Abstract
Gaucher disease (GD) causes the accumulation of glucocerebrosides in various organs, resulting in hepatosplenomegaly, anemia, decreased platelet counts, and bone disorders. Glucosylsphingosine accumulates in the brain and causes central nervous system (CNS) disorders. GD can be classified into types I (without CNS disorders), II, and III. Substrate reduction therapy (SRT) is an oral therapy that improves patients' quality of life; however, its effect on type III GD is unknown. We administered SRT to GD types I and III patients and found it effective. Malignancy is a late complication of GD, but this is the first report of Barrett adenocarcinoma.
Keywords: Gaucher disease, glucocerebrosidase, glucocerebroside, glucosyl sphingosine, enzyme replacement therapy, substrate reduction therapy
Introduction
Gaucher disease (GD) is caused by mutations in the glucocerebrosidase gene, resulting in decreased glucocerebrosidase activity. Glucocerebrosidase substrates, mainly glucocerebrosides, accumulate in the reticuloendothelial system of the liver, spleen, and bone marrow, resulting in elevated serum angiotensin-converting enzyme (ACE) and acid phosphatase (ACP) levels, hepatosplenomegaly, anemia, decreased platelet count, bone pain, and pathological fractures (1). Glucosylsphingosine, a deacylated form of glucocerebroside, has been reported to accumulate in the brain and cause central nervous system (CNS) disorders (2), but this effect has not yet been fully established.
GD is an autosomal recessive inherited lysosomal disease. It is classified into types I, II, and III, according to the presence and severity of CNS disorders. GD type I has no CNS symptoms, whereas types II and III have these symptoms. GD type II has a more rapid onset and disease progression and a poorer prognosis than GD type III. The main treatment modalities include enzyme replacement therapy (ERT) and substrate reduction therapy (SRT). ERT is a more common treatment than SRT; however, it requires frequent visits to the hospital for the administration of intravenous infusion every two weeks. In contrast, SRT is administered orally and may improve patient's quality of life (QOL). The efficacy of SRT in GD type I has been reported (3,4); however, its efficacy in GD type III remains unknown.
In the present study, we administered SRT to men with GD types I and III and found it to be effective. We therefore report the possible effectiveness of SRT for GD type III. Malignancy is a late complication that should be considered during GD. We encountered a patient with Barrett adenocarcinoma and report our findings in him. Blood cancers are common malignancies associated with GD, and Barrett adenocarcinoma has not yet been reported.
Case Reports
Case 1
A 61-year-old man had been diagnosed with GD at infancy. He had suffered repeated fractures since infancy and had undergone splenectomy for splenomegaly at approximately seven years old. He had undergone ERT using imiglucerase at another hospital. One year before he visited our hospital, at 59 years old, he had developed symptomatic epilepsy and been referred to our neurosurgery department, where levetiracetam administration was initiated. He was referred to our department to consolidate treatments because he was to be admitted to a facility because of his declining activity of daily living (ADL).
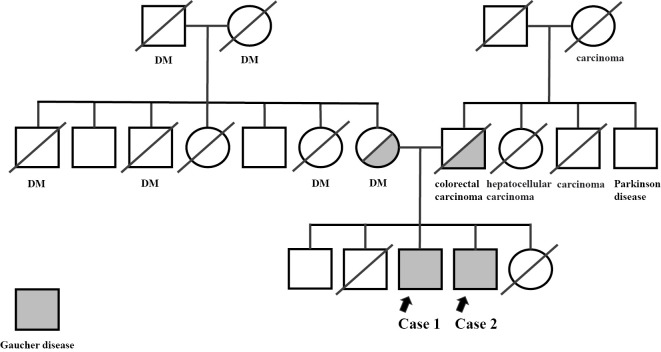
His family history revealed that both of his parents were carriers of beta-glucocerebrosidase (GBA) genes. He was the third son, and his brother, the fourth son, had GD as well (Fig. 1). The patient was receiving levetiracetam at 1,000 mg/day. His height, weight, body mass index (BMI), blood pressure, and pulse were 144.3 cm, 48.1 kg, 23.1 kg/m2, 103/58 mmHg, and 75 beats/min, respectively. Physical examination findings were normal, as were neurological findings, including the absence of ocular motility disorders, such as strabismus and horizontal gaze palsy. Blood tests at the first visit to our hospital showed that the serum ACE, hemoglobin levels and platelet counts were within the normal limits (Table 1). Homozygous L444P (c.1448T & gt; C, p.Leu483Pro) mutations were detected in the GBA gene. The CYP2D6 genotype was an Extensive Metabolizer (EM). Abdominal ultrasonography revealed no abnormal findings. A bone density test showed a lumbar spine value of 0.787 g/cm2, young adult mean (YAM) 79% (-1.3 standard deviations); however, the femoral neck could not be measured.
