Abstract
Rationale & Objective
PRESERVE seeks to provide new knowledge to inform shared decision-making regarding blood pressure (BP) management for pediatric chronic kidney disease (CKD). PRESERVE will compare the effectiveness of alternative strategies for monitoring and treating hypertension on preserving kidney function; expand the National Patient-Centered Clinical Research Network (PCORnet) common data model by adding pediatric- and kidney-specific variables and linking electronic health record data to other kidney disease databases; and assess the lived experiences of patients related to BP management.
Study Design
Multicenter retrospective cohort study (clinical outcomes) and cross-sectional study (patient-reported outcomes [PROs]).
Setting & Participants
PRESERVE will include approximately 20,000 children between January 2009-December 2022 with mild-moderate CKD from 15 health care institutions that participate in 6 PCORnet Clinical Research Networks (PEDSnet, STAR, GPC, PaTH, CAPRiCORN, and OneFlorida+). The inclusion criteria were ≥1 nephrologist visit and ≥2 estimated glomerular filtration rate (eGFR) values in the range of 30 to <90 mL/min/1.73 m2 separated by ≥90 days without an intervening value ≥90 mL/min/1.73 m2 and no prior dialysis or kidney transplant.
Exposures
BP measurements (clinic-based and 24-hour ambulatory BP); urine protein; and antihypertensive treatment by therapeutic class.
Outcomes
The primary outcome is a composite event of a 50% reduction in eGFR, eGFR of <15 mL/min/1.73 m2, long-term dialysis or kidney transplant. Secondary outcomes include change in eGFR, adverse events, and PROs.
Analytical Approach
Longitudinal models for dichotomous (proportional hazards or accelerated failure time) and continuous (generalized linear mixed models) clinical outcomes; multivariable linear regression for PROs. We will evaluate heterogeneity of treatment effect by CKD etiology and degree of proteinuria and will examine variation in hypertension management and outcomes based on socio-demographics.
Limitations
Causal inference limited by observational analyses.
Conclusions
PRESERVE will leverage the PCORnet infrastructure to conduct large-scale observational studies that address BP management knowledge gaps for pediatric CKD, focusing on outcomes that are meaningful to patients.
Plain-Language Summary
Hypertension is a major modifiable contributor to loss of kidney function in chronic kidney disease (CKD). The purpose of PRESERVE is to provide evidence to inform shared decision-making regarding blood pressure management for children with CKD. PRESERVE is a consortium of 16 health care institutions in PCORnet, the National Patient-Centered Clinical Research Network, and includes electronic health record data for >19,000 children with CKD. PRESERVE will (1) expand the PCORnet infrastructure for research in pediatric CKD by adding kidney-specific variables and linking electronic health record data to other kidney disease databases; (2) compare the effectiveness of alternative strategies for monitoring and treating hypertension on preserving kidney function; and (3) assess the lived experiences of patients and caregivers related to blood pressure management.
Index Words: Pediatric, children, chronic kidney disease, blood pressure, hypertension, kidney function
Pediatric chronic kidney disease (CKD) is rare and has substantial effects on current and future health. Hypertension occurs in 50% of children with CKD,1,2 and is a major risk factor for decline in kidney function.2, 3, 4, 5 Blood pressure (BP) control is a cornerstone of CKD management. Several clinical practice guidelines provide BP management recommendations for pediatric CKD.6, 7, 8, 9 However, because the evidence base is insufficient, conflicting recommendations and substantial practice variation exist.10 Available data derive largely from 2 studies: the ESCAPE trial5 and the Chronic Kidney Disease in Children (CKiD) cohort study.11,12
ESCAPE, which concluded 15 years ago, was a European study of 385 children with CKD randomized to intensified or conventional BP control with an angiotensin-converting enzyme inhibitor (ACEi). After 5 years, 30% of the intensified versus 42% of the conventional group met the primary composite end point of a 50% decline in estimated glomerular filtration rate (eGFR), eGFR <10 mL/min/1.73 m2 or kidney replacement therapy (KRT).5 ESCAPE’s generalizability to routine clinical settings was limited by the use of ambulatory blood pressure monitoring (ABPM) every 6 months, which is not feasible for most children. All participants received a moderately high dose of ramipril and were treated without regard to baseline BP level.
The ongoing CKiD study has enrolled >1,000 children and followed them annually.11,13 CKiD has identified the important impact of hypertension14,15 and proteinuria15, 16, 17 on kidney function decline. The effect of elevated BP was greater in participants with glomerular versus nonglomerular CKD etiologies, suggesting the need to study these 2 groups separately. Use of ACEi or angiotensin receptor blockers (ARBs) was associated with a 21%-37% reduction in the risk of KRT.18 The accelerated decline in GFR demonstrated in the 18 months preceding KRT19 underscores the importance of early intervention, which informed PRESERVE’s focus on mild-moderate CKD.
The goal of PRESERVE is to provide new knowledge to inform shared decision-making about BP management in pediatric CKD. PRESERVE will use the National Patient-Centered Clinical Research Network (PCORnet)20,21 to address the following evidence gaps:
-
(1)
At what level of clinic-assessed BP should antihypertensive treatment be started to best preserve kidney function?
-
(2)
What level of clinic-assessed BP should be targeted over time to best preserve kidney function?
-
(3)
Should antihypertensive treatment initiation thresholds and targets be tailored to the cause of CKD or magnitude of proteinuria?
-
(4)
Given the existing experimental evidence for the renoprotective effects of ACEi/ARB, do electronic health record (EHR) data support their first-line use for BP control in all children with CKD?
