Abstract
Objective
Despite aggressive therapeutic interventions during the acute phase of branch atheromatous disease (BAD)-type cerebral infarction, many patients, even those with a mild condition at the onset, experience neurological deterioration after hospitalization and develop serious deficits. We compared the therapeutic efficacy of multiple antithrombotic therapies for BAD between patients who received a clopidogrel loading dose (loading group; LG) and those without loading (non-loading group; NLG).
Patients
Between January 2019 and May 2022, patients with BAD-type cerebral infarction in the lenticulostriate artery admitted within 24 h of the onset were recruited. This study included 95 consecutive patients who received combination argatroban and dual antiplatelet therapy (aspirin and clopidogrel).
Methods
Patients were classified into the LG and NLG according to whether or not a loading dose of clopidogrel (300 mg) had been administered on admission. Changes in neurological severity [National Institutes of Health Stroke Scale (NIHSS) score] during the acute phase were retrospectively evaluated.
Results
There were 34 (36%) and 61 (64%) patients in the LG and NLG, respectively. On admission, the median NIHSS score was similar between the groups [LG: 2.5 (2-4) vs. NLG: 3 (2-4), p=0.771]. At 48 h following admission, the median NIHSS scores were 1 (0.25-4), and 2 (1-5) in the LG and NLG, respectively (p=0.045). Early neurological deterioration (END; defined as worsening of the NIHSS score by ≥4 points at 48 h after admission) occurred in 3% of LG and 20% of NLG patients (p=0.028).
Conclusion
Administration of a clopidogrel loading dose with combination antithrombotic therapy for BAD reduced END.
Keywords: ischemic stroke, branch atheromatous disease, clopidogrel, loading, neurological deterioration
Introduction
Many patients with branch atheromatous disease (BAD)-type cerebral infarction, even those with a mild condition at the onset, experience neurological deterioration after hospitalization and develop serious deficits (1-4). Therefore, aggressive antithrombotic therapy should be considered at presentation during the acute phase of BAD.
Since arteriosclerosis underlies the pathology of BAD, dual antiplatelet therapy (DAPT) combining two antiplatelet drugs can potentially prevent progression of the disease and is often administered in clinical settings (5-8). The guidelines also strongly recommend the administration of DAPT with aspirin and clopidogrel for non-cardioembolic ischemic stroke within 24 h of the onset (9).
The mechanism underlying symptom progression in BAD is believed to be associated with fibrin clots. In Japan, when BAD is diagnosed, a prevalent therapeutic strategy is antithrombotic therapy using multiple agents, including the anticoagulant argatroban (a selective synthetic thrombin inhibitor), in addition to DAPT (6,7). Indeed, argatroban is administered to 78.4% of patients with BAD and 68.4% with DAPT in clinical practice in Japan (10).
Clopidogrel therapy initiated with a loading dose of 300 mg was approved to prevent recurrence of “non-cardiogenic cerebral infarction in the acute phase” in Japan in 2018. Clopidogrel is metabolized in two stages by cytochrome P450; therefore, clopidogrel exhibits a delayed onset of action. However, this delay can be mitigated via the administration of an initial clopidogrel loading dose (11). However, another study on BAD with a smaller sample size reported that administering clopidogrel loading doses did not prevent neurological deterioration (12).
In the present study, we examined the therapeutic efficacy of intensive treatment whereby a clopidogrel loading dose was added to multiple antithrombotic therapies (DAPT+argatroban).