Figure 1.
Family tree. Half-gray and full-gray individuals are Gaucher disease carriers and developers, respectively. Slashes indicate deceased persons. Case 1 (arrow on the left). Case 2 (arrow on the right). DM: diabetes mellitus
Table 1.
Laboratory Findings at the First Visit for Case 1.
| Hematology | Cr | 0.68 | mg/dL | ||
| WBC | 5,740 | /μL | eGFR | 91 | mL/min |
| RBC | 448×104 | /μL | UA | 5.5 | mg/dL |
| Hb | 15.4 | g/dL | Ca | 9.5 | mg/dL |
| Ht | 44.5 | % | IP | 3.3 | mg/dL |
| Plt | 25.3×104 | /μL | CK | 49 | U/L |
| Amylase | 123 | U/L | |||
| Biochemistry | Na | 142 | mEq/L | ||
| TP | 6.6 | g/dL | K | 4 | mEq/L |
| Alb | 3.7 | g/dL | Cl | 107 | mEq/L |
| T-Bil | 0.6 | mg/dL | ACE | 23.1 | IU/L |
| ALP | 284 | U/L | HbA1c | 5.5 | % |
| γ-GTP | 23 | U/L | BG | 114 | mg/dL |
| AST | 16 | U/L | |||
| ALT | 13 | U/L | |||
| TG | 86 | mg/dL | Urinalysis | ||
| HDL-C | 61 | mg/dL | Protein | (-) | |
| LDL-C | 121 | mg/dL | Glucose | (-) | |
| BUN | 10.7 | mg/dL | Occult blood | (-) |
WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Ht: hematocrit, Plt: platelet, TP: total protein, Alb: albumin, T-Bil: total bilirubin, ALP: alkaline phosphatase, γ-GTP: gamma-glutamyl transpeptidase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, TG: triglyceride, HDL-C: high- density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, BUN: blood urea nitrogen, eGFR: estimated- glomerular filtration rate, ACE: angiotensin-converting enzyme, HbA1c: glycated hemoglobin, BG: blood glucose
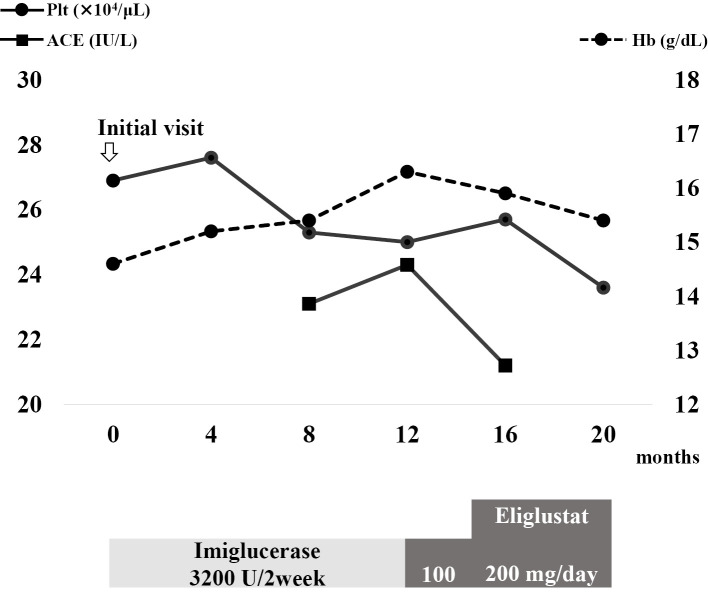
During its course, GD changed from type I to III due to the onset of epilepsy. The blood test results are shown in Fig. 2. After 12 months, the patient was switched to SRT with eliglustat as an initial dose of 100 mg/day. The dose was increased to 200 mg/day after no adverse effect was observed. His serum ACE level tended to decrease, whereas hemoglobin levels and platelet counts were maintained. Hepatomegaly was not observed, and the bone mineral density did not decrease.