-
(5)
What are the trade-offs among BP control, potential harm as evidenced by adverse clinical events, and patients’ symptoms, functioning, and quality of life?
Methods
Aims and Objectives
PRESERVE has 4 aims, each with objectives that guide their scope of work (Box 1).
Box 1. PRESERVE Aims and Objectives.
|
Aim 1: Enhance the PCORnet CDM for pediatric and rare kidney disease research. Objectives
Objectives
Objectives
Objectives
|
Abbreviations: ABPM, ambulatory blood pressure monitoring; BP, blood pressure; CDM, common data model; CKD, chronic kidney disease; CKiD, Chronic Kidney Disease in Children; eGFR, estimated glomerular filtration rate; EHR, electronic health record; USRDS, United States Renal Data System.
Study Setting and Organizational Structure
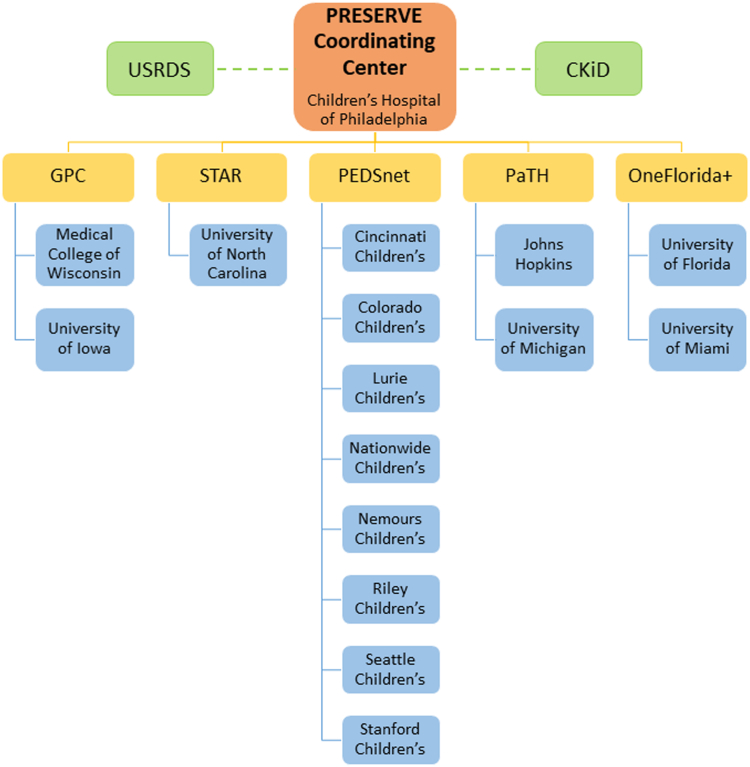
PRESERVE includes 16 institutions in PCORnet, a national network-of-networks that conducts patient-centered outcomes research (Fig 1).21 PCORnet’s infrastructure includes a novel collaboration platform of comprehensive clinical data that is standardized, analysis-ready, and derived from medical institutions and health plans; common network and data governance; streamlined contracting and regulatory agreements; and resources for engaging patients.22
Figure 1.
PRESERVE study setting and organizational structure. PRESERVE includes 16 institutions from 5 PCORnet networks: (1) Children’s Hospital of Philadelphia (Coordinating Center), Cincinnati Children’s Hospital Medical Center, Children’s Hospital Colorado, Indiana University/Riley Hospital for Children, Lurie Children’s Hospital, Nationwide Children’s Hospital, Nemours Children’s Health, Seattle Children’s Hospital, and Stanford Children’s Health (PEDSnet); (2) University of North Carolina (STAR); (3) Medical College of Wisconsin/Children’s Wisconsin and University of Iowa Stead Family Children’s Hospital (GPC); (4) University of Michigan/C.S. Mott Children’s Hospital and Johns Hopkins Children’s Center (PaTH); and (5) University of Florida/Shands Children’s Hospital and University of Miami/Holtz Children’s Hospital (OneFlorida+). PRESERVE also includes linkages with the US Renal Data System (USRDS) and Chronic Kidney Disease in Children (CKiD) study.
PRESERVE is led by PEDSnet (pedsnet.org),23,24 the only PCORnet network devoted exclusively to children. PEDSnet institutions contributing data include Children’s Hospital of Philadelphia, Cincinnati Children’s Hospital Medical Center, Children’s Hospital Colorado, Lurie Children’s Hospital, Nationwide Children’s Hospital, Nemours Children’s Health, Seattle Children’s Hospital, and Stanford Children’s Health. An additional 7 institutions (from 4 other PCORnet networks) are participating: University of North Carolina (STAR), Medical College of Wisconsin/Children’s Wisconsin and University of Iowa Stead Family Children’s Hospital (GPC), University of Michigan/C.S. Mott Children’s Hospital and Johns Hopkins Children’s Center (PaTH), University of Florida/Shands Children’s Hospital, and University of Miami/Holtz Children’s Hospital (OneFlorida+). The study protocol was approved by the Children’s Hospital of Philadelphia Institutional Review Board (IRB #21-018814), the central institutional review board for all participating sites.
Study Design and Population
Most objectives address clinical outcomes using a retrospective cohort study design. The study period is January 2009 to December 2022. Inclusion criteria are an outpatient, emergency department, or inpatient visit with a medical provider during the study period and 2 or more eGFR values of 30 to <90 mL/min/1.73 m2 (using the CKiD U25 formula)25 at least 90 days apart. The cohort entrance date (CED) is defined as the day of the first qualifying eGFR value of 30 to <90 mL/min/1.73 m2. Exclusion criteria are eGFR ≥ 90 ml/min/1.73 m2 between the 2 qualifying eGFRs, age <1 or ≥18 years at cohort entrance, no nephrologist visit at any time during the study period, and long-term dialysis or kidney transplant on or before CED. Outcomes are assessed for as long as patients have visits at a participating institution.
