Abstract
For this work, iron oxide nanoparticles are synthesized by the co-precipitation method with stoichiometric amounts of Fe2+ and Fe3+ salts in a 1:2 ratio in distilled water and the pH is raised by adding an aqueous ammonia solution by controlled dripping. Nanoparticles precipitating after the reaction time are magnetically filtered and stored in ethanol for further analysis. Superparamagnetic Fe3O4 nanoparticles with a slight deviation from the stoichiometry are obtained, with sizes between 7.4 and 12.8 nm and saturation magnetization between 40 and 78 emu/gr. At pH 6, rod-shaped nanoparticles are obtained in addition to spherical ones. With a statistical design, it is shown how the morphological, structural and magnetic properties of the resulting nanoparticles can be manipulated by the synthesis parameters, offering many possibilities to tailor the materials to a wide range of applications.
Keywords: Nanoparticles, Superparamagnetic: magnetite, Co-precipitation method, Experimental design
Highlights
-
•
Experimental design study to influence nanoparticle properties by the synthesizing parameters.
-
•
Morphological, structural and magnetic characterization of superparamagnetic nanoparticles.
-
•
The results offer many possibilities to tailor materials for a wide range of applications.
1. Introduction
Magnetic nanoparticles (MNP) are versatile materials that have practical uses in fields like magnetic recording, drug delivery, bioimaging, hyperthermia, photothermal therapy, sensors, water purification, and magnetic separation [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]]. Different kinds of iron oxide nanoparticles are promising, with Fe3O4 and γ-Fe2O3 being the most extensively explored [12]. In particular, magnetite (Fe3O4) displays the highest level of magnetism and saturation magnetization (Ms) at room temperature, measuring 84 emu/g.
One important aspect of nanoscale materials is their significantly larger surface area relative to bulk matter, which leads to distinct physicochemical properties. Specifically, magnetite nanoparticles with diameters less than 30 nm demonstrate superparamagnetic behavior, exhibiting a strong response to external magnetic fields with no residual magnetization and reduced agglomeration tendencies [4].
For most applications, nanoparticles need to have uniform size and shape while being well dispersed in a solvent. The synthesis method typically controls the size and shape of the nanoparticles. Various techniques are available to synthesize magnetite nanoparticles, including co-precipitation, thermal decomposition, hydrothermal and solvothermal, sol-gel and polyol, microemulsion, electrospray, sonochemical and microwave-assisted, and biosynthesis methods [2,[8], [9], [10], [11], [12], [13], [14]]. Due to its rapid processing, efficient reaction conditions, simplicity and high yield, wet chemical synthesis by co-precipitation is widely used [13]. In general, novel phenomena can be achieved by careful control of particle size and dispersion in composites. Accordingly, synthesis represents the critical parameter in developing crystalline iron-oxide polymorphs that have desirable and distinctive properties well-suited for various technological applications [15].
While numerous articles discuss the co-precipitation synthesis of magnetite, there is no consensus regarding the regulation of properties through synthesis parameters. Instead, there is a preference for the conventional use of the Massart procedure [16], accompanied by slight modifications that do not account for parameter interrelationships. Additionally, a particular case study with a statistical approach that covers only some of these parameters has been considered [13].
The aim of this study is to synthesize iron oxide nanoparticles while varying conditions, such as the drip rate of the alkaline solution. These modifications may affect the properties of the nanoparticles, leading to differences in the resulting product. It is, therefore, crucial to analyze the impact of such changes on the properties of the nanoparticles.
We used a statistical approach to describe and forecast the influence of certain vital synthesis parameters, which is crucial in producing tailored MNPs for numerous applications. We examined the morphological, structural, and magnetic characteristics of the synthesized magnetite nanoparticles. A model was generated to predict particle properties accurately within the studied range of synthesis parameters, offering numerous possibilities for material customization across a wide range of applications.
2. Materials and methods
2.1. General information
This study optimized nanoparticle synthesis variables via iron co-precipitation using the Box-Behnken design (BBD) and response surface methodology (RSM). Magnetic properties of MNP were characterized with a vibrating sample magnetometer (VSM), whereas morphological, chemical, and structural properties were analyzed by transmission electron microscopy (TEM) and powder X-ray diffraction (XRD). All experiments were conducted using STATGRAPHICS Centurion XVI software, based on the "design of experiments" (DoE) methodology. The resulting models were further validated through additional experiments.
2.2. Experimental design and statistical analysis
To identify the optimal combination of co-precipitation variables for synthesizing MNP, we utilized a three-level, four-factor Box-Behnken design (BBD) based on the preliminary range of co-precipitation variables established through single-factor experiments. The BBD constitutes a rotating spherical response surface that features a center point, as well as midpoints between the corners, all of which are circumscribed on a sphere. This design, determined by the process requirements, can optimize multiple chemical and physical processes with a suitable number of experiments [[17], [18], [19], [20]].
We selected the alkalization drip rate (VNH4), reaction time (Tres), temperature (Temp), and stirring rate (Stirr) as the parameters of interest. All syntheses were executed in a precisely controlled reaction setup.
The Box-Behnken design was then applied to an expanded study range that covered the preliminary parameters. Thus, the VNH4 factor was evaluated between 10 and 90 ml/h, Tres between 10 and 50 min, Temp between 30 and 90 °C, and Stirr between 300 and 900 rpm (Table 1).
