Abstract
OBJECTIVES
This study describes coronary revascularization strategies used by sex and age in the USA.
METHODS
A sex-stratified cohort study from the National Inpatient Sample from the Agency for Healthcare Research and Quality (USA) including patients admitted for coronary revascularization with primary or secondary diagnoses of chronic coronary syndrome or non-ST elevation myocardial infarction who underwent ≥3-vessel coronary artery bypass grafting or percutaneous coronary intervention from January 2019 to December 2020. The primary outcome was the use rate of coronary artery bypass grafting or multivessel percutaneous coronary intervention. Prespecified subgroups included age and non-ST elevation myocardial infarction.
RESULTS
Among 121 150 patients (21.7% women), there were no sex differences in age (women: 66.6 [66.5–66.7], men: 67.6 [67.5–67.7], standardized mean difference: 0.1) or non-ST elevation myocardial infarction incidence (women: 37.4%, men: 45.7%, standardized mean difference: 0.17). The majority of women (74.2%) and men (84.9%) underwent bypass grafting, which was unaffected by age, race or non-ST elevation myocardial infarction. Women were less likely to undergo bypass grafting than percutaneous intervention (adjusted odds ratio 0.49, 95% confidence interval 0.44–0.54; P < 0.001) and a disparity most pronounced in patients >80 years old (adjusted odds ratio 0.31, 95% confidence interval 0.22–0.45; P < 0.001).
CONCLUSIONS
Most patients with multivessel coronary artery disease needing revascularization undergo bypass grafting, irrespective of sex, age or clinical presentation. The sex disparity in the use of bypass grafting is mostly seen among patients >80 years old.
Keywords: Sex differences, Women’s health, Coronary artery bypass grafting, Percutaneous coronary intervention, Multivessel coronary artery disease
Multivessel coronary artery disease (CAD) is highly prevalent among CAD patients [1] and is associated with worse outcomes when compared with less extensive CAD [2–4].
INTRODUCTION
Multivessel coronary artery disease (CAD) is highly prevalent among CAD patients [1] and is associated with worse outcomes when compared with less extensive CAD [2–4]. Revascularization strategies for the management of multivessel CAD include surgical [coronary artery bypass grafting (CABG)] and interventional [multivessel percutaneous coronary intervention (PCI)] revascularization. Current clinical practice guidelines [5, 6] recommend both CABG and PCI for multivessel CAD; as surgery has better outcomes in patients with severe disease [7–14], recommendations shift towards CABG with increasing complexity of coronary anatomy. While previous studies have described the relative use of CABG and PCI in the general population [15–18], there is limited evidence on physician revascularization choices by age and sex. This is particularly important as women and older patients have specific preoperative risk profiles that may drive referral to one or another coronary revascularization modality.
In the present analysis, we utilize a national database with the aim of evaluating modalities of coronary revascularization for multivessel CAD by sex and age in the current US clinical practice.
METHODS
Ethics statement
The study was reviewed by the Weill Cornell Medicine Institutional Review Board (# 23-09026517-01) and it was determined that the proposed study was not research involving human subjects per to Code of Federal Regulations on the Protection of Human Subjects and the need for further review was waived and thus individual consent was not needed.
Study design and data collection
This retrospective cohort study follows the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines [19], and the checklist is provided in Supplementary Material, Table S1.
The data were obtained via the National Inpatient Sample (NIS) from the Agency for Healthcare Research and Quality, which is the largest publicly available all-payer inpatient database in the USA [20]. It is comprised of ∼13 million discharges from the index years 2019–2020, representing 20% of all inpatient stays in the USA. The NIS includes information on demographics, inpatient diagnoses and procedures and hospital characteristics and has been used in the past to investigate sex differences in CAD [16, 21]. The Agency for Healthcare Research and Quality provides sampling weights, which are used to obtain national estimates of the sample size [20].
Study population
The NIS was queried for all adult (≥18 years) hospital stays with a primary or secondary diagnosis of non-ST elevation myocardial infarction (NSTEMI) or chronic coronary syndrome (CCS), and who underwent multivessel PCI or CABG (3 or more coronary arteries) from January 2019 to December 2020. The International Classification of Diseases, 10th Edition codes that were utilized to identify patients can be found in Supplementary Material, Table S2. While the standard definition of multivessel CCS encompasses the presence of obstructive disease in 2 or more epicardial coronary arteries, we specifically included PCI codes that indicate 3 or more arteries, as including two-vessel PCI in the study would have introduced additional complexity in interpreting the results. Patients were included if they underwent multivessel (3 or more vessels) PCI or CABG. Patients were excluded if they had concomitant CABG and PCI or if they had ST-elevation myocardial infarction. If a hospital stay had CCS and NSTEMI co-diagnoses, these patients were assigned to the NSTEMI group. Patients who did not have NSTEMI or CCS as their primary or secondary diagnosis code were excluded.
Outcomes
The primary outcome was the use rate of CABG or PCI.
Statistical analyses
Baseline demographics were stratified by sex. Categorical variables were reported as frequency counts and percentages, while continuous variables were reported as means with standard deviations. Age, PCI and CABG volumes were all analysed as continuous variables.
