Abstract
Shiga toxin-converting bacteriophages are involved in the pathogenicity of some enteric bacteria, such as Escherichia coli O157:H7, but data on the occurrence and distribution of such phages as free particles in nature were not available. An experimental approach has been developed to detect the presence of the Shiga toxin 2 (Stx 2)-encoding bacteriophages in sewage. The Stx 2 gene was amplified by PCR from phages concentrated from 10-ml samples of sewage. Moreover, the phages carrying the Stx 2 gene were detected in supernatants from bacteriophage enrichment cultures by using an Stx 2-negative E. coli O157:H7 strain infected with phages purified from volumes of sewage as small as 0.02 ml. Additionally, the A subunit of Stx 2 was detected in the supernatants of the bacteriophage enrichment cultures, which also showed cytotoxic activity for Vero cells. By enrichment of phages concentrated from different volumes of sewage and applying the most-probable-number technique, it was estimated that the number of phages infectious for E. coli O157:H7 and carrying the Stx 2 gene was in the range of 1 to 10 per ml of sewage from two different origins. These values were approximately 1% of all phages infecting E. coli O157:H7.
Bacterial virulence factors such as toxins are often encoded by bacteriophages. Among other examples, factors encoded by phages have been described in some of the emerging or reemerging pathogens, including the pyrogenic exotoxin A production in group A streptococci (16), the cholera toxin production in Vibrio cholerae (38), and enterotoxin production in enterohemorrhagic Escherichia coli (EHEC) strains (25, 33). Importantly, different serotypes of E. coli, including O157:H7, and a few non-E. coli species of enteric bacteria produce enterotoxins and cause serious human diseases (3, 11, 17). E. coli O157:H7 and other EHEC strains can produce cytotoxins that closely resemble the toxins produced by Shigella dysenteriae type 1 strains. These E. coli serotypes produce at least two immunologically distinct Shiga toxins, designated 1 (Stx 1) and 2 (Stx 2), which are encoded by phages (25, 33). Phages that contain the structural genes for Stx 1 and Stx 2 have been isolated from O157 strains, and their morphologies, genome sizes, and restriction fragment length polymorphisms have been characterized (24, 29, 36). However, there is still a lack of knowledge regarding the role of O157 phages in the epidemiology and pathogenesis of EHEC O157 infections. Little is known about the actual incidence of active gene transfer by phages in natural conditions, although transduction has been shown to occur at significant rates in simulated natural conditions (32), and it has recently been shown that transduction may be even greater in natural conditions than in the laboratory (22, 38). Thus, there are reasons to believe that transduction and consequent phage conversion between native bacteria occurs in natural environments including, for example, food and water, both of which are associated with the transmission of pathogens such as E. coli O157:H7 (21). It may also be theoretically possible that ingestion of phages themselves, either by humans or by animals, can cause infection of the existing E. coli flora. To determine the role of phages in the transfer of Stx genes in natural environments or in the human intestinal tract, more information on the abundance of phages carrying such genes in natural environments such as contaminated water and food is needed. We present data here on the prevalence of phages carrying the gene coding for Stx 2 in sewage.
MATERIALS AND METHODS
Bacterial strains, bacteriophages, and media.
The bacterial strains used in this study were as follows. E. coli O157:H7 ATCC 43888, which does not produce either Shiga toxin 1 or 2 and does not possess the genes for these toxins, was used as a negative control in all experiments. E. coli O157:H7 ATCC 43889, which produces Shiga toxin 2, and bacteriophage 933W were used as positive controls. Bacteriophage 933W was obtained by lysogenic induction with mitomycin C of E. coli C600(933W) as indicated by Mühldorfer et al. (23). E. coli CN13 (26) was used for the detection of somatic coliphages.
Tryptic soy agar was used to grow bacteria for bacterial DNA extraction. Modified Scholten’s broth (10 g of peptone, 3 g of yeast extract, 12 g of meat extract, 3 g of NaCl, 0.7 g of Na2CO3, and 1.25 mmol of magnesium in final volume of 1 liter) or Modified Scholten’s agar were used for the enrichment and detection of phages infecting the different strains of E. coli (12).
Bacterial enumeration.
The enumeration of fecal coliforms present in the sewage samples studied was performed according to standard methods (2).
Bacteriophage enumeration.
Somatic coliphages, defined as those infecting strain E. coli CN13 (26), and bacteriophages infecting strain ATCC 43888 of E. coli O157:H7 were enumerated by the double-agar-layer method described by Adams (1).
Sewage samples.
This study was performed with sewage samples collected during a 12-month period (January 1996 to January 1997). Raw sewage samples were collected from the influent raw urban sewage at two different wastewater treatment plants. Treatment plant 1 serves a large population of approximately 1,400,000 inhabitants, and treatment plant 2 receives sewage from a population of approximately 400,000 inhabitants. No remarkable incidence of enterocolitis was reported during the study period in these areas.
Enrichment cultures.