Table 1.
BBD experiments parameters.
| PH | V_NH4 | T_reac | Temp | Stirr | |
|---|---|---|---|---|---|
| BBD01 | 9 | 50 | 30 | 30 | 300 |
| BBD02 | 9 | 50 | 30 | 60 | 600 |
| BBD03 | 9 | 10 | 30 | 30 | 600 |
| BBD04 | 9 | 10 | 30 | 60 | 900 |
| BBD05 | 9 | 10 | 30 | 90 | 600 |
| BBD06 | 9 | 90 | 10 | 60 | 600 |
| BBD07 | 9 | 10 | 30 | 60 | 300 |
| BBD08 | 9 | 50 | 30 | 90 | 300 |
| BBD09 | 9 | 50 | 30 | 60 | 600 |
| BBD10 | 9 | 90 | 30 | 60 | 300 |
| BBD11 | 9 | 50 | 50 | 30 | 600 |
| BBD12 | 9 | 10 | 10 | 60 | 600 |
| BBD13 | 9 | 90 | 30 | 90 | 600 |
| BBD14 | 9 | 90 | 50 | 60 | 600 |
| BBD15 | 9 | 50 | 30 | 90 | 900 |
| BBD16 | 9 | 50 | 50 | 90 | 600 |
| BBD17 | 9 | 50 | 50 | 60 | 900 |
| BBD18 | 9 | 50 | 30 | 30 | 900 |
| BBD19 | 9 | 50 | 50 | 60 | 300 |
| BBD20 | 9 | 50 | 30 | 60 | 600 |
| BBD21 | 9 | 90 | 30 | 60 | 900 |
| BBD22 | 9 | 50 | 10 | 30 | 600 |
| BBD23 | 9 | 50 | 10 | 90 | 600 |
| BBD24 | 9 | 90 | 30 | 30 | 600 |
| BBD25 | 9 | 50 | 10 | 60 | 300 |
| BBD26 | 9 | 10 | 50 | 60 | 600 |
| BBD27 | 9 | 50 | 10 | 60 | 900 |
The chosen variables for synthesizing the nanoparticles were: mean diameter of the resulting nanoparticles (NPsize and NPxrd), expressed in nm; the distribution of the nanoparticle sizes (Disp), expressed as a percentage of NPsize; the agglomeration of the nanoparticles (Aglo), expressed in arbitrary units.; the saturation magnetization (Ms), expressed in emu/gr; the coercive field (Hc), expressed in Oerted; the remnant magnetization (Mr), expressed in emu/gr; and the oxidation state of magnetite (3-x of F3-xO4). To minimize the effects of unanticipated variables, all experiments were conducted in random order.
The generalized polynomial model of the second order that is used in response surface analysis is described in Eq. (1).
| (1) |
where Y represents the response variable; Ao, Ai, Aii, and Aij are the regression coefficients for the intercept, linear, quadratic, and interaction terms, respectively; and Xi and Xj are the independent variables where i is not equal to j. A positive or negative sign indicates a direct or inverse effect of the variable on the target, respectively. The models were used to assess the impact of each independent variable on the responses.
STATGRAPHICS Centurion XVI (version 16.1.03) was used for the analysis of the experimental design and the calculation of the predicted data. The effectiveness of the polynomial model equations was measured via the coefficient of determination R2 using analysis of variance (ANOVA). This method was utilized to assess variable impacts, interactions, and the statistical significance of the models. The optimal synthesis conditions were estimated through regression analysis and 3D response surface plots. To confirm the experimental statistical strategies' validity, we conducted three additional experiments. (See Ref. [21] for more information on experimental design and statistical analysis.).
In general, our results are statistically significant for the resulting effects, which indicates that the fitted models explain approximately 70 % or more of the variability in all the cases.This implies that the data have high variability, but still show a significant trend. This trend indicates that even though the data points are far from the regression line, the predictor variable is still providing information about the response.
2.3. Syntesis of iron oxide nanoparticles
In theory, the co-precipitation synthesis method entails the addition of a base, typically NaOH or NH3 (aq), to a distilled water solution containing Fe+2 and Fe+3 salts in a 1:2 stoichiometric ratio, which matches the stoichiometry for magnetite precisely. This process aims to produce magnetite nanoparticles with high purity. Eq. (2) shows the resulting reaction.
| (2) |
In this work, a specific set of 27 magnetite NPs samples were synthesized by co-precipitation according to a Box-Behnken experimental design.
For each synthesis, 486 mg of FeCl3.6.H2O and 250 mg of FeSO4.7.H2O were specifically diluted in a total of 60 mL of distilled water. The resulting mixture was added to a three-necked glass flask with an additional 60 ml of heated distilled water, reaching the specified temperature on a hotplate in a silicone bath. The final volume of the solution was 120 ml. The system is used sealed with septum plugs through which a constant flow of N2 is injected to prevent oxidation during the synthesis. At the same time, the mixture is stirred at Stirr speed with a mechanical PTFE stirrer with four 45° angled blades. Once the desired temperature has been reached, a 28 % ammonia solution is injected with a programmable syringe pump at VNH4 until a pH of 9 is achieved. The system maintains these conditions until the indicated Tres time has elapsed. The resulting solution is magnetically filtered using a neodymium permanent magnet. Then, 100 ml of deionized water is added again to wash the nanoparticles, followed by sonication for 1 min. This process is repeated thrice, and in the final step, the water is replaced by 40 ml of ethanol. Finally, each sample is labeled and stored in two clear glass vials with a capacity of 20 mL. One of the vials is then oven-dried at 30 °C.
