Abstract
We herein report a patient with KRAS wild-type non-small-cell lung cancer (NSCLC) with concurrent STK11 and KEAP1 mutations. A 53-year-old man visited a local doctor with a complaint of left shoulder swelling and pain. He was diagnosed with NSCLC cT4N0M1c stage IVB. A comprehensive genome profile test revealed mutations in STK11 and KEAP1 but no KRAS mutations. The patient was refractory to radiotherapy, immunotherapy, and chemotherapy. Thus, STK11 and KEAP1 mutations can be considered resistance mutations that confer resistance to various anticancer therapies in KRAS wild-type NSCLC.
Keywords: non-small-cell lung cancer (NSCLC), therapy resistance, CGP test, KRAS mutation, STK11 mutation, KEAP1 mutation
Introduction
The development of lung cancer treatment using immune checkpoint inhibitors (ICIs) and their combinations have increased the number of long-term survivors. However, some patients are resistant to treatments and experience rapid cancer progression, possibly due to treatment-resistant mutations.
Comprehensive genome profile (CGP) testing has enabled us to comprehensively screen for cancer-related gene mutations in patients with cancer, and studies on resistance mutations are vital as well. Serine/threonine kinase 11 (STK11) and kelch-like ECH-associated protein 1 (KEAP1) mutations are known to cause resistance to lung cancer therapy. Previous studies have reported that STK11 and/or KEAP1 mutations were enriched in men diagnosed at a young age who had a smoking history or a high tumor mutation burden (TMB) (1). Recently, it was reported that KRAS mutation-positive non-small-cell lung cancer (NSCLC) patients with the combination of STK11 and KEAP1 demonstrated resistance to treatment with various drugs (2-4).
We herein report a KRAS wild-type NSCLC patient with co-mutations of STK11/KEAP1 refractory to radiotherapy, combined immunotherapy, and chemotherapy.
Case Report
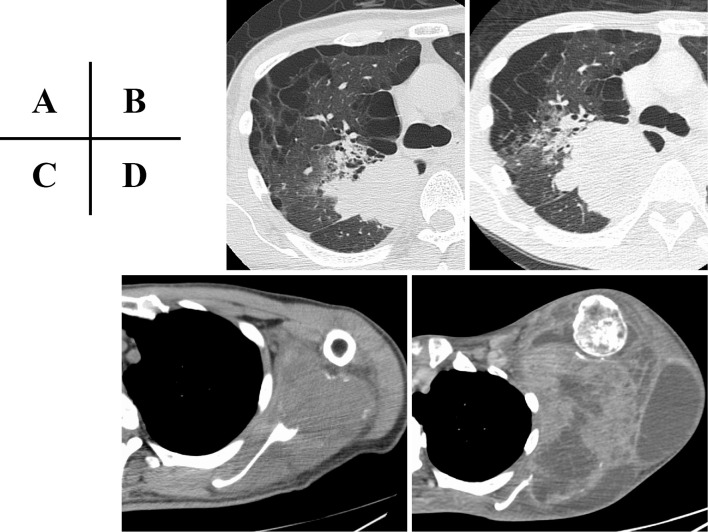
A 53-year-old man visited his local doctor with a complaint of left shoulder swelling and pain from the left shoulder to the left wrist joint since October 2020. Computed tomography (CT) revealed a mass in the right upper lobe of the lung and a large mass in the left scapula (Fig. 1A, C). A CT-guided needle biopsy was performed on the left scapular mass, and anaplastic non-small-cell carcinoma cells were detected (Fig. 2). A pathological evaluation revealed that the cells were negative for thyroid transcription factor-1 and p40. A blood test showed a high neutrophil/lymphocyte ratio (NLR) that was thought to have been caused by the STK11 mutation (5). Furthermore, multiple tumor markers were screened, including beta-human chorionic gonadotropin, but no trend implicating a specific histological subtype was observed (Table 1). Later, the patient was diagnosed with NSCLC cT4N0M1c stage IVB without any driver gene mutations showing 2% programmed death ligand-1 (PD-L1) expression. As the left scapular mass grew rapidly, accompanied by worsening pain, the patient was subjected to palliative irradiation (25 Gy/10 fr) starting in January 2021 prior to chemotherapy. However, both the left scapular mass and the right upper lobe lung mass grew eventually (Fig. 1B, D), with no improvement in his pain and a worsening of his general condition.
Figure 1.
Chest CT at the first visit depicting a 49×43-mm large mass in the upper lobe of the right lung (A) and a 76×60-mm metastatic bone mass with osteolytic changes in the left scapula (C). Two months later, the massed had grown to 80×45 mm (B) and 188×148 mm (D), respectively. CT: computed tomography
Figure 2.

