Summary
Single-cell assay for transposase-accessible chromatin with sequencing (scATAC-seq) resolves the heterogeneity of epigenetic states across cells but does not typically capture exonic mutations, which limits our knowledge of how somatic mutations alter chromatin landscapes. Here, we present a plate-based approach coupling high-sensitivity genotyping of genomic loci with high-content scATAC-seq libraries from the same single cells. We first describe steps for optimization of genotyping primers, followed by detailed guidance on the preparation of both scATAC-seq and single-cell genotyping libraries, fully automated on high-throughput liquid handling platforms.
For complete details on the use and execution of this protocol, please refer to Turkalj, Jakobsen et al.1
Subject areas: Bioinformatics, Cancer, Genetics, Genomics, Molecular Biology, Sequence Analysis, Sequencing, Single Cell, Stem Cells
Graphical abstract

Highlights
-
•
scATAC-seq coupled with high-sensitivity mutation capture at multiple genomic loci
-
•
Link genetic and epigenetic evolution in malignant and pre-malignant tissues
-
•
Guidance on barcoding, automation, and liquid handling for high-throughput applications
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Single-cell assay for transposase-accessible chromatin with sequencing (scATAC-seq) resolves the heterogeneity of epigenetic states across cells but does not typically capture exonic mutations, which limits our knowledge of how somatic mutations alter chromatin landscapes. Here, we present a plate-based approach coupling high-sensitivity genotyping of genomic loci with high-content scATAC-seq libraries from the same single cells. We first describe steps for optimization of genotyping primers, followed by detailed guidance on the preparation of both scATAC-seq and single-cell genotyping libraries, fully automated on high-throughput liquid handling platforms.
Before you begin
Institutional permissions
Patient samples were collected with informed consent under ethically approved protocols (MREC 06/Q1606/110 or NHS REC 17/YH/0382). Written informed consent was obtained in accordance with the Declaration of Helsinki. If GTAC is performed on primary human samples, before the collection of clinical biopsies, the study requires the approval by the Ethics Committee or by an equivalent organization. Informed consent should be collected from all patients.
Preparation one: Determination of the optimal number of PCR cycles for the specific tissue type
Timing: 2 days
Before the execution of GTAC on primary samples of interest, we strongly recommend performing a pilot experiment on a sample derived from the same tissue type first, to define the optimal number of PCR cycles required for single-cell amplification of open chromatin fragments (part 3, point 36). For preparation one, it is sufficient to perform plate-based scATAC-seq,2,4 without the addition of target-specific genotyping primers. During these steps, you will sort single tagmented nuclei into multiple 96-well plates. One plate will be used for preparation one, to define the optimal number of PCR cycles. The remaining plates (stored at −80°C) will be used in preparation three, to test genotyping primers in single cells.
CRITICAL: These pilot experiments can help avoid several potential issues (troubleshooting).
Note: PCR cycle numbers stated throughout the protocol apply to healthy and leukemic primary human bone marrow mononuclear cells, but these numbers may vary across tissue types.
Note: Before starting with preparation one, prepare all buffers listed in materials and equipment. Order generic i7 and i5 primers and reconstitute them at 100 μM in TE buffer (10 mM Tris-HCl pH 8.0, 0.1 mM EDTA).
Optional: Depending on the tissue type (solid versus cell suspension) and preservation state (fresh versus cryopreserved) of your sample, you may need to prepare different buffers for sample preparation. Follow the considerations in part 2, point 7 and prepare buffers according to the sample type. If FACS staining and pre-sorting prior to Omni-ATAC are required, prepare FACS buffer, thawing media, and antibody and fluorescence-minus one (FMO) mixes.
Note: Points 1, 4, and 5a should be performed in a designated pre-PCR clean area, ideally in a biosafety cabinet, prior to PCR.
-
1.Prepare 2 mL of generic i7 and i5 20 μM primer dilutions in nuclease-free water. Prepare generic lysis buffer for 10 96-well plates, according to the table below.Generic lysis buffer
Reagent 1 reaction 1150 reactions (10 96-well plates + 20% dead volume) Tris HCl pH 8.0 1 M 0.2 μL 230 μL NaCl 5 M 0.04 μL 46 μL SDS 10% (wt/vol) 0.08 μL 92 μL Generic i7 primer 20 μM 1 μL 1150 μL Generic i5 primer 20 μM 1 μL 1150 μL Nuclease-free water 1.68 μL 1932 μL TOTAL 4 μL 4600 μL -
a.Aliquot 4 μL of generic lysis buffer into each well of a fresh 96-well plate.
-
b.When a plate is aliquoted, cover it with aluminum adhesive seals, centrifuge in a plate spinner for 10 s at 500 × g, and place on wet ice. Repeat for all plates.
-
a.
-
2.
Prepare the sample and perform Omni-ATAC as detailed in part 2, points 7–26.
Note: Follow the considerations in part 2, point 7, regarding the optimal course of action for different tissue types!
-
3.Sort single tagmented nuclei into plates prepared in point 1:
-
a.For sorter calibration and sorting panel setup, follow part 2, points 27–29, but calibrate the machine for sorting into 96-well plates.
-
b.Centrifuge lysis buffer plates in a plate spinner for 10 s at 500 × g.
-
c.Sort single nuclei in each column, keeping 1–2 wells of the plate empty.
-
d.After nuclei have been sorted into the whole plate, cover it with an aluminum adhesive seal, centrifuge immediately in a plate spinner for 20 s at 500 × g, and snap freeze the plate by placing it on dry ice.
-
a.
Pause point: After the sort, plates can be stored at −80°C for up to 3 months. Some sorted plates will also be used in preparation three.
-
4.Using one sorted 96-well plate, proceed with lysis and Tween 20 quenching:
-
a.Incubate the plate at 65°C for 15 min in a thermal cycler, with the lid heated to 105°C.
-
b.Add 2 μL of Tween 20 10% (vol/vol) to each well using an 8 × 20 μL multichannel pipette.
-
c.Centrifuge the plate in a plate spinner for 10 s at 500 × g and incubate at 21°C for 10 min.
-
a.
-
5.Prepare the generic PCR mix by mixing 960 μL of Q5 High-Fidelity 2× Master Mix with 240 μL of nuclease-free water.
-
a.Dispense 10 μL of this mix to each well using an 8 × 20 μL multichannel pipette. Dispense on the sides of the wells and do not touch the bottom with the tips. Keep the plate and the PCR mix in cold racks. Each well will now contain 16 μL of material.
-
b.Seal the plate with a plastic PCR adhesive seal, centrifuge in a plate spinner for 10 s at 500 × g, and pre-amplify libraries by running 8 cycles of the standard PCR program (part 3, point 36).
-
a.
-
6.After 8 cycles, centrifuge the plate in a plate spinner for 10 s at 500 × g and place in a cold rack.
-
a.Transfer the whole volume from 48 wells (half plate) into a fresh 96-well plate for qPCR. Cover the original plate with a plastic PCR adhesive seal and keep it cold.
-
b.Add SYBR Green to the wells of the new plate to reach 1× final concentration (Figure 1A).
-
c.Perform qPCR with the program below. Use an empty well as negative control.PCR cycling conditions
Steps Temperature Time Cycles Initial Denaturation 98°C 1 min 1 Denaturation 98°C 10 s 30 cycles Annealing 60°C 30 s Extension 72°C 30 s Hold 4°C Hold -
d.Observe the qPCR amplification plot. Each curve represents a single well. Select the number of cycles n at which the curves start rising exponentially (Figure 1B).
-
e.Run n cycles of PCR for the remaining 48 pre-amplified libraries (original plate).Note: You have now defined the optimal number of PCR cycles to run on single cells from this type of tissue. In future GTAC experiments on the same type of tissue, run 8 + n PCR cycles in total.
-
a.
-
7.After PCR, centrifuge the plate in a plate spinner for 10 s at 500 × g and pool all the volume from 48 wells into a 15 mL conical centrifuge Falcon tube. The tube will contain ∼700 μL of material at this point. Purify the pool:
-
a.Add 3500 μL of PB buffer to the pool (5× the volume in the pool). Mix 5–10 times but do not vortex.
-
b.Perform a column purification as described in part 4, points 41–44. Elute in 21 μL of EB buffer or nuclease-free water.
-
c.Perform a double-sided AMPure XP bead size selection, first with a 0.5× beads:sample ratio, followed by a 1.2× beads:sample ratio, as described in part 4, points 46–55. At the end of bead purification, elute in 10 μL of nuclease-free water.
-
a.
CRITICAL: Your sample volume at this stage is half of the volume noted in part 4, point 46. Hence, first add 10.5 μL of beads to the sample, followed by 14.7 μL of beads in the next addition.
-
8.
Quantify the pool using a Qubit dsDNA HS Assay Kit. Using the ng/μL readout, estimate the total mass [ng] of DNA in the pool, and the approximate mass [ng] of DNA per cell.
Note: In our experience, when 16 cycles are performed on human bone marrow CD34+ cells (healthy or leukemic), we obtain ∼0.8–3 ng of DNA per single cell. However, the yield will be sample-specific.
-
9.Check the library size distribution using a microcapillary assay like Agilent Bioanalyzer or TapeStation. Traces indicative of high-quality libraries are shown in Figure 2. Set the region between 100 and 7,000 bp. Calculate the molarity of your library using the average size from the Bioanalyzer or an equivalent assay and the concentration [ng/μL] obtained with Qubit.
-
a.Estimate whether the library amount would be sufficient for sequencing, considering that normally you would have 382 cells (∼8 times more material) in the pool (the minimum library concentration for sequencing on Illumina platforms is 4 nM).
-
i.If the library concentration is sufficient and the fragment size distribution resembles a high-quality ATAC library (Figure 2), use these conditions in the GTAC experiment on the samples of interest.
-
ii.If library concentration is insufficient, repeat another test by increasing the number of PCR cycles.
-
iii.If Bioanalyzer traces are suboptimal, refer to the troubleshooting section for potential causes and solutions.
-
i.
-
a.
Figure 1.
Optimal PCR cycle number titration
(A) Procedures for establishing the optimal number of PCR cycles for a GTAC experiment. Blue numbers indicate the order in which the steps are executed.
(B) SYBR Green-based qPCR was performed after 8 initial cycles of amplification. Each curve represents a single well. The optimal additional number of PCR cycles is highlighted in green.
Figure 2.
Bioanalyzer traces showing optimal scATAC-seq libraries
For each trace, a variable number of single-cell libraries was pooled and purified. Successful libraries display the characteristic ‘wavy’ shape, with periodic rises and drops in signal intensity (nucleosomal pattern).
Preparation two: Design and validation of target-specific pre-amplification and nested genotyping primers using bulk genomic DNA (gDNA)
Timing: 2 days
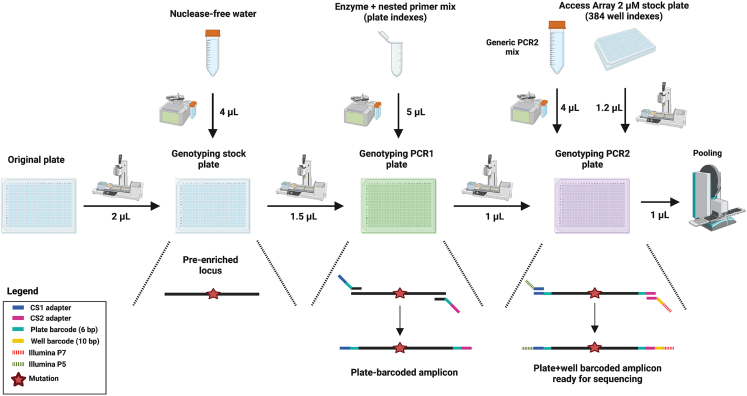
The main advantage of GTAC over classical scATAC-seq is the amplification of multiple genomic loci harboring mutations, in parallel with amplification of open chromatin fragments.1 This is achieved by adding target-specific primers (part 3, point 34) to the PCR (part 3, point 36).
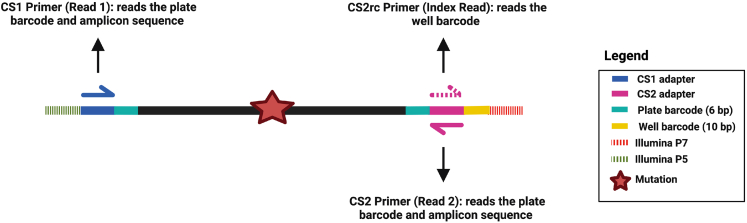
Preparation two and preparation three describe how to design and validate these target-specific primers. Two primer pairs are needed for each locus of interest: one used in the first PCR (part 3, point 36), to pre-enrich for the locus – hereafter, we refer to these primers as “pre-amplification genotyping primers”; the second pair is nested within the pre-amplification amplicon and is used in genotyping PCR1 (part 5, points 61–64), to further enrich for the locus, attach plate barcodes, and universal CS1/CS2 adapters – hereafter, we refer to these primers as “nested genotyping primers”. A schematic overview is shown in Figure 3.
Note: A list of all pre-amplification and nested genotyping primers that we have previously validated for GTAC applications1 is provided in Table S1. We advise to use some of these primer pairs as positive controls in points 12 and 13. Additionally, if some of these primers cover the genomic loci of your interest, there is no need to re-design new primers for these loci.
-
10.Design pre-amplification genotyping primer pairs for each locus using Primer-BLAST5 or another tool for primer design. We recommend designing at least 2 pairs per locus.
-
a.Use the following criteria:
-
i.Aim for an amplicon size < 300 bp, if possible (ideally < 200).
-
ii.Aim for a primer length between 19 bp and 25 bp.
-
iii.Check for primer specificity against a genomic reference. If possible, select those pairs that show no or minimal non-specific binding.
-
iv.Melting temperature should be 57°C–63°C; GC content should be 20%–80%.
-
v.Once at least 2 primer pairs are designed per locus, use the IDT Oligo Analyzer to check for annealing to generic i7 and i5 primers, for each genotyping primer. Exclude genotyping primers for which the predicted secondary structure of the heterodimer formed with the i7 or i5 primer displays ΔG < −9 kcal/mol, if possible.
-
i.
-
b.Order the primers and reconstitute them at 100 μM in TE buffer (10 mM Tris-HCl pH 8.0, 0.1 mM EDTA).
-
c.Prepare 2 μM primer dilutions to be used in point 12.
-
a.
Note: Reconstitute the pre-amplification genotyping primers and prepare the 2 μM primer dilutions in a designated pre-PCR clean area, ideally in a biosafety cabinet. The original 100 μM stock, kept in the pre-PCR area, will also be used in preparation three.
Note: Keeping a short amplicon size minimizes the risk of the Tn5 inserting within the amplicon, which may lead to failed genotyping and allelic dropouts, if the targeted locus is found in open chromatin.
CRITICAL: Pre-amplification genotyping primers for which the predicted secondary structure of the heterodimer formed with the i7 or i5 primer displays ΔG < −9 kcal/mol are likely to interfere with scATAC-seq library generation and should be avoided. Troubleshooting 2.
-
11.Design nested genotyping primer pairs for each locus using Primer-BLAST5 or another tool for primer design. We recommend designing at least 2 pairs per locus.
-
a.Use the following criteria:
-
i.Primers should be nested with respect to those designed in point 10.
-
ii.Aim for an amplicon size of 75–200 bp, if possible (not less than 75 bp).
-
iii.Design the primers so that the beginning of the forward or of the reverse primer is within 69 bp from the mutation. This is crucial when using 75 bp paired-end sequencing, to efficiently cover the nucleotide of interest. For details about sequencing configurations, refer to the Note after part 5, point 84.
-
iv.Aim for primer length between 19 bp and 25 bp.
-
v.Check for primer specificity against a genomic reference. If possible, select those pairs that show no or minimal non-specific binding.
-
vi.Melting temperature should be 57°C–63°C; GC content should be 20%–80%.
-
i.
-
b.Order the primers and reconstitute them at 100 μM in TE buffer (10 mM Tris-HCl pH 8.0, 0.1 mM EDTA). Forward primers should contain the CS1 adapter, while reverse primers should contain the CS2 adapter, at their 5′ ends.
-
c.Prepare 2 μM primer dilutions to be used in point 13.
-
a.
-
12.Validate pre-amplification genotyping primers using bulk gDNA (usually extracted from cell lines):
-
a.Set up the following reaction for each primer pair:Pre-amplification genotyping primer testing reaction (bulk gDNA)
Reagent 1 reaction Q5 High-Fidelity 2× Master Mix 5 μL gDNA template 50–150 ng/μL 0.5 μL Nuclease-free water 3.5 μL Forward pre-amplification primer 2 μM 0.5 μL Reverse pre-amplification primer 2 μM 0.5 μL -
b.Set up a no template negative control reaction and a positive control reaction with primers that display successful amplification at these conditions (Table S1).
-
c.Run the following PCR program:PCR cycling conditions
Steps Temperature Time Cycles Gap-Filling 72°C 10 min 1 Initial Denaturation 98°C 10 min 1 Denaturation 98°C 10 s 40 cycles Annealing 60°C 30 s Extension 72°C 30 s Final extension 72°C 20 s 1 Hold 4°C Hold -
d.Run the PCR product on a 2% agarose gel and select the pair with the strongest and most specific amplification. Examples are shown in Figure 4.
-
a.
-
13.Validate nested genotyping primers using bulk gDNA (usually extracted from cell lines):
-
a.Set up the following reaction for each primer pair:Nested genotyping primer testing reaction (bulk gDNA)
Reagent 1 reaction KAPA2G Robust HS Ready Mix 3.125 μL gDNA template 50–150 ng/μL 1.5 μL Forward genotyping nested primer 2 μM 0.81 μL Reverse genotyping nested primer 2 μM 0.81 μL -
b.Set up a no template negative control reaction and a positive control reaction with primers that display successful amplification at these conditions (Table S1).
-
c.Run the following PCR program:PCR cycling conditions
Steps Temperature Time Cycles Initial Denaturation 95°C 3 min 1 Denaturation 95°C 15 s 35 cycles Annealing 60°C 20 s Extension 72°C 1 min Final extension 72°C 5 min 1 Hold 4°C Hold -
d.Run the PCR product on a 2% agarose gel and select the pair with the strongest and most specific amplification. Examples are shown in Figure 4. Troubleshooting 11.Note: For some loci, it may be challenging to obtain strong and specific amplification with bulk gDNA, even when many pairs are tested. In these cases, we still recommend testing primers in single cells, which may show acceptable amplification despite suboptimal results on bulk gDNA.Note: Given that 2 forward and 2 reverse primers were designed for each locus, we recommend setting up 4 reactions per locus, each testing a different forward/reverse primer combination.Optional: For some genotyping loci, it may be challenging to design an efficient nested primer pair. In these cases, you may use the same primer pair for the pre-amplification PCR (part 3, point 36) and for PCR1 (part 5, points 61–64). In those cases, test the primer pair both with the Q5 High-Fidelity 2× Master Mix (point 12) and with the KAPA2G Robust HS Ready Mix (point 13). However, in our experience, genotyping is more efficient when a nested genotyping primer pair is used for PCR1.Optional: In our applications, we usually genotype point mutations or small indels: hence, the same primer pair amplifies the wild-type (WT) and the mutant alleles. Sometimes, you may need to amplify larger fusion genes – in these cases, you need to design a fusion-specific and a WT-specific primer pair to efficiently capture both alleles. In those cases, remember to test the fusion-specific primer pair on gDNA containing the fusion gene of interest to validate amplification efficiency.
