Abstract
Background: Direct oral anticoagulants (DOACs) are known to have similar efficacy with a decreased risk of bleeding when compared to warfarin for the treatment of venous thromboembolism (VTE). In patients with obesity, there are limited data regarding the safety and efficacy of DOACs. Despite concerns for both under- and over-dosing patients with extremes of body weight, there are no dose adjustment recommendations in the package inserts for any of the DOACs. Objective: To evaluate the safety and efficacy of DOACs versus warfarin for the treatment of VTE in patients with obesity. Methods: This single-center, retrospective cohort study included obese patients initiated on DOAC or warfarin therapy for VTE from January 2015 to January 2022. Patients with cancer, hypercoagulable disorders, end-stage kidney disease, or pregnancy were excluded. The primary endpoint was VTE recurrence. Secondary endpoints included major and minor bleeding. Results: A total of 120 patients met criteria for inclusion. Ninety-two received DOAC therapy and 28 received warfarin. The primary endpoint occurred in 4 patients in the DOAC group and 3 patients in the warfarin group (P = 0.35). Major bleeding occurred in 2 patients. Minor bleeding events occurred in 10 (8.33%) patients. Of those, 6 (6.5%) events occurred in patients receiving a DOAC and 4 (14.3%) events occurred in patients receiving warfarin (P = 0.28). Limitations of this study include the retrospective single-center study design. Conclusions: There was a comparable risk of bleeding and recurrent VTE between DOACs and warfarin in patients initiated on therapy for VTE.
Keywords: DOACs, apixaban, rivaroxaban, obesity, venous thromboembolism
Introduction
Venous thromboembolism (VTE) is a prevalent condition that affects approximately 600,000 people each year in the United States.1,2 Venous thromboembolism events cause significant morbidity and mortality with mortality rates increasing over the last decade.1 -3 Oral anticoagulation therapy is effective in treating VTE but carries a significant risk of bleeding. Therefore, appropriate treatment is essential.
Obesity is a consistent and well-documented risk factor for VTE with the event rates increasing as BMI increases.4,5 Obesity contributes to the development of VTE via chronic inflammation and impaired fibrinolysis that leads to a prothrombotic state. 6
Direct oral anticoagulants (DOACs) are known to have similar efficacy but a decreased risk of bleeding when compared to warfarin for the treatment of VTE. 7 However, there are few studies describing the safety and efficacy of DOACs for the treatment of VTE in patients with obesity. Pharmacokinetic and pharmacodynamics studies of DOACs in the obese patient population have reported a modest effect on reduced drug exposure and lower peak concentrations; however, the clinical significance of this is unknown.8,9 Initial trials demonstrating efficacy of DOACs for the treatment of VTE consistently enrolled few patients who weighed more than 100 kg. Despite an overall lack of data and concerns for both under- and over-dosing patients with extremes of body weight, there are no dose adjustment recommendations in the package inserts for any of the DOAC analogs.10 -13
The International Society on Thrombosis and Hemostasis (ISTH) 2016 guidelines recommended against the use of DOACs in patients with extreme obesity (body mass index [BMI] > 40 kg/m2 or weight > 120 kg) with the stipulation that peak and trough drug levels should be obtained if DOACs were to be used in these patients. 14 These recommendations were based on the paucity of data regarding the safety and efficacy of DOACs in patients with extreme obesity. In 2021, the ISTH published updated guidance which now recommends the use of standard doses of apixaban and rivaroxaban for the treatment of VTE in patients regardless of BMI or weight. 15 The guidelines also now recommend against monitoring peak and trough levels due to insufficient data to influence treatment decisions accordingly.
The severely obese (BMI > 40 kg/m2) population is underrepresented even in studies of obesity. By targeting only patients with a BMI > 40, this study seeks to add significant numbers to the existing literature in this population. Therefore, the purpose of this study was to determine the safety and efficacy of DOACs for the treatment of VTE in patients with severe obesity.
Methods
Study Population
This was a single-center, retrospective study conducted at a 505-bed community teaching hospital between January 2015 and July 2022. This study was approved by the institutional review board and conducted according to their requirements. Electronic medical records of obese patients who were treated with oral anticoagulation for acute VTE were reviewed. Patients were included if they were at least 19 years of age, treated with a DOAC or warfarin for VTE, and had a BMI greater than or equal to 40 kg/m2 or weight greater than 120 kg. Patients were excluded from the study if they had cancer, a hypercoagulable disorder, end-stage kidney disease (ESKD), a glomerular filtration rate of ≤15 mL/min/1.73 m2, were on anticoagulation therapy at the time of index admission, or were pregnant. Patients were contacted via telephone if the necessary information was not able to be extracted from chart review alone. Patients were deemed lost to follow-up if they were unable to be contacted after three attempts or had invalid contact information.