Histopathological findings obtained by a CT-guided needle biopsy from the left scapula showed anaplastic non-small-cell carcinoma cells. CT: computed tomography
Table 1.
Laboratory Report on the First Visit.
| Hematology | Biochemistry | Tumor Marker | ||||||
| WBC | 13,550 | /μL | TP | 5.9 | g/dL | CEA | 12.4 | ng/mL |
| Neut | 86.8 | % | ALB | 1.8 | g/dL | NSE | 26 | ng/mL |
| Ly | 6.9 | % | T-Bil | 0.4 | mg/dL | ProGRP | 28.4 | pg/mL |
| Mo | 6.1 | % | BUN | 10.4 | mg/dL | CYFRA | 15.8 | ng/mL |
| Eo | 0.1 | % | Cr | 0.45 | mg/dL | SCC | 2.3 | ng/mL |
| Ba | 0.1 | % | AST | 38 | IU/L | sIL-2R | 956 | U/mL |
| RBC | 297 | ×104/μL | ALT | 22 | IU/L | β-hCG | 670 | mIU/mL |
| Hb | 7.7 | g/dL | LDH | 573 | IU/L | KL-6 | 1,168 | U/mL |
| Ht | 23.5 | % | Na | 132 | mEq/L | |||
| MCV | 79.1 | fL | K | 4.2 | mEq/L | |||
| MCHC | 32.8 | % | Cl | 94 | mEq/L | |||
| Plt | 59.9 | ×104/μL | Corrected Ca | 10.3 | mEq/L | |||
| CRP | 16.9 | mg/dL | ||||||
In February 2021, carboplatin+nab-paclitaxel+pembrolizumab therapy was administered at a low dose [carboplatin area under the curve (AUC) 4.5 on day 1; nab-paclitaxel 100 mg/m2, on day 1, 8, and 15; and pembrolizumab 200 mg/body, on day 1 and every 4 weeks] because his performance status was 2 based on the Eastern Cooperative Oncology Group scale. In addition, the CGP test (FoundationOneⓇ CDx Cancer Genome Profile Test), which is used in cancer genome medicine, was performed at the beginning of the treatment and identified STK11 and KEAP1 mutations, whereas KRAS was not detected (Table 2). Despite two treatment courses, however, his tumor grew, and his pain worsened. After a discussion with the patient, we decided to provide supportive care, and he died in April 2021.
Table 2.
Gene Mutations Detected in FoundationOne® CDx.
| APC N732fs*58 |
| CUL3 splice site 1207-2A>T |
| KEAP1 G417W |
| MUTYH splice site 892-2A>G |
| RET R77H |
| STK11 STK11(NM_000455) rearrangement exon 1 |
| TP53 R158L |
Discussion
We herein report a patient with KRAS wild-type NSCLC treated for a STK11/KEAP1 mutation who proved refractory to radiotherapy, immunotherapy, and chemotherapy.
Among the multiple gene mutations detected by next generation sequence (NGS) in this case, STK11 and KEAP1 mutations are reported as treatment-resistant mutations in lung cancer therapy. STK11 is a tumor suppressor gene found in 8-39% of advanced NSCLC cases (6). It has been reported that PD-L1 expression decreases when tumor cells acquire the STK11 mutation (7-9), and the overall survival (OS) of NSCLC patients with STK11 mutations declines when they are treated with ICIs (7,8,10). Furthermore, STK11 mutations are known to be involved in radiotherapy resistance acquisition via the suppression of the KEAP1/Nrf2 pathway. In fact, in a previous study or patients with stage III NSCLC treated with radiotherapy, those harboring an STK11 mutation had a higher local recurrence rate and shorter OS than STK11 wild-type patients (11). In contrast, KEAP1 mutations are detected in 11-27% of patients with NSCLC (6). KEAP1 mutations are known to increase chemotherapy and radiotherapy resistance due to the decreased activity of the KEAP1/Nrf2 pathway (12,13). In addition, the KEAP1 mutation is reported to be a poor prognostic factor in patients with NSCLC (7,14).
These mutations are frequently reported as resistance mutations in lung cancer, particularly in patients with KRAS mutation-positive lung cancer. Skoulidis and Arbour report that patients with KRAS mutation-positive lung cancer have STK11 or KEAP1 mutations frequently and have a significantly shorter OS than those without these mutations (2,3).
As mentioned above, mutations in STK11 and KEAP1 can independently cause significant resistance to treatment. Furthermore, the coexistence of these mutations increases resistance to treatment and is associated with a poor prognosis (15-17). Shen et al. reported that the median OS of patients with co-mutations of STK11/KEAP1 in KRAS mutation-positive lung cancer was only 7.3 months (17). As both STK11 and KEAP1 are involved in the KEAP1/Nrf2 pathway, inter-communication may enhance Nrf2 activity, leading to therapy resistance (18).