-
a.
Figure 3.
Schematic overview of primers to be tested prior to the GTAC experiment
Figure 4.
Representative gel of primer testing with bulk gDNA
Left gel: each lane shows the PCR product of a single primer pair used on gDNA. Arrows are color-coded based on the quality of the product. An optimal primer pair displays amplification as indicated by the green arrow. Right gel: a primer pair for which there is prior knowledge of good amplification is used as positive control. A no-template reaction is used as negative control. A 100 bp ladder was used for both gels.
Preparation three: Validation of selected genotyping primers in single cells
Timing: 1–10 days
You now need to test successful pre-amplification and nested genotyping primer pairs selected in preparation two in single cells, to evaluate whether (a) you can efficiently generate genotyping amplicons at single-cell level; (b) scATAC-seq library generation is successful in presence of these primers.
CRITICAL: If you are genotyping multiple loci in the same sample of interest, pre-amplification genotyping primers for all loci need to be added simultaneously prior to the first PCR (point 15).
Note: For Preparation three, it is not necessary to use samples with specific mutations, as efficiency of mutation detection is not being evaluated at this step. An exception to this is the case in which you are genotyping specific fusion genes (see the consideration at the end of preparation two).
Note: Points 14–15 days (prior to PCR) should be performed in a designated pre-PCR clean area, ideally in a biosafety cabinet.
-
14.Using one plate sorted in preparation one, point 3, perform lysis and Tween 20 quenching:
-
a.Incubate the plate at 65°C for 15 min in a thermal cycler, with the lid heated to 105°C.
-
b.Add 2 μL of Tween 20 10% (vol/vol) to each well using an 8 × 20 μL multichannel pipette.
-
c.Centrifuge the plate in a plate spinner for 10 s at 500 × g and incubate at 21°C for 10 min.
-
a.
-
15.Prepare PCR mixes:
-
a.Prepare PCR primer mixes with pre-amplification genotyping primers for 30 reactions, according to the table below. The final concentration of each pre-amplification genotyping primer in each well (16 μL) should be 100 nM. Troubleshooting 11.Note: The number of mixes depends on the number of different conditions you are testing.Note: The table below shows an example with 2 genotyping loci (4 primers). For more loci, add further primer pairs as needed, in place of water. For > 4 loci (> 8 primers), use a higher initial primer concentration, to keep the volume at 10 μL of mix per reaction.PCR mix containing pre-amplification genotyping primers
Reagent 1 reaction 30 reactions Q5 High-Fidelity 2× Master Mix 8 μL 240 μL Pre-amplification forward primer (locus 1 sample 1) 6.4 μM 0.25 μL 7.5 μL Pre-amplification reverse primer (locus 1 sample 1) 6.4 μM 0.25 μL 7.5 μL Pre-amplification forward primer (locus 2 sample 1) 6.4 μM 0.25 μL 7.5 μL Pre-amplification reverse primer (locus 2 sample 1) 6.4 μM 0.25 μL 7.5 μL Nuclease-free water 1 μL 30 μL -
b.Prepare a positive control mix without pre-amplification genotyping primers by mixing 240 μL of Q5 High-Fidelity 2× Master Mix with 60 μL of nuclease-free water.
-
c.Dispense each mix into 24 wells, by adding 10 μL of mix per well (Figure 5).
-
d.Seal the plate with a plastic PCR adhesive seal and centrifuge in a plate spinner for 10 s at 500 × g.
-
e.Run the PCR program as in part 3, point 36, by using the number of cycles estimated in preparation one.
-
a.
-
16.
Aliquot 8 μL of nuclease-free water to a fresh 96-well plate (test genotyping stock plate).
-
17.
When PCR is finished, transfer 4 μL of amplified material to the test genotyping stock plate. Seal the test genotyping stock plate with an aluminum adhesive seal and place it on wet ice.
-
18.Pool the remaining material from each condition into a separate 2 mL microcentrifuge tube (Figure 5). Each tube should contain ∼250 μL of material. Purify the pools as follows:
-
a.Add 1250 μL of PB buffer to each pool. Mix 5–10 times but do not vortex.
-
b.Perform a column purification as described in part 4, points 41–44. Elute each pool in 21 μL of EB buffer or nuclease-free water.
-
c.Perform a double-sided AMPure XP bead size selection, first with a 0.5×, followed by a 1.2× beads:sample ratio, as described in part 4, points 46–55. At the end of bead purification, elute in 8–10 μL of nuclease-free water.
-
a.
CRITICAL: Your sample volume is half that in part 4, point 46. Hence, add first 10.5 μL of beads to sample, followed by 14.7 μL of beads in the next addition.
-
19.
Quantify the pool using a Qubit dsDNA HS Assay Kit. Check library size distribution using a microcapillary assay like Agilent Bioanalyzer or TapeStation. Make sure ATAC libraries are comparable to those in Figure 2. Troubleshooting 2.
CRITICAL: If at this point one or more conditions show sub-optimal results, this is likely due to one or more pre-amplification genotyping primer pairs in that condition interfering with ATAC library amplification. In this case, perform another test with only one primer pair per condition, to identify the problematic pair. Re-design primers for this locus and test them as in preparation two and preparation three.
-
20.Test single-cell genotyping:
-
a.Transfer 1.5 μL aliquot from each well of the test genotyping stock plate generated in point 17 as input for the genotyping PCR1 reaction (table below). Use a minimum of 6 wells per condition.Single-cell genotyping PCR1 testing reaction
Reagent 1 reaction KAPA2G Robust HS Ready Mix 3.125 μL Nuclease-free water 1.25 μL Nested genotyping forward/reverse primer pair 5 μM each primer 0.375 μL Pre-amplified library 1.5 μL -
b.Run the PCR program from preparation two, point 13c.Note: Each amplicon needs to be tested in a separate reaction. Hence, if two loci were amplified in one condition in point 15, each well needs to be used as input for two reactions – one amplifying locus 1, and one amplifying locus 2.
-
a.
-
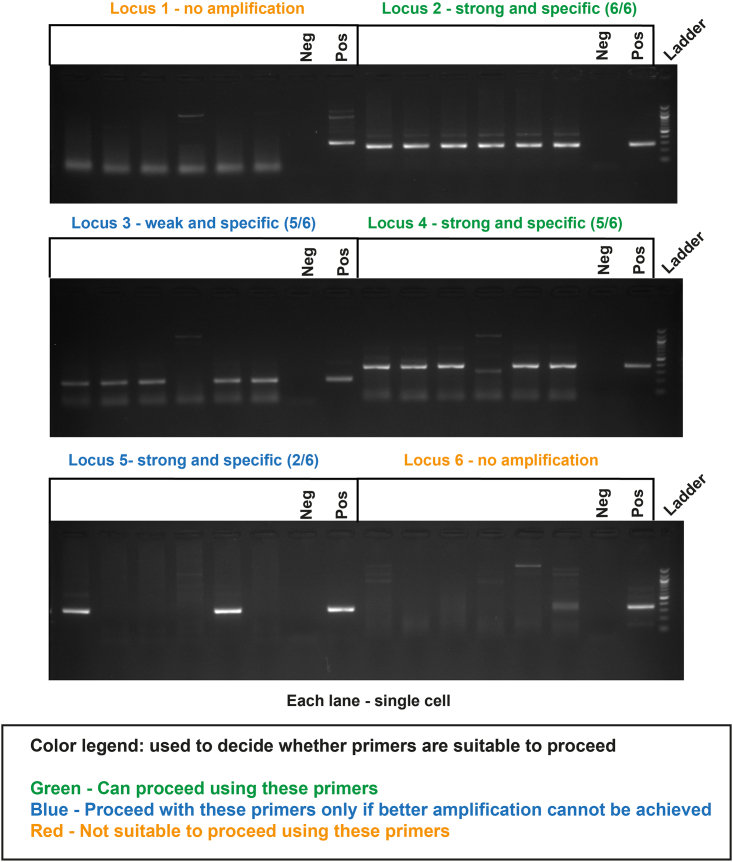
21.Run each reaction on a 2% agarose gel to check how many cells display successful amplification, band intensity for each locus, and amplification specificity. Use gDNA as positive control and a no template reaction as negative control. Representative gels in Figure 6.
-
a.If primers show acceptable single-cell amplification (ideally at least 5/6 strong and specific bands per locus) and if scATAC-seq library generation is successful, these primers can be used in the GTAC experiment on primary samples. Troubleshooting 11.
-
b.Follow preparation two and preparation three to find suitable primers for all loci.
-
a.
Optional: If more than 4 loci are amplified per cell for a single sample of interest, you need to decide which loci will be amplified in the same genotyping PCR1 reaction (part 5). At the genotyping PCR1 reaction stage, we advise genotyping up to 4 different loci in the same reaction. For example, if you are amplifying 6 loci per cell in a single sample of interest, you should split genotyping PCR1 into two reactions. The choice of which loci to amplify together in the same genotyping PCR1 reaction is based on the results of point 21. We advise combining loci that show strong amplification and keeping them separate from loci showing weaker amplification, to avoid amplification of one locus outcompeting the amplification of the other locus. Troubleshooting 11
Figure 5.
Schematic overview of primer testing in single cells
ATAC traces are tested from pools of 24 single cells. Efficiency of single-cell genotyping is tested individually in single cells. Blue numbers indicate the order in which the steps are executed.
Figure 6.
Single-cell genotyping testing results
Primers for each locus were tested in 6 single cells. Each lane represents amplification from a single cell. Depending on the quality and consistency of amplification, primers can be used in a GTAC experiment, or new primers need to be tested for the locus. Bulk gDNA was used as positive control. A no-template reaction was used as negative control.
Preparation four: Preparation of the primers necessary for GTAC
Timing: 1 h
Once all pre-amplification and nested genotyping primers are validated, you are ready for the execution of GTAC on samples of interest. At this stage, order and prepare all primers necessary for the protocol (sequences listed in the key resources table).
-
22.Order primers necessary for the preparation of the barcoded lysis buffer (part 1):
-
a.384 barcoded i7 primers (Table S2) are ordered in plate format, reconstituted at 100 μM in TE buffer (10 mM Tris-HCl pH 8.0, 0.1 mM EDTA).
-
i.Keep the 384 barcoded i7 primers at −80°C prior to use.
-
i.
-
b.Reconstitute barcoded i5 primers (Table S2) at 100 μM in TE buffer.
-
i.The number of different barcoded i5 primers to order is equivalent to the number of different 384-well plates to be pooled and sequenced on the same sequencing run or on the same lane (for details, refer to part 1).
-
ii.Keep the barcoded i5 primers at −20°C prior to use.
-
i.
-
a.
-
23.Order target-specific pre-amplification genotyping primers validated in preparation two and preparation three.
-
a.Reconstitute pre-amplification genotyping primers required for part 3 at 100 μM in TE buffer.
-
b.Keep reconstituted pre-amplification genotyping primers at −20°C prior to use.
-
a.
-
24.Order the Access Array Barcode Library for Illumina Sequencers-384, Single Direction (four 96-well plates containing 40 μL of 2 μM primer pair per well; 384 barcoded primer pairs in total), required for part 5. Hereafter, we refer to these as “barcoded genotyping PCR2 primers”.
-
a.Prepare two fresh 384-well plates.
-
b.Transfer 20 μL of 2 μM barcoded genotyping PCR2 primers into each of the two fresh 384-well plates, following these steps:
-
i.Aliquot each of the four original 96-well plates into a separate quadrant of the fresh 384-well plate (A1 into quadrant 1, A2 into quadrant 2, etc.).
-
ii.Hereafter, we refer to these 384-well plates as “Access Array 2 μM stock plates”.
-
iii.Keep the Access Array 2 μM stock plates at −20°C prior to use.
-
i.
-
a.
Note: Make sure to order sufficient Access Array Barcode Library for Illumina Sequencers-384, Single Direction kits, for the approximate number of plates you plan to sort, considering that each genotyping PCR2 plate (part 5, point 65) requires 1.2 μL of primer pair per well (you might need to prepare multiple Access Array 2 μM stock plates).
-
25.Order the CS1, LCS1, CS2, and CS2rc custom sequencing primers required for genotyping library sequencing (part 5).
-
a.Reconstitute the primers at 100 μM in TE buffer.
-
b.Prepare single-use aliquots for each primer:
-
i.Primers and volumes required depend on the Illumina sequencing platform you will use to sequence genotyping libraries. Refer to part 5, point 84 for primer volumes required per sequencing run on different Illumina sequencing platforms.
-
i.
-
c.Keep reconstituted single-use aliquots at −20°C prior to use.
-
a.
CRITICAL: Handle barcoded i7 and i5 primers, pre-amplification genotyping primers, Access Array 2 μM stock plates, and sequencing primers, in a designated pre-PCR clean area, ideally in a biosafety cabinet, to avoid contamination from PCR products. CS1, CS2, and CS2rc primers contain LNA modifications and should always be aliquoted and stored as single-use aliquots at −80°C.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-human CD45RA (1:40; clone HI100) | BD | Cat# 564552; RRID:AB_2738841 |
| Mouse anti-human CD90 (1:20; clone 5E10) | BioLegend | Cat# 328124; RRID:AB_2561693 |
| Mouse anti-human CD123 (1:40; clone 6H6) | BioLegend | Cat# 306006; RRID:AB_314580 |
| Mouse anti-human CD38 (1:20; clone HIT2) | BioLegend | Cat# 303526; RRID:AB_10983072 |
| Mouse anti-human CD10 (1:40; clone HI10a) | BioLegend | Cat# 312222; RRID:AB_2562157 |
| Mouse anti-human CD34 (1:160; clone 581) | BioLegend | Cat# 343510; RRID:AB_1877153 |
| Mouse anti-human CD117 (1:80; clone 104D2) | BioLegend | Cat# 313238; RRID:AB_2629837 |
| Mouse anti-human CD2 (1:160; clone RPA-2.10) | BioLegend | Cat# 300210; RRID:AB_314034 |
| Mouse anti-human CD3 (1:320; clone HIT3a) | BioLegend | Cat# 300310; RRID:AB_314046 |
| Mouse anti-human CD4 (1:160; clone RPA-T4) | BioLegend | Cat# 300510; RRID:AB_314078 |
| Mouse anti-human CD8a (1:320; clone RPA-T8) | BioLegend | Cat# 301010; RRID:AB_314128 |
| Mouse anti-human CD20 (1:160; clone 2H7) | BioLegend | Cat# 302308; RRID:AB_314256 |
| Mouse anti-human CD235ab (1:320; clone HIR2) | BioLegend | Cat# 306606; RRID:AB_314623 |
| Chemicals, peptides, and recombinant proteins | ||
| Fetal bovine serum, heat inactivated (or equivalent) | Sigma-Aldrich | Cat# F9665-100ML |
| IMDM, no phenol red | Gibco | Cat# 21056023 |
| DPBS, no calcium, no magnesium | Thermo Fisher Scientific | Cat# 14190169 |
| Bovine serum albumin solution 30% (wt/vol) | Sigma-Aldrich | Cat# A8577 |
| Digitonin | Promega | Cat# G9441 |
| IGEPAL CA-630 | Sigma-Aldrich | Cat# I8896 |
| Tween 20 10% (or equivalent polysorbate 20) | Sigma-Aldrich | Cat# 655205-250ML |
| UltraPure 1 M Tris-HCl, pH 8.0 | Thermo Fisher Scientific | Cat# 15568025 |
| Sodium chloride solution 5 M | Sigma-Aldrich | Cat# S6546 |
| MgCl2 (1 M) | Thermo Fisher Scientific | Cat# AM9530G |
| N,N-dimethylformamide (DMF) | Sigma-Aldrich | Cat# D4551-250ML |
| Magnesium acetate solution 1 M | Sigma-Aldrich | Cat# 63052-100ML |
| Potassium acetate solution 5 M | Sigma-Aldrich | Cat# 95843-100ML-F |
| Illumina Tagment DNA Enzyme and Buffer Small Kit | Illumina | Cat# 20034197 |
| EDTA (0.5 M), pH 8.0, RNase-free | Thermo Fisher Scientific | Cat# AM9260G |
| SDS solution, molecular biology grade (10% w/v) | Promega | Cat# V6551 |
| DAPI solution (1 mg/mL) | Thermo Fisher Scientific | Cat# 62248 |
| UltraPure agarose | Thermo Fisher Scientific | Cat# 16500500 |
| UltraPure ethidium bromide, 10 mg/mL | Thermo Fisher Scientific | Cat# 15585011 |
| Q5 high-fidelity 2× master mix | New England Biolabs | Cat# M0492L |
| Nuclease-free water | Thermo Fisher Scientific | Cat# AM9932 |
| Buffer PB, 500 mL binding buffer | QIAGEN | Cat# 19066 |
| Buffer PE (concentrate, 100 mL) | QIAGEN | Cat# 19065 |
| Sodium acetate (3 M), pH 5.5, RNase-free | Thermo Fisher Scientific | Cat# AM9740 |
| KAPA2G Robust HotStart ReadyMix, 6.25 mL | Sigma-Aldrich | Cat# KK5702 |
| FastStart high fidelity PCR system, dNTPack | Sigma-Aldrich | Cat# 4738292001 |
| TE buffer | Thermo Fisher Scientific | Cat# 12090015 |
| Buffer EB (250 mL) | QIAGEN | Cat# 19086 |
| Ethanol absolute | VWR | Cat# 20821.310 |
| Critical commercial assays | ||
| DNeasy Blood & Tissue Kit | QIAGEN | Cat# 69506 |
| Qubit dsDNA Quantification, High Sensitivity Assay Kit | Thermo Fisher Scientific | Cat# Q32854 |
| Agilent TapeStation HS D5000 ScreenTape | Agilent | Cat# 5067-5592 |
| Agilent TapeStation HS D5000 Reagents | Agilent | Cat# 5067-5593 |
| Agilent TapeStation HS D1000 ScreenTape | Agilent | Cat# 5067-5583 |
| Agilent TapeStation HS D1000 Reagents | Agilent | Cat# 5067-5584 |
| Agilent High Sensitivity DNA Kit | Agilent | Cat# 5067-4626 |
| Oligonucleotides | ||
| Target-specific genotyping primers (standard desalting) | IDT (designed by the user) | N/A |
| Target-specific nested barcoded genotyping PCR1 primers (standard desalting) | IDT (designed by the user) | N/A |
| See Table S2 for 384 indexed i7 primers (standard desalting) | IDT (design: Xu et al.4) | https://www.nature.com/articles/s41596-021-00583-5 |
| See Table S2 for barcoded i5 primers (HPLC purified) | IDT | N/A |
| Access Array barcode library for Illumina sequencers-384, single direction, 2 μM | Standard BioTools | Cat# 100-4876 |
| CS1 sequencing primer (HPLC purified): A+CA+CTG+A CGACATGGTTCTACA |
IDT | https://www.sciencedirect.com/science/article/pii/S266616672030112X |
