib and an NSAI or placebo plus NSAI. Cohort B included patients who experienced disease progression after ET. Patients were given abemaciclib plus fulvestrant or placebo plus fulvestrant. In cohort A, PFS was not reached in the abemaciclib arm. However, PFS of the placebo arm was 14.7 months. In cohort B, PFS was 11.5 months in the abemaciclib arm compared to 5.6 months in the placebo arm. Overall, the change in tumor size was higher in the abemaciclib arm compared to the placebo arm in both cohorts. The most common adverse events in both cohorts in the abemaciclib arms were neutropenia and diarrhea. 25
MonarchE
An open-label phase 3 study performed from 2017 to 2020 compared the benefit of abemaciclib and ET versus ET alone. It included female and male patients with HR+/HER2−, high-risk recurrence, node-positive early breast cancer, following surgery, with or without radiotherapy or chemotherapy. A high risk of recurrence was indicated by 1 of the following: 4 or more positive axillary lymph nodes, tumor size of at least 5 cm, tumor grade 3, or ki-67 index score of less than or equal to 20%. The patient population consisted of females with a median age of 51 and postmenopausal. “A total of 95.4% of patients had received radiotherapy, and 95.4% of patients had received prior chemotherapy (37.0% neoadjuvant, 58.3% adjuvant, 3.5% received both, and all patients who received both were counted in the neoadjuvant total).” 26 The most common adverse events were diarrhea and neutropenia. The invasive disease-free survival (IDFS) rate of abemaciclib and ET group was 92.2% compared to 88.7% in the ET alone group. In 2021, an additional follow-up study for monarchE was completed. Results of the study revealed a 5.4% improvement in 3-year ISFS rates in the abemaciclib and ET group (88.8%) compared to the ET group alone (86.1%). In addition, the FDA approved abemaciclib in 2021 as the first CDK4/6 inhibitor for patients with high-risk early breast cancer due to the results of the monarchE study. 27
Emerald
The EMERALD trial was a multicenter randomized, open-label, phase 3 clinical study performed from 2019 to 2021. The objective of the study was to compare the efficacy and safety of elacestrant, an investigational oral selective estrogen receptor degrader and modulator, in women with HR+/HER2− with recurrent or MBC with standard of care (SOC) therapy. Patients in the study included postmenopausal women or men aged 18 and older with HR+/HER2− with recurrent or MBC. Patients in this study included those with progression or relapse within 28 days of 1 or 2 lines of ET treatment, or progression or relapse during or 12 months within completing aromatase inhibitor (AI) or fulvestrant and CDK4/6 inhibitor adjuvant treatment. Patients could have had treatment with or without one line of chemotherapy regimen. Finally, ESR1 mutation status was determined before randomization of 1:1 patients to each treatment group. One group was given an elacestrant 400 mg daily.28,29 Another group was given SOC therapy of fulvestrant, anastrozole, letrozole, or exemestane monotherapy and dosed according to labeling. Fulvestrant was recommended in patients who had not been treated with the medication previously. Results of the study revealed that 6-month and 12-month PFS rates were significantly higher in the elacestrant groups in all patients and in patients with ESR1 mutation. The 12-month PFS rate in the elacestrant group was 22.3% vs 9.4% in the SOC group. In addition, the 6-month PFS rate in the SOC group was 34.3% in the elacestrant group vs 20.4 % in the SOC group. The most common adverse effect for the elacestrant group and the SOC group was nausea 35% vs 18.8%, respectively. Based on the results, elacestrant significantly improved PFS compared to SOC therapy in all patients and in patients with ESR1 mutations. 29 The FDA approval for elacestrant in January 2023 followed the results of the EMERALD trial. Elecestrant was approved for use in postmenopausal women or men with HR+/HER2− recurrent or MBC after disease progression with at least one line of ET. 30 In addition, ESR1 mutations are common in ER+ breast cancer and cause resistance to aromatase inhibitor therapy such as letrozole, exemestane, and anastrozole. 31 The FDA approval provided a new therapy option in patients with this genetic mutation.