Figure 2.
Changes in blood test results and treatment since the initial visit. The solid line with circular joints indicates the platelet count. The dotted line with circular joints indicates the hemoglobin level. The solid line with square joints indicates the serum ACE level. Plt: platelet, Hb: hemoglobin, ACE: angiotensin-converting enzyme
Case 2
A 57-year-old man, the younger brother of the patient in Case 1, was suspected of having congenital GD at infancy. He underwent splenectomy for splenomegaly at approximately three years old. He had repeatedly suffered pathological fractures at 10 and 20 years old, and he had undergone amputation of his right lower limb due to osteomyelitis at 29 years old. He was an occasional drinker and an ex-smoker. He had undergone ERT with imiglucerase at another hospital, and one year before he visited our hospital, he had been switched to SRT with eliglustat. He was therefore referred to our department.
At his initial visit, he was receiving eliglustat 200 mg/day and famotidine 20 mg/day. His height, weight, BMI, blood pressure, and pulse were 159.5 cm, 64.7 kg, 25.4 kg/m2, 135/96 mmHg, and 82 beats/min, respectively. Physical examination findings were normal, as were neurological findings, including the absence of ocular motility disorders, such as strabismus and horizontal gaze palsy. Blood tests at the first visit to our hospital showed that serum ACE and hemoglobin levels were within the normal limits, and the platelet count was mildly decreased (Table 2). Homozygous L444P mutations were detected in the GBA gene. The CYP2D6 genotype was an EM. Abdominal ultrasonography showed a fatty liver. A bone density test showed a lumbar spine and left femoral neck densities of 0.944 g/cm2 (YAM 94%, -0.4 standard deviations) and 0.890 g/cm2 (YAM 121%, 1.4 standard deviations), respectively. The patient was therefore diagnosed with GD type I without CNS disorders.
Table 2.
Laboratory Findings at the First Visit for Case 2.
| Hematology | Cr | 0.73 | mg/dL | ||
| WBC | 13,410 | /μL | eGFR | 87 | mL/min |
| RBC | 482×104 | /μL | UA | 7 | mg/dL |
| Hb | 14.7 | g/dL | Ca | 9.4 | mg/dL |
| Ht | 42 | % | IP | 2.8 | mg/dL |
| Plt | 13.2×104 | /μL | CK | 458 | U/L |
| Amylase | 65 | U/L | |||
| Biochemistry | Na | 138 | mEq/L | ||
| TP | 7.7 | g/dL | K | 3.9 | mEq/L |
| Alb | 3.7 | g/dL | Cl | 107 | mEq/L |
| T-Bil | 0.9 | mg/dL | ACE | 29.9 | IU/L |
| ALP | 493 | U/L | HbA1c | 6.1 | % |
| γ-GTP | 38 | U/L | BG | 105 | mg/dL |
| AST | 34 | U/L | |||
| ALT | 20 | U/L | |||
| TG | 50 | mg/dL | Urinalysis | ||
| HDL-C | 32 | mg/dL | Protein | (-) | |
| LDL-C | 95 | mg/dL | Glucose | (-) | |
| BUN | 15.4 | mg/dL | Occult blood | (-) |
WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Ht: hematocrit, Plt: platelet, TP: total protein, Alb: albumin, T-Bil: total bilirubin, ALP: alkaline phosphatase, γ-GTP: gamma-glutamyl transpeptidase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, TG: triglyceride, HDL-C: high- density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, BUN: blood urea nitrogen, eGFR: estimated- glomerular filtration rate, ACE: angiotensin-converting enzyme, HbA1c: glycated hemoglobin, BG: blood glucose