Outcomes
The primary outcome was the occurrence of VTE within 12 months of the index admission date while on VTE treatment. Secondary outcomes included the occurrence of pulmonary embolism (PE), deep vein thrombosis (DVT), major bleeding, and minor bleeding. Major bleeding was defined as fatal bleeding, symptomatic bleeding in a critical area or organ, or a drop in hemoglobin of greater than or equal to 2 mg/dL or requiring greater than or equal to 2 units of packed red blood cells. Minor bleeding was defined as any bleeding event not classified as major bleeding.
Statistical Analysis
Categorical data were represented with descriptive statistics. The chi-square test was used to evaluate differences in the primary and secondary endpoints with a P < 0.05 indicating statistical significance. Owing to the limited number of expected cases, a power analysis was not completed.
Results
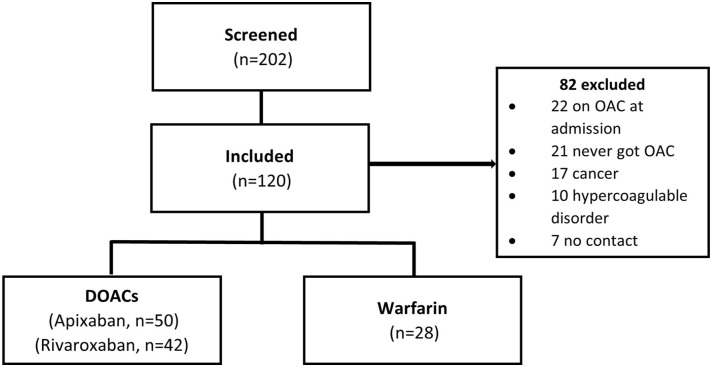
A total of 202 patients with acute VTE on oral anticoagulation met the criteria for screening (Figure 1). Eighty-two patients were excluded. The reasons for exclusion were the presence of oral anticoagulation on admission (22), never receiving oral anticoagulation (21), concomitant cancer (17), hypercoagulable disorder (10), lost to follow-up (7), and ESKD (5). A total of 120 patients met inclusion criteria for the study. Baseline characteristics are shown in Table 1. There were 92 patients in the DOAC treatment group and 28 patients in the warfarin treatment group. Of those in the DOAC treatment group, 50 received apixaban, 42 received rivaroxaban, and no patients received edoxaban, betrixaban, or dabigatran. Overall, the mean age was 54.5 years, 64.2% were black, and 45% were male.
Figure 1.
Patients evaluated for inclusion.
Abbreviations: DOAC, direct oral anticoagulant; OAC, oral anticoagulant.
Table 1.
Baseline Characteristics.
| Characteristic | Apixaban (n = 50) | Rivaroxaban (n = 42) | Warfarin (n = 28) |
|---|---|---|---|
| Age (years), mean ± SD | 56.5 ± 14.1 | 53.7 ± 14.0 | 51.9 ± 14.8 |
| Male, n (%) | 18 (36) | 12 (28.6) | 15 (53.5) |
| Race, n (%) | |||
| African American | 35 (70) | 26 (61.9) | 16 (57.1) |
| Weight (kg), mean ± SD | 141.1 ± 19.4 | 141.2 ± 16.6 | 158.2 ± 28.9 |
| BMI (kg/m2), mean ± SD | 47.5 ± 6.5 | 46.3 ± 5.3 | 54.8 ± 12.1 |
| 40.0-49.9 kg/m2, n (%) | 39 (78) | 32 (76.2) | 13 (46.4) |
| 49.9-50.0 kg/m2, n (%) | 8 (16) | 10 (23.8) | 8 (28.6) |
| ≥50.0 kg/m2, n (%) | 3 (6) | 0 (0) | 7 (25) |
| Serum creatinine (mg/dL) – admission, mean ± SD | 1.26 ± 0.9 | 1.14 ± 0.52 | 1.90 ± 1.81 |
| Serum creatinine (mg/dL) – discharge, mean ± SD | 1.04 ± 0.53 | 0.97 ± 0.36 | 1.57 ± 1.36 |
| Chronic kidney disease, n (%) | 11 (22) | 7 (16.6) | 8 (28.5) |
| History of VTE, n (%) | 6 (13.6) | 11 (26.2) | 5 (17.8) |
| History of atrial fibrillation, n (%) | 2 (4) | 2 (4.76) | 2 (0.7) |
Abbreviations: BMI, body mass index; SD, standard deviation; VTE, venous thromboembolism.