We calculated the standardized mean differences for continuous and categorical variables using the following formulas [22], respectively:
where μ stands for mean, σ stands for standard deviation, n1 and n2 stand for sample sizes and P stands for proportion [23]. These standardized mean differences are identical to Cohen's size effect, therefore standardized mean differences (SMDs) of <0.20, 0.20, 0.50 and 0.80 are considered small, medium and large [24].
CABG and PCI use rates were compared between groups using the chi-squared test and univariable logistic regression. A multivariable logistic regression model was fit for PCI and CABG use and included preoperative patient factors such as comorbidity, demographics and socioeconomic status. Covariate selection was based on clinical relevance and inclusion in commonly used risk assessment scores, such as the Society of Thoracic Surgeons score. Given the clinical relevance of these factors, we deemed it important to adjust for them, irrespective of whether collinearity was present or not. Results were reported as odds ratios and adjusted odds ratios with 95% confidence intervals (CIs). The factors included in risk adjustment are presented in Supplementary Material, Table S3. Significance was set at P-value 0.05 without multiplicity adjustment.
Given the described relationship between mortality and institutional volume of PCI and CABG [25–27], as a sensitivity analysis, another multivariable logistic regression model was fit for PCI and CABG use that included all the factors in the first model and added institutional volume of PCI and CABG. An additional sensitivity analysis was stratified by race (white versus non-white patients). Finally, we provided E-values for adjusted odds ratios to assess the potential effect of unmeasured confounders [28]. The E-value quantifies the odds ratio that an unmeasured confounder would need to have to nullify the observed odds ratio. A higher E-value indicates a lower likelihood of the existence of such a confounding factor.
Pre-specified subgroup analyses included analysis by NSTEMI status (non-NSTEMI versus NSTEMI) and by age (4 age subgroups: ≤60, 61–70, 71–80 and >80 years).
Missing data accounted for <1.0% of the overall dataset; therefore, no imputation was performed. Stata 16 was used for all statistical analyses [29].
RESULTS
Study cohort and baseline characteristics
Between January 2019 and December 2020, 121 150 patients (26 280 [21.7%] women) were admitted with primary or secondary diagnoses of NSTEMI or CCS and underwent multivessel PCI or CABG. The baseline patient and hospital characteristics are summarized in Table 1. All differences between sexes in race, age (women: 66.6 [66.5–66.7], men: 67.6 [67.5–67.7], SMD: 0.1), incidence of NSTEMI (women: 37.4%, men: 45.7%, SMD: 0.17), household income status, hospital teaching status, admission at large hospital, Medicare/Medicaid insurance status, elective hospital admission, incidence of prior sternotomy, prior cerebrovascular accident, congestive heart failure, valvular disease, hypertension, stage III or IV chronic kidney disease, peripheral vascular disease, coagulopathy, malignancy, liver disease, diabetes, chronic pulmonary disease, anaemia, obesity and pre-existing arrhythmia were very small.
Table 1:
Comparison of baseline characteristics between men and women
| Baseline characteristics | Overall | Women | Men | SMD (absolute) |
|---|---|---|---|---|
| NE = 121 150 | NE = 26 280 | NE = 94 870 | ||
| Demographic and hospital characteristics | ||||
| Age (years), mean (SD) | 66.8 (66.74–66.85) | 66.6 (66.5–66.7) | 67.6 (67.5–67.7) | 0.1 |
| Caucasian race (%) | 75.6 | 76.9 | 71.0 | 0.14 |
| Lowest quartile household income (%) | 28.3 | 27.1 | 32.7 | 0.13 |
| Medicare/Medicaid insurance (%) | 35.9 | 37.5 | 30.2 | 0.15 |
| Large hospital bed size (%) | 58.6 | 58.8 | 57.7 | 0.02 |
| Teaching hospital (%) | 82.7 | 82.9 | 82.3 | 0.01 |
| Elective admission (%) | 42.8 | 44.6 | 36.2 | 0.17 |
| Patient comorbidity (%) | ||||
| Congestive heart failure | 40.2 | 39.0 | 44.5 | 0.11 |
| NSTEMI | 39.2 | 37.4 | 45.7 | 0.17 |
| Prior sternotomy | 4.7 | 4.8 | 4.5 | 0.02 |
| Arrhythmia | 47.1 | 49.0 | 40.6 | 0.17 |
| Valvular disease | 19.8 | 19.0 | 22.6 | 0.09 |
| Diabetes mellitus | 51.4 | 49.5 | 58.4 | 0.18 |
| Hypertension | 47 | 45.7 | 51.6 | 0.12 |
| Chronic kidney disease (stage III–IV) | 11.5 | 11.1 | 13.1 | 0.06 |
| Peripheral vascular disease | 13.5 | 13.4 | 14.0 | 0.02 |
| Cerebrovascular accident | 2.1 | 1.9 | 3.0 | 0.08 |
| Chronic pulmonary disease | 20.7 | 19.0 | 26.9 | 0.2 |
| Coagulopathy | 21.4 | 22.1 | 19.4 | 0.07 |
| Anaemia | 3.2 | 2.7 | 5.1 | 0.13 |
| Malignancy | 2.6 | 2.7 | 2.4 | 0.02 |
| Liver disease | 1.8 | 1.7 | 2.3 | 0.05 |
| Obesity | 28.9 | 27.7 | 33.6 | 0.13 |
NE: national estimates; NSTEMI: non-ST elevation myocardial infarction; SD: standard deviation; SMD: standardized mean difference.