In order to determine the presence in sewage of bacteriophages carrying the Stx 2 gene that were detectable by DNA amplification and able to infect E. coli O157:H7, bacteriophage enrichment cultures were prepared as follows. Sewage samples were centrifuged at 12,000 × g for 30 min to remove particulate material, filtered through a 0.22-μm-pore-size low-protein-binding membrane (Millex-GV; Millipore Corp.) to eliminate bacteria, and then treated, as indicated below, with DNase to remove the free DNA. Then volumes of 100, 10, 1, 0.2, 0.1, 0.02, 0.01, and 0.001 ml were added to 100-ml liquid cultures of logarithmic-growth-phase strain ATCC 43888 of E. coli O157:H7 (which does not possess the gene for the Stx 2) that contained approximately 2 × 108 cells per ml. Then, culture medium was added to a final volume of 250 ml. After overnight incubation, aliquots of the cultures were centrifuged at 12,000 × g for 30 min. Next, the presence of the Stx 2 gene sequence in the bacteriophages in the supernatants of these cultures was determined. Some of the supernatants were also tested for the presence of the subunit A of Stx 2 and also for the presence of Vero cell cytotoxicity.
Purification of bacteriophages from sewage and from supernatants of bacteriophage enrichment cultures.
Bacteriophages were recovered from sewage and partially purified as follows. First, 100-, 10-, and 1-ml samples of sewage were centrifuged for 3 h at 48,000 × g. The pellet was resuspended in 0.25 N glycine buffer (pH 9.5), shaken at 4°C for 30 min to disrupt the bacteriophage clumps, buffered to pH 7.4 with double-concentrated phosphate-buffered saline (PBS), and again centrifuged at 12,000 × g for 20 min. The supernatant was centrifuged for an additional 1 h at 171,000 × g. The pellet was then resuspended in 100 μl of PBS. To ensure that recovered DNA belonged exclusively to the bacteriophages and was not free DNA, the resuspended phages were treated with 10 U of DNase per ml for 30 min as described by Sambrook et al. (31). In addition, in the first 10 tests the phage suspension was divided into two portions after DNA digestion. The bacteriophages in one of the portions were further purified by CsCl centrifugation at 60,000 × g. The band in which we expected the bacteriophages (8), which corresponded to a density of 1.45 ± 0.02 g·ml−1, was collected and dialyzed to remove the CsCl. Bacteriophage enumeration was performed to verify that phages were present at the above-mentioned densities. The two subsamples were then processed as described below for DNA extraction and amplification. No differences were observed in the results for the two subsamples with respect to PCR amplification. Therefore, CsCl centrifugation was considered unnecessary and thus was not applied after the first 10 experiments.
The bacteriophages present in the supernatants of the bacteriophage enrichment cultures were obtained as follows. Supernatants were first filtered through a 0.22-μm-pore-size low-protein-binding membrane and then treated with 10 U of DNase per ml for 30 min according to the method of Sambrook et al. (31).
DNA extraction.
DNA was extracted from bacteriophages as described by Sambrook et al. (31). Briefly, proteinase K digestion at a final concentration of 50 μg·ml−1 was performed, followed by an extraction with phenol-cloroform-isoamyl alcohol (25:24:1), and the DNA was then recovered by precipitation with ethanol. The precipitated DNA was dried and redissolved in 20 μl of doubly distilled water; 1 μl of the DNA solution was then used for the PCR amplification.
Chromosomal DNA from bacterial controls was extracted as described by Hofmann and Brian (13). Briefly, a colony grown on tryptic soy agar was suspended in 25 μl of a solution containing 2.5 μl of 10× PCR Buffer II (Gene-AMP; Perkin-Elmer), 2 μl of MgCl2 solution, and 20.5 μl of double-distilled sterile water. The suspension was incubated at 95°C for 15 min and immediately transferred to ice-cold absolute alcohol. After centrifugation at 16,000 × g for 5 min, 1 μl of the supernatant was used for the amplification.
PCR DNA amplification.
First-round PCR DNA amplification for specific detection of the Stx 2 gene was performed with the Amplitaq DNA Polymerase Kit (Perkin-Elmer) according to the instructions of the manufacturer. Two primers were selected from the DNA sequence of the Stx 2 gene, aligned with previously published sequences, and evaluated against the sequences of the EMBL gene data bank by the FastA program of the Genetics Computer Group package (7). The primers were as follows: upper primer, 5′-GCGTTTTGACCATCTTCGT-3′; and lower primer, 5′-ACAGGAGCAGTTTCAGACAG-3′. Both primers were synthesized by MWG Biotech (Boehringer Mannheim). Briefly, the conditions for amplification were 1 cycle at 94°C; 35 cycles at 94°C for 1.5 min, 55°C for 1 min, and 72°C for 1 min; and finally 1 cycle at 72°C for 4 min. Then 5 μl of the amplified DNA mixture was analyzed for amplification products by gel electrophoresis on 2% agarose and stained with ethidium bromide.