| CS2 sequencing primer (HPLC purified): T+AC+GGT+A GCAGAGACTTGGTCT |
IDT | https://www.sciencedirect.com/science/article/pii/S266616672030112X |
| CS2rc sequencing primer (HPLC purified): A+GAC+CA+ AGTCTCTGCTACCGTA |
IDT | https://www.sciencedirect.com/science/article/pii/S266616672030112X |
| LCS1 sequencing primer (HPLC purified): GGCGACCA CCGAGATCTACACTGACGACATGGTTCTACA |
IDT | https://www.sciencedirect.com/science/article/pii/S266616672030112X |
| Generic i7 primer (standard desalting): CAAGCAGAAG ACGGCATACGAGATTCGCCTTAGTCTCGTGGGCTC GGAGATGT |
IDT | N/A |
| Generic i5 primer (standard desalting): AATGATACGG CGACCACCGAGATCTACACTCGTCGGCAGCGTCA GATGTG |
IDT | N/A |
| Software and algorithms | ||
| Primer-BLAST | Ye et al.5 | https://bmcbioinformatics.biomedcentral.com/articles/10.1186/1471-2105-13-134 |
| TARGET-seq genotyping pipeline | Rodriguez-Meira et al.6 | https://github.com/albarmeira/TARGET-seq |
| Bcl2fastq (version 2.20) | Illumina | https://emea.support.illumina.com/content/dam/illumina-support/documents/documentation/software_documentation/bcl2fastq/bcl2fastq2-v2-20-software-guide-15051736-03.pdf |
| Snakemake scATAC-seq pre-processing pipeline | Chen et al.2 | https://github.com/dbrg77/scATAC_snakemake |
| R (version 4.1.3) | R-Project | |
| ArchR (version 1.0.2) | Granja et al.7 | https://www.nature.com/articles/s41588-021-00790-6 |
| FlowJo (version 9 or 10) | FlowJo | https://www.bdbiosciences.com/en-gb/products/software/flowjo-v10-software |
| infSCITE | Jahn et al.8 | https://genomebiology.biomedcentral.com/articles/10.1186/s13059-016-0936-x |
| Custom scripts for downstream GTAC analysis | Turkalj, Jakobsen et al.1 | https://github.com/sventurkalj/GTAC/tree/v1.0.0 |
| Other | ||
| 12.5 mL GRIPTIP, sterile, filter | INTEGRA Biosciences | Cat# 6455 |
| High volume MANTIS chip | Formulatrix | Cat# MCHVSMR6 |
| Agencourt AMPure XP beads (or equivalent) | Beckman Coulter | Cat# A63881 |
| FrameStar PCR plate 384-well, skirted | Azenta Life Sciences | Cat# 4ti-0384/C |
| FrameStar 96-well semi-skirted PCR plate | Azenta Life Sciences | Cat# 4ti-0900/C |
| MicroAmp optical 96-well reaction plate | Thermo Fisher Scientific | Cat# N8010560 |
| Axygen 96-well clear V-bottom 500 μL polypropylene deep well plate | Corning | Cat# P-96-450V-C |
| Adhesive PCR plate seals (plastic) | Thermo Fisher Scientific | Cat# AB0558 |
| Self-adhesive plate seal, aluminum, thick 60 μm | Starlab (UK) Ltd | Cat# E2796-0792 |
| qPCR adhesive seal | Azenta Life Sciences | Cat# 4ti-0560 |
| 20 mL extender tube (or equivalent) | Angen Biotech | Cat# D50071 |
| QIAvac 24 Plus (or equivalent) | QIAGEN | Cat# 19413 |
| Falcon 5 mL round bottom polystyrene test tube, with cell strainer snap cap (or equivalent) | Corning | Cat# 352235 |
| QIAquick spin column | QIAGEN | Cat# 28104 |
| DNA LoBind tube 1.5 mL | Eppendorf | Cat# 022431021 |
| NucleoCounter NC-3000 (or equivalent) | ChemoMetec | Cat# 991-3001 |
| NC-slide A8 (or equivalent) | ChemoMetec | Cat# 942-0003 |
| Solution 13 (or equivalent) | ChemoMetec | Cat# 910-3013 |
| 25 mL disposable reagent reservoir, sterile (or equivalent) | Corning | Cat# RES-V-25-S |
| CoolRack XT PCR384 thermoconductive tube rack for 384-well PCR plates (or equivalent) | Azenta Life Sciences | Cat# BCS-538 |
| ThermoMixer C (or equivalent) | Thermo Fisher Scientific | Cat# 15158953 |
| Centrifuge 5430 R (or equivalent) | Eppendorf | Cat# 5428000655 |
| Centrifuge 5910 R (or equivalent) | Eppendorf | Cat# 5943000061 |
| MPS 1000 mini plate spinner (or equivalent) | Labnet | Cat# C1000 |
| ProFlex 96-well PCR system (or equivalent) | Thermo Fisher Scientific | Cat# 4484075 |
| ProFlex 384-well PCR system (or equivalent) | Thermo Fisher Scientific | Cat# 4484077 |
| QuantStudio 3 real-time PCR system, 96-well, 0.2 mL, laptop (or equivalent) | Thermo Fisher Scientific | Cat# A28567 |
| Invitrogen Qubit 3 fluorometer (or equivalent) | Thermo Fisher Scientific | Cat# 15387293 |
| Qubit assay tubes (or equivalent) | Thermo Fisher Scientific | Cat# Q32856 |
| 2100 bioanalyzer instrument | Agilent | Cat# G2939BA |
| 4200 TapeStation system (or equivalent) | Agilent | Cat# G2991BA |
| Magnetic stand-96 (or equivalent) | Thermo Fisher Scientific | Cat# AM10027 |
| Magnetic separation rack, 0.2 mL tubes (or equivalent) | EpiCypher | Cat# 10-0008 |
| GelDoc Go gel imaging system (or equivalent) | Bio-Rad | Cat# 12009077 |
| PowerPac basic power supply (or equivalent) | Bio-Rad | Cat# 1645050 |
| Fisherbrand Midi Plus horizontal gel system (or equivalent) | Thermo Fisher Scientific | Cat# 11833293 |
| MA900 multi-application cell sorter (or equivalent) | Sony | N/A |
| MANTIS automated liquid dispenser (or equivalent) | Formulatrix | N/A |
| Mosquito HTS nanolitre liquid handler (or equivalent) | SPT Labtech | N/A |
| VIAFLO 96/384 electronic pipette (or equivalent) | Integra Biosciences | N/A |
Materials and equipment
Note: FACS buffer and thawing media recipes listed below apply to thawing and staining procedures involving human cryopreserved bone marrow/peripheral blood mononuclear cells.
FACS buffer
| Reagent | Amount |
|---|---|
| Fetal Bovine Serum (FBS) | 50 mL |
| DNase I (10 mg/mL) | 500 μL |
| IMDM (no phenol red) | 450 mL |
| Total | 500.5 mL |
Store at 4°C for a maximum of two weeks.
Thawing media for cryopreserved human bone marrow/peripheral blood mononuclear cells
| Reagent | Amount |
|---|---|
| Fetal Bovine Serum (FBS) | 5 mL |
| DNase I (10 mg/mL) | 500 μL |
| FACS buffer | 45 mL |
| Total | 50.5 mL |
Store at 4°C for a maximum of two weeks.
Note: Pass the FACS buffer and thawing media through a sterile filter prior to storage.
Note: Prepare FACS buffer without DNase I (below) only if FACS staining and sample pre-sorting prior to Omni-ATAC are required. Label it appropriately to distinguish from normal FACS buffer.
-
•
FACS buffer without DNase I: mix 50 mL of FBS with 450 mL of IMDM (no phenol red). Cells will be pre-sorted into this buffer prior to Omni-ATAC.
Store at 4°C for a maximum of 1 month.
-
•
Prepare DPBS with 0.5% BSA by mixing 500 μL of BSA 10% (wt/vol) with 9.5 mL of DPBS 1×.
Store at -20°C for a maximum of 1 month.
Note: Regardless of the starting tissue type, prepare the buffers according to the recipes below, originally provided by Xu et al., 20214 and Xu et al., 2022.9
Nucleus dilution buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| BSA 10% (wt/vol) | 0.5% | 500 μL |
| DPBS 1× | 0.475× | 4.75 mL |
| Nuclease-free water | N/A | 4.75 mL |
| Total | N/A | 10 mL |
Store at -20°C for a maximum of 1 month.
Omni-RSB buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris-HCl 1 M pH 8 | 10 mM | 500 μL |
| NaCl 5 M | 10 mM | 100 μL |
| MgCl2 1 M | 3 mM | 150 μL |
| Nuclease-free water | N/A | 49.25 mL |
| Total | N/A | 50 mL |
Store at 4°C for a maximum of two months.
4× THS-TD buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris-HCl 1 M pH 8 | 132 mM | 132 μL |
| Potassium acetate 5 M | 264 mM | 52.8 μL |
| Magnesium acetate 1 M | 40 mM | 40 μL |
| N,N-Dimethylformamide (DMF) | 64% | 640 μL |
| Nuclease-free water | N/A | 135.2 μL |
| Total | N/A | 1 mL |
Store at −20°C for a maximum of one month.
CRITICAL: Handle DMF under a fume hood.
2× tagmentation stop buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris-HCl 1 M pH 8 | 10 mM | 100 μL |
| EDTA 0.5 M | 20 mM | 400 μL |
| BSA 10% (wt/vol) | 2% | 2 mL |
| Nuclease-free water | N/A | 7.5 mL |
| Total | N/A | 10 mL |
Store at −20°C for a maximum of two months.
Step-by-step method details
Part 1: Barcoded lysis buffer preparation
Timing: 3–5 h
In this section, we describe how to prepare 384-well plates containing the fully barcoded lysis buffer, which is done one day before the single nucleus sort (Figure 7). At the end of the section, each well will contain one of the 384 uniquely barcoded i7 primers (well indexes) and each plate will contain a different plate-specific barcoded i5 primer (plate index). i7 and i5 primers amplify and fully barcode tagmented fragments of open chromatin (scATAC-seq fragments).
CRITICAL: Decide in advance how many plates will be sequenced on the same sequencing run (or in the same lane), to estimate the required number of different i5 indexes. Refer to the note after part 4, point 60, for considerations about the required sequencing depth per cell for scATAC-seq libraries.
Optional: Here, we describe the procedure for generating five lysis buffer 384-well plates, but more plates can be prepared at the same time for convenience and stored at −80°C for at least 2 months.
Note: 384-well or 96-well plates can be used. Here, we describe the procedure for 384-well plates. If 96-well plates are used instead, we recommend doubling all the volumes.
Note:Part 1 should be performed in a designated pre-PCR clean area, ideally in a biosafety cabinet, to avoid contaminations from PCR products.
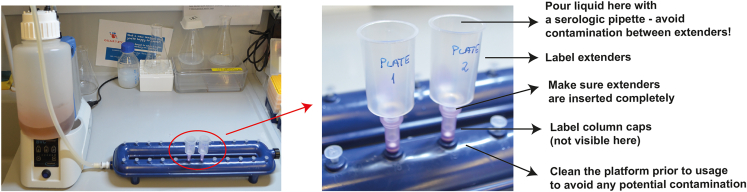
Note: In the following steps, we describe the usage of the MANTIS and of the INTEGRA VIAFLO 96/384 electronic pipettor in detail. These liquid handling platforms will also be used throughout the later stages, but for detailed instructions about their setup and usage please refer to these initial steps. We advise getting familiar with the working procedures of these platforms in advance (Figures 8 and 9 and Methods videos S1, S2, S3, and S4). Note that the use of these platforms is not essential, but drastically increases throughput. Finally, note that alternative equivalent liquid handling platforms can be used.
-
1.Prepare 50 mL of 1.1× lysis buffer as outlined in the table below:1.1× lysis buffer
Reagent Amount Tris-HCl 1 M pH 8 550 μL NaCl 5 M 110 μL SDS 10% (wt/vol) 1100 μL Nuclease-free water 48.25 mL -
a.Mix well using a serological pipette. Keep the buffer cold.
-
a.
-
2.Dispense 27 μL of 1.1× lysis buffer into each well of a fresh 384-well plate.Note: Here, we describe the procedure using the MANTIS liquid handling platform (alternative platforms can be used).
-
a.Switch on the MANTIS.
-
i.Clean the MANTIS plate holder and chip holder with 70% ethanol.
-
ii.Open the MANTIS software.
-
iii.Insert a High Volume (HV) chip into the chip holder (Methods video S1).
-
i.
-
b.Perform manual priming of the chip:
-
i.Insert a 1 mL non-filtered tip into the chip.
-
ii.Pipette 500 μL of nuclease-free water into the tip (Figure 8A).
-
iii.Make sure “HV” is selected next to the “Reagent Name” column (Figure 8B).
-
iv.Click on the chip position number: this indicates to the MANTIS arm to grab the chip at the indicated position (Figure 8B).
-
v.Click on the “Manual Priming Input on MANTIS Arms” icon (Figure 8B) and wait until all water passes through the chip. When there is no liquid coming out, stop the manual priming.
-
vi.Click the position number again to release the chip, which is now ready to be used for lysis buffer dispensing.
-
vii.Remove the tip from the chip (Methods video S1).
-
i.
-
c.Create a new Dispense List (Figure 8C) and name it appropriately. Make sure the program is calibrated for the appropriate plate type and height of the plate rack.
-
i.Insert “GTAC 1.1× lysis buffer” as name of your reagent.
-
ii.In the plate display, select all wells and input 27 μL in the volume window above.
-
iii.Drag the name of your reagent to the “Reagent Name” column (Figure 8C).
-
iv.Click HV to the left to specify the chip options. In the menu, set “Pre-dispense” to 15 μL; “Recovery” to 6 μL; “Enable pause for reagent refill”; “Tip volume” to 1300 μL; “Wash Station 1” and “Wash Station 2” both to 500 μL (Figure 8D).
-
i.
-
d.Place a 384-well cold rack onto the MANTIS plate holder and place a fresh 384-well plate into the rack. Make sure the plate sits well inside the rack.
-
i.Insert a new non-filtered 1 mL tip into the chip.
-
ii.Pipette ∼1500 μL into the tip. Ensure that there are no bubbles at the bottom of the tip.
-
iii.Click the green start icon (Figure 8C) to start dispensing (Methods video S1). Depending on the “Tip volume” you set, the dispense will pause after a given time and allow you to refill the tip with 1.1× lysis buffer.
-
i.
-
e.Once the dispense is done, remove the plate rack from the MANTIS, cover the plate with an aluminum or plastic adhesive seal, and centrifuge in a plate spinner for 10 s at 500 × g. You can leave the plate at 21°C.
-
f.Replace the 1 mL tip on the chip with an empty clean tip for washing.
-
i.Click the chip position number to grab the chip.
-
ii.Click the “Wash Input on MANTIS Arms” icon (Figure 8E) – this will flush the chip with 500 μL of 70% ethanol and 500 μL of water.
-
iii.When done, click the “Recover Input on MANTIS Arms” icon (Figure 8E) 1–2 times to aspirate any remaining volume from the chip.
-
iv.Click on the chip position again to release the chip.
-
v.Remove the tip.Note: We perform the water priming step (point 2b) each time we start using the MANTIS. Hereafter, we refer to this step as ‘manual priming with water’.Note: We recommend using one MANTIS HV chip exclusively for lysis buffer preparation steps.
 CRITICAL: Set a high enough “Pre-dispense” volume (at least 15 μL) to get air bubbles outside of the chip prior to dispensing. This avoids insufficient volume being dispensed inside the first wells. Hence, whenever using the MANTIS, prepare at least ∼15% of dead volume of the dispensed mix.
CRITICAL: Set a high enough “Pre-dispense” volume (at least 15 μL) to get air bubbles outside of the chip prior to dispensing. This avoids insufficient volume being dispensed inside the first wells. Hence, whenever using the MANTIS, prepare at least ∼15% of dead volume of the dispensed mix. CRITICAL: Make sure to have enough 70% ethanol and nuclease-free water in the washing tubes to properly flush the chip. Always use nuclease-free water.
CRITICAL: Make sure to have enough 70% ethanol and nuclease-free water in the washing tubes to properly flush the chip. Always use nuclease-free water.
-
i.
-
a.
-
3.Transfer 3 μL of the 100 μM barcoded i7 indexes to the 1.1× lysis buffer plate, to obtain a stock plate of 10 μM barcoded i7 indexes in 1× lysis buffer.Note: We perform this step using the INTEGRA VIAFLO electronic pipettor with a 384-well 12.5 μL pipetting head (Figure 9), but this step can be performed with any multichannel pipette system.
-
a.Switch on the VIAFLO. When prompted, press the RUN key to home the instrument, and when prompted press the RUN key again to home the pipettor.
-
b.Make sure the 384-well pipetting head is installed. If you need to switch heads:
-
i.Scroll to “Toolbox” on the display with the touch wheel, select it with the OK key, and select “Change Head” with the OK key.
-
ii.Follow the instructions on the display (Methods video S2).
-
i.
-
c.Select “Pipet” from the display. Set the pipetting volume to 3 μL, Aspirate Speed to 4, and Dispense Speed to 1.
-
d.Load the tips (Methods video S3):
-
i.Make sure the stage lever is set to the central position (Figure 9).
-
ii.Place a new 384 tip box in the plate holder position B.
-
iii.By holding the controller, lower the pipetting unit towards the tips until the tip loading button starts flashing.
-
iv.Push the tip loading button while keeping the pipetting unit down. When the tip loading button stops flashing, pull up the pipetting unit. Tips should now be fully loaded on the head.
-
i.
-
e.Place the 100 μM barcoded i7 index plate and the 1.1× lysis buffer plate in two adjacent plate holders (positions A and AB, respectively).
-
f.Insert the tips close to the bottom of the 100 μM barcoded i7 index plate and aspirate 3 μL by pressing the RUN key on the controller.
-
i.Move the pipetting unit up and insert the tips into the 1.1× lysis buffer plate.
-
ii.Press the RUN key again to release the volume and wait until the display signals “Done”. This will generate a 10 μM barcoded i7 index plate at a total volume of 30 μL per well (Methods video S4).