Future Studies and Treatment Beyond CDK4/6 Inhibitors
Ribob
New studies of the CDK4/6 inhibitors continue to be done to include different drug therapy combinations, different patient populations, and different breast cancer stages. One of these studies is RIBOB. The study began recruitment in 2019, and it is a prospective, open-label, single-arm trial on the efficacy and safety of ribociclib plus letrozole in women 70 years and older without previous hormone treatment. In this study, 150 patients will be enrolled. 32 There are few studies on older patients with other comorbidities. The study also includes a population that is prevalent in clinical practice.
EarLEE-2
Another study that is pending completion is EarLEE-2. The multicenter randomized double-blind, phase 3 placebo-controlled clinical study will test the efficacy and safety of ribociclib in patients with HR+/HER2−, intermediate risk breast cancer as adjuvant treatment with ET. Patients will receive similar treatment interventions in the EarLEE-1 clinical trial. The primary objective of the study IDFS. The start date of the study is 2018 and the estimated completion date is 2025. 33
Natalee
Another phase 3, multicenter, randomized open-label trial that started in 2018 is NATALEE. The study’s objective is to test the efficacy and safety of ribociclib in patients with HR+/HER2−, early breast cancer as adjuvant treatment with ET. Patients in the study are pre- or postmenopausal women and men 18 and up with HR+/HER2− breast cancer. The primary objective of the study is invasive disease-free survival (iDFS). In the first arm, patients received ribociclib 400 mg on days 1 to 21, followed by 7 days off and continuous ET. The second arm received ET only once daily continuously. 34 The primary results of the study revealed clinically significant longer iDFS in patients who were given ribociclib plus ET versus patients who were given ET alone (P = 0.0014). 35 This study provided breakthrough results that could expand FDA indications for ribociclib in MBC and early breast cancer.
Veritac1/veritac2
Another study that showcased a new medication that could be used after CDK4/6 inhibitor treatment in patients with ESR1 mutation was the VERITAC1/VERITAC2 trials. The phase 2 trial compared the efficacy of ARV-471 in patients with advanced HR+/HER2− advanced/MBC who received prior treatment with one or more CDK4/6 inhibitors.22,36 The results presented by Dr. Anne Schott at the San Antonio Breast Cancer Symposium revealed that the clinical benefit rate (CBR) was 37.1% in the 200 mg arm and 38.9% in the 500 mg arm. In patients with ESR1 mutation, the CBR was 47.4% in the 200 mg arm and 54.5% in the 500 mg arm. The results signified the possible treatment option of proteolysis targeting chimeras (PROTACs) engineered to degrade the estrogen receptor in HR+/HER2− breast cancer as an alternative to ET due to ERS1 mutations. 36
CAPItello-291
Another trial that showcased an alternative to SOC therapy, specifically CDK4/6 inhibitors, included capivasertib. Capivasertib works by targeting the PI3K-AKT-mTOR intracellular pathway. 37 The phase 3 study evaluated the efficacy of capivasertib, an AKT inhibitor plus fulvestrant versus placebo and fulvestrant in pre- and or postmenopausal women and adult males with metastatic or advanced HR+/HER2− breast cancer. Results of the study revealed that the PFS of the capivasertib arm was twice that of the placebo arm (7.2 months vs. 3.6 months). 37