Nested PCR was performed according to the instructions of the manufacturer (Perkin-Elmer). Internal primers were selected as described above for the external ones. The primers were as follows: upper primer, 5′-TAATACGGCAACAAATACT-3′; and lower primer, 5′-TGATGAAACCAGTGAGTGA-3′. A 1-μl portion of the first-round PCR was used for the nested PCR. The amplification program was as described above but with an annealing temperature of 50°C. Amplified DNA mixture (5 μl) was analyzed for amplification products by gel electrophoresis on 2% agarose and stained with ethidium bromide.
Hybridization.
Hybridization to confirm PCR results was performed by both dot blotting (5) and Southern blotting (35) of the amplification mixture. The internal probe for hybridization was selected from the DNA sequence of the Stx 2 gene, aligned with previously published sequences, and evaluated against the sequences of the EMBL gene data bank as described above (14). The sequence of the probe is 5′-ATGACAACGGACAGCAGTTATACCAC-3′. The probe was synthesized by MWG Biotech and labeled with [γ-32P]ATP by using T4 kinase according to the method of Sambrook et al. (31).
Sequencing.
Further confirmation of the nature of the amplified DNA of some of the samples was achieved by sequencing. Amplified DNA products from the nested PCR experiments were sequenced by using the ABI PRISM 377 DNA Sequencer (Perkin-Elmer) according to the standard methods described by the manufacturer, with the same primers as those used for the nested PCR. Sequences of the 169-bp fragment included among the primers used for the nested PCR are situated between positions 551 and 720 of the genetic sequence of bacteriophage 933W Stx 2 gene deposited in the EMBL gene data bank with the code X07865 (14). Forward and reverse sequencing was performed in parallel for each amplified DNA.
Western blotting.
The presence of the toxin in the supernatants of the bacteriophage enrichment cultures was assessed by Western blotting (27) as follows. First, supernatants were concentrated 10-fold by passage through 10K cutoff filtration membrane microconcentrators (Microsep) with centrifugation at 16,000 × g at 4°C. Then, 15 μl samples of each concentrated supernatant was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 12% polyacrylamide slab gels. Proteins were electrophoretically transferred (1 h at 100 V) to nitrocellulose membrane (Trans-Blot Transfer Medium; Bio-Rad); which was then blocked at 4°C overnight with 2 mM Tris–28 mM NaCl containing 0.02% Tween 20 and 3% serum albumin. Next, the nitrocellulose sheet was incubated by swirling in an orbital incubator for 1.5 h at room temperature with undiluted hybridoma culture supernatant (11E10 ATCC CRL 1907), which produces an immunoglobulin G (monoclonal antibody [MAb] 11E10) against the A subunit of Stx 2, and then washed three times by rocking for 10 min in 2 mM Tris–28 mM NaCl containing 0.02% Tween 20. Bound MAb was detected after incubation for 1.5 h in a 1:1,000 dilution of an anti-mouse alkaline phosphatase-conjugated antibody followed by addition of the substrate.
Next, a strip of the SDS-PAGE slab gel corresponding to the site of migration of the A subunit of Stx 2 was carefully cut out and electrophoretically eluted. The partially purified toxin was then concentrated and processed as indicated above for the raw supernatants.
Vero cell cytotoxicity.
The presence of Vero cell cytotoxicity in the supernatants of the bacteriophage enrichment cultures was analyzed by using the microtiter cytotoxicity assay and the cytotoxin-neutralizing abilities of the specific MAb mentioned above. Trypsinized Vero cells were counted and suspended to the desired concentration in growth medium, and 0.1 ml was pipetted into 96-well microtiter plates. Monolayers were established after 24 h of incubation at 37°C in a 5% CO2 incubator. Then 100-μl portions of serial 10-fold dilutions in cell culture medium of the supernatants of the bacteriophage enrichment cultures were added to each of the microwells. Supernatants of cultures of E. coli ATCC 43888 were tested to exclude their potential effect on Vero cells. A 100-μl sample of sewage was also tested to exclude other cytotoxins that might have been present. After 48 h of incubation at 37°C, the remaining cells were fixed with a 2% solution of formalin in 0.067 M PBS (pH 7.2) for 1 min; the fixative was then removed by rinsing with water, and the plates were stained with crystal violet in 5% ethanol–2% formalin for 10 min. Excess stain was removed by water rinsing, and the plates were air dried.
To verify that the observed cytotoxicity was due to Stx 2, a neutralization test was performed. Mixtures (1:1) of 10-fold dilutions of the supernatants of bacteriophage enrichment cultures and antibody (MAb 11E10) were incubated at 37°C for 1 h. Then, 100-μl portions of the mixtures were added to the microwells. The subsequent procedure was as described above.
Estimation of numbers of phages carrying the Stx gene.
The number of phages carrying the Stx 2 gene in sewage was estimated by considering all of the sewage samples as a single sample and then quantifying them by the most-probable-number method (6).
RESULTS
Numbers of bacterial indicators and bacteriophages in the studied samples.
The numbers of fecal coliforms and somatic coliphages in the studied sewage samples (Table 1) are in the range of numbers of such microbes found in sewage of Western countries (9, 37). The numbers of bacteriophages infecting E. coli O157:H7 ATCC 43888 were approximately 2 orders of magnitude lower than the values of somatic coliphages (Table 1).