Coronary artery bypass grafting versus percutaneous coronary intervention
The vast majority of both women (74.2%) and men (84.9%) underwent CABG, while a minority of women (25.8%) and men (15.1%) underwent multivessel PCI. After adjustment for both patient and hospital factors, women were less likely than men to undergo CABG [adjusted odds ratio (aOR) 0.49, 95% CI 0.44–0.54; P < 0.001]. Among CABG patients, the on-pump technique was used in 70.45% in males and 61.6% in females (P < 0.001). Use rates of CABG and PCI are summarized in Table 2. In-hospital mortality rates per age group, CABG versus PCI and NSTEMI/CCS status are available in Supplementary Material, Table S4.
Table 2:
Use rate of coronary artery bypass grafting and multivessel percutaneous coronary intervention
| Men (%) | Women (%) | P-value | Unadjusted ORa | P-value | Adjusted ORa,b | P-value | E-value | |
|---|---|---|---|---|---|---|---|---|
| All ages | ||||||||
| Overall | NE = 94 870 | NE = 26 280 | ||||||
| MV-PCI | 15.1 | 25.8 | <0.001 | 0.47 (0.43–0.51) | <0.001 | 0.49 (0.44–0.54) | <0.001 | 3.5 |
| CABG | 84.9 | 74.2 | ||||||
| CCS | NE = 59 380 | NE = 14 275 | ||||||
| MV-PCI | 9.1 | 17.5 | <0.001 | 0.44 (0.38–0.51) | <0.001 | 0.45 (0.39–0.53) | <0.001 | 3.87 |
| CABG | 90.9 | 82.5 | ||||||
| NSTEMI | NE = 35 490 | NE = 12 005 | ||||||
| MV-PCI | 25.1 | 35.7 | <0.001 | 0.53 (0.47–0.6) | <0.001 | 0.5 (0.44–0.58) | <0.001 | 3.4 |
| CABG | 74.9 | 64.3 | ||||||
| <61 years | ||||||||
| Overall | NE = 24 735 | NE = 6125 | ||||||
| MV-PCI | 15.9 | 24.7 | <0.001 | 0.53 (0.44–0.63) | <0.001 | 0.53 (0.43–0.66) | <0.001 | 3.2 |
| CABG | 84.1 | 75.3 | ||||||
| CCS | NE = 13 985 | NE = 3110 | ||||||
| MV-PCI | 9.1 | 16.7 | <0.001 | 0.45 (0.33–0.61) | <0.001 | 0.44 (0.3–0.63) | <0.001 | 4 |
| CABG | 90.9 | 83.3 | ||||||
| NSTEMI | NE = 10 750 | NE = 3015 | ||||||
| MV-PCI | 24.7 | 32.8 | <0.001 | 0.6 (0.47–0.76) | <0.001 | 0.57 (0.43–0.75) | <0.001 | 2.9 |
| CABG | 75.3 | 67.2 | ||||||
| 61–70 years | ||||||||
| Overall | NE = 34 760 | NE = 8990 | ||||||
| MV-PCI | 11.8 | 21.0 | <0.001 | 0.46 (0.39–0.54) | <0.001 | 0.49 (0.41–0.59) | <0.001 | 3.5 |
| CABG | 88.2 | 79.0 | ||||||
| CCS | NE = 22 350 | NE = 5135 | ||||||
| MV-PCI | 6.5 | 14.5 | <0.001 | 0.35 (0.27–0.45) | <0.001 | 0.39 (0.29–0.52) | <0.001 | 4.6 |
| CABG | 93.5 | 85.5 | ||||||
| NSTEMI | NE = 12 410 | NE = 3855 | ||||||
| MV-PCI | 21.3 | 29.6 | <0.001 | 0.6 (0.48–0.75) | <0.001 | 0.56 (0.44–0.7) | <0.001 | 2.7 |
| CABG | 78.7 | 70.4 | ||||||
| 71–80 years | ||||||||
| Overall | NE = 28 470 | NE = 8565 | ||||||
| MV-PCI | 14.1 | 22.9 | <0.001 | 0.51 (0.44–0.6) | <0.001 | 0.52 (0.44–0.63) | <0.001 | 3.3 |
| CABG | 85.9 | 77.1 | ||||||
| CCS | NE = 19 040 | NE = 4885 | ||||||
| MV-PCI | 9.0 | 15.3 | <0.001 | 0.52 (0.41–0.67) | <0.001 | 0.59 (0.45–0.78) | <0.001 | 2.8 |
| CABG | 91.0 | 84.7 | ||||||
| NSTEMI | NE = 9430 | NE = 3680 | ||||||
| MV-PCI | 24.4 | 33.0 | <0.001 | 0.58 (0.46–0.73) | <0.001 | 0.48 (0.37–0.62) | <0.001 | 3.6 |
| CABG | 75.6 | 67.0 | ||||||
| >80 years | ||||||||
| Overall | NE = 6905 | NE = 2600 | ||||||
| MV-PCI | 32.9 | 55.2 | <0.001 | 0.34 (0.26–0.44) | <0.001 | 0.31 (0.22–0.45) | <0.001 | 5.9 |
| CABG | 67.1 | 44.8 | ||||||
| CCS | NE = 4005 | NE = 1145 | ||||||
| MV-PCI | 24.0 | 43.2 | <0.001 | 0.33 (0.22–0.51) | <0.001 | 0.2 (0.1–0.4) | <0.001 | 9.5 |
| CABG | 76.0 | 56.8 | ||||||
| NSTEMI | NE = 2900 | NE = 1455 | ||||||
| MV-PCI | 45.2 | 64.6 | <0.001 | 0.39 (0.27–0.56) | <0.001 | 0.37 (0.24–0.59) | <0.001 | 4.8 |
| CABG | 54.8 | 35.4 | ||||||
OR of undergoing CABG versus multivessel PCI (men are the reference group).