It is important to note that all samples were replicated consistently to achieve accurate results for proper analysis.
2.4. Characterization of nanoparticles
2.4.1. TEM
Transmission electron microscopy was utilized to assess the morphology of the nanoparticles. Since the nanoparticles are agglomerated, the measure of the average size of each sample were made measuring hundreds of nanoparticle diameters individually in multiple TEM images. These data are used to create a histogram, which is finally satisfactorily distributed according to a function f(D) described by a log-linear distribution (Eq. (3)).
| (3) |
where D0 is the mean value of particle diameter and σ2 is the variance.
2.4.2. XRD
Powder XRD patterns were obtained with PAN analytical, X'pert PRO using 40 kV, 30 mA, Cu Kα radiation (λ = 1.5405 Å) in the scanning range of 10°–65° (2θ).
X-ray diffraction was used for structural characterization, considering that the patterns provide information on particle size while the relative intensities of the peaks provide the information to identify the crystalline phase.
The Debye-Scherrer equation (Eq. 4) is a widely used tool to calculating the mean crystallite size, considering the broadening of a peak in the spectrum at half height.
| (4) |
where D is the average diameter of the nanoparticles, k is Sherrer's constant which can be assumed to be 0.89 for magnetite, B is the full width at half maximum or the broadening of the diffraction line in radians, λ is the wavelength of the X-ray used, and 2ϴ is the diffraction angle.
2.4.3. VSM
The magnetic properties of MNP were characterized using a vibrating sample magnetometer, wich provides information on the magnetic moment as a function of the applied magnetic field. This information represents the corresponding magnetization curve, wich was used to obtain the saturation magnetization (Ms), remnant magnetization (Mr) and coercivity (Hc).
3. Results and discussion
3.1. Effect of the pH on iron oxide
In a preliminary analysis, varying the pH, we obtained specific results for pH 6, 9, and 11. In the TEM images, Fig. 1 (a, b, c), it can be seen that in all three cases spherical nanoparticles were synthesized, except that in the case of pH 6, that were observed mixed with nanorods. In the TEM images it is also possible to visualize the lattice spacing of individual nanoparticles for each case. In Fig. 1, part (a) displays a nanorod having a lattice spacing of 0.46 and 0.18 nm that corresponds to twice the (200) and (211) goethite planes, respectively. Moreover, there is a rhombohedron that can be visualized with a lattice spacing of 0.44 and 0. Three types of nanoparticles were identified through XRD diffractogram analysis of the samples, as shown in Fig. 2 (a, b): (a) Rods measuring 15 nm corresponded to the (210) and (310) planes of goethite, each occurring twice; (b) A nanoparticle with a lattice spacing of 0.58 nm corresponds to two (200) magnetite planes; and (c) Another nanoparticle with a lattice spacing of 0.52 nm corresponds to 2 times (311) magnetite plane. Therefore, it is possible to determine that the identified rods are made of goethite.
Fig. 1.
TEM images of the nanoparticles for (a) pH 6, (b) pH 9, (c) pH 11.
Fig. 2.
XRD diffractograms of the resulting nanoparticles for (a) pH 6, (b) pH 9 and pH 11, with their structural pattern identification.
This study presents a clear demonstration of the reaction and formation pathways in the co-precipitation of magnetite nanoparticles. The addition of base to the iron solution resulted in the nuclei and growth of nanoparticles passing through the goethite phase before reaching the magnetite phase, corroborating previous findings [13,[22], [23], [24], [25]]. In the diffractograms obtained for pH9 and pH11 it is possible to observe that the peak positions are slightly shifted towards higher 2θ values, indicating that the lattice parameter is contracted potentially due to the eventual oxidation of magnetite (This will be discussed later in this article.). On the other hand, the Ms measurements obtained for the samples (Fig. 3), with values corresponding to 41.5, 77.8 and 68.2 emu/g, for the samples at pH 6, 9 and 11, respectively. The magnetic properties of the nanoparticles decrease as pH increases above 9. Thus, it is evident that pH plays a critical role in the formation pathway, rather than a specific ammonia dilution (molar ratio between ammonia and iron ions) as stated in a recent paper [3].
Fig. 3.
VMS of samples. Magnetic moment vs the applied magnetic field.
Based on the data, pH 9 was chosen as it guarantees the creation of a nanoparticulate material with exceptional magnetic properties [26]. Likewise, after some tests prior to those presented in this work, in which an ambient environment was used, with the presence of oxygen, vs. a controlled environment, with nitrogen injection, it was decided to work in a controlled environment in order to generate an oxygen-free environment to avoid uncontrolled oxidation of the material.
It should be noted that even the manipulation of the synthesis parameters for the specific obtention of goethite could be particularly useful in some applications taking advantage of its shape [3].