Objectives 2.1 and 2.2 use cross-sectional study designs to develop and evaluate BP z scores and operational definitions for hypertension. These analyses will use data from children aged between 3 and <18 years in the PEDSnet population with outpatient visits with valid values for systolic and diastolic BP as well as associated height and weight.
EHR Data Quality
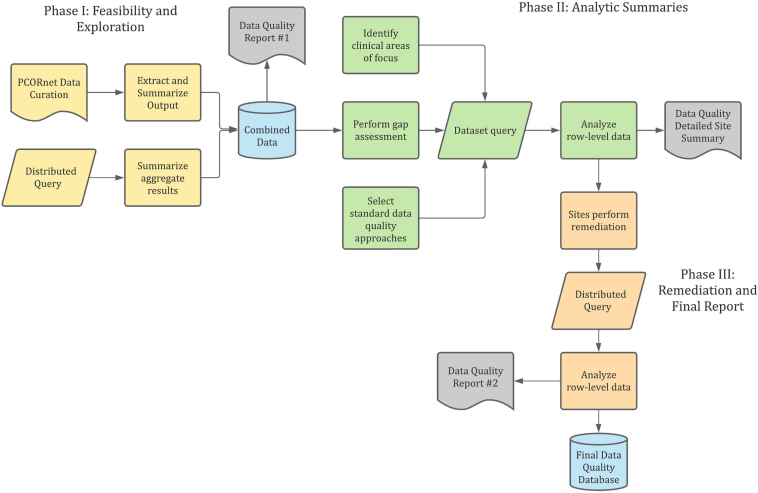
Data quality assessment for PRESERVE applies the systematic framework detailed in Razzaghi et al26 and the principles outlined in Kahn et al27 (Fig 2). Phase 1 leverages results from PCORnet’s network-wide data curation as well as data quality analyses assessing eligibility criteria and summary statistics for principal data elements, focusing on ranges and measures of central tendency for quantitative values and tabulation for categorical values across institutions. It consists of 79 checks across 10 domains: anthropometrics, BP, cohort definition, cohort entry, diagnoses, follow-up, laboratory tests, medications, nephrology provider specialty, and procedures. Data quality checks are categorized as testing completeness (data attributes are sufficient), conformance (data comply to database and PCORnet standards), or correctness (data are plausible or have face validity). We examine attrition, outliers, length of follow-up and cohort entry, demographic characterizations, mismapped codes (eg, urine creatinine in source system represented as serum creatinine), missingness of major variables, outlier laboratory test values, duplication errors, and trends over time. Phase 2 applies to patient-level data and comprises 68 additional checks that identify issues related to sequencing of clinical events and temporal trends, missing clinical results and variables, anomalous clinical distributions, and misrepresentation of variables. It includes an additional category for concordance, which measures the agreement of clinical data across multiple data elements (eg, correlation between systolic and diastolic BP for all values at an institution). Phase 2 focuses on patient characteristics before and following cohort entry, granularity of geocoded address data, days between height and serum creatinine measurements for eGFR computation, distribution of quantitative laboratory test values and BP measurements, and usage of coding terminologies. In both phases, results are benchmarked across participating institutions to detect potential anomalies, categorized as counts high, counts low, values high, values low, values discordant, outlier values, missingness, atypical code utilization, or mapping issues. All findings identified are communicated to participating institutions via reports, a tracking system, and a systematic data quality catalog prioritizing issues. Institutional informatics teams provide information about whether the finding is remediable before submission of the finalized dataset. Where findings are not remediable, known limitations can be considered in analysis plans.
Figure 2.
Study-specific data quality assessment for PRESERVE.
Chart Review
We will use random sampling, stratified by CKD stage at CED, to select 1,500 patients (100 per institution) for chart review to evaluate selection criteria, CKD etiology, long-term dialysis, kidney transplant, and hypertension classification. To increase sample size for ABPM data, we will oversample patients most likely to have undergone an ABPM for 75% of the chart review subcohort.
Patient and Parent Engagement
An important goal of PRESERVE is to develop meaningful engagement of parents/caregivers in the research process. The Glomerular Learning Network (GLEAN; glomerularlearningnetwork.org) serves as the leading rare disease partner for PRESERVE. GLEAN includes 9 pediatric health systems (8 of which are in PCORnet). GLEAN improves the health of children with glomerular disease by conducting research and quality improvement. PRESERVE leverages advances in the data quality of kidney-specific variables that resulted from GLEAN and PEDSnet studies.28, 29, 30, 31 Three GLEAN Parent Partners are coinvestigators in PRESERVE and lead the PRESERVE Patient and Parent Workgroup.
Each institution identified parents of patients and youth with CKD to participate in this workgroup. Partners received training through FYREworks (fyreworkstraining.com), a set of interactive, web-based trainings (developed in PEDSnet) that helps parents and patients understand their role in the research process and the importance of their expertise to a research team.32 Responsibilities of this workgroup include development and implementation of approaches for evaluating engagement; coproduction of the protocol for the patient survey, recruitment strategy, and study materials; review and interpretation of results for all aims; and creation of approaches for dissemination of aggregate study results to patient communities.