-
iii.Change the pipetting volume to 12 μL on the display while the tips are still inserted in the plate.
-
iv.Aspirate and dispense using the RUN key 3–5 times to properly mix the i7 indexes with the lysis buffer (Methods video S4).
-
i.
-
g.Once mixing is complete:
-
i.Remove the 100 μM barcoded i7 index plate from the holder, cover well with two aluminum adhesive seals, and place on dry ice for snap freezing.
-
ii.Remove the stock 10 μM i7 index plate, cover with an aluminum or plastic adhesive seal, and centrifuge in a plate spinner for 20 s at 500 × g.
-
iii.Label the plate as “i7 10 μM stock” and let it rest for 10 min at 21°C.
-
iv.Place the plate in the fridge or on a cold rack.
-
i.
-
h.Finally, remove the tips from the pipetting head: place the empty 384 tip box on a plate holder position B, insert the tips into the box (do not touch the bottom of the box), and press the tip ejection key twice (Methods video S3). Dispose of the tips.
 CRITICAL: Take absolute care to avoid any cross-well contamination of i7 indexes at this point, especially when handling the 100 μM barcoded i7 index plate.Note: When dispensing the 384 barcodes, visually inspect the volume in the tips to make sure all the barcodes were aspirated.Note: When using the VIAFLO to transfer or mix viscous solutions (as the 1.1× lysis buffer), keep the Dispense Speed low, to avoid retention of the volume inside the tips.
CRITICAL: Take absolute care to avoid any cross-well contamination of i7 indexes at this point, especially when handling the 100 μM barcoded i7 index plate.Note: When dispensing the 384 barcodes, visually inspect the volume in the tips to make sure all the barcodes were aspirated.Note: When using the VIAFLO to transfer or mix viscous solutions (as the 1.1× lysis buffer), keep the Dispense Speed low, to avoid retention of the volume inside the tips.
-
a.
-
4.Dilute the 100 μM i5 plate barcodes in 1.1× lysis buffer:
-
a.Mix 50 μL of 100 μM i5 plate barcode with 450 μL of 1.1× lysis buffer, to obtain a 10 μM i5 plate barcode/1× lysis buffer mix.
-
b.Mix well and keep cold.
-
c.Repeat for all i5 barcodes.
-
a.
CRITICAL: Avoid any cross-contamination of different i5 barcodes. Clean the pipettes between dispenses with 70% ethanol, to avoid traces of the previous i5 barcode contaminating the next one. Also, make sure to use filtered tips! If you notice cross-contamination, do not use these barcodes!
Note: The number of different 10 μM i5 mixes depends on the number of 384-well plates that will be multiplexed on the same sequencing run. We usually multiplex 5 plates on the same sequencing run; hence, we prepare 5 different 10 μM i5 plate barcode mixes.
Note: The volumes here are sufficient to prepare one 384-well plate per i5 barcode. If you are preparing more 384-well plates with the same i5 barcode, prepare more 10 μM i5 plate barcode/1× lysis buffer mix accordingly.
-
5.Transfer 1 μL of the 10 μM barcoded i7 indexes from the i7 10 μM stock plate into fresh 384-well plates. Prepare five fresh 384-well plates.Note: Here, we describe the procedure using the VIAFLO in Repeat Dispense pipetting mode.
-
a.Load a new set of 384 tips (Methods video S3).
-
b.Place the i7 10 μM stock plate and the first fresh 384-well plate on two plate holders of the VIAFLO. Keep the stage lever at the central position.
-
c.In the display, select “Repeat Dispense”. Set “Dispense” to 1 μL; “Count” to 6; “Aspiration Speed” to 1; “Dispense Speed” to 1.
-
d.Aspirate 6 μL from the i7 10 μM stock plate.
-
e.Transfer 1 μL into the fresh 384-well plate (insert the tips close to the well bottom).
-
f.Remove the aliquoted plate from the holder and cover with a plastic or aluminum adhesive seal.
-
g.Repeat points 5e and 5f for the remaining fresh 384-well plates.
-
h.Dispense the remaining volume into the i7 10 μM stock plate with the PURGE key.
-
i.Remove the tips (Methods video S3).
-
j.Centrifuge all the plates in a plate spinner for 10 s at 500 × g. Snap freeze the i7 10 μM stock plate on dry ice. Place the five aliquoted 384-well plates in the fridge. Switch off the VIAFLO.
 CRITICAL: When aliquoting small volumes into empty plates as described here, make sure that the tips are in close proximity of the well bottom. Otherwise, the liquid may be retained on the tips.
CRITICAL: When aliquoting small volumes into empty plates as described here, make sure that the tips are in close proximity of the well bottom. Otherwise, the liquid may be retained on the tips. CRITICAL: After all plates are dispensed, visually inspect the bottom of each plate to make sure that each well contains the i7 index. Sometimes, a few wells are not dispensed due to liquid retention on the tip. If this happens, manually transfer 1 μL from the i7 10 μM stock plate to the missing wells.
CRITICAL: After all plates are dispensed, visually inspect the bottom of each plate to make sure that each well contains the i7 index. Sometimes, a few wells are not dispensed due to liquid retention on the tip. If this happens, manually transfer 1 μL from the i7 10 μM stock plate to the missing wells.
-
a.
-
6.Finally, aliquot 1 μL of 10 μM i5 index (point 4) into the five plates containing 1 μL of 10 μM i7 indexes (point 5), to obtain 2 μL of fully barcoded lysis buffer per well. A different 10 μM i5 index will be aliquoted into each plate.Note: Here, we describe the procedure using the MANTIS.
-
a.Create a new Dispense List to aliquot 1 μL of into each well. Re-use the HV chip from point 2.
-
b.Place a new non-filtered 1 mL tip into the chip and transfer 500 μL of the first 10 μM i5 index into the tip.
-
c.Place the first 10 μM i7 index plate on the cold rack onto the MANTIS plate holder.
-
d.Aliquot 1 μL of 10 μM i5 index into each well. This will generate a 5 μM i7/i5 indexed 1× lysis buffer plate used for sorting (final volume of 2 μL per well).
-
e.When the dispense is done, remove the cold rack from the plate holder.
-
i.Label the plate with the name of the appropriate i5 index.
-
ii.Seal carefully with an aluminum adhesive seal.
-
iii.Centrifuge in a plate spinner for 20 s at 500 × g and freeze on dry ice.
-
i.
-
f.Replace the tip on the HV chip and wash the HV chip as in point 2f.
-
g.Carefully clean the HV chip on the outside:
-
i.Remove the HV chip from the holder.
-
ii.Wipe the chip on the bottom side with paper inserted in 70% ethanol.
-
iii.Clean the HV chip with dry paper. This removes traces of index contamination.
-
i.
-
h.Repeat points 6a–6g for all plates, using different 10 μM i5 indexes.
-
i.Wash the HV chip, store it in a 50 mL conical centrifuge Falcon tube, and switch off the MANTIS.
 CRITICAL: Always wash and clean the HV chip properly between different i5 indexes, to avoid contaminating plates with the wrong plate index.
CRITICAL: Always wash and clean the HV chip properly between different i5 indexes, to avoid contaminating plates with the wrong plate index. CRITICAL: When freezing plates, always use aluminum instead of plastic adhesive seals.Optional: If two people are working, one person can perform point 5 and the other person can simultaneously perform point 6. This setup will speed up the execution of these steps.
CRITICAL: When freezing plates, always use aluminum instead of plastic adhesive seals.Optional: If two people are working, one person can perform point 5 and the other person can simultaneously perform point 6. This setup will speed up the execution of these steps. Pause point: You can store the frozen 5 μM i5/i7 indexed 1× lysis buffer plates at −80°C until the sort or, if you prepare more plates than needed, for at least 6 months.
Pause point: You can store the frozen 5 μM i5/i7 indexed 1× lysis buffer plates at −80°C until the sort or, if you prepare more plates than needed, for at least 6 months.
-
i.
-
a.
Figure 7.
The automated preparation of barcoded lysis buffer
Liquid handling platforms used at individual steps, as well as volumes transferred, are indicated next to the arrows. Blue numbers indicate the order in which the steps are executed.
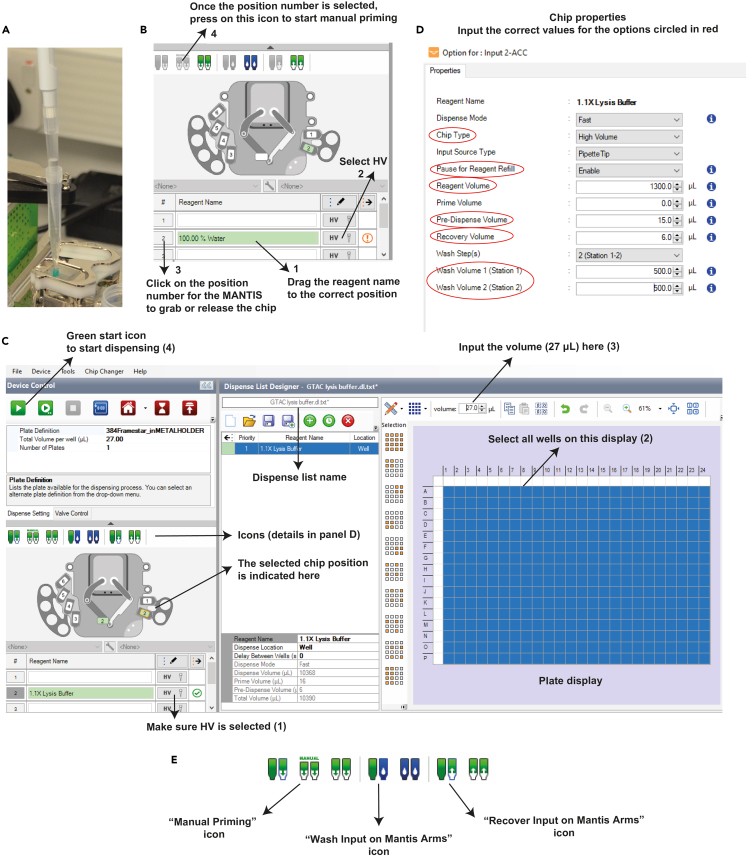
Figure 8.
MANTIS operating instructions
(A) Pipetting liquid into a non-filtered tip inserted into the HV chip.
(B) Steps for setting up manual priming with water. Numbers indicate the order in which steps are executed.
(C) Dispense list display. Numbers in brackets indicate the order in which steps are executed.
(D) Chip properties display. Red circles indicate the parameters that need to be defined by the user.
(E) Icons used for priming, washing, and volume recovery from the chip.
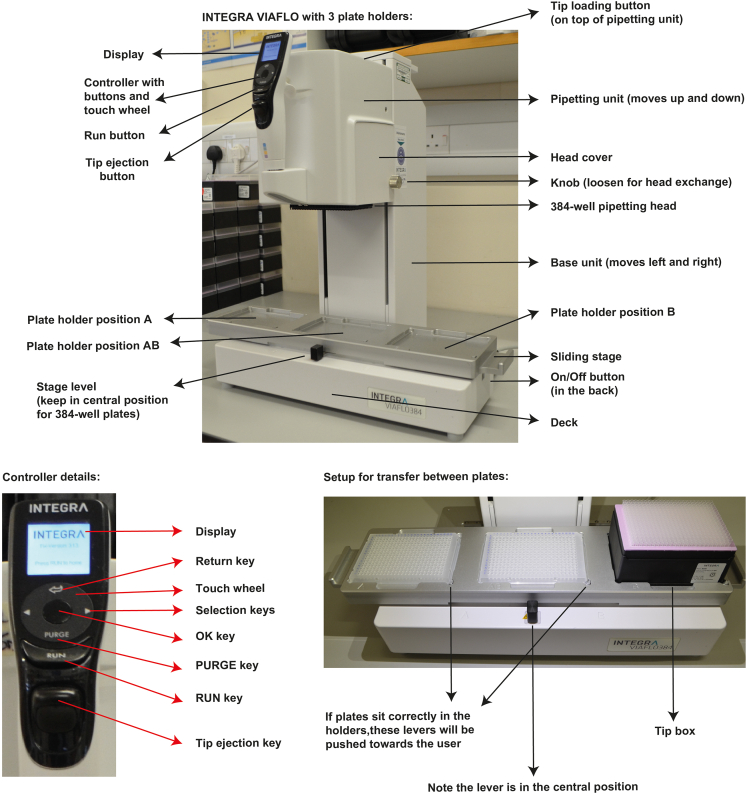
Figure 9.
INTEGRA VIAFLO components
Top: components of the VIAFLO. Bottom left: components of the VIAFLO controller. Bottom right: setup for volume transfer between plates. Note that this instrument has 3 plate holders, but an instrument with 2 plate holders can also be used.
Part 2: Sample preparation, pre-sorting, tagmentation, and single-nucleus sorting
Timing: 1 day
In this section, we describe the protocol for sample processing, pre-sorting of populations of interest, Omni-ATAC on cell populations, and single-nucleus sorting of tagmented nuclei into 5 μM i5/i7 indexed 1× lysis buffer plates prepared in part 1.
Note: In our applications, usually only a fraction of cells from the initial sample is of interest for GTAC analysis. Hence, we first pre-sort cells of interest into a tube, perform Omni-ATAC on these cells, and only then sort single tagmented nuclei. Points 8–17 describe in detail our custom thawing and FACS staining procedures for pre-sorting, optimized for cryopreserved human bone marrow (BM)/peripheral blood (PB) hematopoietic cells, adapted from Rodriguez-Meira et al., 2020.6 An example of 2× antibody mixes and 2× FMO control mixes that we use to stain human BM mononuclear cells can be found in Table S3.
-
7.If you are working with cryopreserved human BM or PB mononuclear cells and need to pre-sort cell population of interest prior to Omni-ATAC using FACS, proceed directly to point 8. For other scenarios and/or tissues, follow one of the points below, depending on sample type:
-
a.If you work with a different tissue type (fresh or cryopreserved), just a fraction of the sample is of interest, and you use FACS to pre-enrich for the desired cell fraction.
-
i.Follow in-house protocols for tissue dissociation (and/or thawing) and FACS staining (instead of following points 8–18).
-
ii.After staining, proceed with point 19 (pre-sorting).
-
i.
-
b.If just a fraction of the sample (fresh or cryopreserved) is of interest and you use a pre-enrichment strategy different from FACS sorting:
-
i.Follow in-house protocols for tissue dissociation (and/or thawing) and pre-enrichment (instead of following points 8–22).
-
ii.Proceed directly with point 23 (preparation for Omni-ATAC).
-
i.
-
c.If the whole sample (fresh or cryopreserved) is of interest (hence, any kind of pre-enrichment is not necessary prior to Omni-ATAC):
-
i.Follow in-house protocols for tissue dissociation (and/or thawing) followed by nuclei extraction (instead of following points 8–24f).
-
ii.Proceed directly with point 24 g (nuclei centrifugation and tagmentation).Note: Prior to point 24g, for optimal GTAC results, you need to have 20,000–100,000 nuclei per microcentrifuge tube. < 20,000 cells might result in fewer sorted plates, while > 100,000 cells may lead to suboptimal tagmentation. Supplementary Protocol 2 (points 1–14) from Corces et al., 201710 provides guidelines on nuclei isolation from solid cryopreserved tissues, prior to Omni-ATAC.
-
i.
-
d.If you are working with cultured cells and pre-enrichment is not necessary:
-
i.Transfer 20,000–100,000 cells into a microcentrifuge tube and start at point 23 (instead of following points 8–22). Cell viability should ideally be > 85% prior to Omni-ATAC procedures.Note: If cell viability is lower, refer to Supplementary Protocol 1 from Corces et al., 2017,10 for guidelines on how to avoid suboptimal results in case of low viability.Optional: Regardless of the sample preparation procedures, if cell numbers are high, you can split cells into multiple microcentrifuge tubes with 20,000–100,000 cells per tube prior to Omni-ATAC and run several tagmentation reactions in parallel.
 CRITICAL: To have appropriate single-cell genotyping controls and to control for batch effects in scATAC-seq, we sort cells from a WT sample into one column of each plate. Hence, in parallel with the sample of interest, we always process a cryopreserved control sample too. This control sample is also used for FMOs and sorting gate settings. Make sure not to cross-contaminate samples.
CRITICAL: To have appropriate single-cell genotyping controls and to control for batch effects in scATAC-seq, we sort cells from a WT sample into one column of each plate. Hence, in parallel with the sample of interest, we always process a cryopreserved control sample too. This control sample is also used for FMOs and sorting gate settings. Make sure not to cross-contaminate samples. CRITICAL: During all steps of sample preparation, pipette mix gently not to lyse fragile cells. Perform points 9–18 under a sterile laminar flow cabinet and use filtered tips.
CRITICAL: During all steps of sample preparation, pipette mix gently not to lyse fragile cells. Perform points 9–18 under a sterile laminar flow cabinet and use filtered tips.
-
i.
-
a.
-
8.Prepare for cryopreserved sample processing:
-
a.Prepare a box of wet ice and place FACS buffer on ice.
-
b.Bring cryopreserved samples from liquid nitrogen on dry ice.
-
c.Set the water bath to 37°C and warm the thawing media and FBS.
-
d.Set up the FACS sorter (we use the Sony MA900 Multi-Application Cell Sorter, but other machines can be used).
-
a.
-
9.Thaw the samples. Work with a maximum of two samples at a time:
-
a.Place samples in the water bath and wait until they are 70%–80% liquid. Proceed when there is still a small piece of frozen tissue inside the cryovial.
-
b.Dropwise, add 1 mL of warm FBS to the sample using a P1000.
-
c.Pipette mix very gently and transfer all the volume into a 15 mL conical centrifuge Falcon tube.
-
d.Dropwise, add 1 mL of warm thawing media to the tube. Hand-mix gently.
-
e.Wash the cryovial with 1 mL of thawing media and add dropwise to the tube.
-
f.Slowly, add 6 mL of thawing media to the tube to reach a total of 10 mL. After every 2 mL added, hand-mix gently.
-
g.Place the tube on ice.
-
a.
-
10.
When all samples are ready, centrifuge at 350 × g for 10 min. Remove supernatant, ideally by pouring it gently and then blotting the tube on dry paper, to remove traces of liquid.
-
11.Resuspend cells in 1 mL of ice-cold FACS buffer and mix gently.
-
a.Pass cells through a 35 μm filtered FACS tube.
-
b.Wash the 15 mL tube with 1 mL of FACS buffer. If starting cell number was > 20 million, add an additional 3 mL of FACS buffer, for a total of 5 mL.
-
a.
-
12.Count cells either with trypan blue and a hemocytometer or using an automatic cell counter.
-
a.Take note of total cell numbers and viability.
-
b.Based on the number of FMOs used, set aside an aliquot of control cells, and place them on ice.
-
a.
-
13.
Centrifuge other samples at 350 × g for 5 min. Remove supernatant as in point 10. ∼50 μL of supernatant will remain in the tube.