3.2. Experimental design results
3.2.1. Effects on NP
The average diameters of all resulting nanoparticles ranged from 7.4 to 12.8 nm, as determined from the TEM images. Using the presented statistical tool we obtained the estimated effects and interactions of the selected parameters. We use the Pareto Chart of the standardized effects (Fig. 4). In this graph, the effects are represented as horizontal bars, in decreasing order of importance. In addition, a vertical blue line is displayed. Crossing this line with an effect signifies a significant difference from zero with 95 % confidence. Thus, it can be seen that the relationship between VNH4 and Tres is the most important factor in determining the NPsize. Moreover, the greater the correlation value, the bigger the size of the nanoparticles. The explanation rests on the fact that the lower the VNH4 the longer it takes for the reaction to come to completion. As a result, less subsequent Tres is required for the reaction to reach completion.
Fig. 4.
Standardized Pareto chart for NPsize. Parameters of interest: (A) V_NH4, alkalizing drip rate; (B) T_res, reaction time; (C) Temp, temperature; and (D) Stirr, stirring rate.
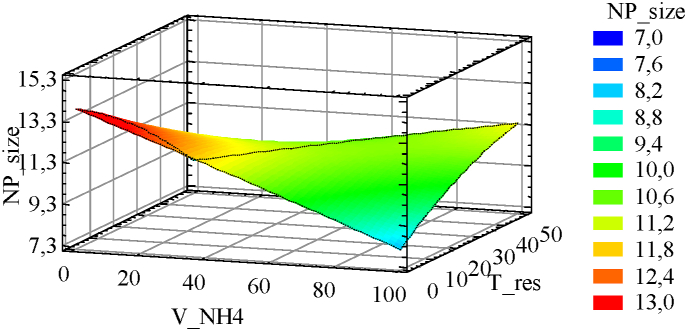
Taking into account the aforementioned interrelationship, observing the estimated response surface of Fig. 5, it is possible to predict that the largest nanoparticle size will be obtained when both VNH4 and Tres are at their minimum values. This means that the aforementioned intermediate times, relative to VNH4, favor particle formation and growth to a greater extent than Tres. Furthermore, the rapid addition of the ammonia solution to the reaction solution favors continuous nucleation with respect to growth, thus allowing the formation of small particles [27], and a slow addition of the base allows to promote growth with respect to nucleation. Thus, Three would correspond mostly to time associated with the continuity of particle growth rather than particle nucleation.
Fig. 5.
Estimated response surface of NPsize with Temp and Stirr set to 60 and 600 respectively.
From the XRD patterns, it was observed that all the resulting nanoparticles had average diameters between 7.5 and 17.8 nm. The Pareto Chart of the standardized effects (Fig. 6) shows that the interrelationship between VNH4 and Tres is the most important factor in determining the NPXRD. The higher the value of VNH4 and Tres interrelationship, the larger the nanoparticle size.
Fig. 6.
Standardized Pareto chart for NPXRD. Parameters of interest: (A) V_NH4, alkalizing drip rate; (B) T_res, reaction time; (C) Temp, temperature; and (D) Stirr, stirring rate.
Although it is possible to observe a difference between the sizes obtained by the two analysis systems, particularly visible towards the larger sizes, it is possible to confirm that the main dependence is related to the interrelation between VNH4 and Tres. The similarity of the low size values between the two measurement systems confirms that the peak broadening is essentially due to size effects and that microtensions can be neglected. The difference in larger sizes can be explained by considering a slight broadening of the diffraction peaks due to a partial shift caused by partial oxidation of magnetite. Something similar has been proposed earlier [28] and will be corroborated later in this article.
The results of the model obtained through experimental design are presented in Eq. (4).The adjusted equation predicts NPsize values between 8.6 and 14.1 nm.
With this achievable size range, we have nanoparticles that at least double their specific surface area, which allows for size management to optimize the specific surface area according to the needs of each application.
| (4) |
3.2.2. Effects on disp
Assuming a size dispersion dependent on the NP obtained, expressed as a percentage thereof, all the design results correspond to dispersions between 23.9 and 43.4 %. In this case, the Pareto Chart of the standardized effects (Fig. 7) shows that the square of VNH4 is the most important factor determining the Disp. The higher the value of this correlation, the greater the dispersion of the nanoparticles obtained.
Fig. 7.
Standardized Pareto chart for Disp. Parameters of interest: (A) V_NH4, alkalizing drip rate; (B) T_res, reaction time; (C) Temp, temperature; and (D) Stirr, stirring rate.
Considering this and observing its effects in Fig. 8, it is possible to predict that the minimum dispersion is obtained when VNH4 is at its middle value, around 50 ml/h. This means that a medium value of VNH4 favors the homogeneous nucleation and growth of the particles. Abruptly adding the base solution can cause pH inhomogeneity in the reaction medium before complete mixing, which alters the reaction rate and pathway [23], favoring particle nucleation over growth. Conversely, adding the base solution very slowly favors particle growth over nucleation.
Fig. 8.
Estimated response surface of Disp with Temp and Stirr set to 60 and 600 respectively.
The results of the model obtained are shown in Eq. (5). The adjusted equation predicts Disp values between 15.7 and 45.6 %, and can be optimized according to the needs of the application.
| (5) |
3.2.3. Effects on aglo
The agglomeration of the nanoparticles was qualitatively analyzed in relation to their total size observed in the TEM images, using an arbitrary scale from 0 to 10. From the results, an analysis of the effect of the parameters was also performed. According to Fig. 9, the level of particle agglomeration is primarily determined by the interaction between VNH4 and Tres. A lower value of this relationship leads to smaller Aglo.