TABLE 1.
Numbers of coliform bacteria and phages infecting different strains of E. coli in the sewage samples studied
| Sample source | Avg. no. (range)a of:
|
||
|---|---|---|---|
| Fecal coliforms (CFU/ml) | Somatic coliphagesb (PFU/ml) | Phages infecting E. coli O157:H7c (PFU/ml) | |
| Sewage plant 1 (n = 10) | 2.1 × 105 (7.2 × 104–3.2 × 105) | 2.5 × 104 (1.1 × 104–4.3 × 104) | 5.5 × 102 (1.2 × 102–8.0 × 102) |
| Sewage plant 2 (n = 7) | 1.6 × 105 (4.9 × 104–2.6 × 105) | 1.8 × 104 (1.1 × 104–3.2 × 104) | 5.1 × 102 (1.0 × 102–1.4 × 103) |
The average is the arithmetic mean. The range represents the maximum and minimum values.
Somatic coliphages defined as those infecting strain E. coli CN13.
Bacteriophages detected in strain E. coli O157:H7 ATCC 43888.
PCR DNA amplification of DNA from bacteriophages purified from sewage.
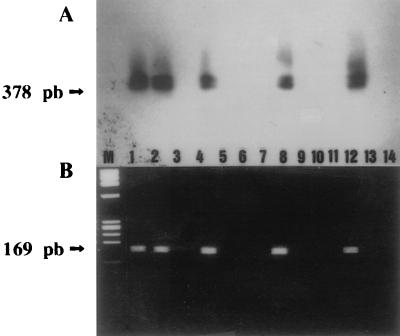
Positive controls, both bacteria and bacteriophages, showed amplified DNAs of 378 bp, as expected according to the position of the primers used for amplification of the Stx 2 gene after first-round PCR. In contrast, neither negative controls nor phages concentrated from sewage showed amplified DNAs by simple staining with ethidium bromide. However, amplified DNA was recognized in many samples of phages concentrated from sewage after hybridization with a specific probe (Table 2), by both dot and Southern blotting (Fig. 1A). Moreover, Southern blotting verified that the amplified DNA recognized by the internal probe had the expected size, 378 bp, according to the primers used for the PCR amplification (Fig. 1A). Also, both the positive controls of bacteria and bacteriophages and the sewage samples that gave positive amplification detected by hybridization showed amplified DNAs of 169 bp (Fig. 1B) after nested PCR, as expected from the position of the internal primers in the Stx 2 gene.
TABLE 2.
PCR analysis of sewage samples for phages carrying the Stx 2 gene
| Sample source | No. positive/total no. testeda in a water sample of:
|
||
|---|---|---|---|
| 100 ml | 10 ml | 1 ml | |
| Sewage plant 1 | 10/10 | 13/13 | 0/12 |
| Sewage plant 2 | 7/7 | 4/4 | 0/2 |
The number of positive samples in nested PCR analyses compared to the total number of samples studied.
FIG. 1.
(A) Southern blot analysis of the agarose gel of first-round PCR products hybridized with the Stx 2-radiolabeled probe (35). (B) Agarose gel electrophoresis and ethidium bromide staining of nested PCR products, with the first-round PCR products as templates. Lanes: 1, DNA from E. coli ATCC 43889, which bears Stx 2; 2, DNA from bacteriophage 933W, which bears Stx 2 (23); 4, 8, and 12, DNA of bacteriophages purified from 10 ml of sewage; 5, 9, and 13, DNA of bacteriophages purified from 1 ml of sewage water; 3, 6, and 10, DNA from E. coli ATCC 43888, which does not bear Stx 2; 7, 11, and 14, negative controls containing no template; M, molecular weight marker, φX-174-RF DNA HaeIII digest.
The sequence of the Stx 2 gene studied here was always purified from DNA extracted from phages purified from 100- and 10-ml samples of sewage but not from 1-ml samples (Table 2).
PCR DNA amplification of DNA from bacteriophages purified from supernatants of bacteriophage enrichment cultures.
To verify that the sequences of Stx 2 detected in phages in sewage belonged to bacteriophages able to infect E. coli O157:H7, the presence of the sequences was determined in supernatants of bacteriophage enrichment cultures as described above. Amplified DNA was recognized in many of the samples (Table 3) of phages from the supernatants of bacteriophage enrichment cultures after nested PCR and hybridization with a specific probe. Nested PCR and Southern blotting showed again that the amplified DNA had the expected size according to the primers used for first-round PCR and nested PCR.
TABLE 3.
Levels of bacteriophages carrying the Stx 2 gene and infecting E. coli O157:H7 in sewage
| Sample source | No. positive/total no. testeda in a water sample of:
|
MPN/1 ml (lower limit–upper limit)b | |||||
|---|---|---|---|---|---|---|---|
| 10 ml | 1 ml | 0.2 ml | 0.1 ml | 0.02 ml | 0.01 ml | ||
| Sewage plant 1 | 9/9 | 6/9 | 2/2 | 9/9 | 1/2 | 0/7 | 3.4 (1.7–7.0) |
| Sewage plant 2 | 2/2 | 2/2 | 1/1 | 2/2 | 0/1 | 0/2 | 21.3 (5.3–84.6)c |
The number of positive samples in nested PCR analyses compared to the total number of samples studied.