The mean weight was 141 kg in the patients receiving DOAC therapy and 158 kg in the patients receiving warfarin. The mean BMI was approximately 47 kg/m2 in patients receiving DOAC therapy and 54 kg/m2 in patients receiving warfarin. Approximately 25% of patients in the warfarin group had a BMI of ≥50 kg/m2. Twenty-six patients (21.6%) had a history of chronic kidney disease, 22 patients (18.3%) had a history of VTE, and 6 patients (5%) had a history of atrial fibrillation.
Venous thromboembolism recurrence occurred in 4 patients (4.35%) in the DOAC group and in 3 patients (10.71%) in the warfarin group (P = 0.35) (Table 2). Of the patients receiving DOAC therapy, 3 of the events occurred in patients receiving apixaban, and 1 event occurred in a patient receiving rivaroxaban. Recurrent DVT occurred in 6 (5%) patients. Two (4%) patients were receiving apixaban, 1 (2.38%) patient was receiving rivaroxaban, and 3 (10.7%) patients were receiving warfarin. Recurrent PE occurred in 1 (2%) patient, and that patient was receiving apixaban. Major bleeding events occurred in 2 (1.67%) patients. Of those, 1 (0.83%) patient was receiving rivaroxaban and 1 (3.57%) patient was receiving warfarin (p = 0.41). The patient receiving rivaroxaban experienced a gastrointestinal bleed requiring 3 units of packed red blood cells, and the patient receiving warfarin (INR 2.8) experienced gross hematuria requiring multiple blood transfusions and surgical intervention. Minor bleeding events occurred in 10 (8.33%) patients. Of those, 6 (6.5%) events occurred in patients receiving a DOAC and 4 (14.3%) events occurred in patients receiving warfarin (P = 0.28). The international normalized ratio (INR) ranged from 2.4 to 7.6 in patients with minor bleeding. The most common location of minor bleeding was gastrointestinal (6), followed by uterine (2), vaginal (1), and hematoma (1).
Table 2.
Efficacy and Safety Results.
| Outcome | DOACs (n = 92) |
Warfarin (n = 28) |
P value |
|---|---|---|---|
| VTE recurrence, n (%) | 4 (4.4) | 3 (10.7) | 0.35 |
| DVT occurrence, n (%) | 3 (3.2) | 3 (10.7) | – |
| PE occurrence, n (%) | 1 (1.1) | 0 (0) | – |
| Major bleeding event, n (%) | 1 (1.1) | 1 (3.6) | 0.41 |
| Minor bleeding event, n (%) | 6 (6.5) | 4 (14.3) | 0.28 |
Abbreviations: DOACs, direct oral anticoagulants; DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Discussion
In this retrospective study, using DOACs for the treatment of VTE in patients with severe obesity did not result in an increased incidence of VTE recurrence when compared to warfarin. There was also no significant difference in major and minor bleeding events between groups. These findings are consistent with previous investigations into the use of DOACs in severe obesity. This article adds an additional cohort to studies previously conducted in this population.
To date, there have been no randomized controlled trials examining the efficacy and safety of DOACs in obese and morbidly obese patients. Therefore, the use of DOACs in this patient population has been guarded. A systematic review comprised of five observational studies (6585 patients) in morbidly obese patients (body weight >120 kg or BMI >40 kg/m2) found DOAC analogs to be noninferior to warfarin for the primary efficacy outcome of VTE recurrence (odds ratio [OR]: 1.07, 95% confidence interval [CI]: 0.93-1.23) and the primary safety outcome of major bleeding (OR: 0.80, 95% CI: 0.54-1.17). 16
A retrospective matched cohort study of 1840 patients with a primary admission diagnosis of acute VTE who were treated with either a DOAC (apixaban, dabigatran, or rivaroxaban [632 patients] or warfarin [1208 patients]) and who had a body weight greater than 100 kg and less than 300 kg, found no significant difference in the rate of VTE recurrence (6.5% vs 6.4%, P = 0.93) between groups. In addition, the authors found no significant difference in the occurrence of PE and DVT between the DOAC and warfarin groups (3.7% vs 3.8%, p = 0.94, and 3% vs 3.5%, p = 0.56, respectively). Bleeding occurred in 1.7% of patients in the DOAC group and in 1.2% of patients in the warfarin group (p = 0.31). The authors concluded that DOACs should be considered a reasonable alternative to warfarin for the treatment of acute VTE in obese patients. 17
Perino et al conducted a retrospective cohort study including 51 871 Veterans Health Administration patients with first-time VTE from 2013 to 2018 that were treated with a DOAC or warfarin. Patients were stratified by weight and BMI and included 4227 patients with BMI of 40 kg/m2 or greater. The authors found that DOAC prescriptions were not associated with major bleeds, clinically relevant nonmajor bleeds, or recurrent VTE in patients with a higher weight and BMI when compared to warfarin. 18
The findings of the aforementioned studies align with those of this study. Limitations of this study include a small sample size at a single center and those inherent to a retrospective study design, including reliance on accurate electronic medical record documentation. In addition, the inability to determine time spent in therapeutic range for patients on warfarin may have affected results. Finally, there was a higher average BMI in the warfarin group compared to the DOAC group. This is likely due to prescribers preferring warfarin in patients at the very extremes of weight and that confidence with using DOACs in obese patients developed over time. It is unlikely this difference in weight impacted the outcomes, as all patients were severely obese.