Fig. 9.
Standardized Pareto chart for Aglo. Parameters of interest: (A) V_NH4, alkalizing drip rate; (B) T_res, reaction time; (C) Temp, temperature; and (D) Stirr, stirring rate.
Observing the response surface in Fig. 10, it is possible to conclude that the synthesis time is the parameter with the highest incidence in the agglomeration of the nanoparticles. This in turn implies that agglomeration is inversely related to particle size, reaching the minimum when VNH4 and Tres are at their minimum values.
Fig. 10.
Estimated response surface of Aglo with Temp and Stirr set to 60 and 600 respectively.
The resulting model is shown in Eq. (6). With this model Aglo values can be optimized as a function of the application.
| (6) |
So far, we can say that if we want low dispersion and less agglomerated nanoparticles, the parameters to use during the synthesis would be with low VNH4 and Tres, without the values of Temp or Stirr having a greater incidence. This could be very good for application cases where there is no option to increase the temperature, either for feasibility or cost savings.
3.2.4. Effects on Ms
From the VSM results, it was observed that all the nanoparticles have superparamagnetic behavior with Ms between 40.2 and 72 emu/gr. All of these values are excellent as they facilitate the effortless collection of all nanoparticles using a standard magnet in just one step [26]. Using the statistical effects and interactions (Fig. 11), it is possible to observe that the interrelation between VNH4 and Tres is the most important factor in determining the Ms. Furthermore, the higher the value of said interrelation, the greater the saturation of the magnetization. This agrees with previous research as larger sizes of magnetite nanoparticles within the range where superparamagnetic behavior occurs exhibit greater saturation magnetization [24,29].
Fig. 11.
Standardized Pareto chart for Ms. Parameters of interest: (A) V_NH4, alkalizing drip rate; (B) T_res, reaction time; (C) Temp, temperature; and (D) Stirr, stirring rate.
In fact, observing the response surface in Fig. 12, it is possible to notice that it is very similar to that observed for NPsize, impling that the Ms is directly related to the NP. The explanation of this will be the subject of discussion later in this article, after the topic of nanoparticle stoichiometry has been discussed.
Fig. 12.
Estimated response surface of Ms with Temp and Stirr set to 60 and 600 respectively.
The model results obtained are shown in Eq. (7). The adjusted equation predicts Ms values between 46 and 76.2 emu/gr, and can be optimized depending on the application.
| (7) |
For example, how to obtain 10 nm nanoparticles with almost 80 emu/gr and almost no temperature application, which would be ideal for industrial applications, has already been demonstrated at this point.
3.2.5. Effects on Mr
In the case of Mr, values between 1.1 and 5.4 emu/gr were obtained, which are quite low values and allow us to determine that MNPs are obtained that maintain their superparamagnetic properties. This enables their quick redistribution after the external magnetic field is eliminated [30]. Using the statistical effects and interactions (Fig. 13) it is possible to observe that the Tres x Temp and VNH4 x Tres interrelationships are the most important factors in determining the Mr. Moreover, the lower the value of Tres x Temp and the higher the value of VNH4 x Tres interrelationships, the higher the remnant magnetization. The second interaction in turn implies that Mr depends on Ms and therefore on the particle size.
Fig. 13.
Standardized Pareto chart for Mr. Parameters of interest: (A) V_NH4, alkalizing drip rate; (B) T_res, reaction time; (C) Temp, temperature; and (D) Stirr, stirring rate.
Considering this and observing its effects in Fig. 14, it can be predicted that the minimum remnant magnetization is obtained when Tres and Temp are in their maximum values. This means that the high temperature and the long reaction time during the process favor the stability of the superparamagnetic properties of the nanoparticles. This could be explained considering that the reaction time favors particle growth and the temperature facilitates structural ordering, thus allowing its correct and complete formation.
Fig. 14.
Estimated response surface mesh plot of Mr with Stirr set to 600.
The resulting model is shown in Eq. (8). The adjusted equation predicts Mr values from 0.8 to 6.9 emu/gr, that can be optimized depending on the needs of the application.
| (8) |
3.2.6. Effects on Hc
In the case of Hc, values between 8.9 and 37.2 Oersted were obtained, which is very good in all cases because they are very small values, which ensures the obtaining of superparamagnetic nanoparticles because very small coercivity is often regarded as a signature of superparamagnetism [31]. The Pareto Chart of the standardized effects (Fig. 15) shows that, as in the case of Mr, there is a strong dependence on Ms, as well as the interrelation between Tres and Temp, but there is also an inverse relationship with Temp.
Fig. 15.
Standardized Pareto chart for Hc. Parameters of interest: (A) V_NH4, alkalizing drip rate; (B) T_res, reaction time; (C) Temp, temperature; and (D) Stirr, stirring rate.
Observing the response surface in Fig. 16, it is possible to establish that the minimum coercitivity is obtained when Tres and Temp are in their maximum values. This confirm what was mentioned above, that a high temperature during the process and a long reaction time favor the completion of the reaction and thus the stability of the superparamagnetic properties of the nanoparticles.