Results
Outcomes
The primary outcome in PRESERVE will be a 4-part composite of initiation of long-term dialysis, kidney transplant, an eGFR of <15 mL/min/1.73 m2 or a decrease in eGFR of >50% (Table 1). This variable has been extensively used in pediatric CKD research.5,14, 15, 16,33,34 Kidney function is the most important outcome for patients with pediatric CKD and their families and clinicians reported in the literature35,36 and described in our Parent Partner Engagement Studio, which occured during study planning.
Table 1.
PRESERVE Outcomes
| Name of Outcome | Specific Measure | Timepoints | Powered | |
|---|---|---|---|---|
| Primary | Kidney function decline composite | Composite event of 50% reduction in eGFR, kidney replacement therapy, or eGFR < 15 mL/min/1.73 m2 | From CED to end of follow-up | Yes |
| Secondary | Patient-reported outcome profile | PROMIS Pediatric measures for fatigue, pain, sleep health, anxiety, life satisfaction, and peer relationships Other measures of health status deemed important by parent and youth partners; experience with home BP monitoring |
Single time point (date of patient survey) | Yes |
| Secondary | GFR trajectory | Change in eGFR per unit time | From CED to end of follow-up | Yes |
| Exploratory | BP treatment adverse events | Diagnosis-based adverse events: hypotension, dizziness, cough, stomatitis, tonsillitis, urinary tract infection, nocturnal enuresis, gastrointestinal symptoms, fatigue, edema, hair loss, respiratory tract infection, pyelonephritis, headache, pericarditis, syncope Laboratory-based adverse events: hyperkalemia, increase in liver enzymes, leukocytopenia, anemia, acidosis Other adverse events: death, ED visit for hypertension, hospitalization for hypertension |
From CED to end of follow-up | No |
Abbreviations: BP, blood pressure; CED, cohort entrance date; ED, emergency department; eGFR, estimated glomerular filtration rate.
Although a 50% decline in eGFR has become standard, we will run sensitivity analyses varying the level of decline (eg, 30%-70%)10 to test the sensitivity of the composite outcome to these thresholds. The secondary outcome will model kidney function using eGFR change over time. Patient-reported outcomes (PROs) will include measures of health status deemed important by parent and youth partners. Exploratory outcomes will be adverse events associated with hypertension management.
Analysis Plan
Aim 1: Enhance the PCORnet Common Data Model for Pediatric and Rare Kidney Disease Research
PRESERVE will improve the PCORnet infrastructure by defining and evaluating the quality of new data elements and variables for kidney-related research: pediatric-specific BP z scores, hypertension (defined by diagnosis terms and recorded BP), eGFR computed for children and young adults, proteinuria, CKD etiologies, antihypertensive medications, long-term dialysis, and kidney transplant. We will develop technical specifications for these variables and create a reusable code for all derived variables as well as data quality analyses.
Linkage with CKiD will supplement EHR data with adjudicated data related to etiology of kidney disease, measured GFR, and ABPM. The US Renal Data System (USRDS, usrds.org) provides near complete national capture of dialysis and transplantation. Overlapping data elements will be assessed for concordance to estimate error rates in linkage or source data. Where variables conflict, analyses can prespecify which data source should be used (eg, USRDS will take precedence over the EHR for long-term dialysis).
Aim 2: Describe and Examine the Effectiveness of Consistent BP and Urine Protein Monitoring for Preserving Kidney Function
Reference values for age-specific BP were obtained using standardized protocols in research settings and secondary data analyses of these assessments. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents repurposed data from 11 studies to assemble a sample of 63,000 children,9 including children with overweight and obesity. To develop data unbiased by weight, Rosner et al37 excluded individuals in the Fourth Report’s database who had a body mass index ≥85th percentile and recomputed BP z scores using quantile regression. This approach was used for the normative BP values included in the 2017 American Academy of Pediatrics Clinical Practice Guideline on high BP in children.6 Using clinic-based assessments from the EHR, we will replicate the methodology used by Rosner et al37 to compute systolic and diastolic BP z scores by age, sex, and height percentile for children aged 3-17 years without chronic medical conditions, seen for general pediatric care in outpatient settings, and whose body mass index is <85th percentile. We will contrast the results from these analyses with the age-sex-height specific z scores in the American Academy of Pediatrics Clinical Practice Guideline.
We propose to develop and test a set of alternative algorithms for identifying hypertension defined by actual BP values using EHR data from the clinical setting. These algorithms will include BP z score categories and will consider time between measurements and number of measurements. We will evaluate their validity by examining rates of diagnostic coding for hypertension, treatment of hypertension, and characteristics of the patients identified as hypertensive using the alternative definitions.
For the approximately 300 CKiD participants in the PRESERVE database, we will contrast annual CKiD ABPM mean wake BP with a similar measure using all clinic BP assessments within 12 months before the ABPM. We will compute correlations and 95% confidence intervals (95% CIs), accounting for within patient clustering. We will replicate these analyses using ABPM data derived from institutional sources.
We will characterize the frequency and variability, overall and based on patient and institutional characteristics, for BP (clinic-based and ABPM) and urine protein (total protein and microalbumin, adjusted for urine creatinine) monitoring. For each patient, we will compute the proportion of encounters for which a valid BP z score can be estimated. For those individuals with hypertension, we will determine whether they have annual urine protein and ABPM assessments. We will adapt continuity of care indices,38 such as the usual provider ratio,39 to create BP and urine protein assessment continuity variables. These predictors will be used to test the hypothesis that consistent evaluation of BP and urine protein during the early phase of CKD can reduce risk of subsequent kidney function decline. We will generate institution-specific reports that show levels of adherence to guidelines for the measures, overall, by calendar time and by patient characteristics (eg, age and eGFR), and identify opportunities for practice improvement. We will describe differences in measured BP, hypertension, proteinuria, BP monitoring, and urine protein monitoring ecologically by (deidentified) institution and area-level deprivation measures and individually by age, gender, race/ethnicity, CKD etiology, and CKD stage.