Model was adjusted for age, diabetes mellitus, hypertension, prior sternotomy, chronic heart failure, peripheral vascular disease, chronic kidney disease, coagulopathy, liver disease, obesity, anaemia, cancer, dementia, race, insurance type and household income.
CABG: coronary artery bypass grafting; CCS: chronic coronary syndrome; MV-PCI: multivessel percutaneous coronary intervention; NE: national estimate; NSTEMI: non-ST elevation myocardial infarction; OR: odds ratio; PCI: percutaneous coronary intervention.
Subgroup analyses
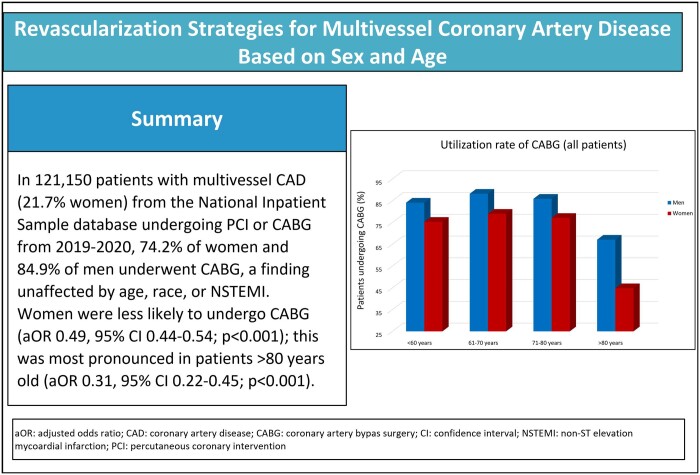
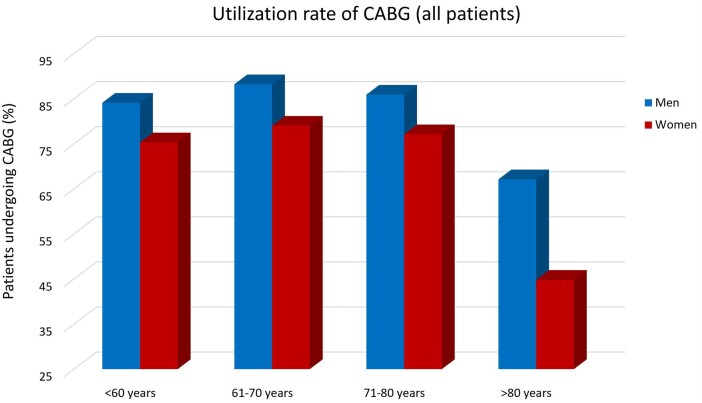
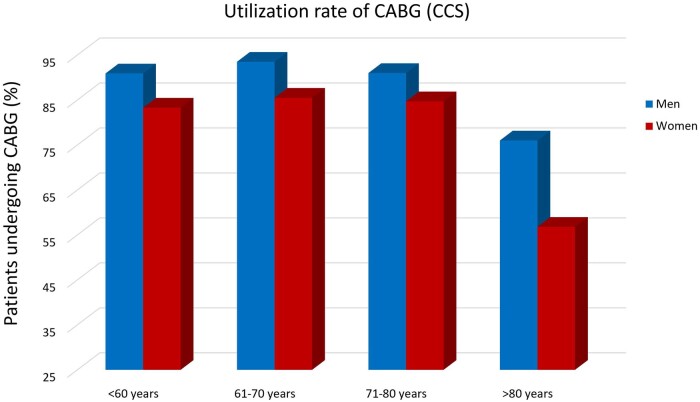
Stratified by all age groups, the majority of the patients of both sexes underwent CABG rather than PCI (Fig. 1). In patients younger than 61 years old, 75.3% of women and 84.1% of men underwent CABG, in patients aged 61–70 years old, 79.0% of women and 88.2% of men underwent CABG, in patients aged 71–80 years, 77.1% of women and 85.9% of men underwent CABG, and in the oldest patients (age >80), 44.8% of women and 67.1% of men underwent CABG. In all age groups, women were less likely than men to undergo CABG, but this disparity was most pronounced among the oldest patients (>80 years, aOR 0.31, 95% CI 0.22–0.45; P < 0.001). These results were consistent in the CCS and NSTEMI cohorts (Figs 2 and 3). Results of coronary revascularization strategies by age and clinical presentation are summarized in Table 2.