Material and Methods
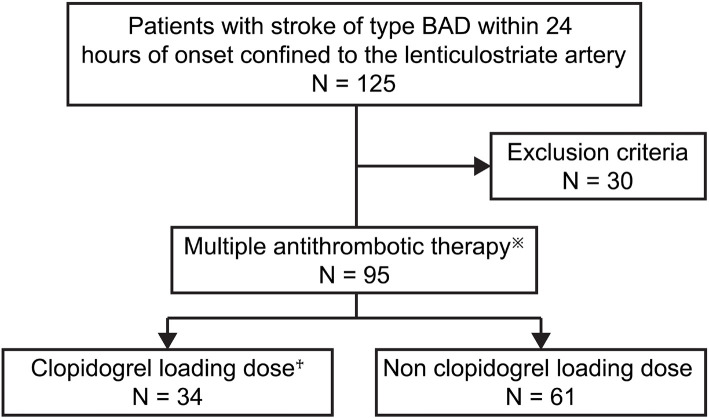
Between January 2019 and May 2022, 125 patients with BAD-type cerebral infarction in the lenticulostriate artery (LSA) were admitted to our department within 24 h of the onset. This study included 95 consecutive patients who received combination therapy with argatroban and DAPT and had been independent in their activities of daily living with a modified Rankin Scale (mRS) score of ≤1 before admission.
The patients were classified into the loading group (LG), who received an initial clopidogrel loading dose of 300 mg on the day of admission, and those without loading (the non-loading group; NLG) (Fig. 1). BAD was defined based on diffusion-weighted imaging at the time of arrival as infarction involving ≥3 horizontal slices (section thickness/intersection gap, 5/1.5 mm) in the LSA territory and on magnetic resonance angiography as no stenosis (≥50%) or occlusion of a major artery of the branch artery and no cardioembolic source (e.g. atrial fibrillation) (1,3).
Figure 1.
Baseline characteristics of patients in the clopidogrel loading and non-loading groups. Argatroban: 60 mg/day for 2 days, followed by 10 mg twice daily for 5 days). Aspirin: 100 mg/day. Clopidogrel: 75 mg/day. †300 mg on day 1, followed by 75 mg once daily.
This study was also designed to evaluate the effect of clopidogrel loading. Therefore, DAPT was limited to the combined usage of aspirin and clopidogrel, and patients who used other combinations, such as aspirin+cilostazol, clopidogrel+cilostazol, or ozagrel sodium (an intravenous antiplatelet drug), were excluded. In addition, patients who used anticoagulants other than argatroban or who received thrombolytic therapy were also excluded from the study. Aspirin was used at a fixed dose of 100 mg per day from the start of treatment.
Between the LG and NLG, we compared the age, sex, risk factors for cerebral infarction (hypertension, diabetes mellitus, hyperlipidemia, and smoking history), history of previous ischemic stroke, history of coronary artery disease, body weight (BW), laboratory test results on admission [blood glucose level (BG), hemoglobin A1c level (HbA1c), creatinine (Cr), creatinine clearance (CrCl) calculated using the Cockcroft-Gault equation (13), triglyceride (TG), total cholesterol (T-CHO), low-density lipoprotein cholesterol (LDL-C)], blood pressure (systolic and diastolic) on admission, antithrombotic drug use at the time of the stroke onset, National Institutes of Health Stroke Scale (NIHSS) score (on admission, 48 h after admission, and 1 week after admission) (14), infarct volume, rate of statin (HMG CoA reductase inhibitors) and edaravone (free radical scavenger) use, duration of hospital stay, occurrence of symptomatic intracerebral hemorrhaging (sICH), and clinical outcomes at hospital discharge.
Neurological deterioration was classified as early neurological deterioration (END) ΔN4 when the NIHSS score at 48 h worsened by ≥4 points compared to the score on admission and ENDΔN1 when the NIHSS score worsened by ≥1 point. The infarct volume of the selected slice with the largest number of lesions visually observed from diffusion weighted imaging (DWI) was measured using the ABC/2 method (15,16): 0.5×diameter of the length×diameter of the width×(0.5×number of DWI slices of acute infarction). sICH was defined as ICH associated with worsening of any neurological symptom (17). Clinical outcomes were assessed using the mRS score at discharge (18).