Aim 3: Compare the Effectiveness of BP Medication Strategies for Preserving Kidney Function
We will examine rates of the dichotomous composite outcome and the continuous outcome of decline in eGFR over time by level of BP when antihypertensive treatment was initiated. The former will use survival analysis methods, while the latter will employ generalized linear models to assess trajectories. A BP percentile will be assigned based on the maximum of the systolic and diastolic z scores closest to the day of initiation. We will explore categorization of BP by every 5th percentile (e.g., ≥95th, 90-94th, 85-89th,..., <50th), which is enabled by our large sample size.
Using clinic BP assessments, BP control will be allowed to vary with each visit, and the mean of the systolic and diastolic z-scores6 will be selected as the measure of a visit’s BP level and categorized into a BP quantile. Although larger quantile bands of BP control have been used in prior studies,14 the size of our sample allows us to refine our analytic approach by separating into smaller categories. We will estimate the cumulative survival for each BP category using the counting process formulation.40 Participants enter the risk set at a visit when BP is assessed and exit when they change BP category at a subsequent visit, are lost to follow-up, or experience the composite event. Regarding Kaplan-Meier plots, patients will be allowed to remain in or change their risk set between encounters. Because the comparator is time-dependent, it is likely that the assumptions of the proportional hazards model will not be met, in which case we will explore the extended cox model which allows for inclusion of time-dependent covariates.41 We will also explore use of accelerated failure time models. In a prior CKiD study, the accelerated failure time model was selected as the preferred model for evaluating kidney function decline because of accelerated decline among patients whose kidney function was nearing kidney failure.16
We will contrast the effects of ACEi and ARBs with those of other antihypertensive medications used as first-line treatments and with each other on kidney function decline. Models will control for factors related to first-line therapy decision-making, such as age and gender, serum potassium, asthma, cardiac disease, and eGFR. We will compare different dynamic treatment strategies for minimizing kidney function decline through 2 approaches. The first will use latent class analysis to determine if there is a parsimonious set of pathways for antihypertensive treatment. If so, we will contrast rates of kidney function decline by treatment pathway. In the second approach, we will fit marginal structural models, which accommodate observational study designs that require adjustment for time-varying covariates and outcomes that dynamically interact with one another, which is the case for BP level and antihypertensive treatment.42,43 We will evaluate heterogeneity of treatment effect by CKD stage, CKD etiology, and proteinuria. We will evaluate disparities by sociodemographic characteristics. Table 2 shows the characteristics of the study sample.
Table 2.
Characteristics of the Study Cohort
| Characteristic | N (%) |
|---|---|
| Sex | |
| Female | 10,100 (51.1%) |
| Male | 9,646 (48.9%) |
| Race and ethnicity | |
| Asian or Pacific Islander/Other/Unknown | 2,111 (10.7%) |
| Black or African-American | 4,188 (21.2%) |
| Hispanic | 2,854 (14.5%) |
| White | 10,593 (53.6%) |
| Age at cohort entry (y) | |
| Mean (SD) | 9.4 (5.3) |
| Median [Q1, Q3] | 10.0 [4.4, 14.3] |
| Calendar year of cohort entry | |
| 2009-2012 | 6,974 (35.3%) |
| 2013-2015 | 4,959 (25.1%) |
| 2016-2018 | 4,943 (25.0%) |
| 2019-2021 | 2,870 (14.5%) |
| Qualifying serum creatinine measurement (mg/dL) at CED | |
| Mean (SD) | 0.74 (0.33) |
| Median [Q1, Q3] | 0.70 [0.50, 0.90] |
| Qualifying eGFR measurement (mL/min/1.73 m2) at CED (using U25) | |
| Mean (SD) | 71.4 (15.3) |
| Median [Q1, Q3] | 76.0 [63.3, 83.4] |
| eGFR measurements per person-year (between CED to last in-person visit) | |
| Mean (SD) | 5.8 (19.9) |
| Median [Q1, Q3] | 1.7 [0.8, 4.3] |
| At least 1 glomerular disease diagnosis | |
| N | 16,656 (84.4%) |
| Y | 3,090 (15.6%) |
| At least 1 hypertension diagnosis | |
| N | 10,455 (52.9%) |
| Y | 9,291 (47.1%) |
| At least 1 elevated blood pressure (4th report blood pressure z scores) | |
| N | 5,950 (30.1%) |
| Y | 13,796 (69.9%) |
| Height z score at CED (CDC z scores – age ≥2 y) | |
| Mean (SD) | −0.4 (1.4) |
| Median [Q1, Q3] | −0.3 [−1.3, 0.5] |
| Missing | 2514 (12.7%) |
| Years from CED to last in-person visit | |
| Mean (SD) | 6.6 (3.6) |
| Median [Q1, Q3] | 6.1 [3.6, 9.3] |
| Nephrology visits per person-year (between CED to last in-person visit) | |
| Mean (SD) | 4.3 (8.3) |
| Median [Q1, Q3] | 1.8 [0.5, 4.8] |
Note: Preliminary data from 19,746 patients meeting cohort inclusion criteria from January 2009 to December 2021 across 15 institutions contributing data to PRESERVE. Data extracted for cohort through December 2022.