Fig. 16.
Estimated response surface mesh plot of Hc with Stirr set to 600.
The resulting model obtained are shown in Eq. (9). The adjusted equation predicts Hc values from 5 to 48 Oersted that can be optimized depending on the application.
| (9) |
3.2.7. Effects on Fe3-xO4
Most of the authors do not clarify the obtaining of magnetite or maghemite, but simply assume the phase obtained [32]. This is because it is difficult to distinguish these phases using classical characterizations. Nevertheless, there are authors who have shown that when the size is less than 20 nm, the magnetic hyperfine structure can be described as magnetite with a slight deviation from the stoichiometry and the overall formula can be written as Fe3-xO4 [32,33]. Considering this, we perform an XRD analysis of the obtained oxide nanoparticles to study possible variations and their possibility of control. Values between 2.886 and 3 were obtained for the relative deviation from the stoichiometry of Fe3O4, (3-x). The Pareto diagram of the standardized effects (Fig. 17) shows that Tres, Temp and VNH4 are determining factors in the stoichiometry of the magnetite obtained. The higher the values, in the presented order, the lower the stoichiometric variation obtained.
Fig. 17.
Standardized Pareto chart for (3-x). Parameters of interest: (A) V_NH4, alkalizing drip rate; (B) T_res, reaction time; (C) Temp, temperature; and (D) Stirr, stirring rate.
Considering the above effects and looking at the estimated response surface of Fig. 18, it can be seen that the most correct stoichiometric magnetite is obtained when Tres, Temp and VNH4 are at their maximum values. This implies that the stoichiometric deviation of the resulting magnetite decreases with increasing particle size and formation temperature. Furthermore, it is possible to explain that Tres is the most important factor, since it is precisely the time that favors growth over particle formation. This in turn can explain what has been observed for the magnetization, since if the larger the size, the more complete the stoichiometry, the closer its magnetic behavior will be to that of magnetite, and if its stoichiometry is more displaced, its magnetic behavior will increasingly resemble that of maghemite.
Fig. 18.
Estimated response surface mesh plot of (3-x) with Stirr set to 600.
The model results obtained are shown in Eq. (10). The adjusted equation predicts Fe3-xO4 with 3-x from 2.8 to 3, that can be optimized depending on the application.
| (10) |
3.2.8. Validation experiments
Finally, three validation experiments were carried out (Table 2). These experiments were chosen to be interesting to verify because they imply extremes in the parameters that turned out to be the most important.
Table 2.
Validation experiments parameters.
| PH | V_NH4 | T_res | Temp | Stirr | |
|---|---|---|---|---|---|
| M1 | 9 | 100 | 30 | 77 | 600 |
| M2 | 9 | 10 | 0 | 50 | 300 |
| M3 | 9 | 2 | 50 | 50 | 500 |
The first case M1 corresponds to extreme maximum VNH4. The result obtained for the NP was 12.6 nm, which corresponds quite well to the 13.1 that our model would predict for this case, with less than 4 % of error. In parallel, the Disp result of 35.1 % while the model predict 35.4 %, less than 1 % of error. For the M2 case, we extreme de minimum Tres value and use a low VNH4 to obtain a high value of Ms and high stoichiometry of the resulting magnetite. According to the corresponding model, the Ms should be of 74.3 emu/gr, and the experiment results in a value of 69.3 emu/gr, which is equivalent to 7.2 % of error. In parallel, the (3-x) result 2.939 while the model predict 2.887, 1.8 % of error. In the last case M3 we prove a extreme mínimum value of VNH4, which give us very good camparative results, since the NP varied by only 0.9 % (11.2 exp. vs 11.4 model) and the Ms by 26 % (65.3 exp. vs 48.3 model).
These results are completely satisfactory for the control of the properties of the resulting nanoparticles by managing the synthesis parameters. Even more so considering that in the case of magnetic properties, where we have the greatest differences, specifically at VNH4 extreme values, is where probably the speed of addition of the base solution would make it possible to modify which reaction pathway is dominant, thus favoring the obtaining of one oxide.
It should be noted that although this article did not carry out a complete study of the efficiency of the synthesis, the samples made after optimization of the parameters show a visually complete recovery of the material with a totally transparent remaining solution.
4. Conclusions
A statistical technique, rooted in "Design of Experiments" (DoE), was employed to design a reaction setup for co-precipitating specific iron oxide nanoparticles. The primary interest was to determine how reaction conditions affected the size and saturation magnetization of the output. The nanoparticles synthesized underwent analysis through XRD, TEM, and VSM methods.
The synthesis yields mainly spherical magnetite morphology, with pH as the decisive factor in the formation process. All samples display superparamagnetic behavior at room temperature. Successful synthesis of iron oxide nanoparticles is achieved in the size range of 7.4–12.8 nm with dispersion in the range of 21.4–42.1%. The nanoparticles demonstrate varying magnetic properties, featuring saturation magnetization ranging from 46 to 78 emu/g, a remarkably low coercive field, and remanent magnetization ranging from 7 to 37 Oe and 1–5 emu/g, respectively. The oxide nanoparticles are characterized as Fe3-xO4 with the alkali drop rate serving as the critical parameter for modifying "x." Overall, the alkalization drop rate and reaction time can be manipulated to control particle size and magnetic properties. The magnetic properties vary due to both size and composition, as the oxidation rate increases with decreasing size.
