Abstract
The determination of plasma oestradiol has numerous applications in epidemiology, reproductive medicine and breast cancer management. Commercially available analytical methods, which measure the hormone levels without prior purification, have been successfully developed for measuring oestradiol in premenopausal women. The application of these methodologies to the quantification of the very low levels of oestradiol in postmenopausal women is more problematic in terms of accuracy and interpretation. The importance of using appropriate methodology is discussed and illustrated with data demonstrating the disparity in the results obtained when low levels of oestradiol were quantified using direct and indirect methods.
Keywords: immunoassays, oestradiol, postmenopausal
Over recent years the measurement of plasma oestrogen levels, particularly oestradiol levels, in postmenopausal women has made important contributions to an improved understanding of the aetiology and treatment of breast cancer. Current studies aim to build on this by determining whether such measurements can be usefully incorporated into the evaluation of breast cancer risk in relation to the implementation of risk reduction strategies.
Plasma oestradiol analysis has also provided a key pharmacodynamic endpoint in the development of inhibitors of aromatase, the enzyme responsible for oestrogen synthesis [1]. These drugs are now recognised as the most effective endocrine agents in postmenopausal breast cancer and are becoming ever more widely used [2]. Studies of the effects of these agents on other body systems and how this relates to oestrogen suppression and the measurement of compliance with aromatase inhibitor treatment require further analysis of oestrogen levels.
Accurate assessment of plasma oestradiol levels is, however, not straightforward. This is most particularly the case in postmenopausal women where the mean untreated levels are approximately 25 pmol/l (7 pg/ml). The problems associated with accurate assessment are, unfortunately, ill-understood and they may lead to erroneous conclusions being made in research studies and routine evaluation in the clinic if inappropriate assays are conducted.
The assessment of oestradiol has until recently not been an important issue in postmenopausal women other than in research laboratories, but it has been essential for the assessment of infertility and of strategies for ovulation induction. Analytical methodologies have thus focused on supplying results in the range of 100–1200 pmol/l as found in normal premenopausal women, or higher still as found during ovulation induction. The estimation of these levels is far less demanding than those in postmenopausal women. Compromises in the methodologies have thus been possible that allow simplicity, automation, precision, the use of small sample volumes and a rapid turn-round. These may generate substantially inaccurate results but in this context this has little detrimental effect on decision-making.
The major compromise has been to conduct the immunoassay on serum or plasma that has not been subject to pretreatments that extract the oestradiol from the sample (e.g. with an organic extractant such as diethyl ether). Extraction of this type was universally employed in the assay of oestradiol (and other plasma steroids) until about 15 years ago and this allowed the analyte to be quantified in a purified form. The application of the newer, so-called direct analytical approaches to the assessment of postmenopausal oestradiol levels can lead to gross and misleading inaccuracies.
The immunoassay is universally used in the routine laboratory assessment of oestradiol. The inaccuracies result largely from two aspects of the immunoassay's application. First, oestradiol binds avidly (association constant, 0.68 × 109 l/mol) to sex hormone binding globulin such that, if antiserum is added directly to plasma samples, the sex hormone binding globulin effectively competes with the antibody for oestradiol. The direct assays attempt to rectify this by adding displacing agents to the analytical reagents. The second problem is the presence in the blood of very high concentrations of water-soluble conjugated steroids, at concentrations that are orders of magnitude above those of oestradiol. These may cross-react to a minor degree with the oestradiol antiserum but their concentration and the sum effect of numerous such compounds can lead to substantial biases. General issues of the matrix of plasma that differ between samples and can only be approximated in assay standards also contribute to error.
An overview study by the Endogenous Hormones and Breast Cancer Collaborative Group of prospective collections of plasma and subsequent measurements of oestradiol and associated steroids in women that eventually developed breast cancer illustrates both the importance of plasma oestradiol in postmenopausal women and the inaccuracies of analysis [3]. Inconsistent results had been published on the relationship between plasma oestradiol levels and breast cancer risk, but this overview analysis of nine studies revealed a highly significant relationship (Fig. 1) in which there was an overall increase in relative risk of 1.29 (95% confidence interval, 1.15–1.44; P < 0.001) for every doubling of oestradiol concentration. The true risk is probably higher since the measurements were made on single samples and it has been estimated that taking account of within-subject variability by taking multiple samples may lead to a doubling of estimates of relative risk [4]. The problems in analysis are illustrated by the mean oestradiol levels in the postmenopausal controls reported by the various laboratories varying about fivefold, and ranging from 21.7 pmol/l to 101 pmol/l. Four of the nine studies used different direct radioimmunoassays and the other five studies used an organic extraction prior to radioimmunoassay. Quoted detection limits ranged in value from 3 pmol/l to 37 pmol/l.