Aim 4: Assess Patients’ Lived Experiences Related to BP Management
A Delphi survey of patients, caregivers and healthcare professionals in 48 countries found top priorities to include kidney function, mortality, life satisfaction, and BP;36 each of these outcomes will be assessed in PRESERVE. We will build on prior work that demonstrated content validity of the Patient-Reported Outcomes Measurement Information System (PROMIS) measures in prospective studies of children with chronic disease, including 212 children with CKD.44
PRO measures in the PRESERVE survey will be selected based on input from the Patient and Parent Workgroup and expert clinicians. The survey will be completed by youth with CKD aged 8-21 years and parents of youth aged 8-17 years with CKD (target enrollment of 750 patients). The survey data will enable us to examine patient experiences with ABPM and PROs by level of BP control and medication management approaches. The coordinating center will identify a subset of the PRESERVE cohort at each site meeting inclusion criteria for the survey (≥1 hypertension diagnosis code and ≥1 exposure to an antihypertensive medication). Methods of recruitment will include emails, telephone calls, in-person conversations, letters, EHR portal messages, and text messages.
Discussion
PRESERVE will provide key information on kidney function preservation and evaluation of clinical BP measures using an unprecedented large EHR cohort for pediatric CKD and with comprehensive stakeholder engagement. This study builds on the epidemiologic data from CKiD and leverages a large volume of point-of-care measures to model kidney function trajectory and time to kidney failure. There are limited longitudinal data on outcomes in children with CKD after transitioning to adult care. Linkage of PCORnet data with USRDS will enable ascertainment of this primary outcome for the study population beyond their pediatric care at participating centers. Moreover, PRESERVE will contrast management practice benefits (preservation of kidney function) with potential harms (adverse events and PROs).
Although 24-hour ABPM is considered the gold standard for assessing BP control, it is challenging to use repeatedly in children outside of the research setting. A recent study demonstrated that protocolized clinic systolic BPs were not inferior to ABPM systolic BPs for risk discrimination of outcomes, including left ventricular hypertrophy and kidney failure.45 The volume of longitudinal clinic BP measurements available in this study represents a unique opportunity to define optimal BP thresholds and targets for treatment initiation and longitudinal control. The incorporation of ABPM data in our study enables classification and predictive accuracy assessments of clinic measurements, which will facilitate new guidelines based on real-world patterns of care.
The ongoing engagement of patient/parent and clinician partners and dissemination of findings both within participating centers and to the broader pediatric nephrology community will accelerate their incorporation into clinical practice and new evidence-based guidelines.
In contrast to prospective cohort studies and clinical trials, our study includes an unselected sample focusing on children with mild-moderate CKD, when BP management practices may have the largest impact. The sample is also large enough for subgroup analyses to describe the heterogeneity of treatment effect based on kidney disease etiology, baseline proteinuria and eGFR, and other clinical factors.
Observational methods require causal inference methodology because unobserved covariates may affect assignment to the comparator groups and outcome.46 Although we have selected covariates based on clinical input, we will mention this limitation in all manuscripts and provide sufficient detail (following STROBE criteria) for others to judge validity. For objectives evaluating BP and urine protein monitoring, confounding by indication may be a challenge. BP and urine protein are likely to be monitored more frequently because of greater CKD severity and risk of CKD progression. We will consider use of propensity scores to address this issue.
PRESERVE includes source EHR data across 16 institutions, each with different clinical workflows and changes to their EHR over the study period, which introduce heterogeneity and possible measurement error into the data. PCORnet has tried to mitigate this by implementing a common data model (CDM) and robust data characterization program. Although these processes produce analysis-ready data, they do not change fundamental differences in clinical workflows across institutions.
The study sample is drawn from 16 regional children’s hospitals and may not be representative of all children with this rare condition in the US. These referral centers may serve children with greater levels of comorbid or other health conditions that complicate their care. However, 73% of the US pediatric nephrology workforce is linked to academic medical centers.47 We will evaluate representativeness by contrasting the distribution of BP in the general pediatric population in PEDSnet with normative data from the National High BP Education Program6; comparing demographic and clinical characteristics of the PRESERVE population at initiation of KRT with USRDS; contrasting patient populations and rates of outcomes across institutions, as cross-institutional convergence strengthens external validity; performing a PCORnet-wide query and contrasting patients in our sample with those not in our sample.
In summary, PRESERVE will leverage the PCORnet infrastructure to conduct observational studies that address BP management knowledge gaps in an unprecedented large, national cohort of children with CKD, focusing on outcomes that are meaningful to patients. Enhancements to the PCORnet CDM will produce a sustainable infrastructure for future research in pediatric CKD and other rare pediatric conditions for which BP and kidney function are important measures.
Article Information
Authors’ Full Names and Academic Degrees
Michelle R. Denburg, MD, MSCE, Hanieh Razzaghi, MPH, Amy J. Goodwin Davies, PhD, Vikas Dharnidharka, MD, MPH, Bradley P. Dixon, MD, Joseph T. Flynn, MD, MS, Dorey Glenn, MD, MPH, Caroline A. Gluck, MD, MTR, Lyndsay Harshman, MD, MS, Aneta Jovanovska, MS, Chryso Pefkaros Katsoufis, MD, Amy L. Kratchman, BA, Mark Levondosky, ADCJ, Rebecca Levondosky, AAS, Jill McDonald, MA, Mark Mitsnefes, MD, MS, Zubin J. Modi, MD, MS, Jordan Musante, MPH, Alicia M. Neu, MD, Cynthia G. Pan, MD, Hiren P. Patel, MD, Larry T. Patterson, MD, Julia Schuchard, PhD, Priya S. Verghese, MBBS, MPH, Amy C. Wilson, MD, Cynthia Wong, MD, and Christopher B. Forrest, MD, PhD.