Figure 1:
Use rate of CABG in the included cohort. CABG: coronary artery bypass grafting.
Figure 2:
Use rate of CABG in patients with chronic coronary syndrome. CABG: coronary artery bypass grafting; CCS: chronic coronary syndrome.
Figure 3:
Use rate of CABG in patients with NSTEMI. CABG: coronary artery bypass grafting; NSTEMI: non-ST elevation myocardial infarction.
Within each sex, patients aged 61–70 and 71–80 years were equally, if not more, likely than the youngest patients (age <60 years) to undergo CABG. In both sexes, however, the oldest patient cohort (age >80 years) was less likely to undergo CABG when compared with patients aged <60 years (men: aOR 0.35, 95% CI 0.30–0.41; P < 0.001, women: aOR 0.22, 95% CI 0.17–0.29, P < 0.001). This finding was consistent in the CCS and NSTEMI cohorts. Results of within-sex comparisons of CABG use rates are presented in Table 3.
Table 3:
Use rate of coronary artery bypass grafting and multivessel percutaneous coronary intervention within each sex across different age groups
| Unadjusted ORa | P-value | Adjusted ORa,b | P-value | E-value | |
|---|---|---|---|---|---|
| Overall (CCS and NSTEMI) | |||||
| Men | |||||
| <60 years (reference) | 1 | NA | 1 | NA | |
| 61–70 years | 1.46 (1.3–1.64) | <0.001 | 1.49 (1.28–1.73) | <0.001 | 2.3 |
| 71–80 years | 1.15 (1.02–1.29) | 0.026 | 1.26 (1.05–1.51) | 0.015 | 1.6 |
| >80 years | 0.35 (0.3–0.41) | <0.001 | 0.4 (0.32–0.51) | <0.001 | 4.4 |
| Women | |||||
| <60 years (reference) | 1 | NA | 1 | NA | |
| 61–70 years | 1.38 (1.12–1.71) | 0.003 | 1.52 (1.16–1.99) | 0.002 | 2.1 |
| 71–80 years | 1.19 (0.96–1.47) | 0.106 | 1.41 (1.06–1.89) | 0.019 | 1 |
| >80 years | 0.22 (0.17–0.29) | <0.001 | 0.29 (0.2–0.42) | <0.001 | 8.6 |
| CCS | |||||
| Men | |||||
| <60 years (reference) | 1 | NA | 1 | NA | |
| 61–70 years | 1.52 (1.25–1.84) | <0.001 | 1.32 (1.03–1.68) | 0.026 | 2 |
| 71–80 years | 0.98 (0.81–1.19) | 0.864 | 0.88 (0.65–1.17) | 0.37 | 1 |
| >80 years | 0.27 (0.21–0.34) | <0.001 | 0.25 (0.18–0.36) | <0.001 | 7.5 |
| Women | |||||
| <60 years (reference) | 1 | NA | 1 | NA | |
| 61–70 years | 1.25 (0.89–1.75) | 0.202 | 1.23 (0.8–1.89) | 0.353 | 1 |
| 71–80 years | 1.16 (0.83–1.63) | 0.388 | 1.22 (0.77–1.92) | 0.401 | 1 |
| >80 years | 0.19 (0.12–0.3) | <0.001 | 0.17 (0.09–0.32) | <0.001 | 11.2 |
| NSTEMI | |||||
| Men | |||||
| <60 years (reference) | 1 | NA | 1 | NA | |
| 61–70 years | 1.25 (1.07–1.46) | 0.006 | 1.54 (1.27–1.88) | <0.001 | 2.4 |
| 71–80 years | 1.06 (0.89–1.26) | 0.504 | 1.53 (1.2–1.95) | 0.001 | 2.4 |
| >80 years | 0.34 (0.27–0.43) | <0.001 | 0.47 (0.35–0.65) | <0.001 | 3.7 |
| Women | |||||
| <60 years (reference) | 1 | NA | 1 | NA | |
| 61–70 years | 1.28 (0.97–1.68) | 0.082 | 1.62 (1.15–2.27) | 0.006 | 2.6 |
| 71–80 years | 1.04 (0.79–1.39) | 0.766 | 1.51 (1.03–2.21) | 0.036 | 2.4 |
| >80 years | 0.22 (0.15–0.33) | <0.001 | 0.38 (0.23–0.61) | <0.001 | 4.7 |
OR of undergoing CABG versus multivessel PCI.