MPN, most probable number. The confidence limit was 95%.
The number of samples was too low to be meaningful.
The Stx sequence was detected in the supernatants of cultures infected with phages purified from sewage volumes as small as 0.02 ml but never from smaller volumes (Table 3).
Sequences of the amplified DNAs.
The sequences of the 169-bp amplimers of bacteriophages from two supernatants corresponding to enrichment cultures infected with 0.1 ml of sewage were sequenced to confirm the Stx 2 gene amplification. Both samples had the same sequence as Stx 2 (code X07865) as described in the EMBL gene data bank.
Presence of the toxin protein in the supernatants of bacteriophage enrichment cultures.
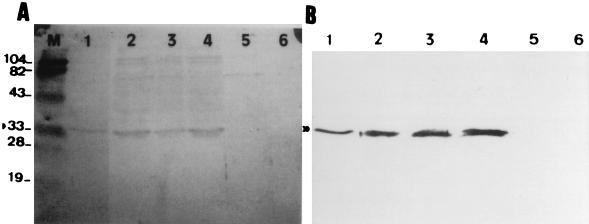
A band reflecting an estimated molecular mass of 33 kDa (Fig. 2A), which corresponds to the A subunit of Stx 2 (27), was detected in the supernatants of some bacteriophage enrichment cultures in which phages carrying the Stx 2 gene had been detected by PCR. A band showing a mobility similar to that of the bacteriophage enrichment cultures appeared in the lysate of strain ATCC 43889 of E. coli O157:H7, which produces Stx 2. In contrast, this band did not appear either in the lysate of uninfected cultures of strain E. coli O157:H7 ATCC 43888 or in the sewage sample used to make the bacteriophage enrichment cultures. A second Western blotting was performed after elution, concentration, and partial purification of the portion present in the strip of the SDS-PAGE slab gel in which the 33-kDa band appeared. In this case a unique clear band of 33 kDa was seen on the gels (Fig. 2B).
FIG. 2.
(A) Western blot (with anti-Stx 2 A subunit) of crude concentrated supernatants of cultures of E. coli O157:H7 ATCC 43888 infected with: 10, 1, and 0.1 ml of sewage that had been centrifuged, filtered, and DNase treated (lanes 2, 3, and 4, respectively); sonically derived lysates of E. coli O157:H7 ATCC 43889 (lane 1) and ATCC 43888 (lane 5); sewage that had been centrifuged, filtered, and DNase treated (lane 6); and prestained protein size standards in kDa (lane M). (B) Lanes are as in panel A, after cutting a strip of the SDS-PAGE slab gel of the zone to which the A subunit migrates, eluting the strips electrophoretically, and concentrating the eluates by passage through 10K cutoff filtration membrane microconcentrators (Microsep) by centrifugation at 16,000 × g at 4°C.
Presence of Vero cell cytotoxicity in the supernatants of bacteriophage enrichment cultures.
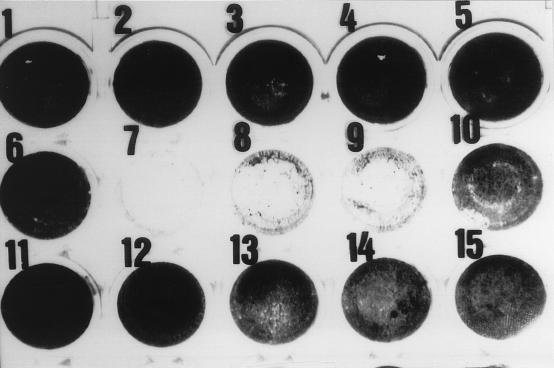
The presence of Vero cell cytotoxicity in the supernatants of enrichment cultures was analyzed by using the microtiter cytotoxicity assay and the cytotoxin-neutralizing abilities of a specific MAb (11E10). Supernatants of cultures that were positive for amplified DNA and for the A subunit of the toxin showed cytotoxicity on Vero cells, which caused the destruction of the cell monolayer (Fig. 3). This cytotoxicity was neutralized by the specific antibody 11E10. During the same period of incubation, the monolayers of either control cells or those directly inoculated with sewage did not experience any destruction.
FIG. 3.
Visual estimation of Vero cell cytotoxicity. Wells 1 to 5 were inoculated with growth medium; well 6 was inoculated with sewage that had been centrifuged, filtered, and DNase treated; wells 7, 8, 9, and 10 were inoculated with 10-fold dilutions of a crude supernatant of E. coli O157:H7 ATCC 43888 infected with 10 ml of sewage that had been centrifuged, filtered, and DNase treated. Wells 11, 12, 13, 14, and 15 were like wells 6, 7, 8, 9, and 10, respectively, but with the addition of anti-Stx 2 subunit A MAb 11E10.