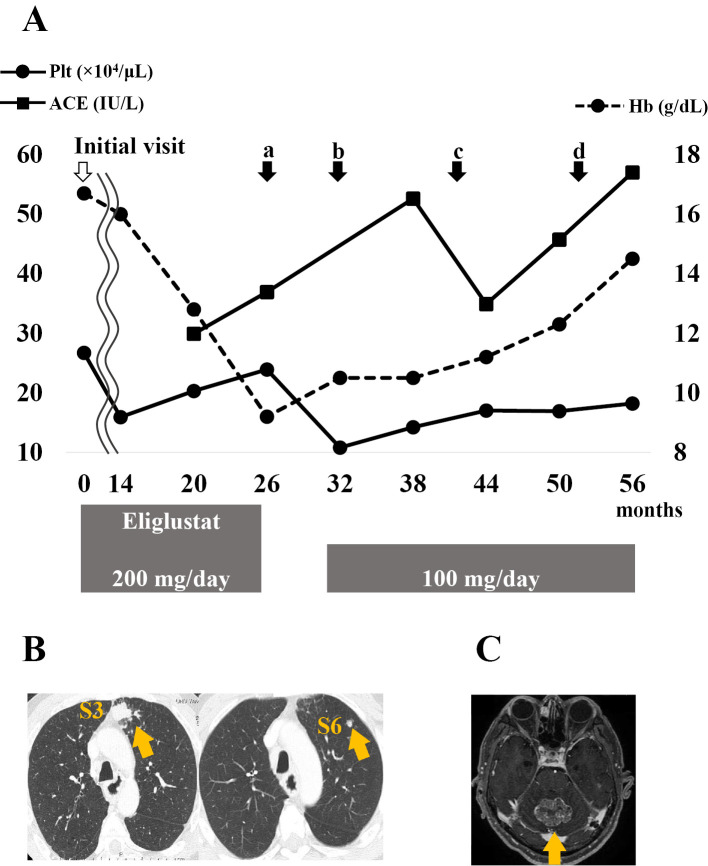
The blood test results are shown in Fig. 3. Hemoglobin levels tended to decrease over 26 months. Barrett adenocarcinoma was discovered, and thoracoscopic subtotal esophagectomy was performed after 26 months (Fig. 3A). The patient self-interrupted eliglustat therapy for six months postoperatively. He was administered clarithromycin for chronic sinusitis at 32 months, and the dose of eliglustat was reduced from 200 mg/day to 100 mg/day owing to its interaction with clarithromycin (Fig. 3A). After the eliglustat dose reduction, the serum ACE level increased and later decreased; however, it increased again at 56 months. The hemoglobin level and platelet count showed a gradually increasing trend.
Figure 3.
Changes in blood test results, events, and treatment since the initial visit. The solid line with circular joints indicates the platelet count. The dotted line with circular joints indicates the hemoglobin level. The solid line with square joints indicates the serum ACE level. (a) Subtotal esophagectomy for Barrett adenocarcinoma, (b) initiation of clarithromycin treatment for chronic sinusitis, and detection of (c) a lung tumor in S3 followed by that in S6 and (d) a cerebellar tumor. B: Computed tomography image of the lung tumors in S3 (left) and S6 (right) (arrows). C: Magnetic resonance imaging of the cerebellar tumor (arrow). Plt: platelet, Hb: hemoglobin, ACE: angiotensin-converting enzyme
The patient was followed up with computed tomography (CT) every 3 months after surgery for Barrett adenocarcinoma. Chest CT performed at 41 months showed a 12-mm mass in superior segment 3 (S3) of the left lung. During follow-up, the mass grew to 23 mm, and a new nodule was observed in S6 (Fig. 3B). A transbronchial lung biopsy showed no malignant cells, and the tumor markers for lung cancer were negative. However, based on the clinical signs, lung cancer was suspected, and thoracoscopic partial resection of the left lung upper lobe was performed at 52 months.
A pathological examination revealed a metastatic lung tumor from Barrett adenocarcinoma. The patient presented with lightheadedness, and magnetic resonance imaging showed a 40-mm mass in the cerebellum (Fig. 3C). This tumor was considered a metastatic brain tumor from Barrett adenocarcinoma. CyberKnife therapy with 37.1 Gy/7 fr for the cerebellum was performed, and tegafur/gimeracil/oteracil potassium was administered as chemotherapy. After treatment, the tumor in the cerebellum shrank.
Discussion
The number of GD cases worldwide is estimated to be approximately 5,000-10,000, but in Japan, the number is approximately 150, which is extremely small. GD is a rare disease that cannot be accurately diagnosed or treated. The accumulation of cases and dissemination of information are important considerations. The possibility of GD should be considered when hepatosplenomegaly, anemia, a decreased platelet count, or bone or CNS disorders are observed or when there are family members with GD. A definitive diagnosis can be made by confirming decreased glucocerebrosidase activity in cultured dermal fibroblasts or lymphocytes or by genetic testing for specific genetic mutations.
