Abstract
Adjuvant chemotherapy has been proven to reduce significantly the risk for relapse and death in women with operable breast cancer. Nevertheless, the prognosis for patients presenting with extensive axillary lymph node involvement remains suboptimal. In an attempt to improve on the efficacy of existing chemotherapy, a phase III intergroup trial led by the Cancer and Leukemia Group B (CALGB 97-41) was designed, which tested a mathematical model of tumor growth based on the Norton–Simon hypothesis. This hypothesis, developed about 3 decades ago, and the kinetic model derived from it, created the basis of the concepts of dose density and sequential therapy, both of which were tested in CALGB 97-41. This large prospective randomized trial demonstrated that shortening the time interval between each chemotherapy cycle while maintaining the same dose size resulted in significant improvements in disease-free and overall survival in patients with node-positive breast carcinoma. This finding is highly relevant and has immediate implications for clinical practice.
Introduction
Breast cancer is the most commonly diagnosed form of cancer and is the second most common cause of cancer-related death in women, both in Europe and in the USA. It was estimated that in the year 2004 about 40,100 patients would die from metastatic breast carcinoma in the USA [1,2]. Although increased patient awareness and improved screening techniques (including mammography, ultrasound, and breast magnetic resonance imaging) now permit early detection of localized and resectable tumors, many women still die from recurrent breast carcinoma, suggesting that a substantial number of patients already have distant micrometastasis at the time of diagnosis. With time, a significant number of patients will develop metastatic disease, even after seemingly curative surgery and radiotherapy. Systemic adjuvant chemotherapy for early-stage breast cancer is utilized to eradicate microscopic deposits of cancer cells that may have spread or metastasized from the primary breast cancer, and so it is recommended in large groups of patients to prevent or delay progression, based on risk assessment.
In the 1990s, the Early Breast Cancer Trialists' Collaborative Group published overviews, consolidating data from the randomized trials using meta-analytical techniques. This methodology has the advantage of being able to combine data from many small under-powered trials, consideration of the individual results from which might have led to negative or positive conclusions [3]. Adjuvant chemotherapy has been proven to reduce significantly the risk for relapse and death in women with operable breast cancer, and therapy with anthracycline-containing regimens has been shown to confer a small but significant benefit over the classic CMF (cyclophosphamide, methotrexate, and 5-fluorouracil) combinations (Table 1). The most recent Early Breast Cancer Trialists' Collaborative Group meta-analysis [3] showed reductions in the annual hazard rate for recurrence and death of 23.5% and 14.3%, respectively. However, the absolute magnitude of the effect is modest and the risk for relapse remains significant, especially in patients with metastatic involvement of axillary lymph nodes.
Table 1.
Impact of adjuvant chemotherapy on reducing annual odds of recurrence and death
| Comparison (n) | Recurrence | Death |
| CMF versus nil (8150) | +24 ± 3 | +14 ± 4 |
| CMF+ versus nil (3218) | +20 ± 5 | +15 ± 5 |
| Anthracyclines+ versus CMF (6950) | +12 ± 4 | +11 ± 5 |
| Longer versus shorter (6104) | +7 ± 4 | -1 ± 5 |
Values are expressed as percentage ± standard deviation. Data from Early Breast Cancer Trialists' Collaborative Group [3]. CMF, cyclophosphamide, methotrexate and 5-fluorouracil.
The Oxford overview [3] confirmed that polychemotherapy (more than two agents) offers a survival advantage compared with single agents in the adjuvant setting. Four to six courses of therapy (3–6 months) appear to confer optimal benefit, with the administration of additional courses adding to toxicity without substantially improving overall benefit.
As previously mentioned, the available data indicate that adjuvant chemotherapy with an anthracycline-containing regimen results in a small but statistically significant improvement in survival compared with regimens that do not contain an anthracycline. The current standard adjuvant anthracycline-containing regimens are as follows: doxorubicin and cyclophosphamide (AC); epirubicin and cyclophosphamide (EC); cyclophosphamide, doxorubicin, and 5-fluorouracil; 5-fluorouracil, doxorubicin or epirubicin, and cyclophosphamide (FAC/FEC); AC followed by CMF; doxorubicin or epirubicin followed by CMF; AC followed by paclitaxel; and docetaxel, doxorubicin, and cyclophosphamide. Although CMF is commonly utilized for lower risk, node-negative breast carcinomas, regimens containing an anthracycline and/or taxanes are usually recommended for higher risk, node-positive breast carcinomas. Taxanes, including paclitaxel and docetaxel, were recently introduced in the adjuvant setting for breast carcinoma.