This retrospective study was approved by the Human Research Ethics Committee of Saitama Medical University International Medical Center (approval No: 2022-50).
Statistical analyses
Data were analyzed using the IBM SPSS Statistics software program for Windows, version 20.0 (IBM, Armonk, USA). The age, systolic/diastolic blood pressure, BW, HbA1c, TG, T-CHO, LDL-C, BG, Cr, and CrCl values and the infarct volume were compared between groups using Student's t-test. The NIHSS score, duration of hospital stay, and mRS scores were compared using the Mann-Whitney U test, and ratios were compared using Fisher's exact test (two-sided). p values <0.05 were considered statistically significant.
Results
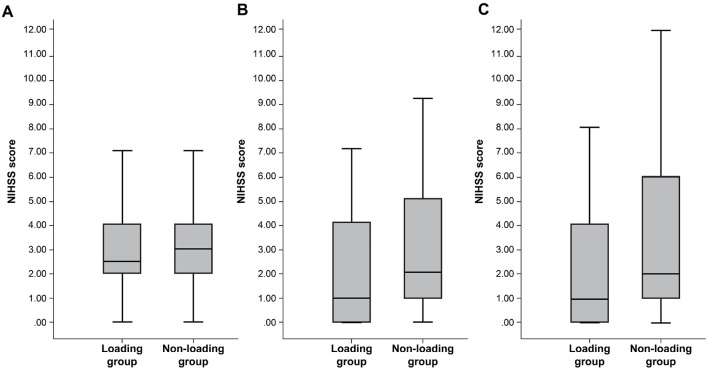
Table summarizes the patient characteristics of the LG and NLG. There were 34 (36%) and 61 (64%) patients in the LG and NLG, respectively. There were no significant between-group differences in background factors, such as the age, sex, NIHSS score on admission, or infarct volume. Changes in the NIHSS score are shown in Fig. 2. The NIHSS score was significantly lower in the LG than in the NLG at 48 h after admission [median (interquartile range (IQR)) LG: 1 (0.25-4) vs. NLG: 2 (1-5), p=0.045]. The NIHSS score at 1 week after admission was lower in the LG than in the NLG but not to a significant degree [LG: 1 (0-3.75) vs. NLG: 2 (1-6); p=0.083]. The limb movement items (NIHSS scores 0-8) were compared at admission and 48 h after admission. The NIHSS scores at admission did not differ markedly between the groups [LG: 2 (2-2.75) vs. NLG: 2 (2-3); p=0.217], but the NIHSS score at 48 h was significantly lower in the LG than in the NLG [LG: 1 (0.25-2) vs. NLG: 2 (1-4); p=0.009]. In contrast, there was no significant difference between the two groups in the NIHSS score, excluding the limb movement items on admission and 48 h after admission [on admission, LG: 0 (0-1) vs. NLG: 0 (0-1); p=0.324, 48 h after admission, LG: 0 (0-1) vs. NLG: 0 (0-1); p=0.909].
Table.
Patient Characteristics of Loading and Non-loading Groups.