Figure 1.
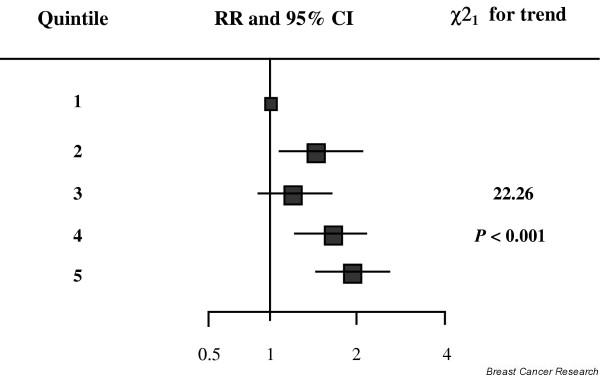
Relative risk (RR) of breast cancer by increasing quintiles of oestradiol concentration from a pooled analysis of nine studies (modified from [3]). The position of each square indicates the magnitude of the RR, and the area of the square is proportional to the amount of statistical information available. The length of the horizontal line through the square indicates the 95% confidence interval (CI).
The way in which the performance of most direct assays varies from the performance of an assay that uses organic extraction, and has been sensitised and validated for use in postmenopausal women, is vividly demonstrated by the comparisons made in Fig. 2. Each panel in Fig. 2 demonstrates the results obtained when pairs of samples taken from 10 postmenopausal breast cancer patients were analysed for oestradiol using four different methods. For each patient the first sample was taken before, and the second sample during, the use of anastrozole, an aromatase inhibitor. Anastrozole inhibits aromatase, the sole source of steroidal oestrogens, by a mean 97% [5]. However, in Fig. 2a (Estradiol 33540; Beckman Coulter Access Immunoassay system, Fullerton, CA, USA) and in Fig. 2b (3rd Generation Estradiol Radioimmunoassay, DSL-39100; Diagnostic Systems Laboratories Inc., Webster, TX, USA) the application of two types of direct assays gives values that fall by a mean 25% and 34%, respectively, and in some cases patients show no suppression in oestradiol levels at all. This observation contrasts markedly with the situation in Fig. 2c, which shows the results from applying a sensitive extraction assay [6]. All these patients show almost complete suppression of oestradiol levels, mostly to below the sensitivity limit of the assay (3 pmol/l). By designating samples with values below the detection limit as having values of 3 pmol/l a mean suppression of 88% may be calculated, but this is inevitably an underestimate. Revealingly, the application of an extraction step prior to use of the DSL-39100 kit (used in its normal direct fashion in Fig. 2b) leads to results that now resemble those derived from the extraction assay.
Figure 2.
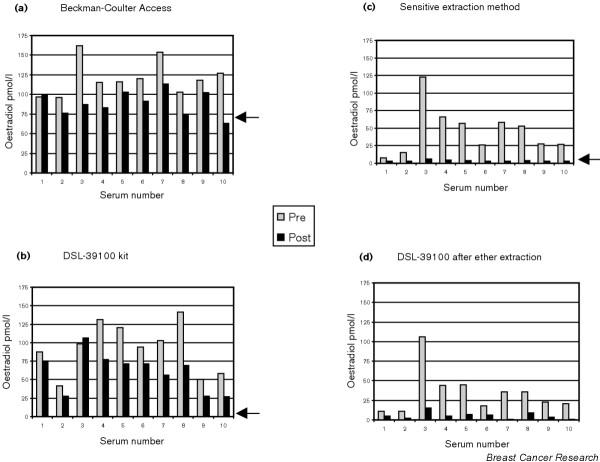
Each panel shows the results of analysing pairs of samples from 10 postmenopausal breast cancer patients. Light column, measured serum oestradiol before treatment (Pre); dark column, result during the use of the aromatase inhibitor, anastrozole. (a), (b) Results obtained when the oestradiol was measured by direct assay: (a) Beckman Coulter Access Immumoassay system Estradiol 33540, and (b) DSL 3rd Generation Estradiol Radioimmunoassay DSL-39100. (c), (d) Results when the oestradiol is measured after a pre-extraction with an organic solvent: (c) extraction and radioimmunoassay (as used at the Royal Marsden [6]), and (d) the DSL-39100 kit after pre-extraction of serum samples with diethyl ether. Arrows indicate the detection limits for the assays; no arrow in (d) since this analysis was performed for the present study only and no detection limit was determined.