Resistance Pathways of CDK4/6 Inhibitors
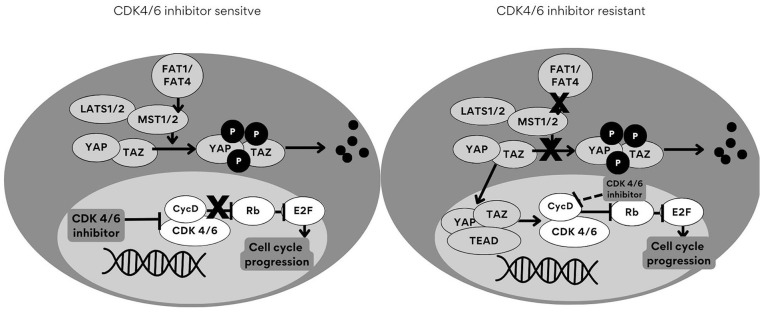
Recent discoveries of CDK4/6 inhibitor agents have allowed the PFS to double in patients with HR+/HER2− advance in breast cancer. The use of these agents has changed the approach to managing breast cancer. However, overuse of these agents leads to an increase in resistance to the inhibitors. 38 More research needs to be conducted due to the evolving nature of these pathways. Resistance is caused by the increased expression of CDK6, which then leads to a decreased response to CDK4/6 inhibitors. Although the use of CDK4/6 inhibitors has improved PFS in patients with HR+/HER2− breast cancer, studies have shown that resistance pathways can cause cells to be insensitive to CDK4/6 inhibitors, leading to continued cell proliferation. A few resistance pathways that affect CDK4/6 activity include FAT1, YAP/TAZ, and TP53.38,39 In previous studies, the loss of FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway, which regulates transcriptional coactivators. 39 A summary of the FAT1 resistance pathway and components of the Hippo signaling pathway is found in Figure 2. A study by Li et al, revealed that FAT1 loss led to CDK6 overexpression and increased minimum inhibitory concentration of CDK4/6 inhibitors by 4 to 6 folds. This was proven following the knockdown methods of CDK6. Loss of FAT1 induced CDK6 expression and increased drug insensitivity to CDK4/6 inhibitors. CDK6 knockdown restored abemaciclib’s ability to inhibit cell proliferation. 5 According to the study by Yang et al, MCF-7 cells (cell lines derived from human breast tissue) were exposed to abemaciclib (LY5219) for 21 weeks to produce cells resistant to CDK4/6 inhibitors. The cells (designated MCF-7 resistant [MR]) had a significantly higher half maximal inhibitory concentration (IC50) of (231 nm) for inhibition of proliferation compared with parental cells (27.2 nm). Parent cells and resistant cells underwent treatment with palbociclib (PD0332991) and ribociclib (LEE011) as well. In both cases, the resistant cells required significantly higher drug concentrations (6-to-8-fold) for growth suppression, as summarized in Table 2. 40 Strategies such as CRISPR/Cas9 gene editing to overcome resistance pathways are still being studied. 41
Figure 2.
FAT1 and FAT4 are involved in the inhibition of Yes-associated protein (YAP)-mediated cell proliferation. 40 FAT1/4 induces the phosphorylation of YAP and its paralog transcriptional coactivator, TAZ followed by degradation as seen on the left (CDK4/6 inhibitor sensitive pathway). When FAT1 is active, cell growth will be perturbed due to the degradation of YAP/TAZ, which are major components of the Hippo signaling pathway. 39 The loss of FAT1, as seen on the right (CDK4/6 inhibitor resistant pathway), will not have any effect on YAP/TAZ phosphorylation. The YAP/TAZ complex translocates to the nucleus and binds to TEADs (major DNA binding transcription factors), which further activate the expression of CDK6 to maintain cell proliferation.38,39
Abbreviations: CDK, cyclin-dependent kinase; FAT1, FAT atypical cadherin 1; LATS, large tumor supressor; MST, mammilian STE20-like kinase; TAZ, transcriptonal co-activator with PDZ- binding motif; TEAD, transcriptional enhanced associate domain.
Table 2.