| Loading group (n=34) | Non-loading group (n=61) | p value | |
|---|---|---|---|
| Age, years | 71.8±13.3 | 71.7±11.2 | 0.958 |
| Female sex, no. (%) | 13 (38) | 29 (48) | 0.399 |
| Hypertension, no. (%) | 22 (65) | 50 (82) | 0.081 |
| Diabetes mellitus, no. (%) | 9 (26) | 23 (38) | 0.366 |
| Hyperlipidemia, no. (%) | 16 (47) | 29 (48) | 1.000 |
| Coronary heart disease, no. (%) | 4 (12) | 6 (10) | 0.742 |
| Cerebral infarction, no. (%) | 5 (15) | 6 (10) | 0.515 |
| Smoking history, no. (%) | 6 (18) | 14 (23) | 0.609 |
| Systolic blood pressure, mmHg | 180.9±30.9 | 191.3±30.8 | 0.118 |
| Diastolic blood pressure, mmHg | 97.7±16.6 | 95.4±21.3 | 0.584 |
| BW, kg | 61.8±13.9 | 58.7±11.8 | 0.261 |
| Glucose level, mg/dL | 135.9±70.3 | 144.8±77.8 | 0.582 |
| Hemoglobin A1c, % | 6.1±1.6 | 6.4±1.7 | 0.315 |
| Creatinine, mg/dL | 1.07±1.43 | 0.85±0.60 | 0.283 |
| Creatinine clearance, mL/min | 72.6±37.4 | 73.7±31.2 | 0.879 |
| Triglyceride, mg/dL | 116.0±50.3 | 130.0±74.3 | 0.268 |
| Total cholesterol, mg/dL | 208.0±49.7 | 219.0±51.7 | 0.462 |
| Low-density lipoprotein cholesterol, mg/dL | 144.0±40.1 | 139.0±42.8 | 0.581 |
| NIHSS score on admission | 2.5 (2-4) | 3 (2-4) | 0.771 |
| Infarct volume, mL | 1.08±1.71 | 0.91±1.06 | 0.548 |
| Treatment with antithrombotic drugs on admission | 5 (18) | 7 (11) | 0.750 |
| Aspirin | 2 | 4 | |
| Clopidogrel | 2 | 3 | |
| Cilostazol | 1 | ||
| Concomitant medication | |||
| Statin, no. (%) | 27 (79) | 39 (64) | 0.163 |
| Edaravone, no. (%) | 30 (88) | 55 (90) | 0.742 |
| Duration of hospitalization, days | 17.5 (14.25-28) | 19 (12-29) | 0.855 |
Data are shown as mean±SD, median (interquartile range), or number (%). BA: basilar artery, BMI: body mass index, BW: body weight, DWI-ASPECTS: Alberta stroke program early computed tomography score on diffusion-weighted imaging, ICA: internal carotid artery, MCA: middle cerebral artery, NIHSS: National Institutes of Health Stroke Scale, rt-PA: recombinant tissue plasminogen activator
Figure 2.
Changes in the National Institutes of Health Stroke Scale (NIHSS) score. (A) On admission, (B) 48 h after admission, and (C) 1 week after admission. The NIHSS score was significantly lower in the loading group than in the non-loading group at 48 h following admission. Data are presented as the median (interquartile ranges) [on admission: 2.5 (2-4) vs. 3 (2-4), p=0.771; 48 h after admission: 1 (0.25-4) vs. 2 (1-5), p=0.045; 1 week after admission: 1 (0-3.75) vs. 2 (1-6), p=0.083].
ENDΔN1 was less frequent in the NLG than in the LG (18% in the LG and 31% in the NLG) but not to a significant degree (p=0.244). In contrast, the rate of ENDΔN4 was 3% in the LG and 20% in the NLG, showing significantly less deterioration of the NIHSS score ≥4 points in the LG than in the NLG (p=0.028).
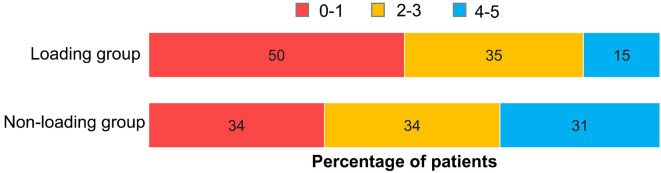
Fig. 3 shows the mRS score at discharge. The proportion of patients with mRS 0-1 at discharge was 50% in the LG and 34% in the NLG, showing no significant difference (p=0.190); however, the LG had a higher proportion of patients with a favorable outcome than the NLG. There was no sICH in either group.
Figure 3.
Modified Rankin Scale (mRS) score. At hospital discharge, patients with an mRS score of 0-1 comprised 50.0% (17 of 34 patients) of the loading group and 34.4% (21 of 61 patients) of the non-loading group. The outcomes showed no significant between-group difference (p=0.190).