Currently available phase III data with adjuvant paclitaxel-anthracycline combinations demonstrate their significant superiority in terms of clinical outcome when compared with doxorubicin-based, non-taxane-containing combinations. The Cancer and Leukemia Group B (CALGB) 93-44 trial reported a significant improvement in disease-free survival (DFS) at 5 years (70% versus 65%) and overall survival (OS; 80% versus 77%) for the sequential addition of paclitaxel to AC [4]. The National Surgical Adjuvant Breast and Bowel Project (NSABP) trial B28 reported similar results [5]. In the Breast Cancer International Research Group 001 trial [6] the docetaxel, doxorubicin, and 5-fluorouracil combination was significantly superior to FAC.
Dosing and dose escalation
In an attempt to improve the efficacy of chemotherapy in breast cancer, dose intensification has been evaluated extensively over the past decade. Dose intensity is calculated by dividing the total dose of drug given per surface area by the duration of treatment. It is expressed as mg/m2 per week [7]. The most widely used method of increasing dose intensity has been dose escalation. Dose escalation is tested by comparing higher doses with lower ones. In some dose ranges escalation improves efficacy, and in some dose ranges it does not.
The dose escalation concept was based on the Skipper–Schabel–Wilcox model, also termed the log-kill model – the first significant proliferation model in clinical oncology [8]. According to this model, enough cycles of enough drugs at high enough doses should be able to kill a high percentage, if not all, of the cells. Unfortunately, this has not been clinically proven to be true for breast carcinoma. Dose escalation randomized trials have demonstrated a threshold effect for the AC combination, with doses of 60 mg/m2 doxorubicin and 600 mg/m2 cyclophosphamide appearing optimal. Moderate to marked dose escalation of cyclophosphamide did not prove beneficial in two NSABP trials, and, disturbingly, there was a report of 21 cases of myeloproliferative disorders in the NSABP B25 trial [9,10]. Similar results were suggested for doxorubicin in the report of the CALGB 93-44 trial [4]. Here, a 50% increase (60 mg/m2, 75 mg/m2, and 90 mg/m2) in doxorubicin dose and dose intensity (administered every 21 days) did not improve outcome.
There is currently no convincing evidence demonstrating that that more dose escalated treatment regimens (e.g. high-dose chemotherapy with peripheral stem cell support) result in overall improved outcomes compared with the administration of polychemotherapy programs at standard dose levels [11].
Dose density
Tumor cell growth kinetics show that human solid tumors do not exhibit an exponential growth pattern, but rather are better fit by a sigmoid growth pattern, for which the Gompertzian equation is the most commonly used. In the Gompertzian equation, the doubling time is not constant but rather increases with increasing tumor size, up to a certain mass/volume [12].
An alternative model to the Skipper–Schabel–Wilcox one, the Norton–Simon model, predicts that the best way to destroy an heterogeneous mix of cancer cells is to eradicate the numerically dominant, faster growing cells first, followed by eradication of the more slow growing, resistant cells [11]. This is termed sequential therapy, which has been proven to be clinically superior to alternating therapy. The sequential schedule increases the intensity of drug exposure, and is predicted to minimize tumor cell regrowth between cycles. The total impact of therapy could relate to the cell kill for each dose, the duration of drug administration, and the rate of tumor growth between each treatment. If so, then a fixed cell kill achieved at shorter time intervals should improve the overall impact of therapy. This concept is termed 'dose density' [13]; regrowth of resistant cells during and between cycles of chemotherapy is theoretically a principal cause of treatment failure. Suppression of cancer cell regrowth could be accomplished by repetitive cycles of chemotherapy. The increased dose exposure is a consequence of shortened inter-treatment intervals for each drug or combination and not of increased doses, and such treatment is therefore called more 'dose dense' (Fig. 1).
Figure 1.