Model was adjusted for age, diabetes mellitus, hypertension, prior sternotomy, chronic heart failure, peripheral vascular disease, chronic kidney disease, coagulopathy, liver disease, obesity, anaemia, cancer, dementia, race, insurance type and household income.
CABG: coronary artery bypass grafting; CCS: chronic coronary syndrome; MV-PCI: multivessel percutaneous coronary intervention; NA: not applicable; NE: national estimate; NSTEMI: non-ST elevation myocardial infarction; OR: odds ratio.
Sensitivity analysis
The sensitivity analysis adjusted for institutional PCI and CABG volume was consistent with the main analysis (aOR for women to undergo CABG 0.49, 95% CI 0.44–0.54; P < 0.001). This finding was similar for patients with CCS (aOR 0.0.45, 95% CI 0.39–0.53; P < 0.001) and for those with NSTEMI (aOR 0.5, 95% CI 0.44–0.57; P < 0.001). The sensitivity analysis for race was consistent with the main analysis (aOR for white women to undergo CABG 0.47, 95% CI 0.42–0.53; P < 0.001, aOR for non-white women to undergo CABG 0.51, 95% CI 0.42–0.62; P < 0.001).
DISCUSSION
In this analysis of 121 150 patients (26 280 [21.7%] women) from a US national database, the majority of men and women with multivessel CAD who underwent revascularization received CABG rather than PCI. This finding was consistent at all ages including octogenarians, and among patients presenting with NSTEMI, and was consistent among white and non-white patients.
Overall, women received CABG less frequently than men, but the absolute difference was most evident in the subgroup of patients aged over 80 years. This sex disparity was not associated with large differences in demographics, socioeconomic factors, comorbidities, or hospital procedural volumes between the sexes, suggesting a possible systematic bias in the referral of women for surgical revascularization. CABG has been associated with worse outcomes in women compared with men, including shorter-term mortality and major adverse cardiac events [30–33], which may discourage healthcare providers from offering CABG to women. However, overall, the vast majority of women received CABG, demonstrating that CABG remains recognized as the standard of care for multivessel CAD in both sexes. It is also reassuring to note that in 2014, the sex disparity in CABG use among Medicare beneficiaries in the USA was more pronounced than in our analysis (127 per 100 00 person-years for women and 392 per 100 person-years for men) [33], and this may be the signal of a progressive reduction of the sex-related gap in referral to surgery.
The difference between sexes in CABG use rate was greatest in the oldest patients (80 years and older). It is important to note that there is evidence that the sex difference in adverse CABG outcomes disappears in older patients [30]. An individual patient data meta-analysis including 4 randomized trials and 13 193 patients (2714 women) found that overall, women had a significantly higher risk of major adverse cardiac and cerebrovascular events (adjusted hazard ratio 1.12, 95% CI 1.04–1.21; P = 0.004) [30], but age was a significant sex effect modifier (P for interaction <0.001), and the excess risk was not seen in women aged 75 and above (adjusted hazard ratio 1.29, 95% CI 1.17–1.43 for women <75 years and 0.94, 95% CI 0.83–1.05 for women 75 years and above) [30]. This finding may be related to the lower prevalence of non-atherosclerotic CCS in older women [34, 35] and highlights the need to investigate the factors that deny older women access to the potentially life-prolonging surgery.
Limitations
The substantially lower number of women versus men included in this analysis suggests referral bias that preceded the diagnosis of multivessel CAD. In addition, the NIS does not provide detailed coronary artery anatomical or echocardiographic information, and therefore data that may predispose referral to 1 revascularization approach (for instance, target vessel and conduit quality, ejection fraction) were not captured. The NIS also fails to capture data on patient frailty, which may have predisposed physicians to refer patients to a less invasive revascularization strategy. The NIS also does not capture patient preference, although, in the Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) trial [7], anatomical inappropriateness and patient refusals accounted for only 16% of patients unsuitable for CABG. National estimates reported in this study are based on the stratified sampling design of the NIS and are subject to the inherent uncertainty associated with sampling. The NIS data are collected from billing claims, another possible source of error or bias. Lastly, the NIS captures in-hospital admissions only and PCIs that were performed in the outpatient setting are not captured in this database.
CONCLUSIONS
In this nationwide cohort study of 121 150 patients and 26 280 women with multivessel CAD, both men and women were more likely to undergo CABG rather than PCI for revascularization. This finding did not vary by age, race and clinical presentation. A sex disparity in the utilization of CABG was mostly evident among patients 80 years and older.
Supplementary Material
Glossary
ABBREVIATIONS
- aOR
Adjusted odds ratio
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- CCS
Chronic coronary syndrome
- CI
Confidence interval
- NIS
National Inpatient Sample
- PCI
Percutaneous coronary intervention
- SMDs
Standardized mean differences
Contributor Information
Samian Sulaiman, Division of Cardiology, West Virginia University, Morgantown, WV, USA; Department of Cardiovascular Disease, Mayo Clinic, Rochester, MN, USA.