Interestingly, our patient did not have any KRAS mutations. While STK11 and KEAP1 mutations have mainly been reported as factors associated with treatment resistance in KRAS mutation-positive patients, the frequency of coexistence of STK11 and KEAP1 mutations in patients with KRAS wild-type NSCLC was recently reported to be 3.9% (7). In addition, Wohlhieter et al. showed that the coexistence of STK11 and KEAP1 mutations causes resistance to various anticancer therapies, even in patients with KRAS wild-type lung cancer (18). Thus, we considered the coexistence of STK11 and KEAP1 mutations to be the crucial cause of treatment resistance development in this case. There are currently no effective treatments for lung cancer cases with these mutations. This highlights the need for the development of novel drugs that can selectively inhibit these genes.
Conclusions
This case report indicates that the combination of STK11 and KEAP1 mutations causes resistance to radiotherapy and combined immunotherapy and chemotherapy, even in patients with KRAS wild-type lung cancer. Thus, it should be emphasized that the presence of STK11 and KEAP1 co-mutations inflicts treatment resistance, regardless of the KRAS mutation status.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported by JSPS KAKENHI (Grant Number 20K17182).
References
- 1. Papillon-Cavanagh S, Doshi P, Dobrin R, et al. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open 5: e000706, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 8: 822-835, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arbour KC, Jordan E, Kim HR, et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res 24: 334-340, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riely GJ, Jordan E, Kim HR, et al. Association of outcomes and co-occurring genomic alterations in patients with KRAS-mutant non-small cell lung cancer. J Clin Oncol 34: 9019, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Proulx-Rocray F, Routy B, Mohamad Nassabein R, et al. The prognostic impact of KRAS, TP53, STK11 and KEAP1 mutations and the influence of the NLR in NSCLC patients with immunotherapy. J Clin Oncol 39: e21010, 2021. [DOI] [PubMed] [Google Scholar]
- 6. Di Federico A, De Giglio A, Parisi C, Gelsomino F. STK11/LKB1 and KEAP1 mutations in non-small cell lung cancer: prognostic rather than predictive? Eur J Cancer 157: 108-113, 2021. [DOI] [PubMed] [Google Scholar]
- 7. Cordeiro de Lima VC, Corassa M, Saldanha E, et al. STK11 and KEAP1 mutations in non-small cell lung cancer patients: descriptive analysis and prognostic value among Hispanics (STRIKE registry-CLICaP). Lung Cancer 170: 114-121, 2022. [DOI] [PubMed] [Google Scholar]
- 8. Wang H, Guo J, Shang X, Wang Z. Less immune cell infiltration and worse prognosis after immunotherapy for patients with lung adenocarcinoma who harbored STK11 mutation. Int Immunopharmacol 84: 106574, 2020. [DOI] [PubMed] [Google Scholar]
- 9. Koyama S, Akbay EA, Li YY, et al. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T cell activity in the lung tumor microenvironment. Cancer Res 76: 999-1008, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shire NJ, Klein AB, Golozar A, et al. STK11 (LKB1) mutations in metastatic NSCLC: prognostic value in the real world. PLOS ONE 15: e0238358, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sitthideatphaiboon P, Galan-cobo A, Negrao MV, et al. LKB1 mutations in NSCLC are associated with KEAP1/NRF2-dependent radiotherapy resistance targetable by glutaminase inhibition. Clin Cancer Res 27: 1720-1733, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohta T, Iijima K, Miyamoto M, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res 68: 1303-1309, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Jeong Y, Hoang NT, Lovejoy A, et al. Role of KEAP1/NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiotherapy response prediction. Cancer Discov 7: 86-101, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goeman F, De Nicola F, Scalera S, et al. Mutations in the KEAP1-NFE2L2 pathway define a molecular subset of rapidly progressing lung adenocarcinoma. J Thorac Oncol 14: 1924-1934, 2019. [DOI] [PubMed] [Google Scholar]
- 15. Arbour K, Shen R, Plodkowski A, et al. MA19.09 concurrent mutations in STK11 and KEAP1 is associated with resistance to PD-(L)1 blockade in patients with NSCLC despite high TMB. J Thorac Oncol 13: S424, 2018. [Google Scholar]
- 16. Papillon-Cavanagh S, Doshi P, Dobrin R, Szustakowski J, Walsh AM. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open 5: e000706, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen R, Martin A, Ni A, et al. Harnessing clinical sequencing data for survival stratification of patients with metastatic lung adenocarcinomas. JCO Precis Oncol 3: PO.18.00307, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wohlhieter CA, Richards AL, Uddin F, et al. Concurrent mutations in STK11 and KEAP1 promote ferroptosis protection and SCD1 dependence in lung cancer. Cell Rep 33: 108444, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]