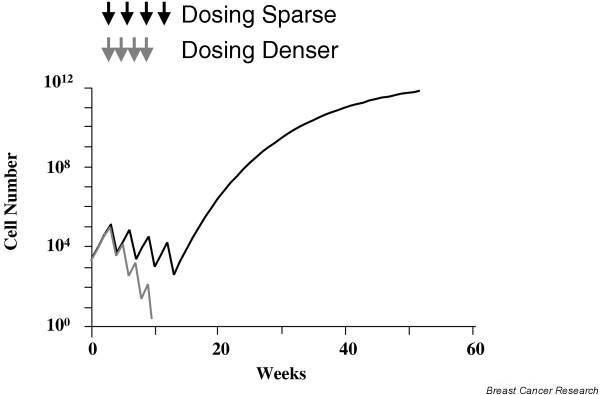
More frequent dosing can prevent cancer cell regrowth.
The randomized trial of doxorubicin and CMF from the National Cancer Institute of Milan [14] can be interpreted as a 'proof of principle' of the dose dense hypothesis. In that study, adjuvant sequential chemotherapy with doxorubicin for four cycles followed by CMF was compared with the same regimen delivered in an alternating manner. Interestingly, the authors expected the alternating regimen to prove superior, based on the Goldie–Coldman hypothesis [15]. Instead, the sequential (more dose dense) plan was associated with significantly improved DFS and OS. Bonadonna and coworkers [14] concluded that, although in the alternating plan the four cycles of doxorubicin were spread over 27 weeks, in the sequential plan they were administered within 9 weeks, and therefore the increased dose intensity of doxorubicin could account for the superiority of the latter plan. Functionally, the intensity of the treatment was increased by shortening the intervals without increasing dose levels of doxorubicin, providing clinical support for exploration of the hypothesis that more dose dense chemotherapy can improve outcomes.
Because of the availability of hematopoietic growth factors to reduce granulocytopenia and risk for infections, the 'dose dense' approach could be evaluated in a series of pilot adjuvant trials at Memorial Sloan-Kettering Cancer Center [16-18], with promising results. It was subsequently tested in a large phase III adjuvant trial in node-positive breast cancer; the CALGB study 97-41 [19] compared sequential doxorubicin, paclitaxel, and cyclophosphamide at standard dose levels of 60 mg/m2, 175 mg/m2, and 600 mg/m2, respectively, against concurrent AC, followed by paclitaxel, at the same dose levels. A separate randomization in this trial compared every 2 weeks with every 3 weeks dosing to assess the relative efficacy and toxicity of more or less dose dense therapy (Fig. 2). The every 2 weeks schedule was delivered with granulocyte colony-stimulating factor (CSF) support. This trial is particularly informative because it was a pure test of the dose density concept; all patients received the same drugs, the same number of drug cycles, at the same cumulative dose and the same individual doses, in all arms of the trial. A total of 2005 patients were randomized, all with node-positive resected breast carcinoma.
Figure 2.
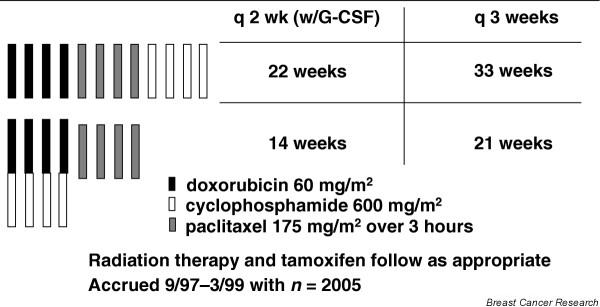
Cancer and Leukemia Group B (CALGB) 97-41 node-positivetrial: 2 × 2 factorial design. G-CSF, granulocyte colony-stimulating factor.
In that study, the incidence of grade IV neutropenia and treatment delays due to hematologic toxicity was significantly reduced and the efficacy was increased with the every 2 weeks schedule as compared with the every 3 weeks schedule. All four treatment schedules were proven to be feasible and safe. However, at a median follow up of 36 months DFS and OS were both statistically superior for the every 2 weeks, dose dense arm (3-year DFS: 85% versus 81%, P = 0.01; 3-year OS: 92% versus 90%, P = 0.013), regardless of predictive factors such as the number of positive nodes, tumor size, menopausal status, and tumor estrogen receptor status, thus translating increased dose density into increased clinical benefit (Figs 3 and 4). There was no difference in DFS or OS between the sequential and the concurrent arms. As mentioned above, the DFS and OS advantages of dose density were not accompanied by an increase in toxicity. Indeed, the use of filgrastim in the every 2 weeks, dose dense regimens resulted in a statistically significant decrease in granulocyte toxicity. Furthermore, the study utilized a baseline granulocyte count of 1000/μl (rather than the traditional 1500/μl) for administering chemotherapy, and this was proven to be safe. CALGB 97-41 showed not only the feasibility of this approach, but also the superiority of dose dense over conventional chemotherapy. These findings are exciting and are consistent with previous mathematical model predictions that shortening the interval between chemotherapy cycles could result in more effective eradication of malignant cells, potentially improving survival.