Supernatants of E. coli ATCC 43888 also have no effect on the Vero cells monolayer, a finding which, together with the neutralization, excludes the possibility that toxicity on Vero cells could be due to other molecules (e.g., lipopolysaccharides).
Numbers of Stx 2-carrying bacteriophages in sewage.
The numbers of bacteriophages that bear the Stx 2 gene and are infectious for E. coli O157:H7 were estimated according to the results shown in Table 3 for the sewage samples of the sewage treatment plant 1, from which a significant number of data were available. The most probable number of bacteriophages was 3.4 per ml. Because of the more-limited data available from treatment plant 2, the estimated most probable number is not very reliable, but it can be estimated to be similar or higher than that for plant 1.
DISCUSSION
As indicated in the Introduction, bacteriophages carry genes involved in the pathogenicity of bacteria that they convert. In the case of Stx 2 genes, such knowledge has been acquired through the lysogenic induction of phages from the pathogenic bacterial strains (39), but up to now, data on the occurrence of such phages as free particles in nature were not available. By a PCR-based method frequently used for the detection in water of human viruses and bacteriophages (28, 30), bacteriophages bearing sequences of the Stx 2 gene were detected in raw sewage which, on the basis of the numbers of fecal bacteria and bacteriophages, can be considered to be representative of the sewage from cities in developed countries (9, 37). The studied sequence of the Stx 2 gene was always amplified from DNA extracted from phages purified from 10-ml volumes of sewage and not from smaller volumes. However, taking into consideration that in the process of phage purification and DNA extraction we ended up with 20 μl of DNA suspension regardless of the initial volume of sewage tested and that only 1 μl of the DNA solution was used for the PCR, we may estimate that phages are present in volumes of sewage smaller than 10 ml. Precautions taken in the process of purification of bacteriophages from sewage, i.e., treatment with DNase and cesium chloride centrifugation, clearly indicate that the amplified sequences belong to DNA extracted from the bacteriophages.
However, DNA amplification directly from bacteriophages purified from sewage does not provide information on either the infectiousness of the phages or their ability to infect a particular host, e.g., E. coli O157:H7. The results of the amplification of the DNA of bacteriophages purified from the supernatants of the bacteriophage enrichment cultures clearly show that bacteriophages carrying the Stx 2 gene present in sewage were able to replicate in E. coli O157:H7 ATCC 43888, which does not bear the Stx 2 gene. Indeed, small sewage volumes, 0.1 ml in most cases and 0.02 ml in one sample, gave rise after a 1:2,500 or 1:12,500 dilution and enrichment to supernatants from which bacteriophages carrying the Stx 2 gene were obtained. It should be considered that in the same sewage samples the selected sequence of the Stx 2 gene could not be amplified from bacteriophages purified from 1-ml samples and so data from these enrichments clearly show that phages carrying the studied sequence of the Stx 2 gene replicated in E. coli O157:H7.
The sequences of the studied amplimers of 169 bp, which correspond to a conserved region of the Stx 2 sequence, provide further confirmation that the DNA sequences detected in the phages purified from supernatants of bacteriophage enrichment cultures belong to the Stx 2 gene. Indeed, a comparison of sequenced amplimers of the Stx 2 with published sequences of the same fragment of the Stx 2 gene showed homologies almost identical to the homologies described among different available sequences of Stx 2 genes (14).
Nevertheless, the detection of a partial sequence of the Stx 2 gene does not prove that the detected bacteriophages transport either the full gene or the functional gene. Since the production of Stx 2 is markedly increased after the lysogenic induction of strains converted by phages carrying the Stx 2 gene (23, 39), it was to be expected that even a low number of bacteria with replicating bacteriophages carrying the Stx 2 gene will produce enough toxin to be detectable in the supernatant of the enrichment cultures. With this hypothesis in mind, the presence of Stx 2 was tested in the supernatant of some enrichment cultures in order to assess whether the bacteriophages, or a part of them, carried the functional gene. The presence of subunit A of the toxin protein and the Vero cell cytotoxicity in the supernatants of bacteriophage enrichment cultures that indicated bacteriophage replication as detected by PCR both demonstrate that the Stx 2 genes carried by infectious bacteriophages, or at least by some of them, are complete and functional. In summary, the results regarding the detection of the Stx gene, the subunit A of the toxin protein, and the Vero cell activity in the supernatants of bacteriophage enrichment cultures show the presence of phages infectious for E. coli O157:H7 and carrying functional Stx 2 genes in sewage volumes as small as 0.02 ml.
Although the estimation of the numbers of phages by the application of the most-probable-number technique should only be taken as a rough estimation, it does indicate high levels of phages carrying the Stx 2 gene in urban sewage that remain infectious and that are able to infect O157 strains. These values, which range from 1 to 10 per ml, represent around 1% of all phages infecting E. coli O157:H7. Data on the presence in sewage or polluted waters of EHEC strains carrying Stx 2 is limited and difficult to compare (10, 19, 20), and data on the prevalence of E. coli O157:H7 in sewage are lacking. However, available data on PCR analysis show that the Stx 2 gene in bacteria in sewage is not as abundant as it is shown here for phages (10). The high levels of Stx genes in bacteriophages suggest that many E. coli strains and even other enterobacteria may be shedding bacteriophages that carry the Stx 2 gene and that may infect E. coli O157:H7.