-
14.
Work without light. Add 2× antibody full-stain mixes to each sample. We add 50 μL of antibody mix if cell number is < 10 million or 100 μL of antibody mix for higher cell numbers.
Place samples at 4°C, in the dark, and incubate for 30–40 min.
Note: Some cells clump during this step and cannot be properly resuspended in the antibody mix. We tend to eliminate these clumps with the pipette tip, given that they may lead to suboptimal staining.
-
15.Take the control cell aliquot set aside in point 12b and mix gently.
-
a.Add cells to each 2× FMO mix and mix gently.
-
b.Place FMO tubes at 4°C, in the dark.
-
c.Incubate for 30–40 min.
-
a.
-
16.
Prepare single-staining controls used to set the correct voltages for each fluorochrome.
Note: To save precious cells, we use commercial beads for single-staining controls. Ideally, control cells should be used for single stains, to more closely match sample fluorescence characteristics.
-
17.When incubation is complete, wash samples and FMOs with 1 mL of ice-cold FACS buffer and centrifuge at 350 × g for 5 min at 8°C.
-
a.During centrifugation, mix 10 mL of ice-cold FACS buffer with 1 μL of DAPI to obtain FACS buffer with 1/104 DAPI.
-
a.
-
18.Remove supernatant as in point 10.
-
a.Resuspend samples in 700 μL-1 mL of FACS buffer with 1/104 DAPI.
-
b.Resuspend FMOs in 100 μL of FACS buffer with 1/104 DAPI, apart from the FMO for the DAPI channel, which should be resuspended in 100 μL of FACS buffer.
-
c.Keep cells cold, in the dark.
-
a.
Optional: You can use a nuclear dye other than DAPI. In that case, it is crucial to keep the same dye for successive single-nucleus sorting! Refer to Xu et al., 20214 for a benchmark of several dyes.
Note: For samples with high cell numbers or displaying visible clumping, repeat cell filtering.
-
19.Set up the pre-sorting FACS panel:
-
a.Use single stains to set up voltages.
-
b.Run each FMO control and perform “Manual Compensation” to optimize the compensation matrix.
-
c.Using a combination of FMO readouts and the full-stained control sample, set up the sorting gates.
-
a.
- 20.
-
21.
Put 200 μL of FACS buffer without DNase I in 1.5 mL microcentrifuge tubes to which cells will be sorted. Place tubes on ice. Set the collection and sample chambers to 4°C.
CRITICAL: To minimize cell loss, pre-coat tubes with 1 mL of FACS buffer without DNase I for 5 min.
CRITICAL: At this point, populations of interest will be sorted into tubes. Each tube will subsequently be subject to Omni-ATAC, which erases most surface protein information due to membrane lysis. If you wish to retain FACS population information for successive single-nucleus sorting, sort different populations into different tubes and label tubes according to the population sorted.
CRITICAL: Aim to sort 20,000–100,000 cells into each tube. Keep different samples in separate tubes.
-
22.Sort single, live cells of interest into microcentrifuge tubes with 200 μL of FACS buffer without DNase I:
-
a.Set the sorting mode according to cell numbers and desired purity.
-
b.After the sort is finished, place tubes on ice, in the dark.
-
a.
-
23.After sorting, proceed immediately with the following steps:
-
a.Pre-chill a fixed-angle centrifuge to 4°C and heat the thermomixer to 37°C.
-
b.Check the viability of the sorted samples. If the viability is < 85%, we advise to consider the indications by Corces et al., 2017.10
-
c.Prepare digitonin 0.1% by mixing 1 μL of digitonin 2% (wt/vol) with 19 μL of nuclease-free water and keep on ice.
-
d.Prepare Omni-RSB-T buffer by mixing 10 mL of Omni-RSB buffer with 100 μL of Tween 20 10% (vol/vol) and keep on ice.
-
e.Prepare the Omni-RSB-DTN buffer and the tagmentation mix (without the Tn5 enzyme) according to the tables below and keep on ice.
-
a.
Omni-RSB-DTN buffer
| Reagent | Volume |
|---|---|
| Omni-RSB buffer | 487.5 μL |
| Tween 20 10% (vol/vol) | 5 μL |
| IGEPAL CA-630 10% (vol/vol) | 5 μL |
| Digitonin 2% (wt/vol) | 2.5 μL |
Tagmentation mix (no Tn5 enzyme)
| Reagent | Volume for 1 sample |
|---|---|
| 4× THS TD buffer | 12.5 μL |
| Digitonin 0.1% (wt/vol) | 5 μL |
| Nuclease-free water | 27.5 μL |
Note: Avoid freezing and thawing digitonin 2% more than three times. Prepare aliquots of digitonin 2% in advance, prior to storage at −20°C.
CRITICAL: Work quickly and keep your samples on ice. Always keep track of the microcentrifuge tube orientation in the fixed-angle centrifuge. For very small cells or for low cell numbers, it may not always be possible to see the cell pellet. It is crucial not to disturb pellets when aspirating supernatants, which drastically reduces the nuclei yield prior to sorting. Work next to a light source, to carefully observe any movement of cell pellets.
-
24.Perform Omni-ATAC on the sorted samples following the steps below:
-
a.Centrifuge samples at 500 × g for 5 min at 4°C in the pre-chilled fixed-angle centrifuge.
-
b.Carefully aspirate the supernatant. Use two pipetting steps: collect most supernatant with a P1000 tip and then collect the remaining liquid with a P200 pipette, by fitting a non-filtered 20 μL tip on top of a 200 μL tip. Keep the bottom of the tip on the opposite side of the pellet.
-
c.Resuspend the pellet in 1 mL of cold DPBS with 0.5% BSA and centrifuge samples at 500 × g for 5 min at 4°C in the pre-chilled fixed-angle centrifuge.
-
d.Remove 900 μL of supernatant with a P1000 tip and the remaining 100 μL as in point 24b.
-
e.Resuspend cells in 50 μL of Omni-RSB-DTN buffer. Pipette gently up to 6 times and leave on ice for exactly 3 min. Troubleshooting 5 and 8.
-
f.Add 1 mL of Omni-RSB-T buffer to dilute the lysing detergents. Invert the tube 3 times gently with your hand.
-
g.Centrifuge nuclei at 1,000 × g for 5 min at 4°C in the pre-chilled fixed-angle centrifuge. During centrifugation, for each sample, add 5 μL of Tn5 enzyme to 45 μL of tagmentation mix, pipette mix 10 times, and keep on ice.
-
h.Remove 950 μL of supernatant with a P1000 tip and the remaining 100 μL as in point 24b. Take care not to disturb the pellet. Troubleshooting 7 and 10.
-
a.
Optional: If cell numbers are low, Omni-ATAC may result in severe cell loss and sorting multiple plates may not be feasible. In these cases, an alternative strategy is to perform FAST-ATAC instead of Omni-ATAC. For details about FAST-ATAC procedures prior to single nuclei sorting, refer to the detailed guide by Xu et al., 20214 (specifically, point 6B). However, note that this may result in lower scATAC-seq library complexity and higher percentage of mitochondrial reads.
CRITICAL: Considerable nuclei loss can occur at this step. The nuclei pellet is usually harder to see than a cellular pellet and, sometimes, a spread of nuclei can be observed instead of a round pellet. If pellets start falling toward the bottom of the tube, leave a few μL on the bottom. However, do not leave excessive liquid in the tube – this causes Tn5 dilution and retention of mitochondrial DNA.
Note: When working with many samples, pellets drop slowly toward the bottom of the tube. This is usually not a point of concern if you can still see them, but make sure not to aspirate pellets.
-
25.Resuspend nuclei in 50 μL of Tn5-tagmentation mix and pipette mix gently ∼20 times. Place tubes on the pre-heated thermomixer and incubate at 37°C for 30 min with 800 rpm shaking.
-
a.During incubation, thaw the 5 μM i5/i7 indexed 1× lysis buffer plates (part 1) at 21°C.
-
b.Prepare a box of dry ice next to the sorter.
-
a.
-
26.
After 30 min, add 50 μL of 2× tagmentation stop buffer to the nuclei and mix gently up to 10 times. Keep on ice for ≥ 10 min. Nuclei can stay in this buffer for ∼3–4 h. Troubleshooting 4.
-
27.Proceed immediately with sorter calibration for 384-well plate sorting:
-
a.Cover an empty 384-well plate (same model as the ones you will be sorting nuclei into) with a plastic PCR adhesive seal and insert into the collection platform.
-
b.Select 384-well plate sorting:
-
i.In “Sort Settings”, select “Plate Adjustment”.
-
ii.Sort 30 droplets of sheath fluid on positions A1, A24, P1, P24, and I13 (“Four Corners and Centre Well”).
-
iii.Adjust the droplet position and repeat the process iteratively to center all the droplets in the middle of the wells (Figure 10C).
-
i.
-
c.Unseal the plate and repeat point 27b by collecting the droplets inside the wells. If needed, adjust the positions iteratively until the droplets are collected to the bottom of each well (Figure 10D). Make sure there is no splashing on the sides of the wells.
-
d.Finally, select the “One Fourth of All Wells” option to sort 30 droplets of sheath fluid into every fourth well. Check the positions of most droplets are optimal (Figure 10D). Troubleshooting 1, 3, and 9.
-
a.
CRITICAL: Improper calibration will result in empty wells.
Optional: For safety, calibration can be re-checked using the control sample instead of the sheath fluid. We recommend doing that when already satisfied with sheath droplet positions.
-
28.Add 500–900 μL of nucleus dilution buffer to all samples, mix gently 10–20 times, and transfer all the volume into FACS tubes.
-
a.Run each sample for a few seconds at lowest pressure to observe the event rate for each sample.
-
b.Dilute samples enough to obtain an event rate of 5–10 events/s. Add more nucleus dilution buffer to samples accordingly.
-
a.
-
29.Run the control sample for a few seconds and record events, to define gates for nuclei, singlets, and DAPI-negative events (Figure 11).
-
a.Add 1 μL of DAPI to 99 μL of nucleus dilution buffer (DAPI 1/100).
-
b.Add DAPI 1/100 to the control sample for a final DAPI concentration of 1/104. Mix gently and wait for 5–10 min.
-
c.Run the control sample to define the DAPI-positive gate (Figure 11). Troubleshooting 9.
-
d.Stain all samples for a final 1/104 DAPI concentration.
-
a.
Note: We recommend stringency when setting the DAPI-positive gate, to capture only fully lysed nuclei. Two consecutive singlet gates should also be stringent enough to remove most clumps.
CRITICAL: After adding DAPI, mix gently and wait for ∼10 min for full nuclear staining to take place.
-
30.Centrifuge all the 5 μM i5/i7 indexed 1× lysis buffer plates in a plate spinner for 20 s at 500 × g. Carefully unseal the first plate and proceed to single-nucleus sorting:
-
a.Keep the event rate < 10 events/s, if possible.
-
b.Sort DAPI-positive events into all the columns. Sort WT control cells into ∼10% of the wells (we usually use column 24). Keep two wells as empty controls.
-
a.
Note: We use a sorting mode in which 50% of the droplet before and after the target droplet must be empty, and in which the target event needs to be within the 75% central part of the droplet.
Optional: Using a more stringent sorting mode will result in fewer doublets but fewer sorted plates.
CRITICAL: Avoid splashes when unsealing lysis buffer plates to prevent cross-well contamination!
-
31.
Once finished, cover the plate carefully with an aluminum adhesive seal, centrifuge in a plate spinner for 20 s at 500 × g, and snap freeze on dry ice. Repeat points 30–31 for all plates.
Pause point: Sorted plates can be stored at −80°C for up to 3 months. Tagmented DNA is still not gap-filled at this stage, however. It is hence ideal to start with the next steps as soon as possible.
Figure 10.
Sony MA900 cell sorter calibration
(A–D) Panels A and B provide the visual guidelines for calibrating the sorter for sorting into microcentrifuge tubes. Panels C and D provide the visual guidelines for calibrating the sorter for sorting into 384-well plates.
Figure 11.
FACS gating strategy for single-nucleus sorting
Part 3: Plate processing, PCR, and genotyping stock preparation
Timing: 2–3 h
In this section, we describe how to process the sorted 384-well plates. After Tn5 release and Tween 20 quenching, each plate undergoes a PCR reaction in order to amplify tagmented fragments of open chromatin. Crucially, target-specific pre-amplification genotyping primers validated in preparation two and preparation three are added to the PCR reaction to pre-enrich for genomic loci of interest. After PCR, a portion of the material will be transferred to a new genotyping stock plate which will be used for single-cell genotyping library construction (part 5). The remaining material will be pooled in order to purify the fully barcoded scATAC-seq libraries (part 4).
Note: Points 32–35 should be performed in a designated pre-PCR clean area, ideally in a biosafety cabinet, prior to PCR.
-
32.
Thaw plates at 21°C. Incubate plates at 65°C for 15 min in a thermal cycler, with the lid heated to 105°C. This step releases the Tn5 from tagmented DNA.
-
33.Aliquot 1 μL of Tween 20 10% (vol/vol) into each well, to quench SDS:Note: We perform this step using the MANTIS liquid handler platform (for details on MANTIS usage refer to part 1) and using an HV chip designated for GTAC pre-PCR steps.
-
a.Manually prime the MANTIS with 500 μL of nuclease-free water.
-
b.Unseal the plate (avoiding splashes) and aliquot 1 μL of Tween 20 10% (vol/vol) into each well.
-
c.When a plate is aliquoted, seal it with an aluminum or plastic adhesive seal, centrifuge in a plate spinner for 10 s at 500 × g, and leave at 21°C for 10 min.
-
d.Repeat points 33a–33c for all plates.
-
e.After all plates are aliquoted, wash the HV chip.
-
a.
-
34.
Prepare the PCR mix as detailed in the table below. Mix well and keep on a cold rack.
PCR mix containing pre-amplification genotyping primers
| Reagent | 1 reaction | 800 reactions (2 plates) |
|---|---|---|
| Q5 High-Fidelity 2× Master Mix | 4 μL | 3200 μL |
| Nuclease-free water | 0.5 μL | 400 μL |
| Forward primer 1 6.4 μM | 0.125 μL | 100 μL |
| Reverse primer 1 6.4 μM | 0.125 μL | 100 μL |
| Forward primer 2 6.4 μM | 0.125 μL | 100 μL |
| Reverse primer 2 6.4 μM | 0.125 μL | 100 μL |
Note: This mix contains target-specific primers (100 nM final concentration per primer) required for initial amplification of genotyping loci, simultaneously with amplification of tagmented DNA. Note that here, we show an example with 2 genotyping loci (4 primers). For more loci, add further primer pairs as needed, replacing the equivalent volume of water. For > 4 loci (> 8 primers), use a higher initial primer concentration, to keep the volume of mix at 5 μL per reaction.
CRITICAL: Make sure all genotyping primers were appropriately tested prior to this step.
Optional: Alternatively, you can use the NEBNext High-Fidelity 2× PCR Master Mix (optimized for NGS libraries; cat #M0541L), but make sure that primers were tested with the enzyme you are using at this stage.
Optional: We use a final concentration of 100 nM per genotyping primer. However, in case of suboptimal genotyping, this concentration may be increased up to 400 nM, with prior testing (preparation three).
-
35.Using the MANTIS, dispense 5 μL of PCR mix into each well of each 384-well plate.
-
a.When a plate is aliquoted, seal well with a plastic PCR adhesive seal, centrifuge in a plate spinner for 10 s at 500 × g, and place on wet ice.
-
b.When all plates are aliquoted, wash the HV chip, place it in a 50 mL conical centrifuge Falcon tube, and switch off the MANTIS. Troubleshooting 2.
-
a.
Note: If aliquoting the same PCR mix into multiple plates, there is no need to wash the HV chip between plates. However, if different mixes are aliquoted, wash the HV chip between plates.
CRITICAL: Use the plate sealer to tightly seal the plate with a plastic PCR adhesive seal, to avoid evaporation. For all the following points, work outside the pre-PCR clean area.
-
36.
Outside of the pre-PCR clean area, incubate the plates using the following PCR program: troubleshooting 1, 3, 4, and 11.
PCR cycling conditions
| Steps | Temperature | Time | Cycles |
|---|---|---|---|
| Gap-Filling | 72°C | 10 min | 1 |
| Initial Denaturation | 98°C | 1 min | 1 |
| Denaturation | 98°C | 10 s | Cycles defined in preparation one (we use 16–18) |
| Annealing | 60°C | 30 s | |
| Extension | 72°C | 30 s | |
| Final extension | 72°C | 5 min | 1 |
| Hold | 4°C | Hold | |
CRITICAL: The PCR will fail if the first 72°C gap-filling step is not performed.
-
37.While the PCR is running, prepare the number of fresh 384-well plates corresponding to the number of plates undergoing PCR.
-
a.Aliquot 4 μL of nuclease-free water into every well of each plate using the MANTIS.
-
b.Label each plate “genotyping stock”. Seal the plates.
-
a.
-
38.Transfer 2 μL of amplified material from the PCR plate into the genotyping stock plate.Note: Here, we describe the procedure using the VIAFLO.
-
a.When PCR is finished, centrifuge all the plates in a plate spinner for 20 s at 500 × g.
-
b.Carefully unseal the first plate and place in a plate holder of the VIAFLO.
-
c.Place one genotyping stock plate in another plate holder, with the stage lever set to the central position.
-
d.Transfer 2 μL and mix a few times.
-
e.Seal the genotyping stock plate, label appropriately, centrifuge in a plate spinner for 10 s at 500 × g, and snap freeze on dry ice.
-
f.Repeat points 38a–38e for all plates, changing tips each time.
-
g.Store genotyping stock plates at −80°C.
 CRITICAL: scATAC-seq libraries are fully barcoded at this stage but genotyping amplicons are not yet barcoded. Avoid any cross-well contamination while preparing genotyping stock plates.
CRITICAL: scATAC-seq libraries are fully barcoded at this stage but genotyping amplicons are not yet barcoded. Avoid any cross-well contamination while preparing genotyping stock plates. Pause point: Genotyping stocks, used in part 5, can be stored at −80°C for 6 months.
Pause point: Genotyping stocks, used in part 5, can be stored at −80°C for 6 months.
-
a.
Part 4: Single-cell ATAC-seq library pooling, purification, and preparation for sequencing
Timing: 3–4 h
In this section, we describe how to pool and purify scATAC-seq libraries from the same plate, perform quality control (QC) using capillary electrophoresis, and how to pool libraries from different plates at equimolar ratios to prepare the final scATAC-seq library for sequencing. At this point, scATAC-seq libraries are fully barcoded, due to the unique combination of i7 well index and i5 plate index. Prior to QC, each plate is pooled into a different tube.
CRITICAL: Incubate an aliquot of AMPure XP beads at 21°C for at least 30 min. These beads will be used in point 46.