Figure 3.
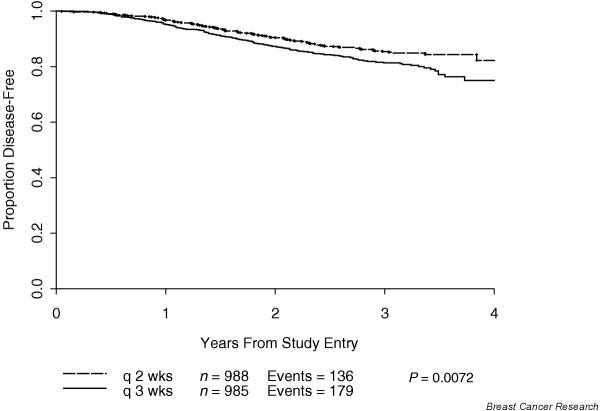
Disease-free survival by density. Adapted from Citron and coworkers [19].
Figure 4.
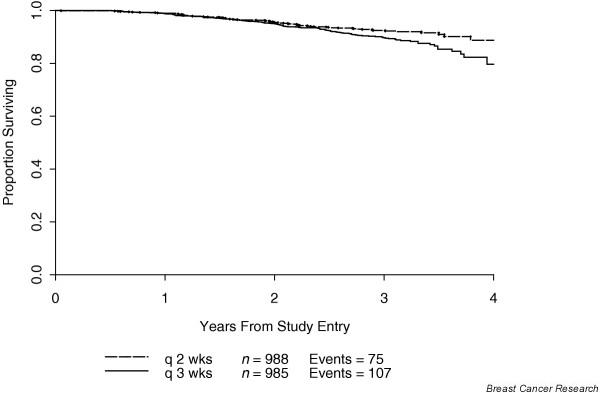
Overall survival by density. Adapted from Citron and coworkers [19].
Evidence from other trials
Other trials have tested the dose dense hypothesis, with positive results; however, not all of them are 'clean' tests of the dose density concept. In fact, the interpretability of studies may often be confounded by the design, in that the two arms are not equal in terms of the number of cycles or total drug dose, or even different drugs are delivered [20].
Möbus and colleagues [21] from the Arbeitsgemeinschaft für Gynaekologische Onkologie group recently demonstrated the superiority of adjuvant dose dense epirubicin, paclitaxel, and cyclophosphamide (ETC), every 2 weeks with filgrastim support, over sequential EC followed by paclitaxel every 3 weeks (Fig. 5). The authors defined this trial as a comparison of both dose dense and dose intense chemotherapy versus standard chemotherapy: in fact, the experimental ETC arm had higher cumulative doses of chemotherapy than did the 3-weekly arm. The study revealed a benefit in terms of DFS and OS for the ETC arm, although, given the higher doses delivered, it is not clear whether the superiority was purely conveyed by dose density alone.
Figure 5.
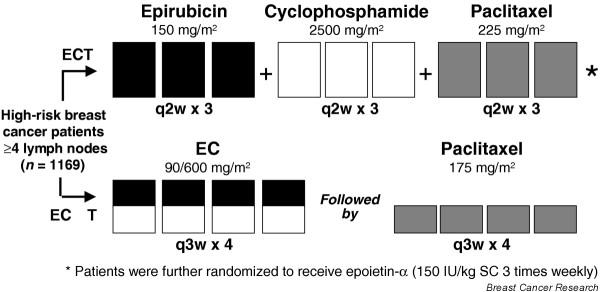
Phase III, prospective, randomized, multicenter Arbeitsgemeinschaft für Gynaekologische Onkologie group trial. Adapted from Möbus and coworkers [21].