The high levels of phages carrying the Stx 2 gene in urban sewage indicate a great abundance of such phages circulating among the human population. Despite these high levels, the results do not yet allow us to evaluate either the role of bacteriophages in the maintenance of bacteria carrying the Stx 2 gene in nature or the role of bacteriophages in the transmission of the Stx 2 gene among different enterobacteria. However, taking into account that coliphages are more resistant than E. coli to disinfection (34), water treatment (15), and natural inactivation in freshwater (4) and seawater (18), the data presented here suggest that bacteriophages may have a crucial role as a reservoir of the Stx 2 gene in nature.
We believe that this study opens interesting perspectives for the study of phages carrying genes that code for Shiga toxin genes. Future studies will seek to determine the importance of phages in the transfer of such genes between different strains and different species of enterobacteria in natural conditions, to determine the role of bacteriophages in the storage of such genes in nature, and to determine whether the ingestion of phages themselves, by either humans or animals, can cause conversion of the existing E. coli flora. A better understanding of these issues should provide important information on the role of current practices in urban sewage and slurry treatment and disposal, as well as in food management, in the horizontal transfer of such genes and on their role in generating strains of enteric bacteria that carry multiple virulence factors.
ACKNOWLEDGMENTS
We thank Alison O’Brien from Department of Microbiology of the Uniformed Services University of the Health Sciences, Bethesda, Md.; Helge Karch from Institut für Hygiene und Mikrobiologie der Universität Würzburg, Wurzburg, Germany; and Inge Mühldorfer from Lehrstuhl für Molekulare Infektionsbiologie der Universität Würzburg (Germany), who provided some of the strains used in this study.
This study was partially supported by a grant (1995 SGR 00415) of the Generalitat de Catalunya. We thank the Serveis Científico-Tècnics of the University of Barcelona for their help in sequencing.
REFERENCES
- 1.Adams M H. Bacteriophages. New York, N.Y: Interscience Publishers, Inc.; 1959. pp. 27–30. [Google Scholar]
- 2.American Public Health Association. Standard methods for the examination of water and wastewater. 18th ed. Washington, D.C: American Public Health Association; 1992. [Google Scholar]
- 3.Anonymous. Enterohaemorrhagic Escherichia coli infection in Japan. Weekly Epidemiol Rec. 1996;30:229. [Google Scholar]
- 4.Bell R G. The limitation of the ratio of fecal coliforms to total coliphages as a faecal pollution index. Water Res. 1976;10:743–748. [Google Scholar]
- 5.Brown T A. Essential molecular biology: a practical approach. In: Brown T A, editor. The practical approach series. I-II. Oxford, United Kingdom: Oxford University Press; 1991. [Google Scholar]
- 6.De Man J C. The probability of the most probable number. Eur J Appl Microbiol. 1975;1:72–77. [Google Scholar]
- 7.Devereux J P, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francki R I B, Fauquet C M, Knudson D L, Brown F. Fifth report of the International Committee on Taxonomy of Viruses. Basel, Switzerland: S. Karger AG; 1991. Classification and nomenclature of viruses. [Google Scholar]
- 9.Grabow W O K, Coubrough P, Nupen E M, Bateman B W. Evaluation of coliphages as indicators of the virological quality of sewage-polluted water. Water SA. 1984;10:7–14. [Google Scholar]
- 10.Grant S B, Pendroy C P, Mayer C L, Bellin J K, Palmer C J. Prevalence of enterohemorrhagic Escherichia coli in raw and treated municipal sewage. Appl Environ Microbiol. 1996;62:3466–3469. doi: 10.1128/aem.62.9.3466-3469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin P M, Tauxe R V. The epidemiology of infections caused by E. coli O157:H7, other enterohaemorrhagic E. coli and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 12.Havelaar A H, Hogeboom W H. Factors affecting the enumeration of coliphages in sewage and sewage-polluted waters. Antonie Leeuwenhoek. 1983;49:387–397. doi: 10.1007/BF00399318. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann M A, Brian D A. Sequencing PCR DNA amplified directly from a bacterial colony. BioTechniques. 1991;11:30–31. [PubMed] [Google Scholar]
- 14.Jackson M P, Neill R J, O’Brien A D, Holmes R K, Newland J W. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol Lett. 1987;44:109–114. doi: 10.1016/0882-4010(87)90106-9. [DOI] [PubMed] [Google Scholar]
- 15.Jofre J, Oller E, Ribas F, Vidal A, Lucena F. Potential usefulness of bacteriophages that infect Bacteroides fragilis as model organisms for monitoring virus removal in drinking water treatment plants. Appl Environ Microbiol. 1995;61:3227–3231. doi: 10.1128/aem.61.9.3227-3231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson L P, Tomai M A, Schlievert P M. Bacteriophage involvement in group A streptococcal pyrogenic exotoxin A production. J Bacteriol. 1986;166:623–627. doi: 10.1128/jb.166.2.623-627.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kott Y, Ben Ari H. Bacteriophages as marine pollution indicators. Rev Int Oceanogr Med. 1968;9:207–217. [Google Scholar]