Three points are noteworthy in these two cases. First, Case 1 developed epilepsy during the course of the disease and transitioned from GD type I to type III. While 92% of patients overseas have GD type I (5), only approximately half of the patients in Japan have GD type I at the time of the initial diagnosis, and some cases develop GD type II or type III due to the development of CNS disorder during the course of the disease (6). The N370S mutation accounts for approximately 70% of all genetic mutations in Jews and is associated with the development of GD type I (7). In Japan, the N370S mutation has not been identified, but the L444P and F213I mutations are common (7,8). In the present two cases, the men had homozygous L444P mutations. The differences in genetic mutations between non-Japanese and Japanese populations may be related to differences in the distribution of GD types. We believe that the investigation of more cases will provide useful information concerning the correlation between the genotype and phenotype, prediction of complications and the prognosis, and the selection and development of treatments.
Second, SRT can be used effectively and safely in patients with GD Type III. In Japan, the two main therapies are ERT with imiglucerase or veraglucerase alfa and SRT with eliglustat. ERT breaks down accumulated glucocerebrosides, whereas SRT suppresses glucocerebroside synthesis and reduces its accumulation. Treatment endpoints include improvement in anemia, platelet counts, and serum ACE and ACP levels; hepatosplenomegaly resolution; and bone density improvement. Neither ERT nor SRT is effective for CNS disorder, and symptomatic treatment, such as antiepileptic drugs, is the main treatment modality (9).
Imiglucerase was approved in Japan in 1998 and has since become the mainstay of treatment. An eight-year efficacy and safety study of imiglucerase in Japanese GD types I, II, and III reported improvements in hemoglobin levels, platelet counts, liver and spleen volumes, and serum ACE and ACP levels. Adverse effects were observed in approximately 27% of patients; however, most cases were mild hypersensitivities, such as urticaria and a fever, and no instances of anaphylaxis were observed (10). These results showed that imiglucerase is effective and safe for all forms of GD in Japanese patients.
In addition, eliglustat was approved in Japan in 2015. A study on its effects on GD type I showed an improvement in hemoglobin levels, platelet counts, liver and spleen volumes, and bone densities and confirmed its non-inferiority to ERT (3,4). Its efficacy has only been reported for GD type I and is yet to be confirmed for GD type III. The available drug information on eliglustat states, “Since there is no experience in the use of this drug for GD type II and type III, it should be administered only when the benefits are judged to outweigh the risks, after a full explanation to the patient.” Important adverse effects include arrhythmia and syncope owing to cardiotoxicity. As eliglustat is mainly metabolized by CYP2D6, the CYP2D6 genotype should be confirmed before administration, since the genotype determines whether or not the drug can be administered and its dosage. In addition, the dosage and administration should be further adjusted if a concomitant drug has CYP2D6 or CYP3A inhibitory effects. In the present case, the CYP2D6 genotype in both brothers was an EM, and eliglustat was recommended at 200 mg/day. The patient in Case 2 received clarithromycin during his treatment, which may have slowed down the metabolism of eliglustat due to CYP3A inhibition, so a reduction in the dose of eliglustat was recommended. The patient in Case 1 had GD type III and was on imiglucerase; however, his declining ADL made frequent hospital treatment difficult; therefore, he was switched to eliglustat. After the switch, there was no progression of hemoglobin levels or platelet count decline, and serum ACE levels showed a trend toward improvement. This drug has been used safely and has no adverse effects. Antiepileptic drugs were administered to treat the CNS disorders, and the patient was seizure-free. This case is a valuable report of the effective treatment of GD type III with eliglustat. In the patient in Case 2, the decrease in hemoglobin levels despite treatment with eliglustat may have been due to gastrointestinal bleeding induced by Barrett adenocarcinoma and not due to deterioration of GD.
Third, the patient in Case 2 developed Barrett adenocarcinoma. Malignancy is a late complication that should be considered during GD. Hypergammaglobulinemia develops relatively early in GD (11). This can progress to hematologic cancers, such as multiple myeloma and non-Hodgkin B-cell lymphoma (12,13). Blood cancers are more common; however, other cancer types, such as breast, liver, pancreas, kidney, colon, lung, skin, and prostate cancers, have equally been reported (11,14). To our knowledge, this is the first report of esophageal cancer associated with GD.