Venturini and coworkers [22], from the Gruppo Oncologico del Nord-Ovest group, recently reported on the comparative efficacy of adjuvant standard versus accelerated FEC for patients with early breast carcinoma. That trial was similar to CALGB 97-41 in that it was designed in a manner that provided a pure test of the 'dose dense' concept; patients were randomly assigned to receive six cycles of FEC (fluorouracil 600 mg/m2, epirubicin 60 mg/m2, and cyclophosphamide 600 mg/m2) given either every 3 weeks (FEC21) or every 2 weeks (FEC14) with CSF support (Fig. 6). All patients received the same number of cycles, the same drugs, and the same drug levels. After a median follow-up period of 6.7 years, there was a statistically nonsignificant reduction in the risk for death in the FEC14 arm (hazard ratio 0.82, 95% confidence interval 0.6–1.12; P = 0.22). The hazard ratio was in the same direction as that of CALGB 97-41, but it did not reach statistical significance.
Figure 6.
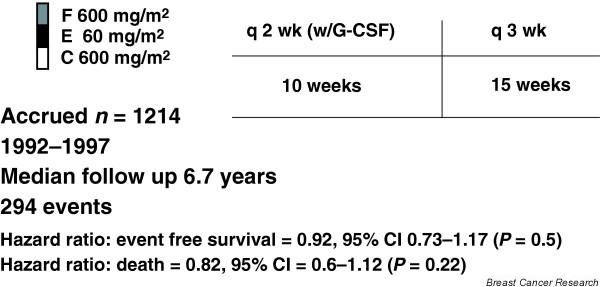
Phase III adjuvant trial comparing standard versus accelerated 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) regimen in early breast cancer patients. Results from the Gruppo Oncologico del Nord-Ovest group MIG1 study. G-CSF, granulocyte colony-stimulating factor. Adapted from Venturini and coworkers [22].
There were, however, several important differences between the Venturini and CALGB 97-41 trials, including the sample size (1214 versus 2005 patients, respectively), selection criteria (34% of patients in the Venturini trial had node-negative disease), and the chemotherapy regimens utilized. The Venturini trial was also relatively under-powered. Furthermore, it is preferable to use the sequential AC followed by paclitaxel therapy, as used in the CALGB 97-41 study, rather than FEC14, as utilized in the Venturini trial. In fact, previous studies have demonstrated that the FEC100 regimen (containing 100 mg/m2 epirubicin) is more effective than FEC50 (containing 50 mg/m2 epirubicin) when given every 3 weeks in early-stage breast cancer [23]. The assumption could be made that the use of the FEC100 regimen given every 2 weeks with granulocyte CSF may provide benefit. However, in a pilot trial [24] we recently reported that FEC100 regimen delivered every 2 weeks was not feasible because of nonhematologic toxicity, suggesting that caution should be exercised in adopting this approach for different regimens.
Future avenues
We await the results of multiple ongoing trials currently testing the concept of dose density. The Eastern Cooperative Group adjuvant trial 11–99 is a large 4-arm, phase III trial with 4 arms: patients were treated with 3-weekly AC × 4 cycles followed by either 3-weekly paclitaxel × 4 cycles, or weekly paclitaxel × 12 cycles, or 3-weekly docetaxel × 4 cycles, or weekly docetaxel × 12 cycles That study completed accrual in January 2002 and will answer the question regarding whether dose dense weekly taxane is superior to conventionally administered taxane (and which one). The National Cancer Institute of Canada MA.21 trial is currently comparing three regimens: standard AC for four cycles followed by paclitaxel for four cycles every 3 weeks; cyclophosphamide, epirubicin, and 5-fluorouracil for six cycles; and biweekly EC for six cycles followed by paclitaxel for four cycles every 3 weeks. Interestingly, in the latter regimen only EC is dose dense, and results will reveal whether there is any therapeutic benefit in accelerating part of a regimen. At Memorial Sloan Kettering Cancer Center, we are currently exploring the feasibility of EC and paclitaxel at 10–11 days (denser) intervals, with filgrastim support, in an attempt to take the concept of dose density to a further degree (Fig. 7).
Figure 7.
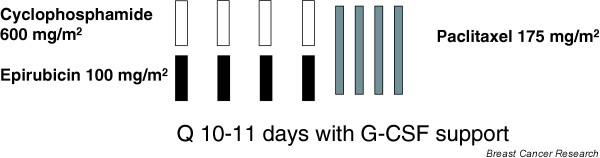
Memorial Sloan-Kettering Cancer Center Pilot trial of epirubicin and cyclophosphamide followed by paclitaxel at 10–11 day intervals. G-CSF, granulocyte colony-stimulating factor.