Finally, it has been demonstrated that optimizing the achieved models enables a selective choice of the synthesis parameters, depending on the preferred characteristics of the final nanoparticles. This creates new opportunities for more precise investigations in each application case.
Data availability
Data will be available on request.
CRediT authorship contribution statement
María José Inestrosa-Izurieta: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition. Diego Vilches: Investigation, Methodology. Julio I. Urzúa: Funding acquisition, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research has not receive any grants from public, commercial, or nonprofit funding agencies. The Chilean Nuclear Energy Commission provided resources to support this work. The authors express their gratitude to the Center for the Development of Nanoscience and Nanotechnology (CEDENNA) staff for their assistance with TEM and VSM measurements.
References
- 1.Sangaiya P., Jayaprakash R. A review on iron oxide nanoparticles and their biomedical applications. J. Supercond. Nov. Magn. 2018;31:3397–3413. doi: 10.1007/s10948-018-4841-2. [DOI] [Google Scholar]
- 2.Ansari S., Ficiarà E., Ruffinatti F., Stura I., Argenziano M., Abollino O., Cavalli R., Guiot C., D'Agata F. Magnetic iron oxide nanoparticles: synthesis, characterization and functionalization for biomedical applications in the central nervous system. Materials. 2019;12:465. doi: 10.3390/ma12030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han H., Sun W., Sun W., Hu Y. Magnetic separation of impurities from hydrometallurgy solutions and waste water using magnetic iron ore seeding. Iron Ores, IntechOpen. 2021:13. doi: 10.5772/intechopen.93728. [DOI] [Google Scholar]
- 4.Molina-Calderón L., Basualto-Flores C., Paredes-García V., Venegas-Yazigi D. Advances of magnetic nanohydrometallurgy using superparamagnetic nanomaterials as rare earth ions adsorbents: a grand opportunity for sustainable rare earth recovery. Sep. Purif. Technol. 2022;299 doi: 10.1016/j.seppur.2022.121708. [DOI] [Google Scholar]
- 5.Singh M., Dhiman S., Debnath N., Das S. Sustain. Nanotechnol. Environ. Remediat. Elsevier; 2022. Magnetic nanoparticles and their application in sustainable environment; pp. 457–483. [DOI] [Google Scholar]
- 6.Misra A., Zambrzycki C., Kloker G., Kotyrba A., Anjass M.H., Franco Castillo I., Mitchell S.G., Güttel R., Streb C. Water purification and microplastics removal using magnetic polyoxometalate‐supported ionic liquid phases (magPOM‐SILPs) Angew. Chem. Int. Ed. 2020;59:1601–1605. doi: 10.1002/anie.201912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi H.M., De M., Richter F., He J., V Prasad P., Dravid V.P. Effect of silica shell thickness of Fe3O4–SiOx core–shell nanostructures on MRI contrast. J. Nanoparticle Res. 2013;15:1448. doi: 10.1007/s11051-013-1448-1. [DOI] [Google Scholar]
- 8.Wegmann M., Scharr M. Precis. Med. Elsevier; 2018. Synthesis of magnetic iron oxide nanoparticles; pp. 145–181. [DOI] [Google Scholar]
- 9.Pinho S.L.C., Sereno J., Abrunhosa A.J., Delville M., Rocha J., Carlos L.D., Geraldes C.F.G.C. Gd- and Eu-loaded iron Oxide@Silica core–shell nanocomposites as trimodal contrast agents for magnetic resonance imaging and optical imaging. Inorg. Chem. 2019;58:16618–16628. doi: 10.1021/acs.inorgchem.9b02655. [DOI] [PubMed] [Google Scholar]
- 10.Condomitti U., Almeida S., Silveira A., de Melo F., Toma H. Green processing of strategic elements based on magnetic nanohydrometallurgy. J. Braz. Chem. Soc. 2018;29:948–959. doi: 10.21577/0103-5053.20180009. [DOI] [Google Scholar]
- 11.Popescu R.C., Andronescu E., Vasile B.S. Recent advances in magnetite nanoparticle functionalization for nanomedicine. Nanomaterials. 2019;9:1–31. doi: 10.3390/nano9121791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dar M.I., Shivashankar S.A. Single crystalline magnetite, maghemite, and hematite nanoparticles with rich coercivity. RSC Adv. 2014;4:4105–4113. doi: 10.1039/c3ra45457f. [DOI] [Google Scholar]
- 13.Roth H., Schwaminger S.P., Schindler M., Wagner F.E., Berensmeier S. Influencing factors in the CO-precipitation process of superparamagnetic iron oxide nano particles: a model based study. J. Magn. Magn Mater. 2015;377:81–89. doi: 10.1016/j.jmmm.2014.10.074. [DOI] [Google Scholar]
- 14.Petcharoen K., Sirivat A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B. 2012;177:421–427. doi: 10.1016/j.mseb.2012.01.003. [DOI] [Google Scholar]