-
39.Pool one plate at a time following these steps:
-
a.Centrifuge plates containing amplified scATAC-seq libraries in a plate spinner for 10 s at 500 × g.
-
b.Pool all the material (∼6 μL) from each well using a multichannel pipette:
-
i.Pool the material first into a strip of 8 PCR tubes.
-
ii.When all the wells of a single plate are pooled in the strip, transfer all the volume from the strip into a 15 mL conical centrifuge Falcon tube.
-
i.
-
c.Repeat points 39a–39b for each plate, pooling each plate into a separate tube. You should have ∼ 2 mL of material in each tube.
-
a.
Optional: For a quicker pooling strategy, refer to step 23 of the protocol by Xu et al., 2021.4
-
40.
Add 10 mL of PB buffer (5 × sample volume) to each tube. Mix 5–10 times with the serological pipette. Do not vortex.
Optional: Add 10–30 μL of sodium acetate pH 5.5 to each pool to increase DNA adsorption.
-
41.
Install fresh QIAquick columns on a vacuum and install a 20 mL extender tube on top of each column (Figure 12). Label extender tubes and columns with the appropriate plate name. Load each pool through a column and wait until the entire volume has passed through the column.
-
42.
Wash each column with 5–10 mL of PE wash buffer and wait until the entire volume has passed through the column.
CRITICAL: Remember to add ethanol to a new bottle of PE buffer and label the bottle in advance.
-
43.
Remove extender tubes from the columns. Remove columns from the vacuum and put each column into a collection tube. Centrifuge at 18,000 × g for 1 min to remove traces of PE buffer.
-
44.Discard collection tubes and place columns in fresh DNA LoBind 1.5 mL microcentrifuge tubes (or equivalent), labeled on the sides.
-
a.Pipette 21 μL of EB buffer directly on the membrane of each column, without touching the membrane.
-
b.Incubate at 21°C for 1 min.
-
c.Centrifuge at 18,000 × g for 1 min.
-
a.
-
45.
Repeat the elution with 21 μL of EB buffer (point 44) to maximize DNA yield. At the end of this step, each microcentrifuge tube will contain 42 μL of material.
-
46.Vortex AMPure XP beads thoroughly.
-
a.Add 21 μL of beads (0.5× beads:sample ratio) to each pool and mix 20–30 times. Avoid bubbles.
-
b.Incubate at 21°C for at least 5 min – this will allow large unwanted DNA fragments to bind the beads.
-
c.During incubation, prepare fresh 80% (vol/vol) ethanol by mixing 24 mL of ethanol 100% with 6 mL of nuclease-free water.
-
a.
Note: Depending on magnet type, bead purification can be performed in microcentrifuge tubes, PCR tubes, or on a V-bottom 96-well plate. The choice will usually depend on the number of pools to purify. For > 8 pools, we use the V-bottom 96-well plate and a plate magnet.
-
47.
Place tubes on a magnetic stand. Beads will accumulate towards the magnet. Wait for the liquid to become completely clear of beads (minimum 3 min). At this point, unwanted large fragments of DNA are bound to the beads, while fragments of interest are in the supernatant.
-
48.When the supernatant is completely devoid of beads:
-
a.Transfer the whole 63 μL of supernatant into new tubes, labeled appropriately.
-
b.Add 29.4 μL of AMPure XP beads to each pool and mix 20–30 times. Avoid bubbles.
-
c.Incubate at 21°C for at least 5 min.
-
a.
-
49.
Place the tubes on a magnetic stand and wait for the liquid to become completely clear of beads (minimum 3 min). This time, the fragments of interest are bound to the beads. Points 50–52 must be performed with the tubes placed on the magnetic stand all the time.
-
50.
Carefully remove and discard all supernatant (∼93 μL), taking care not to aspirate any beads.
-
51.Proceed immediately with 80% ethanol washing:
-
a.Dropwise, add 200 μL of 80% ethanol to each pool, not disturbing the beads.
-
b.Incubate for 30 s.
-
c.Remove and discard all 80% ethanol, not disturbing the beads.
-
d.Repeat points 51a–51c 1–2 more times.
-
e.After the final wash, carefully remove any trace of ethanol using 20 μL tips.
-
a.
-
52.
Let the beads air-dry for a few minutes (usually 3–5 min, depending on the volume of the beads). The beads are dry enough when the surface of the pellet changes from shiny to matt. While waiting, pre-aliquot nuclease-free water or EB buffer to PCR strips.
CRITICAL: If the bead pellet starts cracking, proceed to point 53 immediately. If beads over-dry, DNA yield will be reduced, and beads will be challenging to resuspend. However, it is important to dry them sufficiently, given that traces of ethanol could interfere with subsequent QC steps.
-
53.
Remove tubes from the magnet. Resuspend beads in 22 μL of nuclease-free water or EB buffer. Mix thoroughly 20–30 times to resuspend the beads and incubate at 21°C for 3 min.
-
54.
Place the tube back on the magnet and wait for 2–3 min for the supernatant to be completely clear of beads. The DNA fragments of interest are now in the supernatant.
-
55.
Transfer 22 μL of clear supernatant to fresh DNA LoBind 1.5 mL microcentrifuge tubes (or equivalent), labeled appropriately. Take care not to transfer any beads. Troubleshooting 6.
-
56.
Measure the concentration of each pool using the Qubit dsDNA HS Assay Kit. Use 1 μL of each library. Take note of the pool concentration [ng/μL].
-
57.For each pool, check scATAC-seq library size distribution using a microcapillary assay such as Agilent Bioanalyzer. If pool concentration [ng/μL] is > 20 ng/μL, dilute it prior to running the Bioanalyzer. Check average library size by choosing the region between 100 and 7,000 bp. Representative good quality libraries can be found in Figure 2. A “wavy” shape is expected, with variable peak heights.
-
a.If you observe excessive traces of primers or primer dimers, perform an additional AMPure XP bead purification using 1.2× bead:sample ratio.
-
b.If a large high-bp peak (details in Figure 18) is present, repeat the AMPure XP double-side exclusion (points 46–55).
-
a.
Note: If running many pools, we recommend using either the Agilent 4200 TapeStation System with a High Sensitivity D5000 Kit in 96-well plate format, or the Agilent Fragment Analyzer HS NGS Fragment Analysis Kit (1–6000 bp).
-
58.
Once you obtain the concentration [ng/μL] and the average size for each pool, combine equimolar ratios of each pool that will be sequenced on the same run into a fresh DNA LoBind 1.5 mL microcentrifuge tube (or equivalent). Use Table S4 as pooling template.
-
59.Quantify the final pool using the Qubit dsDNA HS Assay Kit and estimate its average size with Agilent Bioanalyzer or TapeStation.
-
a.Input the results into appropriate columns in Table S4 to estimate library molarity.
-
b.Dilute the library to 4 nM for sequencing on Illumina platforms.
-
a.
CRITICAL: Be precise when diluting the final library for sequencing. Excessive library will result in flow cell over-clustering, which might cause the sequencing run to fail. If the library is too diluted, under-clustering will result in low data amount, and excessive under-clustering may lead to a failed run.
-
60.
Sequence the libraries on an Illumina platform.
Note: The choice of platform and kit depends on the desired sequencing depth. We usually aim for 200,000 reads/cell, and thus sequence 5 pooled 384-well plates on an Illumina NextSeq platform, using a High Output kit. If more plates are sequenced together, a HiSeq or a NovaSeq platform might be more suitable. We usually perform 75 bp paired-end sequencing (150 cycles), but it is also possible to sequence using fewer cycles. Classic Illumina primers from the kits are used for sequencing.
Note: Different samples and tissues display variable library complexity. While 200,000 reads/cell might be suitable for some tissue types, this may be either excessive or insufficient for others. PCR duplication values are usually a good metric of whether you sequenced the libraries to saturation.
Figure 12.
Setup for scATAC-seq library purification via QIAquick columns with extender tubes
Figure 18.
Representative suboptimal ATAC traces
(A)–(I) Bioanalyzer traces displaying failed or suboptimal ATAC library amplification, which requires troubleshooting.
Part 5: Single-cell genotyping library preparation
Timing: 2 days for 5–20 genotyping plates; 3 days for 20–40 genotyping plates
In this section, we describe the procedures for generating Illumina-compatible libraries for single-cell genotyping, starting from the genotyping stock plates prepared in part 3, point 38. For each cell, non-barcoded genotyping amplicons were generated in part 3, point 36 by target-specific genotyping primers. Now, genotyping amplicons need to be further enriched and fully barcoded, to be able to assign them to single cells. This is achieved via two additional PCR steps (Figure 13).
Figure 13.
Single-cell genotyping procedures
Liquid handling platforms used at individual steps, as well as volumes transferred, are indicated next to the arrows. A schematic overview of the amplification and barcoding strategy at each step is shown below.
The first step (genotyping PCR1) uses primers that are nested within the original amplicon(s) and which contain universal CS1 (forward primer) and CS2 (reverse primer) adapters. Plate-specific 6 bp barcode sequences are incorporated into these primers, between the CS adaptors and the target-specific sequence, enabling libraries from different plates to be pooled together later. The second step (genotyping PCR2) uses primers that bind to the CS1/CS2 adapters (from the Access Array Barcode Library for Illumina Sequencers-384, Single Direction kit) and that attach Illumina-compatible sequencing adapters to genotyping amplicons. The primer that binds to the CS2 adapter also contains a 10 bp index, serving as cell barcode, which is well-specific. The combination of plate and cell barcode is unique for each cell across all plates, allowing all amplicons to be sequenced together. After genotyping PCR2, libraries are pooled, purified, and quantified for sequencing.
Note: The steps described in part 5 are performed using the MANTIS, INTEGRA VIAFLO, and the Mosquito HTS Nanolitre Liquid Handler (Figure 13). However, users might automate the protocol on other liquid handling platforms, such as the Biomek FxP Liquid Handling Platform (Beckman Coulter).6
-
61.Order barcoded genotyping PCR1 primers, validated in preparation two and preparation three (nested genotyping primers), for each genotyping locus of interest. Examples on how to design barcoded genotyping PCR1 primers6 are provided in Table S5.
-
a.Reconstitute barcoded genotyping PCR1 primers at 100 μM in TE buffer, in a designated pre-PCR clean area, ideally in a biosafety cabinet.
-
b.Keep reconstituted primers at −20°C prior to use or proceed directly with point 62.
-
a.
Note: The plate barcode is defined by the unique combination of the forward and the reverse 6 bp barcodes on the genotyping PCR1 primers. The number of different barcoded combinations of genotyping PCR1 primer pairs to order for each locus depends on the number of genotyping stock plates that you have. For example, if you sorted the sample into 10 plates, you need 10 different barcoded genotyping PCR1 primer pairs for each genotyping locus. For details, refer to Table S5.
CRITICAL: When ordering barcoded genotyping PCR1 primers, name each primer according to the locus and barcode, to avoid confusion. We also advise labelling the tube caps.
-
62.Prepare 770 μL of each genotyping PCR1 primer mix: add 8.58 μL of each 100 μM genotyping PCR1 primer to the respective mix and top up with nuclease-free water to 770 μL. A template for genotyping PCR1 primer mix preparation can be found in Table S6.Note: At this point, the final concentration of each primer in the primer mix should be 1.11 μM.Note: The total number of primer mixes depends on (a) the number of genotyping stock plates, and (b) the number of genotyped loci per cell. Each genotyping stock plate requires a different primer mix, due to different plate barcodes. Furthermore, in preparation three, point 21, you defined how many different genotyping reactions are required per single cell, which depends on the number of targeted loci. Usually, up to 4 different genotyping amplicons per cell can be combined in the same reaction. To provide examples:
-
a.If you have 5 genotyping plates and amplify 1 locus/cell, prepare 5 primer mixes;
-
b.if you have 5 genotyping plates and amplify 4 loci/cell, prepare 5 primer mixes;
-
c.if you have 5 genotyping plates and amplify 6 loci/cell, prepare 10 primer mixes;
-
d.if you have 10 genotyping plates and amplify 8 loci/cell, prepare 20 primer mixes.
Note: All primers used to amplify targets from a single initial genotyping stock plate need to have the same plate barcode, even if they are not all in the same genotyping PCR1 reaction (given that they barcode the same “original” plate). To summarize:-
a.no of different plate barcodes = no of genotyping stock plates;
-
b.no of primer mixes = no of genotyping stock plates × no of reactions per plate.
-
a.
Note: We recommend preparing primer mixes in advance; this step can take several hours. We prepare primer mixes in a designated pre-PCR clean area to avoid contamination.
CRITICAL: If barcoded primers are added to the wrong mix, genotyping amplicon reads will be assigned to the wrong plate.
-
63.Prepare for genotyping PCR1:
-
a.Clean all working surfaces with 70% ethanol.
-
b.Thaw enough KAPA2G Robust HS Ready Mix and keep on ice.
-
c.Vortex genotyping PCR1 primer mixes and keep on ice.
-
d.Thaw genotyping stock plates prepared in part 3, point 38 and centrifuge them at 3,000 × g for 3 min at 21°C.
-
e.Prepare sufficient fresh 384-well plates for the number of genotyping PCR1 reactions.
-
f.Switch on the MANTIS liquid handling platform and the VIAFLO.
-
i.Prepare a HV MANTIS chip used exclusively for PCR1.
-
ii.Manually prime the chip with 500 μL of nuclease-free water (Methods video S1).
-
i.
-
a.
Optional: Keep sorting plates, genotyping stock plates, genotyping PCR1, and genotyping PCR2 plates, all in different colors, to avoid confusion.
CRITICAL: Always centrifuge plates at 21°C and not at 4°C to avoid condensation on the plate cover, which may lead to cross-well contamination.
-
64.Set up the genotyping PCR1. Set up two genotyping PCR1 plates at a time as follows:Note: Here, we describe the procedure using the MANTIS and the VIAFLO liquid handling platforms.
-
a.Prepare the enzyme-primer mix for one plate: mix 1300 μL of KAPA2G Robust HS Ready Mix with 700 μL of PCR1 primer mix (Table S6) and pipette mix 10 times.
-
b.Aliquot 5 μL of enzyme-primer mix into each well of a fresh genotyping PCR1 plate, using the MANTIS.
-
c.Place the aliquoted genotyping PCR1 plate and the genotyping stock plate on two plate holders of the VIAFLO, with the stage lever set to the central position (Figure 9).
-
i.Transfer 1.5 μL of material from the genotyping stock plate into the genotyping PCR1 plate.
-
ii.Mix 3 times using the same tips.
-
iii.Discard the tips (Methods video S4).
-
i.
-
d.Seal the genotyping stock plate with an aluminum adhesive seal and the genotyping PCR1 plate with a plastic PCR adhesive seal. Centrifuge both plates in a plate spinner for 10 s at 500 × g. Snap freeze the genotyping stock plate on dry ice. Label the genotyping PCR1 plate appropriately and place it on wet ice.
-
e.Change the non-filtered tip on the MANTIS HV chip.
-
i.Wash with 600 μL of ethanol 70% and 600 μL of nuclease-free water.
-
ii.Recover 6 μL 2–3 times.
-
iii.Discard the tip.
-
iv.Take the chip out of the holder and use tissue inserted in 70% ethanol to wipe both the bottom part of the chip and the place where the chip sits, to eliminate any trace of barcode.
-
v.Dry the chip and the chip holder.
-
vi.Return the chip to the holder and insert a fresh non-filtered tip into the chip. You are now ready to aliquot the next plate.
-
i.
-
f.Repeat points 64a–64e to prepare the second PCR1 plate. Use the same genotyping stock plate if you are preparing the second genotyping reaction for the same original sorting plate or switch genotyping stock plate if you are preparing the genotyping reaction for the next original sorting plate.
-
g.When two genotyping PCR1 plates are ready, place them in a thermal cycler with the lid heated at 105°C and run the following PCR1 program:PCR cycling conditions
Steps Temperature Time Cycles Initial Denaturation 95°C 3 min 1 Denaturation 95°C 15 s 40 - no cycles in first PCR (22–24 cycles in our case) Annealing 60°C 20 s Extension 72°C 1 min Final extension 72°C 5 min 1 Hold 4°C Hold -
h.Repeat points 64a–64g to prepare all genotyping PCR1 plates.
 CRITICAL: Wash the MANTIS chip carefully between every genotyping PCR1 plate, to avoid contaminating the next genotyping PCR1 plate with plate barcodes from the previous plate.Optional: If two people are working, we suggest that one person operates the MANTIS while the other operates the VIAFLO. This drastically speeds up the protocol. We also recommend processing all genotyping PCR1 plates before proceeding to generation of genotyping PCR2 plates.
CRITICAL: Wash the MANTIS chip carefully between every genotyping PCR1 plate, to avoid contaminating the next genotyping PCR1 plate with plate barcodes from the previous plate.Optional: If two people are working, we suggest that one person operates the MANTIS while the other operates the VIAFLO. This drastically speeds up the protocol. We also recommend processing all genotyping PCR1 plates before proceeding to generation of genotyping PCR2 plates. Pause point: When genotyping PCR1 is done, you can proceed directly with genotyping PCR2, or you can snap freeze genotyping PCR1 plates and store them at −80°C for at least 3 months.
Pause point: When genotyping PCR1 is done, you can proceed directly with genotyping PCR2, or you can snap freeze genotyping PCR1 plates and store them at −80°C for at least 3 months.
-
a.
-
65.Prepare plates containing 1.2 μL of 2 μM barcoded PCR2 primers: these will be genotyping PCR2 plates.Note: Here, we describe the procedure using the VIAFLO.
-
a.Prepare fresh 384-well plates (the number equivalent to that of genotyping PCR1 plates).
-
b.Thaw the Access Array 2 μM stock plate (preparation four) and centrifuge it at 3,000 × g for 3 min at 21°C.
-
c.Aliquot 1.2 μL of material from the Access Array 2 μM stock plate into each fresh 384-well plate, using the Repeat Dispense pipetting mode. As soon as four plates are aliquoted, cover them with adhesive aluminum seals, centrifuge in a plate spinner for 10 s at 500 × g, and place on wet ice.
 CRITICAL: Aliquoted plates need to be covered and kept on ice, to avoid evaporation.
CRITICAL: Aliquoted plates need to be covered and kept on ice, to avoid evaporation. Pause point: You can either proceed with genotyping PCR2, or you can snap freeze plates prepared in point 65 on dry ice and store them at −80°C for at least 2 weeks.
Pause point: You can either proceed with genotyping PCR2, or you can snap freeze plates prepared in point 65 on dry ice and store them at −80°C for at least 2 weeks.
-
a.
-
66.
Prepare enough genotyping PCR2 mix for the number of genotyping PCR2 plates you have, according to the table below. Keep the mix on ice.