- 19.Lang A L, Tsai Y-L, Mayer C L, Patton K C, Palmer C J. Multiplex PCR for detection of the heat-labile toxin gene and shiga-like toxin I and II genes in Escherichia coli isolated from natural waters. Appl Environ Microbiol. 1994;60:3145–3149. doi: 10.1128/aem.60.9.3145-3149.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins M T, Rivera I G, Clark D L, Olson B H. Detection of virulence factors in culturable Escherichia coli isolates from water samples by DNA probes and recovery of toxin-bearing strains in minimal o-nitrophenol-β-d-galactopyranoside-4-methylumbelliferyl-β-d-glucuronide media. Appl Environ Microbiol. 1992;58:3095–3100. doi: 10.1128/aem.58.9.3095-3100.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGowan K L, Wickersham E, Strockbine N A. Escherichia coli O157:H7 from water. Lancet. 1989;i:967–968. doi: 10.1016/s0140-6736(89)92559-2. [DOI] [PubMed] [Google Scholar]
- 22.Mel S, Mekalanos J J. Modulation of horizontal gene transfer in pathogenic bacteria by in vivo signals. Cell. 1996;87:795–798. doi: 10.1016/s0092-8674(00)81986-8. [DOI] [PubMed] [Google Scholar]
- 23.Mühldorfer I, Hacker J, Keusch G T, Acheson D W, Tschäpe H, Kane A V, Ritter A, Ölschläger T, Donohue-Rolfe A. Regulation of the Shiga-like toxin II operon in E. coli. Infect Immun. 1996;64:495–502. doi: 10.1128/iai.64.2.495-502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newland J W, Neill R J. DNA probes for Shiga-like toxin I and II and for toxin converting bacteriophages. J Clin Microbiol. 1988;26:1292–1297. doi: 10.1128/jcm.26.7.1292-1297.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien A D, Newland J W, Miller S F, Holmes R K, Smith H W, Formal S B. Shiga-like toxin-converting phages from E. coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 26.Payment P, Franco E. Elimination of coliphages, Clostridium perfringens, and human enteric viruses during drinking water treatment. Appl Environ Microbiol. 1993;59:2418–2424. doi: 10.1128/aem.59.8.2418-2424.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera L P, Marqués L R M, O’Brien A D. Isolation and characterization of monoclonal antibodies to Shiga-like toxin II enterohemorrhagic E. coli and use of the monoclonal antibodies in a colony enzyme-linked immunosorbent assay. J Clin Microbiol. 1988;26:2127–2131. doi: 10.1128/jcm.26.10.2127-2131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puig M, Jofre J, Lucena F, Allard A, Wadell G, Girones R. Detection of adenoviruses and enterovirus in polluted waters by nested PCR amplification. Appl Environ Microbiol. 1994;60:2963–2970. doi: 10.1128/aem.60.8.2963-2970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rietra P J, Willshaw G A, Smith H R, Field A M, Scotland S M, Rowe B. Comparison of Vero-cytotoxin-encoding phages from Escherichia coli of human and bovine origin. J Gen Microbiol. 1989;135:2307–2318. doi: 10.1099/00221287-135-8-2307. [DOI] [PubMed] [Google Scholar]
- 30.Rose J B, Zhow X, Griffin D W, Paul J H. Comparison of PCR and plaque assay for detection and enumeration of coliphages in polluted marine waters. Appl Environ Microbiol. 1997;63:4564–4566. doi: 10.1128/aem.63.11.4564-4566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Saye D J, Ogunseitan O, Sayler G S, Miller R V. Transduction of linked chromosomal genes between Pseudomonas aeruginosa during incubation in situ in a freshwater habitat. Appl Environ Microbiol. 1990;56:140–145. doi: 10.1128/aem.56.1.140-145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scotland S M, Smith H R, Willshaw G A, Rowe B. Verocytotoxin production in strain of Escherichia coli is determined by genes carried on bacteriophage. Lancet. 1983;ii:216. doi: 10.1016/s0140-6736(83)90192-7. [DOI] [PubMed] [Google Scholar]
- 34.Sobsey M P. Inactivation of health-related microorganisms in water disinfection processes. Water Sci Technol. 1989;21:179–195. [Google Scholar]
- 35.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 36.Strockbine N A, Marques L R, Newland J W, Smith H W, Holmes R K, O’Brien A D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986;53:135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tartera C, Lucena F, Jofre J. Human origin of Bacteroides fragilis bacteriophages present in the environment. Appl Environ Microbiol. 1989;55:2696–2701. doi: 10.1128/aem.55.10.2696-2701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 39.Yee A J, De Grandis S, Gyles C L. Mitomycin-induced synthesis of a Shiga-like toxin from enteropathogenic Escherichia coli H.I.8. Infect Immun. 1993;61:4510–4513. doi: 10.1128/iai.61.10.4510-4513.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]