Results From the Study by Yang et al Revealed That Cells Resistant to CDK4/6 Inhibitors had a Higher Maximal Inhibitory Concentration (IC50) Compared to Cells not Resistant to CDK4/6 Inhibitors (Parental Cells). Resistant Cells Required 6- to 8-fold Higher Drug Concentrations to Inhibit Cell Proliferation, as Summarized in the Table. 40
| Palbociclib | Ribociclib |
|---|---|
| MCF-7 (parental cells) IC50: 45.7 nM | MCF-7 IC50: 129.3 nM |
| MR (resistant cells) IC50: 359.5 nM | MR IC50: 646.6 nM |
Abbreviations: IC, inhibitory concentration; MCF-7, Michigan Cancer Foundation – 7; MR, Michigan Cancer Foundation 7 resistant.
In addition, a recent study presented at the 2022 San Antonio Breast Cancer Symposium by Guarducci revealed more information about CDK4/6 inhibitor resistance. 22 The study used DNA barcoded cell lines and determined that resistance to abemaciclib, palbociclib, and ESR1 mutations occurs due to pre-existing mutations that occur in subsets of tumor cells. The study signified the need to determine the patient’s genetic predisposition to resistance before CDK4/6 inhibitor treatment to prevent acquired resistance and failure of therapy.
Furthermore, studies on genes involved in resistance pathways have revealed that certain ethnic minorities have higher mutation rates in certain genes. Recent studies have shown African Americans with head and neck squamous cell carcinoma have worse prognosis compared to their Caucasian counterparts, even with adjusting for socioeconomic factors. 42 A deeper dive into African American genomics and immunologic factors reveals that there is a higher mutation in their FAT1, CASP8, and HRAS genes compared to their white counterparts. In addition, African Americans exhibited low infiltration effector immune cells with shorter survival rates than whites. 42
Conclusion
The FDA approval of palbociclib in 2016 led to various studies of CDK4/6 inhibitors that changed the treatment of breast cancer. In addition, clinical studies on the use of CDK4/6 inhibitors are underway in the treatment of head and neck cancer and other cancers due to the crosstalk of CDK4/6 and other signaling pathways in cancer. Studies completed with investigational drugs have expanded the possible alternatives to CDK4/6 inhibitors. Knowledge of CDK4/6 inhibitors and their resistance pathways and the prevalence of certain genes that affect resistance in ethnic minorities compared to Caucasian counterparts can help improve patient outcomes in breast cancer patients, increase PFS rates and close the gap in health disparities.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kristen J. Asprer  https://orcid.org/0009-0003-0336-3691
https://orcid.org/0009-0003-0336-3691
References
- 1. Goel S, DeCristo MJ, McAllister SS, Zhao JJ. CDK4/6 inhibition in cancer: beyond cell cycle arrest. Trends in Cell Biol. 2018;28(11):911-925. doi: 10.1016/j.tcb.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rhind N, Russell P. Signaling pathways that regulate cell division. Cold Spring Harb Perspect Biol. 2012;4(10):a005942. doi: 10.1101/cshperspect.a005942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding L, Cao J, Lin W, et al. The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. Int J Mol Sci. 2020;21(6):1960. doi: 10.3390/ijms21061960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doan A, Arand J, Gong D, et al. RB depletion is required for the continuous growth of tumors initiated by loss of RB. PLOS Genet. 2021;17(12):e1009941. doi: 10.1371/journal.pgen.1009941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huppert LA, Gumusay O, Idossa D, Rugo HS. Systemic therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative early stage and metastatic breast cancer. CA Cancer J Clin. 2023;73:480-515. doi: 10.3322/caac.21777 [DOI] [PubMed] [Google Scholar]