Genotyping PCR2 mix
| Reagent | 1 reaction | 1 plate (400 reactions) | Kit |
|---|---|---|---|
| FastStart Buffer 10× (labeled 3) | 0.6 μL | 240 μL | FastStart High Fidelity PCR System |
| MgCl2 (labeled 4) | 1.08 μL | 432 μL | FastStart High Fidelity PCR System |
| DMSO (labeled 5) | 0.3 μL | 120 μL | FastStart High Fidelity PCR System |
| Nucleotide mix (labeled 6) | 0.12 μL | 48 μL | FastStart High Fidelity PCR System |
| Enzyme (labeled 1) | 0.06 μL | 24 μL | FastStart High Fidelity PCR System |
| Nuclease-free water | 1.84 μL | 736 μL | N/A |
Note: Thaw DMSO at 21°C – it tends to form aggregates if kept on ice.
-
67.Prepare for genotyping PCR2:
-
a.Clean all working surfaces with 70% ethanol.
-
b.Centrifuge genotyping PCR1 plates (point 64) and plates containing 1.2 μL of 2 μM barcoded PCR2 primers (point 65) at 3,000 × g for 2 min at 21°C.
-
c.Switch on the MANTIS and the VIAFLO.
-
d.Prepare a HV MANTIS chip used exclusively for PCR2. Manually prime the chip with 500 μL of nuclease-free water.
-
a.
-
68.Set up two genotyping PCR2 plates at a time.Note: Here, we describe the procedure using the MANTIS and the VIAFLO.
-
a.Place the plate containing 1.2 μL of 2 μM barcoded PCR2 primers on a cold rack and on the MANTIS plate holder, and aliquot 4 μL of genotyping PCR2 mix into this plate. Hereafter, we refer to the aliquoted plate as to a genotyping PCR2 plate.
-
b.Cover the plate with an aluminum or plastic adhesive seal and centrifuge in a plate spinner for 10 s at 500 × g.
-
c.Place one genotyping PCR1 plate and the aliquoted genotyping PCR2 plate on two plate holders of the VIAFLO with the stage lever set to the central position (Figure 9).
-
i.Transfer 1 μL of material from the genotyping PCR1 plate to the genotyping PCR2 plate.
-
ii.Mix 3 times using the same tips.
-
iii.Discard the tips.
-
i.
-
d.Seal the genotyping PCR1 plate with an aluminum adhesive seal and the genotyping PCR2 plate with a plastic PCR adhesive seal. Centrifuge both plates in a plate spinner for 10 s at 500 × g, snap freeze the genotyping PCR1 plate, and place the genotyping PCR2 plate on wet ice.
-
e.Repeat points 68a–68d to prepare a second genotyping PCR2 plate, using a second genotyping PCR1 plate. There is no need to wash the MANTIS chip in between plates.
-
f.When two genotyping PCR2 plates are done, place them in a thermal cycler with the lid heated at 105°C and run the following PCR2 program:PCR cycling conditions
Steps Temperature Time Cycles Initial Denaturation 95°C 10 min 1 Denaturation 95°C 15 s 10 cycles Annealing 60°C 30 s Extension 72°C 1 min Final extension 72°C 5 min 1 Hold 4°C Hold -
g.Repeat points 68a–68f to prepare all genotyping PCR2 plates.Optional: If two people are working, we suggest that one person operates the MANTIS while the other operates the VIAFLO. This setup will drastically speed up the execution of the protocol. We also recommend preparing all genotyping PCR2 plates before proceeding to the next points.Note: Wipe the chip with paper inserted in 70% ethanol between every 4 genotyping PCR2 plates, to clean potential splashes of barcoded PCR2 primers.
 Pause point: When genotyping PCR2 is done, you can proceed, or you can snap freeze genotyping PCR2 plates on dry ice and store them at −80°C for at least 3 months. We usually process ∼40 genotyping PCR2 plates per day.Note: Each well of each genotyping PCR2 plate is now fully barcoded with a combination of well and plate barcode. However, we pool and purify each genotyping PCR2 plate separately, for QC purposes.
Pause point: When genotyping PCR2 is done, you can proceed, or you can snap freeze genotyping PCR2 plates on dry ice and store them at −80°C for at least 3 months. We usually process ∼40 genotyping PCR2 plates per day.Note: Each well of each genotyping PCR2 plate is now fully barcoded with a combination of well and plate barcode. However, we pool and purify each genotyping PCR2 plate separately, for QC purposes.
-
a.
-
69.Pool the wells from genotyping PCR2 plates (1 μL from each well).Note: Here, we describe the procedure using the Mosquito HTS Nanolitre Liquid Handler.
-
a.Centrifuge all genotyping PCR2 plates in a plate spinner for 10 s at 500 × g and keep them on ice.
-
b.Prepare a fresh 384-well plate, used for genotyping PCR2 plate pooling, and label it as “PCR2 pooling plate”. The goal is to pool genotyping PCR2 plates into alternate columns of the PCR2 pooling plate, in which each column will contain material from one full genotyping PCR2 plate (Figure 14A).
-
c.Switch on the Mosquito and set up a program as in Figure 14B: this will pool an entire PCR2 plate into column 1 of the PCR2 pooling plate, taking 1 μL from each well of genotyping PCR2 plate.
-
d.Insert one genotyping PCR2 plate and the PCR2 pooling plate into magnetic racks for the Mosquito (Figure 14C).
-
e.Place the genotyping PCR2 plate at position 1 and the PCR2 pooling plate at position 2 of the Mosquito (Figure 14C).
-
f.Run the program.
-
g.When the program is done, cover the genotyping PCR2 plate with an aluminum adhesive seal and snap freeze.
-
h.Repeat points 69d–69g for the second genotyping PCR2 plate, this time pooling into column 3 of the PCR2 pooling plate (Figure 14A). The third genotyping PCR2 plate will be pooled into column 5, etc.Note: Given that you pool into every second column of the PCR2 pooling plate, you can pool up to 12 genotyping PCR2 plates into one pooling plate.Note: Switch Mosquito tips between each genotyping PCR2 plate to maintain pipetting accuracy.Optional: Genotyping PCR2 plate pooling can also be performed with a multichannel pipette or with an alternative liquid handling platform.
-
a.
-
70.
When the PCR2 pooling plate is ready, pool all the wells of a single column into a fresh DNA LoBind 1.5 mL microcentrifuge tube (or equivalent), labeled according to the genotyping PCR2 plate, using a P200. The total number of tubes equals the number of genotyping PCR2 plates.
Optional: If you have many columns to pool, we suggest using a 12 × 200 μL multichannel pipette and transferring the material into a V-bottom 96-well plate. Keep track of which pool is in which well.
-
71.Prepare for bead purification of the pools using V-bottom 96-well plates:
-
a.Incubate AMPure XP beads for 30 min at 21°C. After 30 min, vortex thoroughly and pour into a clean reservoir, labeled “beads”.
-
b.Prepare 80% (vol/vol) ethanol by mixing 40 mL of ethanol 100% and 10 mL of nuclease-free water. Pour 80% ethanol into a clean reservoir, labeled “EtOH”.
-
c.Pour nuclease-free water into a second clean reservoir, labeled “H2O”.
-
d.Transfer 100 μL of each pool to a well of a fresh V-bottom 96-well plate.
-
a.
Optional: You can also bead-purify in microcentrifuge tubes or in PCR strips, but for higher numbers of libraries, using an 8 × 200 μL multichannel pipette and the V-shaped 96-well plates is more practical.
-
72.Add 120 μL of beads (1.2× beads:sample ratio) to each pool and mix 20–30 times. Avoid bubbles.
-
a.Incubate at 21°C for at least 5 min – this will allow DNA fragments of interest to bind to the beads.
-
a.
-
73.
Place the plate on a magnetic stand for plates. Beads will accumulate towards the magnet. Wait for the liquid to become completely clear of beads (minimum 3 min).
-
74.
When the supernatant is completely clear, carefully remove and discard all supernatant (∼220 μL), taking care not to aspirate the beads.
-
75.Proceed immediately with 80% ethanol washing:
-
a.Dropwise, add 200 μL of 80% ethanol to each pool, not disturbing the beads.
-
b.Incubate for 30 s.
-
c.Remove and discard all 80% ethanol, not disturbing the beads.
-
d.Repeat points 75a–75c 1–2 more times.
-
e.After the final wash, carefully remove traces of ethanol using 20 μL tips.
-
a.
-
76.
Let the beads air-dry for a few minutes (usually 4–5 min at this step). The beads are dry enough when the surface of the pellet changes from shiny to matt.
-
77.
Remove the plate from the magnet. Resuspend beads in 50 μL of nuclease-free water. Mix thoroughly 20–30 times to completely resuspend the beads and incubate at 21°C for 3 min.
-
78.
Place the plate back on the magnet and wait for 2–3 min for the supernatant to be completely devoid of beads. Libraries are now in the supernatant.
-
79.
Transfer 48–50 μL of clear supernatant to fresh DNA LoBind 1.5 mL microcentrifuge tubes (or equivalent), labeled accordingly. Take care not to transfer any beads.
-
80.
Measure the concentration of each pool using the Qubit dsDNA HS Assay Kit. Use 1 μL of each library. Take note of the pool concentration [ng/μL].
-
81.
For each pool, check genotyping library size distribution using a microcapillary assay such as Agilent Bioanalyzer or TapeStation with a D1000 Kit. Check that each pool contains peaks at the expected size and record the average library size by choosing the region between 50 and 900 bp. Representative good quality libraries can be found in Figure 15.
Note: Barcodes and adapter sequences add a total of 115 extra bp to the genotyping amplicon.
Pause point: You can freeze the pools and keep them at −20°C for 6 months.
-
82.
Combine equimolar ratios of each pool to a fresh DNA LoBind 1.5 mL microcentrifuge tube (or equivalent), for pools that will be sequenced on the same sequencing run. When pooling, consider the average library size, the concentration [ng/μL], and the number of amplicons per pool. Use Table S7 as template.
Optional: At this stage, you may perform a double-size AMPure XP bead selection on the final pool, by using a 0.6×, followed by a 1.2× beads:sample ratio. This step can help remove or reduce the high-bp peak which is likely a trace of the scATAC-seq library. However, do not perform this step if you expect a genotyping amplicon to be of that size!
-
83.Measure the concentration [ng/μL] of the final pool with Qubit and the average library size with Agilent TapeStation D1000.
-
a.Dilute the library to 4 nM for sequencing (Table S7).
-
a.
-
84.Sequence the library on either a MiSeq platform, using custom sequencing CS1 (read 1), CS2 (read 2), and CS2rc (index read) primers, or on a NextSeq platform, using custom sequencing LCS1 (read 1), CS2 (read 2), and CS2rc (index read) primers (Figure 16). Single-use primer aliquots were prepared in preparation four.Note: The choice of kit depends on the desired sequencing depth: we usually aim for 2,000 reads per cell per amplicon.
-
a.For the MiSeq:
-
i.Pierce ports 12, 13 and 14 containing Illumina sequencing primers with separate 1 mL tips. Aspirate the entire volumes into 3 tubes, labeled I12, I13, and I14.
-
ii.Mix 10 μL of 100 μM CS1 with 10 μL of 100 μM CS2 to obtain 20 μL of CS1/CS2 50 μM (we refer to CS1/CS2 50 μM as to “FL1”).
-
iii.Mix 7 μL of FL1 with 200 μL of I12 and with 493 μL of HT1 buffer. Vortex and centrifuge briefly. Transfer the entire volume into port 18.
-
iv.Mix 3.5 μL of CS2rc 100 μM with 200 μL of I13 and with 496.5 μL of HT1 buffer. Vortex and centrifuge briefly. Transfer the entire volume into port 19.
-
v.Mix 7 μL of FL1 with 200 μL of I14 and with 493 μL of HT1 buffer. Vortex and centrifuge briefly. Transfer the entire volume into port 20.
-
vi.Denature and dilute the 4 nM library from point 83a following the Illumina guidelines. Spike in 5%–10% of PhiX.
-
i.
-
b.For the NextSeq:
-
i.Pierce ports 20, 21 and 22 containing Illumina sequencing primers with separate 1 mL tips. Aspirate the entire volumes into 3 tubes, labeled I20, I21, and I22.
-
ii.Mix 6 μL of LCS1 100 μM primer with 800 μL of I20 and with 1194 μL of HT1. Vortex and centrifuge briefly. Transfer the entire volume into port 7.
-
iii.Mix 6 μL of CS2 100 μM primer with 800 μL of I21 and with 1194 μL of HT1. Vortex and centrifuge briefly. Transfer the entire volume into port 8.
-
iv.Mix 6 μL of CS2rc 100 μM primer with 1994 μL of HT1. Vortex and centrifuge briefly. Transfer the entire volume into port 9.Note: I22 is not required for sequencing PhiX and does not need to be added to the custom CS2rc index primer.
-
v.Denature and dilute the 4 nM library from point 83a following the Illumina guidelines. Spike in 5%–10% of PhiX.Note: For primer sequences, refer to the key resources table. Use the following sequencing configuration: 76 cycles (read 1) + 10 cycles (index read) + 76 cycles (read 2).
 CRITICAL: Make sure to perform enough cycles to read the mutant locus at least with read 1 or read 2.
CRITICAL: Make sure to perform enough cycles to read the mutant locus at least with read 1 or read 2. CRITICAL: Make use of a PhiX spike-in (5%–10%), given the low genotyping library complexity! If PhiX is not used, the sequencing run is likely to fail.Note: CS1/LCS1 and CS2 primers will bind to CS1 and CS2 adaptors inserted during PCR1, allowing the amplicon and plate barcodes to be sequenced. The CS2rc primer is the reverse complement of the CS2 adaptor allowing the 10 bp cell index inserted during PCR2 to be sequenced.Optional: Given that nested genotyping amplicons are usually short (100–200 bp for most amplicons), we perform 75 bp paired-end sequencing, to avoid read 1 or read 2 sequencing the CS adapters. However, in case of longer amplicons, you can use a different configuration – the key is that either read 1 or read 2 successfully cover the mutant locus of interest.
CRITICAL: Make use of a PhiX spike-in (5%–10%), given the low genotyping library complexity! If PhiX is not used, the sequencing run is likely to fail.Note: CS1/LCS1 and CS2 primers will bind to CS1 and CS2 adaptors inserted during PCR1, allowing the amplicon and plate barcodes to be sequenced. The CS2rc primer is the reverse complement of the CS2 adaptor allowing the 10 bp cell index inserted during PCR2 to be sequenced.Optional: Given that nested genotyping amplicons are usually short (100–200 bp for most amplicons), we perform 75 bp paired-end sequencing, to avoid read 1 or read 2 sequencing the CS adapters. However, in case of longer amplicons, you can use a different configuration – the key is that either read 1 or read 2 successfully cover the mutant locus of interest.
-
i.
-
a.
Figure 14.
Mosquito setup for genotyping PCR2 plate pooling
(A) Schematic overview of the procedure for pooling genotyping PCR2 plates.
(B) Mosquito program for genotyping PCR2 plate pooling.
(C) Setup of plates on the Mosquito.
Figure 15.
Quality control of genotyping libraries
(A) TapeStation bands showing genotyping amplicons for each plate (382 pooled cells). Amplicons of expected size should be observed.
(B) A TapeStation trace showing intensity of signal as function of size in bp.
Figure 16.
Sequencing strategy for genotyping libraries
CS2rc is the reverse complement of the CS2 sequence. Note that, if sequencing on the NextSeq, a longer version of the CS1 primer (LCS1) is used instead of the CS1 primer.
Expected outcomes
Single-cell ATAC-seq library generation: after part 4, point 57, you should expect to generate scATAC-seq library traces comparable to those in Figure 2. A sign of a good quality library is the presence of multiple peaks displaying the characteristic nucleosomal pattern in a wavy shape. Peak heights may be variable and depend on sample and tissue type. After bead purification, when libraries are eluted in 22 μL, we usually obtain ∼10–30 ng/μL of DNA. Note that these numbers apply to human bone marrow samples processed with 16 cycles of PCR. We observed higher yields when processing cell lines. In our experience, library shape is more informative of successful library generation than the concentration. If sequencing on the NextSeq, you should expect at least 85% of bases > Q30.
After running the pre-processing pipeline, you should expect ∼90% or more wells passing QC filters. A successful human bone marrow cell sequenced at the depth of ∼200,000 reads usually displays: > 95% mapping rate; 40,000–150,000 unique nuclear fragments/cell; 0.5%–10% of reads mapping to the mitochondrial genome; 50%–80% of mapped reads in called peaks; TSS enrichment > 8 as computed by ArchR.7 A duplication level of > 70% indicates that libraries are sequenced close to saturation.
Single-cell genotyping library generation: after part 5, point 80, each genotyping library should contain 5–100 ng/μL of DNA. Note that the yield is likely to depend on the number of loci amplified in each reaction. After part 5, point 81, for each genotyping library, you should observe bands of the expected size, as shown in Figure 15. We frequently observed an additional low-intensity band between ∼400 bp and 500 bp – this is usually not problematic. In the case of multiple amplicons in the same mix, it is common to observe that some bands are more intense than others, due to differences in amplification efficiency for different loci. This is usually not a point of concern, and lower intensity bands still allow for efficient genotyping. If sequencing on the NextSeq, you should expect at least 85% of bases > Q30. In a good quality run, 95%–100% of cells that pass scATAC-seq QC should also be successfully genotyped for the given amplicon.
Quantification and statistical analysis
-
1.Pre-processing of scATAC-seq data:
-
a.For scATAC-seq library demultiplexing, prepare a sample sheet containing i7 and i5 barcode information for each single cell, according to Illumina guidelines. Troubleshooting 12.
-
b.Demultiplex raw sequencing data with bcl2fastq, which generates two FASTQ files per cell (R1 and R2).
-
c.These files are used as input for the previously published pre-processing pipeline2 (https://github.com/dbrg77/scATAC_snakemake).
-
i.We use a slightly modified version of this pipeline, in which we do not remove duplicate reads from the merged BAM file (we still remove them from BAM files corresponding to single cells).
-
ii.Note that you may use other pre-processing strategies, which may involve a different read mapping tool, for example.
-
i.
-
d.The pipeline generates a fragment file, containing coordinates and cell barcodes of all nuclear fragments.
-
e.This file is used as input for downstream analysis pipelines, like ArchR.7 Alternatively, you may use Signac11 or other packages for downstream analysis.
-
i.You can find our scripts for downstream analysis using ArchR and Signac at https://github.com/sventurkalj/GTAC/tree/v1.0.0.
-
ii.At this step, filtering of low-quality cells is necessary prior to downstream analysis. Filtering thresholds will depend on sample quality and are usually estimated on per-sample basis. In our experiments, we exclude inferred doublets (usually 1%–5% of total cells) and include cells displaying between 10,000 and 120,000 unique nuclear fragments, and TSS enrichment > 8. These parameters are rather stringent and can be relaxed based on data distribution and sequencing depth. However, we advise excluding at least doublets and cells with < 1,000 unique nuclear fragments and TSS enrichment < 4.