Conclusion
Breast cancer remains a worldwide public health concern, despite the fact that mortality rates have been declining in the USA, Canada, and Europe [25] Advances in detection, diagnosis, and treatment of breast cancer have certainly contributed to this decline, but the search for optimal regimens and their applications is still ongoing. Results with dose-dense chemotherapy in CALGB 97-41 are exciting, showing improved DFS and OS and less grade 4 neutropenia. The findings of that study suggest that dose dense scheduling with appropriate chemotherapy regimens and CSF support is 'ready for prime time' and can replace conventional dosing as the new standard of care in primary breast cancer. On the basis of current data, practicing oncologists should consider treating patients with breast cancer in this dose dense manner. However, extrapolating these data to all regimens outside a clinical trial setting should be done with caution, because unexpected toxicities may emerge. These findings suggest important avenues for future research in both breast cancer and other chemosensitive tumors, and confirmatory studies are encouraged; in this regard, it is of paramount importance to continue to support clinical trials.
Abbreviations
AC = doxorubicin and cyclophosphamide; CALGB = Cancer and Leukemia Group B; CMF = cyclophosphamide, methotrexate, and 5-fluorouracil; CSF = colony-stimulating factor; DFS = disease-free survival; EC = epirubicin and cyclophosphamide; ETC = epirubicin, paclitaxel, and cyclophosphamide; FAC/FEC = 5-fluorouracil, doxorubicin or epirubicin, and cyclophosphamide; NSABP = National Surgical Adjuvant Breast and Bowel Project; OS = overall survival.
Competing interests
The author(s) declare that they have no competing interests.
References
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ, American Cancer Society Cancer Statistics 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- Levi F, Lucchini F, Negri E, Zatonski W, Boyle P, La Vecchia C. Trends in cancer mortality in the European Union and accession countries, 1980–2000. Ann Oncol. 2004;15:1425–1431. doi: 10.1093/annonc/mdh346. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group Polychemotherapy for early breast cancer: an overview of the randomized trials. Lancet. 1998;352:930–942. doi: 10.1016/S0140-6736(98)03301-7. [DOI] [PubMed] [Google Scholar]
- Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes DF, Tkaczuk KH, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- Mamounas EP, Bryant J, Lembersky BC, Fisher B, Atkins JN, Fehrenbacher L, Raich PC, Yothers G, Soran A, Wolmark N. Paclitaxel following doxorubicin/cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer [abstract 12] Proc Am Soc Clin Oncol. 2003;22:4. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- Nabholtz JM, Pienkowski T, Mackey J, et al. Phase III trial comparing TAC (docetaxel, doxorubicin, cyclophosphamide) with FAC (5-fluorouracil, doxorubicin, cyclophosphamide) in the adjuvant treatment of node positive breast cancer patients: interim analysis of the BCIRG 001 study [abstract 141] Proc Am Soc Clin Oncol. 2002;21:36a. [Google Scholar]
- Hryniuk W, Levine MN. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer [abstract] J Clin Oncol. 1986;4:1162–1170. doi: 10.1200/JCO.1986.4.8.1162. [DOI] [PubMed] [Google Scholar]
- Skipper HE. Laboratory models: some historical perspective. Cancer Treat Rep. 1986;70:3–7. [PubMed] [Google Scholar]
- Fisher B, Anderson S, Wickerham DL, DeCillis A, Dimitrov N, Mamounas E, Wolmark N, Pugh R, Atkins JN, Meyers FJ, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B22. J Clin Oncol. 1997;15:1858–1869. doi: 10.1200/JCO.1997.15.5.1858. [DOI] [PubMed] [Google Scholar]
- Fisher B, Anderson S, DeCillis A, Dimitrov N, Atkins JN, Fehrenbacher L, Henry PH, Romond EH, Lanier KS, Davila E, et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel project B-25. J Clin Oncol. 1999;17:3374–3388. doi: 10.1200/JCO.1999.17.11.3374. [DOI] [PubMed] [Google Scholar]