Authors’ Contributions
Research idea and study design: MRD, HR, JTF, AJ, ALK, ML, RL, MM, AMN, JS, CBF; data acquisition: MRD, HR, AJGD, BD, JTF, DG, CAG, LH, CPK, J McDonald, MM, J Musante, ZJM, AMN, CGP, HPP, LTP, PSV, ACW, CW, CBF; data analysis/interpretation: MRD, HR, AJGD, VD, BD, JTF, DG, CAG, LH, CPK, MM, ZJM, AMN, CGP, HPP, LTP, PSV, ACW, CW, CBF; statistical analysis: MRD, HR, AJGD, CBF; supervision or mentorship: MRD, HR, J McDonald, CBF. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This study is funded through PCORI Award #RD-2020C2-20338. The funder of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Financial Disclosure
J. Schuchard is employed by Janssen Pharmaceuticals, Inc. A. Jovanovska is employed by Merck. R. Levondosky is employed by Virtua Mount Holly Hospital. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received February 20, 2023. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form July 10, 2023.
Footnotes
Complete author and article information provided before references.
References
- 1.Flynn J.T., Mitsnefes M., Pierce C., et al. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52(4):631–637. doi: 10.1161/HYPERTENSIONAHA.108.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitsnefes M., Ho P.L., McEnery P.T. Hypertension and progression of chronic renal insufficiency in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) J Am Soc Nephrol. 2003;14(10):2618–2622. doi: 10.1097/01.asn.0000089565.04535.4b. [DOI] [PubMed] [Google Scholar]
- 3.VanDeVoorde R.G., Mitsnefes M.M. Hypertension and CKD. Adv Chronic Kidney Dis. 2011;18(5):355–361. doi: 10.1053/j.ackd.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Wingen A.M., Fabian-Bach C., Schaefer F., Mehls O. Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet. 1997;349(9059):1117–1123. doi: 10.1016/s0140-6736(96)09260-4. [DOI] [PubMed] [Google Scholar]
- 5.ESCAPE Trial Group. Wühl E., Trivelli A., et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361(17):1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 6.Flynn J.T., Kaelber D.C., Baker-Smith C.M., et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3) doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1–S290. [PubMed] [Google Scholar]
- 8.Lurbe E., Cifkova R., Cruickshank J.K., et al. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens. 2009;27(9):1719–1742. doi: 10.1097/HJH.0b013e32832f4f6b. [DOI] [PubMed] [Google Scholar]
- 9.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. doi: 10.1542/peds.114.2.S2.555. [DOI] [PubMed] [Google Scholar]
- 10.Dionne J.M. Evidence-based guidelines for the management of hypertension in children with chronic kidney disease. Pediatr Nephrol. 2015;30(11):1919–1927. doi: 10.1007/s00467-015-3077-7. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson M.A., Ng D.K., Warady B.A., Furth S.L., Flynn J.T. The CKiD study: overview and summary of findings related to kidney disease progression. Pediatr Nephrol. 2021;36(3):527–538. doi: 10.1007/s00467-019-04458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong C.J., Moxey-Mims M., Jerry-Fluker J., Warady B.A., Furth S.L. CKiD (CKD in children) prospective cohort study: a review of current findings. Am J Kidney Dis. 2012;60(6):1002–1011. doi: 10.1053/j.ajkd.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furth S.L., Cole S.R., Moxey-Mims M., et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds B.C., Roem J.L., Ng D.K.S., et al. Association of time-varying blood pressure with chronic kidney disease progression in children. JAMA Netw Open. 2020;3(2) doi: 10.1001/jamanetworkopen.2019.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warady B.A., Abraham A.G., Schwartz G.J., et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the Chronic Kidney Disease in Children (CKiD) Cohort. Am J Kidney Dis. 2015;65(6):878–888. doi: 10.1053/j.ajkd.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furth S.L., Pierce C., Hui W.F., et al. Estimating time to ESRD in children with CKD. Am J Kidney Dis. 2018;71(6):783–792. doi: 10.1053/j.ajkd.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong C.S., Pierce C.B., Cole S.R., et al. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol. 2009;4(4):812–819. doi: 10.2215/CJN.01780408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham A.G., Betoko A., Fadrowski J.J., et al. Renin-angiotensin II-aldosterone system blockers and time to renal replacement therapy in children with CKD. Pediatr Nephrol. 2017;32(4):643–649. doi: 10.1007/s00467-016-3512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong Y., Munoz A., Schwartz G.J., Warady B.A., Furth S.L., Abraham A.G. Nonlinear trajectory of GFR in children before RRT. J Am Soc Nephrol. 2014;25(5):913–917. doi: 10.1681/asn.2013050487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins F.S., Hudson K.L., Briggs J.P., Lauer M.S. PCORnet: turning a dream into reality. J Am Med Inform Assoc. 2014;21(4):576–577. doi: 10.1136/amiajnl-2014-002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrest C.B., McTigue K.M., Hernandez A.F., et al. PCORnet® 2020: current state, accomplishments, and future directions. J Clin Epidemiol. 2021;129:60–67. doi: 10.1016/j.jclinepi.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selby J.V., Grossman C., Zirkle M., Barbash S. Multistakeholder engagement in PCORnet, the national patient-centered clinical research network. Med Care. 2018;56(10 suppl 10 suppl 1):S4–S5. doi: 10.1097/MLR.0000000000000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrest C.B., Margolis P., Seid M., Colletti R.B. PEDSnet: how a prototype pediatric learning health system is being expanded into a national network. Health Aff (Millwood) 2014;33(7):1171–1177. doi: 10.1377/hlthaff.2014.0127. [DOI] [PubMed] [Google Scholar]