Conclusion
Patients treated with DOAC therapy for VTE were found to have similar rates of recurrent VTE and major and minor bleeding within 1 year compared to patients treated with warfarin. While a randomized, prospective trial is greatly needed for this population, this study adds to and supports the findings of previous investigations in the severely obese population.
Footnotes
Authors’ Note: The methodology and preliminary results of this study were presented at the poster session of the 2023 ASHP Midyear Clinical Meeting, Las Vegas, NV.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Quinn Hattaway  https://orcid.org/0000-0003-0061-6895
https://orcid.org/0000-0003-0061-6895
Jessica A. Starr  https://orcid.org/0000-0003-2704-4372
https://orcid.org/0000-0003-2704-4372
Nathan A. Pinner  https://orcid.org/0000-0003-1938-5951
https://orcid.org/0000-0003-1938-5951
References
- 1. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi: 10.1038/nrcardio.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501. doi: 10.1016/j.amepre.2009.12.017 [DOI] [PubMed] [Google Scholar]
- 3. Martin KA, Molsberry R, Cuttica MJ, Desai KR, Schimmel DR, Khan SS. Time trends in pulmonary embolism mortality rates in the United States, 1999 to 2018. J Am Heart Assoc. 2020;9(17):e016784. doi: 10.1161/JAHA.120.016784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93-102. doi: 10.1161/circulationaha.107.709204 [DOI] [PubMed] [Google Scholar]
- 5. Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen city heart study. Circulation. 2010;121(17):1896-1903. doi: 10.1161/CIRCULATIONAHA.109.921460 [DOI] [PubMed] [Google Scholar]
- 6. Blokhin IO, Lentz SR. Mechanisms of thrombosis in obesity. Curr Opin Hematol. 2013;20(5):437-444. doi: 10.1097/MOH.0b013e3283634443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510. doi: 10.1056/NEJMoa1007903 [DOI] [PubMed] [Google Scholar]
- 8. Upreti VV, Wang J, Barrett YC, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76(6):908-916. doi: 10.1111/bcp.12114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alalawneh M, Awaisu A, Rachid O. Rivaroxaban pharmacokinetics in obese subjects: a systematic review. Clin Pharmacokinet. 2022;61(12):1677-1695. doi: 10.1007/s40262-022-01160-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eliquis (apixaban) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2019. Accessed August 4, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202155s024lbl.pdf [Google Scholar]
- 11. Pradaxa (dabigatran) [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2020. Accessed August 4, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022512s039lbl.pdf [Google Scholar]
- 12. Savaysa (edoxaban) [package insert]. Basking Ridge, NJ: Daiichi Sankyo, Inc; 2020. Accessed August 4, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/206316s016lbl.pdf [Google Scholar]
- 13. Xarelto (rivaroxaban) [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2021. Accessed August 4, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022406s036,202439s036lbl.pdf [Google Scholar]
- 14. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313. doi: 10.1111/jth.13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin KA, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC subcommittee on control of anticoagulation. J Thromb Haemost. 2021;19(8):1874-1882. doi: 10.1111/jth.15358 [DOI] [PubMed] [Google Scholar]
- 16. Elshafei MN, Mohamed MFH, El-Bardissy A, et al. Comparative effectiveness and safety of direct oral anticoagulants compared to warfarin in morbidly obese patients with acute venous thromboembolism: systematic review and a meta-analysis. J Thromb Thrombolysis. 2021;51(2):388-396. doi: 10.1007/s11239-020-02179-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coons JC, Albert L, Bejjani A, Iasella CJ. Effectiveness and safety of direct oral anticoagulants versus warfarin in obese patients with acute venous thromboembolism. Pharmacotherapy. 2020;40(3):204-210. doi: 10.1002/phar.2369 [DOI] [PubMed] [Google Scholar]
- 18. Perino AC, Fan J, Schmitt S, et al. Anticoagulation treatment and outcomes of venous thromboembolism by weight and body mass index: insights from the veterans health administration. Circ Cardiovasc Qual Outcomes. 2021;14(11):e008005. doi: 10.1161/circoutcomes.121.008005 [DOI] [PubMed] [Google Scholar]