- Rodenhuis S, Bontenbal M, Beex LV, Wagstaff J, Richel DJ, Nooij MA, Voest EE, Hupperets P, van Tinteren H, Peterse HL, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancer. N Engl J Med. 2003;349:7–16. doi: 10.1056/NEJMoa022794. [DOI] [PubMed] [Google Scholar]
- Norton L. A Gompertzian model of human breast cancer growth. Cancer Res. 1988;48:7067–7071. [PubMed] [Google Scholar]
- Surbone A, Gilewski T, Dang CT, Norton L. Cytokinetics. In: Holland JF, Frei E, Bast RC, editor. Cancer Medicine. 5. Baltimore, MD: Williams and Wilkins; 2000. pp. 491–519. [Google Scholar]
- Bonadonna G, Zambetti M, Valagussa P. Sequential or alternating doxorubicin and CMF regimens in breast cancer with more tan three positive nodes. JAMA. 1995;273:542–547. doi: 10.1001/jama.273.7.542. [DOI] [PubMed] [Google Scholar]
- Goldie JH, Coldman JA. A mathematical model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979;63:1727–1733. [PubMed] [Google Scholar]
- Hudis C, Seidman A, Baselga J, Raptis G, Lebwohl D, Gilewski T, Moynahan M, Sklarin N, Fennelly D, Crown JP, et al. Sequential dose-dense doxorubicin, paclitaxel, and cyclophosphamide for respectable high-risk breast cancer: feasibility and efficacy. J Clin Oncol. 1999;17:93–100. doi: 10.1200/JCO.1999.17.1.93. [DOI] [PubMed] [Google Scholar]
- Hudis C, Fornier M, Riccio L, Lebwohl D, Crown J, Gilewski T, Surbone A, Currie V, Seidman A, Reichman B, et al. 5-year result of dose-intensive sequential adjuvant chemotherapy for women with high-risk node-positive breast cancer: a phase II study. J Clin Oncol. 1999;17:1118. doi: 10.1200/JCO.1999.17.4.1118. [DOI] [PubMed] [Google Scholar]
- Fornier MN, Seidman AD, Theodoulou M, Moynahan ME, Currie V, Moasser M, Sklarin N, Gilewski T, D'Andrea G, Salvaggio R, et al. Doxorubicin followed by sequential paclitaxel and cyclophosphamide vs. concurrent paclitaxel and cyclophosphamide: 5-year results of a phase II randomized trial of adjuvant dose-dense chemotherapy for women with node-positive breast carcinoma. Clin Cancer Res. 2001;7:3934–3941. [PubMed] [Google Scholar]
- Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- Therasse P, Mauriac L, Welnicka-Jaskiewicz M, Bruning P, Cufer T, Bonnefoi H, Tomiak E, Pritchard KI, Hamilton A, Piccart MJ, EORTC Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study. J Clin Oncol. 2003;21:843–850. doi: 10.1200/JCO.2003.05.135. [DOI] [PubMed] [Google Scholar]
- Möbus VJ, Untch M, Du Bois A, Lueck H-J, Thomssen C, Kuhn W, Kurbacher C, Nitz U, Kreienberg R, Jackisch C. Dose-dense sequential chemotherapy with epirubicin(E), paclitaxel (T) and cyclophosphamide (C) (ETC) is superior to conventional dosed chemotherapy in high-risk breast cancer patients (≥ 4 +LN). First results of an AGO-trial [abstract 513] Proc Am Soc Clin Oncol. 2004.
- Venturini M, Aitini E, Del Mastro L, Sertoli MR, Conte P, Olmeo N, Mammoliti S, Cavazzini G, Pastorino S, Bruzzi P, Rosso R. Phase II adjuvant trial comparing standard versus accelerated FEC regimen I early breast cancer patients. Results from GONO-MIG1 study [abstract 12] Breast Cancer Res Treat. 2003. p. S9.
- French Epirubicin Study Group Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol. 2001;19:602–611. doi: 10.1200/JCO.2001.19.3.602. [DOI] [PubMed] [Google Scholar]
- Dang CT, D'Andrea GM, Moynahan ME, Dickler MN, Seidman AD, Fornier M, Robson ME, Theodoulou M, Lake D, Currie VE, et al. Phase II study of feasibility of dose-dense FEC followed by alternating weekly taxanes in high-risk, four or more node-positive breast cancer. Clin Cancer Res. 2004;10:5754–5761. doi: 10.1158/1078-0432.CCR-04-0634. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK, eds . SEER Cancer Statistics Review, 1975–2001. Bethesda, MD: National Cancer Institute; 2004. http://seer.cancer.gov/csr/1975_2001/ [Google Scholar]