There are some theories as to why GD develops into malignant tumors: (1) when glucocerebrosidase deficiency causes glucosylsphingosine to accumulate, its byproduct sphingosine also increases intracellularly, which directly activates carcinogenic signals and contributes to carcinogenesis (15); and (2) interleukin-6 secreted by macrophages that phagocytose glucocerebrosides activates a signaling system that leads to the development of multiple myeloma (16). However, no conclusions have been reached. Treatment with eliglustat reduces myeloma protein and B-cell malignancy (17). Currently, the relationship between GD and esophageal cancer remains unclear, so further studies on the pathogenesis of this disease with more cases are needed.
Conclusions
We encountered two men with GD. At present, SRT has been proven effective only for GD type I; however, it may be equally effective for GD type III. We administered SRT to GD type I and III patients and found it effective. It should be noted that malignancy may develop during the course of the disease.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We gratefully acknowledge the work of the past and present members of our department.
References
- 1. Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab 83: 6-15, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Nilsson O, Svennerholm L. Accumulation of glucosylceramide and glucosylsphingosine (psychosine) in cerebrum and cerebellum in infantile and juvenile Gaucher disease. J Neurochem 39: 709-718, 1982. [DOI] [PubMed] [Google Scholar]
- 3. Lukina E, Watman N, Dragosky M, et al. Eliglustat, an investigational oral therapy for Gaucher disease type 1: Phase 2 trial results after 4 years of treatment. Blood Cells Mol Dis 53: 274-276, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Cox TM, Drelichman G, Cravo R, et al. Eliglustat compared with imiglucerase in patients with Gaucher's disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomized, open-label, non-inferiority trial. Lancet 385: 2355-2362, 2015. [DOI] [PubMed] [Google Scholar]
- 5.The International Collaborative Gaucher Group. Gaucher Registry 2010 Annual Report (Data cutoff 31 Dec. 2009). Gen-zyme 2010. [Google Scholar]
- 6. Tajima A, Yokoi T, Ariga M, et al. Clinical and genetic study of Japanese patients with type 3 Gaucher disease. Mol Genet Metab 97: 272-277, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Horowitz M, Tzuri G, Eyal N, et al. Prevalence of nine mutations among Jewish and non-Jewish Gaucher disease patients. Am J Hum Genet 53: 921-930, 1993. [PMC free article] [PubMed] [Google Scholar]
- 8. Ida H, Rennert OM, Kawame H, Maekawa K, Eto Y. Mutation prevalence among 47 unrelated Japanese patients with Gaucher disease: identification of four novel mutations. J Inherit Metab Dis 20: 67-73, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Vaca GF, Lenz T, Knight EMP, Tuxhorn I. Gaucher disease: successful treatment of myoclonic status epilepticus with levetiracetam. Epileptic Disord 14: 155-158, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Ida H, Eto Y, Tanaka A. [Drug Clinics Efficacy and safety of Cerezyme in Japanese patients with Gaucher disease (types I, II, and III) based on the results of an 8-year post-marketing surveillance]. Shounikasinryou (J Pediatr Pract) 76: 1325-1334, 2013. (in Japanese). [Google Scholar]
- 11. Taddei TH, Kacena KA, Yang M, et al. The underrecognized progressive nature of N370S Gaucher disease and assessment of cancer risk in 403 patients. Am J Hematol 84: 208-214, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenbloom BE, Weinreb NJ, Zimran A, Kacena KA, Charrow J, Ward E. Gaucher disease and cancer incidence: a study from the Gaucher Registry. Blood 105: 4569-4572, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Zimran A, Liphshitz I, Barchana M, Abrahamov A, Elstein D. Incidence of malignancies among patients with type I Gaucher disease from a single referral clinic. Blood Cells Mol Dis 34: 197-200, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Biegstraaten M, Cox TM, Belmatoug N, et al. Management goals for type I Gaucher disease: an expert consensus document from the European working group on Gaucher disease. Blood Cells Mol Dis 68: 203-208, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139-150, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Cheung WC, Van Ness B. Distinct IL-6 signal transduction leads to growth arrest and death in B cells or growth promotion and cell survival in myeloma cells. Leukemia 16: 1182-1188, 2002. [DOI] [PubMed] [Google Scholar]
- 17. Pavlova EV, Archer J, Wang S, et al. Inhibition of UDP-glucosylceramide synthase in mice prevents Gaucher disease-associated B-cell malignancy. J Pathol 235: 113-124, 2015. [DOI] [PubMed] [Google Scholar]