There were significant correlations between the pretreatment results obtained with the sensitive extraction assay and those obtained with the other three assays: r = 0.51 for the DSL-39100 kit, r = 0.73 for the Beckman Coulter Access 33540 and r = 0.98 for the DSL-39100 kit after organic extraction.
The results of the present study with the aromatase inhibitor suggest that at least 70% of the oestradiol measured by the two direct assays in postmenopausal women is an artifact. These assays are probably no worse in this respect than other direct immunoassays, and may in fact be better. It should particularly be noted that the Beckman method has a detection limit of 73 pmol/l and that values below this level should be quoted as < 73 pmol/l. The results indicate that the use of direct assays to check compliance of women on aromatase inhibitors or to conduct research studies in postmenopausal women is inappropriate and is likely to give aberrant guidance. Such assays are also not suitable to help distinguish postmenopausal women from premenopausal women; this is occasionally necessary in tamoxifen-treated patients where confounding effects of the drug on gonadotropin levels make these levels unsuitable for the determination of menopausal status.
This variability between methodological approaches obviously did not prevent the Endogenous Hormones and Breast Cancer Collaborative Group from making the very valuable epidemiological observation of the relationship between plasma oestradiol levels and breast cancer risk. Indeed, these methods may be considered satisfactory for some epidemiological purposes where limitations of sample volume may be a major consideration. The methodological limitations almost certainly affected their ability to define accurately the strength of the relationship, however, and will continue to do so if implemented in future studies.
A number of investigators now view this relationship between plasma oestradiol levels and breast cancer risk as one that has important potential for inclusion in algorithms for predicting breast cancer risk, particularly in association with antihormonal strategies for breast cancer prevention [7]. The implementation of such an approach will require the use of accurate, well-validated, rugged assays that operate alongside well-developed risk relationships for different populations. It is possible that new, more definitive nonimmunoassay approaches to the oestradiol assay (e.g. with tandem mass spectrometry) may allow accurate and precise assay of oestradiol [8]. However, when immunoassays are applied it seems inevitable that we will have to employ labour-intensive and inconvenient extraction procedures for the accurate analysis that these new opportunities demand.
Competing interests
The author(s) declare that they have no competing interests.
Acknowledgments
Acknowledgement
The analyses performed in this study were supported by the Daniel and Phyllis Da Costa International Fund for Breast Cancer Prevention.
References
- Dowsett M. Drug and hormone interactions of aromatase inhibitors. Endocr Relat Cancer. 1999;6:181–185. doi: 10.1677/erc.0.0060181. [DOI] [PubMed] [Google Scholar]
- Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- The Endogenous Hormones and Breast Cancer Collaborative Group Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3 year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–654. [PubMed] [Google Scholar]
- Geisler J, Haynes B, Anker G, Dowsett M, Looning PE. Influence of letrozole and anastrozole on total body aromatisation and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomised, cross-over study. J Clin Oncol. 2002;20:751–757. doi: 10.1200/JCO.20.3.751. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Goss PE, Powles TJ, Hutchinson G, Brodie AM, Jeffcoate SL, Coombes RC. Use of the aromatase inhibitor 4-hydroxyandrostenedione in postmenopausal breast cancer: optimization of therapeutic dose and route. Cancer Res. 1987;47:1957–1961. [PubMed] [Google Scholar]
- Dowsett M. Clinical trials with aromatase inhibitors for the prevention of breast cancer. In: Ingle JN, Dowsett M, editor. In Advances in Endocrine Therapy of Breast Cancer. New York: Marcel Dekker; 2004. pp. 189–201. [Google Scholar]
- Nelson RE, Grebe SK, O'Kane DJ, Ravinder JS. Liquid chromatography–tandem mass spectrometry assay for simultaneous measurement of oestradiol and estrone in human plasma. Clin Chem. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]