- 6. Li Z, Zou W, Zhang J, et al. Mechanisms of CDK4/6 inhibitor resistance in luminal breast cancer. Front Pharmacol. 2020;11:580251. doi: 10.3389/fphar.2020.580251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Z, Razavi P, Li Q, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell. 2018;34(6):893-905.e8. doi: 10.1016/j.ccell.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niu Y, Xu J, Sun T. Cyclin-dependent kinases 4/6 inhibitors in breast cancer: current status, resistance, and combination strategies. J Cancer. 2019;10(22):5504-5517. doi: 10.7150/jca.3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev. 2006;20(18):2513-2526. doi: 10.1101/gad.1446006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925-1936. doi: 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 11. Cristofanilli M, Rugo HS, Im S, et al. Overall survival (OS) with palbociclib (PAL) + fulvestrant (FUL) in women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC): updated analyses from PALOMA-3. J Clin Oncol. 2021;39(15):1000. doi: 10.1200/JCO.2021.39.15_suppl.1000 [DOI] [Google Scholar]
- 12. Cristofanilli M, Rugo HS, Im SA, et al. Overall survival with palbociclib and fulvestrant in women with HR+/HER2- ABC: updated exploratory analyses of PALOMA-3, a double-blind, phase III randomized study. Clin Cancer Res. 2022;28(16):3433-3442. doi: 10.1158/1078-0432.CCR-22-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yardley DA, Hart L, Favret A, et al. Efficacy and safety of ribociclib with letrozole in US patients enrolled in the MONALEESA-2 study. Clin Breast Cancer. 2019;19(4):268-277.e1. doi: 10.1016/j.clbc.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 14. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465-2472. doi: 10.1200/JCO.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 15. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904-915. doi: 10.1016/S1470-2045(18)30292-4(12) [DOI] [PubMed] [Google Scholar]
- 16. De Laurentiis M, Borstnar S, Campone M, et al. Full population results from the core phase of CompLEEment-1, a phase 3b study of ribociclib plus letrozole as first-line therapy for advanced breast cancer in an expanded population [published correction appears in Breast Cancer Res Treat. 2021 Oct 8]. Breast Cancer Res Treat. 2021;189(3) 689-699. doi: 10.1007/s10549-021-06334-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adjuvant ribociclib with endocrine therapy in hormone receptor+/HER2- high risk early breast cancer (EarLEE-1). ClinicalTrials.gov; identifier: NCT03078751. Updated October 11, 2021. Accessed July 20, 2022. https://clinicaltrials.gov/ct2/show/results/NCT03078751?view=results EarLEE [Google Scholar]
- 18. Prat A, Saura C, Pascual T, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):33-43. doi: 10.1016/S1470-2045(19)30786-7 [DOI] [PubMed] [Google Scholar]
- 19. Rauh S, ed. SABCS 2022: San Antonio Breast Cancer Symposium. MediCom Medical Publishers; 2023. Accessed March 1, 2023. https://conferences.medicom-publishers.com/wp-content/uploads/2023/02/E_MCR-SABCS-2022.pdf [Google Scholar]
- 20. Palbociclib after CDK and endocrine therapy (PACE)—full text view- ClinicalTrials.gov. Accessed March 28, 2023. https://clinicaltrials.gov/ct2/show/NCT03147287.
- 21. Study to compare the combination of ribociclib plus goserelin acetate with hormonal therapy versus combination chemotherapy in premenopausal or perimenopausal patients with advanced or metastatic breast cancer—full text view. ClinicalTrials.gov. Accessed March 28, 2023. https://www.clinicaltrials.gov/ct2/show/NCT03839823 [Google Scholar]
- 22. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer [published correction appears in Clin Cancer Res. 2018;24(21):5485]. Clin Cancer Res. 2017;23(17): 5218-5224. doi: 10.1158/1078-0432.CCR-17-0754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sledge GW, Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884. doi: 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 24. Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646. doi: 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 25. Zhang QY, Sun T, Yin YM, et al. MONARCH plus: abemaciclib plus endocrine therapy in women with HR+/HER2- advanced breast cancer: the multinational randomized phase III study. Ther Adv Med Oncol. 2020;12:1758835920963925. doi: 10.1177/1758835920963925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987-3998. doi: 10.1200/JCO.20.02514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32(12):1571-1581. doi: 10.1016/j.annonc.2021.09.015 [DOI] [PubMed] [Google Scholar]