- 15.Kumar S., Kumar M., Singh A. Synthesis and characterization of iron oxide nanoparticles (Fe2O3, Fe3O4): a brief review. Contemp. Phys. 2021;62(3):144–164. doi: 10.1080/00107514.2022.2080910. [DOI] [Google Scholar]
- 16.Massart R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981;17:1247–1248. [Google Scholar]
- 17.Goktas S., Köse M.D., Bayraktar O. A screening experimental design to develop high extraction yield of flavonoids in Ginkgo biloba leaves. J. Pharm. Appl. Chem. 2017;3:33–39. doi: 10.18576/jpac/030104. [DOI] [Google Scholar]
- 18.Peng X., Yang G., Shi Y., Zhou Y., Zhang M., Li S. Box–Behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci. Rep. 2020;10:3595. doi: 10.1038/s41598-020-60339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhawan H., Upadhyayula S., Sharma D.K. Design of experiments to optimize the extraction parameters of a power grade Indian coal. Int. J. Coal. Sci. Technol. 2018;5:417–429. doi: 10.1007/s40789-018-0216-3. [DOI] [Google Scholar]
- 20.Da Silva Sousa C., Jr., Nascimento M., Yokoyama L., Galvão Caldas Da Cunha O. Experimental design in solvent extraction: a study for divalent metals separation in D2EHPA/isoparaffin system. Engineering. 2012;4:816–825. doi: 10.4236/eng.2012.411104. [DOI] [Google Scholar]
- 21.NIST/SEMATECH E-Handbook of Statistical Methods. 2013. https://www.itl.nist.gov/div898/handbook/pri/section3/pri3.htmhttps://www.itl.nist.gov/div898/handbook/ [Google Scholar]
- 22.Ahn T., Kim J.H., Yang H.M., Lee J.W., Kim J.D. Formation pathways of magnetite nanoparticles by coprecipitation method. J. Phys. Chem. C. 2012;116:6069–6076. doi: 10.1021/jp211843g. [DOI] [Google Scholar]
- 23.Baumgartner J., Dey A., Bomans P.H.H., Le Coadou C., Fratzl P., Sommerdijk N.A.J.M., Faivre D. Nucleation and growth of magnetite from solution. Nat. Mater. 2013;12:310–314. doi: 10.1038/nmat3558. [DOI] [PubMed] [Google Scholar]
- 24.Ra’ad Z., Alsahib N.K.A., Al-Karam L.Q. Synthesis of iron oxide nanoparticles using precipitation method with different pH values. AIP Conf. Proc. 2021;2372 doi: 10.1063/5.0067088. [DOI] [Google Scholar]
- 25.Jolivet J., Chanéac C., Tronc E. Iron oxide chemistry. From molecular clusters to extended solid networks. Chem. Commun. 2004;5:477–483. doi: 10.1039/B304532N. [DOI] [PubMed] [Google Scholar]
- 26.Basualto C., Gaete J., Molina L., Valenzuela F., Yañez C., Marco J.F. Lanthanide sorbent based on magnetite nanoparticles functionalized with organophosphorus extractants. Sci. Technol. Adv. Mater. 2015;16 doi: 10.1088/1468-6996/16/3/035010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascolo M.C., Pei Y., Ring T.A. Room temperature Co-precipitation synthesis of magnetite nanoparticles in a large ph window with different bases. Materials. 2013;6:5549–5567. doi: 10.3390/ma6125549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mürbe J., Rechtenbach A., Töpfer J. Synthesis and physical characterization of magnetite nanoparticles for biomedical applications. Mater. Chem. Phys. 2008;110:426–433. doi: 10.1016/j.matchemphys.2008.02.037. [DOI] [Google Scholar]
- 29.Wen X., Yang J., He B., Gu Z. Preparation of monodisperse magnetite nanoparticles under mild conditions. Curr. Appl. Phys. 2008;8:535–541. doi: 10.1016/j.cap.2007.09.003. [DOI] [Google Scholar]
- 30.Alfaro I., Molina L., González P., Gaete J., Valenzuela F., Marco J.F., Sáez C., Basualto C. Silica-coated magnetite nanoparticles functionalized with betaine and their use as an adsorbent for Mo(VI) and Re(VII) species from acidic aqueous solutions. J. Ind. Eng. Chem. 2019;78:271–283. doi: 10.1016/j.jiec.2019.06.002. [DOI] [Google Scholar]
- 31.Andersson R.L., Cabedo L., Hedenqvist M.S., Olsson R.T., Ström V. Superparamagnetic [sic] nanofibers by electrospinning. RSC Adv. 2016;6:21413–21422. doi: 10.1039/c5ra27791d. [DOI] [Google Scholar]
- 32.Carvalho M.D., Henriques F., Ferreira L.P., Godinho M., Cruz M.M. Iron oxide nanoparticles: the Influence of synthesis method and size on composition and magnetic properties. J. Solid State Chem. 2013;201:144–152. doi: 10.1016/j.jssc.2013.02.024. [DOI] [Google Scholar]
- 33.Santoyo Salazar J., Perez L., De Abril O., Truong Phuoc L., Ihiawakrim D., Vazquez M., Greneche J.M., Begin-Colin S., Pourroy G. Magnetic iron oxide nanoparticles in 10-40 nm range: composition in terms of magnetite/maghemite ratio and effect on the magnetic properties. Chem. Mater. 2011;23:1379–1386. doi: 10.1021/cm103188a. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available on request.