Discussion
The results of this study demonstrated that multiple antithrombotic therapies (DAPT+argatroban), which are currently believed to be the most potent therapies for BAD occurring in the LSA territory, significantly reduced END (especially worsening of movement symptoms) when these therapies were administered in combination with an initial loading of clopidogrel, compared with their administration alone.
When clopidogrel is administered at an initial loading dose of 300 mg in combination with aspirin, the antithrombotic effect appears within 90 min of the initial administration, reaches potency at 6 h following the initial administration (61% and 75% of platelet and fibrin reduction, respectively), and subsequently achieve steady state. Initial administration of a clopidogrel loading dose of 300 mg accelerated the onset of the antithrombotic effect of combination therapy with clopidogrel and acetylsalicylic acid (11).
BAD is characterized by END, and symptom progression up to three days after the onset is reportedly a factor associated with a poor prognosis (19). Therefore, it is important to aggressively treat BAD in order to prevent symptom progression during the early stages. The results of the present study suggest that the administration of clopidogrel loading doses led to the onset of a potent antithrombotic effect, which contributed to the prevention of symptom progression in the early stages after the onset.
However, the prevalence of CYP2C19*2 and *3 polymorphisms, which are associated with poor antithrombotic agent metabolizers, is higher in the Japanese population (approximate prevalence of 20%) than in Europeans and Americans (20,21). CYP2C19 polymorphisms strongly affect the antiplatelet action of clopidogrel, which has been demonstrated in the real-world setting. A previous report showed that the clopidogrel loading effects on pharmacokinetics vary among CYP2C19 genetic polymorphisms (22). Since this study cannot rule out the possibility of genetic polymorphisms affecting the therapeutic effects, the impact of genetic polymorphisms needs to be verified in future studies.
Several limitations associated with the present study warrant mention. First, we used a retrospective study design to obtain data. Second, the included cohort was small. Third, the base therapy was limited to DAPT (aspirin+clopidogrel) plus argatroban, and this therapy was not compared with other combination therapies. Finally, only BAD localized in the LSA territory was targeted; therefore, other types of BAD, such as that occurring in the paramedian pontine artery territory, were not examined.
There is no clear evidence concerning the efficacy and safety of BAD treatment, and no therapeutic strategies have yet been established. However, the present findings suggest that the addition of clopidogrel loading doses to conventional multiple antithrombotic therapy for BAD might prevent END compared with therapy without clopidogrel loading doses. The further accumulation of cases and detailed investigation of various treatments, including the loading effect of clopidogrel on BAD, will facilitate the development of more effective therapeutic strategies for BAD.
Conclusion
The combination of clopidogrel initial loading with multiple antithrombotic therapies using DAPT (aspirin+clopidogrel) plus argatroban for BAD occurring in the LSA territory may prevent symptom progression effectively in the early stages after the onset.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Nakase T, Yamamoto Y, Takagi M, Japan Branch Atheromatous Disease Registry Collaborators. The impact of diagnosing branch atheromatous disease for predicting prognosis. J Stroke Cerebrovasc Dis 24: 2423-2428, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Yamamoto Y, Ohara T, Hamanaka M, et al. Predictive factors for progressive motor deficits in penetrating artery infarctions in two different arterial territories. J Neurol Sci 288: 170-174, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Deguchi I, Hayashi T, Kato Y, et al. Treatment outcomes of tissue plasminogen activator infusion for branch atheromatous disease. J Stroke Cerebrovasc