Lamia Harik, Department of Cardiothoracic Surgery, Weill Cornell Medicine, New York, NY, USA.
C Noel Bairey Merz, Department of Cardiology, Barbra Streisand Women’s Heart Center, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Stephen E Fremes, Department of Surgery, Schulich Heart Centre, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
Ruth Masterson Creber, Columbia University School of Nursing, New York, NY, USA.
Lisa Q Rong, Department of Anesthesia, Weill Cornell Medicine, New York, NY, USA.
Mohamad Alkhouli, Department of Cardiovascular Disease, Mayo Clinic, Rochester, MN, USA.
Mario Gaudino, Department of Cardiothoracic Surgery, Weill Cornell Medicine, New York, NY, USA.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
No funding is reported for this article.
Conflict of interest: Mario Gaudino receives research grants from the National Institutes of Health, the Canadian Health and Research Institutes and the Starr Foundation. C. Noel Bairey Merz is supported by the National Institutes of Health R01HL124649, U54 AG065141, the Edythe L. Broad and the Constance Austin Women's Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women's Health Research (SWHR), Washington, DC, the Linda Joy Pollin Women's Heart Health Program, the Erika Glazer Women's Heart Health Project and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California. Lisa Q. Rong is supported by the National Institutes of Health K23 HL153836. Lamia Harik is supported by the National Heart, Lung, and Blood Institute T32HL160520-01A1. Stephen E. Fremes receives research grants from the National Institutes of Health, the Canadian Institutes of Health Research, Medtronic, Boston Scientific and Amgen. Ruth Masterson Creber receives research funding from the National Institutes of Health (R01HL161458, R01NS123639, R01HL152021) and PCORI. The other authors report no conflict of interest.
DATA AVAILABILITY
The data were obtained via the National Inpatient Sample (NIS) from the Agency for Healthcare Research and Quality (AHRQ), which is the largest publicly available all-payer inpatient database in the USA (https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp) [20]. The data underlying this article will shared on reasonable request to the corresponding author.
Author contributions
Samian Sulaiman: Data curation; Formal analysis; Methodology; Software; Writing—original draft; Writing—review & editing. Lamia Harik: Conceptualization; Data curation; Methodology; Visualization; Writing—original draft; Writing—review & editing. C. Noel Bairey Merz: Methodology; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Stephen E. Fremes: Methodology; Supervision; Validation; Writing—original draft; Writing—review & editing. Ruth Masterson Creber: Methodology; Validation; Visualization; Writing—original draft; Writing—review & editing. Lisa Q. Rong: Methodology; Supervision; Validation; Writing—review & editing. Mohamad Alkhouli: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing—original draft; Writing—review & editing. Mario Gaudino: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Ari Mennander and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. D'Ascenzo F, Presutti DG, Picardi E, Moretti C, Omedè P, Sciuto F. et al. Prevalence and non-invasive predictors of left main or three-vessel coronary disease: evidence from a collaborative international meta-analysis including 22 740 patients. Heart 2012;98:914–9. [DOI] [PubMed] [Google Scholar]
- 2. Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C. et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J 2007;28:1709–16. [DOI] [PubMed] [Google Scholar]
- 3. Weintraub WS, King SB, Douglas JS, Kosinski AS.. Percutaneous transluminal coronary angioplasty as a first revascularization procedure in single-, double- and triple-vessel coronary artery disease. J Am Coll Cardiol 1995;26:142–51. [DOI] [PubMed] [Google Scholar]
- 4. Giustino G, Baber U, Aquino M, Sartori S, Stone GW, Leon MB. et al. Safety and efficacy of new-generation drug-eluting stents in women undergoing complex percutaneous coronary artery revascularization. JACC Cardiovasc Interv 2016;9:674–84. [DOI] [PubMed] [Google Scholar]
- 5. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM. et al. 2021 ACC/AHA/SCAI Guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e4–e17. https://www.ahajournals.org/doi/10.1161/CIR.0000000000001039 [DOI] [PubMed] [Google Scholar]
- 6. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U. et al. ; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165.30165437 [Google Scholar]
- 7. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ. et al. ; SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–72. [DOI] [PubMed] [Google Scholar]
- 8. Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A. et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629–38. [DOI] [PubMed] [Google Scholar]
- 9. Thuijs DJFM, Kappetein AP, Serruys PW, Mohr FW, Morice MC, Mack MJ. et al. ; SYNTAX Extended Survival Investigators. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019;394:1325–34. [DOI] [PubMed] [Google Scholar]
- 10. Gul B, Shah T, Head SJ, Chieffo A, Hu X, Li F. et al. Revascularization options for females with multivessel coronary artery disease. JACC Cardiovasc Interv 2020;13:1009–10. [DOI] [PubMed] [Google Scholar]
- 11. Head SJ, Milojevic M, Daemen J, Ahn JM, Boersma E, Christiansen EH et al Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 2018;391:939–48. [DOI] [PubMed] [Google Scholar]