- 24.Forrest C.B., Margolis P.A., Bailey L.C., et al. PEDSnet: a National Pediatric Learning Health System. J Am Med Inform Assoc. 2014;21(4):602–606. doi: 10.1136/amiajnl-2014-002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce C.B., Muñoz A., Ng D.K., Warady B.A., Furth S.L., Schwartz G.J. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99(4):948–956. doi: 10.1016/j.kint.2020.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razzaghi H., Greenberg J., Bailey L.C. Developing a systematic approach to assessing data quality in secondary use of clinical data based on intended use. Learn Health Syst. 2022;6(1) doi: 10.1002/lrh2.10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn M.G., Callahan T.J., Barnard J., et al. A harmonized data quality assessment terminology and framework for the secondary use of electronic health record data. EGEMS (Wash DC) 2016;4(1):1244. doi: 10.13063/2327-9214.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denburg M.R., Razzaghi H., Bailey L.C., et al. Using electronic health record data to rapidly identify children with glomerular disease for clinical research. J Am Soc Nephrol. 2019;30(12):2427–2435. doi: 10.1681/ASN.2019040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodwin Davies A.J., Xiao R., Razzaghi H., et al. Skeletal outcomes in children and young adults with glomerular disease. J Am Soc Nephrol. 2022;33(12):2233–2246. doi: 10.1681/asn.2021101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenderfer S.E., Chang J.C., Goodwin Davies A., et al. Using a multi-institutional pediatric learning health system to identify systemic lupus erythematosus and lupus nephritis: development and validation of computable phenotypes. Clin J Am Soc Nephrol. 2022;17(1):65–74. doi: 10.2215/cjn.07810621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone H.K., Mitsnefes M., Dickinson K., et al. Clinical course and management of children with IgA vasculitis with nephritis. Pediatr Nephrol. 2023;38(11):3721–3733. doi: 10.1007/s00467-023-06023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellison J.S., Lorenzo M., Beck H., et al. Comparative effectiveness of paediatric kidney stone surgery (the PKIDS trial): study protocol for a patient-centred pragmatic clinical trial. BMJ Open. 2022;12(4) doi: 10.1136/bmjopen-2021-056789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong L.S.H., Sautenet B., Tong A., et al. Range and heterogeneity of outcomes in randomized trials of pediatric chronic kidney disease. J Pediatr. 2017;186:110–117 e11. doi: 10.1016/j.jpeds.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 34.van den Belt S.M., Heerspink H.J.L., Gracchi V., de Zeeuw D., Wuhl E., Schaefer F., ESCAPE Trial Group Early proteinuria lowering by angiotensin-converting enzyme inhibition predicts renal survival in children with CKD. J Am Soc Nephrol. 2018;29(8):2225–2233. doi: 10.1681/asn.2018010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson C.S., Gutman T., Craig J.C., et al. Identifying important outcomes for young people with CKD and their caregivers: a nominal group technique study. Am J Kidney Dis. 2019;74(1):82–94. doi: 10.1053/j.ajkd.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 36.Logeman C., Guha C., Howell M., et al. Developing consensus-based outcome domains for trials in children and adolescents with CKD: an international Delphi survey. Am J Kidney Dis. 2020;76(4):533–545. doi: 10.1053/j.ajkd.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Rosner B., Cook N., Portman R., Daniels S., Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653–666. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 38.Steinwachs D.M. Measuring provider continuity in ambulatory care: an assessment of alternative approaches. Med Care. 1979;17(6):551–565. doi: 10.1097/00005650-197906000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Forrest C.B., Starfield B. Entry into primary care and continuity: the effects of access. Am J Public Health. 1998;88(9):1330–1336. doi: 10.2105/ajph.88.9.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersen P.K., Gill R.D. Cox’s regression model for counting processes: a large sample study. Ann Statist. 1982;10(4):1100–1120. doi: 10.1214/aos/1176345976. [DOI] [Google Scholar]
- 41.Kleinbaum D.G., Klein M. Springer; 2011. Survival analysis: a self-learning text. [Google Scholar]
- 42.Hernan M.A., Hernandez-Diaz S., Robins J.M. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 43.Suarez D., Haro J.M., Novick D., Ochoa S. Marginal structural models might overcome confounding when analyzing multiple treatment effects in observational studies. J Clin Epidemiol. 2008;61(6):525–530. doi: 10.1016/j.jclinepi.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Forrest C.B., Forrest K.D., Clegg J.L., et al. Establishing the content validity of PROMIS Pediatric pain interference, fatigue, sleep disturbance, and sleep-related impairment measures in children with chronic kidney disease and Crohn's disease. J Patient Rep Outcomes. 2020;4(1):11. doi: 10.1186/s41687-020-0178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ku E., McCulloch C.E., Warady B.A., Furth S.L., Grimes B.A., Mitsnefes M.M. Twenty-four-hour ambulatory blood pressure versus clinic blood pressure measurements and risk of adverse outcomes in children with CKD. Clin J Am Soc Nephrol. 2018;13(3):422–428. doi: 10.2215/cjn.09630917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenbaum P.R. Harvard University Press; 2017. Observation & experiment: an introduction to causal inference. [Google Scholar]
- 47.Primack W.A., Meyers K.E., Kirkwood S.J., Ruch-Ross H.S., Radabaugh C.L., Greenbaum L.A. The US pediatric nephrology workforce: a report commissioned by the American Academy of Pediatrics. Am J Kidney Dis. 2015;66(1):33–39. doi: 10.1053/j.ajkd.2015.03.022. [DOI] [PubMed] [Google Scholar]