- 28. Phase 3 trial of elacestrant vs. standard of care for the treatment of patients with ER+/HER2- Advanced Breast Cancer. ClinicalTrials.gov. Accessed September 25, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT03778931
- 29. Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246-3256. doi: 10.1200/JCO.22.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Center for Drug Evaluation and Research. FDA approves elacestrant for ER-positive HER2-negative, ESR1-mutated advanced or metastatic breast cancer. U.S. Food and Drug Administration. Published 2023. Accessed February 10, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer [Google Scholar]
- 31. Brett JO, Spring LM, Bardia A, et al. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23:85. doi: 10.1186/s13058-021-01462-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kenis C, Ponde NF, Decoster L, et al. 385TiP—RIBOB: a study on the efficacy and safety of ribociclib in combination with letrozole in older women (≥70 years) with hormone receptor-positive (HR+) HER2-negative (HER2-) advanced breast cancer (ABC) with no prior systemic therapy for advanced disease. Ann Oncol. 2019;30(5):v140. 10.1093/annonc/mdz242.080 [DOI] [Google Scholar]
- 33. Adjuvant ribociclib with endocrine therapy in hormone receptor+/HER2- intermediate risk early breast cancer (EarLEE-2). ClinicalTRials.gov; identifier: NCT03081234. Updated March 15, 2018. Accessed July 22, 2022. https://classic.clinicaltrials.gov/ct2/show/NCT03081234 [Google Scholar]
- 34. A phase III multi-center, randomized, open-label trial to evaluate efficacy and safety of ribociclib with endocrine therapy as an adjuvant treatment in patients with hormone receptor-positive, HER2-negative early breast cancer (New Adjuvant TriAl with Ribociclib [LEE011]: NATALEE). Clinicaltrials.gov. Published February 2022. Accessed July 25, 2022. https://clinicaltrials.gov/ct2/show/NCT03701334
- 35. Slamon DJ, Stroyakovskiy D, Yardley DA, et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: Primary results from the phase III NATALEE trial. J Clin Oncol. 2023;41(17):LBA500. doi: 10.1200/JCO.2023.41.17_suppl.LBA500 [DOI] [Google Scholar]
- 36. Berberabe T. Clinical benefit for ARV-471 continues to impress in expansion cohort in ER+/HER2– breast cancer. Targeted Oncology. Accessed March 6, 2023. https://www.targetedonc.com/view/clinical-benefit-for-arv-471-continues-to-impress-in-expansion-cohort-in-er-her2-breast-cancer
- 37. Capivasertib+fulvestrant vs placebo+fulvestrant as treatment for locally advanced (inoperable) or metastatic HR+/HER2- breast cancer (CAPItello-291). ClinicalTrials.gov; identifier: NCT04305496. Updated February 2, 2023. Accessed March 3, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT04305496 [Google Scholar]
- 38. McCartney A, Migliaccio I, Bonechi M, et al. Mechanisms of resistance to cdk4/6 inhibitors: potential implications and biomarkers for clinical practice. Front Oncol. 2019;9:666. doi: 10.3389/fonc.2019.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li R, Shao J, Jin YJ, et al. Endothelial FAT1 inhibits angiogenesis by controlling YAP/TAZ protein degradation via E3 ligase MIB2. Nat Commun. 2023;14(1):1980. doi: 10.1038/s41467-023-37671-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang C, Li Z, Bhatt T, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36(16):2255-2264. doi: 10.1038/onc.2016.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vaghari-Tabari M, Hassanpour P, Sadeghsoltani F, et al. CRISPR/Cas9 gene editing: a new approach for overcoming drug resistance in cancer. Cell Mol Biol Lett. 2022;27:49. doi: 10.1186/s11658-022-00348-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chaudhary S, Dam V, Ganguly K, et al. Differential mutation spectrum and immune landscape in African Americans versus Whites: a possible determinant to health disparity in head and neck cancer. Cancer Let. 2020;492:44-53. doi: 10.1016/j.canlet.2020.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]