-
i.
-
a.
-
2.For single-cell genotyping pre-processing, we use a previously published pipeline used for TARGET-seq3,6 (https://github.com/albarmeira/TARGET-seq):
-
a.The GenoDemux_Fastq.sh script will perform demultiplexing. As input, you need:
-
i.The generic “SampleSheet_byWell_TARGET.csv” file containing the 384 well barcodes inserted during genotyping PCR2.
-
ii.The metadata file containing information about plate and well barcode for each single cell. Here, it is important to keep cell IDs (names) consistent with the ones used for scATAC demultiplexing, to be able to later link genotypes to accessibility profiles of single cells. Troubleshooting 12.
-
i.
-
b.Once two FASTQ files have been generated per cell (R1 and R2), proceed with the SCgenotype.pl script, following the instructions. Note that, in GTAC, we do not use mRNA primers, hence you can leave the mRNA primer files blank. The pre-processing pipeline will generate a summary file of nucleotide counts per single cell, for each locus of interest.
-
c.These summary files are used as input for our custom genotyping analysis scripts (https://github.com/sventurkalj/GTAC/tree/v1.0.0):
-
i.We first compute the total locus coverage per cell and exclude cells with less than 30 reads across the locus.
-
ii.We then compute single cell variant allele frequencies (scVAF) and mutant read numbers across control WT cells to set thresholds for mutation calling based on these values.1 This will result in a cell being called mutant or WT at the locus.
-
iii.If multiple loci are screened, we repeat this procedure for all loci. Next, we integrate mutation calls across all loci for each single cell and generate a matrix in order to run infSCITE, which identifies the most likely clonal hierarchy. Note that alternative approaches for tree inference can be used.
-
iv.The second custom script then assigns each cell to a clone, based on the combination of mutations in that cell. Here, the conditions set for clonal assignment are driven by the infSCITE result. For a small proportion of cells, a clone cannot be confidently assigned due to failed genotyping on multiple loci – we exclude these cells from downstream analysis (for details refer to Turkalj, Jakobsen et al., 20231).
-
i.
-
a.
-
3.Note that, while the pre-processing pipeline can readily detect SNVs, indels may be challenging to count accurately.
-
a.In those cases, to get WT and mutant counts from single cells, you can apply the grep function in Unix directly to the FASTQ files, counting the number of WT and indel sequences observed in each file.
-
i.If the indel is covered both by R1 and R2, grep the WT and mutant sequences from both FASTQ files. Note that, for R2, you need to provide the reverse complement sequences.
-
ii.In those cases, the total WT and mutant coverage is given by the sum of reads from the R1 and R2 files.
-
i.
-
b.For specific indels, like FLT3-ITD, specialized pipelines12 can be adapted.
-
a.
-
4.Finally, clone information for each single cell is merged with its scATAC-seq metadata via cell IDs. This will allow clone-specific analysis of chromatin accessibility in downstream steps.
-
a.Our custom pipelines (https://github.com/sventurkalj/GTAC/tree/v1.0.0) provide examples of code used for downstream clone-specific analysis of chromatin accessibility.
-
a.
Limitations
At present, GTAC has several limitations. First, in terms of cell numbers, GTAC provides lower throughput compared to droplet-based strategies. At best, one tagmentation reaction on 50,000–100,000 cells can yield up to ten FACS sorted 384-well plates. If starting cell numbers are high, you can decide to use multiple tagmentation reactions, to sort more plates. Even if almost completely automated, the method requires around 5 days to fully process ten 384-well plates. However, the time required increases with increasing numbers of genotyped loci, given that the number of genotyping PCR1 and PCR2 reactions per cell increases. Altogether, data from 10,000–12,000 cells can be generated in a few weeks. However, most sorted nuclei usually pass QC filters, both for genotyping and scATAC-seq libraries, partially compensating for lower throughput. Other methods achieving higher throughput13 are available but currently suffer from lower genotyping sensitivity and scATAC-seq library content.
Second, GTAC relies on targeted analysis of mutations previously detected in the same samples by either targeted panels or whole exome sequencing of cell populations, meaning that a portion of the initial sample needs to be used for driver mutation discovery prior to GTAC.
Third, for genetically complex tumors, in which hundreds of mutations are present within the same sample, it would be necessary to limit GTAC to a subset of mutations. In our study,1 we successfully genotyped 7 genomic loci simultaneously in a primary sample. Notably, the scATAC-seq library shape was unaltered even when 10, 15, or 20 loci were amplified in parallel (Figure 17). However, applying GTAC to large numbers of loci would require further validation.
Figure 17.
Bioanalyzer traces showing ATAC libraries when 10, 15, or 20 genomic loci were co-amplified
Human CD34+ bone marrow cells were used. Each trace derives from 96 pooled single cells.
Finally, GTAC does not enable the capture of RNA from the same cells, limiting our understanding of how chromatin variation, associated with different mutations, alters gene expression. If multiple vials from the same sample are available, methods integrating single-cell genotyping or clonal inference with transcriptomic readouts can be applied,3,14,15,16,17 and scRNA and scATAC datasets can be integrated computationally. However, future methods integrating single-cell genotyping, chromatin accessibility, and transcriptome readouts from the same cells would be beneficial to tackle these limitations.
Troubleshooting
Problem 1
scATAC-seq library yield is very low and insufficient for sequencing (part 4, points 56–57; Figure 18A).
Potential solution
-
•
The number of PCR cycles (part 3, point 36) is insufficient. Increase the number of PCR cycles based on the results obtained in preparation one. If your scATAC-seq library yield is still low, if possible, perform a qPCR specifically on the problematic sample, to define the optimal number of cycles for that specific sample, using a few columns of one sorted plate.
-
•
The sorter is not properly calibrated (part 2, point 27), resulting in excessive empty wells. Calibrate carefully until the droplets are collected at the center of the well. You can also estimate the sorting accuracy by simulating a single-cell genotyping test on your sorted plate, as in preparation three (use 16–32 cells from few random columns) and checking how many wells show a genotyping band.
Problem 2
Bioanalyzer trace shows a large low-bp peak. ATAC trace is either of low intensity or absent (part 4, point 57; Figures 18B and 18C).
Potential solution
-
•
It is likely that some of your pre-amplification genotyping primers bind with high affinity to the i7 and/or i5 primers, thus interfering with scATAC-seq library amplification. Test all pre-amplification genotyping primers individually as described in preparation three, to identify the problematic pair. Avoid primers for which the predicted secondary structure of the heterodimer formed with the i7 or i5 primer displays ΔG < −9 kcal/mol (IDT Oligo Analyzer; preparation two). Make sure you are not using excessive primer concentration.
Problem 3
Bioanalyzer shows no ATAC trace at all (part 4, point 57; Figure 18D).
Potential solution
-
•
Make sure to use a Tn5 enzyme which is not expired. Make sure to add enough Tn5 enzyme to the tagmentation reaction (part 2, points 24g and 25).
-
•
Make sure to have sufficient MgCl2 in the tagmentation mix.
-
•
Make sure to include the 72°C gap-filling step in the PCR (part 3, point 36).
-
•
Make sure that the i7 and i5 primer sequences that you are using are correct.
-
•
Make sure the sorter is properly calibrated (refer to potential solution of problem 1).
Problem 4
ATAC trace displays only a prominent non-nucleosomal peak (Figure 18E) or only a non-nucleosomal peak and a lower, mono-nucleosomal peak (part 4, point 57; Figure 18F). This is likely to result in low library complexity.
Potential solution
-
•
Increase the elongation time of the PCR (part 3, point 36) up to 1 min. Some samples show a preponderance of non-nucleosomal compared to nucleosomal fragments and may require PCR optimization. Having only the non-nucleosomal peak will reduce library content.
-
•
This might also indicate over-tagmentation. Make sure to add 2× tagmentation stop buffer after tagmentation (part 2, point 26). Make sure that the EDTA concentration in the 2× tagmentation stop buffer is appropriate.
Problem 5
ATAC trace does not display the typical wavy shape: periodic drops of intensity are not prominent. (part 4, point 57; Figure 18G). This is likely to result in low library complexity. Sequencing may show a high percentage of mitochondrial reads. Signal-to-noise ratio (FRiP) may be low.
Potential solution
-
•
It is likely your detergent lysis conditions are suboptimal, leading to excessive lysis and disruption of nucleosomes prior to tagmentation. Remake all detergent solutions, taking care not to add excessive detergents.
-
•
Make sure your thermal mixer is set at 37°C during tagmentation.
-
•
When resuspending cells and nuclei, pipette gently not to disrupt the structure of cells or nuclei.
Problem 6
ATAC trace displays a very intense high-bp peak (part 4, point 57; Figures 18H and 18I).
Potential solution
Problem 7
A very low number of nuclei in the tube prior to single-nucleus sorting (part 2, point 28).
Potential solution
-
•
Take extreme care when aspirating the pellets during the Omni-ATAC procedure (part 2, point 24). Do not disturb pellets. Make sure to use a fixed-angle centrifuge. If you can’t see the nuclear pellet, leave a few μL of supernatant inside the tube.
Problem 8
Many non-lysed cells prior to single-nucleus sorting (part 2, point 28).
Potential solution
-
•
Make sure to use the correct concentration of all detergents, and to lyse the cells for 3 min, when performing the Omni-ATAC procedure (part 2, point 24).
Problem 9
Sequencing shows many empty wells (quantification and statistical analysis).
Potential solution
Problem 10
Sequencing shows excessive mitochondrial read percentage (quantification and statistical analysis).
Potential solution
-
•
Collect all, or almost all, supernatant when aspirating liquid during the Omni-ATAC procedure (part 2, point 24h). Mitochondrial DNA likely resides mostly in the supernatant.
Problem 11
Many cells show a successful scATAC-seq library but poor or failed genotyping at one or more loci (quantification and statistical analysis).
Potential solution
-
•
This indicates that, while the sort and scATAC-seq library construction went well, there is a problem in genotyping library construction. Revisit your primer testing results (preparation two and preparation three) and evaluate genotyping bands for the problematic loci. If bands are faint, design new primer pairs and select those that generate bright bands.
-
•
Increase pre-amplification genotyping primer concentration up to 400 nM and test whether bands are brighter (preparation three, point 15).
-
•
Change the organization of your genotyping PCR1 reactions: if there is one problematic locus, amplify that locus separately from the remaining loci, in a separate genotyping PCR1 reaction.
Problem 12
Empty control wells show many sequencing reads, and/or the control WT sample shows many mutant reads, on one or several plates (quantification and statistical analysis).
Potential solution
-
•
This likely indicates cross-well contamination in those plates. Data from these plates should not be used and cannot be trusted. Always centrifuge plates prior to unsealing. When unsealing, take care not to generate any liquid splashes (keep the plate firmly against the bench). Always centrifuge plates at 21°C to avoid condensation of the seals.
-
•
Alternatively, you might be assigning sequencing reads to the wrong wells (due to a mistake in the sample sheet or in the genotyping metadata; quantification and statistical analysis).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Paresh Vyas (paresh.vyas@imm.ox.ac.uk).
Materials availability
The list of previously validated target-specific genotyping primers1 can be found in Table S1. The list of barcoded i73 and i5 oligo sequences used for scATAC-seq library amplification can be found in Table S2.
Acknowledgments
P.V. acknowledges funding from the Medical Research Council Molecular Haematology Unit Programme Grant (MC_UU_00029/8), Blood Cancer UK Programme Continuity Grant 13008, NIHR Senior Fellowship, and the Oxford BRC Haematology Theme. S.T. is supported by a Scatcherd European Scholarship in partnership with the Medical Research Council/Radcliffe Department of Medicine and the Clarendon Fund. N.A.J. is supported by a Medical Research Council and Leukaemia UK Clinical Research Training Fellowship (MR/R002258/1). A.G. is supported by DPhil studentship funding from the Lady Tata Memorial Trust. F.A.R. is supported by the Oxford – Sir David Weatherall Scholarship from Green Templeton College and the WIMM Prize Studentship from the MRC Weatherall Institute of Molecular Medicine. The authors thank Dr. Felix Dingler, Dr. Assunta Adamo, and Mr. Ramy Slama for insightful discussions. The authors also acknowledge the MRC WIMM Flow Cytometry Facility and Single Cell Facility. Figures, or part of Figures 1, 3, 5, 7, 13, 14, and 16, as well as the Graphical abstract, were created using BioRender.
Author contributions
Conceptualization, S.T., N.A.J., and P.V.; methodology, S.T., N.A.J., and A.G.; investigation, S.T., N.A.J., and A.G.; formal analysis, S.T. and N.A.J.; visualization, S.T. and F.A.R.; supervision, P.V.; writing – original draft, S.T., N.A.J., A.G., and F.A.R.; writing – review and editing, all authors.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102641.
Contributor Information
Sven Turkalj, Email: sven.turkalj@rdm.ox.ac.uk.
Paresh Vyas, Email: paresh.vyas@imm.ox.ac.uk.
Supplemental information
Data and code availability
The code for GTAC analysis can be accessed at GitHub: https://github.com/sventurkalj/GTAC/tree/v1.0.0. The code is also provided in an open access disposition at Zenodo: https://doi.org/10.5281/zenodo.7817225. Python and Perl scripts used for scATAC-seq or single-cell genotyping data pre-processing were published previously2,3 and are available at the respective GitHub pages. DOIs are listed in the key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Turkalj S., Jakobsen N.A., Groom A., Metzner M., Riva S.G., Gür E.R., Usukhbayar B., Salazar M.A., Hentges L.D., Mickute G., et al. GTAC enables parallel genotyping of multiple genomic loci with chromatin accessibility profiling in single cells. Cell Stem Cell. 2023;30:722–740.e11. doi: 10.1016/j.stem.2023.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Chen X., Miragaia R.J., Natarajan K.N., Teichmann S.A. A rapid and robust method for single cell chromatin accessibility profiling. Nat. Commun. 2018;9:5345. doi: 10.1038/s41467-018-07771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Meira A., Buck G., Clark S.A., Povinelli B.J., Alcolea V., Louka E., McGowan S., Hamblin A., Sousos N., Barkas N., et al. Unravelling Intratumoral Heterogeneity through High-Sensitivity Single-Cell Mutational Analysis and Parallel RNA Sequencing. Mol. Cell. 2019;73:1292–1305.e8. doi: 10.1016/j.molcel.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu W., Wen Y., Liang Y., Xu Q., Wang X., Jin W., Chen X. A plate-based single-cell ATAC-seq workflow for fast and robust profiling of chromatin accessibility. Nat. Protoc. 2021;16:4084–4107. doi: 10.1038/s41596-021-00583-5. [DOI] [PubMed] [Google Scholar]
- 5.Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., Madden T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinf. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Meira A., O'Sullivan J., Rahman H., Mead A.J. TARGET-Seq: A Protocol for High-Sensitivity Single-Cell Mutational Analysis and Parallel RNA Sequencing. STAR Protoc. 2020;1 doi: 10.1016/j.xpro.2020.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granja J.M., Corces M.R., Pierce S.E., Bagdatli S.T., Choudhry H., Chang H.Y., Greenleaf W.J. ArchR is a scalable software package for integrative single-cell chromatin accessibility analysis. Nat. Genet. 2021;53:403–411. doi: 10.1038/s41588-021-00790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahn K., Kuipers J., Beerenwinkel N. Tree inference for single-cell data. Genome Biol. 2016;17:86. doi: 10.1186/s13059-016-0936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu W., Yang W., Zhang Y., Chen Y., Hong N., Zhang Q., Wang X., Hu Y., Song K., Jin W., Chen X. ISSAAC-seq enables sensitive and flexible multimodal profiling of chromatin accessibility and gene expression in single cells. bioRxiv. 2022 doi: 10.1101/2022.01.16.476488. Preprint at. [DOI] [PubMed] [Google Scholar]
- 10.Corces M.R., Trevino A.E., Hamilton E.G., Greenside P.G., Sinnott-Armstrong N.A., Vesuna S., Satpathy A.T., Rubin A.J., Montine K.S., Wu B., et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods. 2017;14:959–962. doi: 10.1038/nmeth.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuart T., Srivastava A., Madad S., Lareau C.A., Satija R. Single-cell chromatin state analysis with Signac. Nat. Methods. 2021;18:1333–1341. doi: 10.1038/s41592-021-01282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blätte T.J., Schmalbrock L.K., Skambraks S., Lux S., Cocciardi S., Dolnik A., Döhner H., Döhner K., Bullinger L. getITD for FLT3-ITD-based MRD monitoring in AML. Leukemia. 2019;33:2535–2539. doi: 10.1038/s41375-019-0483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers R.M., Izzo F., Kottapalli S., Prieto T., Dunbar A., Bowman R.L., Mimitou E.P., Stahl M., El Ghaity-Beckley S., Arandela J., et al. Integrated Single-Cell Genotyping and Chromatin Accessibility Charts JAK2 V617F Human Hematopoietic Differentiation. bioRxiv. 2022 doi: 10.1101/2022.05.11.491515. Preprint at. [DOI] [Google Scholar]
- 14.Nam A.S., Kim K.T., Chaligne R., Izzo F., Ang C., Taylor J., Myers R.M., Abu-Zeinah G., Brand R., Omans N.D., et al. Somatic mutations and cell identity linked by Genotyping of Transcriptomes. Nature. 2019;571:355–360. doi: 10.1038/s41586-019-1367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velten L., Story B.A., Hernández-Malmierca P., Raffel S., Leonce D.R., Milbank J., Paulsen M., Demir A., Szu-Tu C., Frömel R., et al. Identification of leukemic and pre-leukemic stem cells by clonal tracking from single-cell transcriptomics. Nat. Commun. 2021;12:1366. doi: 10.1038/s41467-021-21650-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beneyto-Calabuig S., Merbach A.K., Kniffka J.-A., Antes M., Szu-Tu C., Rohde C., Waclawiczek A., Stelmach P., Gräßle S., Pervan P., et al. Clonally resolved single-cell multi-omics identifies routes of cellular differentiation in acute myeloid leukemia. Cell Stem Cell. 2023;30:706–721.e8. doi: 10.1016/j.stem.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Jakobsen N.A., Turkalj S., Zeng A.G.X., Stoilova B., Metzner M., Nagree M.S., Shah S., Moore R., Usukhbayar B., Salazar M.A., et al. Selective advantage of mutant stem cells in clonal hematopoiesis occurs by attenuating the deleterious effects of inflammation and aging. bioRxiv. 2023 doi: 10.1101/2023.09.12.557322. Preprint at. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code for GTAC analysis can be accessed at GitHub: https://github.com/sventurkalj/GTAC/tree/v1.0.0. The code is also provided in an open access disposition at Zenodo: https://doi.org/10.5281/zenodo.7817225. Python and Perl scripts used for scATAC-seq or single-cell genotyping data pre-processing were published previously2,3 and are available at the respective GitHub pages. DOIs are listed in the key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.