- 12. Holm NR, Mäkikallio T, Lindsay MM, Spence MS, Erglis A, Menown IBA. et al. ; NOBLE investigators. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet 2020;395:191–9. [DOI] [PubMed] [Google Scholar]
- 13. Park SJ, Ahn JM, Kim YH, Park DW, Yun SC, Lee JY. et al. ; BEST Trial Investigators. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204–12. [DOI] [PubMed] [Google Scholar]
- 14. Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL.. Everolimus-eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med 2015;372:1213–22. [DOI] [PubMed] [Google Scholar]
- 15. Gudnadottir GS, Andersen K, Thrainsdottir IS, James SK, Lagerqvist B, Gudnason T.. Gender differences in coronary angiography, subsequent interventions, and outcomes among patients with acute coronary syndromes. Am Heart J 2017;191:65–74. [DOI] [PubMed] [Google Scholar]
- 16. Desai R, Singh S, Fong HK, Goyal H, Gupta S, Zalavadia D. et al. Racial and sex disparities in resource utilization and outcomes of multi-vessel percutaneous coronary interventions (a 5-year nationwide evaluation in the United States). Cardiovasc Diagn Ther 2019;9:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fink N, Nikolsky E, Assali A, Shapira O, Kassif Y, Barac YD. et al. Revascularization strategies and survival in patients with multivessel coronary artery disease. Ann Thorac Surg 2019;107:106–11. [DOI] [PubMed] [Google Scholar]
- 18. Huckaby LV, Seese LM, Sultan I, Gleason TG, Wang Y, Thoma F. et al. The impact of sex on outcomes after revascularization for multivessel coronary disease. Ann Thorac Surg 2020;110:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 20.NIS Database Documentation [Internet]. [cited 2023 Jun 14]. Available from: https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp
- 21. Alkhouli M, Alqahtani F, Jneid H, Al Hajji M, Boubas W, Lerman A.. Age-stratified sex-related differences in the incidence, management, and outcomes of acute myocardial infarction. Mayo Clin Proc 2021;96:332–41. [DOI] [PubMed] [Google Scholar]
- 22.Stephanie Glen. “Pooled Standard Deviation—Statistics How To.” from StatisticsHowTo.com: Elementary Statistics for the rest of us! [Internet]. [cited 2023 Sep 28]. Available from: https://www.statisticshowto.com/pooled-standard-deviation/.
- 23. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Academic Press, 2013, 459 p. [Google Scholar]
- 25. Saito Y, Tateishi K, Kanda M, Shiko Y, Kawasaki Y, Kobayashi Y. et al. Volume‐outcome relationships for percutaneous coronary intervention in acute myocardial infarction. J Am Heart Assoc 2022;11:e023805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim LK, Looser P, Swaminathan RV, Minutello RM, Wong SC, Girardi L et al Outcomes in patients undergoing coronary artery bypass graft surgery in the United States based on hospital volume, 2007 to 2011. J Thorac Cardiovasc Surg 2016;151:1686–92. [DOI] [PubMed] [Google Scholar]
- 27. Gutacker N, Bloor K, Cookson R, Gale CP, Maynard A, Pagano D. et al. ; as part of the ECHO collaboration. Hospital surgical volumes and mortality after coronary artery bypass grafting: using international comparisons to determine a safe threshold. Health Serv Res 2017;52:863–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. VanderWeele T. Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford, New York: Oxford University Press, 2015, 728 p. [Google Scholar]
- 29. StataCorp. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC, 2019. [Google Scholar]
- 30. Gaudino M, Di Franco A, Alexander JH, Bakaeen F, Egorova N, Kurlansky P. et al. Sex differences in outcomes after coronary artery bypass grafting: a pooled analysis of individual patient data. Eur Heart J 2021;43:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi D, Zhang B, Motamed M, Lee S, Wang P, McLaren C. et al. Higher mortality in women after coronary artery bypass: meta-analysis and bias analysis of confounding. Ann Thorac Surg 2022;113:674–80. [DOI] [PubMed] [Google Scholar]
- 32. Enumah ZO, Canner JK, Alejo D, Warren DS, Zhou X, Yenokyan G. et al. Persistent racial and sex disparities in outcomes after coronary artery bypass surgery: a retrospective clinical registry review in the drug-eluting stent era. Ann Surg 2020;272:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Angraal S, Khera R, Wang Y, Lu Y, Jean R, Dreyer RP. et al. Sex and race differences in the utilization and outcomes of coronary artery bypass grafting among medicare beneficiaries, 1999–2014. J Am Heart Assoc 2018;77:e009014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Norris CM, Yip CYY, Nerenberg KA, Clavel M, Pacheco C, Foulds HJA et al State of the science in women’s cardiovascular disease: a Canadian perspective on the influence of sex and gender. J Am Heart Assoc 2020;9:e015634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barrett-Connor E. Menopause, atherosclerosis, and coronary artery disease. Curr Opin Pharmacol 2013;13:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data were obtained via the National Inpatient Sample (NIS) from the Agency for Healthcare Research and Quality (AHRQ), which is the largest publicly available all-payer inpatient database in the USA (https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp) [20]. The data underlying this article will shared on reasonable request to the corresponding author.