Dis 22: e168-e172, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Petrone L, Nannoni S, Del Bene A, Palumbo V, Inzitari D. Branch atheromatous disease: a clinically meaningful, yet unproven concept. Cerebrovasc Dis 41: 87-95, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Huang YC, Lee JD, Weng HH, Lin LC, Tsai YH, Yang JT. Statin and dual antiplatelet therapy for the prevention of early neurological deterioration and recurrent stroke in branch atheromatous disease: a protocol for a prospective single-arm study using a historical control for comparison. BMJ Open 11: e054381, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamamoto Y, Nagakane Y, Makino M, et al. Aggressive antiplatelet treatment for acute branch atheromatous disease type infarcts: a 12-year prospective study. Int J Stroke 9: E8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagakane Y, Tanaka E, Ashida S, et al. Safety of dual antiplatelet therapy with argatroban in patients with acute ischemic stroke. Brain Nerve 70: 557-562, 2018. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 8. Kimura T, Tucker A, Sugimura T, et al. Ultra-early combination antiplatelet therapy with cilostazol for the prevention of branch atheromatous disease: a multicenter prospective study. Cerebrovasc Dis Extra 6: 84-95, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powers WJ, Rabinstein AA, Ackerson T, the American Heart Association Stroke Council, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50: e344-e418, 2019. [DOI] [PubMed] [Google Scholar]
- 10. Editorial committee of Japan Stroke Data Bank 2021 in National Cerebral and Cardiovascular Center. In: Japan Stroke Data Bank 2021. Nakayama Shoten, Tokyo, 2021: 68-71 (in Japanese). [Google Scholar]
- 11. Cadroy Y, Bossavy JP, Thalamas C, Sagnard L, Sakariassen K, Boneu B. Early potent antithrombotic effect with combined aspirin and a loading dose of clopidogrel on experimental arterial thrombogenesis in humans. Circulation 101: 2823-2828, 2000. [DOI] [PubMed] [Google Scholar]
- 12. Ikeda-Sakai Y, Sasaki M, Nakase T. Effects with and without clopidogrel loading treatment for acute ischemic cerebrovascular disease patients: a retrospective cohort study. J Stroke Cerebrovasc Dis 26: 2901-2908, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 16: 31-41, 1976. [DOI] [PubMed] [Google Scholar]
- 14. Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20: 864-870, 1989. [DOI] [PubMed] [Google Scholar]
- 15. Tokuda K, Hanada K, Takebayashi T, Koyama T, Fujita T, Okita Y. Factors associated with prognosis of upper limb function in branch atheromatous disease. Clin Neurol Neurosurg 218: 107267, 2022. [DOI] [PubMed] [Google Scholar]
- 16. Sananmuang T, Dejsiripongsa T, Keandoungchun J, Apirakkan M. Reliability of ABC/2 method in measuring of infarct volume in magnetic resonance diffusion-weighted image. Asian J Neurosurg 14: 801-807, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333: 1581-1587, 1995. [DOI] [PubMed] [Google Scholar]
- 18. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19: 604-607, 1988. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi Y, Yamashita T, Morihara R, et al. Different characteristics of anterior and posterior branch atheromatous diseases with or without early neurologic deterioration. J Stroke Cerebrovasc Dis 26: 1314-1320, 2017. [DOI] [PubMed] [Google Scholar]
- 20. Jinnai T, Horiuchi H, Makiyama T, et al. Impact of CYP2C19 polymorphisms on the antiplatelet effect of clopidogrel in an actual clinical setting in Japan. Circ J 73: 1498-1503, 2009. [DOI] [PubMed] [Google Scholar]
- 21. Furuta T, Sugimoto M, Shirai N, Ishizaki T. CYP2C19 pharmacogenomics associated with therapy of Helicobacter pylori infection and gastro-esophageal reflux diseases with a proton pump inhibitor. Pharmacogenomics 8: 1199-1210, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi M, Kajiwara M, Hasegawa S. A randomized study of the safety, tolerability, pharmacodynamics, and pharmacokinetics of clopidogrel in three different CYP2C19 genotype groups of healthy Japanese subjects. J Atheroscler Thromb 22: 1186-1196, 2015. [DOI] [PubMed] [Google Scholar]